Abstract
Combination of chemopreventive agents with distinct molecular mechanisms is considered to offer a potential for enhancing cancer prevention efficacy while minimizing toxicity. Here we report two chemopreventive agents, selenite and genistein, that have synergistic effects on apoptosis, cell cycle arrest, and associated signaling pathways in p53-expressing LNCaP and p53-null PC3 prostate cancer cells. We show that selenite induced apoptosis only, whereas genistein induced both apoptosis and G2/M cell cycle arrest. Combination of these two agents exhibited enhanced effects, which were slightly greater in LNCaP than PC3 cells. Selenite or genistein alone upregulated protein levels of p53 in LNCaP cells only and p21waf1 and Bax in both cell lines. Additionally, genistein inhibited AKT phosphorylation. Downregulation of AKT by siRNA caused apoptosis and G2/M cell cycle arrest and masked the effects of genistein. Treatment with insulin-like growth factor I (IGF-I) elevated levels of total and phosphorylated AKT and suppressed the effects of genistein. Neither downregulation of AKT nor IGF-I treatment altered the cellular effects of selenite. Our study demonstrates that selenium and genistein act via different molecular mechanisms and exhibit enhanced anticancer effects, suggesting that a combination of selenium and genistein may offer better efficacy and reduction of toxicity in prostate cancer prevention.
INTRODUCTION
Prostate cancer is the most commonly diagnosed cancer and the second leading cause of cancer deaths in men in the United States and Europe (1,2). Studies have shown that prostate cancer incidence may be reduced by chemopreventive strategies (3). Selenium is an essential element in maintenance of the activity of some antioxidant enzymes and redox-regulatory proteins. Epidemiological studies have shown an inverse association between serum selenium levels and cancer risk in humans (4,5). Previous studies have documented that selenium accumulated preferentially in the human prostate gland (6,7). The most compelling findings relating selenium to prostate cancer prevention were from a double-blind, placebo-controlled, randomized cancer prevention trial (8). The study showed that selenium supplement reduced prostate cancer incidence. The results of this study have led to a current larger Phase III, double-blind, placebo-controlled clinical trial, the Selenium and Vitamin E Chemoprevention Trial (SELECT) (9).
The anticancer mechanisms of selenium are still not fully understood. Several mechanisms have been proposed, which include maintenance of glutathione peroxidase (GPx) activity to protect against oxidative damage, detoxification of intermediate metabolites of chemical carcinogens, stimulation of the immune system, induction of cell cycle arrest and apoptosis, and inhibition of angiogenesis (4,10–12). Studies have shown that selenium induced prostate cancer cell apoptosis and cell cycle arrest, processes that have been postulated to be critical for cancer chemoprevention by selenium (13–15). However, the toxicity of selenium to normal organs may limit the utilization of this agent in cancer chemoprevention. Selenium has been reported to induce DNA damage, particularly DNA strand breaks and base damage, at high doses (16,17). Dose-dependent fetocidal effects and fetal growth retardation were observed in pregnant mice injected subcutaneously with selenite (9,18). Therefore, toxic effects of selenium might be a problem if it is used at higher doses that are required for cancer prevention. Combination strategies utilizing two or more chemopreventive agents may be more effective and require lower doses of each agent to minimize toxicity. This strategy is currently being used in the SELECT trial (9,19).
Epidemiological studies have shown that Asian men who consume diets rich in soy isoflavones have low incidence of prostate cancer (20). Genistein (4′, 5, 7-trihydroxyisoflavone), the most abundant isoflavone present in soy, has been shown to inhibit growth of both androgen-dependent and -independent prostate cancer cells in vitro (21). Prostate cancer incidence was significantly reduced in chemically induced animal cancer models after ingestion of genistein in the diet at nutritionally relevant concentrations (22,23). Several mechanisms have been proposed for genistein anticarcinogenic activity. These include induction of apoptosis, inhibition of angiogenesis, inhibition of protein tyrosine kinases, inhibition of DNA topoisomerase II , inhibition of NF-kappa B, downregulation of transforming growth factor-beta (TGF-β) and inhibition of epidermal growth factor (EGF) (24–28). Safety and efficacy are also issues for the use of genistein in cancer chemoprevention (29).
There are no reports on combined use of selenium and genistein in prostate cancer chemoprevention, although they have been shown to have anticancer activity individually. Previous studies have shown that these two agents affect both similar and different signaling pathways in prostate cancer cells (14,30). It is not known whether these two agents may have synergistic effects in cancer cells. In this study, we investigated the effects of genistein and selenite alone and in combination on cell cycle arrest and apoptosis and analyzed the underlying mechanisms of these two chemopreventive agents in prostate cancer cells.
MATERIALS AND METHODS
Chemicals and Antibodies
Sodium selenite, genistein, insulin-like growth factor-1 (IGF-1), anti-β-actin antibody, and Annexin V Apoptotic Analysis Kit were purchased from Sigma Chemical Co. (St. Louis, MO). Anti-p53, anti-Bax, antiphosphorylated p53 (serine 15) antibodies, and SignalSilence Akt siRNA (#6211) were purchased from Cell Signaling Technology (Beverly, MA). siRNA Duplex Control (nonsilencing) and RNAiFect Transfection Reagent were purchased from QIAGEN (Valencia, CA). SuperSignal West Pico Stable Peroxide and Luminol/Enhancer Solutions, M-PER Mammalian Protein Extraction Reagent, and Mitochondria Isolation Kit were purchased from Pierce Biotechnology, Inc. (Rockford, IL). Anti-p21waf1 (C-19) antibody was purchased from Santa Cruz Biotechnology, Inc. (Santa Cruz, CA).
Cell Culture
LNCaP and PC3 cells were obtained from the American Type Culture Collection (Rockville, MD) and routinely maintained in 100-mm tissue culture dishes (Corning, NY) in RPMI 1640 supplemented with 5% heat-inactivated fetal bovine serum and 1% antibiotic antimycotic (Life Technologies, Inc., Rockville, MD) at 37°C in a humidified atmosphere of 95% air and 5% CO2. For biochemical analyses, cells were collected by rinsing in PBS 3 times, scraping with a rubber policeman in 10-ml PBS, and then centrifuging at 2,000 rpm for 5 min. After removing the PBS, cell pellets were stored at −80°C until use.
Cell Viability Assay
Cells were seeded at 5 × 104 cells/well in 24-well plates overnight before treatment with different agents and then allowed to grow for an additional 5 days. For the MTT assay, MTT solution (10 µl; 5 mg/ml in PBS) was added to each well of the plates and incubated for 3 h at 37°C. MTT lysis buffer (100 µl of 10% SDS, 45% dimethyl formamide, adjusted to pH 4.5 by glacial acid) was then added to dissolve the formazan. The optical density was measured at 570 nm using a Beckman DU-640 Spectrophotometer (Beckman Coulter, Fullerton, CA). The percentage of viable cells was calculated as the relative optical density compared to the control.
Flow Cytometric Analysis
Cell samples were prepared and analyzed as described previously (14). Cell cycle was analyzed with the FITC BrdU Kit (BD Pharmingen, San Diego, CA) according to the manufacturer’s instructions. Cells were incubated with indicated concentrations of agents for 24 h and subsequently pulsed with BrdU for 30 min at 37°C. The cells were washed in a staining buffer [1 × Dulbecco’s phosphate-buffered saline (DPBS) +3% FBS), fixed/permeabilized with Cytofix/Cytoperm buffer, and then washed with Perm/Wash buffer. After permeabilization, cells were treated with 30 µg DNAse for 1 h at 37°C and then stained with FITC-conjugated anti-BrdU antibody and 7-AAD before flow cytometric analysis. DNA content was analyzed using a FACScan flow cytometer (BD Bioscience, San Jose, CA). Annexin V-FITC Apoptosis Detection Kit was used for apoptosis assay. Cells were washed with a calcium-supplemented PBS buffer after removal from the growth plates with an EDTA-free trypsin solution. The cell suspension was then centrifuged at 500 g for 7 min. Cells were washed in cold PBS and centrifuged again. The supernatant was discarded and FITC-Annexin V was added to a final concentration of 0.5 µg/ml plus 2 µg/ml PI. Cells were incubated for 30 min in the dark and then analyzed using a FACScan flow cytometer.
Western Blot Analysis
Cell pellets were lysed with M-PER mammalian protein extraction reagent and protein concentrations were determined using the Bradford assay. Cell lysates (20–50 µg) were electrophoresed in 12.5% SDS polyacrylamide gels and then transferred onto nitrocellulose membranes. After blotting in 5% nonfat dry milk in Tween 20 Tris-buffered saline (TTBS), the membranes were incubated with primary antibodies at 1:1,000 to 2,000 dilutions in TTBS overnight at 4°C and then secondary antibodies conjugated with horseradish peroxidase at 1:10,000 dilution in TTBS for 1 h at room temperature. Protein bands were visualized on X-ray film using an enhanced chemiluminescence system.
siRNA Transfection
Cells were seeded at 2 × 105 cells/well in 6-well plates and allowed to grow to 60% confluence. Cells were transfected with 50 nM AKT siRNA with 2 µl RNAiFect™ Transfection reagent in 1 ml serum-free medium for 12 h, and then 1 ml fresh medium with 10% FBS was added to each well for 24 h before treatments. Cells were also transfected with negative control siRNA.
IGF-1 Treatment
Cells were seeded at 5 × 105 cells/well in 100-mM plates or at 5 × 104 cells/well in 24-well plates and allowed to grow to 60% confluence. Cells were treated with 20 nM IGF-1 for 12 h, and then 1 ml fresh medium with 10% FBS was added to each well for 24 h before other treatments.
Statistical Analysis
All data are presented as the mean ± SD from at least 3 sets of independent experiments. Data were analyzed by 1-, 2- or 3-way ANOVA followed by pairwise or post hoc comparisons using SPSS (Version 10.0.1; Chicago, IL). Differences were considered significant at P < 0.05.
RESULTS
Induction of Cell Death, G2/M Cell Cycle Arrest, and Apoptosis by Selenite and Genistein
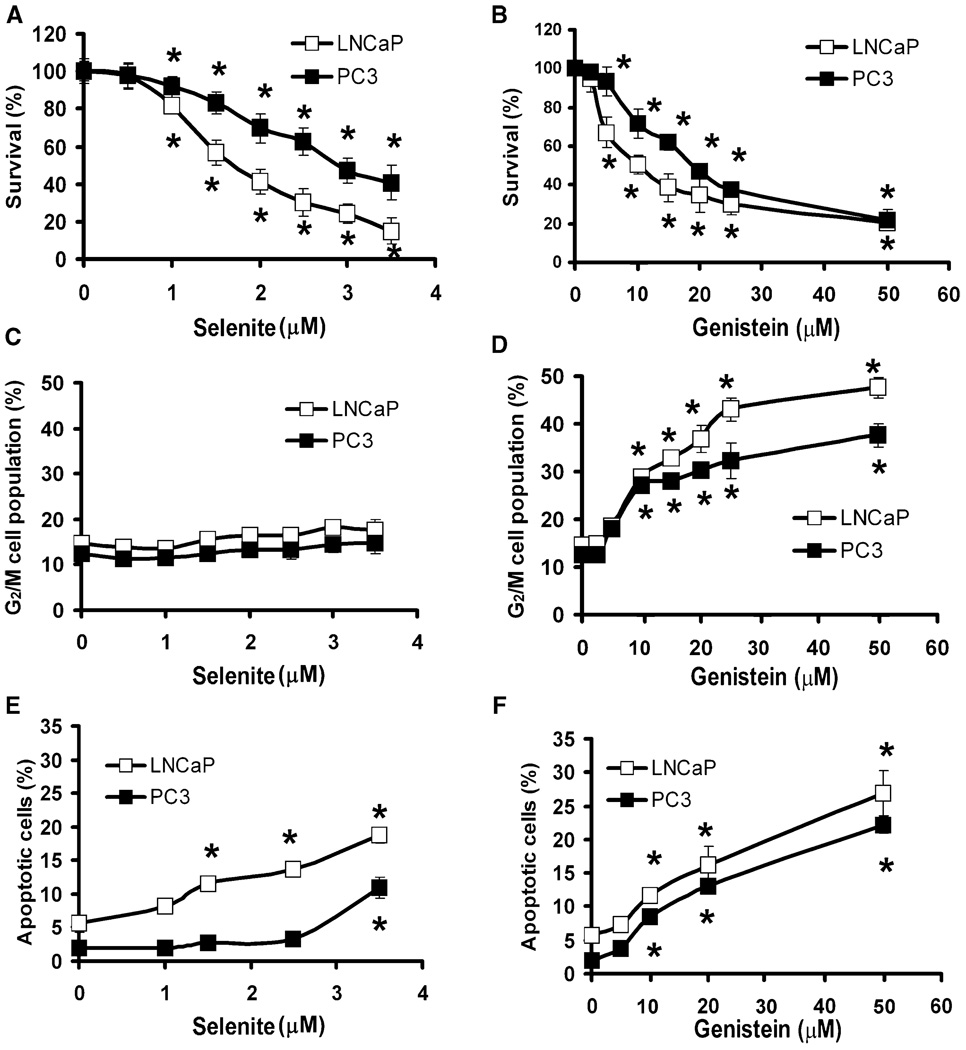
LNCaP and PC3 cells were treated with different doses of selenite or genistein for 5 days, and cell viability was assessed by the MTT assay. As shown in Figs. 1A and 1B, selenite and genistein decreased cell viability of both cell lines in a dose-dependent manner. Significant cell viability decreases occurred in cells treated with 1.0 µM and higher doses of selenite (Fig. 1A) or 5.0 µM and higher doses of genistein (Fig. 1B). Reduction of cell viability by 50% (IC50) required 1.7 µM selenite or 10 µM genistein for LNCaP cells and 3.0 µM selenite or 20 µM genistein for PC3 cells (Figs. 1A and 1B); therefore, PC3 cells were almost twofold more resistant to selenite or genistein than LNCaP cells (Figs. 1A and 1B). The different sensitivity between LNCaP and PC3 cells remained in selenite in the doses above IC50 but disappeared in genistein. Flow cytometric analysis showed that selenite did not induce significant G2/M cell cycle arrest in either cell line. Selenite treatment at 3.5 µM concentration only had a slight effect (a 1.2-fold increase) on G2/M cell cycle arrest in both cell lines (Fig. 1C). In contrast, genistein induced G2/M cell cycle arrest in a dose-dependent manner in both LNCaP and PC3 cells after 24 h treatment. LNCaP or PC3 cells treated with 10 µM genistein for 24 h showed a 1.8-fold increase (from 15% to 28%) or a 2.3-fold increase (from 12% to 27%) in the G2/M phase cell population (Fig. 1D). Selenite had no significant effect on G0/G1 and S phases of the cell cycle except for 3.5 µM selenite, which decreased S phase only in LNCaP cells (supplemental Fig. 1). Genistein decreased G0/G1 in a dose-dependent manner, but only 50 µM genistein decreased S phase in both cell lines (supplemental Fig. 1S). These data suggest that G2/M phase is the primary target of genistein. Flow cytometry analysis showed that selenite induced LNCaP cell apoptosis in a dose-dependent manner after 48 h treatment (Fig. 1E). LNCaP cells treated with 2.5 µM selenite for 48 h showed a 2.6-fold increase (from 5% to 13%) in apoptosis compared to cells without treatment. In contrast to LNCaP cells, PC3 cells were more resistant to selenite, and a significant increase (from 2% to 10%) in apoptosis was observed only with 3.5 µM selenite treatment at 48 h (Fig. 1E). Genistein induced cell apoptosis in a dose-dependent manner after 48 h treatment in both cell lines (Fig. 1F). Treatment with 10 µM genistein induced a 2.2-fold increase (from 5% to 11%) and fourfold increase (2% to 8%) in apoptosis in LNCaP and PC3 cells, respectively, compared to cells without treatment (Fig. 1F).
FIG. 1.
Induction of cell death, G2/M cell cycle arrest, and apoptosis by selenite or genistein in LNCaP and PC3 cells. A: MTT assay of cell viability of LNCaP and PC3 cells treated with different doses of selenite for 5 days. B: The MTT assay of cell viability of LNCaP and PC3 cells treated with different doses of genistein for 5 days. C: Flow cytometric analysis of the G2/M population in LNCaP and PC3 cells treated with different doses of selenite for 24 h. D: Flow cytometric analysis of the G2/M population in LNCaP and PC3 cells treated with different doses of genistein for 24 h. E: Flow cytometric analysis of Annexin V-positive apoptotic cell population in LNCaP and PC3 cells treated with different doses of selenite for 48 h. F: Flow cytometric analysis of Annexin V-positive apoptotic cell population in LNCaP and PC3 cells treated with different doses of genistein for 48 h. Data were obtained from 3 independent experiments and the results shown are mean ± SD. *P < 0.05 compared with 0 µM.
Enhanced Effects on Cell Death, G2/M Cell Cycle Arrest, and Apoptosis by Combined Selenite and Genistein
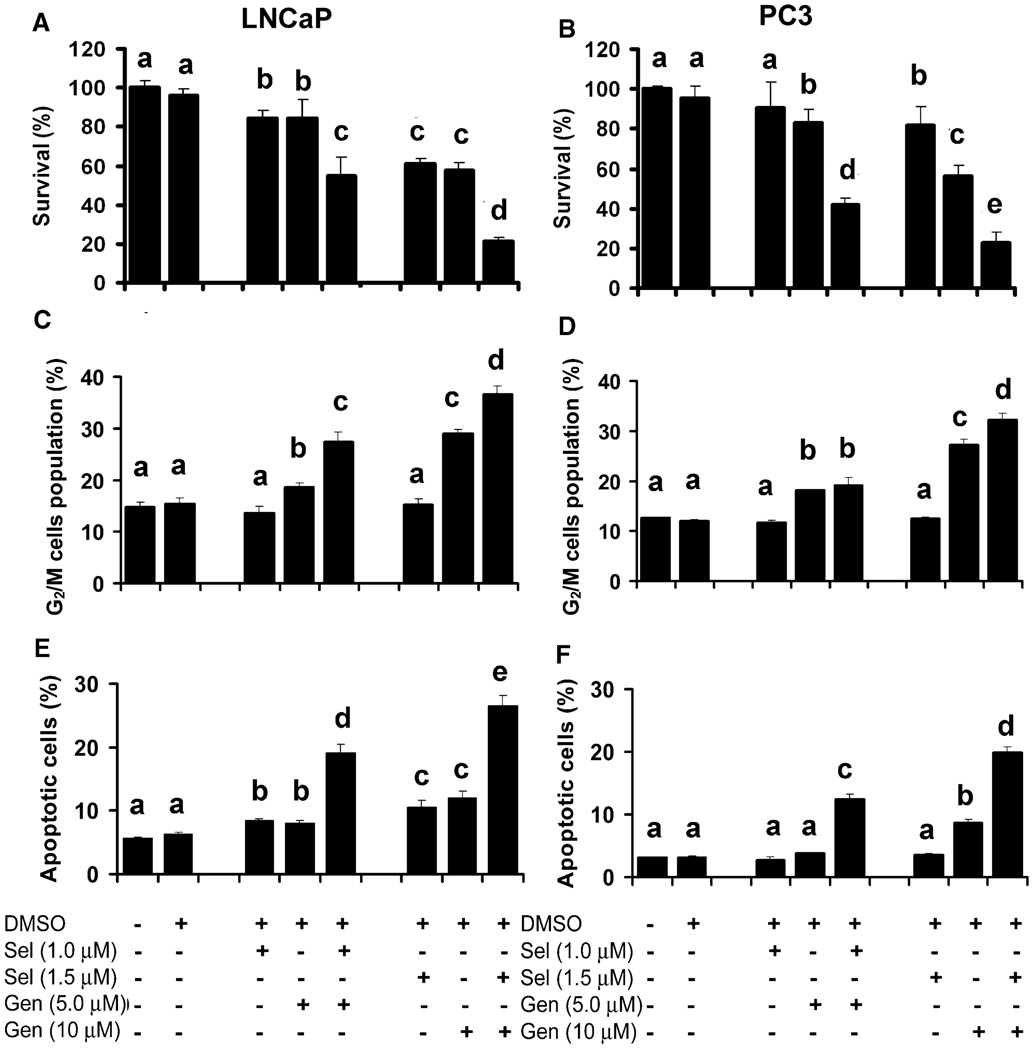
To test combined effects of selenite and genistein, cells were treated with 1 or 1.5 µM selenite and/or 5 or 10 µM genistein. These two concentrations of selenite and genistein were chosen because they exhibited only low to moderate cellular effects and allowed assessment of possible synergistic effects between selenite and genistein. As shown in Fig. 2A, 1 µM selenite or 5 µM genistein induced 15% cell death in LNCaP cells after 5 days treatment, whereas a combination resulted in 45% cell death. Treatment with 1.5 µM selenite or 10 µM genistein exhibited 38% or 43% cell death, respectively, whereas combined treatment resulted in 78% cell death. Similar to LNCaP cells, PC3 cells also showed a synergistic effect between selenite and genistein. As shown in Fig. 2B, 1 µM selenite or 5 µM genistein alone induced only 10% or 18% cell death in PC3 cells after 5 days treatment, but the combination resulted in 58% cell death. Similar to the low-dose treatment, 1.5 µM selenite or 10 µM genistein alone induced 18% or 43% cell death, respectively, whereas the combination resulted in 78% cell death. Cotreatment with selenite and genistein also exhibited synergistic or additive effects on G2/M cell cycle arrest and apoptosis. As shown in Figs. 2C and D, selenite at 1.0 or 1.5 µM did not induce G2/M cell cycle arrest in LNCaP and PC3 cells after 24 h treatment, whereas 5 or 10 µM genistein induced significant G2/M cell cycle arrest in both cell lines in a dose-dependent manner. Genistein at the 5 µM concentration resulted in 18% of cells arrested in the G2/M phase, and 10 µM genistein induced 29% and 27% LNCaP and PC3 cells arrested in the G2/M phase, respectively. The effect of combined treatment was also dose dependent, but it was greater in LNCaP cells than PC3 cells. Combined treatment with 5 µM genistein and 1 µM selenite resulted in 27% LNCaP cells arrested in the G2/M phase of the cell cycle, whereas 19% PC3 cells showed G2/M cell cycle arrest. Flow cytometric analysis demonstrated that 1 µM selenite or 5 µM genistein alone did not induce significant apoptosis in LNCaP cells after 48 h treatment, but the combination resulted in a 2.4-fold increase (from 8% to 19%) in apoptosis (Fig. 2E). Cotreatment with 1.5 µM selenite and 10 µM genistein also showed a synergistic effect (29% apoptotic cells) on induction of apoptosis in LNCaP cells compared to 1.5 µM selenite or 10 µM genistein alone, which induced apoptosis in 10% or 12% cells, respectively (Fig. 2E). In contrast, 1 or 1.5 µM selenite or 5 µM genistein treatment alone did not increase apoptosis in PC3 cells (Fig. 2F). Ten µM genistein alone induced 9% apoptosis, whereas a combination with 1.5 µM selenite induced 20% apoptosis in PC3 cells (Fig. 2F). The combined effects in PC3 cells were weaker than those in LNCaP cells. The data demonstrate synergistic effects between selenite and genistein, which is more potent in LNCaP cells than PC3 cells.
FIG. 2.
Induction of G2/M cell cycle arrest and apoptosis by cotreatment with selenite and genistein in LNCaP and PC3 cells. A and B: The MTT assay of cell viability of LNCaP cells and PC3 cells treated with selenite (Sel), genistein (Gen), or in combination for 5 days. C and D: Flow cytometry analysis of the G2/M population in LNCaP cells and PC3 cells treated with selenite, genistein, or in combination for 24 h. E and F: Flow cytometric analysis of Annexin V-positive apoptotic cell population in LNCaP cells and PC3 cells treated with selenite, genistein, or in combination for 48 h. Data were obtained from 3 independent experiments and the results shown are mean ± SD. Means with different letters above bars indicate significant differences (P < 0.05) in each panel. DMSO, dimethyl sulfoxide.
Involvement of AKT in Cell Death, G2/M Cell Cycle Arrest, and Apoptosis Induced by Selenite and Genistein
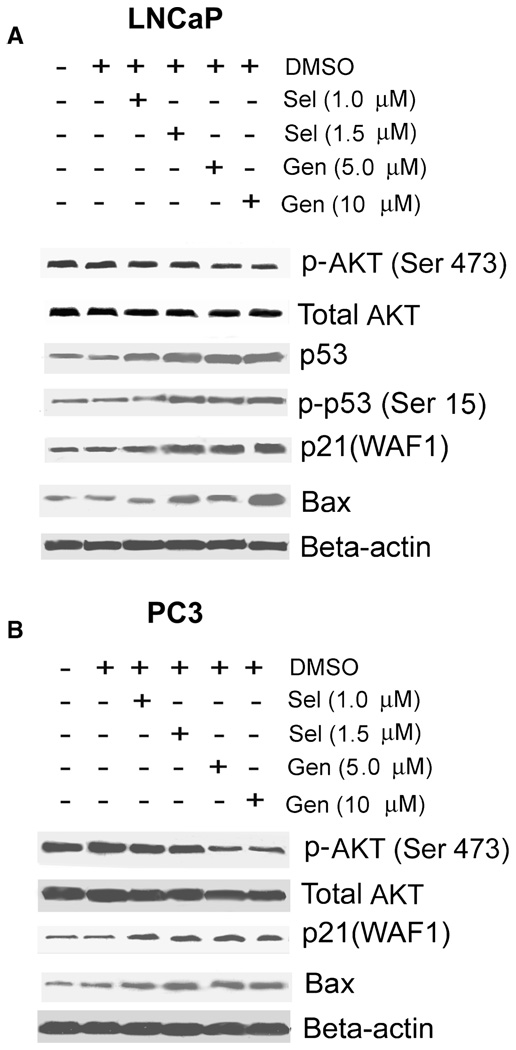
Since AKT and p53 are postulated to be involved in cancer chemoprevention by selenite and genistein, we next determined effects of selenite and genistein on levels of these two proteins and the cell cycle regulatory protein p21waf1 and the proapoptotic protein Bax by Western blot analysis. As shown in Fig. 3A, 1.5 µM selenite increased both total and phosphorylated p53 on serine15 (Ser 15) and p53 target genes p21waf1 and Bax but had no effect on AKT in wild type p53-expressing LNCaP cells after 24 h treatment. A slight decrease in phosphorylated AKT on serine 473 (Ser 473) was observed in LNCaP cells treated with 2.5 µM selenite (data not shown). In addition to upregulation of both total p53 and phosphorylated p53 (Ser 15), both 5 and 10 µM genistein also decreased phosphorylated AKT (Ser 473), but not total AKT (Fig. 3A). In p53-null PC3 cells, both selenite and genistein increased p21waf1 and Bax, but only genistein decreased phosphorylated AKT (Ser 473; Fig. 3B). Similar to the effect in LNCaP cells, genistein did not change protein levels of total AKT in PC3 cells. The results indicate that both selenite and genistein upregulate p53, but only genistein inhibits AKT phosphorylation. The results also indicate that both selenite and genistein can regulate p21waf1 and Bax via a p53-independent pathway.
FIG. 3.
Effects of genistein and selenite on Akt, p53, p21waf1, and Bax in LNCaP and PC3 cells. A and B: Western blot analysis of total AKT, phosphorylated AKT at serine 473 [p-AKT(Ser 473)], total p53, phosphorylated p53 at serine 15 [p-p53(Ser 15)], p21waf1, and Bax in LNCaP cells and PC3 cells. Cells were treated with selenite (Sel) or genistein (Gen) for 24 h, and 50 µg proteins were loaded for the assay. The results shown here is one representative of 3 repeated experiments. DMSO, dimethyl sulfoxide.
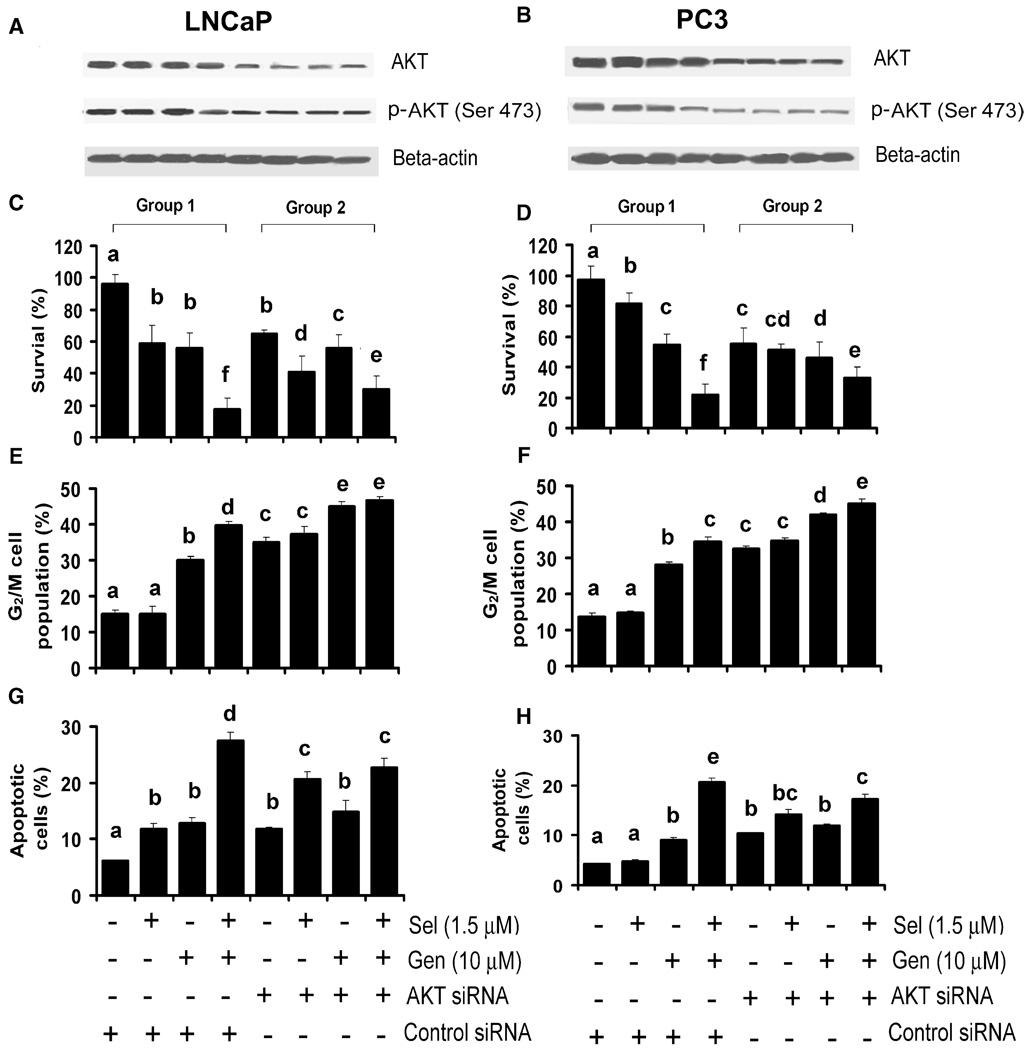
To determine the role of AKT, cells were transfected with the AKT specific siRNA or control (scrambled) siRNA. As shown in Figs. 4A and 4B, both AKT and phosphorylated AKT (Ser 473) were decreased in both LNCaP and PC3 cells transfected with 50 nM AKT siRNA for 48 h, but there were no changes in cells transfected with the control siRNA. Cotreatment with selenite and genistein resulted in decreased phosphorylated AKT (Ser 473) only in cells transfected with the control siRNA. There were no further decreases in total or phosphorylated AKT (Ser 473) by genistein in cells transfected with the AKT siRNA. AKT siRNA transfection alone decreased cell viability by 30% in LNCaP cells (Fig. 4C). AKT siRNA transfection plus selenite treatment increased cell death to 55% in LNCaP cells compared to 40% by selenite with control siRNA transfection (Fig. 4C). There was no enhanced effect between the AKT siRNA and genistein. AKT siRNA transfection had a slightly greater effect on cell death in PC3 cells than LNCaP cells, 45% vs. 30%, but combined AKT siRNA and genistein plus selenite had no further additive effect in PC3 cells (Fig. 4D). In contrast, AKT siRNA transfection decreased cell death induced by cotreatment with genistein and selenite in both LNCaP and PC3 cells. AKT siRNA transfection increased G2/M cell cycle arrest in both LNCaP (35%) and PC3 (32%) cells (Figs. 4E and 4F) and slightly enhanced the effect of genistein alone (45% in LNCaP, 41% in PC3) or combined with selenite (46% in LNCaP, 45% in PC3) but did not change the effect of selenite alone. AKT siRNA transfection also increased apoptosis in both LNCaP (11%) and PC3 (10%) cells and enhanced the apoptotic effect of selenite in LNCaP cells only (Figs. 4G and 4H). AKT siRNA transfection caused 20% apoptosis in LNCaP cells treated with 1.5 µM selenite compared with 11% apoptosis in cells transfected with the control siRNA but had no enhancement effect on genistein-induced apoptosis and slightly decreased (27% to 22% in LNCaP cells; 20% to 17% in PC3 cells) the apoptotic effect by cotreatment with selenite and genistein in both cell lines. Our previous study demonstrated that downregulation of p53 decreased apoptosis induced by selenite in LNCaP cells, whereas reexpression of p53 enhanced apoptosis by selenite in PC3 cells [13]. However, downregulation of p53 by p53 siRNA transfection in LNCaP cells or reexpression of p53 by adenoviral p53 constructs in PC3 cells did not alter cellular sensitivity to genistein (data not shown). The results suggest that genistein is more dependent on the AKT signaling pathway, whereas selenite is dependent on the p53 pathway.
FIG. 4.
Effects of downregulation of AKT on selenite- and genistein-induced cell cycle arrest and apoptosis in LNCaP and PC3 cells. A and B: Western blot analysis of effects of AKT siRNA transfection on protein levels of total AKT and phosphorylated AKT (p-AKT at Ser 473) in LNCaP and PC3 cells. Cells were transfected with 50 nM AKT siRNA for 24 h and then treated with selenite (Sel), genistein (Gen), or in combination for another 24 h. Fifty µg of protein were loaded for the assay. C and D: MTT assay of cell viability of LNCaP and PC3 cells treated with selenite, genistein or in combination for 5 days after transfection with 50 nM AKT siRNA for 24 h. E and F: Flow cytometric analysis of the G2/M population of LNCaP cells and PC3 cells treated with selenite, genistein, or in combination after transfection with AKT siRNA. Cells were treated with selenite, genistein, or in combination for 24 h after transfection with 50 nM AKT siRNA for 24 h. G and H: Flow cytometric analysis of apoptosis in LNCaP and PC3 cells treated with selenite, genistein, or in combination for 48 h after transfection with 50 nM AKT siRNA for 24 h. Data were obtained from 3 independent experiments, and the results shown are mean ± SD. Group 1, control siRNA transfection. Group 2, AKT siRNA transfection. Means with different letters above bars indicate significant differences (P < 0.05) in each panel.
Inhibition of Genistein-Induced Cell Death, G2/M Cell Arrest, and Apoptosis by IGF-I
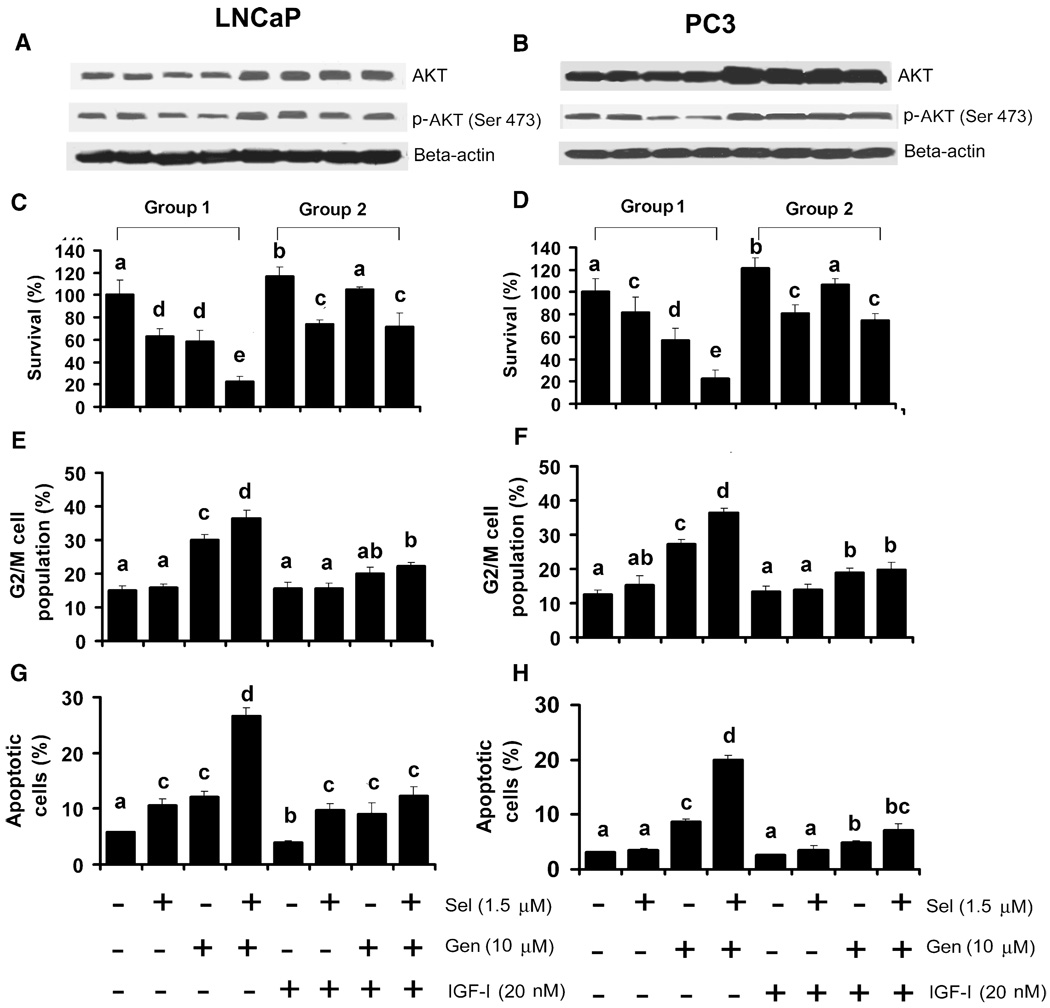
IGF-I is known to promote cell survival and proliferation by activation of the AKT signaling pathway. To test whether IGF-I would inhibit the effects of selenite and genistein on cell survival, G2/M cell cycle arrest, and apoptosis, cells were pretreated with 20 nM IGF-I for 24 h followed by treatment with selenite and/or genistein for another 24 h. IGF-I treatment significantly increased protein levels of both total AKT and phosphorylated AKT (Ser 473) and suppressed inhibition of AKT phosphorylation by genistein or combined selenite and genistein treatment (Figs. 5A and B). The MTT assay showed that IGF-I slightly stimulated proliferation of both LNCaP (118% viability) and PC3 cells (117%) and decreased genistein-induced cell death in both cell lines (Figs. 5C and D). However, IGF-I did not inhibit selenite-induced cell death, but decreased cell death induced by the combination of selenite and genistein to levels of selenite alone, suggesting that IGF-I only inhibited effects of genistein. Like the effect on cell viability, IGF-I pretreatment suppressed G2/M cell cycle arrest and apoptosis induced by genistein alone or combined with selenite in both cell lines (Figs. 5E–5H). However, IGF-I did not inhibit apoptosis by selenite in LNCaP cells (Fig. 5G). The results suggest that IGF-I may protect cells from genistein-induced death, G2/M cell cycle arrest, and apoptosis by upregulation of total AKT and phosphorylated AKT (Ser 473), whereas selenite-induced cell death and apoptosis are independent of the AKT signaling pathway.
FIG. 5.
Inhibition of genistein-induced cell cycle arrest and apoptosis by insulin-like growth factor-I (IGF-I). A and B: Western blot analysis of effects of IGF-I on expression of total AKT and p-AKT (Ser 473) in LNCaP and PC3 cells. Cells were treated with 20 nM IGF-I for 24 h followed by treatment with selenite, genistein or in combination for another 24 h. Fifty µg of protein were loaded for the assay. C and D: MTT assay of cell viability of LNCaP and PC3 cells treated with selenite, genistein, or in combination for 5 days after pretreatment with 20 nM IGF-I for 24 h. E and F: Flow cytometric analysis of the G2/M population of LNCaP cells and PC3 cells treated with selenite, genistein, or in combination for 24 h after pretreatment with IGF-I for 24 h. G and H: Flow cytometric analysis of apoptosis in LNCaP cells and PC3 cells treated with selenite, genistein, or in combination for 48 h after pretreatment with IGF-I for 24 h. The data were obtained from 3 independent experiments and the results shown are mean ± SD. Group 1, without IGF-I pretreatment. Group 2, with IGF-I pretreatment. Means with different letters above bars indicate significant differences (P < 0.05) in each panel.
DISCUSSION
Our study documents that selenite and genistein synergistically induced cell death, G2/M cell cycle arrest, and apoptosis in two human prostate cancer cell lines. Selenite was more effective in wild-type p53-expressing LNCaP cells than p53-null PC3 cells, whereas genistein at the doses above IC50 was almost equally effective in both cell lines. In addition, selenite induced apoptosis only, whereas genistein induced apoptosis and G2/M cell cycle arrest. Our study also showed that genistein inhibited AKT phosphorylation. Downregulation of AKT masked the effects of genistein, and upregulation of AKT by IGF-I antagonized genistein. Unlike genistein, selenite at the tested doses did not modify AKT, and changes in the AKT status by siRNA transfection or IGF-I pretreatment in cells did not alter the effects of selenite. Combined selenite and genistein resulted in synergistic or additive effects on cell death, G2/M cell cycle arrest, and apoptosis in both prostate cancer cell lines.
AKT plays a role in regulation of cell growth and survival by modification of some proteins essential for cell cycle regulation and apoptosis through phosphorylation modifications (31). Studies have showed that constitutive activation of AKT in some cancer cells overcame cell-growth arrest in the G2/M phase and apoptosis induced by certain anticancer agents (32,33). In contrast, inactivation of AKT inhibited cell proliferation by arresting the cells in the G2/M phase (34). It has been shown that genistein regulates cell signaling genes that are critical for cell proliferation, apoptosis, oncogenesis, and transcriptional regulation (35). Studies have demonstrated that genistein inhibited cancer growth and induced apoptosis through inhibition of the AKT signaling pathway (36,37). In our study, we found that genistein treatment induced G2/M cell cycle arrest and apoptosis in both LNCaP and PC3 cells. Cells treated with genistein showed decreased levels of phosphorylated AKT. These results suggest that the cellular effects of genistein observed in our study are likely through inhibition of AKT phosphorylation. If this is true, downregulation of AKT should result in similar cellular effects to genistein treatment. Indeed, our study showed that downregulation of AKT using siRNA transfection resulted in G2/M cell cycle arrest and apoptosis, results similar to those observed following genistein treatment. Additionally, downregulation of AKT masked the effects of genistein. One possibility is that downregulation of AKT by siRNA suppressed the signaling pathway for genistein action. Another possibility is that AKT-mediated cellular effects had already reached maximum by AKT siRNA transfection, and therefore no further cellular effects could be observed in cells treated with genistein. On the other hand, cells treated with IGF-I, a growth factor that has been shown to promote cancer cell growth and inhibit apoptosis (38), increased protein levels of total and phosphorylated AKT and inhibited the cellular effects of genistein. This is most likely due to upregulation of AKT by IGF-I with resultant suppression of genistein effects. These results suggest that inhibition of AKT is an underlying mechanism by which genistein induces G2/M cell cycle arrest and apoptosis in prostate cancer cells.
Unlike genistein, selenite treatment did not change levels of AKT or its phosphorylation status. Wu et al. (39) reported that methylseleninic acid (MSA) downregulated phosphorylated AKT by inhibition of the activity of phosphatidylinositol 3-kinase (PI3K) in PC3 cells (39). This discrepancy may be due to the different chemical forms of selenium used in the two studies. It has been reported that cellular effects of these two selenium compounds are different (40). Our study shows that alterations of AKT by siRNA transfection or IGF-I pretreatment did not change the cellular effects of selenite. Additionally, selenite treatment induced apoptosis only, and wild-type p53-expressing LNCaP cells were more sensitive to selenite than p53-null PC3 cells. These results indicate that the cellular effects of selenite and genistein are different. Genistein acts at least partially via the AKT signaling pathway, whereas selenite acts via p53 and other signaling pathways (12–15). Zu et al. (41) demonstrated that a combination of MSA and D-α-tocopheryl succinate (VES) synergistically induced apoptosis in PC3 cells but did not alter the distribution of cell cycle phases. MSA induced apoptosis via mitochondrial-independent pathways by activation of caspases 8 and 12, whereas VES caused apoptosis through the mitochondrial-dependent pathway by activation of caspase 9. This synergistic effect required 5 µM MSA and 20 µM VES. Similar to the study by Zu et al. (41), our study shows that the combination of selenite and genistein had a greater effect on induction of apoptosis than single agents in both androgen-sensitive LNCaP cells and androgen-insensitive PC3 cells. However, our study also demonstrates that the combination required 1 to 1.5 µM selenite and 5 to 10 µM genistein, dosages that were lower compared to the combination of SMA and VES. In contrast, our study demonstrates that the combination of selenite and genistein also enhanced cell cycle arrest. In addition to combinations of selenium with other chemopreventive agents, recent studies have shown that selenium compounds also enhanced the effects of radiation and chemotherapeutic agents on cancer cells. Results from ours and others suggest that Se may have the potential to enhance the efficacy of other chemopreventive or chemotherapeutic agents (42–46).
Our previous studies demonstrated that LNCaP cells were more sensitive than PC3 cells to selenite, and induction of apoptosis by selenite or combined selenomethionine and methioninase in LNCaP cells was associated with induction of p53 phosphorylation and elevation of total p53 (14,15). Down-regulation of p53 by siRNA transfection decreased the sensitivity of LNCaP cells to selenite and selenomethionine. Conversely, reexpression of wild-type p53 increased the sensitivity of PC3 cells to these two selenium compounds. These studies have demonstrated that the apoptotic effect of selenium was mediated by superoxide via the p53-dependent mitochondrial pathway. Similar results have also been observed by other investigators (16,47). Selenium-induced and p53-mediated G2/M cell cycle arrest and apoptosis were also observed in colon cancer cells (48). Although genistein treatment induced p53 phosphorylation and upregulated p53, its cellular effects were almost equal in both p53-null PC3 cells and wild-type p53 expressing LNCaP cells. Restoration of wild-type p53 in PC3 cells by transduction of adenoviral p53 cDNA constructs or downregulation of p53 in LNCaP cells by transfection of p53 siRNA did not change the cellular effects of genistein (data not shown). These results indicate that the genistein actions are p53 independent.
In addition to AKT and p53, p21waf1 and Bax may also be involved in the effects of genistein and selenite. Our study shows that p21waf1 and Bax were upregulated by genistein or selenite in both p53-expressing LNCaP and p53-null PC3 cells. This indicates that p21waf1 and Bax can be regulated via p53-dependent and -independent pathways, which may be the mechanism for cell cycle arrest and apoptosis induced by combined genistein and selenite. Other mechanisms may also be involved in the actions of selenite and genistein in prostate cancer cells. Studies have shown that selenium downregulated androgen receptor in prostate cancer cells, resulting in cell growth inhibition (49,50). Genistein regulated the androgen receptor in LNCaP cells (51). Further studies are needed to clarify whether androgen receptor or other signaling pathways also participate in the cellular effects of combined selenite and genistein.
Clinical cancer therapies usually utilize a combination of chemotherapeutic agents to gain better anticancer effects and reduce side effects from each agent. A combination of two or more agents for cancer chemoprevention have also been used in clinical trials (9,52). Here we demonstrate that a combination of selenite and genistein required lower doses of each agent to achieve better effects on cell cycle arrest and apoptosis than single agents in prostate cancer cells. This approach may avoid toxic side effects of each agent used in clinical trials in humans. Our results indicate that selenite and genistein induce G2/M cell cycle arrest and apoptosis via different molecular mechanisms. Therefore, combined use of selenium and genistein may be an effective strategy for prostate cancer chemoprevention. Studies to test the combined use of selenium and genistein in animals or humans are warranted since they have been used individually and shown to be promising chemopreventive agents against prostate cancer in some clinical trials.
ACKNOWLEDGMENTS
This work was supported by NIH Grants CA114281 and CA73612, and the Office of Research and Development, Biomedical Laboratory Research and Development Service, Department of Veterans Affairs.
Footnotes
Full terms and conditions of use: http://www.informaworld.com/terms-and-conditions-of-access.pdf
This article may be used for research, teaching and private study purposes. Any substantial or systematic reproduction, re-distribution, re-selling, loan or sub-licensing, systematic supply or distribution in any form to anyone is expressly forbidden.
Publisher's Disclaimer: The publisher does not give any warranty express or implied or make any representation that the contents will be complete or accurate or up to date. The accuracy of any instructions, formulae and drug doses should be independently verified with primary sources. The publisher shall not be liable for any loss, actions, claims, proceedings, demand or costs or damages whatsoever or howsoever caused arising directly or indirectly in connection with or arising out of the use of this material.
Contributor Information
Rui Zhao, Department of Pathology and Laboratory Medicine, University of Wisconsin School of Medicine and Public Health, Madison, Wisconsin, USA.
Nong Xiang, Department of Pathology and Laboratory Medicine, University of Wisconsin School of Medicine and Public Health, Madison, Wisconsin, USA.
Fredrick E. Domann, Free Radical and Radiation Biology Graduate Program, University of Iowa, Iowa City, Iowa, USA
Weixiong Zhong, Department of Pathology and Laboratory Medicine, University of Wisconsin School of Medicine and Public Health, Madison, Wisconsin, and Pathology and Laboratory Medicine Service, William S. Middleton Memorial Veterans Hospital, Madison, Wisconsin, USA.
REFERENCES
- 1.Albano JD, Ward E, Jemal A, Anderson R, Cokkinides VE, et al. Cancer mortality in the United States by education level and race. J Natl Cancer Inst. 2007;99:1384–1394. doi: 10.1093/jnci/djm127. [DOI] [PubMed] [Google Scholar]
- 2.Ferlay J, Autier P, Boniol M, Heanue M, Colombet M, et al. Estimates of the cancer incidence and mortality in Europe in 2006. Ann Oncol. 2007;18:581–592. doi: 10.1093/annonc/mdl498. [DOI] [PubMed] [Google Scholar]
- 3.Thorpe JF, Jain S, Marczylo TH, Gescher AJ, Steward WP, et al. A review of phase III clinical trials of prostate cancer chemoprevention. Ann R Coll Surg Engl. 2007;89:207–211. doi: 10.1308/003588407X179125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Combs GF, Jr, Gray WP. Chemopreventive agents: selenium. Pharmacol Ther. 1998;79:179–192. doi: 10.1016/s0163-7258(98)00014-x. [DOI] [PubMed] [Google Scholar]
- 5.Schrauzer GN, White DA, Schneider CJ. Cancer mortality correlation studies—III: statistical associations with dietary selenium intakes. Bioinorg Chem. 1977;7:23–31. doi: 10.1016/s0006-3061(00)80126-x. [DOI] [PubMed] [Google Scholar]
- 6.Clark LC, Cantor KP, Allaway WH. Selenium in forage crops and cancer mortality in U.S. counties. Arch Environ Health. 1991;46:37–42. doi: 10.1080/00039896.1991.9937427. [DOI] [PubMed] [Google Scholar]
- 7.Sabichi AL, Lee JJ, Taylor RJ, Thompson IM, Miles BJ, et al. Selenium accumulation in prostate tissue during a randomized, controlled short-term trial of l-selenomethionine: a Southwest Oncology Group Study. Clin Cancer Res. 2006;12:2178–2184. doi: 10.1158/1078-0432.CCR-05-0937. [DOI] [PubMed] [Google Scholar]
- 8.Yoshizawa K, Willett WC, Morris SJ, Stampfer MJ, Spiegelman D, et al. Study of prediagnostic selenium level in toenails and the risk of advanced prostate cancer. J Natl Cancer Inst. 1998;90:1219–1224. doi: 10.1093/jnci/90.16.1219. [DOI] [PubMed] [Google Scholar]
- 9.Klein EA, Thompson IM, Lippman SM, Goodman PJ, Albanes D, et al. SELECT: the next prostate cancer prevention trial. Selenum and Vitamin E Cancer Prevention Trial. J Urol. 2001;166:1311–1315. doi: 10.1016/s0022-5347(05)65759-x. [DOI] [PubMed] [Google Scholar]
- 10.Combs GF., Jr Status of selenium in prostate cancer prevention. Br J Cancer. 2004;91:195–199. doi: 10.1038/sj.bjc.6601974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ganther HE. Selenium metabolism, selenoproteins, and mechanisms of cancer prevention: complexities with thioredoxin reductase. Carcinogenesis. 1999;20:1657–1666. doi: 10.1093/carcin/20.9.1657. [DOI] [PubMed] [Google Scholar]
- 12.Whanger PD. Selenium and its relationship to cancer: an update. Br J Nutr. 2004;91:11–28. doi: 10.1079/bjn20031015. [DOI] [PubMed] [Google Scholar]
- 13.Zhong W, Oberley TD. Redox-mediated effects of selenium on apoptosis and cell cycle in the LNCaP human prostate cancer cell line. Cancer Res. 2001;61:7071–7078. [PubMed] [Google Scholar]
- 14.Zhao R, Xiang N, Domann FE, Zhong W. Expression of p53 enhances selenite-induced superoxide production and apoptosis in human prostate cancer cells. Cancer Res. 2006;66:2296–2304. doi: 10.1158/0008-5472.CAN-05-2216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhao R, Domann FE, Zhong W. Apoptosis induced by selenomethionine and methioninase is superoxide mediated and p53 dependent in human prostate cancer cells. Mol Cancer Ther. 2006;5:3275–3284. doi: 10.1158/1535-7163.MCT-06-0400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jiang C, Hu H, Malewicz B, Wang Z, Lu J. Selenite-induced p53 Ser-15 phosphorylation and caspase-mediated apoptosis in LNCaP human prostate cancer cells. Mol Cancer Ther. 2004;3:877–884. [PubMed] [Google Scholar]
- 17.Zhou N, Xiao H, Li TK, Nur EKA, Liu LF. DNA damage-mediated apoptosis induced by selenium compounds. J Biol Chem. 2003;278:29532–29537. doi: 10.1074/jbc.M301877200. [DOI] [PubMed] [Google Scholar]
- 18.Plasterer MR, Bradshaw WS, Booth GM, Carter MW, Schuler RL, et al. Developmental toxicity of nine selected compounds following prenatal exposure in the mouse: naphthalene, p-nitrophenol, sodium selenite, dimethyl phthalate, ethylenethiourea, and four glycol ether derivatives. J Toxicol Environ Health. 1985;15:25–38. doi: 10.1080/15287398509530633. [DOI] [PubMed] [Google Scholar]
- 19.Lowe JF, Frazee LA. Update on prostate cancer chemoprevention. Pharmacotherapy. 2006;26:353–359. doi: 10.1592/phco.26.3.353. [DOI] [PubMed] [Google Scholar]
- 20.Adlercreutz CH, Goldin BR, Gorbach SL, Hockerstedt KA, Watanabe S, et al. Soybean phytoestrogen intake and cancer risk. J Nutr. 1995;125 doi: 10.1093/jn/125.3_Suppl.757S. 757S–770S. [DOI] [PubMed] [Google Scholar]
- 21.Naik HR, Lehr JE, Pienta KJ. An in vitro and in vivo study of antitumor effects of genistein on hormone refractory prostate cancer. Anticancer Res. 1994;14:2617–2619. [PubMed] [Google Scholar]
- 22.Mentor-Marcel R, Lamartiniere CA, Eltoum IE, Greenberg NM, Elgavish A. Genistein in the diet reduces the incidence of poorly differentiated prostatic adenocarcinoma in transgenic mice (TRAMP) Cancer Res. 2001;61:6777–6782. [PubMed] [Google Scholar]
- 23.Wang J, Eltoum IE, Lamartiniere CA. Dietary genistein suppresses chemically induced prostate cancer in Lobund-Wistar rats. Cancer Lett. 2002;186:11–18. doi: 10.1016/s0304-3835(01)00811-4. [DOI] [PubMed] [Google Scholar]
- 24.Alhasan SA, Pietrasczkiwicz H, Alonso MD, Ensley J, Sarkar FH. Genistein-induced cell cycle arrest and apoptosis in a head and neck squamous cell carcinoma cell line. Nutr Cancer. 1999;34:12–19. doi: 10.1207/S15327914NC340102. [DOI] [PubMed] [Google Scholar]
- 25.Fotsis T, Pepper M, Adlercreutz H, Fleischmann G, Hase T, et al. Genistein, a dietary-derived inhibitor of in vitro angiogenesis. Proc Natl Acad Sci USA. 1993;90:2690–2694. doi: 10.1073/pnas.90.7.2690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Luke C, Krott R, Luke M, Lebek J, Walter P, et al. Effects of protein tyrosine kinase inhibitor genistein on retinal function in superfused vertebrate retina. J Ocul Pharmacol Ther. 2001;17:151–158. doi: 10.1089/10807680151125474. [DOI] [PubMed] [Google Scholar]
- 27.Markovits J, Linassier C, Fosse P, Couprie J, Pierre J, et al. Inhibitory effects of the tyrosine kinase inhibitor genistein on mammalian DNA topoisomerase II. Cancer Res. 1989;49:5111–5117. [PubMed] [Google Scholar]
- 28.Brown NM, Wang J, Cotroneo MS, Zhao YX, Lamartiniere CA. Prepubertal genistein treatment modulates TGF-alpha, EGF and EGF-receptor mRNAs and proteins in the rat mammary gland. Mol Cell Endocrinol. 1998;144:149–165. doi: 10.1016/s0303-7207(98)00106-3. [DOI] [PubMed] [Google Scholar]
- 29.Faqi AS, Johnson WD, Morrissey RL, McCormick DL. Reproductive toxicity assessment of chronic dietary exposure to soy isoflavones in male rats. Reprod Toxicol. 2004;18:605–611. doi: 10.1016/j.reprotox.2004.02.013. [DOI] [PubMed] [Google Scholar]
- 30.McCarty MF. Targeting multiple signaling pathways as a strategy for managing prostate cancer: multifocal signal modulation therapy. Integr Cancer Ther. 2004;3:349–380. doi: 10.1177/1534735404270757. [DOI] [PubMed] [Google Scholar]
- 31.Du K, Tsichlis PN. Regulation of the Akt kinase by interacting proteins. Oncogene. 2005;24:7401–7409. doi: 10.1038/sj.onc.1209099. [DOI] [PubMed] [Google Scholar]
- 32.Holzbeierlein JM, McIntosh J, Thrasher JB. The role of soy phytoestrogens in prostate cancer. Curr Opin Urol. 2005;15:17–22. doi: 10.1097/00042307-200501000-00005. [DOI] [PubMed] [Google Scholar]
- 33.Li Y, Upadhyay S, Bhuiyan M, Sarkar FH. Induction of apoptosis in breast cancer cells MDA-MB-231 by genistein. Oncogene. 1999;18:3166–3172. doi: 10.1038/sj.onc.1202650. [DOI] [PubMed] [Google Scholar]
- 34.Mansour A, McCarthy B, Schwander SK, Chang V, Kotenko S, et al. Genistein induces G2 arrest in malignant B cells by decreasing IL-10 secretion. Cell Cycle. 2004;3:1597–1605. doi: 10.4161/cc.3.12.1293. [DOI] [PubMed] [Google Scholar]
- 35.Li Y, Sarkar FH. Gene expression profiles of genistein-treated PC3 prostate cancer cells. J Nutr. 2002;132:3623–3631. doi: 10.1093/jn/132.12.3623. [DOI] [PubMed] [Google Scholar]
- 36.Barnes S. Effect of genistein on in vitro and in vivo models of cancer. J Nutr. 1995;125 doi: 10.1093/jn/125.3_Suppl.777S. 777S–783S. [DOI] [PubMed] [Google Scholar]
- 37.Gong L, Li Y, Nedeljkovic-Kurepa A, Sarkar FH. Inactivation of NF-kappaB by genistein is mediated via Akt signaling pathway in breast cancer cells. Oncogene. 2003;22:4702–4709. doi: 10.1038/sj.onc.1206583. [DOI] [PubMed] [Google Scholar]
- 38.Smith GD, Gunnell D, Holly J. Cancer and insulin-like growth factor-I: a potential mechanism linking the environment with cancer risk. BMJ. 2000;321:847–848. doi: 10.1136/bmj.321.7265.847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wu Y, Zu K, Warren MA, Wallace PK, Ip C. Delineating the mechanism by which selenium deactivates Akt in prostate cancer cells. Mol Cancer Ther. 2006;5:246–252. doi: 10.1158/1535-7163.MCT-05-0376. [DOI] [PubMed] [Google Scholar]
- 40.Ip C, Hayes C, Budnick RM, Ganther HE. Chemical form of selenium, critical metabolites, and cancer prevention. Cancer Res. 1991;51:595–600. [PubMed] [Google Scholar]
- 41.Zu K, Ip C. Synergy between selenium and vitamin E in apoptosis induction is associated with activation of distinctive initiator caspases in human prostate cancer cells. Cancer Res. 2003;63:6988–6995. [PubMed] [Google Scholar]
- 42.Shin SH, Yoon MJ, Kim M, Kim JI, Lee SJ, et al. Enhanced lung cancer cell killing by the combination of selenium and ionizing radiation. Oncol Rep. 2007;17:209–216. [PubMed] [Google Scholar]
- 43.Dorr W. Effects of selenium on radiation responses of tumor cells and tissue. Strahlenther Onkol. 2006;182:693–695. doi: 10.1007/s00066-006-1595-8. [DOI] [PubMed] [Google Scholar]
- 44.Husbeck B, Peehl DM, Knox SJ. Redox modulation of human prostate carcinoma cells by selenite increases radiation-induced cell killing. Free Radic Biol Med. 2005;38:50–57. doi: 10.1016/j.freeradbiomed.2004.09.022. [DOI] [PubMed] [Google Scholar]
- 45.Hu H, Li GX, Wang L, Watts J, Combs GF, Jr, et al. Methylseleninic acid enhances taxane drug efficacy against human prostate cancer and down-regulates antiapoptotic proteins Bcl-XL and survivin. Clin Cancer Res. 2008;14:1150–1158. doi: 10.1158/1078-0432.CCR-07-4037. [DOI] [PubMed] [Google Scholar]
- 46.Li Z, Carrier L, Rowan BG. Methylseleninic acid synergizes with tamoxifen to induce caspase-mediated apoptosis in breast cancer cells. Mol Cancer Ther. 2008;7:3056–3063. doi: 10.1158/1535-7163.MCT-07-2142. [DOI] [PubMed] [Google Scholar]
- 47.Li GX, Hu H, Jiang C, Schuster T, Lu J. Differential involvement of reactive oxygen species in apoptosis induced by two classes of selenium compounds in human prostate cancer cells. Int J Cancer. 2007;120:2034–2043. doi: 10.1002/ijc.22480. [DOI] [PubMed] [Google Scholar]
- 48.Goel A, Fuerst F, Hotchkiss E, Boland CR. Selenomethionine induces p53 mediated cell cycle arrest and apoptosis in human colon cancer cells. Cancer Biol Ther. 2006;5:529–535. doi: 10.4161/cbt.5.5.2654. [DOI] [PubMed] [Google Scholar]
- 49.Husbeck B, Bhattacharyya RS, Feldman D, Knox SJ. Inhibition of androgen receptor signaling by selenite and methylseleninic acid in prostate cancer cells: two distinct mechanisms of action. Mol Cancer Ther. 2006;5:2078–2085. doi: 10.1158/1535-7163.MCT-06-0056. [DOI] [PubMed] [Google Scholar]
- 50.Dong Y, Lee SO, Zhang H, Marshall J, Gao AC, et al. Prostate specific antigen expression is down-regulated by selenium through disruption of androgen receptor signaling. Cancer Res. 2004;64:19–22. doi: 10.1158/0008-5472.can-03-2789. [DOI] [PubMed] [Google Scholar]
- 51.Bektic J, Berger AP, Pfeil K, Dobler G, Bartsch G, et al. Androgen receptor regulation by physiological concentrations of the isoflavonoid genistein in androgen-dependent LNCaP cells is mediated by estrogen receptor beta. Eur Urol. 2004;45:245–251. doi: 10.1016/j.eururo.2003.09.001. [DOI] [PubMed] [Google Scholar]
- 52.Gwyn K, Sinicrope FA. Chemoprevention of colorectal cancer. Am J Gastroenterol. 2002;97:13–21. doi: 10.1111/j.1572-0241.2002.05435.x. [DOI] [PubMed] [Google Scholar]