Abstract
We examined the expression and influence of IL-10 during influenza infection. We find that IL-10 does not impact sublethal infection, heterosubtypic immunity, or the maintenance of long-lived influenza antigen depots. However, IL-10-deficient mice display dramatically increased survival compared to wild-type mice when challenged with lethal doses of virus, correlating with increased expression of several Th17-associated cytokines in the lungs of IL-10-deficient mice during the peak of infection, but not with unchecked inflammation or with increased cellular responses. Foxp3− CD4 T effectors at the site of infection represent the most abundant source of IL-10 in wild-type mice during high dose influenza infection, and the majority of these cells co-produce IFNγ. Finally, as compared to predominant Th1 responses in WT mice, virus-specific T cell responses in the absence of IL-10 display a strong Th17 component in addition to a strong Th1 response and we show that Th17-polarized CD4 T cell effectors can protect naïve mice against an otherwise lethal influenza challenge and employ unique mechanisms to do so. Our results show that IL-10 expression inhibits development of Th17 responses during influenza infection and that this is correlated with compromised protection during high dose primary, but not secondary, challenge.
This is an author-produced version of a manuscript accepted for publication in The Journal of Immunology (The JI). The American Association of Immunologists, Inc. (AAI), publisher of The JI, holds the copyright to this manuscript. This version of the manuscript has not yet been copyedited or subjected to editorial proofreading by The JI; hence, it may differ from the final version published in The JI (online and in print). AAI (The JI) is not liable for errors or omissions in this author-produced version of the manuscript or in any version derived from it by the U.S. National Institutes of Health or any other third party. The final, citable version of record can be found at www.jimmunol.org.
Keywords: rodent, T cell, viral, cytokine, lung
Introduction
IL-10 was initially described as a Th2-associated factor involved in regulating pro-inflammatory signals generated by antigen presenting cell (APC) populations, thereby inhibiting Th1 responses (1). It is now known that IL-10 is a complex anti-inflammatory cytokine produced by several different cell types (2). Given this more complete understanding, the impact of IL-10 production has been investigated in several mouse models of human disease. These studies have revealed important but varied impacts of IL-10 expression (3).
Studies employing Toxoplasma, Trypanosome, Plasmodium and Listeria infection models highlight a crucial role for IL-10 in limiting pathology caused by otherwise unchecked T cell responses (4–9). These results are similar to observations of naturally occurring enterocolitis in IL-10-deficient (IL-10 KO) mice caused by uncontrolled T cell responses directed against gut flora (10, 11). Limiting immunopathology associated with strong immune responses thus represents a central regulatory role of IL-10. Recent observations have established that an important source of IL-10 during such responses are CD4 T cells capable of co-producing IFNγ and IL-10 (12, 13), or a distinct population of induced antigen-specific regulatory CD4 T cells (Tr1) producing mainly IL-10 (14). But while IL-10 can act to limit collateral damage, its expression can have the concomitant effect of dampening inflammation and lessening the effectiveness of strong Th1-polarized immunity. For example, eliminating IL-10 signaling by employing blocking antibodies or IL-10 KO mice can increase resistance in mycobacterial (15–17) and bacterial (18–20) challenge models. A detrimental impact of IL-10 in immunity against Hepatitis B and C viruses, and HIV has also been proposed based on human studies (21).
Recent observations have also defined a profound role for IL-10 in allowing persistent infection. Both in Leishmania (22) and Lymphocytic Choriomeningitis Virus (LCMV) (23, 24) models, blocking IL-10 receptor-dependent signaling can lead to increased T cell responses and the effective clearance of otherwise chronic infections. While sterilizing immunity after Leishmania infection is associated with loss of long-term protective memory (25), memory responses against LCMV remain intact following IL-10 receptor blockade. IL-10, however, plays a strong role in promoting protective Listeria-specific memory CD8 T cells (26).
Production of IL-10 has been described following infection with influenza (flu) virus (27–30) but little is known regarding the impact of IL-10 on the outcome of primary or secondary flu responses. In addition, we have demonstrated the existence of long-lived depots of flu antigen, detectable several weeks after the resolution of primary infection (31). It is unknown whether presence of antigen in this case reflects an extremely low level of chronic infection, which might be limited to a replicative niche in part defined by IL-10 expression as has been described for LCMV or Leishmania, or if instead sterilizing immunity prevails but antigen persists.
Here, we investigate multiple parameters of the anti-flu response in WT and IL-10 KO mice. We find that following low dose infection, WT and IL-10 KO mice respond similarly in terms of weight loss, subsequent recovery, and viral clearance. IL-10 does not impact presentation of long-lived flu antigen, detectable by CFSE-dilution of a reporter population of naïve A/PR8-specific TCR transgenic (Tg) CD4 T cells transferred several weeks following primary infection. IL-10 also does not impact protective heterosubtypic immunity.
However, following high dose challenge IL-10 KO mice display a significant survival advantage compared to WT mice and we show that administration of IL-10 receptor blocking antibodies to WT mice results in a similar survival advantage. We find that highly activated virus-specific FoxP3− CD4 T cell effectors, present in the lung during the peak of the anti-flu response and capable of co-producing IFNγ are the major source of IL-10 during flu infection. Most strikingly, we report that flu-specific T cell responses develop a stronger Th17 component in the absence of IL-10 and that well-polarized in vitro-generated Th17 effector CD4 T cells can protect otherwise naïve mice against lethal flu challenge via unique mechanisms independent of helper function, perforin-mediated cytotoxicity, IFNγ, and IL-17A. Our results thus show that production of IL-10 is detrimental during high-dose primary influenza challenge, and furthermore, show an unexpected protective role for virus-specific Th17 responses.
Materials and Methods
Mice
BALB/c, BALB/c IL-10 KO, or C57BL/6.FoxP3.GFP mice were at least 8 wk old at time of infection. Naïve CD4 T cells were obtained from 5–8 wk old HNT.Thy1.1, HNT IL-10 KO, or HNT IFNγ/Perforin KO mice on a BALB/c background. HNT mice express a TCR (Vα15, Vβ8.3) recognizing aa 126–138 (HNTNGVTAACHSE) of A/PuertoRico/8/34 (A/PR8) hemagglutinin (HA) presented on I-Ad (32). All mice were obtained from the animal breeding facility at Trudeau Institute. Experimental animal procedures were conducted in accordance with the Trudeau Institute Animal Care and Use Committee guidelines.
Naïve CD4 T cell isolation, effector and memory CD4 T cell generation, cell transfer and antibody administration
Naïve CD4 T cells were obtained from pooled spleen and lymph nodes as previously described (33). Resulting TCR Tg cells were routinely >97% HNT TCR+ and expressed a characteristic naive phenotype (small size, CD62Lhi, CD44lo and CD25lo). In some experiments, CD4 T cells were CFSE labeled, as previously described (34).
Th1-polarized effectors were generated as previously described (35). Th17-polarized effectors were generated by culturing HNT cells and APC (both at 2×105/mL) for 4 days with peptide and IL-2 as previously described (35), and IL-6 (20ng/mL), IL-21 (50ng/mL), IL-23 (25 ng/mL), TNF (10 ng/mL), IL-1β (10 ng/mL), TGFβ (0.5 ng/mL), anti-IL-4 (11B11) (2 µg/mL), and anti-IFNγ (XMG1.2) (2 µg/mL).
In vitro-generated memory cells were obtained by thoroughly washing Th1 effectors and re-culturing in fresh media for 3 days in the absence of antigen and cytokine as described previously (35). Naïve or memory cells were adoptively transferred in 200 µl PBS by i.v. injection.
In certain experiments, mice were injected i.p. with 500 µg of αThy1.2 (30H12), αCD8 (TIB210), or αCD4 (GK1.5) depleting antibodies 1 day before infection. In other experiments, mice were treated every 2 days, beginning 1 day before infection with 500 µg of IL-10 receptor blocking antibody (1B1.3A) or an isotype control (rat IgG1) i.p. In other experiments, mice were treated every 2 days, beginning 1 day before infection with 200 µg of α-IL-17 neutralizing antibody (R&D), or an isotype control (rate IgG2a) i.p.
Virus stocks and infections
Influenza A/PR8 (H1N1) virus was produced in the allantoic cavity of embryonated hen eggs from stock originating at St. Jude Children’s Hospital and the egg infective dose (EID50) characterized. Influenza A/Philippines/2/82/x-79 (H3N2) virus was similarly prepared and characterized from a stock originating from S. Epstein (NIH, Bethesda, MD). Mice were infected i.n. under light isoflurane anesthesia (Webster Veterinary Supply) with stated doses of virus in 50 µl PBS.
Real Time-PCR
Viral titers were determined by quantitation of viral RNA. RNA was prepared from whole lung homogenates using TRIzol (Sigma-Aldrich), and 2.5 µg of RNA was reverse transcribed into cDNA using random hexamer primers and Superscript II Reverse Transcriptase (Invitrogen). Quantitative PCR was performed to amplify the polymerase (PA) gene of A/PR8 using an ABI Prism 7700 Sequence Detector (Applied Biosystems) with 50 ng of cDNA per reaction and the following primers and probe: forward primer, 5'-CGGTCCAAATTCCTGCTGA-3'; reverse primer, 5'CATTGGGTTCCTTCCATCCA-3'; probe, 5'-6-FAM-CCAAGTCATGAAGGAGAGGGAATACCGCT-3'. Data were analyzed with Sequence Detector v1.7a (Applied Biosystems). The copy number of the PA gene per 50 ng of cDNA was calculated using a PA-containing plasmid of known concentration as a standard.
Alternatively, RNA samples were reverse-transcribed and cDNA was amplified with Taqman reagents on the ABI Prism 7700 sequence detection system (Applied Biosystems). The 'fold increase' in signal relative to that of uninfected samples was determined with the 'DeltaDeltaCT' (change in cycling threshold) calculation recommended by Applied Biosystems.
Cytokine Quantification
Levels of cytokine in lung homogenates and culture supernatant was determined using mouse luminex kits (Invitrogen and Millipore) read on a Luminex 100 reader (Luminex Corp.)
Tissue preparation
At different time points after virus infection, mice were euthanized by cervical dislocation followed by exsanguinated by perforation of the abdominal aorta. Lungs were perfused by injecting 10 ml of PBS in the left ventricle of the heart. Lungs, spleen, and draining mediastinal lymph node (dLN) were prepared into single cell suspensions by mechanical disruption of organs and passage through a nylon membrane.
For assessment of immunopathology following flu infection, lung lobes were isolated and immediately fixed in 10% neutral buffered formalin. Lung samples were subsequently processed, embedded in paraffin, sectioned, placed on L-lysine-coated slides, and stained with Hemotoxylin and Eosin (H&E) using standard histological techniques. Sections were graded blindly from 0 to 4, on the basis of the extent of mononuclear cell infiltration and tissue damage as depicted in Supplemental Fig 3.
Flow cytometry
Cell suspensions were washed, resuspended in FACS buffer (PBS plus 0.5% BSA and 0.02% sodium azide (NaN3); Sigma-Aldrich) and incubated on ice with 1 µg anti-FcR (2.4G2) followed by saturating concentrations of fluorochrome-labeled Abs for surface staining. For intracellular cytokine analysis, see below. FoxP3 expression was determined by intracellular staining as per manufacturer’s instructions employing a FoxP3 staining kit (bioscience). FACS analysis was performed using Becton Dickson FACS Scan (BD Biosciences) and FlowJo (Tree Star) analysis software.
Detection of cytokine producing cells
Intracellular cytokine staining was performed as previously described (33). Briefly, CD4 T cells were stimulated for 16 h with HNT-pulsed APC. After 2h, 10 µg/ml Brefeldin A (Sigma) was added. Alternatively, cells were treated with PMA and Ionomycin for 4 hours, with Brefeldin A added after 2 h. Cells were then surface stained and fixed for 20 min in 4% paraformaldehyde. After washing, cells were permeablized by 10 min incubation in 0.1% saponin buffer (PBS plus 1% FBS, 0.1% NaN3 and 0.1% saponin; Sigma-Aldrich) and stained for cytokine by the addition of anti-IFNγ, -IL-10, and -IL-17, and granzyme B fluorescently labeled Ab for 20 minutes.
Peptide-dependent IFNγ IL-2, and IL-17 secreting cells were assayed with a standard ELISPOT assay. Ninety-six-well, multiscreen nitrocellulose-flat bottom plates (Millipore) were coated overnight at 4° C with purified anti-IFNγ (R4–6A2), anti-IL-2 (JES6–1A12) monoclonal Ab (Pharmingen), or anti-IL-17 (50101.111; R&D) at 10 µg/ml in 1M bicarbonate buffer. Plates were washed and 105 lung, 106 spleen or lymph node cells, 106 irradiated (1500 rad) syngeneic APC/well, and 10 µg/mL of relevant peptide were added. Plates were incubated overnight at 37°C in 5% CO2. Plates were thoroughly washed prior to the addition of biotinylated anti-IFNγ (XMG1.2), anti-IL-2 (JES6–5H4) (BD PharMingen), or anti-IL-17 (R&D) in PBS-tween (PBST) overnight at 4°C. After washing, plates were incubated 2 h at room temperature with alkaline-phosphatase strepavidin (0.2 µg/mL) in PBST. After final washes with ddH20, NBT/BCIP Stock Solution (Nitro blue tetrazolium chloride/5-Bromo-4-Chloro-3-indolyl phosphate, toluidine salt, (Sigma-Aldrich) was added and the reaction stopped with dH20. Spots were enumerated with an Immunospot reader (Cellular Technology).
Detection of flu-specific antibody and antibody secreting cells
The level of flu-specific total IgG and hemagglutination inhibition (HAI) titer in convalescent sera, and the number of flu-specific antibody secreting cells (ASC) on d21 post infection were determined as described (36, 37).
Measurement of Respiratory Mechanics
Non-invasive whole body plethsymography (WBP) (Buxco) was employed to measure respiratory rates (breaths/min.) and minute volumes (mL/min.) on conscious, unrestrained animals following flu infection. The minute volume is defined as the volume of air exchanged during a 1-min. interval and is calculated as follows [respiratory rate X tidal volume].
Statistical analysis
Unpaired, two-tailed, Students t-tests, ∝ = 0.05, were used to assess whether the means of two normally distributed groups differed significantly. One-way ANOVA analysis with Bonferroni’s multiple comparison post-test was employed to compare multiple means. Two-way ANOVA analysis with repeated measures was also employed in some experiments. The Log Rank test was used to test for significant differences in Kaplan-Meier survival curves. All error bars represent the standard deviation.
Results
Minimal impact of IL-10 on low dose flu infection and long-lived flu antigen
To assess the impact of IL-10 expression during primary flu infection, we first challenged naïve aged-matched WT or IL-10 KO BALB/c mice with a low dose (0.1 LD50 or 500 EID50) of A/PR8. We observed no significant differences in weight loss or subsequent weight gain, correlating with similar kinetics of viral clearance (Supplemental Fig 1A and B). We also observed no differences in titer, isotype-spread, or hemagglutination inhibition activity of flu-specific antibodies when convalescent serum or ASC cells from WT and IL-10 KO mice were analyzed (Supplemental Fig 1C–E), consistent with a minimal role for IL-10 in directing murine B cell responses (2).
As mice can completely clear otherwise chronic LCMV infection in the absence of IL-10 signaling (23, 24), we reasoned that IL-10 might impact long-lived presentation of flu antigen if it were dependent on live virus. To test this hypothesis, we employed a sensitive readout of flu antigen presentation following the resolution of primary infection (31, 38). Naïve CFSE-labeled TCR Tg CD4 T cells specific for an epitope of the A/PR8 hemagglutinin (HNT) were transferred to Thy-disparate WT or IL-10 KO mice that had been infected with A/PR8 2, 3, or 4 weeks previously. CFSE profiles of HNT cells in both hosts were similar when analyzed 7 d post-transfer and the level of division declined similarly as cells were transferred at later time points (Supplemental Fig 2). These results show that IL-10 does not impact the generation or maintenance of long-lived flu antigen depots following primary infection.
IL-10 negatively impacts high dose flu challenge
We next sought to determine if higher doses of virus, against which stronger immune responses might be required to control infection, but which might also cause more immunopathology, would reveal an impact of IL-10, either positive, by controlling immunopathology, or negative, by interfering with T cell responses. Upon challenge with 1 LD50 A/PR8 (10,000 EID50), IL-10 KO mice displayed similar patterns of weight loss and weight gain, but survival was significantly enhanced compared to WT mice (Fig 1A). IL-10 KO mice also displayed a significant survival advantage after 2 LD50 challenge (unpublished observations). As IL-10 KO mice can develop significant gut inflammation when housed under standard conditions which might account, at least in part, for the differential survival of WT and IL-10 KO mice, we treated WT mice with IL-10 receptor (IL-10R) blocking antibody or an isotype control and challenged with 1 LD50 A/PR8. As seen in Fig 1 B, mice treated with IL-10R blocking antibody displayed similar weight loss and weight gain, but significantly enhanced survival as compared to isotype antibody treated mice. As with WT and IL-10 KO mice (Fig 1 C), the magnitude of infection and the kinetics of viral clearance were similar in isotype and IL-10R antibody treated mice (data not shown).
Figure 1.
IL-10 expression negatively influences high-dose primary flu challenge. Age-matched WT or IL-10 KO mice were challenged with 1LD50 A/PR8 (5000 EID50). (A) weight loss and conditional survival (n=15 mice / group, and * = P < 0.05 from 1 of 5 similar experiments (exp)). (B) WT mice infected with 1 LD50 A/PR8 were treated with either IL-10 receptor blocking antibody or an isotype control, as described, and weight loss and conditional survival monitored (n=8, and * = P < 0.05 from 1 of 2 independent exp). Weight loss in A-B is of all surviving animals. (C) On stated days post-infection, viral titers were determined by quantitative PCR (n=5 / group / day from 1 of 3 similar exp), and (D) respiratory rates and minute volumes monitored (n= at least 7, * = P < 0.05, and *** = P < 0.001 from 1 of 2 independent exp). (E) H&E stained lung sections were scored blindly for levels of immunopathology (n=3–7 mice / group / day from 1 of 2 exp).
Interestingly, IL-10 KO mice displayed significantly enhanced lung function as compared to WT mice during lethal infection as assessed both by respiratory rate and minute volume (Fig 1 D). To determine if increased lung function reflected differential pathology in WT versus IL-10 KO mice, lungs were examined histologically and scored as outlined in Supplemental Fig 3. High dose challenge in both strains was associated with extensive perivascular lymphocytic inflammation and diffuse infiltration of the alveoli, swelling of the bronchial wall, and hyperplasia of the bronchial epithelium with associated epithelial infiltration of lymphocytes. This intraepithelial inflammation is characteristic of T cell-mediated reactions, such as those seen in viral exanthems and contact dermatitis (39). The cause of death in WT mice does not appear to be related to the degree of inflammation in the lung as pathologic examination revealed that the degree of inflammation is, if anything, greater in IL-10 KO than WT mice as graded on changes apparent in the pulmonary artery, bronchi, and alveoli (Fig 1 E). Since mice began to reach cutoffs of conditional survival by day 9 after 1LD50 challenge, comparative analysis ceased at this time-point. These findings show that increased lung function and survival observed in IL-10 KO mice following high dose flu challenge do not correlate with reduced immunopathology compared to WT mice, and suggest that elements of the inflammatory response generated against flu in the absence of IL-10 enhance protection.
IL-10 does not impact the magnitude of cellular responses against flu
It is possible that a stronger anti-viral T cell response in IL-10 KO mice, either in terms of the number of responding cells or in terms of per-cell cytokine production, is responsible for increased protection against morbidity, as studies employing LCMV have shown a more robust T cell response in the absence of IL-10 signaling (23, 24). We first quantified bulk CD44high T cells and observed similar kinetic accumulation and absolute numbers of CD4 and CD8 T cells in lungs of WT and IL-10 KO mice following 1 LD50 infection (Supplemental Fig 4A). Focusing on IFNγ and IL-2 production traditionally associated with strong Th1 responses and protection against flu, we observed similar flu-specific cytokine responses from day 5 through day 9 post-infection (Supplemental Fig 4B). Thus, the magnitude of flu-specific Th1 responses is not affected by IL-10 expression. Furthermore, we observed no differences in several classes of leukocytes, including neutrophils, NK cells, dendritic cells, and macrophages in the lungs of WT and IL-10 KO mice throughout the course of 1 LD50 A/PR8 infection (Supplemental Fig 5).
IL-10 selectively impacts expression of Th17-associated cytokines during flu infection
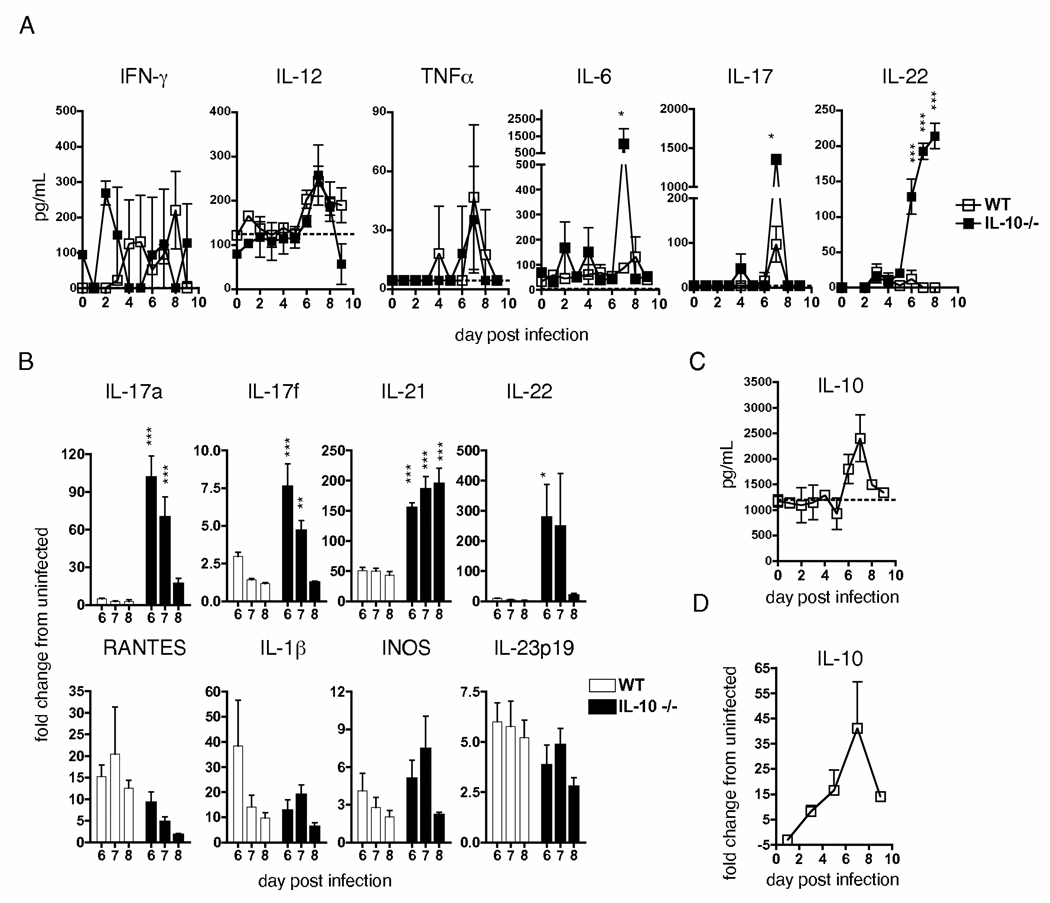
Given the ability of IL-10 to impact inflammation, and since detrimental IL-10-mediated effects are likely to be via regulation of factors not typically associated with protective flu responses, we next investigated the effect of IL-10 deficiency on lung inflammation during lethal flu infection. We analyzed lungs from WT and IL-10 KO mice infected with 1 LD50 A/PR8 for expression of over 20 cytokines and chemokines kinetically through d 9 of 1 LD50 A/PR8 challenge. While we did not observe significant differences in several major inflammatory molecules, including IFNγ, IL-12, and TNF, we did observe significantly elevated levels of IL-6 and IL-17, and IL-22 in IL-10 KO mice during the peak period of the anti-flu T cell response (Fig 2A and Supplemental Fig 6).
Figure 2.
Increased expression of Th17-associated cytokines in the absence of IL-10. WT or IL-10 KO mice were challenged with 1LD50 A/PR8 and on stated days, lungs isolated and analyzed for (A and C) protein, or (B and D) message for stated cytokines, as described (n=3 mice / group / time point for A and C; n=5 for B and D). Dotted lines in A and C represent average level of protein in uninfected mice. Data presented is representative of two similar independent exp separately measuring protein and message (* = P < 0.05, ** = P < 0.01, and *** = P < 0.001).
We further analyzed RNA message in WT and IL-10 KO mice for additional Th17-associated products during the peak period of lung inflammation. IL-10 KO mice displayed dramatically enhanced levels of IL-17A, IL-17F, IL-21, and IL-22 message as compared to WT mice during d 6–8 post-infection (Fig 2B) while no significant differences in IL-1, INOS, RANTES, or IL-23 mRNA expression were observed, all of which have been implicated in affecting responses against flu (40–42). Thus, IL-10 deficiency does not result in unchecked inflammation, but rather leads to increased expression of Th17-associated cytokines during flu infection. In support of this hypothesis, we observed peak expression of IL-10 in WT mice when maximal Th17-associated cytokine levels were observed in IL-10 KO mice (Fig. 2C and D).
FoxP3− CD4 T cells co-producing IFNγ are an important source of IL-10
The kinetics of IL-10 expression in Fig 2C and D suggest that activated T cells that migrate to the lung and are re-exposed to flu antigen there (33) are a likely source of IL-10. To determine the extent of IL-10 production from T cells during flu infection, we depleted all T cells or CD4+/CD8+ subsets from WT mice and infected with 1LD50 A/PR8. As seen in Fig 3A, expression of IL-10 was greatly reduced in the absence of T cells on d 7 post-infection. Depletion of CD4+ cells reduced IL-10 expression nearly as much as T cell depletion while depletion of CD8+ cells had little impact (Fig 3A), indicating that most IL-10 production was CD4 T cell dependent.
Figure 3.
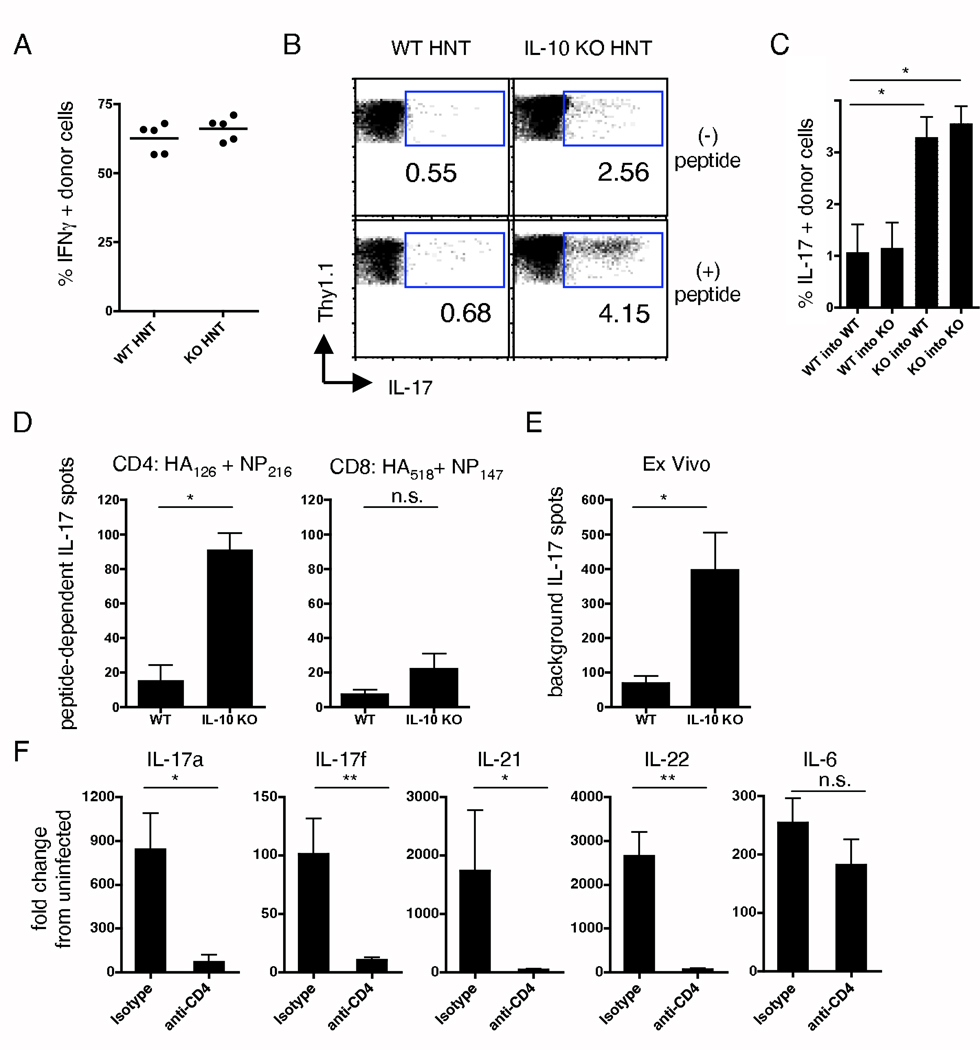
Effector CD4 T cells co-producing IFNγ are the primary source of IL-10 during flu infection. (A) WT mice were treated with depleting or isotype control antibodies and infected with 1 LD50 A/PR8. On d7, lungs were analyzed for IL-10 expression (n=5, and * = P < 0.05). (B) 2×106 naïve HNT.Thy1.1 CD4 T cells were transferred to WT hosts then infected with 1 LD50 A/PR8, and IL-10+ donor cells determined by ICCS on d7 (n=5). (C) Representative examples of donor cell IL-10/IFNγ and isotype staining +/− peptide stimulation. (D) Stated numbers of donor cells were transferred and ICCS performed as in (C); the ratio of IFNγ+/IL-10− to IFNγ+/IL-10+ donor cells in lungs is shown (n=3). (E) Representative example of IL-10/IFNγ staining gated on CD4 T cells from DLN and lung of WT mice in the absence of adoptive transfer. (F) CD25/FoxP3 staining of host and donor lung CD4 T cells on d 7 post-infection. (G) Total IL-10+ T cells from FoxP3.GFP mice were separated on the basis of GFP expression (* = P < 0.05). Data is representative of 2 independent exp for A, D, G and greater than 5 for B, C, E, F.
To investigate the nature of IL-10 expression by flu-specific CD4 T cells, we transferred HNT CD4 T cells to naïve Thy-disparate mice and infected with 1 LD50 A/PR8. On d 7, donor cell IL-10 production was determined by intracellular cytokine staining (ICCS). Nearly 20% of lung-resident HNT cells produced IL-10 compared to 5% or less of donor cells in the DLN and spleen (Fig 3B). Cells isolated from bronchoalveolar lavage (BAL) resembled cells obtained from whole lungs (unpublished observations). Multiparameter ICCS revealed that the majority of IL-10+ cells were also IFNγ+ (Fig 3C). The pattern of IL-10/IFNγ-producing donor cells over a broad nearly 100-fold range of donor cell input was similar (Fig 3D), suggesting that the number of monoclonal T cells did not impact the IL-10-producing phenotype. Furthermore, endogenous CD4 T cells in the absence of adoptive transfer displayed a similar pattern of IL-10 production as HNT cells with higher IL-10 production in the lung than lymphoid organs, particularly by IL-10/IFNγ producing cells (Fig 3E).
While FoxP3+ CD4 T cells did accumulate in infected lungs, very few HNT cells converted to a FoxP3+ phenotype (Fig 3F). We further employed FoxP3-GFP reporter mice to more definitively examine the relationship between FoxP3 expression and IL-10 production. We found that the majority of IL-10+ lymphocytes present in the lungs of lethally infected mice were FoxP3− (Fig 3G). Thus, highly activated FoxP3− effector CD4 T cells at the site of infection, capable of co-producing IL-10 and IFNγ, represent the major source of IL-10 during flu challenge.
Absence of IL-10 promotes a strong Th17 component in flu-specific CD4 T cell responses
We next transferred WT HNT cells to WT hosts, or IL-10 KO HNT cells to IL-10 KO hosts and infected with 1 LD50 A/PR8 to investigate in more detail the impact of IL-10 deficiency on CD4 T cell responses. In agreement with polyclonal responses (Supplemental Fig 4), HNT populations reached similar numbers in lungs by d 7, with similar CFSE dilution in all organs tested (unpublished observations), and similar percentages of HNT cells stained IFNγ+ in the presence or absence of IL-10 (Fig 4A). Thus, production, or lack thereof, of IL-10 by responding CD4 T cells did not alter their expansion or the generation of IFNγ-producing effectors capable of migration to the lung.
Figure 4.
Th1/Th17 phenotype of responding flu-specific IL-10 KO T cells. 2×106 naïve WT or IL-10 KO HNT cells were transferred to WT or IL-10 KO hosts, respectively, then infected with 1LD50 A/PR8. 7 d post-challenge, lung HNT cells were analyzed for (A) IFNγ or (B) IL-17 +/− peptide stimulation (n=5). (C) Peptide-dependent IL-17 production from WT or IL-10 KO HNT cells transferred to WT or IL-10 KO hosts (n=3, and * = P < 0.05). WT or IL-10 KO mice were challenged with 1LD50 A/PR8 and ELISPOT analysis performed on d 7 for IL-17. (D) CD4 and CD8 peptide-specific, and (E) non-antigen elicited IL-17 spots per lung (n=5, and * = P < 0.05). (F) WT mice were treated with CD4 depleting or isotype control antibodies and infected with 1 LD50 A/PR8. On d 7, lungs were analyzed for stated cytokines (n=5, * = P < 0.05 and ** = P < 0.01). A-C are representative of 3 independent exp, E-F of 2.
Strikingly, however, significantly more IL-17-producing cells were observed in transferred IL-10 KO versus WT HNT cells both with and without peptide stimulation (Fig 4B), consistent with observations of increased Th17-associated cytokines in lungs of infected IL-10 KO mice (Fig 2). IL-10 KO HNT cells displayed similar enhanced flu-specific IL-17 production when transferred to either IL-10 KO or WT hosts (Fig 4C). Thus, autocrine IL-10 produced by responding CD4 T cells appears to play a critical role in regulating the generation of Th17-polarized CD4 T cell effectors during flu infection.
To confirm increased IL-17 production from polyclonal CD4 T cells in the absence of IL-10, we performed ELISPOT analysis on lung-resident lymphocytes from WT and IL-10 KO mice on d 7 post-1LD50 A/PR8 challenge. We observed significantly enhanced peptide-specific IL-17 production, predominantly from CD4 T cells, in IL-10 KO mice (Fig. 4D). Similar to ICCS results (Fig 4B), IL-17 production was observed from IL-10 KO lymphocytes plated without additional stimulation (Fig 4E), suggesting active recognition of antigen and IL-17 secretion by T cells in vivo, although other populations, such as NK or γδ T cell, could also contribute to enhanced IL-17 production in IL-10 KO mice. To determine if CD4 T cells are the major source of IL-17 detected in IL-10 KO mice, we treated IL-10 KO mice with either CD4-depleting antibody or an isotype control and assessed IL-17 message on d 7 post-infection. Not only was the level of IL-17A message dramatically lower in anti-CD4 treated mice, but we also observed dramatic reductions in mRNA for IL-17F, IL-21, and IL-22, but not for IL-6 (Fig 4F). These results show that while IL-17-producing CD4 T cells are generated during primary flu infection in WT mice, a dramatically enhanced Th17 response develops in the absence of IL-10 signaling that may contribute to the increased resistance of IL-10 KO mice to high-dose challenge.
Th-17 Polarized CD4 T cell effectors can protect against lethal flu infection
We generated Th17-polarized effectors from naïve HNT cells in vitro using published protocols with modifications based on preliminary studies (43), to directly investigate whether such effectors could contribute to protection against flu infection. As expected, Th17 effectors produced substantial IL-17 with negligible IFNγ while Th1 effectors displayed the reverse pattern as assessed both by ICCS and ELISA (Fig 5A and B). We also observed substantial production of IL-21 and IL-22 by Th17 but not Th1 or Th2 effectors (Fig 5B). Furthermore, Taqman PCR analysis revealed strong, mutually exclusive expression of T-bet and ROR-γt in Th1 and Th17 effector cultures, respectively (Fig 5C). Interestingly, compared to strong Granzyme B expression by Th1-polarized effectors, Th17 effectors were Granzyme B negative (Fig. 5D, left panel). An identical Granzyme B staining pattern was observed with CD8 T cell-derived Tc1 and Tc17 effectors, correlating with cytotoxic function of the former but not the latter (44).
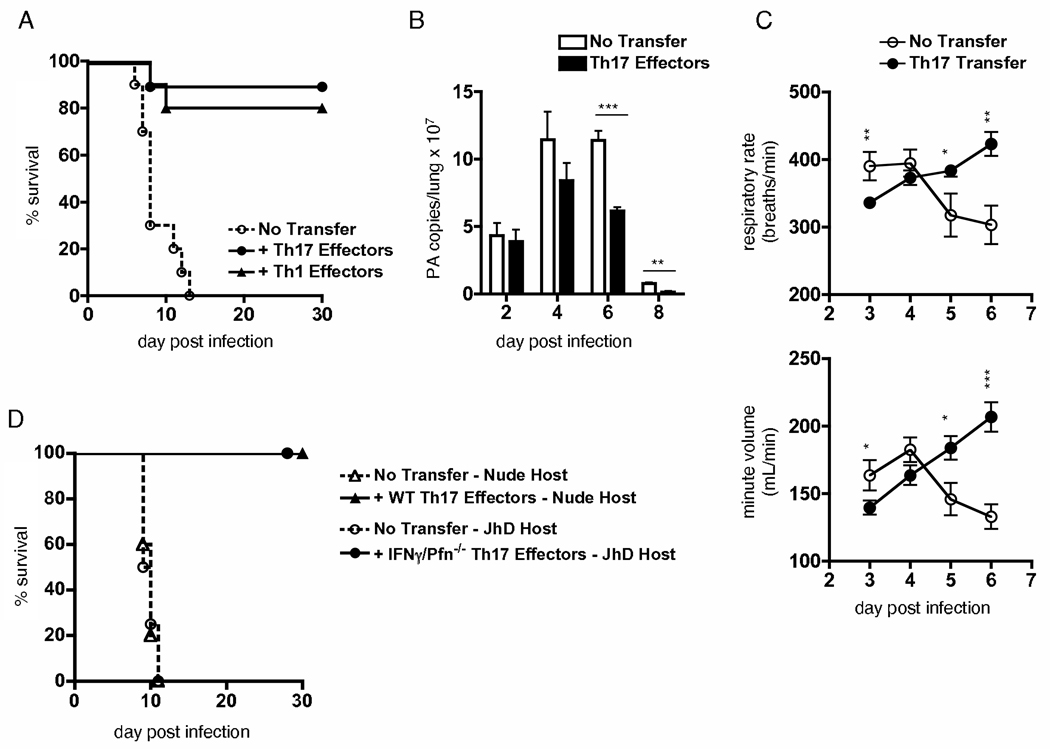
Figure 5.
Th17-polarized effectors retain defining aspects of their phenotype in vivo following flu challenge. (A) IFNγ and IL-17 staining of Th1 and Th17 effectors +/− peptide stimulation, and (B) IFNγ, IL-4, IL-17, IL-21 and IL-22 measured in 24 h supernatants of indicated effectors stimulated with APC and peptide. (C) Message for T-bet, GATA-3, and ROR-γt in polarized effectors. (D) Granzyme B staining of Th1 and Th17 effectors prior to and d3 following adoptive transfer and flu infection. (E) IFNγ and IL-17 staining of Th1 and Th17 HNT effectors in lungs 3d after transfer and flu infection. A-B are representative of 4 independent exp, D-E of 3 similar exp.
To verify that effectors retained their phenotypes in vivo, we transferred Th1 or Th17 HNT effectors to naïve BALB/c hosts and infected with flu. Lung-resident effectors were analyzed d 3 post-infection, found in previous studies to coincide with maximal responses of transferred flu-specific Th1 effectors (45). Th1 and Th17 effectors retained similar granzyme B staining patterns as in vitro cultures (Fig. 5D). Responding Th17 effectors in vivo also retained a significant IL-17+ population, smaller than that observed in vitro, and were slightly but consistently enriched for IFNγ+ cells (Fig 5E). This IFNγ+ population, however, was only a fraction of the IFNγ+ cells observed in responding Th1 effectors, which did not contain a significant IL-17+ population (Fig 5B). Thus, in vitro-polarized effectors retained defining aspects of the Th17 phenotype when responding to flu in vivo. In agreement with recent studies (46, 47), we found that IL-12 significantly impacted the proportion of IFNγ+ Th17 effectors responding to flu, as transfer into IL-12 (p35)-deficient hosts resulted in no increase of IFNγ+ cells while IL-17+ cells remained stable (unpublished observations).
To directly test the protective potential of Th17 effectors, we transferred Th17-polarized HNT effectors to naïve mice and then infected with 2LD50 A/PR8. We transferred a number equal to the number of Th1-polarized HNT effectors previously shown to be required for protection of WT mice against this dose of flu (45). As seen in figure 6A, Th17 effectors completely protected naïve mice and were as effective as Th1 effectors. While transfer of Th1 effectors led to significantly reduced viral titers on d 4 post-infection (45), we observed a slightly delayed but significant impact on viral clearance after transfer of Th17 effectors (Fig 6B). Interestingly, Th17 effectors initially led to decreases in respiratory function compared to infected mice not receiving cells but by d 5 post-infection promoted enhanced lung function compared to controls (Fig 6C). Thus, protection against flu mediated by Th17-polarized CD4 T cells is correlated both with enhanced viral clearance and with enhanced lung function. This is consistent with the concept that the greater resistance against flu seen in the absence of IL-10 is due to the increased induction of Th17 responses in addition to a strong Th1 response.
Figure 6.
Th17-polarized effectors protect against lethal flu challenge employing novel mechanisms. 5×106 Th1 or Th17 HNT effectors were transferred to naïve BALB/c mice subsequently infected with 2 LD50 A/PR8 (10,000 EID50) and (A) conditional survival monitored (n=10 mice per group). (B) On stated days, viral titers were determined by quantitative PCR (n=5 mice / group / day, ** = P < 0.01, and *** = P < 0.001). (C) Respiratory rate and minute volume was determined as described (n=10 mice / group / time-point, * = P < 0.05, ** = P < 0.01, and *** = P < 0.001). (D) 5×106 Th17 effectors generated from WT or INFγ-/Perforin KO HNT cells were transferred to nude or JHD hosts, respectively. All mice were subsequently infected with a lethal dose (1500 EID50) of A/PR8. Conditional survival is shown (n=10 mice / group). A-C representative of at least 2 independent exp, D is a summary of 2 independent exp for each condition, with n=5 mice per group.
We next sought to better characterize how Th17-polarized CD4 T cells protect against flu. To determine whether a cooperative mechanism was involved between IL-17-producing HNT effectors and endogenous virus-specific Th1 cells, as has been reported for optimal protection against tuberculosis (48), we transferred Th17-polarized HNT cells to T cell-deficient nude hosts. When challenged with a dose of virus lethal to unprimed nude mice, mice receiving Th17 HNT effectors were completely protected (Fig 6D), suggesting that Th17 effectors are capable of protecting independently of other T cells.
We have previously shown that Th1-polarized HNT effectors protect against flu by providing help for B cell responses and by direct perforin-mediated cytolytic activity, and that perforin-deficient effectors could not protect B cell-deficient (JHD) hosts from lethal A/PR8 infection (45). Furthermore, the Th17 effectors transferred produce IL-21 when stimulated in vitro, which is implicated in B cell help. Strikingly, we found that perforin/IFNγ-double-deficient Th17-polarized HNT effectors completely protected JHD mice against otherwise lethal flu challenge (Fig 6D), strongly arguing against the possibility that contaminating Th1 or Th1-like cells within Th17 populations are responsible for protection, or that identical protective mechanisms are employed by Th17 and Th1 effectors. Additionally, administration of neutralizing antibody against IL-17A had no impact on weight loss patterns or protection of JHD mice receiving perforin/IFNγ-double-deficient Th17 effectors, nor did such treatment impact weight loss or enhanced survival of IL-10 KO compared to WT mice (data not shown). These results show that Th17 effectors mediate protection against flu via novel mechanisms independent of IFNγ, helper function, perforin-mediated cytotoxicity, and IL-17A, and further strengthen the hypothesis that IL-10 impedes optimal responses to higher doses of flu in part by inhibiting the development of Th17-polarized effectors.
Reduced expression and impact of IL-10 during heterosubtypic challenge
Finally, we investigated the impact of IL-10 on heterosubtypic flu infection. WT and IL-10 KO mice primed with 0.1 LD50 A/PR8 (H1N1) 25, 50, or 100 d previously were challenged with a lethal dose (300 LD50) of A/Philippines (H3N2). The robustness of heterosubtypic immunity decreased with time after primary challenge, as expected (49), but no differences in weight loss or subsequent recovery were observed between WT and IL-10 KO mice, and all primed mice survived (Fig 7 A).
Figure 7.
Reduced IL-10 expression during secondary flu responses. (A) WT or IL-10 KO mice were infected with 0.1LD50 A/PR8 (H1N1) and on stated days post primary infection, mice were challenged with 300 LD50 A/Philippines (H3N2) and monitored for weight loss (n=5 mice / group). No primed mice succumbed to secondary challenge. (B) After naïve HNT cell transfer, lung and dLN resident donor cells were assayed for IL-10 production by ICCS on stated days post 0.1LD50 A/PR8 challenge. (C) 2×106 naïve or memory HNT cells (Th1-polarized rested effectors) were transferred to naïve hosts subsequently infected with 1 LD50 A/PR8. On stated days, lungs (n=3 / group) were analyzed for IL-10 message. (D) Lung homogenates (n=3 mice / day) from naïve mice infected with 1 LD50 A/PR8 and from mice primed 60 days previously with A/PR8 and challenged with 300 LD50 A/Philippines were analyzed for IL-10 expression. Data in B-D is representative of 2 independent exp.
Given the dramatic impact of IL-10 on high dose primary, but not secondary flu infection, we investigated IL-10 expression by flu-specific memory CD4 T cells present after the resolution of primary infection. We first assessed IL-10 expression by HNT cells transferred to naïve mice and primed with 0.1 LD50 A/PR8. Upon stimulation, donor cells showed similar patterns of IL-10 production 7 d after either 1LD50 or 0.1LD50 challenge (Fig 3B and Fig 7B), but following viral clearance, the capacity for IL-10 production by HNT cells was dramatically reduced (Fig 7B), suggesting that secondary responses against flu are much less influenced by IL-10 as compared to primary infection. In support of this hypothesis, we observed much reduced IL-10 expression following flu infection in mice that had received Th1-polarized HNT rested effectors, that are virtually identical to memory CD4 T cells (35), compared to mice receiving an equal number of naïve HNT cells (Fig 7C). Finally, WT mice primed with 0.1LD50 A/PR8 and challenged with A/Philippines also displayed only very low levels of IL-10 as compared to naïve mice infected with 0.1LD50 A/PR8 (Fig 7D). These results demonstrate much reduced expression and impact of IL-10 on flu-specific memory responses.
Discussion
The importance of IL-10 expression during immune responses has largely been studied in models of autoimmunity, tolerance, and chronic infection. Comparatively little is known regarding the impact of IL-10 during acute viral infection. Here we investigated the nature of and influence of IL-10 expression during primary and secondary infection with highly pathogenic flu viruses. We show that IL-10 interferes with optimal protection against flu, but emerges as a negative factor only during higher dose challenge. We show that FoxP3− effector CD4 T cells co-producing IFNγ in the lung represent the most abundant source of IL-10 during primary infection, and that increased resistance against lethal challenge in the absence of IL-10 correlates, surprisingly, with the emergence of a strong Th17 component of the flu-specific T cell response. These results suggest that IL-10-producing cells amongst effector T cells serve to dampen production of Th17-associated cytokines that in the case of high dose flu infection, is counter protective. In support of this hypothesis, we demonstrate that transfer of Th17-polarized CD4 T cell effectors can protect otherwise naïve mice against lethal flu infection employing novel mechanisms independent of established Th1 effector-mediated protection.
Increased inflammation during flu infection in the absence of IL-10 correlates kinetically with peak IL-10 expression in WT mice. Our observations are thus consistent with the hypothesis that IL-10 acts to limit strong inflammation coinciding with strong pathogen-specific immune responses. A similar mode of action for IL-10 has been described during acute Toxoplasma gondii and Trypanosoma cruzi infection (5, 7, 8). However, while in these studies unchecked immunopathology in IL-10-deficient mice led to earlier mortality as compared to WT mice, we noted no impact of IL-10-deficiency on lung immunopathology and significantly increased survival in the absence of IL-10 following high dose flu challenge. We suggest that it is likely that the localized, self-limiting, and acute nature of flu infection prevents the excessive and lethal immunopathology observed in more systemic or protracted infections. Our findings are unexpected also given the correlation of higher levels of inflammation (the H5N1 induced “cytokine storm”) with increased severity of disease (50), as we observed enhanced lung function in IL-10 KO mice during the peak period of inflammatory responses. However, putative positive versus negative roles for select inflammatory molecules during flu infection are not clear (51, 52), and our observations show a similar cellular influx and upregulation of only select factors in the absence of IL-10, not an unchecked inflammatory response.
Similar to recent reports employing intracellular protozoan infection models (53, 54), we show that activated virus-specific Foxp3− CD4 T cells co-producing IFNγ represent an important source of IL-10 during flu infection. Expression of IL-10 is transient, correlating with high viral titers and the peak of T cell responses, suggesting that, in agreement with these previous studies, a strong pro-Th1 inflammatory environment is a critical factor in its generation. It remains to be determined whether IL-10+/IFNγ+ CD4 T cells revert to a resting state, loosing expression of IL-10, or if the IL-10+/IFNγ+ phenotype correlates with a terminally differentiated phenotype (55) and that this population undergoes apoptosis following resolution of primary infection. Several factors, including IL-12, IL-6, IL-27, and IFNα, have been implicated in promoting IL-10 expression in T cell effectors (56–61), and expression of all of these cytokines are increased in the lung during primary flu infection (unpublished observations). These observations warrant further study as regards the possibility of enhancing immunity against flu through modification of IL-10 expression by T cell effectors.
In other studies we have found that despite the high number of IFNγ-producing T cells that develop in response to flu challenge, protection mediated by flu-specific CD4 T cell effectors is completely IFNγ-independent (45). This suggests that other CD4 T cell effector subsets and diverse, possibly unidentified, effector mechanisms may play key roles in protection. Our results support this hypothesis and reveal that the flu-specific CD4 T cell effector response in the lung is more complex and heterogeneous than previously appreciated. Certainly the majority of responding CD4 T cells produce IFNγ, but our results indicate that in WT mice a substantial fraction of effectors are IL-10/IFNγ co-producing cells. Also, in WT mice a small population of effectors produce IL-17, and we suggest that this population is normally kept to a low level via the action of IL-10, but expands when IL-10 is absent. Surprisingly, we show that IL-10 KO CD4 T cells transferred to WT mice develop enhanced Th17 responses against flu, suggesting autocrine regulation of Th17 cytokine responses by IL-10 in activated effectors. Though IL-10 KO mice express significantly more IL-6 than WT mice following flu challenge, the induction of Th17 responses in IL-10 KO CD4 T cells responding in WT hosts also suggests that stronger virus-specific Th17 responses in IL-10 KO mice do not depend upon the enhanced expression of factors, such as TGF-β, that have been shown to promote Th17 development. While not addressed in our studies, it is possible that a similar mechanism of regulation of Tc17 responses by IL-10 operates in effector CD8 T cells.
As it focuses attention solely on the ability to produce IL-17, the term ‘Th17’ can minimize the perceived importance of other factors produced by this subset of activated effectors, including, but not limited to, IL-17F, IL-21, and IL-22 (62). In agreement with others, we also show that in comparison to relatively homogeneous in vitro populations, heterogeneity amongst CD4 T cells capable of producing Th17 cytokines is observed in vivo (46, 47, 63). Our results show that Th17-polarized CD4 T cell effectors can protect against flu in an IL-17A- and IFNγ-independent fashion, distinct from Th1 effector-mediated mechanisms of protection including helper function and perforin-mediated cytotoxicity.
Optimal immunity against flu is multivariate and remarkably redundant, as high degrees of protection are often observed in models in which major constituents of the WT response, such as CD4 and CD8 T cells or B cells are deficient (64). Further studies will thus be required to determine the specific mechanisms and interactions responsible for Th17 effector-mediated protection against influenza, but it is tempting to speculate that non-inflammatory properties of Th17-associated cytokines are involved. For example, IL-17 has been shown to increase mucin production from and increase proliferation of lung epithelial cells (65). A similar impact on epithelial cell proliferation and resistance to injury has been shown for IL-22 (66), and IL-6 has been found to suppress T regulatory cell activity (67), rescue lymphocytes from apoptosis, and, somewhat paradoxically, to be involved in resolution of acute inflammation (68). Furthermore, IL-17 and IL-22 have been shown to regulate the production of a variety of antimicrobial proteins from epithelial cells (69).
Our observations of similar viral titers and similar Th1/Tc1 responses but improved lung function in IL-10 KO versus WT mice suggest that flu-specific Th17 responses may enhance protection by impacting the severity of lung damage and/or rate of lung repair rather than through direct anti-viral effects. Our results support the hypothesis that enhanced resistance against flu in the absence of IL-10 is due to the activities of a population of Th17 cells acting in conjunction with mechanisms associated with protective anti-viral responses against flu such as strong Th1/Tc1 responses. The kinetics of maximal Th17 responses following lethal infection (d 7–8) further supports the hypothesis that protective elements of the anti-flu response revealed by IL-10 deficiency occur during a short timeframe during the resolution phase of infection. This late impact of the Th17 response may help to explain the similar weight loss patterns observed in WT and IL-10 KO mice.
In contrast to our results, a recent report by Sun et al. found that blocking IL-10 signaling caused increased morbidity in flu infected mice, and administration of IL-10r blocking antibody correlated with a broad increase in lung inflammation but a minimal increase in IL-17 (70). It is possible that the different impact of IL-10 in our studies is due to the differential ability of mouse colonies, even of the same strain, to generate strong Th17 responses (71, and unpublished observations). Thus, our mice may be more prone to generate stronger Th17 responses. It is also possible that differences in the IL-10r antibody treatment regimes can account, at least in part, for the different outcomes observed. Another contrast between the two studies is that Sun et al. reported that lung-resident effector CD8 T cells were the major source of IL-10 during primary flu infection. In contrast in our studies there was minimal IL-10 production from lung CD8 T cells. As our studies employ a much higher lethal challenge dose, we suggest that the kinetics and/or differential contribution of effector CD4 and CD8 T cell IL-10 production might be influenced viral dose.
Although we noted robust IL-10 expression during the peak of the primary anti-flu response, we observed little IL-10 during heterosubtypic challenge or when flu-specific memory CD4 T cells were transferred to naïve hosts infected with virus. Our findings are consistent with observations of reduced IL-10 expression in responding memory as compared to naïve T cells (35, 53, 72), and support the hypothesis that regulation through IL-10 expression by Th1-polarized CD4 T effectors is a mechanism mostly restricted to primary responses. Our findings further show that autocrine IL-10 production by highly activated effectors may also serve to inhibit the development of a Th17 component within responding T cells. Alternatively, the accelerated T cell response and earlier viral clearance characteristic of heterosubtypic flu challenge may not facilitate the development of an inflammatory environment required for the generation of IFNγ+/IL-10+ effector CD4 T cells. While our studies do not establish direct significance of lower IL-10 expression in protective secondary responses against flu, it is interesting that the lung inflammatory environment in IL-10 KO mice following primary infection resembles that of WT mice following heterosubytpic challenge in terms of upregulated expression of IL-6 and Th17-associated cytokines (unpublished observations).
In contrast to T cell memory following Listeria monocytogenes infection (26), our results suggest that optimal flu-specific memory T cell responses do not depend on IL-10 expression during priming as equivalent and long-lasting heterosubtypic protection was observed in WT and IL-10 KO mice. Our previous studies demonstrated that strong heterosubtypic immunity is dependent on both virus-specific memory CD4 and CD8 T cells (49). Furthermore, the presence of flu antigen depots is not influenced by IL-10, although this does not rule out a longer-term IL-10-independent survival niche for the virus following resolution of primary infection. Given the potential of residual depots of flu antigen to impact the generation and maintenance of virus-specific memory T cells (73), understanding the nature of, and factors controlling long-lived flu antigen remain important areas of study.
Our in vivo observations of increased production of Th17-associated cytokines during flu infection in the absence of IL-10 are consistent with and extend recent studies suggesting an inhibitory role for IL-10 in the development of Th17 cytokine responses in vitro (74). It is tempting to speculate that pathogen-specific T cell response phenotypes in diverse studies linking increased survival and IL-10-deficincy may also display elements of a Th17 response, and that Th17-polarized effectors in these circumstances may also contribute to protection.
Supplementary Material
Acknowledgements
We thank Drs. Georgia Perona-Wright and Andrea Cooper for helpful discussions, and the Trudeau Institute Imaging Core.
This work was supported by NIH grants AI-46530 and AI067294 (to S.L. Swain) and NS06104 (to C. Teuscher), by Department of Defense HR+3222, and by Trudeau Institute. The authors have no conflicting financial interests.
Abbreviations
- WT
wildtype
- IL-10 KO
IL-10 deficient
- A/PR8
A/PuertoRico/8/34
- EID
egg infective dose
- dLN
draining lymph node
- Tc
T cytotoxic cell
- exp
experiment
References
- 1.Fiorentino DF, Bond MW, Mosmann TR. Two types of mouse T helper cell. IV. Th2 clones secrete a factor that inhibits cytokine production by Th1 clones. J Exp Med. 1989;170:2081–2095. doi: 10.1084/jem.170.6.2081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Moore KW, de Waal Malefyt R, Coffman RL, O'Garra A. Interleukin-10 and the interleukin-10 receptor. Annu Rev Immunol. 2001;19:683–765. doi: 10.1146/annurev.immunol.19.1.683. [DOI] [PubMed] [Google Scholar]
- 3.Couper KN, Blount DG, Riley EM. IL-10: the master regulator of immunity to infection. J Immunol. 2008;180:5771–5777. doi: 10.4049/jimmunol.180.9.5771. [DOI] [PubMed] [Google Scholar]
- 4.Li C, Corraliza I, Langhorne J. A defect in interleukin-10 leads to enhanced malarial disease in Plasmodium chabaudi chabaudi infection in mice. Infect Immun. 1999;67:4435–4442. doi: 10.1128/iai.67.9.4435-4442.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hunter CA, Ellis-Neyes LA, Slifer T, Kanaly S, Grunig G, Fort M, Rennick D, Araujo FG. IL-10 is required to prevent immune hyperactivity during infection with Trypanosoma cruzi. J Immunol. 1997;158:3311–3316. [PubMed] [Google Scholar]
- 6.Holscher C, Mohrs M, Dai WJ, Kohler G, Ryffel B, Schaub GA, Mossmann H, Brombacher F. Tumor necrosis factor alpha-mediated toxic shock in Trypanosoma cruzi-infected interleukin 10-deficient mice. Infect Immun. 2000;68:4075–4083. doi: 10.1128/iai.68.7.4075-4083.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Suzuki Y, Sher A, Yap G, Park D, Neyer LE, Liesenfeld O, Fort M, Kang H, Gufwoli E. IL-10 is required for prevention of necrosis in the small intestine and mortality in both genetically resistant BALB/c and susceptible C57BL/6 mice following peroral infection with Toxoplasma gondii. J Immunol. 2000;164:5375–5382. doi: 10.4049/jimmunol.164.10.5375. [DOI] [PubMed] [Google Scholar]
- 8.Gazzinelli RT, Wysocka M, Hieny S, Scharton-Kersten T, Cheever A, Kuhn R, Muller W, Trinchieri G, Sher A. In the absence of endogenous IL-10, mice acutely infected with Toxoplasma gondii succumb to a lethal immune response dependent on CD4+ T cells and accompanied by overproduction of IL-12, IFN-gamma and TNF-alpha. J Immunol. 1996;157:798–805. [PubMed] [Google Scholar]
- 9.Deckert M, Soltek S, Geginat G, Lutjen S, Montesinos-Rongen M, Hof H, Schluter D. Endogenous interleukin-10 is required for prevention of a hyperinflammatory intracerebral immune response in Listeria monocytogenes meningoencephalitis. Infect Immun. 2001;69:4561–4571. doi: 10.1128/IAI.69.7.4561-4571.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kuhn R, Lohler J, Rennick D, Rajewsky K, Muller W. Interleukin-10-deficient mice develop chronic enterocolitis. Cell. 1993;75:263–274. doi: 10.1016/0092-8674(93)80068-p. [DOI] [PubMed] [Google Scholar]
- 11.Kullberg MC, Ward JM, Gorelick PL, Caspar P, Hieny S, Cheever A, Jankovic D, Sher A. Helicobacter hepaticus triggers colitis in specific-pathogen-free interleukin-10 (IL-10)-deficient mice through an IL-12- and gamma interferon-dependent mechanism. Infect Immun. 1998;66:5157–5166. doi: 10.1128/iai.66.11.5157-5166.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Trinchieri G. Interleukin-10 production by effector T cells: Th1 cells show self control. J Exp Med. 2007;204:239–243. doi: 10.1084/jem.20070104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.O'Garra A, Vieira P. T(H)1 cells control themselves by producing interleukin-10. Nature reviews. 2007;7:425–428. doi: 10.1038/nri2097. [DOI] [PubMed] [Google Scholar]
- 14.Roncarolo MG, Gregori S, Battaglia M, Bacchetta R, Fleischhauer K, Levings MK. Interleukin-10-secreting type 1 regulatory T cells in rodents and humans. Immunological reviews. 2006;212:28–50. doi: 10.1111/j.0105-2896.2006.00420.x. [DOI] [PubMed] [Google Scholar]
- 15.Denis M, Ghadirian E. IL-10 neutralization augments mouse resistance to systemic Mycobacterium avium infections. J Immunol. 1993;151:5425–5430. [PubMed] [Google Scholar]
- 16.Bermudez LE, Champsi J. Infection with Mycobacterium avium induces production of interleukin-10 (IL-10), and administration of anti-IL-10 antibody is associated with enhanced resistance to infection in mice. Infect Immun. 1993;61:3093–3097. doi: 10.1128/iai.61.7.3093-3097.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Murray PJ, Young RA. Increased antimycobacterial immunity in interleukin-10-deficient mice. Infect Immun. 1999;67:3087–3095. doi: 10.1128/iai.67.6.3087-3095.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wagner RD, Maroushek NM, Brown JF, Czuprynski CJ. Treatment with anti-interleukin-10 monoclonal antibody enhances early resistance to but impairs complete clearance of Listeria monocytogenes infection in mice. Infect Immun. 1994;62:2345–2353. doi: 10.1128/iai.62.6.2345-2353.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dai WJ, Kohler G, Brombacher F. Both innate and acquired immunity to Listeria monocytogenes infection are increased in IL-10-deficient mice. J Immunol. 1997;158:2259–2267. [PubMed] [Google Scholar]
- 20.Brown JP, Zachary JF, Teuscher C, Weis JJ, Wooten RM. Dual role of interleukin-10 in murine Lyme disease: regulation of arthritis severity and host defense. Infect Immun. 1999;67:5142–5150. doi: 10.1128/iai.67.10.5142-5150.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Blackburn SD, Wherry EJ. IL-10, T cell exhaustion and viral persistence. Trends in microbiology. 2007;15:143–146. doi: 10.1016/j.tim.2007.02.006. [DOI] [PubMed] [Google Scholar]
- 22.Belkaid Y, Hoffmann KF, Mendez S, Kamhawi S, Udey MC, Wynn TA, Sacks DL. The role of interleukin (IL)-10 in the persistence of Leishmania major in the skin after healing and the therapeutic potential of anti-IL-10 receptor antibody for sterile cure. J Exp Med. 2001;194:1497–1506. doi: 10.1084/jem.194.10.1497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Brooks DG, Trifilo MJ, Edelmann KH, Teyton L, McGavern DB, Oldstone MB. Interleukin-10 determines viral clearance or persistence in vivo. Nature medicine. 2006;12:1301–1309. doi: 10.1038/nm1492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ejrnaes M, Filippi CM, Martinic MM, Ling EM, Togher LM, Crotty S, von Herrath MG. Resolution of a chronic viral infection after interleukin-10 receptor blockade. J Exp Med. 2006;203:2461–2472. doi: 10.1084/jem.20061462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Uzonna JE, Wei G, Yurkowski D, Bretscher P. Immune elimination of Leishmania major in mice: implications for immune memory, vaccination, and reactivation disease. J Immunol. 2001;167:6967–6974. doi: 10.4049/jimmunol.167.12.6967. [DOI] [PubMed] [Google Scholar]
- 26.Foulds KE, Rotte MJ, Seder RA. IL-10 is required for optimal CD8 T cell memory following Listeria monocytogenes infection. J Immunol. 2006;177:2565–2574. doi: 10.4049/jimmunol.177.4.2565. [DOI] [PubMed] [Google Scholar]
- 27.Baumgarth N, Brown L, Jackson D, Kelso A. Novel features of the respiratory tract T-cell response to influenza virus infection: lung T cells increase expression of gamma interferon mRNA in vivo and maintain high levels of mRNA expression for interleukin-5 (IL-5) and IL-10. J Virol. 1994;68:7575–7581. doi: 10.1128/jvi.68.11.7575-7581.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sarawar SR, Doherty PC. Concurrent production of interleukin-2, interleukin-10, and gamma interferon in the regional lymph nodes of mice with influenza pneumonia. J Virol. 1994;68:3112–3119. doi: 10.1128/jvi.68.5.3112-3119.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sarawar SR, Carding SR, Allan W, McMickle A, Fujihashi K, Kiyono H, McGhee JR, Doherty PC. Cytokine profiles of bronchoalveolar lavage cells from mice with influenza pneumonia: consequences of CD4+ and CD8+ T cell depletion. Regional immunology. 1993;5:142–150. [PubMed] [Google Scholar]
- 30.Carding SR, Allan W, McMickle A, Doherty PC. Activation of cytokine genes in T cells during primary and secondary murine influenza pneumonia. J Exp Med. 1993;177:475–482. doi: 10.1084/jem.177.2.475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jelley-Gibbs DM, Brown DM, Dibble JP, Haynes L, Eaton SM, Swain SL. Unexpected prolonged presentation of influenza antigens promotes CD4 T cell memory generation. J Exp Med. 2005;202:697–706. doi: 10.1084/jem.20050227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Scott B, Liblau R, Degermann S, Marconi LA, Ogata L, Caton AJ, McDevitt HO, Lo D. A role for non-MHC genetic polymorphism in susceptibility to spontaneous autoimmunity. Immunity. 1994;1:73–83. doi: 10.1016/1074-7613(94)90011-6. [DOI] [PubMed] [Google Scholar]
- 33.Roman E, Miller E, Harmsen A, Wiley J, Von Andrian UH, Huston G, Swain SL. CD4 effector T cell subsets in the response to influenza: heterogeneity, migration, and function. J Exp Med. 2002;196:957–968. doi: 10.1084/jem.20021052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lyons AB, Parish CR. Determination of lymphocyte division by flow cytometry. J Immunol Methods. 1994;171:131–137. doi: 10.1016/0022-1759(94)90236-4. [DOI] [PubMed] [Google Scholar]
- 35.McKinstry KK, Golech S, Lee WH, Huston G, Weng NP, Swain SL. Rapid default transition of CD4 T cell effectors to functional memory cells. J Exp Med. 2007;204:2199–2211. doi: 10.1084/jem.20070041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kamperschroer C, Dibble JP, Meents DL, Schwartzberg PL, Swain SL. SAP is required for Th cell function and for immunity to influenza. J Immunol. 2006;177:5317–5327. doi: 10.4049/jimmunol.177.8.5317. [DOI] [PubMed] [Google Scholar]
- 37.Cottey R, Rowe CA, Bender BS. Influenza Virus. In: Coligan GE, Kruisbeek AM, Shevach EM, Stober W, editors. Current Protocols in Immunology. John Wiley & Sons; 2001. pp. 1–32. [DOI] [PubMed] [Google Scholar]
- 38.Jelley-Gibbs DM, Dibble JP, Brown DM, Strutt TM, McKinstry KK, Swain SL. Persistent depots of influenza antigen fail to induce a cytotoxic CD8 T cell response. J Immunol. 2007;178:7563–7570. doi: 10.4049/jimmunol.178.12.7563. [DOI] [PubMed] [Google Scholar]
- 39.Sell S. Immunology, Immunopathology, and Immunity. 6th Edition ed. Washington: ASM Press; 2001. T-cell mediated cytotoxicity; pp. 401–430. [Google Scholar]
- 40.Williman J, Young S, Buchan G, Slobbe L, Wilson M, Pang P, Austyn J, Preston S, Baird M. DNA fusion vaccines incorporating IL-23 or RANTES for use in immunization against influenza. Vaccine. 2008;26:5153–5158. doi: 10.1016/j.vaccine.2008.03.084. [DOI] [PubMed] [Google Scholar]
- 41.Schmitz N, Kurrer M, Bachmann MF, Kopf M. Interleukin-1 is responsible for acute lung immunopathology but increases survival of respiratory influenza virus infection. J Virol. 2005;79:6441–6448. doi: 10.1128/JVI.79.10.6441-6448.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Jayasekera JP, Vinuesa CG, Karupiah G, King NJ. Enhanced antiviral antibody secretion and attenuated immunopathology during influenza virus infection in nitric oxide synthase-2-deficient mice. The Journal of general virology. 2006;87:3361–3371. doi: 10.1099/vir.0.82131-0. [DOI] [PubMed] [Google Scholar]
- 43.Zhou L, Lopes JE, Chong MM, Ivanov II, Min R, Victora GD, Shen Y, Du J, Rubtsov YP, Rudensky AY, Ziegler SF, Littman DR. TGF-beta-induced Foxp3 inhibits T(H)17 cell differentiation by antagonizing RORgammat function. Nature. 2008;453:236–240. doi: 10.1038/nature06878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Liu SJ, Tsai JP, Shen CR, Sher YP, Hsieh CL, Yeh YC, Chou AH, Chang SR, Hsiao KN, Yu FW, Chen HW. Induction of a distinct CD8 Tnc17 subset by transforming growth factor-beta and interleukin-6. Journal of leukocyte biology. 2007;82:354–360. doi: 10.1189/jlb.0207111. [DOI] [PubMed] [Google Scholar]
- 45.Brown DM, Dilzer AM, Meents DL, Swain SL. CD4 T cell-mediated protection from lethal influenza: perforin and antibody-mediated mechanisms give a one-two punch. J Immunol. 2006;177:2888–2898. doi: 10.4049/jimmunol.177.5.2888. [DOI] [PubMed] [Google Scholar]
- 46.Shi G, Cox CA, Vistica BP, Tan C, Wawrousek EF, Gery I. Phenotype switching by inflammation-inducing polarized Th17 cells, but not by Th1 cells. J Immunol. 2008;181:7205–7213. doi: 10.4049/jimmunol.181.10.7205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lee YK, Turner H, Maynard CL, Oliver JR, Chen D, Elson CO, Weaver CT. Late Developmental Plasticity in the T Helper 17 Lineage. Immunity. 2008 doi: 10.1016/j.immuni.2008.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Khader SA, Bell GK, Pearl JE, Fountain JJ, Rangel-Moreno J, Cilley GE, Shen F, Eaton SM, Gaffen SL, Swain SL, Locksley RM, Haynes L, Randall TD, Cooper AM. IL-23 and IL-17 in the establishment of protective pulmonary CD4+ T cell responses after vaccination and during Mycobacterium tuberculosis challenge. Nature immunology. 2007;8:369–377. doi: 10.1038/ni1449. [DOI] [PubMed] [Google Scholar]
- 49.Powell TJ, Strutt T, Reome J, Hollenbaugh JA, Roberts AD, Woodland DL, Swain SL, Dutton RW. Priming with cold-adapted influenza A does not prevent infection but elicits long-lived protection against supralethal challenge with heterosubtypic virus. J Immunol. 2007;178:1030–1038. doi: 10.4049/jimmunol.178.2.1030. [DOI] [PubMed] [Google Scholar]
- 50.de Jong MD, Simmons CP, Thanh TT, Hien VM, Smith GJ, Chau TN, Hoang DM, Chau NV, Khanh TH, Dong VC, Qui PT, Cam BV, Ha do Q, Guan Y, Peiris JS, Chinh NT, Hien TT, Farrar J. Fatal outcome of human influenza A (H5N1) is associated with high viral load and hypercytokinemia. Nature medicine. 2006;12:1203–1207. doi: 10.1038/nm1477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Salomon R, Hoffmann E, Webster RG. Inhibition of the cytokine response does not protect against lethal H5N1 influenza infection. Proceedings of the National Academy of Sciences of the United States of America. 2007;104:12479–12481. doi: 10.1073/pnas.0705289104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Szretter KJ, Gangappa S, Lu X, Smith C, Shieh WJ, Zaki SR, Sambhara S, Tumpey TM, Katz JM. Role of host cytokine responses in the pathogenesis of avian H5N1 influenza viruses in mice. J Virol. 2007;81:2736–2744. doi: 10.1128/JVI.02336-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Jankovic D, Kullberg MC, Feng CG, Goldszmid RS, Collazo CM, Wilson M, Wynn TA, Kamanaka M, Flavell RA, Sher A. Conventional T-bet+Foxp3- Th1 cells are the major source of host-protective regulatory IL-10 during intracellular protozoan infection. J Exp Med. 2007 doi: 10.1084/jem.20062175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Anderson CF, Oukka M, Kuchroo VJ, Sacks D. CD4+CD25-Foxp3- Th1 cells are the source of IL-10-mediated immune suppression in chronic cutaneous leishmaniasis. J Exp Med. 2007 doi: 10.1084/jem.20061886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wu CY, Kirman JR, Rotte MJ, Davey DF, Perfetto SP, Rhee EG, Freidag BL, Hill BJ, Douek DC, Seder RA. Distinct lineages of T(H)1 cells have differential capacities for memory cell generation in vivo. Nature immunology. 2002;3:852–858. doi: 10.1038/ni832. [DOI] [PubMed] [Google Scholar]
- 56.Awasthi A, Carrier Y, Peron JP, Bettelli E, Kamanaka M, Flavell RA, Kuchroo VK, Oukka M, Weiner HL. A dominant function for interleukin 27 in generating interleukin 10-producing anti-inflammatory T cells. Nature immunology. 2007;8:1380–1389. doi: 10.1038/ni1541. [DOI] [PubMed] [Google Scholar]
- 57.Meyaard L, Hovenkamp E, Otto SA, Miedema F. IL-12-induced IL-10 production by human T cells as a negative feedback for IL-12-induced immune responses. J Immunol. 1996;156:2776–2782. [PubMed] [Google Scholar]
- 58.Peritt D, Aste-Amezaga M, Gerosa F, Paganin C, Trinchieri G. Interleukin-10 induction by IL-12: a possible modulatory mechanism? Ann N Y Acad Sci. 1996;795:387–389. doi: 10.1111/j.1749-6632.1996.tb52701.x. [DOI] [PubMed] [Google Scholar]
- 59.Stumhofer JS, Silver JS, Laurence A, Porrett PM, Harris TH, Turka LA, Ernst M, Saris CJ, O'Shea JJ, Hunter CA. Interleukins 27 and 6 induce STAT3-mediated T cell production of interleukin 10. Nature immunology. 2007;8:1363–1371. doi: 10.1038/ni1537. [DOI] [PubMed] [Google Scholar]
- 60.Levings MK, Sangregorio R, Galbiati F, Squadrone S, de Waal Malefyt R, Roncarolo MG. IFN-alpha and IL-10 induce the differentiation of human type 1 T regulatory cells. J Immunol. 2001;166:5530–5539. doi: 10.4049/jimmunol.166.9.5530. [DOI] [PubMed] [Google Scholar]
- 61.Kadowaki N, Antonenko S, Lau JY, Liu YJ. Natural interferon alpha/beta-producing cells link innate and adaptive immunity. J Exp Med. 2000;192:219–226. doi: 10.1084/jem.192.2.219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ouyang W, Kolls JK, Zheng Y. The biological functions of T helper 17 cell effector cytokines in inflammation. Immunity. 2008;28:454–467. doi: 10.1016/j.immuni.2008.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Muranski P, Boni A, Antony PA, Cassard L, Irvine KR, Kaiser A, Paulos CM, Palmer DC, Touloukian CE, Ptak K, Gattinoni L, Wrzesinski C, Hinrichs CS, Kerstann KW, Feigenbaum L, Chan CC, Restifo NP. Tumor-specific Th17-polarized cells eradicate large established melanoma. Blood. 2008;112:362–373. doi: 10.1182/blood-2007-11-120998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Brown DM, Roman E, Swain SL. CD4 T cell responses to influenza infection. Seminars in immunology. 2004;16:171–177. doi: 10.1016/j.smim.2004.02.004. [DOI] [PubMed] [Google Scholar]
- 65.Inoue D, Numasaki M, Watanabe M, Kubo H, Sasaki T, Yasuda H, Yamaya M, Sasaki H. IL-17A promotes the growth of airway epithelial cells through ERK-dependent signaling pathway. Biochemical and biophysical research communications. 2006;347:852–858. doi: 10.1016/j.bbrc.2006.06.137. [DOI] [PubMed] [Google Scholar]
- 66.Aujla SJ, Chan YR, Zheng M, Fei M, Askew DJ, Pociask DA, Reinhart TA, McAllister F, Edeal J, Gaus K, Husain S, Kreindler JL, Dubin PJ, Pilewski JM, Myerburg MM, Mason CA, Iwakura Y, Kolls JK. IL-22 mediates mucosal host defense against Gram-negative bacterial pneumonia. Nature medicine. 2008;14:275–281. doi: 10.1038/nm1710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Pasare C, Medzhitov R. Science. Vol. 299. New York, NY: 2003. Toll pathway-dependent blockade of CD4+CD25+ T cell-mediated suppression by dendritic cells; pp. 1033–1036. [DOI] [PubMed] [Google Scholar]
- 68.Jones SA. Directing transition from innate to acquired immunity: defining a role for IL-6. J Immunol. 2005;175:3463–3468. doi: 10.4049/jimmunol.175.6.3463. [DOI] [PubMed] [Google Scholar]
- 69.Kolls JK, McCray PB, Jr, Chan YR. Cytokine-mediated regulation of antimicrobial proteins. Nature reviews. 2008;8:829–835. doi: 10.1038/nri2433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Sun J, Madan R, Karp CL, Braciale TJ. Effector T cells control lung inflammation during acute influenza virus infection by producing IL-10. Nature medicine. 2009;15:277–284. doi: 10.1038/nm.1929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Ivanov II, Frutos Rde L, Manel N, Yoshinaga K, Rifkin DB, Sartor RB, Finlay BB, Littman DR. Specific microbiota direct the differentiation of IL-17-producing T-helper cells in the mucosa of the small intestine. Cell host & microbe. 2008;4:337–349. doi: 10.1016/j.chom.2008.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Dong J, Ivascu C, Chang HD, Wu P, Angeli R, Maggi L, Eckhardt F, Tykocinski L, Haefliger C, Mowes B, Sieper J, Radbruch A, Annunziato F, Thiel A. IL-10 is excluded from the functional cytokine memory of human CD4+ memory T lymphocytes. J Immunol. 2007;179:2389–2396. doi: 10.4049/jimmunol.179.4.2389. [DOI] [PubMed] [Google Scholar]
- 73.Jelley-Gibbs DM, Strutt TM, McKinstry KK, Swain SL. Influencing the fates of CD4 T cells on the path to memory: lessons from influenza. Immunology and cell biology. 2008;86:343–352. doi: 10.1038/icb.2008.13. [DOI] [PubMed] [Google Scholar]
- 74.Gu Y, Yang J, Ouyang X, Liu W, Li H, Yang J, Bromberg J, Chen SH, Mayer L, Unkeless JC, Xiong H. Interleukin 10 suppresses Th17 cytokines secreted by macrophages and T cells. Eur J Immunol. 2008 doi: 10.1002/eji.200838331. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.