Abstract
The snail Biomphalaria glabrata (Gastropoda, Mollusca) is an important intermediate host for the human parasite Schistosoma mansoni (Digenea, Trematoda). Anti-pathogen responses of B. glabrata were studied towards a better understanding of snail immunity and host-parasite compatibility. Open reading frame ESTs (ORESTES) were sampled from different transcriptomes of M line strain B. glabrata, 12 hours post challenge with Escherichia coli (Gram-negative), Micrococcus luteus (Gram-positive) bacteria or compatible S. mansoni, and controls. The resulting 3123 ORESTES represented 2129 unique sequences (373 clusters, 1756 singletons). Of these, 175 (8.1%) were putative defense factors, including lectins, antimicrobial peptides and components of various immune effector systems. Comparison of biological processes (GO-terms) within different transcriptomes indicated that B. glabrata increased oxygen transport and metal binding in reaction to all challenges. Comprehensive comparisons of transcriptomes revealed that responses of B. glabrata against bacteria were similar to each other and differed from the ineffective response to S. mansoni. Furthermore, the response to S. mansoni infection was less comprehensive than that to bacteria. Many novel (unknown) sequences were recovered in association with particular challenges. Biomphalaria glabrata possesses multi-faceted, potent immune defenses. This agrees with the notion that S. mansoni is capable of immune-evasion and prevents effective host defense responses in order to survive in B. glabrata. Future analysis of the numerous unknown sequences recovered from challenged snails may reveal novel immune factors and provide increased understanding of immunity of B. glabrata in relation to parasite-host compatibility.
Keywords: Biomphalaria glabrata, Schistosoma mansoni, Escherichia coli, Micrococcus luteus, parasite-host compatibility, snail immunity, ORESTES, comparative transcriptomics
Introduction
Biomphalaria glabrata is one of the major snail species that contributes to the transmission of schistosomiasis to humans. To date it remains unclear why B. glabrata is such a suitable intermediate host for S. mansoni. Incompatible schistosome parasites evoke a rapid defense response after penetration into a B. glabrata (Nowak et al., 2004; Sullivan and Richards, 1981) and are killed with a combination of humoral and cellular cytotoxicity (Adema and Loker, 1997; Lockyer et al., 2004). The intramolluscan survival and successful development of the parasite depend critically on the ability of S. mansoni to avoid immuno-elimination by the internal defense system of the snail host. Depending on the genetic background of both parasite and host, S. mansoni can achieve immuno-compatibility with B. glabrata in particular combinations that may be restricted to strain and even individual level (Lewis et al., 2001; Theron and Coustau, 2005).
Schistosoma mansoni is thought to escape elimination by avoiding recognition as non-self in order to prevent activation of host defenses; additionally the parasite may interfere with the efficiency of host defenses (Lie, 1982; Loker and Adema, 1995). The exact mechanisms employed by the parasite to effect these survival strategies remain to be elucidated. Biological properties of the snail host also play a role. Quantitative differences in cytotoxic factors produced by internal defenses of snails, such as the ratio of individual species of reactive oxygen radicals, may contribute to resistance or susceptibility of specific strains of B. glabrata relative to PR-1 strain S. mansoni (Bender et al., 2007). It remains unclear, however, how B. glabrata snails can be simultaneously resistant to one strain of schistosomes and susceptible to another (Frandsen, 1979; Richards, 1975; Richards and Shade, 1987; Webster et al., 2004; Webster and Woolhouse, 1998)or how snails with assumed inferior defenses can persist in nature while being exposed continuously to other pathogens in addition to S. mansoni.
Most studies of snail immunity involve Biomphalaria glabrata, a major intermediate host snail for Schistosoma mansoni in the New World (Morgan et al., 2001). The distribution of B. glabrata largely defines the areas that are endemic for schistosomiasis in South and Central America (Dejong et al., 2003), but only a low proportion of snails in the field is patently infected with schistosomes (de Souza et al., 1994) and some B. glabrata are naturally resistant (Paraense and Correa, 1963). Increasingly, experimental study of B. glabrata as intermediate host of schistosomes and related digenetic trematode parasites incorporates characterization of the genome (Adema et al., 2006; DeJong et al., 2004; Raghavan and Knight, 2006), the proteome (Bouchut et al., 2006b; Vergote et al., 2005) and especially the transcriptome (Bouchut et al., 2006a; Jung et al., 2005; Knight et al., 1999; Lockyer et al., 2007a; Lockyer et al., 2007b; Miller et al., 2001; Mitta et al., 2005; Nowak et al., 2004; Raghavan et al., 2003) of B. glabrata.
Molecular studies have demonstrated that B. glabrata is capable of highly complex responses to pathogens (Adema et al., 1999; Zhang et al., 2004) but the determinant factors for resistance or susceptibility of B. glabrata regarding schistosome infection remain to be characterized. Previous studies of immune-interactions between schistosomes and susceptible or resistant strains of B. glabrata have identified several immune mechanisms that contribute to the elimination of parasites (Bender et al., 2007; van der Knaap and Loker, 1990). The bias toward using digenean trematodes as experimental pathogens may have limited insights into the immune capabilities of B. glabrata because some digeneans interfere with or avoid host responses (Lie, 1982; Loker and Adema, 1995). The experimental use of other types of pathogens such as bacteria may provide a more comprehensive insight into the internal defense system of B. glabrata. Previous reports regarding interactions of B. glabrata with bacteria did not provide molecular sequence data (Adema et al., 1999; Ducklow et al., 1979; Ducklow et al., 1980; Matricon-Gondran and Letocart, 1999).
The characterization of the immune capabilities of B. glabrata in the context of parasite-snail compatibility also has great potential to inform on the evolution of internal defense systems of animals in general. As a mollusk, B. glabrata represents the lophotrochozoa. The information on the immunology of this major branch of (invertebrate) protostome bilaterians is limited, especially in comparison to ecdysozoa and the deuterostomes (Loker et al., 2004). Likely, B. glabrata employs some immune factors in common with other bilateria due to their shared evolutionary history. But in the more than 500 million years since divergence from a common ancestor, lophotrochozoa may have elaborated unique and novel defense components that remain to be identified.
In this study, M line strain B. glabrata snails (highly susceptible to S. mansoni) were subjected to schistosome infection or to injection with bacteria; either Gram-negative (G−) Escherichia coli or Gram-positive (G+) Micrococcus luteus. As is the case with injection, also schistosome infection causes wounding and damage to snails tissues; miracidia apply mechanical force (Pan, 1965) and proteolytic enzymes (Pino-Heiss et al., 1985; Yoshino et al., 1993) to penetrate through the epithelia of the body wall and colonize the tissues of snails. Thus the transcription profiles representing the responses of this single strain of B. glabrata to these distinct challenges were each sampled using open reading frame ESTs (ORESTES, seede Souza et al., 2000). The resulting sequences provide a broader insight into the gene complement of B. glabrata, and will help the interpretation of data stemming from the ongoing B. glabrata genome sequencing project (Raghavan and Knight, 2006). Data analysis was performed to identify both known and novel candidate immune factors (unknown sequences associated with responses to particular pathogens), and to compare immune-related and general aspects of the transcriptome responses of B. glabrata to different immune challenges.
Methods
Live material and experimental treatments
M-line strain Biomphalaria glabrata snails are maintained at the University of New Mexico (UNM, Loker and Hertel, 1987), adult snails (shell diameter 10–12mm) were used in this study. Schistosoma mansoni (PR-1 strain) was obtained from the Schistosomiasis Resource Center (NIAID Contract N01-A1-30026) at the Biomedical Research Institute (Rockville, Maryland). Escherichia coli and Micrococcus luteus, ATCC 14948 and 9341, respectively, were available from the microbiology teaching facility at UNM. These bacteria species were selected because they are common in nature, their genomes have been characterized, and they have been used frequently and are recommended as model infectious organisms in invertebrates (Hetru and Bulet, 1997).
The 3 experimental groups consisted of pools of 20 snails; 35 snails were pooled for the control group. For management and annotation of sequence data, groups were designated to identify the contributing laboratory (CA for C Adema), the strain of B. glabrata (M for M line), the experimental treatment (C, M, N, P for control, and exposure to S. mansoni, Gram negative or Gram positive bacteria, respectively) and type of sequence data (O for ORESTES).
Individual B. glabrata were exposed to 50 S. mansoni miracidia in artificial spring water (ASW, Loker and Hertel, 1987) for 12 hours. Snails in the bacteria-exposed groups were each injected in the headfoot with 50 µl of bacterial culture in LB medium (OD600 of 1.0 = 8 × 108 cells/ml ) of E. coli or M. luteus using a G27 hypodermic needle, and kept on the bench for 5 minutes before being placed into 24 well plates with ASW for 12 hours (Adema et al., 1999). Control snails were placed into 24 well plates with ASW for 12 hours.
mRNA purification
The shell was removed and whole body tissues of individual snails were disrupted in a 1.5µl tube with a plastic pestle (Kimble/Kontes Glass Co., Vineland, NJ) in 1 ml Trizol®-Reagent (Invitrogen, Carlsbad, CA). Samples were extracted with 0.2ml chloroform and centrifuged (12,000g, 15 minutes, 4°C). The RNA was precipitated using 0.5ml of isopropanol, pelleted (12,000g, 10 minutes, 4°C), washed (75% ethanol), air dried, and dissolved in 50µl deionized water.
All bacteria-challenged snails were considered infected. To confirm S. mansoni infection of M line B. glabrata, the absence of miracidia in the wells after exposure was confirmed microscopically and a nested PCR method was used to detect the presence of S. mansoni 18S rDNA in individual snails (Hanelt et al., 1997). In addition to RNA, DNA was harvested from Trizol®-Reagent extractions from each snail that was exposed to S. mansoni. Briefly, DNA was precipitated (using 0.3ml 100% ethanol) from the interphase that remained after removal of the aqueous phase (RNA), pelleted (2,000g, 5 minutes, 4°C), washed twice in 1ml 0.1M sodium citrate, 10% ethanol, and dissolved in 50µl 8mM NaOH. Only RNA samples from snails that tested positive for parasite DNA were used.
Total RNA samples from individual snails were treated with DNA-free® (Ambion, Inc, Austin, Texas). The absence of residual DNA was tested by PCR (AmpliTaq Gold®, Applied Biosystems, Foster City, California) relative to a positive control, using primers: BCML-ACT.FOR1 (GTGATGGTTGGTATGGGTCAGAAGG) and BCML-ACT.REV1 (GAAGCTGTAGCCCCTCTCAGTG) to target a cytoplasmic actin gene (GenBank#: AF329436, Adema, 2002) . The PCR conditions were: 95°C, 10 minutes; 30 cycles at: 95°C, 1 minute, 55°C, 1 minute, 72°C, 1 minute; 72°C, 7 minutes. mRNA was purified from pooled RNA samples with the Poly(A)Purist™ MAG kit (Ambion, Inc., Austin, Texas) and stored at −70°C.
ORESTES mini-libraries
The open reading frame EST (ORESTES) strategy (Camargo et al., 2001) was chosen for the propensity to sample the central (coding) regions of transcripts (as compared to ESTs derived from poly-A primed cDNA). The recovery of coding (conserved) sequence increases chances for relevant identification by similarity searches against databases. Also the use of random priming for the generation of cDNA benefits the recovery of less frequently expressed sequences (de Souza et al., 2000). ORESTES were generated as follows. Nine sets of ORESTES primers, either 25mers (sets A-E) or 18mers (sets F-I) were designed using a random number generator. Set A consisted of 10 primers; the subsequent primer sets consisted of sets of 8 (Supplement S1). For each mRNA template (representing controls or experimental groups), RT-PCR was performed with these sets of ORESTES primers, and the resulting cDNAs were cloned to create a total of 612 mini-libraries (10 forward primers × 10 reverse primers × 1 primer set = 100, plus 8 forward primers × 8 reverse primers × 8 primer sets = 512).
For each sample, 40ng of mRNA was reverse transcribed (42°C, 1h) with 50pmol of appropriate primer using the Advantage® RT-for-PCR kit (Clontech, Mountain View, California). The resulting cDNA (100µl final volume) was used as template in PCR reactions with the same (RT) primer and one other primer within an ORESTES primer set, ultimately using all possible combinations within a given primer set. Every 50µl PCR reaction consisted of 5µl cDNA template, 200µM of each dNTP, 4mM MgCl2, 50pmol of each primer, and 1.25U AmpliTaq Gold® DNA polymerase. The temperature profile was 95°C, 10 minutes; 15 cycles at 95°C, 30 seconds, 54°C, 30 seconds, 72°C, 1 minute; 25 cycles at: 95°C, 30 seconds, 50°C, 30 seconds, 72°C, 1 minute; 72°C, 15 minutes. Unused PCR reagents and PCR products smaller than ~80bp were removed with the Perfectprep® PCR Cleanup 96 kit (Eppendorf, Hamburg, Germany). The resulting RT-PCR products were diluted in 30µl deionized water and were cloned by incubating 1.5µl of product with 0.5µl TOPO-TA® pCR2.1 (Invitrogen, Carlsbad, California) and 0.5µl salt solution (room temperature, 30 minutes). Each reaction received 25µl of TOP10 chemically competent cells and was kept on ice for 30 minutes, heat shocked (42°C, 30 seconds) and incubated in 125µl of SOC for 2 hours at 37°C. Eighty µl of each cloning reaction was plated for blue-white screening overnight (37°C). From each mini-library, 24 transformants were grown up in wells of a 96 well tissue culture plate containing 100µl LB, 50µg/ml kanamycin (37°C, 180rpm, 1 hour) and stored as (40%) glycerol cultures at −30°C.
Clone selection for insert sequencing
The plasmid insert of 24 clones from each mini-library was PCR amplified (AmpliTaq Gold®) using 0.5 µl of glycerol stock as template with M13-20 and M13rev primers. A pilot study indicated that sampling 20 clones per library maximized the discovery of new sequence diversity. We expanded this set to 24 clones to facilitate the 8 × 12, 96 well plate format. Amplicons were visualized using the E-Gel® 96 well high-throughput agarose electrophoresis system (Invitrogen, Carlsbad, California). Clones were selected for sequencing based on: 1) presence of a single insert; 2) insert size ≥ 100bp; 3) no size-duplicate inserts for each mini library. Initial tests indicated that some primer combinations yielded high numbers of cDNA inserts derived from ribosomal RNA. This was not unexpected as previously observed; rRNA copurifies with mRNA of B. glabrata and is also reverse transcribed when using random primers (Lockyer et al., 2007a; Nowak et al., 2004). Particular primer combinations with great affinity for rDNA sequences were not used further.
Plasmid isolation and sequencing
All transformants selected for sequencing were regridded as glycerol stocks into 96 well tissue culture plates, assigned a clone number consisting of plate number and well, and archived at −70C. Plasmid DNA was extracted from each transformant with the Perfectprep® Plasmid 96 Vac Direct Bind kit (Eppendorf), using 50µl warm deionized water for elution and the Perfectprep® PCR Cleanup 96 (Eppendorf). Single pass sequencing was done using the ABI PRISM® BigDye® Terminator version 3.1 Cycle Sequencing Kit (ABI, Foster City, California) with the following conditions: 3.3pM primer (M13-20), 0.5µl BDT, 1.75µl 5x buffer, 3.7µl water, and 4µl plasmid DNA (200–400ng per reaction); 96°C, 10 minutes; 25 cycles at: 96°C, 30 seconds, 52°C, 15 seconds, 60°C, 4 minutes. Extension products were read on an ABI 3100 sequencer.
Sequence analysis
Sequence chromatograms were edited by hand, and vector and primer sequences were removed using Sequencher™ (Genecodes Corp., Ann Arbor, Michigan). Insert sequences shorter than 95bp and identical sequences derived from the same template and primer combination were discarded. Putative IDs were assigned to sequences based on BLAST analysis against protein and nucleotide databases (PROSITE, non-redundant GenBank databases, B. glabrata-derived dbEST entries). The cut-off for significance was e = 1.0×10−5. Sequences with similarity to rDNA or mitochondrial genes (accession number NC_005439, DeJong et al., 2004) of B. glabrata, or to sequences from S. mansoni or bacteria were not included in transcriptome analyses. Resulting ORESTES were submitted to GenBank. CAP3 (Huang and Madan, 1999) was used with default settings to cluster ORESTES. All clusters were BLASTED to confirm the putative IDs previously assigned to individual ORESTES. Each EST was analyzed for gene function using the KAAS - KEGG database (http://www.genome.jp/kegg/kaas/). The KAAS - KEGG database is able to link a set of ESTs with a network of interacting molecules (Kanehisa and Goto, 2000). Blast2GO (Conesa et al., 2005) was used to assign Gene Ontology (GO) terms to ORESTES (http://www.blast2go.de/). This program also assessed differential gene expression by comparing the distribution of GO term processes between the control and treatment groups, generating Fisher’s Exact test P-values for representation of each GO term. Comparative analysis of representation of particular ORESTES among treatment groups and control snails was done with Pymood™ software (Allometra, Davis, California).
Results
Immune challenge of snails
All B. glabrata snails in the control group and the 24 snails exposed to S. mansoni were alive 12 hours after they had been exposed or sham exposed and isolated in separate wells. Nested PCR targeting parasite 18S DNA identified 20 snails that harbored S. mansoni for use in this study. Bacterial exposure, effected by injection of 50 µl bacterial suspension into the tissues of the head foot of snails, did not adversely impact snail viability; all snails were alive and active at 12 hours post challenge. The mRNA was extracted and pooled from 20 snails of each challenge group and from 35 untreated controls.
Sequence data compilation and annotation
The mRNA samples from the 4 groups of B. glabrata (3 experimental and 1 control) were used to generate 2448 mini-libraries by use of paired random primers (Supplement S1) and low-stringency RT-PCR. After screening an initial 58752 clones against small or duplicate insert size, 7331 clones were selected for unidirectional single-pass sequencing of inserts. Of the resulting sequences, 2303 inserts (31%) derived from rDNA genes (http://biology.unm.edu/biomphalaria-genome/rdnabg.html) or the mitochondrial genome (DeJong et al., 2004). This considerable reduction in yield of sequence data was anticipated; rRNA sequences from B. glabrata are A-rich and copurify with mRNA. This rRNA is also converted to cDNA during experimental reverse transcription to generate ESTs (Lockyer et al., 2007b; Nowak et al., 2004). After removal of 840 failed reads, 542 short reads (< 90nt), 476 identical replicates within mini-libraries, and 47 sequences from plant, yeast or bacteria, ultimately 3123 valid ORESTES (322 nt average length) were obtained. Of these, 1028 sequences were derived from the control group, 707 and 678 resulted from snails exposed to E. coli or M. luteus, respectively, and 716 represented the transcriptome of S. mansoni-infected B. glabrata. BLAST analyses revealed that 1928 (61%) of these ORESTES had no similarity to any of the 18016 ESTs from B. glabrata contained within GenBank (June 2007), 380 (12%) had similarity to both B. glabrata ESTs and ESTs from other mollusks and 5% had similarity to ESTs reported only from other mollusks but not B. glabrata. Additionally, 75 singleton ESTs and 71 ORESTES clusters without a putative ID had similarity to unidentified mollusk ESTs in GenBank (see Supplement S2 for complete list). Putative identities based on BLAST similarity were included with the submissions to GenBank. [GenBank:CF216472-CF216486, CK327217-CK327247, CK386771-CK386814, CK640644-CK640684, CK725226-CK725261, CK768266-CK768306, CK800769-CK800816, CK84999-CK85025, CN013290-CN013346, CN445741-CN445897, CN476057-CN476119, CN549142-CN549191, CN655000-CN655058, CN779671-CN779736, CX820337-CX836382, CX727730-CX727806, CX820337-CX820343, CX836372-CX836382, DN966827-DN967024, CO635864-CO635934, CO654001-CO654074, CV022343-CV022409, CV042520-CV042595, CV173977-CV174081, CV627118-CV627165, CV997188-CV997219, CX160605-CX160630, CX727807-CX727810, CX820344-CX820347, DN837171-DN837237, DN952341-DN952395, DN967025-DN967047, EE722305-EE723011, EE723012-EE723689].
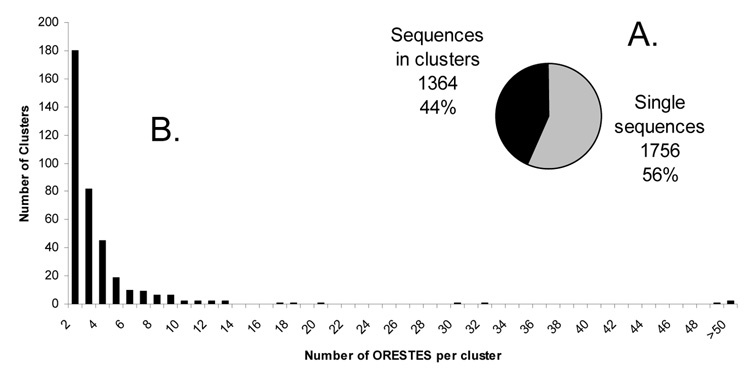
Clustering of the 3123 individual ORESTES using CAP3 software (Huang and Madan, 1999) yielded 373 clusters (assembled out of 1367 ORESTES; Figure 1A), leaving 1756 singletons. Most clusters consisted of between 2–6 sequences but the largest clusters comprised over 50 members (Figure 1B). The largest clusters included sequences with similarity to hemoglobin, myoglobin and myosin. BLAST searches provided putative IDs for 1668 (78 %) of the 2129 unique sequences (clusters and singletons). In several cases a cluster provided a putative ID for a cluster mate sequence that did not share significant BLAST similarity to known sequences. The putative identity of several ORESTES provided new views of the general biology of B. glabrata (Supplement S3). The remaining 22% of the unique sequences (461 clusters and singletons, representing 993 ORESTES) were novel sequences that lacked significant similarities to the databases.
Figure 1. Size distribution of ORESTES clusters within the Biomphalaria glabrata data set.
A. Proportion of clusters versus singletons. Note: the 1364 clustered sequences fall into 373 clusters. B. Distribution of ORESTES clusters by size.
Functional classification
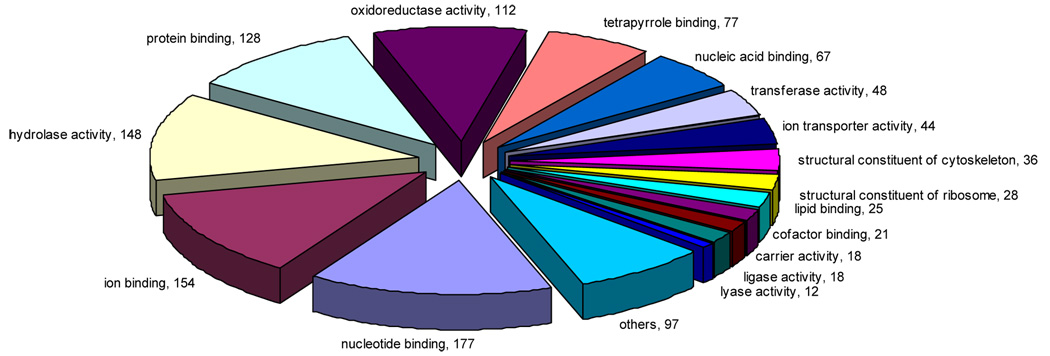
KAAS - KEGG analysis assigned gene function to 509 (16.3%) of all ORESTES. Based on significant similarity with known sequences (BLAST e-values < e−5), the Blast2GO utility (http://www.blast2go.de) assigned level 3 Gene Ontology classifications to 1210 ORESTES (39%) of the original 3123 ORESTES. More than half of the classified ORESTES (619) were involved in binding and nearly a third (332) were involved in catalytic activity (Figure 2). Between both analyses, functions were assigned to 1354 of the 3123 ORESTES.
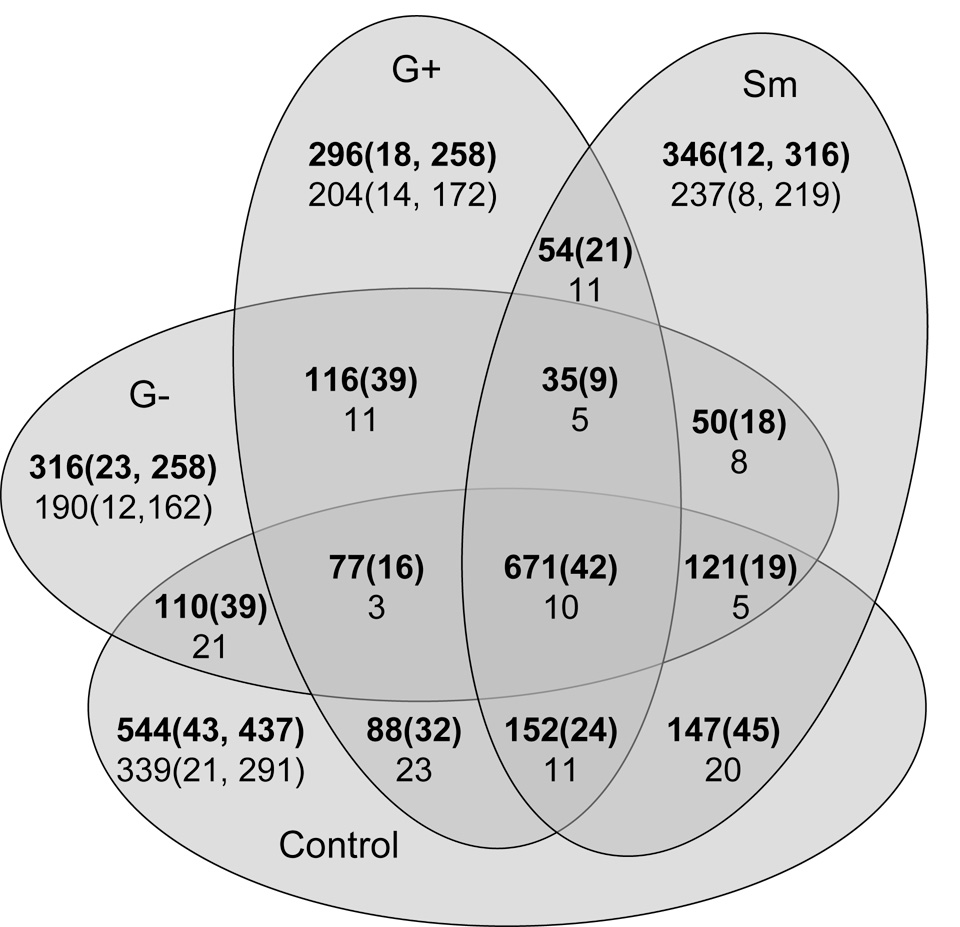
Figure 2. Patterns of Biomphalaria glabrata ORESTES across experimental groups.
ORESTES recovered from particular treatments groups are presented within individual ovals. Non-overlapping areas of ovals represent ORESTES unique to that group (Control: unexposed control, G−: Escherichia coli-exposed, G+ Micrococcus luteus-exposed, Sm: Schistosoma mansoni-exposed). Overlap of ovals contains ORESTES shared by groups. Numbers in bold are the total numbers of ORESTES; non-bolded numbers represent unknowns (without GenBank sequence similarity). The numbers of clusters are indicated in brackets, delimited by a comma from the number of remaining singlets.
Immune factors
A total of 766 ORESTES representing 179 clusters and singletons (8.5% of all unique sequences), was assigned a putative immune function, based on sequence similarity with previously characterized immune response genes using results from BLAST, BLAST2GO and KAAS – KEGG searches and confirmation by hand. These immune factors were categorized into the following functional groups: 1) pattern recognition, 2) cell adhesion, 3) signal transduction, 4) metal binding, 5) stress response, 6) (anti)oxidant-related, 7) proteases, 8) serpins, 9) inflammatory response, 10) apoptosis-related and 11) other putative immune factors. A twelfth group lists unknown clusters and singletons with similarity to sequences identified in other studies as differentially expressed in B. glabrata in response to pathogens. Several sequences were recovered uniquely from pathogen-challenged B. glabrata. Among sequences not previously documented from B. glabrata were several chitin-binding factors, as well as components of cell-mediated cytotoxicity and RNA interference (see Table 1). The number of ORESTES associated with putative immune function varied between control and exposed snails (control: 21.3%, M. luteus-exposed: 26.3%, E. coli-exposed: 26.3%, S. mansoni-exposed: 25.6%; Table 1).
Table 1. Immune-related ORESTES generated from Biomphalaria glabrata.
Putative immune-factors as identified by BLAST searches (e < 10−5). The number of ORESTES recovered from each of the transcriptomes are indicated. The unknown category includes sequences that did not yield significant BLAST scores but that have similarity to transcripts reported as immune-relevant genes in other studies.
| #ESTs | |||||
|---|---|---|---|---|---|
| Characteristic or function | Sequence Product ID | Control | G+ -exposed | G− -exposed | S.m.- exposed |
| Pattern recognition/ binding | Angiopoietin-like 1 Fibrinogen-like | 1 | 0 | 0 | 0 |
| Beta-1,3-glucan-binding protein | 1 | 3 | 0 | 1 | |
| Calcium binding protein† | 0 | 5 | 0 | 0 | |
| Cellulase | 0 | 0 | 2 | 3 | |
| Chitin binding Peritrophin-A | 0 | 1 | 0 | 0 | |
| Chitin-binding protein | 0 | 1 | 1 | 0 | |
| Chitotriosidase | 1 | 1 | 0 | 1 | |
| C-type lectin‡ | 4 | 0 | 0 | 2 | |
| DEC-1† | 2 | 1 | 1 | 2 | |
| DEC-3† | 0 | 0 | 0 | 1 | |
| Fibrillin Biomphalaria glabrata chitin-binding† | 4 | 0 | 0 | 1 | |
| FREP 20 | 0 | 2 | 1 | 0 | |
| FREP 21 | 1 | 0 | 0 | 0 | |
| FREP 23 | 0 | 1 | 0 | 0 | |
| FREP 24 | 1 | 0 | 0 | 0 | |
| FREP 25 | 0 | 0 | 0 | 1 | |
| FREP 26 | 0 | 0 | 1 | 0 | |
| FREP 3 † | 0 | 1 | 0 | 0 | |
| FREP 5 | 0 | 0 | 1 | 0 | |
| FREP 7 | 0 | 0 | 0 | 1 | |
| Galectin | 2 | 1 | 0 | 1 | |
| Peptidoglycan-recognition protein (PGRP) | 0 | 1 | 0 | 0 | |
| Sialic acid binding lectin | 1 | 1 | 0 | 0 | |
| Thrombospondin-family Echinonectin | 0 | 0 | 0 | 1 | |
| Thrombospondin-family hemolectin | 1 | 0 | 0 | 1 | |
| Cell Adhesion | Cadherin Desmocollin | 0 | 1 | 0 | 0 |
| Cadherin Fat tumor suppressor protein‡ | 0 | 0 | 0 | 5 | |
| Dermatopontin 1 | 0 | 0 | 1 | 0 | |
| Dermatopontin A† | 1 | 1 | 3 | 0 | |
| Dermatopontin B | 1 | 0 | 0 | 0 | |
| Dermatopontin C | 0 | 1 | 0 | 0 | |
| Ependymin-related protein† | 1 | 3 | 3 | 0 | |
| Fatty acid binding protein | 0 | 1 | 1 | 0 | |
| Hyaluronan binding protein | 0 | 1 | 0 | 0 | |
| Integrin | 0 | 0 | 0 | 1 | |
| Matrilin† | 1 | 2 | 1 | 0 | |
| Signal transduction | DCC-interacting protein | 0 | 0 | 1 | 0 |
| Diguanylate cyclase/phosphodiesterase | 0 | 0 | 0 | 1 | |
| F-box-like/WD repeat protein repeat area of protein | 0 | 0 | 0 | 1 | |
| Frizzled | 1 | 0 | 0 | 0 | |
| Guanine nucleotide-binding protein | 1 | 0 | 1 | 1 | |
| Hepatocyte growth factor | 0 | 0 | 0 | 1 | |
| Hypocretin receptor 2 | 0 | 0 | 0 | 1 | |
| IkappaB kinase complex-associated protein | 0 | 1 | 0 | 0 | |
| MAP kinase-interacting serine/threonine kinase 2 | 2 | 0 | 0 | 0 | |
| Nucleoporin (nuclear pore complex protein, I-kappaB kinase regulation) | 0 | 0 | 1 | 1 | |
| Protein phosphatase | 0 | 1 | 0 | 0 | |
| Ras-like protein GTP-binding nuclear protein Ran (GTPase Ran) | 0 | 2 | 0 | 4 | |
| Ras-like protein rac 1 | 1 | 0 | 0 | 0 | |
| Ras-like protein Ran-binding protein 7 (Importin 7) | 0 | 0 | 1 | 0 | |
| Ras-like protein Ras-related protein Rab 1 | 1 | 1 | 2 | 1 | |
| Ras-like protein Ras-related protein Rab 6 | 1 | 0 | 0 | 0 | |
| Ribosomal protein S6 kinase | 0 | 1 | 0 | 0 | |
| Scr-like A-2 | 1 | 0 | 0 | 0 | |
| Signal transducer and activator of transcription | 1 | 0 | 0 | 0 | |
| Thrombospondin-family Bone morphogenetic protein | 0 | 0 | 0 | 1 | |
| Tumor necrosis factor, alpha-induced protein 3 | 1 | 0 | 0 | 0 | |
| Ubiquitin | 1 | 0 | 0 | 0 | |
| Ubiquitin Activating enzyme (E1) | 0 | 1 | 0 | 0 | |
| Ubiquitin Bg poly-ubiquitin gene | 3 | 2 | 1 | 2 | |
| Ubiquitin Conjugating enzyme | 0 | 0 | 1 | 1 | |
| Ubiquitin ligase | 2 | 0 | 0 | 0 | |
| Ubiquitin Specific protease 9 | 1 | 0 | 0 | 0 | |
| Ubiquitin Specific proteinase 34 | 0 | 0 | 0 | 1 | |
| Ubiquitin Ubiquitin conjugating enzyme | 0 | 1 | 0 | 0 | |
| vav 3 oncogene | 1 | 0 | 0 | 0 | |
| Antioxidant related | Catalase | 1 | 0 | 0 | 0 |
| Chorion oxidase/peroxidase | 3 | 1 | 0 | 3 | |
| Peroxinectin | 0 | 0 | 0 | 2 | |
| Peroxiredoxin | 0 | 1 | 2 | 0 | |
| Superoxide dismutase [Cu-Zn] | 4 | 0 | 0 | 1 | |
| Thioredoxin | 1 | 1 | 2 | 0 | |
| Apoptosis related | 24-dehydrocholesterol reductase | 1 | 0 | 0 | 0 |
| Amyloid beta binding protein Ubiquitin-Like | 0 | 0 | 0 | 1 | |
| Calreticulin | 1 | 0 | 0 | 0 | |
| Cullin 1 | 1 | 0 | 0 | 0 | |
| Cyclin | 1 | 0 | 0 | 0 | |
| Death-associated protein kinase | 0 | 0 | 0 | 1 | |
| Discoidin-Coagulation factor | 0 | 0 | 1 | 0 | |
| Endonuclease G | 4 | 0 | 1 | 1 | |
| Glyceraldehyde-3-phosphate dehydrogenase | 0 | 4 | 3 | 0 | |
| Inhibitor of apoptosis protein (IAP) | 2 | 0 | 0 | 1 | |
| Metabotropic X receptor | 0 | 2 | 0 | 0 | |
| Neurogenic locus notch homolog protein | 1 | 0 | 0 | 0 | |
| Neutral ceramidase | 0 | 0 | 1 | 0 | |
| p53 inducible protein isoform 1 | 1 | 0 | 0 | 1 | |
| Peflin (Penta-EF hand domain-containing protein 1) | 0 | 0 | 0 | 1 | |
| Phosphoinositide-3-kinase | 0 | 0 | 0 | 1 | |
| Protein tyrosine phosphatase | 1 | 0 | 0 | 0 | |
| Sphingomyelin phosphodiesterase | 0 | 0 | 2 | 1 | |
| Syntaxin-7 | 0 | 2 | 0 | 0 | |
| Thrombospondin-family Bg thrombospondin domain | 3 | 0 | 1 | 1 | |
| Thrombospondin-family Bg VWD domain-containing protein† | 4 | 1 | 3 | 1 | |
| Thrombospondin-family Hemocytin | 1 | 1 | 2 | 0 | |
| Thrombospondin-family Mucin 19 | 3 | 0 | 0 | 0 | |
| Thrombospondin-family Mucin 4 | 1 | 0 | 0 | 0 | |
| Thrombospondin-family Thrombospondin† | 0 | 0 | 2 | 0 | |
| Thrombospondin-family Zonadhesin† | 1 | 0 | 0 | 0 | |
| Translationally controlled tumor protein | 1 | 0 | 0 | 0 | |
| WD repeat protein | 2 | 2 | 0 | 1 | |
| Stress response | AMPK-related protein kinase | 0 | 0 | 0 | 1 |
| Heat shock protein 60 | 1 | 0 | 0 | 0 | |
| Heat shock protein 1 | 1 | 5 | 5 | 1 | |
| Heat shock protein 70 | 1 | 2 | 4 | 8 | |
| Heat shock protein 90 | 1 | 0 | 0 | 0 | |
| Heat shock protein beta-1 * | 1 | 0 | 0 | 0 | |
| Proteinase inhibitor I25 Cystatin | 0 | 0 | 0 | 1 | |
| Serine/threonine protein kinase | 1 | 2 | 3 | 2 | |
| Stress-induced-phosphoprotein 1 | 0 | 1 | 0 | 0 | |
| TFIIH basal transcription factor | 1 | 0 | 0 | 0 | |
| Universal stress protein | 0 | 1 | 1 | 0 | |
| Metal binding | Amiloride-sensitive amine oxidase | 1 | 0 | 1 | 2 |
| Copper-transporting ATPase | 0 | 0 | 0 | 1 | |
| Cysteine-rich motor neuron | 0 | 2 | 1 | 0 | |
| Cytochrome oxidase subunit II (COII, mitochondrial) | 1 | 8 | 7 | 1 | |
| Cytochrome P450* | 1 | 0 | 0 | 2 | |
| Dihydropyrimidinase | 1 | 0 | 1 | 0 | |
| Ferritin Soma 1 § | 5 | 2 | 0 | 9 | |
| Ferritin Soma 2 | 2 | 11 | 5 | 2 | |
| Ferritin Yolk 1 | 0 | 0 | 1 | 0 | |
| Ferritin Yolk 2 | 1 | 0 | 0 | 0 | |
| Hemoglobin type 1 | 23 | 30 | 45 | 42 | |
| Hemoglobin type 2† | 1 | 2 | 5 | 1 | |
| Iron responsive element binding protein | 1 | 0 | 0 | 0 | |
| Monooxygenase DBH-like 1 | 5 | 0 | 2 | 0 | |
| Myoglobin | 3 | 13 | 13 | 4 | |
| NADH dehydrogenase | 2 | 0 | 0 | 1 | |
| Phenylalanine hydroxylase | 0 | 0 | 1 | 0 | |
| Serine/threonine protein phosphatase PP1-beta | 1 | 0 | 0 | 1 | |
| Serpins | Serine Proteinase Inhibitors (serpins)(antithrombin) | 2 | 2 | 2 | 0 |
| Serine Proteinase Inhibitors (serpins)(antithrombin) Antithrombin-III | 1 | 3 | 1 | 0 | |
| Serine Proteinase Inhibitors (serpins)(antithrombin) BG serine protease alpha | 4 | 0 | 0 | 0 | |
| Serine Proteinase Inhibitors (serpins)(antithrombin) clade B (ovalbumin) | 1 | 0 | 2 | 1 | |
| Serine Proteinase Inhibitors (serpins)(antithrombin) fibrinolytic protease | 1 | 0 | 0 | 4 | |
| Serine Proteinase Inhibitors (serpins)(antithrombin) kazal-type proteinase inhibitor | 1 | 0 | 0 | 0 | |
| Serine Proteinase Inhibitors (serpins)(antithrombin) Serpin | 0 | 1 | 0 | 0 | |
| Serine Proteinase Inhibitors (serpins)(antithrombin) Serpin B | 0 | 1 | 0 | 1 | |
| Serine Proteinase Inhibitors (serpins)(antithrombin) Serpin I4 ‡ | 3 | 2 | 3 | 1 | |
| Serine Proteinase Inhibitors (serpins)(antithrombin) SerpinF | 0 | 0 | 0 | 1 | |
| Serine Proteinase Inhibitors (serpins)(antithrombin) Thrombospondin 2 | 1 | 0 | 0 | 0 | |
| Proteases | Cathepsin B | 1 | 4 | 0 | 0 |
| Cathepsin C | 0 | 0 | 2 | 0 | |
| Cathepsin D | 2 | 1 | 2 | 2 | |
| Cathepsin F | 0 | 0 | 0 | 1 | |
| Cathepsin L† | 2 | 3 | 4 | 1 | |
| Cathepsin Z | 0 | 2 | 0 | 0 | |
| Inflammatory response | ADAM | 1 | 0 | 1 | 1 |
| ADAM 17-like protease | 2 | 0 | 0 | 0 | |
| Secreted protein acidic and rich in cysteine (SPARC) | 0 | 3 | 0 | 0 | |
| Tryptase-2 | 0 | 0 | 1 | 0 | |
| Vacuolar protein sorting protein | 1 | 0 | 0 | 0 | |
| Other putative immune factors | Alpha-2-macroglobulin | 1 | 0 | 3 | 0 |
| Aplysianin-A‡ | 5 | 1 | 2 | 6 | |
| Bactericidal permeability-increasing protein (BPI)/Lipopolysaccharide-binding protein (LBP) | 0 | 0 | 0 | 1 | |
| Beta-1,4-endoglucanase 1†# | 2 | 0 | 0 | 2 | |
| Beta-catenin | 0 | 0 | 1 | 0 | |
| Contactin-associated protein-like 2 | 1 | 1 | 0 | 3 | |
| Complement: Utp21 specific WD40 | 0 | 0 | 1 | 0 | |
| Complement control protein containing SUSHI repeat | 1 | 0 | 0 | 0 | |
| Defense protein | 0 | 1 | 0 | 0 | |
| Developmentally regulated albumen gland gene protein | 21 | 2 | 6 | 2 | |
| Endo-1,4-mannanase† | 1 | 0 | 0 | 1 | |
| Fibulin | 1 | 1 | 0 | 1 | |
| G-type lysozyme | 1 | 0 | 0 | 0 | |
| IMMUNE-RESPONSIVE PROTEIN 1 | 1 | 0 | 0 | 0 | |
| Lipase | 3 | 0 | 0 | 0 | |
| Low density lipoprotein receptor-related protein | 4 | 0 | 1 | 2 | |
| Piwi-like | 0 | 0 | 0 | 1 | |
| S-adenosylhomocysteine hydrolase# | 0 | 1 | 0 | 0 | |
| Zinc Carboxypeptidase A# | 1 | 0 | 0 | 1 | |
| Cystatin, type 2 | 0 | 0 | 0 | 1 | |
| Unknowns | |||||
| Ucluster0156* | 0 | 0 | 4 | 0 | |
| UCluster0465* | 0 | 1 | 1 | 0 | |
| UCluster0600* | 0 | 0 | 2 | 0 | |
| UCluster0061† | 0 | 2 | 1 | 1 | |
| UCluster0007§ | 19 | 12 | 5 | 13 | |
| UCluster0525* | 0 | 0 | 2 | 0 | |
| P021C02§ | 0 | 0 | 0 | 1 | |
| P069B06* | 0 | 1 | 0 | 0 | |
Significantly upregulated in Biomphalaria glabrata susceptible to Echinostoma caproni(Bouchut et al. (2006a, b))
Significantly upregulated in B. glabrata resistant to E. caproni (Bouchut et al. (2006a, b))
Recovered from a supression substractive hybridization library enriched for Schistosoma mansoni-exposed BS90 B. glabrata (Nowak et al. (2004))
Recovered from a supression substractive hybridization library enriched for E. paraensei-exposed M-line B. glabrata (Jung et al. (2005))
Recovered from a supression substractive hybridization library enriched for B. glabrata resistant to S. mansoni (Lockyer et al. (2007))
Comparison of Transcriptomes
BLAST2GO comparison of the biological processes represented within the separate transcriptomes revealed several significant differences (Table 2). First, each treatment group differed significantly from the control group at several Directed Acyclic Graph (DAG) nodes. Second, the responses relative to the control of bacteria-challenged groups are highly similar to each other and differ from the response of S. mansoni-exposed B. glabrata.
Table 2. Biological processes affected by different immune challenges.
Significant differences in GO categories of Biomphalaria glabrata treatment groups compared with control. P-values in bold denote significant down regulation, all other values indicate upregulation. GO clusters refer to GO terms which are directly linked (related) and often contain the same populations of ESTs (see Table S2).
| p-value (treatment v. control) | |||||||
|---|---|---|---|---|---|---|---|
| GO cluster | GO level | GO type | GO number | GO term | Gram− | Gram+ | S. mansoni |
| 1 | 5 | BP | GO:0015669 | gas transport | <0.01 | <0.01 | <0.01 |
| 6 | BP | GO:0015671 | oxygen transport | <0.01 | <0.01 | <0.01 | |
| 3 | MF | GO:0046906 | tetrapyrrole binding | <0.01 | <0.01 | <0.01 | |
| 4 | MF | GO:0020037 | heme binding | ||||
| 2 | 4 | MF | GO:0046872 | metal ion binding | <0.05 | <.0.01 | <0.05 |
| 5 | MF | GO:0046914 | transition metal ion binding | <.0.01 | <.0.01 | <0.05 | |
| 6 | MF | GO:0005506 | iron ion binding | <.0.01 | <.0.01 | <.0.01 | |
| 3 | 6 | BP | GO:0046164 | alcohol catabolism | <0.05 | <0.05 | NS |
| 7 | BP | GO:0046365 | monosaccharide catabolism | <0.05 | <0.05 | NS | |
| 8 | BP | GO:0019320 | hexose catabolism | <0.05 | <0.05 | NS | |
| 9 | BP | GO:0006007 | glucose catabolism | <0.05 | <0.05 | NS | |
| 10 | BP | GO:0006096 | glycolysis | <0.05 | <0.05 | NS | |
| 4 | 2 | MF | GO:0005198 | structural molecule activity | <.0.01 | NS | NS |
| 3 | MF | GO:0005200 | structural constituent of cytoskeleton | <.0.01 | <0.05 | NS | |
| 8 | CC | GO:0015629 | actin cytoskeleton | <.0.01 | <0.05 | NS | |
| 5 | 3 | MF | GO:0016787 | Hydrolase activity | <.0.01 | NS | NS |
| 6 | 3 | MF | GO:0008289 | lipid binding | <.0.01 | NS | <.0.01 |
| 7 | 3 | BP | GO:0006936 | Muscle contraction | <.0.01 | <0.05 | <.0.01 |
| 8 | 2 | BP | GO:0006807 | nitrogen compound metabolic process | <.0.01 | <0.05 | NS |
| 3 | BP | GO:0009308 | amine metabolic process | <.0.01 | <0.05 | NS | |
| 9 | 3 | MF | GO:0016563 | transcriptional activator activity | NS | NS | <.0.01 |
| 4 | MF | GO:0003713 | transcription coactivator activity | NS | NS | <.0.01 | |
| 10 | 3 | MF | GO:0003682 | chromatin binding | NS | NS | <.0.01 |
Snails challenged with any pathogen showed higher levels of ‘gas transport’, (‘oxygen gas transport’), and ‘metal ion binding’ (‘iron ion transport’). Snails challenged with bacteria showed increased alcohol and sugar catabolism, glycolysis, and ‘structural molecule activity’, relating to actin. Bacteria-challenged snails also showed a decrease in ‘amine metabolic processes’, whereas challenge only with E. coli indicated lower ‘hydrolase activity’. By contrast, the transcriptome of S. mansoni–exposed snails showed decreased levels of ‘transcriptional activator activity’ and ‘chromatin binding’ (Table 2). None of the biological pathways associated with defense was represented by adequate numbers of sequences to reveal significant differences with the BLAST2GO analysis.
Comparison of transcriptomes using all sequence data
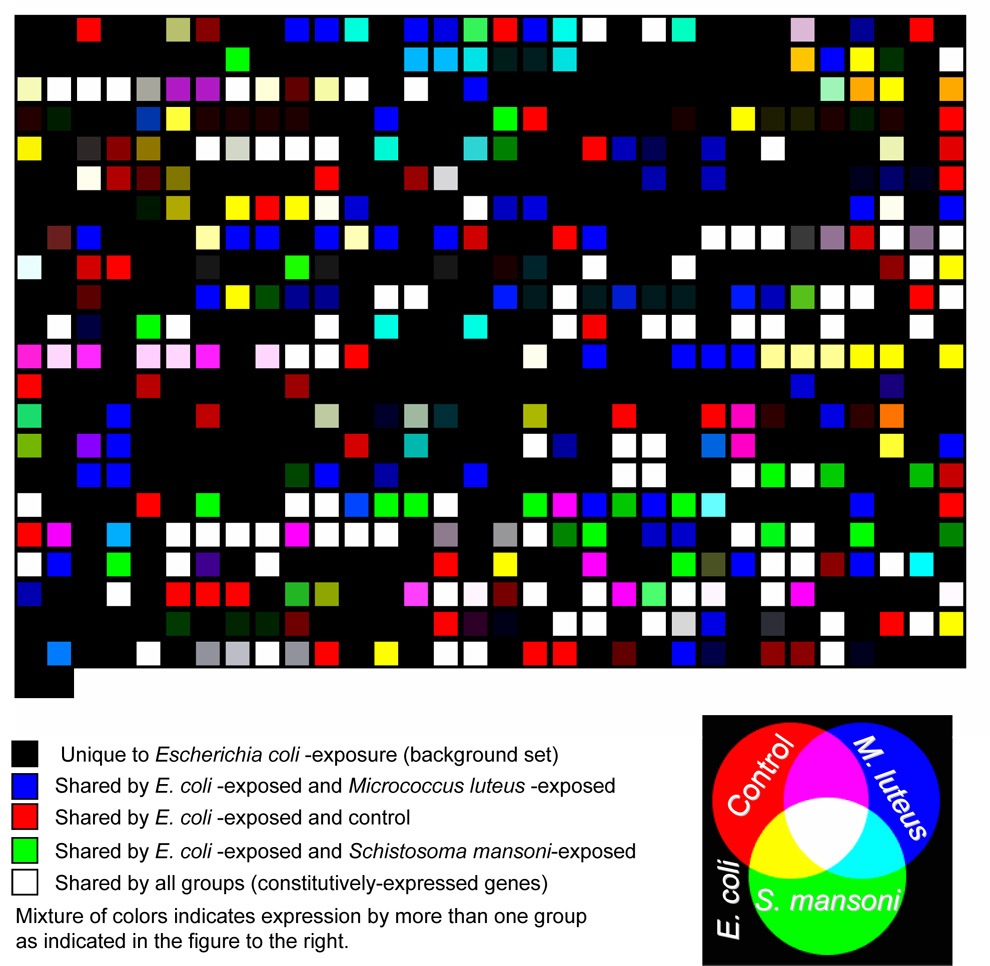
The use of PyMood software for a sequence-based similarity comparison (BLAST) of all 3123 individual ORESTES (regardless of cluster status, putative ID, or experimental group from which they originated) identified 1630 ORESTES that were shared by two or more transcriptome groups, 1502 sequences were unique to a particular single transcriptome group (Figure 3). Interestingly, the 671 ORESTES shared by all groups assembled into only 42 clusters, of which 10 were unknowns. These constitutively expressed transcripts may include candidate housekeeping genes, but this will require future confirmation by determination of expression level (Table 3). The 544 individual ORESTES recovered only from untreated B. glabrata were more diverse; they represented 432 clusters and singletons. These sequences were not evoked by any specific immune challenge; therefore defense factors included in this set are assumed to be expressed constitutively. A great diversity of 250 unique sequences not seen from untreated controls was recovered from experimentally challenged snails; several of these may represent general response factors to stress or injury. Each of the transcriptome sets sampled from B. glabrata contained a large proportion (between 47 and 55%) of novel sequences that could not be assigned a putative ID. Comparatively, the expression profiles from snails exposed to G− or G+ bacteria were more similar to each other than to expression profiles of Schistosoma mansoni-exposed snails or controls (Figures 4a and 4b).
Figure 3. Distribution of shared transcripts based on sequence similarity and putative ID.
Virtual microarray view of Biomphalaria glabrata ORESTES data. Black squares represent ORESTES uniquely recovered from G--exposed snails. ORESTES shared by G--exposed snails and one or more other groups are identified as colored squares as indicated in the legend. Gradations of these colors indicate: 1) degree of similarity (saturation of color: darker squares indicate less similar ORESTES), and 2) sharing of ORESTES by more than 2 groups (mixture of colors; for example, pink indicates ORESTES shared by G--exposed, G+-exposed, and control). Note the numerous blue spots compared with the relatively few green spots. This indicates that the response of B. glabrata to both bacterial exposures (blue) was much more similar than the response of S. mansoni-exposed was to that of G--exposed snails.
Table 3. Constitutively expressed ORESTES in B. glabrata.
ORESTES were placed in each of these contigs by sequence and name similarity. Number of recovered sequences is indicated for each experimental group.
| # ESTs | |||||
|---|---|---|---|---|---|
| Sequence ID | Control | S.m.-exposed | G+-exposed | G--exposed | Total |
| Actin | 6 | 10 | 9 | 15 | 40 |
| Aplysianin-A | 5 | 6 | 2 | 1 | 14 |
| ATP synthase | 1 | 1 | 2 | 1 | 5 |
| Carbonic anhydrase | 1 | 1 | 1 | 1 | 4 |
| Cathepsin D | 2 | 2 | 2 | 1 | 7 |
| Cathepsin L | 2 | 1 | 4 | 3 | 10 |
| Coiled-coil-helix-coiled-coil-helix domain | 1 | 1 | 1 | 1 | 4 |
| Cytochrome oxidase subunit II (COII, mitochondrial) | 1 | 1 | 7 | 8 | 17 |
| DEC-1 | 2 | 2 | 1 | 1 | 6 |
| Developmentally regulated albumen gland gene protein | 21 | 2 | 6 | 2 | 31 |
| F-actin capping protein | 2 | 1 | 1 | 3 | 7 |
| Ferritin Soma 2 | 2 | 2 | 5 | 11 | 20 |
| Heat shock protein 1 | 1 | 1 | 5 | 5 | 12 |
| Heat shock protein 70 | 1 | 8 | 4 | 2 | 15 |
| Hemoglobin type 1 | 23 | 42 | 45 | 30 | 140 |
| Hemoglobin type 2 | 1 | 1 | 5 | 2 | 9 |
| Myc homolog | 1 | 1 | 4 | 1 | 7 |
| Myoglobin | 3 | 4 | 13 | 13 | 33 |
| Myosin | 4 | 8 | 7 | 11 | 30 |
| Polyadenylate-binding protein | 5 | 7 | 1 | 2 | 15 |
| Proteasome | 2 | 1 | 1 | 1 | 5 |
| Ras-like protein Ras-related protein Rab 1 | 1 | 1 | 2 | 1 | 5 |
| Ribosomal protein S2 | 1 | 2 | 2 | 7 | 12 |
| Serine Proteinase Inhibitors (serpins, antithrombin) Serpin I4 | 3 | 1 | 3 | 2 | 9 |
| Serine/threonine protein kinase | 1 | 2 | 3 | 2 | 8 |
| Thrombospondin-family Bg VWD domain-containing protein | 4 | 1 | 3 | 1 | 9 |
| Translation elongation factor (EF1-alpha) | 2 | 1 | 4 | 2 | 9 |
| Translation elongation factor (EF-2) | 3 | 2 | 3 | 1 | 9 |
| Tubulin Beta | 3 | 3 | 5 | 3 | 14 |
| Twitchin | 1 | 1 | 1 | 1 | 4 |
| Ubiquitin Bg poly-ubiquitin gene | 3 | 2 | 1 | 2 | 8 |
| UCluster0007 | 19 | 13 | 5 | 12 | 49 |
| UCluster0018 | 8 | 7 | 9 | 6 | 30 |
| UCluster0025 | 1 | 1 | 1 | 3 | 6 |
| UCluster0030 | 3 | 8 | 1 | 4 | 16 |
| UCluster0052 | 1 | 1 | 1 | 1 | 4 |
| UCluster0058 | 1 | 3 | 1 | 1 | 6 |
| UCluster0067 | 1 | 2 | 5 | 4 | 12 |
| UCluster0085 | 2 | 3 | 1 | 4 | 10 |
| UCluster0102 | 1 | 1 | 1 | 2 | 5 |
| UCluster0221 | 1 | 1 | 3 | 4 | 9 |
| WD repeat protein | 2 | 1 | 1 | 2 | 6 |
Figure 4. Gene Expression profile of Biomphalaria glabrata using Gene Ontology.
Values indicate the number of ORESTES categorized into the third molecular function ontology level, and include data from all groups. Only the sixteen most abundant categories are presented. Analysis based on 1210/3123 ORESTES assigned a GO term.
Finally, when considering a comparison of the expression profiles resulting from each treatment, the responses of bacteria exposed snails are more similar to each other than to that of S. mansoni exposed snails (first 3 rows of Table 4). The expression profile of control snails is more similar to that of S. mansoni exposed snails than to those of either bacterial exposed snails (last 3 rows of Table 4).
Table 4. Summary of ORESTES shared by groups.
Number of ORESTES shared by pairs of challenged groups and of each challenged group to control. Numbers indicate total number of ESTs; numbers in brackets indicate the number of clusters made by assembling ORESTES.
| All ORESTES | Putative immune related ORESTES | |
|---|---|---|
| E. coli and M. luteus | 116(39) | 24(8) |
| E. coli and S. mansoni | 50(18) | 8(2) |
| M. luteus and S. mansoni | 54(21) | 12(4) |
| Control and E. coli | 110(39) | 7(2) |
| Control and M. luteus | 88(32) | 13(3) |
| Control and S. mansoni | 147(45) | 44(13) |
Discussion
Previous investigations of the immunobiology of B. glabrata have aimed to characterize the differences between snail strains that are resistant or susceptible to S. mansoni. This study differs by characterizing responses of one single B. glabrata strain (M line) to different challenges with either compatible S. mansoni or bacteria (incompatible).
Bacterial infections are rare in wild populations of gastropods and experimentally injected bacteria are cleared from the circulation within hours and then eliminated by phagocytic hemocytes (Bayne, 1982; van der Knaap et al., 1981). Snails, including B. glabrata, routinely survive exposure to bacteria (Adema et al., 1999). Now, gene discovery disclosed aspects of the transcriptomes associated with the predictably effective antibacterial defense responses of B. glabrata, including 533 individual sequences that were uniquely recovered following bacterial challenge.
The snails were sampled at 12 hours post challenge to capture both the antibacterial responses of B. glabrata and the initial response efforts of the snails to S. mansoni. This relatively early time point was chosen to complement those used in other studies (Bouchut et al., 2006b; Jung et al., 2005; Knight et al., 1999; Lockyer et al., 2007a; Lockyer et al., 2007b; Miller et al., 2001; Mitta et al., 2005; Nowak et al., 2004; Raghavan et al., 2003). Another consideration was that the rapid encapsulation of penetrating (incompatible) schistosomes by snail hemocytes indicates that crucial aspects of mounting defense responses are orchestrated early during pathogen-snail interactions, even if the actual elimination may take 72–96 hours (Loker et al., 1982; Nowak et al., 2004; Sullivan and Richards, 1981).
Among the putative immune factors that were identified was a PIWI-like sequence. This indicates that B. glabrata has gene silencing abilities for defense of its genome against mobile elements (Thalia et al., 2008), as also demonstrated by functional RNA interference in B. glabrata (Jiang et al., 2006). A collection of chitin-interacting factors (Peritrophin A, Chitotriosidase, DEC-1, DEC-3, and Fibrillin), including a Chitin-binding protein previously described as a putative defense factor of B. glabrata (Mitta et al., 2005), fits well with a report that chitin response factors are part of immune defenses in mollusks and other organisms (Badariotti et al., 2007). Other sequences that are expressed by B. glabrata in response to immune challenge may be either defense factors, part of responses to secondary infections, or stress-response factors. Considered together, independent of the challenging pathogen, the immune-relevant ORESTES revealed aspects of many stages of a functional defense response in B. glabrata ranging from recognition to regulation, stress responses and both cellular and humoral immune-effector systems.
The size of this dataset and the current lack of large-scale analysis methods (e.g. microarrays) made the analysis of expression patterns of individual transcripts impractical. Therefore, this study relies on computational analyses (Blast2GO and Pymood) to make the data accessible for functional interpretation.
The Blast2GO program has an ‘enrichment analysis’ component for holistic comparison of datasets. Each ORESTES was placed into a higher functional context, so that all ORESTES in a given treatment group can be compared to the control group. Though the Blast2GO analysis was limited since only 39% of the ORESTES could be assigned GO annotations, several intereting features were none-the-less noted from the different groups of immune-challenged snails. Oxygen transport and heme binding (specifically the upregulation of hemoglobin 1, 2, and myoglobin) were elevated in B. glabrata following exposure to all pathogens, suggesting that a higher respiratory demand is associated with responses to infection. Previous studies that focused on hemocytes rather than the whole body of B. glabrata also reported elevated metabolic rates following exposure to S. mansoni (Raghavan et al., 2003; Yoshino and Lodes, 1988). Increased respiration has also been noted in leaf cutter ants in response to pathogenic fungus (Poulsen et al., 2002). Similarly, increased metal ion binding (specifically iron) was noted from challenged snails. The sequestering of iron in response to pathogens by vertebrates and invertebrates is considered an ancient host defense response (Beck et al., 2002; Weinberg and Weinberg, 1995). Finally, the consistency of differences in GO annotation between treatment and control group lends confidence to the validity of this method of transcriptome comparison.
Given that the genome of a protostome invertebrate may contain up to 20000 genes (Doolittle, 2002) and considering the absence of several sequences expected to be expressed in B. glabrata (e.g. enzymes that generate toxic oxygen radicals, Bender et al., 2007; Lockyer et al., 2007a), it is clear that the 2129 unique sequences reported here do not represent a complete sampling of the transcriptome. However, the subsets of transcriptomes do provide relevant information; two independent and different large scale analyses (BLAST2GO and PyMood) each revealed similar patterns among the four different complex transcriptome assemblies. Both methods consistently distinguished antibacterial responses from those of the S. mansoni-exposed B. glabrata, and from untreated snails. Furthermore, sampling bias was avoided by using identical methods to generate and characterize randomly selected ORESTES from each group. Thus the patterns within the transcriptome data were analyzed to generate testable hypotheses regarding the responses of B. glabrata to the challenges applied in this study.
The data presented here strongly suggests that the responses of B. glabrata to E. coli and M. luteus are much more similar to each other than to S. mansoni, based on either all ORESTES or only those ORESTES deemed immunologically relevant (Table 4). The data also suggest that the response to S. mansoni is different (Table 2), and more similar to the expression profile of control snails (Table 4) when compared with a response to bacteria. These observations are consistent with the notion that B. glabrata has effective internal defenses but fails to mobilize a successful responses when interacting with a compatible schistosome parasite (Lie, 1982; Loker and Adema, 1995). Taken together, the expression profiles may allow us to begin putting together the ‘recipe’ used by snails for an effective immune response and to identify ‘ingredients’ that are lacking from unsuccessful responses.
The large number of unknown sequences makes sweeping conclusions difficult; the data collected may contain a number of candidate immune factors that require functional characterization before meaningful interpretation of immune capabilities of B. glabrata is possible. Likely, the function of unknown sequences evoked by S. mansoni contributes little to an effective defense response, although several of these gene products may be part of a frustrated or incomplete response. Several of the unknown sequences that were recovered from B. glabrata following challenge with bacteria were expressed during the rapid clearance of incompatible pathogens by the internal defenses of snails and could therefore be involved in killing pathogens. New tools, such as microarrays will help evaluate the expression and characterization of these factors. In fact many of the sequences reported here have contributed to the design and production of cDNA arrays and oligo arrays for more detailed analysis of transcriptome reactions of B. glabrata. With the recent insights that immune defense of various phylogenetic groups differ dramatically from each other and from the mammalian paradigm (Litman et al., 2005; Loker et al., 2004), the many unknown sequences collected from B. glabrata may inform regarding lophotrochozoan immunity, in ways that further elucidate the immunobiology of B. glabrata and the underlying mechanisms for compatibility of S. mansoni/B. glabrata associations.
Supplementary Material
Sequences are listed 5’ to 3’.
Putative IDs of sequences contributing to GO clusters as giving in Table 1.
Result of BLAST comparison of unknown ORESTES sequences to all molluscan or B. glabrata ESTs in GenBank (July 2007). * indicates immune relevant ORESTES.
Acknowledgements
We thank Dr. E. Sam Loker for hosting this research project in his laboratory at the Biology Department, University of New Mexico, and for helpful discussions. Technical support for sequencing and computational analysis of ORESTES was provided by Mr. George Rosenberg and Mrs. Jennifer Hathaway of the Molecular Biology Facility, (Biology, UNM) which is supported by NIH grant number 1P20RR18754 from the Institute Development Award (IDeA) Program of the National Center for Research. Dr. Fred A. Lewis from the Schistosomiasis Resource Center (NIAID Contract N01-A1-30026) provided Schistosoma mansoni (PR-1 strain) for these experiments. Additional technical support was provided by Ms. Amena Ishak and Mr. Steven A. Saenz (SAS was supported by the NIH Minority Access to Research Careers program at the UNM). This study was supported by NIH R01 AI052363 (CMA).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Ben Hanelt, Email: bhanelt@unm.edu.
Cheng Man Lun, Email: bonster@unm.edu.
Coen M Adema, Email: coenadem@unm.edu.
References
- Adema CM. Comparative study of cytoplasmic actin DNA sequences from six species of Planorbidae (Gastropoda : Basommatophora) J Mollus Stud. 2002;68:17–23. [Google Scholar]
- Adema CM, Hertel LA, Loker ES. Evidence from two planorbid snails of a complex and dedicated response to digenean (echinostome) infection. Parasitology. 1999;119:395–404. doi: 10.1017/s0031182099004850. [DOI] [PubMed] [Google Scholar]
- Adema CM, Loker ES. Specificity and immunobiology of larval digenean-snail associations. In: Fried B, Graczyk TK, editors. Advances in trematode biology. Boca Raton: CRC; 1997. pp. 230–263. [Google Scholar]
- Adema CM, Luo MZ, Hanelt B, Hertel LA, Marshall JJ, Zhang SM, DeJong RJ, Kim HR, Kudrna D, Wing RA, Soderlund C, Knight M, Lewis FA, Caldeira RL, Jannotti-Passos LK, Carvalho OD, Loker ES. A bacterial artificial chromosome library for Biomphalaria glabrata, intermediate snail host of Schistosoma mansoni. Mem I Oswaldo Cruz. 2006;101:167–177. doi: 10.1590/s0074-02762006000900027. [DOI] [PubMed] [Google Scholar]
- Badariotti F, Thuau R, Lelong C, Dubos MP, Favrel P. Characterization of an atypical family 18 chitinase from the oyster Crassostrea gigas: evidence for a role in early development and immunity. Dev Comp Immunol. 2007;31:559–570. doi: 10.1016/j.dci.2006.09.002. [DOI] [PubMed] [Google Scholar]
- Bayne CJ. Molluscan immunobiology: isolation of an Aeromonas formicans which escapes the internal defense system of Helix pomatia. Dev Comp Immunol. 1982;6:675–682. [PubMed] [Google Scholar]
- Beck G, Ellis TW, Habicht GS, Schluter SF, Marchalonis JJ. Evolution of the acute phase response: iron release by echinoderm (Asterias forbesi) coelomocytes, and cloning of an echinoderm ferritin molecule. Dev Comp Immunol. 2002;26:11–26. doi: 10.1016/s0145-305x(01)00051-9. [DOI] [PubMed] [Google Scholar]
- Bender RC, Goodall CP, Blouin MS, Bayne CJ. Variation in expression of Biomphalaria glabrata SOD1: A potential controlling factor in susceptibility/resistance to Schistosoma mansoni. Dev Comp Immunol. 2007;31:874–878. doi: 10.1016/j.dci.2006.12.005. [DOI] [PubMed] [Google Scholar]
- Bouchut A, Roger E, Coustau C, Gourbal B, Mitta G. Compatibility in the Biomphalaria glabrata/Echinostoma caproni model: Potential involvement of adhesion genes. Int J Parasitol. 2006a;36:175–184. doi: 10.1016/j.ijpara.2005.09.009. [DOI] [PubMed] [Google Scholar]
- Bouchut A, Sautiere PE, Coustau C, Mitta G. Compatibility in the Biomphalaria glabrata/Echinostoma caproni model: Potential involvement of proteins from hemocytes revealed by a proteomic approach. Acta Tropica. 2006b;98:234–246. doi: 10.1016/j.actatropica.2006.05.007. [DOI] [PubMed] [Google Scholar]
- Camargo AA, Samaia HP, Dias-Neto E, Simao DF, Migotto IA, Briones MR, Costa FF, Nagai MA, Verjovski-Almeida S, Zago MA, Andrade LE, Carrer H, El-Dorry HF, Espreafico EM, Habr-Gama A, Giannella-Neto D, Goldman GH, Gruber A, Hackel C, Kimura ET, Maciel RM, Marie SK, Martins EA, Nobrega MP, Paco-Larson ML, Pardini MI, Pereira GG, Pesquero JB, Rodrigues V, Rogatto SR, da Silva ID, Sogayar MC, Sonati MF, Tajara EH, Valentini SR, Alberto FL, Amaral ME, Aneas I, Arnaldi LA, de Assis AM, Bengtson MH, Bergamo NA, Bombonato V, de Camargo ME, Canevari RA, Carraro DM, Cerutti JM, Correa ML, Correa RF, Costa MC, Curcio C, Hokama PO, Ferreira AJ, Furuzawa GK, Gushiken T, Ho PL, Kimura E, Krieger JE, Leite LC, Majumder P, Marins M, Marques ER, Melo AS, Melo MB, Mestriner CA, Miracca EC, Miranda DC, Nascimento AL, Nobrega FG, Ojopi EP, Pandolfi JR, Pessoa LG, Prevedel AC, Rahal P, Rainho CA, Reis EM, Ribeiro ML, da Ros N, de Sa RG, Sales MM, Sant'anna SC, dos Santos ML, da Silva AM, da Silva NP, Silva WA, Jr, da Silveira RA, Sousa JF, Stecconi D, Tsukumo F, Valente V, Soares F, Moreira ES, Nunes DN, Correa RG, Zalcberg H, Carvalho AF, Reis LF, Brentani RR, Simpson AJ, de Souza SJ. The contribution of 700,000 ORF sequence tags to the definition of the human transcriptome. Proc Natl Acad Sci U S A. 2001;98:12103–12108. doi: 10.1073/pnas.201182798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conesa A, Gotz S, Garcia-Gomez JM, Terol J, Talon M, Robles M. Blast2GO: a universal tool for annotation, visualization and analysis in functional genomics research. Bioinformatics. 2005;21:3674–3676. doi: 10.1093/bioinformatics/bti610. [DOI] [PubMed] [Google Scholar]
- de Souza CP, Araujo N, Jannotti-Passos LK, Guimaraes CT. Production of Schistosoma mansoni cercariae by Biomphalaria glabrata from a focus in Belo Horizonte, Minas Gerais. Rev Inst Med Trop Sao Paulo. 1994;36:485–489. doi: 10.1590/s0036-46651994000600002. [DOI] [PubMed] [Google Scholar]
- de Souza SJ, Camargo AA, Briones MR, Costa FF, Nagai MA, Verjovski-Almeida S, Zago MA, Andrade LE, Carrer H, El-Dorry HF, Espreafico EM, Habr-Gama A, Giannella-Neto D, Goldman GH, Gruber A, Hackel C, Kimura ET, Maciel RM, Marie SK, Martins EA, Nobrega MP, Paco-Larson ML, Pardini MI, Pereira GG, Pesquero JB, Rodrigues V, Rogatto SR, da Silva ID, Sogayar MC, de Fatima Sonati M, Tajara EH, Valentini SR, Acencio M, Alberto FL, Amaral ME, Aneas I, Bengtson MH, Carraro DM, Carvalho AF, Carvalho LH, Cerutti JM, Correa ML, Costa MC, Curcio C, Gushiken T, Ho PL, Kimura E, Leite LC, Maia G, Majumder P, Marins M, Matsukuma A, Melo AS, Mestriner CA, Miracca EC, Miranda DC, Nascimento AN, Nobrega FG, Ojopi EP, Pandolfi JR, Pessoa LG, Rahal P, Rainho CA, da Ros N, de Sa RG, Sales MM, da Silva NP, Silva TC, da Silva W, Jr, Simao DF, Sousa JF, Stecconi D, Tsukumo F, Valente V, Zalcbeg H, Brentani RR, Reis FL, Dias-Neto E, Simpson AJ. Identification of human chromosome 22 transcribed sequences with ORF expressed sequence tags. Proc Natl Acad Sci U S A. 2000;97:12690–12693. doi: 10.1073/pnas.97.23.12690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeJong RJ, Emery AM, Adema CM. The mitochondrial genome of Biomphalaria glabrata (Gastropoda: Basommatophora), intermediate host of Schistosoma mansoni. J Parasitol. 2004;90:991–997. doi: 10.1645/GE-284R. [DOI] [PubMed] [Google Scholar]
- Dejong RJ, Morgan JA, Wilson WD, Al-Jaser MH, Appleton CC, Coulibaly G, D'Andrea PS, Doenhoff MJ, Haas W, Idris MA, Magalhaes LA, Mone H, Mouahid G, Mubila L, Pointier JP, Webster JP, Zanotti-Magalhaes EM, Paraense WL, Mkoji GM, Loker ES. Phylogeography of Biomphalaria glabrata and B. pfeifferi, important intermediate hosts of Schistosoma mansoni in the New and Old World tropics. Mol Ecol. 2003;12:3041–3056. doi: 10.1046/j.1365-294x.2003.01977.x. [DOI] [PubMed] [Google Scholar]
- Doolittle RF. The parasite genome: The grand assault. Nature. 2002;419:493–494. doi: 10.1038/419493a. [DOI] [PubMed] [Google Scholar]
- Ducklow HW, Boyle PJ, Maugel PW, Strong C, Mitchell R. Bacterial flora of the schistosome vector snail Biomphalaria glabrata. Appl Environ Microbiol. 1979;38:667–672. doi: 10.1128/aem.38.4.667-672.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ducklow HW, Tarraza HM, Jr, Mitchell R. Experimental pathogenicity of Vibrio parahaemolyticus for the schistosome-bearing snail Biomphalaria glabrata. Can J Microbiol. 1980;26:503–506. doi: 10.1139/m80-084. [DOI] [PubMed] [Google Scholar]
- Frandsen F. Studies of the relationship between Schistosoma and their intermediate hosts. III. The genus Biomphalaria and Schistosoma mansoni from Egypt, Kenya, Sudan,Uganda, West Indies (St. Lucia) and Zaire (two different strains: Katanga and Kinshasa) J Helminthol. 1979;53:321–348. doi: 10.1017/s0022149x00006179. [DOI] [PubMed] [Google Scholar]
- Hanelt B, Adema CM, Mansour MH, Loker ES. Detection of Schistosoma mansoni in Biomphalaria using nested PCR. J Parasitol. 1997;83:387–394. [PubMed] [Google Scholar]
- Hetru C, Bulet P. Strategies for the isolation and characterization of antimicrobial peptides of invertebrates. In: Schafer WM, editor. Antibacterial peptide protocols. Totowa, New Jersey: Humana Press; 1997. [DOI] [PubMed] [Google Scholar]
- Huang XQ, Madan A. CAP3: A DNA sequence assembly program. Genome Res. 1999;9:868–877. doi: 10.1101/gr.9.9.868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang Y, Loker ES, Zhang SM. In vivo and in vitro knockdown of FREP2 gene expression in the snail Biomphalaria glabrata using RNA interference. Dev Comp Immunol. 2006;30:855–866. doi: 10.1016/j.dci.2005.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung Y, Nowak TS, Zhang SM, Hertel LA, Loker ES, Adema CM. Manganese superoxide dismutase from Biomphalaria glabrata. J Invertebr Pathol. 2005;90:59–63. doi: 10.1016/j.jip.2005.06.014. [DOI] [PubMed] [Google Scholar]
- Kanehisa M, Goto S. KEGG: kyoto encyclopedia of genes and genomes. Nucleic Acids Res. 2000;28:27–30. doi: 10.1093/nar/28.1.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knight M, Miller AN, Patterson CN, Rowe CG, Michaels G, Carr D, Richards CS, Lewis FA. The identification of markers segregating with resistance to Schistosoma mansoni infection in the snail Biomphalaria glabrata. Proc Natl Acad Sci U S A. 1999;96:1510–1515. doi: 10.1073/pnas.96.4.1510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis FA, Patterson CN, Knight M, Richards CS. The relationship between Schistosoma mansoni and Biomphalaria glabrata: genetic and molecular approaches. Parasitology. 2001;123 Suppl:S169–S179. doi: 10.1017/s0031182001007831. [DOI] [PubMed] [Google Scholar]
- Lie KJ. Survival of Schistosoma mansoni and other trematode larvae in the snail Biomphalaria glabrata - a discussion of the Interference Theory. Trop Geogr Med. 1982;34:111–122. [PubMed] [Google Scholar]
- Litman GW, Cannon JP, Dishaw LJ. Reconstructing immune phylogeny: new perspectives. Nat Rev Immunol. 2005;5:866–879. doi: 10.1038/nri1712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lockyer AE, Jones CS, Noble LR, Rollinson D. Trematodes and snails: an intimate association. Can J Zool. 2004;82:251–269. [Google Scholar]
- Lockyer AE, Spinks J, Noble LR, Rollinson D, Jones CS. Identification of genes involved in interactions between Biomphalaria glabrata and Schistosoma mansoni by suppression subtractive hybridization. Mol Biochem Parasitol. 2007a;151:18–27. doi: 10.1016/j.molbiopara.2006.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lockyer AE, Spinks JN, Walker AJ, Kane RA, Noble LR, Rollinson D, Dias-Neto E, Jones CS. Biomphalaria glabrata transcriptome: identification of cell-signalling, transcriptional control and immune-related genes from open reading frame expressed sequence tags (ORESTES) Dev Comp Immunol. 2007b;31:763–782. doi: 10.1016/j.dci.2006.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loker ES, Adema CM. Schistosomes, echinostomes and snails: comparative immunobiology. Parasitol Today. 1995;11:120–124. [Google Scholar]
- Loker ES, Adema CM, Zhang SM, Kepler TB. Invertebrate immune systems--not homogeneous, not simple, not well understood. Immunol Rev. 2004;198:10–24. doi: 10.1111/j.0105-2896.2004.0117.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loker ES, Bayne CJ, Buckley PM, Kruse KT. Ultrastructure of encapsulation of Schistosoma mansoni mother sporocysts by hemocytes of juveniles of the 10-R2 strain of Biomphalaria glabrata. J Parasitol. 1982;68:84–94. [PubMed] [Google Scholar]
- Loker ES, Hertel LA. Alterations in Biomphalaria glabrata plasma induced by infection with the digenetic trematode Echinostoma paraensei. J Parasitol. 1987;73:503–513. [PubMed] [Google Scholar]
- Matricon-Gondran M, Letocart M. Internal defenses of the snail Biomphalaria glabrata. J Invertebr Pathol. 1999;74:248–254. doi: 10.1006/jipa.1999.4878. [DOI] [PubMed] [Google Scholar]
- Miller AN, Raghavan N, FitzGerald PC, Lewis FA, Knight M. Differential gene expression in haemocytes of the snail Biomphalaria glabrata: effects of Schistosoma mansoni infection. Int J Parasitol. 2001;31:687–696. doi: 10.1016/s0020-7519(01)00133-3. [DOI] [PubMed] [Google Scholar]
- Mitta G, Galinier R, Tisseyre P, Allienne JF, Girerd-Chambaz Y, Guillou F, Bouchut A, Coustau C. Gene discovery and expression analysis of immune-relevant genes from Biomphalaria glabrata hemocytes. Dev Comp Immunol. 2005;29:393–407. doi: 10.1016/j.dci.2004.10.002. [DOI] [PubMed] [Google Scholar]
- Morgan JA, Dejong RJ, Snyder SD, Mkoji GM, Loker ES. Schistosoma mansoni and Biomphalaria: past history and future trends. Parasitology. 2001;123 Suppl:S211–S228. doi: 10.1017/s0031182001007703. [DOI] [PubMed] [Google Scholar]
- Nowak TS, Woodards AC, Jung Y, Adema CM, Loker ES. Identification of transcripts generated during the response of resistant Biomphalaria glabrata to Schistosoma mansoni infection using suppression subtractive hybridization. J Parasitol. 2004;90:1034–1040. doi: 10.1645/GE-193R1. [DOI] [PubMed] [Google Scholar]
- Pan CT. Studies on the host-parasite relationship between Schistosoma mansoni and the snail Australorbis glabratus. Am J Trop Med Hyg. 1965;14:931–976. doi: 10.4269/ajtmh.1965.14.931. [DOI] [PubMed] [Google Scholar]
- Paraense WL, Correa LR. Variation in susceptibility of populations of Australorbis glabratus to a strain of Schistosoma mansoni. Rev Inst Med Trop Sao Paulo. 1963;5:15–22. [PubMed] [Google Scholar]
- Pino-Heiss S, Brown M, McKerrow JH. Schistosoma mansoni: degradation of host extracellular matrix by eggs and miracidia. Exp Parasitol. 1985;59:217–221. doi: 10.1016/0014-4894(85)90075-x. [DOI] [PubMed] [Google Scholar]
- Poulsen M, Bot ANM, Nielsen MG, Boomsma JJ. Experimental evidence for the costs and hygienic significance of the antibiotic metapleural gland secretion in leaf-cutting ants. Behav Ecol Sociobiol. 2002;52:151–157. [Google Scholar]
- Raghavan N, Knight M. The snail (Biomphalaria glabrata) genome project. Trends Parasitol. 2006;22:148–151. doi: 10.1016/j.pt.2006.02.008. [DOI] [PubMed] [Google Scholar]
- Raghavan N, Miller AN, Gardner M, FitzGerald PC, Kerlavage AR, Johnston DA, Lewis FA, Knight M. Comparative gene analysis of Biomphalaria glabrata hemocytes pre- and post-exposure to miracidia of Schistosoma mansoni. Mol Biochem Parasitol. 2003;126:181–191. doi: 10.1016/s0166-6851(02)00272-4. [DOI] [PubMed] [Google Scholar]
- Richards CS. Genetic factors in susceptibility of Biomphalaria glabrata for different strains of Schistosoma mansoni. Parasitology. 1975;70:231–241. doi: 10.1017/s0031182000049696. [DOI] [PubMed] [Google Scholar]
- Richards CS, Shade PC. The genetic variation of compatibility in Biomphalaria glabrata and Schistosoma mansoni. J Parasitol. 1987;73:1146–1151. [PubMed] [Google Scholar]
- Sullivan JT, Richards CS. Schistosoma mansoni, NIH-SM-PR-2 strain, in susceptible and nonsusceptible stocks of Biomphalaria glabrata: comparative histology. J Parasitol. 1981;67:702–708. [PubMed] [Google Scholar]
- Thalia A, Farazi TA, Juranek SA, Tuschl T. The growing catalog of small RNAs and their association with distinct Argonaute/Piwi family members. Development. 2008;135:1201–1214. doi: 10.1242/dev.005629. [DOI] [PubMed] [Google Scholar]
- Theron A, Coustau C. Are Biomphalaria snails resistant to Schistosoma mansoni? J Helminthol. 2005;79:187–191. doi: 10.1079/joh2005299. [DOI] [PubMed] [Google Scholar]
- van der Knaap WP, Loker ES. Immune mechanisms in trematode-snail interactions. Parasitol Today. 1990;6:175–182. doi: 10.1016/0169-4758(90)90349-9. [DOI] [PubMed] [Google Scholar]
- van der Knaap WP, Sminia T, Kroese FG, Dikkeboom R. Elimination of bacteria from the circulation of the pond snail Lymnaea stagnalis. Dev Comp Immunol. 1981;5:21–32. doi: 10.1016/s0145-305x(81)80004-3. [DOI] [PubMed] [Google Scholar]
- Vergote D, Bouchut A, Sautiere PE, Roger E, Galinier R, Rognon A, Coustau C, Salzet M, Mitta G. Characterisation of proteins differentially present in the plasma of Biomphalaria glabrata susceptible or resistant to Echinostoma caproni. Int J Parasitol. 2005;35:215–224. doi: 10.1016/j.ijpara.2004.11.006. [DOI] [PubMed] [Google Scholar]
- Webster JP, Gower CM, Blair L. Do hosts and parasites coevolve? Empirical support from the Schistosoma system. Am Nat. 2004;164:33–51. doi: 10.1086/424607. [DOI] [PubMed] [Google Scholar]
- Webster JP, Woolhouse MEJ. Selection and strain specificity of compatibility between snail intermediate hosts and their parasitic schistosomes. Evolution. 1998;52 doi: 10.1111/j.1558-5646.1998.tb02243.x. [DOI] [PubMed] [Google Scholar]
- Weinberg ED, Weinberg GA. The Role of Iron in Infection. Curr Opin Infect Dis. 1995;8:164–169. [Google Scholar]
- Yoshino TP, Lodes MJ. Secretory protein biosynthesis in snail hemocytes: in vitro modulation by larval schistosome excretory-secretory products. J of Parasitol. 1988;74:538–547. [PubMed] [Google Scholar]
- Yoshino TP, Lodes MJ, Rege AA, Chappell CL. Proteinase activity in miracidia, transformation excretory-secretory products, and primary sporocysts of Schistosoma mansoni. J of Parasitol. 1993;79:23–31. [PubMed] [Google Scholar]
- Zhang SM, Adema CM, Kepler TB, Loker ES. Diversification of Ig superfamily genes in an invertebrate. Science. 2004;305:251–254. doi: 10.1126/science.1088069. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Sequences are listed 5’ to 3’.
Putative IDs of sequences contributing to GO clusters as giving in Table 1.
Result of BLAST comparison of unknown ORESTES sequences to all molluscan or B. glabrata ESTs in GenBank (July 2007). * indicates immune relevant ORESTES.