Abstract
We recently found that an orally directed stretch of the esophagus activates a neurally mediated relaxation of the lower esophageal sphincter (LES). Goals of our study were to characterize the neural mechanisms responsible for axial and transverse stretch-activated responses in the LES. LES pressure was monitored in anesthetized and artificially ventilated mice. Sutures were placed in the esophagus to exert graded stretch in the longitudinal and transverse directions. Effects of bilateral vagotomy and pharmacological agents on the stretch-activated LES responses were investigated. The relationship between vagally stimulated axial stretch and LES relaxation was also studied. Stretch in the longitudinal and transverse directions caused a dose-dependent LES relaxation and contraction, respectively, that were not affected by bilateral vagotomy and sympathectomy but were blocked by tetrodotoxin. In bilateral vagotomized animals, hexamethonium, atropine, pyridoxalphosphate-6-azophenyl-2′,4′ disulfonic acid (PPADS), and ondansetron did not block the stretch-activated LES relaxation and contraction. Axial stretch-activated LES relaxation was blocked by nitric oxide inhibitor and transverse stretch-activated LES contraction was blocked by a combination of atropine and substance P antagonist. Electrical stimulation of the vagus nerve induced LES relaxation and axial stretch on the LES, both of which were blocked by rocuronium. Axial and transverse stretch-activated LES relaxation and contraction were present in the W/Wv mice that lack interstitial cells of Cajal (ICC). Stretch-activated LES relaxation and contraction are mediated through mechanosensitive neurons located in the myenteric plexus, which involves neither synaptic transmission nor ICC.
Keywords: mechanosensitive neuron, excitatory motor neuron, inhibitory motor neurons
lower esophageal sphincter (LES) muscle is in a state of tonic contraction due to myogenic and neurogenic elements of the LES (17). In response to swallow and esophageal distension, the LES relaxes through an inhibitory pathway located in the vagus nerve (16, 27, 28). The current understanding is that the vagal inhibitory efferent fibers synapse with the myenteric inhibitory neurons located in the wall of the esophagus and LES to mediate LES relaxation. Upon activation, these myenteric inhibitory neurons release nitric oxide to induce relaxation of the LES (25, 33, 39).
Swallow, esophageal distension, and transient LES relaxation (TLESR) are associated with the movement of the LES in oral direction (9, 11, 26). Animal and human studies show that with swallow, esophageal distension, and TLESR the LES moves 2–3 cm in the oral direction. The latter is due to the esophageal longitudinal muscle contraction that occurs concurrently with the LES relaxation. We recently found that an axial stretch of the LES in the oral direction induces a neurologically mediated LES relaxation in the opossum (10). The goals of our study were to further characterize the stretch-activated neuronal pathway to the LES. We wanted to determine 1) whether neuronal synapse or interstitial cells of Cajal (ICC) are involved in the stretch-activated LES responses and 2) whether axial stretch is involved in the vagally activated relaxation of the LES.
METHODS
Studies were performed in mice of either sex, weighing between 20 and 30 g (C57BL/6J, WBB6F1/J-KitW/KitW-v, and background mice purchased from Jackson Laboratory, Bar Harbor, ME). The animal safety committee of the San Diego Veterans Affairs HealthCare System approved the study protocol. Animals were sedated with an intraperitoneal injection of 0.1–0.19 ml stock solution. The stock solution contained 3.75 ml ketamine solution (concentration 100 mg/ml), 0.4 ml xylazine solution (concentration 100 mg/ml), 0.75 ml acepromazine solution (concentration 10 mg/ml), and 20 ml sterile water. Twenty percent of the initial induction dose was injected as needed during the experiment. A venous cannula was placed in the internal jugular vein (for injection of pharmacological agents), an arterial line was placed in the carotid artery (to measure blood pressure), and a tracheotomy was performed for tracheal intubation and ventilation. Mice were ventilated at 41 strokes/min, at a tidal volume of 0.5 ml using a mouse ventilator (Harvard Pump, model 683, Holliston, MA).
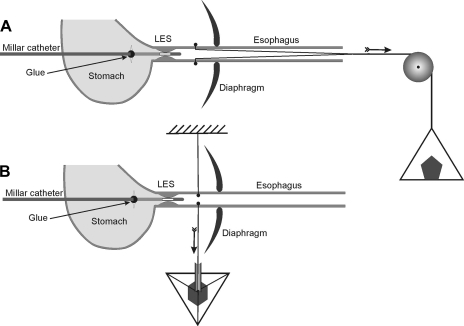
A midline laparotomy was performed and a 20-cm length of polyethylene (PE)-10 tubing (Fisher Scientific, Pittsburgh, PA) was passed through the mouth into the esophagus. A curved RB-1 needle with a 20-cm long, 5-0 silk suture was passed through the esophageal wall (2–3 mm above the LES), PE tube (placed in the lumen of the esophagus), and the opposite esophageal wall. The needle was removed and silk sutures of ∼10-cm length were left on each side of the esophageal wall. Knots were placed at the both ends of sutures and PE tubing along with the sutures was pulled out from the mouth for stretching of the LES in the oral direction. For exerting transverse stretch on the esophagus, the sutures were pulled back through the laparotomy, just above the LES. Figure 1 shows the schematic of the technique to apply axial and transverse stretch on the esophageal wall. A 2-Fr, solid-state Millar pressure transducer catheter (Millar Instruments, Houston, TX) was passed through a small incision, located 2–3 mm below the LES on the stomach wall. A small amount of tissue adhesive (NEXABAND S/C manufactured by Closure Medical and distributed by Abbott, North Chicago, IL) was used to glue the pressure transducer catheter with the stomach wall. The above arrangement prevented a relative movement between the LES and pressure transducer during axial and transverse stretch of the esophagus. Sutures exiting through the mouth and esophagus were passed over a pulley (attached to the end of the table) and weights were applied to exert graded stretch on the esophageal wall in the oral (Fig. 1A) and transverse directions (Fig. 1B).
Fig. 1.
Schematic diagram of experimental design. A 2-Fr solid-state Millar transducer is located in the lower esophageal sphincter (LES). The catheter is glued to the stomach wall. Two silk sutures were tied outside the esophageal wall to exert oroaxial stretch (A) and inside the esophageal wall to exert transverse stretch (B).
Study protocol.
Oroaxial (longitudinal) and transverse stretches were applied to the esophageal wall with weights of 2, 4, 6, and 8 g. The 8-g weight usually produced the maximal LES relaxation and contraction. Several observations were made with each weight. The effect of increasing the duration of stretch was determined by applying the weight for durations of 20, 40, and 60 s. The effects of axial and transverse stretch on the LES pressure were studied under following conditions: 1) control, 2) following unilateral and bilateral vagotomy, and 3) following bilateral sympathectomy. Right and left vagus nerves were identified in the carotid sheaths in the neck, and bilateral cervical vagotomy was performed. For bilateral sympathectomy, all nerve branches traversing along the blood vessels to the distal esophagus and adjacent to the stomach were transected. The effects of following pharmacological agents, either alone or in various combinations, were investigated: 1) nitric oxide synthase inhibitor [NG-nitro-l-arginine methyl ester (l-NAME) 100 mg/kg] (32), 2) tetrodotoxin (TTX, 60 μg/kg) (15), 3) atropine (30 μg/kg) (16), 4) nicotine acetylcholine receptor antagonist (hexamethonium 20 mg/kg) (16), 5) pyridoxalphosphate-6-azophenyl-2′,4′ disulfonic acid (PPADS, P2xpurinoreceptor antagonist, 5 μg/per mouse) (34), 6) ondansetron (5-HT3 receptor antagonist, 1 mg/kg) (7), and 7) CP-96345 (substance P antagonist, 2.5 mg/kg) (3). The doses of various receptor antagonists were the maximal doses used for the in vivo studies reported in the literature. All pharmacological agents were purchased from the Sigma-Aldrich (Milwaukee, WI).
In a separate group of animals, effects of vagal stimulation on the LES pressure and LES cranial displacement were studied. Following bilateral vagotomy, the peripheral end of one of the vagus nerves was placed on two silver hook electrodes and electrical pulses of various frequencies were delivered via Grass pulse generator (model S48 Astro-Med, West Warwick, RI). Cranial displacement of the LES during vagal stimulation was recorded by using two 1-mm piezoelectric crystals (1, 31) (Sonometrics, London, ON, Canada). One crystal was anchored on the upper edge of LES with a suture (moving crystal) and the other was mounted on a wooden stick and placed in the abdomen close to LES (fixed crystal). The technique has been validated and used extensively before (1, 31); it allows measurement of change in the distance between two points with accuracy of 0.01 mm. At the end of each experiment, euthanasia was administered with an IV injection of potassium chloride solution (2 mmol/kg) in accordance with the euthanasia guidelines of the San Diego Veterans Affairs Animal Committee (4).
Data analysis.
The LES pressure was measured as end-expiratory pressure above the gastric pressure. Percent LES relaxation and LES contraction as well as absolute LES pressure were determined. Duration of LES relaxation was determined from the time when the LES pressure dropped to 50% of the prestretch value to the time when it returned to 50% of the prestretch value. The effects of various pharmacological agents on LES relaxation and contraction were quantitated. Effect of vagal stimulation on the esophageal contraction pressure, LES cranial movement, and LES relaxation was determined.
Statistical analysis.
Differences between the LES pressure during axial and transverse stretches and the effects of various pharmacological agents were determined by paired t-test and one-way or two-way ANOVA, as appropriate. Data are presented as means ± SE. P < 0.05 was considered statistically significant.
RESULTS
Basal LES pressure and effects of vagotomy and sympathectomy.
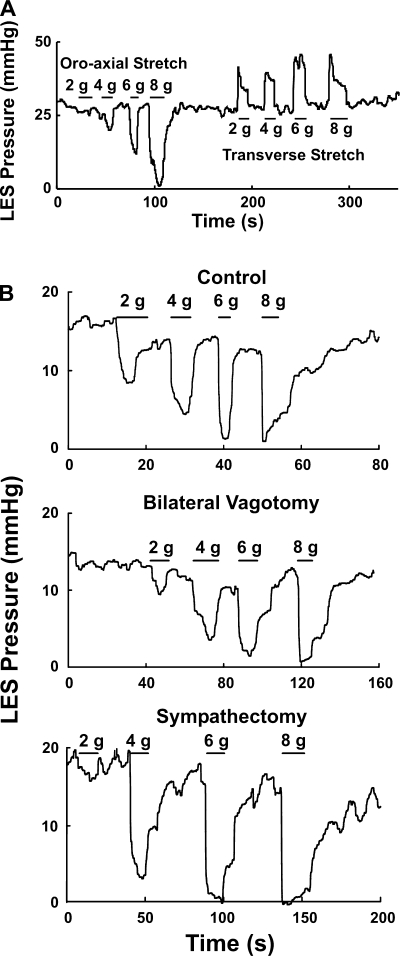
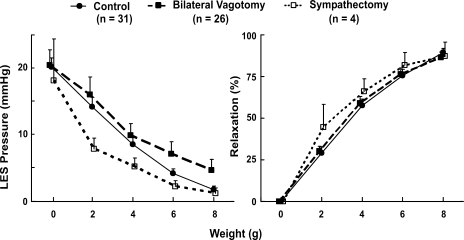
The basal LES pressures in C57BL/6J mice were normally distributed with a mean pressure of 24 ± 2 mmHg. One minute after left vagotomy the LES pressure was 23 ± 3 mmHg, and 1 min following bilateral vagotomy it was 27 ± 3 mmHg. Similar to bilateral vagotomy, bilateral sympathectomy had no influence on the basal LES pressure (Figs. 2 and 3).
Fig. 2.
A: effect of axial and transverse stretch on LES. B: effects of before bilateral vagotomy, bilateral vagotomy, and bilateral vagotomy plus bilateral sympathectomy on the axial stretch-activated LES relaxation. Note that neither vagotomy nor sympathectomy had any influence on the oroaxial stretch-activated LES relaxation.
Fig. 3.
Summary of the effects of before bilateral vagotomy (control; n = 31), bilateral vagotomy (n = 26), and bilateral vagotomy plus bilateral sympathectomy (n = 4) on the oroaxial stretch-induced LES relaxation. There is no significant difference among 3 groups.
Effects of longitudinal (oroaxial) and transverse stretch on the LES pressure.
Oroaxial stretch with the application of weights caused LES relaxation, and with the removal of weight there was prompt recovery of the basal LES pressure (Fig. 2). The latency of response between the application of weight and the onset of LES relaxation was less than 1 s. The degree of LES relaxation increased and the residual LES pressure decreased as the applied weight increased. Minimal weight that induced any LES relaxation was 1–2 g, and an 8-g stretch produced 93 ± 5% relaxation (Fig. 3). Increasing the duration of stretch resulted in an increase in the duration of LES relaxation. The duration of axial stretch and duration of LES relaxation were almost the same. Bilateral vagotomy had no influence on the oroaxial stretch-activated LES relaxation. Similarly, sympathectomy did not affect the axial stretch-activated LES relaxation (Figs. 2 and 3).
Transverse stretch on the esophagus, on the other hand, resulted in an increase in the LES pressure in a dose-dependent fashion (Fig. 2). Bilateral vagotomy and sympathectomy had no influence on the transverse stretch-activated LES pressure increases.
Effects of TTX on the stretch-activated LES relaxation and contraction.
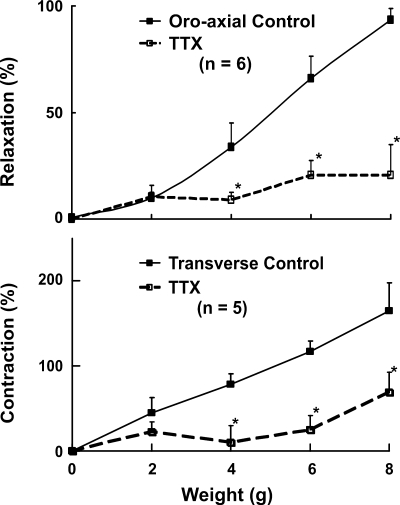
All of these experiments were conducted in the mice following bilateral vagotomy. The effects of TTX on the stretch-induced changes in the LES pressure are shown in Fig. 4. There was a slight decrease in the basal LES pressure following TTX (from 21 ± 4 to 19 ± 4 mmHg), but the difference was not statistically significant. Oroaxial and transverse stretch-activated changes in the LES pressure were significantly blocked by the TTX. The LES relaxation induced by 8-g oroaxial stretch was blocked from 93 ± 5% before to 21 ± 14% after the TTX, P < 0.01. Similarly, the LES contraction induced by 8-g transverse stretch was blocked, from a 165 ± 33% increase to 69 ± 30% by the TTX (n = 5, P < 0 .01) (Fig. 4). These observations suggest that both LES relaxation induced by axial stretch and LES contraction induced by transverse stretch are neurally mediated responses.
Fig. 4.
Effects of tetrodotoxin (TTX) (60 μg/kg) on the oroaxial and transverse stretch-activated changes in the LES pressure, n = 5–6 animals. Data are means ± SE. Note that TTX blocks both oroaxial stretch-activated LES relaxation and transverse stretch-activated LES contraction. *P < 0.05.
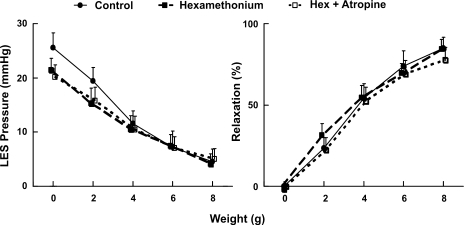
Effects of hexamethonium and atropine on the axial stretch-activated LES relaxation.
These experiments were conducted to determine whether neural synapse is involved in the stretch-activated LES relaxation. Hexamethonium (nicotinic receptor blocker) had no effect on the basal LES pressure. On the other hand, addition of atropine (muscarinic receptor blocker) reduced the basal LES pressure by ∼24%. Neither hexamethonium nor atropine when applied individually had any influence on the oroaxial stretch-activated LES relaxation. Furthermore, simultaneous administration of hexamethonium and atropine did not significantly affect the oroaxial stretch-activated LES relaxation (Fig. 5).
Fig. 5.
Effects of hexamethonium (Hex) and atropine on the oroaxial stretch-activated LES relaxation. Hexamethonium alone did not affect the basal LES pressure and the axial stretch-induced relaxation. Addition of atropine reduced the basal LES pressure by ∼24% but did not affect the axial stretch-induced LES relaxation (n = 7).
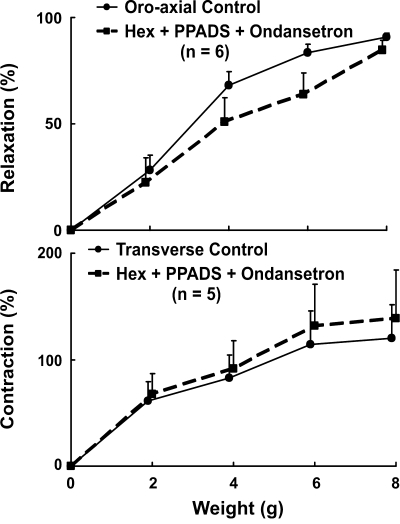
Effects of hexamethonium, PPADS, and ondansetron on the stretch-activated LES relaxation and contraction.
These experiments were conducted to determine whether the stretch-activated LES relaxation and contraction involve purinergic and serotonergic synaptic transmission. The effects of a combination of hexamethonium, PPADS (purinergic receptor blocker), and ondansetron (5-HT3 receptor blocker) on the stretch-activated LES responses were studied. The effects of combined administration of these agents on the basal LES pressure were variable, but there was consistently no effect on the axial stretch-induced LES relaxation (Fig. 6, top) and contraction (Fig. 6, bottom). These data suggest either that there is no synaptic transmission in the stretch-activated reflex pathways to the LES or that the synaptic transmission does not involve cholinergic nicotinic, purinergic, and 5-HT3 receptors.
Fig. 6.
Effect of combined hexamethonium (nicotinic), PPADS (purinergic), and ondansetron (5-HT3 antagonists) on the oroaxial stretch-activated LES relaxation (top, n = 6) and transverse stretch-activated LES contraction (bottom, n = 5). There was no difference before and after the administration of agents.
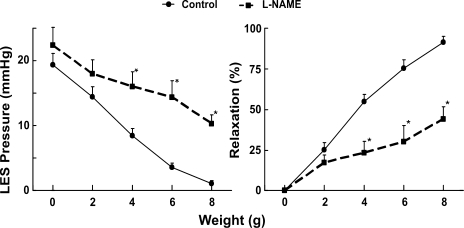
Effects of l-NAME on the oroaxial stretch-activated relaxation.
The effect of l-NAME administration on the stretch-activated LES relaxation is shown in Fig. 7. Administration of l-NAME resulted in an increase in the basal LES pressure, from 19 ± 2 to 22 ± 3 mmHg (P = 0.2). Importantly, the oroaxial stretch-activated LES relaxation was significantly blocked by l-NAME. The degree of LES relaxation induced by 8 g axial stretch was reduced from 91 ± 3 to 44 ± 7% by l-NAME (P = 0.01).
Fig. 7.
Effects of NG-nitro-l-arginine methyl ester (l-NAME; nitric oxide inhibitor) on the oroaxial stretch-activated LES relaxation. Left: LES pressure in mmHg. Right: percent relaxation. Note that l-NAME increased the LES pressure and blocked the oroaxial stretch-activated LES relaxation significantly, *P < 0.05 (n = 12).
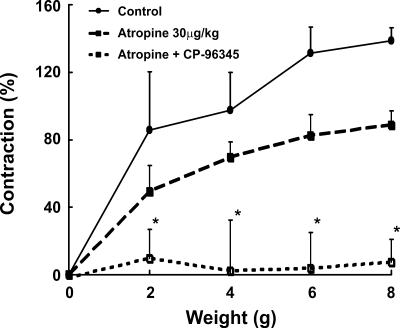
Effect of atropine and substance P antagonist on the transverse stretch-activated LES contraction.
The effect of atropine and substance P antagonist on the transverse stretch-activated LES contraction is shown in Fig. 8. Atropine reduced the contraction induced by transverse stretch, but the differences were not statistically different from the controls. A combination of atropine and CP-96345 (substance P antagonist) almost abolished the LES contraction induced by transverse stretch.
Fig. 8.
Effect of atropine and substance P antagonist CP-96345 on the transverse stretch-activated LES contraction. Atropine reduced the contraction, but the difference is not statistically different from control. A combination of atropine and CP-96345 abolished the stretch-activated contraction completely, *P < 0.001 (n = 5).
Effect of rocuronium on the vagus nerve-stimulated relaxation of the LES.
Electrical stimulation of the peripheral end of the cervical vagus nerve induced frequency-dependent relaxation of the LES and cranial displacement of the LES (Fig. 9). The maximal LES displacement was 0.44 ± 0.19 mm. Electrical stimulation of the vagus nerve also induced a frequency-dependent increase in the esophageal pressure (recorded by a transducer placed 10 mm above the LES). There is a close temporal correlation between the onset of esophageal contraction, LES cranial displacement, and LES relaxation. Rocuronium (0.2 mg/kg), a selective nicotinic blocker at the neuromuscular junction of the skeletal muscle, abolished esophageal contraction, cranial movement of the LES, and LES relaxation (Fig. 9).
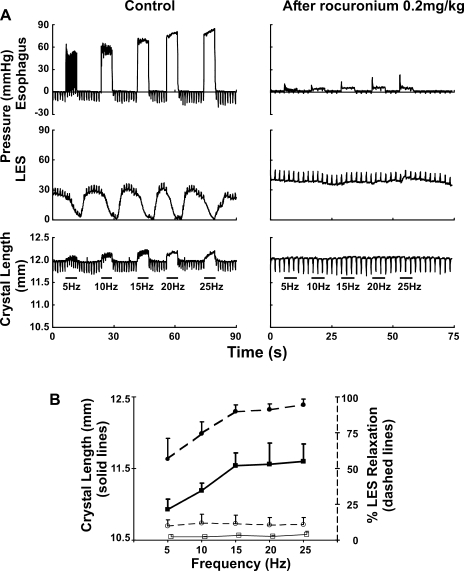
Fig. 9.
Relationship between vagally stimulated LES relaxation and axial stretch on the LES. Axial stretch on the LES was recorded by using peizoelectric crystals (see methods). Following bilateral cervical vagotomy, the peripheral end of one of the vagus nerves was electrically stimulated for 3 s with pulse duration of 0.5 ms, amplitude of 10 V, and frequencies of 5, 10, 15, 20, and 25 Hz. A: note that electrical stimulation of the vagus nerve induced a frequency-dependent esophageal contraction (first tracing left and right), relaxation of the LES (second tracing left and right), and cranial displacement of the LES (third tracing left and right). Rocuronium (0.02 mg/kg), a selective blocker of nicotinic receptors at the neuromuscular junction, abolished esophageal contraction, LES relaxation, and cranial LES displacement. B: summary data from 4 mice (P < 0.001 by paired t-test).
Effect of oroaxial and transverse stretch on LES relaxation and contraction in the W/WV−/− mice.
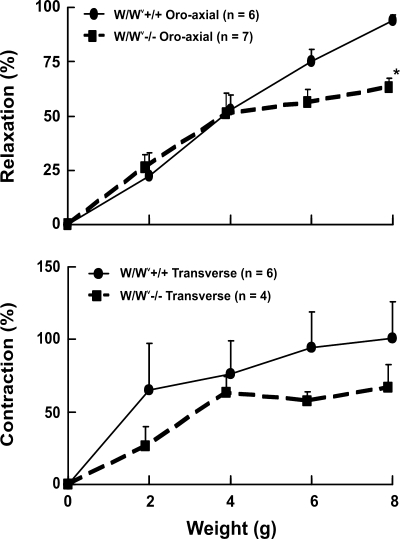
The effects of oroaxial and transverse stretch on the LES relaxation and contraction in the W/WV−/− mice that lack ICC in the myenteric plexus of the LES are shown in Fig. 10. The control for these experiments was W/WV+/+ mice from the Jackson Laboratory. These experiments were conducted to determine whether the ICC are involved in stretch-activated LES relaxation and contraction. Both LES relaxation and contraction were preserved in the W/WV−/− mice, albeit the amplitude of responses was slightly reduced compared with their control littermates. The LES relaxation at 8-g stretch was significantly lower in the ICC-deficient mice.
Fig. 10.
Effects of oroaxial and transverse stretch on LES activities in W/WV−/− and W/WV+/+ mice. Both relaxation in response to axial stretch and contraction in response to transverse stretch were present in the interstitial cells of Cajal-deficient W/WV−/− mice.
DISCUSSION
In summary, our data show the following: 1) oroaxial and transverse stretch of the esophagus just above the LES cause a neurologically mediated LES relaxation and contraction, respectively; 2) bilateral vagotomy and sympathectomy have no influence on the stretch-activated LES responses but these responses are blocked by TTX; 3) hexamethonium and atropine, either alone or in combination, do not influence the oroaxial stretch-activated LES relaxation; 4) a combination of hexamethonium, ondansetron, and PPADS does not affect the stretch-activated LES relaxation and contraction; 5) oroaxial stretch-activated LES relaxation is blocked by nitric oxide inhibitor (l-NAME) and the transverse stretch-activated LES contraction is abolished by a combination of atropine and substance P antagonist; 6) vagally stimulated LES relaxation is associated with an axial stretch on the LES, both of which are abolished by rocuronium; and, finally, 7) stretch-activated LES relaxation and contraction responses are mostly intact in the W/WV−/− mice that lack ICC. Our observations indicate that stretch-activated responses in the LES are mediated through the direct activation of excitatory and inhibitory motor neurons in the myenteric plexus of the LES. Our observations also indicate that the vagally stimulated LES relaxation is mediated through axial stretch on the LES exerted by the longitudinal muscle contraction of the esophagus.
Various investigators have used different species [cats (5), dogs (24), ferrets (6), rabbit (19, 22), and opossum (12, 17)] to study the LES. For several reasons, opossum has been used most extensively because the LES and distal esophagus in the opossums, similar to humans, consist of smooth muscle. Mouse, as an experimental model to study the LES, has also been used but to a limited extent (21, 29, 32). The challenge with a small animal like mice is the difficulty in recording LES pressure. Goyal and colleagues (32) used microinfusion manometry to record the LES pressure in mice. On the other hand, we used a solid-state transducer to record the LES pressure successfully. Another important issue is the potential problem of axial movement between the transducer and the LES during axial stretch, which may appear as LES relaxation. To avoid the relative movement between the LES and pressure transducer we placed the pressure transducer via a gastrotomy, located just below (2 mm) the LES. The catheter where it exited the stomach was glued to the stomach wall with the thinking that the LES and the catheter will move together with axial stretch. The LES relaxation induced by axial stretch in our experiment was blocked by TTX and l-NAME, which proves there is no relative movement between the transducer and the LES.
Clearly, there are several advantages in using mice as an animal model to study the LES, the most important of which is the availability of genetically altered mice. Similar to humans and opossum, the LES in mice also consists of smooth muscle. Our data show a large number of functional similarities between the mouse and opossum LES. The LES tone is mostly myogenic in both species because bilateral vagotomy and TTX had minimal influence on the basal LES pressure. Furthermore, nitric oxide inhibitor blocks neurologically mediated LES relaxation in both species.
In an earlier study conducted in opossums, we found that an oroaxial stretch of the esophagus caused a neurologically mediated LES relaxation (10). In the present study, we found the same to be true in the mice. In addition, we extended our observations to show that there are differences in the LES responses to oroaxial and transverse stretches: the former causes LES relaxation and the latter LES contraction. One may argue that the amounts of weight used to induce LES relaxation and contraction are high (8 g for a 20- to 30-g animal) and not physiological. We believe that the reason for the high weight requirement may be related to the fact that in our experimental model we apply axial stretch on the LES using only two strings. On the other hand, under physiological conditions the axial stretch on the LES is exerted by a large number of longitudinal muscle fibers of the esophagus, each one of which may act in parallel to magnify the actual stretch. Our study shows that axial stretch and transverse stretch-activated LES responses are both neurologically mediated because they are blocked by TTX. Furthermore, these effects are not affected by bilateral vagotomy, suggesting that they do not require participation of brain stem and are mediated at the level of myenteric plexus. We also investigated whether neural synapse was involved in the neural pathway of the stretch-activated LES responses. Synaptic transmission in the myenteric plexus, throughout the majority of gastrointestinal tract, is mediated predominantly through the nicotinic cholinergic and to a lesser degree by purinergic (P2X) and serotonin (5-HT3) receptors (14). The lack of effect of stretch-induced LES relaxation and contraction by hexamethonium, ondansetron, and PPADS suggests that nicotinic, purinergic, and serotonegeric neurotransmissions are not involved in the stretch-activated LES responses. In the opossum LES, vagally mediated LES relaxation requires synaptic transmission that involves cholinergic nicotinic and muscarinic receptors (16). Therefore, we also conducted studies with hexamethonium and atropine and found that neither agent individually nor a combination of the two agents had any influence on the oroaxial stretch-activated LES relaxation and transverse stretch-activated LES contraction.
A reflex by definition requires sensory and motor neurons and myenteric plexus contains large number of sensory and motor neurons (13). Kunze and colleagues (23) found that mechanical stretch activates the intrinsic primary afferent neuron (IPAN), which in turn activates the motor neuron to induce contraction and relaxation of the muscles in the small intestine. Therefore, our initial hypothesis was that axial and transverse stretch activates the sensory neuron first, possibly IPAN, in the myenteric plexus, which in turn stimulates the inhibitory and excitatory motor neuron. However, we did not find that to be the case: oroaxial stretch-activated LES relaxation and transverse stretch-activated LES contraction were not blocked by any of the pharmacological agents known to block the neural synaptic transmission in the myenteric plexus of the gastrointestinal tract. We used maximal doses of pharmacological agents that are shown to be effective in blocking synaptic transmission in the various animal preparations. Even though we can't completely exclude the possibility of an unknown synaptic neurotransmitter, it appears that there is no synapse involved in the stretch-activated LES responses.
A large number of studies suggest that the ICC are interposed between the myenteric motor neurons and smooth muscle cells, and they mediate excitatory and inhibitory neurotransmission (35–37) in the gastrointestinal tract. Furthermore, it is also suggested that the ICC may act as stretch sensors in the gastrointestinal tract (38). In our studies, we found that stretch-activated LES relaxation and contraction were only slightly impaired in the W/WV−/− mice that are known to lack myenteric ICC in the LES (36). Therefore, our studies do not support a major role of the ICC in either the sensory or the motor neurotransmission function in the mice LES.
Nitric oxide is the major neurotransmitter of inhibitory motor neurons and acetylcholine along with the substance P (18) are the major neurotransmitters of the excitatory myenteric motor neurons. Accordingly, we found that the axial stretch-activated LES relaxation was blocked by l-NAME and atropine along with substance P antagonists blocked the transverse stretch-activated LES contraction. We believe our data raise the possibility that longitudinal stretch activates the inhibitory motor neurons directly to release nitric oxide and transverse stretch activates the excitatory motor neuron directly to release acetylcholine and substance P. An important question therefore is whether the motor neurons in the myenteric plexus can perform both sensory and motor functions. In a recent comprehensive book of the enteric nervous system we did not find any description of such neurons (13). However, Dickson and colleagues (8) recently found that longitudinal stretch inhibits the transverse stretch-activated peristaltic contractions in the guinea pig colon. The authors propose that the longitudinal stretch-activated inhibitory reflex in the guinea pig colon is mediated through the nitric oxide-containing interneuron. However, it may be that this nitric oxide-containing interneuron in the colon is actually the inhibitory motor neuron of the LES.
What is the physiological relevance of stretch-activated LES relaxation? Animal and human studies show that all types of LES relaxation, i.e., swallow, esophageal distension, and vagally stimulated LES relaxation, are associated with an oral movement of the LES. The latter is caused by the contraction of longitudinal muscles of the esophagus, which pulls the LES in the oral direction. Recent studies in humans also show a unique pattern of longitudinal muscle contraction in the distal esophagus during transient lower esophageal sphincter relaxation. The latter starts prior to and persists during the entire duration of transient LES relaxation (2). Rocuronium, a selective nicotinic blocker at the neuromuscular junction of skeletal muscle, blocked longitudinal muscle contraction of the esophagus (revealed by loss of cranial displacement of the LES during vagal stimulation in our experiments). In accordance with our hypothesis, rocuronium also abolished the LES relaxation completely in our experiments. We propose that further studies are needed to determine the role of stretch-sensitive inhibitory motor neurons in the swallow-induced as well as other types of LES relaxations. Fundoplication is an effective surgery to treat gastroesophageal reflux disease; however, the mechanism of its actions is not clear. One of the proposed mechanism is that fundoplication renders transient LES relaxations (major mechanism of reflux) incomplete (20, 30). We propose that fundoplication by preventing axial stretch on the LES interferes with LES relaxation. If future studies reveal that is indeed true, it may be possible to design novel therapeutic strategies that prevent axial stretch on the LES to treat gastroesophageal reflux disease.
GRANTS
This work was supported by National Institute of Diabetes and Digestive and Kidney Diseases Grant RO-1 DK60733.
REFERENCES
- 1.Armstrong SR, McCullough JL, Beattie DT. Measurement of 5-HT4 receptor-mediated esophageal responses by digital sonomicrometry in the anesthetized rat. J Pharmacol Toxicol Methods 53: 198–205, 2006. [DOI] [PubMed] [Google Scholar]
- 2.Babaei A, Bhargava V, Korsapati H, Zheng WH, Mittal RK. A unique longitudinal muscle contraction pattern associated with transient lower esophageal sphincter relaxation. Gastroenterology 134: 1322–1331, 2008. [DOI] [PubMed] [Google Scholar]
- 3.Bang R, Sass G, Kiemer AK, Vollmar AM, Neuhuber WL, Tiegs G. Neurokinin-1 receptor antagonists CP-96,345 and L-733,060 protect mice from cytokine-mediated liver injury. J Pharmacol Exp Ther 305: 31–39, 2003. [DOI] [PubMed] [Google Scholar]
- 4.Beaver BVRW, Leary S, McKiernam S, Bain S, Schulz R. 2000 report of the AVMA panel on euthanasia. JAVMA 218: 669–696, 2001. [DOI] [PubMed] [Google Scholar]
- 5.Behar J, Kerstein M, Biancani P. Neural control of the lower esophageal sphincter in the cat: studies on the excitatory pathways to the lower esophageal sphincter. Gastroenterology 82: 680–688, 1982. [PubMed] [Google Scholar]
- 6.Blackshaw LA, Staunton E, Lehmann A, Dent J. Inhibition of transient LES relaxations and reflux in ferrets by GABA receptor agonists. Am J Physiol Gastrointest Liver Physiol 277: G867–G874, 1999. [DOI] [PubMed] [Google Scholar]
- 7.Brierley SM, Carter R, Jones W 3rd, Xu L, Robinson DR, Hicks GA, Gebhart GF, Blackshaw LA. Differential chemosensory function and receptor expression of splanchnic and pelvic colonic afferents in mice. J Physiol 567: 267–281, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dickson EJ, Spencer NJ, Hennig GW, Bayguinov PO, Ren J, Heredia DJ, Smith TK. An enteric occult reflex underlies accommodation and slow transit in the distal large bowel. Gastroenterology 132: 1912–1924, 2007. [DOI] [PubMed] [Google Scholar]
- 9.Dodds WJ, Stewart ET, Hodges D, Zboralske FF. Movement of the feline esophagus associated with respiration and peristalsis. An evaluation using tantalum markers. J Clin Invest 52: 1–13, 1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dogan I, Bhargava V, Liu J, Mittal RK. Axial stretch: a novel mechanism of the lower esophageal sphincter relaxation. Am J Physiol Gastrointest Liver Physiol 292: G329–G334, 2007. [DOI] [PubMed] [Google Scholar]
- 11.Edmundowicz SA, Clouse RE. Shortening of the esophagus in response to swallowing. Am J Physiol Gastrointest Liver Physiol 260: G512–G516, 1991. [DOI] [PubMed] [Google Scholar]
- 12.Fox JA, Daniel EE. Role of Ca2+ in genesis of lower esophageal sphincter tone and other active contractions. Am J Physiol Endocrinol Metab Gastrointest Physiol 237: E163–E171, 1979. [DOI] [PubMed] [Google Scholar]
- 13.Furness J The Enteric Nervous System. Malden, MA: Blackwell, 2006.
- 14.Galligan JJ Pharmacology of synaptic transmission in the enteric nervous system. Curr Opin Pharmacol 2: 623–629, 2002. [DOI] [PubMed] [Google Scholar]
- 15.Goyal RK, Rattan S. Genesis of basal sphincter pressure: effect of tetrodotoxin on lower esophageal sphincter pressure in opossum in vivo. Gastroenterology 71: 62–67, 1976. [PubMed] [Google Scholar]
- 16.Goyal RK, Rattan S. Nature of the vagal inhibitory innervation to the lower esophageal sphincter. J Clin Invest 55: 1119–1126, 1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Goyal RK, Rattan S. Neurohumoral, hormonal, and drug receptors for the lower esophageal sphincter. Gastroenterology 74: 598–619, 1978. [PubMed] [Google Scholar]
- 18.Grider JR Neurotransmitters mediating the intestinal peristaltic reflex in the mouse. J Pharmacol Exp Ther 307: 460–467, 2003. [DOI] [PubMed] [Google Scholar]
- 19.Henry MA, Pelissari CE, Carvalho LR. Morphological and functional evaluation of distal esophagus of rabbits submitted to esophageal infusion with caustic soda. Acta Cir Bras 23: 16–21, 2008. [DOI] [PubMed] [Google Scholar]
- 20.Ireland AC, Holloway RH, Toouli J, Dent J. Mechanisms underlying the antireflux action of fundoplication. Gut 34: 303–308, 1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kim CD, Goyal RK, Mashimo H. Neuronal NOS provides nitrergic inhibitory neurotransmitter in mouse lower esophageal sphincter. Am J Physiol Gastrointest Liver Physiol 277: G280–G284, 1999. [DOI] [PubMed] [Google Scholar]
- 22.Kohjitani A, Miyawaki T, Funahashi M, Higuchi H, Matsuo R, Shimada M. Ketamine and midazolam differentially inhibit nonadrenergic noncholinergic lower esophageal sphincter relaxation in rabbits: role of superoxide anion and nitric oxide synthase. Anesthesiology 98: 449–458, 2003. [DOI] [PubMed] [Google Scholar]
- 23.Kunze WA, Clerc N, Bertrand PP, Furness JB. Contractile activity in intestinal muscle evokes action potential discharge in guinea-pig myenteric neurons. J Physiol 517: 547–561, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lehmann A, Blackshaw LA, Branden L, Carlsson A, Jensen J, Nygren E, Smid SD. Cannabinoid receptor agonism inhibits transient lower esophageal sphincter relaxations and reflux in dogs. Gastroenterology 123: 1129–1134, 2002. [DOI] [PubMed] [Google Scholar]
- 25.Murray J, Du C, Ledlow A, Bates JN, Conklin JL. Nitric oxide: mediator of nonadrenergic noncholinergic responses of opossum esophageal muscle. Am J Physiol Gastrointest Liver Physiol 261: G401–G406, 1991. [DOI] [PubMed] [Google Scholar]
- 26.Pandolfino JE, Zhang QG, Ghosh SK, Han A, Boniquit C, Kahrilas PJ. Transient lower esophageal sphincter relaxations and reflux: mechanistic analysis using concurrent fluoroscopy and high-resolution manometry. Gastroenterology 131: 1725–1733, 2006. [DOI] [PubMed] [Google Scholar]
- 27.Rattan S, Goyal RK. Neural control of the lower esophageal sphincter: influence of the vagus nerves. J Clin Invest 54: 899–906, 1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ryan JP, Snape WJ Jr, Cohen S. Influence of vagal cooling on esophageal function. Am J Physiol Endocrinol Metab Gastrointest Physiol 232: E159–E164, 1977. [DOI] [PubMed] [Google Scholar]
- 29.Sang Q, Goyal RK. Lower esophageal sphincter relaxation and activation of medullary neurons by subdiaphragmatic vagal stimulation in the mouse. Gastroenterology 119: 1600–1609, 2000. [DOI] [PubMed] [Google Scholar]
- 30.Scheffer RC, Tatum RP, Shi G, Akkermans LM, Joehl RJ, Kahrilas PJ. Reduced TLESR elicitation in response to gastric distension in fundoplication patients. Am J Physiol Gastrointest Liver Physiol 284: G815–G820, 2003. [DOI] [PubMed] [Google Scholar]
- 31.Shirato K, Shabetai R, Bhargava V, Franklin D, Ross J Jr. Alteration of the left ventricular diastolic pressure-segment length relation produced by the pericardium. Effects of cardiac distension and afterload reduction in conscious dogs. Circulation 57: 1191–1198, 1978. [DOI] [PubMed] [Google Scholar]
- 32.Sivarao DV, Mashimo HL, Thatte HS, Goyal RK. Lower esophageal sphincter is achalasic in nNOS(−/−) and hypotensive in W/W(v) mutant mice. Gastroenterology 121: 34–42, 2001. [DOI] [PubMed] [Google Scholar]
- 33.Tottrup A, Svane D, Forman A. Nitric oxide mediating NANC inhibition in opossum lower esophageal sphincter. Am J Physiol Gastrointest Liver Physiol 260: G385–G389, 1991. [DOI] [PubMed] [Google Scholar]
- 34.Tsuda M, Ueno S, Inoue K. In vivo pathway of thermal hyperalgesia by intrathecal administration of alpha,beta-methylene ATP in mouse spinal cord: involvement of the glutamate-NMDA receptor system. Br J Pharmacol 127: 449–456, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ward SM, Beckett EA, Wang X, Baker F, Khoyi M, Sanders KM. Interstitial cells of Cajal mediate cholinergic neurotransmission from enteric motor neurons. J Neurosci 20: 1393–1403, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ward SM, Morris G, Reese L, Wang XY, Sanders KM. Interstitial cells of Cajal mediate enteric inhibitory neurotransmission in the lower esophageal and pyloric sphincters. Gastroenterology 115: 314–329, 1998. [DOI] [PubMed] [Google Scholar]
- 37.Ward SM, Sanders KM. Involvement of intramuscular interstitial cells of Cajal in neuroeffector transmission in the gastrointestinal tract. J Physiol 576: 675–682, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Won KJ, Sanders KM, Ward SM. Interstitial cells of Cajal mediate mechanosensitive responses in the stomach. Proc Natl Acad Sci USA 102: 14913–14918, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yamato S, Saha JK, Goyal RK. Role of nitric oxide in lower esophageal sphincter relaxation to swallowing. Life Sci 50: 1263–1272, 1992. [DOI] [PubMed] [Google Scholar]