Abstract
Proprotein convertase subtilisin/kexin type 9 (PCSK9) is predominantly expressed in liver and regulates cholesterol metabolism by down regulating liver LDL receptor (LDLR) proteins. Here we report transgenic overexpression of human PCSK9 in kidney increased plasma levels of PCSK9 and subsequently led to a dramatic reduction in liver LDLR proteins. The regulation of LDLR by PCSK9 displayed tissue specificity, with liver being the most responsive tissue. Even though the PCSK9 transgene was highly expressed in kidney, LDLR proteins were suppressed to a lower extent in this tissue than in liver. Adrenal LDLR proteins were not regulated by elevated plasma PCSK9. hPCSK9 transgene expression and subsequent reduction of liver LDLR led to increases in plasma total cholesterol, LDL cholesterol, and ApoB, which were further increased by a high-fat, high-cholesterol diet. We also observed that the size distribution of hPCSK9 in transgenic mouse plasma was heterogeneous. In chow-fed mice, the majority of PCSK9 proteins were in free forms; however, feeding a high-fat, high-cholesterol diet resulted in a shift of hPCSK9 distribution toward larger complexes. PCSK9 distribution in human plasma also exhibited heterogeneity and individual variability in the percentage of PCSK9 in free form and in large complexes. We provide strong evidence to support that human PCSK9 proteins secreted from extrahepatic tissue are able to promote LDLR degradation in liver and increase plasma LDL. Our data also suggest that LDLR protein regulation by PCSK9 has tissue specificity, with liver being the most responsive tissue.
Keywords: HFHC, low density lipoprotein receptor, proprotein convertase subtilisin/kexin type 9
Proprotein convertase subtilisin/kexin type 9 (PCSK9) was identified by its cosegregation with autosomal dominant hypercholesterolemia (1). Gain-of-function mutations in PCSK9 cause hypercholesterolemia in humans (1–4), whereas loss-of-function mutations in humans lead to a reduction in LDL cholesterol and a marked decrease in the risk of coronary heart disease (5–7). PCSK9 is a member of the subtilisin serine protease family and the proteinase K subfamily. The protein is synthesized as a proprotein that autocatalytically cleaves to generate mature protein (8). PCSK9 protein is secreted as a complex containing cleaved N-terminal prodomain, which remains associated with the catalytic domain (8). The catalytic activity is required for PCSK9 maturation and secretion (9) but appears not to be essential for the reduction of LDLR (LDL receptor) by secreted PCSK9 (10).
PCSK9 is predominantly expressed in liver, intestine, and to a lesser extent, in kidney (8). PCSK9 gene expression is regulated by cholesterol via sterol regulatory element-binding protein (SREBP) pathways (11, 12). In mice, PCSK9 gene expression is downregulated by dietary cholesterol and dramatically upregulated by hepatic overexpression of the transactivation domain of SREBP-1a and SREBP-2 (12). Deletion of PCSK9 in mice leads to elevated liver LDLR protein (but not mRNA) and to reduced plasma total cholesterol, mainly HDL cholesterol (13). Furthermore, PCSK9 deficiency increases LDL clearance and enhances the response to statin (13). Knockdown of PCSK9 by antisense approach also decreases total cholesterol in high-fat–fed mice in an LDLR dependent manner (14). Hepatic overexpression of murine or human PCSK9 by adenovirus (9, 15–16) or transgene (17) results in the reduction of liver LDLR protein and elevation of LDL cholesterol. Transgenic mice overexpressing PCSK9 in liver accumulated PCSK9 to a level of 100–400 μg/ml in plasma and reduced liver LDLR protein in wild-type mice that are parabiotically joined with transgenic mice (18). Grefhorst et al. demonstrated that infusion of recombinant hPCSK9 at 32 μg/hour gave rise to about 1 μg/ml of plasma PCSK9 and preferentially promoted LDLR degradation over a six h period (19). Mounting evidence suggests that PCSK9 functions extracellularly to promote the degradation of LDLR by directly interacting with LDLR on the cell surface, resulting in reduced receptor recycling by unknown mechanisms (18, 20–22).
In the following studies, we generated transgenic mice which primarily overexpress PCSK9 in kidney to address the function of circulating PCSK9 in the regulation of plasma lipoproteins. We provide evidence to support that plasma PCSK9 expressed in extrahepatic tissue is able to promote LDLR degradation in liver and raise plasma LDL. The data also suggest that LDLR protein regulation by PCSK9 has tissue specificity, with liver being the most responsive tissue. Finally, we analyzed the plasma distribution of PCSK9 protein in transgenic mice and in humans.
MATERIALS AND METHODS
Generation of PCSK9 transgenic mice
Full-length human PCSK9 cDNA with a C-terminal V5 tag was expressed under the transcriptional control of a composite human albumin promoter containing a 235 bp SV40 enhancer element and a 213 bp albumin promoter element (from pDRIVE-SV40-hAlb, InvivoGen, San Diego, CA). A chimeric intron (from pRL-SV40, Promega, Madison, Wisconsin) was inserted between the promoter and PCSK9 cDNA to enhance the expression. The expression cassette is flanked by a 1.2 kb insulator sequences from the chicken β-globin gene at both the 5′ and 3′ ends of the transgene (23). The human PCSK9 expression construct containing V5 tag was injected into the pronucleus of fertilized eggs from the C57BL6J/N strain as described (24). Transgenic founders and their transgenic offspring were identified by a PCR product amplified from the insulator sequences.
Animals
hPCSK9 transgenic mice and wild-type littermates were used in this study. Mice were maintained on a 12-h light/dark cycle and fed Purina Rodent Lab chow 5001 pellets (Ralston Purina Co. St. Louis, MO) or AIN-76A high-fat, high-cholesterol (HFHC) western diet (TestDiet, 20% fat, 0.15% cholesterol and 34% sucrose). The animals were fed HFHC diet for eight weeks beginning at four weeks of age. The study protocol was approved by Pfizer Institutional Animal Care and Use Committee. All animals received humane care according to the criteria stated by the National Academy of Sciences National Research Council.
Lipid measurement and lipoprotein isolation
Blood was collected from mice fasted for 4 h. Total cholesterol levels in plasma or lipoproteins were assayed by enzymatic methods (Wako Pure Chemical Industries Ltd.). Lipoprotein profiles were analyzed by fast protein liquid chromatography (FPLC). Pooled mouse plasma (200 ul) from six mice was loaded onto tandem Superose 6 columns (Pharmacia LKB Biotechnology, Piscataway, NJ) and eluted with lipoprotein separation buffer (154 mM NaCl, 1 mM EDTA and 0.02% NaN3) as described previously (25). Fresh human plasma samples from overnight-fasted healthy volunteers were analyzed with the same FPLC procedure. Informed consent and blood samples were obtained for all subjects, and studies were approved by an institutional review committee (Pfizer Research Blood Donor Program). Mouse ApoB ELISA was performed as described previously (26). Purified PCSK9 (2 μg) was mixed with 2 mg of HBS (Human Serum Albumin) and fractioned using the same FPLC procedure.
Gene expression analysis
Euthanized mice were subjected to whole-body perfusion with PBS, and tissues were harvested. RNA was isolated from tissues using Qiagen RNeasy kit as described by the manufacturer’s manual and then subjected to reverse transcription to synthesize cDNA using Reverse Transcription kit (Applied Biosystems). Three individual liver or kidney RNA samples and pooled adrenal RNA samples from three animals were used for RT-PCR. Real-time quantitative PCR analysis was performed using TaqMan mix and an ABI-7700HT Sequence Detection System (Applied Biosystems). Expression was normalized to a cyclophilin control.
Plasma PCSK9 protein quantification by ELISA
Rabbit polyclonal antibody CRN6 against a PCSK9 peptide (PEEDGTRFHRQASK) and rabbit polyclonal antibody 5761 against full-length human PCSK9 protein were used as capture and detection antibodies, respectively. Purified full-length human PCSK9 protein was used as protein standard for the ELISA (27). Ninety-six-well plates (Immulon 4HBX #3855, Thermo Electron Corp., Waltham, MA) were coated with 100 μl of CRN6 capture antibody (1 μg/ml) in phosphate buffered saline (PBS) at 4°C overnight, followed by blocking with 300 μl of 0.5% BSA (BSA) in PBS buffer. 100 μl of mouse plasma samples with a 1:500 dilution or 100 μl of purified human PCSK9 protein standards from 1 ng/ml to 128 ng/ml were incubated with capture antibody-coated plates at room temperature (RT) for 1 h. Captured PCSK9 protein was incubated with 100 μl of 1 μg/ml biotinylated (Sulfo-NHS-LC-Biotin, #21327, Pierce) rabbit polyclonal antibody 5761 for 1 h followed by detection with 1:200 diluted streptavidin-HRP (R and D, #DY998) for 45 min and 100 μl of TMB substrate (Sigma, #T4444) for about 2 min. After stopping color development with 100 μl of 2N H2SO4, the plates were read on the SpectraMax plate reader at OD450. Plasma PCSK9 levels were calculated using a standard curve generated with purified human PCSK9 protein by four-parameter fit. Concentration of plasma PCSK9 was expressed as mean ± standard deviation.
Immunoblot analysis of hepatic LDLR protein level
Euthanized mice were subjected to whole-body perfusion with PBS, and tissues were harvested. Hepatic and kidney total proteins were extracted from individual frozen tissue samples. Adrenal tissues were pooled for protein extraction. Tissues were homogenized in lysis buffer (10 mM Tris HCl, 1 mM MgCl2, 1% NP40, 1 mM EDTA, 0.5% Deoxycholate, pH 7.4) with a polytron homogenizer. The supernatants from a centrifugation at 10,000 g were collected. Individual liver or kidney protein extracts were pooled at equal amount of protein for each study group. Total proteins (30 μg) were separated by electrophoresis using NuPAGE Novex gel (4%–12% Bis-Tris, Invitrogen NP0321) and transferred to Nitrocellulose membrane (Invitrogen LC2001). Mouse LDLR protein was detected with a goat anti-mouse LDLR antibody (R and D Systems Cat# AF2255), and human PCSK9 protein in transgenic mice was detected using antibody against V5-tag (Sigma).
RESULTS
Human PCSK9 transgene expression
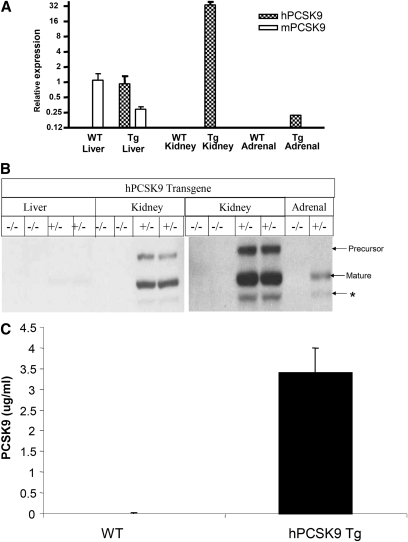
Tissue distribution of human PCSK9 (hPCSK9), transgene, and endogenous mouse PCSK9 (mPCSK9) was analyzed by quantitative PCR. Mouse PCSK9 was predominantly expressed in the liver of both wild-type and transgenic mice (Fig. 1A). Although hPCSK9 transgene expression was designed to be driven by an albumin promoter, the hPCSK9 transgene was principally expressed in kidney. Although the transcriptional mechanisms mediating kidney expression in this model are unknown, it may be dictated by the context of the SV40 enhancer and the human albumin promoter. As the high-level expression in kidney is observed in more than one founder lines, it is unlikely that it results from the genomic integration site.
Fig. 1.
Human PCSK9 transgene expressed predominately in kidney in transgenic mice. A: mRNA expression of hPCSK9 transgene and endogenous mouse PCSK9 in liver, kidney, and adrenal. Gene expression was analyzed by quantitative PCR as described in Materials and Methods. The relative gene expression was presented relative to endogenous mPCSK9 of wild-type mice. n = 3 for each group. B: Human PCSK9 protein levels in liver, kidney, and adrenal of transgenic mice. Pooled tissue extracts from wild-type and transgenic mice fed with chow diet were subjected to immunoblot analysis visualized by immunoblotting using antibody against V5-tag (n = 3). PCSK9 precursor, mature form, and a truncated form (*) are detected. C: Plasma levels of human PCSK9 in transgenic mice. Plasma PCSK9 levels were measured by ELISA as described in Materials and Methods. PCSK9, proprotein convertase subtilisin/kexin type 9.
The mRNA expression levels of hPCSK9 in liver were low and appeared to be similar to the levels of endogenous mPCSK9 in wild-type mice. We noted that hepatic endogenous mPCSK9 gene expression was reduced in hPCSK9 transgenic mice (Fig. 1A); therefore, total liver PCSK9 expression in transgenic mice may remain similar to that in wild-type controls. Very low levels of hPCSK9 transgene expression were detected in adrenal glands. Human PCSK9 protein levels were high in kidney and barely detectable in liver, consistent with mRNA expression results (Fig. 1B). A truncated form of PCSK9 was also detected and may represent the furin-cleaved product suggested by Benjannet et al (28). Human PCSK9 protein in transgenic mice was secreted and resulted in elevation of plasma PCSK9 levels to about 3 μg/ml, which is roughly ten times the levels found in human plasma (Fig. 1C).
LDL receptor protein levels in transgenic mice
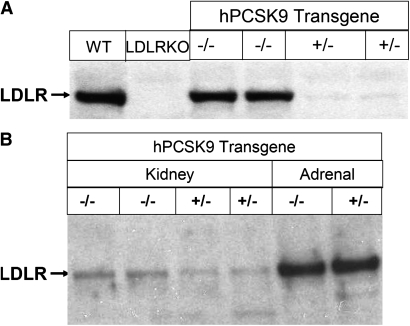
It has been shown that hepatic expression or injection of recombinant PCSK9 reduced liver LDLR protein (15, 18, 19, 29, 30). We analyzed liver LDLR protein expression to determine whether elevated plasma PCSK9 from an extrahepatic source can affect liver LDLR. Extrahepatic expression of hPCSK9 nearly abolished LDLR protein in liver (Fig. 2A). However, elevated plasma PCSK9 levels did not affect adrenal LDLR protein levels (Fig. 2B). Although kidney has the highest expression levels of hPCSK9 transgene, LDLR protein was only reduced by about 50% (Fig. 2B). Because LDLR mRNA was not significantly altered in the transgenic mice (data not shown), the decrease in the LDLR protein is most likely due to posttranscriptional mechanisms.
Fig. 2.
Effects of overexpressed human PCSK9 on LDLR protein levels in transgenic mice fed with chow diet. A: hPCSK9 expression markedly diminished liver LDLR proteins. Liver extracts from chow fed mice were pooled for immunoblot analysis. n = 3 for each group. B: hPCSK9 expression reduced kidney LDLR protein levels and did not alter adrenal LDLR proteins. Tissue extracts were pooled from chow diet fed wild-type and hPCSK9 trangenic mice as described in Materials and Methods and were subjected to immunoblot analysis. n = 3 for each group. LDLR, LDL receptor; PCSK9, proprotein convertase subtilisin/kexin type 9.
Plasma lipids and lipoprotein distribution
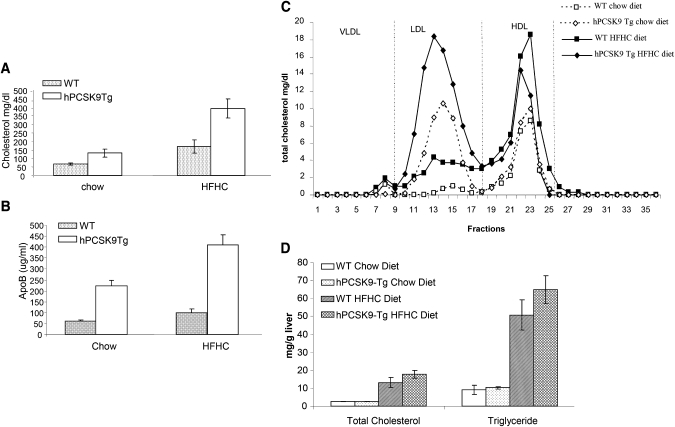
To understand the regulation of plasma lipids and lipoprotein metabolism by PCSK9 in the state of diet-induced hypercholesterolemia, hPCSK9 transgenic and wild-type mice were fed a HFHC diet for eight weeks. Similar to previous reports of hepatic overexpression of PCSK9 (9, 15, 17), extrahepatic overexpression of hPCSK9 elevated plasma total cholesterol in regular-chow-diet–fed mice by 2-fold (Fig. 3A). HFHC diet feeding increased total cholesterol in wild-type mice by about 2-fold, and hPCSK9 expression led to a further increase in plasma cholesterol to about 400 mg/dl. ApoB levels, measured by ELISA, were increased 4-fold in hPCSK9 transgenic mice compared with wild-type mice, irrespective of the diet conditions (Fig. 3B), signifying that increased cholesterol was attributable to the increase in ApoB containing LDL. Using FPLC fractionation to analyze plasma lipoprotein distribution in chow-fed transgenic mice, we confirmed that PCSK9 overexpression markedly elevated LDL cholesterol (fractions 10–18) but not HDL cholesterol (Fig. 3C). HFHC diet increased LDL and HDL in wild-type mice. PCSK9 transgene expression further increased LDL but had no significant effect on HDL (Fig. 3C). Elevation of plasma PCSK9 did not significantly change liver total cholesterol and triglyceride levels (Fig. 3D).
Fig. 3.
Effects of human PCSK9 transgene expression on plasma levels of total cholesterol, ApoB, and lipoproteins in transgenic mice. Plasma total cholesterol levels (A) and ApoB levels (B) in wild-type and hPCSK9 transgenic mice fed with either regular chow or HFHC diet for 8 weeks (n = 6 for each group). C: FPLC analysis of plasma lipoprotein profiles in wild-type and hPCSK9 transgenic mice fed with regular chow diet or HFHC diet. Pooled plasma from each group was fractionated by FPLC and total cholesterol levels in each fraction were measured to represent the lipoprotein profile. D: hPCSK9 expression did not alter liver total cholesterol and triglyceride levels. Liver lipid levels were analyzed from liver homogenate of wild-type and hPCSK9 transgenic mice fed with regular chow or HFHC diet. FPLC, fast-protein liquid chromatography; HFHC, high fat, high cholesterol; PCSK9, proprotein convertase subtilisin/kexin type 9.
PCSK9 distribution in transgenic mice and human individuals
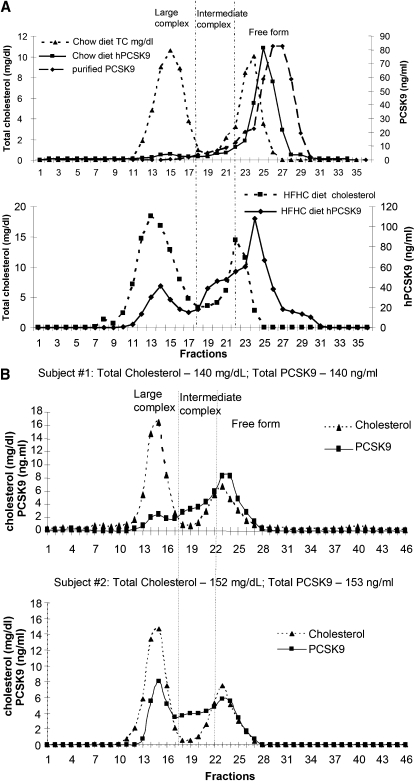
We analyzed plasma distribution of hPCSK9 protein in transgenic mice by FPLC fractionation. PCSK9 protein levels in FPLC fractions were determined by ELISA as described in Materials and Methods. In mice fed with regular chow diet, the majority of PCSK9 constituted the main peak (fractions 22–29) and overlapped with the distribution of purified hPCSK9 (Fig. 4A, upper panel), indicating the majority of overexpressed hPCSK9 proteins circulate as free forms without associating with any complexes. Only a small fraction of PCSK9 (up to 7%) was in larger complexes and coeluted with LDL fractions (fractions 10–18) (Fig. 4A). HFHC diet feeding shifted hPCSK9 distribution toward larger complexes (Fig. 4A, lower panel). The size distribution is gradual and not distinct. For the convenience of characterization and description, the complexes were categorized into two species: LDL-sized large complexes overlapping in size with LDL (fractions 10–18), and intermediate-sized complexes (fractions 19–21) that by size fell between free forms (fractions 22–29) and large complexes. Upon HFHC feeding, nearly 20% and 30% of hPCSK9 eluted in LDL-sized large complexes and intermediate complexes, respectively, and only 50% hPCSK9 proteins remained as free forms (fractions 22–29) (Fig. 4A, lower panel).
Fig. 4.
Size distribution of plasma PCSK9. A: Distribution profile of plasma hPCSK9 in transgenic mice. Pooled plasma samples from transgenic mice fed with either chow (upper panel) or HFHC diet (lower panel) were analyzed by FPLC fractionation. PCSK9 protein levels in FPLC fractions were determined by ELISA and cographed with the cholesterol profiles. 2ug of purified human PCSK9 protein was fractionated similarly to define the size distribution of free proteins. The size distribution of hPCSK9 was categorized into three forms: LDL-sized large complex, intermediate complex, and free form. (B) Representative size distribution profiles of plasma PCSK9 from two healthy human subjects. Human plasma samples were analyzed by FPLC fractionation, and PCSK9 protein levels were determined by ELISA. FPLC, fast-protein liquid chromatography; HFHC, high fat, high cholesterol; PCSK9, proprotein convertase subtilisin/kexin type 9; TC, total cholesterol.
To determine the size distribution of plasma PCSK9 in humans, we analyzed PCSK9 plasma samples from ten healthy individuals. We noticed that human HDL, spanning fractions 19–28, had broader size distribution than mouse HDL (fractions 19–24) (Figs. 4A, 4B). PCSK9 in human plasma displayed a wide indistinct size distribution and individual variability. The major difference among individual samples analyzed was the percentage of PCSK9 in free forms and in LDL-sized large complexes. Examples of PCSK9 profiles from two individuals, who have similar levels of plasma PCSK9, total cholesterol, and lipoprotein profile, are shown in Fig. 4B. The PCSK9 profile of Subject #1 was similar to that obtained in transgenic mice fed with HFHC diet. There was approximately 50% and 16% of PCSK9 in free forms (fractions 22–29) and in LDL-sized large complexes, respectively, (Fig. 4B, upper panel). Compared with the first profile, Subject #2 had more PCSK9 (nearly 38%) in LDL-sized large complexes and less free forms (only 28%) (Fig. 4B, lower panel). The percentages of intermediate-sized complexes were similar in the two profiles.
DISCUSSION
In this study, we generated transgenic mice that overexpress human PCSK9 in kidney and have increased circulating PCSK9 levels. We provided strong evidence to support that human PCSK9 proteins secreted from extrahepatic tissue are able to promote LDLR degradation in liver and increase plasma LDL. Several studies have shown that hepatic overexpression of murine or human PCSK9 results in increased LDL cholesterol by inhibiting LDL clearance via reduction of liver LDLR proteins (9, 15–17). The LDLR-lowering function of circulating PCSK9 was first suggested by a parabiosis study reported by Horton’s group in which plasma PCSK9 and LDL levels were increased in the recipients (18). Recently, they further confirmed the function of extracellular PCSK9 by demonstrating that infusion of recombinant hPCSK9 preferentially promoted liver LDLR degradation (19). However, no LDL elevation was observed in this study. Cao’s group demonstrated transient LDLR lowering and LDL elevation following injection of 30–300 μg/mice of recombinant PCSK9 in mice (30, 31). The studies from both groups showed that recombinant PCSK9 proteins are rapidly cleared from plasma. The high clearance rate of injected PCSK9 may explain the transient effects on LDLR and plasma LDL. Generation of a mouse model that overexpresses hPCSK9 in kidney provided a tool for us to study the regulation of lipoprotein metabolism by persistently elevated circulating PCSK9. In our transgenic mice, expression of hPCSK9 in liver markedly reduced endogenous mPCSK9 mRNA levels, so there appears to be no overall change in liver PCSK9 expression. Purified mPCSK9 and hPCSK9 were able to similarly degrade hLDLR in human hepatoma cells (data not shown), suggesting that hPCSK9 and mPCSK9 may not have significant species specificity in terms of promoting LDLR degradation. These data support that degradation of liver LDLR in these transgenic mice is mainly attributable to elevated circulating PCSK9. Although kidney expressed high levels of hPCSK9 transgene, the extent of LDLR reduction was lower than that in liver, confirming that circulating PCSK9 preferentially reduces liver LDLR (Fig. 2B) (19). LDLR in adrenal was not regulated by PCSK9, consistent with the results reported by Horton’s group (19). Our studies suggest that LDLR regulation by PCSK9 has tissue specificity. There are several possibilities to explain the response in kidney: (1) the high levels of expression in kidney could trigger an intracellular pathway of LDLR degradation; (2) there may be a differential response of different tissues to extracellular PCSK9, with liver being the most responsive tissue; or (3) secreted hPCSK9 in kidney may have an increased autocrine effect. The mechanisms that mediate the tissue-specific response to PCSK9 warrant further investigation.
We analyzed the plasma distribution of PCSK9 protein by FPLC fractionation. In transgenic mice fed with chow diet, the majority of hPCSK9 was located in fractions that overlapped with purified free PCSK9, and only a small percentage of plasma hPCSK9 proteins were found in large complexes overlapping in size with LDL (Fig. 4A). HFHC feeding markedly raised the amount of PCSK9 in complexes larger than the size of purified PCSK9 (Fig. 4A). This occurred even though plasma total PCSK9 levels remained unaffected by HFHC diet feeding because hPCSK9 expression is driven by a constitutive promoter. The PCSK9 containing complexes that coeluted with LDL may not associate with LDL directly. We were unable to demonstrate the direct binding of PCSK9 and LDL. The large complexes containing hPCSK9 may represent self-multimers or complexes associated with other plasma proteins. A recent report showed that PCSK9 protein can self-associate to form dimers or trimers, and lipoproteins appear to facilitate this multimer formation (32). The mechanisms mediating HFHC diet-induced hPCSK9 complex formation and the nature of the PCSK9 containing complexes are unknown and are under further investigation. Recently, Fan et al. showed that the majority of PCSK9 was associated with LDL fractions in their human transgenic mice on normal chow diet (32), which is different from our observation. The expression levels and the distribution of transgene or genetic background of the mice may contribute to the differences.
PCSK9 in human plasma exhibited a broad indistinct size distribution and individual variability in both the percentage of PCSK9 in free forms and in LDL-sized large complexes. The mechanisms contributing to the differential distribution remain to be elucidated. With limited sample size, we were unable to obtain any correlation between PCSK9 distribution and plasma LDL/HDL or PCSK9 levels. Studies using more samples with a wider range of LDL/HDL cholesterol and PCSK9 levels may help to shed light on the mechanisms.
Acknowledgments
The authors thank Diane Nadeau for technical assistance to generate transgenic mice.
Footnotes
Abbreviations:
- apoB
- apolipoprotein B
- HFHC
- high fat and high cholesterol
- LDLR
- low density lipoprotein receptor
- PCSK9
- proprotein convertase subtilisin/kexin type 9
- SREBP
- sterol regulatory element-binding protein
REFERENCES
- 1.Abifadel M., Varret M., Rabes J. P., Allard D., Ouguerram K., Devillers M., Cruaud C., Benjannet S., Wickham L., Erlich D., et al. 2003. Mutations in PCSK9 cause autosomal dominant hypercholesterolemia. Nat. Genet. 34: 154–156 [DOI] [PubMed] [Google Scholar]
- 2.Sun X. M., Eden E. R., Tosi I., Neuwirth C. K., Wile D., Naoumova R. P., Soutar A. K. 2005. Evidence for effect of mutant PCSK9 on apolipoprotein B secretion as the cause of unusually severe dominant hypercholesterolaemia. Hum. Mol. Genet. 14: 1161–1169 [DOI] [PubMed] [Google Scholar]
- 3.Leren T. P.2004. Mutations in the PCSK9 gene in Norwegian subjects with autosomal dominant hypercholesterolemia. Clin. Genet. 65: 419–422 [DOI] [PubMed] [Google Scholar]
- 4.Timms K. M., Wagner S., Samuels M. E., Forbey K., Goldfine H., Jammulapati S., Skolnick M. H., Hopkins P. N., Hunt S. C., Shattuck D. M. 2004. A mutation in PCSK9 causing autosomal-dominant hypercholesterolemia in a Utah pedigree. Hum. Genet. 114: 349–353 [DOI] [PubMed] [Google Scholar]
- 5.Cohen J. C., Boerwinkle E., Mosley T. H., Jr., Hobbs H. H. 2006. Sequence variations in PCSK9, low LDL, and protection against coronary heart disease. N. Engl. J. Med. 354: 1264–1272 [DOI] [PubMed] [Google Scholar]
- 6.Cohen J., Pertsemlidis A., Kotowski I. K., Graham R., Garcia C. K., Hobbs H. H. 2005. Low LDL cholesterol in individuals of African descent resulting from frequent nonsense mutations in PCSK9. Nat. Genet. 37: 161–165 [DOI] [PubMed] [Google Scholar]
- 7.Kotowski I. K., Pertsemlidis A., Luke A., Cooper R. S., Vega G. L., Cohen J. C., Hobbs H. H. 2006. A spectrum of PCSK9 alleles contributes to plasma levels of low-density lipoprotein cholesterol. Am. J. Hum. Genet. 78: 410–422 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Seidah N. G., Benjannet S., Wickham L., Marcinkiewicz J., Jasmin S. B., Stifani S., Basak A., Prat A., Chretien M. 2003. The secretory proprotein convertase neural apoptosis-regulated convertase 1 (NARC-1): liver regeneration and neuronal differentiation. Proc. Natl. Acad. Sci. USA. 100: 928–933 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Benjannet S., Rhainds D., Essalmani R., Mayne J., Wickham L., Jin W., Asselin M. C., Hamelin J., Varret M., Allard D., et al. 2004. NARC-1/PCSK9 and its natural mutants: zymogen cleavage and effects on the low density lipoprotein (LDL) receptor and LDL cholesterol. J. Biol. Chem. 279: 48865–48875 [DOI] [PubMed] [Google Scholar]
- 10.McNutt M. C., Lagace T. A., Horton J. D. 2007. Catalytic activity is not required for secreted PCSK9 to reduce low density lipoprotein receptors in HepG2 cells. J. Biol. Chem. 282: 20799–20803 [DOI] [PubMed] [Google Scholar]
- 11.Park S. W., Moon Y. A., Horton J. D. 2004. Post-transcriptional regulation of low density lipoprotein receptor protein by proprotein convertase subtilisin/kexin type 9a in mouse liver. J. Biol. Chem. 279: 50630–50638 [DOI] [PubMed] [Google Scholar]
- 12.Maxwell K. N., Soccio R. E., Duncan E. M., Sehayek E., Breslow J. L. 2003. Novel putative SREBP and LXR target genes identified by microarray analysis in liver of cholesterol-fed mice. J. Lipid Res. 44: 2109–2119 [DOI] [PubMed] [Google Scholar]
- 13.Rashid S., Curtis D. E., Garuti R., Anderson N. N., Bashmakov Y., Ho Y. K., Hammer R. E., Moon Y. A., Horton J. D. 2005. Decreased plasma cholesterol and hypersensitivity to statins in mice lacking PCSK9. Proc. Natl. Acad. Sci. USA. 102: 5374–5379 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Graham M. J., Lemonidis K. M., Whipple C. P., Subramaniam A., Monia B. P., Crooke S. T., Crooke R. M. 2007. Antisense inhibition of proprotein convertase subtilisin/kexin type 9 reduces serum LDL in hyperlipidemic mice. J. Lipid Res. 48: 763–767 [DOI] [PubMed] [Google Scholar]
- 15.Maxwell K. N., Breslow J. L. 2004. Adenoviral-mediated expression of PCSK9 in mice results in a low-density lipoprotein receptor knockout phenotype. Proc. Natl. Acad. Sci. USA. 101: 7100–7105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lalanne F., Lambert G., Amar M. J., Chetiveaux M., Zair Y., Jarnoux A. L., Ouguerram K., Friburg J., Seidah N. G., Brewer H. B., Jr., et al. 2005. Wild-type PCSK9 inhibits LDL clearance but does not affect apoB-containing lipoprotein production in mouse and cultured cells. J. Lipid Res. 46: 1312–1319 [DOI] [PubMed] [Google Scholar]
- 17.Zaid A., Roubtsova A., Essalmani R., Marcinkiewicz J., Chamberland A., Hamelin J., Tremblay M., Jacques H., Jin W., Davignon J., et al. 2008. Proprotein convertase subtilisin/kexin type 9 (PCSK9): hepatocyte-specific low-density lipoprotein receptor degradation and critical role in mouse liver regeneration. Hepatology. 48: 646–654 [DOI] [PubMed] [Google Scholar]
- 18.Lagace T. A., Curtis D. E., Garuti R., McNutt M. C., Park S. W., Prather H. B., Anderson N. N., Ho Y. K., Hammer R. E., Horton J. D. 2006. Secreted PCSK9 decreases the number of LDL receptors in hepatocytes and in livers of parabiotic mice. J. Clin. Invest. 116: 2995–3005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Grefhorst A., McNutt M. C., Lagace T. A., Horton J. D. 2008. Plasma PCSK9 preferentially reduces liver LDL receptors in mice. J. Lipid Res. 49: 1303–1311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fisher T. S., Lo Surdo P., Pandit S., Mattu M., Santoro J. C., Wisniewski D., Cummings R. T., Calzetta A., Cubbon R. M., Fischer P. A., et al. 2007. Effects of pH and low density lipoprotein (LDL) on PCSK9-dependent LDL receptor regulation. J. Biol. Chem. 282: 20502–20512 [DOI] [PubMed] [Google Scholar]
- 21.Zhang D. W., Lagace T. A., Garuti R., Zhao Z., McDonald M., Horton J. D., Cohen J. C., Hobbs H. H. 2007. Binding of proprotein convertase subtilisin/kexin type 9 to epidermal growth factor-like repeat A of low density lipoprotein receptor decreases receptor recycling and increases degradation. J. Biol. Chem. 282: 18602–18612 [DOI] [PubMed] [Google Scholar]
- 22.Cameron J., Holla O. L., Ranheim T., Kulseth M. A., Berge K. E., Leren T. P. 2006. Effect of mutations in the PCSK9 gene on the cell surface LDL receptors. Hum. Mol. Genet. 15: 1551–1558 [DOI] [PubMed] [Google Scholar]
- 23.Chung J. H., Whiteley M., Felsenfeld G. 1993. A 5′ element of the chicken beta-globin domain serves as an insulator in human erythroid cells and protects against position effect in Drosophila. Cell. 74: 505–514 [DOI] [PubMed] [Google Scholar]
- 24.Nagy A., Gertsenstein M., Vintersten K., Behringer R. 2003. Manipulating the Mouse Embryo: A Laboratory Manual Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY [Google Scholar]
- 25.Francone O. L., Haghpassand M., Bennett J. A., Royer L., McNeish J. 1997. Expression of human lecithin:cholesterol acyltransferase in transgenic mice: effects on cholesterol efflux, esterification, and transport. J. Lipid Res. 38: 813–822 [PubMed] [Google Scholar]
- 26.Ueda Y., Royer L., Gong E., Zhang J., Cooper P. N., Francone O., Rubin E. M. 1999. Lower plasma levels and accelerated clearance of high density lipoprotein (HDL) and non-HDL cholesterol in scavenger receptor class B type I transgenic mice. J. Biol. Chem. 274: 7165–7171 [DOI] [PubMed] [Google Scholar]
- 27.Cunningham D., Danley D. E., Geoghegan K. F., Griffor M. C., Hawkins J. L., Subashi T. A., Varghese A. H., Ammirati M. J., Culp J. S., Hoth L. R., et al. 2007. Structural and biophysical studies of PCSK9 and its mutants linked to familial hypercholesterolemia. Nat. Struct. Mol. Biol. 14: 413–419 [DOI] [PubMed] [Google Scholar]
- 28.Benjannet S., Rhainds D., Hamelin J., Nassoury N., Seidah N. G. 2006. The proprotein convertase (PC) PCSK9 is inactivated by furin and/or PC5/6A: functional consequences of natural mutations and post-translational modifications. J. Biol. Chem. 281: 30561–30572 [DOI] [PubMed] [Google Scholar]
- 29.Maxwell K. N., Fisher E. A., Breslow J. L. 2005. Overexpression of PCSK9 accelerates the degradation of the LDLR in a post-endoplasmic reticulum compartment. Proc. Natl. Acad. Sci. USA. 102: 2069–2074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Qian Y. W., Schmidt R. J., Zhang Y., Chu S., Lin A., Wang H., Wang X., Beyer T. P., Bensch W. R., Li W., et al. 2007. Secreted PCSK9 downregulates low density lipoprotein receptor through receptor-mediated endocytosis. J. Lipid Res. 48: 1488–1498 [DOI] [PubMed] [Google Scholar]
- 31.Schmidt R. J., Beyer T. P., Bensch W. R., Qian Y. W., Lin A., Kowala M., Alborn W. E., Konrad R. J., Cao G. 2008. Secreted proprotein convertase subtilisin/kexin type 9 reduces both hepatic and extrahepatic low-density lipoprotein receptors in vivo. Biochem. Biophys. Res. Commun. 370: 634–640 [DOI] [PubMed] [Google Scholar]
- 32.Fan D., Yancey P. G., Qiu S., Ding L., Weeber E. J., Linton M. F., Fazio S. 2008. Self-association of human PCSK9 correlates with its LDLR-degrading activity. Biochemistry. 47: 1631–1639 [DOI] [PMC free article] [PubMed] [Google Scholar]