Abstract
Our previous finding, that the capsaicin- and KCl-induced Ca2+-dependent production of the intra- and intercellular signaling molecule N-arachidonoyl ethanolamine (anandamide) in cultured primary sensory neurons could be abolished and reduced by ∼2/3 by capsaicin-induced degeneration of capsaicin-sensitive neurons, respectively suggests that a major sub-population of capsaicin-sensitive cells together with a group of non-capsaicin-sensitive cells should express enzymes involved in Ca2+-dependent anandamide synthesis. N-acyl phosphotidylethanolamine phospholipase D (NAPE-PLD) is known to be involved in Ca2+-dependent anandamide production. Hence, here, we used reverse transcriptase and quantitative real time polymerase chain reaction to study NAPE-PLD expression in dorsal root ganglia and to clarify the sub-population of cells expressing this enzyme. Cultures prepared from mouse dorsal root ganglia were grown either in the absence or presence of the neurotoxin, capsaicin (10 μM) overnight. We report, that NAPE-PLD is expressed both in dorsal root ganglia and cultures prepared from dorsal root ganglia and grown in the absence of capsaicin. Furthermore, we also report that capsaicin application downregulates the expression of NAPE-PLD as well as the capsaicin receptor, transient receptor potential vanilloid type 1 ion channel, by about 70% in the cultures prepared from dorsal root ganglia. These findings indicate that a major sub-population of capsaicin-sensitive primary sensory neurons expresses NAPE-PLD, and suggest that NAPE-PLD is expressed predominantly by capsaicin-sensitive neurons in dorsal root ganglia. These data also suggest that NAPE-PLD might be a target to control the activity and excitability of a major sub-population of nociceptive primary sensory neurons.
Key words: anandamide, dorsal root ganglion, transient receptor vanilloid type 1, TRPV1, nociceptive, pain
Abbreviations: anandamide, N-arachidonoyl ethanolamine; CB1, cannabinoid 1; CB2, cannabinoid 2; DRG, dorsal root ganglia; FAAH, fatty acid amide hydrolase; GAPDH, glyceraldehyde-3-phosphate dehydrogenase; NAPE-PLD, N-acyl phosphotidylethanolamine phospholipase D; PCR, polymerase chain reaction; RT, reverse transcriptase; TRPV1, transient receptor potential vanilloid type 1
The great majority of nociceptive primary sensory neurons are sensitive to capsaicin, the agent that is responsible for the hotness of the chili pepper (Porszasz and Jancso, 1959; Jancso et al., 1977; Lynn and Carpenter, 1982; Heyman and Rang, 1985; Wood et al., 1988). Capsaicin-sensitivity of cells is underlain by the expression of the capsaicin receptor, transient receptor potential vanilloid type 1 ion channel (TRPV1; Caterina et al., 1997, 2000; Davis et al., 2000). In addition to capsaicin, TRPV1 is also responsive to a series of other activators such as moderate heat, protons, post-translational modifications, depolarization and other exogenous and endogenous agents, including N-arachidonoyl ethanolamine (anandamide; Caterina et al., 1997; Zygmunt et al., 1999; Huang et al., 2002; Shin et al., 2002; Chu et al., 2003; Voets et al., 2004; Movahed et al., 2005). Anandamide, in addition to TRPV1, is also an activator of other receptors, including the cannabinoid 1 (CB1) and cannabinoid 2 (CB2) receptors, and the orphan G protein-coupled receptor 55 (GPR 55) (Matsuda et al., 1990; Devane et al., 1992; Munro et al., 1993; Zygmunt et al., 1999; Ryberg et al., 2007). Of these, the CB1 and CB2 receptors are co-expressed with TRPV1 in a major sub-population of primary sensory neurons (Ahluwalia et al., 2000; Ross et al., 2001; Agarwal et al., 2007; Anand et al., 2008). Previous findings suggest that anandamide, by activating TRPV1 and the cannabinoid receptors, may be involved in the regulation of the activity and excitability of the TRPV1/CB1 receptor-expressing cells (Ellington et al., 2002; Ahluwalia et al., 2003a; Németh et al., 2003; Anand et al., 2008).
Interestingly, primary sensory neurons, including the capsaicin-sensitive cells are capable of producing anandamide (Ahluwalia et al., 2003b; van der Stelt et al., 2005; Vellani et al., 2008). Anandamide-production in primary sensory neurons could depend on, or could be independent of, Ca2+ (Ahluwalia et al., 2003b; van der Stelt et al., 2005; Vellani et al., 2008). The Ca2+-dependent anandamide production could be triggered by capsaicin, KCl-induced depolarization or by Ca2+ release from the intracellular stores (Ahluwalia et al., 2003b; van der Stelt et al., 2005). While the capsaicin-induced anandamide production is completely abolished, the KCl-induced anandamide synthesis is reduced to about one third of the control value, by overnight capsaicin treatment, which induces Ca2+-dependent cytotoxicity and cellular death (Jancso et al., 1977, 1995; Gamse et al., 1982; Chard et al., 1995; Wood et al., 1988; Olah et al., 2001; Ahluwalia et al., 2003b).
The enzyme, N-acyl phosphotidylethanolamine phospholipase D (NAPE-PLD), belongs to the zinc metallohydrolase family of the β-lactamase fold (Okamoto et al., 2004) and is known to produce various long and medium chain bioactive N-acylethanolamines, including anandamide in a Ca2+-dependent fashion (Okamoto et al., 2004; Sun et al., 2004; Leung et al., 2006; Liu et al., 2006; Simon and Cravatt, 2006, 2008). Therefore, we hypothesized that NAPE-PLD could be expressed by a major sub-population of capsaicin-sensitive cells in addition to a group of non-capsaicin-sensitive cells, which could belong to the non-nociceptive sub-population of primary sensory neurons. Accordingly, here, we studied the expression of NAPE-PLD in dorsal root ganglia (DRG) and cultures prepared from DRG. In order to find out whether NAPE-PLD is expressed by the capsaicin-sensitive cells, half of the cultures were grown in the presence of capsaicin.
Experimental procedures
Animals and preparation of primary sensory neuronal cultures
All procedures in this work were performed in accordance with the UK Animals (Scientific Procedures) Act 1986, and its ethical guidelines and every effort was taken to minimize the number and suffering of animals used. Measurements were performed on tissue homogenates of DRG, heart and total brain, collected from 10- to 12-week-old male C57BL/6 mice and on cultures, which were prepared from DRG collected also from 10- to 12-week-old male C57BL/6 mice.
Tissues were quickly dissected out from terminally anesthetized (Enflurane; Abbott Laboratories, Kent, UK) animals and chopped into small pieces in RNAlater (QIAGEN, Crawley, UK). Tissue samples were stored in RNAlater at 4 °C until they were used for RNA isolation.
Cultures were prepared following terminal anesthesia (Enflurane, Abbott Laboratories) as described previously (Nagy and Rang, 1999). Briefly, ganglia were removed from the first cervical to the first sacral segment from both sides and placed into Dulbecco's Modified Eagle's Medium F12 (Sigma, Gillingham, UK) supplemented with 2 mM l-glutamine (Invitrogen, Paisley, UK), 5000 IU/ml penicillin (Invitrogen, Paisley, UK), 5000 μg/ml streptomycin (Invitrogen, Paisley, UK) and 2% Ultroser G (BioSepra SA, Cergy-Pontoise, France). Connective tissues in the DRG were digested by 0.125% type IV collagenase (Lorne Diagnostics, Bury St. Edmunds, UK) for 3 h at 37 °C in 5% CO2. Ganglia were triturated with a fire-polished Pasteur pipette, and cells were plated on poly dl-ornithine-coated glass coverslips (Sigma). Cells were cultured at 37 °C in a humidified atmosphere gassed with 5% CO2 for a day in the supplemented F12 medium to which 50 ng/ml nerve growth factor was added (Promega, Southampton, UK). Half of the cultures prepared from three mice were grown in the presence of 10 μM capsaicin, which was dissolved in dimethyl sulfoxide (final concentration 3 mM).
Isolation of mRNA and reverse transcriptase (RT) reaction
Tissue samples were weighed and homogenized by a tissue homogenizer. Cell lysates were further homogenized using QIAshredder columns (QIAGEN, Crawley, UK). Cultured cells were scraped from the coverslips and homogenized using QIAshredder columns. RNA from the homogenates was extracted using the RNeasy Mini Kit (QIAGEN) according to the manufacturer's instructions. Following elution the RNA was quantified and stored at −80 °C until further use.
Total RNA (600 ng) was reverse transcribed with SuperScript II (Invitrogen), using oligo (dT)15 primer (Promega), dNTP (Promega), SUPERasin (Ambion, Huntington, UK), first-strand buffer (Invitrogen) and DTT (Invitrogen).
Polymerase chain reaction (PCR)
Primers were designed to amplify mouse NAPE-PLD and the housekeeping gene, glyceraldehyde-3-phosphate dehydrogenase (GAPDH). The sequences of the primers (MWG Biotech, Ebersberg, Germany) were as follows: NAPE-PLD: forward 5′-GGC CAA CAT GGA AAA ACA TC-3′; reverse: 5′-ATG AGC TCG TCC ATT TCC AC-3′; GAPDH: forward: 5′-GGT GAA GGT CGG AGT CAA CG-3′; reverse: 5′-CAA AGT TGT CAT GGA TTG ACC-3′. The predicted product sizes were 222 and 370 bp for NAPE-PLD and GAPDH, respectively. The PCR reaction mixture contained 3 mM MgCl2, 1× reaction buffer (5 mM Tris–HCl, 50 mM KCl, 1.5 mM MgCl2, pH 8.3), 0.2 mM deoxynucleotide mix and 1.25 U of Go-Taq Flexi DNA polymerase (Promega). The amplification reaction consisted of 30 cycles with 30 s of denaturation at 96 °C, 1 min annealing, and 3 min extension at 72 °C in a thermal cycler (Eppendorf-Mastercycler Personal, UK). The annealing temperatures for both NAPE-PLD and GAPDH were 55 °C. PCR products were separated on agarose gels and visualized with ethidium bromide.
Quantitative real time PCR
For quantitative real-time PCR, specific assays were obtained (PrimerDesign, Ltd., UK). The primers were designed and validated by the manufacturer. In these experiments we assessed the effect of capsaicin treatment on the expression of NAPE-PLD and TRPV1 relative to the expression of GAPDH. The primers for TRPV1 were designed against the GenBank sequence NM_001001445 and were as follows; forward: 5′-CCT GCA TTG ACA CCT GTG AA-3′; reverse: 5′-AGT CGG TTC AAG GGT TCC A-3′. Primers for NAPE-PLD were designed using GenBank sequence NM_178728: forward: 5′-GGG CGG CTC TCA CTT TCT A-3; reverse: 5′-ACA CTT GTG CTT ATA GGT CAT TTA AT-3′. For GAPDH a pre-designed primer set was provided by the manufacturer. The reaction was performed in triplicate using Precision qPCR master mix with SYBR green and ROX (PrimerDesign, Ltd.) on an ABI 7900HT real-time PCR machine. These reactions were enzyme-activated by heating at 95 °C for 10 min (hot start), then denaturing at 95 °C for 15 s followed by cooling to 60 °C for data collection (50 cycles). The “crossover” threshold (ct) was determined in each sample for each DNA. The average GAPDH measurement in each sample was used to establish the relative expression of NAPE-PLD and TRPV1 in the respective sample.
Results
First, we aimed to establish whether NAPE-PLD is expressed in DRG and in cultures prepared from DRG. In addition to cDNA from DRG and cultures, cDNA from the heart, where NAPE-PLD has been cloned from (Okamoto et al., 2004), and the brain, where NAPE-PLD expression has been reported recently (Morishita et al., 2005; Leung et al., 2006), was also included in the reaction, for control.
RT-PCR produced distinct products with sizes between 350 and 400 bp, and 200 and 250 bp in all samples (Fig. 1A, B). While the larger product (Fig. 1A) corresponded with the predicted size of the GAPDH, the smaller product (Fig. 1B) corresponded with that expected for the NAPE-PLD RT-PCR product. These findings indicated that NAPE-PLD is expressed both in DRG and cultures prepared from DRG.
Fig. 1.
RT-PCR analysis of NAPE-PLD gene expression. (A) Agarose gel electrophoresis of RT-PCR products for GAPDH (370 bp) from cDNA made to RNA from brain, heart, DRG and cultures prepared from DRG (lanes 1, 2, 3, and 4, respectively). (B) Agarose gel electrophoresis of RT-PCR products for NAPE-PLD (222 bp) from cDNA made to RNA from brain, heart, DRG and cultures prepared from DRG (lanes 1, 2, 3, and 4, respectively). Note that NAPE-PLD is expressed in all the tissues we examined. (C) NAPE-PLD (lane 1) and GAPDH (lane 4) gene expression in mouse dorsal root ganglion cultures grown under control conditions (without capsaicin). Lane 2 shows NAPE-PLD expression in a brain sample collected from the same animal used to derive the cultures analyzed in lanes 1 and 4. Lane 3 is a control PCR reaction, where RNA equivalent to the cDNA used in lane 1 has been used as template. (D) NAPE-PLD (lane 1) and GAPDH (lane 4) gene expression in mouse dorsal root ganglion cultures grown in the presence of 10 μM capsaicin overnight. Lane 2 shows NAPE-PLD expression in the brain sample collected from the same animal. Lane 3 is a control PCR reaction, where RNA equivalent to the cDNA used in lane 1 has been used as template. Note that the treatment of cultures with capsaicin significantly downregulated NAPE-PLD expression (lane 1 in C and D.)
In addition to primary sensory neurons, both DRG and cultures prepared from DRG contain cells other then primary sensory neurons. However, only a sub-population of primary sensory neurons is susceptible to degeneration by the neurotoxin, capsaicin in both intact DRG and cultures prepared from DRG (Porszasz and Jancso, 1959; Jancso et al., 1977; Lynn and Carpenter, 1982; Heyman and Rang, 1985; Wood et al., 1988). Thus, in order to find out the cell type expressing NAPE-PLD, we induced capsaicin-evoked degeneration of capsaicin-sensitive cells by growing the cultures in 10 μM of this neurotoxin overnight (Gamse et al., 1982; Chard et al., 1995; Jancso et al., 1995). In agreement with results of the previous experiment, RT-PCR produced NAPE-PLD amplicon when cDNA of control cultures served as a template (Fig. 1C, lane 1). However, very little PCR product was detected when cDNA from the capsaicin-treated cultures was used as a template for the reaction (Fig. 1D, lane 1).
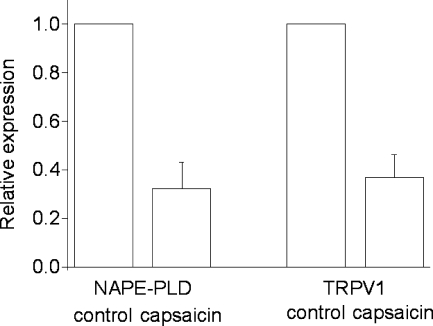
In order to ascertain that capsaicin treatment indeed downregulated NAPE-PLD expression, we next measured the relative expression of NAPE-PLD, and TRPV1, which mediates the capsaicin-induced neurotoxic Ca2+ influx (Caterina et al., 1997), in control and capsaicin-treated cultures with real time quantitative PCR. We found that capsaicin treatment reduced NAPE-PLD mRNA expression by about 70% (Fig. 2). The capsaicin treatment downregulated TRPV1 mRNA expression by the same extent.
Fig. 2.
Relative gene expression of NAPE-PLD and TRPV1 in cultures prepared from DRG and grown either in the absence (control) or presence of 10 μM capsaicin overnight. The expression of the NAPE-PLD and TRPV1 mRNA was normalized to that of GAPDH mRNA. Each set shows an average from three cultures, each prepared from different animals, with standard errors of the mean shown. Note that the treatment of cultures with capsaicin downregulated both TRPV1 and NAPE-PLD-expression relative to that seen in untreated cultures.
Discussion
At present, TRPV1 is the only molecule, which is known to selectively and specifically respond to capsaicin (Caterina et al., 1997, 2000; Davis et al., 2000). Immunohistochemical and functional data show that only a sub-population of neurons expresses TRPV1 in DRG and cultures prepared from DRG (Nagy et al., 1993; Caterina et al., 1997, 2000; Guo et al., 1999; Michael and Priestley, 1999; Ahluwalia et al., 2000; Singh Tahim et al., 2005). Thus, capsaicin treatment can induce degeneration only in the TRPV1-expressing sub-population of neurons in cultures prepared from DRG.
We found in the present study that overnight exposure of cultured primary sensory neurons to capsaicin reduced TRPV1 mRNA expression by ∼70%. This degree of downregulation in TRPV1 mRNA expression is comparable with the ∼70%–80% reduction in TRPV1 protein expression and in the number of TRPV1-expressing neurons produced by prolonged (≥48 h) application of the ultrapotent TRPV1 activator, resiniferatoxin to primary sensory neurons (Olah et al., 2001; Tender et al., 2005). In parallel with the reduction in TRPV1 mRNA expression, NAPE-PLD mRNA expression was also reduced by ∼70%. These findings indicate that a major proportion of capsaicin-sensitive primary sensory neurons expresses NAPE-PLD, and suggest that the majority of the NAPE-PLD-expressing primary sensory neurons are capsaicin sensitive. However, assuming that the TRPV1 mRNA we detected in our cultures following the capsaicin treatment, was expressed in neurons, which, in spite of their TRPV1 expression, were not responsive to capsaicin, or alternatively, they were responsive to capsaicin but resistant to the subsequent excitotoxicity, it is tempting to propose that TRPV1 and NAPE-PLD could be co-expressed to a high degree, in primary sensory neurons.
Based on the similarity between the overnight 10 μM capsaicin exposure-evoked downregulation of NAPE-PLD mRNA expression, and the previously demonstrated downregulation in KCl-evoked anandamide release (∼60%–70%; Ahluwalia et al., 2003b), it is also tempting to propose that only NAPE-PLD could be responsible for Ca2+-dependent anandamide production in primary sensory neurons. However, in addition to the Ca2+-evoked anandamide synthesis (Ahluwalia et al., 2003b; van der Stelt et al., 2005), primary sensory neurons, also produce anandamide in a Ca2+-independent manner (Vellani et al., 2008). Therefore, NAPE-PLD must represent only one of the anandamide-synthesizing enzymatic pathways present in these cells. To the best of our knowledge, this is the first report demonstrating the expression of an enzyme that is involved in anandamide synthesis in primary sensory neurons, and it is not known which other anandamide-producing enzymatic pathways are expressed, and whether any of the other pathways are involved in anandamide synthesis in a Ca2+-dependent fashion, in these neurons (Di Marzo and Petrosino, 2007). Therefore, further studies are needed to elucidate these issues.
Nevertheless, the presence of NAPE-PLD in capsaicin-sensitive primary sensory neurons raises the question, what role does this enzyme play in these neurons. Several of the NAPE-PLD products, including oleoylethanolamide, linoleoylethanolamide and anandamide, activate TRPV1 (Zygmunt et al., 1999; Okamoto et al., 2004; Movahed et al., 2005). However, the finding that among these products, anandamide also activates a series of inhibitory receptors, which are co-expressed with TRPV1 in primary sensory neurons (Matsuda et al., 1990; Devane et al., 1992; Munro et al., 1993; Zygmunt et al., 1999; Ahluwalia et al., 2000; Ross et al., 2001; Agarwal et al., 2007; Anand et al., 2008) suggests that anandamide production by NAPE-PLD could be the most important in relation to regulating the activity and excitability of a major sub-population of capsaicin-sensitive, thus, nociceptive cells.
The expression pattern of the anandamide-responding receptors in nociceptive primary sensory neurons (Ahluwalia et al., 2000; Ross et al., 2001; Agarwal et al., 2007; Anand et al., 2008) together with previous functional data (Ellington et al., 2002; Ahluwalia et al., 2003b; Németh et al., 2003; Nagy et al., 2006; Anand et al., 2008) suggests that NAPE-PLD activity may provide a CB1- and/or CB2-mediated brake on the responsiveness and activity of, the cells, and TRPV1. Alternatively, NAPE-PLD might be part of a signal amplification pathway in TRPV1-expressing cells, which has been suggested recently by van der Stelt and Di Marzo (2005). The presence of such an amplification system in capsaicin-sensitive primary sensory neurons is supported by recent findings. First, van der Stelt and co-workers (2005) have reported that the Ca2+-dependent anandamide production results in TRPV1 activity in cultured primary sensory neurons. Second, we found that inhibition of the anandamide-hydrolysing enzyme, fatty acid amide hydrolase (FAAH), which is also expressed by a major sub-population of TRPV1-expressing primary sensory neurons results in TRPV1 activity (Lever et al., 2009). Third, repeated application of anandamide to TRPV1 sensitizes the responses of this ion channel (Premkumar and Ahern, 2000).
In primary sensory neurons, TRPV1 seems to have a pivotal role in signaling peripheral inflammatory events to the CNS through getting activated directly or indirectly by inflammatory mediators, which are produced and released from the inflamed tissues (Caterina et al., 2000; Davis et al., 2000; Ji et al., 2004; Ma and Quirion, 2007; Nagy et al., 2008, 2009). A series of inflammatory mediators induces Ca2+ influx into primary sensory neurons, including into the capsaicin-sensitive cells (Thayer et al., 1998; Cesare et al., 1999; Smith et al., 2000; Moriyama et al., 2005). Comparison of the increase in the intracellular Ca2+ concentration produced by some of the inflammatory mediators, including bradykinin and prostaglandin E2, to those which can evoke anandamide production (van der Stelt et al., 2005) suggests that inflammatory mediators should be capable of inducing anandamide production. In addition to inducing Ca2+ influx, inflammatory mediators also induce post-translational changes in TRPV1 (for references see Nagy et al., 2008, 2009). These changes together with the sensitizing effect of anandamide on TRPV1 (Premkumar and Ahern, 2000) result in a significant increase in the otherwise modest efficacy and potency of anandamide on TRPV1 (Zygmunt et al., 1999; Ahluwalia et al., 2003b; Singh Tahim et al., 2005). Thus, anandamide produced even in small concentrations inside the TRPV1-expressing cells can induce a significant increase in TRPV1 open probability and subsequent action potential generation. In addition to anandamide, however, the production of other TRPV1-activating N-acylethanolamines by NAPE-PLD may also contribute to TRPV1 activity in inflammatory conditions (Movahed et al., 2005).
Based on the considerations above, we propose that NAPE-PLD expression and activity in capsaicin-sensitive primary sensory neurons could serve as a vital part of the anandamide-mediated signal amplification process (van der Stelt and Di Marzo, 2005). That signal amplification, hence NAPE-PLD activity, could be fundamental for the development of increased responsiveness of these cells in pathological conditions, and subsequently, for sending information about inflammatory processes at the periphery to the CNS. However, the signal amplification process by NAPE-PLD may not be unique in capsaicin-sensitive primary sensory neurons, because increasing anandamide concentration, for example by inhibiting FAAH activity, also results in TRPV1 activity in the brain (Maione et al., 2006; Morgese et al., 2007). Thus, NAPE-PLD may also play a pivotal role in signal amplification in neurons expressing both NAPE-PLD and TRPV1 (Cristino et al., 2006, 2008), in various areas of the CNS. Nevertheless, if anandamide produced by NAPE-PLD indeed has a fundamental amplification role, which contributes to the development of TRPV1 activation and sensitization, targeting this enzyme in primary sensory neurons may provide a novel approach to reduce the activity and excitability of capsaicin-sensitive primary sensory neurons, thus, to reduce pain, in inflammatory conditions.
Acknowledgments
Andrew Photiou has been supported by the Wellcome Trust (061637/Z/06/Z). Cleoper C. Paule has been supported by a BBSRC-GSK CASE award (BBS/N/204/11514).
References
- Agarwal N., Pacher P., Tegeder I., Amaya F., Constantin C.E., Brenner G.J., Rubino T., Michalski C.W., Marsicano G., Monory K., Mackie K., Marian C., Batkai S., Parolaro D., Fischer M.J., Reeh P., Kunos G., Kress M., Lutz B., Woolf C.J., Kuner R. Cannabinoids mediate analgesia largely via peripheral type 1 cannabinoid receptors in nociceptors. Nat Neurosci. 2007;10:870–879. doi: 10.1038/nn1916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahluwalia J., Urban L., Bevan S., Nagy I. Anandamide regulates neuropeptide release from capsaicin-sensitive primary sensory neurons by activating both the cannabinoid 1 receptor and the vanilloid receptor 1 in vitro. Eur J Neurosci. 2003;7:2611–2618. doi: 10.1046/j.1460-9568.2003.02703.x. [DOI] [PubMed] [Google Scholar]
- Ahluwalia J., Yaqoob M., Urban L., Bevan S., Nagy I. Activation of capsaicin-sensitive primary sensory neurones induces anandamide production and release. J Neurochem. 2003;84:585–591. doi: 10.1046/j.1471-4159.2003.01550.x. [DOI] [PubMed] [Google Scholar]
- Ahluwalia J., Urban L., Capogna M., Bevan S., Nagy I. Cannabinoid 1 receptors are expressed in nociceptive primary sensory neurons. Neuroscience. 2000;100:685–688. doi: 10.1016/s0306-4522(00)00389-4. [DOI] [PubMed] [Google Scholar]
- Anand U., Otto W.R., Sanchez-Herrera D., Facer P., Yiangou Y., Korchev Y., Birch R., Benham C., Bountra C., Chessell I.P., Anand P. Cannabinoid receptor CB2 localisation and agonist-mediated inhibition of capsaicin responses in human sensory neurons. Pain. 2008;138:667–680. doi: 10.1016/j.pain.2008.06.007. [DOI] [PubMed] [Google Scholar]
- Caterina M.J., Leffler A., Malmberg A.B., Martin W.J., Trafton J., Petersen-Zeitz K.R., Koltzenburg M., Basbaum A.I., Julius D. Impaired nociception and pain sensation in mice lacking the capsaicin receptor. Science. 2000;288:306–313. doi: 10.1126/science.288.5464.306. [DOI] [PubMed] [Google Scholar]
- Caterina M.J., Schumacher M.A., Tominaga M., Rosen T.A., Levine J.D., Julius D. The capsaicin receptor: a heat-activated ion channel in the pain pathway. Nature. 1997;389:816–824. doi: 10.1038/39807. [DOI] [PubMed] [Google Scholar]
- Cesare P., Dekker L.V., Sardini A., Parker P.J., McNaughton P.A. Specific involvement of PKC-epsilon in sensitization of the neuronal response to painful heat. Neuron. 1999;23:617–624. doi: 10.1016/s0896-6273(00)80813-2. [DOI] [PubMed] [Google Scholar]
- Chard P.S., Bleakman D., Savidge J.R., Miller R.J. Capsaicin-induced neurotoxicity in cultured dorsal root ganglion neurons: involvement of calcium-activated proteases. Neuroscience. 1995;65:1099–1108. doi: 10.1016/0306-4522(94)00548-j. [DOI] [PubMed] [Google Scholar]
- Chu C.J., Huang S.M., De Petrocellis L., Bisogno T., Ewing S.A., Miller J.D., Zipkin R.E., Daddario N., Appendino G., Di Marzo V., Walker J.M. N-oleoyldopamine, a novel endogenous capsaicin-like lipid that produces hyperalgesia. J Biol Chem. 2003;278:13633–13639. doi: 10.1074/jbc.M211231200. [DOI] [PubMed] [Google Scholar]
- Cristino L., de Petrocellis L., Pryce G., Baker D., Guglielmotti V., Di Marzo V. Immunohistochemical localization of cannabinoid type 1 and vanilloid transient receptor potential vanilloid type 1 receptors in the mouse brain. Neuroscience. 2006;139:1405–1415. doi: 10.1016/j.neuroscience.2006.02.074. [DOI] [PubMed] [Google Scholar]
- Cristino L., Starowicz K., De Petrocellis L., Morishita J., Ueda N., Guglielmotti V., Di Marzo V. Immunohistochemical localization of anabolic and catabolic enzymes for anandamide and other putative endovanilloids in the hippocampus and cerebellar cortex of the mouse brain. Neuroscience. 2008;151:955–968. doi: 10.1016/j.neuroscience.2007.11.047. [DOI] [PubMed] [Google Scholar]
- Davis J.B., Gray J., Gunthorpe M.J., Hatcher J.P., Davey PT Overend P., Harries M.H., Latcham J., Clapham C., Atkinson K., Hughes S.A., Rance K., Grau E., Harper A.J., Pugh P.L., Rogers D.C., Bingham S., Randall A., Sheardown S.A. Vanilloid receptor-1 is essential for inflammatory thermal hyperalgesia. Nature. 2000;405:183–187. doi: 10.1038/35012076. [DOI] [PubMed] [Google Scholar]
- Devane W.A., Hanus L., Breuer A., Pertwee R.G., Stevenson L.A., Griffin G., Gibson D., Mandelbaum A., Etinger A., Mechoulam R. Isolation and structure of a brain constituent that binds to the cannabinoid receptor. Science. 1992;258:1946–1949. doi: 10.1126/science.1470919. [DOI] [PubMed] [Google Scholar]
- Di Marzo V., Petrosino S. Endocannabinoids and the regulation of their levels in health and disease. Curr Opin Lipidol. 2007;18:129–140. doi: 10.1097/MOL.0b013e32803dbdec. [DOI] [PubMed] [Google Scholar]
- Ellington H.C., Cotter M.A., Cameron N.E., Ross R.A. The effect of cannabinoids on capsaicin-evoked calcitonin gene-related peptide (CGRP) release from the isolated paw skin of diabetic and non-diabetic rats. Neuropharmacology. 2002;42:966–975. doi: 10.1016/s0028-3908(02)00040-0. [DOI] [PubMed] [Google Scholar]
- Gamse R., Petsche U., Lembeck F., Jancsò G. Capsaicin applied to peripheral nerve inhibits axoplasmic transport of substance P and somatostatin. Brain Res. 1982;239:447–462. doi: 10.1016/0006-8993(82)90521-2. [DOI] [PubMed] [Google Scholar]
- Guo A., Vulchanova L., Wang J., Li X., Elde R. Immunocytochemical localization of the vanilloid receptor 1 (VR1): Relationship to neuropeptides, the P2X3 purinoceptor and IB4 binding sites. Eur J Neurosci. 1999;11:946–958. doi: 10.1046/j.1460-9568.1999.00503.x. [DOI] [PubMed] [Google Scholar]
- Heyman I., Rang H.P. Depolarizing responses to capsaicin in a subpopulation of rat dorsal root ganglion cells. Neurosci Lett. 1985;56:69–75. doi: 10.1016/0304-3940(85)90442-2. [DOI] [PubMed] [Google Scholar]
- Huang S.M., Bisogno T., Trevisani M., Al-Hayani A., De Petrocellis L., Fezza F., Tognetto M., Petros T.J., Krey J.F., Chu C.J., Miller J.D., Davies S.N., Geppetti P., Walker J.M., Di Marzo V. An endogenous capsaicin-like substance with high potency at recombinant and native vanilloid VR1 receptors. Proc Natl Acad Sci U S A. 2002;99:8400–8405. doi: 10.1073/pnas.122196999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jancsó G., Kiraly E., Jancsó-Gábor A. Pharmacologically induced selective degeneration of chemosensitive primary sensory neurones. Nature. 1977;270:741–743. doi: 10.1038/270741a0. [DOI] [PubMed] [Google Scholar]
- Jancsó G., Király E., Joó F., Such G., Nagy A. Selective degeneration by capsaicin of a subpopulation of primary sensory neurons in the adult rat. Neurosci Lett. 1995;59:209–214. doi: 10.1016/0304-3940(85)90201-0. [DOI] [PubMed] [Google Scholar]
- Ji R.R. Peripheral and central mechanisms of inflammatory pain, with emphasis on MAP kinases. Curr Drug Targets Inflamm Allerg. 2004;3:299–303. doi: 10.2174/1568010043343804. [DOI] [PubMed] [Google Scholar]
- Leung D., Saghatelian A., Simon G.M., Cravatt B.F. Inactivation of N-acyl phosphatidylethanolamine phospholipase D reveals multiple mechanisms for the biosynthesis of endocannabinoids. Biochemistry. 2006;45:4720–4726. doi: 10.1021/bi060163l. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lever I., Robinson M., Cibelli M., Paule C., Santha P., Hunt S.H., Cravatt B.F., Elphick M.R., Nagy I., Rice A.S.C. Localisation of the endocannabinoid-degrading enzyme fatty acid amide hydrolase in rat dorsal root ganglion cells and its regulation in pathological conditions. J Neurosci. 2009;29:3766–3780. doi: 10.1523/JNEUROSCI.4071-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J., Wang L., Harvey-White J., Osei-Hyiaman D., Razdan R., Gong Q., Chan A.C., Zhou Z., Huang B.X., Kim H.Y., Kunos G. A biosynthetic pathway for anandamide. Proc Natl Acad Sci U S A. 2006;103:13345–13350. doi: 10.1073/pnas.0601832103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynn B., Carpenter S.E. Primary afferent units from the hairy skin of the rat hind limb. Brain Res. 1982;238:29–43. doi: 10.1016/0006-8993(82)90768-5. [DOI] [PubMed] [Google Scholar]
- Ma W., Quirion R. Inflammatory mediators modulating the transient receptor potential vanilloid 1 receptor: therapeutic targets to treat inflammatory and neuropathic pain. Expert Opin Ther Targets. 2007;11:307–320. doi: 10.1517/14728222.11.3.307. [DOI] [PubMed] [Google Scholar]
- Maione S., Bisogno T., de Novellis V., Palazzo E., Cristino L., Valenti M., Petrosino S., Guglielmotti V., Rossi F., Di Marzo V. Elevation of endocannabinoid levels in the ventrolateral periaqueductal grey through inhibition of fatty acid amide hydrolase affects descending nociceptive pathways via both cannabinoid receptor type 1 and transient receptor potential vanilloid type-1 receptors. J Pharmacol Exp Ther. 2006;316:969–982. doi: 10.1124/jpet.105.093286. [DOI] [PubMed] [Google Scholar]
- Matsuda L.A., Lolait S.J., Brownstein M.J., Young A.C., Bonner T.I. Structure of a cannabinoid receptor and functional expression of the cloned cDNA. Nature. 1990;346:561–564. doi: 10.1038/346561a0. [DOI] [PubMed] [Google Scholar]
- Michael G.J., Priestley J.V. Differential expression of the mRNA for the vanilloid receptor subtype 1 in cells of the adult rat dorsal root and nodose ganglia and its downregulation by axotomy. J Neurosci. 1999;19:1844–1845. doi: 10.1523/JNEUROSCI.19-05-01844.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgese M.G., Cassano T., Cuomo V., Giuffrida A. Anti-dyskinetic effects of cannabinoids in a rat model of Parkinson's disease: role of CB(1) and TRPV1 receptors. Exp Neurol. 2007;208:110–119. doi: 10.1016/j.expneurol.2007.07.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morishita J., Okamoto Y., Tsuboi K., Ueno M., Sakamoto H., Maekawa N., Ueda N. Regional distribution and age-dependent expression of N-acylphosphatidylethanolamine-hydrolyzing phospholipase D in rat brain. J Neurochem. 2005;94:753–762. doi: 10.1111/j.1471-4159.2005.03234.x. [DOI] [PubMed] [Google Scholar]
- Moriyama T., Higashi T., Togashi K., Iida T., Segi E., Sugimoto Y., Tominaga T., Narumiya S., Tominaga M. Sensitization of TRPV1 by EP1 and IP reveals peripheral nociceptive mechanism of prostaglandins. Mol Pain. 2005;1:3. doi: 10.1186/1744-8069-1-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Movahed P., Jönsson B.A., Birnir B., Wingstrand J.A., Jørgensen T.D., Ermund A., Sterner O., Zygmunt P.M., Högestätt E.D. Endogenous unsaturated C18 N-acylethanolamines are vanilloid receptor (TRPV1) agonists. J Biol Chem. 2005;280:38496–38504. doi: 10.1074/jbc.M507429200. [DOI] [PubMed] [Google Scholar]
- Munro S., Thomas K.L., Abu-Shaar M. Molecular characterization of a peripheral receptor for cannabinoids. Nature. 1993;365:61–65. doi: 10.1038/365061a0. [DOI] [PubMed] [Google Scholar]
- Nagy I., Pabla R., Matesz C., Dray A., Woolf C.J., Urban L. Cobalt uptake enables identification of capsaicin- and bradykinin-sensitive subpopulations of rat dorsal root ganglion cells in vitro. Neuroscience. 1993;56:241–246. doi: 10.1016/0306-4522(93)90576-2. [DOI] [PubMed] [Google Scholar]
- Nagy I., Paule C., White J.P.M. Molecular mechanisms of TRPV1-mediated pain. In: Jancso G., editor. Neuroimmune biology, Vol. 8, neurogenic inflammation in health and disease. Elsevier BV; Amsterdam: 2009. pp. 75–99. [Google Scholar]
- Nagy I., Paule C., White J., Urban L. Functional molecular biology of the transient receptor potential vanilloid type 1 (TRPV1) ion channel. In: Kofalvi A., editor. Cannabinoids and the brain. Springer Verlag; New York: 2008. pp. 101–130. [Google Scholar]
- Nagy I., Rang H.P. Similarities and differences between the responses of rat sensory neurons to noxious heat and capsaicin. J Neurosci. 1999;19:10647–10655. doi: 10.1523/JNEUROSCI.19-24-10647.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagy I., Reeh P., Srubek Tomassy G., Santha P. Control of activity: relationship between excitatory and inhibitory receptors expressed by nociceptors. In: Flor H., Kalso E., Dostrovsky J.O., editors. Proceedings of the 11th World Congress on Pain. IASP Press; Seattle: 2006. pp. 109–110. [Google Scholar]
- Németh J., Helyes Z., Thán M., Jakab B., Pintér E., Szolcsányi J. Concentration-dependent dual effect of anandamide on sensory neuropeptide release from isolated rat tracheae. Neurosci Lett. 2003;336:89–92. doi: 10.1016/s0304-3940(02)01221-1. [DOI] [PubMed] [Google Scholar]
- Okamoto Y., Morishita J., Tsuboi K., Tonai T., Ueda N. Molecular characterization of a phospholipase D generating anandamide and its congeners. J Biol Chem. 2004;279:5298–5305. doi: 10.1074/jbc.M306642200. [DOI] [PubMed] [Google Scholar]
- Olah Z., Szabo T., Karai L., Hough C., Fields R.D., Caudle R.M., Blumberg P.M., Iadarola M.J. Ligand-induced dynamic membrane changes and cell deletion conferred by vanilloid receptor 1. J Biol Chem. 2001;276:11021–11030. doi: 10.1074/jbc.M008392200. [DOI] [PubMed] [Google Scholar]
- Porszasz J., Jancso N. Studies on the action potentials of sensory nerves in animals desensitized with capsaicin. Acta Physiol Acad Sci Hung. 1959;16:299–306. [PubMed] [Google Scholar]
- Premkumar L.S., Ahern G.P. Induction of vanilloid receptor channel activity by protein kinase C. Nature. 2000;408:985–990. doi: 10.1038/35050121. [DOI] [PubMed] [Google Scholar]
- Ross R.A., Coutts A.A., McFarlane S.M., Anavi-Goffer S., Irving A.J., Pertwee R.G., MacEwan D.J., Scott R.H. Actions of cannabinoid receptor ligands on rat cultured sensory neurones: implications for antinociception. Neuropharmacology. 2001;40:221–232. doi: 10.1016/s0028-3908(00)00135-0. [DOI] [PubMed] [Google Scholar]
- Ryberg E., Larsson N., Sjögren S., Hjorth S., Hermansson N.O., Leonova J., Elebring T., Nilsson K., Drmota T., Greasley P.J. The orphan receptor GPR55 is a novel cannabinoid receptor. Br J Pharmacol. 2007;152:1092–1101. doi: 10.1038/sj.bjp.0707460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin J., Cho H., Hwang S.W., Jung J., Shin C.Y., Lee S.Y., Kim S.H., Lee M.G., Choi Y.H., Kim J., Haber N.A., Reichling D.B., Khasar S., Levine J.D., Oh U. Bradykinin-12-lipoxygenase-VR1 signaling pathway for inflammatory hyperalgesia. Proc Natl Acad Sci U S A. 2002;99:10150–10155. doi: 10.1073/pnas.152002699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon G.M., Cravatt B.F. Endocannabinoid biosynthesis proceeding through glycerophospho-N-acyl ethanolamine and a role for alpha/beta-hydrolase 4 in this pathway. J Biol Chem. 2006;281:26465–26472. doi: 10.1074/jbc.M604660200. [DOI] [PubMed] [Google Scholar]
- Simon G.M., Cravatt B.F. Anandamide biosynthesis catalyzed by the phosphodiesterase GDE1 and detection of glycerophospho-N-acyl ethanolamine precursors in mouse brain. J Biol Chem. 2008;283:9341–9349. doi: 10.1074/jbc.M707807200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh Tahim A., Sántha P., Nagy I. Inflammatory mediators convert anandamide into a potent activator of the vanilloid type 1 transient receptor potential receptor in nociceptive primary sensory neurons. Neuroscience. 2005;136:539–548. doi: 10.1016/j.neuroscience.2005.08.005. [DOI] [PubMed] [Google Scholar]
- Smith J.A., Davis C.L., Burgess G.M. Prostaglandin E2-induced sensitization of bradykinin-evoked responses in rat dorsal root ganglion neurons is mediated by cAMP-dependent protein kinase A. Eur J Neurosci. 2000;12:3250–3258. doi: 10.1046/j.1460-9568.2000.00218.x. [DOI] [PubMed] [Google Scholar]
- Sun Y.X., Tsuboi K., Okamoto Y., Tonai T., Murakami M., Kudo I., Ueda N. Biosynthesis of anandamide and N-palmitoylethanolamine by sequential actions of phospholipase A2 and lysophospholipase D. Biochem J. 2004;380:749–756. doi: 10.1042/BJ20040031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tender G.C., Walbridge S., Olah Z., Karai L., Iadarola M., Oldfield E.H., Lonser R.R. Selective ablation of nociceptive neurons for elimination of hyperalgesia and neurogenic inflammation. J Neurosurg. 2005;102:522–525. doi: 10.3171/jns.2005.102.3.0522. [DOI] [PubMed] [Google Scholar]
- Thayer S.A., Perney T.M., Miller R.J. Regulation of calcium homeostasis in sensory neurons by bradykinin. J Neurosci. 1998;18:4089–4097. doi: 10.1523/JNEUROSCI.08-11-04089.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Stelt M., Di Marzo V. Anandamide as an intracellular messenger regulating ion channel activity. Prostaglandins Other Lipid Mediat. 2005;77:111–122. doi: 10.1016/j.prostaglandins.2004.09.007. [DOI] [PubMed] [Google Scholar]
- van der Stelt M., Trevisani M., Vellani V., De Petrocellis L., Schiano Moriello A., Campi B., McNaughton P., Geppetti P., Di Marzo V. Anandamide acts as an intracellular messenger amplifying Ca2+ influx via TRPV1 channels. EMBO J. 2005;24:3026–3037. doi: 10.1038/sj.emboj.7600784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vellani V., Petrosino S., De Petrocellis L., Valenti M., Prandini M., Magherini P.C., McNaughton P.A., Di Marzo V. Functional lipidomics: Calcium-independent activation of endocannabinoid/endovanilloid lipid signalling in sensory neurons by protein kinases C and A and thrombin. Neuropharmacology. 2008;55:1274–1279. doi: 10.1016/j.neuropharm.2008.01.010. [DOI] [PubMed] [Google Scholar]
- Voets T., Droogmans G., Wissenbach U., Janssens A., Flockerzi V., Nilius B. The principle of temperature-dependent gating in cold- and heat-sensitive TRP channels. Nature. 2004;430:748–754. doi: 10.1038/nature02732. [DOI] [PubMed] [Google Scholar]
- Wood J.N., Winter J., James I.F., Rang H.P., Yeats J., Bevan S. Capsaicin-induced ion fluxes in dorsal root ganglion cells in culture. J Neurosci. 1988;8:3208–3220. doi: 10.1523/JNEUROSCI.08-09-03208.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zygmunt P.M., Petersson J., Andersson D.A., Chuang H., Sørgård M., Di Marzo V., Julius D., Högestätt E.D. Vanilloid receptors on sensory nerves mediate the vasodilator action of anandamide. Nature. 1999;400:452–457. doi: 10.1038/22761. [DOI] [PubMed] [Google Scholar]