Abstract
High density lipoprotein cholesterol is thought to represent a preferred source of sterols secreted into bile following hepatic uptake by scavenger receptor class B type I (SR-BI). The present study aimed to determine the metabolic effects of an endothelial lipase (EL)–mediated stimulation of HDL cholesterol uptake on liver lipid metabolism and biliary cholesterol secretion in wild-type, SR-BI knockout, and SR-BI overexpressing mice. In each model, injection of an EL expressing adenovirus decreased plasma HDL cholesterol (P < 0.001) whereas hepatic cholesterol content increased (P < 0.05), translating into decreased expression of sterol-regulatory element binding protein 2 (SREBP2) and its target genes HMG-CoA reductase and LDL receptor (each P < 0.01). Biliary cholesterol secretion was dependent on hepatic SR-BI expression, being decreased in SR-BI knockouts (P < 0.001) and increased following hepatic SR-BI overexpression (P < 0.001). However, in each model, biliary secretion of cholesterol, bile acids, and phospholipids as well as fecal bile acid and neutral sterol content, remained unchanged in response to EL overexpression. Importantly, hepatic ABCG5/G8 expression did not correlate with biliary cholesterol secretion rates under these conditions. These results demonstrate that an acute decrease of plasma HDL cholesterol levels by overexpressing EL increases hepatic cholesterol content but leaves biliary sterol secretion unaltered. Instead, biliary cholesterol secretion rates are related to the hepatic expression level of SR-BI. These data stress the importance of SR-BI for biliary cholesterol secretion and might have relevance for concepts of reverse cholesterol transport.
Keywords: bile, EL, HDL, liver, LXR, metabolism, phospholipase, SREBP
Plasma levels of high density lipoprotein cholesterol are inversely associated with the risk of atherosclerotic cardiovascular disease (1, 2). This protective effect of HDL is largely ascribed to the role of this lipoprotein in reverse cholesterol transport (RCT), a process comprising the movement of excess cholesterol from the periphery back to the liver for subsequent secretion into the bile (3, 4). Within the plasma compartment, substantial remodeling of HDL particles occurs. A factor exerting a major impact in this regard is endothelial lipase (EL).
EL has recently been identified as a member of the triacylglycerol lipase gene family. It is expressed in endothelial cells as well as in macrophages and hepatocytes (5, 6). Remarkably, EL possesses merely phospholipase activity (7). EL expression is upregulated in vitro by proinflammatory stimuli (8, 9), and EL plasma levels correlate with the levels of proinflammatory cytokines in human populations (10, 11). In experimental animals, both overexpression (5, 12) as well as loss-of-function models (13–15) have established EL to be a negative regulator of plasma HDL cholesterol levels by increasing HDL catabolism. Moreover, accumulating evidence points to a comparable role of EL in human HDL metabolism (15–17).
Consistent with the role of HDL in RCT, HDL is thought to represent a preferred source of sterols that are subsequently secreted into the bile (3, 4, 18, 19). Currently, no data are available regarding the effect of an acute decrease of plasma HDL cholesterol levels on biliary sterol excretion caused by a single physiologically relevant stimulus. This study aimed to test the hypothesis that an acute, substantial decrease of plasma HDL cholesterol levels by EL overexpression impacts liver cholesterol metabolism and biliary cholesterol secretion. Our data demonstrate that in wild-type mice, virtual elimination of HDL cholesterol by EL overexpression results in hepatic cholesterol accumulation but not in increased biliary cholesterol secretion. In scavenger receptor class B type I (SR-BI) knockout mice and SR-BI overexpressing mice, EL decreased plasma HDL cholesterol and increased hepatic cholesterol content. However, the rate of biliary cholesterol secretion depended on the hepatic SR-BI expression level, indicating that, at least under these conditions, SR-BI is involved in control of biliary cholesterol secretion independent of ABCG5/G8.
EXPERIMENTAL PROCEDURES
Animals
C57BL/6J mice were obtained from Charles River (Sulzfeld, Germany). SR-BI knockout mice were obtained from Jackson (Bar Harbor, ME) and backcrossed to the C57BL/6J genetic background for a total of eight generations. The animals were caged in animal rooms with alternating 12-h periods of light (from 7:30 AM to 7:30 PM) and dark (from 7:30 PM to 7:30 AM), with ad libitum access to water and mouse chow diet (Arie Blok, Woerden, The Netherlands). Animal experiments were performed in conformity with PHS policy and in accordance with the national laws. All protocols were approved by the responsible ethics committee of the University of Groningen.
Generation of recombinant adenoviruses
The human EL cDNA was amplified from HepG2 cells (ATCC via LGC Promochem, Teddington, UK) by PCR using specific primers according to the human EL sequence (NM_006033, GenBank) and subcloned into pcDNA3.1 (Invitrogen, Carlsbad, CA,). Recombinant adenovirus (AdhEL) was generated using the Adeno-X kit (Clontech, Mountain View, CA) according to the manufacturers instructions. An empty adenovirus (AdNull) was used as control. In pilot experiments we ensured that the control adenovirus AdNull did not significantly impact on plasma HDL levels or hepatic cholesterol content compared with saline receiving control mice (see supplementary Fig. I). Generation of the murine SR-BI expressing adenovirus AdSR-BI as well as the empty control adenovirus AdNull has been described previously (20). Recombinant adenoviruses were amplified and purified as described (21). For in vivo studies using EL overexpression alone, mice were injected with 1 × 1011 particles/mouse of either AdhEL or the control virus AdNull. For in vivo experiments to explore the effects of SR-BI as well as EL overexpression, mice were injected with a total of 2 × 1011 particles/mouse consisting of 1 × 1011 particles of AdSR-BI with the addition of 1 × 1011 particles of either AdhEL or the control virus AdNull. The experiments described were carried out on day 5 following injection of the recombinant adenoviruses. On day 5 the vast majority of expression of human EL was in the liver; little expression was detectable in muscle, spleen, and adrenals; and expression was almost absent in other organs (see supplementary Fig. II).
Plasma lipid and lipoprotein analysis
Mice were bled from the retro-orbital plexus after a 4-h fast using heparinized capillary tubes. Aliquots of plasma were stored at − 80°C until analysis, and commercially available reagents were used to measure plasma total cholesterol (Roche Diagnostics, Basel, Switzerland), triglycerides, and phospholipids (Wako Pure Chemical Industries, Neuss, Germany). HDL cholesterol was assessed by precipitating apoB-containing lipoproteins using 0.36% phosphotungstic acid (Sigma, St. Louis, MO) followed by cholesterol determination in the supernatant as described above. Pooled plasma samples were subjected to fast protein liquid chromatography (FPLC) gel filtration using a superose 6 column (GE Healthcare, Uppsala, Sweden) as described (22). Individual fractions were assayed for cholesterol concentrations as described above.
Analysis of liver lipid composition
Liver lipids were extracted following homogenization according to the method of Bligh and Dyer as described (23). Total and free cholesterol, phospholipids, and triglycerides in the liver were then determined as previously described (23, 24).
Analysis of gene expression by real-time quantitative PCR
Total RNA from mouse livers was extracted using TriReagent (Sigma, St. Louis, MO) and quantified using a NanoDrop ND-1000 UV-Vis spectrophotometer (NanoDrop Technologies, Wilmington, DE). cDNA synthesis was performed from 1 μg of total RNA using reagents from Applied Biosystems (Darmstadt, Germany). Real-time quantitative PCR was carried out on an Applied Biosystems 7700 sequence detector with the default settings (23). Primers and probes were designed with the Primer Express Software (Applied Biosystems) and synthesized by Eurogentec (Seraing, Belgium). mRNA expression levels presented were calculated relative to the housekeeping gene cyclophilin and further normalized to the relative expression level of the respective controls (23).
Western blot analysis of endothelial lipase expression
Livers were disrupted by sonication on ice in phosphate-buffered saline containing CompleteTM protease inhibitors (Roche, Mannheim, Germany) followed by the addition of Triton X-100 to a final concentration of 1%. Plasma membranes of primary hepatocytes were isolated as previously described (23). Protein concentrations were determinend with the bicinchoninic acid (BCA) assay (Pierce Biotechnology, Inc., Rockford, IL). In the case of plasma, 0.25µl of mouse plasma was loaded per lane. Proteins were separated by SDS-PAGE and blotted onto nitrocellulose (GE Healthcare Bio-Sciences Corp., Piscataway, NJ). Polyclonal rabbit anti-human EL antibodies cross-reacting with endogenous mouse EL (Novus Biologicals, Littleton, CO) were used to detect protein expression followed by the appropriate secondary antibody.
Bile collection and assessment of biliary excretion of cholesterol, phospholipids, and bile acids
Bile was collected by cannulation of the gallbladder under hypnorm (fentanyl/fluanisone; 1 ml/kg) and diazepam (10 mg/kg) anesthesia using a humidified incubator to maintain body temperature (23). Bile was collected for 30 min, and production was determined gravimetrically (23). Biliary bile salt, cholesterol, and phospholipid concentrations were determined, and the respective biliary excretion rates calculated as described previously (23, 24).
Fecal sterol analysis
Mice were housed in groups, and feces were collected over a period of 24 h and separated from the bedding. Fecal samples were lyophilized and weighed. Aliquots thereof were used for determination of neutral and acidic sterol content by gas liquid chromatography as described (23).
Statistical analysis
Statistical analysis was performed using the statistical package for social sciences (SPSS, SPSS Inc., Chicago, IL). Data are presented as means ± SEM. Statistical analysis was performed using the Mann-Whitney U-test to compare different groups. Statistical significance for all comparisons was assigned at P < 0.05.
RESULTS
Hepatic EL expression results in substantially decreased plasma HDL cholesterol levels
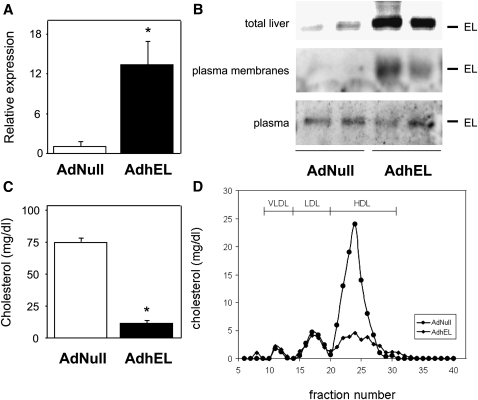
At day 5 following injection of the hEL adenovirus, hepatic mRNA expression of EL (Fig. 1A) as well as protein expression of EL in total liver and on plasma membranes of hepatocytes was markedly increased, while plasma levels of EL remained unchanged (Fig. 1B). In parallel, plasma total cholesterol levels were significantly lower in wild-type AdhEL-injected mice compared with controls (11 ± 2 versus 75 ± 3 mg/dl, respectively; P < 0.001) (Fig. 1C). FPLC analysis revealed that this drop in total cholesterol was mainly due to the virtual absence of HDL cholesterol in plasma of AdhEL-injected mice (Fig. 1D). Consistent with a role of EL as a phospholipase, plasma phospholipids were also significantly lower in AdhEL injected mice compared with AdNull injected controls (17 ± 11 versus 171 ± 40 mg/dl, respectively; P < 0.001). In addition, plasma triglycerides were decreased in response to EL overexpression compared with control mice (12 ± 5 versus 51 ± 10 mg/dl, respectively; P < 0.05).
Fig. 1.
Effect of hepatic EL overexpression on plasma cholesterol levels in C57BL/6 mice. A: Hepatic mRNA expression of EL determined by quantitative real-time PCR. B: Western blot analysis of total hepatic EL protein levels, EL protein associated with plasma membranes of isolated primary hepatocytes, and EL in mouse plasma on day 5 after injection of the respective adenoviruses. C: Plasma total cholesterol levels on day 5 after injection of either AdhEL or the control adenovirus AdNull. D: FPLC cholesterol profiles on day 5 after injection of either AdhEL (diamonds) or the control adenovirus AdNull (circles). Pooled plasma samples were subjected to gel filtration using a Superose 6 column, and cholesterol levels in each fraction were determined. The relative elution positions of the different lipoprotein subclasses are indicated. All measurements were performed as described under Experimental Procedures. Data in (A) and (C) are presented as means ± SEM; * indicates statistically significant differences between groups (P < 0.001) as assessed by Mann-Whitney U-test; n = 6–7 mice for each group. AdhEL, recombinant adenovirus; AdNull; empty adenovirus; EL, endothelial lipase.
Since SR-BI is involved in HDL cholesterol selective uptake at the basolateral membrane of the hepatocyte (25), we further explored the role of SR-BI by overexpressing EL in SR-BI knockout mice as well as overexpressing EL together with SR-BI in wild-type mice. In SR-BI knockout mice, EL overexpression resulted in a 71% decrease in plasma HDL cholesterol levels (52 ± 5 versus 182 ± 6 mg/dl, respectively; P < 0.001). Plasma phospholipids (102 ± 4 versus 267 ± 6 mg/dl, respectively; P < 0.001) and triglycerides (64 ± 7 versus 124 ± 12 mg/dl, respectively; P < 0.001) were also lower in response to EL overexpression.
Next, we addressed the effects of EL overexpression in wild-type mice receiving AdhEL or AdNull together with AdSR-BI. Also under these conditions EL overexpression resulted in a significant decrease in plasma HDL cholesterol levels (8 ± 1 versus 14 ± 2 mg/dl, respectively; P < 0.01), while plasma phospholipids (21 ± 4 versus 25 ± 5 mg/dl, respectively, NS) and triglycerides (14 ± 3 versus 18 ± 4 mg/dl, respectively; NS) did not change.
EL expression increases hepatic cholesterol content independent of hepatic SR-BI expression levels
Body weight and liver weight were virtually identical when comparing mice injected with AdhEL with AdNull-receiving controls in all experiments performed. None of the given significances or conclusions drawn changed when liver lipid data were expressed per gram of tissue instead of whole liver.
In wild-type mice, hepatic total cholesterol content increased by 61% in response to EL overexpression (19.4 ± 2.8 versus 12.1 ± 1.3 µmol/liver, respectively; P < 0.05) (Fig. 2A). Particularly, the hepatic free cholesterol content was almost doubled in EL overexpressing mice (8.3 ± 1.7 versus 4.4 ± 0.8 µmol/liver, respectively; P < 0.05), while the hepatic cholesterol ester content was slightly increased (8.7 ± 0.7 versus 7.0 ± 0.3 µmol/liver, respectively; P < 0.05). Hepatic triglyceride content as well as phospholipid content did not differ significantly between AdhEL and AdNull injected wild-type mice.
Fig. 2.
Hepatic cholesterol levels in response to hepatic EL expression in wild-type mice (A), SR-BI knockout mice (B) and mice with hepatic SR-BI overexpression (C). On day 5 following injection of the respective mouse models with either an adenovirus expressing human EL (AdhEL) or an empty control adenovirus (AdNull), livers were homogenized, lipids were extracted and total cholesterol (TC) was determined as described under Experimental Procedures. Data are presented as means ± SEM. * indicates statistically significant differences between groups (at least P < 0.05) as assessed by Mann-Whitney U-test. n = 4-7 mice per group. AdhEL, recombinant adenovirus; AdNull; empty adenovirus; EL, endothelial lipase; SR-BI, scavenger receptor class B type I.
In SR-BI knockout mice, hepatic total cholesterol content increased significantly by 32% in response to EL overexpression (16.8 ± 1.3 versus 12.7 ± 0.8 µmol/liver, respectively; P < 0.05) (Fig. 2B), while hepatic free cholesterol (8.3 ± 1.3 versus 6.1 ± 0.7 µmol/liver, respectively, NS) as well as cholesterol ester content (8.5 ± 1.0 versus 6.6 ± 0.9 µmol/liver, respectively, NS) were higher in AdhEL injected mice, however, not significantly. Hepatic triglyceride content as well as phospholipid content remained unchanged.
In mice receiving AdSR-BI together with AdhEL or AdNull, hepatic total cholesterol content was already higher due to hepatic SR-BI overexpression (P < 0.001 compared with wild-type AdNull injected mice) and increased even more in response to EL overexpression (24.4 ± 1.8 versus 17.2 ± 2.1 µmol/liver, respectively; P < 0.05) (Fig. 2C). Hepatic free cholesterol content was significantly increased in mice receiving AdEL (13.2 ± 2.1 versus 7.8 ± 1.6 µmol/liver, respectively; P < 0.05), while cholesterol ester content (11.2 ± 2.9 versus 9.4 ± 2.3 µmol/liver, respectively, NS) increased, however, not significantly. Hepatic triglyceride content as well as phospholipid content remained unchanged.
EL modification of the HDL particle results in increased selective uptake via SR-BI and in increased holoparticle uptake independent of SR-BI in primary mouse hepatocytes in vitro
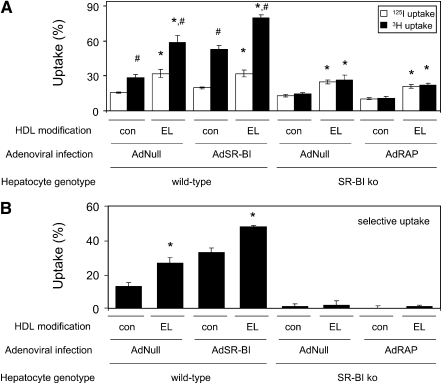
To further investigate the underlying mechanism of increased hepatic cholesterol content in response to EL overexpression in all experimental models used, a series of in vitro studies using primary mouse hepatocytes was performed. In wild-type hepatocytes infected with AdNull (i.e., expressing endogenous SR-BI), EL modification of the HDL particle resulted in increased uptake of 125I-TC-labeled HDL apolipoproteins (15.6 ± 0.6 versus 31.9 ± 3.5%; P < 0.01) and, to an even further extent, increased uptake of 3H-cholesteryl linoleyl ether, suggesting increased uptake of cholesteryl esters (28.5 ± 2.8 versus 58.6 ± 6.2%; P < 0.01) leading to an increased net selective uptake (12.9 ± 2.3 versus 26.6 ± 3.2%; P < 0.05) (Figs. 3A, 3B). SR-BI overexpression in wild-type hepatocytes increased 3H-cholesteryl linoleyl ether uptake from control HDL (52.7 ± 3.4%; P < 0.01) without affecting 125I-TC-labeled apolipoprotein uptake (19.9 ± 0.8%, NS), resulting in increased selective uptake (32.8 ± 2.7%; P < 0.05) (Figs. 3A, 3B). In addition, SR-BI overexpression in hepatocytes did not affect 125I-TC-labeled HDL protein uptake from EL-modified HDL (31.8 ± 3.0%, NS), but 3H-cholesteryl linoleyl ether uptake increased even further (79.6 ± 2.7%; P < 0.01), resulting in even higher selective uptake (47.7 ± 0.7%; P < 0.01) (Figs. 3A, 3B). These data indicate that EL modification of HDL increases selective uptake as well as holoparticle uptake.
Fig. 3.
EL modification of the HDL particle enhances selective as well as holoparticle uptake in primary hepatocytes in vitro. HDL was isolated from AdNull (con) or AdhEL (EL) injected C57BL/6 mice as indicated and double labeled in the apolipoprotein (125I) as well as cholesteryl ester (3H) moieties as detailed in Experimental Procedures. Labeled HDL preparations were incubated with primary hepatocytes from either wild-type C57BL/6 (wild-type) or SR-BI knockout (SR-BI ko) mice infected with the indicated adenoviral constructs. Then (A) uptake of 125I-TC-labeled HDL apolipoproteins and 3H-cholesteryl linoleyl ether from HDL was determined separately, and from these data (B) selective uptake was calculated. Data are presented as means ± SEM; * indicates statistically significant differences between HDL isolated from AdNull versus AdhEL injected mice; # indicates statistically significant differences between uptake of HDL 3H-cholesteryl linoleyl ether and 125I-TC-labeled HDL apolipoproteins (each at least P < 0.05); n = 4 wells for each respective experimental condition. AdhEL, recombinant adenovirus; AdNull, empty adenovirus; EL, endothelial lipase; SR-BI, scavenger receptor class B type I.
In hepatocytes from SR-BI knockout mice lacking endogenous SR-BI expression, no selective uptake from control HDL was discernible, indicating that all selective uptake by hepatocytes is mediated through SR-BI. However, uptake of 125I-TC-labeled apolipoproteins (13.1 ± 0.8 versus 24.6 ± 1.6%; P < 0.01) as well as 3H-cholesteryl linoleyl ether (14.3 ± 1.2 versus 26.6 ± 4.0%; P < 0.01) from EL-modified HDL was increased compared with control HDL, substantiating the notion that EL modification increases the affinity of the HDL particle for holoparticle uptake (Fig. 3A). To explore whether LRP-1 is involved in this uptake process, SR-BI knockout hepatocytes were in addition to AdNull infected with a receptor-associated protein (RAP) expressing adenovirus (AdRAP) (26). RAP is inhibiting ligand binding to receptors from the LDLR family. However, RAP expression had no impact on the HDL holoparticle uptake of either control or EL-modified HDL, indicating that LRP-1 might not be involved in this process (Fig. 3A and B).
Biliary cholesterol secretion and fecal sterol excretion remain unchanged in response to EL expression
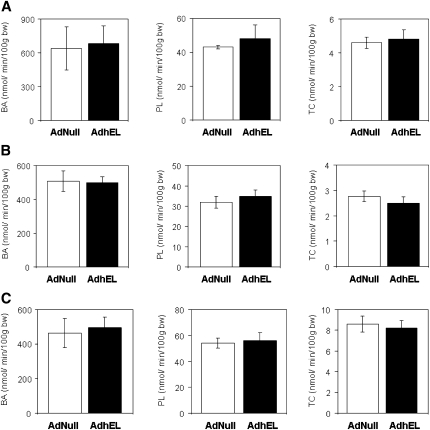
As biliary sterols are supposedly derived to a major part from HDL cholesterol (4, 18), we next investigated whether the dramatic decrease in plasma HDL cholesterol levels as well as the increased hepatic cholesterol content in response to EL overexpression would translate into altered biliary sterol secretion rates. Bile flow remained unchanged in response to EL overexpression compared with AdNull-injected controls in all respective experiments. In wild-type mice, there was no difference between AdhEL and AdNull injected mice regarding the biliary secretion rates of bile acids (680 ± 163 versus 637 ± 194 nmol/min/100g BW, respectively, NS) and phospholipids (47.7 ± 7.6 versus 43.1 ± 1.1 nmol/min/100g BW, respectively, NS) (Fig. 4A). Also the biliary secretion of cholesterol did not differ between mice overexpressing EL and controls (4.77 ± 0.56 versus 4.60 ± 0.33 nmol/min/100g BW, respectively, NS) (Fig. 4A). Consistent with the unaffected biliary secretion rates, EL overexpression had no effect on fecal bile salt content or fecal neutral sterol content (Table 1).
Fig. 4.
Biliary lipid secretion rates in response to hepatic EL expression in wild-type mice (A), SR-BI knockout mice (B) and mice with hepatic SR-BI overexpression (C). On day 5 following injection of the respective mouse models with either an adenovirus expressing human EL (AdhEL) or an empty control adenovirus (AdNull), bile was collected continuously and biliary output rates of bile acids (BA), phospholipids (PL), and cholesterol (TC) were determined as described under Experimental Procedures. Data are presented as means ± SEM; n = 4–6 mice for each group. SR-BI, scavenger receptor class B type I.
TABLE 1.
Fecal bile acid and neutral sterol content in response to EL overexpression
| C57BL/6 |
SR-BI knockout |
C57BL/6 + AdSR-BI |
||||
|---|---|---|---|---|---|---|
| AdNull | AdhEL | AdNull | AdhEL | AdNull | AdhEL | |
| Bile acids (µmol/g) | 2.6 ± 0.3 | 2.5 ± 0.3 | 2.3 ± 0.7 | 1.8 ± 0.5 | 4.2 ± 0.6 | 4.0 ± 0.5 |
| Neutral sterols (µmol/g) | 3.9 ± 0.4 | 4.0 ± 0.3 | 2.7 ± 0.4 | 2.9 ± 0.3 | 7.4 ± 0.8 | 6.7 ± 0.9 |
Mice of the indicated genotypes administered the respective adenoviruses were group-housed, feces were collected over a period of 24 h and separated from the bedding. Fecal samples were lyophilized and weighed. Aliquots were used to determine neutral and acidic sterol content by gas liquid chromatography as detailed in Experimental Procedures. Values are means ± SEM; n = 3–5 groups of mice for each condition. AdhEL, recombinant adenovirus; AdNull; empty adenovirus; SR-BI, scavenger receptor class B type I.
In SR-BI knockout mice, there was no difference between AdhEL and AdNull injected mice regarding the biliary secretion rates of bile acids (501 ± 32 versus 508 ± 60 nmol/min/100g BW, respectively, NS) and phospholipids (35 ± 3 versus 32 ± 3 nmol/min/100g BW, respectively, NS) (Fig. 4B). Consistent with previous reports (27), the biliary secretion of cholesterol was lower in Adnull injected SR-BI knockout mice compared with AdNull injected wild-type mice (P < 0.001); however, was not different between SR-BI knockout mice overexpressing EL and SR-BI knockout controls (2.49 ± 0.26 versus 2.76 ± 0.21 nmol/min/100g BW, respectively, NS) (Fig. 4B). In addition, EL overexpression had no effect on fecal bile salt content or fecal neutral sterol content (Table 1).
In mice injected with AdSR-BI together with AdhEL or AdNull, hepatic EL overexpression did not impact on the biliary secretion rates of bile acids (495 ± 62 versus 463 ± 84 nmol/min/100g BW, respectively, NS) and phospholipids (56 ± 6 versus 54 ± 4 nmol/min/100g BW, respectively, NS) (Fig. 4C). As seen in the models with normal or absent hepatic SR-BI expression, the biliary secretion of cholesterol also remained unchanged in response to EL overexpression (8.2 ± 0.7 versus 8.6 ± 0.8 nmol/min/100g BW, respectively) (Fig. 4C). In addition, EL overexpression did not increase fecal bile salt or neutral sterol content (Table 1).
Hepatic gene expression profile in response to EL overexpression is consistent with increased cholesterol uptake and content
In wild-type mice overexpressing EL, hepatic mRNA expression of HMG-CoA reductase was decreased by 49% (1.00 ± 0.05 versus 0.51 ± 0.08, respectively; P < 0.01) and expression of the LDLR was reduced by 30% (1.00 ± 0.07 versus 0.70 ± 0.07, respectively; P = 0.01) compared with control virus-injected mice (Table 2). Consistent with these changes, expression of sterol-regulatory element binding protein 2 (SREBP2) was significantly lower in mice injected with AdhEL (1.00 ± 0.04 versus 0.73 ± 0.06, respectively; P < 0.01) (Table 2). On the other hand, compared with controls, increasing EL expression had no significant effect on the hepatic mRNA expression of the transport proteins ABCG5, ABCG8, bile salt export pump (BSEP), ABCB11, multidrug resistance protein 2 (MDR2), ABCB4, or SR-BI (Table 2). These data are in line with the unchanged biliary secretion rates of cholesterol, bile acids, and phospholipids.
TABLE 2.
Hepatic mRNA expression levels measured by quantitative real-time PCR in response to EL overexpression
| C57BL/6 |
SR-BI knockout |
C57BL/6 + AdSR-BI |
||||
|---|---|---|---|---|---|---|
| AdNull | AdhEL | AdNull | AdhEL | AdNull | AdhEL | |
| HMG-CoAR | 1.00 ± 0.05 | 0.51 ± 0.08a | 1.00 ± 0.07 | 0.56 ± 0.06a | 1.00 ± 0.06 | 0.64 ± 0.07a |
| LDLR | 1.00 ± 0.07 | 0.70 ± 0.07a | 1.00 ± 0.09 | 0.67 ± 0.05a | 1.00 ± 0.06 | 0.63 ± 0.06a |
| SREBP-2 | 1.00 ± 0.04 | 0.73 ± 0.06a | 1.00 ± 0.05 | 0.62 ± 0.06a | 1.00 ± 0.04 | 0.76 ± 0.05a |
| ABCG5 | 1.00 ± 0.13 | 0.78 ± 0.16 | 1.00 ± 0.12 | 0.55 ± 0.03a | 1.00 ± 0.08 | 0.74 ± 0.07a |
| ABCG8 | 1.00 ± 0.09 | 0.72 ± 0.11 | 1.00 ± 0.15 | 0.50 ± 0.06a | 1.00 ± 0.10 | 0.76 ± 0.11 |
| LXRα | 1.00 ± 0.10 | 0.91 ± 0.12 | 1.00 ± 0.09 | 0.93 ± 0.08 | 1.00 ± 0.12 | 0.86 ± 0.13 |
| BSEP | 1.00 ± 0.09 | 0.70 ± 0.10 | 1.00 ± 0.10 | 0.86 ± 0.11 | 1.00 ± 0.12 | 0.96 ± 0.11 |
| MDR2 | 1.00 ± 0.11 | 0.84 ± 0.10 | 1.00 ± 0.09 | 0.91 ± 0.10 | 1.00 ± 0.09 | 0.92 ± 0.10 |
| SR-BI | 1.00 ± 0.11 | 0.82 ± 0.10 | nd | nd | 1.00 ± 0.14 | 0.99 ± 0.12 |
Livers of mice with the indicated genotypes administered the respective adenoviruses were harvested on day 5 following adenovirus injection and snap-frozen in liquid nitrogen. mRNA expression levels were determined by real-time quantitative PCR as described in Experimental Procedures. Values are means ± SEM. Within each set of experiments, gene expression levels are related to the respective AdNull-injected controls. nd, not detectable. AdhEL, recombinant adenovirus; AdNull; empty adenovirus; EL, endothelial lipase; SR-BI, scavenger receptor class B type I; SREBP, sterol-regulatory element binding protein.
Significantly different from AdNull injected controls (at least P < 0.05).
Comparable to the data obtained in wild-type mice, hepatic EL overexpression in SR-BI knockout mice resulted in decreased hepatic mRNA expression of HMG-CoA reductase (1.00 ± 0.07 versus 0.56 ± 0.06; P < 0.01) and the LDLR (1.00 ± 0.09 versus 0.67 ± 0.05; P < 0.01) (Table 2). In addition, expression of SREBP2 was significantly lower in mice injected with AdhEL (1.00 ± 0.05 versus 0.62 ± 0.06; P < 0.01) (Table 2). In contrast to the changes in hepatic mRNA expression in wild-type mice, increasing EL expression in SR-BI knockout mice resulted in decreased mRNA levels of ABCG5 (1.00 ± 0.12 versus 0.55 ± 0.03; P < 0.01) and ABCG8 (1.00 ± 0.15 versus 0.50 ± 0.06; P < 0.01) (Table 2). BSEP and MDR2 expression remained unchanged (Table 2).
As seen in mice with normal or absent SR-BI expression, hepatic EL expression resulted also in mice overexpressing SR-BI in decreased hepatic mRNA levels of HMG-CoA reductase (1.00 ± 0.06 versus 0.64 ± 0.07; P < 0.01) and LDLR (1.00 ± 0.06 versus 0.63 ± 0.06; P < 0.01) (Table 2). In addition, expression of SREBP2 was significantly lower in mice injected with AdhEL (1.00 ± 0.04 versus 0.76 ± 0.05; P < 0.01) (Table 2).
Injection of AdhEL together with AdSR-BI resulted in a significant decrease in hepatic mRNA levels of ABCG5 (1.00 ± 0.08 versus 0.74 ± 0.07; P < 0.05), while ABCG8 expression was also lower, however, not significantly (1.00 ± 0.10 versus 0.76 ± 0.11, NS) (Table 2). BSEP, MDR2, and SR-BI expression remained essentially unaffected by EL overexpression (Table 2).
Biliary cholesterol secretion in mice with altered hepatic expression levels of EL and SR-BI occurs independent of ABCG5 expression
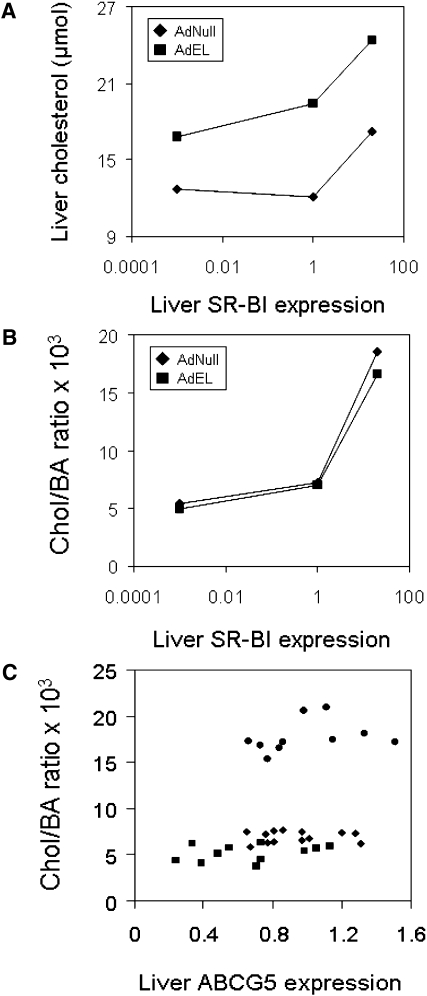
Fig. 5 summarizes the effects of EL related to hepatic SR-BI and ABCG5 expression. Independent of the hepatic SR-BI expression state (knockout, wild-type, or overexpression), EL expression increases hepatic cholesterol content (Fig. 5A). Biliary cholesterol secretion independent of bile acid secretion (chol/BA ratio) increases with increasing hepatic SR-BI expression. However, EL expression and the EL-mediated increase in hepatic cholesterol content do not have an additional effect on biliary cholesterol secretion over the SR-BI-mediated effect (Fig. 5B). Interestingly, in this experimental setting, bile acid–corrected biliary cholesterol secretion is not correlated with the hepatic expression levels of ABCG5 (Fig. 5C) and ABCG8 (data not shown).
Fig. 5.
Biliary cholesterol secretion follows the hepatic SR-BI expression level independent of EL or ABCG5 expression. A: The effect of EL overexpression on the hepatic cholesterol content dependent on the hepatic SR-BI expression level. B: The effect of EL overexpression on bile salt-independent biliary cholesterol secretion (chol/BA ratio) dependent on the hepatic SR-BI expression level. For these graphs a logarithmic scale was chosen, the hepatic SR-BI expression in the SR-BI knockout mice was assigned the value 0.001, and expression in wild-type mice was set to 1. C: Correlation between hepatic ABCG5 mRNA expression and bile salt-independent biliary cholesterol secretion (chol/BA ratio) including all mice investigated in this study (AdNull as well as AdhEL injected). Squares represent SR-BI knockout mice, diamonds wild-type mice, and circles mice with hepatic SR-BI overexpression. For this graph, the hepatic ABCG5 expression of wild-type mice injected with the control adenovirus AdNull was set to 1, and mRNA expression in the other groups was expressed relative to this value. AdhEL, recombinant adenovirus; EL, endothelial lipase; AdNull; empty adenovirus; SR-BI, scavenger receptor class B type I.
DISCUSSION
This study demonstrates that an acute decrease in HDL cholesterol by a single physiologically relevant stimulus (i.e., the action of the phospholipase EL) results in hepatic cholesterol accumulation while biliary cholesterol secretion remains unchanged. Our results further indicate that under these conditions, the hepatic SR-BI expression level is a determinant of biliary cholesterol secretion independent of ABCG5/G8.
EL has been identified as a negative regulator of plasma HDL cholesterol levels in a number of studies employing overexpression (5, 12), gene knockout (14, 15), and antibody-mediated inhibition of EL activity (13). HDL particles are substrates for EL in in vitro assays (7, 28, 29). In vivo, EL overexpression has been shown to increase the catabolic rate of HDL apolipoproteins as the underlying metabolic mechanism of decreased HDL cholesterol plasma levels (12). Analogous to other lipases, also for EL a nonlipolytic ligand function has been demonstrated that might represent an alternative mechanistic basis contributing to the results obtained in our study. However, the liganding function of EL might be less relevant for the in vivo effect of EL on HDL metabolism compared with the lipolytic activity of EL (30). Therefore, as a working model, EL-mediated hydrolysis of HDL phospholipids has been proposed to result in destabilization of the HDL particle, followed by shedding of poorly lipidated apoA-I molecules that are then more rapidly cleared by the kidneys (9). Our present study confirms and extends these observations by showing that, besides uniformly mediating decreased plasma HDL cholesterol levels in all models used, hepatic EL expression also results in a net increase in hepatic cholesterol content by enhancing HDL selective as well as holoparticle uptake. A likely candidate system to mediate HDL holoparticle uptake in the absence of SR-BI is the recently described complex containing the ectopic β -chain of ATP synthase (31). This enzyme generates extracellular ADP upon HDL binding, which then activates the nucleotide receptor P2Y13 resulting in clathrin-dependent HDL holoparticle endocytosis (32). However, independent of the underlying mechanism, biliary cholesterol secretion is apparently unaffected by an acute influx of HDL-derived cholesterol.
Interestingly, in the three models with different hepatic SR-BI expression used, the EL-mediated increase in hepatic cholesterol content did not affect the gene expression levels of the heterodimer ABCG5/G8. ABCG5/G8 are LXR target genes and were recently identified to play a key role in biliary cholesterol secretion (33). In ABCG5/G8 knockout mice, biliary cholesterol secretion is severely reduced (34, 35), whereas it is significantly increased in response to ABCG5/G8 overexpression in hepatocytes (36). The lack of an increase in mRNA levels of these key proteins mediating biliary cholesterol secretion in our models is consistent with the physiological data we obtained. However, ABCG5/G8–independent biliary cholesterol secretion pathways have been suggested to occur. While ABCG5 knockout mice have residual cholesterol secretion that is subject to stimulation (37), correlation studies in different mouse models (38) as well as in humans (39) indicated that biliary cholesterol secretion might be independent from the expression of ABCG5/G8 within liver. In addition, a recent study demonstrated that in ATP8B1-deficient mice increased biliary cholesterol secretion is independent from the expression of ABCG8 (40).
Interestingly, in our study hepatic ABCG5/G8 expression did not correlate with biliary cholesterol secretion, and hepatic mRNA levels of the LXR target gene ABCG5/G8 even decreased in response to EL, which remarkably occurred in the face of an increased hepatic cholesterol content. These data indicate that HDL-derived cholesterol might be partitioned into an intrahepatic pool not accessible toward LXR cholesterol sensing. On the other hand, the increase in hepatic cholesterol content mediated by EL was reflected by a decreased expression of SREBP2, which is conceivably also the underlying basis for a significantly lower expression of HMG-CoA reductase as well as the LDLR (41). These changes are predicted to translate into reduced cholesterol uptake via the LDL pathway and reduced endogenous cholesterol synthesis, adaptive mechanisms to adjust for the increase in hepatic cholesterol. Taken together, our data suggest that within the hepatocyte, distinct cholesterol pools exist for HDL-derived cholesterol destined for storage and direct secretion into bile. The storage pool is apparently differentially accessible toward cholesterol-sensing mechanisms of the hepatocyte, recognized by the SREBP2/SCAP/INSIG system (however, not by LXR). SR-BI might play a major role in directing cholesterol toward biliary secretion. Thus far studies tracing the cellular fate of HDL cholesterol taken up via SR-BI have largely focused on HDL-associated free sterols. These studies established that in polarized cells, uptake of free sterols via SR-BI and their respective transport to the bile canaliculus occurs in a rapid, nonvesicular, and largely energy-independent manner (42, 43). However, the bulk of HDL cholesterol enters the cell via SR-BI as cholesteryl ester, and it has been acknowledged as a limitation to the above-mentioned studies that there might be fundamental differences in the intracellular routing of cholesteryl esters (42). Further research will be required to delineate these pathways. Increased knowledge on this topic could conceivably contribute to develop novel therapeutic strategies against atherosclerotic cardiovascular disease.
Previously, we reported that in mice with transgenic overexpresion of group IIA secretory phospholipase A2 plasma HDL cholesterol levels are decreased and selective uptake via SR-BI is increased, while hepatic SR-BI expression was unchanged (23). Consistent with our present study, group IIA secretory phospholipase A2 transgenic mice had an increased hepatic cholesterol content, while biliary cholesterol secretion remained unaltered.
Biliary cholesterol secretion has been investigated in two mouse models with chronic low or virtually absent plasma HDL cholesterol levels due to decreased HDL formation. ApoA-I knockout mice have significantly decreased plasma HDL cholesterol, unchanged hepatic SR-BI expression, and unaltered biliary cholesterol secretion (44, 46). ABCA1 knockout mice display almost absent plasma HDL cholesterol, but they also have unchanged hepatic SR-BI expression levels (24). Biliary cholesterol secretion rates in ABCA1-deficient mice are virtually identical compared with controls (24). The main difference of these two previous reports compared with our present study is that apoA-I knockout mice as well as ABCA1 knockout mice have a defect in HDL formation, whereas with overexpression of EL, we induce a decrease in HDL cholesterol levels by increasing HDL catabolism, resulting in increased hepatic cholesterol content.
Experimental animal models in which hepatic SR-BI expression is modulated might represent an exception from the observation that plasma HDL levels are independent from biliary cholesterol secretion. Hepatic SR-BI overexpression resulted in decreased plasma HDL cholesterol levels due to increased catabolism and, in this case, increased biliary cholesterol levels were also noticed (20). Conversely, in mice with attenuated or absent SR-BI expression, plasma HDL cholesterol levels are increased, while biliary cholesterol secretion is moderately but significantly reduced (27, 44). In our model, biliary cholesterol secretion rates changed with altering hepatic SR-BI expression; however, there was no additional effect of EL. In addition, hepatic SR-BI has been shown to increase RCT from macrophages independent of plasma HDL cholesterol levels (47). These data point toward an important role of the SR-BI expression level within the hepatocyte to provide a link between plasma HDL cholesterol metabolism, hepatic cholesterol uptake, and the subsequent secretion of cholesterol into the bile. Combined, these studies suggest that plasma HDL cholesterol levels independent from production or catabolism might not be directly related to biliary cholesterol secretion when SR-BI expression is not affected.
In summary, our data demonstrate that a virtual elimination of plasma HDL cholesterol due to increased EL-mediated catabolism causes hepatic cholesterol accumulation; however, it does not influence biliary cholesterol secretion. In this setting, biliary cholesterol secretion rates are independent of ABCG5/G8 and depend instead on the hepatic expression level of SR-BI. These data indicate an important role for hepatocyte SR-BI in directing cholesterol toward biliary secretion and stress the relevance of SR-BI in RCT.
Supplementary Material
Acknowledgments
The authors thank Dr. Karen Kozarsky (GlaxoSmithKline, King of Prussia, PA) for providing the SR-BI expressing adenovirus AdSR-BI used in this study. The authors are grateful to Drs. Joachim Herz (University of Texas Southwestern, Dallas, TX) and Bart van Vlijmen (Leiden University Medical Center, Leiden, The Netherlands) for providing the AdRAP adenovirus. The authors thank Rick Havinga for expert technical assistance.
Footnotes
Abbreviations:
- AdhEL
- recombinant adenovirus
- AdNull
- empty adenovirus
- EL
- endothelial lipase
- NS
- not significant
- RCT
- reverse cholesterol transport
- SR-BI
- scavenger receptor class B type I
- SREBP
- sterol-regulatory element binding protein
This work was supported by the Netherlands Organization for Scientific Research VIDI Grant 917-56-358 (U.J.F.T.).
The online version of this article (available at http://www.jlr.org) contains supplementary data in the form of two figures.
REFERENCES
- 1.Assmann G., Gotto A. M., Jr.2004. HDL cholesterol and protective factors in atherosclerosis. Circulation. 109: III8–III14 [DOI] [PubMed] [Google Scholar]
- 2.Linsel-Nitschke P., Tall A. R. 2005. HDL as a target in the treatment of atherosclerotic cardiovascular disease. Nat. Rev. Drug Discov. 4: 193–205 [DOI] [PubMed] [Google Scholar]
- 3.Duffy D., Rader D. J. 2006. Emerging therapies targeting high-density lipoprotein metabolism and reverse cholesterol transport. Circulation. 113: 1140–1150 [DOI] [PubMed] [Google Scholar]
- 4.Lewis G. F.2006. Determinants of plasma HDL concentrations and reverse cholesterol transport. Curr. Opin. Cardiol. 21: 345–352 [DOI] [PubMed] [Google Scholar]
- 5.Jaye M., Lynch K. J., Krawiec J., Marchadier D., Maugeais C., Doan K., South V., Amin D., Perrone M., Rader D. J. 1999. A novel endothelial-derived lipase that modulates HDL metabolism. Nat. Genet. 21: 424–428 [DOI] [PubMed] [Google Scholar]
- 6.Hirata K., Dichek H. L., Cioffi J. A., Choi S. Y., Leeper N. J., Quintana L., Kronmal G. S., Cooper A. D., Quertermous T. 1999. Cloning of a unique lipase from endothelial cells extends the lipase gene family. J. Biol. Chem. 274: 14170–14175 [DOI] [PubMed] [Google Scholar]
- 7.McCoy M. G., Sun G. S., Marchadier D., Maugeais C., Glick J. M., Rader D. J. 2002. Characterization of the lipolytic activity of endothelial lipase. J. Lipid Res. 43: 921–929 [PubMed] [Google Scholar]
- 8.Jin W., Sun G. S., Marchadier D., Octtaviani E., Glick J. M., Rader D. J. 2003. Endothelial cells secrete triglyceride lipase and phospholipase activities in response to cytokines as a result of endothelial lipase. Circ. Res. 92: 644–650 [DOI] [PubMed] [Google Scholar]
- 9.Broedl U. C., Jin W., Rader D. J. 2004. Endothelial lipase: a modulator of lipoprotein metabolism upregulated by inflammation. Trends Cardiovasc. Med. 14: 202–206 [DOI] [PubMed] [Google Scholar]
- 10.Badellino K. O., Wolfe M. L., Reilly M. P., Rader D. J. 2006. Endothelial lipase concentrations are increased in metabolic syndrome and associated with coronary atherosclerosis. PLoS Med. 3: e22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Paradis M. E., Badellino K. O., Rader D. J., Deshaies Y., Couture P., Archer W. R., Bergeron N., Lamarche B. 2006. Endothelial lipase is associated with inflammation in humans. J. Lipid Res. 47: 2808–2813 [DOI] [PubMed] [Google Scholar]
- 12.Maugeais C., Tietge U. J. F., Broedl U. C., Marchadier D., Cain W., McCoy M. G., Lund-Katz S., Glick J. M., Rader D. J. 2003. Dose-dependent acceleration of high-density lipoprotein catabolism by endothelial lipase. Circulation. 108: 2121–2126 [DOI] [PubMed] [Google Scholar]
- 13.Jin W., Millar J. S., Broedl U., Glick J. M., Rader D. J. 2003. Inhibition of endothelial lipase causes increased HDL cholesterol levels in vivo. J. Clin. Invest. 111: 357–362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ishida T., Choi S., Kundu R. K., Hirata K., Rubin E. M., Cooper A. D., Quertermous T. 2003. Endothelial lipase is a major determinant of HDL level. J. Clin. Invest. 111: 347–355 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ma K., Cilingiroglu M., Otvos J. D., Ballantyne C. M., Marian A. J., Chan L. 2003. Endothelial lipase is a major genetic determinant for high-density lipoprotein concentration, structure, and metabolism. Proc. Natl. Acad. Sci. USA. 100: 2748–2753 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.deLemos A. S., Wolfe M. L., Long C. J., Sivapackianathan R., Rader D. J. 2002. Identification of genetic variants in endothelial lipase in persons with elevated high-density lipoprotein cholesterol. Circulation. 106: 1321–1326 [DOI] [PubMed] [Google Scholar]
- 17.Lamarche B., Paradis M. E. 2007. Endothelial lipase and the metabolic syndrome. Curr. Opin. Lipidol. 18: 298–303 [DOI] [PubMed] [Google Scholar]
- 18.von Eckardstein A., Nofer J. R., Assmann G. 2001. High density lipoproteins and arteriosclerosis. Role of cholesterol efflux and reverse cholesterol transport. Arterioscler. Thromb. Vasc. Biol. 21: 13–27 [DOI] [PubMed] [Google Scholar]
- 19.Toth P. P.2003. Reverse cholesterol transport: high-density lipoprotein’s magnificent mile. Curr. Atheroscler. Rep. 5: 386–393 [DOI] [PubMed] [Google Scholar]
- 20.Kozarsky K. F., Donahee M. H., Rigotti A., Iqbal S. N., Edelman E. R., Krieger M. 1997. Overexpression of the HDL receptor SR-BI alters plasma HDL and bile cholesterol levels. Nature. 387: 414–417 [DOI] [PubMed] [Google Scholar]
- 21.Tietge U. J. F., Maugeais C., Lund-Katz S., Grass D., deBeer F. C., Rader D. J. 2002. Human secretory phospholipase A2 mediates decreased plasma levels of HDL cholesterol and apoA-I in response to inflammation in human apoA-I transgenic mice. Arterioscler. Thromb. Vasc. Biol. 22: 1213–1218 [DOI] [PubMed] [Google Scholar]
- 22.Tietge U. J. F., Maugeais C., Cain W., Grass D., Glick J. M., de Beer F. C., Rader D. J. 2000. Overexpression of secretory phospholipase A(2) causes rapid catabolism and altered tissue uptake of high density lipoprotein cholesteryl ester and apolipoprotein A-I. J. Biol. Chem. 275: 10077–10084 [DOI] [PubMed] [Google Scholar]
- 23.Tietge U. J. F., Nijstad N., Havinga R., Baller J. F., van der Sluijs F. H., Bloks V. W., Gautier T., Kuipers F. 2008. Secretory phospholipase A2 (sPLA2) expression in transgenic mice results in increased hepatic SR-BI-mediated selective HDL cholesteryl ester uptake but does not affect biliary cholesterol secretion or gallstone formation. J. Lipid Res. 49: 563–571 [DOI] [PubMed] [Google Scholar]
- 24.Groen A. K., Bloks V. W., Bandsma R. H., Ottenhoff R., Chimini G., Kuipers F. 2001. Hepatobiliary cholesterol transport is not impaired in Abca1-null mice lacking HDL. J. Clin. Invest. 108: 843–850 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Trigatti B. L., Krieger M., Rigotti A. 2003. Influence of the HDL receptor SR-BI on lipoprotein metabolism and atherosclerosis. Arterioscler. Thromb. Vasc. Biol. 23: 1732–1738 [DOI] [PubMed] [Google Scholar]
- 26.Willnow T. E., Sheng Z., Ishibashi S., Herz J. 1994. Inhibition of hepatic chylomicron remnant uptake by gene transfer of a receptor antagonist. Science. 264: 1471–1474 [DOI] [PubMed] [Google Scholar]
- 27.Mardones P., Quinones V., Amigo L., Moreno M., Miquel J. F., Schwarz M., Miettinen H. E., Trigatti B., Krieger M., VanPatten S., et al. 2001. Hepatic cholesterol and bile acid metabolism and intestinal cholesterol absorption in scavenger receptor class B type I-deficient mice. J. Lipid Res. 42: 170–180 [PubMed] [Google Scholar]
- 28.Duong M., Psaltis M., Rader D. J., Marchadier D., Barter P. J., Rye K. A. 2003. Evidence that hepatic lipase and endothelial lipase have different substrate specificities for high-density lipoprotein phospholipids. Biochemistry. 42: 13778–13785 [DOI] [PubMed] [Google Scholar]
- 29.Griffon N., Budreck E. C., Long C. J., Broedl U. C., Marchadier D. H., Glick J. M., Rader D. J. 2006. Substrate specificity of lipoprotein lipase and endothelial lipase: studies of lid chimeras. J. Lipid Res. 47: 1803–1811 [DOI] [PubMed] [Google Scholar]
- 30.Broedl U. C., Maugeais C., Marchadier D., Glick J. M., Rader D. J. 2003. Effects of nonlipolytic ligand function of endothelial lipase on high density lipoprotein metabolism in vivo. J. Biol. Chem. 278: 40688–40693 [DOI] [PubMed] [Google Scholar]
- 31.Martinez L. O., Jacquet S., Esteve J. P., Rolland C., Cabezon E., Champagne E., Pineau T., Georgeaud V., Walker J. E., Terce F., et al. 2003. Ectopic beta-chain of ATP synthase is an apolipoprotein A-I receptor in hepatic HDL endocytosis. Nature. 421: 75–79 [DOI] [PubMed] [Google Scholar]
- 32.Jacquet S., Malaval C., Martinez L. O., Sak K., Rolland C., Perez C., Nauze M., Champagne E., Terce F., Gachet C., et al. 2005. The nucleotide receptor P2Y13 is a key regulator of hepatic high-density lipoprotein (HDL) endocytosis. Cell. Mol. Life Sci. 62: 2508–2515 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Berge K. E., Tian H., Graf G. A., Yu L., Grishin N. V., Schultz J., Kwiterovich P., Shan B., Barnes R., Hobbs H. H. 2000. Accumulation of dietary cholesterol in sitosterolemia caused by mutations in adjacent ABC transporters. Science. 290: 1771–1775 [DOI] [PubMed] [Google Scholar]
- 34.Yu L., Gupta S., Xu F., Liverman A. D., Moschetta A., Mangelsdorf D. J., Repa J. J., Hobbs H. H., Cohen J. C. 2005. Expression of ABCG5 and ABCG8 is required for regulation of biliary cholesterol secretion. J. Biol. Chem. 280: 8742–8747 [DOI] [PubMed] [Google Scholar]
- 35.Yu L., Hammer R. E., Li-Hawkins J., Von Bergmann K., Lutjohann D., Cohen J. C., Hobbs H. H. 2002. Disruption of Abcg5 and Abcg8 in mice reveals their crucial role in biliary cholesterol secretion. Proc. Natl. Acad. Sci. USA. 99: 16237–16242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yu L., Li-Hawkins J., Hammer R. E., Berge K. E., Horton J. D., Cohen J. C., Hobbs H. H. 2002. Overexpression of ABCG5 and ABCG8 promotes biliary cholesterol secretion and reduces fractional absorption of dietary cholesterol. J. Clin. Invest. 110: 671–680 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Plösch T., van der Veen J. N., Havinga R., Huijkman N. C., Bloks V. W., Kuipers F. 2006. Abcg5/Abcg8-independent pathways contribute to hepatobiliary cholesterol secretion in mice. Am. J. Physiol. Gastrointest. Liver Physiol. 291: G414–G423 [DOI] [PubMed] [Google Scholar]
- 38.Kosters A., Frijters R. J., Schaap F. G., Vink E., Plösch T., Ottenhoff R., Jirsa M., De Cuyper I. M., Kuipers F., Groen A. K. 2003. Relation between hepatic expression of ATP-binding cassette transporters G5 and G8 and biliary cholesterol secretion in mice. J. Hepatol. 38: 710–716 [DOI] [PubMed] [Google Scholar]
- 39.Geuken E., Visser D. S., Leuvenink H. G., de Jong K. P., Peeters P. M., Slooff M. J., Kuipers F., Porte R. J. 2005. Hepatic expression of ABC transporters G5 and G8 does not correlate with biliary cholesterol secretion in liver transplant patients. Hepatology. 42: 1166–1174 [DOI] [PubMed] [Google Scholar]
- 40.Groen A., Kunne C., Jongsma G., van den Oever K., Mok K. S., Petruzzelli M., Vrins C. L., Bull L., Paulusma C. C., Oude Elferink R. P. 2008. Abcg5/8 independent biliary cholesterol excretion in atp8b1-deficient mice. Gastroenterology. 134: 2091–2100 [DOI] [PubMed] [Google Scholar]
- 41.Brown M. S., Goldstein J. L. 1997. The SREBP pathway: regulation of cholesterol metabolism by proteolysis of a membrane-bound transcription factor. Cell. 89: 331–340 [DOI] [PubMed] [Google Scholar]
- 42.Wustner D.2005. Mathematical analysis of hepatic high density lipoprotein transport based on quantitative imaging data. J. Biol. Chem. 280: 6766–6779 [DOI] [PubMed] [Google Scholar]
- 43.Wustner D., Mondal M., Huang A., Maxfield F. R. 2004. Different transport routes for high density lipoprotein and its associated free sterol in polarized hepatic cells. J. Lipid Res. 45: 427–437 [DOI] [PubMed] [Google Scholar]
- 44.Ji Y., Wang N., Ramakrishnan R., Sehayek E., Huszar D., Breslow J. L., Tall A. R. 1999. Hepatic scavenger receptor BI promotes rapid clearance of high density lipoprotein free cholesterol and its transport into bile. J. Biol. Chem. 274: 33398–33402 [DOI] [PubMed] [Google Scholar]
- 45.Plump A. S., Azrolan N., Odaka H., Wu L., Jiang X., Tall A., Eisenberg S., Breslow J. L. 1997. ApoA-I knockout mice: characterization of HDL metabolism in homozygotes and identification of a post-RNA mechanism of apoA-I up-regulation in heterozygotes. J. Lipid Res. 38: 1033–1047 [PubMed] [Google Scholar]
- 46.Wang N., Weng W., Breslow J. L., Tall A. R. 1996. Scavenger receptor BI (SR-BI) is up-regulated in adrenal gland in apolipoprotein A-I and hepatic lipase knock-out mice as a response to depletion of cholesterol stores. In vivo evidence that SR-BI is a functional high density lipoprotein receptor under feedback control. J. Biol. Chem. 271: 21001–21004 [DOI] [PubMed] [Google Scholar]
- 47.Zhang Y., Da Silva J. R., Reilly M., Billheimer J. T., Rothblat G. H., Rader D. J. 2005. Hepatic expression of scavenger receptor class B type I (SR-BI) is a positive regulator of macrophage reverse cholesterol transport in vivo. J. Clin. Invest. 115: 2870–2874 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.