Abstract
This study was designed to investigate the role of matrix metalloproteinases (MMPs) and tissue inhibitors of MMPs (TIMPs) in the reabsorption of neovessels in collagen gel cultures of rat and mouse aortic rings. Aortic angiogenesis was associated with collagen lysis and production of the matrix-degrading enzymes MMP-2, MMP-9, and membrane-type MMP (MT1-MMP, or MMP-14). Vascular growth and regression were not affected by disruption of MMP-2 or MMP-9. In addition, no effect on vascular regression was observed by blocking plasmin, a protease implicated in the activation of MMPs, with ɛ-aminocaproic acid or by adding plasminogen, which caused a modest increase in vascular proliferation. Conversely, angiogenesis was blocked and vessels stabilized by inhibiting MT1-MMP with neutralizing antibodies, TIMP-2, TIMP-3, or TIMP-4. TIMP-1, which blocks MMP-2 and MMP-9 but is a poor inhibitor of MT1-MMP, had no antiangiogenic effect. However, TIMP-1 prolonged the survival of neovessels following angiogenesis. Vascular regression was accelerated in aortic cultures from TIMP-1- and TIMP-2-deficient mice. The vascular survival effect of anti-MT1-MMP antibodies and TIMPs with MT1-MMP inhibitory activity was associated with complete inhibition of collagen lysis. In contrast, TIMP-1 had no anticollagenolytic effect. These results indicate that MT1-MMP plays a critical role not only in angiogenesis but also in vascular regression and demonstrate that TIMPs with anti-MT1-MMP activity have opposite effects on angiogenic outcomes depending on the stage of the angiogenic process. This study also suggests the existence of a TIMP-1-mediated alternate pathway of vascular survival that is unrelated to MT1-MMP inhibitory activity.
Keywords: neovascularization, matrix metalloproteinase-14, collagen, endothelial cells, aorta, rarefaction, membrane type 1 matrix metalloproteinase, tissue inhibitors of matrix metalloproteinases
angiogenesis, the formation of neovessels from preexisting blood vessels, is a multistep process characterized by endothelial migration and proliferation, capillary tube formation, and pericyte recruitment (19). To penetrate the basement membrane and invade the interstitial matrix, endothelial cells and pericytes produce a variety of proteolytic enzymes with different substrate specificities (46). Among these, matrix metalloproteinases (MMPs) have been recognized as critical regulators of the angiogenic process (18, 46, 49).
MMPs are zinc-dependent endopeptidases that are modulated at multiple levels including transcription, secretion, proenzyme activation, and functional blockade by tissue inhibitors of metalloproteinases (TIMPs) (5, 20). The MMP family comprises at least 24 enzymes, but particularly critical in the regulation of angiogenesis is a subgroup of MMPs known as membrane-type MMPs (MT-MMPs) (14, 44). Because they are tethered to the membrane, MT-MMPs can selectively focus matrix-degrading activity around filopodia and invadopodia of migrating cells, where matrix breakdown is needed for forward extension of cytoplasmic processes (13). MT1-MMP (MMP-14), the most widely studied of the MT-MMPs, accomplishes this task by directly degrading interstitial collagen and other matrix molecules (14, 39). MT1-MMP also activates pro-MMP-2, a gelatinase capable of breaking down basement membrane molecules (56). MT1-MMP biological activity is not limited to extracellular matrix (ECM) proteolysis, since MT1-MMP interacts with integrin receptors, which regulate endothelial adhesion to the ECM (4, 23), and degrades tissue transglutaminase, a cross-linking enzyme that stabilizes matrix molecules and promotes integrin-dependent adhesion and spreading of cells (6).
The activity of MMPs including MT1-MMP is counteracted by TIMPs (5). TIMPs have the capacity to inhibit angiogenic sprouting but also ensure that excessive degradation of the ECM does not lead to loss of adhesive substrates that are critical for endothelial cell survival. Four TIMPs have been currently isolated and characterized: TIMP-1, TIMP-2, TIMP-3, and TIMP-4, which differ in solubility, interaction with different MMPs, and regulation of gene expression. TIMP-1, TIMP-2, and TIMP-4 are soluble, whereas TIMP-3 is closely associated with the ECM (5, 34). TIMP-1 forms a preferential complex with pro-MMP-9, whereas TIMP-2 binds primarily to and facilitates the MT1-MMP-mediated activation of pro-MMP-2. TIMP-4 binds to pro-MMP-2 but, unlike TIMP-2, is not involved in MMP-2 activation by MT1-MMP. TIMP-3 has the capacity to associate with both pro-MMP-2 and pro-MMP-9. TIMP-2, TIMP-3, and TIMP-4 are strong inhibitors of MT1-MMP, whereas TIMP-1 has no significant effect on MT1-MMP function (9).
Most studies on the role of MMPs and TIMPs in angiogenesis have focused on the early stages of the angiogenic process (1, 14, 22, 25, 32). Formation of neovessels is, however, followed by vascular remodeling, which results in the stabilization of some vessels and the regression of others. Vascular regression is required for proper embryonic development (27), postnatal retinal maturation (7), corpus luteum involution (38), and granulation tissue reabsorption (16). Conversely, vascular stabilization contributes to the progression of angiogenesis-dependent disorders such as cancer, diabetic retinopathy, complicated atherosclerosis, and rheumatoid arthritis. Although outcomes of angiogenic responses depend on neovessel survival, the mechanisms regulating vascular regression or stabilization and the role of MMPs and TIMPs in these processes have not been fully investigated and remain poorly understood.
The aortic ring model of angiogenesis reproduces ex vivo all the stages of the angiogenic process, including vascular regression. Angiogenesis in this system is regulated by MMP activity (61), but the role of specific MMPs and TIMPs in the reabsorption or survival of vessels following angiogenesis has not been characterized. In this article we present data demonstrating that in aortic cultures, MT1-MMP is an important regulator not only of angiogenesis but also of vascular regression. We demonstrate that TIMP-1, TIMP-2, TIMP-3, and TIMP-4 are all capable of inhibiting the reabsorption of vessels following angiogenesis but that only TIMP-2, TIMP-3, and TIMP-4, which block MT1-MMP, have the capacity to inhibit angiogenesis. TIMPs with anti-MT1-MMP activity are more effective than TIMP-1 in promoting vascular survival and have the added capacity to inhibit collagen lysis. Thus MT1-MMP-mediated degradation of the ECM occurs throughout the angiogenic process and continues after vessels have formed, contributing to their reabsorption. The dual role of MT1-MMP and TIMPs in vessel growth and regression underscores the context-dependent nature of angiogenic regulation by MMPs. The complexity of this system represents a challenge for the design of therapeutic strategies aimed at treating angiogenesis-dependent disorders through inhibition of MMP activity.
MATERIALS AND METHODS
Materials.
Endothelial cell basal medium (EBM) was obtained from Lonza (Walkersville, MD). Mouse anti-human-MT1-MMP monoclonal antibody (MAB3317, clone 113-5B7), which cross-reacts with rat MT1-MMP, was purchased from Millipore Biosciences (Temecula, CA). LEM-1/58 and LEM-2/15 monoclonal antibodies directed against the MT1-MMP catalytic domain were a gift from Dr. A. G. Arroyo (University of Madrid, Madrid, Spain) (22). Recombinant human TIMPs and control nonimmune mouse IgGs were obtained from R&D Systems (Minneapolis, MN). Synthetic MMP inhibitor GM6001 was purchased from EMD Biosciences (Calbiochem; La Jolla, CA). Alexa Fluor 568-conjugated Griffonia simplicifolia isolectin-B4 (IB4) and Alexa Fluor 488-conjugated goat anti-mouse IgG secondary antibodies were obtained from Molecular Probes (Eugene, OR). We obtained 10% neutral buffered formalin from Biochemical Sciences (Swedesboro, NJ). Collagen was isolated from rat tails as described previously (40).
Collagen gel culture of rat and mouse aortas.
All animal procedures were approved by the Veterans Affairs Puget Sound Health Care System Institutional Animal Care and Use Committee and were performed in accordance with the Guide for the Care and Use of Laboratory Animals, published by the National Institutes of Health (NIH Publication No. 85-23, Revised 1996). Thoracic aortas were dissected from euthanized 2-mo-old Fischer 344 male rats (Harlan, Indianapolis, IN), cleaned of fibroadipose tissue and blood, and serially cross-sectioned into ∼1- to 2-mm rings, as described previously (43). For studies with mouse rings, genetically modified animals with disrupted genes were obtained from the following investigators: B. T. Baxter (University of Nebraska, Lincoln, NE), MMP-2−/− (31); M. Reidy (University of Washington, Seattle, WA), MMP-9−/− (55); A. Eddy (Children's Hospital, Seattle, WA), TIMP-1−/− (52); and D. Madtes (University of Washington), TIMP-2−/− (56). Aortas from age-matched control animals were used as controls in each experiment. Rat aortic rings were embedded in collagen gels cast in four-well dishes (43, 63). Mouse aortic cultures were prepared in 96-well dishes as described previously (2). All aortic rings were cultured in serum-free medium with or without reagents of interest. Cultures were maintained at 37°C and 5% CO2 in a humidified incubator, and medium was replaced three times a week starting from day 3. For MMP inhibition studies, GM6001 was dissolved in dimethyl sulfoxide (DMSO; Sigma, St. Louis, MO) and added to the culture medium at a final concentration of 20 μM. Control cultures received an equivalent amount of DMSO. For antibody blocking experiments, azide-free antibodies were added to serum-free EBM at a final concentration of 10–20 μg/ml. Control cultures received an equivalent concentration of nonimmune IgG. Recombinant TIMPs were added to cultures at a final concentration of 10 μg/ml.
Inhibition of plasmin was obtained by treating cultures with 300 μg/ml ɛ-aminocaproic acid (EACA; Sigma). This dose of EACA was previously shown to completely block the fibrinolytic activity of aortic rings (43). Recombinant rat plasminogen (American Diagnostica, Stamford, CT) was added to the culture medium at concentrations ranging from 10 to 200 ng/ml.
Measurement of angiogenesis and collagen lysis.
The angiogenic response of living aortic cultures was measured by counting the number of neovessels over time (43). Images of live or formalin fixed cultures were captured with an Olympus MagnaFire S99800 digital camera (Olympus American, Melville, NY) mounted on a Leitz Laborlux K microscope or with a 35-mm camera mounted on an IMT-inverted Olympus microscope. Collagen breakdown was quantitated by using image analysis (Bioquant, Nashville, TN) to measure the halo of periaortic collagen lysis during vascular regression.
Confocal microscopy.
Expression of MT1-MMP in angiogenic outgrowths was evaluated in whole mount preparations of formalin-fixed cultures by using double-immunofluorescence staining followed by confocal microscopy as described previously (63). Double staining was obtained by reacting the cultures with the endothelial cell marker IB4 (Molecular Probes) and the anti-MT1-MMP antibody (MAB3317; Millipore Biosciences). Briefly, cultures were blocked with 5% goat serum in Tris-buffered saline (pH 8.0) containing 0.1% Tween 20 (Sigma) for 1 h at room temperature and reacted for 1 h with mouse anti-MT1-MMP monoclonal antibody, followed by incubation for 1 h with a cocktail of Alexa Fluor 568-conjugated IB4 and Alexa Fluor 488-conjugated goat anti-mouse secondary antibody. Samples were mounted in Gelvatol (Monsanto, St. Louis, MO) on glass slides and examined with a Leica TCS-SP laser scanning microscope. Confocal images were obtained by Z-plane analysis followed by projection and overlay using Leica software.
Western blotting.
Freshly isolated rat aortas were snap frozen in liquid nitrogen, crushed in a Biopulverizer (Biospec Products, Bartlesville, OK), and solubilized on ice with 100 μl of ice-cold lysis buffer (50 mM Tris·HCl, pH 7.5, 1% Triton X-100, 150 mM NaCl, 10 mM EDTA, 2 mM EGTA, 0.02% sodium azide, 50 mM NaF, and 2 mM vanadate, supplemented with protease inhibitors). The resulting slurry was sonicated for 10 s on ice and centrifuged at 1,000 g for 10 min at 4°C. Protein concentration of the supernatant was measured with the bicinchoninic acid assay (BCA; Pierce, Rockford, IL). Samples were boiled for 10 min in SDS-PAGE sample buffer (62.5 mM Tris·HCl, pH 6.8, 10% glycerol, 2% SDS, 0.65 mM DTT, 0.06% bromophenol blue, and 5% 2-mercaptoethanol), and 25 μg of protein were loaded in each lane of a 4–20% gradient polyacrylamide gel. Proteins were transferred to nylon membranes and probed with monoclonal mouse-anti-human MT1-MMP antibody (MAB3317; Millipore Biosciences). Specific antibody binding was detected with the enhanced chemiluminescence system (ECL; Amersham, Piscataway, NJ). Blots were stripped and reprobed with an anti-β-actin antibody (Sigma) to evaluate protein loading.
Reverse transcriptase-polymerase chain reaction.
Total RNA was isolated from three to six rat aortic rings after snap freezing in liquid nitrogen and manual pulverization. RNA was extracted with Trizol reagent (Invitrogen) followed by further purification with the RNeasy micro kit (Qiagen, Valencia, CA). Total RNA was dissolved in a final volume of 13 μl of diethyl pyrocarbonate-treated water and examined for quality with a Bioanalyzer 2100 (Agilent, Palo Alto, CA). cDNA templates for standard and real-time PCR were synthesized by reverse transcription. Briefly, 100 ng of total RNA were incubated for 5 min at 95°C with random hexamer primers (Promega, Madison, WI), cooled to 42°C, and incubated for 1 h in the presence of 50 units of SuperScript III (Invitrogen), 5 mM each dNTP (Promega), 50 mM DTT, 20 units of RNase inhibitor (Promega), and 1× first-strand buffer (Invitrogen) in a final volume of 50 μl. Duplicate reactions were carried out lacking the SuperScript enzyme to act as negative controls. Standard PCR was carried out using 2 μl of the RT reactions as template with 1.5 mM MgCl2, 50 mM KCl, 10 pM each primer, and 1 unit of Taq polymerase (Promega) in a final reaction volume of 20 μl. The following primers were used: MT1-MMP, 5′-GATACCCAATCCCATTGGCCA-3′ and 5′-CCATTGGGCATCCAGAAGAGAGC-3′; and GAPDH, 5′-CCTCTGGAAAGCTGTGGCGT-3′ and 5′-TTGGAGGCCATGTAGGCCAT-3′. Thirty cycles of PCR were carried out, with each cycle consisting of 45 s at 95°C, 45 s at 58°C, and 1 min at 72°C. PCR products were visualized on 1.2% agarose gels.
Real-time PCR analysis.
To examine the relative expression of MT1-MMP at multiple time points between days 3 and 14 of the angiogenic response, we used the two-step quantitative real-time RT-PCR (qRT-PCR) SYBR green method (Applied Biosystems, Foster City, CA). Random-primed reverse transcription was carried out as described above using total RNA isolated from aortic cultures. Ten to twelve cultures were used for each time point analyzed; 1/50 of the final RT reaction was used as template in qRT-PCR reactions containing oligonucleotide primers (Invitrogen). The following primers were used: MT1-MMP, 5′-GGATACCCAATGCCCATTGGCCA-3′ and 5′-CCATTGGGCATCCAGAAGAGAGC-3′; and β-actin, 5′-GGGAAATCGTGCGTGACATT-3′ and 5′-GCGGCAGTGGCCATCTC-3′. Melt curve analysis showed that each primer set produced a single product. Relative quantification was carried out on an ABI 7000 thermal cycler (Applied Biosystems) in reactions containing 1× Universal SYBR green master mix (Applied Biosystems), 300 nM forward and reverse primers, and 5 μl of cDNA template. The PCR cycling profile was 2 min at 50°C and 10 min at 95°C, followed by 35 cycles of 95°C for 15 s and 60°C for 1 min. Each PCR reaction was carried out at least in triplicate. qRT-PCR data were analyzed with Prizm software (Applied Biosystems). Relative ratios of fluorescent intensities of products from each treatment group were calculated using the 2−ΔΔCt method (35). The mRNA expression levels of each sample were normalized to the levels of β-actin expression measured in the same sample. The relative expression of MT1-MMP at each time point was compared with expression at day 3, which was set to 1.
Electron microscopy.
For ultrastructural studies, collagen gel cultures were fixed in 2.5% glutaraldehyde-0.1 M Na-cacodylate (pH 7.4) and processed for embedding in EPON-812 (Ted Pella, Redding, CA). Thin sections were stained with uranyl acetate and lead citrate and examined with a transmission electron microscope (Jeol, Peabody, MA). Collagen fibril density around neovessels was quantified with the histogram function of Adobe Photoshop (62) by counting pixels representing fibrillar collagen in 10 random 10,000 pixel2 fields for each treatment group.
RESULTS
Disruption of MMP-2 or MMP-9 genes has no effect on angiogenic sprouting and vascular regression in the aortic ring model.
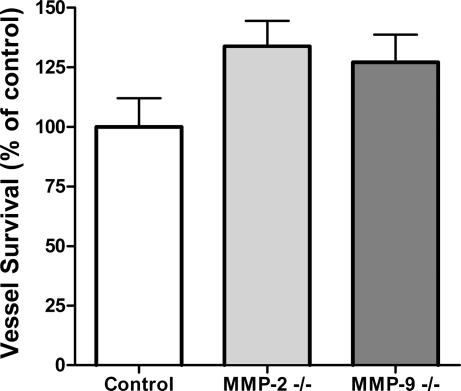
Angiogenesis in collagen cultures of rat aorta is a self-limited process characterized by an initial growth phase of 7–8 days followed by vascular regression in the 2nd and 3rd weeks. Vessel growth and regression are associated with progressive collagen lysis and accumulation in both matrix and culture medium of MMPs. Previous studies demonstrated that MMP-2 and MMP-9 were the most abundant MMP species in this system (61). However, experiments with genetically modified mice showed no significant difference in angiogenic sprouting between aortic rings deficient in MMP-2 or MMP-9 and control rings (data not shown) as reported (14, 36). In addition, lack of MMP-2 or MMP-9 function had no effect on the rate of vascular regression (Fig. 1).
Fig. 1.
Loss of matrix metalloproteinase (MMP)-2 or MMP-9 does not affect vessel survival. Vascular survival in collagen gel cultures of aortic rings from mice deficient in MMP-2 or MMP-9 (2nd wk of culture) was not significantly different from vascular survival in control cultures. Values are means ± SE; n = 4.
Vascular regression in collagen gel cultures of rat aorta is not influenced by plasmin activity.
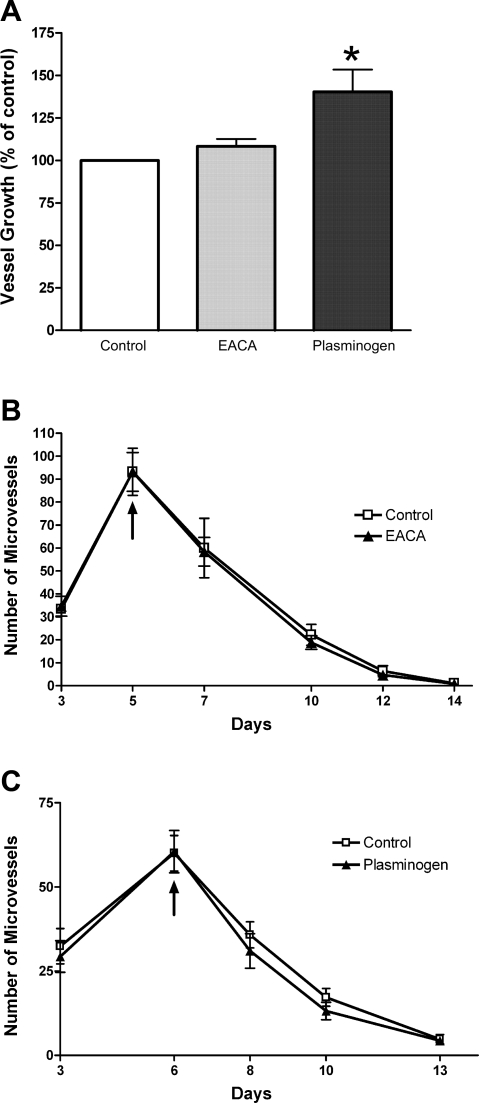
To evaluate the possible role of plasmin, a protease implicated in the activation of MMPs, in the formation and regression of aortic vessels, we treated aortic cultures with the plasmin inhibitor EACA or with plasminogen. These compounds were added to the growth medium from the beginning of the experiment or at the peak of growth. EACA had no effect on the angiogenic response or the rate of vascular regression, regardless of the time of treatment. Plasminogen caused a modest stimulation of angiogenesis when added from the beginning of the experiment but had no effect on the rate of vascular regression in either treatment protocol (Fig. 2). Neither EACA or plasminogen inhibited collagen lysis (data not shown).
Fig. 2.
Effect of ɛ-aminocaproic acid (EACA) and plasminogen on vascular growth and regression. Angiogenesis in collagen gel cultures of rat aorta (peak of vessel growth) was slightly stimulated by plasminogen, whereas the plasmin inhibitor EACA had no effect (A). Neither EACA (300 μg/ml; B) or plasminogen (200 μg/ml; C) had any effect on the rate of vascular regression when added to cultured medium following angiogenesis. Arrows in B and C indicate the day treatment was started. Values are means ± SE; n = 4. *P < 0.05.
Neovessels of rat aortic ring cultures express MT1-MMP.
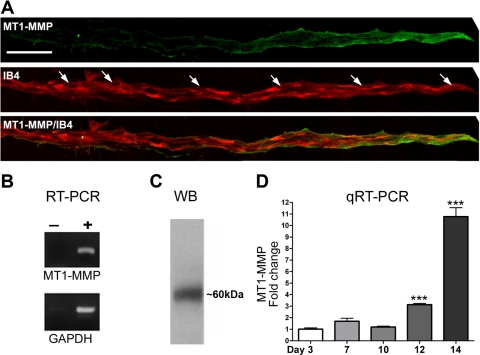
Previous reports have implicated MT1-MMP as a critical regulator of angiogenic sprouting (26, 28), but no data are available on the possible role of this enzyme in the breakdown of the neovasculature in the aortic ring model. Immunostaining of sprouting microvessels revealed the highest expression of MT1-MMP in endothelial tip cells (Fig. 3A). The membrane localization of MT1-MMP and its distribution gradient along the vascular axis strongly suggested that MT1-MMP was actively involved in endothelial sprouting and vascular remodeling. RT-PCR and Western blot studies confirmed that MT1-MMP mRNA and protein were expressed in rat aortic explants (Fig. 3, B and C). qRT-PCR studies showed that MT1-MMP expression markedly increased over time, becoming severalfold higher during vascular regression compared with the peak of vessel growth (Fig. 3D).
Fig. 3.
Membrane type 1 (MT1)-MMP expression in angiogenic outgrowths. A: confocal images of a representative neovessel from a collagen gel culture of rat aorta double-stained with an anti-MT1-MMP antibody (green) and an endothelial cell marker (IB4; red). Note: there is a gradient of MT1-MMP that is strongly expressed in the endothelial tip cells and barely detectable at the root of the neovessel. Arrows in the IB4-stained panel indicate nuclei (scale bar, 30 μm). B: photographs of ethidium bromide-stained gels showing PCR products from RT-PCR performed with (+) and without (−) reverse transcriptase on RNA isolated from rat aortic ring cultures and primers for MT1-MMP and GAPDH. C: photograph of a Western blot (WB) showing MT1-MMP expression in rat aortic cultures. A single band of ∼60 kDa was detected with anti-MT1-MMP antibody. D: quantitative real-time RT-PCR (qRT-PCR) of aortic ring cultures demonstrated progressive increase of MT1-MMP expression over time. Values are means ± SE; n = 3, ***P < 0.001.
MT1-MMP blockade inhibits angiogenesis and prevents vascular regression.
To specifically block MT1-MMP activity, aortic ring cultures were treated with three different monoclonal antibodies directed against MT1-MMP: one was against the hemopexin domain, which has been implicated in the regulation of MT1-MMP catalytic and migration-inducing activities (12), and the other two were against the catalytic domain of MT1-MMP and shown to specifically inhibit the enzymatic function of this MMP (22).
When aortic cultures were treated from the beginning of the experiment, the anti-MT1-MMP antibodies significantly inhibited angiogenesis (Fig. 4, A, C, E). Aortic rings cultured under conditions of continuous inhibition eventually developed a few neovessels, which survived longer than control neovessels. If administered after the growth phase, the anti-MT1-MMP antibodies prevented vascular breakdown (Fig. 4, B, D, F). Neovessels in MT1-MMP antibody-treated cultures became more stable, maintaining their length and matrix contact for periods of several weeks, whereas control neovessels regressed over time, becoming reabsorbed within 10–14 days.
Fig. 4.
MT1-MMP blockade inhibits angiogenesis and prevents vascular regression. Representative collagen gel cultures of rat aorta treated with nonimmune IgG or anti-MT1-MMP antibody (MAB3317) either from the beginning of the experiment (A and C) or starting at day 6 (B and D). Cultures in A and C were photographed on day 6; cultures in B and D were photographed on day 10. E: quantification of neovessels at day 6 in the presence of 3 different anti-MT1-MMP antibodies or control IgG. F: growth curve of aortic cultures treated from day 6 with anti-MT1-MMP antibody MAB3317. Note: early MT1-MMP blockade had an antiangiogenic effect (C, E), whereas blockade of MT1-MMP after the angiogenic growth phase had a vascular survival effect (D, F). Vascular regression in control cultures was associated with the formation of a periaortic halo of collagen lysis (asterisk in B), whereas collagen lysis was not observed in cultures treated with anti-MT1-MMP antibody (D). Scale bar, 500 μm. Values are means ± SE; n = 4. **P < 0.01; ***P < 0.001.
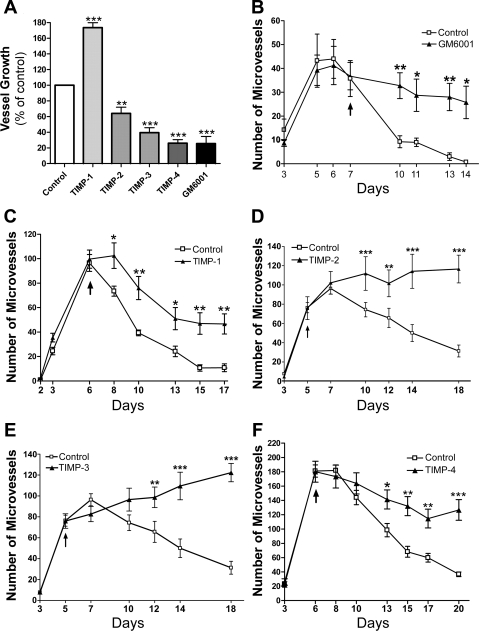
TIMP-2, TIMP-3, and TIMP-4 inhibit angiogenesis.
To further evaluate the possible role of MT1-MMP in the angiogenic response of the rat aorta, we studied the effects of TIMP-1, TIMP-2, TIMP-3, and TIMP-4 on the growth and survival of neovessels. When added to cultures of rat aorta from the beginning of the experiment, TIMP-2, TIMP-3, and TIMP-4 all inhibited angiogenesis, whereas TIMP-1 had no antiangiogenic effect (Fig. 5). TIMP-1 instead promoted neovessel growth (Fig. 5A). These findings corroborate the idea that angiogenesis is regulated by MT1-MMP, because the activity of this enzyme is inhibited by TIMP-2, TIMP-3, and TIMP-4 but not by TIMP-1 (57), which instead blocks MMP-2 and MMP-9.
Fig. 5.
Tissue inhibitors of metalloproteinase (TIMP)-2, TIMP-3, and TIMP-4 inhibit angiogenesis and stabilize neovessels, whereas TIMP-1 stabilizes neovessels but promotes angiogenesis. A: angiogenesis in collagen gel cultures of rat aorta was inhibited by TIMP-2, TIMP-3, TIMP-4, and the synthetic MMP inhibitor GM6001 but not by TIMP-1, which had a proangiogenic effect. Change in angiogenic response at the peak of microvessel growth is expressed as percentage of control. When treatment was started following the angiogenic growth phase (days 5 and 6), vessels were effectively stabilized by the synthetic MMP inhibitor GM6001 (B), TIMP-2 (D), TIMP-3 (E), and TIMP-4 (F) and to a lesser degree by TIMP-1 (C). As a result, GM6001- or TIMP-treated neovessels survived longer than untreated control neovessels. Values are means ± SE; n = 4. *P < 0.05; **P < 0.01; ***P < 0.001.
TIMP-1, TIMP-2, TIMP-3, and TIMP-4 stabilize neovessels.
To evaluate the role of TIMPs in vessel survival, we separately added each of the four TIMPs to aortic cultures after vessels had formed. Treatment with TIMP-1, TIMP-2, TIMP-3, and TIMP-4 stabilized existing vessels, producing a net proangiogenic effect over time (Fig. 5, C–F). The vascular survival effect over control values after 2–3 wk of culture was 100% or higher for TIMP-2 and TIMP-3, 80% for TIMP-4, and 50% for TIMP-1. Effects on vessel growth and survival obtained with anti-MT1-MMP antibodies and TIMPs (Fig. 5B) were comparable to results obtained with synthetic broad-spectrum inhibitors of MMPs (58, 61).
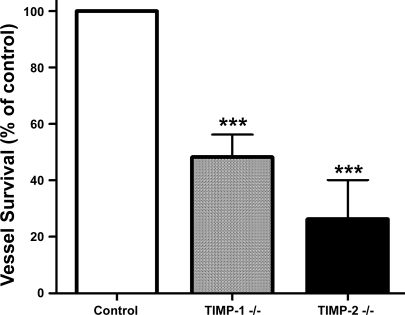
Neovessels regress faster in aortic cultures from TIMP-1- and TIMP-2-deficient mice.
To explore the role of endogenous TIMPs in vascular survival, we performed experiments with aortic rings from mice with disrupted TIMP-1 or TIMP-2 genes. In both cases, aortic rings generated angiogenic outgrowths comparable to normal controls. However, neovessels lacking TIMP-1 or TIMP-2 genes became fragmented and regressed at a faster rate than control vessels (Fig. 6). Vessel survival compared with controls at the end of the experiment was 50% in TIMP-1-deficient cultures and 25% in cultures with disrupted TIMP-2.
Fig. 6.
Neovessel survival is reduced in cultures of aortic rings from TIMP-1- or TIMP-2-deficient mice. The survival of neovessels following angiogenesis was significantly reduced in collagen gel cultures of aortic rings with disrupted TIMP-1 or TIMP-2 genes compared with cultures of normal rings. Values are means ± SE; n = 4. ***P < 0.001.
Neovessel stabilization correlates with inhibition of collagen lysis and perivascular condensation of collagen fibrils.
Collagen lysis was markedly inhibited in aortic ring cultures treated with anti-MT1-MMP antibodies or recombinant TIMP-2, TIMP-3, or TIMP-4. Conversely, TIMP-1 had no anticollagenolytic activity (Fig. 7A). Ultrastructural studies of cultures treated with anti-MT1-MMP antibody or TIMP-2 showed that endothelial cells of stabilized neovessels were tightly anchored to the underlying collagen. Perivascular spaces in these cultures contained more densely packed collagen fibrils than control neovessels. The abluminal surface of these stabilized vessels was flat and firmly adherent to the underlying collagen. Conversely, regressing microvessels of control cultures were surrounded by a loose matrix of sparse collagen fibrils. Their abluminal surface was poorly adherent to the underlying collagen and exhibited many blebs and vesicles (Fig. 7, B–E).
Fig. 7.
Neovessel stabilization correlates with inhibition of collagen lysis. A: image analysis measurement of periaortic collagen lysis revealed marked anticollagenolytic effect by the synthetic MMP inhibitor GM6001, the anti-MT1-MMP antibody LEM2/15, TIMP-2, TIMP-3, and TIMP-4 but not by TIMP-1. Values are means ± SE; n = 4. ***P < 0.001. B–D: electron micrographs (scale bars, 2 μm) show cross sections of representative microvessels from untreated control aortic ring culture (B) and cultures treated with anti-MT1-MMP antibody 3317 (C) or recombinant TIMP-2 (D). Note: microvessels stabilized by the anti-MT1-MMP antibody and TIMP-2 are surrounded by a dense matrix of tightly packed collagen fibrils (asterisks in C and D), whereas the regressing microvessel of the untreated control is loosely associated with only sparse collagen fibrils (B); cytoplasmic blebs in areas of endothelial cell detachment from the underlying extracellular matrix are indicated by arrows in B. E: image analysis measurement of perivascular collagen density in electron micrographs of representative neovessels. Treatment with MT1-MMP blocking antibody or recombinant TIMP-2 resulted in 2- to 3-fold increased collagen fibril density along the abluminal surface of neovessels. Values are means ± SE; n = 10. *P < 0.05.
DISCUSSION
MT1-MMP and TIMPs have previously been shown to play an important role in regulating endothelial sprouting during angiogenesis (14, 48, 54). In this article, we have provided evidence that these molecules also control the fate of neovessels following the initial angiogenic growth phase. These observations were made possible by the use of the aortic ring model, which recapitulates ex vivo all the stages of the angiogenic process, including vascular regression. Our conclusions are based on the following observations: 1) aorta-derived angiogenic outgrowths produce MT1-MMP, which is highly expressed at the tip of the vessel sprouts; 2) MT1-MMP expression is detected throughout the angiogenic process and increases during the vascular regression phase, when there is extensive collagen lysis; 3) neutralizing anti-MT1-MMP antibodies and recombinant TIMPs with anti-MT1-MMP activity (TIMP-2, TIMP-3, and TIMP-4) block collagen lysis; 4) neutralizing anti-MT1-MMP antibodies and TIMPs with anti-MT1-MMP activity inhibit angiogenesis but also stabilize neovessels if administered after vessels have formed; 5) recombinant TIMP-1, a weak inhibitor of MT1-MMP, has no antiangiogenic activity and does not block collagen lysis but has vascular stabilizing properties, although it is less effective than TIMP-2, TIMP-3, and TIMP-4; and 6) disruption of the TIMP-1 and TIMP-2 genes results in accelerated vascular regression.
Our studies with blocking antibodies and TIMPs indicate that MT1-MMP is required not only for the formation of blood vessels (14, 25) but also for vascular regression. The observation that blockade of MT1-MMP at different time points has opposite effects on angiogenic outcomes indicates that this enzyme plays different roles depending on the stage of the angiogenic process. Blocking MT1-MMP with specific antibodies or recombinant TIMPs before vessel formation results in marked inhibition of angiogenesis, but the same treatment stabilizes neovessels and prevents their regression if it is initiated after the angiogenic growth phase.
The observation that vascular regression in the absence of MT1-MMP inhibitors is associated with perivascular collagen lysis, whereas MT1-MMP inhibitor-mediated vascular stabilization is characterized by inhibition of collagen lysis and perivascular condensation of collagen fibrils, strongly implicates extracellular matrix-degrading events in the MT1-MMP-mediated regulation of neovessel breakdown following angiogenesis. In our experiments, collagen lysis can be blocked by treating neovessels with the same antibodies or TIMPs that cause inhibition of angiogenesis when administered early. MT1-MMP can degrade collagen either directly or indirectly by promoting the activation of pro-MMP-2 (15, 39, 56). MT1-MMP forms a complex with TIMP-2, which in turn binds pro-MMP-2, making it available for activation by adjacent TIMP-2-free MT1-MMP molecules (56). MMP-2 also localizes on the cell surface by binding to the αvβ3-integrin receptor (11) or to collagen fibrils that adhere to β1-integrin receptors (24). MMP-2 attached to the cell surface degrades many ECM molecules, including type IV collagen, which is otherwise resistant to MT1-MMP (20). There also is evidence that MMP-2 can digest native collagen fibrils (33). Aortic ring cultures produce abundant MMP-2, including its active form (61). Experiments presented in this article and previously reported by others (14, 36), however, show that disruption of the MMP-2 gene has no effect on angiogenesis. In this report, we present the novel finding that MMP-2 deficiency has no effect on the rate of vascular regression.
Aortic cultures produce MMP-9, which is among the MMPs implicated in the formation of blood vessels (8, 61). Using aortic rings from MMP-9-deficient mice, we found that disruption of this enzyme does not influence the angiogenic response or the reabsorption of neovessels. In addition, TIMP-1, a strong inhibitor of MMP-2 and MMP-9 (5, 30), has no antiangiogenic activity in this system and is unable to block collagen lysis. Together, experiments with MMP-2- and MMP-9-deficient mouse aortas or with normal aortas treated with TIMP-1 indicate that MMP-2 and MMP-9 are not required for the formation or regression of neovessels in the aortic ring model. This is consistent with similar observations made by other groups (14, 36). Conversely, previous studies with aortic rings from MT1-MMP-deficient mice (14) and our results with blocking antibodies and TIMPs with anti-MT1-MMP activity implicate MT1-MMP as a critical regulator of angiogenesis in a collagen matrix.
Since plasmin can promote vascular regression through activation of MMP-1 and MMP-9 (17), we tested the role of this enzyme in our model by treating aortic cultures with the plasmin inhibitor EACA or by adding exogenous plasminogen to the system. Addition of these molecules had no effect on the rate of vascular regression. The inability of plasminogen to accelerate vascular regression in aortic cultures may be due to the presence of pericytes, which can protect endothelial tubes from the destabilizing effects of serine proteases (50). However, the failure of EACA to stabilize vessels suggests that plasmin does not play a significant role in the regression of microvessels in our model. The modest stimulatory effect of plasminogen on the number of microvessels formed in the aortic cultures may be related to the activation of this molecule by urokinase-type plasminogen, which is highly expressed by the angiogenic outgrowths (3, 60).
The observation that recombinant TIMPs inhibit vascular regression and prolong the survival of neovessels in aortic cultures is in keeping with a previous report that bovine retinal pericytes stabilize capillary tubes formed by human umbilical vein endothelial cells by producing TIMP-3 and by inducing production of TIMP-2 by the endothelial cells themselves (50). Our data confirm and expand these findings in a developmental model of angiogenesis with native cells of the same species that have not been modified by repeated passages in culture. They also provide a direct correlation between the vessel stabilization effect of recombinant TIMP-1 and TIMP-2 and the accelerated vascular regression in cultures of TIMP-deficient aortas.
Although we found a direct correlation between inhibition of collagen lysis and stabilization of neovessels by TIMPs, it is possible that these molecules also may have promoted vascular survival through mechanisms independent of MMP inhibition. For example, TIMPs may directly regulate endothelial cell growth (45). This mechanism may play a role in the effect of TIMP-1, which promotes angiogenesis and neovessel survival without influencing collagen lysis or MT1-MMP activity. TIMP-3 has been shown to inhibit angiogenesis by interfering with the binding of vascular endothelial growth factor to VEGF-R2 (47). In addition, a mutated form of TIMP-2 without MMP inhibitory activity has been shown to suppress endothelial cell proliferation and angiogenesis in vivo through a protein tyrosine phosphate-dependent mechanism (45, 51). However, this mutated TIMP-2 reportedly has no effect on the ability of sprouting endothelial cells to invade collagen gels (14).
The finding that MT1-MMP is highly expressed at the tip of neovessels in aortic ring cultures indicates that this enzyme is most concentrated in regional domains of the neovasculature where matrix-degrading events are most needed for endothelial cell invasion, as previously reported in other systems (13, 59). Furthermore, MT1-MMP may facilitate the migration of endothelial tip cells through its ability to bind directly to β1- and β3-integrin receptors (11, 23).
MT1-MMP also is expressed in the membrane of trailing endothelial cells and associated pericytes, where continuous activity of this enzyme in the absence of a forward motion of the vascular sprouts, i.e., under stationary conditions, eventually results in gradual rarefaction of the collagen substrate around the neovessels. In addition, MT1-MMP can break down tissue transglutaminase, a cross-linking enzyme that stabilizes ECM molecules and promotes endothelial adhesion to the ECM (6). Unbalanced proteolysis of the ECM continuing undisturbed after neovessels have formed and endothelial cells are no longer migrating may lead to loss of matrix support, endothelial apoptosis due to integrin disengagement from the ECM, and vascular regression. Conversely, perivascular accumulation of collagen and other ECM molecules under condition of MT1-MMP inhibition following angiogenesis may provide survival signals to endothelial cells through integrin receptors (10, 21, 41, 42).
The observation that aortic ring cultures treated with MT1-MMP inhibitors eventually develop neovessels, although in markedly reduced numbers and with a significant delay, suggests that redundant proteolytic mechanisms exist, capable of rescuing the angiogenic process. Similar redundant mechanisms may operate during vascular regression. The existence of MMP redundancy explains the observation that ablation of the MT1-MMP gene in mice results in a significantly shortened life span but is not embryonically lethal (29) and that endothelial tube formation in vitro is associated with upregulated expression not only of MT1-MMP but also of MT2-MMP and MT3-MMP (32).
In conclusion, our results indicate that MT1-MMP and TIMPs play a critical role in both neovessel sprouting and vascular regression during angiogenesis in the aortic ring model. The finding that MT1-MMP inhibition has opposite effects on the outcome of the angiogenic process depending on the time of treatment raises important questions about the therapeutic use of broad-spectrum synthetic MMP inhibitors. The failure of MMP inhibitors in cancer clinical trials has been attributed to the multifunctional nature of the MMPs and the complexity of the MMP system (37, 53). To these important variables we now add the temporal context in which MMP inhibition takes place. Our results predict that currently available MMP inhibitors will block neovessel formation but also stabilize vessels that have already developed, thereby producing random and possibly paradoxical results in patients with an already established disease. They also suggest that the fate of neovessels may be influenced by a delicate balance of MT1-MMP and TIMP activities. To gain further insight into this hypothesis, future studies should focus on the mechanisms by which MT1-MMP and TIMPs regulate vascular survival. Understanding how these molecules influence the stability of a neovasculature following angiogenesis may help design antiangiogenic drugs with improved target specificity for a more effective treatment of cancer and other angiogenesis-dependent disorders.
GRANTS
This work was supported by National Heart, Lung, and Blood Institute Grant HL52585 (R. F. Nicosia) and the Medical Research Service, Department of Veterans Affairs (R. F. Nicosia).
Acknowledgments
We thank Debbie Jones, Division of Pathology and Laboratory Medicine, VA Puget Sound Health Care System, Seattle, WA, for excellent technical assistance with the electron microscopy studies.
REFERENCES
- 1.Anand-Apte B, Pepper MS, Voest E, Montesano R, Olsen B, Murphy G, Apte SS, Zetter B. Inhibition of angiogenesis by tissue inhibitor of metalloproteinase-3. Invest Ophthalmol Vis Sci 38: 817–823, 1997. [PubMed] [Google Scholar]
- 2.Aplin AC, Fogel E, Zorzi P, Nicosia RF. The aortic ring model of angiogenesis. Methods Enzymol 443: 119–136, 2008. [DOI] [PubMed] [Google Scholar]
- 3.Bacharach E, Itin A, Keshet E. In vivo patterns of expression of urokinase and its inhibitor PAI-1 suggest a concerted role in regulating physiological angiogenesis. Proc Natl Acad Sci USA 89: 10686–10690, 1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Baciu PC, Suleiman EA, Deryugina EI, Strongin AY. Membrane type-1 matrix metalloproteinase (MT1-MMP) processing of pro-alpha v integrin regulates cross-talk between alpha v beta 3 and alpha 2 beta 1 integrins in breast carcinoma cells. Exp Cell Res 291: 167–175, 2003. [DOI] [PubMed] [Google Scholar]
- 5.Baker AH, Edwards DR, Murphy G. Metalloproteinase inhibitors: biological actions and therapeutic opportunities. J Cell Sci 115: 3719–3727, 2002. [DOI] [PubMed] [Google Scholar]
- 6.Belkin AM, Akimov SS, Zaritskaya LS, Ratnikov BI, Deryugina EI, Strongin AY. Matrix-dependent proteolysis of surface transglutaminase by membrane-type metalloproteinase regulates cancer cell adhesion and locomotion. J Biol Chem 276: 18415–18422, 2001. [DOI] [PubMed] [Google Scholar]
- 7.Benjamin LE, Hemo I, Keshet E. A plasticity window for blood vessel remodelling is defined by pericyte coverage of the preformed endothelial network and is regulated by PDGF-B and VEGF. Development 125: 1591–1598, 1998. [DOI] [PubMed] [Google Scholar]
- 8.Bergers G, Brekken R, McMahon G, Vu TH, Itoh T, Tamaki K, Tanzawa K, Thorpe P, Itohara S, Werb Z, Hanahan D. Matrix metalloproteinase-9 triggers the angiogenic switch during carcinogenesis. Nat Cell Biol 2: 737–744, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bigg HF, Morrison CJ, Butler GS, Bogoyevitch MA, Wang Z, Soloway PD, Overall CM. Tissue inhibitor of metalloproteinases-4 inhibits but does not support the activation of gelatinase A via efficient inhibition of membrane type 1-matrix metalloproteinase. Cancer Res 61: 3610–3618, 2001. [PubMed] [Google Scholar]
- 10.Bonanno E, Iurlaro M, Madri JA, Nicosia RF. Type IV collagen modulates angiogenesis and neovessel survival in the rat aorta model. In Vitro Cell Dev Biol Anim 36: 336–340, 2000. [DOI] [PubMed] [Google Scholar]
- 11.Brooks PC, Stromblad S, Sanders LC, von Schalscha TL, Aimes RT, Stetler-Stevenson WG, Quigley JP, Cheresh DA. Localization of matrix metalloproteinase MMP-2 to the surface of invasive cells by interaction with integrin alpha v beta 3. Cell 85: 683–693, 1996. [DOI] [PubMed] [Google Scholar]
- 12.Cao J, Kozarekar P, Pavlaki M, Chiarelli C, Bahou WF, Zucker S. Distinct roles for the catalytic and hemopexin domains of membrane type 1-matrix metalloproteinase in substrate degradation and cell migration. J Biol Chem 279: 14129–14139, 2004. [DOI] [PubMed] [Google Scholar]
- 13.Chen WT, Wang JY. Specialized surface protrusions of invasive cells, invadopodia and lamellipodia, have differential MT1-MMP, MMP-2, and TIMP-2 localization. Ann NY Acad Sci 878: 361–371, 1999. [DOI] [PubMed] [Google Scholar]
- 14.Chun TH, Sabeh F, Ota I, Murphy H, McDonagh KT, Holmbeck K, Birkedal-Hansen H, Allen ED, Weiss SJ. MT1-MMP-dependent neovessel formation within the confines of the three-dimensional extracellular matrix. J Cell Biol 167: 757–767, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.D'ortho MP, Will H, Atkinson S, Butler G, Messent A, Gavrilovic J, Smith B, Timpl R, Zardi L, Murphy G. Membrane-type matrix metalloproteinases 1 and 2 exhibit broad-spectrum proteolytic capacities comparable to many matrix metalloproteinases. Eur J Biochem 250: 751–757, 1997. [DOI] [PubMed] [Google Scholar]
- 16.Darby IA, Bisucci T, Pittet B, Garbin S, Gabbiani G, Desmouliere A. Skin flap-induced regression of granulation tissue correlates with reduced growth factor and increased metalloproteinase expression. J Pathol 197: 117–127, 2002. [DOI] [PubMed] [Google Scholar]
- 17.Davis GE, Pintar Allen KA, Salazar R, Maxwell SA. Matrix metalloproteinase-1 and -9 activation by plasmin regulates a novel endothelial cell-mediated mechanism of collagen gel contraction and capillary tube regression in three-dimensional collagen matrices. J Cell Sci 114: 917–930, 2001. [DOI] [PubMed] [Google Scholar]
- 18.Davis GE, Saunders WB. Molecular balance of capillary tube formation versus regression in wound repair: role of matrix metalloproteinases and their inhibitors. J Invest Dermatol 126, Suppl: 44–56, 2006. [DOI] [PubMed] [Google Scholar]
- 19.Folkman J Fundamental concepts of the angiogenic process. Curr Mol Med 3: 643–651, 2003. [DOI] [PubMed] [Google Scholar]
- 20.Freije JM, Balbin M, Pendas AM, Sanchez LM, Puente XS, Lopez-Otin C. Matrix metalloproteinases and tumor progression. Adv Exp Med Biol 532: 91–107, 2003. [DOI] [PubMed] [Google Scholar]
- 21.Frisch SM, Ruoslahti E. Integrins and anoikis. Curr Opin Cell Biol 9: 701–706, 1997. [DOI] [PubMed] [Google Scholar]
- 22.Galvez BG, Matias-Roman S, Albar JP, Sanchez-Madrid F, Arroyo AG. Membrane type 1-matrix metalloproteinase is activated during migration of human endothelial cells and modulates endothelial motility and matrix remodeling. J Biol Chem 276: 37491–37500, 2001. [DOI] [PubMed] [Google Scholar]
- 23.Galvez BG, Matias-Roman S, Yanez-Mo M, Sanchez-Madrid F, Arroyo AG. ECM regulates MT1-MMP localization with beta 1 or alpha v beta 3 integrins at distinct cell compartments modulating its internalization and activity on human endothelial cells. J Cell Biol 159: 509–521, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Guo C, Piacentini L. Type I collagen-induced MMP-2 activation coincides with up-regulation of membrane type 1-matrix metalloproteinase and TIMP-2 in cardiac fibroblasts. J Biol Chem 278: 46699–46708, 2003. [DOI] [PubMed] [Google Scholar]
- 25.Haas TL, Davis SJ, Madri JA. Three-dimensional type I collagen lattices induce coordinate expression of matrix metalloproteinases MT1-MMP and MMP-2 in microvascular endothelial cells. J Biol Chem 273: 3604–3610, 1998. [DOI] [PubMed] [Google Scholar]
- 26.Haas TL, Madri JA. Extracellular matrix-driven matrix metalloproteinase production in endothelial cells: implications for angiogenesis. Trends Cardiovasc Med 9: 70–77, 1999. [DOI] [PubMed] [Google Scholar]
- 27.Hallmann R, Feinberg RN, Latker CH, Sasse J, Risau W. Regression of blood vessels precedes cartilage differentiation during chick limb development. Differentiation 34: 98–105, 1987. [DOI] [PubMed] [Google Scholar]
- 28.Hiraoka N, Allen E, Apel IJ, Gyetko MR, Weiss SJ. Matrix metalloproteinases regulate neovascularization by acting as pericellular fibrinolysins. Cell 95: 365–377, 1998. [DOI] [PubMed] [Google Scholar]
- 29.Holmbeck K, Bianco P, Caterina J, Yamada S, Kromer M, Kuznetsov SA, Mankani M, Robey PG, Poole AR, Pidoux I, Ward JM, Birkedal-Hansen H. MT1-MMP-deficient mice develop dwarfism, osteopenia, arthritis, and connective tissue disease due to inadequate collagen turnover. Cell 99: 81–92, 1999. [DOI] [PubMed] [Google Scholar]
- 30.Hotary KB, Allen ED, Brooks PC, Datta NS, Long MW, Weiss SJ. Membrane type I matrix metalloproteinase usurps tumor growth control imposed by the three-dimensional extracellular matrix. Cell 114: 33–45, 2003. [DOI] [PubMed] [Google Scholar]
- 31.Itoh T, Ikeda T, Gomi H, Nakao S, Suzuki T, Itohara S. Unaltered secretion of beta-amyloid precursor protein in gelatinase A (matrix metalloproteinase 2)-deficient mice. J Biol Chem 272: 22389–22392, 1997. [DOI] [PubMed] [Google Scholar]
- 32.Lafleur MA, Handsley MM, Knauper V, Murphy G, Edwards DR. Endothelial tubulogenesis within fibrin gels specifically requires the activity of membrane-type-matrix metalloproteinases (MT-MMPs). J Cell Sci 115: 3427–3438, 2002. [DOI] [PubMed] [Google Scholar]
- 33.Lauer-Fields JL, Tuzinski KA, Shimokawa K, Nagase H, Fields GB. Hydrolysis of triple-helical collagen peptide models by matrix metalloproteinases. J Biol Chem 275: 13282–13290, 2000. [DOI] [PubMed] [Google Scholar]
- 34.Leco KJ, Khokha R, Pavloff N, Hawkes SP, Edwards DR. Tissue inhibitor of metalloproteinases-3 (TIMP-3) is an extracellular matrix-associated protein with a distinctive pattern of expression in mouse cells and tissues. J Biol Chem 269: 9352–9360, 1994. [PubMed] [Google Scholar]
- 35.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2{↑−ΔΔCT} method. Methods 25: 402–408, 2001. [DOI] [PubMed] [Google Scholar]
- 36.Masson V, de la Ballina LR, Munaut C, Wielockx B, Jost M, Maillard C, Blacher S, Bajou K, Itoh T, Itohara S, Werb Z, Libert C, Foidart JM, Noel A. Contribution of host MMP-2 and MMP-9 to promote tumor vascularization and invasion of malignant keratinocytes. FASEB J 19: 234–236, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Matter H, Schudok M. Recent advances in the design of matrix metalloprotease inhibitors. Curr Opin Drug Discov Devel 7: 513–535, 2004. [PubMed] [Google Scholar]
- 38.Modlich U, Kaup FJ, Augustin HG. Cyclic angiogenesis and blood vessel regression in the ovary: blood vessel regression during luteolysis involves endothelial cell detachment and vessel occlusion. Lab Invest 74: 771–780, 1996. [PubMed] [Google Scholar]
- 39.Monea S, Roberts B, Marcus SG, Shamamian P, Mignatti P. Roles of MT1-MMP in the regulation of cell surface proteolysis. Ann NY Acad Sci 878: 703–706, 1999. [DOI] [PubMed] [Google Scholar]
- 40.Nicosia RF, Zhu WH. Rat aortic ring assay of angiogenesis. In: Methods in Endothelial Cell Biology, edited by Augustin HG. Berlin: Springer, 2004, p. 125–144.
- 41.Nicosia RF, Bonanno E, Smith M. Fibronectin promotes the elongation of microvessels during angiogenesis in vitro. J Cell Physiol 154: 654–661, 1993. [DOI] [PubMed] [Google Scholar]
- 42.Nicosia RF, Bonanno E, Smith M, Yurchenco P. Modulation of angiogenesis in vitro by laminin-entactin complex. Dev Biol 164: 197–206, 1994. [DOI] [PubMed] [Google Scholar]
- 43.Nicosia RF, Ottinetti A. Growth of microvessels in serum-free matrix culture of rat aorta. A quantitative assay of angiogenesis in vitro. Lab Invest 63: 115–122, 1990. [PubMed] [Google Scholar]
- 44.Noel A, Maillard C, Rocks N, Jost M, Chabottaux V, Sounni NE, Maquoi E, Cataldo D, Foidart JM. Membrane associated proteases and their inhibitors in tumour angiogenesis. J Clin Pathol 57: 577–584, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Oh J, Seo DW, Diaz T, Wei B, Ward Y, Ray JM, Morioka Y, Shi S, Kitayama H, Takahashi C, Noda M, Stetler-Stevenson WG. Tissue inhibitors of metalloproteinase 2 inhibits endothelial cell migration through increased expression of RECK. Cancer Res 64: 9062–9069, 2004. [DOI] [PubMed] [Google Scholar]
- 46.Pepper MS Role of the matrix metalloproteinase and plasminogen activator-plasmin systems in angiogenesis. Arterioscler Thromb Vasc Biol 21: 1104–1117, 2001. [DOI] [PubMed] [Google Scholar]
- 47.Qi JH, Ebrahem Q, Moore N, Murphy G, Claesson-Welsh L, Bond M, Baker A, Anand-Apte B. A novel function for tissue inhibitor of metalloproteinases-3 (TIMP3): inhibition of angiogenesis by blockage of VEGF binding to VEGF receptor-2. Nat Med 9: 407–415, 2003. [DOI] [PubMed] [Google Scholar]
- 48.Raffetto JD, Khalil RA. Matrix metalloproteinases and their inhibitors in vascular remodeling and vascular disease. Biochem Pharmacol 75: 346–359, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ray JM, Stetler-Stevenson WG. The role of matrix metalloproteases and their inhibitors in tumour invasion, metastasis and angiogenesis. Eur Respir J 7: 2062–2072, 1994. [PubMed] [Google Scholar]
- 50.Saunders WB, Bohnsack BL, Faske JB, Anthis NJ, Bayless KJ, Hirschi KK, Davis GE. Coregulation of vascular tube stabilization by endothelial cell TIMP-2 and pericyte TIMP-3. J Cell Biol 175: 179–191, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Seo DW, Li H, Guedez L, Wingfield PT, Diaz T, Salloum R, Wei BY, Stetler-Stevenson WG. TIMP-2 mediated inhibition of angiogenesis: an MMP-independent mechanism. Cell 114: 171–180, 2003. [DOI] [PubMed] [Google Scholar]
- 52.Soloway PD, Alexander CM, Werb Z, Jaenisch R. Targeted mutagenesis of Timp-1 reveals that lung tumor invasion is influenced by Timp-1 genotype of the tumor but not by that of the host. Oncogene 13: 2307–2314, 1996. [PubMed] [Google Scholar]
- 53.Stetler-Stevenson WG Matrix metalloproteinases in angiogenesis: a moving target for therapeutic intervention. J Clin Invest 103: 1237–1241, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Stetler-Stevenson WG, Seo DW. TIMP-2: an endogenous inhibitor of angiogenesis. Trends Mol Med 11: 97–103, 2005. [DOI] [PubMed] [Google Scholar]
- 55.Vu TH, Shipley JM, Bergers G, Berger JE, Helms JA, Hanahan D, Shapiro SD, Senior RM, Werb Z. MMP-9/gelatinase B is a key regulator of growth plate angiogenesis and apoptosis of hypertrophic chondrocytes. Cell 93: 411–422, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wang Z, Juttermann R, Soloway PD. TIMP-2 is required for efficient activation of proMMP-2 in vivo. J Biol Chem 275: 26411–26415, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Will H, Atkinson SJ, Butler GS, Smith B, Murphy G. The soluble catalytic domain of membrane type 1 matrix metalloproteinase cleaves the propeptide of progelatinase A and initiates autoproteolytic activation. Regulation by TIMP-2 and TIMP-3. J Biol Chem 271: 17119–17123, 1996. [DOI] [PubMed] [Google Scholar]
- 58.Yamamoto M, Tsujishita H, Hori N, Ohishi Y, Inoue S, Ikeda S, Okada Y. Inhibition of membrane-type 1 matrix metalloproteinase by hydroxamate inhibitors: an examination of the subsite pocket. J Med Chem 41: 1209–1217, 1998. [DOI] [PubMed] [Google Scholar]
- 59.Yana I, Sagara H, Takaki S, Takatsu K, Nakamura K, Nakao K, Katsuki M, Taniguchi S, Aoki T, Sato H, Weiss SJ, Seiki M. Crosstalk between neovessels and mural cells directs the site-specific expression of MT1-MMP to endothelial tip cells. J Cell Sci 120: 1607–1614, 2007. [DOI] [PubMed] [Google Scholar]
- 60.Yasunaga C, Nakashima Y, Sueishi K. A role of fibrinolytic activity in angiogenesis. Quantitative assay using in vitro method. Lab Invest 61: 698–704, 1989. [PubMed] [Google Scholar]
- 61.Zhu WH, Guo X, Villaschi S, Nicosia RF. Regulation of vascular growth and regression by matrix metalloproteinases in the rat aorta model of angiogenesis. Lab Invest 80: 545–555, 2000. [DOI] [PubMed] [Google Scholar]
- 62.Zhu WH, Han J, Nicosia RF. Requisite role of p38 MAPK in mural cell recruitment during angiogenesis in the rat aorta model. J Vasc Res 40: 140–148, 2003. [DOI] [PubMed] [Google Scholar]
- 63.Zhu WH, Nicosia RF. The thin prep rat aortic ring assay: a modified method for the characterization of angiogenesis in whole mounts. Angiogenesis 5: 81–86, 2002. [DOI] [PubMed] [Google Scholar]