Abstract
Endothelial activation and surface expression of cell adhesion molecules (CAMs) is critical for binding and recruitment of circulating leukocytes in tissues during the inflammatory response. Endothelial CAM expression plays a critical role in the intestinal microvasculature in inflammatory bowel disease (IBD), as blockade of leukocyte α4-integrin binding by gut endothelial CAM ligands has therapeutic benefit in IBD. Mechanisms underlying expression of vascular cell adhesion molecule (VCAM)-1, a ligand for α4-integrin in primary cultures of human intestinal microvascular endothelial cells (HIMEC) has not been defined. We investigated the effect of curcumin, phosphatidylinositol 3-kinase (PI 3-kinase)/protein kinase B (Akt), and mitogen-activated protein kinase (MAPK) inhibitors on VCAM-1 expression and function in HIMEC. CAM expression was assessed and HIMEC-leukocyte adhesion was visualized under static and flow conditions. Western blotting and in vitro kinase assays were used to assess Akt and MAPK activation. Nuclear factor-κB (NF-κB) activation and nuclear translocation of its p65 subunit were determined. Tumor necrosis factor (TNF)-α/lipopolysaccharide (LPS)-induced VCAM-1 expression in HIMEC was suppressed by Akt small-interfering RNA, curcumin, and inhibitors of NF-κB (SN-50), p38 MAPK (SB-203580) and PI 3-kinase/Akt (LY-294002). VCAM-1 induction was partially suppressed by p44/42 MAPK (PD-098059) but unaffected by c-Jun NH2-terminal kinase (SP-600125) inhibition. Curcumin inhibited Akt/MAPK/NF-κB activity and prevented nuclear translocation of the p65 NF-κB subunit following TNF-α/LPS. At physiological shear stress, curcumin attenuated leukocyte adhesion to TNF-α/LPS-activated HIMEC monolayers. In conclusion, curcumin inhibited the expression of VCAM-1 in HIMECs through blockade of Akt, p38 MAPK, and NF-κB. Curcumin may represent a novel therapeutic agent targeting endothelial activation in IBD.
Keywords: phosphatidylinositol 3-kinase, protein kinase B, mitogen-activated protein kinase, nuclear factor-κB
in the dysregulated, chronic inflammation that characterizes human inflammatory bowel disease [IBD; Crohn's disease (CD) and ulcerative colitis (UC)], alterations in intestinal microvascular endothelium have been identified at the morphological and functional levels, including increased expression of cell adhesion molecules (CAM) and enhanced leukocyte recruitment (4). To further characterize microvascular endothelial changes in gut inflammation, human intestinal microvascular endothelial cells (HIMEC) cultures have been generated, demonstrating inducible expression of the molecules E-selectin, intercellular adhesion molecule (ICAM)-1, MAdCAM-1, and vascular cell adhesion molecule (VCAM)-1 following activation with inflammatory cytokines [i.e., tumor necrosis factor (TNF)-α, interleukin-1β] and lipopolysaccharide (LPS) (4). Characterizing mechanisms underlying gut microvascular endothelial activation and CAM expression represents an essential area for defining mechanisms of IBD pathogenesis as well as developing novel targets for therapy.
VCAM-1 is involved in firm adhesion and transmigration of leukocytes expressing the α4-integrin ligand. α4-Integrin-expressing leukocytes play a critical role in the pathogenesis of rodent models of IBD and a spontaneous form of chronic colitis that occurs in primates (i.e., cotton-top tamarin). Recent clinical trials have confirmed the importance of α4-leukocyte trafficking in the intestine in human IBD, as natalizumab and MLN02 (10, 30) have demonstrated benefit in CD and UC respectively. The clinical efficacy of α4-integrin inhibition is believed to result from interruption of interaction with MAdCAM-1, but at present it is not known whether an alternative ligand, VCAM-1, may also be playing a role in IBD chronic inflammation.
VCAM-1 upregulation by proinflammatory cytokines has been reported in various cells (6, 7, 35). Induction of VCAM-1 in endothelial cells by TNF-α requires nuclear factor (NF)-κB, activator protein-1, phospholipase C, and phosphatidylinositol 3-kinase (PI 3-kinase) activation (16, 23). Downregulation of VCAM-1 expression with subsequent impairment of endothelial-leukocyte adhesion in rodents was linked to inhibition of PI 3-kinase/protein kinase B (Akt) signaling (19–21, 25, 32). Endothelial expression of VCAM-1 in both human and animal models of IBD has been difficult to confirm at a histological level. Smooth muscle and mesenchymal expression of VCAM-1 has been readily demonstrated, but endothelial expression in tissue histology has been less definitive. However, specific targeting of VCAM-1 in rodent models of IBD has demonstrated clear evidence of benefit, suggesting that this molecule is playing an important role in IBD chronic gut inflammation.
Curcuma longa Linn, Zingiberaceae (curcumin), the major yellow coloring pigment found in the household spice turmeric, has been used for centuries in food preparation and Ayurvedic traditional medicine to treat inflammatory disorders (34). Curcumin has low toxicity, has treated chronic gut inflammation in animal models, and has shown benefit in a randomized, multicenter crossover trial in the treatment of UC (12). Mechanistically, curcumin inhibits: 1) production of reactive oxygen species; 2) nitric oxide synthase activity; 3) lipoxygenase activity; and 4) cyclooxygenase-2 activity during inflammation (9, 5, 17, 29).
Prior work by our group has shown that expression of the α4-integrin ligand MAdCAM-1 in HIMECs following activation with TNF-α/LPS is regulated through the PI 3-kinase/Akt pathway but neither E-selectin nor ICAM-1 expression was affected by this signaling mechanism (24). In this study, we demonstrate that, in addition to activation of p38 mitogen-activated protein kinase (MAPK) and NF-κB, VCAM-1 expression in TNF-α/LPS-stimulated HIMEC also requires signaling through PI 3-kinase/Akt. Moreover, VCAM-1 expression and leukocyte adhesion in TNF-α/LPS-activated HIMEC were inhibited by curcumin, LY-294002 (PI 3-kinase/Akt), SN-50 (NF-κB), and SB-203580 (p38 MAPK) pretreatment. The present data further define the intracellular mechanisms of PI 3-kinase/Akt-mediated activation in gut microvascular endothelium and leukocyte recruitment through α4-integrin binding, identifying potential endothelial targets for IBD therapy.
MATERIALS AND METHODS
Reagents.
TNF-α and antibodies to ICAM-1, VCAM-1, E-selectin, and the Tissue-Cell-Staining kit were purchased from R&D Systems (Minneapolis, MN). Nonradioactive In vitro MAPK Kinase Assay Kits and MAPK antibodies were from Cell Signaling Technology (Danvers, MA). The nonradioactive in vitro Akt Kinase Assay Kit was obtained from Upstate Cell Signaling (Lake Placid, NY). PD-098059, SB-203580, SP-600125, SN-50, and LY-294002 inhibitors were obtained from Calbiochem (La Jolla, CA). RNA Cell Protect reagent and RNeasy Plus Mini Kits were obtained from Qiagen (Valencia, CA). Akt primers catalog no. PPH0008A were obtained from SA Biosciences (Frederick, MD). Akt small-interfering RNA (siRNA) (target siRNA), glyceraldehyde-3-phosphate dehydrogenase (GAPDH) siRNA (positive control), and nontargeting siRNA (negative control) were obtained from Dharmacon (Chicago, IL). The iScript cDNA synthesis kit, Sybr Green Master Mix, iQ5 software, and all other electrophoresis reagents were obtained from Bio-Rad (Hercules, CA). Unless otherwise indicated, all other chemicals used in this study were purchased from Sigma Chemical (St. Louis, MO).
HIMEC isolation and culture.
All experiments were approved by the Human Research Review Committee of the Medical College of Wisconsin. HIMECs were isolated and cultured as previously described (4). Experiments were performed on three independent HIMEC lines unless otherwise specified. All images displayed were a representative result of one of the three independent experiments.
Activation and pharmacological modulation of HIMEC.
HIMEC activation was achieved following TNF-α (100 U/ml)/LPS (1 μg/ml) stimulation for specified time periods. Curcumin (10 μM), SN-50 (18 μM), LY-294002 (10 μM), SB-203580 (10 μM), PD-098059 (10 μM), SP-600125 (10 μM), and vitamin E (25 μM) were used to determine the effect of these inhibitors on VCAM-1 expression in HIMEC. Of note, we have performed dose-response experiments for all inhibitors stated, and the most potent but nontoxic dose for our experimental analysis was chosen (data not shown).
Assessment of CAM surface expression and leukocyte-HIMEC adhesion assay.
CAM surface expression in HIMEC and U-937-HIMEC interaction were assessed as described previously (4, 26, 28).
Semiquantitative RT-PCR.
VCAM-1 mRNA expression was determined as described previously (24), using VCAM-1 (forward: 5′-GGA ACC TTG CAG CTT ACA GTG ACA GAG CTC CC-3′, reverse: 5′-CAA GTC TAC ATA TCA CCC AAG-3′)- and β-actin (forward: 5′-TGA CGG GGT CAC CCA CAC TGT GCC CAT CTA-3′, reverse: 5′-CTA GAA GCA TTG CGG TGG ACG ATG GAG GG-3′)-specific primers. RNA without reverse transcription was used as negative control (no RT), and expression of β-actin served as an internal control.
MAdCAM-1 mRNA expression was determined using real-time PCR as described previously (11, 27), using MAdCAM (forward: 5′-GAA GTG ATC CCA ACA GGC TC-3′, reverse: 5′-TTC CAG AGG TGA TAG GTG GG-3′) and GAPDH (forward: 5′-TGC ACC ACC AAC TGC TTA GC-3′, reverse: 5′-GGC ATG GAC TGT GGT CAT GAG-3′) specific primers. Normalized gene expression was analyzed using iQ5 software.
Western blot analysis.
SDS-PAGE and Western blot analysis were performed using antibodies to VCAM-1, Akt, and MAPK as described previously (28).
Akt in vitro kinase activity assay.
Active Akt was immunoprecipitate from cell extracts and then an in vitro kinase assay was performed using glycogen synthase kinase (GSK)-3 Fusion Protein as a substrate according to the manufacturer's instructions. Phosphorylation of GSK-3 was measured using phospho-GSK-3α/β (Ser21/9) antibody.
Akt siRNA transfection and VCAM-1 expression.
Transfection was done using Amaxa's primary endothelial transfection kit and Nucleofector device according to the manufacturer's instructions. HIMEC were transfected with 100 nM of either Akt siRNA (target gene), GAPDH siRNA (positive control), or nontargeting siRNA (negative control). Pooled siRNAs for each gene were obtained from Dharmacon. After transfection (24 h), cells were either unstimulated or stimulated with 100 U/ml TNF-α and 1 μg/ml LPS for 6 h. RNA was then isolated using Qiagen's RNeasy Plus Mini Kit according to the manufacturer's instructions. Reverse transcription was done with 1 μg of RNA using Bio-Rad's iScript cDNA synthesis kit. Akt gene knockdown and VCAM gene expression were analyzed via real-time PCR using Bio-Rad's Sybr Green Master Mix, 2 μl of cDNA, and 250 nM primer in 25-μl reactions. Cycling parameters were 95°C for 3 min and then 45 cycles of 95°C for 10 s and 60°C for 30 s. Generation of a single product was confirmed with a melt cycle. Real-time data were analyzed using Bio-Rad's iQ5 software. Primer sequences were as follows: α-actin forward CTG GAA CGG TGA AGG TGA CA and reverse AAG GGA CTT CCT GTA ACA ATG CA, VCAM forward TGT TGA GAT CTC CCC TGG AC and reverse CGC TCA GAG GGC TGT CTA TC, and GAPDH forward TGC ACC ACC AAC TGC TTA GC and reverse GGC ATG GAC TGT GGT CAT GAG. Akt primers were obtained from SA Biosciences.
c-Jun NH2-terminal kinase and p38 MAPK in vitro kinase activity.
c-Jun NH2-terminal kinase (JNK) and p38 MAPK activity were determined as previously described (28).
p44/42 MAPK in vitro kinase activity.
Active p44/42 MAPK was immunoprecipitated from cell extracts, using an immobilized phospho-p44/42 MAPK (Thr202/Tyr204) antibody. Next, an in vitro kinase assay is performed using Elk-1 protein as a substrate. Phosphorylation of Elk-1 was detected by Western blotting using phospho-Elk-1 (Ser383) Antibody.
Assay of transcription factor NF-κB.
Using TransAM NF-κB and Nuclear Protein Extraction Kits, NF-κB activity was measured according to the manufacturer's protocol (Active Motif, Carlsbad, CA). The samples were analyzed in a 96-well plate containing the immobilized NF-κB consensus site (5′-GGG ACT TTCC-3′) oligonucleotide. The activated form of NF-κB in nuclear extract binds to this oligonucleotide. Use of an antibody against NF-κB p65 subunit and a horseradish peroxidase (HRP)-conjugated secondary antibody results in a colorimetric readout, which was quantified at 450 nm using a Beckman DU-650 spectrophotometer. Data from triplicate wells were expressed as a means ± SD.
Immunofluorescence staining.
Immunofluorescence staining was performed using NF-κB p65 subunit antibody and a FITC-conjugated secondary antibody as described previously (24).
Immunohistological analysis.
Specimens from human colon were prepared as described previously using VCAM-1 antibody (13, 15).
RESULTS
TNF-α/LPS induces expression of VCAM-1 gene and protein in HIMEC.
We have previously demonstrated that TNF-α/LPS activation of HIMEC significantly increased ICAM-1, E-selectin, and VCAM-1 expression (3, 26). In this study, we characterized the signaling pathways underlying VCAM-1 gene and protein expression in HIMEC following TNF-α/LPS activation.
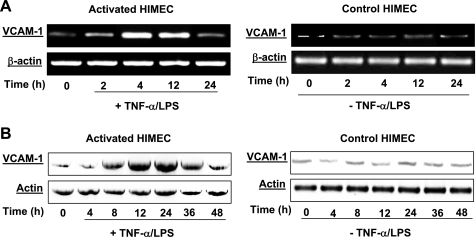
VCAM-1 mRNA expression was determined by semiquantitative RT-PCR. VCAM-1 mRNA expression in HIMEC following TNF-α/LPS stimulation was time-dependent, and the maximum response was achieved at 4 h, lasted for 12 h, and then returned to basal levels by 24 h (Fig. 1A, left). Corresponding to the VCAM-1 mRNA expression, Western blot analysis demonstrated that TNF-α/LPS activation of HIMEC induced increased VCAM-1 protein significantly by 12 h, which was sustained up to 24 h, and then decreased by 36 h and returned to basal levels by 48 h (Fig. 1B, left). Time-matched controls for VCAM-1 mRNA and protein expression are shown in Fig. 1, A and B, right. Individual blots were stripped and reprobed with an anti-actin antibody to ensure equal protein loading.
Fig. 1.
Effect of tumor necrosis factor (TNF)-α/lipopolysaccharide (LPS) on vascular cell adhesion molecule (VCAM)-1 mRNA and protein expression in human intestinal microvascular endothelial cells (HIMEC). The effect of TNF-α/LPS on VCAM-1 mRNA and protein expression was determined. A: detection of VCAM-1 mRNA in HIMEC by semiquantitative reverse transcriptase-PCR using specific primers. HIMEC does not constitutively express mRNA for VCAM-1. Stimulation of HIMEC with a combination of TNF-α (100 IU/ml) and LPS (1 μg/ml) led to marked upregulation of VCAM-1. VCAM-1 mRNA expression in HIMEC following TNF-α/LPS activation was time dependent; the maximum response was achieved by 4 h, which was lasted for 12 h and then returned to the basal level by 24 h (left). β-Actin served as an internal loading control. B: TNF-α/LPS enhanced VCAM-1 protein expression in HIMEC. VCAM-1 protein expression in HIMEC following TNF-α/LPS activation was assessed using Western blot analysis. Total cell lysates from cultured HIMEC stimulated with TNF-α (100 IU/ml) and LPS (1 μ g/ml) were subjected to SDS-PAGE and immunoblotted with VCAM-1 antibody. TNF-α/LPS activation of HIMEC significantly increased VCAM-1 protein expression by 12 h, was sustained through 24 h, decreased at 36 h, and returned to baseline by 48 h (left). Time-matched controls for VCAM-1 mRNA and protein are shown on right. The same blot was stripped and reprobed with an anti-actin antibody to ensure equivalent protein loading. Data shown are from one of three independent experiments.
Effect of curcumin on Akt signaling in HIMEC.
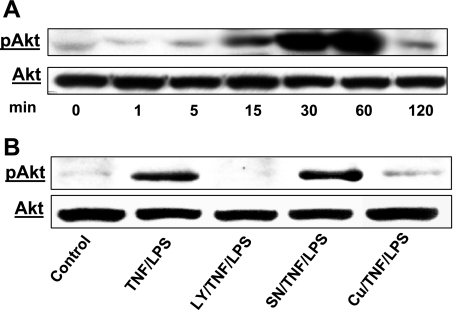
TNF-α/LPS stimulation of HIMEC resulted in phosphorylation of Akt in a time-dependent manner, which was initially evident at 15 min, lasted up to 60 min, and declined by 120 min (Fig. 2A). No Akt phosphorylation was detected in unstimulated control HIMEC at any given time point (data not shown). Pretreatment of HIMEC for 30 min with either curcumin (10 μM) or the PI 3-kinase inhibitor LY-294002 (10 μM) before TNF-α/LPS stimulation completely abolished the Akt phosphorylation (Fig. 2B), whereas SN-50, an NF-κB inhibitor, did not affect the Akt phosphorylation. Total nonphosphorylated Akt was probed to demonstrate equal protein loading.
Fig. 2.
Effect of TNF-α/LPS on protein kinase B (Akt) phosphorylation (p) in HIMEC. A: TNF-α/LPS induces Akt phosphorylation in HIMEC. Activation of Akt in HIMEC by TNF-α/LPS was assessed using Western blot analysis. Total cell lysates from cultured HIMEC stimulated with TNF-α (100 IU/ml) and LPS (1 μg/ml) were subjected to SDS-PAGE and immunoblotted with total and phospho-(Ser473)-Akt antibodies. TNF-α/LPS induced phosphorylation of Akt in a time-dependent fashion. TNF-α/LPS stimulation of HIMEC resulted in a strong increase in Akt phosphorylation at 30 min that lasted up to 60 min and declined by 120 min compared with total Akt. Time-matched controls did not show any changes in Akt phosphorylation at any given time points (data not shown). B: pretreatment of HIMEC for 30 min with either curcumin (Cu, 10 μM) or PI 3-kinase inhibitor LY-294002 (LY, 10 μM) before TNF-α/LPS stimulation abolished the Akt phosphorylation, whereas SN-50 (SN, NF-κB inhibitor) did not. Stripping and reprobing of the blot with total Akt antibody demonstrates equivalent protein loading. GSK, glycogen synthase kinase. Data shown are from one of three independent experiments.
Akt in vitro kinase activity assay.
The active Akt was immunoprecipitated from TNF-α/LPS-activated HIMEC by immobilized Akt antibody. The Akt in vitro kinase assay was performed using GSK-3 fusion protein as a substrate. GSK-3 underwent phosphorylation in TNF-α/LPS-activated HIMEC but not in control samples using phospho-GSK-3α/β (Ser21/9) antibody and Western blotting. Curcumin and LY-294002 pretreatment of HIMEC resulted in inhibition of GSK-3 phosphorylation and Akt activity (Fig. 3).
Fig. 3.
Effect of curcumin on Akt in vitro kinase activity. The inhibitory effect of curcumin on phosphatidylinositol 3-kinase (PI 3-kinase)/Akt activation in TNF-α/LPS-activated HIMEC was assessed at a functional level. Active Akt from TNF-α/LPS-stimulated HIMEC was immunoprecipitated by immobilized phospho-(Ser473)-Akt antibody. GSK-3 fusion protein was used as a substrate to demonstrate Akt enzymatic activity. In TNF-α/LPS-activated HIMEC, the GSK-3 was phosphorylated as detected by a specific phospho-GSK-3α/β (Ser21/9) antibody. Both curcumin and LY-294002 pretreatment of HIMEC inhibited GSK-3 phosphorylation and Akt activity. Data shown are from one of three independent experiments.
Effect of curcumin on MAPK signaling pathways in HIMEC.
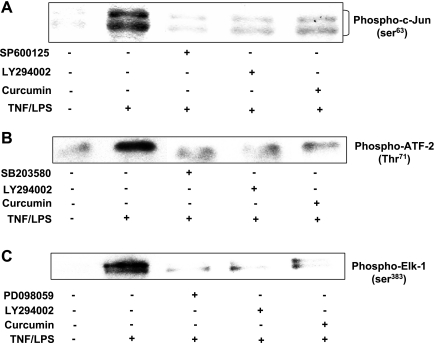
The effect of curcumin on the kinase activity of the individual MAPK was investigated using an in vitro kinase activity system. As shown in Fig. 4A, TNF-α/LPS activation of HIMEC increased the JNK activity, which was evident by c-Jun phosphorylation using phospho (Ser63)-c-Jun antibody. Pretreatment of HIMEC with curcumin, SP-600125, and LY-294002 before TNF-α/LPS activation abolished the JNK activity. Figure 4B demonstrates that TNF-α/LPS activation of HIMEC increased the p38 MAPK activity, which was evident by ATF-2 phosphorylation. Phosphorylation of ATF-2 at Thr71 was measured by Western blotting using phospho-ATF-2 (Thr71) antibody. Pretreatment of HIMEC with SB-203580, LY-294002, and curcumin before TNF-α/LPS activation inhibited the p38 MAPK activity. As shown in Fig. 4C, TNF-α/LPS activation of HIMEC increased the p44/42 MAPK activity, which was evident by Elk-1 phosphorylation detected by Western blotting using phospho-Elk-1 Antibody. Pretreatment of HIMEC with curcumin, PD-098059, and LY-294002 before TNF-α/LPS activation inhibited the p44/42 MAPK activity.
Fig. 4.
Effect of curcumin on mitogen-activated protein kinase (MAPK) in vitro kinase activity in HIMEC. A: c-Jun NH2-terminal kinase (JNK) activity was assayed in cell lysates using a nonradioactive kinase activity assay kit. Briefly, active JNK was immunoprecipitated from lysates with immobilized phospho-JNK (Thr183/Try185) antibody. JNK in vitro kinase activity in HIMEC by TNF-α/LPS was evident by c-Jun phosphorylation using phospho-(Ser63)-c-Jun antibody. Pretreatment of HIMEC with SP-600125, LY-294002, and curcumin before TNF-α/LPS activation abolished the JNK activity. B: p38 MAPK activity was assayed in cell lysates using a nonradioactive kinase activity assay kit. Briefly, active p38 MAPK was immunoprecipitated from lysates with immobilized phospho-p38 MAPK (Thr180/Try182) antibody. p38 MAPK in vitro kinase activity in HIMEC by TNF-α/LPS was evident by activating transcription factor-2 phosphorylation using phospho (Thr71)-ATF-2 antibody. Pretreatment of HIMEC with SB-203580, LY-294002, and curcumin before TNF-α/LPS activation abolished the p38 MAPK activity. C: p44/42 MAPK activity was assayed in cell lysates using a nonradioactive kinase activity assay kit. Briefly, active p44/42 MAPK was immunoprecipitated from lysates with immobilized phospho-p44/42 MAPK (Thr202/Try204) antibody. p44/42 MAPK in vitro kinase activity in HIMEC by TNF-α/LPS was evident by Elk-1 phosphorylation using phospho (Ser383)-Elk-1 antibody. Pretreatment of HIMEC with PD-098059, LY-294002, and curcumin before TNF-α/LPS activation abolished the p44/42 MAPK kinase activity. Data shown are from one of three independent experiments.
VCAM-1 expression in HIMEC is dependent on Akt/p38 MAPK/NF-κB activation.
The contribution of the PI 3-kinase pathway to VCAM-1 and ICAM-1 expression in human monocytes has been previously demonstrated (1). Downregulation of VCAM-1 expression and impairment of monocyte-endothelial cell adhesion in rodents through inhibition of the PI 3-kinase/Akt pathway with lovastatin has been previously reported (25). However, the role of the PI 3-kinase/Akt pathway, and its potential contribution to TNF-α/LPS-induced VCAM-1 expression in HIMEC had not been specifically defined. The next series of experiments focused on the PI 3-kinase/Akt signaling pathway in HIMEC, since this signaling mechanism is intimately involved in cellular differentiation and was previously demonstrated to play a critical role in α4-integrin-expressing leukocyte binding through MAdCAM-1 expression in HIMEC. Interestingly, HIMEC expression of E-selectin and ICAM-1 did not involve PI 3-kinase/Akt, suggesting that specific signaling pathways underlie specific CAM expression during inflammation (24).
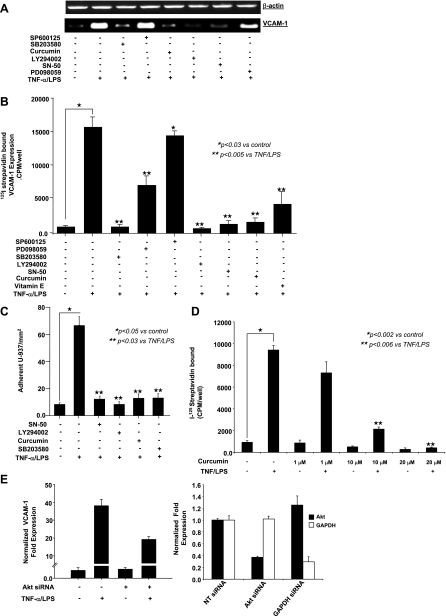
As demonstrated in Fig. 5, A–C, LY-294002 (PI 3-kinase/Akt), SB-203580 (p38 MAPK), SN-50 (NF-κB) inhibitors, vitamin E, and curcumin effectively inhibited VCAM-1 mRNA, protein, cell surface expression, and leukocyte binding, whereas SP-600125 (JNK) inhibitor had no effect on VCAM-1 expression and PD-098059 (p44/42 MAPK) inhibitor only partially decreased VCAM-1 mRNA, protein, and cell surface expression (Fig. 5, A–C). Curcumin inhibited VCAM-1 expression in a dose-dependent fashion (Fig. 5D). These findings suggest that activation of Akt/p38 MAPK/NF-κB and possibly p44/42 MAPK may play a role in TNF-α/LPS-induced VCAM-1 expression in HIMEC, and curcumin was a potent inhibitor of all of these inflammatory pathways.
Fig. 5.
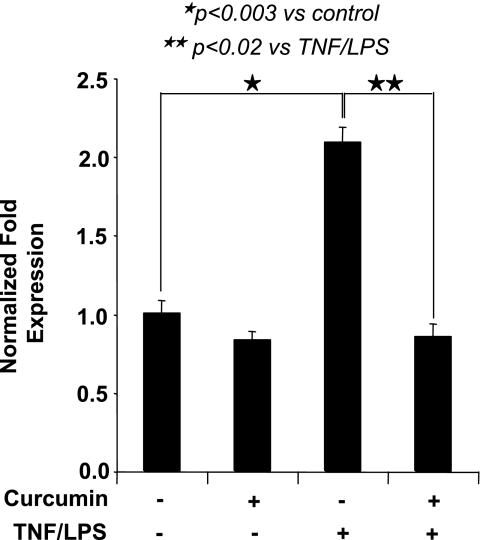
Effect of pharmacological inhibitors on VCAM-1 expression. A: VCAM-1 mRNA expression in TNF-α/LPS-activated HIMEC was completely inhibited by SB-203580 (p38 MAPK inhibitor), LY-294002 (PI 3-kinase/Akt inhibitor), SN-50 [nuclear factor (NF)-κB inhibitor], and curcumin. Pretreatment of HIMEC with PD-098059 (p44/42 MAPK inhibitor) resulted in partial inhibition of VCAM-1 mRNA, whereas SP-600125 (JNK inhibitor) did not alter VCAM-1 expression following TNF-α/LPS. B: VCAM-1 cell surface expression was significantly inhibited by SB-203580, curcumin, LY-294002, SN-50, and vitamin E (lipid-soluble antioxidant) but not SP-600125 and was partially blocked by PD-098059. C: function of VCAM-1 expression in TNF-α/LPS-activated HIMEC was assessed using low shear stress flow adhesion assays and binding of U-937 monocytes, which are known to express α4-integrins. U-937 monocyte adhesion to TNF-α/LPS-activated HIMEC was inhibited by SN-50, LY-294002, curcumin, and SB-203580 pretreatment. D: inhibitory effect of curcumin on VCAM-1 expression was dose dependent, and a maximal inhibitory effect was achieved by 10 μM of curcumin pretreatment of HIMEC 30 min before TNF-α/LPS activation. E: Akt gene silencing in HIMEC. With the use of appropriate controls [glyceraldehyde-3-phosphate dehydrogenase [GAPDH small-interfering RNA (siRNA) as a positive control and nontargeting (NT) siRNA as a negative control], specific knockdown of Akt by Akt siRNA was demonstrated by real-time PCR (right). After significant silencing of Akt expression was confirmed, the effect on VCAM-1 expression after TNF-α/LPS stimulation was measured by real-time PCR (left). Data are expressed as the mean normalized fold expression above baseline of 3 individual experiments ± SD. Data shown are from one of three independent experiments.
Gene silencing of Akt in HIMEC.
To confirm whether a functional effect of VCAM-1 expression was linked to enhanced Akt expression in HIMEC, we performed gene-silencing experiments using transfection with siRNA specific for Akt. With the use of appropriate controls (GAPDH siRNA as a positive control and nontargeted siRNA as a negative control), specific knockdown of Akt by Akt siRNA was demonstrated by real-time PCR (Fig. 5E, right). After confirming significant silencing of Akt expression, the effect on VCAM-1 expression after TNF-α/LPS stimulation was measured by real-time PCR (Fig. 5E, left).
Effect of curcumin on MAdCAM-1 gene expression in HIMEC.
Involvement of PI 3-kinase/Akt activation in MAdCAM-1 expression following TNF-α/LPS stimulation of HIMEC was previously demonstrated by our group (24). Our data suggest that VCAM-1 and MAdCAM-1 expression in HIMEC share similar signaling pathways following TNF-α/LPS activation. Given the potent inhibitory effect of curcumin on VCAM-1 expression, we determined its effect on MAdCAM-1 expression in HIMEC. As demonstrated in Fig. 6, curcumin significantly inhibited MAdCAM-1 mRNA expression.
Fig. 6.
Effect of curcumin on MAdCAM-1 gene expression in HIMEC. Curcumin pretreatment of HIMEC before TNF-α/LPS activation abolished the MAdCAM-1 mRNA expression as determined by real-time PCR. Data are expressed as the mean normalized fold expression above baseline ± SD of 3 individual experiments.
NF-κB inhibitor attenuates TNF-α/LPS-induced VCAM-1 expression.
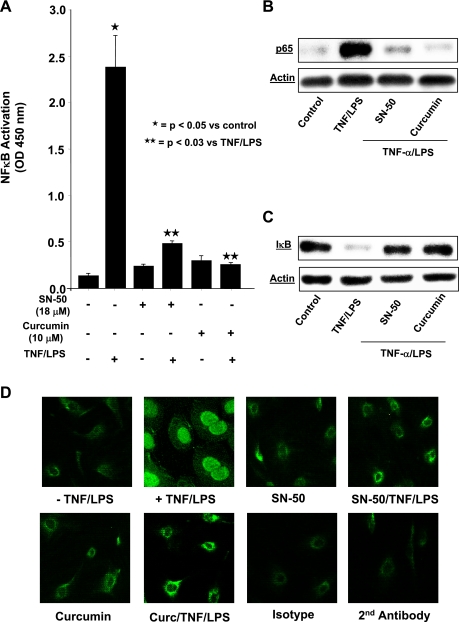
Involvement of NF-κB activation in E-selectin and ICAM-1 expression following TNF-α/LPS stimulation of HIMEC has been previously demonstrated (24). We investigated whether NF-κB activation also plays a role in VCAM-1 expression in TNF-α/LPS-activated HIMEC. As shown above in Fig. 5, A–C, pretreatment of HIMEC with the NF-κB inhibitor (SN-50) attenuated VCAM-1 gene, cell surface expression, and leukocyte binding. Figure 7A demonstrates that NF-κB-DNA binding activity was completely inhibited by SN-50 and curcumin pretreatment of HIMEC before TNF-α/LPS activation, using a cell-based ELISA-NF-κB assay. Western blot analysis from nuclear protein fractions of TNF-α/LPS-activated HIMEC show the immunoreactivity of NF-κB subunit p65, which was also inhibited by both SN-50 and curcumin (Fig. 7B). Furthermore, Western blotting showed that inhibitory factor κB-α is rapidly degraded in TNF-α/LPS-activated HIMEC in <30 min and then recovers by 60 min, resulting in NF-κB activation (Fig. 7C). Translocation of NF-κB subunit p65 in the nucleus was effectively blocked with both SN-50 pretreatment and curcumin (Fig. 7D).
Fig. 7.
Effect of curcumin on NF-κB activation in HIMEC. TransAM ELISA-based assay was performed to determine the NF-κB activity in control and TNF-α/LPS stimulated HIMEC nuclear protein. Briefly, 5 μg of nuclear extracts were used to assay NF-κB activity, and activated transcription factor binds to the immobilized NF-κB consensus site (5′-GGG ACT TTCC-3′) oligonucleotide. The activated form of NF-κB in nuclear extract binds to this oligonucleotide. Use of an antibody against NF-κB p65 subunit and a horseradish peroxidase (HRP)-conjugated secondary antibody results in a colorimetric readout, which was quantified at 450 nm using a Beckman DU-650 spectrophotometer. Data from triplicate wells were expressed as means ± SD. A: NF-κB-DNA binding activity was inhibited by both SN-50 and curcumin before TNF-α/LPS activation of HIMEC. B: similarly, Western blot analysis from nuclear protein of TNF-α/LPS-activated HIMEC show the inhibition of p65 subunit of NF-κB by both SN-50 and curcumin. C: Western blotting showed that inhibitory factor κB (IκB)-α is rapidly degraded in TNF-α/LPS-activated HIMEC in <30 min and then recovers by 60 min, resulting in NF-κB activation. D: immunofluorescence staining of TNF-α/LPS-activated HIMEC demonstrated the nuclear translocation of NF-κB subunit p65, which was effectively blocked with SN-50 pretreatment and curcumin. Data shown are from one of three independent experiments.
Together these results suggest that PI 3-kinase/Akt, MAPK, and NF-κB are the key regulatory pathways for VCAM-1 expression in HIMEC following TNF-α/LPS activation.
Immunohistochemical localization of VCAM-1 in colonic microvessels.
In frozen sections from non-IBD resected human colon (i.e., diverticular disease, colon cancer resection margins), mucosal microvascular endothelial VCAM-1 expression was assessed by immunohistochemistry using a diaminobenzidine-HRP-based substrate system. VCAM-1 immunoreactivity (shown by dark brown precipitate) is evident in select mucosal and submucosal microvessels (Fig. 8). Of note, not all microvessels showed positive immunoreactivity in these colonic specimens.
Fig. 8.
Immunohistochemical localization of VCAM-1 in colonic microvasculature. VCAM-1 expression was assessed by immunohistochemistry using a diaminobenzidine- and HRP-based substrate system. VCAM-1 immunoreactivity (shown by dark brown precipitate) is evident in selective colonic mucosal and submucosal microvessels (arrows).
Schematic of Akt activation leading to VCAM-1 expression.
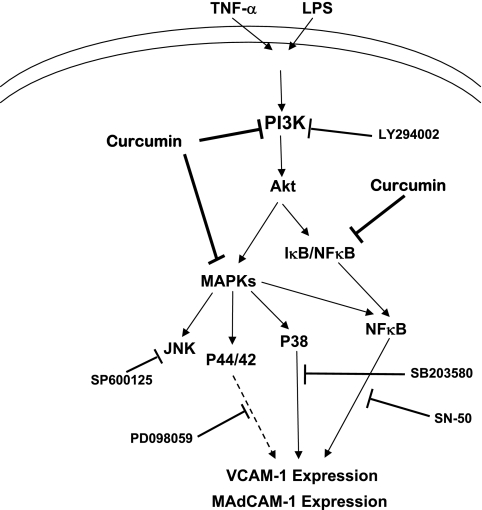
We hypothesize that TNF-α/LPS activation of HIMEC results in PI 3-kinase activation and subsequent Akt phosphorylation, as demonstrated in the summary figure (Fig. 9). Activated Akt will in turn activate MAPK cascades and NF-κB pathways, which will ultimately result in increased gene and protein expression of MAdCAM-1 and VCAM-1, the two major endothelial ligands for α4-expressing leukocytes, which preferentially home to the mucosal immune compartment in the intestine.
Fig. 9.
Akt pathway activation leading to VCAM-1 expression in HIMEC. Summary figure of hypothesized signaling pathways underlying VCAM-1 and MAdCAM-1 expression in HIMEC following TNF-α/LPS activation via Akt activation.
DISCUSSION
The present study has confirmed that TNF-α/LPS stimulation of HIMEC resulted in activation of MAPKs and NF-κB and increased expression of VCAM-1 along with MAdCAM-1, E-selectin, and ICAM-1 (24, 26). We defined the mechanisms and signaling pathways that underlie VCAM-1 expression in TNF-α/LPS-activated HIMECs. Our findings indicate that, in addition to activation of the MAPK and NF-κB, the PI 3-kinase/Akt signaling pathway is integral to VCAM-1 expression in TNF-α/LPS-activated HIMEC.
VCAM-1 expression in HIMEC required p38 MAPK, NF-κB, and PI 3-kinase/Akt activation, which is in marked contrast to the signaling cascades required for E-selectin and ICAM-1 expression, which only required NF-κB but not PI 3-kinase/Akt activation (24). Our findings are paralleled by studies that have shown that the PI 3-kinase/Akt inhibitor LY-294002 did not exert inhibitory effects on either E-selectin or ICAM-1 expression in human umbilical vein endothelial cells (22). These results raise the question of selectively blocking PI 3-kinase/Akt as a therapeutic target that would interrupt α4-mediated leukocyte transmigration to the gut endothelium in the treatment of IBD, while not affecting other cell adhesion pathways involved in cellular homeostasis and leukocyte recruitment.
PI 3-kinase consists of two subunits (p85 and p110), and, upon activation, p110 catalyzes the phosphorylation of membrane phosphatidylinositol 4,5-bisphosphate to generate phosphatidylinositol 3,4,5-trisphosphate, leading to Thr308 and Ser473 Akt phosphorylation and activation (31). The PI 3-kinase/Akt pathway plays a role in multiple aspects of cell physiology and homeostasis, since Akt activation will result in cell proliferation, differentiation, chemotaxis, survival, enhanced glucose metabolism, and nitric oxide production (18). In the present study, we demonstrate that VCAM-1 expression in TNF-α/LPS-activated HIMEC is also regulated by the PI 3-kinase/Akt pathway. The importance of PI 3-kinase/Akt signaling in expression of endothelial ligands for leukocyte α4-integrin, a critical molecular homing mechanism that is specific to the gut, suggests that this signaling cascade plays an essential role in gut-specific differentiation of these endothelial cells. However, PI 3-kinase/Akt activation in large-vessel endothelial populations has also been reported (22), which implies that additional signaling pathways will likely be required to achieve the complete gut-specific differentiation exhibited by HIMEC.
Previous attempts to target the signaling cascades involved in inflammatory activation in IBD have met with mixed success. The initial success of a p38 MAPK inhibitor in a pilot study (14) has yet to demonstrate success in a pivotal multicenter trial. Two other inhibitors of inflammatory signal transduction have failed to demonstrated therapeutic benefit (8). The reasons underlying the failure of these inhibitors of inflammatory signaling cascades are unclear. The inhibitors may have too broad of an effect on diverse cell physiology. Another factor could be the pharmacokinetic issues involving drug delivery to areas of inflammation. An additional concern regarding the therapeutic potential of signal transduction inhibitors in inflammation centers on the timing of their use in complex disorders such as IBD. Selective inhibitors of signal transduction may not be effective for both induction and maintenance of remission in a chronic inflammatory disease but may be more efficacious for one of these two phases in the disease process. Given that most IBD clinical trials will initially focus on induction of remission, agents that may preferentially function in the maintenance of remission may not be assessed with the best approach. A prime example of the potential selective efficacy of an agent that will function in maintenance of remission is highlighted by the compound curcumin. Although curcumin has been shown to be effective in the treatment of animal models of IBD where it prevented development of gut inflammation and decreased morbidity and mortality in both dextran sulfate sodium and trinitrobenzene sulfonic acid colitic mice (33), its major benefit in human trials has been demonstrated in maintenance of remission. Curcumin has been shown in a multicenter crossover trial to offer therapeutic benefit for maintenance of remission in UC patients who were concomitantly on 5ASA therapy (12). Because this agent has been historically used as a component of the spice turmeric in food preparation, one can hypothesize that its therapeutic effect may be more subtle, and this may underlie its demonstrated success in maintaining remission over time. Therefore, curcumin may not be successful in inducing remission in severe active inflammation but offer benefit as a long-term agent for the maintenance of colitis remission.
At present, there are emerging therapeutic agents that specifically target the endothelium for IBD treatment. Work over the past 15 years has demonstrated the central importance of the α4-integrin pathway in leukocyte recruitment to the gut in IBD inflammation. Initial therapeutic observations in animal models of IBD have been translated into human trials with several selective and nonselective α4-integrin inhibitors. Natalizumab, the first anti-α4-integrin inhibitor, has demonstrated significant therapeutic benefit for the treatment of CD and multiple sclerosis. This agent is believed to block the interaction of α4-expressing circulating leukocytes with their endothelial integrin ligands MAdCAM-1 and VCAM-1. Although this agent demonstrated a weaker induction effect, responding patients demonstrated a strong and durable remission extending beyond one year of treatment. At present, there are ongoing trials with multiple anti-α4-integrin inhibitors (i.e., natalizumab, MLN02) for the treatment of both CD and UC (2).
In summary, we have demonstrated that HIMEC express mRNA and protein for VCAM-1 in response to TNF-α/LPS. The regulation of VCAM-1 gene expression in HIMEC is dependent on NF-κB activation, which is similar to ICAM-1 and E-selectin expression. However, VCAM-1 expression is also dependent on PI 3-kinase/Akt signaling, which resembles MAdCAM-1 signaling patterns in HIMEC. VCAM-1 functions as a ligand for the α4-integrin, which is a shared function with MAdCAM-1, the molecule that is felt to play the central role in leukocyte recruitment to the gut. Both VCAM-1 and MAdCAM-1 expression is linked to the PI 3-kinase/Akt activation, suggesting that α4-leukocyte recruitment to the gut is linked to endothelial activation of this signaling cascade, and curcumin inhibited the expression of both molecules. These data suggest that inhibitors of endothelial PI 3-kinase/Akt activation may function to inhibit enhanced CAM expression and warrant evaluation for the treatment of IBD.
GRANTS
This work was supported by National Institute of Diabetes and Digestive and Kidney Diseases Grant DK-065948 and by the Digestive Disease Center of the Medical College of Wisconsin.
REFERENCES
- 1.Amin MA, Haas CS, Zhu K, Mansfield PJ, Kim MJ, Lackowski NP, Koch AE. Migration inhibitory factor up-regulates vascular cell adhesion molecule-1 and intercellular adhesion molecule-1 via Src, PI3 kinase, and NFkappaB. Blood 107: 2252–2261, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Baumgart DC, Sandborn WJ. Inflammatory bowel disease: clinical aspects and established and evolving therapies. Lancet 369: 1641–1657, 2007. [DOI] [PubMed] [Google Scholar]
- 3.Binion DG, Otterson MF, Rafiee P. Curcumin inhibits VEGF mediated angiogenesis in human intestinal microvascular endothelial cells through COX-2 and MAPK inhibition. Gut 57: 1509–1517, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Binion DG, West GA, Ina K, Ziats NP, Emancipator SN, Fiocchi C. Enhanced leukocyte binding by intestinal microvascular endothelial cells in inflammatory bowel disease. Gastroenterology 112: 1895–1907, 1997. [DOI] [PubMed] [Google Scholar]
- 5.Brouet I, Ohshima H. Curcumin, an anti-tumour promoter and anti-inflammatory agent, inhibits induction of nitric oxide synthase in activated macrophages. Biochem Biophys Res Commun 206: 533–540, 1995. [DOI] [PubMed] [Google Scholar]
- 6.Carter RA, O'Donnell K, Sachthep S, Cicuttini F, Boyd AW, Wicks IP. Characterization of a human synovial cell antigen: VCAM-1 and inflammatory arthritis. Immunol Cell Biol 79: 419–428, 2001. [DOI] [PubMed] [Google Scholar]
- 7.Carter RA, Wicks IP. Vascular cell adhesion molecule 1 (CD106): a multifaceted regulator of joint inflammation. Arthritis Rheum 44: 985–994, 2001. [DOI] [PubMed] [Google Scholar]
- 8.Danese S, Semeraro S, Armuzzi A, Papa A, Gasbarrini A. Biological therapies for inflammatory bowel disease: research drives clinics. Mini Rev Med Chem 6: 771–784, 2006. [DOI] [PubMed] [Google Scholar]
- 9.Das KC, Das CK. Curcumin (diferuloylmethane), a singlet oxygen ((1)O(2)) quencher. Biochem Biophys Res Commun 295: 62–66, 2002. [DOI] [PubMed] [Google Scholar]
- 10.Feagan BG, Greenberg GR, Wild G, Fedorak RN, Pare P, McDonald JW, Dube R, Cohen A, Steinhart AH, Landau S, Aguzzi RA, Fox IH, Vandervoort MK. Treatment of ulcerative colitis with a humanized antibody to the alpha4beta7 integrin. N Engl J Med 352: 2499–2507, 2005. [DOI] [PubMed] [Google Scholar]
- 11.Floer M, Binion DG, Nelson VM, Manley S, Wellner M, Sadeghi S, Behmaram B, Sewell C, Otterson MF, Kucharzik T, Rafiee P. Role of MutS homolog 2 (MSH2) in intestinal myofibroblast proliferation during Crohn's disease stricture formation. Am J Physiol Gastrointest Liver Physiol 295: G581–G590, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hanai H, Iida T, Takeuchi K, Watanabe F, Maruyama Y, Andoh A, Tsujikawa T, Fujiyama Y, Mitsuyama K, Sata M, Yamada M, Iwaoka Y, Kanke K, Hiraishi H, Hirayama K, Arai H, Yoshii S, Uchijima M, Nagata T, Koide Y. Curcumin maintenance therapy for ulcerative colitis: randomized, multicenter, double-blind, placebo-controlled trial. Clin Gastroenterol Hepatol 4: 1502–1506, 2006. [DOI] [PubMed] [Google Scholar]
- 13.Heidemann J, Ogawa H, Rafiee P, Lugering N, Maaser C, Domschke W, Binion DG, Dwinell MB. Mucosal angiogenesis regulation by CXCR4 and its ligand CXCL12 expressed by human intestinal microvascular endothelial cells. Am J Physiol Gastrointest Liver Physiol 286: G1059–G1068, 2004. [DOI] [PubMed] [Google Scholar]
- 14.Hommes D, van den Blink B, Plasse T, Bartelsman J, Xu C, Macpherson B, Tytgat G, Peppelenbosch M, Van Deventer S. Inhibition of stress-activated MAP kinases induces clinical improvement in moderate to severe Crohn's disease. Gastroenterology 122: 7–14, 2002. [DOI] [PubMed] [Google Scholar]
- 15.Horowitz S, Binion DG, Nelson VM, Kanaa Y, Javadi P, Lazarova Z, Andrekopoulos C, Kalyanaraman B, Otterson MF, Rafiee P. Increased arginase activity and endothelial dysfunction in human inflammatory bowel disease. Am J Physiol Gastrointest Liver Physiol 292: G1323–G1336, 2007. [DOI] [PubMed] [Google Scholar]
- 16.Inoue K, Kobayashi M, Yano K, Miura M, Izumi A, Mataki C, Doi T, Hamakubo T, Reid PC, Hume DA, Yoshida M, Aird WC, Kodama T, Minami T. Histone deacetylase inhibitor reduces monocyte adhesion to endothelium through the suppression of vascular cell adhesion molecule-1 expression. Arterioscler Thromb Vasc Biol 26: 2652–2659, 2006. [DOI] [PubMed] [Google Scholar]
- 17.Kang G, Kong PJ, Yuh YJ, Lim SY, Yim SV, Chun W, Kim SS. Curcumin suppresses lipopolysaccharide-induced cyclooxygenase-2 expression by inhibiting activator protein 1 and nuclear factor kappab bindings in BV2 microglial cells. J Pharmacol Sci 94: 325–328, 2004. [DOI] [PubMed] [Google Scholar]
- 18.Katso R, Okkenhaug K, Ahmadi K, White S, Timms J, Waterfield MD. Cellular function of phosphoinositide 3-kinases: implications for development, homeostasis, and cancer. Annu Rev Cell Dev Biol 17: 615–675, 2001. [DOI] [PubMed] [Google Scholar]
- 19.Lee CW, Lin CC, Luo SF, Lee HC, Lee IT, Aird WC, Hwang TL, Yang CM. Tumor necrosis factor-alpha enhances neutrophil adhesiveness: induction of vascular cell adhesion molecule-1 via activation of Akt and CaM kinase II and modifications of histone acetyltransferase and histone deacetylase 4 in human tracheal smooth muscle cells. Mol Pharmacol 73: 1454–1464, 2008. [DOI] [PubMed] [Google Scholar]
- 20.Lin WN, Luo SF, Wu CB, Lin CC, Yang CM. Lipopolysaccharide induces VCAM-1 expression and neutrophil adhesion to human tracheal smooth muscle cells: involvement of Src/EGFR/PI3-K/Akt pathway. Toxicol Appl Pharmacol 228: 256–268, 2008. [DOI] [PubMed] [Google Scholar]
- 21.Lu Y, Wahl LM. Production of matrix metalloproteinase-9 by activated human monocytes involves a phosphatidylinositol-3 kinase/Akt/IKKalpha/NF-kappaB pathway. J Leukoc Biol 78: 259–265, 2005. [DOI] [PubMed] [Google Scholar]
- 22.Madge LA, Pober JS. A phosphatidylinositol 3-kinase/Akt pathway, activated by tumor necrosis factor or interleukin-1, inhibits apoptosis but does not activate NFkappaB in human endothelial cells. J Biol Chem 275: 15458–15465, 2000. [DOI] [PubMed] [Google Scholar]
- 23.Min JK, Kim YM, Kim SW, Kwon MC, Kong YY, Hwang IK, Won MH, Rho J, Kwon YG. TNF-related activation-induced cytokine enhances leukocyte adhesiveness: induction of ICAM-1 and VCAM-1 via TNF receptor-associated factor and protein kinase C-dependent NF-kappaB activation in endothelial cells. J Immunol 175: 531–540, 2005. [DOI] [PubMed] [Google Scholar]
- 24.Ogawa H, Binion DG, Heidemann J, Theriot M, Fisher PJ, Johnson NA, Otterson MF, Rafiee P. Mechanisms of MAdCAM-1 gene expression in human intestinal microvascular endothelial cells. Am J Physiol Cell Physiol 288: C272–C281, 2005. [DOI] [PubMed] [Google Scholar]
- 25.Prasad R, Giri S, Nath N, Singh I, Singh AK. Inhibition of phosphoinositide 3 kinase-Akt (protein kinase B)-nuclear factor-kappa B pathway by lovastatin limits endothelial-monocyte cell interaction. J Neurochem 94: 204–214, 2005. [DOI] [PubMed] [Google Scholar]
- 26.Rafiee P, Johnson CP, Li MS, Ogawa H, Heidemann J, Fisher PJ, Lamirand TH, Otterson MF, Wilson KT, Binion DG. Cyclosporine A enhances leukocyte binding by human intestinal microvascular endothelial cells through inhibition of p38 MAPK and iNOS. Paradoxical proinflammatory effect on the microvascular endothelium. J Biol Chem 277: 35605–35615, 2002. [DOI] [PubMed] [Google Scholar]
- 27.Rafiee P, Nelson VM, Manley S, Wellner M, Floer M, Binion DG, Shaker R. Effect of curcumin on acidic pH-induced expression of IL-6 and IL-8 in human esophageal epithelial cells (HET-1A): role of PKC, MAPKs, and NF-kappaB. Am J Physiol Gastrointest Liver Physiol 296: G388–G398, 2009. [DOI] [PubMed] [Google Scholar]
- 28.Rafiee P, Ogawa H, Heidemann J, Li MS, Aslam M, Lamirand TH, Fisher PJ, Graewin SJ, Dwinell MB, Johnson CP, Shaker R, Binion DG. Isolation and characterization of human esophageal microvascular endothelial cells: mechanisms of inflammatory activation. Am J Physiol Gastrointest Liver Physiol 285: G1277–G1292, 2003. [DOI] [PubMed] [Google Scholar]
- 29.Ruby AJ, Kuttan G, Babu KD, Rajasekharan KN, Kuttan R. Anti-tumour and antioxidant activity of natural curcuminoids. Cancer Lett 94: 79–83, 1995. [DOI] [PubMed] [Google Scholar]
- 30.Sandborn WJ, Colombel JF, Enns R, Feagan BG, Hanauer SB, Lawrance IC, Panaccione R, Sanders M, Schreiber S, Targan S, van Deventer S, Goldblum R, Despain D, Hogge GS, Rutgeerts P. Natalizumab induction and maintenance therapy for Crohn's disease. N Engl J Med 353: 1912–1925, 2005. [DOI] [PubMed] [Google Scholar]
- 31.Shiojima I, Walsh K. Role of Akt signaling in vascular homeostasis and angiogenesis. Circ Res 90: 1243–1250, 2002. [DOI] [PubMed] [Google Scholar]
- 32.Strassheim D, Asehnoune K, Park JS, Kim JY, He Q, Richter D, Kuhn K, Mitra S, Abraham E. Phosphoinositide 3-kinase and Akt occupy central roles in inflammatory responses of Toll-like receptor 2-stimulated neutrophils. J Immunol 172: 5727–5733, 2004. [DOI] [PubMed] [Google Scholar]
- 33.Sugimoto K, Hanai H, Tozawa K, Aoshi T, Uchijima M, Nagata T, Koide Y. Curcumin prevents and ameliorates trinitrobenzene sulfonic acid-induced colitis in mice. Gastroenterology 123: 1912–1922, 2002. [DOI] [PubMed] [Google Scholar]
- 34.Surh Y Molecular mechanisms of chemopreventive effects of selected dietary and medicinal phenolic substances. Mutat Res 428: 305–327, 1999. [DOI] [PubMed] [Google Scholar]
- 35.Wang CC, Lin WN, Lee CW, Lin CC, Luo SF, Wang JS, Yang CM. Involvement of p42/p44 MAPK, p38 MAPK, JNK, and NF-kappaB in IL-1beta-induced VCAM-1 expression in human tracheal smooth muscle cells. Am J Physiol Lung Cell Mol Physiol 288: L227–L237, 2005. [DOI] [PubMed] [Google Scholar]