Abstract
Adrenergic stimulation of electrogenic K+ secretion in isolated mucosa from guinea pig distal colon required activation of two β-adrenergic receptor subtypes (β-AdrR). Addition of epinephrine (epi) or norepinephrine (norepi) to the bathing solution of mucosae in Ussing chambers increased short-circuit current (Isc) and transepithelial conductance (Gt), consistent with this cation secretion. A β-adrenergic classification was supported by propranolol antagonism of this secretory response and the lack of effect by the α-AdrR antagonists BE2254 (α1-AdrR) and yohimbine (α2-AdrR). Subtype-selective antagonists CGP20712A (β1-AdrR), ICI-118551 (β2-AdrR), and SR59320A (β3-AdrR) were relatively ineffective at inhibiting the epi-stimulated Isc response. In combination, CGP20712A and ICI-118551 inhibited the response, which supported a synergistic action by β1-AdrR and β2-AdrR. Expression of mRNA for both β1-AdrR and β2-AdrR was indicated by RT-PCR of RNA from colonic epithelial cells. Protein expression was indicated by immunoblot showing bands at molecular weights consistent with monomers and oligomers. Immunoreactivity (ir) for β1-AdrR and β2-AdrR was prominent in basolateral membranes of columnar epithelial cells in the crypts of Lieberkühn as well as intercrypt surface epithelium. Cells in the pericryptal sheath also had β1-AdrRir but did not have discernable β2-AdrRir. The adrenergic sensitivity of K+ secretion measured by Isc and Gt was relatively low as indicated by EC50s of 41 ± 7 nM for epi and 50 ± 14 nM for norepi. Adrenergic activation of electrogenic K+ secretion required the involvement of both β1-AdrR and β2-AdrR, occurring with an agonist sensitivity reduced compared with reported values for either receptor subtype.
Keywords: epinephrine, norepinephrine, isoproteranol, CGP20712A, ICI-118551
the sympathetic nervous system acts on the colonic mucosa primarily to inhibit fluid secretion, an influence that counters the excitatory input from the enteric nervous system (8, 11). Changes in this sympathetic influence adjust for various physiological stimuli and also contribute to clinical conditions ranging from colitis (39) to cancer (6). These sympathetic nerves inhibit both cholinergic and peptidergic secretomotor nerves in the submucosal plexus of the enteric nervous system via adrenergic receptors (AdrRs) such that the suppression of this neural drive for fluid secretion occurs together with the vasoconstriction and slowing of gut motility that characterizes a sympathetic response. In the distal colon, direct actions of adrenergic stimuli on transepithelial secretion also have been noted, including activation (4, 13, 24, 35, 40, 44) as well as inhibition (4, 22, 24, 30, 35). Although the adrenergic influences on blood flow, muscle contraction, and the enteric nerve plexuses have been studied extensively (11), the direct adrenergic actions on transepithelial ion flow are comparatively less defined.
The major ion secretory event characterized for colonic epithelia is electrogenic Cl− secretion. However, in vitro measurements indicate that adrenergic stimulation with epinephrine (epi) primarily activates a sustained electrogenic K+ secretion via a cellular mechanism similar to that for electrogenic Cl− secretion (13, 21, 31). The major difference from the Cl− secretory model is that K+ secretion occurs via the concomitant activation of apical membrane K+ conductance. The range in ion secretory output from the colonic epithelium can be grouped into three quantitatively distinct modes (17, 26). Many secretagogs such as PGE2 and VIP stimulate Cl− secretion that has both transient and sustained components. Because this mode of activation produces significant fluid secretion, it is well described as the flushing mode (14). The other extensively studied mode of Cl− secretion occurs when two secretagogs (commonly PGE2 and a cholinergic agonist) are present simultaneously and produce more Cl− secretion than the additive amount expected for each, which has been described as synergistic (9, 17, 45). The third and least studied mode is distinct from the other two modes by exhibiting Cl− secretion that is only transient, lasting a few minutes, along with a sustained electrogenic K+ secretion as exemplified by β-adrenergic activation. This mode has been termed modulatory (17, 26) to indicate the relatively low rate of secretion and the action to alter the luminal cation composition toward higher K+ concentration. Because modulatory mode K+ secretion activated by epi is dependent entirely on the activity of basolateral membrane Na+:K+:2Cl− cotransporters (13, 31), the distinctions among these three modes essentially become the intracellular signaling that controls activation of K+ channels and Cl− channels in the apical membrane. The character of the AdrRs present is essential for determining how the secretory cell responds to sympathetic stimulation.
Although it has long been appreciated that electrogenic K+ secretion in the distal colon is activated via the β-AdrR class as indicated by sensitivity to the β-antagonist propranolol (12), little is known about the receptor subtypes involved or the mucosal location of these receptors. The intestinal epithelial cells responding directly to adrenergic stimuli will be found within the two major zones of the mucosa, the tubular glands of the crypts of Lieberkühn and the intercrypt surface epithelium. In the colon, this surface epithelium is relatively flat in contrast to the large protruding villi of the small intestine. Renewal of the epithelium occurs from stem cells at the base of the crypts with cell proliferation within the crypt that moves cells along the structure onto the surface followed ultimately by senescence (7). Two major cell types comprise the colonic epithelium, columnar and goblet, roughly in equal proportion, along with a minority population of enteroendocrine cells. The goblet cells secrete mucin, and the columnar cells become the absorptive cells in the surface with distinct brush-border apical membranes. Studies to localize secretory activity indicate that crypt columnar cells respond in a manner consistent with both K+ and Cl− secretion (16, 18). Localization of the Na+:K+:2Cl− cotransporter to the crypt also supports this epithelial zone as a site for K+ secretion as well as Cl− secretion (27, 32). A definition of the receptor types present on these epithelial cells is needed to determine the modes of direct adrenergic response that are possible.
The present study examined the involvement of AdrRs required for activation of the electrogenic K+ secretion characteristic of modulatory mode secretion. Many in vitro studies of ion secretion in colonic mucosa are most related to the synergistic secretory mode because of neural and paracrine release of secretagog substances that result in secretory states with variable levels of activation. To examine the modulatory mode in isolation, secretagogs arriving from neural and paracrine sources must be quelled. The methods used in this study isolated the direct adrenergic actions by three means to allow a better understanding of the cellular events involved in activating electrogenic K+ secretion. Mechanical separation of the mucosa from the underlying muscle and ganglia limited neural input, chemical inhibition of cyclo-oxygenases reduced paracrine production of prostaglandins, and extensive rinsing reduced the concentration of other compounds released during the mucosal isolation process. The results presented here demonstrate comprehensively the expression of mRNA for β-receptor subtypes, the mucosal localization of these receptors, as well as functional evidence for the receptors necessary to activate secretion. Specifically, adrenergic stimulation of modulatory secretion involved both β1- and β2-AdrRs that coordinated to activate the signaling cascades necessary for electrogenic K+ secretion.
MATERIALS AND METHODS
Male guinea pigs (500–800 g body wt, Hartley strain; Harlan Laboratories, Indianapolis, IN) received standard chow and water ad libitum and were housed on site at least 2 wk before experiments. Guinea pigs were euthanized with an animal decapitator (Harvard Apparatus, Holliston, MA) in accordance with a protocol approved by the Wright State University Laboratory Animal Care and Use Committee. Distal colon was removed, cut open along the mesenteric line, and flushed with ice-cold Ringer solution to remove fecal pellets. The colonic segment used was the ∼20-cm-long portion ending roughly 5 cm from the peritoneal border. The mucosa was separated from underlying submucosa and muscle layers using a glass slide such that the plane of dissection occurred between the base of the crypts and the muscularis mucosa with only mucosal components immediately adherent to the epithelium remaining. These isolated colonic mucosal sheets were used for measurement of transepithelial electrical parameters, RNA isolation, and protein detection by immunoblot.
Detection of mRNA with RT-PCR.
Total RNA was extracted by RNeasy Mini Kit (Qiagen, Valencia, CA) from EDTA-released epithelial cells (25). A 20-mg portion of epithelial cells (pelleted at 4,000 g for 5 min) was used per spin column. Total RNA was treated with the recombinant DNase-I (Ambion, Austin, TX) to remove contaminating DNA, used for RT-PCR, or stored at −70°C. RT-PCR was performed using Gene Amp Gold RNA PCR Reagent Kit (Applied Biosystems, Foster City, CA). First-strand cDNA was synthesized using 1 μg total RNA [20 μl reaction mixture containing: 15 units MultiScribe Reverse Transcriptase, 1.25 μM Oligo d(T)16 primer, 1 mM dNTP, 10 units RNase inhibitor, 10 mM DTT, 2.5 mM MgCl2, and 1× RT-PCR buffer]. RT was carried out at 42°C for 30 min and then at 70°C for 7 min. The PCR mixture (50 μl) contained the following: 3 μl first-strand cDNA reaction, 2.5 units AmpliTaq Gold DNA polymerase, 50 pmol forward and reverse gene-specific primers, 1.75 mM MgCl2, 0.8 mM dNTP, and 1× RT-PCR buffer. cDNA was amplified in a thermal cycler (2720 Thermal Cycler, Applied Biosystems) with the following: initial denaturing 10 min at 95°C, 40 cycles 30 s denaturation at 94°C, 30 s annealing at 55°C, 30 s extension at 60°C, final extension for 7 min at 72°C to ensure completion. Each PCR included a negative control reaction using total RNA instead of cDNA as a template to assure that the total RNA was free of genomic DNA. PCR products were analyzed by electrophoresis in a 1.5% agarose gel, visualized by ethidium bromide. DNA products were excised from the gel, purified using QIAquick Gel Extraction Kit (Qiagen), and sequenced (Agencourt Bioscience, Beverly, MA).
Primers specific for β-AdrRs were designed by alignment of those in a previous report (43) with nucleotide sequences for β1-AdrR (GenBank accession NM_012701, rat Adrb1) and β2-AdrR (GenBank accession AJ459814, guinea pig Adrb2). In positions where the nucleotide sequences were not homologous, bases were changed to correspond to the rat β1-AdrR sequence and the guinea pig β2-AdrR sequence. Primers for β1-AdrR were (forward primer) 5′-tcg tgt gca cag tgt ggg cc-3′ (533–552) and (reverse primer) 5′-agg aag cgg cgc tcg cag ctg tcg-3′ (774–797). Primers for β2-AdrR were (forward primer) 5′-cac caa cta ctt cat cac ctc-3′ (201–221) and (reverse primer) 5′-ggc aat cct gaa atc tgg gct ccg gca g-3′ (977–1004). Primers for GAPDH gene were (forward primer) 5′-gtg aag gtc gga gtc aac gga ttt-3′ and (reverse primer) 5′-cac agt ctt ctg ggt ggc agt gat-3′, used as an internal control.
Tissue fixation and immunolocalization.
Colonic tissues were fixed after isolation, as described previously (26). Briefly, isolated mucosal sheets were pinned in a Sylgard-coated dish and immersed in fixation solutions (30–40 min, room temperature). These mucosal tissues were prepared for immunofluorescence by dehydration, sectioning, and mounting on gelatin-coated slides. Sections were permeabilized, blocked, and then incubated for 48 h (4°C) with primary antibody. Antibodies for β-AdrRs were obtained from Santa Cruz Biotechnology (Santa Cruz, CA) and were polyclonal anti-β1-AdrR [4 ng/μl; COOH-terminal epitope (V-19) of mouse Adrb1] and polyclonal anti-β2-AdrR [4 ng/μl; residues 338–413 (H-73) of human Adrb2]. A donkey-anti-rabbit IgG antibody, conjugated to AlexaFluor 488 (Invitrogen, Carlsbad, CA), was used to detect immunoreactivity (ir) (4 ng/μl, 2 h, room temperature). Sections were washed and mounted in Vectashield (Vector Laboratories, Burlingame, CA), and fluorescence was visualized with an Olympus BX60 epifluorescence microscope in the Microscopy Core Facility of the Comprehensive Neuroscience Center.
Detection of proteins by immunoblot.
Isolated colonic mucosa was disrupted on ice by sonication in a buffered solution containing protease inhibitors (26). The isolation solution contained (in mM): 178 Na+, 1.5 Mg2+, 153 Cl−, 50 HEPES, 10 EDTA, 10% glycerol, 1% Triton X-100, protease inhibitor cocktail (one tablet/.10 ml) (Roche Applied Science, Indianapolis, IN). Samples were centrifuged at 6,000 g (10 min, 4°C) followed by centrifugation of the resulting supernatant at 100,000 g (60 min, 4°C) to obtain a membrane sample; protein content was determined by the Bradford method. Proteins were electrophoresed by SDS-PAGE and transferred to PVDF membranes. These membranes were blocked with 10% non-fat dry milk in TBS with 0.1% TWEEN 20 followed by incubation with specific primary antibody and then with horseradish peroxidase-conjugated secondary antibody (Jackson ImmunoResearch Laboratories, West Grove, PA). PVDF membranes were developed (90 s) with LumiGLO (Cell Signaling Technology, Beverly, MA) before imaging to detect product with a Fuji LAS3000 ImageReader in the Center for Genomics Research.
Transepithelial current measurement.
Isolated mucosal sheets were used for measurement of transepithelial current and conductance (15). Four mucosal sheets from each guinea pig were mounted in Ussing chambers (0.64 cm2 aperture), supported on the serosal face by Nuclepore filters (∼10 μm thick, 5-μm pore diameter; Whatman, Clifton, NJ). Bathing solutions (10 ml) were circulated by gas lift through water-jacketed reservoirs (38°C). Standard Ringer's solution contained (in mM): 145 Na+, 5.0 K+, 2.0 Ca2+, 1.2 Mg2+, 125 Cl−, 25 HCO3−, 4.0 H2PO4−, and 10 d-glucose. Solution pH was maintained at 7.4 by continual gassing with 95% O2 and 5% CO2. Connection of chambers to automatic voltage clamps (Physiologic Instruments, San Diego, CA) permitted compensation for solution resistance and continuous measurement of short-circuit current (Isc). Transepithelial electrical potential difference was measured by paired calomel electrodes connected to the chambers by Ringer-agar bridges, and current was passed across the tissue through two Ag-AgCl electrodes connected by Ringer-agar bridges. Isc was referred to as positive for cation flow across the epithelium from mucosal to serosal side. Transepithelial conductance (Gt) was calculated from currents resulting from bipolar square voltage pulses imposed across the mucosa (± 5 mV, 3-s duration, 1-min intervals).
Mucosal responses to secretagogs and inhibitors were examined after producing a quiescent basal condition through suppression of neural and paracrine activators persisting in the isolated colonic mucosa (15). The mucosal preparation removes the influences from enteric plexuses, such that only mucosal nerve endings remain, which have only minimal contribution to the stimulation by secretagogs. Prostanoid production was suppressed by the COx-1 inhibitor SC560 (1 μM) and the COx-2 inhibitor CAY10404 (1 μM) added to both bathing solutions. Other compounds released from mucosal cells into the bathing solutions as a result of mucosal isolation were reduced in concentration (∼8,000-fold) by replacing the solutions three times after mounting the mucosa in the chamber. Amiloride (10 μM) was added to the mucosal bath to inhibit electrogenic Na+ absorption and its contribution to Isc.
SC560 and CAY10404 were obtained from Cayman Chemical (Ann Arbor, MI); CGP20712A, CL316243, ICI-118551, procaterol, SR59320A, yohimbine, and 2-{[b-(4-hydroxyphenyl)ethyl]aminomethyl}-1-tetralone (BE2254) from Tocris Bioscience (Ellisville, MO); epi from Elkins-Sinn (Cherry Hill, NJ). All other chemicals were obtained from Sigma Chemical (St. Louis, MO). Drugs were added in small volumes from concentrated stock solutions (15).
Data analysis.
Responses of Isc and Gt to secretagogs and antagonists were obtained from adjacent mucosae in each colon to permit direct comparisons. Recordings of Isc were digitized at 10-s intervals to examine the secretory time course. Concentration responses of Isc and Gt were fit to Henri-Michaelis-Menten binding curves using a nonlinear least-squares procedure. Results were reported as means ± SE with the number of animals (n) indicated. Statistical comparisons were made using a two-tailed Student's t-test for paired responses, with significant difference accepted at P < 0.05.
RESULTS
Adrenergic activation of secretion.
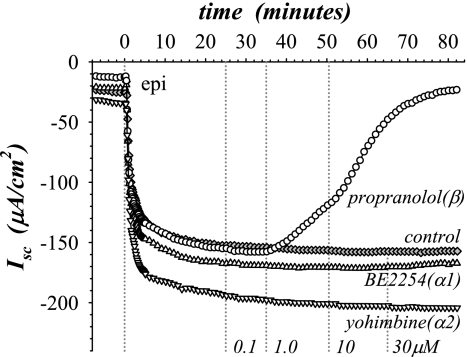
Adrenergic activation of ion secretion was elicited by adding epi to the serosal bathing solution of mucosae in the standard basal state and measured by changes in Isc. The Isc typically decreased with epi to a stable negative value within 20 min (Fig. 1), consistent with electrogenic K+ secretion as measured previously using radioisotopic unidirectional fluxes in conjunction with Isc (13, 21, 31). Involvement of the α- and β-adrenergic types of receptors (α-AdrR and β-AdrR) in this secretory activation was examined using selective antagonists. Propranolol, a β-selective antagonist, added during epi stimulation inhibited the negative Isc (Fig. 1), whereas α-selective antagonists BE2254 (α1-AdrR) and yohimbine (α2-AdrR) did not alter Isc even at a 30-fold excess of the epi concentration, which was consistent with a specific requirement for the β-AdrR type.
Fig. 1.
Secretory activation was sensitive to β-adrenergic antagonism. Isolated mucosae were stimulated by epinephrine (epi) addition (t = 0 min) to the serosal bath (1 μM), from the standard basal condition (see materials and methods). Electrogenic secretion was monitored by the short-circuit current (Isc), measured in 4 adjacent mucosae during stimulation by epi as adrenergic receptor (AdrR) antagonists were added at increasing concentrations (0.1 μM, 25 min; 1 μM, 35 min; 10 μM, 50 min; 30 μM, 65 min) to the serosal bath. The antagonists used were BE2254 (▵, α1-AdrR), yohimbine (▿, α2-AdrR), and propranolol (○, β-AdrR); no antagonist control (⧫). The responses were representative of 3 similar experiments (epiΔIsc: BE2254, −94 ± 14 μA/cm2; yohimbine, −93 ± 17 μA/cm2; propranolol, −8 ± 2 μA/cm2; no antagonist, −86 ± 15 μA/cm2; only the propranolol response was significantly different from epi alone and was not significantly different from zero, P < 0.05).
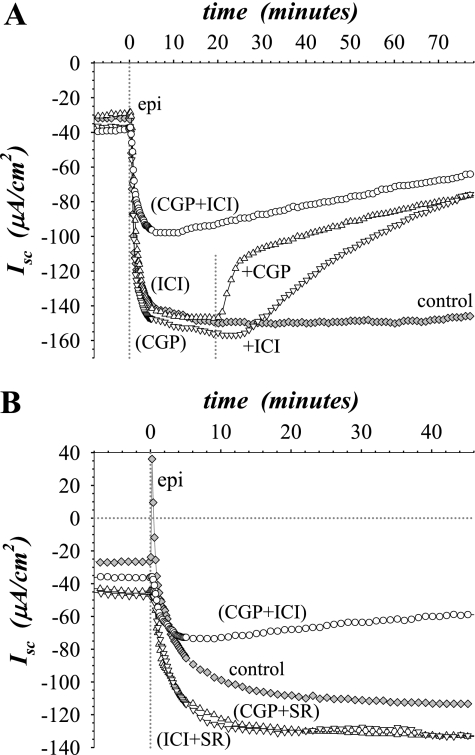
To determine which β-AdrR subtype transduced the secretory response, the subtype-selective antagonists CGP20712A (β1-AdrR), ICI-118551 (β2-AdrR), and SR59320A (β3-AdrR) were used. Prior addition of CGP20712A or ICI-118551 at 1 μM, a threefold excess to epi, did not alter the secretory response (Fig. 2A), which was in stark contrast to the inhibitory action of the general β-selective antagonist propranolol (Fig. 1). Similarly, adding SR59320A at 1 μM before or during the secretory response did not alter Isc (data not shown). On the basis of the known kds for these receptor subtypes (37, 43), CGP20712A and ICI-118551 would have been anticipated to inhibit the response by >90% and SR59320A by >50% if the response involved that receptor subtype. Increasing the concentration of these antagonists to 10 μM only reduced the size of the epi response by 5% to 20% (data not shown), and any expectation of subtype selectivity was lost. However, when either CGP20712A or ICI-118551 was added at 1 μM as a second antagonist during the epi response so that both β1-AdrR and β2-AdrR would be antagonized (total antagonist concentration of 2 μM), Isc declined toward zero (Fig. 2A). A simple additive presence of these two antagonists would have been expected to increase inhibition by <10% in this situation. The slower change with ICI-118551 was consistent with the low on-rate for this antagonist (5). Also, the presence of both antagonists before stimulation blunted the initial response by ∼50% followed by a steady decline in Isc toward zero (Fig. 2A). Because the efficacy of these antagonists was reduced dramatically compared with values reported for β-AdrRs (37, 43), the observed synergistic antagonism by CGP20712A and ICI-118551 suggested an interaction between β1-AdrR and β2-AdrR. In contrast to the combined addition of CGP20712A and ICI-118551, adding the β3-selective antagonist SR59320A in combination with either CGP20712A or ICI-118551 did not alter the secretory response to epi (Fig. 2B). These results supported an activation of electrogenic K+ secretion that required involvement from both β1-AdrR and β2-AdrR subtypes.
Fig. 2.
Combination of β1 and β2 selective antagonists increased efficacy. Isolated mucosae were stimulated by epi (0.3 μM) from the standard basal condition as in Fig 1. A: Isc was measured in 4 adjacent mucosae after pretreatment for 20 min with subtype-selective antagonists CGP20712A (CGP, ▿, β1-AdrR) and ICI-118551 (ICI, ▵, β2-AdrR) added at 1 μM either alone or together (○) to the serosal bath (⧫, no antagonist control). After epi stimulation was near maximal (∼20 min), the second antagonist was added at 1 μM to the serosal bath such that both receptor subtypes were antagonized. B: Isc was measured in 4 adjacent mucosae after pretreatment for 20 min with subtype-selective antagonists CGP20712A, ICI-118551, and SR59320A (SR, β3-AdrR) added in combination at 1 μM each to the serosal bath, CGP + ICI (○), CGP + SR (▵), ICI + SR (▿), and no antagonist control (⧫). The responses were representative of 4 similar experiments (epiΔIsc: CGP + ICI, −18 ± 3 μA/cm2; CGP + SR, −83 ± 19 μA/cm2; ICI + SR, −91 ± 2 μA/cm2; CGP, −86 ± 13 μA/cm2; ICI, −82 ± 7 μA/cm2; no antagonist, −95 ± 7 μA/cm2; only the CGP + ICI response was significantly different from epi alone, and all were significantly different from zero, P < 0.05).
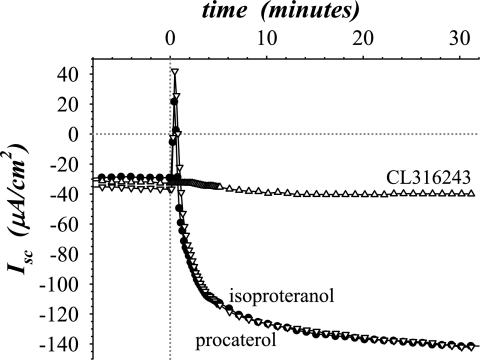
The β-AdrR subtype involvement was tested further with subtype-selective agonists, procaterol (β2-AdrR), CL316243 (β3-AdrR), and isoproteranol (β-AdrR). A suitably selective β1-AdrR agonist was not available. Both isoproteranol and procaterol reproduced the epi response, but CL316243 was without effect (Fig. 3). In the mucosa from some guinea pigs and as seen in this experiment, Isc briefly changed in the positive direction before reversing and attaining a negative value, consistent with a transient component of electrogenic Cl− secretion (31). Typically, the guinea pigs in this study lacked transient positive Isc responses. The ability of isoproteranol to activate both the transient positive Isc and sustained negative Isc (Fig. 3) indicated that both the Cl− secretory and the K+ secretory components of the epi response were transduced via β-AdrR. In addition, the α-AdrR antagonists BE2254 and yohimbine did not alter the transient positive Isc (data not shown), further indicating the lack of involvement by α-AdrR in the secretory response to epi. Together these results with subtype-selective antagonists and agonists supported the ability of β2-AdrR to activate electrogenic K+ secretion but also indicated a requirement for β1-AdrR.
Fig. 3.
Subtype-selective β-adrenergic agonists activated secretion differentially. Isolated mucosae were stimulated by subtype-selective agonists (0.3 μM) from the standard basal condition as in Fig 1. Isc was measured in 3 adjacent mucosae during addition of isoproteranol (•, β-AdrR), procaterol (▿, β2-AdrR), and CL316243 (▵, β3-AdrR) to the serosal bath. A fourth adjacent mucosa was stimulated with epi (0.3 μM) and exhibited a response similar to the mucosae receiving isoproteranol or procaterol (omitted for clarity). The responses were representative of 3 similar experiments (agonistΔIsc: isoproteranol, −91 ± 8 μA/cm2; procaterol, −83 ± 5 μA/cm2; CL316243, −6 ± 3 μA/cm2; epi, −102 ± 9 μA/cm2; only the CL316243 response was significantly different from epi and was not significantly different from zero, P < 0.05).
Identification of β-AdrRs in distal colonic mucosa.
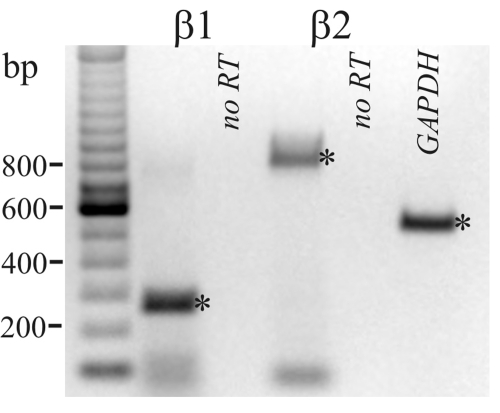
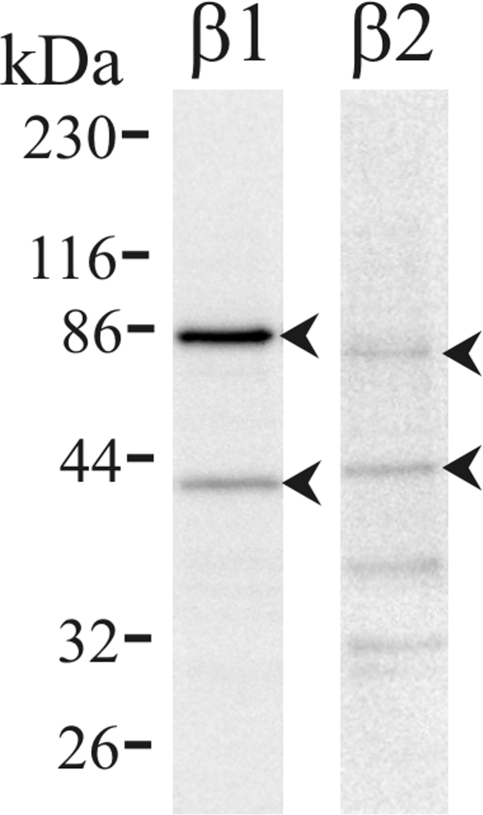
The presence of mRNA for β-AdrR proteins (β1-AdrR and β2-AdrR) in distal colonic epithelial cells was confirmed using RT-PCR (Fig. 4). Both PCR products were purified and sequenced which verified identity with the reported sequence for these receptor proteins, 94% with rat β1-AdrR (Adrb1) and 99% with guinea pig β2-AdrR (Adrb2). The presence of β1-AdrR and β2-AdrR also was examined by immunoblot, and immunoreactive bands consistent with the β1-AdrR and β2-AdrR were observed (Fig. 5). The bands at ∼43 kDa were similar to the anticipated size of unmodified monomeric forms for the receptors (3, 19, 34), 50 kDa for β1-AdrR and 47 kDa for β2-AdrR. The smaller apparent size was consistent with membrane proteins that do not completely unfold with SDS and therefore have a higher mobility in the gel than expected. Larger-sized bands noted at ∼80 kDa were consistent with posttranslational modification or undissociated oligomerization of β-AdrRs (29). These results support the presence of both β1-AdrR and β2-AdrR in colonic mucosal epithelial cells.
Fig. 4.
β-AdrR mRNA detected by RT-PCR. RNA isolated from distal colonic epithelial cells was used to amplify β1-AdrR and β2-AdrR products by RT-PCR. Products were obtained at sizes predicted from the position of the forward and reverse primers (indicated by asterisks), 265 base pairs for β1-AdrR and 804 base pairs for β2-AdrR; and, amplification of a GAPDH product (555 base pairs) served as a positive control for RNA isolation. The negative control obtained by not including reverse transcriptase (no RT) indicated the lack of contamination by genomic DNA in the sample. The faint bands smaller than 100 bp likely were due to unused primers from the PCR.
Fig. 5.
β-AdrR proteins detected by immunoblot. Protein isolated from distal colonic mucosa was immunoblotted with antibodies against the β1-AdrR and β2-AdrR proteins. Immunoreactive bands occurred at 42 kDa and 83 kDa for β1-AdrR and 43 kDa and 79 kDa for β2-AdrR (arrowheads), consistent with monomeric and possible oligomeric forms; fainter bands apparent at lower molecular weights likely represented receptor forms degraded after retrieval from the plasma membrane. Use of the secondary antibody alone eliminated all bands (data not shown), indicating that the primary antibodies were necessary for the observed results. Further support for the specificity of the anti-β1-AdrR labeling was indicated by elimination of bands by preabsorption of the primary antibody with the antigenic peptide (data not shown).
Localization of β-AdrRs in distal colonic mucosa.
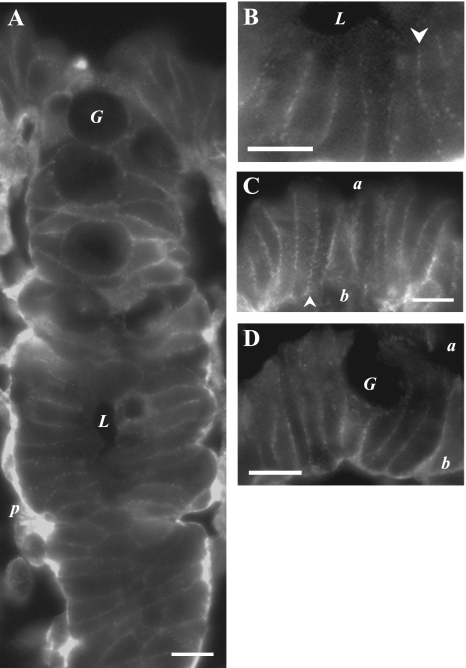
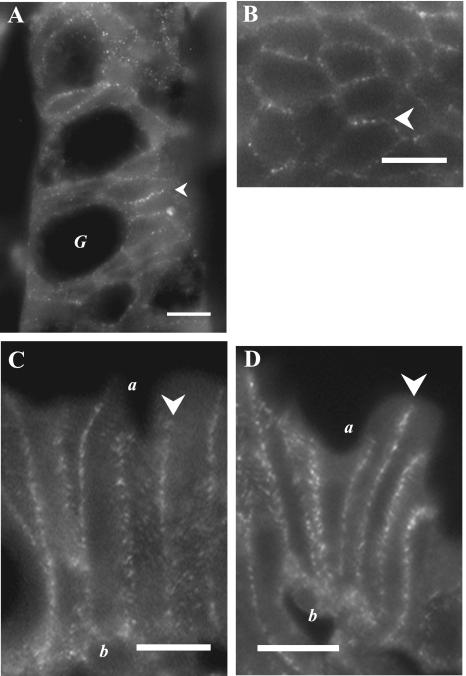
Ir for β1-AdrR (Fig. 6) and β2-AdrR (Fig. 7) was detected in mucosal locations consistent with the plasma membrane of colonic epithelial cells. Prominent labeling was seen in the lateral membrane of crypt and surface epithelial cells. The luminal margins of epithelial cells were not labeled, indicating an absence from the apical membrane. In addition, the uniform lateral labeling in crypts supported the possible presence of β1-AdrR and β2-AdrR in goblet cells as well as in columnar cells. The pericryptal sheath had distinct β1-AdrRir labeling but not β2-AdrRir, indicating a potential difference in adrenergic sensitivity between the epithelial cells and the surrounding myoepithelial cells.
Fig. 6.
β1-AdrR proteins localized in the colonic mucosa. β1-AdrR protein was detected by immunofluorescence (anti-β1-AdrR) in distal colonic mucosa. A. crypts showed distinct lateral membrane β1-AdrRir labeling, but luminal margins did not show labeling (lumen, L). The pericryptal sheath (p) also was prominently labeled. B. portion of the crypt in A was enlarged to show lateral labeling (arrowhead). C and D: surface epithelial cells had prominent β1-AdrRir labeling of lateral membranes (arrowheads) with indications of basal labeling (b), but labeling for apical membranes (a) was not apparent. Apically located goblet granule masses were apparent as dark voids (G). Use of the secondary antibody alone eliminated all membrane labeling (data not shown), indicating that primary antibodies were necessary for the observed results, and preabsorption of the primary antibody with the antigenic peptide also eliminated labeling (data not shown). Scale bars = 10 μm.
Fig. 7.
β2-AdrR proteins localized in the colonic mucosa. β2-AdrR protein was detected by immunofluorescence (anti-β2-AdrR) in distal colonic mucosa. A and B: crypts showed distinct lateral membrane labeling for β2-AdrRir (arrowheads). C and D: surface epithelial cells had prominent β2-AdrRir labeling of lateral membranes (arrowheads) without apparent labeling of apical (a) or basal (b) membranes. Use of the secondary antibody alone eliminated all membrane labeling (data not shown), indicating that primary antibodies were necessary for the observed results. Scale bars = 10 μm.
Sensitivity to epi and norepinephrine.
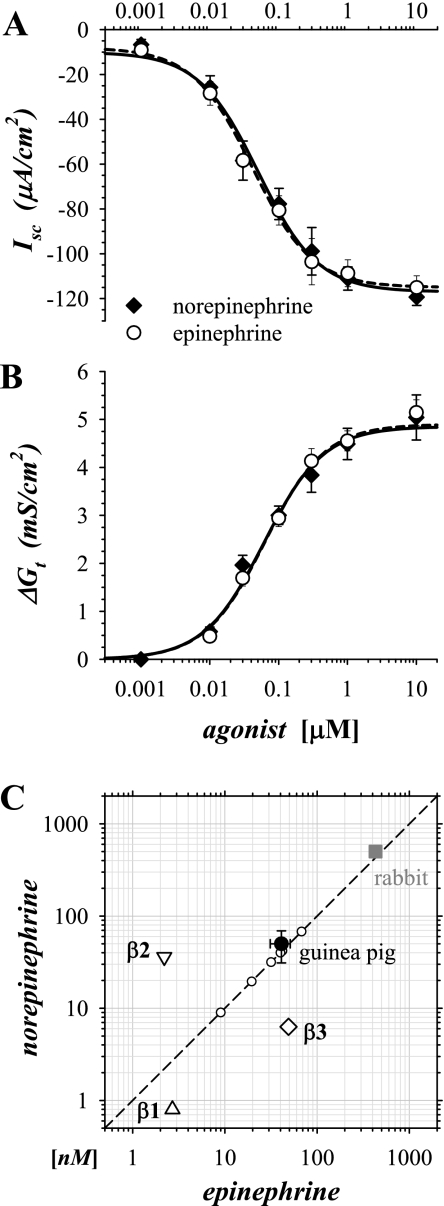
Sensitivity of secretory activation to epi and norepinephrine (norepi) was determined by measuring the concentration dependence for activation of Isc and Gt (Fig. 8). Responses were obtained in adjacent mucosae from each guinea pig in duplicate. These paired EC50s for epi and norepi were nearly identical whether determined from changes in Isc or Gt. This observed sensitivity to epi and norepi did not conform to the sensitivity for any of the three subtypes of β-AdrR (Fig. 8C), indicating that additional factors were present that altered receptor responsiveness in colonic epithelial cells.
Fig. 8.
Sensitivity of secretory activation to epi and norepinephrine (norepi) was similar. Isolated mucosae were stimulated by adding either epi or norepi cumulatively to the standard basal condition, as in Fig 1. The resulting concentration dependences of Isc (A) and Gt (B) were shown (n = 6), and the data were fit by a nonlinear method to Henri-Michaelis-Menton kinetics for a single binding site, norepi (solid line), epi (dashed line). The resulting EC50s from Isc were 41 ± 7 nM for epi and 50 ± 14 nM for norepi; maximal Isc was −115 ± 5 μA/cm2 for epi and −117 ± 4 μA/cm2 for norepi. The EC50s obtained from the fit of Gt were 63 ± 9 nM for epi and 61 ± 17 nM for norepi; maximal Gt was 4.90 ± 0.27 mS/cm2 for epi and 4.85 ± 0.47 mS/cm2 for norepi. Values for epi and norepi were not significantly different (P < 0.05). C: EC50s of the guinea pig Isc response to norepi and epi (•) were compared with EC50s from rabbit distal colonic mucosa (▪; 38) and the 3 β-AdrRs (▵,▿, ◊; 37). Error bars were calculated from variability between groups (N = 6 for epi, N = 3 for norepi). epiEC50s for each distinct group of guinea pigs were shown as points (○) on the line of identity (dashed line).
A large part of the observed variability in EC50s was associated with differences between groups of guinea pigs obtained from the supplier, generally arriving at 2–3-wk intervals; variability within a group was about one-half of the variability between groups. Among these groups (N = 6), the EC50 for epi averaged 35 ± 8 nM, and individually epiEC50 ranged from 6 nM to 81 nM (n = 13). Although the level of receptor expression was not examined, the size of the maximally activated epiIsc did not correlate with the variations in epiEC50 (avgIsc = −116 ± 6 μA/cm2, avgEC50 = 37 ± 6 nM, r2 = 0.033, n = 13), suggesting that the capacity to secrete was not limited by the sensitivity or number of receptors available for intracellular signaling. This variation in sensitivity to epi suggested that the original in vivo state of the guinea pig had a persistent influence on the adrenergic response in vitro; the contribution of Y-neuropeptide receptors to the observed variability has been examined in the companion paper (47).
DISCUSSION
Electrogenic K+ secretion is stimulated by epi in distal colon (13, 21, 31), as seen by a negative Isc that reaches large values, which is a defining characteristic for the modulatory mode of secretion. The capacity for modulatory secretion is larger in herbivores such as the guinea pig (31) and rabbit (13, 38) compared with omnivores such as the rat (26, 44) both in the maximal rate and the length of the distal segment of the colon. The physiological role served by modulatory secretion is not well determined but likely extends beyond a contribution to whole body K+ balance. The action of both subtype-selective antagonists and agonists supported a crucial role for β1-AdrR and β2-AdrR in activation of this secretory mode (Figs. 1, 2, and 3), And mRNA expression of β1-AdrR and β2-AdrR by RT-PCR confirmed a presence in epithelial cells (Figs. 4 and 5). The distribution of these receptors also was consistent with localization to the basolateral membrane of epithelial cells in both the crypt and surface regions, which would permit neural activation (Figs. 6 and 7). The possibility that the predominant location of electrogenic K+ secretion is within the crypts (16, 18) suggests that the β-AdrRs observed in the surface epithelium control other cell functions during the brief 1–2-day duration that epithelial cells spend within the surface zone. In the rat distal colon, an additional function for β-AdrRs is to stimulate electrogenic Na+ absorption that likely occurs in the surface epithelium (41). Together, the evidence from expression and the pharmacological sensitivity provided strong support for the ability of β1-AdrR and β2-AdrR to transduce the activation of modulatory secretion in the guinea pig distal colon.
AdrRs activating colonic ion secretion.
Previous studies provide a consistent but patchwork case for AdrRs in the colonic mucosa. Using labeled receptor ligands, the rat distal colon has been shown to have both β1-AdrR and β2-AdrR in the mucosa (46), and the presence of β1-, β2-, and β3-AdrR was indicated by mRNA expression (10). Human colonic mucosa expresses mRNA for β1-AdrR and β2-AdrR but not for β3-AdrR (33), and the lack of β3-AdrR is supported by an immunohistochemical localization study (2). Although the presence of β3-AdrR was not excluded for guinea pig colonic mucosa, the lack of effects by either a specific agonist or antagonist strongly supported the absence of a functional connection to modulatory secretion (Figs. 2 and 3). The location of the AdrR involved in modulatory secretion is likely on the epithelial cells because the in vitro response is not altered by attempts to block interaction with the other cells remaining in the isolated mucosa. Specifically, the response to epi is not altered by blocking excitability of remnant nerve fibers with TTX or by blunting prostaglandin production via inhibition of cyclo-oxygenases with indomethacin (13, 31, 38). Because epithelial cells were isolated to obtain total RNA, the RT-PCR results for guinea pig distal colon (Fig. 4) directly supported the immunofluorescence localization (Figs. 6 and 7) of β1-AdrR and β2-AdrR to epithelial cells.
Use of subtype-selective antagonists and agonists provides the most immediate and direct means to indicate which receptors are coupled to the modulatory response stimulated by epi. Although α-AdrRs have been detected in colonic mucosa (36, 42), the inability of α-AdrR antagonists to blunt electrogenic K+ secretion together with the lack of stimulation by α-AdrR agonists indicated that this class of receptor was not coupled to signaling pathways that activate the modulatory mode of secretion (13, 38, Fig. 1). Involvement of β-AdrRs in activating K+ secretion is indicated by sensitivity to the β-blocker propranolol, both in rabbit distal colon (13, 38) and in guinea pig distal colon (21, Fig. 1), which supports an intracellular signaling cascade with cAMP production as a key component (1, 48). A previous study using rabbit distal colon to identify the β-AdrR subtype producing K+ secretion used two agonists with selectivity for either β1-AdrR or β2-AdrR (38). The greater efficacy of dobutamine over terbutaline supports the assignment of β1-AdrR as the subtype involved. However, since that time, dobutamine has been observed to have efficacy at both β1-AdrR and β2-AdrR (5), so that the stimulation of K+ secretion may have occurred because of its ability to act at both β1-AdrR and β2-AdrR. Results with subtype-selective antagonists supported a synergistic involvement of β1-AdrR and β2-AdrR because the combined presence of CGP20712A and ICI-118551 was required for effective inhibition (Fig. 2). Overall, the results indicated that the K+ secretory response was initiated by β-AdrRs, likely requiring both β1-AdrR and β2-AdrR subtypes.
A second type of adrenergic action inhibits secretagog stimulated Cl− secretion, which is distinct from the modulatory activation of ion secretion examined in the present study. This inhibitory response is observed as a negative deflection in Isc and alterations in radioisotopic unidirectional Cl− fluxes, with both α-AdrR and β-AdrR implicated in transducing the response (13, 20, 22, 30, 35). In some cases, either TTX or indomethacin can reduce this action of epi, suggesting a neural or paracrine stimulatory pathway (35), consistent with a contribution by the AdrRs present on enteric nerves (28). Thus the inhibition of Cl− secretion by epi may include AdrR on mucosal cells other than the epithelial cells, whereas modulatory secretion, which is characterized most prominently by sustained electrogenic K+ secretion, was activated by β-AdrR on epithelial cells.
Sympathetic responsiveness of modulatory secretion.
AdrRs respond to adrenergic stimuli, such that a sympathetic response can be sensed both from direct neural signaling via norepi and blood circulation carrying adrenal released epi. Because each β-AdrR subtype has a characteristic sensitivity to norepi and epi (Fig. 8C), a cell type with just one of these receptor subtypes will respond preferentially to either the neural or circulatory route of signaling. Modulatory secretion was equally sensitive to norepi and epi in guinea pig and rabbit distal colon, suggesting an equivalent responsiveness to these two routes. This observed ratio of sensitivities was most like β1-AdrR, except that the absolute sensitivity was ∼30-fold lower than β1-AdrR behavior. A source for this difference in sensitivity could be that the β-AdrR action was modified by interaction with other signaling proteins in the colonic epithelial cells, similar to reports for β-AdrRs in other cell types (23, 29). Further support for the possibility of modifications in receptor sensitivity was the observed variability in norepi and epi sensitivity between groups of guinea pigs, which was the subject of the companion paper (47). Presumably, the modulatory response in rabbit distal colon (38) also may be desensitized by a set of interactions particular to the in vitro conditions present at the time of the measurement. Even with a roughly similar sensitivity to epi and norepi, the balance in detecting neural vs. circulatory stimuli may favor nerves because of the potential proximity of sympathetic nerves to epithelial cells (11, 47).
For guinea pig distal colon, the sensitivity to antagonists was decreased in addition to the agonist desensitization. The inhibitory constants of CGP20712A for β1-AdrR and of ICI-118551 for β2-AdrR are in the range of 0.1 nM to 10 nM (5, 23, 43), so that the addition of 1 μM antagonist should have produced a significant inhibition (Fig. 2A) if either β-AdrR was acting alone and unmodified. Also, if each β-AdrR subtype independently controlled a portion of the K+ secretory capacity, then the subtype-selective antagonist would have reduced the secretory Isc in proportion to its overall contribution, contrary to the observed synergy. The requirement that both of these antagonists must be present to produce a significant blunting of the epi response suggested that these two receptor subtypes act together, thus providing two sites for antagonist binding that would allow synergism to be expressed. Because both β1-AdrR and β2-AdrR were present in epithelial cells possibly as oligomers (Fig. 5), the synergistic action of the antagonists could result from a mandatory activation of both receptor subtypes to initiate the appropriate signaling cascades for modulatory secretion. Given that β1-AdrRs also were present on pericryptal cells (Fig. 6C), the mandatory β1-AdrR activation also could have been on these adjacent cells such that they released another agonist essential for epithelial cells to produce modulatory secretion. Without an identified requirement for an additional extracellular signal being released and received, the likeliness instead of a combined β1-AdrR and β2-AdrR activation within the epithelial cells seems more probable. In addition, evidence for heterodimers of β1-AdrR and β2-AdrR has been found in other cell types (23) and may be a general feature of G protein-coupled receptors (29). Overall, the modulatory response consisting of sustained electrogenic K+ secretion occurred via the action of β-AdrRs, but these receptors likely had undergone posttranslational modification (1, 29, 34, 48) specific for the requirements and conditions existing within the distal colonic epithelial cells.
GRANTS
This study was supported by a grant from the National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases (DK65845).
REFERENCES
- 1.Anderson GP Current issues with β2-adrenoceptor agonists: pharmacology and molecular and cellular mechanisms. Clin Rev Allergy Immunol 31: 119–130, 2006. [DOI] [PubMed] [Google Scholar]
- 2.Anthony A, Schepelmann S, Guillaume JL, Strosberg AD, Dhillon AP, Pounder RE, Wakefield AJ. Localization of the β3-adrenoceptor in the human gastrointestinal tract: an immunohistochemical study. Aliment Pharmacol Ther 12: 519–525, 1998. [DOI] [PubMed] [Google Scholar]
- 3.Benovic JL, Shorr RG, Caron MG, Lefkowitz RJ. The mammalian β2-adrenergic receptor: purification and characterization. Biochemistry 23: 4510–4518, 1984. [DOI] [PubMed] [Google Scholar]
- 4.Brown DR, O'Grady SM. Regulation of ion transport in the porcine intestinal tract by enteric neurotransmitters and hormones. Comp Biochem Physiol A Physiol 118: 309–317, 1997. [DOI] [PubMed] [Google Scholar]
- 5.Bylund DB, Eikenberg DC, Hieble JP, Langer SZ, Lefkowitz RJ, Minneman KP, Molinoff PB, Ruffolo RR Jr, Trendelenburg U. International Union of Pharmacology nomenclature of adrenoceptors. Pharmacol Rev 46: 121–136, 1994. [PubMed] [Google Scholar]
- 6.Chamary VL, Loizidou M, Boulos PB, Taylor I, Burnstock G. Changes in vasoconstrictor and vasodilator neurotransmitters in nerves supplying arterioles in developing colorectal polyps. Colorectal Dis 8: 230–234, 2006. [DOI] [PubMed] [Google Scholar]
- 7.Chang WWL, Leblond CP. Renewal of the epithelium in the descending colon of the mouse. I. Presence of three cell populations: vacuolated-columnar, mucous and argentaffin. Am J Anat 131: 73–99, 1971. [DOI] [PubMed] [Google Scholar]
- 8.Cooke HJ, Christofi FL. Enteric neural regulation of mucosal secretion. In: Physiology of the Gastrointestinal Tract, edited by Johnson LR. New York: Raven, 2006, p. 737–762.
- 9.Dharmsathaphorn K, Pandol SJ. Mechanism of Cl− secretion induced by carbachol in a colonic epithelial cell line. J Clin Invest 77: 348–354, 1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Evans BA, Papaioannou M, Bonazzi VR, Summers RJ. Expression of β3-adrenoceptor mRNA in rat tissues. Br J Pharmacol 117: 210–216, 1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Furness JB The Enteric Nervous System. Malden, MA: Blackwell, 2006, pp. 274.
- 12.Gaginella TS, Halm DR, Hubel KA, Miller RJ. Neuromodulation of intestinal ion transport. Fed Proc 43: 2929–2934, 1984. [PubMed] [Google Scholar]
- 13.Halm DR, Frizzell RA. Active K+ transport across rabbit distal colon: relation to Na+ absorption and Cl− secretion. Am J Physiol Cell Physiol 251: C252–C267, 1986. [DOI] [PubMed] [Google Scholar]
- 14.Halm DR, Halm ST. Secretagogue response of goblet cells and columnar cells in human colonic crypts. Am J Physiol Cell Physiol 277: C501–C522, 1999. [Corrigenda. Am J Physiol Cell Physiol 278(1): 212–233, 2000.] [DOI] [PubMed] [Google Scholar]
- 15.Halm DR, Halm ST. Prostanoids stimulate K+ secretion and Cl− secretion in guinea pig distal colon via distinct pathways. Am J Physiol Gastrointest Liver Physiol 281: G984–G996, 2001. [DOI] [PubMed] [Google Scholar]
- 16.Halm DR, Kirk KL, Sathiakumar KC. Stimulation of Cl− permeability in colonic crypts of Lieberkühn measured with a fluorescent indicator. Am J Physiol Gastrointest Liver Physiol 265: G423–G431, 1993. [DOI] [PubMed] [Google Scholar]
- 17.Halm ST, Liao T, Halm DR. Distinct K+ conductive pathways are required for Cl− and K+ secretion across distal colonic epithelium. Am J Physiol Cell Physiol 291: C636–C648, 2006. [DOI] [PubMed] [Google Scholar]
- 18.Halm DR, Rick R. Secretion of K+ and Cl− across colonic epithelium: cellular localization using electron microprobe analysis. Am J Physiol Cell Physiol 262: C1001–C1011, 1992. [DOI] [PubMed] [Google Scholar]
- 19.Hébert TE, Moffett S, Morello JP, Loisel TP, Bichet DG, Barret C, Bouvier M. A peptide derived from a β2-adrenergic receptor transmembrane domain inhibits both receptor dimerization and activation. J Biol Chem 271: 16384–16392, 1996. [DOI] [PubMed] [Google Scholar]
- 20.Hörger S, Schultheiss G, Diener M. Segment-specific effects of epinephrine on ion transport in the colon of the rat. Am J Physiol Gastrointest Liver Physiol 275: G1367–G1376, 1998. [DOI] [PubMed] [Google Scholar]
- 21.Ishida H, Suzuki Y. Potassium secretion in the guinea pig distal colon. Jpn J Physiol 37: 33–48, 1987. [DOI] [PubMed] [Google Scholar]
- 22.Lam RS, App EM, Nahirney D, Szkotak AJ, Vieira-Coelho MA, King M, Duszyk M. Regulation of Cl− secretion by α2-adrenergic receptors in mouse colonic epithelium. J Physiol 548: 475–484, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lavoie C, Hébert TE. Pharmacological characterization of putative β1-β2-adrenergic receptor heterodimers. Can J Physiol Pharmacol 81: 186–195, 2003. [DOI] [PubMed] [Google Scholar]
- 24.Lennane RJ, Peart WS, Shaw J. Adrenergic influences on the electrical potential across the colonic mucosa of the rabbit. J Physiol 250: 367–372, 1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Li Y, Halm DR. Secretory modulation of basolateral membrane inwardly rectified K+ channel in guinea pig distal colonic crypts. Am J Physiol Cell Physiol 282: C719–C735, 2002. [DOI] [PubMed] [Google Scholar]
- 26.Liao T, Wang L, Halm ST, Lu L, Fyffe REW, Halm DR. The K+ channel KVLQT (Kcnq1) located in the basolateral membrane of distal colonic epithelium is not essential for activating Cl− secretion. Am J Physiol Cell Physiol 289: C564–C575, 2005. [DOI] [PubMed] [Google Scholar]
- 27.Lytle C, Tod TJ, Vo KT, Lee JW, Atkinson RD, Straus DS. The peroxisome proliferator-activated receptor gamma ligand rosiglitazone delays the onset of inflammatory bowel disease in mice with interleukin 10 deficiency. Inflamm Bowel Dis 11: 231–243, 2005. [DOI] [PubMed] [Google Scholar]
- 28.Nasser Y, Ho W, Sharkey KA. Distribution of adrenergic receptors in the enteric nervous system of the guinea pig, mouse, and rat. J Comp Neurol 495: 529–553, 2006. [DOI] [PubMed] [Google Scholar]
- 29.Prinster SC, Hague C, Hall RA. Heterodimerization of G protein-coupled receptors: specificity and functional significance. Pharmacol Rev 57: 289–298, 2005. [DOI] [PubMed] [Google Scholar]
- 30.Racusen LC, Binder HJ. Adrenergic interaction with ion transport across colonic mucosa: role of both α and β adrenergic agonists. In: Mechanisms of Intestinal Secretion, edited by Binder HJ. New York: Alan R Liss, 1979, p. 201–215.
- 31.Rechkemmer G, Frizzell RA, Halm DR. Active K+ transport across guinea pig distal colon: action of secretagogues. J Physiol 493: 485–502, 1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Reynolds A, Parris A, Evans LA, Lindqvist S, Sharp P, Lewis M, Tighe R, Williams MR. Dynamic and differential regulation of NKCC1 by calcium and cAMP in the native human colonic epithelium. J Physiol 582: 507–524 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Roberts SJ, Papaioannou M, Evans BA, Summers RJ. Functional and molecular evidence for β1-, β2- and β3- adrenoceptors in human colon. Br J Pharmacol 120: 1527–1535, 1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rybin VO, Xu X, Lisanti MP, Steinberg SF. Differential targeting of β-adrenergic receptor sub-types and adenylyl cyclase to cardiomyocyte caveolae: a mechanism to functionally regulate the cAMP signaling pathway. J Biol Chem 275: 41447–41457, 2000. [DOI] [PubMed] [Google Scholar]
- 35.Schultheiss G, Diener M. β-Adrenoceptor-mediated secretion across the rat colonic epithelium. Eur J Pharmacol 403: 251–258, 2000. [DOI] [PubMed] [Google Scholar]
- 36.Senard JM, Langin D, Estan L, Paris H. Identification of α2-adrenergic receptors and non-adrenergic idazoxan binding sites in rabbit colon epithelial cells. Eur J Pharmacol 191: 59–68, 1990. [DOI] [PubMed] [Google Scholar]
- 37.Skeberdis VA Structure and function of β3-adrenergic receptors. Medicina (Kaunas) 40: 407–413, 2004. [PubMed] [Google Scholar]
- 38.Smith PL, McCabe RD. Potassium secretion by rabbit descending colon: effects of adrenergic stimuli. Am J Physiol Gastrointest Liver Physiol 250: G432–G439, 1986. [DOI] [PubMed] [Google Scholar]
- 39.Straub RH, Stebner K, Härle P, Kees F, Falk W, Schölmerich J. Key role of the sympathetic microenvironment for the interplay of tumour necrosis factor and interleukin 6 in normal but not in inflamed mouse colon mucosa. Gut 54: 1098–1106, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Traynor TR, Brown DR, O'Grady SM. Regulation of ion transport in porcine distal colon: effects of putative neurotransmitters. Gastroenterology 100: 703–710, 1991. [DOI] [PubMed] [Google Scholar]
- 41.Tsuchiya Y, Suzuki Y. The effect of cAMP on electrogenic Na+ absorption in the rat distal colon. Jpn J Physiol 51: 435–444, 2001. [DOI] [PubMed] [Google Scholar]
- 42.Valet P, Senard JM, Devedjian JC, Planat V, Salomon R, Voisin T, Drean G, Couvineau A, Daviaud D, Denis C, Laburthe M, Paris H. Characterization and distribution of α2-adrenergic receptors in the human intestinal mucosa. J Clin Invest 91: 2049–2057, 1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wangemann P, Liu J, Shimozono M, Scofield MA. β1-adrenergic receptors but not β2-adrenergic or vasopressin receptors regulate K+ secretion in vestibular dark cells of the inner ear. J Membr Biol 170: 67–77 1999. [DOI] [PubMed] [Google Scholar]
- 44.Wegmann M, Kämpen A, Weber S, Seyberth HW, Köckerling A. Effect of hydroxyeicosatetraenoic acids on furosemide-sensitive Cl− secretion in rat distal colon. J Pharmacol Exp Ther 295: 133–138, 2000. [PubMed] [Google Scholar]
- 45.Yajima T, Suzuki T, Suzuki Y. Synergism between Ca++-mediated and cyclic AMP-mediated activation of Cl− secretion in isolated guinea pig distal colon. Jpn J Physiol 38: 427–443, 1988. [DOI] [PubMed] [Google Scholar]
- 46.Yu O, Ouyang A. Distribution of β-adrenoceptor sub-types in gastrointestinal tract of nondiabetic and diabetic BB rats: a longitudinal study. Dig Dis Sci 42: 1146–1153, 1997. [DOI] [PubMed] [Google Scholar]
- 47.Zhang J, Halm ST, Halm DR. Adrenergic activation of electrogenic K+ secretion in guinea pig distal colonic epithelium: desensitization via the Y2-neuropeptide receptor. Am J Physiol Gastrointest Liver Physiol. 297: G278–G291, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zheng M, Zhu W, Han Q, Xiao RP. Emerging concepts and therapeutic implications of β-adrenergic receptor sub-type signaling. Pharmacol Ther 108: 257–268, 2005. [DOI] [PubMed] [Google Scholar]