Abstract
Vasoactive factors that regulate splanchnic hemodynamics include nitric oxide, catecholamines, and possibly extracellular nucleosides/nucleotides (adenosine, ATP). CD39/ectonucleoside triphosphate diphosphohydrolase-1 (NTPDase1) is the major vascular ectonucleotidase that hydrolyzes extracellular nucleotides. CD39 activity may be modulated by vascular injury, inflammation, and altered oxygen tension. Altered Cd39 expression by the murine hepatosplanchnic vasculature may impact hemodynamics and portal hypertension (PHT) in vivo. We noted that basal portal pressures (PPs) were comparable in wild-type and Cd39-null mice (n = 9). ATP infusions resulted in increments in PP in wild-type mice, but, in contrast, this significantly decreased in Cd39-null mice (n = 9) post-ATP in a nitric oxide-dependent manner. We then studied Cd39/NTPDase1 deletion in the regulation of portal hemodynamics, vascular integrity, and intestinal permeability in a murine model of PHT. Partial portal vein ligation (PPVL) was performed in Cd39-null (n = 44) and wild-type (n = 23) mice. Sequential measurements obtained after PPVL were indicative of comparable levels of PHT (ranges 14–29 mmHg) in both groups. There was one death in the wild-type group and eight in the Cd39-null group from intestinal bleeding (P = 0.024). Circulatory stasis in the absence of overt portal vein thrombosis, portal congestion, intestinal hemorrhage, and increased permeability were evident in all surviving Cd39-null mice. Deletion of Cd39 results in deleterious outcomes post-PPVL that are associated with significant microcirculatory derangements and major intestinal congestion with hemorrhage mimicking acute mesenteric occlusion. Absent Cd39/NTPDase1 and decreased generation of adenosine in the splanchnic circulation cause heightened vascular permeability and gastrointestinal hemorrhage in PPVL.
Keywords: portal vein stenosis, splanchnic hemodynamics, gastrointestinal bleeding, nitric oxide, ATP, ectonucleotidase
cd39/ectonucleoside triphosphate diphosphohydrolase-1 (E-NTPDase-1; NTPDase1) is the dominant vascular ectonucleotidase that is highly expressed by endothelial cells and vascular smooth muscle cells. This ectoenzyme efficiently hydrolyzes extracellular ATP and ADP to AMP and ultimately forms adenosine via CD73 (26). This ectoenzymatic action blocks extracellular nucleotide-dependent platelet aggregation and mitigates endothelial cell activation (26). However, under conditions of inflammatory stress, CD39 bioactivity is rapidly compromised. Plasma nucleotide phosphohydrolytic cascades are markedly disordered in the Cd39-null mouse, resulting in aberrant activation of purinergic/pyrimidinergic type-2 nucleotide receptors (P2R). This relative failure to generate adenosine within the vasculature results in decreased type-1 purinergic/adenosine receptor activation and disordered vascular inflammatory responses in the Cd39-null mouse (4).
Acute onset of portal hypertension, as occurs with mesenteric vein thrombosis, may be associated with significant derangements in the microcirculation of the gut that may lead to intestinal ischemia and infarction (18, 19). These changes may be also seen in more subacute processes, such as cirrhosis, and are considered secondary to the release of local vasodilators [including nitric oxide (NO) and adenosine among others], leading to a hyperdynamic circulation (21). Portal hypertension induced by partial portal vein ligation (PPVL) is associated with elevated levels of TNF-α and NO production, both of which can result in oxidant and inflammatory injury (18, 22). The formation of reactive oxygen species may also be important in the pathogenesis of these hemodynamic changes (13, 30). In addition, splanchnic vasodilation in the setting of portal hypertension is associated with intestinal edema, hemorrhage, and permeability changes of the small intestine (2, 15).
We previously demonstrated that Cd39 activity modulates platelet activation, thromboregulation, and vascular permeability/leak during intestinal ischemia reperfusion injury in vivo (3, 16). In addition, we also showed that, in Cd39-null mice, total NTPDase activity in mesenteric tissue is markedly decreased (16). Expression of Cd39/NTPDase1 by the hepatosplanchnic endothelium may be a key element in the regulation of the nucleotide ATP (vasoconstrictor at vascular smooth muscle P2X receptors) to nucleoside adenosine (vasodilator) levels and consequently of portal hemodynamics and intestinal permeability in vivo (8, 16). Lastly, although portal hypertension may be associated with increased intestinal edema and permeability, the exact mechanisms how this occurs have not been completely elucidated (34).
Modulation of extracellular nucleotide levels may play an important role in the pathogenesis of intestinal edema, hemorrhage, and permeability in portal hypertension. Therefore, the aim of this investigation was to examine the role of Cd39/NTPDase1 in the regulation of portal vein hemodynamics, vascular integrity, and intestinal permeability in a murine model of portal hypertension of portal vein stenosis induced by PPVL.
MATERIALS AND METHODS
Animals.
Cd39-null (−/−) and control wild-type (+/+) mice were housed and bred in our own facility on the C57BL6 background and have previously been characterized in detail (5, 11). All experimental animal protocols were approved by the Beth Israel Deaconess Medical Center Animal Care and Use Program (Institutional Animal Care and Use Committee).
Animals were housed in a pathogen-free facility accredited by the American Association for Accreditation of Laboratory Animal Care and compliant with the requirements of humane animal care as stipulated by the United States Department of Agriculture and the Department of Health and Human Services. Animals were maintained on a 12-h light/dark cycle and provided with commercially available rodent chow and tap water ad libitum.
Portal pressure measurements.
Measurement of portal pressure (PP) was performed as described previously (17). A catheter from the portal vein was connected to a highly sensitive pressure transducer (PowerLab 4SP-AD Instruments), and pressure was registered on a multichannel recorder. The external zero reference point was placed at the midportion of the animal. PP was measured by inserting a saline-filled 32-gauge microcannula (cat. BS4 59–8305; Harvard Apparatus, Holliston, MA) into the portal vein toward liver. The insertion was achieved by a one-time penetration with no blood leak or suture ligation. The microcannula was connected to a short length of PE-10 tubing, which in turn was joined to PE-50 tubing and connected to a PowerLab pressure transducer. All readings were performed in triplicate, monitored, and saved on a computer. Baseline PPs were measured in −/− and +/+ mice. In the nonoperated state, both −/− and +/+ mice received ATP, at a dose of 100 μM at 50 μl/min for 30 s, and PPs were recorded. Additionally, PPs were measured in both −/− and +/+ mice before, during, and after adenosine 5 μmol/kg per min for 30 s. Similarly, a NO inhibitor, Nω-nitro-l-arginine (l-NNA), at a dose of 24 μg/g was administered to both −/− and +/+ mice (basal state), and PPs were recorded; afterward, ATP was administered, and PPs were recorded again.
Induction of portal hypertension by PPVL.
PPVL was induced in Cd39-null mice (−/−; n = 44) and wild-type (+/+; n = 23) mice, and sequential PPs were measured at defined time points.
PPVL was performed as previously described elsewhere (17). Animals were anesthetized with ketamine hydrochloride (Ketalar, 100 mg/kg; Parke-Davis, Avon, CT) and xylazine (10 mg/kg; Phoenix Pharmaceutical, St. Joseph, MO) as described elsewhere (5, 16). After a midline abdominal incision, the portal vein was identified and freed, and a ligature (silk gut 6–0) was placed around a 27-gauge blunt-tipped needle alongside the portal vein. After removal of the needle there was a calibrated stenosis of the portal vein. The same procedure was performed in sham mice but without ligature of the portal vein. Afterward, animals were housed in plastic cages and allowed free access to food and water. Pressure studies were performed 1–4 days after the operation with absence of portal vein thrombosis confirmed (1, 17).
Cd39/NTPDase1 expression.
Expression of Cd39 on mesenteric and pancreatic tissue samples was performed after induction of PPVL as previously described by Western blot analysis (16).
Splenectomy.
After euthanasia, splenectomy was performed in both −/− and +/+ animals. Spleens were weighed (g) to calculate spleen/body ratio. This ratio is an estimate of the degree of venous congestion in the splanchnic circulation (33).
Intestinal permeability.
Tissues were harvested from euthanized animals at 2 or 4 days after PPVL following permeability studies (−/−; n = 4) and (+/+; n = 4). Intravenous Evans blue dye was given 10 min before blood-flow release (injection of 8 μl 0.5% dye/g body wt). The portal vein was cut open, and perfusion with prewarmed saline via the left cardiac ventricle was performed until portal outflow was clear. Ileal tissue samples were rinsed with 10 ml of prewarmed saline and snap frozen.
The accumulation of Evans Blue dye in tissue specimens is closely linked to the transport of albumin across endothelial surfaces and therefore is considered a measure of vascular permeability (20). Intestinal vascular permeability was quantified by intravenous administration of Evans Blue as described previously and measured spectrophotometrically (20). In brief, weight of snap-frozen jejunal specimens was determined (∼50 mg), and formamide (1.5 ml; Fisher Scientific, Fairlawn, NJ) extraction was performed for 2 h at 55°C. Light adsorption was read in triplicate at a wavelength of 610 nm and subtraction of reference adsorption at 450 nm was performed. Data were expressed as light adsorption [optical density (OD)/g] in arbitrary units per gram of tissue weight.
Statistics.
All data are expressed as means ± SE. Calculations were performed using the SPSS software package (SPSS 11.0, Chicago, IL). For statistical analysis a Wilcoxon test was used as appropriate. Survival rates were calculated according to the Kaplan-Meier-method and compared using the log-rank-test. A P value of <0.05 was considered statistically significant.
RESULTS
Portal hypertension and hemodynamics.
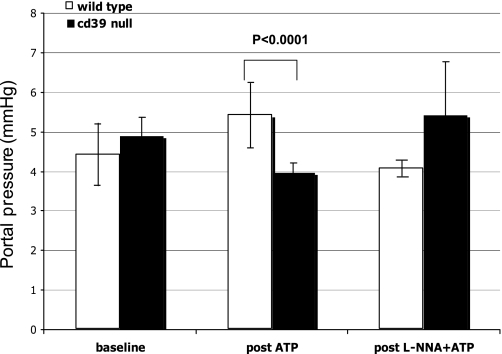
PP recordings after ATP (and prior l-NNA) administration for both −/− (n = 9) and +/+ (n = 9) in quiescent state are shown in Fig. 1. Basal PPs were comparable in both groups. Mean PP before and after ATP infusion was 4.4 ± 0.8 mmHg and 5.4 ± 0.8 mmHg in (+/+) and 4.9 ± 0.5 mmHg and 3.96 ± 0.2 mmHg in −/− animals, respectively (P < 0.0001) (Fig. 1). Under basal conditions, ATP administration alone had minimal effects on PP in +/+ animals but did drop pressures in −/− mice. The effects of adenosine on PP were recorded before and after administration for both −/− (n = 4) and +/+ mice (n = 4), in quiescent state. Wild-type and Cd39-null mice had comparable changes in PP (0.69 ± 0.23 vs. 0.42 ± 0.03 mmHg; P = 0.36) although −/− mice exhibited accelerated responses (19.5 ± 1.5 s) vs. +/+ (86 ± 13 s; P = 0.005). l-NNA administration followed by ATP altered PP in +/+ animals (n = 6) from 4.4. ± 0.8 mmHg to 4.1 ± 0.2 mmHg (P = 0.163). In contrast, PP transiently dropped after l-NNA in −/− animals (n = 5). More importantly, however, this blockade of NO release preceding ATP administration in −/− mice had the effect of increasing PP from 4.9 ± 0.5 mmHg to 5.4 ± 1.3 mmHg (P = 0.137) (Fig. 1).
Fig. 1.
With portal pressure (PP) in quiescent state, baseline pressures were comparable in both wild-type and CD39-null mice. Mean PP before and after ATP infusion was 4.4 ± 0.8 mmHg and 5.4 ± 0.8 mmHg in wild-type and 4.9 ± 0.5 mmHg and 3.96 ± 0.2 mmHg in Cd39-null animals, respectively (P < 0.0001). In wild-type, Nω-nitro-l-arginine (l-NNA) administration after ATP did not significantly alter PP, but PP transiently dropped after l-NNA in Cd39-null animals. Nitric oxide blockade preceding ATP administration in Cd39-null mice increased from 4.9 ± 0.5 mmHg to 5.4 ± 1.3 mmHg (P = 0.137).
A total of 23 +/+ mice and 44 −/− mice were studied for PPVL. At baseline and days 1–4 post-PPVL, there were no significant differences in PP readings between the two groups. Median PP of +/+ was 3.9 ± 0.1 mmHg and the median value of −/− was 4.2 ± 0.1 mmHg; P = 0.939. Mean values were highest on day 2, 20.2 ± 2.8 mmHg vs. 21.8 ± 1.5 mmHg; P = 0.663, (Fig. 2). Cd39-null mice, however, developed more pronounced splenomegaly post-PPVL. The spleen/body ratio of both types of animals is shown in Fig. 2. The spleen/body ratio was significantly higher in the −/− mice at day 4 (0.75 ± 0.1 vs. 0.54 ± 0.1; P < 0.005).
Fig. 2.
Sequential PP measurements after partial portal vein ligation (PPVL) in wild-type and Cd39-null animals days 0–4. There were no significant differences in PP readings between the two groups. Median PP of +/+ was 3.9 ± 0.1 mmHg, and the median value of −/− was 4.2 ± 0.1 mmHg; P = 0.939 (top). The spleen/body ratio of wild-type and Cd39-null animals, days 0–4 after PPVL, was statistically and significantly higher (*P = <0.005) in the Cd39-null mice at day 4 (bottom).
Survival.
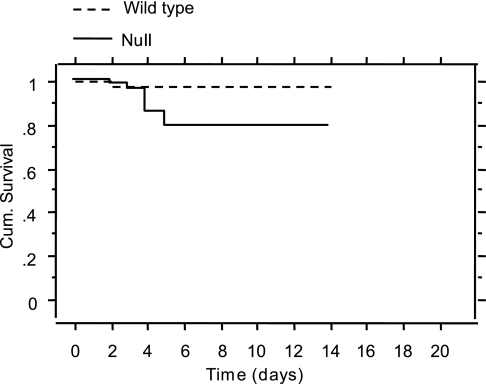
Analyses revealed increased mortality post-PPVL in −/− animals compared with +/+ (Fig. 3). There was one death in +/+ from portal thrombosis and eight deaths in the −/− group all from intestinal bleeding in absence of portal vein thrombosis (P = 0.024).
Fig. 3.
Cumulative (Cum.) survival after PPVL in Cd39-null and wild-type animals. There was significantly increased mortality post-PPVL in Cd39 −/− animals compared with wild-type (P = 0.024).
Cd39/NTPDase1 expression.
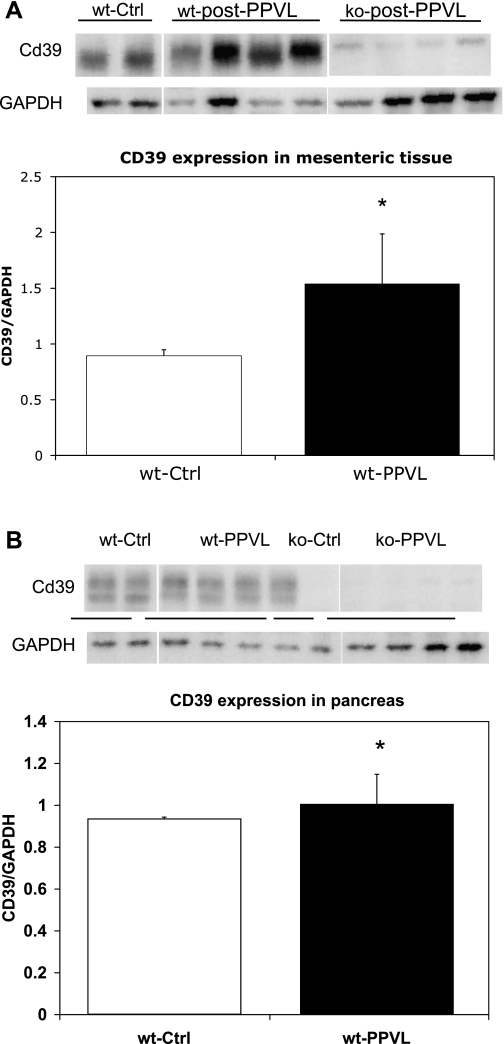
Cd39/NTPDase1 expression in pancreatic and mesenteric tissue revealed an increase in the Cd39 expression normalized for GAPDH expression for wild-type animals (n = 5) (Fig. 4, A and B). In Cd39 −/− animals (n = 5), as expected there was no expression of Cd39/NTPDase1 after PPVL (Fig. 4, A and B). Substantive Cd39/NTPDase1 upregulation in mesenteric tissue was observed after PPVL in the −/− group (n = 5) compared with +/+ group (n = 5) (normalized ratios; P = 0.029).
Fig. 4.
A and B: substantive Cd39/ectonucleoside triphosphate diphosphohydrolase-1 (NTPDase1) normalized for GAPDH expression in mesenteric (top) pancreatic (bottom) tissue after PPVL in wild-type and Cd39-null animals. There was no expression in CD39-null animals. Asterisks denote statistically significant differences at P < 0.05. wt, wild-type; Ctrl, control; ko, knockout.
Intestinal permeability.
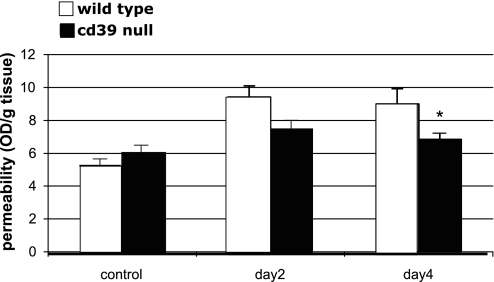
Significant intestinal and microvascular changes in the splanchnic and mesenteric circulation were noticed in −/− after PPVL (days 2–6). There was severe congestion, edema, and tissue hemorrhage of the intestines in all −/− animals (Fig. 5). Evidence of intestinal hemorrhage was evident in a small proportion of −/− animals (n = 2). Intestinal and vascular permeability changes with Evans blue infusion were significantly more pronounced in −/− than in +/+. At baseline (+/+ 6.1 OD/g vs. −/− 5.3 OD/g, P = 0.214), there was no clear difference. At day 2 post-PPVL there was a trend toward differences (+/+ 7.8 OD/g vs. −/− 9.4 OD/g, P = 0.054), and at day 4 there was a significant difference (+/+ 6.5 OD/g vs. −/− 9.0 OD/g, P = 0.025) (Fig. 6).
Fig. 5.
Severe congestion, edema, and tissue hemorrhage of the intestines in Cd39-null animals (right) compared with normal appearing intestines on wild-type animals (left).
Fig. 6.
Intestinal and vascular permeability changes with Evans blue infusion were significantly more pronounced in CD39-null than in wild-type animals. At day 4 there was a significant difference between wild-type 6.5 OD (optical density)/g and Cd39-null animals 9.0 OD/g, *P = 0.025.
DISCUSSION
In portal hypertension, one of the main factors responsible for a sustained increase in portal blood inflow is the presence of splanchnic vasodilation that occurs secondary to the excessive release of putative vasodilators such as NO, adenosine, substance P, and endocannabinoids, among others (7, 14, 18, 24). One of the major deleterious consequences of portal hypertension in humans is the development of gastrointestinal bleeding attributable to gastroesophageal varices. However, local intestinal circulatory changes likely also contribute to bleeding in portal hypertension. To date, the hemodynamic effects of locally generated adenosine (a byproduct of ATP) in an animal model of portal hypertension had not been studied in depth. In this study, we found that Cd39/NTPDase1 is an important vascular factor that plays a key role in the maintenance of vascular integrity and intestinal permeability of animals with PPVL-induced acute portal hypertension.
Extracellular ATP and the phosphohydrolytic products inclusive of adenosine regulate multiple inflammatory pathways. Hypoxia and ischemia, which may occur in the setting of mesenteric occlusion and/or portal hypertensive gastrointestinal bleeding, may reconstitute vascular CD39 immunoreactivity via transcriptional upregulation. Specifically, the human CD39 gene promoter has an important region that induces hypoxia with a critical role for transcription-factor Sp1 associated with hypoxia induction (9). Other pathways may further enhance extracellular adenosine effects, for example netrin-1. It has been shown that netrin-1 attenuates transepithelial leukocyte migration by engaging neutrophil-expressed A2B adenosine receptor. Hypoxia-inducible factor-1α-dependent induction of netrin-1 may be considered to attenuate hypoxia-elicited inflammation (27).
Arterial hemodynamic measurements in Cd39-null mice have shown these mutant mice to be mildly hypertensive and to exhibit far more substantial drops in systemic blood pressure with ATP infusion than matched wild-type animals (10). Our study reveals comparable features with respect to ATP infusion in the portal venous system. In wild-type animals, ATP infusion did not cause any significant change in PP, and l-NNA only caused a minor drop in PP in these animals. In contrast, Cd39-null mice respond to ATP by a fall in PP, likely secondary to endothelial P2Y-mediated release of NO (32). Similarly, adenosine did not induce significant differences in PP in either of the groups. As this is born out post-l-NNA, ATP generates a substantial increment in PPs, likely as a consequence of vascular smooth muscle cell unbalanced P2X-stimulated vasoconstriction (29, 32). These data validate the previously recorded aberrant ATP-mediated hemodynamic effects in Cd39-null mice (10).
PP measurements after PPVL were comparable among quiescent wild-type and Cd39-null animals. Elevations of PP after PPVL in Cd39-null and wild-type animals were also similar, suggesting that Cd39 ectonucleotidase probably does not play a direct role in portal vascular hemodynamics in the portal hypertensive state including a possible role in the development of angiogenesis in portal hypertension, which has been established in CCl4-induced cirrhosis (12). Substantive derangements in the portal microcirculation and vascular integrity of the splanchnic vasculature post-PVL in Cd39-null mice leading to intestinal hemorrhage were associated with increased mortality post-PVL. Increased vascular permeability, a function of altered cAMP, and decreased adenosinergic effects may play a role (25). In addition, increased vascular permeability has been shown to occur in animals and humans with cirrhosis and portal hypertension (15, 23). Proposed mechanisms include structural and functional alterations in the intestinal mucosa that may increase permeability to bacteria, thereby facilitating bacterial translocation, that is, passage of bacteria from the lumen across the mucosa into the mesenteric lymph nodes.
Bacterial translocation, which may occur in acute stress conditions such as trauma, burns, hemorrhagic shock, or liver failure, may further cause increase in cytokine and NO release (31). These alterations are at least partly due to oxidative stress and increased inflammation (31). Previous data have demonstrated that intestinal permeability is increased in patients with cirrhosis (6). This derangement may increase with the degree of liver failure and be associated with significant complications of portal hypertension such as infection and bleeding (28). Our findings demonstrate that intestinal vascular permeability is significantly increased in Cd39-null mice (to day 4). This increased permeability occurred in tandem with significant intestinal edema and bleeding, leading to increased mortality in Cd39-null mice after PPVL, indicating that the Cd39/NTPDase1 and extracellular nucleotides play an important role in vascular integrity and permeability in the setting of portal hypertension.
In conclusion, Cd39-deficient animals develop deleterious outcomes post-PPVL that are associated with severe splanchnic microcirculatory derangements that contribute to gastrointestinal bleeding and the deleterious effects of portal hypertension. Differential expression of Cd39/NTPDase1 and consequent generation of adenosine in the splanchnic circulation may have important implications for vascular permeability and gastrointestinal hemorrhage in the setting of acute portal hypertension.
GRANTS
This work was supported by National Institutes of Health Grant NIH HL076540.
Acknowledgments
The authors thank Dr. Juan G. Abraldes for insightful comments.
REFERENCES
- 1.Albillos A, Colombato LA, Groszmann RJ. Vasodilatation and sodium retention in prehepatic portal hypertension. Gastroenterology 102: 931–935, 1992. [DOI] [PubMed] [Google Scholar]
- 2.Aller MA, Vara E, Garcia C, Palma MD, Arias JL, Nava MP, Arias J. Proinflammatory liver and anti-inflammatory intestinal mediators involved in portal hypertensive rats. Mediators Inflamm 2005: 101–111, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Atkinson B, Dwyer K, Enjyoji K, Robson SC. Ecto-nucleotidases of the CD39/NTPDase family modulate platelet activation and thrombus formation: potential as therapeutic targets. Blood Cells Mol Dis 36: 217–222, 2006. [DOI] [PubMed] [Google Scholar]
- 4.Beldi G, Enjyoji K, Wu Y, Miller L, Banz Y, Sun X, Robson SC. The role of purinergic signaling in the liver and in transplantation: effects of extracellular nucleotides on hepatic graft vascular injury, rejection and metabolism. Front Biosci 13: 2588–2603, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Beldi G, Wu Y, Sun X, Imai M, Enjyoji K, Csizmadia E, Candinas D, Erb L, Robson SC. Regulated catalysis of extracellular nucleotides by vascular CD39/ENTPD1 is required for liver regeneration. Gastroenterology 135: 1751–1760, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Campillo B, Pernet P, Bories PN, Richardet JP, Devanlay M, Aussel C. Intestinal permeability in liver cirrhosis: relationship with severe septic complications. Eur J Gastroenterol Hepatol 11: 755–759, 1999. [DOI] [PubMed] [Google Scholar]
- 7.Cardenas A, Lowe R, Oh S, Bodkin S, Kenney T, Lamorte WW, Afdhal NH. Hemodynamic effects of substance P and its receptor antagonist RP67580 in anesthetized rats with carbon tetrachloride-induced cirrhosis. Scand J Gastroenterol 43: 328–333, 2008. [DOI] [PubMed] [Google Scholar]
- 8.Dranoff JA, Kruglov EA, Robson SC, Braun N, Zimmermann H, Sevigny J. The ecto-nucleoside triphosphate diphosphohydrolase NTPDase2/CD39L1 is expressed in a novel functional compartment within the liver. Hepatology 36: 1135–1144, 2002. [DOI] [PubMed] [Google Scholar]
- 9.Eltzschig HK, Kohler D, Eckle T, Kong T, Robson SC, Colgan SP. Central role of Sp1-regulated CD39 in hypoxia/ischemia protection. Blood 113: 224–232, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Enjyoji K, Kotani K, Thukral C, Blumel B, Sun X, Wu Y, Imai M, Friedman D, Csizmadia E, Bleibel W, Kahn BB, Robson SC. Deletion of cd39/entpd1 results in hepatic insulin resistance. Diabetes 57: 2311–2320, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Enjyoji K, Sevigny J, Lin Y, Frenette PS, Christie PD, Esch JS 2nd, Imai M, Edelberg JM, Rayburn H, Lech M, Beeler DL, Csizmadia E, Wagner DD, Robson SC, Rosenberg RD. Targeted disruption of cd39/ATP diphosphohydrolase results in disordered hemostasis and thromboregulation. Nat Med 5: 1010–1017, 1999. [DOI] [PubMed] [Google Scholar]
- 12.Fernandez M, Vizzutti F, Garcia-Pagan JC, Rodes J, Bosch J. Anti-VEGF receptor-2 monoclonal antibody prevents portal-systemic collateral vessel formation in portal hypertensive mice. Gastroenterology 126: 886–894, 2004. [DOI] [PubMed] [Google Scholar]
- 13.Fernando B, Marley R, Holt S, Anand R, Harry D, Sanderson P, Smith R, Hamilton G, Moore K. N-acetylcysteine prevents development of the hyperdynamic circulation in the portal hypertensive rat. Hepatology 28: 689–694, 1998. [DOI] [PubMed] [Google Scholar]
- 14.Forrest EH, Bouchier IA, Hayes PC. Acute effect of low dose theophylline on the circulatory disturbances of cirrhosis. Gut 40: 139–144, 1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Geerts AM, De Vriese AS, Vanheule E, Van Vlierberghe H, Mortier S, Cheung KJ, Demetter P, Lameire N, De Vos M, Colle I. Increased angiogenesis and permeability in the mesenteric microvasculature of rats with cirrhosis and portal hypertension: an in vivo study. Liver Int 26: 889–898, 2006. [DOI] [PubMed] [Google Scholar]
- 16.Guckelberger O, Sun XF, Sevigny J, Imai M, Kaczmarek E, Enjyoji K, Kruskal JB, Robson SC. Beneficial effects of CD39/ecto-nucleoside triphosphate diphosphohydrolase-1 in murine intestinal ischemia-reperfusion injury. Thromb Haemost 91: 576–586, 2004. [DOI] [PubMed] [Google Scholar]
- 17.Iwakiri Y, Cadelina G, Sessa WC, Groszmann RJ. Mice with targeted deletion of eNOS develop hyperdynamic circulation associated with portal hypertension. Am J Physiol Gastrointest Liver Physiol 283: G1074–G1081, 2002. [DOI] [PubMed] [Google Scholar]
- 18.Iwakiri Y, Groszmann RJ. The hyperdynamic circulation of chronic liver diseases: from the patient to the molecule. Hepatology 43: S121–S131, 2006. [DOI] [PubMed] [Google Scholar]
- 19.Kumar S, Sarr MG, Kamath PS. Mesenteric venous thrombosis. N Engl J Med 345: 1683–1688, 2001. [DOI] [PubMed] [Google Scholar]
- 20.Lange S, Delbro DS, Jennische E. Evans blue permeation of intestinal mucosa in the rat. Scand J Gastroenterol 29: 38–46, 1994. [DOI] [PubMed] [Google Scholar]
- 21.Lee SS, Chilton EL, Pak JM. Adenosine receptor blockade reduces splanchnic hyperemia in cirrhotic rats. Hepatology 15: 1107–1111, 1992. [DOI] [PubMed] [Google Scholar]
- 22.Lopez-Talavera JC, Merrill WW, Groszmann RJ. Tumor necrosis factor alpha: a major contributor to the hyperdynamic circulation in prehepatic portal-hypertensive rats. Gastroenterology 108: 761–767, 1995. [DOI] [PubMed] [Google Scholar]
- 23.Melgar-Lesmes P, Tugues S, Ros J, Fernandez-Varo G, Morales-Ruiz M, Rodes J, Jimenez W. Vascular endothelial growth factor and angiopoietin-2 play a major role in the pathogenesis of vascular leakage in cirrhotic rats. Gut 58: 285–292, 2009. [DOI] [PubMed] [Google Scholar]
- 24.Moezi L, Gaskari SA, Lee SS. Endocannabinoids and liver disease. V. Endocannabinoids as mediators of vascular and cardiac abnormalities in cirrhosis. Am J Physiol Gastrointest Liver Physiol 295: G649–G653, 2008. [DOI] [PubMed] [Google Scholar]
- 25.Narravula S, Lennon PF, Mueller BU, Colgan SP. Regulation of endothelial CD73 by adenosine: paracrine pathway for enhanced endothelial barrier function. J Immunol 165: 5262–5268, 2000. [DOI] [PubMed] [Google Scholar]
- 26.Robson SC, Sevigny J, Zimmermann H. The E-NTPDase family of ectonucleotidases: structure function relationships and pathophysiological significance. Purinergic Signal 2: 409–430, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rosenberger P, Schwab JM, Mirakaj V, Masekowsky E, Mager A, Morote-Garcia JC, Unertl K, Eltzschig HK. Hypoxia-inducible factor-dependent induction of netrin-1 dampens inflammation caused by hypoxia. Nat Immunol 10: 195–202, 2009. [DOI] [PubMed] [Google Scholar]
- 28.Tandon P, Garcia-Tsao G. Bacterial infections, sepsis, and multiorgan failure in cirrhosis. Semin Liver Dis 28: 26–42, 2008. [DOI] [PubMed] [Google Scholar]
- 29.Vial C, Evans RJ. P2X(1) receptor-deficient mice establish the native P2X receptor and a P2Y6-like receptor in arteries. Mol Pharmacol 62: 1438–1445, 2002. [DOI] [PubMed] [Google Scholar]
- 30.Wang JJ, Gao GW, Gao RZ, Liu CA, Ding X, Yao ZX. Effects of tumor necrosis factor, endothelin and nitric oxide on hyperdynamic circulation of rats with acute and chronic portal hypertension. World J Gastroenterol 10: 689–693, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wiest R, Garcia-Tsao G. Bacterial translocation (BT) in cirrhosis. Hepatology 41: 422–433, 2005. [DOI] [PubMed] [Google Scholar]
- 32.Wihlborg AK, Malmsjo M, Eyjolfsson A, Gustafsson R, Jacobson K, Erlinge D. Extracellular nucleotides induce vasodilatation in human arteries via prostaglandins, nitric oxide and endothelium-derived hyperpolarising factor. Br J Pharmacol 138: 1451–1458, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yamaguchi S, Kawanaka H, Yoshida D, Maehara Y, Hashizume M. Splenic hemodynamics and decreased endothelial nitric oxide synthase in the spleen of rats with liver cirrhosis. Life Sci 80: 2036–2044, 2007. [DOI] [PubMed] [Google Scholar]
- 34.Yao GX, Shen ZY, Xue XB, Yang Z. Intestinal permeability in rats with CCl4-induced portal hypertension. World J Gastroenterol 12: 479–481, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]