Abstract
The gene tailless (tlx) encodes a forebrain-restricted transcription factor that is robustly expressed in progenitor cells of the ventricular and subventricular zones during neurogenesis. To investigate the role of tlx in neocortical development we generated a targeted deletion of tlx by homologous recombination. Here we compared the lamination, connectivity and patterning of cortical regions in adult tlx−/− mice and their wild-type littermates. We found first that neocortical thickness is reduced by 20% in mutant animals; most of this reduction is due to a diminution of supragranular layers, while layer I and layers IV through VI are relatively intact cytoarchitecturally. Consistent with this, the cross-sectional area of the corpus callosum is reduced by over 40%. Second, thalamocortical and intrinsic excitatory circuits in tlx−/− mice exhibit an essentially normal distribution from layer IV to the white matter, but are reduced superficial to layer IV. Finally, within parietal cortex of mutant mice a vibrissa-like pattern of cortical barrels is present in the expected rostro-caudal location. These observations indicate that loss of tlx function most severely affects generation and differentiation of neurons destined for superficial cortical layers. Thus, tlx may be important in sustaining the progenitor cell population throughout late prenatal development. Establishment of functional cortical areas, and development of basic patterns of thalamocortical and intra-cortical circuits occurs independently of tlx function.
Introduction
Normal functioning of the cerebral cortex requires a carefully orchestrated sequence of developmental events. Appropriate numbers of cortical neurons are generated in the ventricular zone (VZ) and subventricular zone (SVZ) of the embryonic telencephalon, and postmitotic cells migrate to establish distinct cortical layers, where they subsequently differentiate and form precise patterns of synaptic connections. A critical, early step in this process is correct timing of progenitor cell proliferation, which gives rise to neurons that will populate successively more superficial cortical layers (Angevine and Sidman, 1961; Rakic, 1974; Takahashi et al., 1993, 1995a,b; Brustle et al., 1995; Campbell et al., 1995; Fischell, 1995; Temple and Qian, 1995; Levitt et al., 1997; Kornack and Rakic, 1998; Haydar et al., 2000). Proliferation of progenitor cells is regulated by factors that are both extrinsic and intrinsic to the VZ/SVZ (McConnell and Kaznowski, 1991; Ferri and Levitt, 1995; Eagleson et al., 1996; Ferri et al., 1996; Frantz and McConnell, 1996; Qian et al., 1997; Lillien, 1998). One potential intrinsic factor is encoded by the orphan nuclear receptor gene, tailless (tlx).
tlx is the vertebrate homologue of the Drosophila tailless gene, tll, and is a member of the orphan nuclear receptor gene superfamily that encodes ligand activated transcription factors (Pignoni et al., 1990; Monaghan et al., 1995). In Drosophila, tll initially is expressed in the embryonic termini, where it is critical for normal pattern formation including development of the brain (Pignoni et al., 1990). In mouse (Monaghan et al., 1995, 1997), rat and chick (Yu et al., 1994) and human (Jackson et al., 1998) transcription of tlx is restricted principally to the telencephalon, diencephalon and associated structures including the retina and olfactory placode (Yu et al., 1994; Monaghan et al., 1995, 1997). tlx expression first can be detected ~8.5 days post conception in the VZ of the embryonic mouse forebrain. Beginning around midgestation tlx also is expressed in cells of the SVZ and marginal zone (i.e. future layer I) (Lake et al., 2000; Roy et al., 2001, 2002).
To investigate the role of tlx in development of the vertebrate brain we generated a targeted disruption of the gene by homologous recombination (Monaghan et al., 1997). Animals that are homozygous for the disrupted gene (tlx−/−) are indistinguishable from their normal littermates at birth and are reproductively competent upon reaching sexual maturity (Monaghan et al., 1997). Mutant animals nevertheless display pronounced behavioral abnormalities including severe aggression, stereotypy, altered maternal instincts, late onset epilepsy and reduced learning abilities (Monaghan et al., 1997; Roy et al., 2002). Consistent with these behavioral observations, preliminary analyses have identified structural alterations in the rhinencephalon of tlx−/− animals, which exhibit reductions in size of certain nuclei of the amygdala, the islands of Calleja, the piriform and entorhinal cortices, and the hippocampal dentate gyrus (Monaghan et al., 1997).
How loss of tailless function affects neocortical development is unknown. Robust expression of tlx throughout the dorsal and ventral telencephalic VZ and SVZ from E8.5 until birth suggests a possible role in regulating the generation of cortical neurons, which are born during this time (Angevine and Sidman, 1961; Bayer and Altman, 1991; Levison and Goldman, 1993; Luskin and Boone, 1994; Monaghan et al., 1995, 1997). Preliminary comparisons of proliferation and differentiation in neocortex of tlx−/− mutant versus wild type embryos support this idea. Thus, embryos lacking tailless function exhibit an accelerated proliferation rate from embryonic day (E) 9.5 to E14.5, and an enhanced number of neurons in the marginal zone from E9.5 to E12.5. However, after mid-neurogenesis (i.e. E14.5) the number of progenitor cells is significantly diminished, and their proliferation rate is slower than in wild-type littermates of the same age (Roy et al., 2001, 2002) (Roy et al., submitted).
To identify effects of tlx deletion on cortical development we began a series of experiments aimed at comparing the lamination, connectivity and patterning of cortical regions in adult tlx−/− mice and their wild-type littermates. We found that neocortical thickness is reduced by ~20% in mutant animals, primarily due to thinning of superficial cortical layers. Concomitantly, the corpus callosum is severely reduced in volume. Despite reduced cortical thickness, thalamocortical and intrinsic excitatory circuits exhibit relatively normal patterns of distribution. Finally, we observed a vibrissa-like pattern of cortical barrels within the parietal cortex of all mutant mice. These findings suggest that tlx expression is important for sustaining the progenitor cell population throughout late prenatal development. Loss of tlx function most severely affects the generation and differentiation of neurons destined for the superficial layers of neocortex. The establishment of particular cortical regions, however, occurs independently of tlx function.
Materials and Methods
Animals
Targeted disruption of the tlx gene has been described in detail else-where (Monaghan et al., 1997). Mating heterozygous 129J/C57Bl/6 mice (ninth generation backcrossed into C57Bl/6) generated homozygous and heterozygous animals in the expected ratio. Offspring of these matings were genotyped by PCR as described previously (Monaghan et al., 1997). This study is based upon examination of 18 tlx−/− mice and 16 wild-type (i.e. tlx+/+). Several heterozygous animals also were examined; the brains of these mice appeared identical to wild-type animals in all respects. Animals were born in our breeding colony at the University of Pittsburgh and were 2–6 months of age when they were killed. The care and hand-ling of animals was approved by the University of Pittsburgh Institutional Animal Care and Use Committee and conformed to NIH guidelines.
Histology
Six wild-type and eight mutant animals were anesthetized with IsoVet (isof lurane; Shering-Plough, Union, NJ) and perfused transcardially with 4% paraformaldehyde in 0.1 M sodium phosphate buffer (pH 7.3). Brains were removed, post-fixed overnight then transferred to 30% sucrose in phosphate buffer for cryoprotection. Frozen sections were cut at 50 µm on a sledge microtome and collected in phosphate buffer. Some hemispheres were cut in the coronal plane (i.e. normal to the pial surface). Other hemispheres were flattened by gently pressing them with a microscope slide during the freezing process so that subsequent sections would pass tangential to the pial surface and successively through each cortical layer (Land and Simons, 1985). A 1:2 series of sections was mounted onto chrome-alum gelatinized slides, stained for Nissl with 0.1% thionin, dehydrated in an ascending series of ethanol, cleared in xylene and coverslipped with Permount (Histologic Mounting Media, Fisher Scientific, FairLawn, NJ).
An adjacent series of sections was stained for cytochrome oxidase (CO) activity to reveal the metabolic architecture of the cortex, and in particular to mark the location and arrangement of barrel-like structures denoting the somatosensory cortical area (Woolsey and van der Loos, 1970; Welker and Woolsey, 1974). Brief ly, sections were rinsed in 0.1 M phosphate buffer (3 × 5 min) and incubated at 38°C in a CO reaction mixture described previously (Wong-Riley, 1979; Land and Simons, 1985). The CO reaction was monitored visually and terminated by transferring the sections to chilled 0.1 M buffer, after which they were rinsed in buffer, mounted onto slides, dehydrated and coverslipped.
Immunohistochemistry
An additional six wild-type and six mutant mice were perfused as described above. Thirty micron thick coronal sections through the cortices of four animals (two mice of each genotype) were stained immunohistochemically for vesicular glutamate transporter 2 (VGLUT2) (Synaptic Systems, Gottingen, Germany) diluted 1:20 000. Within the neocortex VGLUT2 (previously identified as ‘differentiation-associated Na+-dependent inorganic phosphate transporter’; DNPI) (Aihara et al., 2000) is localized to the synaptic vesicles of thalamocortical axon terminals, thus marking their location (Fujiyama et al., 2001). The pattern of VGLUT2 immunoreactivity therefore provides insight into the overall inter- and intralaminar distribution of thalamocortical axons in wild-type and mutant cortices. To assess possible layer-specific effects of tailless deletion, cortices from eight mice (four of each genotype) were sectioned at 30 µm in the sagittal plane. Sections in this plane were stained immunohistochemically with the monoclonal antibody SMI-32 (Sternberger Monoclonals, Lutherville, MD) diluted 1:1000 or with antibodies against calbindin D-28k (Sigma-Aldrich, St Louis, MO) diluted 1:200. SMI-32 recognizes nonphosphorylated epitopes on neurofilament protein (Sternberger and Sternberger, 1983) and is enriched in certain populations of large cortical pyramidal neurons, especially in layer V (van der Gucht et al., 2001); calbindin D-28k is a calcium binding protein that, in rodents, is predominantly localized to a subpopulation of cortical non-pyramidal neurons and small pyramidal neurons in layers II/III (Hof et al., 1999).
From each brain a 1:3 series of sections was washed in 0.05 M Tris buffered saline (TBS; pH 7.3; 3 × 10 min) and preincubated for 1 h at room temperature in blocking serum consisting of 0.05 M TBS containing 10% normal goat serum, 1% BSA and 0.4% Triton X-100. Sections then were incubated for 60 h at 4°C in primary antibody diluted in blocking serum. Subsequently they were washed in 0.05 M Tris buffer, and processed using the Vectastain ABC kit (Vector Laboratories, Burlingame, CA) according to the manufacturer’s instructions. After rinsing in buffer, sections were incubated in 0.5 mg/ml 3,3′-diaminobenzidine tetrahydrochloride (DAB) with 0.01% H2O2. DAB stained sections were washed in buffer, mounted onto slides, dehydrated and coverslipped.
Zinc Histochemistry
Cortices from four wild-type and four mutant mice were stained histochemically for synaptic zinc. Zinc histochemistry reveals a discrete population of glutamatergic, presumably excitatory synaptic terminals that are distinguished by the presence of zinc, along with glutamate, within their synaptic vesicles (Frederickson, 1989; Beaulieu et al., 1992; Frederickson et al., 2000). Zinc-containing circuits arise from intrinsic cortical neurons and are distributed heterogeneously among the cortical layers in a highly conserved pattern (Slomianka et al., 1990; Garret and Slomianka, 1992; Dyck and Cynader, 1993; Dyck et al., 1993; Land, 1995; Casanovas-Aguilar et al., 1998; Land and Akhtar, 1999). These circuits thus provide a separate index of cortical lamination for comparison with Nissl- and VGLUT2- stained specimens.
Histochemical localization of synaptic zinc was assessed using the Danscher method (Danscher, 1982). Mice received an intraperitoneal injection of the zinc chelator sodium selenite (20 mg/kg) from a freshly prepared stock solution (20 mg/ml in distilled H2O) and were anesthetized with IsoVet and killed by decapitation 1 h later. The brain was quickly removed, encased in OCT embedding medium (Miles, Elkhart, IN) and rapidly frozen by surrounding it with crushed dry ice. Twenty-micron coronal sections were cut on a cryostat, collected onto warm (38°C) microscope slides and stored with a desiccant at −40°C until they were processed. One series of sections from each hemisphere was stained histochemically for synaptic zinc according to a modification of the Danscher method (Dyck et al., 1993). Brief ly, slides were thawed, allowed to dry at room temperature, and fixed and hydrated in a descending series of ethanol (i.e. 95%, 15 min, 70%, 50%; 2 min each), after which they were rinsed in distilled H2O (3 × 2 min). Rinsed slides were dipped in 0.5% gelatin and dried with a hair dryer. The gelatin-coated slides were immersed in a physical developer prepared from 50% Acacia Gum (120 ml), 2.0 M sodium citrate buffer (20 ml), 0.5 M hydroquinone (30 ml) and 37 mM silver lactate (30 ml). The latter two reagents were added to the incubation medium in the dark. The slides were incubated at room temperature in the dark for ~120 min with agitation. After development, the slides were washed for 30 min in warm (40°C) running tap water in order to remove gelatin and any adhering precipitates, then rinsed in distilled H2O (3 × 2 min). The slides were fixed in 5% sodium thiosulfate for 12 min, rinsed in distilled H2O (4 × 2 min) and postfixed in 70% alcohol for 30 min. The sections then were counterstained with 0.1% thionin or were further dehydrated and coverslipped. Occasional series of adjacent sections were stained for Nissl only or for CO as described above.
Quantitative Measurements
It became obvious early in this study that the mutant cortices were considerably thinner than those of wild-type mice. Regional and laminar specificity of reduced cortical thickness were evaluated quantitatively. To do this we measured the thickness of cortical layers along a radial line extending from the pial surface to the white matter in the frontal, parietal and occipital regions of mutant and wild-type animals. Because mutant cortices are somewhat smaller in overall dimensions than those of wild-type mice and because they have enlarged lateral ventricles (Fig. 1) (Monaghan et al., 1997) we could not assume that stereotaxic location would permit sampling of homologous cortical regions in the two groups. Thus, the measured portions of particular cortical areas were selected as follows from coronal sections. Frontal cortex was defined as a relatively agranular region of cortex immediately lateral to the cingulum bundle at the level of the septal nuclei. In normal mice this corresponds to area 6 of motor cortex (Caviness, 1975). Parietal cortex was defined as a region of granular cortex on the lateral convexity of the hemisphere at the level of the anterior thalamus. In each case measurements were made through the center of one of the cortical barrels, which were plainly visible in layer IV of primary somatosensory cortex (area 3) of mutant and wild-type mice (e.g. see Fig 2, Fig 6 and Fig 7). Occipital cortex was defined as a region of granular cortex caudal to the splenium of the corpus callosum and lateral to its forceps major. We took this to be primary visual cortex (area 17) (Caviness, 1975). The mean value of each of these measures in the mutants (n = 7) was compared with controls (n = 5) and the significance evaluated with t-tests.
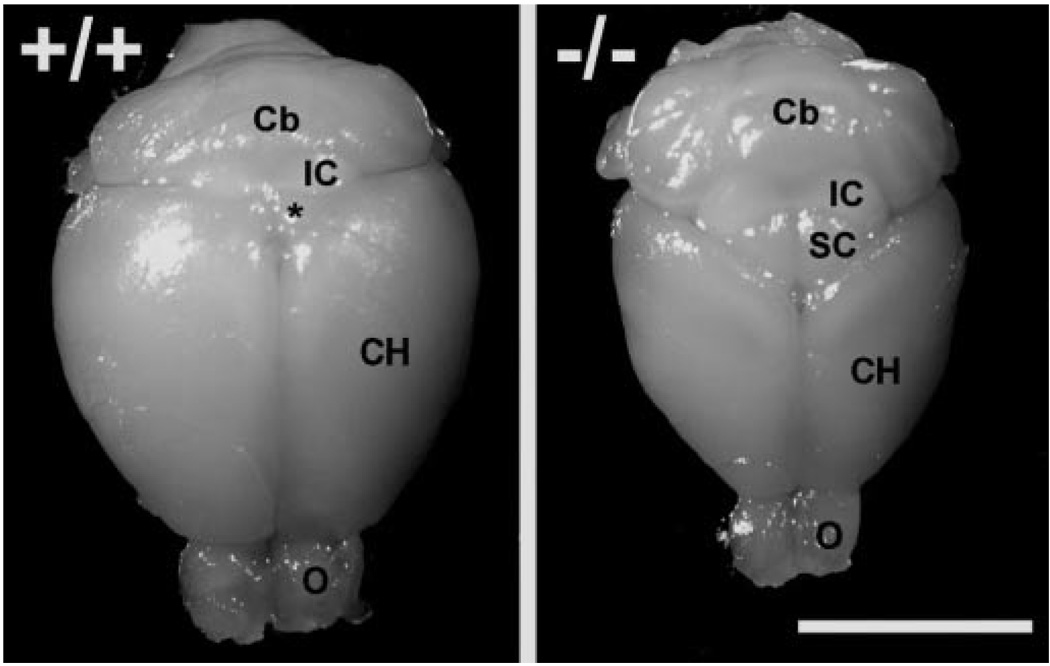
Figure 1.
Deletion of tailless principally affects development of telencephalon. The cerebral hemispheres (CH) and olfactory bulbs (O) of tailless mutant mice (−/−) are visibly reduced in size compared with wild-type littermates (+/+). The gross morphology of subcortical structures including the brainstem and cerebellum (Cb) is relatively unaffected. IC = inferior colliculus, SC = superior colliculus, * = caudo-medial aspect of superior colliculus visible between cerebral hemispheres of wild-type mice. Bar = 1 cm.
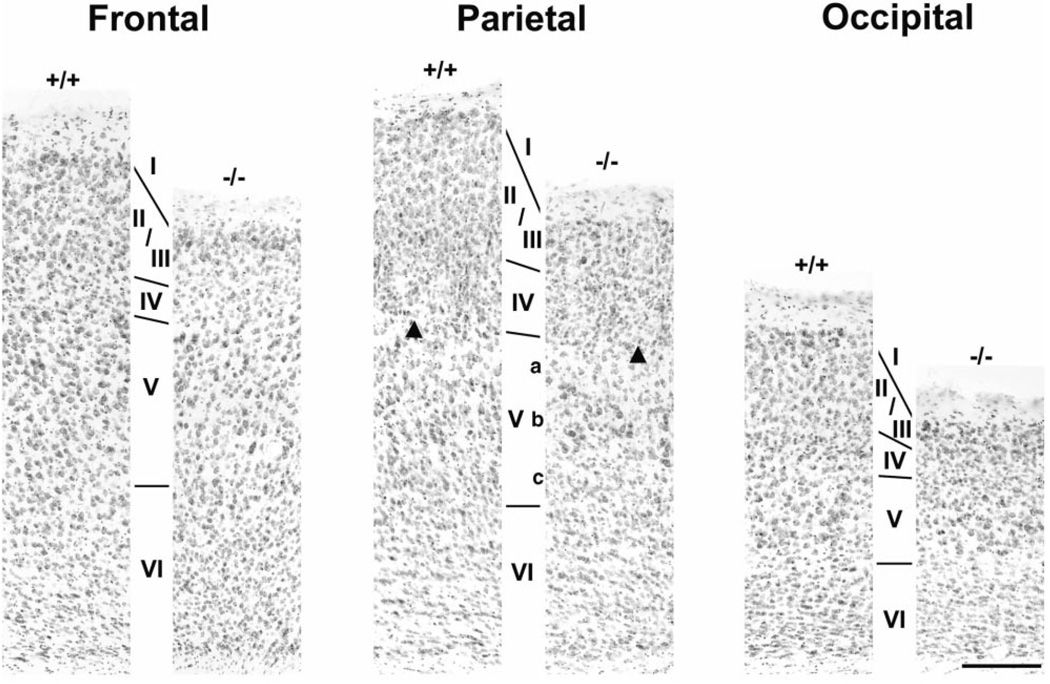
Figure 2.
Neocortex in tlx−/− mice is normally laminated but thinner than in wild-type mice. Panels show pairs of Nissl-stained coronal sections through frontal, parietal and occipital cortical regions of wild-type (+/+) and mutant (−/−) mice. Cortical layers as deduced from cell size and packing density are indicated by Roman numerals I through VI. Individual layers, and in some cases sublayers (e.g. layers Va, Vb and Vc in parietal cortex), are readily recognized in mutant mice. Each cortical region of mutant mice, however, is distinguished by a reduction in overall cortical thickness due principally to abnormally thin layers II/III. Arrowheads = cortical barrel in layer IV of parietal somatosensory region. Bar = 200 µm.
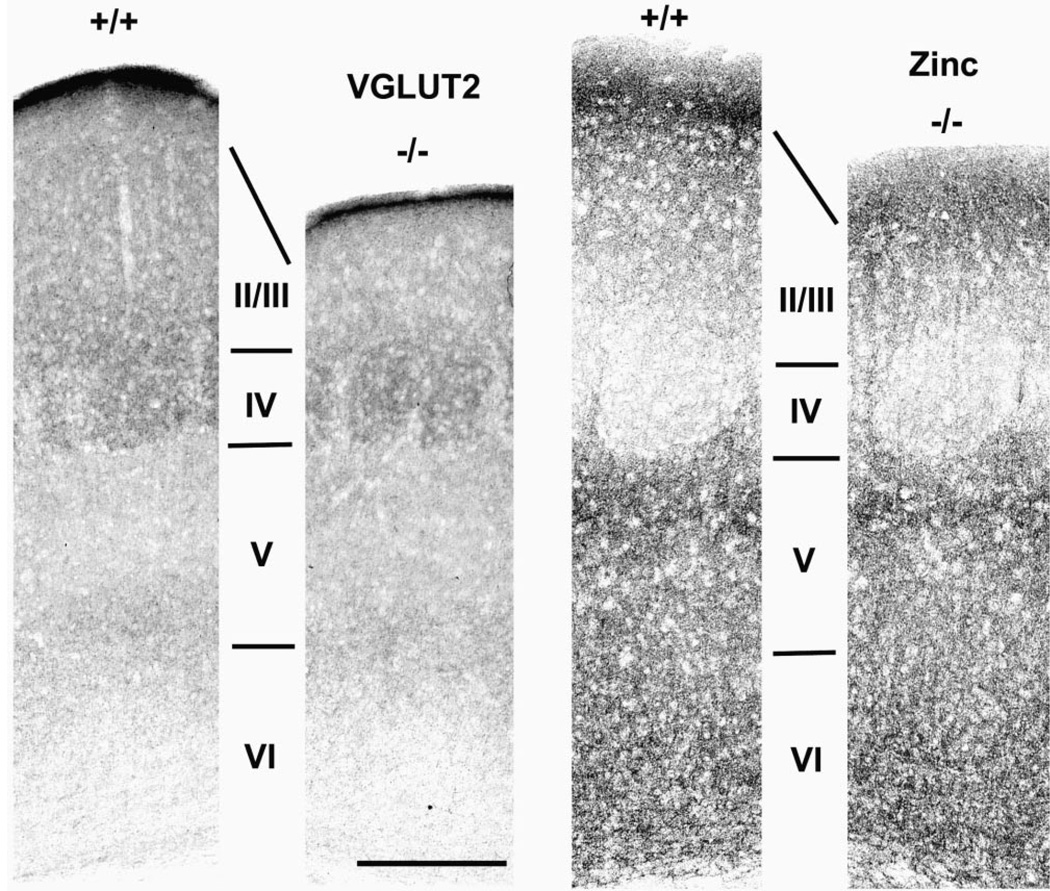
Figure 6.
Cortical circuits reflect cortical cytoarchitecture. Panels show pairs of coronal sections through barrel cortex of wild-type (+/+) and mutant (−/−) mice stained immunohistochemically for VGLUT2 (left pair of panels) or for synaptic zinc (right pair of panels). Each panel is centered on a layer IV barrel as determined from adjacent Nissl-stained sections. Note the complementary density and distribution of axon terminals stained by these two markers. Also note comparable staining patterns in wild-type and mutant cortex for layers IV through VI, and the attenuated thickness of layers II/III. Bar = 300 µm for all panels.
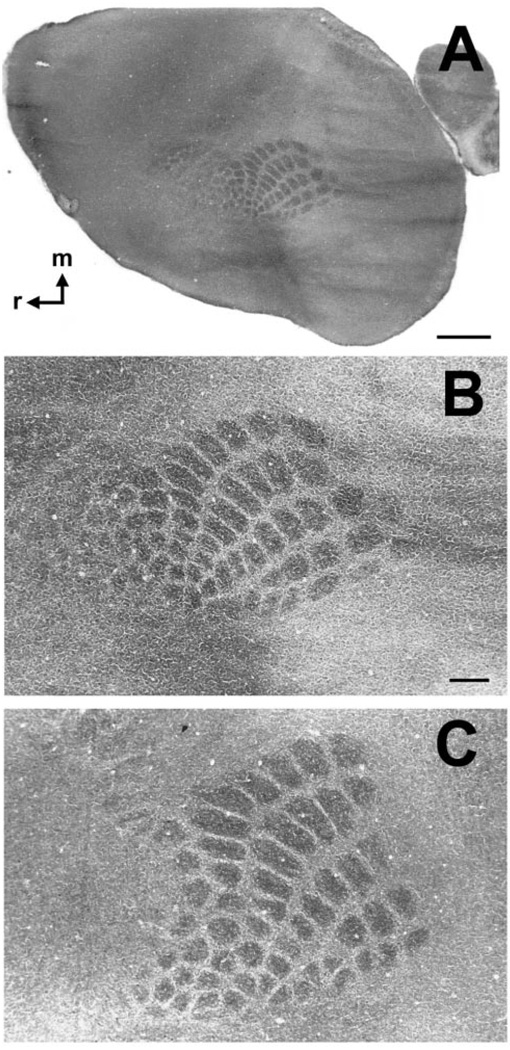
Figure 7.
Area maps develop normally in tlx−/− mice. Panels show single tangential sections through cortical layer IV stained for cytochrome oxidase (CO). (A) CO-stained section from tlx−/− mouse. Patches of dark CO reactivity denoting somatosensory cortical barrels occupy a central location along the rostrocaudal axis of the neocortex, as they do in wild-type animals. Higher magnification of this barrelfield is shown in B. (B, C) Higher magnification photomicrographs of mutant (B) and wild-type barrelfield (C). Note that the pattern of barrel rows and arcs is similar in the two mouse lines. Weathervane in A points rostrally (r) and medially (m). Bar in A = 1 mm; bar in B = 300 µm (also applies to C).
To investigate a potential effect of reduced cortical thickness on interhemispheric connections, we measured the cross-sectional area of the corpus callosum in sagittal sections from four wild-type and four mutant mice. To avoid possible gender-related differences in callosal area (DeLacoste-Utamsing and Holloway, 1982; Kim and Juraska, 1997) all eight mice used in these analyses were females. We used Neurolucida (Microbrightfield, Inc., Williston, VT) to trace the perimeter of the corpus callosum in the first full parasagittal section through one hemisphere of each mouse (usually ~60–90 µm from the mid-sagittal plane). We then determined the cross-sectional area of each corpus callosum using Neuro-Explorer (Microbrightfield) and exported the data into a spreadsheet where we calculated the means and standard deviations for each strain. An unpaired t-test was used to compare the resulting values.
Results
In mice lacking the transcription factor tailless the cerebral hemispheres and olfactory bulbs are visibly reduced in size while major components of the brainstem and the cerebellum appear relatively normal (Fig. 1). Previous studies noted that specific regions of the limbic forebrain in tlx mutants are smaller than in wild-type littermates, due at least in part to reduction in the number of constituent neurons (Monaghan et al., 1997). Because neurons destined to form neocortex also are generated during the period when tlx normally is expressed in the VZ and SVZ we investigated here whether loss of tlx function leads to abnormalities in neocortical development in addition to the conspicuous defects in limbic structures.
Cytoarchitecture of tlx−/− Neocortex
The neocortex of tlx−/− mice is reduced in thickness compared with wild-type littermates (Fig. 2). It nevertheless is possible to identify individual cortical layers in Nissl-stained sections from mutant mice based upon cell size and packing density. Indeed, the overall inside-out pattern of lamination in tlx−/− mice is relatively normal. Compared with the cortical cytoarchitecture of wild-type littermates, however, mutant cortices display a marked attenuation of superficial layers, in particular layers II/III.
Figure 2 illustrates the cytoarchitecture of wild-type and mutant littermates in frontal, parietal and occipital cortical regions. In each location, layer I and layers IV through VI in tlx−/− mutants are similar in organization to the pattern observed in wild-type mice. This is particularly evident in the parietal region where barrel-like structures demarcate layer IV (e.g. arrowheads in Fig. 2; see also below) and where layer V is seen to consist of several relatively distinct sublayers.
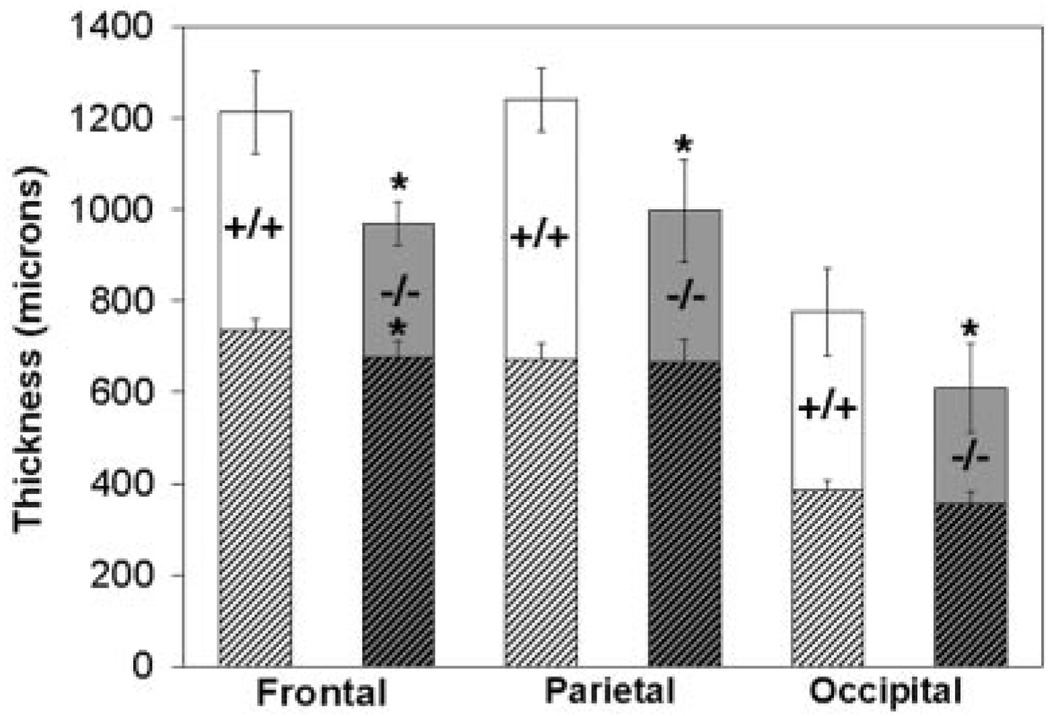
To quantify the effect of tlx deletion on cortical lamination we first measured total cortical thickness (i.e. measured from the pial surface to the white matter) in Nissl-stained sections through frontal, parietal and occipital cortex from five wild-type and seven mutant mice (Materials and Methods). Consistent with qualitative evaluation, the mean total cortical thickness in the mutant mice was significantly reduced compared with wild-type mice in the frontal (P = 0.003), parietal (P < 0.001) and occipital (P = 0.006) regions. On average a particular cortical region in mutant mice is ~80% of normal thickness (frontal, 79.9%; parietal, 80.4%; occipital, 78.6%) (Fig. 3, full columns).
Figure 3.
Loss of tailless function severely affects thickness of superficial cortical layers. Chart compares overall cortical thickness (full columns) or thickness of infragranular layers only (hatched region) in frontal, parietal and occipital cortex of wild-type (+/+; n = 5) and mutant (−/−; n = 7) mice. Each cortical region in mutant mice is ~80% of the thickness of the corresponding region in wild-type mice (frontal= 79.9%; parietal = 80.4%; occipital = 78.6%). An asterisk indicates that total cortical thickness is significantly thinner than in the corresponding region of wild-type animals (frontal, P = 0.003; parietal, P < 0.001; occipital, P = 0.006). Thickness of the infragranular layers (i.e. layers V and VI) in mutant mice is similar to that observed in wild-type mice in parietal (P=0.79) and occipital regions (P=0.15), but thinner in the frontal region (P = 0.02). Bars denote standard deviation.
We then considered separately the superficial versus deep cortical layers (i.e. layers I through IV vs layers V and VI). We chose this parcellation of cortical layers for these measurements because the layer IV/layer V border could be confidently identified in Nissl-stained material from all cortical areas in both strains. We found the thickness of the superficial layers of mutant mice to be significantly reduced compared with wild-type littermates in all regions (frontal P = 0.007, parietal P = 0.003 and occipital P = 0.006, respectively). In contrast, the thickness of the infragranular layers in mutant mice was similar to controls in both the parietal (P = 0.79) and occipital (P = 0.15) regions (e.g. Figure 3, cross-hatched). In the frontal region, however, the thickness of the infragranular layers was significantly less than controls (P = 0.02). The latter finding could indicate a more severe influence of tlx deletion on development of frontal cortex, whose neurons are generated earliest in the rostro-caudal wave of cortical neurogenesis. It may, on the other hand, reflect a shortcoming in our criteria for routinely identifying in mutant animals a frontal cortical area for quantitative study (i.e. see Materials and Methods). Nevertheless, these findings suggest that tlx deletion results in a decrease in cortical thickness that is expressed throughout the neocortical mantle. The data indicate, further, that much of the decrease in cortical thickness is due to attenuation of superficial layers.
Distribution of Cell-type Specific Molecules
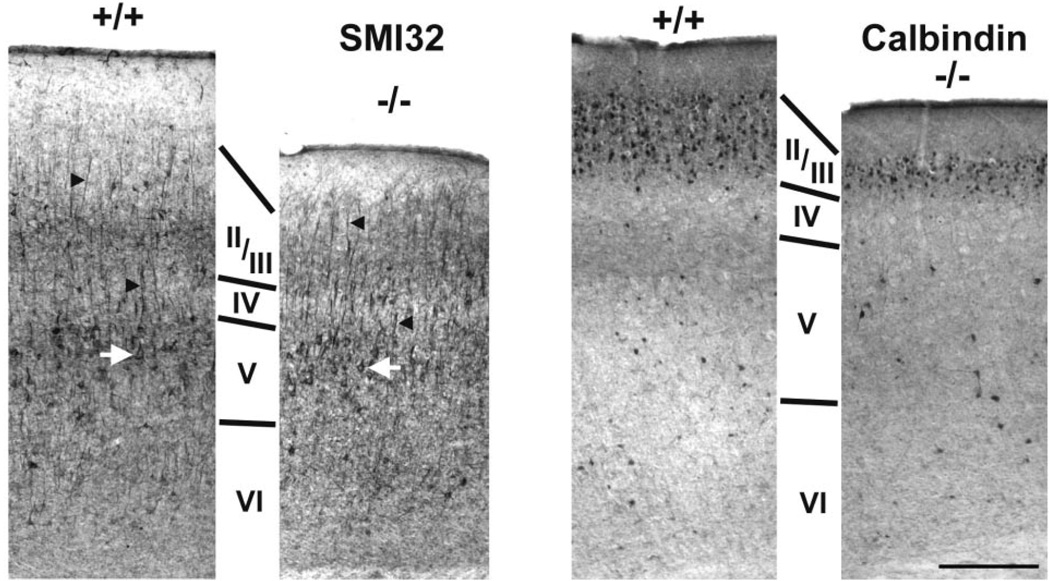
The cytoarchitectural analyses suggest that deep cortical layers in tlx−/− mice are less severely affected than superficial ones, which appear to be reduced in thickness. To further investigate a possible layer-specific effect of tailless deletion on cortical development we examined the expression of two molecules, SMI-32 and calbindin D-28k, which are predominantly localized to neurons in layer V and layer II/III, respectively. In both mouse strains SMI-32 immunostaining reveals a population of large pyramidal neurons (Fig. 4; left panels). Stained neuronal somata are especially prominent in layer V (white arrows), and their thick apical dendrites (e.g. black arrowheads) are seen to pass radially through more superficial cortical layers. Thus, neurons in cytoarchitecturally defined layer V of mutant mice can acquire a molecular phenotype and structural characteristics typical for cells in layer V of wild-type mice.
Figure 4.
Molecular markers highlight layer-specific effects of tailless deletion. Panels show pairs of sagittal sections through parietal cortex of wild-type (+/+) and mutant (−/−) mice stained immunohistochemically for SMI-32 (left pair of panels) or for calbindin D-28k (right pair of panels). Large pyramidal cells immunoreactive for SMI-32 are present in deep layers (i.e. layer V) of both mouse strains (e.g. white arrows). Note also that these neurons support prominent apical dendrites (e.g. black arrowheads), exhibiting a relatively normal morphology in tlx−/− compared with wild-type cortex. Calbindin-D28k-immunoreactive neurons (right panels) are scattered diffusely throughout deep cortical layers. Their numbers are especially enriched, however, in a superficial stratum that coincides with cytoarchitectonically defined layers II/III. Note that calbindin-immunoreactive neurons in tlx−/− mice are distributed in a pattern resembling that observed in wild-type mice, except that the superficial stratum of these cells is abnormally thin. Cortical layers as deduced from nearby sections stained with thionin are indicated by Roman numerals. Bar = 300 µm for all panels.
In wild-type mice calbindin D-28k-immunoreactive neurons are scattered diffusely throughout the cortical layers (Fig. 4; right panels; +/+). Stained neurons are quite numerous, however, in layers II/III, where they form a rather uniform stratum that spans the thickness of those layers. A similar pattern is observed in mutant cortex (Fig. 4; −/−). Notably, as predicted by the above cytoarchitectural analyses, the superficial tier of calbindin-immunoreactive neurons is severely attenuated in tlx−/− mice. Together these findings indicate that diverse neuronal phenotypes are generated in tlx−/− cortex, but mutant mice lack the ability to fully populate superficial cortical layers.
Area of the Corpus Callosum
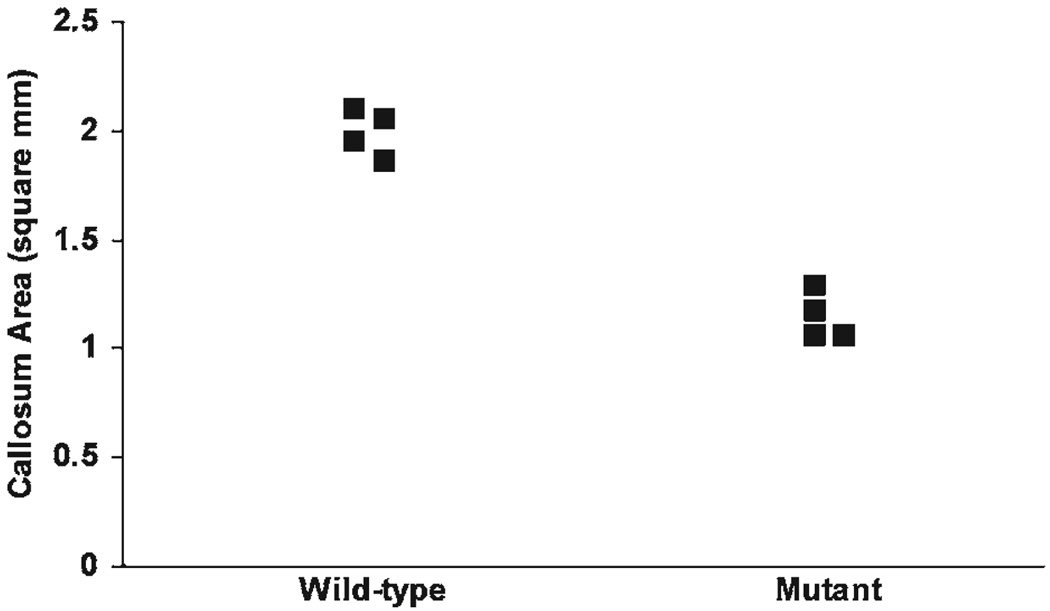
Late-generated cortical neurons, particularly pyramidal cells residing in layers II/III, are a principal source of interhemispheric axons (Wise and Jones, 1976; Jensen and Altman, 1982); see also (Schwartz and Goldman-Rakic, 1991). The apparent diminution of the superficial layers in tlx−/− mice therefore could reduce the overall size of commissural projections. We tested this hypothesis by measuring the cross-sectional area of the corpus callosum in sagitally sectioned hemispheres from four wild-type mice and four mutant mice. In wild-type mice the corpus callosum encompasses 1.992 ± 0.108 mm2, whereas in tlx−/− mice the area is reduced to 1.146 ± 0.112 mm2 (Fig. 5). This 42.5% reduction in cross-sectional area is highly significant (P < 0.0001). The precise magnitude of axonal loss awaits more detailed ultrastructural studies that are beyond the scope of the present investigation. It nevertheless seems likely that inter-hemispheric (and perhaps intrahemispheric) projections are substantially compromised in tailless mutant mice, commensurate with the reduction in size of superficial cortical layers.
Figure 5.
Corpus callosum is significantly reduced in tailless mutants. Scatter-plot shows cross-sectional area of the corpus callosum from four wild-type and four mutant female mice. Several data points are shifted to the right for clarity. Mean callosal area in wild-type mice is significantly larger than in mutant mice (1.992 ± 0.108 mm2 vs 1.146 ± 0.112 mm2; P < 0.0001).
Distribution of Cortical Circuits in tlx−/− Mice
The laminar organization of cortical neurons is reflected in distinct patterns of afferent and intrinsic connections that they receive. This is thought to result from layer-specific expression of molecular cues by cortical neurons that regulate the growth and branching of axons during cortical development (Castellani and Bolz, 1997; Mann et al., 2002). We used markers for specific axonal systems to evaluate the influence of tlx function on establishment of extrinsic and intrinsic cortical circuits.
The thalamus provides the major afferent input to the cortex. We used antibodies to VGLUT2, a glutamate transporter that specifically labels thalamic axon terminals (Fujiyama et al., 2001), to visualize the overall distribution pattern of thalamic projections onto wild-type and mutant cortex. Figure 6 (left panels) shows the pattern of VGLUT2 immunoreactivity in the parietal cortex of wild-type (+/+) and mutant (−/−) mice. As evidenced by the density of VGLUT2-immunoreactive puncta, thalamic axons project heavily onto layer IV, in this case onto a somatosensory cortical barrel, with sparse terminations in the supragranular layers (i.e. layers II/III) and at the layer V/VI border. The overall distribution pattern of thalamic terminals visualized by VGLUT2 staining is virtually identical in normal and mutant mice except that cortex superficial to layer IV is much thinner in tlx−/− mice.
To evaluate the distribution of intrinsic cortical circuits we used histochemistry to localize a prominent subclass of glutamatergic axon terminals that also contain zinc within their synaptic vesicles (Beaulieu et al., 1992). Zinc-sequestering synapses arise from pyramidal neurons in layers II/III and VI, and are distributed heterogeneously among the cortical layers (Slomianka et al., 1990; Garret and Slomianka, 1992; Dyck and Cynader, 1993; Dyck et al., 1993; Land, 1995; Casanovas-Aguilar et al., 1998; Land and Akhtar, 1999). In tlx−/− mice the distribution pattern of zinc-containing circuits is strikingly similar to that observed in wild-type mice. Figure 6 (right panels) shows the distribution of synaptic zinc in the parietal somatosensory cortex. In both tlx+/+ and tlx−/− mice synaptic zinc is densest in layers II/III and upper layer V, intermediate in density in layer VI and extremely light in layer IV, denoted here by a cortical barrel. The distribution pattern of zinc-containing terminals thus is complementary to that of VGLUT2-stained puncta. As noted above for VGLUT2 immunoreactivity, a major distinguishing feature of wild-type vs mutant mice is the thickness of cortical tissue superficial to layer IV. Thus, the overall patterns of afferent and intrinsic cortical circuits match the cortical cytoarchitecture in mutant animals, being similar to wild-type from approximately layer IV to the white matter.
Specification of Cortical Maps in tlx−/− Mice
The neocortex normally contains multiple cytoarchitectonically distinct regions that subserve particular functions. Because the overall size of the cerebrum is reduced in tlx−/− mice we wondered whether functional cortical areas might be missing and/or displaced. To obtain clues as to area fate in tlx−/− mice we prepared f lattened sections through several wild-type and mutant cortices and stained them with cytochrome oxidase. We focused on the somatosensory cortical region that, in rodents, contains a discrete map of the contralateral whisker pad (Woolsey and van der Loos, 1970; Welker and Woolsey, 1974). Whisker-related cortical modules (i.e. the barrels) normally are arranged in a pattern that is homeomorphic with the distribution facial whiskers (Woolsey and van der Loos, 1970), and the ‘barrelfield’ occupies a central position in the cortical mantle (Woolsey and van der Loos, 1970).
Figure 7A illustrates a single section through layer IV of mutant cortex that was stained for cytochrome oxidase. As we observed in all mutant animals, a distinct barrel pattern is visible in the expected location mid-way along the rostro-caudal axis of the cortical sheet. This suggests the (as yet untested) possibility that other functional areas occupy regions of cortex both rostral and caudal to the barrelfield as they do in wild-type mice. The barrelfield from this specimen is shown at higher magnification in Figure 7B. For comparison, the barrelfield from a wild-type littermate is shown at the same magnification in Figure 7C. Note that the overall arrangement of barrel rows and arcs — a reflection of thalamocortical axon distribution — is similar to that observed in wild-type animals. Thus, it seems likely that loss of tlx function does not severely disrupt the partitioning of the neocortex into species-specific area maps.
Discussion
Our main finding is that the lack of tailless function during embryogenesis results in abnormal thinning of the cortical sheet, layers II/III being more severely affected than the deep layers or layer I. The overall distribution pattern of afferent and intrinsic cortical circuits, and the development of cortical area maps in tlx−/− mice are relatively normal. Thus, tlx deletion affects development of neurons destined for superficial cortical layers but does not seem to alter neuronal migration and differentiation, or the spatial patterning of the developing cortex.
Loss of tlx Function Affects Development of Superficial Cortical Layers
The basic inside-out pattern of cortical lamination is preserved in tlx−/− mice, indicating that tlx function is not required for radial neuronal migration per se. Mutant cortices, however, are ~20% thinner than those of wild-type mice. Quantitative analyses reveal that most of the reduced cortical depth in tlx−/− mice is due to attenuation of superficial cell layers. In Nissl-stained sections from all cortical regions of tlx−/− mice it is apparent that layers II/III, in particular, are diminished in thickness. This is supported by our observation that a superficial stratum of neurons immunoreactive for calbindin D-28k, which normally marks a population of small pyramidal and non-pyramidal cells in layers II/III (Hof et al., 1999), is substantially narrower in tlx−/− mice compared with wild-type mice. The extent to which the composition of layer IV (vs II/III) also is affected in mutant animals is less obvious in the absence of a layer-specific marker. However, specialized structures like somatosensory cortical barrels are present in layer IV of all mutant animals (e.g. Figure 2, Figure 6 and Figure 7), suggesting that layer IV may be somewhat less affected by loss of tailless function than layers II/III. A less severe effect of tailless deletion on layer IV would be consistent with preliminary findings of Roy et al. (Roy et al., 2001, 2002) (Roy et al., submitted) who observed in tlx−/− mice that numbers and proliferative capacity of cortical progenitor cells begin to decrease around E16 — an age when most, though perhaps not all neurons destined for cortical layer IV are likely to have left the proliferative epithelium (Polleux et al., 1997; Takahashi et al., 1999).
The majority of local and long-distance horizontal, and inter-hemispheric projections in neocortex arise from neurons in layers II/III (Jensen and Altman, 1982; Lund, 1988; Burkhalter, 1989; Schwartz and Goldman-Rakic, 1991). Diminution of superficial layers in tailless mutants therefore is likely to substantially impact the organization of intracortical connections (see also below). Indeed, in tlx−/− mice we found that the cross-sectional area of the corpus callosum, the largest forebrain commissure, is significantly reduced (i.e. by over 40%) compared with wild-type mice. These quantitative data are consistent with previous qualitative observations that the anterior and hippocampal commissures in mutant mice are smaller than normal (Monaghan et al., 1997). Additional studies are required to establish precisely the degree of axon loss in mutant commissures, and the effect, if any, on interhemispheric projection patterns. The data nevertheless support the idea that many projection neurons are missing from the superficial layers of mutant cortex.
By contrast with effects of the tailless mutation on layers II/III, infragranular layers appear relatively well-formed and have a cytoarchitectonic organization similar to deep layers of wild-type mice. Consistent with this qualitative observation, cortical thickness measured from the white matter to the bottom of layer IV in tlx−/− mice (i.e. layers V and VI) is not significantly different from values obtained in wild-type animals in most areas. There nevertheless is a trend toward slightly thinner layers in mutant animals, which reached significance in the frontal region. There are several possible explanations for the slight but noticeable effect on infragranular layers. For example, it might denote the loss of a small population of late-born neurons that normally settle in the deeper layers (Berry and Rogers, 1965; Hicks and D’Amato, 1968). In addition, it could reflect in part reduced numbers of GABAergic neurons that migrate tangentially from the telencephalic medial and lateral ganglionic eminences (de Carlos et al., 1996; Anderson et al., 1997, 2001; Wichterle et al., 2001), where neurogenesis also is affected by loss of tailless function (Monaghan et al., 1997). Finally, there may be subtle changes in cell size or packing density in deep layers of mutant cortex that could be revealed by more detailed morphometric analyses than those employed in the present investigation. It is interesting to note, nevertheless, that particular subpopulations of deep layer neurons — like large pyramidal neurons expressing SMI-32 identified in this study — are present in mutant cortex, where they assume a normal location and acquire relatively normal morphology.
It is intriguing that loss of tailless function in all forebrain progenitor cells, throughout development, has a more profound effect on superficial than on deep cortical layers. Previous studies suggest that distinct subsets of committed progenitor cells exist in the dorsal VZ before neurogenesis begins (Tan and Breen, 1993; Ware et al., 1999; McCarthy et al., 2001), and give rise to progeny with different laminar fates. The restriction of cortical progenitors to deep versus superficial layers has been investigated by a variety of techniques. For example, the developmental potential of early versus late progenitor cells has been explored by transplanting progenitor cells to hosts of different ages. Such studies demonstrate that laminar fate of early progenitors (i.e. those destined for deep layers) is specified before the last cell division (McConnell and Kaznowski, 1991). Thus, early cortical progenitors transplanted in S-phase of the cell cycle to the cortex of an older host, and allowed to undergo one round of division in the host environment adopted a host (i.e. superficial layer) phenotype, while cells transplanted later in the cell cycle adopted a donor (i.e. deep layer) phenotype. By contrast, progenitor cells from older embryos (i.e. cells destined to form superficial layers) are unable to generate deep layer neurons (Frantz and McConnell 1996). Lineage tracing studies and clonal analyses support these observations (Rakic, 1988; Walsh and Cepko, 1988; Luskin et al., 1988) and indicate that early progenitor cells are multipotent whereas later progenitor cells exhibit a progressive restriction in their laminar potential. These studies also show that restriction of developmental potential of progenitor cells occurs early and that, over time, both environmental and intrinsic signals underlie the different behaviors of these populations [(Luskin et al., 1988; Barbe and Levitt, 1991, 1992, 1995; Parnavelas et al., 1991; Takahashi et al., 1993, 1995a,b; Davis and Temple, 1994; Bohner et al., 1997; Lim et al., 1997; Mayer-Proschel et al., 1997; Qian et al., 1997, 1998, 2000); reviewed in (Lillien, 1998)].
Mechanisms that regulate a progenitor cell’s potential to produce deep- versus superficial-layer progeny are not fully understood. Because tlx is absent from progenitor cells throughout development it will be important to determine whether cortical abnormalities observed here result from primary versus secondary effects. Thus, the alterations observed in superficial cortical laminae could be due to a primary requirement for tlx expression in late progenitor cells (i.e. those that seed superficial cortical layers). On the other hand, the abnormalities observed in late progenitor cells could be a secondary response to the altered environment present in mutant animals. Our observation that deep cortical layers are relatively well-formed in these mice but superficial ones are attenuated suggests that, despite the accelerated pace of neurogenesis, assignment of laminar fate initially proceeds normally. It remains to be established whether tailless is involved directly in promoting neurogenesis (e.g. vs gliogenesis), or whether expression of this gene enables progenitor cells to respond to environmental cues that would signal continued proliferation. Our findings nevertheless suggest that tailless may be an essential regulator of a progenitor cell’s choice to proliferate or differentiate. The data further demonstrate that small changes in cell cycle kinetics early can have profound consequences over time (Kornack and Rakic, 1998). Late developing structures, like superficial cortical layers, are particularly vulnerable to early changes in the rate of proliferation since such changes will become amplified as development proceeds.
Laminar Distribution of Intrinsic and Extrinsic Cortical Circuits Is Preserved in tlx−/− Mice
VGLUT2 immunoreactivity, a marker of thalamocortical axon terminals (Fujiyama et al., 2001), is localized to two tiers in tlx−/− mice, as it is in wild-type mice (i.e. Figure 6). The location of these strata in both mouse lines corresponds to cytoarchitecturally defined layer IV and the layer V/layer VI border. These layers normally are principal targets of primary thalamo-cortical projections. Thalamic axons in mutant mice thus appear to recognize layer-specific signals that guide them to their appropriate cortical locus. Moreover, VGLUT2 staining in the parietal somatosensory region of tlx−/− mice clearly delineates the cell-sparse hollows of cortical barrels. This suggests that mutant cortical neurons, in turn, respond to cues provided by the segregated thalamic axons with preferential dendritic growth and orientation, and cell body displacement that creates the barrel structure (Harris and Woolsey, 1981; Erzurumlu and Jhaveri, 1990).
Zinc-containing terminals, which denote a prominent subset of intracortical circuits, are heterogeneously distributed in tlx−/− mice in a pattern similar to that of wild-type mice, and that resembles the pattern observed for zinc-containing pathways in other cortical areas and in other species (Garret and Slomianka, 1992; Dyck et al., 1993; Land and Akhtar, 1999). Although we have not examined this quantitatively, the overall staining intensity for synaptic zinc in individual layers of mutant cortex appears comparable to that observed in wild-type animals (cf., Fig. 6, above). These findings are particularly interesting because a majority of zinc-sequestering cortical neurons (i.e. neurons whose synaptic terminals bear the neuron-specific zinc transporter, ZnT-3) are pyramidal cells in layers II/III (Garret and Slomianka, 1992; Land, 1995; Palmiter et al., 1996). These layers are markedly attenuated in the absence of tailless function (see above). This suggests that in mutant mice individual layer II/III neurons might compensate for missing cells by expanding their projections within normal target regions. It would be interesting in this regard to examine in greater detail the horizontal and interlaminar connections of zinc-ergic pyramidal neurons in tlx−/− mice. Taken together, these data suggest that molecular cues regulating axo-dendritic growth and the precise matching of presynaptic input with postsynaptic target are expressed in appropriate spatiotemporal patterns by cortical neurons in mutant animals (Castellani and Bolz, 1997; Hevner et al., 2002; Mann et al., 2002).
Tlx−/− Cortex Is Subdivided into Species-specific Area Maps
Somatosensory cortical barrels can be identified in the expected location on the lateral cortical convexity in all mutant animals. This is true irrespective of the staining method employed in a particular experiment. In tangentially sectioned hemispheres it is evident that the somatotopic distribution of barrels and the rostro-caudal position of the barrelfield in mutant mice resemble the arrangement observed in wild-type subjects. This suggests the (as yet untested) possibility that other functional areas occupy regions of cortex both rostral and caudal to the barrel-field as they do in wild-type mice. There are two additional important implications of this finding. First, it indicates that clustered thalamocortical axons, which are believed to convey the periphery-related map onto the cerebral cortex (Erzurumlu and Jhaveri, 1990), encounter sufficient cues in mutant forebrain to maintain their normal distribution patterns and to recruit cortical neurons into barrels. Second, correct placement of the barrelfield along the rostro-caudal axis implies that gradients of signaling molecules thought to contribute to parcellation of neocortex into functional areas (Fukuchi-Shimogori and Grove, 2001; Ragsdale and Grove, 2001) are expressed in normal patterns in the absence of tlx−/− function. These findings were unexpected given that precocious progenitor cell differentiation in the absence of tlx function could alter the environment encountered by ingrowing thalamic axons. In summary, tailless expression is critical for the development of late-generated cortical neurons (i.e. those destined for superficial layers) but is not required for cortical patterning per se or for the laminar and columnar arrangement of cortical circuits.
Manipulating the Tlx Gene Provides a Tool for Dissecting Cortical Neurogenesis
Our findings indicate that tlx deletion does not alter radial neuronal migration or the spatial patterning of the developing cortex. However, preliminary studies indicate that the temporal regulation of proliferation and differentiation are uncoupled in the developing forebrain of tlx−/− mice (Roy et al., 2001, 2002) (Roy et al., submitted). The hypoplasia of superficial cortical layers observed in the present study could thus be a consequence of this dysregulation. Interestingly, other proteins that directly regulate proliferation of progenitor cells also have been shown to alter formation of upper cortical layers. For example, absence of the transcription factor Pax-6 in cortical progenitor cells leads to an enhanced rate of cellular proliferation until mid-gestation. Pax-6 also is required for the differentiation and migration of late-born cortical progenitor cells (Stoykova and Gruss, 1994; Stoykova et al., 1996; Gotz et al., 1998; Warren et al., 1999). In addition, both rats and mice with a mutation in the citron-kinase gene (CK) exhibit altered cytokinesis in progenitor cells, massive apoptosis in proliferative regions, and thinning of superficial cortical layers (Di Cunto et al., 2000; Sarkisian et al., 2002). Together with the results of the present study these findings indicate that cells destined for superficial cortical layers are sensitive to upsetting the balance between proliferation and differentiation. This can occur through altered cell cycle kinetics as in the tlx mutation, altered migration/differentiation as in the Pax-6 mutation or altered cell survival as in the CK mutation.
Until the development of animal models such as the tlx mutation, investigators have relied on pharmacological methods to study the anatomical and behavioral consequences of perturbing proliferation of progenitor cells during development. The cortical abnormalities described here for tlx−/− mice are reminiscent of the phenotype observed following fetal exposure to the cytotoxic alkylating agent methylazoxymethanol acetate (MAM) (Marsumoto et al., 1972; Johnston and Coyle, 1979). However, in contrast with MAM treatment, which has widespread systemic effects, genetic manipulation of the tlx gene specifically targets progenitor cells in the forebrain. Future experiments aimed at controlling the temporal and spatial expression of tlx will enable a dissection of the behavior of both early and late progenitor cell populations. Compared with the above models, the advantage of manipulating the tlx gene to gain insights into mechanisms regulating cortical development is that tlx is restricted to the forebrain, and mutant animals survive enabling functional and behavioral analyses to be performed in adults.
Acknowledgments
We thank Susan Erickson, Cynthia Lance-Jones and Dan Simons for helpful comments on the manuscript, and Kristine Roy for providing the mice. Kristin Bond, Kirsten Boyer and Lorraine Shamalla-Hannah provided expert technical assistance. This study was supported by NIMH grant MH060774, the March of Dimes Basil O’Connor grant S-FY98-756 (APM), and by NIH grant NS41428 and University of Pittsburgh Competitive Medical Research Fund (PWL).
References
- Aihara Y, Mashima H, Onda H, Hisano S, Kasuya H, Hori T, Yamada S, Tomura H, Yamada Y, Inoue L, Kojima I, Takeda J. Molecular cloning of a novel brain-type Na+-dependent inorganic phosphate cotransporter. J Neurochem. 2000;74:2622–2625. doi: 10.1046/j.1471-4159.2000.0742622.x. [DOI] [PubMed] [Google Scholar]
- Anderson SA, Eisenstat DD, Shi L, Rubenstein JL. Interneuron migration from basal forebrain to neocortex: dependence on Dlx genes. Science. 1997;278:474–476. doi: 10.1126/science.278.5337.474. [DOI] [PubMed] [Google Scholar]
- Anderson SA, Marin O, Horn C, Jennings K, Rubenstein JL. Distinct cortical migrations from the medial and lateral ganglionic eminences. Development. 2001;128:353–363. doi: 10.1242/dev.128.3.353. [DOI] [PubMed] [Google Scholar]
- Angevine JB, Sidman RL. Autoradiographic study of cell migration during histogenesis of the cerebral cortex of the mouse. Nature. 1961;192:266–268. doi: 10.1038/192766b0. [DOI] [PubMed] [Google Scholar]
- Barbe MF, Levitt P. The early commitment of fetal neurons in the limbic cortex. J Neurosci. 1991;11:519–533. doi: 10.1523/JNEUROSCI.11-02-00519.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbe MF, Levitt P. Attraction of specific thalamic input by cerebral grafts depends on the molecular identity of the implant. Proc Natl Acad Sci. 1992;89:3706–3710. doi: 10.1073/pnas.89.9.3706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbe MF, Levitt P. Age dependant specification of the cortico-cortical connections of cerebral grafts. J Neurosci. 1995;15:1819–1834. doi: 10.1523/JNEUROSCI.15-03-01819.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bayer SA, Altman J. Neocortical Development. New York: Raven; 1991. [Google Scholar]
- Beaulieu C, Dyck R, Cyander M. Enrichment of glutamate in zinc-containing terminals of the cat visual cortex. Neuroreport. 1992;3:861–864. doi: 10.1097/00001756-199210000-00010. [DOI] [PubMed] [Google Scholar]
- Berry M, Rogers AW. The migration of neuroblasts in the developing cerebral cortex. J Anat. 1965;99:691–709. [PMC free article] [PubMed] [Google Scholar]
- Bohner AP, Akers RM, McConnell SK. Induction of deep layer cortical neurons in vitro. Development. 1997;124:915–936. doi: 10.1242/dev.124.4.915. [DOI] [PubMed] [Google Scholar]
- Brustle O, Maskos U, McKay RD. Host-guided migration allows targeted introduction of neurons into the embryonic brain. Neuron. 1995;15:63–78. doi: 10.1016/0896-6273(95)90007-1. [DOI] [PubMed] [Google Scholar]
- Burkhalter A. Intrinsic connections of rat primary visual cortex laminar organization of axonal projections. J Comp Neurol. 1989;279:171–186. doi: 10.1002/cne.902790202. [DOI] [PubMed] [Google Scholar]
- Campbell K, Olsson M, Bjorklund A. Regional incorporation and site-specific differentiation of striatal precursors transplanted into the embryonic forebrain ventricle. Neuron. 1995;15:1259–1273. doi: 10.1016/0896-6273(95)90006-3. [DOI] [PubMed] [Google Scholar]
- Casanovas-Aguilar C, Reblet C, Perez-Clausell J, Bueno-Lopez JL. Zinc-rich afferents to the rat neocortex: projections to the visual cortex traced with intracerebral selenite injections. J Chem Neuroanat. 1998;15:7–109. doi: 10.1016/s0891-0618(98)00035-0. [DOI] [PubMed] [Google Scholar]
- Castellani V, Bolz J. Membrane-associated molecules regulate the formation of layer-specific cortical circuits. Proc Natl Acad Sci. 1997;94:7030–7035. doi: 10.1073/pnas.94.13.7030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caviness VS. Architectonic map of neocortex of the normal mouse. J Comp Neurol. 1975;164:247–263. doi: 10.1002/cne.901640207. [DOI] [PubMed] [Google Scholar]
- Danscher G. Exogenous selenium in the brain: a histochemical technique for light and electron microscopic localization of catalytic selenium bonds. Histochemistry. 1982;76:281–293. doi: 10.1007/BF00543951. [DOI] [PubMed] [Google Scholar]
- Davis AA, Temple S. A self-renewing multipotential stem cell in embryonic rat cerebral cortex. Nature. 1994;17:263–266. doi: 10.1038/372263a0. [DOI] [PubMed] [Google Scholar]
- de Carlos JA, Lopez-Mascaraque L, Valverde F. Dynamics of cell migration from the lateral ganglionic eminence in the rat. J Neurosci. 1996;16:6146–6156. doi: 10.1523/JNEUROSCI.16-19-06146.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeLacoste-Utamsing C, Holloway RL. Sexual dimorphism in the human corpus callosum. Science. 1982;216:1431–1432. doi: 10.1126/science.7089533. [DOI] [PubMed] [Google Scholar]
- Di Cunto F, Imarisio S, Hirsch E, Broccoli V, Bulfone A, Migheli A, Atzori C, Turco E, Triolo R, Dotto GP, Silengo L, Altruda F. Defective neurogenesis in citron kinase knockout mice by altered cytokinesis and massive apoptosis. Neuron. 2000;28:115–127. doi: 10.1016/s0896-6273(00)00090-8. [DOI] [PubMed] [Google Scholar]
- Dyck RH, Cynader M. An interdigitated columnar mosaic of cytochrome oxidase, zinc, and neurotransmitter-related molecules in cat and monkey visual cortex. Proc Natl Acad Sci. 1993;90:9066–9069. doi: 10.1073/pnas.90.19.9066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dyck RH, Beaulieu C, Cynader M. Histochemical localization of synaptic zinc in the developing cat visual cortex. J Comp Neurol. 1993;329:53–67. doi: 10.1002/cne.903290105. [DOI] [PubMed] [Google Scholar]
- Eagleson KL, Ferri RT, Levitt P. Complementary distribution of collagen type IV and the epidermal growth factor receptor in the rat embryonic telencephalon. Cerebral Cortex. 1996;6:540–549. doi: 10.1093/cercor/6.3.540. [DOI] [PubMed] [Google Scholar]
- Erzurumlu RS, Jhaveri S. Thalamic axons confer a blueprint of the sensory periphery onto the developing rat somatosensory cortex. Dev Brain Res. 1990;56:229–234. doi: 10.1016/0165-3806(90)90087-f. [DOI] [PubMed] [Google Scholar]
- Ferri RT, Levitt P. Regulation of regional differences in the fate of cerebral cortical neurons by EGF family-matrix interactions. Development. 1995;121:1151–1160. doi: 10.1242/dev.121.4.1151. [DOI] [PubMed] [Google Scholar]
- Ferri RT, Eagleson KL, Levitt P. Environmental signals influence expression of a cortical areal phenotype in vitro independent of effects on progenitor cell proliferation. Dev Biol. 1996;175:184–190. doi: 10.1006/dbio.1996.0106. [DOI] [PubMed] [Google Scholar]
- Fischell G. Striatal precursors adopt cortical identities in response to local cues. Development. 1995;121:803–812. doi: 10.1242/dev.121.3.803. [DOI] [PubMed] [Google Scholar]
- Frantz GD, McConnell SK. Restriction of late cerebral cortical progenitor cells to an upper-layer fate. Neuron. 1996;17:55–61. doi: 10.1016/s0896-6273(00)80280-9. [DOI] [PubMed] [Google Scholar]
- Frederickson CJ. Neurobiology of zinc and zinc-containing neurons. Int Rev Neurobiol. 1989;31:145–238. doi: 10.1016/s0074-7742(08)60279-2. [DOI] [PubMed] [Google Scholar]
- Frederickson CJ, Suh SW, Silva D, Frederickson CJ, Thompson RB. Importance of zinc in the central nervous system. J Nutr. 2000;130:71S–83S. doi: 10.1093/jn/130.5.1471S. [DOI] [PubMed] [Google Scholar]
- Fujiyama F, Furuta T, Kaneko T. Immunocytochemical localization of candidates for vesicular glutamate transporters in the rat cerebral cortex. J Comp Neurol. 2001;435:379–387. doi: 10.1002/cne.1037. [DOI] [PubMed] [Google Scholar]
- Fukuchi-Shimogori T, Grove EA. Neocortex patterning by the secreted signaling molecule FGF8. Science. 2001;294:1071–1074. doi: 10.1126/science.1064252. [DOI] [PubMed] [Google Scholar]
- Garret B, Slomianka L. Postnatal development of zinc-containing cells and neuropil in the visual cortex of the mouse. Anat Embryol. 1992;186:487–496. doi: 10.1007/BF00185462. [DOI] [PubMed] [Google Scholar]
- Gotz M, Stoykova A, Gruss P. Pax6 controls radial glia differentiation in the cerebral cortex. Neuron. 1998;21:1031–1044. doi: 10.1016/s0896-6273(00)80621-2. [DOI] [PubMed] [Google Scholar]
- Harris RM, Woolsey TA. Dendritic plasticity in mouse barrel cortex following postnatal vibrissa follicle damage. J Comp Neurol. 1981;196:357–376. doi: 10.1002/cne.901960302. [DOI] [PubMed] [Google Scholar]
- Haydar TF, Wang F, Schwartz ML, Rakic P. Differential modulation of proliferation in the neocortical ventricular and subventricular zones. J Neurosci. 2000;20:5764–5774. doi: 10.1523/JNEUROSCI.20-15-05764.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hevner RF, Miyashita-Lin E, Rubenstein JL. Cortical and thalamic axon pathfinding defects in Tbr1, Gbx2, and Pax6 mutant mice: Evidence that cortical and thalamic axons interact and guide each other. J Comp Neurol. 2002;447:8–17. doi: 10.1002/cne.10219. [DOI] [PubMed] [Google Scholar]
- Hicks SP, D’Amato CJ. Cell migrations to the isocortex in the rat. Anat Rec. 1968;160:619–634. doi: 10.1002/ar.1091600311. [DOI] [PubMed] [Google Scholar]
- Hof PR, Glezer II, Conde F, Flagg RA, Rubin MB, Nimchinsky EA, Weisenhorn DMV. Cellular distribution of the calcium binding proteins parvalbumin, calbindin, and calretinin in the neocortex of mammals: phylogenetic and developmental patterns. J Chem Neuroanat. 1999;16:77–116. doi: 10.1016/s0891-0618(98)00065-9. [DOI] [PubMed] [Google Scholar]
- Jackson A, Panayiotidis P, Foroni L. The human homologue of the Drosophila tailless gene (TLX): Characterization and mapping to a region of common deletion in human lymphoid leukemia on chromosome 6q21. Genomics. 1998;50:34–43. doi: 10.1006/geno.1998.5270. [DOI] [PubMed] [Google Scholar]
- Jensen K, Altman J. The contribution of late-generated neurons to the callosal projection in the rat: a study with prenatal x-irradiation. J Comp Neurol. 1982;209:113–122. doi: 10.1002/cne.902090202. [DOI] [PubMed] [Google Scholar]
- Johnston MV, Coyle JT. Histological and neurochemical effects of fetal treatment with methylazoxymethanol on rat neocortex in adulthood. Brain Res. 1979;170:135–155. doi: 10.1016/0006-8993(79)90946-6. [DOI] [PubMed] [Google Scholar]
- Kim JH, Juraska JM. Sex differences in the development of axon number in the splenium of the rat corpus callosum from postnatal day 15 through 60. Dev Brain Res. 1997;102:77–85. doi: 10.1016/s0165-3806(97)00080-1. [DOI] [PubMed] [Google Scholar]
- Kornack DR, Rakic P. Changes in cell-cycle kinetics during the development and evolution of primate neocortex. Proc Natl Acad Sci USA. 1998;95:1242–1246. doi: 10.1073/pnas.95.3.1242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lake K, Vranich N, Bond K, Monaghan AP. Precocious differentiation of cortical neurons in tailless-deficient animals. Soc Neurosci Abstr. 2000;26:1858. [Google Scholar]
- Land PW. Histochemical localization of zinc-containing neurons in rat somatosensory barrel cortex. Soc Neurosci Abstr. 1995;21:121. [Google Scholar]
- Land PW, Akhtar ND. Experience-dependent alteration of synaptic zinc in rat somatosensory barrel cortex. Somatosen Motor Res. 1999;16:129–140. doi: 10.1080/08990229970573. [DOI] [PubMed] [Google Scholar]
- Land PW, Simons DJ. Cytochrome oxidase staining in the rat SmI barrel cortex. J Comp Neurol. 1985;238:225–235. doi: 10.1002/cne.902380209. [DOI] [PubMed] [Google Scholar]
- Levison SW, Goldman JE. Both oligodendrocytes and astrocytes develop from progenitors in the subventricular zone of postnatal rat forebrain. Neuron. 1993;10:201–212. doi: 10.1016/0896-6273(93)90311-e. [DOI] [PubMed] [Google Scholar]
- Levitt P, Barbe MF, Eagleson KL. Patterning and specification of the cerebral cortex. Annu Rev Neurosci. 1997;20:1–25. doi: 10.1146/annurev.neuro.20.1.1. [DOI] [PubMed] [Google Scholar]
- Lillien L. Neuronal progenitors and stem cells: Mechanisms of progenitor heterogeneity. Curr Opin Neurobiol. 1998;8:37–44. doi: 10.1016/s0959-4388(98)80006-8. [DOI] [PubMed] [Google Scholar]
- Lim DA, Fishell GJ, Alvarez-Buylla AM. Postnatal mouse subventricular zone neuronal precursors can migrate and differentiate within multiple levels of the developing neuraxis. Proc Natl Acad Sci. 1997;94:14832–14836. doi: 10.1073/pnas.94.26.14832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lund JS. Anatomical organization of macaque monkey striate visual cortex. Annu Rev Neurosci. 1988;11:253–288. doi: 10.1146/annurev.ne.11.030188.001345. [DOI] [PubMed] [Google Scholar]
- Luskin MB, Boone MS. Rate and pattern of migration of lineally-related olfactory bulb interneurons generated postnatally in the subventricular zone of the rat. Chem Senses. 1994;19:695–714. doi: 10.1093/chemse/19.6.695. [DOI] [PubMed] [Google Scholar]
- Luskin MB, Pearlman AL, Sanes JR. Cell lineage in the cerebral cortex of the mouse studied in vivo and in vitro with a recombinant retrovirus. Neuron. 1988;1:635–647. doi: 10.1016/0896-6273(88)90163-8. [DOI] [PubMed] [Google Scholar]
- Mann F, Peuckert C, Dehner F, Zhou R, Bolz J. Ephrins regulate the formation of terminal axonal arbors during the development of thalamocortical projections. Development. 2002;129:3945–3955. doi: 10.1242/dev.129.16.3945. [DOI] [PubMed] [Google Scholar]
- Marsumoto H, Spatz M, Laquer GL. Quantitative changes with age in the DNA contentof methylazoxymethanol-induced micro-cephalic rat-brain. J Neurochem. 1972;19:297–306. doi: 10.1111/j.1471-4159.1972.tb01339.x. [DOI] [PubMed] [Google Scholar]
- Mayer-Proschel M, Kalyani AJ, Mujtaba T, Rao MS. Isolation of lineage-restricted neuronal precursor from multipotent neuro-epithelial stem cells. Neuron. 1997;19:773–785. doi: 10.1016/s0896-6273(00)80960-5. [DOI] [PubMed] [Google Scholar]
- McCarthy M, Turnbull DH, Walsh CA, Fishell G. Telencephalic neural progenitors appear to be restricted to regional and glial fates before the onset of neurogenesis. J Neurosci. 2001;21:6772–6781. doi: 10.1523/JNEUROSCI.21-17-06772.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McConnell SK, Kaznowski CE. Cell cycle dependence of laminar determination in developing cerebral cortex. Science. 1991;254:282–285. doi: 10.1126/science.254.5029.282. [DOI] [PubMed] [Google Scholar]
- Monaghan AP, Grau E, Bock D, Schutz G. The mouse homolog of the orphan nuclear receptor tailless is expressed in the developing forebrain. Development. 1995;121:839–846. doi: 10.1242/dev.121.3.839. [DOI] [PubMed] [Google Scholar]
- Monaghan AP, Bock D, Gass P, Schwager A, Wolfer DP, Lipp HP, Schutz G. Defective limbic system in mice lacking the tailless gene. Nature. 1997;390:515–517. doi: 10.1038/37364. [DOI] [PubMed] [Google Scholar]
- Palmiter RD, Cole TB, Quaife CJ, Findley SD. ZnT-3, a putative transporter of zinc into synaptic vesicles. Proc Natl Acad Sci. 1996;93:14934–14939. doi: 10.1073/pnas.93.25.14934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parnavelas JG, Barfield JA, Franke E, Luskin MB. Separate progenitor cells give rise to pyramidal and non pyramidal neurons in the rat telencephalon. Cerebral Cortex. 1991;1:463–468. doi: 10.1093/cercor/1.6.463. [DOI] [PubMed] [Google Scholar]
- Pignoni F, Baldarelli RM, Steingrimsson E, Diaz RJ, Patapoutian A, Merriam JR, Lengyel JA. The Drosophila gene tailless is expressed at the embryonic termini and is a member of the steroid receptor superfamily. Cell. 1990;62:151–158. doi: 10.1016/0092-8674(90)90249-e. [DOI] [PubMed] [Google Scholar]
- Polleux F, Dehay C, Kennedy H. The timetable of laminar neurogenesis contributes to the specification of cortical areas in mouse isocortex. J Comp Neurol. 1997;385:95–116. [PubMed] [Google Scholar]
- Qian X, Davis AA, Goderie SK, Temple S. FGF2 concentration regulates the generation of neurons and glia from multipotent cortical stem cells. Neuron. 1997;18:81–93. doi: 10.1016/s0896-6273(01)80048-9. [DOI] [PubMed] [Google Scholar]
- Qian X, Goderie SK, Shen Q, Stern JH, Temple S. Intrinsic programs of patterned cell lineages in isolated vertebrate CNS ventricular zone cells. Development. 1998;125:3143–3152. doi: 10.1242/dev.125.16.3143. [DOI] [PubMed] [Google Scholar]
- Qian X, Shen Q, Goderie SK, He W, Capela A, Davis AA, Temple S. Timing of CNS cell generation: a programmed sequence of neuron and glial cell production from isolated murine cortical stem cells. Neuron. 2000;28:69–80. doi: 10.1016/s0896-6273(00)00086-6. [DOI] [PubMed] [Google Scholar]
- Ragsdale CW, Grove EA. Patterning the mammalian cerebral cortex. Curr Opin Neurobiol. 2001;11:50–58. doi: 10.1016/s0959-4388(00)00173-2. [DOI] [PubMed] [Google Scholar]
- Rakic P. Neurons in rhesus monkey visual cortex: systematic relation between time of origin and eventual disposition. Science. 1974;183:725–727. doi: 10.1126/science.183.4123.425. [DOI] [PubMed] [Google Scholar]
- Rakic P. Specification of cerebral cortical areas. Science. 1988;241:170–176. doi: 10.1126/science.3291116. [DOI] [PubMed] [Google Scholar]
- Roy K, Kuznicki K, Bond K, Monaghan AP. Role of tailless in neuronal and glial proliferation. Soc Neurosci Abstr. 2001;27:359.3. [Google Scholar]
- Roy K, Thiels E, Monaghan AP. Loss of the tailless gene affects forebrain development and emotional behavior. Physiol Behav. 2002;77:595–600. doi: 10.1016/s0031-9384(02)00902-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarkisian MR, Li W, Di Cunto F, D’Mello SR, LoTurco JJ. Citron-kinase, a protein essential to cytokinesis in neuronal progenitors, is deleted in the f lathead mutant rat. J Neurosci. 2002;22:RC217. doi: 10.1523/JNEUROSCI.22-08-j0001.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz ML, Goldman-Rakic PS. Prenatal specification of callosal connections in rhesus monkey. J Comp Neurol. 1991;307:144–162. doi: 10.1002/cne.903070113. [DOI] [PubMed] [Google Scholar]
- Slomianka L, Danscher G, Frederickson CJ. Labeling of neurons of origin of zinc-containing pathways by intraperitoneal injections of sodium selenite. Neuroscience. 1990;38:843–854. doi: 10.1016/0306-4522(90)90076-g. [DOI] [PubMed] [Google Scholar]
- Sternberger LA, Sternberger NH. Monoclonal antibodies distinguish phosphorylated and nonphosphorylated forms of neurofilaments in situ. Proc Natl Acad Sci. 1983;80:6126–6130. doi: 10.1073/pnas.80.19.6126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoykova A, Gruss P. Roles of Pax-genes in developing and adult brain as suggested by expression patterns. J Neurosci. 1994;14:1395–1412. doi: 10.1523/JNEUROSCI.14-03-01395.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoykova A, Fritsch R, Walther C, Gruss P. Forebrain patterning defects in Small eye mutant mice. Development. 1996;122:3453–3465. doi: 10.1242/dev.122.11.3453. [DOI] [PubMed] [Google Scholar]
- Takahashi T, Nowakowski RS, Caviness VS., Jr Cell cycle parameters and patterns of nuclear movement in the neocortical proliferative zone of the fetal mouse. J Neurosci. 1993;13:820–833. doi: 10.1523/JNEUROSCI.13-02-00820.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi T, Nowakowski RS, Caviness VS., Jr The cell cycle of the pseudostratified ventricular epithelium of the embryonic murine cerebral wall. J Neurosci. 1995a;15:6046–6057. doi: 10.1523/JNEUROSCI.15-09-06046.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi T, Nowakowski RS, Caviness VS., Jr Early ontogeny of the secondary proliferative population of the embryonic murine cerebral wall. J Neurosci. 1995b;15:6058–6068. doi: 10.1523/JNEUROSCI.15-09-06058.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi T, Goto T, Miyama S, Nowakowski RS, Caviness VS., Jr Sequence of neuron origin and neocortical laminar fate: relation to cell cycle of origin in the developing murine cerebral wall. J Neurosci. 1999;19:10357–10371. doi: 10.1523/JNEUROSCI.19-23-10357.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan SS, Breen S. Radial mosaicism and tangential cell dispersion both contribute to mouse neocortical development. Nature. 1993;362:638–640. doi: 10.1038/362638a0. [DOI] [PubMed] [Google Scholar]
- Temple S, Qian X. bFGF, neurotrophins, and the control of cortical neurogenesis. Neuron. 1995;15:249–252. doi: 10.1016/0896-6273(95)90030-6. [DOI] [PubMed] [Google Scholar]
- van der Gucht E, Vandesande F, Arckens L. Neurofilament protein: a selective marker for the architectonic parcellation of the visual cortex in adult cat brain. J Comp Neurol. 2001;441:345–368. doi: 10.1002/cne.1416. [DOI] [PubMed] [Google Scholar]
- Walsh C, Cepko CL. Clonally related cortical cells show several migration patterns. Science. 1988;241:1342–1345. doi: 10.1126/science.3137660. [DOI] [PubMed] [Google Scholar]
- Ware ML, Tavazoie SF, Reid CB, Walsh CA. Coexistence of widespread clones and large radial clones in early embryonic ferret cortex. Cerebral Cortex. 1999;9:636–645. doi: 10.1093/cercor/9.6.636. [DOI] [PubMed] [Google Scholar]
- Warren N, Caric D, Pratt T, Clausen JA, Asavaritikrai P, Mason JO, Hill RE, Price DJ. The transcription factor, Pax6, is required for cell proliferation and differentiation in the developing cerebral cortex. Cereb Cortex. 1999;9:627–635. doi: 10.1093/cercor/9.6.627. [DOI] [PubMed] [Google Scholar]
- Welker C, Woolsey TA. Structure of layer IV in the somatosensory neocortex of the rat: description and comparison with the mouse. J Comp Neurol. 1974;158:437–453. doi: 10.1002/cne.901580405. [DOI] [PubMed] [Google Scholar]
- Wichterle H, Turnbull DH, Nery S, Fishell G, Alvarez-Buylla A. In utero fate mapping reveals distinct migratory pathways and fates of neurons born in the mammalian basal forebrain. Development. 2001;128:3759–3771. doi: 10.1242/dev.128.19.3759. [DOI] [PubMed] [Google Scholar]
- Wise SP, Jones EG. The organization and postnatal development of the commissural projection of the rat somatosensory cortex. Brain Res. 1976;168:313–344. doi: 10.1002/cne.901680302. [DOI] [PubMed] [Google Scholar]
- Wong-Riley M. Changes in the visual system of monocularly sutured or enucleated cats demonstrable with cytochrome oxidase histochemistry. Brain Res. 1979;171:11–28. doi: 10.1016/0006-8993(79)90728-5. [DOI] [PubMed] [Google Scholar]
- Woolsey TA, van der Loos H. The structural organization of layer IV in the somatosensory region (SI) of mouse cerebral cortex: the description of a cortical field composed of discrete cyto-architectonic units. Brain Res. 1970;17:205–242. doi: 10.1016/0006-8993(70)90079-x. [DOI] [PubMed] [Google Scholar]
- Yu RT, McKeown M, Evans RM, Umesono K. Relationship between Drosophila gap gene tailless and a vertebrate nuclear receptor Tlx. Nature. 1994;370:375–379. doi: 10.1038/370375a0. [DOI] [PubMed] [Google Scholar]