Abstract
Long-chain acylcarnitines accumulate in long-chain fatty acid oxidation defects, especially during periods of increased energy demand from fat. To test whether this increase in long-chain acylcarnitines in very long-chain acyl-CoA dehydrogenase (VLCAD−/−) knock-out mice correlates with acyl-CoA content, we subjected wild-type (WT) and VLCAD−/− mice to forced treadmill running and analyzed muscle long-chain acyl-CoA and acylcarnitine with tandem mass spectrometry (MS/MS) in the same tissues. After exercise, long-chain acyl-CoA displayed a significant increase in muscle from VLCAD−/− mice [C16:0-CoA, C18:2-CoA and C18:1-CoA in sedentary VLCAD−/−: 5.95 ± 0.33, 4.48 ± 0.51, and 7.70 ± 0.30 nmol · g−1 wet weight, respectively; in exercised VLCAD−/−: 8.71 ± 0.42, 9.03 ± 0.93, and 14.82 ± 1.20 nmol · g−1 wet weight, respectively (P < 0.05)]. Increase in acyl-CoA in VLCAD-deficient muscle was paralleled by a significant increase in the corresponding chain length acylcarnitine. Exercise resulted in significant lowering of the free carnitine pool in VLCAD−/− muscle. This is the first study demonstrating that acylcarnitines and acyl-CoA directly correlate and concomitantly increase after exercise in VLCAD-deficient muscle.
Keywords: β-oxidation, very long-chain acyl-CoA dehydrogenase, acyl-coenzyme A, acylcarnitine
Very-long-chain acyl-CoA dehydrogenase (VLCAD or ACADVL, EC 1.3.99.3) catalyzes the first step in the β-oxidation pathway of long (C14 or longer) chain fatty acids. Deficiency of this enzyme severely impairs the oxidation of long-chain fatty acids (1). Mutations in the VLCAD gene lead to clinical symptoms in children and young adults. Physiological stressors such as fasting, exercise, and viral illnesses are triggers of disease crises (2, 3). Neonatal screening programs (4, 5) have, however, drastically improved long-term survival and a great number of patients have remained asymptomatic with proper preventive measures during the first years of life (4). However, as there is no prevalent VLCAD mutation and VLCAD mutations cannot be correlated to residual VLCAD activity, as measured in fibroblasts or lymphocytes, the late-onset disease phenotype varies greatly (6). Neonatal screening programs are thus identifying more and more patients carrying VLCAD mutations whose clinical outcome cannot be predicted at the time of diagnosis. Therefore, the effect of possible disease triggers, such as exercise-stress, and their resulting metabolic phenotype (i.e., acylcarnitine and/or acyl-CoA accumulation) need to be identified.
With the generation of a transgenic VLCAD-deficient mouse (VLCAD− / −) that displays a stress-induced phenotype similar to that of humans (7, 8), new possibilities arise to study the effect of physiological stressors, such as fasting and exercise, at tissue level. In these mice it was shown that they have impaired Ca2+ handling (9) and heart rate dysfunction (10). VLCAD− / − soleus muscle showed an increase in mitochondrial density, and mitochondria were more heterogeneous in size and appeared disorganized (7). Similar to VLCADD-patients, the metabolic VLCAD− / − mouse phenotype displays an accumulation of fat droplets (7) and long-chain acylcarnitines (11) in muscle.
Acylcarnitines are known to have unspecific cytotoxic effects (12, 13) and accumulate in VLCAD-deficient patients during crisis due to insufficient oxidation capacity of long-chain fatty acids (14). As cytosolic carnitine palmitoyltransferase (CPT) I and mitochondrial CPT-II catalyze the forward and reverse conjugation of acyl-CoA to carnitine, increased long-chain acylcarnitine content in VLCAD− / − tissue is expected, but not proven, to be accompanied by increased long-chain acyl-CoA levels. Long-chain acyl-CoA accumulation in tissue may well result in lower mitochondrial respiration via inhibition the mitochondrial adenine nucleotide translocator in the μM range (15).
Taken together, although the accumulation of long-chain acylcarnitines in VLCADD-patients and in the corresponding mouse model has been demonstrated, an increase in long-chain acyl-CoA levels has, so far, only been assumed.
For the first time, we are now able to simultaneously measure acyl-CoA and acylcarnitines in muscle from VLCAD-deficient mice after exercise stress. Both measurements are performed by tandem mass spectrometry in the same muscle sample, and therefore, allow accurate and direct correlation of both metabolites in sedentary and exercised muscle.
MATERIALS AND METHODS
Reagents
Heptadecanoyl-CoA (C17-CoA) was purchased from Sigma (Deisenhofen, Germany) as Li+ salts and stored at -20°C. Internal standard heptadecanoyl-CoA was prepared by dissolving in methanol to obtain a concentration of 2.5 mM and stored at − 80°C until use.
Animals
VLCAD-deficient mice were generated as described in detail previously (7). Experiments were performed on second- to third-generation intercrosses of C57BL6+129sv VLCAD genotypes. Littermates served as controls, and genotyping of mice was performed as described previously (16). Mice were fed with a standard chow diet (MZ Extrudat from sniff® containing 4.5% w/w crude fat, corresponding to 13% of total metabolizable energy according to the Atwater System) and received tap water ad libitum. As mice are nocturnal animals, treadmill running was performed during the dark cycle. All animal studies were in accordance with the Heinrich-Heine-University Medical Center and Institutional Animal Care and Use Committee guidelines.
Training protocol
Four-month-old WT and VLCAD− / − animals ran 120 min on a Columbus Instruments Simplex II metabolic rodent treadmill, consisting of four individual lanes, without inclination, with shock plate incentive (3 Hz, 200 msec, 160 V, 1.5 mA). Mice were placed in an exercise chamber, and after an adaptation period of 15 min, initial belt speed was set to 4 m/min and increased every 5 min by 2 m/min to a maximum of 15 m/min. Mice ran until they displayed signs of exhaustion (>2 s spent on the shocker plate without attempting to reengage the treadmill). To test for long-term alterations in skeletal muscle mitochondrial content, WT and VLCAD− / − animals ran for 5 days at an average belt-speed of 8 m/min. The sedentary group consisted of four wild-type and five VLCAD− / − animals, and the exercised group consisted of four wild-type and four VLCAD− / − animals.
Acyl-CoA analysis
Hind leg skeletal muscle (100–200 mg) was taken up in five volumes of extraction buffer (1:1:1 iso-propanol / acetonitrile / 10 mM NH4Ac, pH = 5.0) and minced on ice with scissors. Five nmoles of heptadecanoyl-CoA (C17:0-CoA) were added as internal standard, and the tissue was homogenized using an Ultra-Turrax®. Next, the homogenate was centrifuged at 1000 g for 5 min, the supernatant was collected, and the pellet was rehomogenized in five volumes of extraction buffer. The homogenate was centrifuged at 1000 g for 5 min, and the supernatants were pooled. The pooled supernatants were centrifuged at 1000 g for 5 min to remove any remaining cellular debris. For analysis, sample volumes were reduced by approximately 50% using evaporation at 40°C under air.
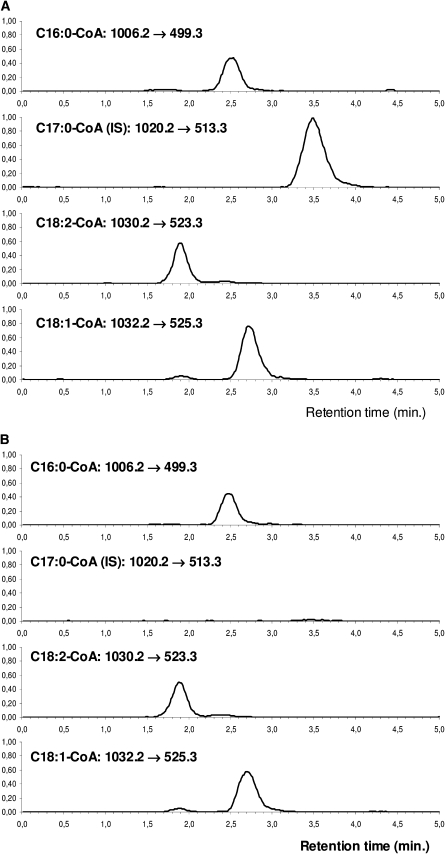
For LC-tandem MS analysis, a Waters 2795 Alliance HPLC system (Waters, Milford, UK) equipped with a thermostated autosampler was used for solvent delivery and sample introduction. Assay samples were placed in a cooled sample tray, and 20 μl were injected onto a reversed-phase Symmetry C18 column (3.5μ; 7.5 × 4.6 mm; WAT066224; Waters), protected by a guard column (SecurityGuard C18 ODS; 4 × 2.0 mm; Phenomenex). C18:2-CoA, C18:1-CoA, C16:0-CoA, and C17:0-CoA were eluted isocratically with 55% (v/v) acetonitrile in 10 mM NH4Ac (pH = 6.5) at a flow rate of 0.6 ml/min. The eluate was delivered into a Quattro Micro API tandem mass spectrometer (Micromass, Cambridge, UK) with an ESI probe in positive-ion mode. The waste valve was used to discard early-eluting salts from contaminating the mass spectrometer. The injection interval was 5 min. Nitrogen was used as drying gas at a flow rate of 700 L/h. The collision energy using argon as collision gas was 35 eV. The declustering potential was 45 V, and the ion source temperature was 120°C. Compounds were detected in the multiple-reaction monitoring (MRM) mode. A representative chromatogram, with (A) and without (B) internal standard C17:0-CoA, is shown in Fig. 1. Specific transitions were used for each metabolite, but HPLC separation remained necessary to remove interfering compounds, such as salts, present in muscle extracts, thereby preventing tandem MS apparatus contamination.
Fig. 1.
Multiple Reactant Monitoring (MRM) chromatograms of a mouse muscle extracts containing long-chain fatty acyl-CoA esters with (A) and without (B) internal standard C17:0-CoA. Monitored transitions, in order of elution: linoleoyl-CoA (C18:2-CoA): 1030.2 → 523.3; palmitoyl-CoA (C16:0-CoA): 1006.2 → 499.3; oleoyl-CoA (C18:1-CoA): 1032.2 → 526.3; heptadecanoyl-CoA (C17:0-CoA) 1020.2 → 513.3.
Acylcarnitine and carnitine analysis
In tissues, analysis of carnitine and acylcarnitines was performed according to van Vlies et al. (17). Briefly, 50 mg of blotted skeletal muscle was lyophilized for 12 h, including internal standards (16.25 nmoles [2H3]carnitine and 0.05 nmoles [2H3]C16-acylcarnitine). The lyophilized tissues were pulverized and dissolved in 1 ml of 80% v/v acetonitrile. After homogenization and centrifugation, the supernatant was dried. Finally, carnitine and acylcarnitines were analyzed by electrospray ionization tandem mass spectrometry as their butyl esters and resuspended in 100 µL ACN/H2O (50/50% v/v).
Citrate synthase activity
Citrate synthase enzyme activity of tissue homogenates was determined to characterize Krebs cycle activity as described previously (18). Samples were assayed in duplicate in flat-bottom microtiter plates, the absorption coefficient was adjusted to the reaction volume (i.e., calculated light path). The media used in the assays were adjusted to 0.1% Triton X-100 to obtain maximal enzyme activities in tissue homogenates.
Cytochrome c oxidase subunit 3 mRNA expression
Expression of mitochondrial DNA encoded cytochrome c oxidase subunit 3 (cox3) mRNA expression was performed as described previously (19) to determine the relative mitochondrial quantity. β-Actin mRNA expression was unaffected by our experimental design and was used as a reference. Cox4 mRNA/cDNA concentration was normalized for the concentrations of β-actine mRNA/cDNA in the same sample. Values are stated as percentage of the sedentary control samples.
Protein determination
Protein concentration of tissue homogenates was determined by the BCA assay (Pierce). The BCA reagent was supplemented with 0.1% (v/v) Triton X-100. BSA was used as standard.
Data analysis and statistics
Data were acquired and analyzed using MassLynx NT v4.0 software (Micromass, UK). Data were analyzed with Origin 6.0 (Microcal Software Inc., Northhampton, MA). If not stated otherwise, reported data are presented as arithmetic means ± standard deviation (SD) with n denoting the number of animals. Statistical analyses were performed using Student’s t-test. Differences between means were considered significant if P < 0.05.
RESULTS
Animal condition and mitochondrial content
In contrast to WT mice, VLCAD− / − mice had difficulty maintaining a running speed of 15 m/min for the full 120 min, resulting in shorter running times for VLCAD− / − mice (Table 1). To stress the mice to their maximal capacity, different running times were needed. Body weights were similar for sedentary WT and VLCAD− / − mice (Table 1). Possible proliferation of muscle mitochondria, due to VLCAD-deficiency and/or exercise, was studied by measuring indirect marker enzyme citrate synthase and the expression of cytochrome c oxidase subunit 3 (cox3) mRNA. Muscle citrate synthase activity (in mU · mg protein− 1) and cox3 expression were not significantly different in WT as well as VLCAD− / − mouse skeletal muscle (Table 1).
TABLE 1.
Animal condition, exercise capacity, and muscle mitochondrial content
| Sedentary |
Exercised |
|||
|---|---|---|---|---|
| Wild-type (n = 4) | VLCAD (n = 5) | Wild-type (n = 4) | VLCAD (n = 4) | |
| Body weight (g) | 25.5 ± 4.2 | 30.0 ± 8.1 | 30.7 ± 9.8 | 24.56 ± 3.2 |
| Running time (min) | — | — | 120 | 95 |
| Citrate synthase activity | 244.7 ± 28.0 | 242.3 ± 59.1 | 258.6 ± 14.0 | 271.7 ± 15.4 |
| Cox3 mRNA (%) | 100 | 99.0 ± 2.7 | 97.5 ± 2.9 | 99.5 ± 1.9 |
Expressed as citrate synthase activity (mU · mg protein1) and Cox3 mRNA expression (as % of wild-type). VLCAD, very long-chain acyl-CoA dehydrogenase.
Muscle acyl-CoA and acylcarnitine content
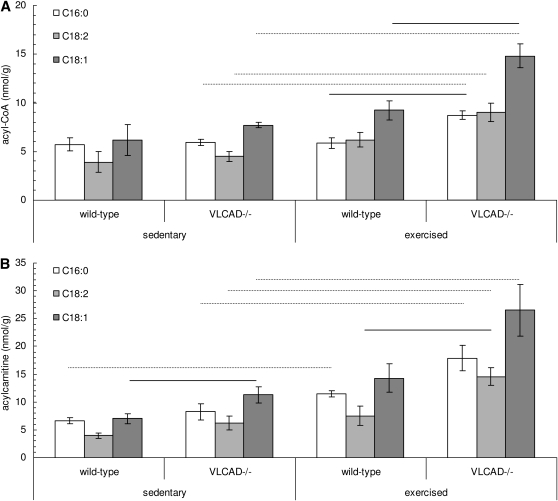
We first measured muscle acylcarnitine and acyl-CoA content in WT and VLCAD− / − mice under sedentary conditions. Overall levels of long-chain acylcarnitines and acyl-CoA were similar in sedentary WT and VLCAD− / − muscle, with the exception of muscle C18:1-carnitine content, which was significantly higher in VLCAD− / − mice compared with WT mice [11.29 ± 3.26 (n = 5) and 7.01 ± 1.74 (n = 4) nmol · g− 1 wet weight, respectively; P < 0.05] (Fig. 2B).
Fig. 2.
Muscle acyl-CoA (A) and acylcarnitine (B) levels in sedentary WT (n = 4) and VLCAD− / − (n = 5) and exercised WT (n = 4) and VLCAD− / − (n = 4) mice. Values are means ± SEM. (—), P < 0.05 compared with WT. (⋯⋯⋯), P < 0.05 compared with sedentary mice. VLCAD, very long-chain acyl-CoA dehydrogenase; WT, wild type.
After exercise stress, consisting of treadmill running at 15 m/min, analysis of long-chain acyl-CoAs revealed a significant increase in VLCAD− / − muscle [C16:0-CoA, C18:2-CoA, and C18:1-CoA in sedentary VLCAD− / −: 5.95 ± 0.74, 4.48 ± 1.14, and 7.70 ± 0.67 nmol · g− 1 wet weight, respectively (n = 5); in exercised VLCAD− / −: 8.71 ± 0.84, 9.03 ± 1.86, and 14.82 ± 2.40 nmol · g− 1 wet weight, respectively; P < 0.05 (n = 4)] (Fig. 2A). This exercise-induced increase in VLCAD-deficient muscle was paralleled by a similar and significant increase in corresponding acylcarnitines with the same acyl-chain length [C16:0-carnitine, C18:2-carnitine, and C18:1-carnitine in sedentary VLCAD− / −: 8.26 ± 3.29, 6.22 ± 2.78, and 11.29 ± 3.27 nmol · g− 1 wet weight, respectively (n = 5); in exercised VLCAD− / −: 17.91 ± 4.62, 14.58 ± 3.08, and 26.50 ± 9.16 nmol · g− 1 wet weight, respectively; P < 0.05 (n = 4)] (Fig. 2B).
Wild-type mice showed a significant increase in C16:0-carnitine after 2 h of belt running, compared with sedentary palmitoyl-carnitine muscle content [6.64 ± 1.14 and 11.46 ± 1.08 nmol · g− 1 wet weight for sedentary versus exercised WT mice; P < 0.05 (n = 4)] (Fig. 2B).
We observed no significant differences between groups in muscle medium-chain octanoyl (C8:0)-carnitine content (data not shown).
Muscle free L-carnitine content
Exercise resulted in lowering of carnitine in both WT and VLCAD− / − muscle. This reduction was significant in VLCAD− / − mice but not in WT mice [carnitine muscle levels in sedentary and exercised VLCAD− / −: 154.3 ± 35.8 (n = 5) and 106.5 ± 13.6 (n = 4) nmol · g− 1 wet weight (P < 0.05), respectively; in sedentary and exercised WT: 199.3 ± 40.4 (n = 4) and 134.7 ± 37.0 (n = 4) nmol · g− 1 wet weight, respectively].
DISCUSSION
In the present study, long-chain acylcarnitines and acyl-CoAs were measured to monitor the effect of exercise stress on VLCAD-deficient muscle. We were able to show for the first time that exercise stress, consisting of treadmill running, resulted in a significant, corresponding increase in C16:0, C18:1, and C18:2-carnitine and -CoA in VLCAD− / − mouse muscle. Accumulation of long-chain acylcarnitines is well documented and has been implicated in the development of rhabdomyolysis in VLCAD-deficient patients (20). However, it is unknown whether, and to what extent, this increase in long-chain acylcarnitines is mirrored by an increase in acyl-CoA esters of corresponding chain length. The mouse model of VLCAD-deficiency also displays acylcarnitine accumulation in tissues during fasting and exercise stress (16) and is, therefore, a suitable animal model to study the effects of VLCAD-deficiency at a cellular level.
First, we measured if mitochondrial content was altered in exercised VLCAD− / − muscle. This is of importance because in heart muscle approximately 95% of the cellular CoA pool is located inside mitochondria (21). Previous studies have shown that mitochondrial density increases in oxidative soleus and heart muscle of VLCAD-deficient mice (7). In glycolytic skeletal gastrocnemius and extensor digitorum longus muscle from WT and VLCAD− / − mice, we compared mitochondrial content, based on citrate synthase enzyme activity, as indirect marker, and Cox3 mRNA expression, and observed that, in contrast to oxidative muscle, mitochondrial content was identical for WT and VLCAD− / − muscle at rest and did not increase due to exercise (Table 1). The observation of similar citrate synthase activity in sedentary and exercised murine skeletal muscle is in line with previous work from Jeneson et al. (22), where treadmill running had no effect on glycolytic fast-twitch extensor digitorum longus citrate synthase content. Taken together, these data suggest that in oxidative muscle mitochondrial proliferation occurs as compensatory mechanism, on the energy supply side of cellular energy homeostasis, in an effort to counteract impaired fatty acid oxidation. In contrast, in glycolytic skeletal muscles, this cellular remodelling appears not to take place.
Long-chain acylcarnitine content, by measuring C16:0, C18:1, and C18:2-carnitine, was slightly higher in VLCAD− / − striated skeletal muscle under sedentary conditions, as published previously (16). However, this increase was only significant for C18:1-carnitine, an observation also reported by Cox et al. (23). In sedentary VLCAD− / − muscle, long-chain C16:0, C18:1, and C18:2 acyl-CoA were not increased, compared with WT muscle. The absolute content of long-chain acyl-CoA (in nmol · g− 1 wet weight) in WT mouse muscle was comparable to reported values in literature, obtained with both HPLC and mass spectrometry techniques (24–28).
Exercise stress significantly increased long-chain acyl metabolites, with C18:1 chain-length having the most pronounced effect. Elevation of C18:1-carnitine and C18:1-CoA was approximately 4-fold and 3-fold, respectively, in exercised VLCAD− / − muscle, as compared with sedentary WT muscle. Unlike WT-exercised muscle, all long-chain acyl CoA esters significantly increased in VLCAD− / − muscle upon exercise (Fig. 2), illustrating the impact of workload on the VLCAD-deficient phenotype and the extent of activated fatty acid oxidation. In addition, it appears that during periods of elevated workload, the carnitine pool of VLCAD-deficient muscle can no longer adequately scavenge acyl moieties, resulting in a significant increase in muscle long-chain acyl-CoA esters. Importantly, this exercise-induced fast increase in long-chain acyl-CoA esters is absent in WT muscle.
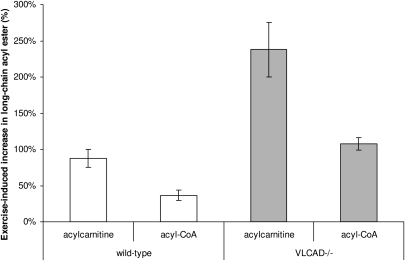
The effect of enhanced workload on long-chain acyl-CoA ester levels can be better illustrated by plotting the exercise-induced increase in acyl-CoA and acylcarnitine as percentage of sedentary levels (Fig. 3). We clearly observe that exercise results in a stronger accumulation of acylcarnitines than acyl-CoAs in both WT as well as VLCAD− / − mice. And although the magnitude by which both long-chain acylcarnitines and acyl-CoAs increase is higher in VLCAD− / − as compared with WT muscle (Fig. 3), importantly in relation to each other, these acyl CoA esters appear to be well equilibrated by the action of carnitine acyltransferases. This is observed in WT and VLCAD− / − muscle, as the exercise-induced increase in long-chain acylcarnitines is approx. 2.5-fold higher than the increase in corresponding acyl-CoA esters (Fig. 3). Furthermore, the proposed role of the carnitine pool functioning as acyl scavenger is supported by our measurements of muscle free carnitine, demonstrating a significant drop in free carnitine in VLCAD− / − muscle upon exercise. Thus, during periods of elevated workload, the acylation state of the cytosolic carnitine pool increases more than that of the mitochondrial pool, indicative of acyl compounds being exported out of the muscle cell (29). This observation fits well with the increased levels of long-chain acylcarnitines in serum and blood from VLCAD− / − mice (16, 23), as acylcarnitines in blood would accumulate over time due to cellular efflux.
Fig. 3.
Exercise-induced increase, as percentage of sedentary levels, in long-chain (C16:0, C18:2, and C18:1) CoA and carnitine esters in WT (n = 4) and VLCAD− / − (n = 5) mouse muscle. Values are means ± SEM. Significances not tested. VLCAD, very long-chain acyl-CoA dehydrogenase; WT, wild type.
Acylcarnitines are known to have unspecific cytotoxic effects (12) and long-chain acyl-CoAs are known to inhibit the mitochondrial adenine nucleotide translocator (15), however, further studies have to determine which of these two metabolites is more cytotoxic in vivo. Thus, in addition to impaired energy production from long-chain fatty acids due to the enzyme defect itself, toxic effects of accumulating acylcarnitines and acyl-CoA esters may play an important role in disease pathogenesis. Interestingly, detailed histological investigation of VLCAD− / − mouse heart has indeed demonstrated an increase in degenerative fibers, collagen deposition, and vacuolated myocytes, which may be indicative of cytotoxicity (7). These cellular alterations in VLCAD-deficient heart were accompanied by changes in mitochondrial ultrastructure, an adaptive response also observed in VLCAD− / − solues muscle cells (7). However, it is currently still unknown whether these changes are the direct consequence of accumulating (long-chain) acylcarnitines and acyl-CoA esters. Of note, calculation of absolute cytosolic concentrations based on tissue content (in nmol · g− 1) should be treated with caution because free long-chain fatty acid and acyl-CoA concentrations are buffered by fatty acid and acyl-CoA binding proteins inside the cell (30), thereby lowering the concentration on free acyl compounds.
In conclusion, we demonstrate for the first time that long-chain acylcarnitines and acyl-CoA esters increase and directly correlate in VLCAD− / − mouse muscle after exercise. However, the question whether exercise-induced accumulation of acyl esters is an important factor in disease pathogenesis cannot be answered at this time.
Acknowledgments
The authors thank Dr. M.D. Laryea, Department of General Pediatrics, Heinrich-Heine-University, Duesseldorf, Germany, for expert advice on liquid-chromatography analysis. We are grateful to Mrs. G. Schmitz and Mr. A. Vogt for technical assistance.
Footnotes
Abbreviations:
- CPT
- cytosolic carnitine palmitoyltransferase
- MS/MS
- tandem mass spectrometry
- WT
- wild type
- VLCAD
- very long-chain acyl-CoA dehydrogenase
REFERENCES
- 1.Wanders R. J., Vreken P., den Boer M. E., Wijburg F. A., van Gennip A. H., Ijlst L. 1999. Disorders of mitochondrial fatty acyl-CoA beta-oxidation. J. Inherit. Metab. Dis. 22: 442–487 [DOI] [PubMed] [Google Scholar]
- 2.Mathur A., Sims H. F., Gopalakrishnan D., Gibson B., Rinaldo P., Vockley J., Hug G., Strauss A. W. 1999. Molecular heterogeneity in very-long-chain acyl-CoA dehydrogenase deficiency causing pediatric cardiomyopathy and sudden death. Circulation. 99: 1337–1343 [DOI] [PubMed] [Google Scholar]
- 3.Strauss A. W., Powell C. K., Hale D. E., Anderson M. M., Ahuja A., Brackett J. C., Sims H. F. 1995. Molecular basis of human mitochondrial very-long-chain acyl-CoA dehydrogenase deficiency causing cardiomyopathy and sudden death in childhood. Proc. Natl. Acad. Sci. USA. 92: 10496–10500 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Liebig M., Schymik I., Mueller M., Wendel U., Mayatepek E., Ruiter J., Strauss A. W., Wanders R. J., Spiekerkoetter U. 2006. Neonatal screening for very long-chain acyl-coA dehydrogenase deficiency: enzymatic and molecular evaluation of neonates with elevated C14:1-carnitine levels. Pediatrics. 118: 1065–1069 [DOI] [PubMed] [Google Scholar]
- 5.Schymik I., Liebig M., Mueller M., Wendel U., Mayatepek E., Strauss A. W., Wanders R. J., Spiekerkoetter U. 2006. Pitfalls of neonatal screening for very-long-chain acyl-CoA dehydrogenase deficiency using tandem mass spectrometry. J. Pediatr. 149: 128–130 [DOI] [PubMed] [Google Scholar]
- 6.Vianey-Saban C., Divry P., Brivet M., Nada M., Zabot M. T., Mathieu M., Roe C. 1998. Mitochondrial very-long-chain acyl-coenzyme A dehydrogenase deficiency: clinical characteristics and diagnostic considerations in 30 patients. Clin. Chim. Acta. 269: 43–62 [DOI] [PubMed] [Google Scholar]
- 7.Exil V. J., Roberts R. L., Sims H., McLaughlin J. E., Malkin R. A., Gardner C. D., Ni G., Rottman J. N., Strauss A. W. 2003. Very-long-chain acyl-coenzyme a dehydrogenase deficiency in mice. Circ. Res. 93: 448–455 [DOI] [PubMed] [Google Scholar]
- 8.Spiekerkoetter U., Tokunaga C., Wendel U., Mayatepek E., Exil V., Duran M., Wijburg F. A., Wanders R. J., Strauss A. W. 2004. Changes in blood carnitine and acylcarnitine profiles of very long-chain acyl-CoA dehydrogenase-deficient mice subjected to stress. Eur. J. Clin. Invest. 34: 191–196 [DOI] [PubMed] [Google Scholar]
- 9.Werdich A. A., Baudenbacher F., Dzhura I., Jeyakumar L. H., Kannankeril P. J., Fleischer S., LeGrone A., Milatovic D., Aschner M., Strauss A. W., et al. 2007. Polymorphic ventricular tachycardia and abnormal Ca2+ handling in very-long-chain acyl-CoA dehydrogenase null mice. Am. J. Physiol. Heart Circ. Physiol. 292: H2202–H2211 [DOI] [PubMed] [Google Scholar]
- 10.Exil V. J., Gardner C. D., Rottman J. N., Sims H., Bartelds B., Khuchua Z., Sindhal R., Ni G., Strauss A. W. 2006. Abnormal mitochondrial bioenergetics and heart rate dysfunction in mice lacking very-long-chain acyl-CoA dehydrogenase. Am. J. Physiol. Heart Circ. Physiol. 290: H1289–H1297 [DOI] [PubMed] [Google Scholar]
- 11.Liebig M., Gyenes M., Brauers G., Ruiter J. P., Wendel U., Mayatepek E., Strauss A. W., Wanders R. J., Spiekerkoetter U. 2006. Carnitine supplementation induces long-chain acylcarnitine production–studies in the VLCAD-deficient mouse. J. Inherit. Metab. Dis. 29: 343–344 [DOI] [PubMed] [Google Scholar]
- 12.Primassin S., ter Veld F., Mayatepek E., Spiekerkoetter U. 2008. Carnitine supplementation induces acylcarnitine production in tissues of very long-chain acyl-CoA dehydrogenase-deficient mice, without replenishing low free carnitine. Pediatr. Res. 63: 632–637 [DOI] [PubMed] [Google Scholar]
- 13.Bonnet D., Martin D., Pascale D. L., Villain E., Jouvet P., Rabier D., Brivet M., Saudubray J. M. 1999. Arrhythmias and conduction defects as presenting symptoms of fatty acid oxidation disorders in children. Circulation. 100: 2248–2253 [DOI] [PubMed] [Google Scholar]
- 14.Spiekerkoetter U., Sun B., Zytkovicz T., Wanders R., Strauss A. W., Wendel U. 2003. MS/MS-based newborn and family screening detects asymptomatic patients with very-long-chain acyl-CoA dehydrogenase deficiency. J. Pediatr. 143: 335–342 [DOI] [PubMed] [Google Scholar]
- 15.Ciapaite J., Bakker S. J., Diamant M., van Eikenhorst G., Heine R. J., Westerhoff H. V., Krab K. 2006. Metabolic control of mitochondrial properties by adenine nucleotide translocator determines palmitoyl-CoA effects. Implications for a mechanism linking obesity and type 2 diabetes. FEBS J. 273: 5288–5302 [DOI] [PubMed] [Google Scholar]
- 16.Spiekerkoetter U., Tokunaga C., Wendel U., Mayatepek E., Ijlst L., Vaz F. M., van Vlies N., Overmars H., Duran M., Wijburg F. A., et al. 2005. Tissue carnitine homeostasis in very-long-chain acyl-CoA dehydrogenase-deficient mice. Pediatr. Res. 57: 760–764 [DOI] [PubMed] [Google Scholar]
- 17.van Vlies N., Tian L., Overmars H., Bootsma A. H., Kulik W., Wanders R. J., Wood P. A., Vaz F. M. 2005. Characterization of carnitine and fatty acid metabolism in the long-chain acyl-CoA dehydrogenase-deficient mouse. Biochem. J. 387: 185–193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shepherd D., Garland P. B. 1969. The kinetic properties of citrate synthase from rat liver mitochondria. Biochem. J. 114: 597–610 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Schafer C., Hoffmann L., Heldt K., Lornejad-Schafer M. R., Brauers G., Gehrmann T., Garrow T. A., Haussinger D., Mayatepek E., Schwahn B. C., et al. 2007. Osmotic regulation of betaine homocysteine-S-methyltransferase expression in H4IIE rat hepatoma cells. Am. J. Physiol. Gastrointest. Liver Physiol. 292: G1089–G1098 [DOI] [PubMed] [Google Scholar]
- 20.Smelt A. H., Poorthuis B. J., Onkenhout W., Scholte H. R., Andresen B. S., van Duinen S. G., Gregersen N., Wintzen A. R. 1998. Very long chain acyl-coenzyme A dehydrogenase deficiency with adult onset. Ann. Neurol. 43: 540–544 [DOI] [PubMed] [Google Scholar]
- 21.Idell-Wenger J. A., Grotyohann L. W., Neely J. R. 1978. Coenzyme A and carnitine distribution in normal and ischemic hearts. J. Biol. Chem. 253: 4310–4318 [PubMed] [Google Scholar]
- 22.Jeneson J. A., de Snoo M. W., Verlinden N. A., Joosten B. J., Doornenbal A., Schot A., Everts M. E. 2007. Treadmill but not wheel running improves fatigue resistance of isolated extensor digitorum longus muscle in mice. Acta Physiol. (Oxf.). 190: 151–161 [DOI] [PubMed] [Google Scholar]
- 23.Cox K. B., Hamm D. A., Millington D. S., Matern D., Vockley J., Rinaldo P., Pinkert C. A., Rhead W. J., Lindsey J. R., Wood P. A. 2001. Gestational, pathologic and biochemical differences between very long-chain acyl-CoA dehydrogenase deficiency and long-chain acyl-CoA dehydrogenase deficiency in the mouse. Hum. Mol. Genet. 10: 2069–2077 [DOI] [PubMed] [Google Scholar]
- 24.Ciapaite J., Bakker S. J. L., van Eikenhorst G., Wagner M. J., Teerlink T., Schalkwijk C. G., Fodor M., Ouwens D. M., Diamante M., Heine R. J., et al. 2007. Functioning of oxidative phosphorylation in liver mitochondria of high-fat diet fed rats. Biochim. Biophys. Acta. 1772: 307–316 [DOI] [PubMed] [Google Scholar]
- 25.Kalderon B., Sheena V., Shachrur S., Hertz R., Bar-Tana J. 2002. Modulation by nutrients and drugs of liver acyl-CoAs analyzed by mass spectrometry. J. Lipid Res. 43: 1125–1132 [DOI] [PubMed] [Google Scholar]
- 26.Sun D., Cree M. G., Wolfe R. R. 2006. Quantification of the concentration and 13C tracer enrichment of long-chain fatty acyl-coenzyme A in muscle by liquid chromatography/mass spectrometry. Anal. Biochem. 349: 87–95 [DOI] [PubMed] [Google Scholar]
- 27.Woldegiorgis G., Spennetta T., Corkey B. E., Williamson J. R., Shrago E. 1985. Extraction of tissue long-chain acyl-CoA esters and measurement by reverse-phase high-performance liquid chromatography. Anal. Biochem. 150: 8–12 [DOI] [PubMed] [Google Scholar]
- 28.Yu C., Chen Y., Cline G. W., Zhang D., Zong H., Wang Y., Bergeron R., Kim J. K., Cushman S. W., Cooney G. J., et al. 2002. Mechanism by which fatty acids inhibit insulin activation of insulin receptor substrate-1 (IRS-1)-associated phosphatidylinositol 3-kinase activity in muscle. J. Biol. Chem. 277: 50230–50236 [DOI] [PubMed] [Google Scholar]
- 29.Ramsay R. R., Arduini A. 1993. The carnitine acyltransferases and their role in modulating acyl-CoA pools. Arch. Biochem. Biophys. 302: 307–314 [DOI] [PubMed] [Google Scholar]
- 30.Faergeman N. J., Knudsen J. 1997. Role of long-chain fatty acyl-CoA esters in the regulation of metabolism and in cell signalling. Biochem. J. 323: 1–12 [DOI] [PMC free article] [PubMed] [Google Scholar]