Abstract
Oxidized LDL (oxLDL) promotes lipid accumulation as well as growth and survival signaling in macrophages. OxLDL uptake is mainly due to scavenger receptors SR-AI/II and CD36. However, other scavenger receptors such as lectin-like oxLDL receptor-1 (LOX-1) may also play a role. We used mice with targeted inactivation of the LOX-1 gene to define the role of this receptor in the uptake of oxLDL and in activation of survival pathways. There was no difference in uptake or degradation of 125I-oxLDL in unstimulated macrophages from wild-type and LOX-1 knockout mice and no difference in the rate of clearance of oxLDL from plasma in vivo. However, when expression of LOX-1 was induced with lysophosphatidylcholine, oxLDL uptake and degradation increased 2-fold in wild-type macrophages but did not change in LOX-1 knockout macrophages. Macrophages lacking LOX-1 showed the same stimulation of PKB phosphorylation and enhancement of survival by oxLDL as wild-type cells. These data show that LOX-1 does not alter the uptake of oxLDL in unstimulated macrophages and is not essential for the pro-survival effect of oxLDL in these cells. However, LOX-1 expression is highly inducible by lysophosphatidylcholine and pro-inflammatory cytokines, and if that occurred in macrophages within atheromas, LOX-1 could substantially increase oxLDL uptake by lesion macrophages.
Keywords: apoptosis, lysophosphatidycholine, macrophage, oxidized low density lipoprotein, scavenger receptor
The lectin-like oxidized LDL receptor (LOX-1) is a 50 kDa type II membrane receptor belonging to the C-type lectin family. It is expressed in vascular smooth muscle cells, macrophages, fibroblasts, and platelets (1, 2). It is reported to be upregulated in pathological conditions including hypertension, diabetes, and atherosclerosis. The potential role of LOX-1 in atherosclerotic process has been based on a number of observations. LOX-1 has the capability to bind, internalize, and degrade oxLDL (3). The activation of LOX-1 by oxLDL induces endothelial dysfunction and apoptosis (4–6). Furthermore LOX-1 is present in atheroma-derived cells and has been identified in animal and human atherosclerotic lesions in vivo (7, 8). Mehta et al. recently reported that in LDL receptor knockout mice, LOX-1 gene inactivation reduces the extent of atherosclerosis (9). The LOX-1 receptor has also been shown to mediate intracellular cell signaling events. For example, the binding of oxLDL to LOX-1 is reported to activate signaling via ERK that modulates the Bax/Bcl2 ratio in vascular smooth muscle cells and to activate NF-B in endothelial cells (10).
LOX-1 is a member of the scavenger receptor family of multiligand receptors that are thought to play a role in innate immunity (11). Other members of this family expressed in macrophages include SRAI/II, CD36, macrosialin/CD68, macrophage receptor with collagenous structure (MARCO), scavenger receptor expressed in endothelial cells (SREC), and a transmembrane chemokine and receptor for phosphatidylserine and oxLDL, SR-PSOX/CXCL16 (11). Previous studies indicate that 30–40% of the degradation of extensively oxidized LDL by mouse peritoneal macrophages is mediated by SR-AI/II and that CD36 accounts for an additional 35% of oxLDL degradation (12, 13). In peritoneal macrophages from mice lacking both SRAI/II and CD36, degradation of oxLDL was reduced by 75% (13). It has been suggested that LOX-1 may also be an important receptor for oxLDL in macrophages (14), but to date no studies have provided a quantitative estimate of the contribution of LOX-1 to oxLDL uptake by macrophages.
We recently showed that oxidized LDL blocks apoptosis in macrophages through activation of the phosphatidylinositol 3-kinase/protein kinase B (PI3K/PKB) cascade, translocation of NF-B to the nucleus, and induction of the anti-apoptotic factor BclXL (15, 16). Our preliminary results in SR-AI/II macrophages (17) and CD36 knockout macrophages (unpublished) suggest that the anti-apoptotic effect of oxLDL does not require either of these two scavenger receptors. LOX-1 was deemed an attractive candidate because its much higher affinity for oxidized LDL compared with acetyl LDL or native LDL is congruent with the finding that oxLDL (but not acetyl LDL or native LDL) can inhibit apoptosis in macrophages and because it has been shown to be involved in survival signaling and NF-B activation (4, 6, 18).
In the present study we describe results of experiments using LOX-1 knockout mice to examine the role the LOX-1 receptor plays in mediating uptake of, and signaling by, ox-LDL in macrophages.
MATERIALS AND METHODS
Materials
Carrier-free Na 125I was purchased from Perkin Elmer (Woodbridge, Ontario). DMEM, αMEM medium, propidium iodide, and RNase A were obtained from Invitrogen (Burlington, ON, Canada). Defined fetal bovine serum (FBS) was from HyClone (Logan, UT). Promega (Madison, WI) supplied 3-(4,5-Dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium, inner salt (MTS). Phenazine methosulfate (PMS) and 1-stearoyl lysophosphatidylcholine were from Sigma Aldrich (St. Louis, MO). Centriplus 20 ultrafilters were purchased from Amicon (Beverly, MA). Molecular Probes (Eugene OR) provided 1,1′-dioctadecyl-3,3,3′,3′-tetramethylindocarbocyanine perchlorate (DiI). Anti-CD68 was purchased from Serotec (Raleigh, NC). Primary antibody against p85 subunit of PI3K was purchased from Santa Cruz Biotechnology (Santa Cruz, CA). Antibodies against total and phosphorylated PKB (Ser470) were from Cell Signaling Technology (Beverly, MA).
Animals
Inactivation of the single-copy murine LOX-1 receptor gene by homologous recombination was performed in the laboratory of Dr. T. Sawamura (9). Offspring with the inactivated gene in germine cells were backcrossed 8 times onto a C57bl/6 background. LOX-1 genotypes were verified by PCR analysis of tail DNA in all breeding animals, as well as in selected experimental animals. DNA was extracted using a Qiagen DNeasy kit. The forward primer for the LOX-1 deletion was 5′-CAGCGAACACAGCTCCGTCTTGAAGG-3′ and the reverse primer was 5′-GGCCAACCATGGCTTGGGAGAATGG-3′. The forward primer for the neomycin-resistance cassette was 5′-CAACGCTATGTCCTGATAGCGGTCC-3′ and the reverse primer was 5′-CGTGTTCCGGCTGTCAGCGCAGG-3′. Wild-type C57bl/6 mice were purchased from The Jackson Laboratory. All animal procedures were in accordance with the guidelines of the Canadian Council on Animal Care and were approved by the Animal Care Committee of the University of British Columbia.
Lipoprotein isolation and labeling
LDL (d = 1.019–1.063 g/ml) was isolated by sequential ultracentrifugation of EDTA-anticoagulated fasting plasma obtained from healthy normolipidemic volunteers (19). Radioiodination was performed using a modification of the iodine monochloride method of MacFarlane (20). The specific activity was between 100 and 150 cpm/ng LDL protein. Iodination was performed before oxidation or acetylation of LDL. Lipoprotein-deficient serum (LPDS) was prepared from the d > 1.25 fraction.
LDL modification
LDL was oxidized by incubating 200 μg/ml LDL in PBS containing 5 μmol/L CuSO4 at 37°C for 20 h (21). OxLDL was then washed and concentrated to about 1 mg/ml with Centriplus 20 ultrafilters (Millipore, Bedford, MA). Agarose gel electrophoresis typically showed a 4-fold increase in electrophoretic mobility for oxidized LDL compared with native LDL. LDL was acetylated by adding four 1-μl aliquots of acetic anhydride at 10 min intervals to 2 mg of LDL in 600 μl of ice-cold 50% saturated sodium acetate (22).
Macrophage isolation and culture
Peritoneal macrophages were obtained from wild-type or LOX-1 knockout C57/bl mice by peritoneal lavage with ice-cold Ca2+-free Dulbecco's PBS. Macrophages were suspended in α-minimal essential medium (α-MEM) with 10% fetal bovine serum (FBS) and plated in 12-well plastic culture plates. Nonadherent cells were removed by medium exchange after 1 h. Adherent macrophages were cultured overnight, washed with α-MEM, and then used for experiments. Bone marrow cells were isolated from the femurs of 6–8-week-old female C57/bl mice as described previously (22). Cells were plated for 24 h in RPMI 1640 containing 10% FBS and 10% L-cell conditioned medium as the source of M-CSF. The nonadherent cells were removed and cultured in the above medium for 5–7 days until 80% confluence was reached.
Reverse transcription polymerase chain reaction analysis
RNA was isolated from BMDM and peritoneal macrophages (PM) from wild-type or LOX-1 knockout mice using Trizol (Invitrogen, Burlington, ON). Total RNA was then used as a template for first strand cDNA synthesis using M-MLV reverse transcriptase from Promega (Madison, WI) according to the manufacturer's instructions. The resulting cDNA was amplified using the forward primer 5′-CGTGTTCCGGCTGTCAGCGCAGG-3′ and the reverse primer 5′-CAACGCTATGTCCTGATAGCGGTCC-3′. These sequences were obtained from the genomic sequence using the algorithm Primer 3 (www-genome.wi.mit.edu/cgi-bin/primer/primer3_www.cgi). PCR was performed with Taq DNA polymerase (Invitrogen, Burlington, ON) in a 25 μl reaction volume containing 10 pmol of each primer. Amplification was performed for 35 cycles at 94°C for 2 min, 94°C for 40s, 60°C for 1 min, and 72°C for 1 min. The final elongation phase was done at 72°C for 10 min. The PCR products were electrophoresed on a 1.5% agarose gel and visualized using ethidium bromide under UV light.
Fluorescent labeling
Fluorescent labeling of oxLDL was performed by adding 100 μg DiI in 60μl dimethyl sulfoxide to 2 mg of oxLDL in the presence of d > 1.21g/ml plasma fraction as a source of lipid transfer activity (24). Following an 8 h incubation period at 37°C, the labeled oxLDL was reisolated by ultracentrifugation at d = 1.1. This procedure typically resulted in an incorporation of 5–15 μg of DiI per mg of LDL protein.
Assays of LDL uptake and degradation in cultured cells
Macrophages were incubated at 37°C with varying concentrations of radioiodinated native, oxidized, or acetylated LDL in α-MEM with 2.5% lipoprotein deficient serum. After 4 h, the medium was removed and the cells were washed. LDL degradation products were quantified by mixing 1 ml of the supernatant medium with 350 μl of 50% trichloroacetic acid and 350 μl of 7.5% AgNO3. The mixture was centrifuged at 2500 rpm for 15 min, and the supernatant was counted on a LKB 1282 γ counter. The cells were removed from the plates with Teflon cell lifters and counted to determine cell-associated LDL.
Uptake of DiI labeled oxLDL by macrophages
Peritoneal macrophages were incubated for 5 h at 37°C with 10 μg/ml DiI-labeled oxidized LDL in α-MEM containing 2.5% LPDS. Cells were washed with PBS, fixed with 2% formaldehyde, and mounted in 90% glycerol, 9.75% PBS, and 0.25% 1,4-diazabicyclo[2,2,2]octane. Cells were examined with a Zeiss Axioskop fluorescence microscope in epifluorescent mode with a fluorescein filter set.
In vivo clearance of LDL
Clearance of oxLDL or native LDL was measured as previously described (25). Briefly, wild-type or LOX-1 deficient mice were anesthetized with 2% isoflurane. The external jugular vein was exposed with the aid of a Zeiss operating microscope (Carl Zeiss Canada Ltd., Don Mills, Ontario) and cannulated with a PE 10 polyethylene catheter (outer diameter 0.61 mm; VWR Canlab), positioned with its tip in the superior vena cava. 125I oxLDL (1-2 million cpm) in 200 μl of 150 mM NaCl was drawn into a 1ml syringe. The syringe was counted before and after the injection to allow an accurate determination of the amount of radioactivity administered. Five or six 20 μl blood samples were collected over 20 min. A second injection was then performed with a similar amount of native LDL, and four or five 20 μl samples were collected over 20 min. For oxLDL, the concentration at zero time was calculated using the injected dose of oxLDL and the volume of distribution of native LDL.
Cell viability assay
Macrophage survival was determined by the MTS-formazan method. This assay is based on the cellular bioreduction of MTS by mitochondrial dehydrogenase enzymes in metabolically active cells. The quantity of formazan product formed was measured by the absorbance at 490 nm and is directly proportional to the number of viable cells in culture (26). MTS-PMS solution (20 µl/well) was added to wells containing 100 µl of culture medium in 96 well plates for 3 h before terminating the experiment. This resulted in final MTS and PMS concentrations of 333 µg/ml and 25 µM, respectively. After 3 h incubation at 37°C in a humidified 5% CO2 atmosphere, the absorbance at 490 nm was recorded with an ELISA plate reader.
Apoptosis assay
Propidium iodide staining and FACS analysis was used to quantitate the subdiploid population. At the end of 24 hr incubation, BMDM from wild-type or LOX-1 knockout mice were fixed and permeabilized with ethanol 70% (v/v) for 1 h at 4°C and washed twice in PBS containing 0.01% glucose. Cells were then resuspended in the same buffer plus RNase A (final concentration 0.1 mg/ml) and propidium iodide (final concentration 0.12 mg/ml). Fluorescence was measured with a BD FACS Canto. The data was analyzed with FCS Express Pro Software Version 3 (De Novo Software, Thornhill, Canada) with gating to exclude debris and cellular aggregates.
Immunoblotting
BMDM were harvested as described above and lysed in ice-cold homogenization buffer [20 mM morpholinepropanesulfonic acid, pH 7.2, 1% Triton X-100, 50 mM β-glycerol phosphate, 5 mM EGTA, 2 mM EDTA, 1 mM sodium vanadate, 25 µM β-methyl aspartic acid, 1 mM DTT, 1 mM phenylmethylsulfonyl fluoride, aprotinin (10 µg/ml), and leupeptin (10 µg/ml)]. Lysates were centrifuged at 14,000 rpm for 10 min, and the protein content of supernatants was quantified using the Bradford protein assay. Fifty micrograms of protein from each sample was loaded and separated by SDS-PAGE using a 10% separating gel. Gels were calibrated with prestained SDS-PAGE low molecular weight standards (Bio-Rad, Hercules, CA). Proteins were then transferred to nitrocellulose membranes which were then blocked for 1 h with 4% skim milk, 0.01% NaN3 in TBS-0.1% Tween 20. The membranes were cut at about the 70 kDa point, and the bottom section was then incubated with anti-phospho(Ser473) PKB antibody overnight in TBS-0.1% Tween 20 at 4°C, washed three times, and then incubated with horseradish peroxidase-conjugated secondary antibody at a 1:5,000 dilution for 1 h. The bands were then visualized by enhanced chemiluminescence. This blot was then stripped in Tris buffer containing SDS (2%) and β-2-mercaptoethanol (100 mM) at 50°C for 20 min, washed, and reprobed with antibody to PKB. The top section of the membrane was incubated with antibody against the p85 subunit of PI3K, and bands were visualized as above.
Analytic procedures
LDL and cell protein was assayed by the method of Lowry (27) in the presence of 0.05% sodium deoxycholate with BSA as the standard. Lipoprotein electrophoresis was done using the Titan gel lipoprotein electrophoresis system (Intermedico, Markham, Ontario). Lipoprotein bands were visualized by staining with Fat red.
Statistical analysis
Statistical analysis was done using ANOVA or Student's t-test as appropriate. P < 0.05 was taken as significant.
RESULTS
LOX-knockout mice
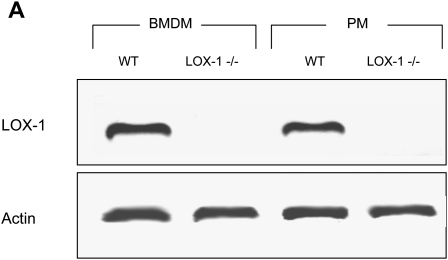
Homozygous LOX-1 deficient animals had no overt phenotype and were fertile. The absence of LOX-1 mRNA was verified by RT-PCR (Fig. 1).
Fig. 1.
Absence of LOX-1 mRNA in macrophages harvested from LOX-1 knockout mice. Absence of LOX-1 mRNA in knockout animals was verified by RT-PCR of total RNA from bone marrow derived macrophages (BMDM) or peritoneal macrophages (PM). An amplicon with migration consistent with the predicted nucleotide sequence is seen in the wild-type cells, whereas no band is seen in knockout cells.
Uptake of fluorescently labeled oxLDL by macrophages
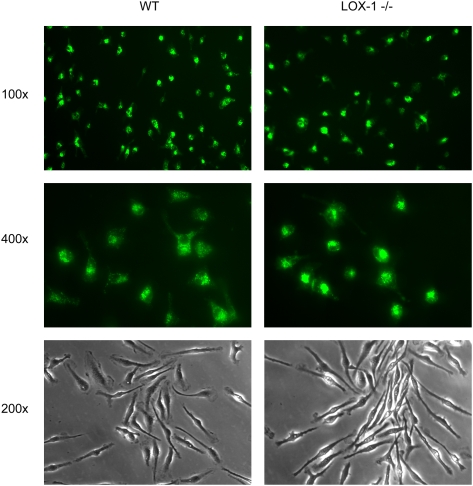
The lipophilic fluorophore diI crosses lysosomal membranes very slowly and so serves as a cumulative marker of the quantity of labeled LDL that is internalized by cells (24, 28). To obtain a morphologic comparison of the internalization of oxLDL in control and LOX-1 deficient macrophages, we incubated peritoneal macrophages grown on coverslips for 5 h with diI-labeled oxLDL. Fluorescence micrographs indicate that there was no apparent difference in the intensity or distribution of fluorescence between wild-type and LOX-1 deficient cells (Fig. 2).
Fig. 2.
Uptake of oxLDL in peritoneal macrophages. Resident peritoneal macrophages from wild-type (WT) and LOX-1 knockout mice were incubated for 5 h with 10μg/ml DiI-labeled oxidized LDL, and then examined using a Zeiss Axioskop fluorescence microscope. No difference in fluorescence intensity is seen.
Uptake and degradation of modified LDLs in wild-type and LOX-1 deficient peritoneal macrophages
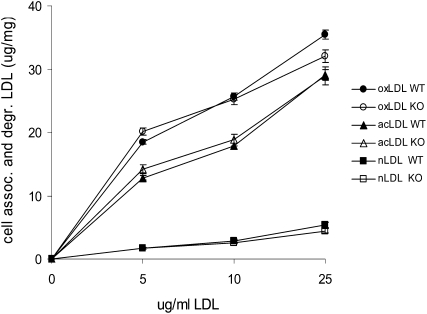
To quantify the relative rates of oxLDL uptake, we incubated macrophages for 5 h with radioiodinated native LDL, oxLDL, or acetyl LDL and then measured rates of LDL uptake and degradation. There was no difference in the rate of uptake of native LDL or either modified LDL in wild-type compared with LOX-1 knockout peritoneal macrophages (Fig. 3).
Fig. 3.
Uptake and degradation of native, oxidized, and acetylated LDL in macrophages. Cultured peritoneal macrophages from wild-type mice (solid symbols) or knockout mice (open symbols) were incubated with the indicated concentration of 125I-labeled native LDL, oxLDL, or acetyl LDL.
Uptake and degradation of oxLDL in wild-type and LOX-1 deficient peritoneal macrophages
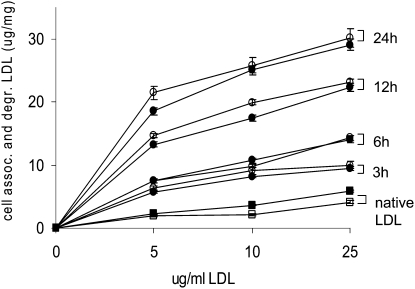
Different scavenger receptors are known to have different affinities for minimally, moderately, or extensively oxidized LDL. We therefore tested the uptake and degradation of radioactively labeled oxLDL in LOX-1 deficient and wild-type macrophages. There was no difference between wild-type and LOX-1 deficient macrophages in uptake or degradation of minimally, moderately, or extensively oxidized LDL (Fig. 4).
Fig. 4.
Uptake and degradation of LDL oxidized to various degrees in wild-type and LOX-1 deficient peritoneal macrophages. Cultured peritoneal macrophages from wild-type mice (solid symbols) or knockout mice (open symbols) were incubated with the indicated concentration of native 125I-LDL (squares) or 125I-LDL oxidized by exposure to 5μM Cu2+ for 3 h, 6 h, 12 h, or 24 h (circles). The electrophoretic mobility of oxLDL was 1.0 (3 h), 1.3 (6 h), 2.2 (12 h) or 3.8 (24 h) times that of native LDL. After 5 h, cells and media were assayed for uptake and degradation of labeled lipoprotein. Results are the sum of cell-associated and degraded LDL expressed as μg LDL per mg cell protein. Values are the means +/− SEM of triplicates.
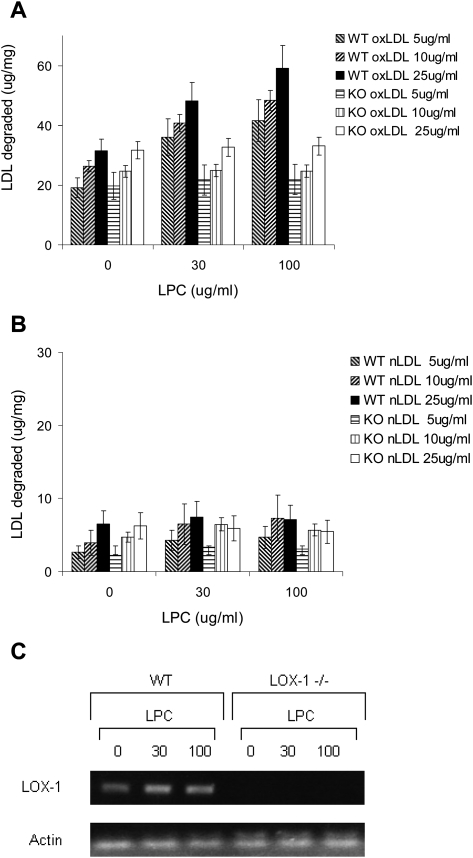
Effect of induction of LOX-1 gene expression on oxLDL uptake and degradation in wild-type macrophages
LOX-1 has been shown to mediate uptake and degradation of oxLDL in endothelial cells, smooth muscle cells, and in transfected cell lines. Hence, it seemed possible that the failure to detect a difference between wild-type and LOX-1 deficient macrophages might be because a high level of expression of SRAI/II and CD36 masked the contribution of LOX-1. To test this, we took advantage of the observation that lysophosphatidylcholine causes a marked and selective (up to 5-fold) induction of LOX-1 in endothelial cells and smooth muscle cells (29, 30). Cultured peritoneal macrophages from wild-type and LOX-1 deficient mice were preincubated for 12 h with varying concentrations of lysophosphatidylcholine, and then incubated for 5 h with 125I oxLDL or 125I LDL (Fig. 5A, 5B). Lysophosphatidylcholine caused a concentration-dependent increase in oxLDL uptake and degradation in wild-type macrophages but had no effect on uptake and degradation in LOX-1 deficient cells. These concentrations of lysophosphatidylcholine are the same as those reported to induce LOX-1 expression in smooth muscle cells and endothelial cells (29). The upregulation of LOX-1 mRNA by these concentrations of lysophosphatidylcholine was confirmed by RT-PCR (Fig. 5C).
Fig. 5.
Effect of induction of LOX-1 with lysophosphatidylcholine on oxLDL uptake and degradation. Cultured peritoneal macrophages from wild-type and LOX-1 knockout mice were preincubated for 12 h with 0, 30, or 100 ug/ml lysophosphatidylcholine. Cells were then washed and incubated for 5 h with varying concentrations of 125I-oxLDL (A) or 125I-LDL (B). Values shown are means ± SD of the sum of cell-associated and degraded oxLDL, reflecting total oxLDL uptake during the incubation. (C) RT-PCR blot showing that LOX-1 mRNA expression was increased in wild-type macrophages by these concentrations of lysophosphatidylcholine.
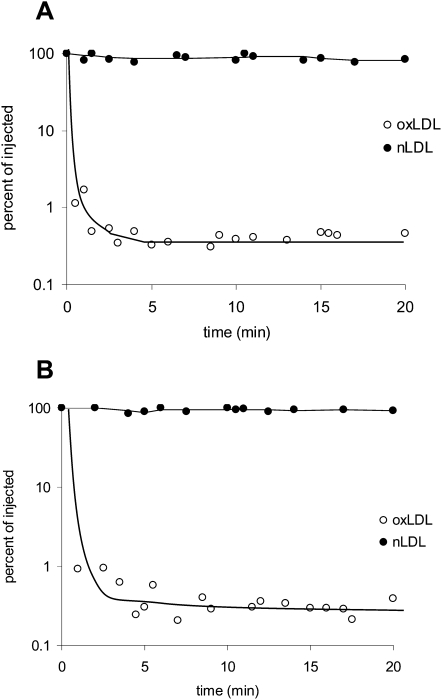
In vivo clearance of oxLDL
To directly assess the role of LOX-1 in oxLDL clearance in vivo, we compared the rates at which oxLDL was removed from plasma from wild-type and LOX-1 deficient mice. Animals were injected first with 125I-oxLDL and then with native 125I-LDL and serial blood samples were assayed for radioactivity. The rates of plasma clearance of oxLDL were very rapid and indistinguishable between wild-type and LOX-1 deficient animals (Fig. 6).
Fig. 6.
In vivo plasma clearance of oxidized and native LDL in wild-type and LOX-1 deficient mice. Wild-type mice (A, n = 3) or LOX-1 knockout mice (B, n = 3) were anesthetized with 2% isoflurane. Oxidized 125I LDL (open circles) or native 125I LDL (solid circles) was injected through a cannula placed in the internal jugular vein. Serial 20 μl blood samples were withdrawn at the indicated time points and radioactivity was measured. Points for all three animals in each case are plotted together. The electrophoretic mobility of oxLDL in this experiment was 4.1-fold that of native LDL.
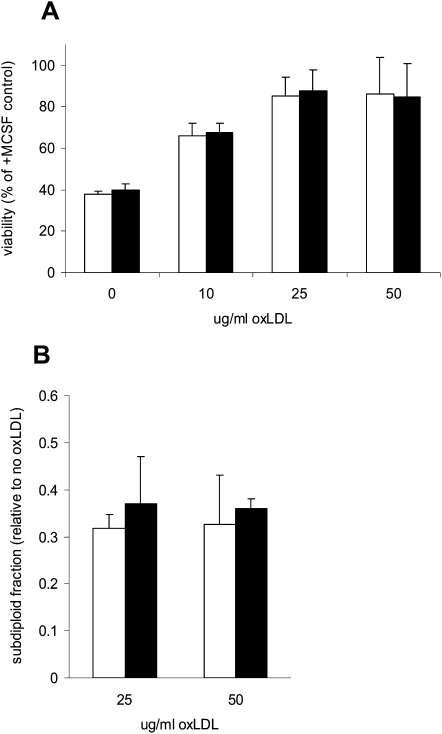
Role of LOX-1 in the ability of oxLDL to increase survival of BMDM
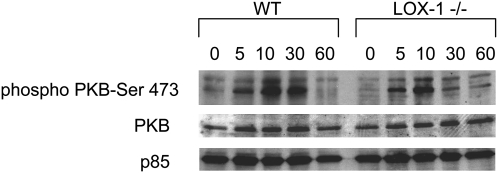
We have shown that oxLDL promotes growth and cytokine-independent survival in macrophages at least in part by activating the PI3K/PKB pro-survival pathway (15–17 26). We previously found that oxLDL promoted survival in SRAI/II-deficient macrophages (31) and in CD36-deficient macrophages (unpublished), so we hypothesized that LOX-1 might be the receptor required for PKB activation. To address this, we compared the ability of oxLDL to promote survival in cytokine-deprived BMDM from wild-type and LOX-1 deficient mice. After 24 h there was no significant difference in the viability of wild-type and LOX-1 deficient cells treated with oxLDL (Fig. 7A). There was no significant difference between wild-type and LOX-1 BMDM in the percentage of sub-diploid cells after incubation with oxLDL (Fig. 7B). Deficiency of LOX-1 did not abolish the ability of oxLDL to promote PKB phosphorylation in BMDM (Fig. 8).
Fig. 7.
LOX-1 deficiency does not impair the ability of oxLDL to inhibit macrophage apoptosis. (A) BMDM from wild-type mice (open bars) or LOX-1 −/− mice (solid bars) were seeded at 3 × 103 cells/well in 96 well plates and cultured for 24 h. They were then washed and incubated for 24 h without M-CSF but with the indicated concentrations of ox-LDL. Viability was then measured by the bioreduction of MTS (described in Materials and Methods) and expressed as a percentage of that in control cells treated with M-CSF. (B) BMDM were plated at 106 cells/well in six well plates and incubated for 24 h in medium containing indicated concentration of oxLDL but no M-CSF. The sub-diploid population was measured by flow cytometry after propidium iodide staining. Results are expressed relative to control cells incubated without CSF, which typically amounted to about 40% of cells. In both panels, results are the means±SD of pooled data from three independent experiments. None of the differences between wild-type and LOX-1 −/−.macrophages was significant.
Fig. 8.
LOX-1 is not essential for induction of PKB phosphorylation by oxLDL in BMDM. Bone marrow-derived macrophages from wild-type (WT) or LOX-1 knockout mice were incubated with or without 25 g/ml oxLDL for 0–60 min. Cell lysates were then analyzed by immunoblotting for phosphorylation of PKB at serine 473. Immunostaining for the p85 subunit of PI3K as well as for total PKB was used to monitor loading.
DISCUSSION
The studies described in this report address two different aspects of the interaction of oxLDL with LOX-1 in macrophages. The first issue is the role of LOX-1 in uptake and degradation of oxLDL by macrophages. LOX-1 clearly has the ability to bind and internalize LDL in endothelial cells and in CHO cells transfected with LOX-1 receptor constructs (3). LOX-1 has a similar ligand specificity as SR-AI/II and CD36, although its affinity for oxLDL is substantially higher than its affinity for acetyl LDL (31). We previously showed that about 70% of the uptake of oxLDL in macrophages was not due to SRAI/II (12). Competition experiments indicated that polyinosinic acid, but not acetyl LDL or native LDL, blocked the SRAI/II-independent uptake of oxidized LDL (12). Although CD36 has been shown to contribute to oxLDL uptake in macrophages, our preliminary findings in CD36 −/− macrophages showed no reduction in the anti-apoptotic effect of oxLDL. Hence it seemed possible that some of the SRAI/II and CD36-independent uptake might be due to a receptor such as LOX-1. This receptor has a reported ligand specificity that is congruent with the ligand specificity that we observed in SRAI/II-deficient macrophages (12). It has been shown that LOX-1 is expressed in monocyte-derived macrophages (14) and in macrophages in atherosclerotic lesions (7). Furthermore, a recent report indicates that LOX-1 accounts for at least half the uptake of oxLDL in PMA-activated HL-60 macrophages (32). However, in the present studies there was no effect of LOX-1 gene inactivation on oxLDL uptake by BMDM or peritoneal macrophages.
One assumption that is implicit in the interpretation of our results is that there is no compensatory increase in the activity of other scavenger receptors in LOX-1 −/− mice and macrophages that exactly balances the effect of the inactivation of LOX-1 gene. A minor contribution of LOX-1 to oxLDL uptake might be masked if there was a high level of nonsaturable uptake mediated by phagocytosis or pinocytosis. However, we have previously shown that at the relatively low concentration of labeled oxLDL used in the present studies, more than 80% of the uptake and degradation of the labeled oxLDL can be competed by 10-fold excess of unlabeled oxLDL or by polyinosinic acid, indicating that the uptake is mediated by a high-affinity saturable mechanism, presumably a receptor(s) (12, 33). Overall, our finding that LOX-1 gene inactivation does not significantly alter oxLDL uptake in unstimulated murine macrophages indicates that LOX-1 accounts for at most 5–10% of oxLDL uptake by these cells. On the other hand, when LOX-1 was upregulated in macrophages by preincubation with lysophosphatidylcholine, internalization of oxLDL increased by more than 40%. It has been shown that pro-inflammatory cytokines such as TNFα (34) and TGFβ (35) upregulate LOX-1 and downregulate SR-AI/II and CD36. Hence, it is possible that in microenvironments where these cytokines are relatively abundant, notably in atherosclerotic lesions, LOX-1 might play a significant role in oxLDL uptake. It is also possible that endothelial cells and smooth muscle cells might rely on LOX-1 as a receptor for the internalization of oxLDL or for mediating oxLDL-induced signal transduction.
The second issue addressed by these studies was the potential role of LOX-1 in survival signaling. The importance of macrophage survival has been recently underlined by a series of studies in different animal models. Currently it is believed that at least in early stages of atherosclerosis, inhibition of macrophage apoptosis or induction of macrophage growth enhances lesion progression (36). It has been shown that oxLDL activates p42/44 MAPK (15), p38MAPK (37), PKC (38), and PI3K/PKB (15, 26). Activation of PI3K/PKB by oxidized LDL promotes growth and survival of macrophages (15, 26). The upstream steps by which oxLDL activates these signaling pathways have not yet been defined. As LOX-1 has been shown to activate PKCα (39), PKCβ (40), p38MAPK (41), and p42/44MAPK (42), it seemed appropriate to determine if it accounted for part of the anti-apoptotic signaling of oxLDL. However, we found no difference in the anti-apoptotic effect of oxLDL in LOX-1 knockout compared with wild-type macrophages.
In contrast to our findings in BMDM, previous studies in smooth muscle cells (10), cultured endothelial cells (43), and chondrocytes (44) reported a pro-apoptotic effect of oxLDL acting at least in part via LOX-1. There are a number of explanations for these apparently discrepant results. In the study by Kataoka et al. in smooth muscle cells (10), oxLDL only induced apoptosis at concentrations of 40 µg/ml or higher, and Fig. 1A in that paper actually shows increased viability of smooth muscle cells at lower concentrations of oxLDL. Also, they incubated cells in medium containing only 1% fetal bovine serum. We have reported a similar biphasic effect of oxLDL in macrophages, with concentrations higher than 100 µg/ml associated with toxicity in macrophages incubated in 10% fetal bovine serum (15). In macrophages incubated in serum-free medium, the threshold for toxicity of oxLDL was much lower, around 20 µg/ml (15). The study by Imanishi and colleagues found that oxLDL upregulated Fas expression and potentiated the effect of an agonistic anti-Fas antibody on apoptosis in endothelial cells (43). This study did not investigate apoptosis induced by oxLDL, only its effect on apoptosis induced by anti-Fas. In this study, endothelial cells were cultured in medium containing only 1% FBS, which would have made them more vulnerable to toxic effects of oxLDL. The study by Nakagawa and coworkers showed that in chondrocytes, oxLDL at concentrations of 40 µg/ml or higher induced apoptosis. Chondrocytes were incubated in serum-free medium, which may explain why the toxic threshold for oxLDL was relatively low. Also, their oxLDL preparations may not have been dialyzed after oxidation, and we have previously shown that this results in a high concentration of toxic water-soluble aldehydes in the oxLDL mixture (45) Although, it appears that LOX-1 plays a role in oxLDL-mediated toxicity in these cells, our results in macrophages provide no evidence that LOX-1 is involved in the anti-apoptotic effect of low concentrations of oxLDL.
Other receptors that are capable of binding oxLDL or some of its components could play a role in pro-survival signaling, including the phosphatidylserine receptor (46), PPARα (47, 48), PPARγ (49–51), TLR4 (52), and FcγRII (53). Studies are under way to determine if any of these play a role in the pro-survival effect of oxLDL in macrophages.
Acknowledgments
Dr. Osamu Cynshi provided LOX-1 knockout mice from a colony maintained at Chugai Pharmaceutical Corp., Tokyo.
Footnotes
Abbreviations:
- acLDL
- acetylated low density lipoprotein
- BMDM
- bone marrow derived macrophages
- lysoPC
- lysophosphatidycholine
- M-CSF
- macrophage colony-stimulating factor
- MTS
- 3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium, inner salt
- oxLDL
- oxidized low density lipoprotein
REFERENCES
- 1.Moriwaki H., Kume N., Sawamura T., Aoyama T., Hoshikawa H., Ochi H., Nishi E., Masaki T., Kita T. 1998. Ligand specificity of LOX-1, a novel endothelial receptor for oxidized low density lipoprotein. Arterioscler. Thromb. Vasc. Biol. 18: 1541–1547 [DOI] [PubMed] [Google Scholar]
- 2.Chen M., Masaki T., Sawamura T. 2002. LOX-1, the receptor for oxidized low-density lipoprotein identified from endothelial cells: implications in endothelial dysfunction and atherosclerosis. Pharmacol. Ther. 95: 89–100 [DOI] [PubMed] [Google Scholar]
- 3.Sawamura T., Kume N., Aoyama T., Moriwaki H., Hoshikawa H., Aiba Y., Tanaka T., Miwa S., Katsura Y., Kita T., et al. 1997. An endothelial receptor for oxidized low-density lipoprotein. Nature. 386: 73–77 [DOI] [PubMed] [Google Scholar]
- 4.Cominacini L., Pasini A. F., Garbin U., Davoli A., Tosetti M. L., Campagnola M., Rigoni A., Pastorino A. M., Lo Cascio V., Sawamura T. 2000. Oxidized low density lipoprotein (ox-LDL) binding to ox-LDL receptor-1 in endothelial cells induces the activation of NF-kappaB through an increased production of intracellular reactive oxygen species. J. Biol. Chem. 275: 12633–12638 [DOI] [PubMed] [Google Scholar]
- 5.Cominacini L., Rigoni A., Pasini A. F., Garbin U., Davoli A., Campagnola M., Pastorino A. M., Lo Cascio V., Sawamura T. 2001. The binding of oxidized low density lipoprotein (ox-LDL) to ox-LDL receptor-1 reduces the intracellular concentration of nitric oxide in endothelial cells through an increased production of superoxide. J. Biol. Chem. 276: 13750–13755 [DOI] [PubMed] [Google Scholar]
- 6.Li D., Mehta J. 2000. Upregulation of endothelial receptor for oxidized LDL (LOX-1) by oxidized LDL and implications in apoptosis of human coronary artery endothelial cells. Evidence from use of antisense LOX-1 mRNA and chemical inhibitors. Arterioscler. Thromb. Vasc. Biol. 20: 1116–1122 [DOI] [PubMed] [Google Scholar]
- 7.Kataoka H., Kume N., Miyamoto S., Minami M., Moriwaki H., Murase T., Sawamura T., Masaki T., Hashimoto N., Kita T. 1999. Expression of lectinlike oxidized low-density lipoprotein receptor-1 in human atherosclerotic lesions. Circulation. 99: 3110–3117 [DOI] [PubMed] [Google Scholar]
- 8.Chen C. H., Jiang W., Via D. P., Luo S., Li T. R., Lee Y. T., Henry P. D. 2000. Oxidized low-density lipoproteins inhibit endothelial cell proliferation by suppressing basic fibroblast growth factor expression. Circulation. 101: 171–177 [DOI] [PubMed] [Google Scholar]
- 9.Mehta J. L., Sanada N., Hu C. P., Chen J., Dandapat A., Sugawara F., Satoh H., Inoue K., Kawase Y., Jishage K., et al. 2007. Deletion of LOX-1 reduces atherogenesis in LDLR knockout mice fed high cholesterol diet. Circ. Res. 100: 1634–1642 [DOI] [PubMed] [Google Scholar]
- 10.Kataoka H., Kume N., Miyamoto S., Minami M., Morimoto M., Hayashida K., Hashimoto N., Kita T. 2001. Oxidized LDL modulates Bax/Bcl-2 through the lectinlike Ox-LDL receptor-1 in vascular smooth muscle cells. Arterioscler. Thromb. Vasc. Biol. 21: 955–960 [DOI] [PubMed] [Google Scholar]
- 11.Greaves D. R., Gordon S. 2005. Thematic review series: The immune system and atherogenesis. Recent insights into the biology of macrophage scavenger receptors. J. Lipid Res. 46: 11–20 [DOI] [PubMed] [Google Scholar]
- 12.Lougheed M., Ming Lum C., Ling W., Suzuki H., Kodama T., Steinbrecher U. P. 1997. High-affinity saturable uptake of oxidized low density lipoprotein by macrophages from mice lacking the scavenger receptor class A type I/II. J. Biol. Chem. 272: 12938–12944 [DOI] [PubMed] [Google Scholar]
- 13.Kunjathoor V. V., Febbraio M., Podrez E. A., Moore K. J., Andersson L., Koehn S., Rhee J. S., Silverstein R., Hoff H. F., Freeman M. W. 2002. Scavenger receptors class A-I/II and CD36 are the principal receptors responsible for the uptake of modified low density lipoprotein leading to lipid loading in macrophages. J. Biol. Chem. 277: 49982–49988 [DOI] [PubMed] [Google Scholar]
- 14.Yoshida H., Kondratenko N., Green S., Steinberg D., Quehenberger O. 1998. Identification of the lectin-like receptor for oxidized low-density lipoprotein in human macrophages and its potential role as a scavenger receptor. Biochem. J. 334: 9–13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hundal R., Salh B., Schrader J., Gómez-Muñoz A., Duronio V., Steinbrecher U. 2001. Oxidized low density lipoprotein inhibits macrophage apoptosis through activation of the PI 3-kinase/PKB pathway. J. Lipid Res. 42: 1483–1491 [PubMed] [Google Scholar]
- 16.Hundal R., Gómez-Muñoz A., Kong J., Salh B., Marotta A., Duronio V., Steinbrecher U. 2003. Oxidized low density lipoprotein inhibits macrophage apoptosis by blocking ceramide generation, thereby maintaining PKB activation and Bcl-XL levels. J. Biol. Chem. 278: 24399–24408 [DOI] [PubMed] [Google Scholar]
- 17.Martens J., Lougheed M., Gomez-Muñoz A., Steinbrecher U. 1999. A modification of apolipoprotein B accounts for most of the induction of macrophage growth by oxidized low density lipoprotein. J. Biol. Chem. 274: 10903–10910 [DOI] [PubMed] [Google Scholar]
- 18.Matsunaga T., Hokari S., Koyama I., Harada T., Komoda T. 2003. NF-kappa B activation in endothelial cells treated with oxidized high-density lipoprotein. Biochem. Biophys. Res. Commun. 303: 313–319 [DOI] [PubMed] [Google Scholar]
- 19.Havel R. J., Eder H. A., Bragdon J. H. 1955. The distribution and chemical composition of ultracentrifugally separated lipoproteins in human serum. J. Clin. Invest. 43: 1345–1353 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bilheimer D. W., Eisenberg S., Levy R. I. 1972. The metabolism of very low density lipoproteins. Biochim. Biophys. Acta. 260: 212–221 [DOI] [PubMed] [Google Scholar]
- 21.Steinbrecher U. P.1987. Oxidation of human low density lipoproteins results in derivatization of lysine residues of apolipoprotein B by lipid peroxide decomposition products. J. Biol. Chem. 262: 3603–3608 [PubMed] [Google Scholar]
- 22.Zhang H., Yang Y., Steinbrecher U. P. 1993. Structural requirements for the binding of modified proteins to the scavenger receptor of macrophages. J. Biol. Chem. 268: 5535–5542 [PubMed] [Google Scholar]
- 23.Hamilton J. A., Myers D., Jessup W., Cochrane F., Byrne R., Whitty G., Moss S. 1999. Oxidized LDL can induce macrophage survival, DNA synthesis, and enhanced proliferative response to CSF-1 and GM-CSF. Arterioscler. Thromb. Vasc. Biol. 19: 98–105 [DOI] [PubMed] [Google Scholar]
- 24.Pitas R. E., Innerarity T. L., Weinstein J. N., Mahley R. W. 1981. Acetoacetylated lipoproteins used to distinguish fibroblasts from macrophages in vitro by fluorescence microscopy. Arteriosclerosis. 1: 177–185 [DOI] [PubMed] [Google Scholar]
- 25.Ling W., Lougheed M., Suzuki H., Buchan A., Kodama T., Steinbrecher U. 1997. Oxidized or acetylated low density lipoprotein are rapidly cleared by the liver in mice with disruption of the scavenger receptor class A type I/type II gene. J. Clin. Invest. 100: 244–252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Martens J., Reiner N., Herrera-Velit P., Steinbrecher U. 1998. Phosphatidylinositol 3-kinase is involved in the induction of macrophage growth by oxidized low density lipoprotein. J. Biol. Chem. 273: 4915–4920 [DOI] [PubMed] [Google Scholar]
- 27.Lowry O. H., Rosebrough N. J., Farr A. L., Randall R. J. 1951. Protein measurement with the Folin phenol reagent. J. Biol. Chem. 193: 265–275 [PubMed] [Google Scholar]
- 28.Stephan Z., Yurachek E. 1993. Rapid fluorometric assay of LDL receptor activity by DiI-labelled LDL. J. Lipid Res. 34: 325–330 [PubMed] [Google Scholar]
- 29.Aoyama T., Fujiwara H., Masaki T., Sawamura T. 1999. Induction of lectin-like oxidized LDL receptor by oxidized LDL and lysophosphatidylcholine in cultured endothelial cells. J. Mol. Cell. Cardiol. 31: 2101–2114 [DOI] [PubMed] [Google Scholar]
- 30.Aoyama T., Chen M., Fujiwara H., Masaki T., Sawamura T. 2000. LOX-1 mediates lysophosphatidylcholine-induced oxidized LDL uptake in smooth muscle cells. FEBS Lett. 467: 217–220 [DOI] [PubMed] [Google Scholar]
- 31.Kakutani M., Ueda M., Naruko T., Masaki T., Sawamura T. 2001. Accumulation of LOX-1 ligand in plasma and atherosclerotic lesions of Watanabe heritable hyperlipidemic rabbits: identification by a novel enzyme immunoassay. Biochem. Biophys. Res. Commun. 282: 180–185 [DOI] [PubMed] [Google Scholar]
- 32.Smirnova I. V., Kajstura M., Sawamura T., Goligorsky M. S. 2004. Asymmetric dimethylarginine upregulates LOX-1 in activated macrophages: role in foam cell formation. Am. J. Physiol. Heart Circ. Physiol. 287: H782–H790 [DOI] [PubMed] [Google Scholar]
- 33.Lougheed M., Steinbrecher U. P. 1996. Mechanism of uptake of copper-oxidized low density lipoprotein in macrophages is dependent on its extent of oxidation. J. Biol. Chem. 271: 11798–11805 [DOI] [PubMed] [Google Scholar]
- 34.Kume N., Moriwaki H., Kataoka H., Minami M., Murase T., Sawamura T., Masaki T., Kita T. 2000. Inducible expression of LOX-1, a novel receptor for oxidized LDL, in macrophages and vascular smooth muscle cells. Ann. N. Y. Acad. Sci. 902: 323–327 [DOI] [PubMed] [Google Scholar]
- 35.Draude G., Lorenz R. L. 2000. TGF-beta1 downregulates CD36 and scavenger receptor A but upregulates LOX-1 in human macrophages. Am. J. Physiol. Heart Circ. Physiol. 278: H1042–H1048 [DOI] [PubMed] [Google Scholar]
- 36.Tabas I.2005. Consequences and therapeutic implications of macrophage apoptosis in atherosclerosis: the importance of lesion stage and phagocytic efficiency. Arterioscler. Thromb. Vasc. Biol. 25: 2255–2264 [DOI] [PubMed] [Google Scholar]
- 37.Salomonsson L., Pettersson S., Englund M. C., Wiklund O., Ohlsson B. G. 2002. Post-transcriptional regulation of VEGF expression by oxidised LDL in human macrophages. Eur. J. Clin. Invest. 32: 767–774 [DOI] [PubMed] [Google Scholar]
- 38.Claus R., Fyrnys B., Deigner H. P., Wolf G. 1996. Oxidized low-density lipoprotein stimulates protein kinase C (PKC) and induces expression of PKC-isotypes via prostaglandin-H-synthase in P388D1 macrophage-like cells. Biochemistry. 35: 4911–4922 [DOI] [PubMed] [Google Scholar]
- 39.Li D., Liu L., Chen H., Sawamura T., Mehta J. L. 2003. LOX-1, an oxidized LDL endothelial receptor, induces CD40/CD40L signaling in human coronary artery endothelial cells. Arterioscler. Thromb. Vasc. Biol. 23: 816–821 [DOI] [PubMed] [Google Scholar]
- 40.Li D., Liu L., Chen H., Sawamura T., Ranganathan S., Mehta J. L. 2003. LOX-1 mediates oxidized low-density lipoprotein-induced expression of matrix metalloproteinases in human coronary artery endothelial cells. Circulation. 107: 612–617 [DOI] [PubMed] [Google Scholar]
- 41.Iwai-Kanai E., Hasegawa K., Sawamura T., Fujita M., Yanazume T., Toyokuni S., Adachi S., Kihara Y., Sasayama S. 2001. Activation of lectin-like oxidized low-density lipoprotein receptor-1 induces apoptosis in cultured neonatal rat cardiac myocytes. Circulation. 104: 2948–2954 [DOI] [PubMed] [Google Scholar]
- 42.Li D., Mehta J. L. 2000. Antisense to LOX-1 inhibits oxidized LDL-mediated upregulation of monocyte chemoattractant protein-1 and monocyte adhesion to human coronary artery endothelial cells. Circulation. 101: 2889–2895 [DOI] [PubMed] [Google Scholar]
- 43.Imanishi T., Hano T., Sawamura T., Takarada S., Nishio I. 2002. Oxidized low density lipoprotein potentiation of Fas-induced apoptosis through lectin-like oxidized-low density lipoprotein receptor-1 in human umbilical vascular endothelial cells. Circ. J. 66: 1060–1064 [DOI] [PubMed] [Google Scholar]
- 44.Nakagawa T., Yasuda T., Hoshikawa H., Shimizu M., Kakinuma T., Chen M., Masaki T., Nakamura T., Sawamura T. 2002. LOX-1 expressed in cultured rat chondrocytes mediates oxidized LDL-induced cell death-possible role of dephosphorylation of Akt. Biochem. Biophys. Res. Commun. 299: 91–97 [DOI] [PubMed] [Google Scholar]
- 45.Steinbrecher U. P., Gomez-Munoz A., Duronio V. 2004. Acid sphingomyelinase in macrophage apoptosis. Curr. Opin. Lipidol. 15: 531–537 [DOI] [PubMed] [Google Scholar]
- 46.Fadok V. A., Xue D., Henson P. 2001. If phosphatidylserine is the death knell, a new phosphatidylserine-specific receptor is the bellringer. Cell Death Differ. 8: 582–587 [DOI] [PubMed] [Google Scholar]
- 47.Delerive P., Furman C., Teissier E., Fruchart J., Duriez P., Staels B. 2000. Oxidized phospholipids activate PPARalpha in a phospholipase A2-dependent manner. FEBS Lett. 471: 34–38 [DOI] [PubMed] [Google Scholar]
- 48.Lee H., Shi W., Tontonoz P., Wang S., Subbanagounder G., Hedrick C. C., Hama S., Borromeo C., Evans R. M., Berliner J. A., et al. 2000. Role for peroxisome proliferator-activated receptor alpha in oxidized phospholipid-induced synthesis of monocyte chemotactic protein-1 and interleukin-8 by endothelial cells. Circ. Res. 87: 516–521 [DOI] [PubMed] [Google Scholar]
- 49.Nagy L., Tontonoz P., Alvarez J., Chen H., Evans R. 1998. Oxidized LDL regulates macrophage gene expression through ligand activation of PPARg. Cell. 93: 229–240 [DOI] [PubMed] [Google Scholar]
- 50.Davies S. S., Pontsler A. V., Marathe G. K., Harrison K. A., Murphy R. C., Hinshaw J. C., Prestwich G. D., Hilaire A. S., Prescott S. M., Zimmerman G. A., et al. 2001. Oxidized alkyl phospholipids are specific, high affinity peroxisome proliferator-activated receptor gamma ligands and agonists. J. Biol. Chem. 276: 16015–16023 [DOI] [PubMed] [Google Scholar]
- 51.Pontsler A. V., St Hilaire A., Marathe G. K., Zimmerman G. A., McIntyre T. M. 2002. Cyclooxygenase-2 is induced in monocytes by peroxisome proliferator activated receptor gamma and oxidized alkyl phospholipids from oxidized low density lipoprotein. J. Biol. Chem. 277: 13029–13036 [DOI] [PubMed] [Google Scholar]
- 52.Miller Y. I., Viriyakosol S., Binder C. J., Feramisco J. R., Kirkland T. N., Witztum J. L. 2003. Minimally modified LDL binds to CD14, induces macrophage spreading via TLR4/MD-2, and inhibits phagocytosis of apoptotic cells. J. Biol. Chem. 278: 1561–1568 [DOI] [PubMed] [Google Scholar]
- 53.Stanton L. W., White R. T., Bryant C. M., Protter A. A., Endemann G. 1992. A macrophage Fc receptor for IgG is also a receptor for oxidized low density lipoprotein. J. Biol. Chem. 267: 22446–22451 [PubMed] [Google Scholar]