Abstract
Cardiolipin (CL) is responsible for modulation of activities of various enzymes involved in oxidative phosphorylation. Although energy production decreases in heart failure (HF), regulation of cardiolipin during HF development is unknown. Enzymes involved in cardiac cardiolipin synthesis and remodeling were studied in spontaneously hypertensive HF (SHHF) rats, explanted hearts from human HF patients, and nonfailing Sprague Dawley (SD) rats. The biosynthetic enzymes cytidinediphosphatediacylglycerol synthetase (CDS), phosphatidylglycerolphosphate synthase (PGPS) and cardiolipin synthase (CLS) were investigated. Mitochondrial CDS activity and CDS-1 mRNA increased in HF whereas CDS-2 mRNA in SHHF and humans, not in SD rats, decreased. PGPS activity, but not mRNA, increased in SHHF. CLS activity and mRNA decreased in SHHF, but mRNA was not significantly altered in humans. Cardiolipin remodeling enzymes, monolysocardiolipin acyltransferase (MLCL AT) and tafazzin, showed variable changes during HF. MLCL AT activity increased in SHHF. Tafazzin mRNA decreased in SHHF and human HF, but not in SD rats. The gene expression of acyl-CoA: lysocardiolipin acyltransferase-1, an endoplasmic reticulum MLCL AT, remained unaltered in SHHF rats. The results provide mechanisms whereby both cardiolipin biosynthesis and remodeling are altered during HF. Increases in CDS-1, PGPS, and MLCL AT suggest compensatory mechanisms during the development of HF. Human and SD data imply that similar trends may occur in human HF, but not during nonpathological aging, consistent with previous cardiolipin studies.
Keywords: acyl-Coenzyme A:lysocardiolipin acyltransferase-1, cardiolipin synthase, cytidinediphosphate-diacylglycerol synthetase, human heart failure, monolysocardiolipin acyltransferase, phosphatidylglycerol phosphate synthase, phospholipid enzyme, spontaneously hypertensive heart failure rat, tafazzin
Congestive heart failure (HF) can occur as a consequence of hypertension, ischemic heart disease, valvular disease, or inherited idiopathic cardiomyopathy (1). According to the American Heart Association Statistical Committee 2008 update, 5,300,000 people suffer from this condition, with 660,000 new cases diagnosed each year (2). With the advent of a wide variety of pharmacological and surgical treatments, the hospital discharge rate of HF patients has improved from 1979 to 2005, but deaths due to HF remain as high in 2004 as in 1994 (3). Therefore, to understand the underlying cause of this serious condition and to prevent its development, it is important to identify the pathophysiologic basis of the disease.
Cardiolipin [CL, bis-(1,2-diacyl-sn-glycero-3-phospho)-1′ -3′ -sn-glycerol] is a major phospholipid of the mitochondrial inner membrane required for the activity of a number of key mitochondrial enzymes involved in cellular energy metabolism (4). During the development of HF, it is believed that mitochondrial energy production is unable to meet the requirements of the hypertrophied heart (5–7). Alterations in CL have been observed in human and experimental models of HF, which correlated closely with a loss of mitochondrial respiratory enzyme activity and disease progression in SHHF rats (8). However, the mechanisms responsible for CL alterations during the development of HF have not been previously investigated.
CL is biosynthesized in a series of steps from phosphatidic acid and must then be remodeled into a form which, in the heart, is linoleic acid (18:2) rich. Using electrospray ionization mass spectroscopy, previous studies in SHHF rats and humans have shown a significant decrease in the form of cardiac CL containing four 18:2 side chains, tetralinoleoyl CL ((18:2)4CL). This is the CL molecular species that comprises 70–80% of cardiac CL in the rat or human heart and is required for the activity of cytochrome oxidase (9, 10). The contents of minor CL species containing oleic acid (18:1), arachidonic acid (20:4), and docosahexaenoic acid (22:6) were increased in failing SHHF rat and humans hearts, indicating that there is a switch to an alternate form of CL remodeling during the development of HF. SD rats, which were free of pathology at the ages used, did not exhibit these drastic changes in CL (9).
In the present study, we examined the activity and mRNA expression of enzymes involved in CL biosynthesis and remodeling in the SHHF rat, a well-established congenital model of idiopathic dilated cardiomyopathy (11). The lean (Mccfacp − / −) male SHHF rats used in this study typically exhibit severe hypertension by 5 months of age, which progresses to marked left ventricular hypetrophy by 15 months, and overt HF by 21–24 months of age (8, 12–14). To complement studies in the SHHF rat, left ventricular (LV) tissue explanted from human HF patients and nonfailing Sprague Dawley (SD) rats were also examined. We hypothesized that alterations in CL biosynthetic and remodeling enzymes contribute to the CL alterations seen in the development of HF and may therefore represent targets for pharmacotherapeautic modulation.
MATERIALS AND METHODS
Materials
[14C]Glycerol-3-phosphate, [5-3H]cytidine 5′ -triphophate, [3H]CDP-DAG, [1,3-3H]glycerol, [1-14C]linoleic acid, phosphatidyl[methyl-3H]choline, and [1-14C]linoleoyl CoA were obtained from either Dupont (Mississauga, ON, Canada), or Amersham (Oakville, ON, Canada). Phosphatidyl[14C]glycerol was synthesized from [14C]glycerol-3-phosphate as described previously (15). Lipid standards were obtained from Serdary Research Laboratories (Englewood Cliffs, NJ). Thin layer chromatographic plates (silica gel G, 0.25 mm thickness) were obtained from Fisher Scientific (Winnipeg, MB, Canada). Ecolite scintillant was obtained from ICN Biochemicals (Montreal, QC, Canada). All other chemicals were certified ACS grade and obtained either from Fisher Scientific or Sigma Chemical Co. (St Louis, MO).
Animal models
Male lean (Mccfacp − / −) SHHF rats were obtained from the breeding colony maintained by S. A. M. at the University of Colorado at Boulder. SD rats were purchased from Harlan (Indianapolis, IN). All animals were treated according to the guidelines established by the University of Colorado Institutional Animal Care and Use Committee and conform to the Guide for the Care and Use of Laboratory Animals published by the National Institutes of Health.
HF Patients
Left ventricular (LV) tissues were obtained from explanted hearts of patients diagnosed with idiopathic dilated cardiomyopathy (IDC; 52 ± 3 years; n = 4 male, 6 female, mean ejection fraction 17 ± 2%). Nonfailing (NF) LV samples were obtained from patients who had no history of cardiac or pulmonary disease (46 ± 5 years; n = 4 male, 6 female; mean ejection fraction 48 ± 2%). All hearts were dissected and flash frozen in a timely manner to assure consistency between samples. There were no significant differences in mean age between the IDC and NF control groups. Nonfailing and failing hearts were obtained via an Institutional review board approved protocol maintained by the University of Colorado Health Sciences Center Cardiac Tissue Bank. Hearts donated for research purposes were obtained under written consent from family members of organ donors whose hearts could not be used for transplantation or by direct written consent from end-stage heart failure patients undergoing cardiac transplantation.
Echocardiographic measurements and assessment of general characteristics
Transthoracic echocardiography was performed on SHHF rats at the ages of 2 months (hypertensive), 15 months (compensated hypertrophy), and 22 months (terminal HF) under inhaled isoflurane anesthesia (5% initial, 2% maintenance) using a 12 MHz pediatric transducer connected to a Hewlett Packard Sonos 5500 Ultrasound system. Short axis M-mode echocardiograms on the left ventricle were obtained for measurement of LV internal diameters at diastole (LVIDd) and systole (LVIDs), LV fractional shortening (FS), ejection fraction (EF), anterior wall thickness (AWT) and posterior wall thickness (PWT) as described previously (12). After echocardiographic measurements, SHHF rats were euthanized and the heart, liver, kidney and lungs were surgically removed and weighed to check the signs of hypertrophy and HF.
Preparation of subcellular fractions and assay of enzyme activities
Subcellular fractions were prepared from myocardial tissue as previously described (8, 16). All isolation procedures were performed at 4°C. A 10% LV homogenate was prepared in buffer containing 0.25 M sucrose, 10 mM Tris-HCl, 0.145 M NaCl (pH 7.4) followed by homogenization with 50 strokes of a tight fitting Dounce A homogenizer. The homogenate was centrifuged at 1,000 g for 10 min, and the resulting supernatant centrifuged at 12,000 g for 15 min. The resulting pellet was resuspended in 0.5 ml of homogenizing buffer by a tight fitting Dounce A homogenizer and used as the source of mitochondrial fraction for assay of mitochondrial enzyme activities. The supernatant was centrifuged at 100,000 g for 1 h. The resulting pellet was resuspended in 0.5 ml of homogenizing buffer by a tight fitting Dounce A homogenizer and used as the source of endoplasmic reticulum fraction to assay cytidinediphosphate-diacylglycerol synthetase (CDS) activity. Phosphatidylglycerophosphate synthase (PGPS) and CL synthase (CLS) activities were determined as previously described (15, 17–19). CDS activity was determined in mitochondrial fractions in accordance with the method given by Rusnak et al. (18). In other experiments, 20 μg of mitochondrial protein was assayed for monolysocardiolipin (MLCL) and phosphatidic acid (PA) and phosphatidylglycerol (PG) production in the presence of 70,000 dpm/nmol [1-14C]linoleneoyl CoA as described previously (19–22). The reaction was performed at 37°C for 40 min following which the [14C]MLCL and [14C]PA+[14C]PG were isolated via thin-layer chromatography.
RNA isolation and real time PCR assay
Standard RNA was prepared from LV tissue samples by the T7 polymerase method as previously described by Young et al. (23). cDNA standards for tafazzin (TAZ) were derived from cDNA clones containing the appropriate assay specific sequences obtained from Invitrogen Corporation (clone ID 7387882 and 7312046, respectively). The absolute quantification for CLS was determined by the input copy number of the transcript of interest with the PCR signal to a standard curve. The same total sample RNA (300 ng) was used for each quantitative RT-PCR reaction and reaction conditions were as previously described (24–26). To quantify PGPS and CDS-1, SYBR Green 1 was used due to the unavailability of specific probes. Since SYBR Green 1 can detect specific and nonspecific PCR products as it can bind to double standard DNA in a sequence independent manner (27), a melting curve analysis was performed at the end of each experiment to confirm the absence of primer-dimers in specific PCR products. Primer sequences are shown in Table 1. Each real time PCR procedure was carried out in duplicates, and at least three separate experiments were performed. All PCR results used mRNA copy number normalized to either the youngest or the nonfailing group to facilitate clear comparisons between species.
TABLE 1.
Sequence of primers used for real time PCR
| Target gene | Species | Accession number | Forward primer | Reverse primer | Probe | Product length (bp) |
|---|---|---|---|---|---|---|
| Phoshatidylglycerolphosphate synthase (PGPS) | rat | BG663105 | 5 -AGA GGT GAA CGG CTT CTT TGG -3 | 5 -CAC TGT AGA ACT GTC GCT CAA TGTG 3 | SYBR Green 1 | 69 |
| CDP-diacylglycerol synthase 1 (CDS-1) | rat | NM_031242 | 5 -GGA GAT TCC GAT GTT CCT GA -3 | 5 -CCA CGA ATC CAC CAG TTC TT- 3 | SYBR Green 1 | 110 |
| CDP-diacylglycerol synthase 1 (CDS-2) | rat | NM_053643.1 | Applied Biosystems Taqman Gene Expression Assay: Assay Location-1057 | 113 | ||
| Cardiolipin synthase(CLS, CRD-1) | rat | NM_001014258.1 | 5 -TGG ATG GAT TTA TTG CTC GAA A -3 | 5 -TGG GAC TGG AAT AAG ATC TGC AT -3 | 5 -FAM-CAG CTT TGG GAA GTG CTC TTG ATC CAC T-TAMRA -3 | 134 |
| Taffazin (TAZ) | rat | NM_001025748.1 | Applied Biosystems Taqman Gene Expression Assay:Assay Location-618 | 106 | ||
| Acyl-CoA:lysocardiolipin acyltransferase-1 (ALCAT) | rat | CO403431 | 5 - CAG AAG GAA CTG ACC TCA CAG AAA A -3 | 5 - GTG GTT CTT GGG TGT AAT ACA TAC TCA-3 | 5 - FAM-TTC TCA GCA AAA TCG-NFG-3 | 123 |
| CDP-diacylglycerol synthase 1 (CDS-2) | human | NM003818.2 | Applied Biosystems Taqman Gene Expression Assay:Assay Location-647 | 83 | ||
| Cardiolipin synthase (CLS) | human | NT011387 | 5 -TGG ATG GAT TTA TTG CTC GAA A -3 | 5 -TGG AAC TGG AAT AAG ATC TGC AT -3 | 5 -FAM-CAG CTT TGG GAA GTG CTC TTG ATC CAC T-TAMRA -3 | 136 |
| Taffazin (TAZ) | human | NM181311.1 | Applied Biosystems Taqman Gene Expression Assay:Assay Location-539 | 87 | ||
Statistical analysis
Data are expressed as means ± SEM. The differences between two groups were evaluated by Student’s t-test. The data from more than two groups were evaluated by one-way ANOVA followed by the Tukey test. Values showing P < 0.05 were considered statistically significant unless otherwise indicated.
RESULTS
Echocardiographic analysis and general characteristics of SHHF rats
Between the ages of 2 and 15 months, echocardiographic data from SHHF rats indicated significant increases in LV chamber diameter and wall thickness with only minor decreases in EF or FS, consistent with the development of compensated LV hypertrophy resulting from chronic hypertension (Table 2). From the age of 15 months to 22 months, hearts exhibited pronounced LV chamber dilatation, anterior wall thickening, and contractile dysfunction. PWT decreased significantly from 15 months to terminal HF. All of these changes are consistent with the late stages of disease in this model.
TABLE 2.
Echocardiographic parameters of 2-, 15- and 22-month-old SHHF rats
| Parameter | 2 months | 15 months | 22 months |
|---|---|---|---|
| LVIDd (mm) | 7.2 ± 0.1 | 8.0 ± 0.3a | 10.4 ± 0.4bd |
| LVIDs (mm) | 4.7 ± 0.2 | 5.3 ± 0.3a | 8.2 ± 0.3b |
| FS (%) | 36 ± 1.0 | 33 ± 2.0 | 21 ± 2.0b |
| EF (%) | 74 ± 1.0 | 70 ± 2.0 | 51 ± 3.0b |
| AWTd (mm) | 1.4 ± 0.1 | 2.0 ± 0.1b | 2.6 ± 0.2bc |
| PWTd (mm) | 1.5 ± 0.1 | 2.3 ± 0.2b | 1.9 ± 0.1ac |
Values are mean ± SEM. AWTd, left ventricular anterior wall thickness in diastole; EF, ejection fraction; FS, fractional shortening; LVIDd, left ventricular internal diameter in diastole; LVIDs, left ventricular internal diameter in systole; PWTd, left ventricular posterior wall thickness in diastole; SHHF, spontaneously hypertensive heart failure.
P < 0.05 vs. 2 month.
P < 0.001 vs. 2 month.
P < 0.01 vs. 15 month
< 0.001 vs. 15 month.
Cardiac hypertrophy was also reflected by increased heart weight, LV, right ventricle, and heart-to-body-weight ratios at 15 and 22 months of age compared with 2-month-old animals (Table 3). The presence of pulmonary edema in 15- and 22-month-old animals was reflected by 24% and 120% increases in the wet weight of lungs, respectively. Significant increases in left and right kidney weights at 15 and 22 months reflect a severely compromised kidney function in these animals. Likewise, the liver weight was increased by 39% and 65% in 15- and 22-month-old SHHF rats, respectively.
TABLE 3.
General characteristics of 2-, 15- and 22-month-old SHHF rats
| Parameter | 2 months | 15 months | 22 months |
|---|---|---|---|
| n | 12 | 6 | 4 |
| BW (g) | 240 ± 4.7 | 396 ± 10.5c | 398 ± 12.0c |
| HW (mg) | 916 ± 85 | 1545 ± 37c | 2442 ± 164c |
| HW/BW (mg/g × 100) | 382 ± 18.1 | 390 ± 3.5 | 613 ± 13.6cf |
| LVW (mg) | 447 ± 15.8 | 750 ± 31.5c | 942 ± 28.5ce |
| RVW (mg) | 210 ± 14.5 | 286 ± 21.0a | 450 ± 29.1 cf |
| Liver wt (g) | 10.7 ± 0.24 | 14.85 ± 0.48c | 17.69 ± 1.47 ce |
| Right kidney wt (g) | 0.91 ± 0.02 | 1.41 ± 0.03 | 2.35 ± 0.48cd |
| Left kidney wt (g) | 0.92 ± 0.03 | 1.39 ± 0.02 | 2.40 ± 0.47 ce |
| Lung wt (g) | 1.50 ± 0.05 | 1.87 ± 0.04a | 3.32 ± 0.19 cf |
Values are mean ± SEM; n = number of rats. BW, body weight; HW, heart weight; HW/BW, heart weight-to-body weight ratio; LVW, left ventricular weight; RVW, right ventricular weight; SHHF, spontaneously hypertensive heart failure.
P < 0.05 vs. 2 month.
P < 0.01 vs. 2 month.
P < 0.001 vs. 2 month.
P < 0.5 vs. 15 month.
P < 0.01 vs. 15 month.
P < 0.001 vs. 15 month.
Alterations in enzyme activities and products involved in CL biosynthesis
Previous studies have shown a decrease in the total amount of CL in interfibrillar mitochondria during the development of HF in SHHF rat hearts and in LV tissue in human HF patients (8). To investigate the biochemical alterations in CL biosynthesis during the development of HF, three enzymes involved in the biosynthetic pathway of CL were studied: CDS, PGPS, and CLS. All studies were carried out with LV tissue as SHHF rats exhibit selective LV contractile dysfunction (28).
CDS.
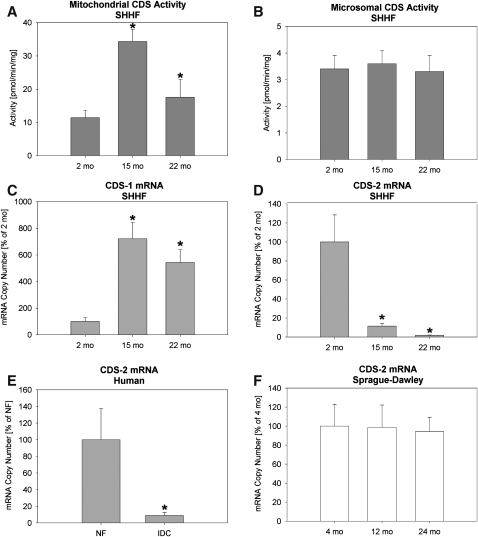
The activity of CDS, a rate limiting enzyme in CL biosynthesis (15, 19, 29), was increased significantly at 15 and 22 months in comparison to 2-month-old SHHF rats (Fig. 1A), while CDS activity in the endoplasmic reticulum-rich microsomal fraction remained unaltered in the progression to HF (Fig. 1B). There are two known genes coding for CDS: CDS-1 and CDS-2. In HF, CDS-1 mRNA levels mimic mitochondrial activity (Fig. 1C), but CDS-2 mRNA levels fall in both SHHF rats and humans (Figs. 1D, 1E) but are unaffected in SD rats (Fig. 1F). Because of limited availability of human heart tissue, no attempt was made to check the status of CDS-1 mRNA in humans. Northern blot studies performed by Lykidis et al. (30) have shown no expression of CDS-1 mRNA in human heart. However, another study indicated the presence of CDS-1 mRNA in human heart (31). These differences may have been due to cross-hybridization of CDS-1 probes with CDS-2 mRNA (32).
Fig. 1.
Cytidinediphosphatediacylglycerol synthetase (CDS) activity and real time PCR from LV tissue. (A) Activity in mitochondrial fraction from SHHF rats; (B) Activity in microsomal endoplasmic reticulum fraction from SHHF rats; (C) CDS-1 mRNA from SHHF rats; (D) CDS-2 mRNA from SHHF rats; (E) CDS-2 mRNA in human nonfailing (NF) or idiopathic dilated cardiomyopathy (IDC); and (F) CDS-2 mRNA from Sprague Dawley rats. All PCR data is expressed as mean ± SEM with the young rats or NF humans as 100%. N = 4 (rats) and 8 (humans) per group. *P < 0.05 vs. 2 month SHHF rats or NF humans. SHHF, spontaneously hypertensive heart failure.
PGPS.
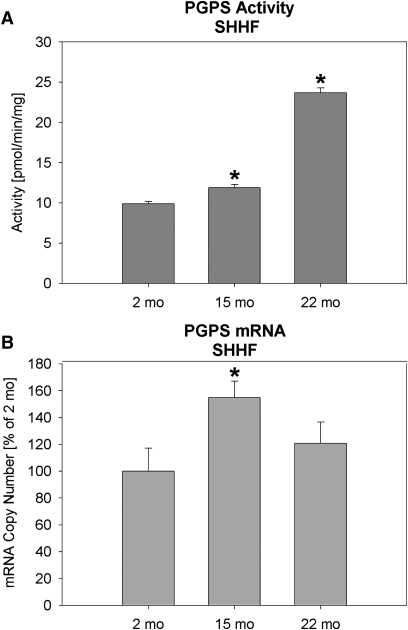
The activity of PGPS, the committed step in CL biosynthesis, in the mitochondrial fraction was increased by 21% and 98% at 15 and 22 months of age in comparison to 2-month-old SHHF rats, respectively (Fig. 2A). The mRNA expression pattern was somewhat different, increasing by 15 months of age in the SHHF rat but decreasing again at 22 months (Fig. 2B). This difference could indicate posttranslational control of PGPS activity. The amount of PA+PG formed, which is an indication of the activity of the enzyme activities up to the CLS step (see Fig. 5), was reduced by 45% and 48% in 15- and 22-months-old SHHF rats, respectively (Table 4).
Fig. 2.
Phosphatidylglycerolphosphate synthase (PGPS) activity and real time PCR from LV tissue. (A) Activity from SHHF rats and (B) mRNA from SHHF rats. PCR data is expressed as mean ± SEM with the young rats as 100%. N = 4, *P < 0.05 vs. 2-month SHHF rats. SHHF, spontaneously hypertensive heart failure.
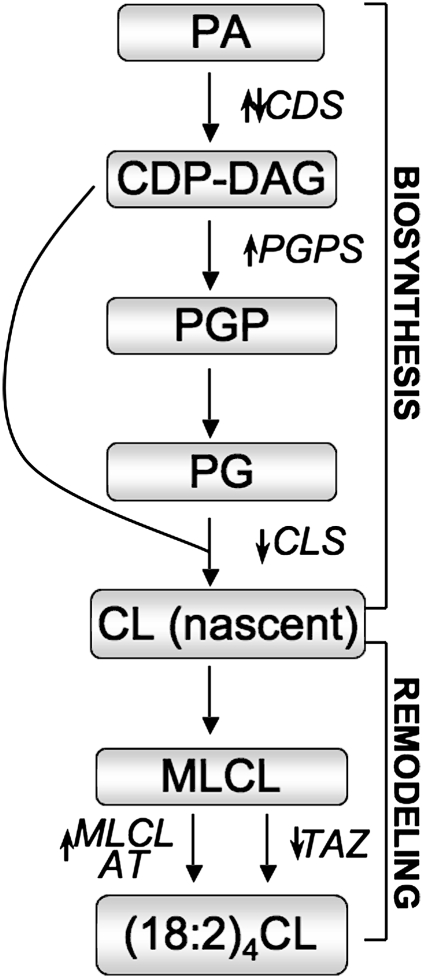
Fig. 5.
Schematic showing changes in CL biosynthesis and remodeling enzymes in heart failure. Small arrows indicate general changes to the enzyme in HF in the present study. CDP-DAG, cytidinediphosphate diacylglycerol; CDS, cytidinediphosphatediacylglycerol synthetase ;CL, cardiolipin; (18:2)4CL, tetralinoleoyl CL; CLS, cardiolipin synthase; MLCL, monolysocardiolipin; MLCL AT, MLCL acyl transferase; PA phosphatidic acid; PG, phosphatidyl glycerol; PGP, phosphatidylglycerolphosphate ; PGPS, PGP synthase ;TAZ, tafazzin.
TABLE 4.
Alterations in mitochondrial products formed in SHHF rat heart during de novo synthesis and remodeling of cardiolipin
| Specific activity (pmol/min/mg) | 2 months | 5 months | 22 months |
|---|---|---|---|
| PA + PG formed | 78.5 ± 5.2 | 43.0 ± 1.3a | 41.1 ± 4.3a |
| MLCL formed | 17.3 ± 1.2 | 8.8 ± 0.3b | 11.0 ± 0.5a |
Values are mean ± SEM. MLCL, monolysocardiolipin; PA, phosphatidic acid; PG, phosphatidylglycerol; SHHF, spontaneously hypertensive heart failure.
P < 0.01 vs. 2 month.
P < 0.001 vs. 2 month.
CLS.
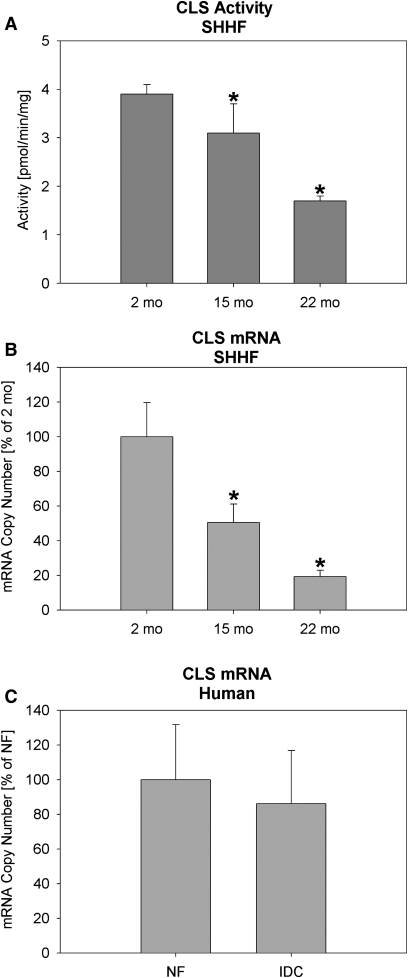
The activity of CLS in the mitochondrial fraction was decreased by 20% at 15 months of age and 57% by 22 months of age (Fig. 3A). Likewise, the gene expression of CLS was decreased by 50% and 80% in 15- and 22-months-old SHHF rats, respectively (Fig. 3B). Human mRNA, however, showed no significant alterations in CLS mRNA (Fig. 3C).
Fig. 3.
CL synthase (CLS) activity and real time PCR from LV tissue. (A) Activity from SHHF rats; (B) CLS mRNA from SHHF rats; and (C) CLS mRNA in human nonfailing (NF) or idiopathic dilated cardiomyopathy (IDC). All PCR data is expressed as mean ± SEM with the young rats or NF humans as 100%. N = 4 (rats) and 8 (humans) per group. *P < 0.05 vs. 2-month SHHF rats or NF humans. SHHF, spontaneously hypertensive heart failure.
Alterations in CL remodeling
There are major alterations in CL composition in SHHF rats and humans during HF (8, 9) suggesting an alteration of CL remodeling.
MLCL AT and acyl-CoA: lysocardiolipin acyltransferase-1 (ALCAT1)
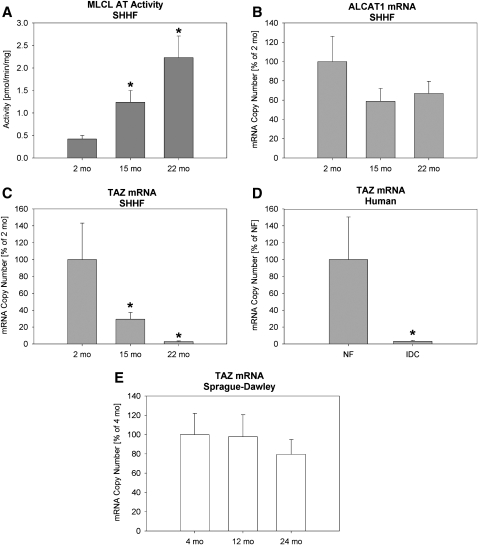
In SHHF rats, the rate of formation of MLCL, an intermediate product in the remodeling of CL (20), was reduced by 50% in 15-months-old SHHF and 36% in 22-months-old SHHF rats compared with 2- months-old animals (Table 4). MLCL AT activity, which can place an acyl group on the MLCL, was increased 3- and 5-fold in 15- and 22-months-old SHHF rats, respectively, compared with 2-months-old rats (Fig. 4A). On the other hand, the gene expression of ALCAT1, an endoplasmic reticulum (ER) MLCL AT (33), remained unaltered at different age groups of SHHF rats (Fig. 4B).
Fig. 4.
CL remodeling enzyme activity and real time PCR from LV tissue. (A) Activity of monolysocardiolipin acyltransferase (MLCL AT) in SHHF rats; (B) Acyl-CoA: lysocardiolipin acyltransferase 1 (ALCAT1) mRNA from SHHF rats; (C) Tafazzin (TAZ) mRNA from SHHF rats; (D) TAZ mRNA in human nonfailing (NF) or idiopathic dilated cardiomyopathy (IDC); and (E) TAZ mRNA from Sprague Dawley rats. All PCR data is expressed as mean ± SEM with the young rats or NF humans as 100%. N = 4 (rats) and 8 (humans) per group. *P 0.05 vs. 2-month SHHF rats or NF humans. SHHF, spontaneously hypertensive heart failure.
TAZ.
Gene expression of tafazzin (TAZ), which encodes a CL transacylase involved in CL remodeling (34), was significantly reduced in 15- and 22-months-old SHHF rats compared with 2-months-old animals (Fig. 4C). It also was decreased in failing human hearts (Fig. 4D). This is a novel finding in that it is the first indication that the CL changes seen in HF may be influenced by the same gene altered in Barth Syndrome. No significant changes in TAZ expression were seen in nonfailing hearts from SD rats (Fig. 4E).
DISCUSSION
In the present study, we observed discordant regulation of CL biosynthetic and remodeling enzymes at the activity and gene expression level in hypertensive, preHF, and HF in SHHF rats. The results are summarized in the pathway shown in Fig. 5. In mammalian heart, de novo biosynthesis of CL begins in the mitochondrial membrane initially by the conversion of PA to cytidine-5′ -diphosphate-1,2-diacylglycerol (CDP-DAG) catalyzed by CDS. Pulse chase labeling studies in the isolated rat heart indicated that CDS catalyzes a rate limiting step in CL de novo biosynthesis (19). Since CDS enzyme activity is localized to both the endoplasmic reticulum and mitochondria, we measured its activity in both these subcellular fractions in the hearts of SHHF rats. Interestingly, the activity of cardiac CDS was increased in the mitochondrial fraction during HF development in SHHF rats. In contrast, there were no alterations in CDS enzyme activity in cardiac microsomal fractions prepared from these animals. A previous study in rat liver concluded that CDS enzyme activity may be regulated differently in endoplasmic reticulum and mitochondria (35). The current study supports that observation and extends alternate regulation of CDS enzyme activity to the heart. Fig. 1 shows that CDS-1 and CDS-2 mRNA were inversely altered during HF development in SHHF rats, suggesting an interplay between these two transcripts. CDS-1, which mimics the mitochondrial CDS activity, may be the larger influence on the activity, although a previous study claimed that CDS-1 did not seem to encode the mitochondrial specific isozyme in COS-7 cells (30). It has been shown previously that an increase in CDS activity was associated with increased secretion of the pro-inflammatory mediators, tumor necrosis factor- α, and interleukin-6, from ECV304 cells upon stimulation with interleukin-1β (36). An increase in tumor necrosis factor- α and interleukin-6 content has been observed in SHHF rats at 15 months of age (12), indicating that increased CDS activity as observed in the present study may be related to the increased secretion of these inflammatory mediators, which are known to cause contractile dysfunction (37). However, further studies are needed to find a direct relationship between contractile dysfunction, CDS activity, and release of pro-inflammatory mediators at compensated and terminal stages of HF in SHHF rats.
The committed step of CL biosynthesis is the condensation of CDP-DAG with sn-glycerol-3-phosphate to form PG phosphate catalyzed by PGPS (38). Both PGPS enzyme activity and its mRNA expression were increased at 15 months in SHHF rats compared with 2-months-old animals. PGPS activity was further increased in 22-months-old SHHF rats. The difference in the pattern of PGPS activity and expression may be related to possible post-translational modification of the enzyme in HF. Due to the unavailability of commercially available immunoprecipitating antibodies for PGPS, the translational level of this transcript was not measured in the present study. A previous study showed, using an epitope-tagged Pgs1p, that PGPS enzyme activity could be modified by phosphorylation in Saccharomyces cerevisiae (39). The possibility of post-translational alteration of PGPS during the development of HF will form the basis for a future study. The final step of mammalian de novo CL biosynthesis involves the conversion of CDP-DAG and PG to CL and is catalyzed by CLS (29, 40). CLS enzyme activity and its mRNA expression were decreased during the development of HF in SHHF rats but not in humans. The decreased enzyme activity of CLS in mitochondria as well as attenuated gene expression of CLS may be responsible for the observed decrease in CL content at 15 and 22 months of HF in SHHF rats. The disparity of the human data from the rat data may be due to large variations in the human subjects, genetic differences between rats and humans, or pharmaceutical interventions that these patients underwent before transplant.
The present study indicates that although CDS and PGPS enzyme activities were increased, the ability to sustain substrates for CL de novo biosynthesis during development of HF in SHHF rats was still compromised, at least in interfibrillar mitochondria. This was confirmed by the decreased amount of [1-14C]linoleate incorporated into PA+PG formed in mitochondria at 15 and 22 months in SHHF rats compared with 2-months-old animals. The decreased availability of CTP, which is known to regulate the CL biosynthesis in isolated heart (40), may account for such a decrease in PA+PG in compensated and terminal stages of HF because CTP is required for CDS activity in vivo. The increased in vitro CDS and PGPS enzyme activities observed in the current study may be a compensatory mechanism for the observed decreased activity and mRNA expression of CLS during the development of HF.
A significant loss of cardiac tetralinoleoyl CL has been demonstrated in SHHF rats and in patients with idiopathic dilated cardiomyopathy (8). Newly synthesized CL undergoes remodeling to form 18:2- rich CL (20, 22, 38). Previous studies have demonstrated that linoleoyl enrichment of CL is achieved by a CL transacylase encoded by the Taz gene (41) or by transfer of linoleoyl-CoA to MLCL by MLCL AT (29, 34). However, we observed a significant increase in MLCL AT activity, which may be a compensatory response to a decrease in CL remodeling by the Taz pathway. Indeed, we observed a remarkable over 90% decrease in TAZ mRNA expression during the development of HF in SHHF rats and humans. Nonfunctional mutations in the TAZ gene lead to decreased L4CL content (32, 42), destabilization of mitochondrial supercomplexes (43), mitochondrial dysfunction, and energy production (44), and are responsible for the severe X-linked cardioskeletal myopathy known as Barth Syndrome (41). In contrast to TAZ, the gene expression of ALCAT1, an enzyme which is capable of the in vitro resynthesis of CL from exogenous MLCL in the ER (33), remained unaltered in all age groups of SHHF rats. CL is synthesized on the inner side of the inner mitochondrial membrane and is localized to mitochondria (19). The physiological significance of ALCAT1 in the ER for the resynthesis of CL remains to be determined. Newly synthesized CL also undergoes hydrolytic degradation via deacylation to MLCL in mammalian tissues via phospholipase A2 (PLA2) (45). Alterations in Ca2+-independent PLA2 (iPLA2) have been shown to contribute to diminished cardiac function in failing hearts due to myocardial infarction (46). Although a recent study by Mancuso et al. (47) has demonstrated that genetic ablation of iPLA2 γ causes a significant decrease in tetra 18:2 CL with abnormal mitochondrial function, no attempt was made to check the status of different PLA2 in SHHF rats. Further studies targeting specifically at the catabolism of CL are needed to address this complex issue in SHHF rats. Nevertheless, this study is the first to suggest that alterations in functional Taz gene expression may contribute to HF in the absence of Barth Syndrome. Factors responsible for regulating Taz gene expression in the healthy and diseased heart are unknown and are currently being investigated in our laboratories.
To our knowledge, this is the first study showing the pattern of alterations in various enzymes involved in CL biosynthesis and remodeling in HF. Since the SHHF rat model has been shown to exhibit common signs of human HF (8, 12), this study provides the preeminent characterization between compensated and later stages of HF. Though the data in this study on humans is limited, it gives an insight into the similarities (and differences in the case of CLS) present between humans and SHHF rats in failure. The SD rat data also serves to distinguish between HF and nonpathological aging, which is an important distinction, especially with CL, which for years was presumed to decrease with age and has now been shown to have mostly pathology-related alterations (8).
Acknowledgments
H. K. Saini-Chohan is a post-doctoral fellow of the CIHR/HSFC-IMPACT Strategic Training Program and the Heart and Stroke Foundation of Canada. G. M. Hatch is a Canada Research Chair in Molecular CL Metabolism.
The authors would like to acknowledge the assistance of Martin E. Young, Ph.D. (Bayor College of Medicine, Houston, TX) in the development of our PCR assays and Michael R. Bristow, M.D., Ph.D., for providing human tissue.
Footnotes
Abbreviations:
- CDS
- cytidinediphosphatediacylglycerol synthetase
- CL
- cardiolipin
- CLS
- cardiolipin synthase
- HF
- heart failure
- MLCL AT
- monolysocardiolipin acyltransferase
- PGPS
- phosphatidylglycerolphosphate synthase
- SD
- Sprague Dawley
- SHHF
- spontaneously hypertensive heart failure
- TAZ
- tafazzin
This work was supported by grants from the Heart and Stroke Foundation of Manitoba and Canadian Institutes of Health Research (G.M.H.), the National Institutes of Health (G.C.S. and D.L.B), the American Heart Association (A.J.C), and the Barth Syndrome Foundation (G.C.S).
REFERENCES
- 1.Massie B. M., Shah N. B. 1997. Evolving trends in the epidemiologic factors of heart failure: rationale for preventive strategies and comprehensive disease management. Am. Heart J. 133: 703–712 [DOI] [PubMed] [Google Scholar]
- 2.Rosamond W., Flegal K., Furie K., Go A., Greenlund K., Haase N., Hailpern S. M., Ho M., Howard V., Kissela B., et al. 2008. Heart disease and stroke statistics–2008 update. (Report from the American Heart Association Statistics Committee and Stroke Statistics Subcommittee.) Circulation. 117: e25–e146 [DOI] [PubMed] [Google Scholar]
- 3.Burt C. W., Schappert S. M. 2004. Ambulatory care visits to physician offices, hospital outpatient departments, and emergency departments: United States, 1999–2000. Vital Health Stat. 13. 157: 1–70 [PubMed] [Google Scholar]
- 4.Chicco A. J., Sparagna G. C. 2007. Role of cardiolipin alterations in mitochondrial dysfunction and disease. Am. J. Physiol. Cell Physiol. 292: C33–C44 [DOI] [PubMed] [Google Scholar]
- 5.Ventura-Clapier R., Garnier A., Veksler V. 2004. Energy metabolism in heart failure. J. Physiol. 555: 1–13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ingwall J. S., Weiss R. G. 2004. Is the failing heart energy starved? On using chemical energy to support cardiac function. Circ. Res. 95: 135–145 [DOI] [PubMed] [Google Scholar]
- 7.Neubauer S.2007. The failing heart–an engine out of fuel. N. Engl. J. Med. 356: 1140–1151 [DOI] [PubMed] [Google Scholar]
- 8.Sparagna G. C., Chicco A. J., Murphy R. C., Bristow M. R., Johnson C. A., Rees M. L., Maxey M. L., McCune S. A., Moore R. L. 2007. Loss of cardiac tetralinoleoyl cardiolipin in human and experimental heart failure. J. Lipid Res. 48: 1559–1570 [DOI] [PubMed] [Google Scholar]
- 9.Sparagna G. C., Johnson C. A., McCune S. A., Moore R. L., Murphy R. C. 2005. Quantitation of cardiolipin molecular species in spontaneously hypertensive heart failure rats using electrospray ionization mass spectrometry. J. Lipid Res. 46: 1196–1204 [DOI] [PubMed] [Google Scholar]
- 10.Schlame M., Ren M., Xu Y., Greenberg M. L., Haller I. 2005. Molecular symmetry in mitochondrial cardiolipins. Chem. Phys. Lipids. 138: 38–49 [DOI] [PubMed] [Google Scholar]
- 11.McCune S. A., Park S., Radin M. J., Jurin R. R. 1995. SHHF/Mcc-facp rat model: a genetic model of congestive heart failure. InMechanisms of Heart Failure Singal P. K., Dixon I. M. C., Beamish R. E., Dhalla N. S., editors Kluwer Acad, Norwell, MA: 91–106 [Google Scholar]
- 12.Heyen J. R., Blasi E. R., Nikula K., Rocha R., Daust H. A., Frierdich G., Van Vleet J. F., De Ciechi P., McMahon E. G., Rudolph A. E. 2002. Structural, functional, and molecular characterization of the SHHF model of heart failure. Am. J. Physiol. Heart Circ. Physiol. 283: H1775–H1784 [DOI] [PubMed] [Google Scholar]
- 13.Chicco A. J., Sparagna G. C., McCune S. A., Johnson C. A., Murphy R. C., Bolden D. A., Rees M. L., Gardner R. T., Moore R. L. 2008. Linoleate-rich high-fat diet decreases mortality in hypertensive heart failure rats compared with lard and low-fat diets. Hypertension. 52: 549–555 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Emter C. A., McCune S. A., Sparagna G. C., Radin M. J., Moore R. L. 2005. Low-intensity exercise training delays onset of decompensated heart failure in spontaneously hypertensive heart failure rats. Am. J. Physiol. Heart Circ. Physiol. 289: H2030–H2038 [DOI] [PubMed] [Google Scholar]
- 15.Hatch G. M., McClarty G. 1996. Regulation of cardiolipin biosynthesis in H9c2 cardiac myoblasts by cytidine 5′ -triphosphate. J. Biol. Chem. 271: 25810–25816 [DOI] [PubMed] [Google Scholar]
- 16.Palmer J. W., Tandler B., Hoppel C. L. 1977. Biochemical properties of subsarcolemmal and interfibrillar mitochondria isolated from rat cardiac muscle. J. Biol. Chem. 252: 8731–8739 [PubMed] [Google Scholar]
- 17.Hatch G. M., Cao S. G., Angel A. 1995. Decrease in cardiac phosphatidylglycerol in streptozotocin-induced diabetic rats does not affect cardiolipin biosynthesis: evidence for distinct pools of phosphatidylglycerol in the heart. Biochem. J. 306: 759–764 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rusnak A., Mangat R., Xu F., McClarty G., Hatch G. M. 1997. Cardiolipin remodeling in a Chinese hamster lung fibroblast cell line deficient in oxidative energy production. J. Bioenerg. Biomembr. 29: 291–298 [DOI] [PubMed] [Google Scholar]
- 19.Hatch G. M.1994. Cardiolipin biosynthesis in the isolated heart. Biochem. J. 297: 201–208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ma B. J., Taylor W. A., Dolinsky V. W., Hatch G. M. 1999. Acylation of monolysocardiolipin in rat heart. J. Lipid Res. 40: 1837–1845 [PubMed] [Google Scholar]
- 21.Taylor W. A., Hatch G. M. 2003. Purification and characterization of monolysocardiolipin acyltransferase from pig liver mitochondria. J. Biol. Chem. 278: 12716–12721 [DOI] [PubMed] [Google Scholar]
- 22.Xu Y., Kelley R. I., Blanck T. J., Schlame M. 2003. Remodeling of cardiolipin by phospholipid transacylation. J. Biol. Chem. 278: 51380–51385 [DOI] [PubMed] [Google Scholar]
- 23.Young M. E., Patil S., Ying J., Depre C., Ahuja H. S., Shipley G. L., Stepkowski S. M., Davies P. J., Taegtmeyer H. 2001. Uncoupling protein 3 transcription is regulated by peroxisome proliferator-activated receptor (alpha) in the adult rodent heart. FASEB J. 15: 833–845 [DOI] [PubMed] [Google Scholar]
- 24.Gibson U. E., Heid C. A., Williams P. M. 1996. A novel method for real time quantitative RT-PCR. Genome Res. 6: 995–1001 [DOI] [PubMed] [Google Scholar]
- 25.Heid C. A., Stevens J., Livak K. J., Williams P. M. 1996. Real time quantitative PCR. Genome Res. 6: 986–994 [DOI] [PubMed] [Google Scholar]
- 26.Zhang F., Pan T., Nielsen L. D., Mason R. J. 2004. Lipogenesis in fetal rat lung: importance of C/EBPalpha, SREBP-1c, and stearoyl-CoA desaturase. Am. J. Respir. Cell Mol. Biol. 30: 174–183 [DOI] [PubMed] [Google Scholar]
- 27.Lekanne Deprez R. H., Fijnvandraat A. C., Ruijter J. M., Moorman A. F. 2002. Sensitivity and accuracy of quantitative real-time polymerase chain reaction using SYBR green I depend on cDNA synthesis conditions. Anal. Biochem. 307: 63–69 [DOI] [PubMed] [Google Scholar]
- 28.Janssen P. M., Stull L. B., Leppo M. K., Altschuld R. A., Marbán E. 2003. Selective contractile dysfunction of left, not right, ventricular myocardium in the SHHF rat. Am. J. Physiol. Heart Circ. Physiol. 284: H772–H778 [DOI] [PubMed] [Google Scholar]
- 29.Hatch G. M.1998. Cardiolipin: biosynthesis, remodeling and trafficking in the heart and mammalian cells. Int. J. Mol. Med. 1: 33–41 [DOI] [PubMed] [Google Scholar]
- 30.Lykidis A., Jackson P. D., Rock C. O., Jackowski S. 1997. The role of CDP-diacylglycerol synthetase and phosphatidylinositol synthase activity levels in the regulation of cellular phosphatidylinositol content. J. Biol. Chem. 272: 33402–33409 [DOI] [PubMed] [Google Scholar]
- 31.Heacock A. M., Uhler M. D., Agranoff B. W. 1996. Cloning of CDP-diacylglycerol synthase from a human neuronal cell line. J. Neurochem. 67: 2200–2203 [DOI] [PubMed] [Google Scholar]
- 32.Mercade A., Sanchez A., Folch J. M. 2007. Characterization and physical mapping of the porcine CDS1 and CDS2 genes. Anim. Biotechnol. 18: 23–35 [DOI] [PubMed] [Google Scholar]
- 33.Cao J., Liu Y., Lockwood J., Burn P., Shi Y. 2004. A novel cardiolipin-remodeling pathway revealed by a gene encoding an endoplasmic reticulum-associated acyl-CoA:lysocardiolipin acyltransferase (ALCAT1) in mouse. J. Biol. Chem. 279: 31727–31734 [DOI] [PubMed] [Google Scholar]
- 34.Hauff K. D., Hatch G. M. 2006. Cardiolipin metabolism and Barth Syndrome. Prog. Lipid Res. 45: 91–101 [DOI] [PubMed] [Google Scholar]
- 35.Mok A. Y., McDougall G. E., McMurray W. C. 1993. Comparative studies of CDP-diacylglycerol synthase in rat liver mitochondria and microsomes. Biochem. Cell Biol. 71: 183–189 [DOI] [PubMed] [Google Scholar]
- 36.Weeks R., Dowhan W., Shen H., Balantac N., Meengs B., Nudelman E., Leung D. W. 1997. Isolation and expression of an isoform of human CDP-diacylglycerol synthase cDNA. DNA Cell Biol. 16: 281–289 [DOI] [PubMed] [Google Scholar]
- 37.Mayer B., Holmer S. R., Hengstenberg C., Lieb W., Pfeifer M., Schunkert H. 2005. Functional improvement in heart failure patients treated with beta-blockers is associated with a decline of cytokine levels. Int. J. Cardiol. 103: 182–186 [DOI] [PubMed] [Google Scholar]
- 38.Hatch G. M.2004. Cell biology of cardiac mitochondrial phospholipids. Biochem. Cell Biol. 82: 99–112 [DOI] [PubMed] [Google Scholar]
- 39.He Q., Greenberg M. L. 2004. Post-translational regulation of phosphatidylglycerolphosphate synthase in response to inositol. Mol. Microbiol. 53: 1243–1249 [DOI] [PubMed] [Google Scholar]
- 40.Cheng P., Hatch G. M. 1995. Inhibition of cardiolipin biosyntheis in the hypoxic rat heart. Lipids. 30: 513–519 [DOI] [PubMed] [Google Scholar]
- 41.Schlame M., Ren M. 2006. Barth syndrome, a human disorder of cardiolipin metabolism. FEBS Lett. 580: 5450–5455 [DOI] [PubMed] [Google Scholar]
- 42.Vreken P., Valianpour F., Nijtmans L. G., Grivell L. A., Plecko B., Wanders R. J., Barth P. G. 2000. Defective remodeling of cardiolipin and phosphatidylglycerol in Barth syndrome. Biochem. Biophys. Res. Commun. 279: 378–382 [DOI] [PubMed] [Google Scholar]
- 43.McKenzie M., Lazarou M., Thorburn D. R., Ryan M. T. 2006. Mitochondrial respiratory chain supercomplexes are destabilized in Barth Syndrome patients. J. Mol. Biol. 361: 462–469 [DOI] [PubMed] [Google Scholar]
- 44.Ma L., Vaz F. M., Gu Z., Wanders R. J., Greenberg M. L. 2004. The human TAZ gene complements mitochondrial dysfunction in the yeast taz1Delta mutant. Implications for Barth syndrome. J. Biol. Chem. 279: 44394–44399 [DOI] [PubMed] [Google Scholar]
- 45.Schlame M., Rustow B. 1990. Lysocardiolipin formation and reacylation in isolated rat liver mitochondria. Biochem. J. 272: 589–595 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.McHowat J., Tappia P. S., Liu S., McCrory R., Panagia V. 2001. Redistribution and abnormal activity of phospholipase A(2) isoenzymes in postinfarct congestive heart failure. Am. J. Physiol. Cell Physiol. 280: C573–C580 [DOI] [PubMed] [Google Scholar]
- 47.Mancuso D. J., Sims H. F., Han X., Jenkins C. M., Guan S. P., Yang K., Moon S. H., Pietka T., Abumrad N. A., Schlesinger P. H., et al. 2007. Genetic ablation of calcium-independent phospholipase A2 gamma leads to alterations in mitochondrial lipid metabolism and function resulting in a deficient mitochondrial bioenergetic phenotype. J. Biol. Chem. 282: 34611–34622 [DOI] [PMC free article] [PubMed] [Google Scholar]