Abstract
Despite being a proinflammatory cytokine, tumor necrosis factor-α (TNF-α) preconditions neurons against various toxic insults. However, underlying molecular mechanisms are poorly understood. The present study identifies the importance of CREB-binding protein (CBP) in facilitating TNF-α-mediated preconditioning in neurons. Treatment of rat primary neurons with fibrillar amyloid-β 1–42 (Aβ) resulted in the loss of CBP protein. However, this loss was compensated by TNF-α preconditioning as the expression of neuronal CBP was upregulated in response to TNF-α treatment. The induction of CBP by TNF-α was observed only in neurons, but not in astroglia and microglia, and it was contingent on the activation of transcription factor NF-κB. Interestingly, antisense knockdown of CBP abrogated TNF-α-mediated preconditioning of neurons against Aβ and glutamate toxicity. Similarly in vivo, pre-administration of TNF-α in mouse cortex prevented Aβ-induced apoptosis and loss of choline acetyl transferase (ChAT)-positive cholinergic neurons. However, co-administration of cbp antisense, but not scrambled oligonucleotides, negated the protective effect of TNF-α against Aβ neurotoxicity. This study illustrates a novel biological role of TNF-α in increasing neuron-specific expression of CBP for preconditioning that may have therapeutic potential against neurodegenerative disorders.
Keywords: Gene Regulation, Neuroimmunology, Amyloid-β, TNF-α, Transcription Factors
Introduction
Fatal neurodegenerative conditions sometimes fail to induce extensive damage due to prior exposure(s) of neurons to similar conditions in milder form. Such instances have been recorded in clinics, where stroke patients with an history of ipsilateral transient ischemic attacks (TIA) experience limited damage than stroke patients without any history of TIA (1, 2). The mechanism underlying such tolerance is referred to as neuronal preconditioning, neuronal tolerance or neurohormesis (3).
Neurons can undergo preconditioning in response to mild ischemia, oxidative stress, seizures, anoxia, excitotoxicity, and pro-inflammatory molecules like, TNF-α (4–6). Treatment of neurons with low to moderate dose of TNF-α, the cytokine with several functions in normal and diseased brain (7, 8), preconditions and protects neurons from metabolic excitotoxicity (9–11), nitric oxide toxicity (12) and Aβ toxicity (13). Additionally, TNF-α also mediates the preconditioning induced by mild hypoxic stress (14), sub-lethal ischemia in vivo (15, 16) and in vitro (17). In addition to murine models, TNF-α also mediates ischemic tolerance in human stroke (18).
However, molecular mechanisms of TNF-α-induced neuronal preconditioning are poorly understood (19). It is known that preconditioning aids in the preservation of mitochondrial membrane integrity thereby blocking or delaying the onset of apoptosis (20). Additionally, significant delay in onset of tolerance after the preconditioning suggests the involvement of extensive transcription in this process. Additionally, preconditioning requires de novo protein synthesis as treatment with cycloheximide (translation blocker) attenuates preconditioning of neurons (21–23). Because co-activator CBP, with inherent acetyl transferase activity, is an integral part of the transcriptional machinery that primes chromatin for transcription in addition to regulating the activity of several signal-induced transcription factors (24), we wondered if CBP was responsible for TNF-α-mediated preconditioning of neurons. CBP was particularly an interesting candidate because it plays an important role in memory consolidation as an acetyl transferase (25, 26) and loss of its function has been associated with Huntington’s disease (27, 28), Rubinstein-Taybi Syndrome (29) and neurodegeneration in general (30). In the course of this study, we have learned that TNF-α induces the expression of CBP both in vitro and in vivo specifically in neurons. Furthermore, we also report that this induction of CBP is necessary for effective preconditioning of neurons by TNF-α.
Material and methods
Reagents
Rat recombinant TNFα (ED50 = 10 – 20 pg/ml) was obtained from R&D Systems (Minneapolis, MN) while Aβ was obtained from Biomol (Plymouth Meeting, PA). Wild type and mutated NBD peptides were also procured from Biomol. Suppliers of various antibodies utilized in this study are mentioned in parenthesis as follow: Anti-CBP (from Upstate; did not cross react with p300), Anti-p50 (Upstate, Charlottesville, VA), Anti-phospho S276 p65, anti-Aβ and anti-MnSOD (Abcam, Cambridge, MA), anti-p65, anti-LaminB, anti-GFAP and anti-Actin (Santacruz Biotechnology, Santa Cruz, CA). The TUNEL assay kit and AlexaFluor conjugated secondary antibodies were obtained from Molecular Probes, Invitrogen (Carlsbad, California).
Isolation of rat cerebellar granule neurons
Rat neurons were prepared as described previously (31) with some modifications. In brief, cerebella of 7-day-postnatal Sprague-Drawly rats were dissociated in Versene solution (1:5000, Invitrogen) and total cells (1 × 106 cells per 35 mm2) were plated in poly-D-Lysine (Sigma, St. Louis, MO) coated plates or dishes for five minutes after which, the non-adherent cell suspension was removed and used for preparing glial cells. Adherent cells were maintained in MEM (Mediatech, Herndon, VA) supplemented with heat-inactivated 10% fetal bovine serum (FBS, Atlas Biologicals, Fort Collins, CO), 25 mM KCl, 3gm/500 glucose (Sigma), and 1% antibiotic-antimycotic solution (Sigma). Cytosine-D-arabinoside (10 μM Ara-C, Sigma) was added to cultures 24 hrs after plating to block the proliferation of non-neuronal cells. These neurons were routinely used during 9–12 days in vitro (DIV). Ara-C was avoided to obtain neuron-glial mix culture (where glial growth was allowed in the neuronal culture). Rat hippocampal and cortical neurons were obtained from E18 embryos. The hippocampi or the cortices were dissected and dissociated in HBSS solution. Then they were plated in Poly-D-Lysine (Sigma) coated plates or dishes in Neurobasal media supplemented with B27 (Invitrogen). These neurons were routinely used during 14–21 days in vitro (DIV).
Isolation of microglia and astrocytes
Rat microglia and astroglia were obtained from the non-adherent cell suspension after plating of neurons. This fraction was then plated in poly-D-lysine-coated flasks or dishes and maintained in culture by replenishment of media every third day. After 9 days, the flasks were shaken at 240 rpm for 2 hours to separate microglia from the more adherent mass of astrocytes. Detailed protocol has been published elsewhere (32).
Preparation of fibrillar Aβ
Fibrillar Aβ1–42 and control reverse peptide Aβ42-1 (Bachem Bioscience, CA) were prepared by incubating freshly solubilized peptides at 50 μM in sterile distilled water at 37°C for 5 d (33).
Treatment of neurons with antisense oligonucleotides
Phosphorothioate-labeled following antisense (ASO) and scrambled oligonucleotides (ScO), which show no homology to any known genes, were synthesized from the Invitrogen (Carlsbad, CA).
ASO: 5′-CAG CAA GTT CTC GGC CAT-3′
ScO: 5′-CGA TGC GTC ATC GAC CAT-3′
In order to modulate the level of CBP, neurons in culture received 2μM oligonucleotides an hour prior to the TNF-α treatment. After 24 h of treatment with TNF-α, cells were challenged with fibrillar Aβ1–42 for another 18 h (for monitoring mRNA) or 24 h (for monitoring protein).
RNA isolation, Reverse Transcriptase (RT)-PCR and quantitative PCR (qPCR)
Total RNA was isolated from neurons using RNA-Easy Qiagen kit following manufactures protocol. To remove any contaminating genomic DNA, total RNA was digested with DNase. Semi-quantitative RT-PCR was carried out as described earlier (34) using oligo(dT)12–18 as primer and MMLV reverse transcriptase (Clontech) in a 20-μl reaction mixture. The resulting cDNA was appropriately diluted and then amplified using Titanium Taq polymerase and following primer sets:
Rat-CBP sense: 5′-CAG AGC TTC TTA GAG GAG GCA-3′
Rat-CBP anti-sense: 5′-GTC TCA GCC AGC ACA CTG CTT-3′
Rat-GAPDH sense: 5′-CGT GGA GTC TAC TGG CGT CTT CAC C-3′
Rat-GAPDH anti-sense: 5′-TGG CAG CAC CAG TGG ATG CA-3′
Band intensities in DNA gels were quantified with the software Typhoon ImageQuant.
For qPCR, cDNA was prepared as mentioned above. This was amplified using the Applied Biosystems Fast SYBR Green master mix in an AB 7500 Fast Real Time machine. The primers used are as follows:
Rat-CBP sense: 5′-AAGCAACAAATTGGGTCACCAGGC-3′
Rat-CBP anti-sense: 5′-TACTAAGGGATGTGGCGATCTGCT-3′
Rat-GAPDH sense: 5′-AGA GAC AGC CGC ATC TTC TTG-3′
Rat-GAPDH anti-sense: 5′-GGT AAC CAG GCG TCC GAT AC-3′
Immunoblotting assays
To obtain whole cell lysate, neurons were lysed in RIPA buffer [1× PBS, 1% NP-40, 0.5% Na-deoxycholate, 0.1% SDS with freshly added 0.5% protease inhibitor cocktail (PIC, Sigma)] in ice. To obtain nuclear lysate, neurons were first treated with a non-ionic detergent buffer [10mM HEPES (pH 7.9), 10mM KCl, 2mM MgCl2, 0.5mM dithiothreitol, 0.1% NP-40] and subsequently the nuclear pellet by lysing them in NETN buffer [0.5% NP-40, 1mM EDTA, 50mM Tris, 120mM NaCl, pH 7.5] freshly supplemented with 0.5% protease inhibitor cocktail (Sigma) and phosphatase inhibitor cocktails (Sigma). Protein content was estimated by Protein assay dye reagent concentrate (Bio-Rad) using manufacturer’s protocol, and immunoblotting was performed as described previously (35). Immuno-blots were probed either by chemiluminescence (PerkinElmer, Waltham, Massachusetts) or by fluorescence detection in Odyssey infrared imaging system (LI-COR Biosciences, Lincoln, NE) and band intensities were quantified with the software Typhoon ImageQuant.
Immunocytochemistry
Cells were fixed with 4% paraformaldehyde and permeabilized with chilled methanol for 5 minutes at −20 degree C. Fixed cells or deparaffinized brain sections were blocked with 3% BSA-PBS for 1 hour at RT followed by incubation with primary antibodies in 1% BSA-PBS for 3 hours at 37°C. Subsequently samples were washed thrice with PBS-Tween solution, incubated with Cy5 tagged secondary antibodies (Jackson ImmunoResearch), and observed under a BioRad MRC1024ES confocal laser-scanning microscope. DAPI (Invitrogen) was added during the final wash step at a dilution of 1:10000.
Electrophoretic mobility shift assay (EMSA) and supershift assay
Nuclear extracts were prepared and EMSA was performed as described previously (35, 36). Briefly, infrared dye (IRDye700)-labeled oligonucleotides containing the consensus binding sequence (underlined) for NF-κB (5′-AGT TGA GGG GAC TTT CCC AGG C-3′) were purchased from Licor Biosciences (Lincoln, NE). Ten microgram of nuclear extract was incubated with binding buffer in ice for 5 minutes prior to incubation with labeled probe for another 15 minutes at room temperature. For the supershift assay, nuclear extract suspended in binding buffer was incubated with 1μg antibody for half hour in ice. Subsequently, samples were separated on a 6% polyacrylamide gel in 0.25 × TBE buffer, which were then scanned with Odyssey infrared imaging system.
Microinjection in mice
Male C57BL/6 mice (8–10 week of age) were anesthetized with ketamine and xylazine and underwent cerebellar operations in a Kopf small animal stereotaxic instrument (Kopf Instruments, Tujunga, CA). Briefly, the animal was mounted in a stereotaxic frame on a heating blanket. Body temperature was maintained at 37 ± 0.5°C during the time of surgery. A midsagittal incision was made to expose the cranium, and a hole <0.5 mm in diameter was drilled with a dental drill over the cerebrum according to the following coordinates: 1.5 mm anterior to lambda, lateral (L) 1.5 mm, and ventral (V) 1 mm. TNF-α (300 ng) dissolved in 3 μl of saline was injected using a 5 μl syringe (Hamilton, Reno, NV) over a period of 2 min, and the needle was held in place for another minute before withdrawing it from the skull to prevent reflux up the needle tract. Similarly, control mice received 3 μl of saline. The incision was closed with surgical staples and covered with a mixture of Bacitracin and Hurricane (20% benzocaine). For Aβ injections, incisions were re-opened after 24 hours and 1 μg Aβ (dissolved in 2 μl saline) was injected in the same incision.
Histological analysis
After 24 h of microinjection of Aβ, mice (n=5) from each groups were anaesthetized with a mixture of ketamine (66.6 mg/kg) and xylazine (6.66 mg/kg) by i.p. injection. Cortex was dissected out from each mice following perfusion with PBS (pH 7.4) and then with 4% (w/v) paraformaldehyde solution in PBS (34). The tissues were further fixed for at least 2 h in the same fixative at room temperature followed by three PBS washes (15 min each). Paraformaldehyde fixed tissues were embedded in paraffin and serial sections (5 μm) were cut. Sections were stained with Haematoxylin-Eosin (H&E) to obtain infiltration and morphological details of cortical tissues..
Fragment end labeling of DNA (TUNEL)
Fragmented DNA was detected in situ by the terminal deoxynucleotidyl transferase (TdT)-mediated binding of 3′-OH ends of DNA fragments generated in response to apoptotic signals, using a commercially available kit from Invitrogen as per manufacturer’s protocol.
MTT assay
Mitochondrial activity (a measure of cellular viability) was measured with the 3-(4,5-dimethylthiazole-2-yl)-2,5-diphenyl tetrazolium (MTT) assay (Sigma) as per manufacturer’s protocol as described previously (33).
Results
TNF-α preconditioning protects rat primary cerebellar granule neurons from Aβ-induced neurotoxicity
In order to test the efficiency of TNF-α in mediating preconditioning in our hands, we treated rat primary cerebellar granule neurons with TNF-α. This cytokine is known to precondition neurons in vitro at various doses ranging from 1–100 ng/ml TNF-α (9, 12, 13). We preferred to test the efficiency of a comparatively lower dose. Thus neurons were pretreated for 24 hours with 10 ng/ml TNF-α. Subsequently, they were challenged with 1μM fibrillar Aβ1–42 for another 24 hours in fresh media and an MTT assay was performed. As seen in Figure 1, treatment with Aβ induced significant degree of cell death, which was eliminated by TNF-α pretreatment. This verifies the TNF-α-induced cross tolerance model against Aβ in our hands.
Figure 1. TNF-α preconditioning protects primary rat cerebellar granule neurons from Aβ toxicity.
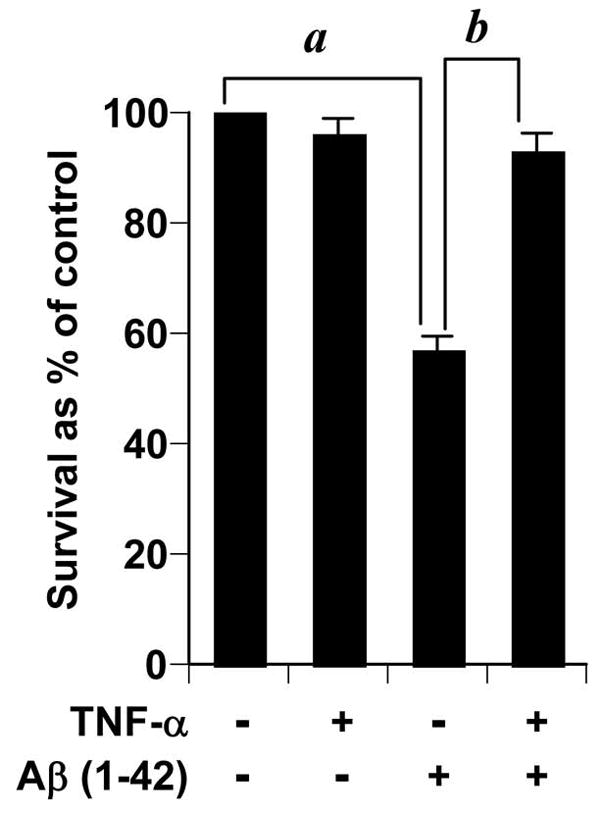
Neurons preconditioned with 10 ng/ml of TNF-α for 24 hours were treated with 2μM 1–42 Aβ for another 24 hours. Subsequently, survival was assessed by MTT assay which was run duplicate. Data from three independent experiments were analyzed by ANOVA. a,b p < 0.01.
Aβ toxicity is marked by the loss of CBP
The loss of CBP in response to neurodegenerative challenges has been observed in various neurodegenerative models and diseases (24). However, the fate of CBP in the face of Aβ-induced neurotoxicity is unknown. Thus, we next investigated the abundance of CBP in neurons 6 hours after fibrillar Aβ challenge. As seen in figure 2A, there was a severe loss of CBP protein in response to Aβ treatment. This loss was however, not manifested at the level of cbp gene expression, which remained unperturbed at the basal level in treated as well as untreated cells (Fig. 2B). Together, it is evident that Aβ-induced loss of CBP was a post-translational event.
Figure 2. Aβ induces the loss of CBP in primary rat cerebellar granule neurons.
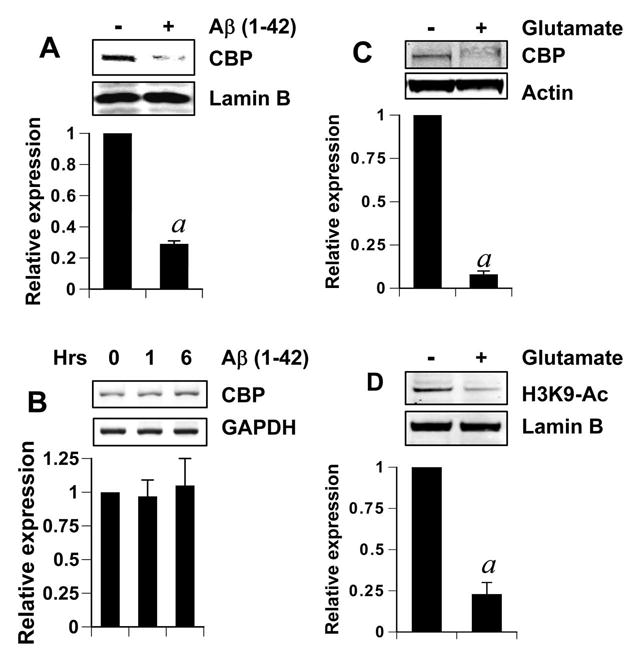
(A) Nuclear extracts were obtained from neurons treated with 2μM Aβ for 6 hours. Sixty microgram nuclear extract of each sample was subjected to gel electrophoresis and western blotting. Blots were probed with anti-CBP antibody (1:500 dilution) and re-probed with nuclear marker LaminB (1:1000 dilution). The relative expression of CBP (CBP/LaminB) was measured after scanning the bands (lower panel). Results represent mean ± SD of three separate attempts. ap < 0.001 versus control. (B) Neurons were treated with 2μM Aβ for indicated time periods and total RNA was obtained from them. RT-PCR was conducted subsequently with primers specific for rat CBP and GAPDH. Data from three independent attempts were quantified and relative expression of CBP (CBP/GAPDH) is shown in lower panel. Results represent mean ± SD of three separate attempts. (C and D) Whole cell lysates (C) or nuclear extract (D) were isolated from cortical neurons that were treated with 100μM glutamate for 6 hours. After gel electrophoresis and western blotting, blots were probed with either anti-CBP antibody (C; 1:500 dilution) or anti-acetylated Histone3 antibody (D; 1:1000 dilution) and re-probed with loading control marker Actin (C) or Lamin B (D). The relative expression of CBP (CBP/Actin) (lower panel of C) and the relative acetylation of histone H3 (acetylated H3/Lamin) (lower panel of D) were measured after scanning the bands. Results represent mean ± SD of three separate attempts. ap < 0.001 versus control.
We also tested CBP levels in neurons treated with excess glutamate for 6 hours. Excess glutamate results in excitotoxic neuronal death and TNF-α preconditioning has been shown to prevent such toxicity previously (9, 11). Neurons treated with glutamate, like ones treated with Aβ, showed distinct loss of CBP (Fig. 2C). It may be noted that whole cell lysate was used in figure 2C to negate any concerns about re-distribution of CBP in non-nuclear spaces in response to toxicity. Similar data were obtained by using whole cell lysate after Aβ treatment (data not shown). This suggests a true loss of CBP protein upon toxic challenges. To see if this loss of CBP had any functional effect, we examined the acetylation level of histone H3, which is known to be acetylated by CBP at its Lysine9 residue. After 6 hours of glutamate treatment, the acetylation level of H3-Lysine9 was significantly reduced in comparison to control (Fig. 2D).
TNF-α upregulates expression of CBP in neurons
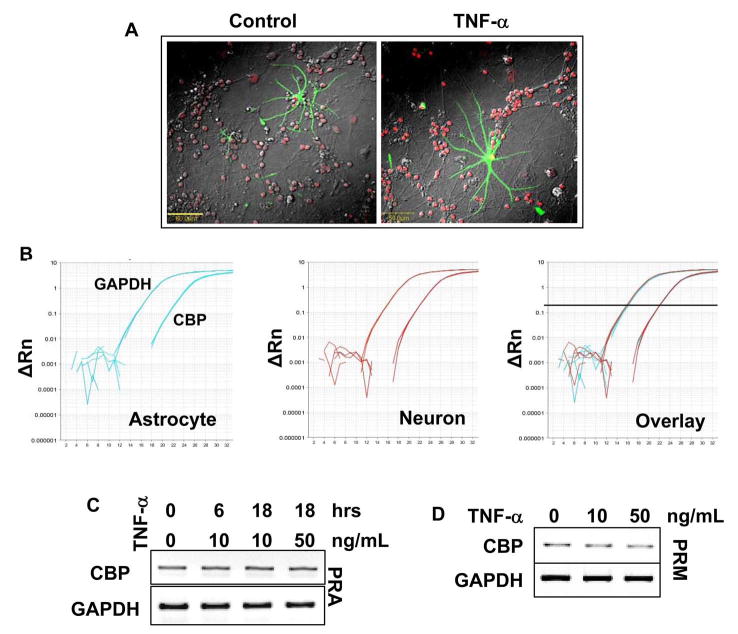
We next investigated if TNF-α-induced neuronal preconditioning involved CBP upregulation. Cells were treated with various doses of TNF-α and nuclear extract was immunoblotted with antibodies against CBP. As seen in figure 3A, treatment with TNF-α upregulated the protein level of CBP with respect to untreated cells. Among all tested doses, 10ng/ml TNF-α was the most effective in inducing CBP than either a higher or a lower dose (Fig. 3A). Next, we performed a time-course of TNF-α treatment where neurons were treated with 10ng/ml TNF-α for different time points. Western blot of these extracts revealed an increase in CBP level at 12 hours which persisted and increased to attain a peak at 24 hours (Fig. 3B). However, CBP was not detected at time points earlier than 12 hours. To confirm our western blot results, we performed immunocytochemical detection of CBP in treated and untreated cerebellar and hippocampal neurons. As seen in figure 3, TNF-α-treated cerebellar (Fig. 3C) and hippocampal neurons (Fig. 3D) showed greater immunoreactivity for CBP than untreated cells that were localized in the nuclear body of neurons. Similar observations were made in rat cortical neurons (data not shown).
Figure 3. TNF-α upregulates CBP.
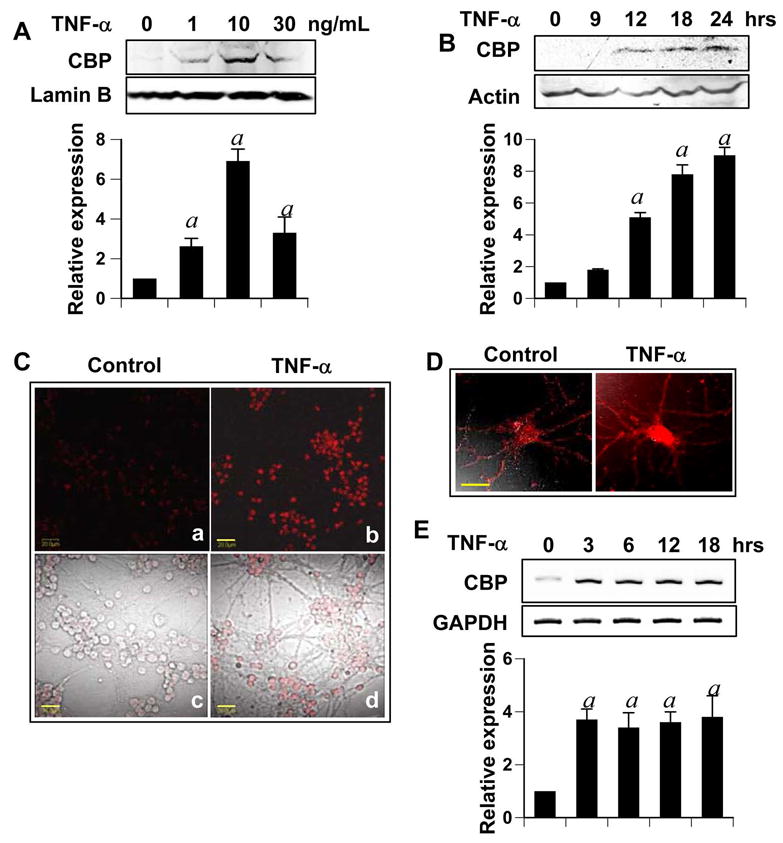
Pure cultures of cerebellar granular neurons were treated with (A) indicated dose of TNF-α for 24 hours, or, (B) 10 ng/ml TNF-α for the times indicated. Nuclear extract (A) or whole cell lysates (B) were subjected to gel electrophoresis and western blotting. Blots were probed with anti-CBP antibody (1:500 dilution) and re-probed with LaminB (1:1000 dilution) for A and Actin (1:1000 dilution) for B. The relative expression of CBP (either CBP/LaminB or CBP/Actin) was measured after scanning the bands (lower panel). Results represent mean ± SD of three separate experiments. ap < 0.001 versus control. (C & D) After treating pure granular (C) or hippocampal (D) neurons for 24 hours with 10 ng/ml TNF-α, cells were fixed, immuno-stained with anti-CBP antibody (1:100 dilution) and were visualized by using a AlexaFluor-conjugated secondary antibody (1:400 dilution). Bars in (C) and (D) are 20μm and 10μm respectively (E) Pure cultures of neurons were treated with 10ng/ml TNF-α for indicated time and RT-PCR was performed from extracted total RNA. Data from three independent attempts were quantified and relative expression of CBP (CBP/GAPDH) is shown in lower panel. ap < 0.001 versus control.
Next we tested whether TNF-α-induced CBP upregulation involved induction of cbp. To this end, we performed RT-PCR with specific primers designed against rat CBP gene. As represented in figure 3D, TNF-α treatment induced the mRNA expression of cbp significantly. Interestingly, although upregulated CBP was not detected until 12 hours post treatment, cbp transcripts were detected as early as 3 hours. Moreover, the level of cbp transcript expression remained high at all tested time points until 18 hours.
TNF-α-induced CBP upregulation is neuron specific
Is the CBP upregulating effect of TNF-α restricted to neurons or is it a global phenomenon in other brain cells like astroglia and microglia? To answer this question, neuron-astrocyte co-cultures were treated with the cytokine followed by immuno-staining with GFAP (astrocyte marker) and CBP. The expression of CBP was enhanced in neurons despite the presence of glial cells, but astrocytes did not show any increased expression of this protein. This could be due to a greater level of basal cbp expression in astrocytes. To verify, we performed qPCR to detect basal level of cbp in pure cultures of astrocytes and neurons. Ct values (22.132 ± 0.01 for astrocytes and 22.142 ± 0.07 for neurons at a threshold of 0.2) and relative basal expression of cbp to gapdh (0.013 ± 0.002 for astrocytes and 0.017 ± 0.005 for neurons) was comparable in these two cell types (Fig. 2B).
To further confirm the lack of cbp induction in astrocytes, we treated pure astrocytes with various doses of TNF-α for various time-points. No significant change in astroglial cbp transcript level was recorded for any dose or time point (Fig. 4C). Next, similar experiments were conducted in microglia, the other brain cell type. As in astrocytes, we did not observe any induction of cbp in these cells as well (Fig. 4D). Together, these studies suggest that TNF-α-mediated upregulation of CBP is a neuron-specific event.
Figure 4. TNF-α upregulates CBP in neurons, but not glia.
(A) Mixed neuronal and glial cultures were treated with 10 ng/ml TNF-α for 24 hours. Subsequently, fixed cells were immuno-stained with anti-CBP (1:100 dilution) and anti-GFAP (1:200 dilution) antibody. GFAP and CBP immunoreactivity were visualized by using a AlexaFluor 488 (green) and AlexaFluor 633 (red) conjugated secondary antibody respectively. (B) To test the basal cbp expression in resting neurons and glia, RNA was obtained from healthy cells and RT-PCR was performed with 2μg RNA from each sample. Subsequently equal amount of RT-PCR product was used for qPCR. Note that CBP Ct values are comparable in neurons and glia as is their relative expression to GAPDH (C and D) Pure astroglia (C; PRA) and microglia (D; PRM) cultures were treated with indicated dose of TNF-α for indicated time. RT-PCR was performed from extracted total RNA with primers specific for rat CBP and GAPDH. Data represents three independent experiments.
TNF-α-dependent expression of CBP is contingent on NF-κB and CREB
Most of the TNF-α-dependent neuroprotective gene regulation is mediated by NF-κB (37). Therefore, to investigate the mechanism of TNF-α-induced CBP expression, we tested if this upregulation was also dependent on NF-κB activation. Rat primary cerebellar granule neurons were treated with TNF-α for different time points and nuclear extracts were immunoblotted to investigate nuclear translocation of NF-κB in neurons (Fig. 5A). We found that neurons responded to TNF-α treatment by localizing NF-κB-p50 and NF-κB-p65 into the nucleus. This localization was most intense at 60 minute. Additionally, we also found that nuclear p65 was phosphorylated at S276, an indicator of the active state for the transcription factor as phosphorylation at this serine residue is critical for DNA binding and gene transcription by NF-κB (38). Therefore, these data suggest nuclear localization of NF-κB subunits in an active state. Next we examined the DNA-binding activity of NF-κB. As seen in figure 5B and 5C, TNF-α treatment induced a slower migrating band, which was ablated by antibody against p50, p65 and phospho-S276-p65, but not by normal IgG. These results suggest that TNF-α was capable of activating the classical NF-κB heterodimer containing active p65.
Figure 5. TNF-α-mediated CBP upregulation is dependent on NF-κB.
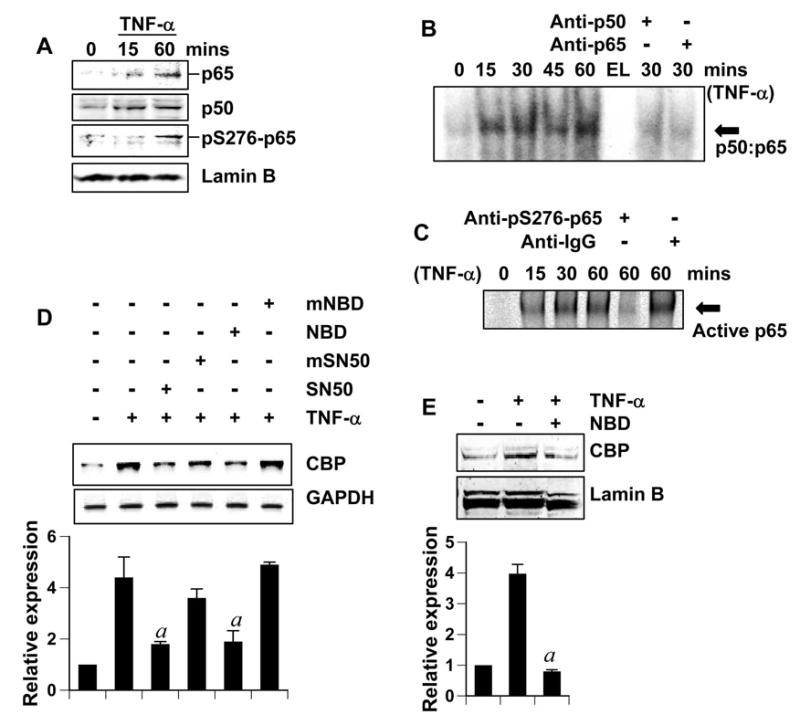
(A) Cerebellar granule neurons were treated with 10 ng/ml TNF-α for indicated times. Subsequently, nuclear extracts were isolated and 25μg nuclear extract was separated by gel electrophoresis and immunoblotted with anti-p65 and anti-p50 (both 1:1000 dilution). Membranes were stripped and reprobed with anti-phosphoS276-p65 (1:500) and anti-LaminB (1:1000) (loading control for nuclear extract). (B and C) Cerebellar granule neurons (B) and cortical neurons (C) were treated with 10 ng/ml TNF-α for indicated minutes. Then nuclear extracts (15μg) were used to perform EMSA with a labeled consensus κB probe. For supershift assay (rightmost lanes), 1μg anti-p65 and anti-p50 antibody (B) or 1μg anti-phosphoS276-p65 and normal IgG (C) was added to the binding mixtures. EL (B): empty lane. (D) Cerebellar granule neurons incubated with 2 μM SN50 (or mutated-SN50) or 5μM NBD peptide (or mutated-NBD peptide) for 2 hours were treated with 10 ng/ml TNFα for 18 hours followed by RT-PCR analysis. The relative expression of CBP (CBP/GAPDH) was measured after scanning the bands (lower panel). Results represent mean ± SD of three separate experiments. ap < 0.001 versus TNF-α. (E) Cerebellar granule neurons incubated with 5μM NBD peptide for 2 hours were treated with 10 ng/ml TNF-α for 24 hours. Nuclear extracts (25μg) were separated by gel electrophoresis and western blotted. The membranes were probed with anti-CBP antibody and then re-probed with anti-LaminB antibody. The relative expression of CBP (CBP/LaminB) was measured after scanning the bands (lower panel). Results represent mean ± SD of three separate experiments. ap < 0.001 versus TNF-α.
Next, we investigated whether TNF-α-mediated upregulation of CBP was dependent on NF-κB. SN50, a synthetic peptide containing signal sequences of Kaposi’s fibroblast growth factor and the nuclear localization sequence of NF-κB p50, has been shown to inhibit the activation of NF-κB (39). Similarly, peptides corresponding to the NF-κB essential modifier (NEMO)-binding domain (NBD) of IκB kinase (IKK) α or IKKβ have been also shown to suppress the induction of NF-κB activation without inhibiting the basal NF-κB activity (40). We used these two specific inhibitors to examine the role of NF-κB in the expression of CBP. Pretreatment of neurons with SN50 and NBD peptide, but not their mutated forms, reversed TNF-α-induced increment in cbp transcript formation (Fig. 5C). Similarly, NBD peptides also blocked TNF-α-induced expression of CBP protein (Fig. 5D). These results suggest that TNF-α stimulates the expression of CBP in neurons via activation of NF-κB.
TNF-α-preconditioning of rat primary cerebellar granule neurons against Aβ toxicity depends on the upregulation of CBP
Because TNF-α induced the expression of CBP in neurons, we were curious if this upregulated CBP was responsible for TNF-α-mediated neuronal preconditioning. To do so, at first, we examined if TNF-α was capable of increasing the level of CBP in Aβ-challenged neurons. Neurons pretreated with TNF-α were challenged with Aβ. It was interesting to observe that while Aβ treatment itself did not induce cbp expression (as shown previously in Fig. 2B), there was increased level of cbp transcript in preconditioned neurons treated subsequently with Aβ (Fig. 6A). This suggests that mechanisms driving upregulation of TNF-induced CBP remain active despite Aβ treatment. At the protein level, abundant CBP was recorded in TNF-α-preconditioned and Aβ-treated neurons with respect to neurons with Aβ treatment only (Fig. 6B). This suggests that these preconditioned neurons could override the Aβ-triggered CBP loss and possess adequate CBP in their system. Next, we investigated the role of induced CBP in neuronal preconditioning. Antisense oligonucleotides provide the most effective tool with which to investigate the in vivo functions of different proteins in primary neurons without altering neuronal characteristics. Therefore, we adopted antisense oligonucleotides against CBP to knock down the protein. This anti-sense and its scrambled control have been previously described (41). As shown in figure 6C, the antisense, but not the scrambled oligonucleotides effectively blocked TNF-α induced CBP expression in neurons. Next, we assessed survival efficiency in TNF-α-preconditioned and non-preconditioned neurons in response to Aβ challenge. The antisense was added to neuronal cultures 1 hour prior to TNFα treatment to target the induced cbp transcript without interfering endogenous basal CBP level significantly (this plan is subsequently referred to as co-treatment). As seen in figure 6D, preconditioned neurons, which have been co-treated with CBP antisense, but not scrambled oligonucleotides, succumbed significantly to Aβ toxicity. This suggests that TNFα-induced CBP is critical for neuronal tolerance against Aβ.
Figure 6. TNF-α preconditioning is dependent on the upregulation of CBP.
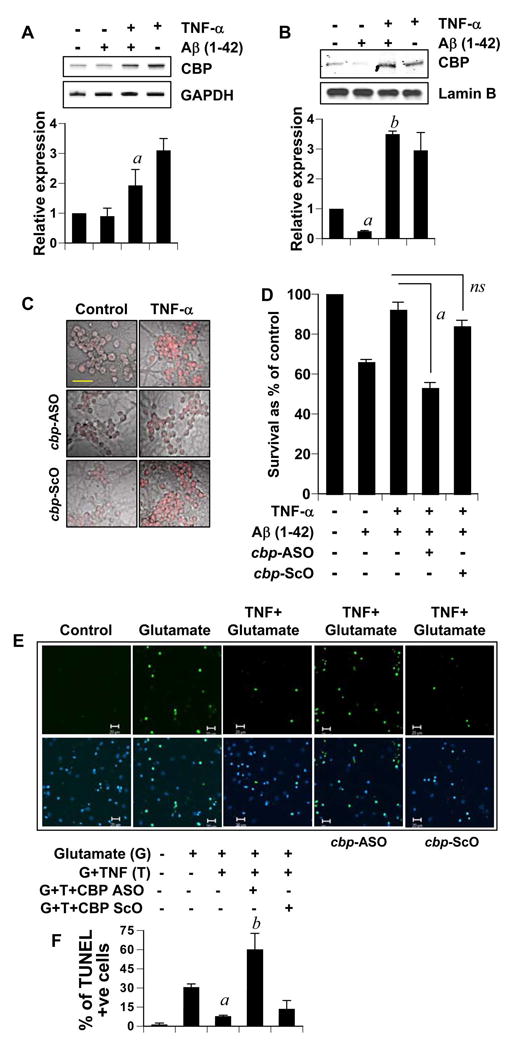
(A) Primary rat cerebellar granule neurons incubated with TNF-α for 24 hours were treated with 1μg/ml Aβ for another 6 hours. Total RNA was used to perform RT-PCR. The relative expression of CBP (CBP/GAPDH) was measured after scanning the bands (lower panel). Results represent mean ± SD of three separate experiments. ap < 0.001 versus Aβ. B) Nuclear extracts (15 μg) obtained from cerebellar granule neurons treated as in (A) were subjected for western blotting. The membrane was probed with anti-CBP antibody and then reprobed with anti-LaminB antibody. Data from three independent attempts were quantified and expressed as relative expression (CBP/LaminB) in lower panel. ap < 0.001 versus control. bp < 0.001 versus Aβ. (C) Cerebellar granule neurons incubated with 2 μM anti-sense against cbp-transcript (cbp-ASO) or a scrambled oligonucleotides (cbp-ScO) for an hour were treated with 10 ng/ml TNFα for 24 hours. CBP level in these cells was detected by immunocytochemistry using anti-CBP antibody (Bar = 20 μM. (D) Cerebellar granule neurons were treated as in (C) and were further subjected to 24 hours treatment with 1μM Aβ. MTT assays were subsequently performed. Results were statistically analyzed with ANOVA and are expressed as mean + SD of three separate experiments (ap<0.001). (E) Rat cortical neurons were treated as in (D), except, cells were treated with 100μM glutamate instead of Aβ for 18 hours. Subsequently, cells were fixed and TUNEL labeled. Bar = 20μm. F) TUNEL positive cells were counted in an Olympus IX81 fluorescence microscope using the MicroSuite™ imaging software and represented as percentage of TUNEL positive cells from a total of DAPI nuclei. Data represent observations made from at least five randomly selected microscopic fields from each of three independent trials. ap < 0.001 versus glutamate; bp < 0.001 versus (glutamate+TNF-α).
To validate the importance of TNF-α-induced CBP in neuroprotection, we tested this further in the glutamate neurotoxicity model. Using TUNEL assay, less number of apoptotic nuclei were detected in preconditioned glutamate-intoxicated cortical neurons in comparison to those that were not preconditioned (Fig. 6E, first 3 panels & Fig. 6F). Next, we tested if upregulation of CBP was related to such tolerance against glutamate excitotoxicity. Neurons were co-treated with either the antisense (ASO) or the scrambled (ScO) oligonucleotides and TNF-α. As illustrated in figure 6E (last two vertical panels) and figure 6F, preconditioned neurons, which had been co-treated with ScO, but not CBP ASO, were significantly more resistant to glutamate toxicity than those without TNF-α preconditioning. Taken together, these observations underline the importance of TNFα-induced CBP in neuronal tolerance against excitotoxicity.
TNF-α increases the expression of manganese superoxide dismutase in neurons via upregulation of CBP
MnSOD (protein from sod2 gene) is a critical regulator of neuronal health during normalcy and neurodegenerative conditions. It has been demonstrated that MnSOD −/+ animals are more susceptible to seizures and kainite-induced neurodegeneration than their wild-type counterparts (28). Consistently, enhanced presence of MnSOD has been shown to protect neurons from neurodegenerative threats both in vitro and in vivo (42, 43). Furthermore, TNF-α-induced MnSOD upregulation pre-conditions neurons against subsequent Aβ or FeSO4 challenge (44). Because transcriptional upregulation of MnSOD has been shown to be dependent on CBP/p300 (45), we wondered whether TNF-α increases the level of MnSOD in neurons via CBP.
To test our hypothesis, we performed following experiment. Neurons were treated with 10ng/ml TNF-α for various time points and RT-PCR was performed for sod2 transcript. Like several previous reports (43, 44, 46), we detected transcriptional upregulation of sod2 after TNF-α treatment (Figure. 7A). As revealed by this time course, new sod2 transcript was not detected prior to 6 hours, but the expression was strongly sustained thereafter. The timing of strong MnSOD expression correlates with the upregulation of CBP by TNF-α (Fig. 2B), suggesting that CBP may have a role to play in sod2 regulation. To test this idea, we challenged CBP antisense-pretreated cells (48 hours; to knock down the endogenous CBP) with TNF-α. As seen in figure 7B, TNF-α was unable to induce the expression of MnSOD in neurons pretreated with antisense, but not scrambled nucleotides, suggesting that TNF-α-induced MnSOD upregulation in neurons is dependent on CBP. To confirm this finding in our preconditioning model, we quantified sod2 transcript levels in Aβ-challenged neurons. Aβ treatment did not induce MnSOD in non-preconditioned neurons, however, TNF-α-preconditioned neurons had greater level of sod2 transcript than untreated controls (Figure. 7C, first 3 lanes). This increase in sod2 gene transcription remained unaltered in cells co-treated with scrambled oligonucleotides. However, antisense knockdown of CBP markedly abrogated TNF-α-induced upregulation of MnSOD in neurons (Fig. 7C, last 2 lanes). Similar trends were recorded for MnSOD protein level, when neurons were immuno-stained for MnSOD (Fig. 7D). Taken together, these data suggest that TNF-α– induced CBP plays a pivotal role in increasing the level of MnSOD during Aβ challenge.
Figure 7. CBP upregulation is critical for TNF-α-induced expression of MnSOD.
(A) CGC neurons were treated with 10ng/ml for indicated times. (B) Neurons were treated with CBP antisense or scrambled oligonucleotides for 48 hours and then treated with 10ng/ml of TNF-α for another 8 hours. (C) Neurons preincubated with CBP antisense or scrambled oligonucleotides for an hour were treated with TNF-α for 24 hours. Subsequently, they were treated with 1μg/ml Aβ for another 6 hours. Total RNA from all these studies (A–C) was used to perform RT-PCR with primers specific for rat MnSOD and GAPDH. The relative expression of MnSOD (MnSOD/GAPDH) in each set was measured after scanning the bands (lower panel). Results represent mean ± SD of three separate experiments. ap < 0.001 versus control in A. bp < 0.05 and ap < 0.001 versus TNF-α only in B. (D) CGC neurons were treated as in (C), fixed and immunostained with antibodies against MnSOD. Figures represent observations made from at least five randomly selected microscopic fields from each of three independent experiments (Bar = 100 μm).
TNF-α preconditioning of mouse cortex against Aβ toxicity depends on the upregulation of CBP
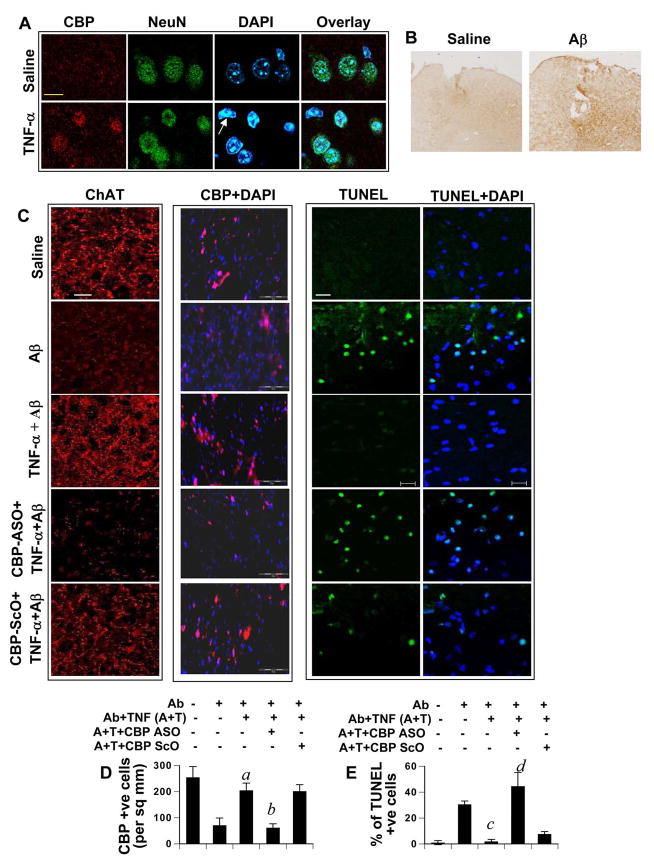
Next, we proceeded to confirm the above observations in vivo within the CNS. First, we verified whether TNF-α was able to upregulate CBP in vivo. To do so, either TNF-α or saline was microinjected into the cortex of male C57/BL6 mice. Animals were sacrificed after 24 hours and brains were perfused and processed for immunocytochemical analysis. Cortical sections were then stained with CBP and NeuN (neuronal marker). As seen in figure 8A, CBP immunostaining was greater in cortical sections of TNF-α-injected animals than that of saline-injected animals. We observed that the staining was restricted to DAPI-stained nuclear bodies of neurons and was not present in any non neuronal nucleus (arrow, figure. 8A). This further highlights that TNF-α-dependent induction of CBP is a neuron-specific event in vivo in the CNS.
Figure 8. CBP upregulation is critical for in vivo preconditioning.
(A) C57/BL6 mice (n=4 in each group) were microinjected with 300 μg TNF-α or saline in the cortex. Brain sections, prepared after 24 hours of the injection, were immunostained with CBP (AlexaFluor 633-red), NeuN (Green-AlexaFluor 488), and DAPI. Yellow bar represents 20 μM. Non-neuronal nucleus with basal level of CBP is indicated with the arrow. (B) Saline and fibrillar Aβ1–42 were microinjected into the cortex of C57/BL6 mice. After 24 h, mice were sacrificed and sections were immunolabelled with anti-Aβ antibody (Abcam) to detect the diffusion of fibrillar Aβ1–42. (C) C57/BL6 mice were microinjected with either saline, or TNF-α, or TNF-α with either cbp anti-sense (ASO) or scrambled (ScO) oligonucleotides on the first day and were followed up with Aβ injection at the same site 24 hours later. After another 24 hours, mice were perfused and brains were processed according to standard paraffin embedding protocols. Brain slices were then immunolabelled with anti-ChAT (AlexaFluor 633-red), anti-CBP (Cy3-red) in individual sets and TUNEL assay was conducted in another. White bars represent 10 μM. (D) Quantification of CBP. CBP-positive cells were counted in four cortical sections (two images per slide) of each of five mice (n=5) in an Olympus IX81 fluorescence microscope using the MicroSuite™ imaging software. Data are represented as CBP positive nuclei per mm2. ap < 0.001 versus Aβ; bp < 0.001 versus (Aβ+TNF-α). (E) Quantification of TUNEL positivity. Graph represents percentage of TUNEL positive cells from a total of DAPI nuclei. Data are representative of four separate sections (two images per slide) from each of five mice. cp < 0.001 versus Aβ; dp < 0.001 versus (Aβ+TNF-α).
Next, we wanted to verify that CBP upregulation was critical for TNF-α-mediated preconditioning in vivo in mouse cortex. At first, we examined the diffusion of fibrillar Aβ1-42 peptides in the cortex after microinjection. As observed in figure 8B, after 24 h of microinjection, Aβ peptides diffused from the injection site.
In a separate set of experiments, mice were microinjected into the cortex with TNF-α in the presence or absence of antisense or scrambled oligonucleotides against cbp. After 24 hours, 1 μg of fibrillar Aβ1–42 was microinjected into the cortex. We have seen that this particular dose Aβ causes the death of choline acetyl transferase (ChAT)-positive neurons in mice (Jana and Pahan, unpublished results). Previously, Aβ has been implicated in inducing the loss of ChAT in axon fibers of cholinergic neurons in the cortex (47). Therefore, this serves as a marker for neuronal loss in the cortex. We therefore investigated the potency of TNF-α preconditioning in negating Aβ-induced loss of cholinergic neurons. As shown in figure 8C (leftmost vertical panel), Aβ induced the loss of ChAT in axonal fibers of the cortex. However, TNF-α preconditioning prevented this loss. Similar to that observed in rat primary neurons, knocking down TNF-induced CBP by using cbp antisense, but not scrambled oligonucleotides, interfered with this resistance resulting in the marked loss of ChAT in these fibers (Figure. 8C). All along, we found a distinct positive co-relation (qualitative) between the presence of ChAT and the level of CBP (Figure 8C, second vertical panel). This suggests the importance of CBP in the maintenance of neuronal health in vivo.
However, a negative correlation was found between the presence of CBP and neuronal death (as measured by TUNEL assay) (Figure 8C, second versus third vertical panel and figure 8D versus 8E). After TUNEL labeling, we scanned the area surrounding the incision (within 100μm radius from the site of microinjection) for TUNEL positive nuclei. As seen in figure 8c, microinjection of Aβ, but not saline, induced apoptosis in the cortex. However, TNF-α preconditioning markedly inhibited Aβ-induced apoptosis in vivo in the cortex (Figure. 8C). On the other hand, antisense but not scrambled oligonucleotides against cbp abrogated this protective effect of TNF-α. Together, these observations suggest that TNF-α preconditioning protects the cortex in vivo against Aβ toxicity via CBP upregulation.
To address any concerns about the loss of tissue integrity in the region surrounding the site of microinjection, we immunostained brain slices with H&E. We did not observe any gross tissue damage (data not presented). Additionally we also checked for any consequential glial inflammatory response in the experimental tissue as a result of the rendered treatment by immunostaining sections with GFAP, a well known marker of gliosis (48). When animals were injected with LPS, a well known gliosis-inducing agent, we observed a notable glial response as marked by reactive astrocytes when compared with saline injected tissue (data not presented). In our experimental groups, we detected a few reactive astrocytes in animals injected with Aβ and Aβ+TNF-α but other groups were found to be largely free of reactive astrocytes (data not presented). Together, these observations suggest that TNF-α preconditioning protects neurons in vitro and in vivo against Aβ toxicity via CBP upregulation and that this protection does not involve any alteration in gliosis.
Discussion
Acetylation homeostasis, the well-preserved stoichiometrical balance of acetyltransferase and deacetylase enzymatic undertakings in normal cells that confers stability to cellular homeostasis by coordinating gene expression and repression on both temporal and spatial basis (24), is a crucial parameter of neuronal survival in several forms of neurodegeneration. In normal neurons, protein dosage and enzymatic activity of HATs (like CBP and p300) and HDACs remain in a harmonized state of balance where adequate available active molecules from either group effectively regulate acetylation of chromatin and transcription factors in a controlled manner. Such equilibrium manifests neuronal homeostasis and is responsible for regulated gene expression leading to normal neuronal functions like, long-term potentiation, learning, memory and survival. However, during neurodegenerative conditions, the neuronal acetylation homeostasis is profoundly impaired (24). Such malfunction is primarily manifested by comprehensive loss of HATs like CBP during various neurodegenerative challenges (28, 30). As is reflected by antecedent deacetylation of histones in apoptotic conditions, this loss is an early event during apoptosis (30). With the loss of HATs, HDACs attain a facilitated gain-of-function, thereby unsettling the acetylation homeostasis and neuronal integrity subsequently (24, 30).
Among several HATs present in neurons, the loss of CBP is widely observed in various forms of neurodegeneration and has been proposed to be pivotal in facilitating neurodegenerative cascade of events (30). Nuclear translocation of expanded polyglutamine-containing neurotoxins (like mutated Huntington protein) selectively enhance ubiquitination and degradation of CBP by proteosomal pathway (27, 28). Alternatively, CBP, but not p300, is redistributed from their normal nuclear location into Huntington aggregates, which compromises their availability for normal functions (27). Furthermore, caspase-6-dependent CBP proteolysis has been reported in low K+ model of neurodegeneration (30). Interestingly, active caspase-6 is also reported in neuropil threads, neuritic plaques and neurofibrillary tracts of Alzheimer brain, suggesting that CBP may be lost in Alzheimer’s disease by caspase cleavage. Additionally, during Alzheimer’s disease progression, Presenilin-1-dependent epsilon-cleavage product N-Cad/CTF2 binds to CBP and conducts its proteosomal degradation (49). In an interesting study with oxidative stress-challenged murine cortical neurons, immuno-cytochemical detection of CBP revealed conspicuous immuno-reactivity only in the nucleus of neurons surviving the hypoxic stress, but not in condensed or fragmented apoptotic nuclei (50). Indeed, the critical nature of CBP loss is illuminated by several over-expression studies of cbp in neurons, all of which demonstrate enhanced neuronal viability in response to various neurodegenerative challenges (27, 30, 51, 52). Taken together, these observations suggest that the loss of CBP is catastrophic for neurons while normalizing its level during crisis may help a neuron over-ride an neurodegenerative insult.
Until now molecular pathways of CBP induction in neurons and its role in preconditioning were not known. However, studies described in this manuscript demonstrate that TNF-α stimulates the expression of CBP in neurons and that TNF-α preconditions neurons via CBP. Our conclusion is based on the following observations. First, TNF-α increased the expression of CBP in neurons. Because TNF-α has profound effects on glial cells like astroglia and microglia, we examined if TNF-α also enhanced the level of CBP in these cells. However, TNF-α had no effect on the level of CBP in either astroglia or microglia. To our knowledge, this is the first report of specific upregulation of the cbp gene in neurons. Second, the upregulated CBP served to compensate for any subsequent CBP loss during neurotoxicity. TNF-α-preconditioned neurons did not experience the loss of CBP after Aβ challenge, as was experienced by unconditioned steady-state cells. Third, TNF-α was unable to precondition neurons against Aβ toxicity when neuronal CBP was knocked down by antisense oligonucleotides. Similarly, pre-administration of TNF-α into the mouse cortex protected cortical neurons against fibrillar Aβ and antisense knockdown of CBP negated this preconditioning effect in vivo in the cortex. These results strongly suggest that upregulation of CBP represents a vital step in TNF-α-mediated neuronal defenses against neurotoxic challenges both in vitro and in vivo. Our proposed pathway is further detailed by our finding that CBP upregulation is required for maintenance of MnSOD expression, an anti-oxidant enzyme that plays a vital role in neuronal defense against neurodegeneration.
Although CBP is an important part the gene expression machinery, very little is known about its regulation. Because NF-κB, a multi-functional transcription factor, is associated closely with neuronal defense against neurotoxicity (53, 54), we investigated its role in the induction of neuronal CBP. By MatInspector analysis we have found that promoters of both rat and human cbp house several putative consensus κB sites. In rat, there are five putative NF-κB sites within 3500 bp upstream of cbp coding region, suggesting a role of NF-κB activation in the transcription of CBP. Our results, that TNF-α treatment caused nuclear translocation of p65 and p50, that TNF-α promoted the phosphorylation of p65 at S276, that TNF-α induced the DNA-binding activity of active NF-κB in neurons, and that specific inhibitors of NF-κB activation (NBD peptides and SN50) abrogated the induction of CBP in TNF-α-treated neurons clearly demonstrate an important role of NF-κB in TNF-α-mediated increase in CBP in neurons. It is noteworthy that CBP is known to acetylate and regulate the functional output of NF-κB (38) and the regulation of cbp expression by NF-κB may represent a feed-back loop.
One perplexing observation that remains unanswered, however, is the neuronal specificity of CBP upregulation. TNF-α elicits NF-κB activation and subsequent responses in all kind of brain cells, including astrocytes and microglia. These responses include upregulation of neurotrophins, anti-apoptotic factors and proteins related to immunity (37, 55, 56). However, TNF-α fails to induce CBP in these cells. This difference in response among these cells may be explained by the presence of a neuron-specific transcription factor which may be recruited by TNF-α trigger. One more possibility involves the REST/Neuron restrictive silencer factor (NRSF), which represses the expression of neuron-specific genes in non-neuronal cells (57), and may therefore restrict TNF-α-induced CBP expression in glial cells. The presence of two NRSF within 3500 bp upstream of the rat cbp coding region entertains this possibility further. Nevertheless, it is possible that the constitutive mechanism of CBP expression differs from the signal-induced mechanism in a way so as to avoid the NRSF restriction in expressing CBP constitutively.
In addition to CBP, this study also re-addresses the role of TNF-α in neurodegeneration. TNF-α, the most widely studied cytokine, plays many roles as a signaling and as an effector molecule in both physiology and pathophysiology of the CNS. On the one hand, TNF-α plays a critical role in brain development, brain physiology, synaptic plasticity, sleep, circadian rhythm, normal behavior, etc (7). On the other, it has been shown to induce the activation of glial cells (astroglia and microglia) and macrophages for the production of a variety of neurotoxins and to initiate the death process in oligodendrocytes and neurons (58). Therefore, TNF-α has been shown to participate in the pathogenesis of stroke and other neurodegenerative diseases and in demyelinating conditions (e.g. multiple sclerosis, experimental allergic encephalopathy, X-Adrenoleukodystrophy) associated with infiltrating macrophages and the production of proinflamatory cytokines (8, 37). The overall role of TNF-α in the grand picture is constantly debated. In the current article, we delineate an important neurotrophic role of TNF-α whereby neuronal preconditioning is mediated via upregulation of neuronal CBP.
In summary, we have demonstrated that TNF-α increases the expression of CBP specifically in neurons and that upregulated CBP mediates TNFα-induced neuronal preconditioning against fibrillar Aβ in addition to other neurotoxins like glutamate (Figure. 9). Although the local concentration of fibrillar Aβ peptides present in the brain microenvironment of AD patients may differ from the concentration we used in primary neurons, and the in vitro situation of rat neurons in culture and the in vivo condition of cholinergic neurons in mouse cortex may not truly resemble the in vivo situation of neurons in the brain of AD patients, our results suggest that TNF-α-mediated upregulation of CBP in neurons may have therapeutic importance in AD and other neurodegenerative disorders.
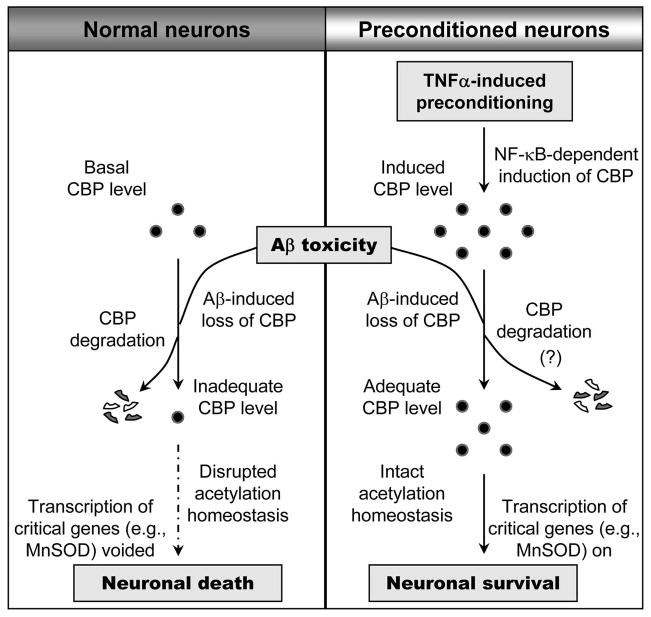
Figure 9. Model representing neuronal preconditioning by TNF-α.
In resting unconditioned neurons, toxicity results in the loss of CBP which impairs the normal pro-survival transcriptional process. This paves the way for neuronal death. However, in preconditioned neurons, TNF-α-induced upregulation of CBP renders neurons with adequate CBP level to sustain normal cell survival associated activities despite any loss due to toxicity.
Acknowledgments
This study was supported by grants from NIH (NS39940 & NS48923) and Alzheimer’s Association (IIRG-07-58684) to KP, the Intramural Research Program of the NIH and the NIEHS and UNMC graduate research assistantship to RNS.
The authors wish to thank Dr. You Zhou (University of Nebraska at Lincoln) and Jeff Tucker (NIEHS, RTP) for assistance with microscopy and Marian Schmid (University of Nebraska Medical Center) for excellent animal care.
References
- 1.Moncayo J, de Freitas GR, Bogousslavsky J, Altieri M, van Melle G. Do transient ischemic attacks have a neuroprotective effect? Neurology. 2000;54:2089–2094. doi: 10.1212/wnl.54.11.2089. [DOI] [PubMed] [Google Scholar]
- 2.Weih M, Kallenberg K, Bergk A, Dirnagl U, Harms L, Wernecke KD, Einhaupl KM. Attenuated stroke severity after prodromal TIA: a role for ischemic tolerance in the brain? Stroke; a journal of cerebral circulation. 1999;30:1851–1854. doi: 10.1161/01.str.30.9.1851. [DOI] [PubMed] [Google Scholar]
- 3.Mattson MP, Cheng A. Neurohormetic phytochemicals: low-dose toxins that induce adaptive neuronal stress responses. Trends Neurosci. 2006 doi: 10.1016/j.tins.2006.09.001. [DOI] [PubMed] [Google Scholar]
- 4.Kirino T. Ischemic tolerance. J Cereb Blood Flow Metab. 2002;22:1283–1296. doi: 10.1097/01.WCB.0000040942.89393.88. [DOI] [PubMed] [Google Scholar]
- 5.Mattson MP. Excitotoxic and excitoprotective mechanisms: abundant targets for the prevention and treatment of neurodegenerative disorders. Neuromolecular medicine. 2003;3:65–94. doi: 10.1385/NMM:3:2:65. [DOI] [PubMed] [Google Scholar]
- 6.Dirnagl U, Simon RP, Hallenbeck JM. Ischemic tolerance and endogenous neuroprotection. Trends Neurosci. 2003;26:248–254. doi: 10.1016/S0166-2236(03)00071-7. [DOI] [PubMed] [Google Scholar]
- 7.Saha RN, Liu X, Pahan K. Up-regulation of BDNF in astrocytes by TNF-alpha: a case for the neuroprotective role of cytokine. J Neuroimmune Pharmacol. 2006;1:212–222. doi: 10.1007/s11481-006-9020-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Allan SM, Rothwell NJ. Cytokines and acute neurodegeneration. Nature reviews. 2001;2:734–744. doi: 10.1038/35094583. [DOI] [PubMed] [Google Scholar]
- 9.Cheng B, Christakos S, Mattson MP. Tumor necrosis factors protect neurons against metabolic-excitotoxic insults and promote maintenance of calcium homeostasis. Neuron. 1994;12:139–153. doi: 10.1016/0896-6273(94)90159-7. [DOI] [PubMed] [Google Scholar]
- 10.Marchetti L, Klein M, Schlett K, Pfizenmaier K, Eisel UL. Tumor necrosis factor (TNF)-mediated neuroprotection against glutamate-induced excitotoxicity is enhanced by N-methyl-D-aspartate receptor activation. Essential role of a TNF receptor 2-mediated phosphatidylinositol 3-kinase-dependent NF-kappa B pathway. The Journal of biological chemistry. 2004;279:32869–32881. doi: 10.1074/jbc.M311766200. [DOI] [PubMed] [Google Scholar]
- 11.Carlson NG, Wieggel WA, Chen J, Bacchi A, Rogers SW, Gahring LC. Inflammatory cytokines IL-1 alpha, IL-1 beta, IL-6, and TNF-alpha impart neuroprotection to an excitotoxin through distinct pathways. J Immunol. 1999;163:3963–3968. [PubMed] [Google Scholar]
- 12.Tamatani M, Che YH, Matsuzaki H, Ogawa S, Okado H, Miyake S, Mizuno T, Tohyama M. Tumor necrosis factor induces Bcl-2 and Bcl-x expression through NFkappaB activation in primary hippocampal neurons. The Journal of biological chemistry. 1999;274:8531–8538. doi: 10.1074/jbc.274.13.8531. [DOI] [PubMed] [Google Scholar]
- 13.Barger SW, Horster D, Furukawa K, Goodman Y, Krieglstein J, Mattson MP. Tumor necrosis factors alpha and beta protect neurons against amyloid beta-peptide toxicity: evidence for involvement of a kappa B-binding factor and attenuation of peroxide and Ca2+ accumulation. Proceedings of the National Academy of Sciences of the United States of America. 1995;92:9328–9332. doi: 10.1073/pnas.92.20.9328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Liu J, Ginis I, Spatz M, Hallenbeck JM. Hypoxic preconditioning protects cultured neurons against hypoxic stress via TNF-alpha and ceramide. American journal of physiology. 2000;278:C144–153. doi: 10.1152/ajpcell.2000.278.1.C144. [DOI] [PubMed] [Google Scholar]
- 15.Ginis I, Jaiswal R, Klimanis D, Liu J, Greenspon J, Hallenbeck JM. TNF-alpha-induced tolerance to ischemic injury involves differential control of NF-kappaB transactivation: the role of NF-kappaB association with p300 adaptor. J Cereb Blood Flow Metab. 2002;22:142–152. doi: 10.1097/00004647-200202000-00002. [DOI] [PubMed] [Google Scholar]
- 16.Wang X, Li X, Erhardt JA, Barone FC, Feuerstein GZ. Detection of tumor necrosis factor-alpha mRNA induction in ischemic brain tolerance by means of real-time polymerase chain reaction. J Cereb Blood Flow Metab. 2000;20:15–20. doi: 10.1097/00004647-200001000-00004. [DOI] [PubMed] [Google Scholar]
- 17.Romera C, Hurtado O, Botella SH, Lizasoain I, Cardenas A, Fernandez-Tome P, Leza JC, Lorenzo P, Moro MA. In vitro ischemic tolerance involves upregulation of glutamate transport partly mediated by the TACE/ADAM17-tumor necrosis factor-alpha pathway. J Neurosci. 2004;24:1350–1357. doi: 10.1523/JNEUROSCI.1596-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Castillo J, Moro MA, Blanco M, Leira R, Serena J, Lizasoain I, Davalos A. The release of tumor necrosis factor-alpha is associated with ischemic tolerance in human stroke. Annals of neurology. 2003;54:811–819. doi: 10.1002/ana.10765. [DOI] [PubMed] [Google Scholar]
- 19.McDunn JE, Cobb JP. That which does not kill you makes you stronger: a molecular mechanism for preconditioning. Sci STKE. 2005;2005:pe34. doi: 10.1126/stke.2912005pe34. [DOI] [PubMed] [Google Scholar]
- 20.Tanaka H, Yokota H, Jover T, Cappuccio I, Calderone A, Simionescu M, Bennett MV, Zukin RS. Ischemic preconditioning: neuronal survival in the face of caspase-3 activation. J Neurosci. 2004;24:2750–2759. doi: 10.1523/JNEUROSCI.5475-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gonzalez-Zulueta M, Feldman AB, Klesse LJ, Kalb RG, Dillman JF, Parada LF, Dawson TM, Dawson VL. Requirement for nitric oxide activation of p21(ras)/extracellular regulated kinase in neuronal ischemic preconditioning. Proceedings of the National Academy of Sciences of the United States of America. 2000;97:436–441. doi: 10.1073/pnas.97.1.436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Barone FC, White RF, Spera PA, Ellison J, Currie RW, Wang X, Feuerstein GZ. Ischemic preconditioning and brain tolerance: temporal histological and functional outcomes, protein synthesis requirement, and interleukin-1 receptor antagonist and early gene expression. Stroke; a journal of cerebral circulation. 1998;29:1937–1950. doi: 10.1161/01.str.29.9.1937. discussion 1950–1931. [DOI] [PubMed] [Google Scholar]
- 23.Ravati A, Ahlemeyer B, Becker A, Klumpp S, Krieglstein J. Preconditioning-induced neuroprotection is mediated by reactive oxygen species and activation of the transcription factor nuclear factor-kappaB. Journal of neurochemistry. 2001;78:909–919. doi: 10.1046/j.1471-4159.2001.00463.x. [DOI] [PubMed] [Google Scholar]
- 24.Saha RN, Pahan K. HATs and HDACs in neurodegeneration: a tale of disconcerted acetylation homeostasis. Cell death and differentiation. 2006;13:539–550. doi: 10.1038/sj.cdd.4401769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Korzus E, Rosenfeld MG, Mayford M. CBP histone acetyltransferase activity is a critical component of memory consolidation. Neuron. 2004;42:961–972. doi: 10.1016/j.neuron.2004.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wood MA, Kaplan MP, Park A, Blanchard EJ, Oliveira AM, Lombardi TL, Abel T. Transgenic mice expressing a truncated form of CREB-binding protein (CBP) exhibit deficits in hippocampal synaptic plasticity and memory storage. Learn Mem. 2005;12:111–119. doi: 10.1101/lm.86605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nucifora FC, Jr, Sasaki M, Peters MF, Huang H, Cooper JK, Yamada M, Takahashi H, Tsuji S, Troncoso J, Dawson VL, Dawson TM, Ross CA. Interference by huntingtin and atrophin-1 with cbp-mediated transcription leading to cellular toxicity. Science. 2001;291:2423–2428. doi: 10.1126/science.1056784. [DOI] [PubMed] [Google Scholar]
- 28.Jiang H, Poirier MA, Liang Y, Pei Z, Weiskittel CE, Smith WW, DeFranco DB, Ross CA. Depletion of CBP is directly linked with cellular toxicity caused by mutant huntingtin. Neurobiol Dis. 2006;23:543–551. doi: 10.1016/j.nbd.2006.04.011. [DOI] [PubMed] [Google Scholar]
- 29.Alarcon JM, Malleret G, Touzani K, Vronskaya S, Ishii S, Kandel ER, Barco A. Chromatin acetylation, memory, and LTP are impaired in CBP+/− mice: a model for the cognitive deficit in Rubinstein-Taybi syndrome and its amelioration. Neuron. 2004;42:947–959. doi: 10.1016/j.neuron.2004.05.021. [DOI] [PubMed] [Google Scholar]
- 30.Rouaux C, Jokic N, Mbebi C, Boutillier S, Loeffler JP, Boutillier AL. Critical loss of CBP/p300 histone acetylase activity by caspase-6 during neurodegeneration. Embo J. 2003;22:6537–6549. doi: 10.1093/emboj/cdg615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bal-Price A, Brown GC. Inflammatory neurodegeneration mediated by nitric oxide from activated glia-inhibiting neuronal respiration, causing glutamate release and excitotoxicity. J Neurosci. 2001;21:6480–6491. doi: 10.1523/JNEUROSCI.21-17-06480.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Saha RN, Jana M, Pahan K. MAPK p38 regulates transcriptional activity of NF-kappaB in primary human astrocytes via acetylation of p65. J Immunol. 2007;179:7101–7109. doi: 10.4049/jimmunol.179.10.7101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jana A, Pahan K. Fibrillar amyloid-beta peptides kill human primary neurons via NADPH oxidase-mediated activation of neutral sphingomyelinase. Implications for Alzheimer’s disease. The Journal of biological chemistry. 2004;279:51451–51459. doi: 10.1074/jbc.M404635200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jana M, Dasgupta S, Saha RN, Liu X, Pahan K. Induction of tumor necrosis factor-alpha (TNF-alpha) by interleukin-12 p40 monomer and homodimer in microglia and macrophages. J Neurochem. 2003;86:519–528. doi: 10.1046/j.1471-4159.2003.01864.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jana M, Anderson JA, Saha RN, Liu X, Pahan K. Regulation of inducible nitric oxide synthase in proinflammatory cytokine-stimulated human primary astrocytes. Free Radic Biol Med. 2005;38:655–664. doi: 10.1016/j.freeradbiomed.2004.11.021. [DOI] [PubMed] [Google Scholar]
- 36.Pahan K, Sheikh FG, Namboodiri AM, Singh I. Lovastatin and phenylacetate inhibit the induction of nitric oxide synthase and cytokines in rat primary astrocytes, microglia, and macrophages. The Journal of clinical investigation. 1997;100:2671–2679. doi: 10.1172/JCI119812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Saha RN, Pahan K. Tumor necrosis factor-alpha at the crossroads of neuronal life and death during HIV-associated dementia. Journal of neurochemistry. 2003;86:1057–1071. doi: 10.1046/j.1471-4159.2003.01942.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhong H, May MJ, Jimi E, Ghosh S. The phosphorylation status of nuclear NF-kappa B determines its association with CBP/p300 or HDAC-1. Mol Cell. 2002;9:625–636. doi: 10.1016/s1097-2765(02)00477-x. [DOI] [PubMed] [Google Scholar]
- 39.Lin YZ, Yao SY, Veach RA, Torgerson TR, Hawiger J. Inhibition of nuclear translocation of transcription factor NF-kappa B by a synthetic peptide containing a cell membrane-permeable motif and nuclear localization sequence. The Journal of biological chemistry. 1995;270:14255–14258. doi: 10.1074/jbc.270.24.14255. [DOI] [PubMed] [Google Scholar]
- 40.May MJ, D’Acquisto F, Madge LA, Glockner J, Pober JS, Ghosh S. Selective inhibition of NF-kappaB activation by a peptide that blocks the interaction of NEMO with the IkappaB kinase complex. Science. 2000;289:1550–1554. doi: 10.1126/science.289.5484.1550. [DOI] [PubMed] [Google Scholar]
- 41.Chaudhary J, Skinner MK. Role of the transcriptional coactivator CBP/p300 in linking basic helix-loop-helix and CREB responses for follicle-stimulating hormone-mediated activation of the transferrin promoter in Sertoli cells. Biology of reproduction. 2001;65:568–574. doi: 10.1095/biolreprod65.2.568. [DOI] [PubMed] [Google Scholar]
- 42.Saha RN, Pahan K. Differential regulation of Mn-superoxide dismutase in neurons and astroglia by HIV-1 gp120: Implications for HIV-associated dementia. Free radical biology & medicine. 2007;42:1866–1878. doi: 10.1016/j.freeradbiomed.2007.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Keller JN, Kindy MS, Holtsberg FW, St Clair DK, Yen HC, Germeyer A, Steiner SM, Bruce-Keller AJ, Hutchins JB, Mattson MP. Mitochondrial manganese superoxide dismutase prevents neural apoptosis and reduces ischemic brain injury: suppression of peroxynitrite production, lipid peroxidation, and mitochondrial dysfunction. J Neurosci. 1998;18:687–697. doi: 10.1523/JNEUROSCI.18-02-00687.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sompol P, Xu Y, Ittarat W, Daosukho C, St Clair D. NF-kappaB-associated MnSOD induction protects against beta-amyloid-induced neuronal apoptosis. J Mol Neurosci. 2006;29:279–288. [PubMed] [Google Scholar]
- 45.Guo Z, Boekhoudt GH, Boss JM. Role of the intronic enhancer in tumor necrosis factor-mediated induction of manganous superoxide dismutase. The Journal of biological chemistry. 2003;278:23570–23578. doi: 10.1074/jbc.M303431200. [DOI] [PubMed] [Google Scholar]
- 46.Bruce-Keller AJ, Geddes JW, Knapp PE, McFall RW, Keller JN, Holtsberg FW, Parthasarathy S, Steiner SM, Mattson MP. Anti-death properties of TNF against metabolic poisoning: mitochondrial stabilization by MnSOD. Journal of neuroimmunology. 1999;93:53–71. doi: 10.1016/s0165-5728(98)00190-8. [DOI] [PubMed] [Google Scholar]
- 47.Oda Y. Choline acetyltransferase: the structure, distribution and pathologic changes in the central nervous system. Pathology international. 1999;49:921–937. doi: 10.1046/j.1440-1827.1999.00977.x. [DOI] [PubMed] [Google Scholar]
- 48.Malhotra SK, Shnitka TK, Elbrink J. Reactive astrocytes--a review. Cytobios. 1990;61:133–160. [PubMed] [Google Scholar]
- 49.Marambaud P, Wen PH, Dutt A, Shioi J, Takashima A, Siman R, Robakis NK. A CBP binding transcriptional repressor produced by the PS1/epsilon-cleavage of N-cadherin is inhibited by PS1 FAD mutations. Cell. 2003;114:635–645. doi: 10.1016/j.cell.2003.08.008. [DOI] [PubMed] [Google Scholar]
- 50.Jin K, Mao XO, Simon RP, Greenberg DA. Cyclic AMP response element binding protein (CREB) and CREB binding protein (CBP) in global cerebral ischemia. J Mol Neurosci. 2001;16:49–56. doi: 10.1385/JMN:16:1:49. [DOI] [PubMed] [Google Scholar]
- 51.McCampbell A, Taye AA, Whitty L, Penney E, Steffan JS, Fischbeck KH. Histone deacetylase inhibitors reduce polyglutamine toxicity. Proceedings of the National Academy of Sciences of the United States of America. 2001;98:15179–15184. doi: 10.1073/pnas.261400698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Taylor JP, Taye AA, Campbell C, Kazemi-Esfarjani P, Fischbeck KH, Min KT. Aberrant histone acetylation, altered transcription, and retinal degeneration in a Drosophila model of polyglutamine disease are rescued by CREB-binding protein. Genes & development. 2003;17:1463–1468. doi: 10.1101/gad.1087503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Mattson MP, Camandola S. NF-kappaB in neuronal plasticity and neurodegenerative disorders. The Journal of clinical investigation. 2001;107:247–254. doi: 10.1172/JCI11916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Mattson MP, Meffert MK. Roles for NF-kappaB in nerve cell survival, plasticity, and disease. Cell death and differentiation. 2006;13:852–860. doi: 10.1038/sj.cdd.4401837. [DOI] [PubMed] [Google Scholar]
- 55.Saha RN, Liu XJ, Pahan K. Up-regulation of BDNF in Astrocytes by TNF-a: A Case for the Neuroprotective Role of Cytokine. J Neuroimmune Pharmacol. 2006;1:212–222. doi: 10.1007/s11481-006-9020-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Brabers NA, Nottet HS. Role of the pro-inflammatory cytokines TNF-alpha and IL-1beta in HIV-associated dementia. European journal of clinical investigation. 2006;36:447–458. doi: 10.1111/j.1365-2362.2006.01657.x. [DOI] [PubMed] [Google Scholar]
- 57.Schoenherr CJ, Anderson DJ. The neuron-restrictive silencer factor (NRSF): a coordinate repressor of multiple neuron-specific genes. Science. 1995;267:1360–1363. doi: 10.1126/science.7871435. [DOI] [PubMed] [Google Scholar]
- 58.Viviani B, Bartesaghi S, Corsini E, Galli CL, Marinovich M. Cytokines role in neurodegenerative events. Toxicology letters. 2004;149:85–89. doi: 10.1016/j.toxlet.2003.12.022. [DOI] [PubMed] [Google Scholar]