Abstract
We examined the role of interleukin (IL)-18 and cytokine-induced neutrophil chemokines (CINC)-1 and CINC-3 in the neutrophil release of superoxide anion (O2−) and elastase following alcohol/ethanol (EtOH) and burn injury. Male rats (∼250 g) were gavaged with EtOH to achieve a blood EtOH level of ∼100 mg/dl before ∼12.5% total body surface area burn or sham injury. Immediately after injury, rats were administered with anti-rat IL-18 antibody (80 μg/kg) or isotype control. After 20 min, anti-IL-18 antibody-treated rats were given either recombinant (r) rat CINC-1 or CINC-3. On day 1 after injury, the combined insult of EtOH and burn injury caused a significant increase in neutrophil elastase and O2− production as well as an increase in neutrophil accumulation, myeloperoxidase activity, and edema in the intestine. Treatment of rats with anti-IL-18 antibody normalized the above parameters. However, administration of rCINC-1 in anti-IL-18 antibody-treated rats increased the above parameters to levels similar to those observed following EtOH and burn injury. In contrast, administration of rCINC-3 did not influence the above parameters except neutrophil elastase. These findings indicate that IL-18 and CINC-1 may independently modulate neutrophil tissue-damaging actions following EtOH and burn injury. However, the finding that the treatment of rats with anti-IL-18 antibodies inhibits CINC-1 and CINC-3 supports the notion that IL-18 plays a critical role in increased neutrophil tissue-damaging action following a combined insult of EtOH intoxication and burn injury.
Keywords: thermal injury, ethanol, reactive oxygen species, proteases, intestine permeability, cytokines
burns and other traumatic injuries remain the leading cause of death in all ages. Furthermore, a significant number of studies have indicated that nearly half of these injuries occur under the influence of alcohol/ethanol (EtOH) intoxication (4, 30, 32, 34, 51). Studies have also suggested that EtOH intoxication at the time of injury potentiates the suppression of host defense and thus can produce infectious complications (5, 19, 23, 31–34, 34, 47). Our previous studies have shown that EtOH intoxication before burn injury exacerbates the suppression of intestinal T cell functions, deteriorates intestinal barrier functions, and increases intestinal bacterial translocation (3, 5, 26–29). Gut epithelial barrier dysfunction and subsequent translocation of bacteria are often implicated in the pathogenesis associated with EtOH exposure, major trauma, and burn injury (5, 17, 24, 43, 48, 49).
Additional findings from our laboratory have shown that the increase in intestinal permeability following a combined insult of EtOH and burn injury was accompanied with an increase in interleukin (IL)-18 production (26, 28, 42). IL-18, like IL-12, was discovered initially to be a cytokine that drives the T cell toward T helper-1 cell subtype and thus was referred as interferon (IFN)-γ-inducing factor (37, 38). However, subsequent studies found that IL-18 is pleiotropic in nature and may cause tissue damage in various inflammatory and disease conditions (7, 20–22, 36, 46, 50, 53). Although many of these studies indicated IL-18-induced IFN-γ to be the cause of tissue damage, we found that IL-18 promotes recruitment of neutrophils to lung and intestine and thus causes tissue damage in those organs (26, 28, 42).
Studies have shown that neutrophils migrate through the endothelium of blood vessels to extravascular inflammatory sites to destroy pathogens by releasing toxic oxygen radical species and proteolytic enzymes. However, excess release of these agents may cause tissue damage in various inflammatory conditions, such as shock, trauma, and burn injury (10, 15, 39, 43, 49). In a recent study, we observed that acute EtOH intoxication potentiates neutrophil release of superoxide anions (O2−) (29). Thus an increase in neutrophil accumulation and the release of O2− and proteolytic enzymes (e.g., elastase) may result in intestinal epithelial damage, capillary leakage, alteration of intestinal permeability, and increase in translocation of bacteria to extraintestinal sites (8, 9, 12, 24, 43). Treatment of animals with antineutrophil antiserum to deplete neutrophils prevented neutrophil-mediated intestinal injury (28). These findings strongly suggest that neutrophils play a critical role in organ damage following EtOH intoxication and burn injury.
Although the mechanism by which EtOH combined with burn injury upregulates neutrophil tissue-damaging actions remains unknown, our recent findings indicate that IL-18 upregulates cytokine-induced neutrophil chemokines (CINC)-1 and CINC-3 and intercellular adhesion molecule 1 in the intestine and lungs following EtOH and burn injury (25, 26, 28). Because neutrophils are known to have receptors for IL-18, the present study investigated the role of IL-18 in increased neutrophil O2− and elastase release. We also examined the role of neutrophil chemokines CINC-1 and CINC-3 in increased neutrophil recruitment to the intestine following EtOH and burn injury. Moreover, to determine whether or not the neutrophil chemokines influence neutrophil activation, we further examined the role of CINC-1 and CINC-3 in IL-18-mediated increase in neutrophil O2− and elastase release following EtOH and burn injury.
MATERIALS AND METHODS
Animals and reagents.
Male Sprague-Dawley rats (225–250 g) were obtained from Charles River Laboratories (Wilmington, MA). Anti-rat IL-18 antibody, recombinant (r) rat CINC-1, and recombinant rat CINC-3 were purchased from R&D Systems (Minneapolis, MN).
Rat model of acute EtOH and burn injury.
Rats were divided into two major groups, saline + sham and EtOH + burn. In the EtOH group, the level of blood EtOH equivalent to 90–100 mg/dl was achieved by gavage feeding of 5 ml 20% EtOH in saline. In the saline group, animals were gavaged with 5 ml of saline. Four hours after gavage, all animals were anesthetized and transferred into a template, which was fabricated to expose ∼12.5% of the total body surface area. For burn injury, rats were immersed in boiling water (∼97°C) for 10–12 s (25, 26, 28, 29). Sham rats were subjected to identical anesthesia and immersed in lukewarm water. Animals were resuscitated intraperitoneally with 10 ml physiological saline, and were administered anti-rat IL-18 antibody (80 μg/kg) or isotype IgG (Santa Cruz Biotechnology, Santa Cruz, CA) intraperitoneally. After 20 min, anti-IL-18 antibody-treated rats were given either recombinant rat CINC-1 (12 μg/kg) or CINC-3 (20 μg/kg). On day 1 after injury, rats were euthanized.
All the experiments were carried out in adherence to the National Institutes of Health Guidelines for the Care and Use of Laboratory Animals and were approved by the University of Alabama at Birmingham and Loyola University Medical Center, Maywood, Animal Institutional Care and Use Committees.
Isolation of neutrophils.
As described in our previous study (29), blood was drawn via cardiac puncture into a heparinized tube. Heparinized whole blood was diluted 1:2 with PBS. The blood was then added slowly to Ficoll Paque (GE Healthcare, Uppsala, Sweden) from the side of the tube and centrifuged at 300 g for 40 min. The pellet containing red blood cells (RBCs) and neutrophil was suspended in PBS and mixed with 3% dextran (Fisher Scientific, Pittsburgh, PA). The cell suspension was left on a plane surface for 1 h at room temperature. Neutrophil-rich supernatant was collected and centrifuged at 300 g for 20 min at 10°C. The RBCs were lysed by the addition of sterile distilled water followed by the addition of 10× HBSS and centrifuged at 300 g for 30 min at 10°C. The purified neutrophils settled at the bottom were resuspended in HBSS and used for subsequent studies.
Measurement of neutrophil O2 anions.
As we have described previously (29), neutrophil superoxide anion release was determined by cytochrome c reduction assay. Briefly, 0.1 ml of neutrophil (5 × 106 cells/ml in HBSS) was incubated with cytochrome c or cytochrome c plus superoxide dismutase for 5 min at 37°C in a 96-well plate. Neutrophil O2− production was initiated by adding phorbol esters (PMA) at a dose of 500 ng/ml. Although we have used lower doses of PMA (50 and 100 ng/ml), a maximum response was obtained with a dose of 500 ng/ml. The absorbance of reduced cytochrome c was measured continuously for 1 h at 550 nm. The peak O2− concentration was achieved ∼20–25 min after neutrophil stimulation with PMA. These peak values were recorded, pooled, and are expressed as means ± SE in results.
Measurement of neutrophil elastase.
Elastase production was measured by activating the neutrophils (2.5 × 106 cells/ml) with PMA (500 ng/ml) at 37°C for 1 h. Cells were washed and lysed, and the supernatant was collected for estimation of elastase activity (54). The lysates (25 μl) were incubated in a 96-well plate at room temperature for 60 min with 1 mM methoxy-succinyl-alanyl-alanyl-prolyl-valyl-p-nitroanilide (Sigma Chemical), 0.1 M HEPES, and 0.5 M NaCl (pH 7.5) in a total volume of 150 μl. Absorbance was measured at 405 nm.
Measurement of intestinal tissue edema.
A piece of small intestine was removed, weighed, and dried for 24 h at 80°C. Water content (%) of intestinal tissue was calculated as (wet weight − dry weight)/wet weight × 100 and was used as a measure of tissue edema (25, 26, 28, 29).
Preparation of intestinal homogenates.
Immediately after the rats were anesthetized, intestine was exposed. Leaving approximately the first 15 cm proximal segment of intestine, a 3-cm length of intestine was removed, cleaned, and snap frozen. Equal weights (100 mg wet weight) of intestine from various groups were suspended in 1 ml PBS and sonicated at 30 cycles, twice, for 30 s on ice. Homogenates were cleared by centrifuging at 10,000 revolution/min (or 10,600 g) at 4°C, and the supernatants were stored at −70°C (25, 26, 28, 29). Protein levels in the homogenates were determined using the Bio-Rad (Hercules, CA) assay kit.
Measurement of intestinal tissue myeloperoxidase levels.
Myeloperoxidase (MPO) activity was measured by incubating intestinal tissue homogenates (10 μl) in a 96-well plate with 290 μl of 50 mM phosphate buffer, 3 μl of substrate solution containing 20 mg/ml o-dianisidine hydrochloride, and 3 μl of 20 mM H2O2. The reaction was stopped by adding 3 μl of 30% sodium azide. Plates were read at 460 nm (25, 26, 28, 29).
Measurement of intestinal tissue IL-18, CINC-1, and CINC-3.
IL-18, CINC-1, and CINC-3 levels in the intestinal tissue homogenates were measured using the ELISA kits (R&D Systems) following the manufacturer's instructions. The concentrations in the samples were determined using the standard curve and by normalizing with protein (25, 26, 28, 29).
Immunohistochemical detection of neutrophils.
Approximately 0.5–1.0-cm-long small intestine rings were fixed in formaldehyde, embedded in paraffin, and cut into ∼5-μm-thick sections using a microtome. After dewaxing and rehydrating, the antigenic site retrieval of the sections was accomplished by boiling each slide for 20 min in 0.01 M citric acid buffer (pH 6.0). Nonspecific staining was blocked with 5% goat serum in PBS buffer. Thereafter, sections were incubated with anti-neutrophil antibody (Accurate Chemical and Scientific, Westbury, NY) for 2 h at room temperature. After being washed in PBS, endogenous peroxidase in intestine sections was blocked using 0.5% hydrogen peroxide solution in PBS. Sections were further incubated with biotinylated goat anti-rabbit secondary antibody. After being washed in PBS buffer, sections were incubated with diaminobenzidine substrate solution with peroxidase enzyme and counterstained with hematoxylin solution (48). Representative sections were selected for the presentation. Some sections were also stained with hematoxylin-eosin for histological analysis (42, 48). Representative sections were selected for the presentation.
Statistical analysis.
These data are presented as means ± SE and were analyzed using the ANOVA statistical program. A P value <0.05 between groups was considered statistically significant.
RESULTS
Intestinal IL-18, CINC-1, and CINC-3 levels.
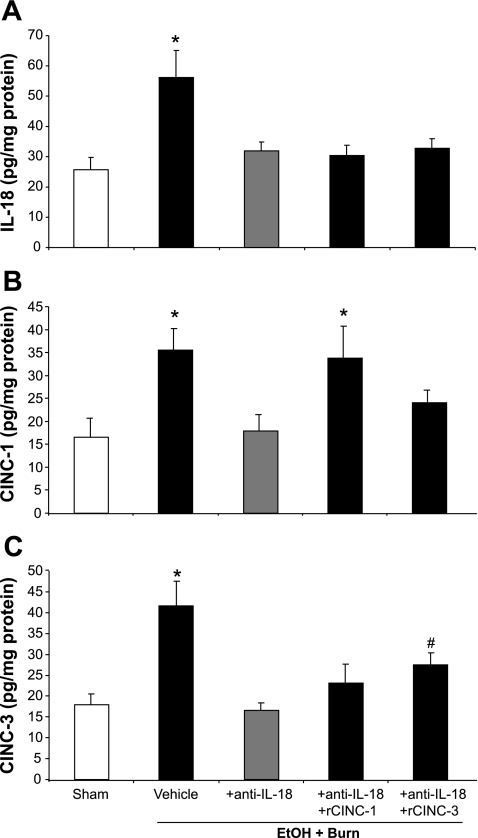
We have shown earlier that on day 1 after EtOH intoxication or burn injury there was no significant change in the intestine tissue levels of IL-18, CINC-1, and CINC-3 compared with shams gavaged with saline (26, 28). However, a significant increase in IL-18, CINC-1, and CINC-3 was observed in the intestine of rats subjected to a combined insult of EtOH intoxication and burn injury compared with shams (Fig. 1). To determine whether the increase in IL-18 is responsible for the increase in CINC-1 and CINC-3, a group of EtOH plus burn-injured rats was treated with anti-IL-18 antibodies, and the effect of this treatment was determined on intestinal levels of IL-18, CINC-1, and CINC-3. The results shown in Fig. 1 clearly indicate that administration of IL-18-neutralizing antibodies (80 μg/kg) decreased the levels of IL-18 (Fig. 1A) as well as CINC-1 (Fig. 1B) and CINC-3 (Fig. 1C) to the levels observed in sham animals. The restitution of CINC-1 or CINC-3 in anti-IL-18 antibody-treated rats did not affect the intestinal IL-18 levels; however, the levels of CINC-1 and CINC-3 were respectively restored to the levels observed following EtOH plus burn injury. Although administration of recombinant CINC-3 also enhanced the levels of CINC-1 in anti-IL-18 antibody-treated rats following EtOH plus burn injury, those levels were not significantly different from sham or the IL-18 antibody-treated group. Similarly, restitution of CINC-1 slightly increased the CINC-3 level, but it was not significantly different from sham or the anti-IL-18 antibody-treated group.
Fig. 1.
Intestinal IL-18, cytokine-induced neutrophil chemokine (CINC)-1, and CINC-3 levels. One day after injury, intestines were collected from all 5 groups, and equal weights of tissue were homogenized. IL-18, CINC-1, and CINC-3 levels were measured using ELISA kit; the values are normalized to the protein and expressed as pg/mg protein. Data are means ± SE from at least 6 animals in each group. *P < 0.05 compared with other groups. #P < 0.05 compared with sham and EtOH + Burn + anti-IL-18. r, recombinant.
Neutrophil superoxide anion production.
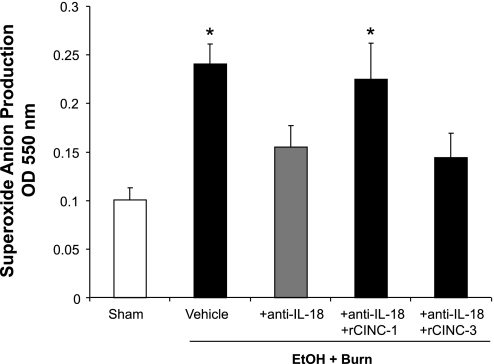
Figure 2 shows that neutrophils from the vehicle-treated EtOH plus burn group have significantly higher O2− production compared with the neutrophils obtained from sham rats. Treatment with anti-IL-18 antibody significantly reduced the level of O2− production. When IL-18 antibody-treated animals were administered with rCINC-1, the level of neutrophil O2− production was restored to the vehicle-treated EtOH plus burn group levels. In contrast, administration of anti-IL-18 antibody-treated animals with rCINC-3 did not alter the level of neutrophil O2− production.
Fig. 2.
Neutrophil superoxide anion production. One day after injury, blood was drawn via cardiac puncture and neutrophils were isolated. Neutrophil (5 × 106 cells/ml) O2− production was measured after stimulation with phorbol myristate acetate (PMA) (500 ng/ml) by using cytochrome c reduction assay. Plates were read at an outer diameter (OD) of 550 nm after every 1 min for 60 min. The peak O2− concentration was achieved ∼20–25 min after neutrophil stimulation with PMA. These peak values were recorded and pooled and are shown as means ± SE from at least 6 animals in each group. *P < 0.05 compared with sham and EtOH + Burn + anti-IL-18.
Neutrophil elastase release.
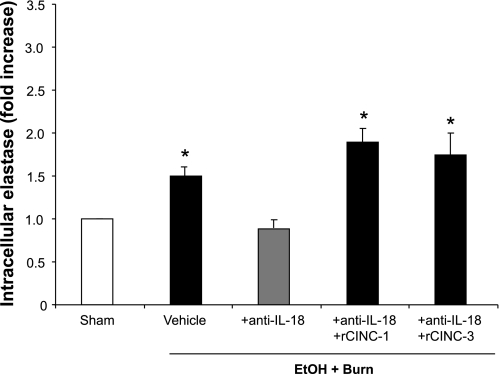
Neutrophils from the vehicle-treated EtOH plus burn group showed significant increase in the intracellular elastase levels compared with the sham group (Fig. 3). Treatment with anti-IL-18 antibody reduced the level of elastase to the sham level. The restitution of CINC-1 or CINC-3 in anti-IL-18 antibody-treated rats restored neutrophil elastase release to the levels observed in vehicle-treated EtOH plus burn group.
Fig. 3.
Neutrophil intracellular elastase levels. One day after injury, blood was drawn via cardiac puncture and neutrophils were isolated. Neutrophil (2.5 × 106 cells/ml) elastase was measured after stimulation with PMA (500 ng/ml) at 37°C for 1 h. Cells were washed and lysed, and supernatant was collected for the estimation of intracellular elastase. Data are shown as means ± SE of fold increase from at least 6 animals in each group. *P < 0.05 compared with sham and EtOH + Burn + anti-IL-18.
Small intestinal neutrophil infiltration.
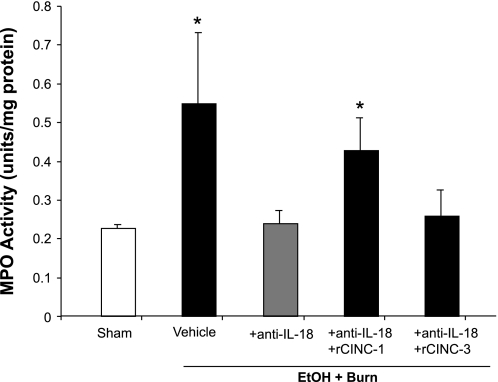
As shown in Fig. 4, MPO activity in the intestine increased significantly in the vehicle-treated EtOH plus burn group compared with shams. Administration of anti-IL-18 antibodies prevented the increase in intestinal MPO activity following EtOH plus burn injury. The restitution of CINC-1 in anti-IL-18-treated animals resulted in a significant increase in the MPO activity compared with shams or anti-IL-18 antibody-treated group. However, there was no increase in the intestinal MPO activity after restitution of CINC-3.
Fig. 4.
Intestinal myeloperoxidase (MPO) activity. One day after injury, intestines were collected from all 5 treatment groups, and equal weights of tissue were homogenized. MPO levels in the homogenates were measured using the procedure described in materials and methods and normalized to the protein. Data are means ± SE from at least 6 animals in each group. *P < 0.05 compared with sham and EtOH + Burn + anti-IL-18.
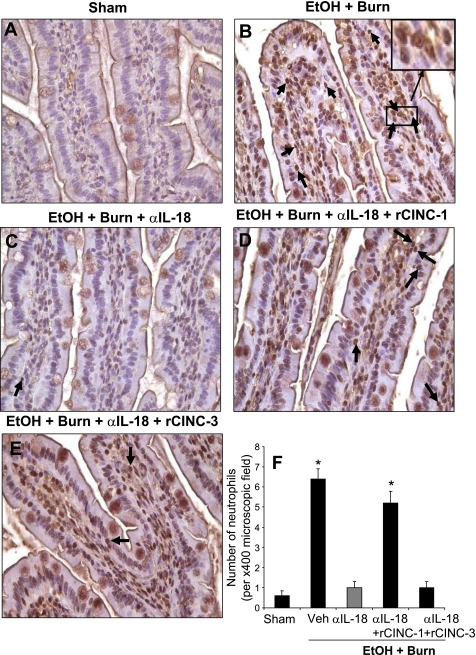
The neutrophil presence was further confirmed by immunohistochemical analysis of intestine sections using anti-neutrophil antibody. The results as shown in Fig. 5 indicate a large number of neutrophils in the intestine sections prepared from rats receiving EtOH plus burn injury compared with shams (Fig. 5). Administration of IL-18 antibody in rats following EtOH and burn injury reduced the number of neutrophils in the intestinal tissue. The restitution of CINC-1 in anti-IL-18-treated animals increased the neutrophil infiltration in the intestine following EtOH and burn injury. Administration of rCINC-3 in anti-IL-18 antibody-treated animals, however, did not affect the neutrophil number in the intestine following EtOH and burn injury.
Fig. 5.
Intestinal neutrophil infiltration as determined by immunoperoxidase staining using anti-neutrophil antibody on day 1 after injury. Representative photomicrographs of intestine from all 5 experimental groups are shown (A–E). The sections were examined with Nikon Eclipse 50i microscope with a magnification of ×400. The sections were photographed with a camera attached to the microscope, and the images were digitally transferred into Photoshop. Inset in B shows digitally enhanced neutrophil infiltrated area. Along with immunopositivity, the presence of neutrophils was also confirmed by their distinct morphological nature with a multilobed nucleus. Each section was scanned for 3–4 microscopic fields, and the data thus obtained from more than 4 animals in each group were pooled and presented in F as means ± SE. *P < 0.05 compared with sham and EtOH + Burn + αIL-18. α, anti; Veh, vehicle.
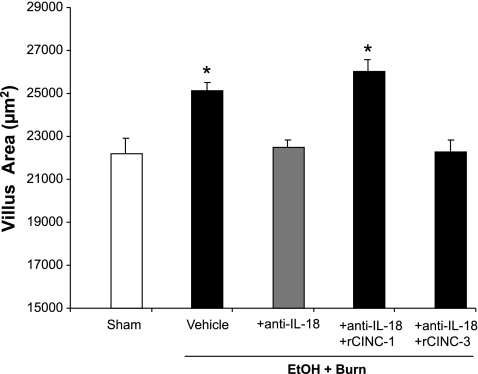
Intestinal villus area and tissue edema.
There was a significant increase in the intestinal villus area of rats receiving EtOH plus burn injury compared with the shams (Fig. 6). Treatment of rats with anti-IL-18 antibody reduced the villus area to the sham level. However, the restitution of CINC-1 and not CINC-3 in anti-IL-18 antibody-treated animals increased the villus area to levels similar to those observed following EtOH and burn injury.
Fig. 6.
Intestinal villus area. One day after injury, intestines were removed and sectioned. The sections were stained with hematoxylin-eosin, and morphometric analysis of villi was done using Nikon eclipse 50i microscope with a magnification of ×400. Each section was scanned for 3–4 microscopic fields with 4 animals in each group. Villus area of 150 villi from more than 4 animals in each group was measured using NIS-Elements AR 2.30 software. Data thus obtained were pooled and presented as means ± SE. *P < 0.05 compared with sham and EtOH + Burn + anti-IL-18.
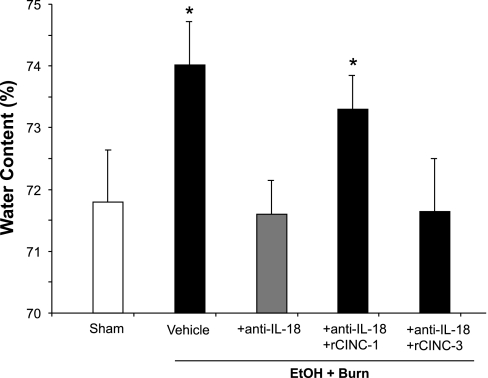
Intestinal tissue edema as determined by water content was significantly increased in rats receiving EtOH plus burn injury (Fig. 7). The treatment of rats with anti-IL-18 antibodies significantly reduced the edema formation following EtOH and burn injury. However, the restitution of CINC-1 in anti-IL-18 antibody-treated animals significantly increased the intestinal tissue water content. In contrast, CINC-3 restitution did not influence the intestinal water content in anti-IL-18 antibody-treated animals following EtOH and burn injury.
Fig. 7.
Intestinal tissue edema formation. One day after injury, intestines were collected from all 5 groups. Intestinal tissue water content was determined by drying the tissue for 24 h at 80°C. Water content (%) was calculated as (wet weight − dry weight)/wet weight × 100. Data are means ± SE from at least 6 animals in each group. *P < 0.05 compared with sham and EtOH + Burn + anti-IL-18.
DISCUSSION
In our previous studies, we have shown that on day 1 after EtOH intoxication or burn injury alone there was no significant change in the intestine tissue levels of IL-18, CINC-1, and CINC-3 compared with shams gavaged with saline (26, 28). Furthermore, neutrophil O2− and its infiltration into the intestine was not found to be significantly different following EtOH intoxication or burn injury alone compared with sham rats gavaged with saline (29). Additionally, unpublished findings from our laboratory indicate that EtOH or burn injury alone did not significantly affect the neutrophil elastase levels (X. Li and M. A. Choudhry). However, a combined insult of EtOH intoxication and burn injury resulted in increased intestinal levels of IL-18, neutrophil chemokines (CINC-1 and CINC-3), neutrophil superoxide anion and elastase, neutrophil accumulation, MPO activity, and intestinal edema. Treatment of rats with anti-IL-18 antibody immediately after injury prevented the increase in the above parameters following EtOH intoxication and burn injury. The restitution of CINC-1 in anti-IL-18 antibody-treated rats markedly increased intestinal levels of CINC-1, neutrophil superoxide anion and elastase, neutrophil accumulation, MPO activity, and intestinal edema. The restitution of CINC-3 in IL-18 antibody-treated animals, on the other hand, did not influence the above parameters except the neutrophil elastase, which was restored to the levels observed after EtOH and burn injury. These findings indicate that, similar to IL-18, CINC-1 may also help in the neutrophil recruitment to the intestine and in the production of superoxide anion and elastase. Nevertheless, the finding that treatment of rats with anti-IL-18 antibodies inhibits CINC-1 and CINC-3 further supports the notion that IL-18 plays a critical role in increased neutrophil tissue-damaging action following a combined insult of EtOH intoxication and burn injury.
IL-18 is a member of the IL-1 cytokine superfamily. It was initially characterized as IFN-γ-inducing factor and thus was recognized as an important player in host defense (37, 38). IL-18 is synthesized as a precursor protein (pro-IL-18), and, in the presence of IL-1β-converting enzyme (or caspase-1), it matures into 18-kDa active protein (6, 13, 35). IL-18 is produced from macrophage-like cells and from epithelial cells, including intestinal epithelial cells (6, 13, 35). In our previous studies, we have reported that EtOH intoxication combined with burn injury increases the level of IL-18 in intestine and lungs (25, 26, 28). We further found that IL-18 helps in the recruitment of neutrophils (25, 26, 28, 29). Jordan et al. (20) have shown that intratracheal administration of IL-18 resulted in increased infiltration of neutrophils and caused significant increase in lung vascular permeability. Conversely, intratracheal instillation of anti-IL-18 antibodies in inflamed lung greatly reduced the recruitment of these cells and prevented increase in vascular permeability (20). Studies have also shown that intratracheal administration of IL-18 bp resulted in suppressed lung vascular permeability and decreased bronchoalveolar lavage content of neutrophils, cytokines, and chemokines (20). Moreover, our recent findings showed that the depletion of neutrophils prevents intestinal and lung tissue damage (25, 28). Altogether, these findings strongly support the suggestion that neutrophils are critical in IL-18-dependent intestinal tissue damage. Similar roles of neutrophils are implicated in other pathological conditions such as acute respiratory distress syndrome and tissue ischemia (11, 14, 40, 43, 48, 55). Because IL-18 receptors are present on neutrophils (35, 54), it is likely that IL-18 can directly recruit neutrophils. Alternatively, it is also possible that IL-18 induces the production of neutrophil chemotactic factors such as CINC, which in turn may play a role in neutrophil recruitment to intestine and other organs in conditions such as EtOH intoxication and burn injury.
Neutrophil infiltration and subsequent edema are the early markers of tissue damage. Earlier studies have shown that EtOH intoxication with or without burn injury activates neutrophils to release O2− and proteases (8, 16). Although such release of O2− and proteases by neutrophils is important for the host defense and the killing of pathogens, excessive release of these products may cause tissue damage. It is widely believed that, after performing their functions, neutrophils undergo apoptosis and are cleared by macrophages. However, studies have shown that following major injury the production of inflammatory mediators not only activates the neutrophils but also enhances their survival. Consistent with these findings, we have recently shown that EtOH intoxication combined with burn injury delays neutrophil apoptosis (29) and thus may further add to the neutrophil-dependent pathology associated with EtOH and burn injury.
Our results indicate that IL-18 plays a role in the increased release of neutrophil O2− and elastase levels after the combined insult of EtOH intoxication and burn injury. We further found that CINC-1 may also independently enhance neutrophil ability to produce O2− and elastase. In contrast, CINC-3, another potent neutrophil chemokine, did not influence the neutrophil effector responses following EtOH and burn injury. Both CINC-1 and CINC-3 are members of the IL-8 family and are potent chemotactic factors for neutrophils (14, 41). Chemotaxis of neutrophils is an important, functional response to chemokines and is a key event in the recruitment of neutrophils in inflammation. CINC is a member of α cysteine-x-cysteine subfamily of chemokines and is classified as CINC-1, CINC-2α, CINC-2β, and CINC-3. Although all three CINCs play roles in neutrophil recruitment, many studies have suggested that CINC-1 and CINC-3 play a predominant role in neutrophil recruitment (14, 25, 26, 28, 41). With the use of CINC antibodies, it was demonstrated that CINC-1 and CINC-3 contribute significantly to the influx of neutrophils in rat inflammation models, including lung injury and lipopolysaccharide-induced inflammation (18, 44). In our studies, we observed that the levels of both CINC-1 and CINC-3 are elevated after a combined insult of EtOH and burn injury. However, the administration of anti-IL-18 antibody prevented the increase in CINC-1 and CINC-3, suggesting a role of IL-18 in their upregulation.
The mechanism by which IL-18 and neutrophil chemokines modulate the neutrophil O2− production and elastase release remains to be investigated. Studies have shown that neutrophils constitutively express IL-18 receptor. Findings from multiple studies have shown that a membrane-bound multicomponent NADPH oxidase consisting of six subunits is responsible for the production of oxygen radicals (1, 45). In resting neutrophils, four of the six subunits, p47phox, p67phox, p40phox, and the small GTPase and Rac2, are localized in the cytoplasm, whereas the remaining two subunits, p22phox and gp91phox, form a heterodimeric membrane-bound flavocytochrome known as cytochrome b558 (1, 45). Upon activation, the cytosolic components p40phox, p47phox, p67phox, and Rac-2 translocate to the membrane where they associate with flavocytochrome b558 to form the active oxidase complex. Previous studies have shown that neutrophil exposure to low levels of cytokines and chemotactic factors (e.g., tumor necrosis factor-α, platelet-activating factor, and lipopolysaccharide) does not fully activate the NADPH oxidase system but results in 10- to 20-fold increase in O2− production, a stage referred to as “neutrophil priming” (45). When these primed neutrophils encounter another signal, they become fully activated to produce reactive oxygen species and elastase. Furthermore, it has also been suggested that at high concentrations the same priming agents may lead to the activation of these cells (2, 43, 54). In our recent study, we found that EtOH combined with burn injury activated the p47phox and p67phox (2, 43, 54). Our findings further indicate that the increase in neutrophil O2− or p47phox and p67phox could result from a decrease in heme oxygenase (HO)-1 expression. HO-1 has an antioxidant role in the cell, and a decrease in HO-1 may hamper the ability of cells to scavenge the O2− and thus contribute to increased neutrophil O2− levels. It is likely that IL-18 directly modulates NADPH assembly and thereby enhances neutrophil O2− production. Recent findings indicate that IL-18 downregulates HO-1 expression in endothelial cells (52). Thus it is also possible that an increase in IL-18 following EtOH and burn injury may cause a decrease in HO-1 and thereby cause an increase in neutrophil O2− under those conditions. However, both these possibilities remain to be investigated. In addition to IL-18, our findings also indicate that restitution of CINC-1 but not CINC-3 activates the neutrophils to produce O2−. In contrast restitution of both CINC-1 and CINC-3 caused an increase in neutrophil elastase release. The precise reason for the observed differences in CINC-1 and CINC-3 action on neutrophils remains to be investigated. Nonetheless, studies have indicated that CINC-1 and CINC-3 may exert different biological activities through distinct G proteins. Several other mediators including leukotrienes, vasoactive peptides, cytokines, and chemokines may activate neutrophils (43).
The present study has utilized a relatively small total body surface area burn injury, which by itself did not cause any change in the neutrophil effector responses nor has produced any deleterious effects on the intestine on day 1 after injury. There is evidence that burn injury size is a critical factor in postburn complications (28). However, other factors such as age, sex, and preclinical manifestation can also influence the outcome of burn patients especially patients with small burn injury. Similarly, EtOH consumption before burn injury has been shown to further confound postburn pathogenesis (5, 19, 23, 32–34, 47). Thus a smaller burn or single dose of EtOH by itself may not have any significant effect on neutrophil effector responses measured in this study. However, when the two insults are combined, they become severe and detrimental.
In summary, results presented in this study suggest a role of IL-18 and neutrophil chemokines in the activation of neutrophils to produce more O2− and elastase. Furthermore, like IL-18, CINC-1 may also modulate neutrophil effector responses following EtOH and burn injury. CINC-3, in contrast, did not appear to influence neutrophil responses. Regardless of the mechanism of neutrophil activation, the resulting release of O2− and elastase may contribute to the intestinal edema formation and tissue damage following EtOH and burn injury.
GRANTS
This study was supported by NIH grants R01AA015731 and R21AA015979 to M. A. Choudhry.
REFERENCES
- 1.Azim AC, Cao H, Gao X, Joo M, Malik AB, van Breemen RB, Sadikot RT, Park G, Christman JW. Regulation of cyclooxygenase-2 expression by small GTPase Rac2 in bone marrow macrophages. Am J Physiol Lung Cell Mol Physiol 293: L668–L673, 2007. [DOI] [PubMed] [Google Scholar]
- 2.Brown GE, Stewart MQ, Bissonnette SA, Elia AE, Wilker E, Yaffe MB. Distinct ligand-dependent roles for p38 MAPK in priming and activation of the neutrophil NADPH oxidase. J Biol Chem 279: 27059–27068, 2004. [DOI] [PubMed] [Google Scholar]
- 3.Choudhry MA, Fazal N, Goto M, Gamelli RL, Sayeed MM. Gut-associated lymphoid T cell suppression enhances bacterial translocation in alcohol and burn injury. Am J Physiol Gastrointest Liver Physiol 282: G937–G947, 2002. [DOI] [PubMed] [Google Scholar]
- 4.Choudhry MA, Gamelli RL, Chaudry IH. Alcohol abuse: a major contributing factor to post-burn/trauma immune complications. In: 2004 Yearbook of Intensive Care and Emergency Medicine, edited by J.-L. Vincent. New York: Springer, 2004, p. 15–26.
- 5.Choudhry MA, Rana SN, Kavanaugh MJ, Kovacs EJ, Gamelli RL, Sayeed MM. Impaired intestinal immunity and barrier function: a cause for enhanced bacterial translocation in alcohol intoxication and burn injury. Alcohol 33: 199–208, 2004. [DOI] [PubMed] [Google Scholar]
- 6.Dinarello CA, Fantuzzi G. Interleukin-18 and host defense against infection. J Infect Dis 187, Suppl 2: S370–S384, 2003. [DOI] [PubMed] [Google Scholar]
- 7.Faggioni R, Cattley RC, Guo J, Flores S, Brown H, Qi M, Yin S, Hill D, Scully S, Chen C, Brankow D, Lewis J, Baikalov C, Yamane H, Meng T, Martin F, Hu S, Boone T, Senaldi G. IL-18-binding protein protects against lipopolysaccharide- induced lethality and prevents the development of Fas/Fas ligand-mediated models of liver disease in mice. J Immunol 167: 5913–5920, 2001. [DOI] [PubMed] [Google Scholar]
- 8.Fazal N, Al Ghoul WM, Schmidt MJ, Choudhry MA, Sayeed MM. Lyn- and ERK-mediated vs. Ca2+ -mediated neutrophil O responses with thermal injury. Am J Physiol Cell Physiol 283: C1469–C1479, 2002. [DOI] [PubMed] [Google Scholar]
- 9.Fazal N, Shamim M, Zagorski J, Choudhry MA, Ravindranath T, Sayeed MM. CINC blockade prevents neutrophil Ca(2+) signaling upregulation and gut bacterial translocation in thermal injury. Biochim Biophys Acta 1535: 50–59, 2000. [DOI] [PubMed] [Google Scholar]
- 10.Fukushima R, Alexander JW, Gianotti L, Pyles T, Ogle CK. Bacterial translocation-related mortality may be associated with neutrophil-mediated organ damage. Shock 3: 323–328, 1995. [PubMed] [Google Scholar]
- 11.Gao X, Xu N, Sekosan M, Mehta D, Ma SY, Rahman A, Malik AB. Differential role of CD18 integrins in mediating lung neutrophil sequestration and increased microvascular permeability induced by Escherichia coli in mice. J Immunol 167: 2895–2901, 2001. [DOI] [PubMed] [Google Scholar]
- 12.Ginzberg HH, Cherapanov V, Dong Q, Cantin A, McCulloch CAG, Shannon PT, Downey GP. Neutrophil-mediated epithelial injury during transmigration: role of elastase. Am J Physiol Gastrointest Liver Physiol 281: G705–G717, 2001. [DOI] [PubMed] [Google Scholar]
- 13.Gracie JA, Robertson SE, McInnes IB. Interleukin-18. J Leukoc Biol 73: 213–224, 2003. [DOI] [PubMed] [Google Scholar]
- 14.Guo RF, Ward PA. Mediators and regulation of neutrophil accumulation in inflammatory responses in lung: insights from the IgG immune complex model. Free Radic Biol Med 33: 303–310, 2002. [DOI] [PubMed] [Google Scholar]
- 15.Hildebrand F, Hubbard WJ, Choudhry MA, Thobe BM, Pape HC, Chaudry IH. Are the protective effects of 17beta-estradiol on splenic macrophages and splenocytes after trauma-hemorrhage mediated via estrogen-receptor (ER)-alpha or ER-beta? J Leukoc Biol 79: 1173–1180, 2006. [DOI] [PubMed] [Google Scholar]
- 16.Hoek J, Thiele GM, Klassen LW, Mandrekar P, Zakhari S, Cook RT, Ray NB, Happel KI, Kolls JK, Kovacs EJ, Szabo G. RSA 2004: combined basic research satellite symposium-mechanisms of alcohol-mediated organ and tissue damage: inflammation and immunity and alcohol and mitochondrial metabolism: at the crossroads of life and death session one: alcohol, cellular and organ damage. Alcohol Clin Exp Res 29: 1735–1743, 2005. [DOI] [PubMed] [Google Scholar]
- 17.Homma H, Hoy E, Xu DZ, Lu Q, Feinman R, Deitch EA. The female intestine is more resistant than the male intestine to gut injury and inflammation when subjected to conditions associated with shock states. Am J Physiol Gastrointest Liver Physiol 288: G466–G472, 2005. [DOI] [PubMed] [Google Scholar]
- 18.Iida M, Watanabe K, Tsurufuji M, Takaishi K, Iizuka Y, Tsurufuji S. Level of neutrophil chemotactic factor CINC/gro, a member of the interleukin-8 family, associated with lipopolysaccharide-induced inflammation in rats. Infect Immun 60: 1268–1272, 1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jones JD, Barber B, Engrav L, Heimbach D. Alcohol use and burn injury. J Burn Care Rehabil 12: 148–152, 1991. [DOI] [PubMed] [Google Scholar]
- 20.Jordan JA, Guo RF, Yun EC, Sarma V, Warner RL, Crouch LD, Senaldi G, Ulich TR, Ward PA. Role of IL-18 in acute lung inflammation. J Immunol 167: 7060–7068, 2001. [DOI] [PubMed] [Google Scholar]
- 21.Joshi VD, Kalvakolanu DV, Hebel JR, Hasday JD, Cross AS. Role of caspase 1 in murine antibacterial host defenses and lethal endotoxemia. Infect Immun 70: 6896–6903, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kashiwamura S, Ueda H, Okamura H. Roles of interleukin-18 in tissue destruction and compensatory reactions. J Immunother 25, Suppl 1: S4–S11, 2002. [DOI] [PubMed] [Google Scholar]
- 23.Kelley D, Lynch JB. Burns in alcohol and drug users result in longer treatment times with more complications. J Burn Care Rehabil 13: 218–220, 1992. [DOI] [PubMed] [Google Scholar]
- 24.Keshavarzian A, Farhadi A, Forsyth CB, Rangan J, Jakate S, Shaikh M, Banan A, Fields JZ. Evidence that chronic alcohol exposure promotes intestinal oxidative stress, intestinal hyperpermeability and endotoxemia prior to development of alcoholic steatohepatitis in rats. J Hepatol 50: 538–547, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Li X, Kovacs EJ, Schwacha MG, Chaudry IH, Choudhry MA. Acute alcohol intoxication increases interleukin 18-mediated neutrophil infiltration and lung inflammation following burn injury in rats. Am J Physiol Lung Cell Mol Physiol 292: L1193–L1201, 2007. [DOI] [PubMed] [Google Scholar]
- 26.Li X, Rana SN, Schwacha MG, Chaudry IH, Choudhry MA. A novel role for IL-18 in corticosterone-mediated intestinal damage in a two-hit rodent model of alcohol intoxication and injury. J Leukoc Biol 80: 367–375, 2006. [DOI] [PubMed] [Google Scholar]
- 27.Li X, Schwacha MG, Chaudry IH, Choudhry MA. A role of PP1/PP2A in mesenteric lymph node T cell suppression in a two-hit rodent model of alcohol intoxication and injury. J Leukoc Biol 79: 453–462, 2006. [DOI] [PubMed] [Google Scholar]
- 28.Li X, Schwacha MG, Chaudry IH, Choudhry MA. Acute alcohol intoxication potentiates neutrophil-mediated intestine tissue damage following burn injury. Shock 29: 377–383, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Li X, Schwacha MG, Chaudry IH, Choudhry MA. Heme oxygenase-1 protects against neutrophil-mediated intestinal damage by down-regulation of neutrophil p47phox and p67phox activity and O2− production in a two-hit model of alcohol intoxication and burn injury. J Immunol 180: 6933–6940, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Maier RV Ethanol abuse and the trauma patient. Surg Infect (Larchmt) 2: 133–141, 2001. [DOI] [PubMed] [Google Scholar]
- 31.Mathis KW, Zambell K, Olubadewo JO, Molina PE. Altered hemodynamic counter-regulation to hemorrhage by acute moderate alcohol intoxication. Shock 26: 55–61, 2006. [DOI] [PubMed] [Google Scholar]
- 32.McGill V, Kowal-Vern A, Fisher SG, Kahn S, Gamelli RL. The impact of substance use on mortality and morbidity from thermal injury. J Trauma 38: 931–934, 1995. [DOI] [PubMed] [Google Scholar]
- 33.McGwin G Jr, Chapman V, Rousculp M, Robison J, Fine P. The epidemiology of fire-related deaths in Alabama, 1992–1997. J Burn Care Rehabil 21: 75–83, 2000. [DOI] [PubMed] [Google Scholar]
- 34.Messingham KA, Faunce DE, Kovacs EJ. Alcohol, injury, and cellular immunity. Alcohol 28: 137–149, 2002. [DOI] [PubMed] [Google Scholar]
- 35.Nakanishi K, Yoshimoto T, Tsutsui H, Okamura H. Interleukin-18 regulates both Th1 and Th2 responses. Annu Rev Immunol 19: 423–474, 2001. [DOI] [PubMed] [Google Scholar]
- 36.Netea MG, Fantuzzi G, Kullberg BJ, Stuyt RJ, Pulido EJ, McIntyre RC Jr, Joosten LA, Van der Meer JW, Dinarello CA. Neutralization of IL-18 reduces neutrophil tissue accumulation and protects mice against lethal Escherichia coli and Salmonella typhimurium endotoxemia. J Immunol 164: 2644–2649, 2000. [DOI] [PubMed] [Google Scholar]
- 37.Okamura H, Kashiwamura S, Tsutsui H, Yoshimoto T, Nakanishi K. Regulation of interferon-gamma production by IL-12 and IL-18. Curr Opin Immunol 10: 259–264, 1998. [DOI] [PubMed] [Google Scholar]
- 38.Okamura H, Tsutsi H, Komatsu T, Yutsudo M, Hakura A, Tanimoto T, Torigoe K, Okura T, Nukada Y, Hattori K. Cloning of a new cytokine that induces IFN-gamma production by T cells. Nature 378: 88–91, 1995. [DOI] [PubMed] [Google Scholar]
- 39.Partrick DA, Moore EE, Offner PJ, Meldrum DR, Tamura DY, Johnson JL, Silliman CC. Maximal human neutrophil priming for superoxide production and elastase release requires p38 mitogen-activated protein kinase activation. Arch Surg 135: 219–225, 2000. [DOI] [PubMed] [Google Scholar]
- 40.Polikandriotis JA, Rupnow HL, Elms SC, Clempus RE, Campbell DJ, Sutliff RL, Brown LA, Guidot DM, Hart CM. Chronic ethanol ingestion increases superoxide production and NADPH oxidase expression in the lung. Am J Respir Cell Mol Biol 34: 314–319, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Quinton LJ, Nelson S, Zhang P, Boe DM, Happel KI, Pan W, Bagby GJ. Selective transport of cytokine-induced neutrophil chemoattractant from the lung to the blood facilitates pulmonary neutrophil recruitment. Am J Physiol Lung Cell Mol Physiol 286: L465–L472, 2004. [DOI] [PubMed] [Google Scholar]
- 42.Rana SN, Li X, Chaudry IH, Bland KI, Choudhry MA. Inhibition of IL-18 reduces myeloperoxidase activity and prevents edema in intestine following alcohol and burn injury. J Leukoc Biol 77: 719–728, 2005. [DOI] [PubMed] [Google Scholar]
- 43.Sayeed MM Exuberant Ca(2+) signaling in neutrophils: a cause for concern. News Physiol Sci 15: 130–136, 2000. [PubMed] [Google Scholar]
- 44.Shanley TP, Schmal H, Warner RL, Schmid E, Friedl HP, Ward PA. Requirement for C-X-C chemokines (macrophage inflammatory protein-2 and cytokine-induced neutrophil chemoattractant) in IgG immune complex-induced lung injury. J Immunol 158: 3439–3448, 1997. [PubMed] [Google Scholar]
- 45.Sheppard FR, Kelher MR, Moore EE, McLaughlin NJ, Banerjee A, Silliman CC. Structural organization of the neutrophil NADPH oxidase: phosphorylation and translocation during priming and activation. J Leukoc Biol 78: 1025–1042, 2005. [DOI] [PubMed] [Google Scholar]
- 46.Siegmund B Interleukin-1beta converting enzyme (caspase-1) in intestinal inflammation. Biochem Pharmacol 64: 1–8, 2002. [DOI] [PubMed] [Google Scholar]
- 47.Silver GM, Albright JM, Schermer CR, Halerz M, Conrad P, Ackerman PD, Lau L, Emanuele MA, Kovacs EJ, Gamelli RL. Adverse clinical outcomes associated with elevated blood alcohol levels at the time of burn injury. J Burn Care Res 29: 784–789, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sir O, Fazal N, Choudhry MA, Gamelli RL, Sayeed MM. Neutrophil depletion prevents intestinal mucosal permeability alterations in burn-injured rats. Am J Physiol Regul Integr Comp Physiol 278: R1224–R1231, 2000. [DOI] [PubMed] [Google Scholar]
- 49.Sir O, Fazal N, Choudhry MA, Goris RJ, Gamelli RL, Sayeed MM. Role of neutrophils in burn-induced microvascular injury in the intestine. Shock 14: 113–117, 2000. [DOI] [PubMed] [Google Scholar]
- 50.Sivakumar PV, Westrich GM, Kanaly S, Garka K, Born TL, Derry JM, Viney JL. Interleukin 18 is a primary mediator of the inflammation associated with dextran sulphate sodium induced colitis: blocking interleukin 18 attenuates intestinal damage. Gut 50: 812–820, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Szabo G, Mandrekar P, Verma B, Isaac A, Catalano D. Acute ethanol consumption synergizes with trauma to increase monocyte tumor necrosis factor alpha production late postinjury. J Clin Immunol 14: 340–352, 1994. [DOI] [PubMed] [Google Scholar]
- 52.Tranter M, Jones WK. Anti-inflammatory effects of HO-1 activity in vascular endothelial cells, commentary on “Carbon monoxide donors or heme oxygenase (HO-1) overexpression blocks interleukin-18-mediated NF-kappaB-PTEN-dependent human cardiac endothelial cell death”. Free Radic Biol Med 44: 261–263, 2008. [DOI] [PubMed] [Google Scholar]
- 53.Wang M, Markel TA, Meldrum DR. Interleukin 18 in the heart. Shock 30: 3–10, 2008. [DOI] [PubMed] [Google Scholar]
- 54.Wyman TH, Dinarello CA, Banerjee A, Gamboni-Robertson F, Hiester AA, England KM, Kelher M, Silliman CC. Physiological levels of interleukin-18 stimulate multiple neutrophil functions through p38 MAP kinase activation. J Leukoc Biol 72: 401–409, 2002. [PubMed] [Google Scholar]
- 55.Yu HP, Yang S, Hsieh YC, Choudhry MA, Bland KI, Chaudry IH. Maintenance of lung myeloperoxidase activity in proestrus females following trauma-hemorrhage: upregulation of hemeoxygenase-1. Am J Physiol Lung Cell Mol Physiol 291: L400–L406, 2006. [DOI] [PubMed] [Google Scholar]