Abstract
The course of intestinal inflammatory responses is tightly coordinated by the extensive communication between the immune system and the enteric nervous system, among which the bidirectional mast cell-neuron interaction within the intestinal wall plays a prominent role. Recent research suggests that somatostatin (SOM) is able to inhibit this self-reinforcing network by simultaneously suppressing the inflammatory activities of both neurons and mast cells. Therefore, we assessed the modulatory effects of SOM on both the short-term and long-term effects induced by the main mast cell mediators histamine (HIS) and 5-HT on spinal sensory neurons. Short-term incubation of dorsal root ganglion cultures with HIS and 5-HT induced neuronal CGRP-release and calcium-mediated activation of both neurons and nonneuronal cells, both of which effects were significantly reduced by SOM. In addition, SOM was also able to suppress the increased neuronal expression of pro- and anti-inflammatory peptides induced by long-term exposure to HIS and 5-HT. Immunocytochemical and molecular-biological experiments revealed the possible involvement of somatostatin receptor 1 (SSTR1) and SSTR2A in these profound SOM-dependent effects. These data, combined with the increased expression of pro- and anti-inflammatory peptides and several SSTRs in murine dorsal root ganglia following intestinal inflammation, reveal that intestinal inflammation not only induces the onset of proinflammatory cascades but simultaneously triggers endogenous systems destined to prevent excessive tissue damage. Moreover, these data provide for the first time functional evidence that SOM is able to directly modulate intestinal inflammatory responses by interference with the coordinating mast cell-neuron communication.
Keywords: dorsal root ganglion, inflammation, neuroimmune interaction, mouse, enteric inflammation
the initial observations of the close anatomical relationship between mast cells and nerve fibers in a multitude of organs and tissues provided the onset for a vast amount of research concerning bidirectional mast cell-neuron communication (1, 33, 34, 44). Because of its vast innervation by a strongly ramified neuronal network, both of intrinsic and extrinsic origin, and of the profound role of mast cells in gastrointestinal (GI) inflammation, the GI tract (GIT) has been subject of many studies investigating the molecular base of neuroimmune interactions (for review, see Ref. 49). Most of these studies have been performed in rodents, in which two distinct subsets of mast cells can be identified based on their serine proteinase content: mucosal mast cells (MMC) and connective tissue mast cells (CTMC) (27). In humans, mast cells are designated either as MCTC for their tryptase and chymase content and, in terms of localization, correspond closely to CTMC, or as MCT, which only contain tryptase and correspond most closely to the rodent MMC. A vast amount of morphological, pharmacological, and functional studies in rodents revealed that more specifically the interactions between enteric nerve fibers and MMC constitute the coordinating core of intestinal responses, especially in pathophysiological conditions and during exposure to antigenic threats. Mast cells are capable of activating neurons through the release of a plethora of preformed and newly synthesized mediators, including histamine (HIS), 5-HT, cytokines, and arachidonic acid metabolites. On the other hand, several mediators (substance P, CGRP, nucleotides, etc.) released from enteric nerve endings activate mast cells or lower their activation threshold for other stimuli. This solid communication network integrates the mast cell's sensitive detection capabilities of potentially noxious substances and the strongly coordinating role of the enteric nervous system, resulting in a fast and coordinated removal of the initiating stimulus.
Almost all mediators shown to be involved in these neuroimmune interactions so far are stimulatory, whereas the need to avoid excess inflammatory responses implies the simultaneous presence of inflammatory-suppressive mediators at the site of inflammation. To obtain maximal efficacy, such inhibitory mediators should be able to simultaneously downregulate the proinflammatory and self-reinforcing activities of both mast cells and neurons. One of the major candidates to exert this protective role is somatostatin (SOM). With the GIT as its major source, this widespread peptide exerts a multitude of functions in the physiological activity of the GIT, including modulation of intestinal motility, fluid secretion, and nutrient resorption. In addition to its abundant presence and activities along the digestive tract, SOM is also considered a potent inflammatory suppressive and antinociceptive mediator (for review, see Ref. 32). The latter activities are exerted not only by direct inhibition of inflammatory cells but also by diminishing the release of chemotactic and proinflammatory neuropeptides from sensory nerve endings. These SOM-dependent effects are mediated by direct activation of high-affinity G protein-coupled receptors, the SOM receptors (SSTRs). This receptor family consists of five SSTR subtypes (named SSTR1 to SSTR5), all existing in one protein isoform, the exception being SSTR2, which is alternatively spliced into SSTR2A and SSTR2B (29).
Previous data reported on the powerful inhibitory effects of SOM on MMC degranulation in the rat intestine, possibly by direct activation of SSTR1 (35, 47). Since SSTR1 and SSTR3 are present on MMC in the inflamed murine intestine, similar inhibitory effects of SOM on MMC degranulation in the mouse are expected (51). Furthermore, evidence is at hand that SOM modulates intestinal inflammatory responses not only by directly targeting MMC but also by modulating neuronal activity during inflammation. The expression of multiple SSTR subtypes in ileal extrinsic nerve fibers and the changes in neuronal SOM and SSTR expression during inflammation suggest that SOM also coordinates inflammatory reactions through modulation of specific neuronal populations (51). We tested this hypothesis by studying the potential modulatory effects of SOM on the MMC mediator-induced changes in spinal sensory neuronal activity, both the short- and long-term effects. Moreover, since sensory neuronal SSTR expression has been shown to be altered in response to inflammation in peripheral tissues, we investigated SSTR expression in spinal ileal afferent neurons in noninflamed and inflamed conditions (2).
MATERIALS AND METHODS
Drugs and Chemicals
All chemicals for setup and maintenance of the dorsal root ganglion (DRG) cultures (Neurobasal-A medium, trypsin, FBS, l-Glutamax, antibiotic/antimycotic solution) were purchased from GIBCO (Invitrogen, Carlsbad, CA). Collagenase, ATP, HIS, 5-HT, and SOM were purchased from Sigma-Aldrich (St. Louis, MO); TRIzol, the Transcriptor First Strand cDNA Synthesis Kit, and the LightCycler FastStartDNA MasterPLUS SYBR Green I kit were from Roche (Mannheim, Germany). Fluorogold was obtained from Fluorochrome (Englewood, CO), pentobarbital sodium from Ceva Sante Animale (Brussels, Belgium), medetomidine from Pfizer (New York, NY), ketamine from Eurovet (Bladel, the Netherlands), the Turbo DNA-free kit from Ambion (Austin, TX), the HotstarTaq Master Mix from Qiagen (Hilden, Germany), fluo-4 AM from Molecular Probes (Invitrogen), the Substance P EIA kit from Cayman Chemical (Ann Arbor, MI), and the Rat CGRP Enzyme Immunoassay Kit from SpiBio (Montigny le Bretonneux, France).
Animals
Adult male C57BL/6J mice (BioServices, Uden, the Netherlands) were given water and a standard pellet diet ad libitum and were kept in a 12:12-h light-dark cycle. National and European principles of laboratory animal care were followed and all experiments were approved by the Medical Ethical Committee on Animal Experimentation of the University of Antwerp. As a model for intestinal inflammation, 8-wk Schistosoma mansoni-infected (8w pi) animals (n = 6) were used. Mice were infected by following previously described protocols (43). This inflammatory model was previously used to investigate the effect of intestinal inflammation on the extrinsic ileal innervation and on the expression of SOM, SSTRs, and several neuropeptides in the murine ileum (14, 50, 51). Moreover, the time- and tissue-dependent recruitment of different mast cell populations and nerve fiber sprouting in the small intestine of S. mansoni-infected mice allow thorough investigations of mast cell-neuron interactions in the GIT. The small intestine of 8w pi animals is characterized by the massive recruitment of MMC to the mucosal and submucosal layers, whereas the 15-wk S. mansoni-infected ileum contains a vast number of CTMC mainly located in the muscle layers (15).
Retrograde Tracings
Mice were anesthetized by intraperitoneal injection of medetomidine (0.4 mg/kg) and ketamine (60 mg/kg) dissolved in physiological solution. When the mice were fully unresponsive, the distal part of the ileum was surgically exteriorized from the abdominal cavity and 2 μl of a 4% Fluorogold solution in sterile water was injected at six to eight sites via a fine-tipped glass micropipette. Excess tracer was carefully dabbed off. The animals were allowed to recover for 1 wk before DRG were removed for immunocytochemical staining.
Isolation of Dorsal Root Ganglia
DRG were isolated, either for immunocytochemistry stainings, RNA isolation, or setting up in vitro DRG cultures. Animals were anesthetized by intraperitoneal injection of an overdose of pentobarbital sodium (Sanofi Benelux, Brussels, Belgium). The spinal cord was dissected and the DRG (level Th8–Th13) were harvested. For RNA isolation, ganglia were immediately frozen in liquid nitrogen, whereas the ganglia for setting up DRG cultures were enzymatically processed (see DRG Cultures). In case of immunocytochemistry, the animals were first transcardially perfused with 4% paraformaldehyde in phosphate buffer (pH 7.4) for 15 min and the isolated ganglia were postfixed in 4% paraformaldehyde for an additional 30 min. Thereafter, DRG were incubated overnight in 0.01 M phosphate-buffered saline (PBS) containing 20% sucrose at 4°C and embedded in optimal cutting temperature (OCT)-embedding medium. Eight-micrometer-thick cryosections were thaw mounted on poly l-lysine-coated slides.
DRG Cultures
After removal of the connective tissue, the ganglia were enzymatically digested at 37°C in 0.5% collagenase for 30 min and in 0.25% trypsin for an additional 15 min. The ganglia were mechanically dissociated by use of fire-polished Pasteur pipettes with decreasing tip diameters and the enzymatic digestion was inhibited by addition of 1 ml fetal bovine serum (FBS). After centrifugation, the pellet was resuspended in Neurobasal-A medium containing 10% FBS, 2 mM Glutamax, and 1% antibiotic-antimycotic solution. The culture medium was refreshed daily. All in vitro experiments were performed when the DRG cultures were 2 days old. Dissociated DRG cell suspensions from each animal were seeded into several culture dishes, enabling comparison of the effects of different incubation conditions against untreated control dishes from the same animal.
Pretreatment of the DRG Cultures
To test the short-term and long-term effects of SOM, HIS, and 5-HT on several aspects of DRG activity, DRG cultures were subjected to incubations with different combinations of these mediators. The long-term effects of HIS, 5-HT, and SOM were tested by quantitative analyses, reverse transcriptase PCR (RT-PCR), and quantitative RT-PCR (qPCR). Following combinations of mediators were added to the DRG cultures from the second day on for 30 h at 2-h intervals following previously described protocols: 1) 10−5 M SOM, 2) 10−5 M HIS and 5-HT, and 3) 10−5 M HIS, 5-HT, and SOM (39, 40). For enzymatic immunoassay (EIA) experiments, cells were exposed to the same combinations of mediators for 15 min, after which the supernatants were collected and stored at −80°C until further analysis. Finally, EIA and calcium life cell imaging (LCI) experiments were also performed on dishes exposed to SOM alone for 30 h (39, 40).
Immunocytochemical Staining
Immunocytochemical stainings were performed, both on isolated DRG and DRG cultures. DRG cultures were fixed for 30 min in 4% paraformaldehyde for PFA at room temperature. All incubations were performed at room temperature and rinsing in PBS was performed between every two incubation steps. Characteristics and sources of the applied primary, secondary and tertiary antibodies are listed in Table 1. Primary antibodies were diluted in 0.1 M PBS with 0.05% thimerosal (PBS#) containing 10% normal horse serum (NHS) and 0.1% Triton X-100. Secondary and tertiary antibodies were diluted in PBS# containing 1% NHS. SSTR2A and SSTR4 were detected by the tyramide signal amplification method according to the manufacturer's guidelines (PerkinElmer Life Science, Boston, MA). Prior to immunocytochemical staining, endogenous peroxidase activity was blocked by hydrogen peroxide (3% in methanol) incubation for 30 min at room temperature. After rinsing, cryosections and the fixed DRG cultures were immersed in PBS# containing 5% bovine serum albumin, 10% NHS, and 1% Triton X-100 for 30 min and incubated with the primary antibody for 16 h, followed by an appropriate secondary/tertiary antibody for 1 h. In double immunocytochemical procedures, cryosections were subjected to additional conventional immunocytochemical protocols. Negative controls, in which the primary antibodies were omitted, and interference control stainings were performed (42). The specificity of all antibodies was verified on murine brain sections by preabsorption with the appropriate antigens purchased from the same supplier as the primary antibodies (1 μg antigen per μg antibody).
Table 1.
Primary, secondary, and tertiary antisera used for immunocytochemical stainings
| Antigen | Host | Dilution | Source | |||
|---|---|---|---|---|---|---|
| Primary Antisera | ||||||
| CGRP | Rabbit | 1/5,000 | Sigma, St. Louis, MO (C8198) | |||
| Glutamine synthetase | Rabbit | 1/20,000 | Abcam, Cambridge, UK (ab49873) | |||
| PECAM-1 | Goat | 1/500 | Santa Cruz Biotechnology, Santa Cruz, CA (sc-1506) | |||
| PGP | Guinea pig | 1/500 | GeneTex, San Antonio, TX (GTX10410) | |||
| SOM | Rat | 1/100 | Biogenesis, Poole, UK (8330-0009) | |||
| SSTR1 | Rabbit | 1/500 | Biotrend, Köln, Germany (SS-840) | |||
| SSTR2A | Rabbit | 1/2,000 | Biotrend (SS-800) | |||
| SSTR4 | Rabbit | 1/500 | Sigma (S0945) | |||
| Substance P | Rat | 1/100 | Biogenesis (8450-0505) | |||
| Secondary and Tertiary Antisera | ||||||
| Cy-3-conjugated donkey-anti-rabbit IgG | 1/1,000 | Jackson Immunoresearch, West Grove, PA | ||||
| Cy-3-conjugated donkey-anti-goat IgG | 1/500 | Jackson Immunoresearch | ||||
| Cy-3-conjugated donkey-anti-guinea pig IgG | 1/500 | Jackson Immunoresearch | ||||
| FITC-conjugated goat anti-rabbit IgG | 1/400 | Jackson Immunoresearch | ||||
| Biotinylated Fab fragments of goat-anti-rabbit IgG | 1/2,000 | Rockland, Gilbertsville, PA | ||||
| FITC-conjugated streptavidin | 1/100 | Rockland | ||||
| Extravidin horseradish peroxidase | 1/1,000 | Sigma | ||||
| Biotin-conjugated tyramide signal amplification kit | PerkinElmer Life Sciences, Boston, MA | |||||
Calcium Live Cell Imaging
Experimental setup, solutions, and perfusion.
Using instantaneous changes in intracellular calcium concentrations ([Ca2+]i) as a measure for cellular activation, the effect of short- and long-term incubation with different mediators on DRG culture activation was measured. All experiments were performed on an inverted microscope (Zeiss Axiovert 200, Carl Zeiss, Jena, Germany), attached to a microlens-enhanced dual spinning disk confocal system (UltraView ER, PerkinElmer) with a ×25 oil immersion lens. Laser intensity was kept to an absolute minimum to avoid photobleaching and phototoxicity. The DRG culture dishes were continuously perfused with a carbogenated (95% O2-5% CO2) physiological solution at room temperature (in mM: 122 NaCl, 2.1 KCl, 1.2 KH2PO4, 1.3 MgSO4·7H2O, 25 NaHCO3, 2.5 CaCl2·2H2O, 11 glucose pH 7.42) through a gravity-fed system with electrically triggered valves, allowing fast exchange of experimental solutions. All experimental substances were dissolved in freshly prepared physiological solution. For Ca2+-free solution, CaCl2 was replaced by 1 mM ethylene tetraacetic acid disodium salt dihydrate (EDTA). The total number of neurons able to respond to different stimuli was determined by perfusing the dishes with physiological solution containing 70 mM KCl, prepared by equimolar substitution of KCl for NaCl. Only neurons showing a clearly detectable increase in [Ca2+]i were included in the experimental analysis. Stimulation of the spinal sensory neurons with KCl was always performed at the end of the experiment to obtain maximal responses to the other mediators tested. Physiological solution containing 50 μM ATP was used as a positive control to identify all viable nonneuronal cells (NNC) in the DRG culture dishes.
Loading procedure and data acquisition.
The DRG cultures were loaded with 1 μM of the fluorescent calcium indicator fluo-4 AM dissolved in Neurobasal-A medium (30 min, 37°C) followed by a washing step and a final deesterification step (15 min, 37°C). Time-lapse images of changes in fluo-4 fluorescence were recorded (2 images/s) by a combined 488-nm laser excitation and FITC emission filter. Fluorescence intensities for individual cells were analyzed offline by Volocity 2 software (Improvision, Coventry, UK). For every cell, the fluorescence intensity, expressed as arbitrary units, was plotted against time. Immediately following each experiment, all neurons within the image were identified by visual inspection, on the basis of shape, diameter, and dimensions of somata and the presence and shapes of neuronal processes. Gray levels were adjusted for the basal level of fluorescence present at the start of the experiment. All graphs are representative of multiple experiments performed under the respective conditions.
EIA Measurements
The release of substance P and CGRP from 2-day-old DRG cultures (n = 4) upon different stimuli was measured by EIA. After pretreatment and stimulation of the DRG cultures, the supernatant of each culture dish was collected, centrifuged at 500 g for 7 min at 4°C, and stored at −80°C until further processing. Substance P and CGRP concentrations in the supernatants were measured by using specific EIA kits according to the manufacturer's instructions. All samples were analyzed in triplicate.
RNA Treatment, RT-PCR, and qPCR
Total RNA from DRG and DRG cultures was isolated by use of TRIzol reagent. Five micrograms of RNA were treated with the Turbo DNA-free kit and 1 μg of DNase-treated RNA was reverse transcribed with the Transcriptor First Strand cDNA synthesis kit. The efficiency of the reverse transcription was verified by use of control RNA and primers included in the reverse transcription kit. DNase-treated RNA samples served as negative controls. Primer sequences and amplification protocols for all SSTRs, SOM, and substance P were published previously (50, 51). Primer characteristics for both isoforms of CGRP, glyceraldehyde-3-phosphate dehydrogenase (GAPDH), hypoxanthine phosphoribosyl transferase (HPRT), and β-actin are listed in Table 2. RT-PCR experiments were performed in a reaction volume of 25 μl containing 1 μl cDNA suspension, 0.4 μM of forward and reverse primer, and 12.5 μl HotStarTaq Master Mix. Amplification products were separated on a 2% agarose gel and visualized under UV illumination. qPCR experiments were performed on a Lightcycler 1.5 machine by using the Lightcycler FastStart DNA MasterPLUS SYBR Green I kit according to the manufacturer's instructions. The mRNA levels of all genes were normalized to the combined mRNA levels of GAPDH, HPRT, and β-actin in each sample.
Table 2.
5′ → 3′ sequence, annealing temperature of the qPCR primers and the temperature at which the fluorescence signal was measured
| Primer | Sequence | TA °C | TM °C |
|---|---|---|---|
| CGRPα forward primer | CATGGCCACTCTCAGTGA | 64 | 82 |
| CGRPα reverse primer | GCTCCCTGGCTTTCATC | ||
| CGRPβ forward primer | CCAGTCAAATATGATGGTGTCT | 60 | 79 |
| CGRPβ reverse primer | CATTGGCTGGATGGCTC | ||
| GAPDH forward primer | TGGCAAAGTGGAGATTGTTGCC | 64 | 83 |
| GAPDH reverse primer | AAGATGGTGATGGGCTTCCCG | ||
| HPRT forward primer | CCTAAGATGAGCGCAAGTTGAA | 60 | 72 |
| HPRT reverse primer | CCACAGGACTAGAACACCTGCTAA | ||
| β-Actin forward primer | ATGCTCCCCGGGCTGTAT | 63 | 72 |
| β-Actin reverse primer | CATAGGAGTCCTTCTGACCCATTC |
TA, annealing temperature; TM, fluorescence measurement temperature.
Quantitative Analyses
The long-term effects of HIS, 5-HT, and SOM on CGRP and substance P expression in DRG were investigated by exposing DRG cultures to different combinations of these mediators for 30 h as described (see above). After pretreatment and fixation, cultures were immunocytochemically double labeled for either CGRP and a panneuronal marker (PGP) or substance P and PGP. The percentage of CGRP- or substance P-immunoreactive (-ir) neurons compared with the entire neuronal population was determined at ×25 magnification in the different experimental groups.
Statistical Analysis
Experimental data from retrograde tracing experiments were analyzed by Student's t-test, quantitative analyses, qPCR, EIA, and LCI experiments by ANOVA following a Bonferroni post hoc test for comparison between different experimental groups and were expressed as means ± SE. For EIA experiments, the release of CGRP in the untreated control dishes was set as 100%. Statistical significance was assumed at P < 0.05.
RESULTS
Effect of Intestinal Inflammation on Pro- and Anti-Inflammatory Neuropeptide Expression in Spinal Sensory Ileal Innervation
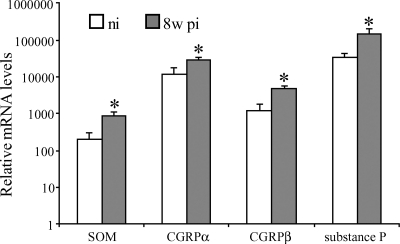
The spinal sensory innervation of the small intestine in rodents originates from the lower thoracic DRG (45). As intestinal inflammation potentially alters the expression of pro- and anti-inflammatory peptides in extrinsic spinal sensory neurons, the expression of substance P, CGRP, and SOM in DRG at level Th8–Th13 of normal and 8w pi mice was investigated. Immunocytochemical detection revealed clear expression of all three peptides in neuronal somata and nerve fibers throughout the ganglia, in both noninflamed and 8w pi conditions (Fig. 1). As identified by retrograde tracings, 11.2 ± 0.5% of the murine Th8–Th13 neurons in the noninfected animals projected to the distal ileum. 6.9 ± 0.4% of this ileum-projecting neuronal population expressed SOM, whereas immunoreactivity for substance P and CGRP was demonstrated in 55.2 ± 1.9 and 68.8 ± 1.7% of the traced neurons, respectively. Intestinal inflammation did not change the percentage of ileum-projecting spinal sensory neurons (9.5 ± 0.4%), although the percentage of SOM-ir traced neurons increased from 6.9 ± 2.7 to 15.1 ± 0.6% (P < 0.001). Accordingly, the percentages of substance P-ir and CGRP-ir Fluorogold-labeled neurons arose to 71.4 ± 1.9% (P < 0.001) and 85.3 ± 1.4% (P < 0.001), respectively. Quantification of mRNA levels for SOM, CGRPα, CGRPβ, and substance P revealed significantly elevated mRNA levels for all four neuropeptides in DRG of 8w pi mice compared with noninflamed conditions, indicative of modulatory effects of intestinal inflammation on the extrinsic ileal innervation (Fig. 2).
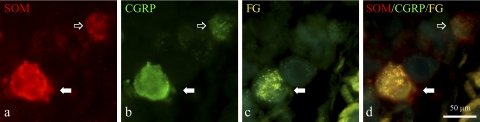
Fig. 1.
Immunocytochemical detection of somatostatin (SOM; a), and CGRP (b) in dorsal root ganglion (DRG) neurons of noninfected mice retrogradely traced from the small intestine with fluorogold (FG; c). Merging SOM and CGRP immunofluorescent stainings (d) reveals partial coexpression of these 2 peptides in neuronal somata (solid arrows), although SOM−/CGRP+ somata are also present (open arrow).
Fig. 2.
Quantitative RT-PCR (qPCR) analysis demonstrated significantly increased mRNA levels for SOM, both isoforms of CGRP, and substance P in murine DRG in response to intestinal inflammation. *Statistically significant difference compared with noninflamed conditions (P < 0.05). ni, Noninfected, 8w pi, 8 wk Schistosoma mansoni-infected.
Effect of HIS, 5-HT, and SOM on DRG Cultures
In view of these inflammation-induced effects on the spinal sensory ileal innervation and of the pivotal role of MMC in intestinal inflammatory responses, we hypothesized that two main MMC mediators, HIS and 5-HT, affect multiple aspects of spinal sensory neuronal activity. Using cultures of individualized DRG cell suspensions (level Th8–Th13), we investigated both acute and chronic effects, together with the potential modulatory effects of SOM on these MMC-mediated neuronal changes.
The short-term effects of HIS and 5-HT on spinal sensory neuronal activity included the release of proinflammatory neuropeptides, analyzed with EIA, and the calcium-mediated neuronal activation, examined using LCI. For the long-term effects of HIS and 5-HT, we investigated the effects of these mediators on the neuronal expression of CGRP and substance P, at both the mRNA and the protein level.
Short-term effects of HIS and 5-HT on spinal sensory neuronal activity.
SOM INHIBITS THE HIS- AND 5-HT-INDUCED RELEASE OF PROINFLAMMATORY PEPTIDES FROM SPINAL SENSORY NEURONS.
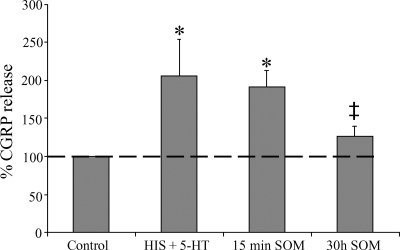
DRG cultures were exposed to 10 μM HIS and 10 μM 5-HT for 15 min, inducing a major CGRP release compared with the unexposed control culture dishes, which was set 100% (Fig. 3). Addition of SOM for 15 min did not alter this HIS- and 5-HT-induced CGRP release. However, pretreatment of the DRG cultures with SOM for 30 h decreased the HIS- and 5-HT-induced CGRP release. In line with previous reports, the release of substance P from spinal sensory neurons remained well below the detection limit, independently from the pretreatments applied (18).
Fig. 3.
Release of CGRP from spinal cultures in response to histamine (HIS) and 5-HT stimulation and the modulatory effects of SOM on this CGRP release. Bars indicate CGRP release in unstimulated spinal cultures (control, 100%), in spinal cultures acutely (15 min) stimulated with 10 μM HIS and 5-HT (HIS + 5-HT), in spinal cultures acutely stimulated with 10 μM HIS, 5-HT, and SOM (15 min SOM), and in cultures stimulated with HIS and 5-HT after a 30 h incubation with SOM (30h SOM). *Statistically significant difference compared with nonstimulated control dishes (P < 0.05); ‡statistically significant difference compared with HIS+5-HT-stimulated dishes (P < 0.05).
SOM INHIBITS THE HIS- AND 5-HT-MEDIATED INCREASES IN [CA2+]I IN SPINAL SENSORY NEURONS.
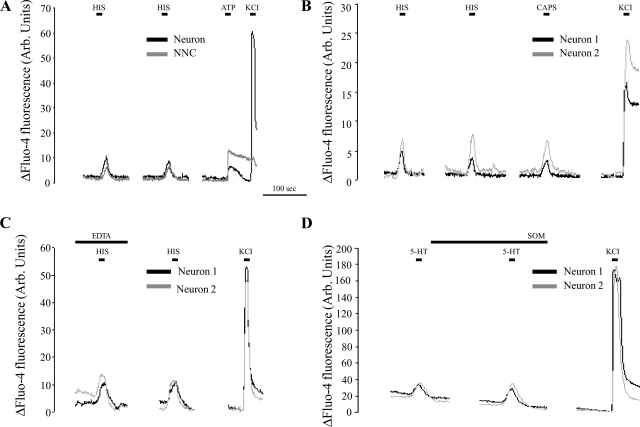
The effects of HIS and 5-HT on the calcium-mediated neuronal activation were studied in fluo-4 AM-loaded spinal sensory cultures. Acute (15 s) application of 10 μM HIS induced a rapid and reversible increase in [Ca2+]i in 64.8 ± 8.0% (n = 109) of the spinal sensory neurons and in 84.7 ± 15.7% (n = 296) of all NNC (Fig. 4A). Similar responses were elicited in 55 ± 6.4% (n = 123) of the spinal sensory neurons and in 83 ± 12.3% (n = 207) of all NNC after a 15-s stimulation with 5-HT. As soon as the agonist was washed away, [Ca2+]i levels declined to baseline levels. When the DRG cultures were reexposed to the same stimulus after a 2-min interval, all neurons and NNC responsive to the first stimulation also responded to the second stimulation, without a significant reduction in response amplitude (Fig. 4A). For further experiments, we focused on the MMC mediator-induced responses in neurons rather than in NNC. All neurons responsive to 5-HT also showed increased [Ca2+]i levels in response to stimulation with 2 μM capsaicin (n = 44), whereas 98% of the HIS-sensitive neurons were also capsaicin sensitive (n = 44) (Fig. 4B).
Fig. 4.
Representative recordings of the normalized changes in fluo-4 fluorescence measured in spinal neurons and nonneuronal cells (NNC) in vitro. A: both neurons and NNC displayed a calcium-dependent activation in response to short-term HIS stimulation. After a 2-min interval, repeated HIS stimulation evoked similar responses without a clear reduction in response amplitude. At the end of the experiment, viable neurons were identified by stimulation with a high K+-containing solution, NNC by stimulation with 50 μM ATP. B: virtually all neurons responsive to HIS and 5-HT also responded to capsaicin (CAPS) stimulation. C: determining the source of the calcium influx in neurons upon HIS- or 5-HT stimulation. For the time indicated, the physiological solution was replaced by a buffer without CaCl2 and containing 1 mM EDTA. Stimulation with 10 μM HIS during this period evoked responses that were not different from the ones after a 2-min interval during which the cells were again superfused with normal physiological solution. D: determining the short-term effects of SOM on neuronal activation by 5-HT. Following the first stimulation, cells were perfused for 2 min with physiological solution containing 10 μM SOM, after which the cells were once again stimulated with physiological perfusate containing both 5-HT and SOM. No decrease in the number of 5-HT-activated neurons or in the evoked response amplitudes was observed. Arb., arbitrary.
Consecutive stimulation of the DRG cultures with 10 μM HIS and 10 μM 5-HT revealed that 75 ± 4.2% (n = 89) of the neurons that were activated by HIS also responded to 5-HT stimulation. No neurons were observed that were responsive to 5-HT but insensitive to HIS.
The source of the calcium influx upon neuronal stimulation with either HIS or 5-HT was determined by using a calcium-free perfusion solution containing 1 mM EDTA (Fig. 4C). DRG cultures were first exposed to either 10 μM HIS or 10 μM 5-HT in this calcium-free solution. After a 2-min interval, cells were reexposed to the same agonists dissolved in normal physiological solution. This way, it was observed that 71.3 ± 8.8% (n = 63) and 92 ± 5.1% (n = 57) of the neurons responsive to HIS and 5-HT, respectively, also elicited an increase in [Ca2+]i upon agonist stimulation in calcium-free conditions, demonstrating the almost complete independence of these responses on extracellular calcium.
In view of its potential suppressive effects on neuronal activation, the effect of SOM on the percentage of spinal sensory neurons responsive to 10 μM HIS and 10 μM 5-HT was investigated. The acute effects of SOM on neuronal excitation were tested by first stimulating the DRG cultures with one of both mast cell mediators. Immediately thereafter, cells were perfused with physiological solution containing 10 μM SOM for 2 min, followed by a new stimulation perfusate containing both SOM and HIS or 5-HT. Since repeated stimulation with HIS or 5-HT after a 2-min interval did not decrease the percentage of activated cells or the amplitude of the evoked responses, this protocol allowed the investigation of the acute effect of SOM on MMC mediator-induced calcium responses in HIS- and 5-HT-responsive neurons. All neurons responding to HIS or 5-HT during the first stimulation also responded during the second stimulation, demonstrating that acute SOM incubation did not affect neuronal calcium-mediated responses to HIS or 5-HT (Fig. 4D). Moreover, no major changes in the amplitude of the responses were observed after SOM incubation.
However, long-term pretreatment (30 h) of the DRG cultures with SOM strongly decreased the percentage of spinal sensory neurons activated by either HIS or 5-HT: 37 ± 4.6% (n = 94) of the spinal sensory neurons and 54.6 ± 6.2% (n = 206) of all NNC showed increased [Ca2+]i levels upon HIS stimulation. These percentages are significantly reduced compared with the untreated cultures (P = 0.006 and 0.041 for the neurons and NNC, respectively). The percentage of spinal sensory neurons responsive to 10 μM 5-HT did not differ significantly after long-term SOM pretreatment (44%, P = 0.3, n = 131), although the percentage of responsive NNC declined from 83 ± 12.3 to 55.4 ± 9.8% (P = 0.004, n = 189).
These experiments confirm that SOM is able to suppress the short-term effects of the mast cell mediators HIS and 5-HT on different cell populations within DRG.
Long-term effects of HIS and 5-HT on spinal sensory neuronal activity.
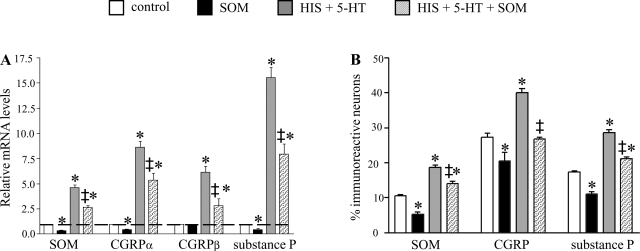
The long-term effects of MMC mediators and SOM on spinal sensory neuronal characteristics were firstly explored by quantification of mRNA levels for different neuropeptides (SOM, substance P, CGRPα, and CGRPβ) in DRG culture dishes incubated with different combinations of HIS, 5-HT, and SOM (Fig. 5A). Compared with untreated control dishes, long-term incubation with SOM significantly decreased mRNA levels of CGRPα, SOM, and substance P, whereas chronic HIS and 5-HT incubation increased the mRNA levels for all four neuropeptides. Combined long-term incubation with HIS, 5-HT, and SOM still increased mRNA levels for all peptides investigated compared with control conditions, although mRNA levels all were significantly lower than in the HIS and 5-HT-incubated dishes.
Fig. 5.
Long-term effects of HIS, 5-HT, and SOM on spinal neuronal characteristics. A: qPCR analysis revealed increased mRNA levels for SOM, both CGRP isoforms, and substance P after long-term incubation with HIS and 5-HT. Coincubation with HIS, 5-HT, and SOM still increased mRNA levels for all peptides investigated, but mRNA levels were reduced compared with the HIS- and 5-HT-incubated dishes. *Statistically significant difference compared with nonstimulated control dishes (P < 0.05); ‡statistically significant difference in HIS+5-HT+SOM stimulated dishes compared with HIS+5-HT stimulated dishes (P < 0.05). B: effect of long-term incubation with HIS, 5-HT, and SOM on the percentages of neurons expressing substance P, CGRP, or SOM. As for the qPCR experiments depicted in A, HIS- and 5-HT-incubation increased the percentages of spinal neurons expressing CGRP, substance P, or SOM. Coincubation with HIS, 5-HT, and SOM still increased the percentages of immunoreactive neurons compared with control dishes, but the percentages were lower than in HIS- and 5-HT incubated dishes. *Statistically significant difference compared with nonstimulated control dishes (P < 0.05); ‡statistically significant difference HIS+5-HT+SOM-stimulated dishes compared with HIS+5-HT-stimulated dishes (P < 0.05).
In the second step, the effects of HIS, 5-HT, and SOM on the protein expression of substance P, CGRP, and SOM were investigated by comparing the percentages of neurons expressing substance P, CGRP, or SOM (Fig. 5B). Long-term incubation of the DRG cultures with SOM decreased the percentages of substance P-, CGRP-, and SOM-ir neurons, whereas incubation with HIS and 5-HT increased the percentages of neurons expressing substance P, CGRP, or SOM. For SOM and substance P, this effect was partly lifted by coincubation with SOM, HIS, and 5-HT, whereas the percentage of CGRP-expressing neurons was normalized after incubation with HIS, 5-HT, and SOM.
Identification of SSTRs Involved in the SOM-Mediated Modulatory Effects
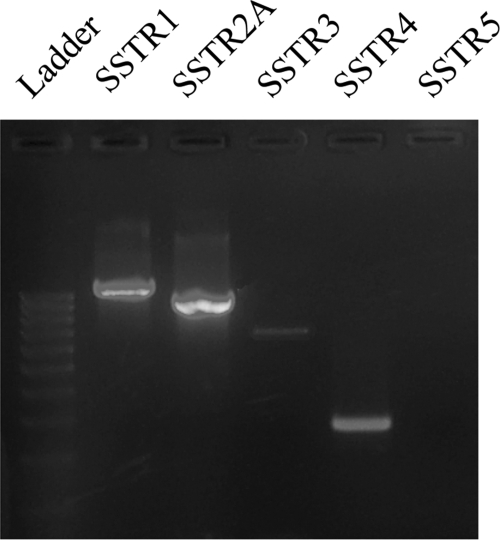
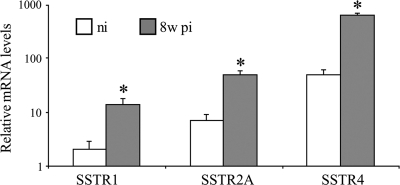
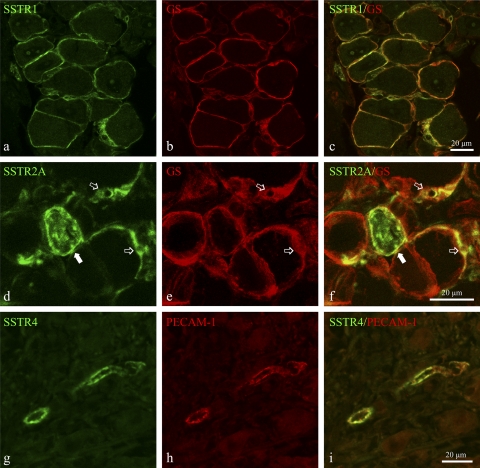
The SSTR subtypes involved in these somatostatinergic effects were identified by RT-PCR. RT-PCR on freshly isolated DRG demonstrated the abundant presence of mRNA for SSTR1, SSTR2A, and SSTR4, in both noninflamed and 8w pi conditions (Fig. 6). Amplification products for SSTR3 were weakly detectable, whereas SSTR5 mRNA could not be detected at all. As demonstrated by qPCR, intestinal inflammation induced significantly elevated mRNA levels for all three SSTR subtypes present in murine DRG level Th8–Th13 (Fig. 7). SSTR1 and SSTR2A were immunocytochemically detected in the vast majority of satellite cells and in some occasional neurons (Fig. 8, a–f). SSTR4 was detected only in endothelial cells lining the blood vessel walls (Fig. 8, g–i). Intestinal inflammation did not alter SSTR distribution.
Fig. 6.
RT-PCR detection of somatostatin receptors (SSTRs) in DRG of noninfected mice. Amplification products for SSTR1, SSTR2A, and SSTR4 were readily detected. SSTR3 was barely detectable, whereas SSTR5 mRNA was undetectable.
Fig. 7.
qPCR analysis of SSTR mRNA levels in murine DRG revealed significantly elevated mRNA levels for SSTR1, SSTR2A, and SSTR4 in response to intestinal inflammation. *Statistically significant difference compared with nonstimulated control dishes (P < 0.05).
Fig. 8.
Immunocytochemical detection of SSTR1 (a–c), SSTR2A (d–f) and SSTR4 (g–i) in DRG cryosections of 8w pi mice. a–c: Abundant expression of SSTR1 (a) in satellite cells, identified by glutathione synthetase (GS) immunoreactivity (b). c: Merged image. SSTR2A was detected both in neuronal somata (solid arrows in d and f) and in multiple satellite cells (open arrows in d–f). f: merged image. g–i show the expression of SSTR4 (g) in endothelial cells identified by PECAM-1 expression (h). i: merged image.
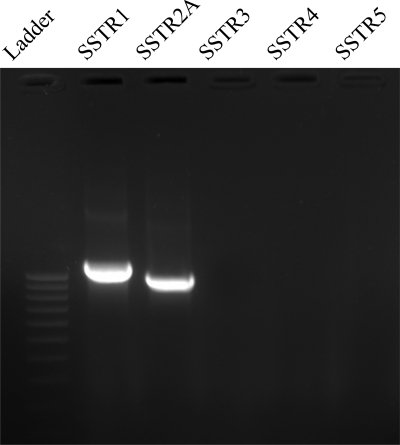
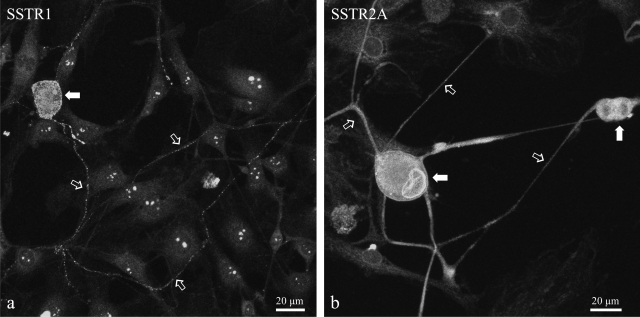
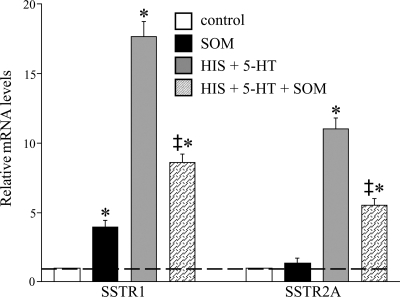
These results are in accordance with RT-PCR data from the DRG cultures. The expression of SSTR1 and SSTR2A was demonstrated with RT-PCR (Fig. 9) and immunocytochemically (Fig. 10). mRNA levels for SSTR1 and SSTR2A were quantified in different experimental conditions, demonstrating that long-term incubation of the DRG cultures with SOM solely induced increased SSTR1 mRNA levels, whereas SSTR2A mRNA levels remained unaltered (Fig. 11). As for the different neuropeptides, chronic HIS- and 5-HT exposure increased mRNA levels for both SSTR subtypes, whereas coincubation with HIS, 5-HT, and SOM lowered their mRNA levels, although they were still higher than in untreated control dishes.
Fig. 9.
RT-PCR detection of SSTRs in control spinal cultures. Only amplification products for SSTR1 and SSTR2A were detected.
Fig. 10.
Immunocytochemical detection of SSTR1 (a) and SSTR2A (b) in spinal cultures revealed the expression of both receptors on neuronal somata (solid arrows in a and b) and in neuronal processes (open arrows in a and b).
Fig. 11.
qPCR analysis of SSTR1 and SSTR2A mRNA levels in spinal cultures in response to incubation with HIS, 5-HT, and SOM. Incubation with SOM alone increased SSTR1 levels, whereas HIS and 5-HT induced elevated mRNA levels for both peptide receptors. Coincubation of HIS, 5-HT, and SOM lowered mRNA levels compared with HIS- and 5-HT-incubated dishes. *Statistically significant difference compared with nonstimulated control dishes (P < 0.05) and statistically significant difference HIS+5-HT+SOM-stimulated dishes compared with HIS+5-HT-stimulated dishes (P < 0.05).
DISCUSSION
This study aimed at determining the effects of intestinal inflammation on peptide expression in spinal sensory neurons and at elucidating the modulatory effects of MMC mediators (HIS and 5-HT) and SOM on spinal sensory neuronal activities.
The neurochemical coding of the ileum-projecting spinal sensory neurons in the mouse was investigated by combined Fluorogold retrograde tracing and immunocytochemistry. Because the spinal sensory innervation of the small intestine in rodents originates from the distal thoracic DRG, we focused on DRG at the level of Th8 until Th13 (45). Quantitative analysis revealed that on average only ∼11% of the DRG neurons at level Th8 to Th13 project to the distal ileum. Substance P- and CGRP IR was demonstrated in 55 and 69% of this ileum-projecting neuronal subpopulation, respectively. These data strongly corroborate previous reports on the neurochemical coding of murine DRG neurons projecting to the jejunum (46). Furthermore, although the expression of SOM in spinal sensory neurons projecting to the lower parts of the GIT has been demonstrated in rats and pigs, this is the first report describing the presence of SOM in spinal sensory neurons projecting to the mouse small intestine (10, 25, 31, 48). Intestinal inflammation did not affect the percentage of spinal sensory neurons projecting to the ileum, although the neurochemical coding of this neuronal population was significantly altered, as demonstrated by qPCR and immunocytochemistry. These data are supported by previous findings on altered peptide expression levels in spinal afferents in response to intestinal inflammation, although species- and inflammatory model-related differences are evident (31, 48).
The second part of this study focused on the direct effects of SOM on the inflammatory responses of spinal sensory neurons. It is generally believed that peripheral inflammation is accompanied by the release of substance P and CGRP from primary afferent nerve endings at the site of inflammation, initiating a series of reactions commonly described as “neurogenic inflammation” (32). Since pharmacological experiments suggested that SOM, released from either the same or different sensory nerve fibers, is able to inhibit this neuropeptide release, we firstly focused on the direct effects of SOM on spinal sensory substance P and CGRP release using EIA (20, 23, 24). Upon short-term HIS and 5-HT stimulation, spinal sensory neurons released detectable amounts of CGRP, whereas simultaneous incubation with SOM did not affect this release. Release of substance P from the cultured neurons was well below the detection limit, in line with recent reports on neuropeptide release from mouse DRG (18). Long-term SOM exposure diminished the HIS- and 5-HT-induced peptide release.
The potential modulatory effects of SOM on the mast cell mediator-induced excitation of spinal sensory neurons were studied by use of calcium LCI. Acute application of HIS or 5-HT induced calcium-mediated neuronal activation in about half of the spinal sensory neurons, in line with a previous report (26). Recently the essential role of mast cell mediators in the exaggerate activation of visceral nociceptive neurons innervating the intestine, inducing visceral hyperalgesia, has been demonstrated (3–5, 13). On the other hand, evidence is at hand that SOM could provide a powerful tool in suppressing exaggerate nociceptive responses. The antinociceptive effects of SOM or its analog octreotide have long been known in humans (28, 30). Extensive research using a wide variety of animal models of inflammation and associated pain led to the conclusion that SOM is a potent peripheral analgesic mediator since it exerts both tonic and short-term inhibitory control over peripheral nociceptors, including the transient receptor potential vanilloid type 1 (TRPV1) (7, 8, 21, 22). However, because SSTR activation also reduces mechanical sensitization, SOM modulates nociception also by affecting other receptors, since TRPV1 is not activated by mechanical stimuli (11). Our experiments extend the conclusions on the antinociceptive effects of SOM even further. Calcium LCI experiments unequivocally demonstrated that SOM is able to decrease the percentages of neurons responsive to short-term stimulation with HIS and 5-HT. Since virtually all HIS- and 5-HT-responsive neurons were capsaicin sensitive and therefore can be assumed to bear TRPV1, SOM appears to be able to suppress nociceptive transmission, after long-term incubation, by simultaneously inhibiting the activity of TRPV1 and the receptors for other inflammatory mediators (HIS and 5-HT) within the same population of sensory neurons (12).
In addition to these immediate HIS- and 5-HT-induced effects, the long-term effects of these mast cell mediators on spinal sensory neurons, together with the modulatory effects of SOM, were investigated. Increased synthesis and transport of the proinflammatory mediators substance P and CGRP in sensory DRG neurons and their neuronal processes appears to be a generalized feature of inflammation in multiple peripheral tissues (6, 16, 19, 36). Moreover, also the expression levels of the receptors for these peptides are strictly regulated in spinal sensory neurons during the course of inflammation (2, 40). Therefore, the degree of nociception during inflammation is fine tuned by the discrete balance between pro- and antinociceptive mediators and their receptors in spinal sensory neurons (2). This hypothesis is supported by our findings that long-term incubation of spinal sensory neurons with HIS and 5-HT not only increases the expression levels of substance P, CGRP, and SOM, at both mRNA and protein levels, but also the expression levels of specific SSTR subtypes. Furthermore, qPCR and quantitative analysis confirmed these data in vitro, since the mRNA levels of substance P, CGRP, SOM, and SSTRs and the proportions of substance P-, CGRP- and SOM-ir neurons were elevated in response to long-term HIS and 5-HT incubation. In line with previous reports, both CGRPα and CGRPβ were detected in DRG, with CGRPα being the predominant isoform (38).
The expression of SSTR2 in rat DRG was repeatedly demonstrated, although mRNA for SSTR1, SSTR3, and SSTR4 also has been detected (2, 9, 37, 41). Our results indicate similar SSTR expression patterns in murine DRG, although we could not detect SSTR4 expression in neuronal profiles, only in endothelial cells. This apparent lack of neuronal SSTR4 expression in murine DRG is supported by the absolute lack of SSTR4 mRNA in DRG cultures. Therefore, it seems plausible to assume that the SOM-induced effects on the DRG cultures are mediated by SSTR1 and/or SSTR2. A possible explanation for the apparent conflicting data between the limited number of SSTR1-expressing neuronal profiles in the DRG and the widespread distribution of SSTR1-bearing extrinsic nerve fibers in the inflamed small intestine could be the preferential expression of SSTR1 on the neuronal processes rather than on the somata (51). The extensive expression of SSTR1 in neuronal processes in the DRG cultures and the limited number of SSTR1-ir neuronal somata underlie this hypothesis.
In addition to the expression of SSTR1 and SSTR2A in DRG neurons, these receptors were also widely expressed in satellite cells, suggesting that locally released SOM also affects satellite cell activity. The precise effects of SOM modulation on satellite cell activity is yet completely unknown. Peripheral inflammation induces increased intercellular satellite cell coupling in the mouse, and indirect evidence is available that blocking these intercellular gap junctions reduces nociceptive transmission (17). Therefore, it is possible that SOM modulates nociception not only by activating neuronal SSTRs in spinal sensory neurons, but also by modulating satellite cell activity. The observation that in vitro SOM not only reduces the numbers of neurons showing calcium-mediated responses to HIS and 5-HT stimulation, but also the number of responsive nonneuronal cells (most likely including satellite cells) supports this conclusion. The molecular base for this satellite cell-to-neuron communication remains largely unexplored. However, the presence of receptors for inflammatory-suppressive mediators on satellite cells opens up windows for evaluating the use of therapeutic components to modulate nociceptive neuronal transmission by targeting satellite cells.
In conclusion, SOM reduces HIS- and 5-HT-induced responses in murine spinal sensory neurons and satellite cells. This somatostatinergic modulatory effect is most probably also important in vivo, since intestinal inflammatory responses are highly dependent on the neuroimmune interactions between MMC and neurons. The previously described inhibitory effects of SOM on MMC degranulation reduce the release of a wide variety of mediators, including HIS and 5-HT (35, 47). We now demonstrate that SOM is able to inhibit both the acute and chronic effects of these MMC mediators on spinal sensory neurons, thereby modulating the mast cell-to-neuron communication. In addition, we demonstrate that SOM suppresses the release of the proinflammatory peptide CGRP from these spinal afferents. Since this peptide stimulates mast cell degranulation, SOM also strongly affects the neuron-to-mast cell communication. Combining these data puts SOM forward as a potentially powerful inhibitor of a complex and self-reinforcing inflammatory cascade coordinated by mast cell-neuron cross talk. Therefore, it is essential to evaluate the use of SOM analogs in the treatment of inflammatory intestinal pathologies. Moreover, since the interactions between mast cells and neurons also occur in other tissues, targeting SOM-mediated pathways could provide new therapies in the treatment of inflammatory pathologies in other organs as well. Finally, it is concluded that intestinal inflammation not only induces the onset of proinflammatory mechanisms, including the release and synthesis of substance P and CGRP, but simultaneously triggers endogenous mechanisms, involving SOM and several SSTR, to avoid excessive tissue damage and pain perception.
GRANTS
This study was supported by a fellowship from the Institute for the Promotion of Innovation by Science and Technology in Flanders (SB53449) to Joeri Van Op den bosch, a concerted research action of the University of Antwerp, and Grant G.0377.04 from the Research Foundation-Flanders.
Acknowledgments
The authors thank the technical staff of all laboratories involved for excellent technical assistance.
REFERENCES
- 1.Alving K, Sundstrom C, Matran R, Panula P, Hokfelt T, Lundberg JM. Association between histamine-containing mast cells and sensory nerves in the skin and airways of control and capsaicin-treated pigs. Cell Tissue Res 264: 529–538, 1991. [DOI] [PubMed] [Google Scholar]
- 2.Bär KJ, Schurigt U, Scholze A, Segond von Banchet G, Stopfel N, Bräuer R, Halbhuber KJ, Schaible HG. The expression and localization of somatostatin receptors in dorsal root ganglion neurons of normal and monoarthritic rats. Neuroscience 127: 197–206, 2004. [DOI] [PubMed] [Google Scholar]
- 3.Barbara G, Stanghellini V, de Giorgio R, Corinaldesi R. Functional gastrointestinal disorders and mast cells: implications for therapy. Neurogastroenterol Motil 18: 6–17, 2006. [DOI] [PubMed] [Google Scholar]
- 4.Barbara G, Stanghellini V, de Giorgio R, Cremon C, Cottrell GS, Santini D, Pasquinelli G, Morselli-Labate AM, Grady EF, Bunnett NW, Collins SM, Corinaldesi R. Activated mast cells in proximity to colonic nerves correlate with abdominal pain in irritable bowel syndrome. Gastroenterology 126: 693–702, 2004. [DOI] [PubMed] [Google Scholar]
- 5.Barbara G, Wang B, Stanghellini V, de Giorgio R, Cremon C, Di Nardo G, Trevisani M, Campi B, Geppetti P, Tonini M, Bunnett NW, Grundy D, Corinaldesi R. Mast cell-dependent excitation of visceral-nociceptive sensory neurons in irritable bowel syndrome. Gastroenterology 132: 26–37, 2007. [DOI] [PubMed] [Google Scholar]
- 6.Carlton SM, Coggeshall RE. Inflammation-induced up-regulation of neurokinin 1 receptors in rat glabrous skin. Neurosci Lett 326: 29–32, 2002. [DOI] [PubMed] [Google Scholar]
- 7.Carlton SM, Du J, Davidson E, Zhou S, Coggeshall RE. Somatostatin receptors on peripheral primary afferent terminals: inhibition of sensitized nociceptors. Pain 90: 233–244, 2001. [DOI] [PubMed] [Google Scholar]
- 8.Carlton SM, Du J, Zhou S, Coggeshall RE. Tonic control of peripheral cutaneous nociceptors by somatostatin receptors. J Neurosci 21: 4042–4049, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Carlton SM, Zhou S, Du J, Hargett RL, Ji G, Coggeshall RE. Somatostatin modulates the transient receptor potential vanilloid 1 (TRPV1) ion channel. Pain 110: 616–627, 2004. [DOI] [PubMed] [Google Scholar]
- 10.Chery-Croze S, Bosshard A, Martin H, Cuber JC, Charnay Y, Chayvialle JA. Peptide immunocytochemistry in afferent neurons from lower guts in rats. Peptides 9: 873–881, 1988. [DOI] [PubMed] [Google Scholar]
- 11.Corsi MM, Ticozzi C, Netti C, Fulgenzi A, Tiengo M, Gaja G, Guidobono F, Ferrerro ME. The effect of somatostatin on experimental inflammation in rats. Anesth Analg 85: 1112–1115, 1997. [DOI] [PubMed] [Google Scholar]
- 12.Cortright DN, Krause JE, Broom DC. TRP channels and pain. Biochim Biophys Acta 1772: 978–988, 2007. [DOI] [PubMed] [Google Scholar]
- 13.De Jonge F, De Laet A, Van Nassauw L, Brown JK, Miller HRP, van Bogaert PP, Timmermans JP, Kroese ABA. In vitro activation of murine DRG neurons by CGRP-mediated mucosal mast cell degranulation. Am J Physiol Gastrointest Liver Physiol 287: G178–G191, 2004. [DOI] [PubMed] [Google Scholar]
- 14.De Jonge F, Van Nassauw L, Adriaensen D, Van Meir F, Miller HR, Van Marck E, Timmermans JP. Effect of intestinal inflammation on capsaicin-sensitive afferents in the ileum of Schistosoma mansoni-infected mice. Histochem Cell Biol 119: 477–484, 2003. [DOI] [PubMed] [Google Scholar]
- 15.De Jonge F, Van Nassauw L, Van Meir F, Miller HRP, Van Marck E, Timmermans JP. Temporal distribution of distinct mast cell phenotypes during intestinal schistosomiasis in mice. Parasite Immunol 24: 225–231, 2002. [DOI] [PubMed] [Google Scholar]
- 16.Donnerer J, Schuligoi R, Stein C. Increased content and transport of substance P and calcitonin gene-related peptide in sensory nerves innervating inflamed tissue: evidence for a regulatory function of nerve growth factor in vivo. Neuroscience 49: 693–698, 1992. [DOI] [PubMed] [Google Scholar]
- 17.Dublin P, Hanani M. Satellite glial cells in sensory ganglia: their possible contribution to inflammatory pain. Brain Behav Immun 21: 592–598, 2007. [DOI] [PubMed] [Google Scholar]
- 18.Eberhardt M, Hoffmann T, Sauer SK, Messlinger K, Reeh PW, Fischer MJM. Calcitonin gene-related peptide release from intact isolated dorsal root and trigeminal ganglia. Neuropeptides 42: 311–317, 2008. [DOI] [PubMed] [Google Scholar]
- 19.Garrett NE, Kidd BL, Cruwys SC, Tomlinson DR. Changes in preprotachykinin mRNA expression and substance P levels in dorsal root ganglion of monoarthritic rats: comparison with changes in synovial substance P levels. Brain Res 675: 203–207, 1995. [DOI] [PubMed] [Google Scholar]
- 20.Green PG, Basbaum AI, Levine JD. Sensory neuropeptide interactions in the production of plasma extravasation in the rat. Neuroscience 50: 745–749, 1992. [DOI] [PubMed] [Google Scholar]
- 21.Heppelmann B, Pawlak M. Inhibitory effect of somatostatin on the mechanosensitivity of articular afferents in normal and inflamed knee joints of the rat. Pain 73: 377–382, 1997. [DOI] [PubMed] [Google Scholar]
- 22.Heppelmann B, Pawlak M. Peripheral application of cyclo-somatostatin, a somatostatin antagonist, increases the mechanosensitivity of rat knee joint afferents. Neurosci Lett 259: 62–64, 1999. [DOI] [PubMed] [Google Scholar]
- 23.Hökfelt T, Elde R, Johansson O, Luft R, Nilsson G, Arimura A. Immunohistochemical evidence for separate populations of somatostatin-containing and substance P-containing primary afferent neurons in the rat. Neuroscience 1: 131–136, 1976. [DOI] [PubMed] [Google Scholar]
- 24.Kashiba H, Ueda Y, Senba E. Coexpression of preprotachykinin-A, alpha-calcitonin gene-related peptide, somatostatin, and neurotrophin receptor family messenger RNAs in rat dorsal root ganglion neurons. Neuroscience 70: 179–189, 1996. [DOI] [PubMed] [Google Scholar]
- 25.Keast JR, De Groat WC. Segmental distribution and peptide content of primary afferent neurons innervating the urogenital tract and colon of male rats. J Comp Neurol 319: 615–623, 1992. [DOI] [PubMed] [Google Scholar]
- 26.Kim BM, Lee SH, Shim WS, Oh U. Histamine-induced Ca(2+) influx via the PLA(2)/lipoxygenase/TRPV1 pathway in rat sensory neurons. Neurosci Lett 361: 159–162, 2004. [DOI] [PubMed] [Google Scholar]
- 27.Metcalfe DD Mast cells. Physiol Rev 77: 1033–1079, 1997. [DOI] [PubMed] [Google Scholar]
- 28.Mollenholt P, Rawal N, Gordh T Jr, Olsson Y. Intrathecal and epidural somatostatin for patients with cancer. Analgesic effects and postmortem neuropathologic investigations of spinal cord and nerve roots. Anesthesiology 81: 534–542, 1994. [DOI] [PubMed] [Google Scholar]
- 29.Patel YC Somatostatin and its receptor family. Front Neuroendocrinol 20: 157–198, 1999. [DOI] [PubMed] [Google Scholar]
- 30.Penn RD, Paice JA, Kroin JS. Octreotride: a potent new non-opiate analgesic for intrathecal infusion. Pain 49: 13–19, 1992. [DOI] [PubMed] [Google Scholar]
- 31.Pidsudko Z, Wasowick K, Sienkiewicz W, Kaleczyc j Czaja K, Lakomy M. The influence of inflammation on the expression of neuropeptides in the ileum-projecting primary sensory neurones in the pig. Folia Morphol (Warsz) 62: 235–237, 2003. [PubMed] [Google Scholar]
- 32.Pinter E, Helyes Z, Szolcsanyi J. Inhibitory effect of somatostatin on inflammation and nociception. Pharmacol Ther 112: 440–456, 2006. [DOI] [PubMed] [Google Scholar]
- 33.Purcell WM, Atterwill CK. Mast cells in neuroimmune function: neurotoxicological and neuropharmacological perspectives. Neurochem Res 20: 521–532, 1995. [DOI] [PubMed] [Google Scholar]
- 34.Rozniecki JJ, Dimitriadou V, Lambracht-Hall M, Pang X, Theoharides TC. Morphological and functional demonstration of rat dura mater mast cell neuron interactions in vitro and in vivo. Brain Res 849: 1–15, 1999. [DOI] [PubMed] [Google Scholar]
- 35.Saavedra Y, Vergara P. Somatostatin inhibits mucosal mast cell degranulation in normal conditions and during mast cell hyperplasia. Regul Pept 111: 67–75, 2003. [DOI] [PubMed] [Google Scholar]
- 36.Schaible HG, Grubb BD. Afferent and spinal mechanisms of joint pain. Pain 55: 5–54, 1993. [DOI] [PubMed] [Google Scholar]
- 37.Schulz S, Schreff M, Schmidt H, Händel M, Prezwlocki R, Höllt V. Immunocytochemical localization of somatostatin receptor sst2A in the rat spinal cord and dorsal root ganglia. Eur J Neurosci 10: 3700–3708, 1998. [DOI] [PubMed] [Google Scholar]
- 38.Schütz B, Mauer D, Salmon AM, Changeux JP, Zimmer A. Analysis of the cellular expression pattern of β-CGRP in α-CGRP-deficient mice. J Comp Neurol 476: 32–43, 2004. [DOI] [PubMed] [Google Scholar]
- 39.Segond von Banchet G, Kiehl M, Schaible HG. Acute and long-term effects of IL-6 on cultured dorsal root ganglion neurones from adult rat. J Neurochem 94: 238–248, 2005. [DOI] [PubMed] [Google Scholar]
- 40.Segond von Banchet G, Scholze A, Schaible HG. Prostaglandin E2 increases the expression of the neurokinin 1 receptor in adult sensory neurones in culture: a novel role of prostaglandins. Br J Pharmacol 139: 672–680, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Senaris RM, Schindler M, Humphrey PP, Emson PC. Expression of somatostatin receptor 3 mRNA in the motorneurones of the rat spinal cord, and the sensory neurones of the spinal ganglia. Brain Res Mol Brain Res 29: 185–190, 1995. [DOI] [PubMed] [Google Scholar]
- 42.Shindler KS, Roth KA. Double immunofluorescent staining using two unconjugated primary antisera raised in the same species. J Histochem Cytochem 44: 1331–1335, 1996. [DOI] [PubMed] [Google Scholar]
- 43.Smithers SR, Terry RJ. Infection of laboratory hosts with cercariae of Schistosoma mansoni and the recovery of adult worms. Parasitology 55: 695–700, 1965. [DOI] [PubMed] [Google Scholar]
- 44.Stead RH, Tomioka M, Quinonez G, Simon GT, Felten SY, Bienenstock J. Intestinal mucosal mast cells in normal and nematode-infected rat intestines are in intimate contact with peptidergic nerves. Proc Natl Acad Sci USA 84: 2975–2979, 1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sternini C Neurochemistry of spinal afferents supplying the gastrointestinal tract and the pancreas. In: Brain-Gut Interactions, edited by Taché A and Wingate D. Boca Raton, FL: CRC, 1991.
- 46.Tan LL, Bornstein JC, Anderson CR. Distinct chemical classes of medium-sized TRPV1 immunoreactive DRG neurons innervate the adult mouse jejunum and colon. Neuroscience 156: 334–343, 2008. [DOI] [PubMed] [Google Scholar]
- 47.Tang C, Lan C, Wang C, Liu R. Amelioration of the development of multiple organ dysfunction syndrome by somatostatin via suppression of intestinal mucosal mast cells. Shock 23: 470–475, 2005. [DOI] [PubMed] [Google Scholar]
- 48.Traub RJ, Hutchcroft K, Gebhart GF. The peptide content of colonic afferents decreases following colonic inflammation. Peptides 20: 267–273, 1999. [DOI] [PubMed] [Google Scholar]
- 49.Van Nassauw L, Adriaensen D, Timmermans JP. The bidirectional communication between neurons and mast cells within the gastrointestinal tract. Auton Neurosci 133: 91–103, 2007. [DOI] [PubMed] [Google Scholar]
- 50.Van Op den bosch J, Lantermann K, Torfs P, Van Marck E, Van Nassauw L, Timmermans J-P. Distribution and expression levels of somatostatin and somatostatin receptors in the ileum of normal and acutely Schistosoma mansoni-infected SSTR2 knockout/lacZ knockin mice. Neurogastroenterol Motil 20: 798–807, 2008. [DOI] [PubMed] [Google Scholar]
- 51.Van Op den bosch J, Van Nassauw L, Lantermann K, Van Marck E, Timmermans J-P. Effects of intestinal inflammation on the cell-specific expression of somatostatin receptor subtypes in the murine ileum. Neurogastroenterol Motil 19: 596–606, 2007. [DOI] [PubMed] [Google Scholar]