Abstract
Genes of the major histocompatibility complex (MHC) show the strongest genetic association with multiple sclerosis (MS) but the underlying mechanisms have remained unresolved.
Here, we asked whether the MS-associated MHC class II molecules, HLA-DRB1*1501, HLA-DRB5*0101, and HLA-DRB1*0401 contribute to autoimmune central nervous system (CNS) demyelination by promoting pathogenic T cell responses to human myelin basic protein (hMBP), using three transgenic (Tg) mouse lines expressing these MHC molecules.
Unexpectedly, profound T cell tolerance to the high-affinity MHC-binding hMBP82-100 epitope was observed in all Tg mouse lines. T cell tolerance to hMBP82-100 was abolished upon backcrossing the HLA-DR Tg mice to MBP-deficient mice. In contrast, T cell tolerance was incomplete for low-affinity MHC-binding hMBP epitopes. Furthermore, hMBP82-100-specific “type B” T cells escaped tolerance in HLA-DRB5*0101 Tg mice. Importantly, T cells specific for low-affinity MHC-binding hMBP epitopes and hMBP82-100-specific “type B” T cells were highly encephalitogenic. Collectively, the results show that MS-associated MHC class II molecules are highly efficient at inducing T cell tolerance to high-affinity MHC-binding epitope, whereas autoreactive T cells specific for the low-affinity MHC-binding epitopes and “type B” T cells can escape the induction of T cell tolerance and may promote MS.
Introduction
Multiple sclerosis (MS) is considered a T cell-mediated autoimmune disease of the central nervous system (CNS) that is triggered by an as of yet unknown environmental event in genetically susceptible individuals (1). Genetic studies indicate that multiple genes contribute to susceptibility to MS, among which the major histocompatibility complex (MHC; human leukocyte antigen (HLA) in humans) has shown the strongest association (2, 3). The HLA-DR2 haplotype consisting of the HLA-DRB1*1501 and DRB5*0101 alleles is most strongly associated with MS in Caucasians, and the HLA-DR4 haplotype (HLA-DRB1*0401) is found with higher prevalence in MS patients from different ethnic groups (4–6). The mechanism by which these HLA-DR alleles confer susceptibility to MS is unknown but several plausible hypotheses have been proposed (7): 1) these MHC molecules could promote autoimmune disease by preferentially presenting pathogenic peptides derived from autoantigens; 2) these molecules could be less efficient in inducing self tolerance to target antigens in MS due to presentation of a limited set of peptides; 3) non-MHC genes located within the MHC region may contribute to disease. Myelin basic protein (MBP) is a potential target antigen in MS because its injection into susceptible rodents and non-human primates induces a MS-like disease, termed experimental autoimmune encephalomyelitis (EAE) (8). Studies of autoimmune T cell responses against MBP in MS patients have identified immunodominant epitopes located in the regions human (h) MBP82-100 and hMBP111-129 in patients with the HLA-DR2 and HLA-DR4 haplotypes, respectively (9–12). Since these MBP regions are contained within Golli-MBP proteins expressed in the human thymus (13), it appears that MBP-reactive T cells, for unknown reasons, escape induction of central and/or peripheral tolerance.
Most of the information on T cell tolerance to MBP has been derived from studies in animal models, in particular in mice (14–16). In one such model, B10.PL mice (H-2u) develop EAE upon immunization with murine (m) MBPAc1-11 peptide (14, 15). By comparing the T cell response to MBP in MBP-expressing and MBP-deficient B10.PL mice, it was shown that the expression of endogenous MBP resulted in the elimination of T cells specific for the mMBP121-150 epitope from the peripheral T cell repertoire, but not of mMBPAc1-11-specific T cells. These two MBP epitopes were found to be distinct in their binding affinity for the I-Au restriction element: mMBPAc1-11 binds weakly to I-Au molecules, whereas mMBP121-150 binds with high affinity. Similar results were obtained by Targoni and colleagues with C3H mice (H-2k), in which elimination of mMBP79-87-specific T cells was conditional on the expression of endogenous MBP (16). It was concluded from these studies that T cells reactive to high-affinity MHC-binding MBP epitopes were efficiently eliminated from the T cell repertoire, whereas T cells specific for low-affinity MHC-binding peptides escaped induction of tolerance. Interestingly, the immunodominant hMBP82-100 epitope in HLA-DR2+ MS patients exhibits a high binding affinity for HLA-DRB1*1501 and HLA-DRB5*0101 molecules (10, 11). Thus, the human data regarding the immunodominance of high-affinity MHC-binding peptides apparently conflict with the data derived on T cell tolerance from animal studies, and the reason for this contradiction has remained unresolved.
In the hierarchy of antigenic epitopes within self-antigens, immunodominant T cell epitopes contrast with cryptic epitopes due to the fact that the latter are only immunogenic if injected as peptides, but that they cannot be recalled upon immunization with protein (17). The crypticity of some T cell epitopes may be attributed to multiple conceivable conformations of a single peptide bound to a specific MHC class II molecule. Along these lines, it has been demonstrated that peptides can be bound to MHC class II molecules in multiple conformations depending on the site of peptide loading within APCs and the presence of H2-DM during MHC-peptide complex formation (18). Intracellular processing of native protein results in loading of peptides on MHC class II molecules in late endosomes under the influence of H2-DM, which favors more stable MHC-peptide conformers (type A). In contrast, loading of exogenous peptides can take place in early endosomes where H2-DM is not present, thus allowing generation of less stable MHC-peptide conformers (type B), as well as type A conformers. “Type A” T cells that recognize type A conformers respond to both native protein and exogenous peptides, while “ type B” T cells recognizing type B (cryptic) conformers respond only to exogenous peptides. Because “type B” T cells are not activated by peptides processed from native protein, it has been hypothesized that they could escape tolerance induction, and could play a role in autoimmune diseases (17, 19, 20). The significance of “type B” T cells has not yet been demonstrated in human autoimmune diseases.
To resolve these issues and provide information on tolerance mechanisms in the context of human MHC class II molecules, we investigated the T cell response to MBP in transgenic (Tg) mice expressing the MS-associated HLA-DR molecules, HLA-DRB1*1501, HLA-DRB5*0101 and HLA-DRB1*0401 (21–24). Our results show that tolerance was induced to the hMBP82-100 epitope in all three Tg mouse lines expressing endogenous MBP. In contrast, T cells specific for the low-affinity MHC-binding hMBP111-129 epitope escaped tolerance in HLA-DRB1*0401 Tg mice. Furthermore, hMBP82-100-specific “type B” T cells were present in HLA-DRB5*0101 Tg mice. Importantly, hMBP111-29-specific T cells and “type B” T cells were highly encephalitogenic in adoptive transfer experiments, suggesting a role for these cells in the pathogenesis of human MS.
Materials and Methods
Mice
HLA-DRB1*1501, HLA-DRB5*0101, and HLA-DRB1*0401 Tg mice were described previously (21–24). Heterozygote C3Fe.SWV MBPshi/+J mice carrying the Shiverer mutation were purchased from the Jackson Laboratory (Bar Harbor, ME, 25). The HLA-DR Tg mice were bred onto the Shiverer background and F1 offspring heterozygous for the Shiverer mutation were intercrossed to obtain MBP-deficient HLA-DR Tg mice (MBP−/−). Peripheral blood mononuclear cells (PBMC) were stained with fluorescence-conjugated antibodies specific for HLA-DR molecules (L243) and murine MHC class II molecules (2G9, AF6-120.1, and 11-5.2) and analyzed by flow cytometry to confirm expression of HLA-DR and the lack of expression of murine MHC class II molecules (BD Bioscience, San Jose, CA). The MBP−/− mice were identified by whole body tremor, and the WT mice (MBP+/+) were discriminated from the heterozygote mice (MBP+/−) using PCR primers that flank the deletion site in the MBP gene in the Shiverer mutation (5’-CAGCCCTGATTTTGCTAAGG-3’; 5’-GCCTGCATGTATGAATGTGC-3’). Mice were maintained under specific pathogen-free conditions and all animal procedures were conducted according to guidelines of the Institutional Care and Use Committee (IACUC) at the University of Texas at San Antonio.
Antigens and cells
hMBP protein was prepared according to the method of Deibler (26). Overlapping 12-mer hMBP peptides spanning the full length of the MBP protein, each of which was shifted by one amino acid, were used for in vitro recall assays. The following MBP peptides were synthesized based on published sequence: hMBP64-78 (ARTAHYGSLPQKSHG), hMBP82-100 (DENPVVHFFKNIVTPRTPP), hMBP111-129 (LSRFSWGAEGQRPGFGYGG), hMBP138-151 (HKGFKGVDAQGTLS), mMBP60-75 (TRTTHYGSLPQKSQHG), mMBP108-126 (LSRFSWGAEGQKPGFGYGG), and mMBP135-149 (HKGFKGAYDAQGTLS). EBV-transformed human B cell line (BCL), HLA-DR4+ (HLA-DRB1*0401+) (27) and HLA-DR2+ (DRB1*1501+/DRB5*0101+) (MGAR, 24), were generously provided by Drs. Sonderstrup and Wucherpfennig, respectively.
Cytokine measurements by ELISPOT and computer-assisted ELISPOT image analysis
Cytokine ELISPOT assays were performed as described previously (28, 29). ELISPOT plates (Multiscreen IP, Millipore, Billerica, MA) were coated with 2 µg/ml IFN-γ-specific capture antibody (AN-18, eBioscience, San Diego, CA) diluted in PBS. The plates were blocked with 1% BSA in PBS for 1 hour at room temperature, and then washed four times with PBS. Cells were added with or without antigen, and incubated for 24 hours at 37°C. Subsequently, the cells were removed by washing 3X with PBS and 4X with PBS/Tween, and IFN-γ-specific biotinylated detection antibody (R4-6A2; 0.5 µg/ml, eBioscience) was added and incubated overnight. The plate-bound second antibody was then incubated with streptoavidin-alkaline phosphatase (Dako, Carpinteria, CA), and cytokine spots were visualized by BCIP/NBT phosphatase substrate (KPL, Gaithersburg, MD). Image analysis of ELISPOT assays was performed on a Series 2 Immunospot analyzer and software (Cellular Technology, Cleveland, OH) as described previously (28, 29). In brief, digitized images of individual well of the ELISPOT plates were analyzed for cytokine spots, based on the comparison of experimental (containing T cells and APC with antigen) and control wells (T cells and APC, no antigen). After separation of spots that were touched or partially overdeveloped, non-specific noise was gated out by applying spot size and circularity analysis as additional criteria. Spots that fell within the accepted criteria were highlighted and counted.
Antigen recall assay
Mice were injected subcutaneously with 100 µg of hMBP protein or MBP peptides in sterile PBS emulsified in Complete Freund adjuvant (CFA) containing 250 µg of heat-killed M. tuberculosis H37 Ra (DIFCO, Detroit, MI). 10 days later, popliteal and inguinal lymph nodes (LN) were removed, and single cell suspensions were adjusted to 5 × 106 cells /ml in HL-1 media (Bio Whittaker, Gaithersburg, MD). 5 × 105 cells per well were placed in ELISPOT plates with or without antigen, and their response was measured by IFN-γ ELISPOT assay.
Generation of antigen-specific T cell line (TCL)
Mice were immunized with 100 µg of MBP peptide in CFA. 10 days after immunization, the draining LN were removed, and single cell suspensions were prepared at 5 × 106 cells /ml in complete DMEM (DMEM supplemented with 10% FCS, 2 mM L-glutamine, non-essential amino acids, 10 mM HEPES, 5 × 10−5M 2-mercaptoethanol, 50 µg/ml gentamycin, 100 U/ml of penicillin and 100 µg/ml of streptomycin). 5 × 106 cells were stimulated with 10 µg/ml of the MBP peptide in 24-well plates and were fed with complete DMEM supplemented with 10 U/ml of recombinant human IL-2 (SIGMA, St. Louis, MO) every 3–4 days. On day 14, 500 cells from each T cell line (TCL) were incubated in ELISPOT plates with or without 10 µg/ml of the peptide in the presence of mitomycin C-treated Tg spleen cells (4 × 105 cells/well) expressing matched HLA-DR molecules, and were tested for antigen-specific IFN-γ-production. TCL that showed more than 3-fold increase of the frequency of IFN-γ-producing cells in response to the antigen with background activity less than 10 spots were regarded as antigen-specific, and were restimulated every 2 weeks for further expansion. The EBV-transformed human BCL (4 × 104 cells/well) were substituted for the mitomycin C-treated spleen cells in other experiments to determine whether MBP epitopes identified in the HLA-DR Tg mice were processed from hMBP protein and presented by human APC. For some TCL, T cell clones (TCC) were generated by limiting dilution method
Induction and assessment of EAE
For adoptive transfer experiments, TCL were activated with 1 µg/ml of hMBP peptide in the presence of mitomycin C-treated Tg spleen cells expressing matched HLA-DR molecules. 3 days later, T cells were collected and 1 × 107 blast cells were transferred intravenously into recipient mice (MBP+/+) that had been irradiated at 4 Gy. Mice were observed daily up to 30 days for development of EAE. The following scoring was used: 0, no disease; 1, limp tail; 2, hind-limb weakness; 3, hind-limb paralysis; 4, hind-limb paralysis and fore-limb paralysis or weakness; 5, moribund.
Results
T cell tolerance induced by endogenous MBP in Tg mice expressing MS-associated HLA-DR molecules
Conceivably, MS-associated human MHC class II molecules could promote autoimmune disease by enhancing the presentation of pathogenic myelin epitopes. To begin to address this issue, we investigated the T cell response to MBP in three HLA-DR Tg mouse lines expressing the MS-associated human MHC class II molecules, HLA-DRB1*1501, HLA-DRB5*0101, and HLA-DRB1*0401 in the absence of endogenous murine MHC class II molecules (21–24).
HLA-DR Tg mice were immunized with hMBP protein emulsified in CFA. Ten days later, single cell suspensions were prepared from draining LN and tested in vitro in IFN-γ ELISPOT assays with overlapping hMBP 12-mer peptides. The overlapping peptides spanned the full length of the hMBP protein including the region hMBP82-100, which has been reported to be immunodominant in MS patients expressing the HLA-DR2 haplotype (9–11).
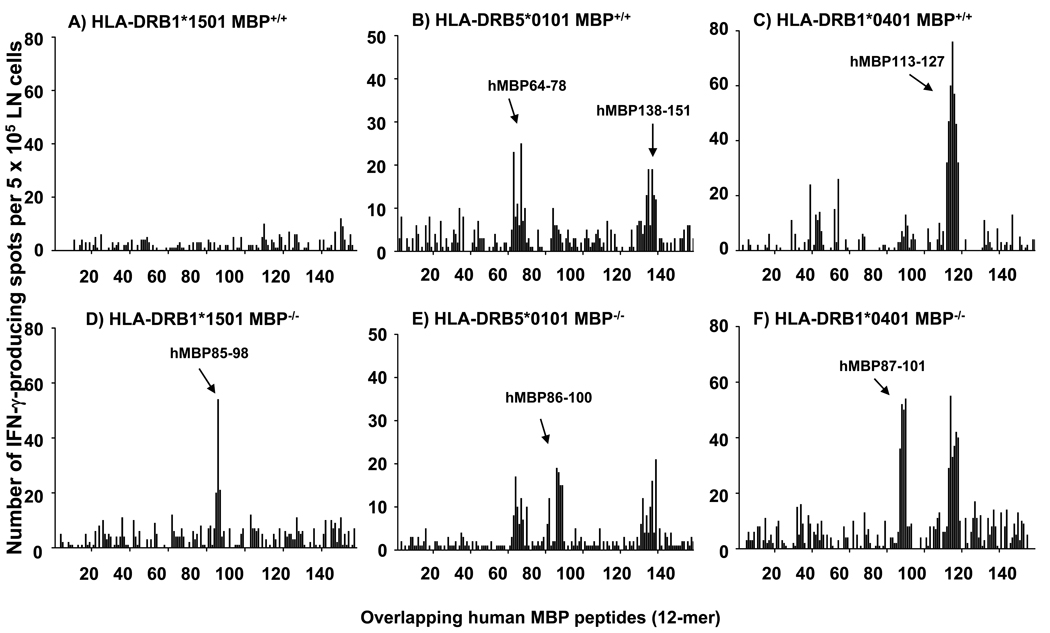
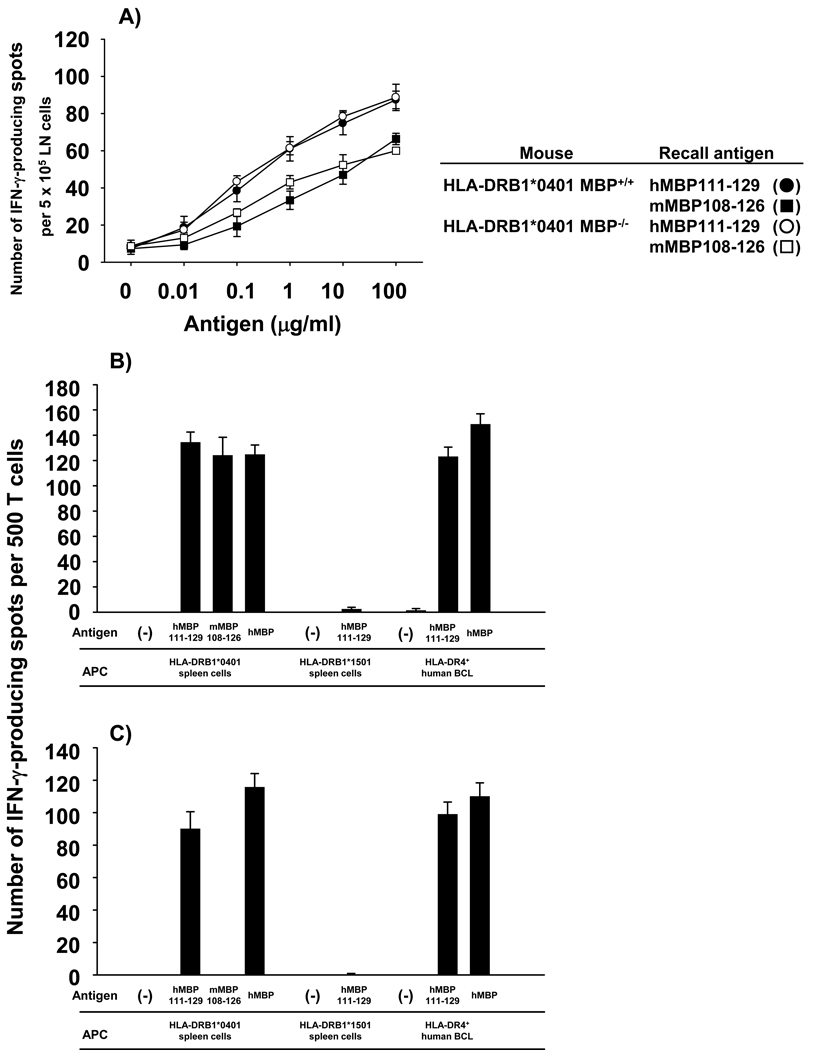
Unexpectedly, HLA-DRB1*1501 Tg mice failed to mount T cell responses to any of the overlapping hMBP peptides, including those of the region hMBP82-100, which had previously been suggested as immunodominant in HLA-DR2+ MS patients (Fig. 1 A). Similarly, HLA-DRB5*0101 and HLA-DRB1*0401 Tg mice did not mount significant T cell responses to this region (Fig. 1 B & C). However, as shown in Fig. 1 B, HLA-DRB5*0101 Tg mice mounted T cell responses to the regions hMBP64-78 (core epitope hMBP67-75, AHYGSLPQ) and hMBP138-151 (core epitope hMBP140-149, GFKGVDAQGT). Furthermore, HLA-DRB1*0401 Tg mice showed T cell responses to the region hMBP113-127 (core epitope hMBP116-124, WGAEGQRPG; Fig. 1 C)
Figure 1. Mapping of T cell epitopes in MBP-expressing and MBP-deficient HLA-DR Tg mice.
MBP-deficient HLA-DR Tg mice (MBP−/−) were generated by crossing HLA-DR Tg mice (MBP+/+) onto the Shiverer background as outlined in Materials and Methods. HLA-DR Tg MBP+/+ (A – C) and MBP−/− (D – F) mice, respectively, were injected with 100 µg of hMBP protein in CFA. 10 days later, the draining LN cells isolated from three mice per group were pooled and tested by IFN-γ ELISPOT assay using a series of 12-mer overlapping peptides that covered the entire human MBP sequences. Data are representative of five experiments.
Importantly, this region of the MBP molecule (hMBP111-129) has been reported by Muraro and colleagues as the immunodominant epitope in MS patients expressing HLA-DRB1*0401 molecules (12). Similar results were obtained by proliferation assay using 3H-thymidine incorporation (data not shown).
Collectively, the data showed that all three HLA-DR Tg mouse lines were unresponsive to the hMBP82-100 epitope previously implicated in MS patients, and that overall only few hMBP epitopes were immunogenic in these animals. This observation was surprising, particularly since hMBP82-100 binds with high affinity to HLA-DRB1*1501, HLA-DRB5*0101, and HLA-DRB1*0401 molecules and should therefore have been presented and induced T cell responses in the Tg mice (10, 11). The results cannot be explained by a defect in antigen presentation in the HLA-DR Tg mice, since previously we and others showed that T cell responses to self and foreign antigens are normal in these animals (KK and TGF, unpublished data, 21–23). Alternatively, the results suggested that central and/or peripheral T cell tolerance to hMBP82-100 was operational in the HLA-DR Tg mice.
To determine whether T cell tolerance was induced by endogenous MBP, we generated MBP-deficient HLA-DRB1*1501, HLA-DRB5*0101, and HLA-DRB1*0401 Tg mice by backcrossing to Shiverer mice, which are deficient for MBP due to a naturally occurring deletion in the last five exons of the MBP gene (25). Subsequently, the MBP-deficient HLA-DR Tg mice (MBP−/−) or their MBP-expressing littermates (MBP+/+) were immunized with hMBP protein and T cell responses to hMBP epitopes were detected by IFN-γ ELISPOT assay using overlapping hMBP peptides.
The results show that in the absence of endogenous MBP robust T cell responses to the region hMBP82-100 were present in all three HLA-DR Tg mouse lines (Fig. 1 D, E, F), but not in the presence of endogenous MBP (Fig. 1 A, B, C). T cell responses to additional MBP epitopes besides hMBP82-100 did not emerge in MBP−/− mice as compared with MBP+/+ mice (Fig. 1).
Collectively, the data show that MS-associated human MHC class II molecules are efficient in inducing T cell unresponsiveness to the hMBP82-100 region in the presence of endogenous MBP.
hMBP82-100-specific “type B” T cells escape tolerance induction in HLA-DRB5*0101 Tg mice
To determine whether hMBP82-100-specific “type B” T cells escaped tolerance induced by MS-associated human MHC class II molecules, we injected HLA-DRB1*1501, HLA-DRB5*0101, and HLA-DRB1*0401 MBP+/+ or MBP−/− mice with hMBP82-100 peptide, and tested recall responses to the peptide after 10 days in draining LN cells by IFN-γ ELISPOT assay (Fig. 2).
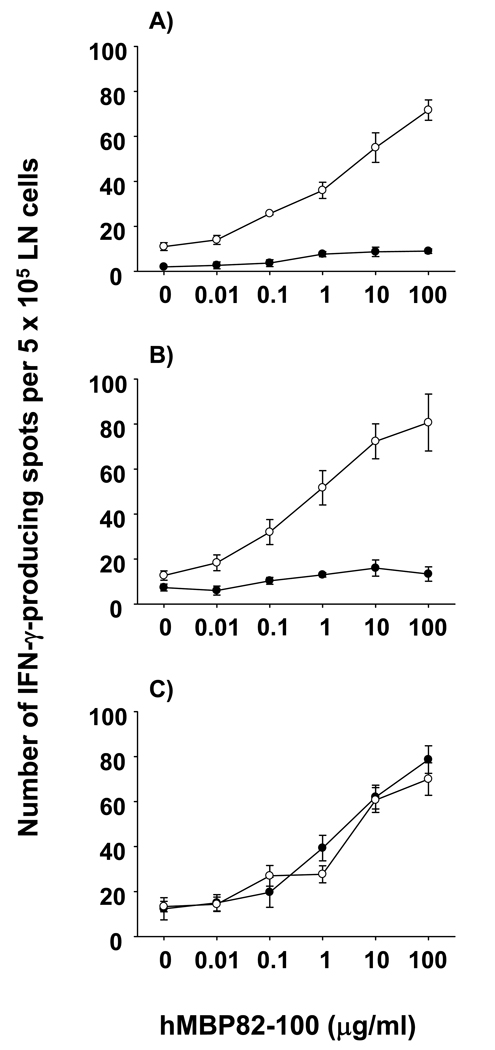
Figure 2. T cell responses to hMBP82-100 peptide in HLA-DR Tg mice in the presence or absence of endogenous MBP.
HLA-DRB1*1501, HLA-DRB1*0401, and HLA-DRB5*0101 MBP+/+ (●) and MBP−/− (○) mice were immunized with 100 µg of hMBP82-100 peptide, and tested on day 10 for the frequency of IFN-γ-producing cells at various concentrations of the peptide (A – C). Data shown are mean ± SD of triplicate wells from individual mice. Data are representative of four to six experiments.
The results show that T cells from hMBP82-100 immunized HLA-DRB1*1501 and DRB1*0401 Tg mice produced negligible IFN-γ upon in vitro recall with this peptide (Fig. 2 A & B, closed symbols), consistent with our earlier results (Fig. 1 A & C). Further confirming our previous findings, vigorous T cell responses were induced by the hMBP82-100 peptide in HLA-DRB1*1501 and HLA-DRB1*0401 MBP−/− mice (Fig. 2 A & B, open symbols). In strong contrast, hMBP82-100-immunized HLA-DRB5*0101 MBP+/+ mice showed vigorous IFN-γ production upon in vitro recall with this peptide, comparable with HLA-DRB5*0101 MBP−/− mice (Fig. 2 C). Dose titration curves showed that the activation threshold of hMBP82-100-reactive T cells obtained from HLA-DRB5*0101 Tg MBP+/+ and MBP−/− mice was comparable (Fig. 2 C). Similar results were obtained using 3H-thymidine-based proliferation assays as the experimental readout (data not shown). Therefore, HLA-DRB5*0101 Tg mice mounted hMBP82-100-specific T cell responses upon immunization with the peptide, but not with the hMBP protein (Fig. 2 C versus Fig. 1 B), consistent with the induction of “type B” T cells.
To confirm whether the hMBP82-100-specific cells were “type B” T cells, we generated TCL from hMBP82-100 peptide-immunized HLA-DRB1*1501, HLA-DRB5*0101, and HLA-DRB1*0401 Tg mice and tested the cells with different APC for their MHC restriction and response to the hMBP82-100 peptide and hMBP protein.
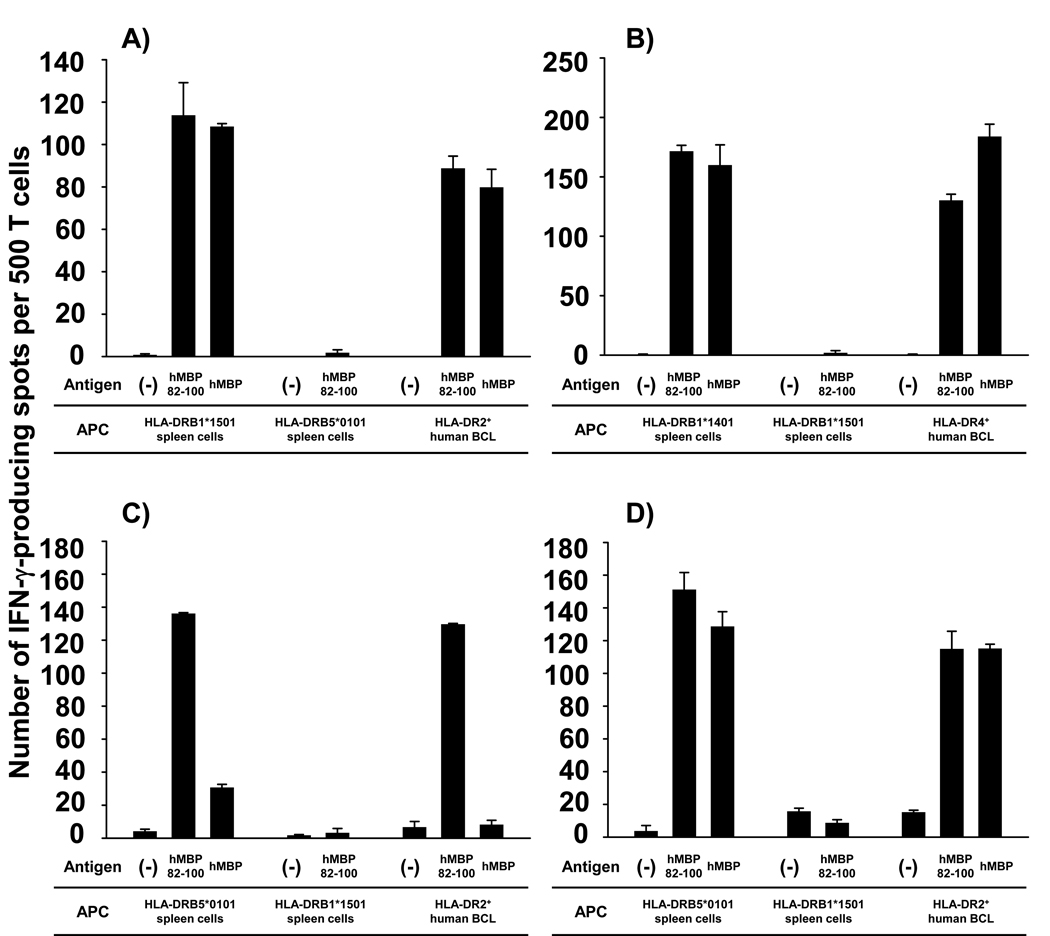
As expected, hMBP82-100-specific TCL could only be generated from HLA-DRB1*1501 and HLA-DRB1*0401 MBP−/− mice (Fig. 3 A & B), but not from MBP+/+ mice (data not shown). In contrast, hMBP82-100-specific TCL could readily be generated from both MBP+/+ and MBP−/− HLA-DRB5*0101 Tg mice (Fig. 3 C & D). hMBP82-100-specific TCL generated from HLA-DRB1*1501 and HLA-DRB1*0401 MBP−/− mice responded to HLA-matched Tg spleen cells pulsed with hMBP 82-100 peptide, as well as hMBP protein (Fig. 3 A & B). T cell responses were abolished with HLA-mismatched Tg spleen cells, and were restored when HLA-matched human BCL were used as APC. Thus, in the context of HLA-DRB1*1501 and HLA-DRB1*0401 molecules, only “type A” TCL were generated, and only from MBP−/− mice. In strong contrast, four out of four hMBP82-100-specific TCL generated from HLA-DRB5*0101 MBP+/+ mice immunized with hMBP82-100 peptide were “type B” T cells (Fig. 3 C).
Figure 3. HLA-DRB5*0101-restricted “Type B” T cells respond to hMBP82-100 peptide but not hMBP protein.
hMBP82-100-specific TCL were generated from HLA-DRB1*1501 MBP−/− (A), HLA-DRB1*0401 MBP−/− (B), and HLA-DRB5*0101 MBP+/+ or MBP−/− mice (C and D) immunized with 100 µg of hMBP82-100 peptide. Antigen specificity and HLA-DR-restriction were determined by IFN-γ cytokine ELISPOT assay of the TCL incubated with 10 µg/ml of hMBP82-100 peptide or 50 µg/ml of hMBP protein in the presence of mitomycin C-treated HLA-matched or HLA-mismatched Tg spleen cells, or HLA-matched human BCL. Similar results were obtained in 3 independent experiments.
These TCL mounted vigorous cytokine production to HLA-DRB5*0101 Tg spleen cells pulsed with hMBP82-100 peptide, but not to spleen cells pulsed with hMBP protein (Fig. 3 C). The TCL responded similarly to the peptide and protein presented by a human HLA-DR2+ BCL (MGAR, HLA-DRB1*1501+/DRB5*0101+), indicating that the hMBP82-100 peptide was also presented by human APCs in the “type B” conformation. Of the five hMBP82-100-specific TCL generated from HLA-DRB5*0101 MBP−/− mice immunized with hMBP82-100 peptide, two TCL were “type B” T cells and three TCL were “type A” T cells (Fig. 3 D).
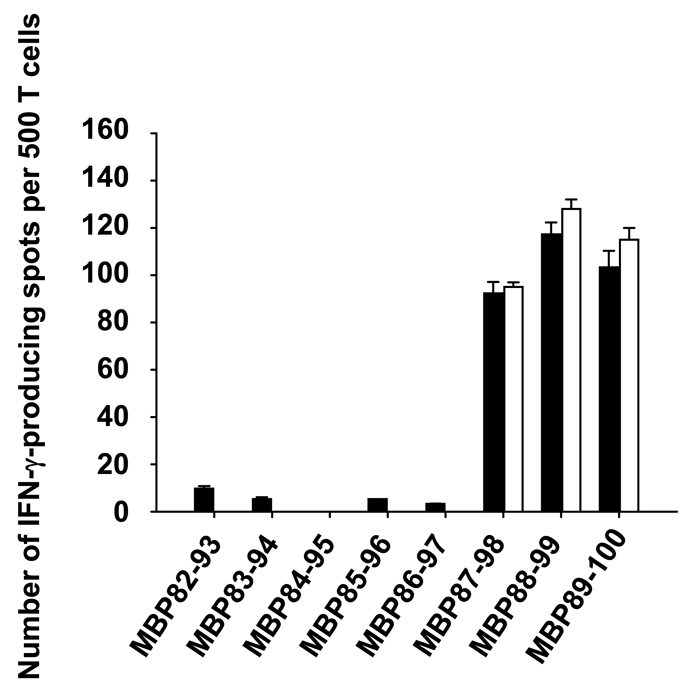
Finally, we asked whether HLA-DRB5*0101-restricted hMBP82-100-specific “type A” or “type B” T cells recognized the same hMBP82-100 core epitope. To address this issue, core epitopes for representative “type A” and “type B” TCL were determined using overlapping hMBP 12-mer peptides spanning the region hMBP82-100. As shown in Fig. 4, both “type A” and “type B” T cells responded to peptides hMBP87-98, hMBP88-99 and hMBP89-100, indicating that the “type A” and “type B” T cells recognize the same core epitope, hMBP89-98 (FFKNIVTPRT).
Figure 4. HLA-DRB5*0101-restricted hMBP82-100-specific “type A” and “type B” TCL recognize the same core epitopes.
500 T cells of HLA-DRB5*0101-restricted, hMBP82-100-specific “type A” (■) or “type B” (□) TCL were incubated with 10 µM of overlapping hMBP peptides (12-mer) spanning the hMBP82-100 region in the presence of mitomycin C-treated spleen cells prepared from HLA-DRB5*0101 Tg mice. T cell response was measured as the frequency of IFN-γ producing cells by IFN-γ ELISPOT assay. Data shown are mean ± SD of triplicate wells from representative of three “type A” and six “type B” TCL. The medium background in the experiment was less than 10 spots.
Collectively, the results show that HLA-DRB1*1501 and HLA-DRB1*0401 molecules were highly efficient at inducing T cell tolerance to the high-affinity MHC-binding hMBP82-100 peptide, whereas “type B” T cells escaped tolerance induction in HLA-DRB5*0101 Tg mice.
Low-affinity MHC-binding MBP peptides induce T cell responses in HLA-DR Tg mice
Recently, evidence has emerged suggesting an important role for T cell responses directed against low-affinity MHC-binding myelin peptides in human MS patients (30). To begin to address whether the T cell responses observed in the HLA-DR Tg MBP+/+ mice were directed against low-affinity MHC-binding peptides, we investigated the binding affinity of the immunodominant peptides in this model.
Binding affinities of hMBP peptides for MS-associated HLA-DR molecules have been published (10–12). Accordingly, hMBP82-100 peptide binds with high affinity to HLA-DRB1*1501, HLA-DRB5*0101, and HLA-DRB1*0401 molecules with an IC50 of 5 nM, 156 nM, and 7 nM, respectively, and we have obtained similar results (data not shown). Thus, the hMBP82-100 epitope is likely to be efficiently presented by these MHC molecules for the induction of central and/or peripheral tolerance, consistent with our data (Fig. 1). In contrast, hMBP64-78 and hMBP111-129, the peptides that were immunodominant in the HLA-DRB5*0101 and HLA-DRB1*0401 Tg mice, respectively, bind weakly to these MHC molecules. Specifically, the binding affinity of hMBP64-78 for HLA-DRB5*0101 molecules is low (IC50 > 20,000 nM; 10, 11). Similarly, hMBP111-129 binds at least 300-fold weaker to HLA-DRB1*0401 molecules (IC50 > 100,000 nM) as compared with the high affinity binding hMBP82-100 peptide (10, 12). The low binding affinity of these peptides for MHC class II molecules suggested that they may be poor at inducing central and/or peripheral T cell tolerance, consistent with previous reports for other low-affinity MHC-binding MBP peptides (14). In contrast, studies on the binding of the hMBP138-151 peptide to HLA-DRB5*0101 molecules suggested that it binds with medium affinity (IC50 1,300 nM), as compared with the higher affinity binding of MBP82-100 with an IC50 of 156 nM (10, 11). Therefore, MBP138-151 could have failed to induce T cell tolerance because of its lower binding affinity for HLA-DRB1*0101 as compared with MBP82-100. However, it was conceivable that species-related differences in amino acid sequence between the human MBP protein used for the immunizations and the endogenous murine MBP inducing tolerance could have affected T cell tolerance for the immunogenic MBP epitopes in the Tg mice. Consistent with this view, the amino acid sequences of hMBP64-78, hMBP111-129, and hMBP138-151 differed in one or more amino acids from those of the corresponding mMBP60-75, mMBP108-126, and mMBP135-149 (Table I). The amino acid sequence of hMBP82-100, however, was identical to the corresponding region MBP79-97 of mMBP.
Table 1.
Comparison of amino acid sequences of human MBP epitopes identified in HLA-DR Tg mice with corresponding murine MBP epitopes.
| Epitop | Sequence |
|---|---|
| Human MBP64-78 | ARTAHYGSLPQKSHG |
| Murine MBP60-75 | TRTTHYGSLPQKSQHG |
| Human MBP 82-100 | DENPVVHFFKNIVTPRTPP |
| Murine MBP 79-97 | DENPVVHFFKNIVTPRTPP |
| Human MBP111-129 | LSRFSWGAEGQRPGFGYGG |
| Murine MBP108-126 | LSRFSWGAEGQKPGFGYGG |
| Human MBP138-151 | HKGFKGVDAQGTLS |
| Murine MBP135-149 | HKGFKGAVDAQGTLS |
The core epitope of human MBP epitopes underlined, and the changes (substitution or insertion) in the murine MBP epitopes in bold.
To experimentally address this issue, HLA-DRB5*0101 and HLA-DRB1*0401 Tg mice were immunized with hMBP64-78, hMBP138-151, and hMBP111-129 peptides, respectively, and draining LN cells were tested 10 days later by IFN-γ ELISPOT assay with the corresponding human and murine MBP peptides.
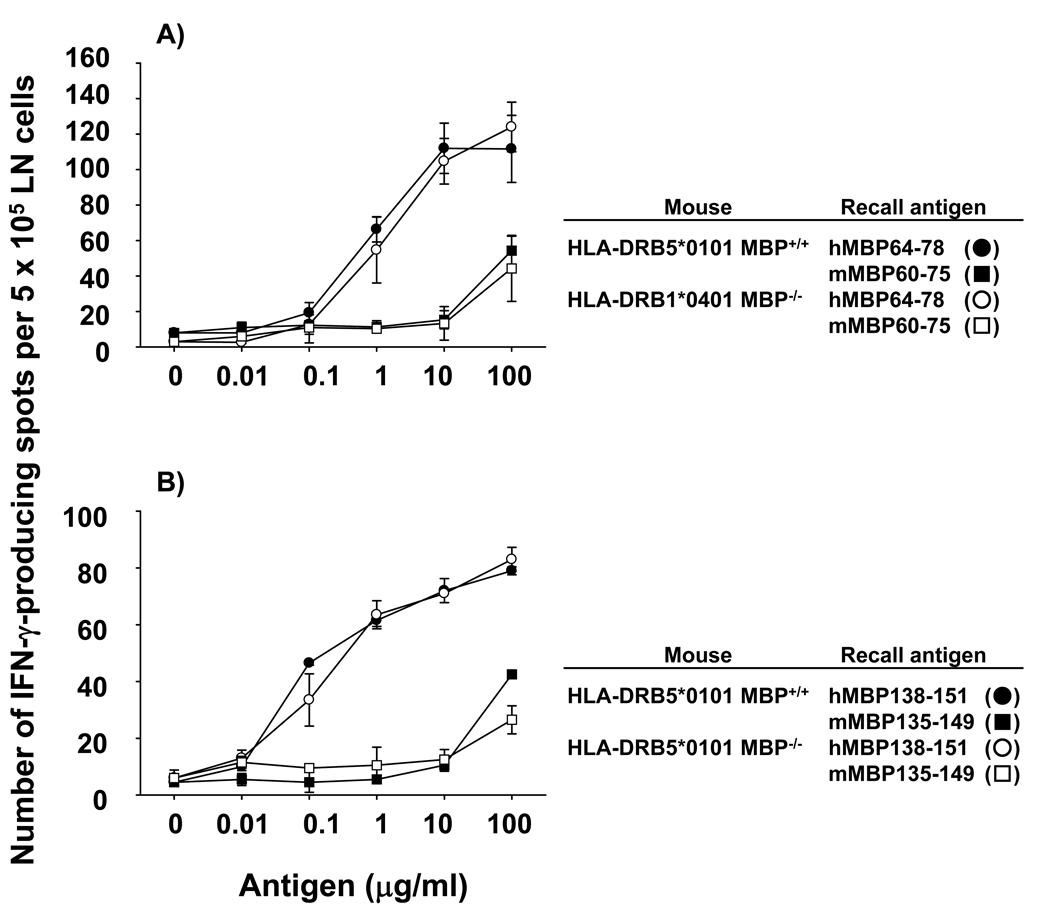
LN cells from hMBP111-129-immunized HLA-DRB1*0401 Tg mice mounted vigorous recall responses to both the human and murine peptides (Fig. 5 A). The results were similar whether the Tg mice were MBP+/+ or MBP−/− and showed that T cells specific for the endogenous mMBP108-126 sequence also escaped tolerance in HLA-DRB1*0401 Tg mice. Of 10 hMBP111-129-specific TCC generated, approximately half were cross-reactive and responded to hMBP111-129 peptide and hMBP protein presented by HLA-DRB1*0401 Tg spleen cells, and showed comparable responses to the mMBP108-126 peptide (Fig. 5 B). The other TCC responded only to the hMBP111-129 peptide and hMBP protein (Fig. 5 C). This observation is consistent with the prediction from the HLA-DRB1*0401 peptide binding motif that the amino acid difference between hMBP111-129 and mMBP108-126 is situated within the TCR contact residues (31, 32). In contrast, LN cells from hMBP64-78-immunized HLA-DRB5*0101 Tg mice mounted vigorous IFN-γ production in response to the human epitope, but T cell responses to the corresponding mMBP60-75 peptide were negligible and only detectable at the highest concentration of the peptide (Fig. 6 A). Similarly, hMBP138-151-specific T cells did not mount substantial responses to the mMBP135-149 peptide, except at the highest concentration (Fig. 6 B). Therefore, the animals were tolerant to the mMBP60-75 and mMBP135-149 peptides, but not the human peptides, suggesting that the lack of tolerance to the hMBP64-78 and hMBP138-151 peptide could have been due to species-specific differences of the peptides. Thus, tolerance mediated by HLA-DRB5*0101 molecules could be even more profound than what we observed in these studies.
Figure 5. Lack of T cell tolerance to endogenous mMBP108-126 in HLA-DRB1*0401 Tg mice.
Draining LN cells from HLA-DRB1*0401 MBP+/+ (closed symbols, ● and ■) and HLA-DRB1*0401 MBP−/− (open symbols, ○ and □) mice immunized with 100 µg of hMBP111-129 peptide were incubated with various concentrations of hMBP111-129 (● and ○) or mMBP108-126 (■ and □) peptides (A). Data shown are mean ± SD of triplicate wells from individual mice and are representative of five experiments. hMBP111-129-specific TCC were generated from HLA-DRB1*0401 MBP+/+ mice immunized with 100 µg of hMBP111-129 peptide. (B, C) Cross-recognition of mMBP108-126 peptide and HLA-DR restriction were tested by measuring IFN-γ production by 500 T cells from individual TCC incubated with 10 µg/ml of hMBP111-129 or mMBP108-126 peptides in the presence of mitomycin C-treated HLA-DRB1*0401 or HLA-DRB1*1501 Tg spleen cells, or HLA-DR4+ (HLA-DRB1*0401+) human BCL, in triplicate wells. Two types of hMBP111-129-specific TCC were identified: TCC crossreative to mMBP 108-126 peptide (B) and non-crossreactive TCC (C).
Figure 6. T cell tolerance to the endogenous murine but not human MBP epitopes in HLA-DRB5*0101 Tg mice.
Draining LN cells from HLA-DRB5*0101 MBP+/+ (closed symbols, ● and ■) and HLA-DRB5*0101 MBP−/− (open symbols, ○ and □) mice immunized with 100 µg of hMBP 64-78 peptide were incubated with various concentrations of hMBP64-78 (● and ○) or mMBP60-75 (■ and □) peptides (A). Data shown are mean ± SD of triplicate wells from individual mice and are representative of five experiments. (B) T cell response of hMBP 138-151 immunized HLA-DRB5*0101 MBP+/+ and MBP−/− mice to the corresponding mMBP135-149 peptide.
Taken together, our data show that hMBP111-129 failed to induce tolerance in HLA-DRB1*0401 Tg mice. We propose that the failure of hMBP111-129 to induce T cell tolerance is due to its low MHC-binding affinity. Importantly, this MBP peptide is immunodominant in MS patients expressing the HLA-DRB1*0401 molecule (12). Overall, these results provide further evidence for the role of low-affinity MHC-binding peptides in MS.
EAE induced by MBP-reactive “type B” T cells and T cells reactive to low-affinity MHC-binding MBP peptides
We asked whether HLA-DRB1*0401-restricted hMBP111-129-specific T cells and HLA-DRB5*0101-restricted hMBP82-100-specific “type B” T cells could induce EAE, since they escaped induction of tolerance in MBP+/+ mice.
Initial studies showed that HLA-DRB1*0401 and HLA-DRB5*0101 Tg mice immunized with hMBP111-129 peptide and hMBP82-100 peptide, respectively, for active EAE induction, did not developed obvious neurological symptoms. Therefore, hMBP111-129-specific TCL were generated and adoptively transferred 3 days after restimulation with peptide into irradiated HLA-DRB1*0401 Tg mice. The data show that hMBP111-129-specific TCL induced classical EAE characterized by ascending paralysis in nine out of eleven HLA-DRB1*0401 Tg mice (Table 2). Dysphagia and abnormal ataxic gait, which was previously reported by another group in HLA-DRB1*0401 Tg mice transferred with hMBP111-129-specific T cells expressing transgenic TCR derived from an HLA-DR4+ MS patient, were not observed in the diseased mice (33). Histological analysis of the brain and spinal cord of mice exhibiting EAE symptoms confirmed infiltration of mononuclear cells and polymorphonuclear cells in the perivascular space and meninges (data not shown).
Table 2.
EAE induced by adoptive transfer of hMBP-specific T cells generated in HLA-DR Tg mic
| Antigen specificity of TCL | Disease frequency | Mean onset day | Mean maximal score | Mortality |
|---|---|---|---|---|
| (diseased/total) | X ± s.d (range) | X ± s.d | ||
| hMBP82-100/ | 6/7 | 7.5 ± 0.6 | 2.7 ± 1.4 | 0/7 |
| HLA-DRB1*1501 | (range 7–8) | |||
| hMBP82-100/ | 9/9 | 4.6 ± 0.7 | 4.6 ± 0.7 | 7/9 |
| HLA-DRB5*0101 (type A) | (range 6–8) | |||
| hMBP82-100/ | 7/11 | 11.6 ± 2.8 | 3.4 ± 1.1 | 2/7 |
| HLA-DRB5*0101 (type B) | (range 9–16) | |||
| hMBP82-100/ | 8/8 | 7 ± 0.5 | 5 | 8/8 |
| HLA-DRB1*0401 | (range 6–8) | |||
| hMBP111-129/ | 9/11 | 8.8 ± 0.9 | 2.6 ± 1.0 | 1/9 |
| HLA-DRB1*0401 | (range 8–10) |
hMBP-specific TCL were stimulated with 1 µg/ml of hMBP peptide for three days, and 1.0 × 107 T cell blasts were adoptively transferred (i.v.) into irradiated (4 Gy) HLA-DR Tg mice (MBP+/+). Mice were observed daily for EAE symptoms for 30 days.
Importantly, HLA-DRB5*0101-restricted hMBP82-100-specific “type B” T cells transferred into irradiated HLA-DRB5*0101 Tg mice were highly encephalitogenic and induced typical ascending paralysis with a mean EAE score of 3.7.
hMBP 82-100-specific “type A” T cells, that were generated from MBP-deficient mice expressing either HLA-DRB1*1501, HLA-DRB5*0101, or HLA-DRB1*0401 molecules, also induced severe classical EAE (Table 2).
Collectively, the results show that HLA-DRB5*0101-restricted MBP-reactive “type B” T cells were highly encephalitogenic. Furthermore, T cells specific for low-affinity HLA-DR- binding peptides induced EAE. The data suggest that these T cells could play a role in the pathogenesis of disease in MS patients expressing these MHC class II molecules.
Discussion
Induction of tolerance to self antigens has been studied in various animal models and important information has been gained (14–16, 34). However, it has not been formally demonstrated whether the principles observed in conventional animal models can be universally applied to immune tolerance in the context of human MHC molecules as well. Recently, Tg mice have been developed that express human MHC class II molecules associated with organ-specific human autoimmune diseases (35–37). The data derived from these models suggest that murine autoreactive T cell responses restricted by Tg human MHC class II molecules faithfully recapitulate human T cell antigen recognition in the context of the respective human MHC class II molecules. Here, we advance this work to study tolerance induction to MBP in Tg mice expressing MS-related HLA-DR molecules.
The presented studies support several conclusions. First, the MS-associated human MHC class II molecules, HLA-DRB1*1501, HLA-DRB5*0101, and HLA-DRB1*0401, were highly efficient in inducing T cell tolerance to MBP, in particular to the high-affinity MHC-binding region hMBP82-100. This was unexpected since the hMBP82-100 epitope had previously been thought to be immunodominant in the context of the HLA-DR2 haplotype in MS patients (9–11). Overall, very few immunogenic MBP epitopes were identified in each HLA-DR Tg mouse line, arguing against the hypothesis that these MHC molecules contribute to autoimmunity by preferentially promoting T cell responses to autoantigens. Second, hMBP82-100-specific “type B” T cells escaped immune tolerance in HLA-DRB5*0101 Tg mice, but not in the context of the other MHC class II molecules. Third, T cells specific for the low-affinity MHC-binding hMBP111-129 epitope escaped tolerance in HLA-DRB1*0401 Tg mice. Importantly, hMBP82-100-specific “type B” T cells and hMBP111-129-specific T cells were highly encephalitogenic. Collectively, the results suggested that MS-associated MHC class II molecules were highly efficient in inducing T cell tolerance to myelin antigens, but that “type B” T cells and T cells specific for low-affinity MHC-binding epitopes could escape tolerance and could differentiate into potentially pathogenic T cells.
A critical role of the MHC-binding affinity of myelin peptides for induction of T cell tolerance was suggested previously in conventional mouse models (14–16). We observed a similar correlation between the binding affinity of MBP peptides for human HLA-DR molecules and the induction of T cell tolerance or the failure thereof. Specifically, the high-affinity MHC-binding hMBP82-100 peptide induced T cell tolerance, presumably central T cell tolerance. However, it is conceivable that this epitope can also induce peripheral tolerance (38). We are currently pursuing studies to further investigate the role of peripheral versus central tolerance in this model.
The high binding affinity of the hMBP82-100 epitope for HLA-DRB1*0401 molecules contrasted with the low binding affinity of the hMBP111-129 peptide and the lack of T cell tolerance to the latter. This was likely not due to differences between the human peptide used for immunization and the endogenous murine MBP sequence, since T cells specific for the mouse sequence were also not tolerized. hMBP111-129 and the corresponding mMBP108-126 epitope differ by one amino acid at position 122, where the arginine in the human MBP sequence is exchanged for lysine in the corresponding murine MBP region (position 119). The arginine at position 122 corresponds to position 7 of the peptide binding motif for the HLA-DRB1*0401 molecule and has been proposed to be a TCR-contact residue (31, 32). This amino acid does not affect binding of hMBP111-129 to the HLA-DRB1*0401 molecule, and the binding affinity of mMBP108-126 for HLA-DRB1*0401 molecules was similarly low to that of hMBP111-129 (TGF, unpublished data). Analysis of more than 10 hMBP111-129-specific TCC generated from HLA-DRB1*0401 MBP+/+ mice revealed that approximately half of the clones recognized mMBP108-126 as well as hMBP111-129. Thus, we conclude that hMBP111-129-specific T cells escaped induction of central and/or peripheral tolerance because of their low binding affinity for HLA-DRB1*0401 molecules, similarly to the results obtained in the I-Au/MBPAc1-11 model in B10.PL mice.
Importantly, Muraro and colleagues reported hMBP111-129 as the immunodominant T epitope in HLA-DR4+ MS patients, whereas responses to the hMBP81-99 epitope were very low (12). Thus, if low-affinity MHC-binding peptides play a greater role in promoting human autoimmune diseases as previously appreciated, design of antigen-specific therapies needs to be reevaluated. Altered peptide ligands based on high-affinity MHC-binding myelin peptides differ fundamentally from variant peptides based on low-affinity MHC-binding peptides, as suggested by the work of Anderton and colleagues (39, 40). A currently unresolved question is how low-affinity MHC-binding peptides may fail to induce thymic T cell tolerance, but can become targets of the autoimmune response by peripheral T cells.
Similarly to HLA-DRB1*0401 MBP+/+ mice, HLA-DRB1*1501 and HLA-DRB5*0101 MBP+/+ mice showed profound T cell tolerance to hMBP82-100 upon immunization with hMBP protein. This finding is in remarkable contrast to previous reports of the immunodominance of the hMBP82-100 epitope in HLA-DR2+ MS patients and the high binding affinity of this peptide for both HLA-DRB1*1501 and HLA-DRB5*0101 molecules (9–11). However, the purported immunodominance of hMBP82-100 in MS patients with the HLA-DR2 haplotype is somewhat of a paradox. Since this peptide binds with high binding affinity to HLA-DRB1*1501, HLA-DRB5*0101, and HLA-DRB1*0401 molecules, it would be predicted to induce central tolerance because Golli-MBP proteins containing this epitope are expressed by human thymic APC (13). This contradiction has been explained by cleavage of the MBP protein at asparagine at position 92 by asparagine endopeptidase (AEP) (41, 42). Since AEP is more abundantly expressed in thymic APC that mediate deletion of autoreactive T cells, as compared with peripheral APC, it was suggested that the cleavage of the hMBP82-100 epitope by AEP may limit its presentation in the thymus, thus leading to the escape of hMBP82-100-specific T cells from tolerance (41). Arguing against this view is the observation that the intact hMBP protein can directly bind to HLA-DRB1*1501 molecules on the cell surface, particularly via the hMBP83-99 region (43, 44). This may suggest that the hMBP82-100 epitope is protected from degradation by AEP. Finally, Bielekova and colleagues recently provided evidence that T cell responses directed against low-affinity MHC-binding myelin peptides are dominant in MS patients, but not healthy controls (30). Using 10- to 100-fold lower antigen concentrations for T cell recall as compared with earlier studies, they found that high-avidity hMBP83-99-specific T cells were virtually undetectable in HLA-DR2+ MS patients. It is conceivable that non-physiologically high concentrations of hMBP82-100 peptide used in previous studies could have obscured that most of these T cells were low-avidity MBP-reactive T cells with a high activation threshold. Recent structural studies on two TCR derived from HLA-DR2+ MS patients, one recognizing the complex of hMBP84-98/DRB1*1501, and the other recognizing the hMBP88-100/DRB5*0101 complex, have revealed unusual TCR binding properties that appeared to result in suboptimal recognition of the MHC-bound self-peptide (45, 46). Thus, our data showing T cell tolerance to hMBP82-100 in the HLA-DR Tg mice is in line with evidence provided by the human studies. It is arguable whether hMBP82-100-reactive T cells in humans can be activated under physiologic conditions in vivo and become pathogenic.
Importantly, the results in the HLA-DRB5*0101 MBP+/+ mice suggested an alternative mechanism by which T cell tolerance to hMBP82-100 could be circumvented. Specifically, “type B” T cells, which emerged upon immunization with hMBP82-100 peptide and could only be reactivated by the hMBP peptide but not the intact hMBP protein, escaped tolerance. Since the MBP82-100-specific “type B” T cells responded to the same core epitope as compared with “type A” T cells generated from HLA-DRB5*0101 MBP−/− mice, the results suggest that these T cells recognize this peptide bound to HLA-DRB5*0101 molecules in a different conformation, similar to the “type B” T cells described by Unanue, Sercarz, and colleagues (17–20). Importantly, the HLA-DRB5*0101-restricted hMBP82-100-specific “type B” T cells were highly encephalitogenic.
We suggest that HLA-DRB5*0101-restricted MBP-reactive “type B” T cells may be relevant for human MS since the “type B” TCL showed the same pattern of recognition when the antigen was presented by human HLA-DR5*0101+B cell lines, i.e. the T cells were activated by hMBP82-100 peptide but not by hMBP protein presented by the APCs. We propose that hMBP82-100-specific “type B” T cells could be present in the peripheral T cell repertoire of MS patients of the HLA-DR2 haplotype and could contribute to the pathogenesis of MS. An open question is whether myelin-specific “type B” T cells occur in the context of HLA-DRB1*1501 and HLA-DRB1*0401 molecules, since we did not detect hMBP82-100-reactive “type B” cells in Tg mice expressing these MHC molecules. However, it is conceivable that “type B” T cells exist for other MBP epitopes or other myelin antigens and we are currently performing experiments to address this issue.
An important question is the issue of immunoregulation in this model. While we favor central or peripheral tolerance as the mechanism underlying T cell unresponsiveness in HLA-DR MBP+/+ mice, it is conceivable that regulatory T cells contributed to the unresponsiveness observed in these studies, which we have not formally ruled out. A limitation of our experiments is that murine T cells restricted by human MHC molecules were studied, and not human T cells directly. Certain features of human T cells, such as the expression of MHC class II molecules upon activation, may contribute to T cell tolerance, which could not be investigated in the setting of the HLA-DR Tg mice. Another issue is that APC in these mice expressed only a single human MHC allele (i.e. HLA-DRB1*1501), whereas human APC can potentially express up to 6 different MHC II alleles (47). Conceivably, co-expression of human MHC molecules could lead to competition for peptides for MHC binding, and could impair T cell tolerance (48). Since hMBP82-100 peptide binds to HLA-DRB1*1501 molecules with higher affinity than to HLA-DRB5*0101 molecules, it would be particularly interesting to see whether competition for the peptide between the two molecules affects tolerance induction to the hMBP 82-100 region. Along these lines, work by Gregersen and colleagues recently suggested that co-expression of HLA-DR2a and HLA-DR2b may modulate disease and the frequencies of myelin-reactive T cells (49).
In conclusion, our results show that MS-associated MHC class II molecules efficiently induce T cell tolerance to MBP. Specifically, T cells specific for the high-affinity MHC-binding hMBP82-100 epitope were efficiently eliminated from the peripheral T cell repertoire in the context of MS-associated HLA-DR molecules. Importantly, T cells specific for the low-affinity MHC-binding hMBP111-129 epitope and “type B” T cells escaped tolerance and became immunodominant. These findings provide evidence that the general mechanisms of tolerance established in conventional animal models also apply to tolerance induction in the context of human MHC class II molecules. Studies of autoreactive T cells specific for low-affinity MHC-binding epitopes, and autoreactive “type B” T cells, could be an area of high yield for advancing the understanding of the pathogenesis of MS and to develop novel antigen-specific therapies. Finally, our results point towards a role for MS-associated MHC class II molecules other than the failure to induce tolerance.
Acknowledgments
We thank Neal A. Guentzel for excellent technical support.
Abbreviations
- AEP
asparagine endopeptidase
- BCL
B cell line
- EAE
experimental autoimmune encephalomyelitis
- LN
lymph node
- MBP
myelin basic protein
- TCC
T cell clone
- TCL
T cell line
- Tg
transgenic
- MS
multiple sclerosis
Footnotes
This work was supported by grant NS-428846 from the National Institute of Health, and grants RG3499 and RG3701 from the National Multiple Sclerosis Society (T.G.F.). K.K is recipient of a Postdoctoral Fellowship from the National Multiple Sclerosis Society
Publisher's Disclaimer: “This is an author-produced version of a manuscript accepted for publication in The Journal of Immunology (The JI). The American Association of Immunologists, Inc. (AAI), publisher of The JI, holds the copyright to this manuscript. This version of the manuscript has not yet been copyedited or subjected to editorial proofreading by The JI; hence, it may differ from the final version published in The JI (online and in print). AAI (The JI) is not liable for errors or omissions in this author-produced version of the manuscript or in any version derived from it by the U.S. National Institutes of Health or any other third party. The final, citable version of record can be found at www.jimmunol.org.”
References
- 1.Martin R, McFarland HF, McFarlin DE. Immunological aspects of demyelinating disease. Annu. Rev. Immunol. 1992;10:153–187. doi: 10.1146/annurev.iy.10.040192.001101. [DOI] [PubMed] [Google Scholar]
- 2.Haines JL, Ter-Minassian M, Bazyk A, Gusella JF, Kim DJ, Terwedow H, Pericak-Vance MA, Rimmler JB, Haynes CS, Roses AD, Lee A, Shaner B, Menold M, Seboun E, Fitoussi R-P, Gartioux C, Reyes C, Ribierre F, Gyapay G, Weissenbach J, Hauser SL, Goodkin DE, Lincoln R, Usuku K, Garcia-Merino A, Gatto N, Young S, Oksenberg JR The Multiple Sclerosis Genetics Group. A complete genomic screen for multiple sclerosis underscores a role for the major histocompatibility complex. Nat. Genet. 1996;13:469–471. doi: 10.1038/ng0896-469. [DOI] [PubMed] [Google Scholar]
- 3.Ebers GC, Kukay K, Bulman DE, Sadvnick AD, Rice G, Anderson C, Armstrong H, Cousin K, Bell RB, Hader W, Paty DW, Hashimoto S, Oger J, Duquette P, Warren S, Gray T, O’Connor P, Nath A, Auty A, Metz L, Francis G, Paulseth JE, Murray TJ, Pryse-Phillips W, Nelson R, Freedman M, Brunet D, Bouchard J-P, Hinds D, Risch N. A full genome search in multiple sclerosis. Nat. Genet. 1996;13:472–476. doi: 10.1038/ng0896-472. [DOI] [PubMed] [Google Scholar]
- 4.Hillert J, Kall T, Vrethem M, Fredrikson S, Ohlson M, Olereup O. The HLA-Dw2 haplotype segregates closely with multiple sclerosis in multiplex families. J. Neuroimminol. 1994;50:95–100. doi: 10.1016/0165-5728(94)90219-4. [DOI] [PubMed] [Google Scholar]
- 5.Marrosu MG, Muntoni F, Murru MR, Spinicci G, Pischedda MP, Goddi F, Cossu P, Pirastu M. Sardinian multiple sclerosis is associated with HLA-DR4: a serologic and molecular analysis. Neurology. 1988;38:1749–1753. doi: 10.1212/wnl.38.11.1749. [DOI] [PubMed] [Google Scholar]
- 6.Kurdi A, Ayesh I, Abdallat A, Maayta U. Different B lumphocyte alloantigens associated with multiple sclerosis in Arabs and North Europeans. Lancet. 1977;309:1123–1125. doi: 10.1016/s0140-6736(77)92383-2. [DOI] [PubMed] [Google Scholar]
- 7.Ridgway WM, Fathman GC. The association of MHC with autoimmune disease: understanding the pathogenesis of autoimmune diabetes. Clin. Immunol. Immunopathol. 1998;86:3–10. doi: 10.1006/clin.1997.4449. [DOI] [PubMed] [Google Scholar]
- 8.Zamvil S, Steinman L. The T Lymphocyte in experimental allergic encephalomyelitis. Annu. Rev. Immunol. 1990;8:579–621. doi: 10.1146/annurev.iy.08.040190.003051. [DOI] [PubMed] [Google Scholar]
- 9.Ota K, Matsui M, Milford EL, Mackin GA, Weiner HL, Hafler DA. T-cell recognition of an immunodominant myelin basic protein epitope in multiple sclerosis. Nature. 1990;346:183–187. doi: 10.1038/346183a0. [DOI] [PubMed] [Google Scholar]
- 10.Valli A, Sette A, Kappos L, Oseroff C, Sidney J, Miescher G, Hochberger M, Albert ED, Adorini L. Binding of myelin basic protein peptides to human histocompatibility leukocyte antigen class II molecules and their recognition by T cells from multiple sclerosis patients. J. Clin. Invest. 1993;91:616–628. doi: 10.1172/JCI116242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Vergelli M, Kalbus M, Rojo SC, Hemmer B, Kalbacher H, Tranquill L, Beck H, McFarland HF, De Mars R, Long EO, Martin R. T cell response to myelin basic protein in the context of the multiple sclerosis-associated HLA-DR15 haplotype: peptide binding, immunodominance and effector functions of T cells. J. Neuroimmunol. 1997;77:195–203. doi: 10.1016/s0165-5728(97)00075-1. [DOI] [PubMed] [Google Scholar]
- 12.Muraro PA, Vergelli M, Kalbus M, Banks DE, Nagle JW, Tranquill LR, Nepom GT, Biddison WE, McFarland HF, Martin R. Immunodominance of a low-affinity major histocompatibility complex-binding myelin basic protein epitope (residues 111–129) in HLA-DR4 (B 1*0401) subjects is associated with a restricted T cell receptor repertoire. J. Clin. Invest. 1997;100:339–349. doi: 10.1172/JCI119539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gotter J, Brors B, Hergenhahn M, Kyewski B. Medullary epithelial cells of the human thymus express a highly diverse selection of tissue-specific genes colocalized in chromosomal clusters. J. Exp. Med. 2004;199:155–166. doi: 10.1084/jem.20031677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Harrington C, Paez A, Hunkapiller T, Mannikko V, Brabb T, Ahearn M, Beeson C, Goverman J. Differential tolerance is induced in T cells recognizing distinct epitopes of myelin basic protein. Immunity. 1998;8:571–580. doi: 10.1016/s1074-7613(00)80562-2. [DOI] [PubMed] [Google Scholar]
- 15.Huesby ES, Sather B, Huesby PG, Goverman J. Age-dependent T cell tolerance and autoimmunity to myelin basic protein. Immunity. 2001;14:471–481. doi: 10.1016/s1074-7613(01)00127-3. [DOI] [PubMed] [Google Scholar]
- 16.Oleg T, Lehmann PV. Endogenous myelin basic protein inactivates the high avidity T cell repertoire. J. Exp. Med. 1998;187:2055–2063. doi: 10.1084/jem.187.12.2055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Moudgil KD, Sercarz EE. Understanding crypticity is the key to revealing the pathogenesis of autoimmunity. Trends Immunol. 2005;26:355–359. doi: 10.1016/j.it.2005.05.007. [DOI] [PubMed] [Google Scholar]
- 18.Pu Z, Lovitch SB, Bikoff EK, Unanue ER. T cells distinguish MHC-peptide complexes formed in separate vesicles and edited by H2-DM. Immunity. 2004;20:467–476. doi: 10.1016/s1074-7613(04)00073-1. [DOI] [PubMed] [Google Scholar]
- 19.Peterson DA, DiPaolo RJ, Kanagawa O, Unanue ER. Quantitative analysis of the T cell repertoire that escape negative selection. Immunity. 1999;11:453–462. doi: 10.1016/s1074-7613(00)80120-x. [DOI] [PubMed] [Google Scholar]
- 20.Lovitch SB, Walters JJ, Gross ML, Unanue ER. APCs present Aβk-derived peptides that are autoantigenic to type B T cells. J. Immunol. 2003;170:4155–4160. doi: 10.4049/jimmunol.170.8.4155. [DOI] [PubMed] [Google Scholar]
- 21.Ito K, Bian H-J, Molina M, Han J, Magram J, Saar E, Belunis C, Bolin DR, Arceo R, Campbell R, Falcioni F, Vidović D, Hammer J, Nagy ZA. HLA-DR4-IE chimeric class II transgenic, murine class II-deficient mice are susceptible to experimental allergic encephalomyelitis. J. Exp. Med. 1996;183:2635–2644. doi: 10.1084/jem.183.6.2635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Forsthuber TG, Shive CL, Wienhold W, de Graaf K, Spack EG, Sublett R, Melms A, Kort J, Racke MK, Weissert R. T cell epitopes of human myelin oligodendrocyte glycoprotein identified in HLA-DR4 (DRB1*0401) transgenic mice are encephalitogenic and are presented by human B cell. J. Immunol. 2001;167:7119–7125. doi: 10.4049/jimmunol.167.12.7119. [DOI] [PubMed] [Google Scholar]
- 23.Rich C, Link JM, Zamora A, Jacobsen H, Meza-Romero R, Offner H, Jones R, Burrows GG, Fugger L, Vandenbark AA. Myelin ologodendrocyte glycoprotein-35-55 peptide induces severe chronic experimental autoimmune encephalomyelitis in HLA-DR2-transgenic mice. Eur. J. Immunol. 2004;34:1251–1261. doi: 10.1002/eji.200324354. [DOI] [PubMed] [Google Scholar]
- 24.Lang HLE, Jacobsen H, Ikezumi S, Andersson C, Harlos K, Madsen L, Hjorth P, Sondergaard L, Svejgaard A, Wucherpfennig K, Stuart DI, Bell JI, Jones EY, Fugger L. A functional and structural basis for TCR cross-reactivity in multiple sclerosis. Nature Immunol. 2002;3:940–943. doi: 10.1038/ni835. [DOI] [PubMed] [Google Scholar]
- 25.Roach A, Takahashi N, Pravtcheva D, Ruddle F, Hood L. Chromosomal mapping of mouse myelin basic protein gene and structure and transcription of the partially deleted gene in shiverer mutant mice. Cell. 1985;42:149–155. doi: 10.1016/s0092-8674(85)80110-0. [DOI] [PubMed] [Google Scholar]
- 26.Deiber GE, Martenson RE, Kies MW. Large scale preparation of myelin basic protein from central nervous tissue of several mammalian species. Prep. Biochem. 1972;2:335–360. doi: 10.1080/00327487208061467. [DOI] [PubMed] [Google Scholar]
- 27.Cognia M, Patel S, Cope AP, De Virgiliis S, Sonderstrup G. T cell epitopes of insulin defined in HLA-DR4 transgenic mice are derived from preproinsulin and proinsulin. Proc. Natl. Acad. Sci. USA. 1998;95:3833–3838. doi: 10.1073/pnas.95.7.3833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shive CL, Hofstetter H, Arredondo L, Shaw C, Forsthuber TG. The enhanced antigen-specific production of cytokines induced by pertussis toxin is due to clonal expansion of T cells and not to altered effector functions of long-term memory cells. Eur. J. Immunol. 2000;20:2422–2431. doi: 10.1002/1521-4141(2000)30:8<2422::AID-IMMU2422>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- 29.Karulin AY, Hesse MD, Tary-Lehmann M, Lehmann PV. Single-cytokine-producing CD4 memory cells predominate in type 1 and type 2 immunity. J. Immunol. 2000;164:1862–1872. doi: 10.4049/jimmunol.164.4.1862. [DOI] [PubMed] [Google Scholar]
- 30.Bielekova B, Sung M-H, Kadom N, Simon R, McFarland HF, Martin R. Expansion and functional relevance of high-avidity myelin-specific CD4+ T cells in multiple sclerosis. J. Immunol. 2004;172:3893–3904. doi: 10.4049/jimmunol.172.6.3893. [DOI] [PubMed] [Google Scholar]
- 31.Hammer J, Belunis C, Bolin D, Papadopoulos J, Walsky R, Higelin J, Danho W, Sinigaglia F, Nazy ZA. High-affnity binding of short peptides to major histocompatibility complex class II molecules by anchor combination. Proc. Natl. Acad. Sci. U.S.A. 1994;91:4456–4460. doi: 10.1073/pnas.91.10.4456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dressen A, Lawrence CM, Cupo S, Zaller DM, Wiley DC. X-ray crystal structure of HLA-DR4 (DRA*0101, DRB1*0401) complexed with a peptide from human collagen II. Immunity. 1997;7:473–481. doi: 10.1016/s1074-7613(00)80369-6. [DOI] [PubMed] [Google Scholar]
- 33.Quandt JA, Baig M, Yao K, Kawamura K, Huh J, Ludwin SK, Bian H-J, Bryant M, Quigley L, Nagy ZA, McFarland HF, Muraro PA, Martin R, Ito K. Unique clinical and pathological features in HLA-DRB1*0401-restricted MBP 111-129-specific humanized TCR transgenic mice. J. Exp. Med. 2004;200:223–234. doi: 10.1084/jem.20030994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Seamons A, Perchellet A, Goverman J. Immune tolerance to myelin proteins. Immunol. Res. 2003;28:201–221. doi: 10.1385/IR:28:3:201. [DOI] [PubMed] [Google Scholar]
- 35.Boyton RJ, Alltmann DM. Transgenic model of autoimmune disease. Clin. Exp. Immunol. 2002;127:4–11. doi: 10.1046/j.1365-2249.2002.01771.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Friese MA, Jensen LY, Willcox N, Fugger L. Humanized mouse models for organ-specific autoimmune disease. Curr. Opin. Immunol. 2006;18:704–709. doi: 10.1016/j.coi.2006.09.003. [DOI] [PubMed] [Google Scholar]
- 37.Mangalam AK, Rajagopalan G, Taneja V, David CS. HLA class II transgenic mice mimic human inflammatory disease. Adv. Immunol. 2008;97:65–147. doi: 10.1016/S0065-2776(08)00002-3. [DOI] [PubMed] [Google Scholar]
- 38.Cabbage SE, Huseby ES, Sather BD, Brabb T, Liggitt D, Goverman J. Regulatory T cells maintain long-term tolerance to myelin basic protein by inducing a novel, dynamic state of T cell tolerance. J. Immunol. 2007;178:887–896. doi: 10.4049/jimmunol.178.2.887. [DOI] [PubMed] [Google Scholar]
- 39.Anderton SM, Radu CG, Lowrey PA, Ward ES, Wraith DC. Negative selection during the peripheral immune response to antigen. J. Exp. Med. 2001;193:1–11. doi: 10.1084/jem.193.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ryan KR, McCue D, Anderton SM. Fas-mediated death and sensory adaptation limit the pathogenic potential of autoreactive T cells after strong antigenic stimulation. J. Leukoc. Biol. 2005;78:43–50. doi: 10.1189/jlb.0205059. [DOI] [PubMed] [Google Scholar]
- 41.Beck H, Schwarz G, Schröter CJ, Deeg M, Baier D, Stevanovic S, Weber E, Driessen C, Kalbacher H. Cathepsin S and an asparagines-specific endopeptidase dominate the proteolytic processing of human myelin basic protein in vitro. Eur. J. Immunol. 2001;31:3726–3736. doi: 10.1002/1521-4141(200112)31:12<3726::aid-immu3726>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- 42.Manoury B, Mazzeo D, Fugger L, Viner N, Ponsford M, Streeter H, Mazza G, Wraith DC, Watts C. Destructive processing by asparagines endopeptidase limits presentation of a dominant T cell epitope in MBP. Nature Immunol. 2002;3:169–174. doi: 10.1038/ni754. [DOI] [PubMed] [Google Scholar]
- 43.Fridkis-Hareli M, Teitelbaum D, Arnon R, Sela M. Symthetic copolymer 1 and myelin basic protein do not require processing prior to binding to class II major histocompatibility complex molecules on living antigen-presenting cells. Cell. Immunol. 1995;163:229–236. doi: 10.1006/cimm.1995.1121. [DOI] [PubMed] [Google Scholar]
- 44.Vergille M, Pinet V, Vogt AB, Kalbus M, Malnati M, Riccio P, Long EO, Martin R. HLA-DR-restricted presentation of purified myelin basic protein is independent of intracellular processing. Eur J. Immunol. 1997;27:941–951. doi: 10.1002/eji.1830270421. [DOI] [PubMed] [Google Scholar]
- 45.Hahn M, Nicholson MJ, Pyrdol J, Wucherpfennig KW. Unconventional topology of self peptide-major histocompatibility complex binding by a human autoimmune T cell receptor. Nature Immunol. 2005;6:490–496. doi: 10.1038/ni1187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Li Y, Huang Y, Lue J, Quandt JA, Martin R, Mariuzza RA. Structure of a human autoimmune TCR bound to a myelin basic protein self-peptide and a multiple sclerosis-associated MHC class II molecule. EMBO J. 2005;24:2968–2979. doi: 10.1038/sj.emboj.7600771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Prat E, Tomaru U, Sabater L, Park DM, Granger R, Kruse N, Ohayon JM, Bettinotti MP, Martin R. HLA-DRB5*0101 and -DRB1*1501 expression in the multiple sclerosis-associated HLA-DR15 haplotype. J. Neuroimmunol. 2005;167:108–119. doi: 10.1016/j.jneuroim.2005.04.027. [DOI] [PubMed] [Google Scholar]
- 48.Sercarz EE, Maverakis E. MHC-guided processing: binding of large antigen fragments. Nature Reviews Immunol. 2003;3:621–629. doi: 10.1038/nri1149. [DOI] [PubMed] [Google Scholar]
- 49.Ø. Gregersen JW, Kranc KR, Ke X, Svendsen P, Madsen LS, Thomsen AR, Cardon LR, Bell JI, Fugger L. Functional epistasis on a common MHC haplotype associated with multiple sclerosis. Nature. 2006;443:574–577. doi: 10.1038/nature05133. [DOI] [PubMed] [Google Scholar]