Abstract
Sall3 is a zinc finger containing putative transcription factor and a member of the Sall gene family. Members of the Sall gene family are highly expressed during development. Sall3-deficient mice die in the perinatal period because of dehydration and display alterations in palate formation and cranial nerve formation (Parrish et al. [2004] Mol Cell Biol 24:7102–7112). We examined the role of Sall3 in the development of the olfactory system. We determined that Sall3 is expressed by cells in the olfactory epithelium and olfactory bulb. Sall3 deficiency specifically alters formation of the glomerular layer. The glomerular layer was hypocellular, because of a decrease in the number of interneurons. The lateral ganglionic eminence and rostral migratory stream developed normally in Sall3-deficient animals, which suggests that Sall3 is not required for the initial specification of olfactory bulb interneurons. Fewer GAD65/67-, Pax6-, calretinin-, and calbindin-positive cells were detected in the glomerular layer, accompanied by an increase in cells positive for these markers in the granule cell layer. In addition, a complete absence of tyrosine hydroxylase expression was observed in the olfactory bulb in the absence of Sall3. However, expression of Nurr1, a marker of dopaminergic precursors, was maintained, indicating that dopaminergic precursors were present. Our data suggest that Sall3 is required for the terminal maturation of neurons destined for the glomerular layer.
Indexing terms: Spalt, 18q deletion syndrome, tyrosine hydroxylase, olfactory bulb
The olfactory bulb (OB) is a laminar structure containing two distinct neuronal populations. Excitatory projection neurons populate the mitral cell layer (MCL), and interneuron populations are located in the glomerular layer (GL) and granule cell layer (GCL), superficial and deep to the MCL respectively. OB neuronal populations are sequentially produced and have distinct origins. Mitral cells are the firstborn OB population (Hinds, 1968). These cells arise in the pallium, and the transcription factor Tbr1 is required for their generation (Bulfone et al., 1998; Moreno et al., 2003; Puelles et al., 2000). OB interneurons arise from an ER81-positive population in the subpallial dorsal lateral ganglionic eminence (LGE) and migrate to the OB via the rostral migratory stream (RMS; Doetsch and Alvarez-Buylla, 1996; Lois and Alvarez-Buylla, 1994; Stenman et al., 2003; Wichterle et al., 1999, 2001).
Previous studies determined that OB interneurons are continuously generated throughout life (Altman, 1969; Altman and Das, 1966; Doetsch and Alvarez-Buylla, 1996; Hinds, 1968; Lois and Alvarez-Buylla, 1994; Luskin, 1993); however, mechanisms that regulate the generation of this diverse cellular population are not well characterized. OB interneurons begin to express mature interneuron markers as they terminally differentiate and radially migrate in the OB. The OB comprises chemically distinct interneuron populations; at least three subtypes have been identified characterized by γ-aminobutyric acid (GABA), calretinin, and calbindin expression (Kosaka et al., 1995; Toida et al., 2000). Cells in the GCL homogenously express GABA (Parrish-Aungst et al., 2007). Within the GL, GABA, calretinin, and calbindin are expressed by ~20–53%, ~20–27%, and ~10% of neurons, respectively (Kosaka et al., 1995; Parrish-Aungst et al., 2007). The remaining interneuron populations in this layer have not yet been chemically distinguished. The GABAergic population can be further divided into dopaminergic and nondopaminergic populations, characterized by the expression of tyrosine hydroxylase (TH), the rate-limiting enzyme in dopamine biosynthesis; ~80% of dopaminergic cells are also GABAergic (Hack et al., 2005; Kosaka et al., 1998; Parrish-Aungst et al., 2007), and 95% of TH-positive cells in the GL are Pax6-positive (Hack et al., 2005). Little overlap is observed between the TH/Pax6 populations and the calretinin (~2% overlap with Pax6)/calbindin (~10% overlap with Pax6)-positive populations (Hack et al., 2005; Kosaka et al., 1995, 1998; Waclaw et al., 2006). In addition, examination of the temporal specification of OB interneurons has identified overlap in the timing of birth of these chemically diverse populations (Tucker et al., 2006). These findings suggest that intrinsic factors play an important role in OB interneuron specification, insofar as diverse interneuron populations born at the same time would be exposed to similar extrinsic cues.
Insight into the mechanisms that regulate production of interneuron populations destined for the OB has been obtained from analyses of murine knockout models (Soria et al., 2004; Stenman et al., 2003; Waclaw et al., 2006; Yoshihara et al., 2005; Yun et al., 2003). These studies have demonstrated that interneuron generation can be divided into the following steps; specification of interneuron progenitors within the LGE, entry from the LGE to the RMS, migration in the RMS, exit from the RMS to the OB, radial migration within the OB, and terminal differentiation. The transcription factors Sp8 (Waclaw et al., 2006) and GSH1/2 (Stenman et al., 2003; Yun et al., 2003) are required for the initial specification of OB progenitors in the LGE. Vax1 is necessary for the transition of cells from the LGE to the RMS (Soria et al., 2004). The secreted protein Slit has been implicated in the migration of inter-neurons via the RMS (Chen et al., 2001; Hu, 1999; Wu et al., 1999), and the homeobox protein ARX is required for the entry of interneurons from the RMS to the OB (Yoshihara et al., 2005). Furthermore, Pax6 has been implicated in the generation of dopaminergic OB interneurons (Hack et al., 2005; Kohwi et al., 2005). Thus, we are beginning to understand components of OB cell type generation and migration within the RMS; however, mechanisms regulating terminal differentiation and maturation within the OB are not well characterized.
We examined the role of Sall3 in the development of the olfactory system. Sall3 is one of four mammalian members of the Sall gene family, which are widely expressed throughout development, in the peripheral and central nervous system as well as in peripheral organs (Al-Baradie et al., 2002; Buck et al., 2000, 2001; Kohlhase et al., 1996, 1999, 2000, 2002a,b; Ott et al., 1996, 2001). Distinct developmental disorders with some overlapping characteristics are associated with mutation or deletion of members of this gene family in humans (Al-Baradie et al., 2002; Kohlhase et al., 1998, 1999, 2002b). SALL3 is one of several genes deleted in 18q deletion syndrome, characterized by hearing loss, mental retardation, midfacial hypoplasia, delayed growth, and limb abnormalities (Jayarajan et al., 2000; Kohlhase et al., 1999; Mahr et al., 1996; Strathdee et al., 1997; Verhoeven et al., 2006; Wilson et al., 1979). The Sall genes are zinc finger-containing putative transcription factors (Kuhnlein et al., 1994). They have been shown to localize to heterochromatin (Netzer et al., 2001; Sakaki-Yumoto et al., 2006; Sato et al., 2004), and it is hypothesized that they can mediate transcriptional repression via recruitment of histone deacetylase (Kiefer et al., 2002; Lauberth and Rauchman, 2006). Members of the Sall gene family can interact at the protein level (Kiefer et al., 2002; Sakaki-Yumoto et al., 2006; Sweetman et al., 2003) and have been implicated in diverse cellular processes, including cell cycle regulation, cell fate specification, neuronal differentiation, migration, and cell adhesion (Barembaum and Bronner-Fraser, 2004; Bohm et al., 2007; Cantera et al., 2002; de Celis et al., 1999; Franch-Marro and Casanova, 2002; Jurgens, 1988; Kuhnlein and Schuh, 1996; Toker et al., 2003). These data suggest the members of the Sall gene family are important developmental regulators.
We have previously shown that Sall3 (previously named msal1) is expressed during olfactory development (Ott et al., 1996, 2001), although a detailed description has not been given. We determined that Sall3 was expressed by cells within the OB and olfactory epithelium. Within the OB, Sall3 was expressed by progenitor cells and subpopulations of differentiated neurons. Sall3-deficient animals die perinatally (Parrish et al., 2004), and we examined development of the olfactory system to P0.5. We identified alterations in interneuron development in Sall3 mutant animals. Our data suggest that Sall3 is required for the terminal maturation of GL interneurons in the developing OB.
Materials and Methods
Animals
Sall3 heterozygous animals were obtained from matings of Sall3 heterozygous mice (129SVJ × C57BL/6J) from the twelfth generation back-cross to C57BL/6J (Parrish et al., 2004). Embryos were obtained from matings of Sall3 heterozygote animals and genotyped as previously described (Parrish et al., 2004). For Sall3 expression studies, embryos were obtained from timed-pregnant CD1 mice from Charles Rivers Laboratories (Wilmington, MA). Embryos were collected via cesarean section at embryonic ages from E13.5 to E18.5. The day of vaginal plug was designated as E0.5; the first postnatal day was designated P0.5. Embryos were fixed in either 4% paraformaldehyde (PFA; pH 7.4; Sigma, St. Louis, MO) and processed through increasing sucrose gradients for cryosectioning, or in Carnoy’s solution (1:3:6 acetic acid:chloroform:100% alcohol), and then processed through a butanol series for paraffin sectioning. P0.5 pups were first perfused with 4% paraformaldehyde (PFA) and then processed as described above. Cryopreserved embryos were embedded in Tissue-Tek O.C.T. compound (EMS, Hatfield, PA) and sectioned at 20 µm. Paraffin-embedded embryos were sectioned at 10 µm. No alterations in the olfactory system were observed in Sall3+/− animals in our study (data not shown), so these animals were also used as controls. Animal protocols and procedures were approved by the Institutional Animal Care and Use Committee at the University of Pittsburgh and adhered to the National Institutes of Health guidelines.
Measurement of OB size
The cortex and OB of P0.5 pups were dissected from the head and visualized on a Nikon (Melville, NY) dissecting microscope, and photographed with a Photometrics (North Reading, MA) Cool Snap digital camera and IP Lab software (Biovision Technologies, Exton, PA). Images were imported into Photoshop 7.0 (Adobe Systems, San Jose, CA) to measure the length of the OB. Statistical analysis of the results was performed using an unpaired t-test with InStat 3 software (GraphPad Software, San Diego, CA).
Immunohistochemisty
Paraffin-processed sections were deparaffinized in xylene, rehydrated through an ethanol series, and washed in 0.1% Triton in phosphate-buffered saline (PBS), pH 7.4. For diaminobenzidine detection, sections were incubated with 3% hydrogen peroxide in methanol for 10 minutes. Slides were subsequently microwaved in 0.1 M sodium citrate solution, pH 6.0, rinsed in PBS, and blocked in 10% heat-inactivated goat serum (Jackson Immunoresearch, West Grove, PA). Primary antibodies were incubated overnight at 4°C. Cryopreserved tissue was rinsed in PBS, blocked in 10% heat-inactivated goat serum, and incubated with primary antibody overnight at 4°C. Antibodies used are listed in Table 1. For each antibody used, a negative control was included, where the sections were incubated without the primary antibody but with the secondary antibody. The tissue was subsequently washed with PBS, incubated with the appropriate biotinylated secondary antibody (Vector Laboratories, Burlingame, CA), and then incubated with a Vectastain Elite ABC kit (Vector Laboratories), according to the manufacturer’s instructions. Staining was visualized by using the nickel-enhanced diaminobenzidine (Sigma) reaction. Sections were counterstained with hematoxylin and eosin (Fisher Scientific, Pittsburgh, PA) or nuclear fast red (Vector Laboratories). Slides were then dehydrated through ethanol, washed in xylene, and mounted in DPX (Fluka, Sigma). For fluorescent detection of signal, the tissue was washed with PBS, incubated with the appropriate Cy3 (Jackson Immunoresearch) and/or Alex Fluor 488 (Invitrogen, Carlsbad, CA) secondary antibody, and counterstained with 1,6-diamidino-2-phenylindole dihydrochloride (DAPI; Sigma) before mounting in fluormount G (Southern Biotechnology Research, Birmingham, AL). For Nissl staining, sections were incubated in 0.5% cresyl violet (Sigma), dehydrated through alcohols, washed in xylene, and mounted in DPX.
TABLE 1.
Antibodies Used in This Study
| Antibody | Species | Concentration | Source | Catalog No. |
|---|---|---|---|---|
| β-Galactosidase | Chicken (polyclonal) | 1:500 | Abcam, Cambridge, MA | Ab9361 |
| c-Fos (Ab-5) | Rabbit (polyclonal) | 1:20,000 | Oncogene, San Diego, CA | PC38 |
| Calretinin | Rabbit (polyclonal) | 1:1,000 | Chemicon, Temecula, CA | AB5054 |
| Calbindin | Rabbit (polyclonal) | 1:1,000 | Abcam | Ab11426 |
| ER81 | Rabbit (polyclonal) | 1:5,000 | Covance, Berkeley, CA | PRB-362C |
| GAD65/67 | Rabbit (polyclonal) | 1:10,000 | Sigma, St. Louis, MO | G 5163 |
| Neurofilament 160 | Mouse (monoclonal) | 1:200 | Sigma | N5264 |
| Pax6 | Rabbit (polyclonal) | 1:500 | Covance | PRB-278P |
| Reelin | Mouse (monoclonal) | 1:500 | Abcam | Ab18569 |
| Sall1 | Mouse (monoclonal) | 1:500 | R&D Systems, Minneapolis, MN | 2ZK9814H |
| Tuj1 | Rabbit (polyclonal) | 1:1,000 | Sigma | T-2200 |
| Tyrosine hydroxylase | Mouse (monoclonal) | 1:5,000 | Sigma | T1299 |
Antibody specificity
The β-galactosidase antibody was raised against purified full-length native protein (Escherichia coli; manufacturer’s technical information). Antibody specificity was verified by the absence of staining in Sall3+/+ animals, which do not contain the β-galactosidase gene (data not shown). The c-Fos antibody was raised against a synthetic peptide (SGFNADYEASSSRC) corresponding to amino acids 4–17 of human c-Fos, and it recognizes the ~55 kDa c-Fos and ~62 kDa v-Fos proteins (manufacturer’s technical information). Previous studies have determined that preabsorption of the c-Fos antibody with a c-Fos peptide eliminates immunoreactivity (Wahlin et al., 2000). c-Fos, an immediate early transcription factor, labels cells in the GL, MCL, and GCL of the OB (Akiba et al., 2007; Guthrie and Gall, 1995). The calretinin antibody was raised against recombinant rat calretinin that recognizes both the calcium-bound and calcium-unbound conformations of calretinin (manufacturer’s technical information). The antibody detects a 32-kDa band by Western blot of purified recombinant calretinin protein (manufacturer’s technical information), and neurons in cerebral cortical layers II/III are immunopositive for this antibody, consistent with calretinin immunoreactivity (Lee et al., 2004; Schmid et al., 2004). Calretinin is expressed by GL, MCL, and GCL cells in the OB (Brinon et al., 1999). The calbindin antibody was raised against 28-kDa calbindin-D protein purified from rat kidney (manufacturer’s technical information). Antibody specificity was confirmed by immunoblot of purified rat calbindin, which detects a single band at 28 kDa (Kurobe et al., 1992). Calbindin is expressed by cells in the GL of the OB (Baimbridge and Miller, 1982; Brinon et al., 1999; Celio, 1990). The ER81 antibody was raised against a mouse ER81 peptide (CNPHPYNEGYVY; manufacturer’s technical information). Antibody specificity was determined by immunohistochemistry staining of COS cells expressing ER81 (Lin et al., 1998). ER81 is expressed by interneurons in the GL and GCL of the OB (Saino-Saito et al., 2007; Stenman et al., 2003). The glutamic acid decarboxylase 65/67 (GAD65/67) antibody was raised against a synthetic peptide corresponding to the C-terminal region of human GAD67 (amino acids 579–594). Anti-GAD65/67 reacts specifically with GAD65 and −67 isoforms (65–67 kDa) derived from rat brain extract by immunoblotting, which is inhibited by incubation with GAD65/67 peptide (manufacturer’s technical information). GAD is expressed by cells in the GL, MCL, and GCL within the adult OB (Parrish-Aungst et al., 2007). The Neurofilament 160 antibody (clone NN18) was produced by fusion of mouse myeloma cells and splenocytes from an immunized mouse using neurofilaments purified from pig spinal cord as the immunogen (manufacturer’s technical information). This antibody specifically stains neurofilaments (Franke et al., 1991), and immunoblot of rat brain extract detects a band of molecular weight 160 kDa (manufacturer’s technical information). The Pax6 antibody was raised against a peptide (QVPGSEPDMSQYWPRLQ) derived from the c-terminus of the mouse Pax6 protein (manufacturer’s technical information). The Pax6 antibody detects two bands of ~50 kDa by immunoblot of tissue from the adult brain, eye, and OB, but not the liver, consistent with previous studies of Pax6 expression (Davis and Reed, 1996; Walther and Gruss, 1991). Pax6 is strongly expressed by interneurons in the GL but is down-regulated by differentiating GCL interneurons within the adult OB (Hack et al., 2005; Stoykova and Gruss, 1994). The epitope for the reelin antibody (clone 142) is located within amino acids 164–189 of the reelin protein, and this antibody specifically labels reelin-expressing Cajal-Retzius cells in the cerebral cortex (de Bergeyck et al., 1998). In the developing OB, reelin is specifically expressed by cells in the MCL (Alcantara et al., 1998). The Sall1 antibody was raised against recombinant human SALL1 (amino acids 258–499; manufacturer’s technical information). Antibody specificity was verified by an absence of staining in Sall1-deficient mice (unpublished observations; Harrison et al., 2007). The Tuj1 antibody was raised against a synthetic peptide corresponding to amino acid residues 441–450 of human β-tubulin III. The Tuj1 antibody recognizes a band of 55 kDa by immunoblotting of whole-mouse-brain extract, which is inhibited by incubation with the immunizing peptide (manufacturer’s technical information). Tuj1 is expressed by neurons shortly after they exit the cell cycle and is maintained in migrating and mature neurons (Lee et al., 1990a,b; Menezes and Luskin, 1994). The TH antibody (clone TH-2) was raised against rat TH and recognizes a 60-kDa band by immunoblotting of rat adrenal phenochromocytoma cell extract (manufacturer’s technical information). This antibody has been previously used to examine TH expression in the OB (Soria et al., 2004). TH is expressed by dopaminergic neurons in the GL of the OB, and the morphology and pattern of staining of clone TH-2 match previous reports of TH expression in the OB, cortex, and midbrain (Halasz et al., 1977; Jacobowitz and Abbott, 1998).
In situ hybridization
The Sall3 probe has previously been described (Ott et al., 2001). To generate a Nurr1 probe, RNA was obtained from the cerebral cortex of an E18.5 wild-type embryo using the RNeasy Protect Mini Kit (Qiagen, Valencia, CA) and reversed transcribed using Advantage RT-for-PCR Kit (Clontech, Palo Alto, CA), according to the manufacturer’s instructions. Nurr1 was amplified from this cDNA using the Advantage cDNA PCR Kit (Clontech) with primers (forward: 5′-CCCGCTTCTCAGAGCTACAG-3′ and reverse: 5′-AGACCCTCATTGGAGGGAGA-3′) designed against nucleotides 5587–6234 of the Mus musculus Nurr1 gene (MMU86783). This PCR product was cloned into pCR-II-Topo (Invitrogen), according to the manufacturer’s instructions. The Nurr1 clone was verified by sequencing. For preparation of antisense digoxigenin-labeled riboprobes, Nurr1 template DNA was linearized with BamH1 and transcribed with T7 RNA polymerase according to the manufacturer’s instructions (Roche Applied Science, Indianapolis, IN). In situ hybridization was conducted as previously described (Schaeren-Wiemers and Gerfin-Moser, 1993), with the following modifications. The sections were treated with proteinase K, 1 µg/ml in 50 mM Tris, pH 7.5, and 5 mM EDTA, at 37°C for 3 minutes (E13.5–E16.5), 7 minutes (E17.5–P0.5) or 15 minutes (adult), followed by postfixation in 4% PFA prior to pre-hybridization. The hybridization buffer was modified to contain 50% formamide, 5× SSC, 5× Denhardt’s solution, 250 µg/ml tRNA, and 250 µg/ml herring sperm DNA. The probe was dissolved at a concentration of 60 ng/ml in hybridization buffer. Hybridization and posthybridization washes were performed at 61°C. A posthybridization treatment with RNase A, 20 µg/ml in 0.5 M NaCl, 10 mM Tris, pH 7.5, 5 mM EDTA buffer, at 37°C for 30 minutes was also added. After color detection, slides were rapidly dehydrated, washed in xylene, and mounted in DPX.
Illustrations
Stained sections were visualized on a Nikon (Melville, NY) fluorescent microscope and photographed with a Photometrics Cool Snap digital camera and IP Lab software. Composite images were prepared in Photoshop 7.0. Contrast, color, and brightness were adjusted in Photoshop 7.0.
Histological identification of OB regions
To assign cellular subtypes and boundaries to OB regions, sections were examined at ×40 magnification. The progenitor population was identified as the cellular region adjacent to the ventricle containing organized parallel arrays of cells with elongated nuclei arranged perpendicular to the ventricular surface. The differentiating population was identified as a region of heavily stained, small cells with round nuclei arranged in a disorganized manner, adjacent to the progenitor population. The MCL was evident from E16.5 and contained cells with large, oval nuclei, perpendicular to the ventricular surface and superficial to the differentiating field. From P0.5, the GL and GCL were identifiable. The GL was defined as the region containing round cells between the MCL and the olfactory nerve layer. The olfactory nerve layer was identified by the presence of elongated cells oriented parallel to the ventricular surface and perpendicular to the MCL. The GL as defined in this study encompasses the GL and the external plexiform layer. The GCL was located on the ventricular side of the MCL and contained small, heavily stained, round cells.
Quantification of glomerular cell number
For quantification of cell number within the GL, P0.5 Nissl-stained OB sections were photographed at ×40 magnification, as described above. The analysis was performed by an observer blind to the genotype. The GL was defined as described above. The boundary of the MCL and outer nerve layer were distinguished at ×200. The total number of cells in a 150-µm-wide window within this region was quantified and analyzed as previously described (Roy et al., 2004). Three sections per animal were counted, and the means of the three sections per animal were compared between genotypes (n = 4 per genotype). The data were subsequently decoded, and statistical analysis of the results was performed using an unpaired t-test in InStat 3 software.
Results
Sall3 is expressed by olfactory progenitors and interneuron populations
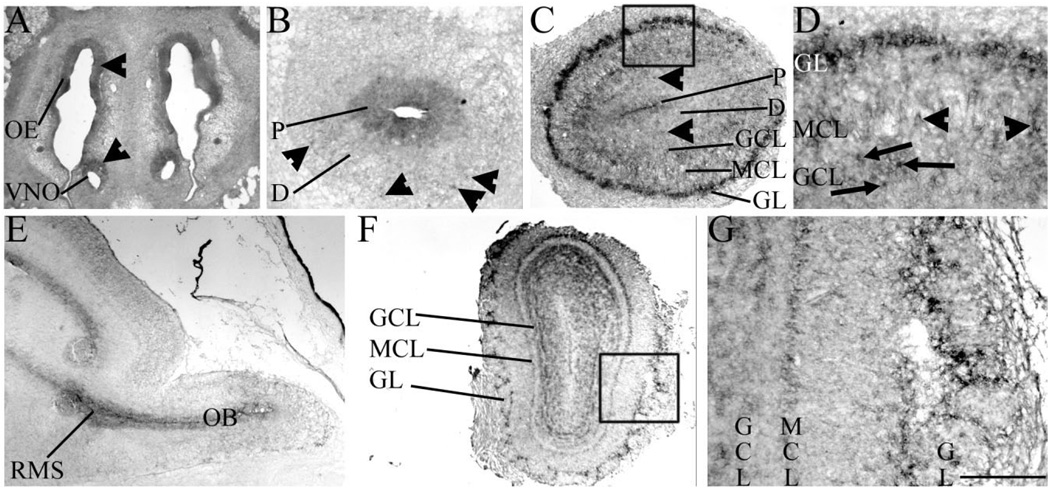
Previous studies have shown that Sall3 is expressed in the developing OB (Ott et al., 1996, 2001); however, a detailed temporal expression pattern has not been described. To understand more precisely the role of Sall3 in the olfactory system, we first characterized its expression in the developing and adult OB by in situ hybridization. OB neurogenesis is initiated on E11.5 in the mouse. At this time, peripheral projections from the olfactory placode contact progenitor cells in the rostral neural epithelium (Gong and Shipley, 1995). These projections are believed to induce differentiation of this population of progenitor cells and to induce OB morphogenesis (Gong and Shipley, 1995). Sall3 was not expressed by cells in the olfactory placode (Ott et al., 2001). The olfactory placode gives rise to components of the peripheral nervous system, such as the vomeronasal organ and olfactory epithelium. By E13.5, Sall3 was expressed by a subpopulation of cells in these structures (arrowheads, Fig. 1A). Expression of Sall3 in the neural epithelium of the rostral most tip of the developing telencephalon was not detected until E11.5 (Ott et al., 1996, 2001). From E13.5, Sall3 was expressed by OB progenitors (P, Fig. 1B) and expression was maintained in this population throughout development (P, Fig. 1C).
Fig. 1.
Sall3 is expressed by olfactory progenitors and interneuron populations. In situ hybridization of Sall3 expression in the developing (A–E) and adult (F,G) olfactory system in coronal (A–D,F,G) and sagittal (E) sections. Sall3 was expressed in the OE and VNO at E13.5 (arrowheads, A). In the olfactory bulb, Sall3 was expressed by progenitor cells and a subpopulation of differentiating (arrowheads) cells at E13.5 (B) and E17.5 (C). At E17.5, Sall3 was additionally expressed by cells in the GL, MCL (arrowheads), and GCL (arrows; D). Sall3 expression was observed in the RMS at E16.5 (E). Expression of Sall3 was maintained in the GL, MCL, and GCL in the adult (F,G). D is the boxed area in C; G is the boxed area in F. OE, olfactory epithelium; VNO, vomeronasal organ; P, progenitor populations; D, differentiating field; GCL, granule cell layer; MCL, mitral cell layer; GL, glomerular layer; RMS, rostral migratory stream; OB, olfactory bulb. Scale bar = 400 µm for A,E; 350 µm for C; 100 µm for D; 800 µm for F; 200 µm for B,G.
Two major olfactory neuronal subtypes are sequentially produced during development, excitatory projection neurons and inhibitory interneurons. Excitatory neurons are born from E11.5 to E13.5 and populate the MCL (Hinds, 1968). At E13.5, Sall3 was expressed by a small subset of differentiating cells destined for the MCL (arrowheads, Fig. 1B). By E17.5, Sall3 was expressed by a few cells in the MCL (arrowheads, Fig. 1D). Inhibitory interneurons arise from an ER81-positive population in the LGE and are born from E14.5 (Hinds, 1968; Stenman et al., 2003). In the developing cerebral cortex, expression of Sall3 is initially restricted to the ventral telencephalon, and, from E12.5, strong expression is observed in the LGE (Ott et al., 1996, 2001). After initial specification within the LGE, neuroblasts destined for the OB migrate to the OB via the RMS. Sall3 expression was observed in the RMS during development (Fig. 1E). Within the OB, interneuron populations are located in the GCL and GL. At E17.5, interneurons destined for these layers are differentiating, and a subset has already reached their final laminar position. Similar to the case at E13.5, relatively few differentiating cells expressed Sall3 at E17.5 (arrowheads, Fig. 1C). However, Sall3 was robustly expressed by a large number of cells in the GL (Fig. 1C,D) and a subpopulation of cells in the GCL (arrows, Fig. 1D). Expression of Sall3 was maintained in the MCL, GCL, and GL of the adult OB (Fig. 1F,G). In summary, Sall3 was expressed in developing olfactory structures from E11.5. Expression was observed in OB progenitors during development and in subpopulations of differentiated cells, predominantly by cells in the GL. These data suggest a role for Sall3 in the development and maintenance of olfactory structures.
The glomerular layer is hypocellular in the absence of Sall3
Sall3 was expressed in the developing and adult olfactory system. To determine whether Sall3 was required for distinct phases of OB development, mice with a targeted disruption of the Sall3 gene were examined. Sall3 homozygous mutant animals (Sall3−/−) die shortly after birth from dehydration and display alterations in palate development and partially penetrant abnormalities in cranial nerves (Parrish et al., 2004). An analysis of gross OB morphology at P0.5 was conducted to determine the effect of loss of Sall3 on OB size. No significant difference in OB length was observed in the absence of Sall3 at P0.5 (1.242 ± 0.01 mm in controls vs. 1.243 ± 0.02 mm in Sall3 mutant animals, n = 4; P = 0.99).
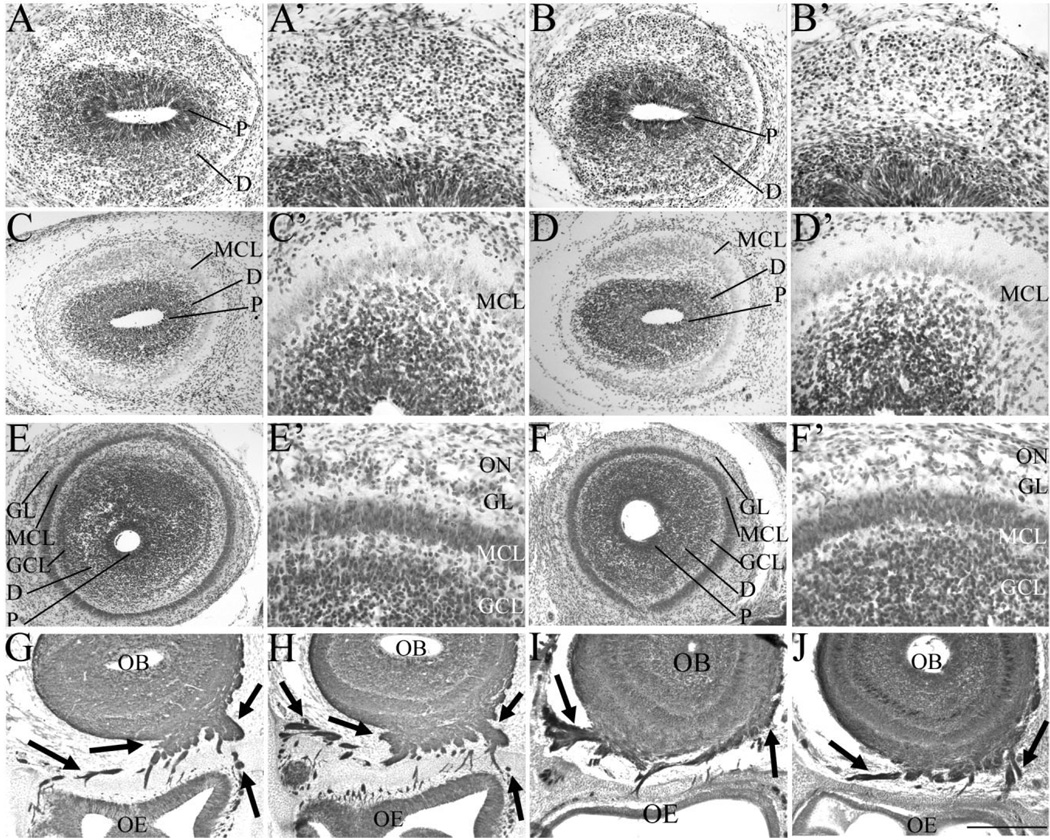
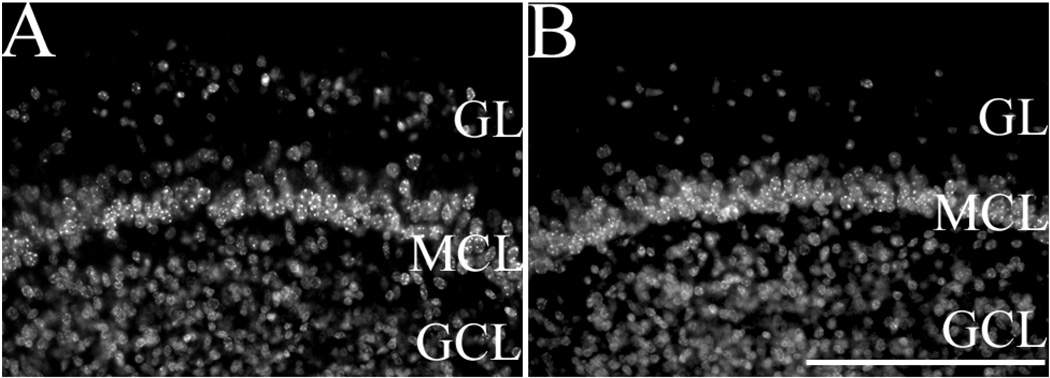
To determine whether Sall3 was required for the development of distinct cell types, coronal sections of control and Sall3−/− animals were examined from E14.5 to P0.5. At E14.5, two distinct histological regions were visible in control OBs, the progenitor population and the differentiating field (Fig. 2A,A′). By E16.5, a layer of differentiated neurons was observed outside the differentiating field. Mitral cells represent the major cellular population that has differentiated at this time and can be distinguished by large, oval cell bodies and light staining with Nissl compared with small, intensely stained cell bodies in the differentiating field. We therefore assigned this population as the MCL (Fig. 2C,C′). No gross alterations in lamination or cellular organization were observed in Sall3 mutant animals at E14.5 or E16.5 (Fig. 2B,B′,D,D′; n = 3, E14.5; n = 2, E16.5). By P0.5, a well-defined MCL was apparent in wild-type and Sall3 mutant animals (n = 3; Fig. 2E–F′). At this age in control animals, a subset of interneurons has differentiated and is located in the GL and GCL (Fig. 2E,E′). In Sall3-deficient animals, the GL appeared hypocellular compared with controls (n = 3; Fig. 2F,F′). We quantified the number of cells in the GL at P0.5 and observed a 32.6% decrease in glomerular cell number in Sall3-deficient animals (125.8 ± 1.8 cells in controls vs. 84.8 ± 5.4 cells in Sall3 mutant animals, n = 4; P< 0.001). These observations suggest a role for Sall3 in glomerular formation.
Fig. 2.
A–J: The glomerular layer is hypocellular in the absence of Sall3. Nissl staining of coronal sections of the olfactory bulb at E14.5 (A–B′), E16.5 (C–D′), and P0.5 (E–F′) and Tuj1 staining at E16.5 (G,H) and P0.5 (I,J) in control (A,A′,C,C′,E,E′,G,I) and Sall3−/− (B,B′,D,D′,F,F′,H,J) animals. Control animals represent images of either Sall3+/+ or Sall3+/− animals. No alterations in early olfactory development were observed in Sall3 mutant animals (B,B′,D,D′). The GL appeared hypocellular at P0.5 in Sall3-deficient animals (F′). The MCL, GCL, and GL were identifiable under high power, by cellular characteristics described in Materials and Methods. Innervation of the olfactory bulb by the olfactory nerve (arrows, G–J) was not altered in Sall3 mutant animals (H,J). P, progenitor populations; D, differentiating field; GCL, granule cell layer; MCL, mitral cell layer; GL, glomerular layer; ON, olfactory nerve; OB, olfactory bulb; OE, olfactory epithelium. Scale bar = 100 µm for A′,B′,C′,D′,E′,F′; 200 µm for A,B; 300 µm for C,D,G,H; 350 µm for E,F,I,J.
Olfactory information is detected by olfactory receptor neurons in the olfactory epithelium, and these neurons project to spatially conserved glomeruli in the OB (Buck and Axel, 1991; Ressler et al., 1993; Sullivan et al., 1995; Vassar et al., 1993). Previous studies have shown that loss of peripheral innervation can lead to a decrease in OB cell number (Cummings and Brunjes, 1997; Fiske and Brunjes, 2001; Mandairon et al., 2003). We therefore investigated whether alterations in the pattern of peripheral innervation were present in Sall3−/− animals. β-Tubulin III (Tuj1) is a pan-neuronal marker that can be used to visualize neuronal projections from the olfactory epithelium to the OB (arrows, Fig. 2G–J). At E16.5, the olfactory nerve has contacted the OB and extended ventrally, medially, and laterally and, by P0.5, encompassed the entire OB. No alteration in olfactory nerve innervation was observed at E16.5 (n = 2; Fig. 2G,H) or P0.5 (n = 4; Fig. 2I,J) in Sall3 mutant animals. Furthermore, protoglomerular structures were observed within the OB in both control and Sall3−/− animals at P0.5 (arrows, Fig. 7G,H). These findings suggest an intrinsic role for Sall3 in the development of OB interneurons.
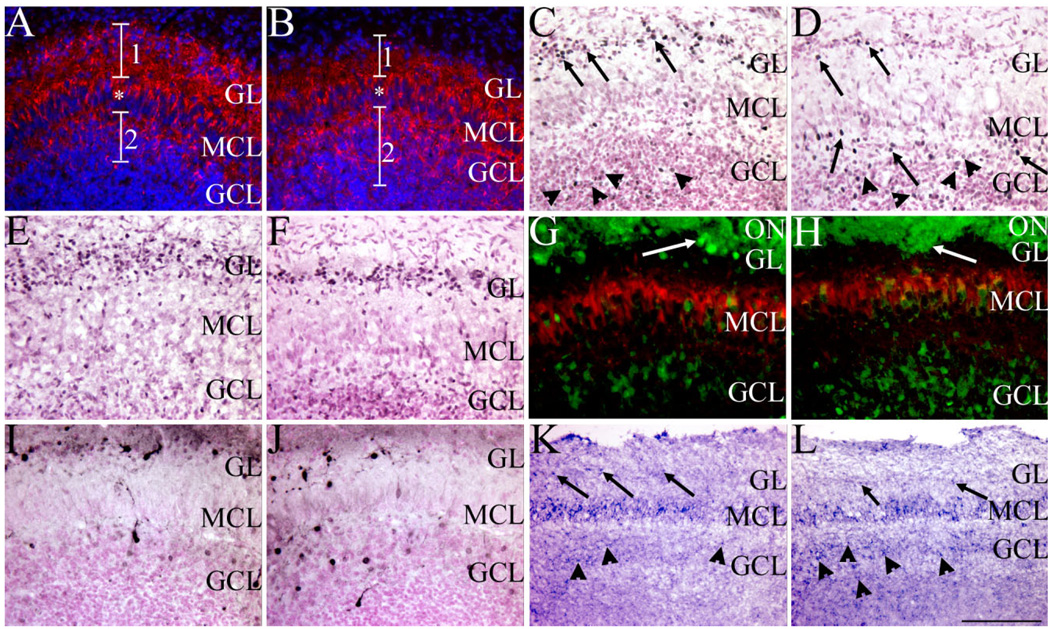
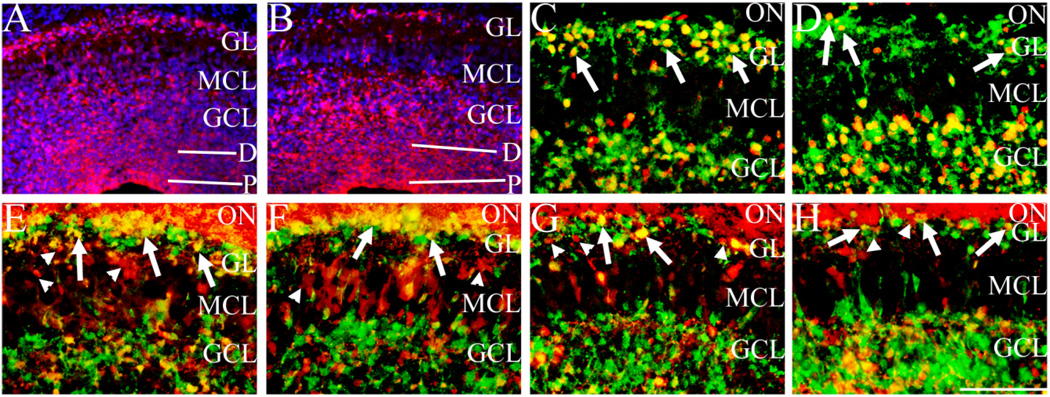
Fig. 7.
Sall3 is required for the terminal maturation of olfactory interneurons. GAD65/67 (red; A,B), Pax6 (black; C,D), ER81 (black; E,F), calretinin (green)/reelin (red; G,H), calbindin (black; I,J), and Nurr1 (blue; K,L) expression in the olfactory bulb in coronal (A–J) and sagittal (K,L) sections at P0.5 of control (A,C,E,G,I,K) and Sall3 mutant (B,D,F,H,J,L) animals. Sections were counterstained with DAPI (blue; A,B) and nuclear fast red (C–F,I,J). Fewer GAD65/67-expressing cells were observed in the GL in Sall3 mutant animals, accompanied by more positive cells in the GCL (B). 1 and 2 demarcate the GL and GCL, respectively; asterisk indicates the GAD65/67-positive projections through the MCL (A,B). In control animals, large Pax6high-expressing cells were observed predominantly in the GL (arrows, C), and small Pax6low-expressing cells were located in the GCL (arrowheads, C). In Sall3 mutant animals, fewer large Pax6high-expressing cells were observed in the GL (arrows, GL, D), and more large Pax6high-expressing cells were observed in the GCL (arrows, GCL, D). No gross difference was observed in the numbers of small Pax6low-expressing cells (arrowheads, D). A decrease in ER81-expressing cells was observed in the GL in Sall3 mutant animals (F). Similar alterations were observed with calretinin and calbindin populations in Sall3-deficient animals (H,J). Glomeruli-like structures were observed in control (arrow, G) and Sall3 mutant animals (arrow, H). Nurr1 expression was observed in the GL (arrows), MCL, and GCL (arrowheads) of control (K) and Sall3 mutant (L) animals, although an increase Nurr1 expression was observed in the GCL in the absence of Sall3 (L). GCL, granule cell layer; MCL, mitral cell layer; GL, glomerular layer; ON, olfactory nerve. Scale bar = 100 µm for A–F,I,J; 75 µm for G,H; 200 µm for K,L.
Absence of olfactory bulb TH expression in Sall3-deficient animals
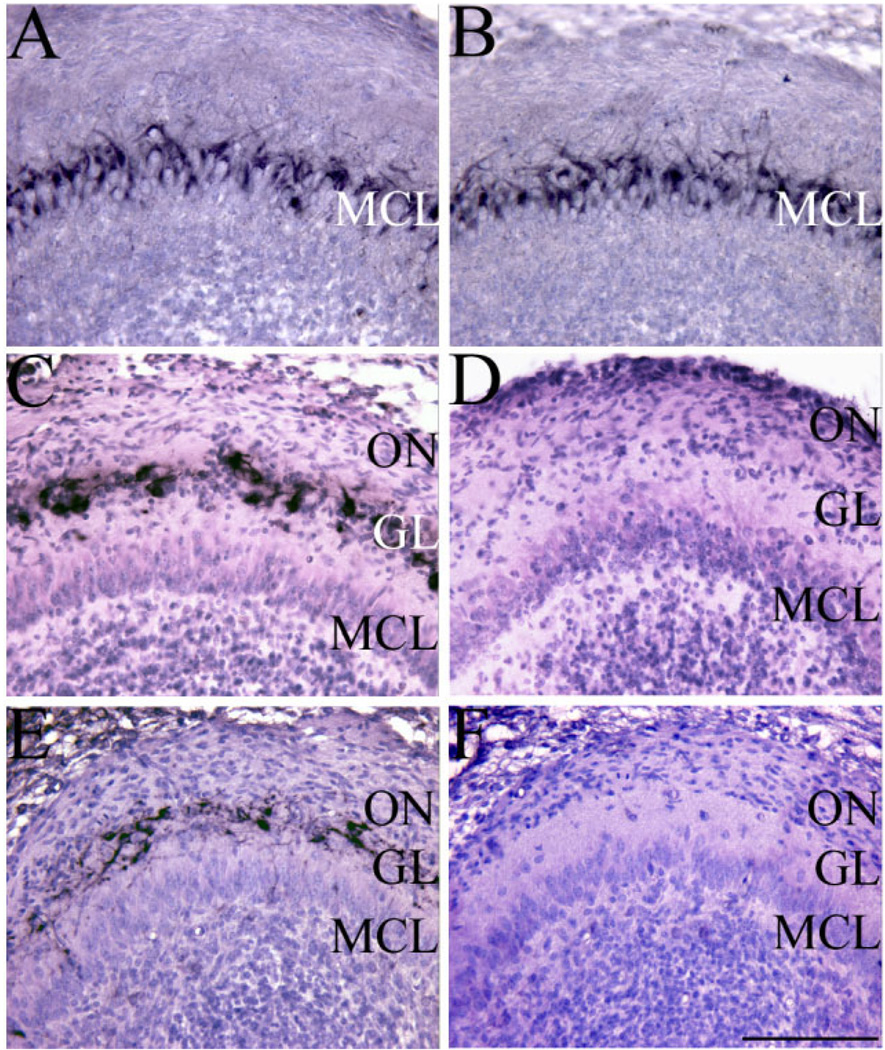
Histological analysis of the developing OB suggested that interneuron populations in the GL were decreased in the absence of Sall3. To characterize these alterations further, a variety of cell-type-specific markers were examined. To identify distinct laminae of the OB and to distinguish between early-and late-generated populations, expression of reelin, a marker of the early-born MCL (Fig. 3A,B), and TH, a marker of the later-born GL (Fig. 3C–F), were examined. Reelin staining indicated that the MCL appeared normal in the absence of Sall3 at P0.5 (n = 3; Fig. 3B). However, in Sall3 mutant animals, TH-positive cells were absent in the GL at P0.5 (n = 6; Fig. 3D), which suggests a requirement for Sall3 in the development of this cellular population. TH expression is first detected in the developing OB at E16.5. To determine whether this cellular population was initially produced and subsequently underwent cell death in the absence of Sall3, we examined expression of TH at this age (Fig. 3E,F). No TH-positive cells were observed at E16.5 in Sall3−/− animals (n = 3; Fig. 3F). To exclude the possibility that cell death contributed to the phenotype observed in Sall3-deficient animals, we examined expression of activated caspase 3 in the OB and LGE at E16.5 and P0.5. No gross difference in cell death was observed, which suggests that Sall3 is not required for cell survival (n = 2 per region per age; data not shown). These findings suggest that Sall3 is required for the generation or specification of dopaminergic GL cells.
Fig. 3.
Olfactory bulb tyrosine hydroxylase (TH) expression is absent in Sall3-deficient animals. Reelin (A,B) and TH (C–F) staining (black) in coronal sections of control (A,C,E) and Sall3 mutant (B,D,F) animals at P0.5 (A–D) and E16.5 (E,F). Sections were counterstained with hematoxylin and eosin. No alterations in reelin expression in the MCL were observed in Sall3-deficient animals (B). TH expression in the GL was absent in Sall3 mutant animals at P0.5 (D) and E16.5 (F). GCL, granule cell layer; MCL, mitral cell layer; GL, glomerular layer; ON, olfactory nerve. Scale bar = 100 µm.
TH expression is maintained in the substantia nigra and nigrostriatal pathway
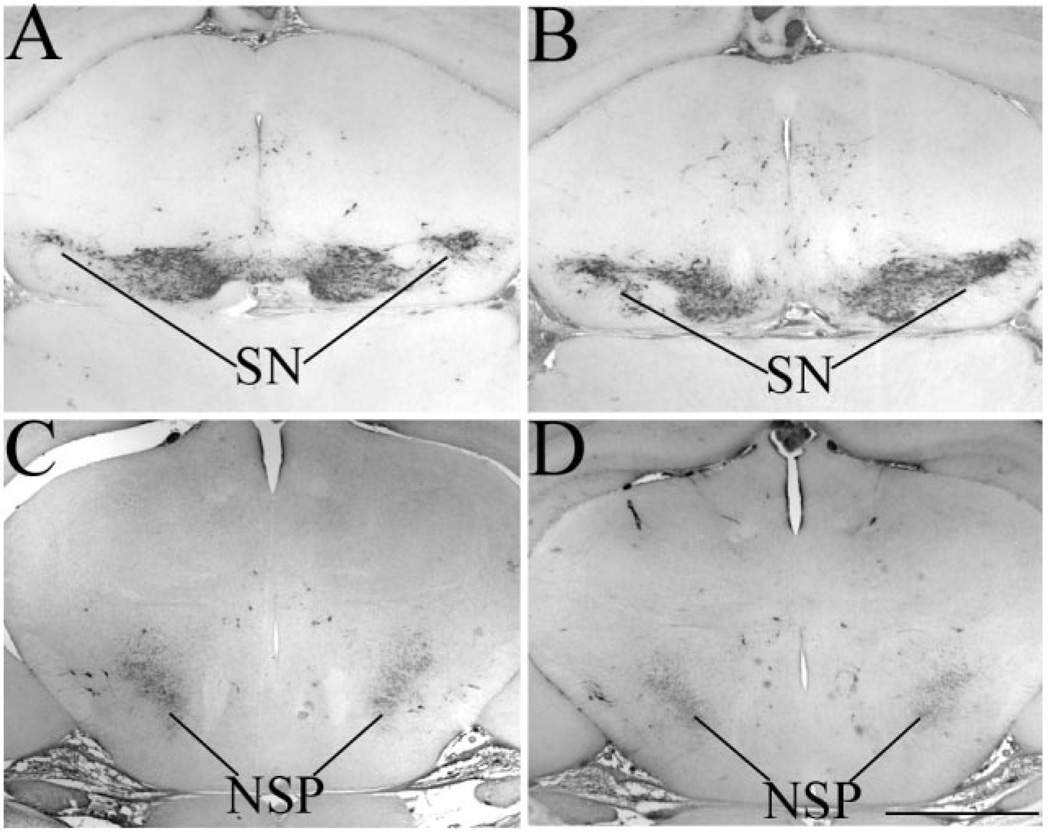
In the absence of Sall3, we observed a decrease in cell number in the GL (Fig. 2F′). In addition, Sall3-deficient animals exhibited a complete absence of TH-positive cells in the OB throughout development (Fig. 3D,F). TH is also expressed by cells in the substantia nigra and their projections through the nigrostriatal pathway of the midbrain (Fig. 4A,C). We have not observed gross histological differences in the developing midbrain in Sall3-deficient mice (Parrish, 2003). Although Sall3 is not expressed by mature cells in the substantia nigra, it is expressed by their progenitors (data not shown). We therefore examined TH expression in the substantia nigra and nigrostriatal pathway to determine whether there was a global deficiency in dopaminergic neurons in the brain. Expression of TH was not altered in the substantia nigra and nigrostriatal pathway in Sall3 mutant animals (n = 3; Fig. 4B,D), which suggests a distinct function for Sall3 in the generation of OB dopaminergic cells.
Fig. 4.
Tyrosine hydroxylase (TH) expression is maintained in the midbrain of Sall3 mutant animals. TH expression (black) in the midbrain of control (A,C) and Sall3−/− animals (B,D) at P0.5. TH expression was maintained in the SN and NSP in the absence of Sall3 (B,D). SN, substantia nigra; NSP, nigrostriatal pathway. Scale bar = 800 µm.
Sall3 mutant animals can detect odor
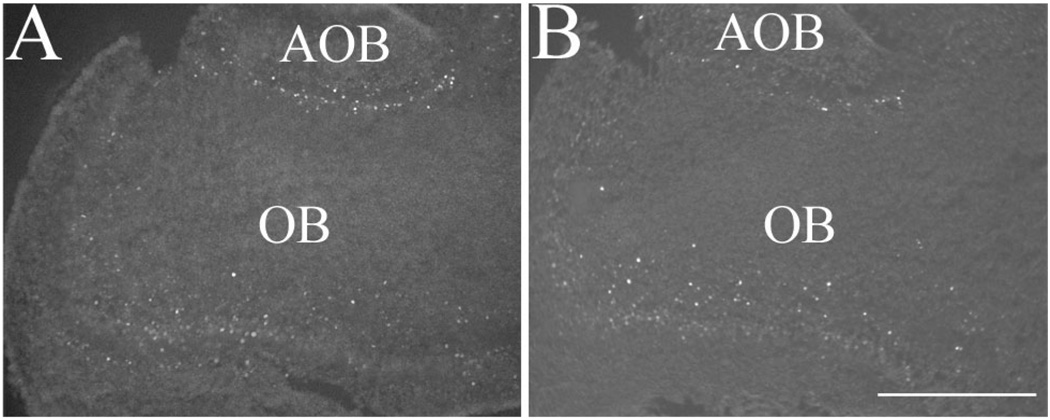
The initiation and maintenance of TH protein expression in the GL are activity dependent (Baker and Farbman, 1993; Baker et al., 1984, 1993; Biffo et al., 1990; McLean and Shipley, 1988; Nadi et al., 1981; Stone et al., 1991). In the absence of Sall3, the OB was innervated from the periphery (Fig. 2H,J), and, though hypocellular, glomeruli-like structures were observed at P0.5 (arrows, Fig. 7H; and data not shown). Rodents use olfactory stimuli to locate nipples during feeding (Bruno et al., 1980; Teicher and Blass, 1976, 1977). Previous studies from our laboratory have determined that Sall3-deficient animals are not anosmic; they can locate nipples on anesthetized dams, although Sall3 mutant animals rapidly detached from the nipples (Parrish et al., 2004). To further verify these findings, we examined the expression of the immediate early transcription factor c-Fos (Fig. 5A,B), which is regulated by neuronal activity (for review see Guthrie and Gall, 1995). Previous studies have shown that TH expression colocalizes with c-Fos expression in the GL and that c-Fos expression is down-regulated, along with TH when input to the OB is altered (Jin et al., 1996; Liu et al., 1999). c-Fos expression was observed in Sall3-deficient animals at P0.5 (n = 2; Fig. 5B). Consistent with the observations of decreased cell number in the GL, c-Fos expression appeared decreased in Sall3−/− animals (Fig. 5B). These data suggest that the absence of TH expression in Sall3−/− animals is not due to a lack of input or activity in the OB. Taken together, these data suggest that olfactory innervation is not altered in the absence of Sall3 and that despite the absence of TH expression sensory information can be detected by Sall3 mutant mice.
Fig. 5.
c-Fos expression is observed in the OB of Sall3−/− animals. Expression of the immediate early gene c-Fos in the OB at P0.5 in sagittal sections of control (A) and Sall3 mutant (B) animals. c-Fos expression, though reduced, was present in the absence of Sall3. AOB, accessory olfactory bulb; OB, olfactory bulb. Scale bar = 400 µm.
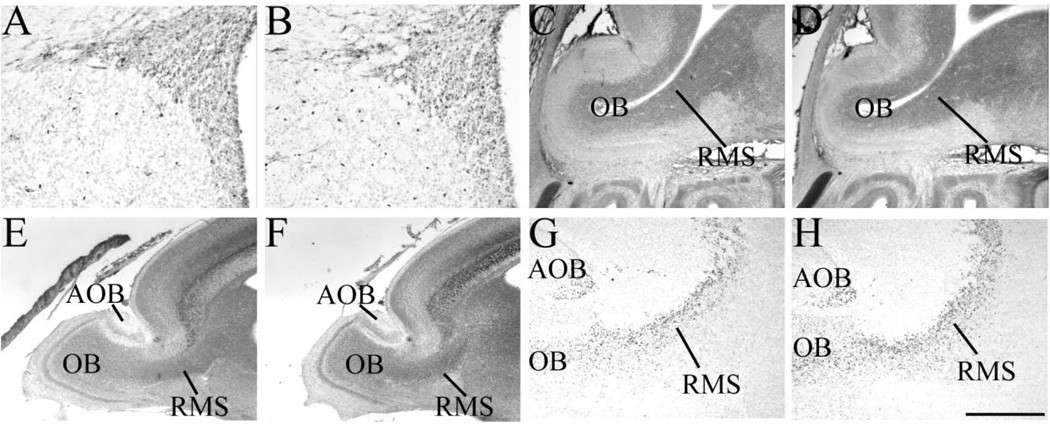
The LGE and RMS are unaltered in Sall3-deficient animals
Our data suggest that Sall3 is required for the development of a population of OB interneurons. OB interneurons are derived from an ER81-expressing population of cells in the LGE (Fig. 6A; Stenman et al., 2003). No alteration in ER81 expression in the LGE was observed at P0.5 in Sall3-deficient animals (n = 3; Fig. 6B), which suggests that Sall3 is not required for the initial specification of these interneuron populations. Cells destined for the OB migrate from the LGE to the OB within the RMS (Doetsch and Alvarez-Buylla, 1996; Lois and Alvarez-Buylla, 1994; Luskin, 1993; Wichterle et al., 1999, 2001). Histological examination of the RMS did not reveal any alterations in this structure in Sall3−/− animals, compared with controls, at E16.5 (n = 2; Fig. 6C,D) or P0.5 (n = 4; Fig. 6E,F). Pax6 is expressed by migrating neuroblasts as they travel along the RMS, and Pax6 has been shown to be required for TH expression (Hack et al., 2005; Kohwi et al., 2005). Pax6-expressing neuroblasts were observed in the RMS in Sall3 mutant animals at P0.5, similar to controls (n = 4; Fig. 6G,H), which suggests that Sall3 is not required for the differentiation or migration of these neuroblasts within the RMS.
Fig. 6.
Sall3 is not required for the initial specification of cells within the LGE or migration within the RMS. Expression of ER81 (A,B), neurofilament (C–F) and Pax6 (G,H) in the lateral ganglionic eminence (A,B) and RMS (C–H) of control (A,C,E,G) and Sall3−/− (B,D,F,H) animals at E16.5 (C,D) and P0.5 (A,B,E–H). ER81 expression was unaltered in the LGE in Sall3 mutant animals (B). Histological examination of the RMS did not reveal any abnormalities in Sall3−/− animals (D,F). Pax6-expressing neuroblasts were observed migrating in the RMS in the absence of Sall3 (H). OB, olfactory bulb; RMS, rostral migratory stream; AOB, accessory olfactory bulb. Scale bar = 150 µm for A,B; 300 µm for E,F; 400 µm for C,D,G,H.
Alterations in other olfactory interneuron subtypes are present in Sall3 mutant animals
Different interneuron subtypes are observed in the OB and can be defined by expression of GABA, calretinin, and calbindin (Kosaka et al., 1995). Expression of these chemical markers is initiated as OB interneurons terminally differentiate and radially migrate in the OB. In Sall3-deficient animals, a 32.6% decrease in cell number in the GL is observed, accompanied by an absence of TH expression. TH-expressing cells account for 11% of cells within the GL (Parrish-Aungst et al., 2007), which suggests that alterations exist in other GL cellular populations in the absence of Sall3. To identify these cellular populations, we examined markers of distinct interneuron subtypes at P0.5. GAD65/67 is expressed by GABAergic cells in the GL (region 1, Fig. 7A) and GCL (region 2, Fig. 7A) as well as the projections of these cells through the MCL (asterisk, Fig. 7A). GAD65/67 is expressed by ~53% of cells within the GL, and ~80% of TH-positive cells are also GAD65/67 positive (Hack et al., 2005; Kosaka et al., 1998; Parrish-Aungst et al., 2007). In Sall3−/− animals, a mix of GAD65/ 67-positive and -negative cells was observed in the GL, although, overall, fewer GAD65/67-positive cells were present in this layer (n = 3; region 1, Fig. 7B). In addition, more GAD65/67-positive cells were observed in the GCL in Sall3-deficient animals (region 2, Fig. 7B). These data suggest that a shift in cell fate specification or an alteration in migration or maturation of interneuron populations exists in the absence of Sall3.
Previous studies have shown that GABA expression overlaps with TH- and Pax6-positive populations in the OB (Hack et al., 2005; Parrish-Aungst et al., 2007). In addition, the majority (95%) of TH-positive cells in the GL are also Pax6 positive, although only 78% of Pax6-positive cells in the GL are TH positive (Hack et al., 2005). Furthermore, Pax6 has been shown to be required for the generation of dopaminergic TH-positive cells in the OB (Hack et al., 2005; Kohwi et al., 2005). To determine whether the absence of TH expression in Sall3 mutant animals was due to a requirement for Pax6, we examined its expression at P0.5. Pax6 expression is down-regulated in the GCL but is highly expressed in the GL (Hack et al., 2005). In control animals, two patterns of Pax6 staining were observed in the GL and GCL, large cells expressing high levels of Pax6, Pax6high (arrows, Fig. 7C), and smaller cells expressing low levels of Pax6, Pax6low (arrowheads, Fig. 7C). Most Pax6high-expressing cells were located in the GL, and Pax6low-expressing cells were observed in the GCL in control animals (Fig. 7C). In Sall3 mutant animals, very few Pax6high-expressing cells were observed in the GL (n = 5; arrows in GL, Fig. 7D). Most of these cells were located in the superficial GCL (arrows in GCL, Fig. 7D). No gross difference in Pax6low-expressing cells in the GCL was observed (arrowheads, Fig. 7D). These finding suggest that the absence of TH-positive cells is not due to a requirement for Pax6 expression. In addition, the presence of large Pax6high-expressing cells in the GCL suggests that these cells are ultimately destined for the GL and that an alteration in the maturation or migration this cellular population occurs in the absence of Sall3. To determine whether radial glia were structurally altered, we examined expression of radial glial markers. GLAST expression appeared normal in Sall3−/− animals at P0.5 (n = 3; data not shown), which suggests that, if a migration deficit is present, it is not due to altered radial glia scaffold.
Recent studies have identified that the Ets transcription factor ER81 is expressed by cells in the LGE, RMS, GL, MCL, and superficial GCL and that OB interneurons are derived from this population (Saino-Saito et al., 2007; Stenman et al., 2003). ER81 expression was unaltered in the LGE (Fig. 6B), and the RMS appeared normal in Sall3-deficient animals (Fig. 6D,F,H), which suggests that the alterations observed in Sall3 mutant animals are downstream of these events. Preliminary unpublished observations by Saino-Saito et al. (2007) suggest that mice deficient for ER81 have a decreased number of TH-positive cells in the OB (Saino-Saito et al., 2007), which suggests a role for this transcription factor in the specification of the dopaminergic cellular population. We examined ER81 expression in the OB to determine whether alterations in expression of this transcription factor contributed to the deficit in Sall3 mutant animals. At P0.5, most cells within the GL are ER81 positive (Fig. 7E). Most cells in the GL were also ER81 positive in Sall3-deficient animals, although this population appeared decreased in number (n = 4; Fig. 7F). Calretinin and calbindin are expressed by independent populations within the OB (Kosaka et al., 1995). To determine whether Sall3 was required for the generation of nondopaminergic OB cellular populations, we examined expression of these markers in Sall3-deficient animals. To distinguish between interneuron and excitatory populations, we examined expression of reelin, a MCL maker, and calretinin, which is expressed by olfactory nerve axons as well as cells within the GL, MCL, and GCL at P0.5 (Fig. 7G). In Sall3-deficient animals, calretinin expression was observed in the GL, MCL, and GCL, although fewer positive cells were located superficial to the MCL, within the GL, and more positive cells were located deep to the MCL, within the GCL (n = 4; Fig. 7H). Calbindin is also expressed by interneurons in the GL and GCL (Fig. 7I). Similar alterations in the distribution of calbindin interneuron populations were observed in Sall3 mutant animals, with fewer positive cells in the GL and more positive cells in the GCL (n = 3; Fig. 7J). These data suggest that Sall3 is required for the maturation of dopaminergic and nondopaminergic cellular populations.
Sall3 is required for the terminal differentiation of dopaminergic olfactory cells
To determine whether the absence of TH expression was due to a failure of dopaminergic cellular maturation, we examined expression of the transcription factor Nurr1 at P0.5. TH-expressing cells arise from Nurr1-positive progenitor-like cells, and, in the ventral midbrain, Nurr1 is required for TH expression (Backman et al., 1999; Castelo-Branco et al., 2003, 2006; Le et al., 1999). Within the OB, TH is coexpressed with Nurr1 in the GL (Backman et al., 1999). In control animals at P0.5, Nurr1-positive cells were observed in the GCL (arrowheads, Fig. 7K), MCL, and GL (arrows, Fig. 7K). Nurr1-positive cells were present in all three layers in Sall3-deficient animals (n = 3; Fig. 7L). However, fewer Nurr1-positive cells were observed in the GL (arrows, Fig. 7L), and more Nurr1-positive cells were observed in the GCL (arrowheads, Fig. 7L). Thus, precursors of TH-expressing cells were present in the OB of Sall3 mutant animals, but mature dopaminergic cells were absent, which suggests a requirement for Sall3 in the terminal differentiation and maturation of Nurr1-positive precursor cells to TH-expressing cells.
Sall3 is not required in a cell autonomous manner for olfactory interneuron development
In Sall3 mutant animals, exon 2 is fused in frame to the β-galactosidase gene (Parrish et al., 2004). This fusion results in a null allele, with deletion of greater than 95% of the coding region of the Sall3 gene. In addition, this construct results in expression of β-galactosidase under the control of the endogenous Sall3 promoter and allows visualization of cells that would normally express Sall3 in Sall3+/− and Sall3−/− animals. To determine whether the absence of TH and other interneuron populations in the GL, observed in Sall3-deficient animals, was due to a cell autonomous effect, we examined expression of β-galactosidase in Sall3+/− and Sall3−/− animals. In Sall3+/− animals, β-galactosidase-positive cells were observed in progenitor cells, differentiating cells, and cells in the GCL, MCL, and GL (n = 3; Fig. 8A). Within the GL and GCL, β-galactosidase was expressed by a subset of cells (Fig. 8A). Similar β-galactosidase expression patterns were observed in Sall3−/− animals (n = 3; Fig. 8B). However, in Sall3-deficient animals, a decrease in the number of β-galactosidase-positive cells in the GL and an increase in the number of β-galactosidase-positive cells in the GCL were observed (Fig. 8B). The presence of β-galactosidase-positive cells in the GL suggests that Sall3-dependent cells can migrate to their laminar position in the absence of Sall3.
Fig. 8.
Sall3 is not required in a cell-autonomous manner for glomerular olfactory interneuron maturation. β-Galactosidase expression (red) in Sall3+/− and Sall3−/− animals at P0.5 (A,B). Expression is observed in progenitor cells, differentiating cells, GCL, MCL, and GL (A,B). Sections were counterstained with DAPI (blue). In Sall3 mutant animals, a decrease in the number of β-galactosidase-expressing cells in the GL and an increase in β-galactosidase-expressing cells in the GCL were observed (B). Pax6 (red; C,D), calretinin (red; E,F), and calbindin (red; G,H) coexpression with β-galactosidase (green) in the P0.5 olfactory bulb in Sall3+/− (C,E,G) and Sall3−/− (D,F,H) animals. Coexpression of β-galactosidase was observed with the majority of Pax6 (arrows, C) cells in the GL. However, a mix of calretinin+ β-galactosidase+ (arrows, E) and calretinin+ β-galactosidase− (arrowheads, E) and calbindin+ β-galactosidase+ (arrows, G) and calbindin+ β-galactosidase− (arrowheads, G) was observed in the GL of Sall3+/− animals. In Sall3 mutant animals, although the number of Pax6-, calretinin-, and calbindin-positive cells in the GL is decreased (D,F,H), cells coexpressing β-galactosidase and Pax6 (arrows, D), β-galactosidase and calretinin (arrows, F), and β-galactosidase and calbindin (arrows, H) were observed. Calretinin and calbindin are additionally expressed by olfactory nerve axons, visible as staining superficial to the GL in E–H. P, progenitor populations; D, differentiating field; GCL, granule cell layer; MCL, mitral cell layer; GL, glomerular layer; ON, olfactory nerve; β-gal, β-galactosidase; CR, calretinin; CB, calbindin. Scale bar = 150 µm for A,B; 100 µm for C–H.
To elucidate further the interneuron subtypes dependent on Sall3 expression, we examined coexpression of Pax6, calretinin, and calbindin with β-galactosidase in the GL of Sall3+/− and Sall3−/− animals at P0.5. In Sall3+/− animals, most Pax6-positive cells were also β-galactosidase positive (n = 3; arrows, Fig. 8C). However, a mix of calretinin+ β-galactosidase+ (arrows, Fig. 8E) and calretinin+ β-galactosidase+ (arrowheads, Fig. 8E) cells and calbindin+ β-galactosidase− (arrows, Fig. 8G) and calbindin+ β-galactosidase+ (arrowheads, Fig. 8G) cells was observed within this layer (n = 3). In Sall3-deficient animals, although the number of cells in the GL expressing these markers was reduced, most cells expressing Pax6 (arrows, Fig. 8D) in the GL were also β-galactosidase positive (n = 3). In addition, similar to the case in Sall3+/− animals, a mix of calretinin+ β-galactosidase+ (arrows, Fig. 8F) and calretinin+ β-galactosidase+ (arrowheads, Fig. 8F) cells and calbindin + β-galactosidase+ (arrows, Fig. 8H) and calbindin+ β-galactosidase− (arrowheads, Fig. 8H) cells was observed in the GL of Sall3−/− animals (n = 3). Thus, Sall3 is not required in a cell-autonomous manner for the migration or differentiation of subpopulations of interneurons that display altered laminar position in Sall3-deficient animals. In summary, Sall3 is expressed by diverse interneuron populations at P0.5, and, in Sall3−/− animals, subpopulations of cells dependent on Sall3 expression can reach their laminar position in the GL.
Sall1 does not compensate for the loss of Sall3
Our studies suggest that Sall3 is required for the terminal maturation of interneurons destined for the GL. Sall family members are highly conserved (Camp et al., 2003; Ott et al., 1996; Sweetman and Munsterberg, 2006), which suggests that a related family member may compensate for loss of Sall3. Sall1 is robustly expressed in the developing OB (Buck et al., 2001; Harrison et al., 2007; Ott et al., 2001), and our studies suggest that loss of Sall1 results in alterations in olfactory development (Harrison et al., 2007). We therefore examined expression of Sall1 in Sall3-deficient OBs at P0.5. Sall1 is robustly expressed by cells in the GCL and MCL in both control and Sall3 mutant animals (Fig. 9A,B). Within the GL, Sall1 was expressed by a subset of cells (Fig. 9A), and, similar to other markers of GL populations examined, this population appeared to show a decrease in Sall3 mutant animals (Fig. 9B). Furthermore, Sall1 expression was not up-regulated in Sall3-deficient animals (Fig. 9A,B). Previous studies identified the fact that Sall family members can interact. Chick Sall3 (cSall3) can interact with cSall1, and this interaction results in the relocation of cSall1 from the nucleus to the cytoplasm (Sweetman et al., 2003). In both control and Sall3 mutant animals, Sall1 expression was nuclear, which suggests that loss of Sall3 does not alter the cellular localization of Sall1 in the developing OB. Together, these findings suggest that Sall1 might not compensate for loss of Sall3.
Fig. 9.
Sall1 does not compensate for loss of Sall3. Sall1 was robustly expressed by cells in the GCL and MCL in both control (A) and Sall3 mutant (B) animals. Within the GL, Sall1 was expressed by a subset of cells (A), and, similar to other GL populations examined, this population appeared to be decreased in Sall3 mutant animals (B). Scale bar = 150 µm.
Discussion
Our study examined the role of Sall3 in the differentiation and maturation of OB interneuron populations. OB interneurons arise from an ER81-positive population of cells located in the LGE and migrate to the OB via the RMS (Doetsch and Alvarez-Buylla, 1996; Lois and Alvarez-Buylla, 1994; Stenman et al., 2003; Wichterle et al., 1999, 2001). Several factors have been identified that influence the initial generation of interneurons from the LGE and their migration via the RMS (Chen et al., 2001; Hu, 1999; Soria et al., 2004; Stenman et al., 2003; Waclaw et al., 2006; Wu et al., 1999; Yoshihara et al., 2005; Yun et al., 2003). Previous studies have suggested that cell type specification is intrinsically regulated (Tucker et al., 2006), but few factors have been identified that regulate the terminal differentiation and specification of these cells within the OB.
We have shown that the zinc finger containing putative transcription factor Sall3 was expressed by olfactory progenitors and a subset of differentiated neurons, predominantly by interneurons in the GL. Within OB interneuron populations, Sall3 was expressed by chemically diverse populations. In the absence of Sall3, no alteration in OB size was observed at P0.5; however, histological examination of the OB identified a decrease in cell number in the GL. TH expression was absent from the OB throughout development. In addition, we observed a decrease in the number of GAD65/67-, Pax6-, calretinin-, and calbindinpositive populations in the GL, accompanied by an increase in the number of positive cells in the GCL. In Sall3+/− animals, Sall3-expressing cells, assayed by the presence of β-galactosidase expression, were observed in the GL and GCL. In Sall3-deficient animals, β-galactosidase-positive cells were also observed in the GL and GCL, although a decrease in the number of β-galactosidase-positive cells in the GL was observed. This indicates that at least a subpopulation of interneurons dependent on Sall3 can appropriately migrate to their laminar position. Furthermore, this suggests that expression of Sall3 in interneuron populations is not essential for their migration to their laminar position. In addition, the morphology of radial glia appeared normal in Sall3-deficient animals, which suggests that, if a migration deficit is present, it is not due to alterations in radial glia. Although we cannot exclude the possibility that Sall3 is required for the migration of subpopulations of interneurons, we hypothesize that Sall3 is required for the terminal maturation of OB interneurons.
In Sall3 mutant animals, the GL appeared hypocellular, and TH expression was absent. Previous studies have implicated Pax6 in the generation of dopaminergic OB cells (Hack et al., 2005; Kohwi et al., 2005). However, Pax6 expression was present in Sall3 mutant animals, which suggests that the absence of TH expression was not due to a requirement for Pax6. Kohwi et al. (2005) proposed that Pax6 instructs a laminar bias toward the GL, as Pax6 is down-regulated by cells in the GCL. Pax6high-expressing cells were observed predominantly in the GCL in Sall3-deficient animals, which suggests that Pax6 is not sufficient to instruct OB laminar position. Furthermore, the presence of Pax6 expression in Sall3 mutant animals suggests that Sall3 is either downstream of the Pax6 signaling pathway or that they represent independent pathways in dopaminergic cellular generation.
In the nigrostriatal pathway TH-positive cells arise from a Nurr1-positive precursor cell (Backman et al., 1999; Castelo-Branco et al., 2003, 2006). In Nurr1-deficient animals, TH expression is absent from the substantia nigra and nigrostriatal pathway but is maintained in the OB (Le et al., 1999). In Sall3 mutant animals, TH expression was maintained in the substantia nigra and nigrostriatal pathway but was absent from the OB. This suggests that independent pathways regulate dopaminergic maturation in distinct neural regions. Expression of the dopaminergic precursor Nurr1 was observed in the GL, MCL, and GCL of Sall3-deficient animals. Nurr1-positive cells were present in reduced numbers in the GL, but an increase in Nurr1 expression in the GCL was observed in the absence of Sall3, which suggests that most of these cells fail to reach their laminar position and express TH. Previous studies have shown that TH expression is context dependent (Baker and Farbman, 1993; Baker et al., 1984, 1993; Biffo et al., 1990; McLean and Shipley, 1988; Nadi et al., 1981; Stone et al., 1991). Therefore, it is possible that these cells cannot express TH because they are not receiving appropriate signals. In Sall3-deficient animals, no alterations in peripheral innervation were observed, and expression of the immediate early protein c-Fos was present. In addition, olfactory perception appeared to be present in Sall3-deficient animals (Parrish et al., 2004). We therefore hypothesize that Sall3 is required for terminal differentiation of Nurr1-positive dopaminergic precursors and expression of a mature dopaminergic TH-positive phenotype.
Our studies have demonstrated that Sall3 is required for the terminal maturation of OB interneurons. We have recently described a role for a related Sall family member, Sall1, in OB development (Harrison et al., 2007). During development, Sall1 and Sall3 are expressed by cells in the olfactory epithelium and the OB. Interestingly, early in development, Sall1 is expressed prior to Sall3 in overlapping cell types in both olfactory epithelium and developing neural epithelium. Sall1 mutant mice die at birth from kidney deficits (Nishinakamura et al., 2001) and have smaller OBs (Harrison et al., 2007). The absence of Sall1 specifically alters mitral cell development and olfactory nerve innervation, whereas interneuron subtypes are relatively normal and mature (Harrison et al., 2007). Furthermore, we identified that Sall1 does not compensate for loss of Sall3 in olfactory development. These findings indicate that Sall family members regulate distinct steps in olfactory bulb neurogenesis.
Our studies, and those of others, demonstrate that independent mechanisms regulate dopaminergic cell maturation in distinct neural regions (Le et al., 1999). In Parkinson’s disease, preferential loss of dopaminergic cells is observed in the substantia nigra pars compacta. However, one of the first symptoms observed in Parkinson’s disease is a loss of olfactory perception (for review see Ponsen et al., 2004), which suggests that global deficiencies in cellular populations exist. Dopaminergic cells are of potential therapeutic value for the treatment of neurodegenerative disorders, such as Parkinson’s disease (for review see Correia et al., 2005; Geraerts et al., 2007; Lindvall and Bjorklund, 2004; Snyder and Olanow, 2005; Sonntag et al., 2005). Several factors have been identified that can induce a dopaminergic phenotype from precursor cells, but these cells do not fully mature (for review see Correia et al., 2005; Sonntag et al., 2005). Our studies have identified a role for the transcription factor Sall3 in the terminal differentiation and maturation of dopaminergic cells derived from the dorsal ganglionic eminence. It may therefore be of therapeutic value to investigate the potential use of Sall3 in promoting a terminal dopaminergic cellular phenotype.
ACKNOWLEDGMENTS
We thank K. Mauro for technical assistance. We also thank Dr. N. Urban for helpful comments on the manuscript and E. Drill and Drs. W. Halfter, C. Lance-Jones, and L. Lillen for helpful discussion.
Grant sponsor: National Institutes of Health; Grant number: NIMH MH60774; Grant number: NIDA AA13004. Mark Parrish’s current address is Stowers Institute of Medical Research, Kansas City, MO 64110.
LITERATURE CITED
- Akiba Y, Sasaki H, Saino-Saito S, Baker H. Temporal and spatial disparity in cFOS expression and dopamine phenotypic differentiation in the neonatal mouse olfactory bulb. Neurochem Res. 2007;32:625–634. doi: 10.1007/s11064-006-9134-7. [DOI] [PubMed] [Google Scholar]
- Al-Baradie R, Yamada K, St. Hilaire C, Chan WM, Andrews C, McIntosh N, Nakano M, Martonyi EJ, Raymond WR, Okumura S, Okihiro MM, Engle EC. Duane radial ray syndrome (Okihiro syndrome) maps to 20q13 and results from mutations in SALL4, a new member of the SAL family. Am J Hum Genet. 2002;71:1195–1199. doi: 10.1086/343821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alcantara S, Ruiz M, D’Arcangelo G, Ezan F, de Lecea L, Curran T, Sotelo C, Soriano E. Regional and cellular patterns of reelin mRNA expression in the forebrain of the developing and adult mouse. J Neurosci. 1998;18:7779–7799. doi: 10.1523/JNEUROSCI.18-19-07779.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altman J. Autoradiographic and histological studies of postnatal neurogenesis. IV. Cell proliferation and migration in the anterior forebrain, with special reference to persisting neurogenesis in the olfactory bulb. J Comp Neurol. 1969;137:433–457. doi: 10.1002/cne.901370404. [DOI] [PubMed] [Google Scholar]
- Altman J, Das GD. Autoradiographic and histological studies of postnatal neurogenesis. I. A longitudinal investigation of the kinetics, migration and transformation of cells incorporating tritiated thymidine in neonate rats, with special reference to postnatal neurogenesis in some brain regions. J Comp Neurol. 1966;126:337–389. doi: 10.1002/cne.901260302. [DOI] [PubMed] [Google Scholar]
- Backman C, Perlmann T, Wallen A, Hoffer BJ, Morales M. A selective group of dopaminergic neurons express Nurr1 in the adult mouse brain. Brain Res. 1999;851:125–132. doi: 10.1016/s0006-8993(99)02149-6. [DOI] [PubMed] [Google Scholar]
- Baimbridge KG, Miller JJ. Immunohistochemical localization of calcium-binding protein in the cerebellum, hippocampal formation and olfactory bulb of the rat. Brain Res. 1982;245:223–229. doi: 10.1016/0006-8993(82)90804-6. [DOI] [PubMed] [Google Scholar]
- Baker H, Farbman AI. Olfactory afferent regulation of the dopamine phenotype in the fetal rat olfactory system. Neuroscience. 1993;52:115–134. doi: 10.1016/0306-4522(93)90187-k. [DOI] [PubMed] [Google Scholar]
- Baker H, Kawano T, Albert V, Joh TH, Reis DJ, Margolis FL. Olfactory bulb dopamine neurons survive deafferentation-induced loss of tyrosine hydroxylase. Neuroscience. 1984;11:605–615. doi: 10.1016/0306-4522(84)90047-2. [DOI] [PubMed] [Google Scholar]
- Baker H, Morel K, Stone DM, Maruniak JA. Adult naris closure profoundly reduces tyrosine hydroxylase expression in mouse olfactory bulb. Brain Res. 1993;614:109–116. doi: 10.1016/0006-8993(93)91023-l. [DOI] [PubMed] [Google Scholar]
- Barembaum M, Bronner-Fraser M. A novel spalt gene expressed in branchial arches affects the ability of cranial neural crest cells to populate sensory ganglia. Neuron Glia Biol. 2004;1:57–63. doi: 10.1017/s1740925x04000080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biffo S, DeLucia R, Mulatero B, Margolis F, Fasolo A. Carnosine-, calcitonin gene-related peptide- and tyrosine hydroxylase-immunoreactivity in the mouse olfactory bulb following peripheral denervation. Brain Res. 1990;528:353–357. doi: 10.1016/0006-8993(90)91682-7. [DOI] [PubMed] [Google Scholar]
- Bohm J, Kaiser FJ, Borozdin W, Depping R, Kohlhase J. Synergistic cooperation of Sall4 and cyclin D1 in transcriptional repression. Biochem Biophys Res Commun. 2007;356:773–779. doi: 10.1016/j.bbrc.2007.03.050. [DOI] [PubMed] [Google Scholar]
- Brinon JG, Martinez-Guijarro FJ, Bravo IG, Arevalo R, Crespo C, Okazaki K, Hidaka H, Aijijon J, Alonso JR. Coexpression of neurocalcin with other calcium-binding proteins in the rat main olfactory bulb. J Comp Neurol. 1999;407:404–414. doi: 10.1002/(sici)1096-9861(19990510)407:3<404::aid-cne8>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- Bruno JP, Teicher MH, Blass EM. Sensory determinants of suckling behavior in weanling rats. J Comp Physiol Psychol. 1980;94:115–127. doi: 10.1037/h0077646. [DOI] [PubMed] [Google Scholar]
- Buck A, Archangelo L, Dixkens C, Kohlhase J. Molecular cloning, chromosomal localization, and expression of the murine SALL1 ortholog Sall1. Cytogenet Cell Genet. 2000;89:150–153. doi: 10.1159/000015598. [DOI] [PubMed] [Google Scholar]
- Buck A, Kispert A, Kohlhase J. Embryonic expression of the murine homologue of SALL1, the gene mutated in Townes-Brocks syndrome. Mech Dev. 2001;104:143–146. doi: 10.1016/s0925-4773(01)00364-1. [DOI] [PubMed] [Google Scholar]
- Buck L, Axel R. A novel multigene family may encode odorant receptors: a molecular basis for odor recognition. Cell. 1991;65:175–187. doi: 10.1016/0092-8674(91)90418-x. [DOI] [PubMed] [Google Scholar]
- Bulfone A, Wang F, Hevner R, Anderson S, Cutforth T, Chen S, Meneses J, Pedersen R, Axel R, Rubenstein JL. An olfactory sensory map develops in the absence of normal projection neurons or GABAergic interneurons. Neuron. 1998;21:1273–1282. doi: 10.1016/s0896-6273(00)80647-9. [DOI] [PubMed] [Google Scholar]
- Camp E, Hope R, Kortschak RD, Cox TC, Lardelli M. Expression of three spalt (sal) gene homologues in zebrafish embryos. Dev Genes Evol. 2003;213:35–43. doi: 10.1007/s00427-002-0284-6. [DOI] [PubMed] [Google Scholar]
- Cantera R, Luer K, Rusten TE, Barrio R, Kafatos FC, Technau GM. Mutations in spalt cause a severe but reversible neurodegenerative phenotype in the embryonic central nervous system of Drosophila melanogaster. Development. 2002;129:5577–5586. doi: 10.1242/dev.00158. [DOI] [PubMed] [Google Scholar]
- Castelo-Branco G, Wagner J, Rodriguez FJ, Kele J, Sousa K, Rawal N, Pasolli HA, Fuchs E, Kitajewski J, Arenas E. Differential regulation of midbrain dopaminergic neuron development by Wnt-1, Wnt- 3a, and Wnt-5a. Proc Natl Acad Sci U S A. 2003;100:12747–12752. doi: 10.1073/pnas.1534900100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castelo-Branco G, Sousa KM, Bryja V, Pinto L, Wagner J, Arenas E. Ventral midbrain glia express region-specific transcription factors and regulate dopaminergic neurogenesis through Wnt-5a secretion. Mol Cell Neurosci. 2006;31:251–262. doi: 10.1016/j.mcn.2005.09.014. [DOI] [PubMed] [Google Scholar]
- Celio MR. Calbindin D-28k and parvalbumin in the rat nervous system. Neuroscience. 1990;35:375–475. doi: 10.1016/0306-4522(90)90091-h. [DOI] [PubMed] [Google Scholar]
- Chen JH, Wen L, Dupuis S, Wu JY, Rao Y. The N-terminal leucine-rich regions in Slit are sufficient to repel olfactory bulb axons and subventricular zone neurons. J Neurosci. 2001;21:1548–1556. doi: 10.1523/JNEUROSCI.21-05-01548.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Correia AS, Anisimov SV, Li JY, Brundin P. Stem cell-based therapy for Parkinson’s disease. Ann Med. 2005;37:487–498. doi: 10.1080/07853890500327967. [DOI] [PubMed] [Google Scholar]
- Cummings DM, Brunjes PC. The effects of variable periods of functional deprivation on olfactory bulb development in rats. Exp Neurol. 1997;148:360–366. doi: 10.1006/exnr.1997.6660. [DOI] [PubMed] [Google Scholar]
- Davis JA, Reed RR. Role of Olf-1 and Pax-6 transcription factors in neurodevelopment. J Neurosci. 1996;16:5082–5094. doi: 10.1523/JNEUROSCI.16-16-05082.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Bergeyck V, Naerhuyzen B, Goffinet AM, Lambert de Rouvroit C. A panel of monoclonal antibodies against reelin, the extracellular matrix protein defective in reeler mutant mice. J Neurosci Methods. 1998;82:17–24. doi: 10.1016/s0165-0270(98)00024-7. [DOI] [PubMed] [Google Scholar]
- de Celis JF, Barrio R, Kafatos FC. Regulation of the spalt/spalt-related gene complex and its function during sensory organ development in the Drosophila thorax. Development. 1999;126:2653–2662. doi: 10.1242/dev.126.12.2653. [DOI] [PubMed] [Google Scholar]
- Doetsch F, Alvarez-Buylla A. Network of tangential pathways for neuronal migration in adult mammalian brain. Proc Natl Acad Sci U S A. 1996;93:14895–14900. doi: 10.1073/pnas.93.25.14895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiske BK, Brunjes PC. Cell death in the developing and sensory-deprived rat olfactory bulb. J Comp Neurol. 2001;431:311–319. [PubMed] [Google Scholar]
- Franch-Marro X, Casanova J. spalt-induced specification of distinct dorsal and ventral domains is required for Drosophila tracheal patterning. Dev Biol. 2002;250:374–382. [PubMed] [Google Scholar]
- Franke FE, Schachenmayr W, Osborn M, Altmannsberger M. Unexpected immunoreactivities of intermediate filament antibodies in human brain and brain tumors. Am J Pathol. 1991;139:67–79. [PMC free article] [PubMed] [Google Scholar]
- Geraerts M, Krylyshkina O, Debyser Z, Baekelandt V. Concise review: therapeutic strategies for Parkinson disease based on the modulation of adult neurogenesis. Stem Cells. 2007;25:263–270. doi: 10.1634/stemcells.2006-0364. [DOI] [PubMed] [Google Scholar]
- Gong Q, Shipley MT. Evidence that pioneer olfactory axons regulate telencephalon cell cycle kinetics to induce the formation of the olfactory bulb. Neuron. 1995;14:91–101. doi: 10.1016/0896-6273(95)90243-0. [DOI] [PubMed] [Google Scholar]
- Guthrie KM, Gall CM. Functional mapping of odor-activated neurons in the olfactory bulb. Chem Senses. 1995;20:271–282. doi: 10.1093/chemse/20.2.271. [DOI] [PubMed] [Google Scholar]
- Hack MA, Saghatelyan A, de Chevigny A, Pfeifer A, Ashery-Padan R, Lledo PM, Gotz M. Neuronal fate determinants of adult olfactory bulb neurogenesis. Nat Neurosci. 2005;8:865–872. doi: 10.1038/nn1479. [DOI] [PubMed] [Google Scholar]
- Halasz N, Ljungdahl A, Hokfelt T, Johansson O, Goldstein M, Park D, Biberfeld P. Transmitter histochemistry of the rat olfactory bulb. I. Immunohistochemical localization of monoamine synthesizing enzymes. Support for intrabulbar, periglomerular dopamine neurons. Brain Res. 1977;126:455–474. doi: 10.1016/0006-8993(77)90597-2. [DOI] [PubMed] [Google Scholar]
- Harrison SJ, Nishinakamura R, Monaghan AP. Sall1 regulates mitral cell development and olfactory nerve extension in the developing olfactory bulb. Cereb Cortex. 2007 doi: 10.1093/cercor/bhm191. (in press). [DOI] [PubMed] [Google Scholar]
- Hinds JW. Autoradiographic study of histogenesis in the mouse olfactory bulb. I. Time of origin of neurons and neuroglia. J Comp Neurol. 1968;134:287–304. doi: 10.1002/cne.901340304. [DOI] [PubMed] [Google Scholar]
- Hu H. Chemorepulsion of neuronal migration by Slit2 in the developing mammalian forebrain. Neuron. 1999;23:703–711. doi: 10.1016/s0896-6273(01)80029-5. [DOI] [PubMed] [Google Scholar]
- Jacobowitz DM, Abbott LC. Chemoarchitectonic atlas of the developing mouse brain. New York: CRC Press; 1998. [Google Scholar]
- Jayarajan V, Swan IR, Patton MA. Hearing impairment in 18q deletion syndrome. J Laryngol Otol. 2000;114:963–966. doi: 10.1258/0022215001904473. [DOI] [PubMed] [Google Scholar]
- Jin BK, Franzen L, Baker H. Regulation of c-Fos mRNA and fos protein expression in olfactory bulbs from unilaterally odor-deprived adult mice. Int J Dev Neurosci. 1996;14:971–982. doi: 10.1016/s0736-5748(96)00044-5. [DOI] [PubMed] [Google Scholar]
- Jurgens G. Head and tail development of the Drosophila embryo involves spalt a novel homeotic gene. EMBO J. 1988;7:189–196. doi: 10.1002/j.1460-2075.1988.tb02799.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiefer SM, McDill BW, Yang J, Rauchman M. Murine Sall1 represses transcription by recruiting a histone deacetylase complex. J Biol Chem. 2002;277:14869–14876. doi: 10.1074/jbc.M200052200. [DOI] [PubMed] [Google Scholar]
- Kohlhase J, Schuh R, Dowe G, Kuhnlein RP, Jackle H, Schroeder B, Schulz-Schaeffer W, Kretzschmar HA, Kohler A, Muller U, Raab-Vetter M, Burkhardt E, Engel W, Stick R. Isolation, characterization, and organ-specific expression of two novel human zinc finger genes related to the Drosophila gene spalt. Genomics. 1996;38:291–298. doi: 10.1006/geno.1996.0631. [DOI] [PubMed] [Google Scholar]
- Kohlhase J, Wischermann A, Reichenbach H, Froster U, Engel W. Mutations in the SALL1 putative transcription factor gene cause Townes-Brocks syndrome. Nat Genet. 1998;18:81–83. doi: 10.1038/ng0198-81. [DOI] [PubMed] [Google Scholar]
- Kohlhase J, Hausmann S, Stojmenovic G, Dixkens C, Bink K, Schulz-Schaeffer W, Altmann M, Engel W. SALL3, a new member of the human spalt-like gene family, maps to 18q23. Genomics. 1999;62:216–222. doi: 10.1006/geno.1999.6005. [DOI] [PubMed] [Google Scholar]
- Kohlhase J, Altmann M, Archangelo L, Dixkens C, Engel W. Genomic cloning, chromosomal mapping, and expression analysis of msal-2. Mamm Genome. 2000;11:64–68. doi: 10.1007/s003350010012. [DOI] [PubMed] [Google Scholar]
- Kohlhase J, Heinrich M, Liebers M, Frohlich Archangelo L, Reardon W, Kispert A. Cloning and expression analysis of SALL4, the murine homologue of the gene mutated in Okihiro syndrome. Cytogenet Genome Res. 2002a;98:274–277. doi: 10.1159/000071048. [DOI] [PubMed] [Google Scholar]
- Kohlhase J, Heinrich M, Schubert L, Liebers M, Kispert A, Laccone F, Turnpenny P, Winter RM, Reardon W. Okihiro syndrome is caused by SALL4 mutations. Hum Mol Genet. 2002b;11:2979–2987. doi: 10.1093/hmg/11.23.2979. [DOI] [PubMed] [Google Scholar]
- Kohwi M, Osumi N, Rubenstein JL, Alvarez-Buylla A. Pax6 is required for making specific subpopulations of granule and periglomerular neurons in the olfactory bulb. J Neurosci. 2005;25:6997–7003. doi: 10.1523/JNEUROSCI.1435-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kosaka K, Aika Y, Toida K, Heizmann CW, Hunziker W, Jacobowitz DM, Nagatsu I, Streit P, Visser TJ, Kosaka T. Chemically defined neuron groups and their subpopulations in the glomerular layer of the rat main olfactory bulb. Neurosci Res. 1995;23:73–88. [PubMed] [Google Scholar]
- Kosaka K, Toida K, Aika Y, Kosaka T. How simple is the organization of the olfactory glomerulus?: the heterogeneity of so-called periglomerular cells. Neurosci Res. 1998;30:101–110. doi: 10.1016/s0168-0102(98)00002-9. [DOI] [PubMed] [Google Scholar]
- Kuhnlein RP, Schuh R. Dual function of the region-specific homeotic gene spalt during Drosophila tracheal system development. Development. 1996;122:2215–2223. doi: 10.1242/dev.122.7.2215. [DOI] [PubMed] [Google Scholar]
- Kuhnlein RP, Frommer G, Friedrich M, Gonzalez-Gaitan M, Weber A, Wagner-Bernholz JF, Gehring WJ, Jackle H, Schuh R. spalt encodes an evolutionarily conserved zinc finger protein of novel structure which provides homeotic gene function in the head and tail region of the Drosophila embryo. EMBO J. 1994;13:168–179. doi: 10.1002/j.1460-2075.1994.tb06246.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurobe N, Inaguma Y, Shinohara H, Semba R, Inagaki T, Kato K. Developmental and age-dependent changes of 28-kDa calbindin-D in the central nervous tissue determined with a sensitive immunoassay method. J Neurochem. 1992;58:128–134. doi: 10.1111/j.1471-4159.1992.tb09287.x. [DOI] [PubMed] [Google Scholar]
- Lauberth SM, Rauchman M. A conserved 12-amino acid motif in Sall1 recruits the nucleosome remodeling and deacetylase corepressor complex. J Biol Chem. 2006;281:23922–23931. doi: 10.1074/jbc.M513461200. [DOI] [PubMed] [Google Scholar]
- Le W, Conneely OM, Zou L, He Y, Saucedo-Cardenas O, Jankovic J, Mosier DR, Appel SH. Selective agenesis of mesencephalic dopaminergic neurons in Nurr1-deficient mice. Exp Neurol. 1999;159:451–458. doi: 10.1006/exnr.1999.7191. [DOI] [PubMed] [Google Scholar]
- Lee JE, Ahn CH, Lee JY, Chung ES, Jeon CJ. Nitric oxide synthase and calcium-binding protein-containing neurons in the hamster visual cortex. Mol Cells. 2004;18:30–39. [PubMed] [Google Scholar]
- Lee MK, Rebhun LI, Frankfurter A. Posttranslational modification of class III beta-tubulin. Proc Natl Acad Sci U S A. 1990a;87:7195–7199. doi: 10.1073/pnas.87.18.7195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee MK, Tuttle JB, Rebhun LI, Cleveland DW, Frankfurter A. The expression and posttranslational modification of a neuron-specific beta-tubulin isotype during chick embryogenesis. Cell Motil Cytoskeleton. 1990b;17:118–132. doi: 10.1002/cm.970170207. [DOI] [PubMed] [Google Scholar]
- Lin JH, Saito T, Anderson DJ, Lance-Jones C, Jessell TM, Arber S. Functionally related motor neuron pool and muscle sensory afferent subtypes defined by coordinate ETS gene expression. Cell. 1998;95:393–407. doi: 10.1016/s0092-8674(00)81770-5. [DOI] [PubMed] [Google Scholar]
- Lindvall O, Bjorklund A. Cell therapy in Parkinson’s disease. NeuroRx. 2004;1:382–393. doi: 10.1602/neurorx.1.4.382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu N, Cigola E, Tinti C, Jin BK, Conti B, Volpe BT, Baker H. Unique regulation of immediate early gene and tyrosine hydroxylase expression in the odor-deprived mouse olfactory bulb. J Biol Chem. 1999;274:3042–3047. doi: 10.1074/jbc.274.5.3042. [DOI] [PubMed] [Google Scholar]
- Lois C, Alvarez-Buylla A. Long-distance neuronal migration in the adult mammalian brain. Science. 1994;264:1145–1148. doi: 10.1126/science.8178174. [DOI] [PubMed] [Google Scholar]
- Luskin MB. Restricted proliferation and migration of postnatally generated neurons derived from the forebrain subventricular zone. Neuron. 1993;11:173–189. doi: 10.1016/0896-6273(93)90281-u. [DOI] [PubMed] [Google Scholar]
- Mahr RN, Moberg PJ, Overhauser J, Strathdee G, Kamholz J, Loevner LA, Campbell H, Zackai EH, Reber ME, Mozley DP, Brown L, Turetsky BI, Shapiro RM. Neuropsychiatry of 18q– syndrome. Am J Med Genet. 1996;67:172–178. doi: 10.1002/(SICI)1096-8628(19960409)67:2<172::AID-AJMG7>3.0.CO;2-U. [DOI] [PubMed] [Google Scholar]
- Mandairon N, Jourdan F, Didier A. Deprivation of sensory inputs to the olfactory bulb up-regulates cell death and proliferation in the subventricular zone of adult mice. Neuroscience. 2003;119:507–516. doi: 10.1016/s0306-4522(03)00172-6. [DOI] [PubMed] [Google Scholar]
- McLean JH, Shipley MT. Postmitotic, postmigrational expression of tyrosine hydroxylase in olfactory bulb dopaminergic neurons. J Neurosci. 1988;8:3658–3669. doi: 10.1523/JNEUROSCI.08-10-03658.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menezes JR, Luskin MB. Expression of neuron-specific tubulin defines a novel population in the proliferative layers of the developing telencephalon. J Neurosci. 1994;14:5399–5416. doi: 10.1523/JNEUROSCI.14-09-05399.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreno N, Bachy I, Retaux S, Gonzalez A. Pallial origin of mitral cells in the olfactory bulbs of Xenopus. Neuroreport. 2003;14:2355–2358. doi: 10.1097/00001756-200312190-00013. [DOI] [PubMed] [Google Scholar]
- Nadi NS, Head R, Grillo M, Hempstead J, Grannot-Reisfeld N, Margolis FL. Chemical deafferentation of the olfactory bulb: plasticity of the levels of tyrosine hydroxylase, dopamine and norepinephrine. Brain Res. 1981;213:365–377. doi: 10.1016/0006-8993(81)90241-9. [DOI] [PubMed] [Google Scholar]
- Netzer C, Rieger L, Brero A, Zhang CD, Hinzke M, Kohlhase J, Bohlander SK. SALL1, the gene mutated in Townes-Brocks syndrome, encodes a transcriptional repressor which interacts with TRF1/PIN2 and localizes to pericentromeric heterochromatin. Hum Mol Genet. 2001;10:3017–3024. doi: 10.1093/hmg/10.26.3017. [DOI] [PubMed] [Google Scholar]
- Nishinakamura R, Matsumoto Y, Nakao K, Nakamura K, Sato A, Copeland NG, Gilbert DJ, Jenkins NA, Scully S, Lacey DL, Katsuki M, Asashima M, Yokota T. Murine homolog of SALL1 is essential for ureteric bud invasion in kidney development. Development. 2001;128:3105–3115. doi: 10.1242/dev.128.16.3105. [DOI] [PubMed] [Google Scholar]
- Ott T, Kaestner KH, Monaghan AP, Schutz G. The mouse homolog of the region specific homeotic gene spalt of Drosophila is expressed in the developing nervous system and in mesoderm-derived structures. Mech Dev. 1996;56:117–128. doi: 10.1016/0925-4773(96)00516-3. [DOI] [PubMed] [Google Scholar]
- Ott T, Parrish M, Bond K, Schwaeger-Nickolenko A, Monaghan AP. A new member of the spalt like zinc finger protein family, Msal-3, is expressed in the CNS and sites of epithelial/mesenchymal interaction. Mech Dev. 2001;101:203–207. doi: 10.1016/s0925-4773(00)00552-9. [DOI] [PubMed] [Google Scholar]
- Parrish M. An investigation in the roles of Spalt homologues during murine development [dissertation] Pittsburgh: University of Pittsburgh; 2003. 220 pp. [Google Scholar]
- Parrish M, Ott T, Lance-Jones C, Schuetz G, Schwaeger-Nickolenko A, Monaghan AP. Loss of the Sall3 gene leads to palate deficiency, abnormalities in cranial nerves, and perinatal lethality. Mol Cell Biol. 2004;24:7102–7112. doi: 10.1128/MCB.24.16.7102-7112.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parrish-Aungst S, Shipley MT, Erdelyi F, Szabo G, Puche AC. Quantitative analysis of neuronal diversity in the mouse olfactory bulb. J Comp Neurol. 2007;501:825–836. doi: 10.1002/cne.21205. [DOI] [PubMed] [Google Scholar]
- Ponsen MM, Stoffers D, Booij J, van Eck-Smit BL, Wolters E, Berendse HW. Idiopathic hyposmia as a preclinical sign of Parkinson’s disease. Ann Neurol. 2004;56:173–181. doi: 10.1002/ana.20160. [DOI] [PubMed] [Google Scholar]
- Puelles L, Kuwana E, Puelles E, Bulfone A, Shimamura K, Keleher J, Smiga S, Rubenstein JL. Pallial and subpallial derivatives in the embryonic chick and mouse telencephalon, traced by the expression of the genes Dlx-2, Emx-1, Nkx-2.1, Pax-6, and Tbr-1. J Comp Neurol. 2000;424:409–438. doi: 10.1002/1096-9861(20000828)424:3<409::aid-cne3>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- Ressler KJ, Sullivan SL, Buck LB. A zonal organization of odorant receptor gene expression in the olfactory epithelium. Cell. 1993;73:597–609. doi: 10.1016/0092-8674(93)90145-g. [DOI] [PubMed] [Google Scholar]
- Roy K, Kuznicki K, Wu Q, Sun Z, Bock D, Schutz G, Vranich N, Monaghan AP. The Tlx gene regulates the timing of neurogenesis in the cortex. J Neurosci. 2004;24:8333–8345. doi: 10.1523/JNEUROSCI.1148-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saino-Saito S, Cave JW, Akiba Y, Sasaki H, Goto K, Kobayashi K, Berlin R, Baker H. ER81 and CaMKIV identify anatomically and phenotypically defined subsets of mouse olfactory bulb interneurons. J Comp Neurol. 2007;502:485–496. doi: 10.1002/cne.21293. [DOI] [PubMed] [Google Scholar]
- Sakaki-Yumoto M, Kobayashi C, Sato A, Fujimura S, Matsumoto Y, Takasato M, Kodama T, Aburatani H, Asashima M, Yoshida N, Nishinakamura R. The murine homolog of SALL4, a causative gene in Okihiro syndrome, is essential for embryonic stem cell proliferation, and cooperates with Sall1 in anorectal, heart, brain and kidney development. Development. 2006;133:3005–3013. doi: 10.1242/dev.02457. [DOI] [PubMed] [Google Scholar]
- Sato A, Kishida S, Tanaka T, Kikuchi A, Kodama T, Asashima M, Nishinakamura R. Sall1, a causative gene for Townes-Brocks syndrome, enhances the canonical Wnt signaling by localizing to heterochromatin. Biochem Biophys Res Commun. 2004;319:103–113. doi: 10.1016/j.bbrc.2004.04.156. [DOI] [PubMed] [Google Scholar]
- Schaeren-Wiemers N, Gerfin-Moser A. A single protocol to detect transcripts of various types and expression levels in neural tissue and cultured cells: in situ hybridization using digoxigenin-labelled cRNA probes. Histochemistry. 1993;100:431–440. doi: 10.1007/BF00267823. [DOI] [PubMed] [Google Scholar]
- Schmid RS, Shelton S, Stanco A, Yokota Y, Kreidberg JA, Anton ES. Alpha3beta1 integrin modulates neuronal migration and placement during early stages of cerebral cortical development. Development. 2004;131:6023–6031. doi: 10.1242/dev.01532. [DOI] [PubMed] [Google Scholar]
- Snyder BJ, Olanow CW. Stem cell treatment for Parkinson’s disease: an update for 2005. Curr Opin Neurol. 2005;18:376–385. doi: 10.1097/01.wco.0000174298.27765.91. [DOI] [PubMed] [Google Scholar]
- Sonntag KC, Simantov R, Isacson O. Stem cells may reshape the prospect of Parkinson’s disease therapy. Brain Res Mol Brain Res. 2005;134:34–51. doi: 10.1016/j.molbrainres.2004.09.002. [DOI] [PubMed] [Google Scholar]
- Soria JM, Taglialatela P, Gil-Perotin S, Galli R, Gritti A, Verdugo JM, Bertuzzi S. Defective postnatal neurogenesis and disorganization of the rostral migratory stream in absence of the Vax1 homeobox gene. J Neurosci. 2004;24:11171–11181. doi: 10.1523/JNEUROSCI.3248-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stenman J, Toresson H, Campbell K. Identification of two distinct progenitor populations in the lateral ganglionic eminence: implications for striatal and olfactory bulb neurogenesis. J Neurosci. 2003;23:167–174. doi: 10.1523/JNEUROSCI.23-01-00167.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stone DM, Grillo M, Margolis FL, Joh TH, Baker H. Differential effect of functional olfactory bulb deafferentation on tyrosine hydroxylase and glutamic acid decarboxylase messenger RNA levels in rodent juxtaglomerular neurons. J Comp Neurol. 1991;311:223–233. doi: 10.1002/cne.903110205. [DOI] [PubMed] [Google Scholar]
- Stoykova A, Gruss P. Roles of Pax-genes in developing and adult brain as suggested by expression patterns. J Neurosci. 1994;14:1395–1412. doi: 10.1523/JNEUROSCI.14-03-01395.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strathdee G, Sutherland R, Jonsson JJ, Sataloff R, Kohonen-Corish M, Grady D, Overhauser J. Molecular characterization of patients with 18q23 deletions. Am J Hum Genet. 1997;60:860–868. [PMC free article] [PubMed] [Google Scholar]
- Sullivan SL, Bohm S, Ressler KJ, Horowitz LF, Buck LB. Target-independent pattern specification in the olfactory epithelium. Neuron. 1995;15:779–789. doi: 10.1016/0896-6273(95)90170-1. [DOI] [PubMed] [Google Scholar]
- Sweetman D, Munsterberg A. The vertebrate spalt genes in development and disease. Dev Biol. 2006;293:285–293. doi: 10.1016/j.ydbio.2006.02.009. [DOI] [PubMed] [Google Scholar]
- Sweetman D, Smith T, Farrell ER, Chantry A, Munsterberg A. The conserved glutamine-rich region of chick csal1 and csal3 mediates protein interactions with other spalt family members. Implications for Townes-Brocks syndrome. J Biol Chem. 2003;278:6560–6566. doi: 10.1074/jbc.M209066200. [DOI] [PubMed] [Google Scholar]
- Teicher MH, Blass EM. Suckling in newborn rats: eliminated by nipple lavage, reinstated by pup saliva. Science. 1976;193:422–425. doi: 10.1126/science.935878. [DOI] [PubMed] [Google Scholar]
- Teicher MH, Blass EM. First suckling response of the newborn albino rat: the roles of olfaction and amniotic fluid. Science. 1977;198:635–636. doi: 10.1126/science.918660. [DOI] [PubMed] [Google Scholar]
- Toida K, Kosaka K, Aika Y, Kosaka T. Chemically defined neuron groups and their subpopulations in the glomerular layer of the rat main olfactory bulb—IV. Intraglomerular synapses of tyrosine hydroxylase-immunoreactive neurons. Neuroscience. 2000;101:11–17. doi: 10.1016/s0306-4522(00)00356-0. [DOI] [PubMed] [Google Scholar]
- Toker AS, Teng Y, Ferreira HB, Emmons SW, Chalfie M. The Caenorhabditis elegans spalt-like gene sem-4 restricts touch cell fate by repressing the selector Hox gene egl-5 and the effector gene mec-3. Development. 2003;130:3831–3840. doi: 10.1242/dev.00398. [DOI] [PubMed] [Google Scholar]
- Tucker ES, Polleux F, LaMantia AS. Position and time specify the migration of a pioneering population of olfactory bulb interneurons. Dev Biol. 2006;297:387–401. doi: 10.1016/j.ydbio.2006.05.009. [DOI] [PubMed] [Google Scholar]
- Vassar R, Ngai J, Axel R. Spatial segregation of odorant receptor expression in the mammalian olfactory epithelium. Cell. 1993;74:309–318. doi: 10.1016/0092-8674(93)90422-m. [DOI] [PubMed] [Google Scholar]
- Verhoeven WM, Feenstra I, Van Ravenswaay-Arts C, Egger JI, Van Beurden JJ, Tuinier S. Neuropsychiatry and deletions of 18q; case report and diagnostic considerations. Genet Counsel. 2006;17:307–313. [PubMed] [Google Scholar]
- Waclaw RR, Allen ZJ, 2nd, Bell SM, Erdelyi F, Szabo G, Potter SS, Campbell K. The zinc finger transcription factor Sp8 regulates the generation and diversity of olfactory bulb interneurons. Neuron. 2006;49:503–516. doi: 10.1016/j.neuron.2006.01.018. [DOI] [PubMed] [Google Scholar]
- Wahlin KJ, Campochiaro PA, Zack DJ, Adler R. Neurotrophic factors cause activation of intracellular signaling pathways in Muller cells and other cells of the inner retina, but not photoreceptors. Invest Ophthalmol Vis Sci. 2000;41:927–936. [PubMed] [Google Scholar]
- Walther C, Gruss P. Pax-6, a murine paired box gene, is expressed in the developing CNS. Development. 1991;113:1435–1449. doi: 10.1242/dev.113.4.1435. [DOI] [PubMed] [Google Scholar]
- Wichterle H, Garcia-Verdugo JM, Herrera DG, Alvarez-Buylla A. Young neurons from medial ganglionic eminence disperse in adult and embryonic brain. Nat Neurosci. 1999;2:461–466. doi: 10.1038/8131. [DOI] [PubMed] [Google Scholar]
- Wichterle H, Turnbull DH, Nery S, Fishell G, Alvarez-Buylla A. In utero fate mapping reveals distinct migratory pathways and fates of neurons born in the mammalian basal forebrain. Development. 2001;128:3759–3771. doi: 10.1242/dev.128.19.3759. [DOI] [PubMed] [Google Scholar]
- Wilson MG, Towner JW, Forsman I, Siris E. Syndromes associated with deletion of the long arm of chromosome 18[del(18q)] Am J Med Genet. 1979;3:155–174. doi: 10.1002/ajmg.1320030207. [DOI] [PubMed] [Google Scholar]
- Wu W, Wong K, Chen J, Jiang Z, Dupuis S, Wu JY, Rao Y. Directional guidance of neuronal migration in the olfactory system by the protein Slit. Nature. 1999;400:331–336. doi: 10.1038/22477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshihara S, Omichi K, Yanazawa M, Kitamura K, Yoshihara Y. Arx homeobox gene is essential for development of mouse olfactory system. Development. 2005;132:751–762. doi: 10.1242/dev.01619. [DOI] [PubMed] [Google Scholar]
- Yun K, Garel S, Fischman S, Rubenstein JL. Patterning of the lateral ganglionic eminence by the Gsh1 and Gsh2 homeobox genes regulates striatal and olfactory bulb histogenesis and the growth of axons through the basal ganglia. J Comp Neurol. 2003;461:151–165. doi: 10.1002/cne.10685. [DOI] [PubMed] [Google Scholar]