Abstract
Background and Purpose
TO901317, a synthetic liver X receptor (LXR) agonist, elevates high-density lipoprotein cholesterol (HDL-C) in mice. We tested the hypothesis that TO901317 treatment of stroke promotes angiogenesis and vascular maturation and improves functional outcome after stroke by increasing endothelial nitric oxide synthase (eNOS) phosphorylation.
Methods
C57BL/6J mice were subjected to middle cerebral artery occlusion (MCAO) and were treated with or without TO901317 (30mg/kg) starting 24h after MCAo and daily for 14 days.
Results
TO901317 significantly increased serum HDL-C level, promoted angiogenesis and vascular stabilization in the ischemic brain and improved functional outcome after stroke. The increased HDL-C level significantly correlated with functional recovery after stroke. TO901317 also increased p-eNOS in the ischemic brain. Mechanisms underlying the TO901317-induced angiogenesis were investigated using eNOS knockout (eNOS−/−) mice. TO901317 treatment of eNOS−/− mice significantly increased HDL-C level but failed to increase angiogenesis and functional outcome after stroke. In vitro studies demonstrated that TO901317 and HDL-C significantly increased capillary tube formation and promoted p-eNOS activity in cultured mouse brain endothelial cells (MBECs) compared to non-treatment control. However, TO901317 and HDL treatment induced capillary tube formation were absented in eNOS-deficient MBEC.
Conclusions
These data indicate that TO901317 treatment increases serum HDL-C level which promotes angiogenesis via eNOS and leads to improvement of functional outcome after stroke.
Keywords: Angiogenesis, eNOS, HDL-C, TO901317, stroke
Introduction
High-density lipoprotein cholesterol (HDL-C) is a heterogeneous group of lipoproteins exhibiting a variety of properties, e.g. decrease in platelet aggregation and inhibition of endothelial cell (EC) apoptosis 1. HDL-C enhances vasorelaxation, promotes EC migration and reendothelialization by increasing endothelial nitric oxide synthase (eNOS) expression and eNOS phosphorylation (p-eNOS) 2–5. Reconstituted HDL treatment of acute myocardial infarction (MI) rats improves cadiac function after MI in rats 6. Niacin treatment of stroke rats increases HDL-C level, upregulates p-eNOS and angiogenesis and improves functional outcome 7. Higher levels of HDL-C are associated with better functional performance in old people 8. Low HDL-C predicts poor cognitive function and worse disability after stroke 9. Clinical studies have shown that statins significantly lower cholesterol, and are not very effective at increasing HDL levels. Thus, agents that increase HDL-C level may be attractive targets as restorative treatments for stroke.
T0901317, a potent non-steroidal synthetic liver X receptor (LXR) agonist, elevates HDL-C and phospholipid and generates enlarged HDL-C particles enriched in cholesterol 10. LXRs control the expression of several genes important for cholesterol homeostasis in the brain 11. LXR knockout (LXR−/−) mice exhibit enlarged brain blood vessels with weak staining of α-smooth muscle actin (a-SMA) and excessive lipid accumulation around the abnormal vessels, which lose their contractile ability and are susceptible to rupture 12. Activation of LXRs promotes neuroprotection and decreases expression of proinflammatory genes and reduces nuclear factor-kappaB transcriptional activity in experimental stroke 13, 14. T0901317 suppresses the vascular inflammatory status and lowers lesional macrophage accumulation 15. However, there are no studies, which evaluate whether TO901317 treatment regulates angiogenesis and promotes neurorestoration after stroke.
In this study, we tested a novel hypothesis, that increasing HDL-C by TO901317 treatment promotes angiogenesis in the ischemic brain as well as improves functional outcome after stroke in mice. In addition, the mechanisms and molecular signaling pathway of TO901317-induced angiogenesis were investigated.
Materials and Methods
Animal middle cerebral artery occlusion (MCAo) model and experimental groups
Adult male wild-type (WT) C57BL/6J mice and C57BL/6J eNOS knockout (eNOS−/−) mice (age 2–3 months, weight 24–28g) were purchased from Charles River (Wilmington, MA). Right temporal (2h) MCAo was induced using the filament model, as previously described 16. Sham-operated mice underwent the same surgical procedure without suture insertion. MCAo and sham-operated WT mice were gavaged starting 24h after surgery with: 1) saline for control; 2) TO901317 (30mg/kg, Cayman Chemical). Our choice of dose was guided by a previous study 17. To test whether eNOS mediates TO901317 induced functional outcome after stroke, eNOS−/− mice were employed. eNOS−/− mice were subjected to 2h MCAo and treated with or without TO901317 (30mg/kg) starting 24h after MCAo daily for 14 days. All mice received daily intra-peritoneal (i.p) injections of bromodeoxyuridine (BrdU, 50mg/kg, Sigma, St, Louis MO), a thymidine analog, which labels newly synthesized DNA, starting at 24h after MCAo daily for identification of cell proliferation. Mice (n=8/group) was sacrificed 14 days after MCAo for immunostaining. Another set of mice (n=3/group) were sacrificed at 5 days after MCAo and ischemic brain tissue from ischemic border (IBZ) was extracted for Western blot assay.
HDL-C measurement
HDL-C was measured at 14 days after MCAo using CardioChek P•A analyzer and HDL-C check strips (Polymer 285 technology System, Inc. Indianapolis, IN), according to the manufacturer’s instructions. The data are presented as mg/dl values.
Foot-Fault functional test 18
Foot-fault test was performed before MCAo, and at 1, 7 and 14 days after MCAo by an investigator who was blinded to the experimental groups. The percentage of Foot-faults of the left paw to total steps were determined.
Histological and immunohistochemical assessment
The brains were fixed by transcardial perfusion with saline, sectioned, tissue processed, and lesion volume calculated, as previously described 19.
For immunostaining, a standard paraffin block was obtained from the center of the lesion (bregma −1mm to +1mm). A series of 6 μm thick sections was cut from the block. Every 10th coronal section for a total 5 sections was used for immunohistochemical staining. Antibody against BrdU, (1:100, Boehringer Mannheim, Indianapolis, IN), Von Willebrand Factor (vWF, a EC marker, 1:400; Dako, Carpenteria, CA), α-SMA (a SMC marker, mouse monoclonal IgG 1:800, Dako), Occludin (Mouse monoclonal lgG antibody, 1:200 dilution, Zymed), were employed 20, 21. Control experiments consisted of staining brain coronal tissue sections as outlined above, but nonimmune serum was substituted for the primary antibody 22. The immunostaining analysis was performed by an investigator blinded to the experimental groups.
Angiogenesis measurement
Brain EC proliferation 23
Five slides from BrdU immunostained coronal sections, were digitized using a 20X objective (Olympus BX40) via the MCID computer imaging analysis system (Imaging Research, St. Catharines, Canada). The number of BrdU immunoreactive ECs within a total of 10 enlarged and thin walled vessels located in the IBZ area were counted 24. Data are presented as the percentage of the number of BrdU immunoreactive cells within 10 vessels/total EC number.
Vascular density measurement
Five slides from vWF immunostained coronal section, with each slide containing 8 fields of view within the IBZ was digitized. The total vascular density in the IBZ was measured in each section, as previously described 24. The total number of vWF positive vessels per mm2 area is presented.
α-SMA positive coated vessels density measurement
The density of α-SMAstained vessels was analyzed with regard to small and large vessels (≥10μm diameter) in the IBZ. Five sections and eight brain regions within each section were acquired and numbers of α-SMA immunoreactive vessels were counted. The total number of α-SMA positive coated vessels per mm2 area is presented.
Mouse brain endothelial cell (MBEC) culture
MBECs (ATCC, CRL-2299), culture was employed. MBECs were treated with: 1) non-treatment control. 2) TO901317 0.1μM and 1μM, respectively (n=3/group). Cells were treated for 12h before harvesting for real-time PCR and Western blot assays.
Real time-PCR
MBECs were harvested and total RNA was isolated following a standard protocol 25. Quantitative PCR was performed on an ABI 7000 PCR instrument (Applied Biosystems, Foster City, CA) using 3-stage program parameters provided by the manufacturer. Each sample was tested in triplicate, and analysis of relative gene expression data using the 2−Δ ΔCT method. The following primers for real-time PCR were designed using Primer Express software (ABI). eNOS: FWD: TTG AAA ATG AGA CTT GTT CAA TGC; RWD: TGC AGA GTA CTG GGT TAC AGA GAG. GAPDH: Fwd: AGA ACA TCA TCC CTG CAT CC; Rev: CAC ATT GGG GGT AGG AAC AC.
Western blot
Equal amounts of cell or brain tissue lysate were subjected to Western blot analysis, as previously described 24. Specific proteins were visualized using a SuperSignal West Pico chemiluminescence kit (Pierce). The primary antibodies were used: anti-β-actin (1:2000; Santa Cruz Biotechnology, Santa Cruz, CA), anti-eNOS (1:1000, Cell Signaling Technology) and anti-phospho-eNOS (Ser1177; 1:1000, Cell Signaling Technology).
eNOS deficit mice brain microvascular endothelial cell (eNOS−/−MBECs) culture
eNOS−/− mouse brains were collected. The cortical brain tissue was isolated and digested in collagenase/dispase, and the microvessels separated by centrifugation in a Percoll (Sigma) gradient. Microvessels were seeded in flasks coated with rat-tail collagen and the medium was changed every 2–3 days. eNOS−/−MBECs were used for tube formation assay.
Capillary-like tube formation assay 26
MBECs (2×104 cells) were incubated in (n=6/group): (a) regular cell culture medium (DMEM) for control; (b) TO9013117 0.1μM, 1μM, and 10μM; (c) HDL-C (80μg/ml and 160μg/ml); (d) eNOS−/−MBECs alone; (e) eNOS−/−MBECs treated with TO901317(1μM) and (f) eNOS−/−MBECs treated with HDL(80μg/ml) for 5h. All assays were performed in triplicate and total length of tube like formation was quantitated.
Statistical analysis
Independent Samples T-Test was used for testing functional outcome, HDL-C, p-eNOS expression, number of vWF- or α-SMA-positive vessels between two groups. One-way ANOVA and Least Significant Difference (LSD) analysis after Post Hoc Test were performed to assess eNOS mRNA, p-eNOS expression and tube formation in vitro. Two-way ANOVA was performed for measurement of BrdU, Occludin and vWF-/α-SMA-positive vessels in the ischemic brain. If an overall treatment group effect was detected at p<0.05, Tukey test after Post Hoc Test was used for multiple comparison. Pearson partial correlation after Bivariate correlation was used to analyze the correlation effect. All data are presented as mean ± Standard Error (SE).
Results
TO901317 treatment improves neurological outcome and increases serum HDL-C level
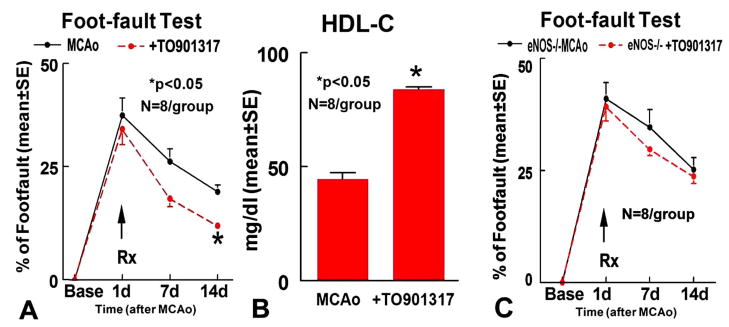
Fig. 1A shows that WT mice treated with TO901317 significantly improved functional recovery in Foot-fault test compared to control MCAo animals (p<0.05). No significant differences of infarct volumes in the TO901317 treated group (14.9%±4.2) were detected compared with MCAo control (18.8%±2.3). Serum of HDL-C significantly increased at 14 days in the TO901317 treated group compared to the non-treatment control MCAo (Fig. 1B, p<0.05). Correlation coefficient analysis shows a strong negative correlation between Foot-fault and HDL-C level at 14 days after treatment (r=−0.78, p<0.05). These data indicate that TO901317 treatment of stroke increases HDL-C and the increased HDL-C correlates with functional outcome after stroke.
Fig. 1.
TO901317 treatment of stroke increases HDL-C and improves functional outcome in WT mice, but fails to improve functional outcome in eNOS−/− mice. A–B: Foot-fault test (A) and HDL-C (B) in WT mice (n=8/group). C: Foot-fault test in eNOS−/− mice;
TO901317 treatment of stroke increases angiogenesis and vascular maturation in the ischemic brain
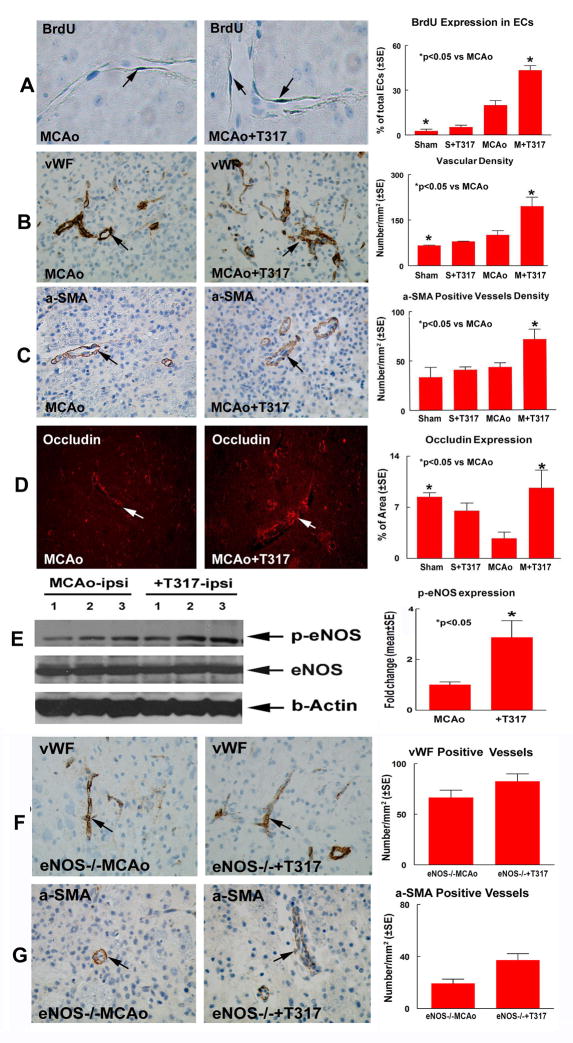
Fig. 2A–B show that BrdU positive ECs (p=0.001, F=12.653, A) and vascular density (p=0.004, F=4.903, B) were significantly increased in the TO901317 treatment groups compared with control animals. Fig. 2C–D show that treatment with TO901317 significantly (p<0.05) increased Occludin expression (p=0.018, F=4.664, D) and α-SMA positive vessels density (p=0.042, F=3.716, C) in the IBZ area compared with the control MCAo mice.
Fig. 2.
TO901317 treatment of stroke increases angiogenesis, vascular maturation and p-eNOS activity in the ischemic brain in WT mice (A–E), but fails to promote angiogenesis and vascular maturation in eNOS−/− mice. A: vascular BrdU positive EC expression and quantitative data. B: vWF immunostaining and vascular density quantitative data. C: α-SMA immunostaining and α-SMA positive vascular density quantitative data. D: Occludin immunostaining and quantitative data. N=8/group. E: Western blot and quantitative data (n=3/group). F: vWF immunostaining and vascular density quantitative data in eNOS−/− mice. G: α-SMA immunostaining and α-SMA positive vascular density quantitative data in eNOS−/− mice. N=8/group.
To test the mechanism that underlies TO901317 induced angiogenesis, eNOS and p-eNOS expression were measured in the ischemic brain. Fig. 2E Shows that TO901317 treatment of stroke significantly increases p-eNOS activity in the ischemic brain (p<0.05).
eNOS is required for TO901317 induced functional outcome after stroke
TO901317 treatment in eNOS−/− mice increases HDL-C level (74.4±5.1mg/dl) compared to eNOS−/− MCAo control (48.4±4.2mg/dl, p<0.05), but fails to improve functional outcome after stroke (Fig. 1C). No significant differences of infarct volumes in the TO901317 treated eNOS−/− mice (18.6%±6.5) were detected compared with eNOS−/− MCAo control (19.3%±2.0). In addition, treatment with TO901317 in eNOS−/− mice did not significantly increase vWF-positive-vessels density (Fig. 2F) and α-SMA-positive-vessels density (Fig. 2G) in the IBZ compared with the eNOS−/− MCAo control.
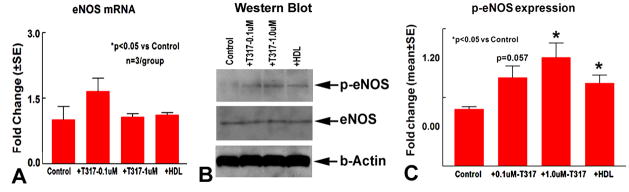
TO901317 and HDL increases MBECs p-eNOS
Fig. 3A–C show that TO901317 and HDL treatment do not regulate eNOS gene and protein expression, but significantly promote p-eNOS activity compared non-treatment control (p<0.05).
Fig. 3.
TO901317 regulates eNOS expression in cultured MBECs. A: eNOS gene expression; B–C: Western blot (B) and quantitative data (C). N=3/group.
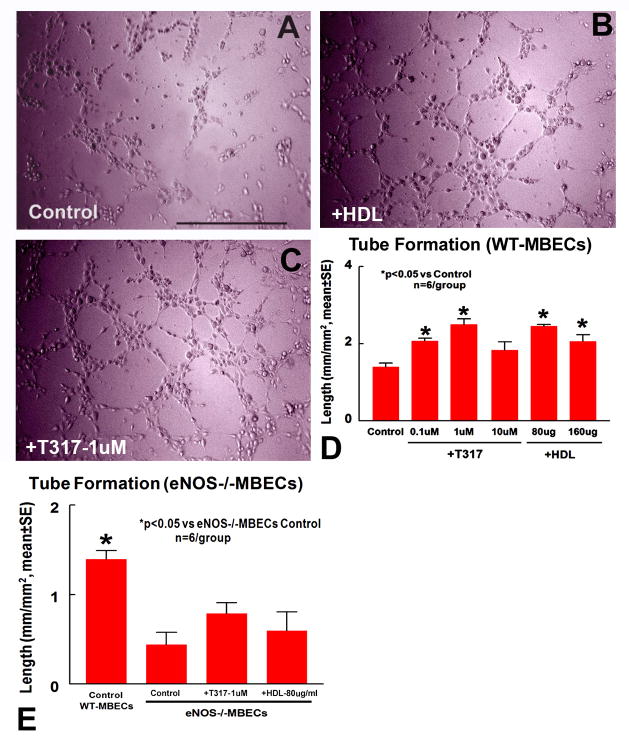
TO901317 and HDL-C induce angiogenesis in vitro
Fig. 4A–D show that TO901317 and HDL-C dose dependently increased capillary tube formation compared with control DMEM medium.
Fig. 4.
TO901317 and HDL regulate capillary-like tube formation. Inhibition of eNOS attenuates TO901317 and HDL induced tube formation. A–D: capillary-like tube formation in WT-MBECs control (A), TO901317(1μm, B) and HDL(80μg/ml, C) and quantitative data (D). E: Quantitative data of capillary-like tube formation in eNOS−/−MBECs. N=6/group. Scale bar in a=0.5mm.
eNOS mediates TO901317 and HDL induced tube formation
Fig. 4E show that capillary tube formation significantly decreased in eNOS−/−MBECs compared to WT-MBECs. TO901317(1μM) and HDL(80μg/ml) do not significantly increase capillary tube formation in eNOS−/−MBECs compared to eNOS−/−MBECs control (p>0.05).
Discussion
TO901317 increases HDL-C and improves functional outcome after stroke
T0901317 is a potent LXR agonist. LXRs activate reverse cholesterol transport including the ATP binding cassette transporter A1 and raise HDL-C levels 27, 28. Intravenous injection of rHDL significantly augments blood flow recovery and increases capillary density in the ischemic leg 29. Stroke patients exposed to power-frequency electromagnetic fields which increases HDL-C show a statistically significant better prognosis compared with the control group 30. In this study, we found that TO901317 treatment significantly increases serum HDL-C, and promotes functional outcome after stroke. Increased HDL-C correlated with functional outcome after stroke. Therefore, increasing HDL-C by TO901317 treatment may contribute to functional outcome after stroke.
TO901317 increase angiogenesis and vascular maturation after stroke
Angiogenesis, involves the sprouting, branching, splitting, and differential growth of vessels in the primary plexus to form the mature vascular system 31. During angiogenic vascular remodeling, supporting cells such as pericytes and SMCs are recruited to the vessels to provide structural support and stability for the vascular walls 32. TO901317 treatment of stroke induces angiogenesis identified by increasing EC proliferation and vascular density and also promotes SMC adhesion to vessel and increases tight junction protein Occludin expression in vessels in the ischemic brain. These data suggest that TO901317 treatment of stroke not only induces angiogenesis, but also promotes vascular maturation. However, recovery of neurological function after stroke is mediated by many coupled events, including vascular remodeling, neurogenesis and synaptogenesis 33. We do not exclude the possibility that other restorative events, in addition to angiogenesis, contribute to recovery of function. Whether TO901317 regulates neurogenesis and synaptogenesis warrants further investigation.
eNOS mediates TO901317 induced angiogenesis after stroke
HDL stimulates eNOS activation 3,34. The anti-atherogenic role of HDL is also related to the increased activity of eNOS 35. HDL-C promotes EC migration and reendothelialization, mediated by activation of eNOS 4. HDL-C promotes eNOS activity by maintaining the lipid environment in caveolae where eNOS is colocalized with partner signaling molecules 36. Enhanced p-eNOS induces a broad range of effects including the promotion of angiogenesis and mural cell recruitment to immature angiogenic sprouts 37. EC-derived NO induces mural cell recruitment as well as subsequent morphogenesis and stabilization of angiogenic vessels 38. Our data show that TO901317 treatment of stroke increases HDL-C level and promotes phosphorylation of eNOS in the ischemic brain. TO901317 and HDL-C treatment of MBECs significantly increase p-eNOS activity as well as promote angiogenesis compared to control. We tested the profile of TO901317 dose-dependent regulation of tube formation in vitro. The reason for why high dose TO901317(10μM) reduces tube formation, warrants further investigation. To elucidate the contribution of eNOS to TO901317-mediated angiogenesis, we employed eNOS−/− mice. We found that TO901317 treatment of stroke in eNOS−/− mice increases HDL-C level, but failed to improve functional outcome after stroke as well as regulate angiogenesis compared to non treatment eNOS−/−mice. These data indicate that eNOS plays critical role in TO901317 induced angiogenesis and functional outcome after stroke.
Summary
We demonstrate that treatment of experimental stroke with TO901317 at 24h after stroke significantly increases HDL-C levels, promotes angiogenesis and improves functional outcome after stroke. eNOS appears to mediate TO901317-induced angiogenesis.
Acknowledgments
The authors wish to thank Qinge Lu for technical assistance. This work was supported by NIA RO1 AG031811, NINDS RO1 NS047682, PO1 NS23393 and American Heart Association grant 0750048Z.
References
- 1.Bermudez V, Cano R, Cano C, Bermudez F, Arraiz N, Acosta L, Finol F, Pabon MR, Amell A, Reyna N, Hidalgo J, Kendall P, Manuel V, Hernandez R. Pharmacologic management of isolated low high-density lipoprotein syndrome. Am J Ther. 2008;15:377–388. doi: 10.1097/MJT.0b013e318169bc0b. [DOI] [PubMed] [Google Scholar]
- 2.Kuvin JT, Ramet ME, Patel AR, Pandian NG, Mendelsohn ME, Karas RH. A novel mechanism for the beneficial vascular effects of high-density lipoprotein cholesterol: Enhanced vasorelaxation and increased endothelial nitric oxide synthase expression. Am Heart J. 2002;144:165–172. doi: 10.1067/mhj.2002.123145. [DOI] [PubMed] [Google Scholar]
- 3.Mineo C, Yuhanna IS, Quon MJ, Shaul PW. High density lipoprotein-induced endothelial nitric-oxide synthase activation is mediated by akt and map kinases. J Biol Chem. 2003;278:9142–9149. doi: 10.1074/jbc.M211394200. [DOI] [PubMed] [Google Scholar]
- 4.Assanasen C, Mineo C, Seetharam D, Yuhanna IS, Marcel YL, Connelly MA, Williams DL, de la Llera-Moya M, Shaul PW, Silver DL. Cholesterol binding, efflux, and a pdz-interacting domain of scavenger receptor-bi mediate hdl-initiated signaling. J Clin Invest. 2005;115:969–977. doi: 10.1172/JCI200523858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Seetharam D, Mineo C, Gormley AK, Gibson LL, Vongpatanasin W, Chambliss KL, Hahner LD, Cummings ML, Kitchens RL, Marcel YL, Rader DJ, Shaul PW. High-density lipoprotein promotes endothelial cell migration and reendothelialization via scavenger receptor-b type i. Circ Res. 2006;98:63–72. doi: 10.1161/01.RES.0000199272.59432.5b. [DOI] [PubMed] [Google Scholar]
- 6.Kiya Y, Miura SI, Imaizumi S, Uehara Y, Matsuo Y, Abe S, Jimi S, Urata H, Rye KA, Saku K. Reconstituted high-density lipoprotein attenuates postinfarction left ventricular remodeling in rats. Atherosclerosis. 2008 doi: 10.1016/j.atherosclerosis.2008.05.056. [DOI] [PubMed] [Google Scholar]
- 7.Chen J, Cui X, Zacharek A, Jiang H, Roberts C, Zhang C, Lu M, Kapke A, Feldkamp CS, Chopp M. Niaspan increases angiogenesis and improves functional recovery after stroke. Ann Neurol. 2007;62:49–58. doi: 10.1002/ana.21160. [DOI] [PubMed] [Google Scholar]
- 8.Landi F, Russo A, Cesari M, Pahor M, Bernabei R, Onder G. Hdl-cholesterol and physical performance: Results from the ageing and longevity study in the sirente geographic area (ilsirente study) Age Ageing. 2007;36:514–520. doi: 10.1093/ageing/afm105. [DOI] [PubMed] [Google Scholar]
- 9.Newman GC, Bang H, Hussain SI, Toole JF. Association of diabetes, homocysteine, and hdl with cognition and disability after stroke. Neurology. 2007;69:2054–2062. doi: 10.1212/01.wnl.0000280457.29680.9c. [DOI] [PubMed] [Google Scholar]
- 10.Cao G, Beyer TP, Yang XP, Schmidt RJ, Zhang Y, Bensch WR, Kauffman RF, Gao H, Ryan TP, Liang Y, Eacho PI, Jiang XC. Phospholipid transfer protein is regulated by liver x receptors in vivo. J Biol Chem. 2002;277:39561–39565. doi: 10.1074/jbc.M207187200. [DOI] [PubMed] [Google Scholar]
- 11.Whitney KD, Watson MA, Collins JL, Benson WG, Stone TM, Numerick MJ, Tippin TK, Wilson JG, Winegar DA, Kliewer SA. Regulation of cholesterol homeostasis by the liver x receptors in the central nervous system. Mol Endocrinol. 2002;16:1378–1385. doi: 10.1210/mend.16.6.0835. [DOI] [PubMed] [Google Scholar]
- 12.Wang L, Schuster GU, Hultenby K, Zhang Q, Andersson S, Gustafsson JA. Liver x receptors in the central nervous system: From lipid homeostasis to neuronal degeneration. Proc Natl Acad Sci U S A. 2002;99:13878–13883. doi: 10.1073/pnas.172510899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Morales JR, Ballesteros I, Deniz JM, Hurtado O, Vivancos J, Nombela F, Lizasoain I, Castrillo A, Moro MA. Activation of liver x receptors promotes neuroprotection and reduces brain inflammation in experimental stroke. Circulation. 2008 doi: 10.1161/CIRCULATIONAHA.108.782300. [DOI] [PubMed] [Google Scholar]
- 14.Sironi L, Mitro N, Cimino M, Gelosa P, Guerrini U, Tremoli E, Saez E. Treatment with lxr agonists after focal cerebral ischemia prevents brain damage. FEBS Lett. 2008 doi: 10.1016/j.febslet.2008.08.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Verschuren L, de Vries-van der Weij J, Zadelaar S, Kleemann R, Kooistra T. Lxr agonist suppresses atherosclerotic lesion growth and promotes lesion regression in apoe*3leiden mice: Time course and potential mechanisms. J Lipid Res. 2008 doi: 10.1194/jlr.M800374-JLR200. [DOI] [PubMed] [Google Scholar]
- 16.Mao Y, Yang GY, Zhou LF, Stern JD, Betz AL. Focal cerebral ischemia in the mouse: Description of a model and effects of permanent and temporary occlusion. Brain Res Mol Brain Res. 1999;63:366–370. doi: 10.1016/s0169-328x(98)00271-x. [DOI] [PubMed] [Google Scholar]
- 17.Riddell DR, Zhou H, Comery TA, Kouranova E, Lo CF, Warwick HK, Ring RH, Kirksey Y, Aschmies S, Xu J, Kubek K, Hirst WD, Gonzales C, Chen Y, Murphy E, Leonard S, Vasylyev D, Oganesian A, Martone RL, Pangalos MN, Reinhart PH, Jacobsen JS. The lxr agonist to901317 selectively lowers hippocampal abeta42 and improves memory in the tg2576 mouse model of alzheimer’s disease. Mol Cell Neurosci. 2007;34:621–628. doi: 10.1016/j.mcn.2007.01.011. [DOI] [PubMed] [Google Scholar]
- 18.Hernandez TD, Schallert T. Seizures and recovery from experimental brain damage. Exp Neurol. 1988;102:318–324. doi: 10.1016/0014-4886(88)90226-9. [DOI] [PubMed] [Google Scholar]
- 19.Swanson RA, Morton MT, Tsao-Wu G, Savalos RA, Davidson C, Sharp FR. A semiautomated method for measuring brain infarct volume. J Cereb Blood Flow Metab. 1990;10:290–293. doi: 10.1038/jcbfm.1990.47. [DOI] [PubMed] [Google Scholar]
- 20.Nourhaghighi N, Teichert-Kuliszewska K, Davis J, Stewart DJ, Nag S. Altered expression of angiopoietins during blood-brain barrier breakdown and angiogenesis. Lab Invest. 2003;83:1211–1222. doi: 10.1097/01.lab.0000082383.40635.fe. [DOI] [PubMed] [Google Scholar]
- 21.Sandhu R, Teichert-Kuliszewska K, Nag S, Proteau G, Robb MJ, Campbell AI, Kuliszewski MA, Kutryk MJ, Stewart DJ. Reciprocal regulation of angiopoietin-1 and angiopoietin-2 following myocardial infarction in the rat. Cardiovasc Res. 2004;64:115–124. doi: 10.1016/j.cardiores.2004.05.013. [DOI] [PubMed] [Google Scholar]
- 22.Li Y, Jiang N, Powers C, Chopp M. Neuronal damage and plasticity identified by microtubule-associated protein 2, growth-associated protein 43, and cyclin d1 immunoreactivity after focal cerebral ischemia in rats. Stroke. 1998;29:1972–1980. doi: 10.1161/01.str.29.9.1972. discussion 1980–1971. [DOI] [PubMed] [Google Scholar]
- 23.Zhang ZG, Zhang L, Jiang Q, Chopp M. Bone marrow-derived endothelial progenitor cells participate in cerebral neovascularization after focal cerebral ischemia in the adult mouse. Circ Res. 2002;90:284–288. doi: 10.1161/hh0302.104460. [DOI] [PubMed] [Google Scholar]
- 24.Chen J, Zhang ZG, Li Y, Wang Y, Wang L, Jiang H, Zhang C, Lu M, Katakowski M, Feldkamp CS, Chopp M. Statins induce angiogenesis, neurogenesis, and synaptogenesis after stroke. Ann Neurol. 2003;53:743–751. doi: 10.1002/ana.10555. [DOI] [PubMed] [Google Scholar]
- 25.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative pcr and the 2(−delta delta c(t)) method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 26.Rikitake Y, Hirata K, Kawashima S, Ozaki M, Takahashi T, Ogawa W, Inoue N, Yokoyama M. Involvement of endothelial nitric oxide in sphingosine-1-phosphate-induced angiogenesis. Arterioscler Thromb Vasc Biol. 2002;22:108–114. doi: 10.1161/hq0102.101843. [DOI] [PubMed] [Google Scholar]
- 27.Schultz JR, Tu H, Luk A, Repa JJ, Medina JC, Li L, Schwendner S, Wang S, Thoolen M, Mangelsdorf DJ, Lustig KD, Shan B. Role of lxrs in control of lipogenesis. Genes Dev. 2000;14:2831–2838. doi: 10.1101/gad.850400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Grefhorst A, Elzinga BM, Voshol PJ, Plosch T, Kok T, Bloks VW, van der Sluijs FH, Havekes LM, Romijn JA, Verkade HJ, Kuipers F. Stimulation of lipogenesis by pharmacological activation of the liver x receptor leads to production of large, triglyceride-rich very low density lipoprotein particles. J Biol Chem. 2002;277:34182–34190. doi: 10.1074/jbc.M204887200. [DOI] [PubMed] [Google Scholar]
- 29.Sumi M, Sata M, Miura SI, Rye KA, Toya N, Kanaoka Y, Yanaga K, Ohki T, Saku K, Nagai R. Reconstituted high-density lipoprotein stimulates differentiation of endothelial progenitor cells and enhances ischemia-induced angiogenesis. Arterioscler Thromb Vasc Biol. 2007 doi: 10.1161/01.ATV.0000259299.38843.64. [DOI] [PubMed] [Google Scholar]
- 30.Deng AW, Yuang XG, Wei D, Zhang JH, Ran CF, Wang M. effect of power-frequency electromagnetic fields on stroke during rehabilitation. Di Yi Jun Yi Da Xue Xue Bao. 2004;24:946–949. 952. [PubMed] [Google Scholar]
- 31.Tallquist MD, Soriano P, Klinghoffer RA. Growth factor signaling pathways in vascular development. Oncogene. 1999;18:7917–7932. doi: 10.1038/sj.onc.1203216. [DOI] [PubMed] [Google Scholar]
- 32.Hughes S, Chan-Ling T. Characterization of smooth muscle cell and pericyte differentiation in the rat retina in vivo. Invest Ophthalmol Vis Sci. 2004;45:2795–2806. doi: 10.1167/iovs.03-1312. [DOI] [PubMed] [Google Scholar]
- 33.Chopp M, Zhang ZG, Jiang Q. Neurogenesis, angiogenesis, and mri indices of functional recovery from stroke. Stroke. 2007;38:827–831. doi: 10.1161/01.STR.0000250235.80253.e9. [DOI] [PubMed] [Google Scholar]
- 34.Ramet ME, Ramet M, Lu Q, Nickerson M, Savolainen MJ, Malzone A, Karas RH. High-density lipoprotein increases the abundance of enos protein in human vascular endothelial cells by increasing its half-life. J Am Coll Cardiol. 2003;41:2288–2297. doi: 10.1016/s0735-1097(03)00481-9. [DOI] [PubMed] [Google Scholar]
- 35.Lee CM, Chien CT, Chang PY, Hsieh MY, Jui HY, Liau CS, Hsu SM, Lee YT. High-density lipoprotein antagonizes oxidized low-density lipoprotein by suppressing oxygen free-radical formation and preserving nitric oxide bioactivity. Atherosclerosis. 2005;183:251–258. doi: 10.1016/j.atherosclerosis.2005.03.029. [DOI] [PubMed] [Google Scholar]
- 36.Mineo C, Deguchi H, Griffin JH, Shaul PW. Endothelial and antithrombotic actions of hdl. Circ Res. 2006;98:1352–1364. doi: 10.1161/01.RES.0000225982.01988.93. [DOI] [PubMed] [Google Scholar]
- 37.Yu J, deMuinck ED, Zhuang Z, Drinane M, Kauser K, Rubanyi GM, Qian HS, Murata T, Escalante B, Sessa WC. Endothelial nitric oxide synthase is critical for ischemic remodeling, mural cell recruitment, and blood flow reserve. Proc Natl Acad Sci U S A. 2005;102:10999–11004. doi: 10.1073/pnas.0501444102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kashiwagi S, Izumi Y, Gohongi T, Demou ZN, Xu L, Huang PL, Buerk DG, Munn LL, Jain RK, Fukumura D. No mediates mural cell recruitment and vessel morphogenesis in murine melanomas and tissue-engineered blood vessels. J Clin Invest. 2005;115:1816–1827. doi: 10.1172/JCI24015. [DOI] [PMC free article] [PubMed] [Google Scholar]