Abstract
Understanding how electrotonic loading of cardiomyocytes by unexcitable cells alters cardiac impulse conduction may be highly relevant to fibrotic heart disease. In this study, we optically mapped electrical propagation in confluent, aligned neonatal rat cardiac monolayers electrotonically loaded with cardiac fibroblasts, control human embryonic kidney (HEK-293) cells, or HEK-293 cells genetically engineered to overexpress the gap junction proteins connexin-43 or connexin-45. Gap junction expression and function were assessed by immunostaining, immunoblotting, and fluorescence recovery after photobleaching and were correlated with the optically mapped propagation of action potentials. We found that neonatal rat ventricular fibroblasts negative for the myofibroblast marker smooth muscle α-actin expressed connexin-45 rather than connexin-43 or connexin-40, weakly coupled to cardiomyocytes, and, without significant depolarization of cardiac resting potential, slowed cardiac conduction to 75% of control only at high (>60%) coverage densities, similar to loading effects found from HEK-293 cells expressing similar levels of connexin-45. In contrast, HEK-293 cells with connexin-43 expression similar to that of cardiomyocytes significantly decreased cardiac conduction velocity and maximum capture rate to as low as 22% and 25% of control values, respectively, while increasing cardiac action potential duration to 212% of control and cardiac resting potential from −71.6 ± 4.9 mV in controls to −65.0 ± 3.8 mV. For all unexcitable cell types and coverage densities, velocity anisotropy ratio remained unchanged. Despite the induced conduction slowing, none of the loading cell types increased the proportion of spontaneously active monolayers. These results signify connexin isoform and expression level as important contributors to potential electrical interactions between unexcitable cells and myocytes in cardiac tissue.
Keywords: optical mapping, cardiac fibroblasts, gap junctions, cell culture, passive cell
direct interactions between cardiomyocytes and endogenous or exogeneously added unexcitable cells may affect electrical function in the healthy and diseased heart. Specifically, fibroblasts are the most numerous unexcitable cell type present in the healthy heart (32). Their primary function is to maintain extracellular matrix homeostasis through highly regulated synthesis and degradation of collagen type I and type III (7). In diseased hearts, however, tissue regions rich with fibroblasts and deposited collagen have been shown to adversely affect impulse conduction by causing delayed activations (20), blocking conduction (47), and anchoring reentrant waves (44). Besides excessive matrix deposition in different pathologies, unexcitable cardiac fibroblasts could also modify the electrophysiological substrate of the heart by direct electrotonic coupling with surrounding cardiomyocytes (22).
In general, the ability of unexcitable cells to electrotonically affect cardiac action potential generation and propagation depends on their coupling strength with cardiomyocytes as well as the size and cell membrane properties of both cardiomyocytes and unexcitable cells. Specifically, because of their relatively depolarized resting potential (8, 15), unexcitable cells weakly coupled to cardiomyocytes could speed up conduction by moderately increasing the myocyte resting potential to effectively decrease excitation threshold (27, 39), or, when well-coupled, could slow cardiac conduction by further increasing the cardiomyocyte resting potential to inactivate sodium gates (27). In addition, through their primarily potassium ion currents (15, 41), unexcitable cells coupled to cardiomyocytes could also alter the time course of cardiac repolarization and the resulting restitution properties (17, 26). Finally, through the effective increase of cardiac cell membrane area (capacitance), coupled unexcitable cells could decrease the density of cardiac excitatory currents and slow down charging and discharging of the cardiac cell membrane, thus slowing conduction and further affecting the shape of the cardiac action potential.
We have previously developed methods to control macroscopic structural and functional anisotropy in uniformly aligned cardiac cell monolayers (5) and used this physiologically relevant setting to study the effect of rapid pacing on the dynamics and acceleration of functional reentry (3, 6). In the current study, we set to explore how electrotonic loading of aligned cardiomyocytes by unexcitable cells affects anisotropic impulse conduction. Specifically, different types of unexcitable cells, including neonatal rat cardiac fibroblasts and genetically engineered human embryonic kidney (HEK-293) cells, were cultured on top of uniformly aligned confluent cardiac monolayers. In this way, unexcitable cells were allowed to contact the top surface of aligned myocytes, approximating in two dimensions the native three-dimensional (3-D) setting of the healthy heart where fibroblasts predominantly border the sides of aligned cardiac fibers (14, 22). In addition, without having unexcitable cells and cardiomyocytes interspersed in a single layer, this configuration allowed us to specifically study the electrotonic action of unexcitable cells as pure current sinks, rather than as combined current sinks and short range conductors (12, 22, 48).
In these cocultures we performed immunohistochemical analysis and fluorescence recovery after photobleaching (FRAP) experiments to assess gap junction expression and the degree of functional coupling of unexcitable cells with cardiomyocytes. Optical and sharp microelectrode recordings of membrane potentials were used to determine the effect of this coupling on cardiac resting potential and anisotropic electrical propagation in cardiac monolayers. Our results reveal that connexin isoform [e.g., connexin-43 (Cx43), connexin-45 (Cx45)] and level of expression are important determinants of the potential electrotonic interactions between electrically unexcitable cells and myocytes in cardiac tissue.
MATERIALS AND METHODS
All animals were treated according to protocols approved by the Duke University Institutional Animal Care and Use Committee.
Fabrication of Microgrooved Substrate For Cell Alignment
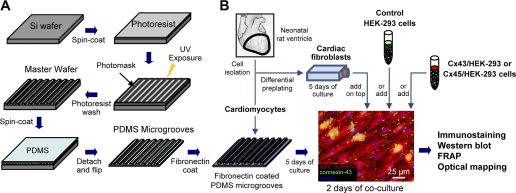
Parallel polydimethylsiloxane (PDMS) microgrooves were prepared using soft lithography techniques by modifying our previously described methods (Fig. 1A) (5). Briefly, silicon wafers were ultraviolet (UV)-ozone cleaned, and a 3-μm-thick layer of photoresist (SU-8 2, Microchem) was spin-coated onto the wafer. After a soft-baking process, a glass photomask with 6-μm-thick chromium lines spaced 6 μm apart was placed over the wafer and exposed to UV light. Following postexposure baking, the uncrosslinked photoresist was washed away with a developer, leaving the desired 6-μm-wide, 3-μm-deep lines on the wafer. This master wafer was silanized overnight to prevent unwanted adhesiveness of PDMS during further processing. PDMS was spin-coated onto the wafer, cured for 2 h at 80°C, peeled off, and cut with a 21-mm diameter circular punch to yield a 200-μm-thick substrate with the 3-μm-deep microgroove pattern cast into its top surface (Fig. 1A). After UV-ozone cleaning, the PDMS microgrooves were coated with fibronectin (25 μg/ml) for 2 h followed by plating of cardiac cells.
Fig. 1.
Schematic of microfabrication and cell culture procedures. A: soft lithography microfabrication technique to create 6-μm-wide, 3-μm-deep polydimethylsiloxane (PDMS) parallel microgrooves. B: confluent aligned culture of cardiomyocytes on fibronectin-coated microgrooves and subsequent coculture with cardiac fibroblasts, control human embryonic kidney (HEK-293) cells, connexin-45-transfected HEK-293 (Cx45/HEK-293) cells, or connexin-43-transfected HEK-293 (Cx43/HEK-293) cells. See text for further details. FRAP, fluorescence recovery after photobleaching; UV, ultraviolet.
Cell Culture
Neonatal rat ventricular cells.
Cardiac cells were isolated from the ventricles of 2- to 30-day-old Sprague-Dawley rats by enzymatic digestion with trypsin and collagenase, as previously described (5). Two 1-h differential preplatings were used to remove faster-attaching nonmyocytes. The remaining cells enriched with cardiomyocytes were resuspended in DMEM/F-12 media (Gibco) supplemented with 10% calf serum (Colorado Serum), 10% horse serum (Hyclone), and penicillin-streptomycin (Gibco) and plated at a density of 2.2 × 105 cells/cm2 on fibronectin-coated PDMS microgrooves, resulting in a confluent, uniformly aligned cardiac cell monolayer (Fig. 1B). To limit endogenous nonmyocyte overgrowth, cardiac cultures were exposed for 2 min to 1,000 rads of gamma radiation from a 137Cs source the day after seeding. After 2 days of culture, the cardiac cell media were changed to a DMEM/F-12-based, serum-free defined media containing 50 U/ml penicillin, 50 μg/ml streptomycin, 2 μg/ml l-thyroxine, 0.1 μg/ml insulin, 0.5 μg/ml transferrin, 2.5 μg/ml ascorbic acid, 1 nM lithium chloride, and 1 nM sodium selenite (Sigma) (33). Flasks of cardiac nonmyocytes obtained during the preplating steps were cultured in medium 199 (Gibco) supplemented with 10% FBS, 1% HEPES, nonessential amino acids, glucose, l-glutamine, vitamin B12, and penicillin G. After 5 days of growth, these nonmyocytes were characterized for their cellular composition by immunostaining and used for loading coculture experiments with cardiomyocytes.
Genetically engineered HEK-293 cells.
To assess the role of connexin isoform in electrotonic loading of cardiomyocytes by unexcitable cells, we expressed connexin-43 or connexin-45 and different fluorescent reporters in HEK-293 cells (CRL-1573, American Type Culture Collection), as previously described (21). Specifically, the rat connexin-43 gene (GJA1) was cloned from neonatal rat cardiac myocytes using primers based on the published rat connexin-43 sequence (PubMed NM_012567), and the amplified PCR product was inserted into a cloning vector and validated by DNA sequencing. Plasmids containing either mCherry (38) and the rat connexin-43 gene separated by an internal ribosomal entry sequence (IRES), mOrange and the mouse connexin-45 gene (kindly provided by Dr. Eric Beyer, University of Chicago) also separated by an IRES sequence, or a fluorescent gene alone [mCherry or enhanced green fluorescent protein (eGFP)] were transformed into bacteria, and 3 μg isolated plasmid DNA was conjugated to GenJet (SignaGen Labs) reagent and used to transfect HEK-293 cells. Positively transfected cells were identified by mCherry, mOrange, or eGFP expression and selected using puromycin. Stable monoclonal cell lines (Cx43/HEK-293 or Cx45/HEK-293) were isolated from the polyclonal population and cultured in DMEM (Gibco) supplemented with 10% FBS and penicillin-streptomycin. In several monoclonal Cx45/HEK-293 lines, expression of connexin-45 protein was assessed by Western blot analysis, and the monoclonal line with connexin-45 expression similar to that of fibroblasts was used in all presented studies. In addition, all structural and functional studies with wild-type, eGFP, and mCherry HEK-293 cells (i.e., cells not transfected with connexin genes) yielded similar results and were pooled together into a control HEK-293 group.
Loading of cardiomyocytes with electrically unexcitable cells.
Neonatal rat ventricular nonmyocytes or different types of genetically engineered HEK-293 cells were detached from plates by trypsinization and seeded on top of 5-day-old confluent cardiac monolayers at densities between 2.2 × 104 and 1.1 × 105 cells/cm2. Experiments were performed after 2 days of coculture in nonmyocyte growth media (Fig. 1B).
Optical Mapping of Action Potential Propagation
Electrical propagation in 2-day-old cocultures (7 days after cardiac cell seeding) was optically mapped in contact fluorescence mode (5, 11) using a bundle of 504 hexagonally arranged optical fibers (RedShirtImaging) providing a spatial resolution of 750 μm in a 19.5-mm field of view. Cocultured monolayers were assessed from 24 different cell isolations with at least two control monolayers (no cells added on top) mapped from each isolation. In total, 204 monolayers were mapped from four different groups: no cells added (n = 57), fibroblasts added (n = 94), control HEK-293 cells added (n = 16), Cx43/HEK-293 cells added (n = 18), and Cx45/HEK-293 cells added (n = 19). For optical mapping (3, 5, 6), the cocultures were incubated for 5 min with a voltage-sensitive dye (di-4 ANEPPS, 15 μM) at room temperature, placed in a heated recording chamber, perfused with Tyrode solution, and illuminated with green excitation light (520 ± 30 nm). The recorded red fluorescence (>590 nm) was transferred through the fiber optic bundle, converted to voltage using photodiodes, amplified, sampled at 2.4 kHz, and stored on a PC. Electrical stimulation, light exposure, and data acquisition were synchronized using custom LabView software. Before the onset of pacing, all monolayers were checked for the presence of spontaneous activity. An XYZ-micropositioned bipolar point platinum electrode was then lowered to within a millimeter of the cell culture surface and used to locally stimulate the center of the monolayer. After the threshold voltage was determined (usually 4–7 V for 10-ms pulses), 1.2 × threshold stimuli were applied at a 2-Hz pacing rate for 1 min. The pacing rate was then increased every minute in steps of 0.5 Hz, and transmembrane potentials were recorded at the end of each step. Maximum capture rate was defined as the maximum rate at which a 1:1 response of monolayers was maintained for at least 30 s of pacing.
Data analysis was performed using custom MATLAB software as previously described (5, 16). Local conduction velocities were calculated using the activation time for each optical fiber relative to those of neighboring fibers (16). Longitudinal (LCV) and transverse conduction velocities (TCV) were determined at the long and short axes of elliptical isochrones excluding recording channels within 1.5 mm of the pacing site. Anisotropy ratio was defined as the ratio of longitudinal-to-transverse conduction velocity. Action potential duration (APD) was measured at 80% repolarization (5).
Immunostaining
Cells were fixed in 2% paraformaldehyde, permeabilized in a 1% solution of Triton X in PBS, and blocked in a 5:1 mixture of 1% BSA and chicken serum (5). Primary antibodies (1 h at room temperature in PBS) used were anti-sarcomeric α-actinin (Sigma, mouse monoclonal), antivimentin (Sigma, mouse monoclonal), anti-connexin-43 (Zymed, rabbit polyclonal), anti-connexin-43 (Millipore, mouse monoclonal), anti-connexin-45 (kindly provided by Prof. Thomas Steinberg, Washington University, St. Louis, rabbit polyclonal), anti-connexin-40 (Zymed, rabbit polyclonal), anti-von Willebrand factor (Abcam, rabbit polyclonal), and anti-smooth muscle α-actin (Abcam, rabbit polyclonal). Secondary antibodies (Alexa Fluor 488, chicken anti-rabbit and Alexa Fluor 594, chicken anti-mouse) were applied in PBS for 1 h at room temperature. Filamentous actin was visualized with FITC-conjugated phalloidin (Sigma), and nuclei were counterstained with DAPI (Sigma). Images were acquired with a charge-coupled device camera (SensiCam QE, Cooke) attached to an inverted fluorescence microscope (Nikon TE2000) or with a confocal microscope (Zeiss LSM 510).
Quantifying the Loading Cell Area Coverage
Cardiac monolayers with ventricular nonmyocytes added on top were fixed immediately after optical mapping and immunostained for vimentin. Similarly, the live fluorescence images of eGFP, mCherry, or mOrange HEK-293 cells on top of cardiac monolayers were obtained immediately before optical mapping. For each monolayer, at least three images were acquired from random locations at ×10 magnification. From the acquired images of vimentin, eGFP, mOrange, or mCherry fluorescence, the total fluorescent area coverage was determined by counting the number of fluorescent pixels above a predetermined threshold. The reported percent coverage by cells added on top of the cardiomyocytes was then obtained by normalizing the measured fluorescence coverage in loaded cultures with that measured in separate, fully confluent monolayers made of the same loading cells.
Western Blot Analysis
Total whole cell protein extracts were assessed with Western blot analysis, as described in our previous studies (4, 37). Briefly, protein lysates from approximately 5 million cells per sample were run on an SDS gel, transferred, blocked, incubated overnight at 4°C in primary antibody (anti-connexin-43, Zymed; anti-connexin-45, Santa Cruz; anti-β-tubulin, Abcam), probed with a horseradish peroxidase-conjugated secondary antibody (Jackson Laboratories) for 1 h at room temperature, incubated in a chemiluminescence solution, and detected on a radiographic film. The intensities of connexin-43 or connexin-45 bands were measured relative to those of β-tubulin using ImageQuant TL software (Amersham Biosciences).
Fluorescence Recovery After Photobleaching
Fluorescence recovery after photobleaching (FRAP) was used to evaluate functional coupling between different cell types similar to previously described studies (1, 45). In this method, fluorescence in a photobleached cell is recovered by passage of the dye from surrounding cells through functional gap junctions, and the speed and level of the recovery reveal the degree of cell coupling. For FRAP analysis, cardiomyocytes were coseeded at a 15:1 ratio with unexcitable loading cells labeled with DiI (Molecular Probes, 2 μM, 30 min at 37°C), a lipophilic membrane stain. After 2 days, the cocultures were stained with calcein AM (Molecular Probes, 0.5 μM, 20 min at 37°C), washed with PBS, and imaged with an upright confocal microscope (Zeiss LSM 510). Individual DiI-labeled cells surrounded by cardiomyocytes were identified by red color and photobleached with a 488-nm argon laser to remove calcein fluorescence from the target cell. Calcein fluorescence recovery in bleached cells was monitored by acquiring an image every 12 s for at least 5 min after bleaching. To verify that dye transfer occurred through gap junctions, the gap junction blocker palmitoleic acid (40 μM, Fluka) was added to selected cocultures. Custom MATLAB software was used to analyze the images and plot the recovery time course after first normalizing the recorded fluorescence to the values immediately before and after bleaching and then correcting for the gradual bleaching of the entire field during image acquisitions (1).
Intracellular Recordings
Cardiomyocyte resting membrane potentials were measured using previously described methods (4) in confluent cardiac monolayers either without unexcitable cells on top (control) or with high coverage (75–100%) of fibroblasts, Cx43/HEK-293, or Cx45/HEK-293 cells. Briefly, sharp microelectrodes were fabricated using a P-97 micropipette puller (Sutter Instruments) to obtain tip resistances between 50 and 100 MΩ. Coverslips were placed into a temperature-controlled (30°C) chamber mounted onto an inverted microscope (Nikon Eclipse TE2000) and perfused with Tyrode solution. Cardiomyocytes were distinguished by specific morphology and/or lack of fluorescence and were impaled through small gaps between unexcitable cells. After the establishment of stable intracellular impalement, resting membrane potential was recorded. Signals were sampled at 20 kHz and low-pass filtered at 4.5 kHz using a 4-pole Butterworth filter in the Multiclamp 700B amplifier (Axon Instruments,). Membrane voltage recordings were digitized using a NIDAQ-MX interface (National Instruments) and analyzed using WinWCP software (Dr. John Dempster, University of Strathclyde, Glasgow, UK). Only the resting potentials that were continuously stable for >1 min were used in data analysis.
Statistical Analysis
Data are expressed as means ± SD and were analyzed using an ANOVA followed by Tukey's multiple-comparison test. Data were considered statistically significant when P < 0.05.
RESULTS
Characterization of Neonatal Rat Ventricular Nonmyocytes
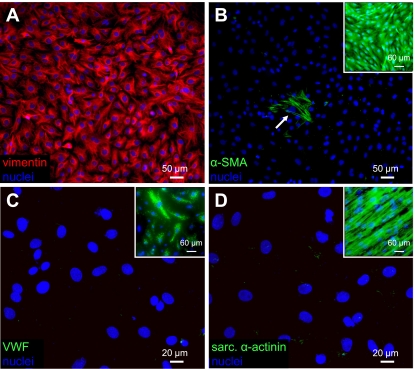
After 5 days of growth in tissue culture flasks, ventricular nonmyocytes were trypsinized, cultured for an additional 3 days on fibronectin-coated coverslips, and immunostained for vimentin, smooth muscle α-actin, von Willebrand factor, and sarcomeric α-actinin. The confluent cultures of nonmyocytes strongly and uniformly expressed vimentin (Fig. 2A), but only a very small percentage of cells (<0.01%) expressed smooth muscle α-actin (Fig. 2B). In addition, no von Willebrand factor (Fig. 2C), or sarcomeric α-actinin (Fig. 2D)-positive cells were observed. On the basis of these immunostainings, the obtained nonmyocyte population was essentially free of smooth muscle cells, endothelial cells, and cardiomyocytes and was composed primarily of cardiac fibroblasts. Furthermore, the sparse positive staining for smooth muscle α-actin also indicated that the vast majority of fibroblasts did not adopt the myofibroblast phenotype.
Fig. 2.
Phenotype of cultured neonatal rat ventricular nonmyocytes. A: cardiac nonmyocytes isolated by differential preplating and cultured on fibronectin-coated coverslips strongly and uniformly expressed vimentin. B: smooth muscle α-actin (α-SMA)-positive staining was only occasionally observed (arrow). Inset: positive control staining in adult rat aortic smooth muscle cells (Cell Applications, San Diego, CA). C: no von Willebrand factor (vWF)-positive cells were observed, indicating the absence of endothelial cells. Inset: positive control staining in human aortic endothelial cells (Cambrex, Walkersville, MD). D: no sarcomeric α-actinin-positive cells were observed, indicating the absence of cardiomyocytes. Inset: positive control staining in cardiomyocytes aligned on PDMS microgrooves. These results indicated that the isolated nonmyocytes represented a virtually pure population of cardiac fibroblasts devoid of other cardiac cells including myofibroblasts.
Structural Assessment of Fibroblast-Cardiomyocyte Cocultures
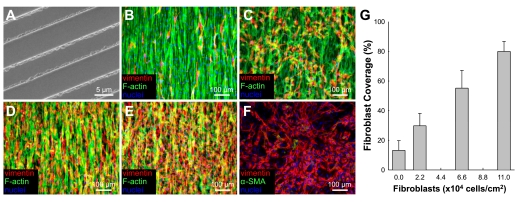
To ensure the reproducibility of aligned cardiac monolayer cultures, all microgrooved PDMS substrates were cast from the same silicon wafer (Figs. 1A and 3A). After 1–2 days of culture, cardiac cells aligned along the direction of the fibronectin-coated microgrooves and formed confluent, uniformly aligned monolayers (Fig. 3B). Endogenous fibroblast overgrowth was limited by gamma irradiation and the use of serum-free media after culture day 2. Gamma irradiation and the use of serum-free medium did not change the conduction velocity, APD, maximum capture rate, or incidence of spontaneous activity in the treated monolayers relative to nontreated controls (data not shown). Endogenous fibroblasts tended to elongate and coalign with surrounding cardiomyocytes (Fig. 3B). In contrast, exogenously added fibroblasts attached and oriented in a random fashion on top of the cardiac monolayers regardless of the number of added cells (Fig. 3, C–E). The added fibroblasts did not adopt the myofibroblast phenotype after 48 h of coculture, remaining vimentin positive and smooth muscle α-actin negative (Fig. 3F). Increasing the number of added fibroblasts yielded a linearly proportional increase in their coverage area (Fig. 3G). Specifically, while endogenous fibroblasts occupied a relatively small area (13.2 ± 6.6%) of the monolayer, the addition of exogenous fibroblasts was systematically varied to yield total fibroblast coverage (endogenous + exogenous) between 2% and 90% (Fig. 3G).
Fig. 3.
Culture of cardiac fibroblasts on top of confluent cardiac monolayers. A: scanning electron micrograph of the silicon wafer used to create the PDMS microgrooved substrates for aligned cardiomyocyte growth. B–E: confluent aligned cardiomyocytes on PDMS microgrooves with 0 (B), 2.2 × 104 (C), 6.6 × 104 (D), or 11.0 × 104 (E) cardiac fibroblasts per cm2 added on top. F: fibroblasts on top of cardiac monolayers remained vimentin positive and smooth muscle α-actin negative. G: the percentage of fibroblast coverage area was linearly proportional to number of added fibroblasts.
Changes in Cardiac Conduction Due to Electrotonic Loading With Cardiac Fibroblasts
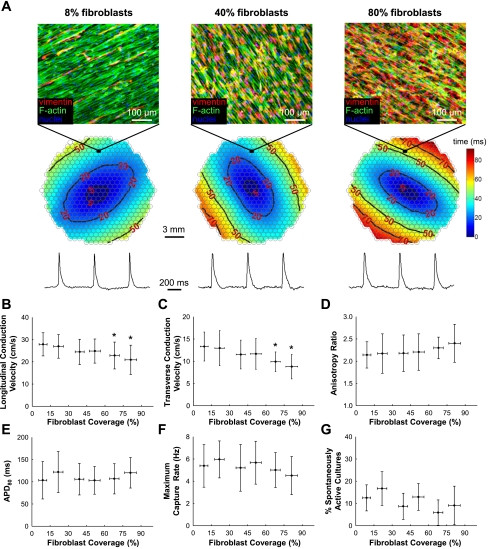
The effect of cardiac fibroblast electrotonic loading on the electrophysiological properties of cardiomyocytes was assessed by optical mapping. To ensure a high degree of structural and functional homogeneity in the monolayers, cultures were inspected before optical mapping experiments by a light microscope and subsequently by immunostaining to confirm the absence of relatively large (>100 × 100 μm2) acellular regions. For all degrees of fibroblast loading, point pacing at 2 Hz yielded uniform anisotropic propagation with smooth elliptical isochrones (Fig. 4A). As the fibroblast coverage area increased from 9.0 ± 3.3% (0–15% range) to 81.3 ± 4.8% (75–100% range), conduction velocity in loaded monolayers decreased from 27.8 ± 5.2 cm/s to 20.9 ± 6.6 cm/s in the longitudinal direction (Fig. 4B) and from 13.3 ± 3.3 cm/s to 8.8 ± 2.7 cm/s in the transverse direction (Fig. 4C), yielding no significant change in anisotropy ratio (mean = 2.21, P = 0.34, Fig. 4D). In both the transverse and the longitudinal directions, only when considerable monolayer area (>60%) was covered by fibroblasts did action potential propagation slow to a significant degree compared with the control (Fig. 4, B and C). Regardless of the percent fibroblast coverage, no significant effect from fibroblast loading was observed on cardiac APD (Fig. 4E), maximum capture rate (Fig. 4F), or the presence of spontaneous contractile activity, which on average was found in a relatively low fraction of all cell cultures (11.6 ± 2.7%, Fig. 4G).
Fig. 4.
Effect of fibroblast electrotonic loading on cardiac electrophysiological parameters. A: representative isochrone maps and action potential traces from monolayers with 8%, 40%, and 80% fibroblast coverage stimulated in the center at 2-Hz pacing rate. Note decreased distance between successive isochrones lines (i.e., conduction slowing) with increased fibroblast coverage. Circles in isochrone maps represent optical recording sites. B–G: measured electrophysiological parameters as a function of fibroblast coverage. The monolayers were separated into six bins based on the percentage of fibroblast coverage: 0–15% (n = 32 monolayers), 15–30% (n = 24), 30–45% (n = 23), 45–60% (n = 31), 60–75% (n = 17), and 75–100% (n = 11). APD80, action potential duration measured at 80% repolarization. *Significant difference from the first bin (0–15% coverage; P < 0.05).
Changes in Cardiac Conduction Due to Electrotonic Loading With HEK-293 Cells
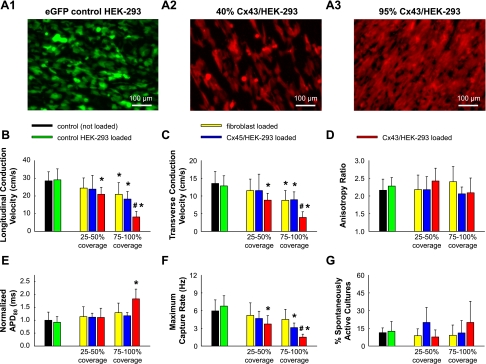
Because of the relatively small effect on cardiac impulse propagation from fibroblast electrotonic loading, we aimed to further clarify the role of connexin isoform by using the same experimental setting to investigate the loading effects from 1) control HEK-293 cells, 2) connexin-43-overexpressing HEK-293 cells (Cx43/HEK-293), and 3) connexin-45-overexpressing HEK-293 cells (Cx45/HEK-293) (Fig. 5, A1–A3). In particular, while electrotonic loading of cardiac monolayers with control HEK-293 cells exerted no effect on cardiac conduction (green bars in Fig. 5, B and C), loading with Cx43/HEK-293 cells at 25–50% coverage reduced LCV, TCV, and maximum capture rate to 73.4 ± 13.9% (Fig. 5B), 65.2 ± 14.3% (Fig. 5C), and 62.7 ± 23.9% (Fig. 5F) of the respective values measured in control (unloaded) cardiac cultures. In comparison, neither cardiac fibroblasts nor Cx45/HEK-293 cells covering the same cardiac monolayer area (25–50%) exerted significant effect on conduction velocity (Fig. 5, B and C). When the coverage density of Cx43/HEK-293 cells was increased to 75–100%, LCV, TCV, and maximum capture rate were further decreased to 28.5 ± 10.9% (Fig. 5B), 29.0 ± 11.8% (Fig. 5C), and 25.2 ± 8.4% (Fig. 5F) of the respective values measured in control (unloaded) cardiac cultures, while APD was increased to 182.8 ± 38.0% of the control value (Fig. 5E). At high coverage densities (75–100%), Cx45/HEK-293 and fibroblast loading yielded similar levels of longitudinal and transverse conduction slowing (in average to 69.1 ± 20.0% and 65.4 ± 18.2%, respectively, of control) which were less than the slowing induced by same coverage Cx43/HEK-293 loading (Fig. 5, B and C). In contrast to Cx43/HEK-293 loading, neither Cx45/HEK-293 nor fibroblast loading affected cardiac APD (Fig. 5E), and the only effect on maximum capture rate was found from the Cx45/HEK-293 loading at 75–100% coverage (Fig. 5F). For all cell types and degrees of loading, no significant effect was found on velocity anisotropy ratio (Fig. 5D) or percentage of spontaneously active cultures (Fig. 5G).
Fig. 5.
Effect of control HEK-293, Cx45/HEK-293, and Cx43/HEK-293 cell loading on cardiac electrophysiological parameters. A1–A3: live fluorescent images of anisotropic cardiac monolayers covered with enhanced green fluorescent protein (eGFP)-transfected control HEK-293 cells (35–50% coverage, n = 16; A1), mCherry and Cx43-transfected HEK-293 cells at medium (25–50%, n = 13; A2)- and high (75–100%, n = 5; A3)-density coverage, or mOrange and Cx45-transfected HEK-293 cells at medium (25–50%, n = 10)- and high (75–100%, n = 9)-density coverage (not shown). B–G: comparison of electrophysiological parameters for low and high coverage loading by cardiac fibroblasts (yellow), Cx45/HEK-293 cells (blue), and Cx43/HEK-293 cells (red) relative to loading by control HEK-293 cells (green) and no loading (black). *Significant difference from the not loaded and control HEK-293-loaded monolayers (P < 0.05). #Significant difference from the same coverage fibroblast-loaded monolayers (P < 0.05).
Expression of Connexin-43 in Unexcitable Cells and Cardiomyocytes
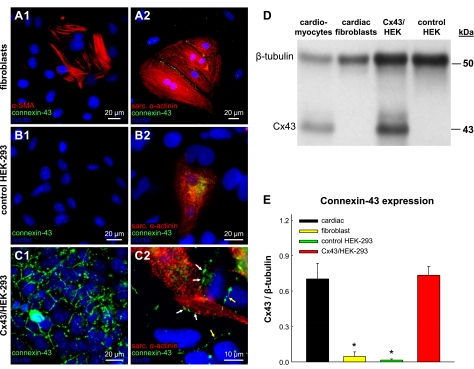
To understand the observed functional differences in cardiac propagation from loading with different unexcitable cell types, we first performed immunostaining and Western blot analyses to assess the distribution and quantify the expression of the major ventricular gap junction protein connexin-43. Cardiac fibroblasts, control HEK-293 cells, and Cx45/HEK-293 cells (not shown) were found to exhibit no detectable presence of connexin-43 junctions when grown in monocultures (Fig. 6, A1 and B1) or cocultures with cardiomyocytes (Fig. 6, A2 and B2). In contrast, Cx43/HEK-293 cells formed abundant gap junctions at their cell borders when cultured alone (Fig. 6C1) or in contact with cardiomyocytes (Fig. 6C2). Consistent with these findings, Western blot analysis (Fig. 6D) revealed negligible levels of connexin-43 protein expression in cardiac fibroblasts and control HEK-293 cells. In contrast, Cx43/HEK-293 cells expressed connexin-43 at levels that were similar to those measured in neonatal rat cardiomyocytes (Fig. 6E).
Fig. 6.
Cx43 expression in unexcitable loading cells and cardiomyocytes. A1, B1, and C1: immunostaining for Cx43 gap junctions in cultures of cardiac fibroblasts (A1), control HEK-293 cells (B1), and Cx43/HEK-293 cells (C1). A2, B2, and C2: immunostaining for Cx43 gap junctions in cocultures of cardiomyocytes with cardiac fibroblasts (A2), control HEK-293 cells (B2), or Cx43/HEK-293 cells (C2). White and yellow arrows in C2 denote heterocellular and homocellular junctions, respectively, in Cx43/HEK-293 cells. No Cx43 junctions were observed in fibroblasts, smooth muscle α-actin-positive nonmyocytes, or control HEK-293 cells. D: an example of a Cx43 Western blot for different unexcitable cells and cardiomyocytes. E: corresponding semiquantitative analysis of Cx43 expression relative to that of β-tubulin. *Significant difference from cardiomyocytes (P < 0.05).
Functional Coupling By FRAP Analysis
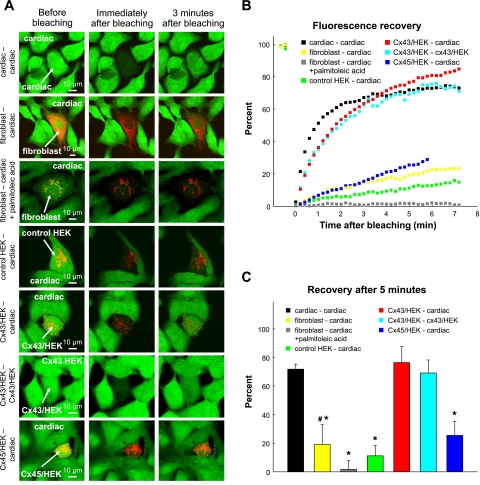
In addition to structural assessment of gap junction coupling by immunostaining and Western blot analysis, FRAP was used to assess and compare the functional coupling of different unexcitable cell types and cardiomyocytes. Individual cardiomyocytes, fibroblasts, or HEK-293 cells were photobleached and monitored for the recovery of green (calcein) fluorescence in different mono- and coculture settings (Fig. 7A). The fluorescence recovery was comparable in cardiomyocytes only, Cx43/HEK-293 cells only, and Cx43/HEK-293 cells surrounded by cardiomyocytes and was found to be considerably slower in cardiac fibroblasts, Cx45/HEK-293 cells, or control HEK-293 cells surrounded by cardiomyocytes (Fig. 7B). Specifically, 5 min after photobleaching, Cx43/HEK-293 cells and cardiomyocytes recovered their fluorescence to levels 2.9- to 4.0-fold higher than those measured in cardiac fibroblasts and Cx45/HEK-293 cells and 6.4- to 6.8-fold higher than in control HEK-293 cells in similar settings (Fig. 7C). In the presence of the gap junction blocker palmitoleic acid (Fig. 7A, third row), the small degree of postbleaching recovery in fibroblasts (and Cx45/HEK-293 cells; not shown) surrounded by cardiomyocytes was completely abolished (Fig. 7, B and C), confirming that these cells were coupled to cardiomyocytes through functional gap junctions and that no nonspecific leakage of calcein dye into the photobleached cells contributed to the observed fluorescence recovery.
Fig. 7.
Analysis of functional gap junctional coupling by FRAP. An individual cell was photobleached with a 488-nm laser and monitored for recovery of calcein dye (green) fluorescence using a confocal microscope. A: each row shows fluorescence before, immediately after, and 3 min after photobleaching in cardiac monocultures (n = 5), cardiac-fibroblast cocultures (n = 7), cardiac-fibroblast cocultures with 40 μM palmitoleic acid added (n = 7), cardiac-control HEK-293 cocultures (n = 3), cardiac-Cx43/HEK-293 cocultures (n = 3), Cx43/HEK-293 monocultures (n = 5), and cardiac-Cx45/HEK-293 cocultures (n = 16). Arrows point to photobleached cells. DiI staining (red) in cocultures was used to distinguish loading cells from cardiomyocytes. B: averaged time courses of fluorescence recovery for each of the settings shown in A. Note no recovery in the presence of palmitoleic acid. C: percent fluorescence recovery 5 min after bleaching. *Significant difference from cardiac-cardiac group (P < 0.05). #Significant difference from the same group in the presence of palmitoleic acid (P < 0.05).
Expression of Connexin-45 and Connexin-40 in Unexcitable Cells and Cardiomyocytes
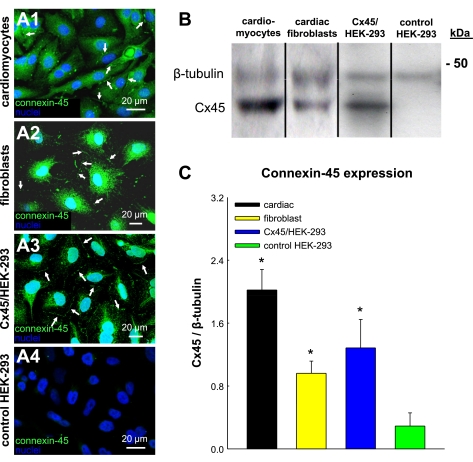
To further elucidate the roles of other connexin isoforms in the measured electrotonic effects, we assessed and compared the expression of the cardiac gap junction proteins connexin-45 and connexin-40 in cardiomyocytes and unexcitable cells. Connexin-45 antibodies used for these studies (24) were selected on the basis of their positive immunostaining and immunoblotting of Cx45/HEK-293 cells and negative immunostaining and immunoblotting of Cx43/HEK-293 cells. Immunostaining of confluent monolayers revealed connexin-45 expression between adjacent cardiomyocytes (Fig. 8 A1), fibroblasts (Fig. 8A2), and Cx45/HEK-293 cells (Fig. 8A3), but not control HEK-293 (Fig. 8A4) or Cx43/HEK-293 cells (not shown). Furthermore, no positive immunostaining for connexin-40 was found in cardiomyocytes or fibroblasts (not shown). Consistent with immunostainings, Western blot analysis showed that cardiomyocytes, fibroblasts, and Cx45/HEK-293 cells exhibited significantly higher connexin-45 expression than control HEK-293 cells (Fig. 8B). In particular, the expression level of connexin-45 relative to β-tubulin in fibroblasts was 2.9 times higher than in control HEK-293 cells and 1.4 times lower than in cardiomyocytes, while connexin-45 expression in Cx45/HEK-293 cells was slightly but not significantly higher than that of fibroblasts (Fig. 8C).
Fig. 8.
Cx45 expression in unexcitable loading cells and cardiomyocytes. A1–A4: immunostaining for Cx45 gap junctions in cultures of cardiomyocytes and loading cells. Note the expression of Cx45 gap junctions in cardiomyocytes, fibroblasts, and Cx45/HEK-293 cells (arrows), but not in control HEK-293 cells. B: an example of a Cx45 Western blot. C: corresponding semiquantitative analysis of Cx45 expression relative to that of β-tubulin. *Significant difference from control HEK-293 cells (P < 0.05).
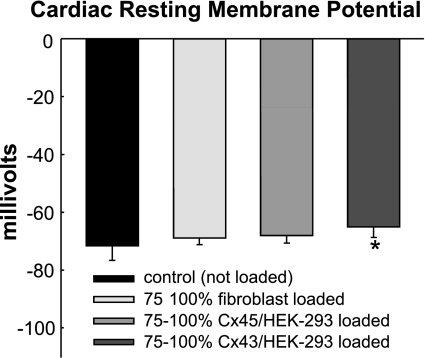
Changes in Cardiomyocyte Resting Membrane Potential Due to Electrotonic Loading
To further understand how electrotonic loading by different unexcitable cells alters cardiac propagation, we measured the cardiomyocyte resting potential in confluent monolayers under different loading conditions (Fig. 9). Consistent with our previous studies (4), control cardiomyocytes without added unexcitable cells exhibited an average resting potential of −71.6 ± 4.9 mV. When Cx43/HEK-293 cells were added at a high coverage density (75–100%), cardiomyocyte resting potential modestly but significantly depolarized to −65.0 ± 3.8 mV. In contrast, high coverage loading with fibroblasts or Cx45/HEK-293 cells caused only a subtle cardiac depolarization (−68.8 ± 2.3 mV and −68.0 ± 2.7 mV, respectively) without reaching statistical significance.
Fig. 9.
Cardiomyocyte resting membrane potential under different loading conditions. Cardiac resting potential is shown from control monolayers with no cells added (n = 14), as well as from monolayers covered 75–100% with fibroblasts (n = 13), Cx45/HEK-293 cells (n = 10), or Cx43/HEK-293 cells (n = 7). *Significant difference from control monolayers (P < 0.05).
DISCUSSION
In this study, we assessed the effects of electrotonic loading by different unexcitable cells on impulse conduction in anisotropic confluent neonatal rat cardiac monolayers. Through the combined use of genetic engineering techniques, structural assays (immunostaining, immunoblotting), and functional assays (FRAP, optical mapping, sharp microelectrode recordings), we demonstrated that the electrophysiological function of cardiomyocytes loaded by unexcitable cells strongly depends on the type and expression level of gap junction proteins. In particular, cardiac fibroblasts and HEK-293 cells that expressed similar levels of connexin-45, but no detectable amount of connexin-43 or connexin-40, marginally slowed cardiac conduction only at high coverage density, while producing no other measured electrophysiological changes in cardiomyocytes. In contrast, HEK-293 cells expressing connexin-43 at a level comparable to that of cardiomyocytes strongly coupled to cardiomyocytes, depolarized cardiac resting membrane potential, significantly slowed impulse propagation, decreased maximum capture rate, and increased action potential duration at high coverage density. None of the studied unexcitable cells significantly altered conduction velocity anisotropy ratio or the relatively low incidence of spontaneous activity of cardiac monolayers at any coverage density.
In the current study, nonmyocytes from the ventricles of neonatal rats cultured on plastic coverslips (Fig. 2A) or on top of cardiomyocytes (Fig. 3F) were found to strongly express vimentin and only coexpress the contractile protein smooth muscle α-actin in a few cells per coverslip (Fig. 2B). Therefore, the studied nonmyocytes were referred to throughout the text as cardiac fibroblasts rather than myofibroblasts, the smooth muscle-like cells found in hypertensive (25) or postinfarction (43) disease. Several studies have shown that cultured fibroblasts can also adopt the myofibroblast phenotype by growth on a rigid substrate (27), application of force (46), exposure to transforming growth factor-β1 (9), or culture in perceived hyperoxic conditions (35). Our study, however, suggests that growth of neonatal rat ventricular fibroblasts on a rigid substrate or on cardiomyocytes is not sufficient for induction of the myofibroblast phenotype, but rather that certain conditions in the cell isolation and culture procedure determine the ability of these cells in vitro to either maintain their in vivo phenotype or become myofibroblasts. Importantly, several different structural and functional assays in this study (Figs. 4–7) consistently supported the finding that neonatal rat cardiac fibroblasts exhibited relatively weak electrical coupling with cardiomyocytes in vitro.
In particular, connexin-43 gap junctions were not observed between fibroblasts and cardiomyocytes or between abutting fibroblasts (Fig. 6, A1 and A2) in immunostainings, a finding that was further confirmed by Western blot analysis (Fig. 6, D and E). However, FRAP experiments revealed that despite the absence of connexin-43 expression, fibroblasts still exhibited limited intercellular communication with cardiomyocytes, which was abolished in the presence of the gap junction blocker palmitoleic acid (Fig. 7). Additional Western blot and immunostaining analyses revealed expression of connexin-45 (Fig. 8), but not connexin-40 (not shown), in cardiac fibroblasts and myocytes. Since connexin-30.2 junctions, if present, have a relatively small conductance (23), it was concluded that connexin-45 was the dominant connexin isoform expressed in fibroblasts which enabled their coupling with cardiomyocytes causing the relatively weak electrotonic loading of monolayers. This conclusion was further supported by the finding that HEK-293 cells engineered to express connexin-45 at a level similar to that of fibroblasts (Fig. 8) 1) functionally coupled to cardiomyocytes at a level similar to that of fibroblasts (Fig. 7) and 2) produced comparable effects on resting membrane potential and impulse conduction in loaded cardiac monolayers to those produced by fibroblasts (Figs. 5 and 9).
In contrast to cardiac fibroblasts and Cx45/HEK-293 cells, Cx43/HEK-293 cells were found to express high levels of connexin-43 (Fig. 6, C1, D, and E), negligible levels of connexin-45 (Fig. 8, A4, B, and C), and to strongly couple among themselves and with cardiomyocytes (Figs. 7 and 6C2). As a result of stronger coupling (17, 18, 36), electrotonic loading by Cx43/HEK-293 cells depolarized cardiac resting potential (Fig. 9) and slowed cardiac conduction more than loading by cardiac fibroblasts or Cx45/HEK-293 cells at equal coverage (Fig. 5, B and C). On the basis of the expression of specific connexin isoforms, fibroblasts and Cx45/HEK-293 cells were expected to electrically couple with cardiomyocytes via homotypic Cx45 and heterotypic or heteromeric Cx45/Cx43 gap junctions (29), while Cx43/HEK-293 cells and cardiomyocytes were expected to couple through predominantly homotypic Cx43 junctions as well as Cx43/Cx45 junctions. Under a simplifying assumption of similar fibroblast and HEK-293 cell membrane properties, a recent modeling study by Jacquemet and Henriquez (17) allows for speculation about the magnitude of coupling conductance between different unexcitable cells and cardiomyocytes in this study. To match the percent longitudinal conduction slowing at 40% cell coverage found in our experiments to the same level of slowing at 40% coverage in the model (17), the average fibroblast-cardiomyocyte or Cx45/HEK-293-cardiomyocyte coupling conductance would have to be 10 nS [i.e., an equivalent of 285 Cx45/Cx45 (31) or 180 Cx45/Cx43 (10) open gap junction channels] while the Cx43/HEK-cardiomyocyte coupling conductance would have to be >80 nS [i.e., an equivalent of >890 Cx43/Cx43 (30) or >1,440 Cx43/Cx45 open gap junction channels]. Therefore, the strong electrotonic effect observed from Cx43/HEK-293 cells compared with other unexcitable cells in this study was likely a result of both the expression of the more conductive connexin isoform and the higher number of gap junction channels that formed between these cells and cardiomyocytes.
On the other hand, factors other than coupling strength including loading cell size, resting potential, and the presence of different ion currents remain to be studied in detail as they are also expected to play important roles in the interactions between cardiomyocytes and unexcitable cells (17, 26, 36). In this study, two different unexcitable cell types (Cx45/HEK-293 cells and fibroblasts) which coupled to cardiomyocytes with similar strengths produced similar changes in cardiac conduction (Fig. 5, B–G), suggesting that coupling strength (at least for relatively low levels of coupling) was the major determinant of the observed electrotonic effects.
Importantly, at the highest coverage densities of Cx43/HEK-293 cells (>95%) the conduction velocity was decreased to as low as 22% of that in unloaded controls without causing conduction block. Previous computational and experimental studies have shown that complete block or inactivation of sodium current in cardiomyocytes can only slow conduction velocity to ∼33% of the normal value (19, 34, 40). Regarding the modest resting potential depolarization of cardiomyocytes loaded by Cx43/HEK-293 cells (Fig. 9), the excessive conduction slowing in the loaded monolayers was likely caused, to a smaller extent, by partial inactivation of sodium channels in depolarized cardiomyocytes (i.e., resistive loading effect) and, to a much larger extent, by slower charging of effectively larger cardiomyocyte membrane area (i.e., capacitive loading effect). Conceivably, in a three-dimensional tissue environment, the larger portion of the cardiac cell membrane available for contact with other cells may further exacerbate the loading effects. In general, with regard to the potential for conduction slowing, the effect of strong electrotonic loading of cardiomyocytes by unexcitable cells appears intermediate between the effects of reduced excitability and reduced coupling (34, 40).
In the present study, we also assessed APD, maximum capture rate, and velocity anisotropy ratio in cardiac monolayers loaded with unexcitable cells (Fig. 4, E–G, and Fig. 5, E–G). In particular, 75–100% coverage with Cx43/HEK-293 cells yielded significant action potential prolongation and decrease in maximum capture rate. This prolongation of the short rat action potential likely occurred because relatively strong coupling current from the depolarized Cx43/HEK-293 cells slowed down the repolarization of the cardiac membrane potential to its resting value (17, 36). In contrast, weaker coupling with cardiac fibroblasts or Cx45/HEK-293 cells exerted no measurable effect on cardiac APD, likely because the resulting electrotonic currents were insufficient to significantly affect the relatively large repolarization current of the triangularly shaped rat action potential. In contrast, cardiac action potentials with a prominent plateau phase are expected to undergo a major APD change even as a result of weak coupling with loading cells because a small plateau current (large input resistance) renders the plateau duration highly sensitive to electrotonic currents of any magnitude (26). In addition, we also found that, for all degrees of electrotonic loading by different unexcitable cells, longitudinal and transverse conduction in cardiac monolayers were slowed to the same degree, yielding no change in velocity anisotropy ratio (Figs. 4D and 5D). The increased cardiac APD and unchanged velocity anisotropy ratio found for strong electrotonic loading in our experiments are consistent with recent computational studies in a microstructural model of anisotropic canine atrial monolayers (18).
Finally, despite the significant slowing of conduction velocity, strong electrotonic loading by Cx43/HEK-293 cells in our study did not increase the incidence of spontaneous activity in cardiac monolayers (Fig. 5G). In general, the ability of cardiomyocytes electrotonically loaded with unexcitable cells to successfully generate and launch spontaneous action potentials will depend on a number of factors including 1) coupling resistance between cardiomyocytes and unexcitable cells, 2) unexcitable cell properties (e.g., cell size, resting potential, input resistance at subthreshold voltages, etc.), 3) cardiomyocyte properties [e.g., cell size, resting potential, input resistance at subthreshold voltages, duration of plateau phase, Na+-Ca2+ exchanger activity (42), etc.], and 4) geometrical loading conditions surrounding a potential focal source [e.g., dimensionality of cardiac network (1-D, 2-D, or 3-D), vicinity to tissue expansions, or narrowings, etc.]. In particular, while strong electrotonic loading by depolarized unexcitable cells could favor spontaneous activity through depolarization of the cardiac resting potential to near-threshold values (resistive loading), it could also suppress spontaneous activity by increasing the membrane area needed to be activated both at and around the focal source (capacitive loading). Therefore, the complex interplay between resistive and capacitive loading effects will determine the occurrence and incidence of spontaneous activity. For example, while a large number of conditions in loaded anisotropic cardiac monolayers explored in this study and in a computational study by Jacquemet and Henriquez (17) resulted in only a few millivolts depolarization of cardiac resting potential and no change in the incidence of spontaneous activity, myofibroblast loading in 1-D cardiac strands in a study by Miragoli et al. (28) yielded cardiomyocyte depolarization by as much as 30 mV and spontaneous activity in virtually all cell cultures. Assuming that electrical properties of cardiomyocytes were similar in the two experimental studies, these major differences in cardiac resistive loading suggest that the myofibroblasts in the studies by Miragoli et al. had a significantly lower input resistance at subthreshold voltages compared with the Cx43/HEK-293 cells used in this study. In addition, paracrine and juxtacrine interactions between loading cells and cardiomyocytes may independently affect the conduction and incidence of spontaneous activity.
In conclusion, this study shows that electrical coupling of cardiomyocytes with unexcitable cells can alter anisotropic action potential propagation in a fashion that significantly depends on the level and isoform of connexin expression by the unexcitable cells. Specifically, the expression of connexin-45, but not connexin-43 or connexin-40, as found in smooth muscle α-actin negative neonatal rat ventricular fibroblasts, or as engineered in HEK-293 cells, was associated with only a weak effect on cardiac impulse conduction. In contrast, the presence of functional connexin-43 gap junctions in unexcitable Cx43/HEK-293 cells at levels comparable to those in cardiomyocytes caused significant conduction slowing and increase of action potential duration, both of which could increase the propensity for conduction block and arrhythmia induction in vivo. It is, however, important to note that the triangular action potential shape, presence of specific ion channels, relatively small membrane area (capacitance), uniform membrane distribution of gap junctions, and expression of specific connexin isoforms (2) characteristic of neonatal rat ventricular myocytes may limit the relevance of these findings for cardiomyocytes of other species and ages. Nevertheless, this study represents a step toward enhanced understanding of the potential electrotonic effects of various unexcitable cells on cardiac tissue function, which in the future is expected to aid the design of novel antiarrhythmic therapies (13) in fibrotic and other heart diseases.
GRANTS
This work was supported by the American Heart Association Predoctoral Fellowship 0715288U (to L. C. McSpadden), the National Science Foundation Graduate Research Fellowship (to R. D. Kirkton), American Heart Association Scientist Development Grant 0530256N, and National Heart, Lung, and Blood Institute Grant HL-083342 (to N. Bursac).
Acknowledgments
The authors acknowledge James Scull for technical assistance with optical mapping studies, Prof. Thomas Steinberg, Washington University, St. Louis, for providing a connexin-45 antibody, and Dr. Eric Beyer, University of Chicago, for providing the connexin-45 plasmid.
REFERENCES
- 1.Abbaci M, Barberi-Heyob M, Stines JR, Blondel W, Dumas D, Guillemin F, Didelon J. Gap junctional intercellular communication capacity by gap-FRAP technique: a comparative study. Biotechnol J 2: 50–61, 2007. [DOI] [PubMed] [Google Scholar]
- 2.Alcolea S, Theveniau-Ruissy M, Jarry-Guichard T, Marics I, Tzouanacou E, Chauvin JP, Briand JP, Moorman AF, Lamers WH, Gros DB. Downregulation of connexin 45 gene products during mouse heart development. Circ Res 84: 1365–1379, 1999. [DOI] [PubMed] [Google Scholar]
- 3.Bursac N, Aguel F, Tung L. Multiarm spirals in a two-dimensional cardiac substrate. Proc Natl Acad Sci USA 101: 15530–15534, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bursac N, Papadaki M, White JA, Eisenberg SR, Vunjak-Novakovic G, Freed LE. Cultivation in rotating bioreactors promotes maintenance of cardiac myocyte electrophysiology and molecular properties. Tissue Eng 9: 1243–1253, 2003. [DOI] [PubMed] [Google Scholar]
- 5.Bursac N, Parker KK, Iravanian S, Tung L. Cardiomyocyte cultures with controlled macroscopic anisotropy: a model for functional electrophysiological studies of cardiac muscle. Circ Res 91: e45–e54, 2002. [DOI] [PubMed] [Google Scholar]
- 6.Bursac N, Tung L. Acceleration of functional reentry by rapid pacing in anisotropic cardiac monolayers: formation of multi-wave functional reentries. Cardiovasc Res 69: 381–390, 2006. [DOI] [PubMed] [Google Scholar]
- 7.Camelliti P, Borg TK, Kohl P. Structural and functional characterisation of cardiac fibroblasts. Cardiovasc Res 65: 40–51, 2005. [DOI] [PubMed] [Google Scholar]
- 8.Chilton L, Ohya S, Freed D, George E, Drobic V, Shibukawa Y, Maccannell KA, Imaizumi Y, Clark RB, Dixon IM, Giles WR. K+ currents regulate the resting membrane potential, proliferation, and contractile responses in ventricular fibroblasts and myofibroblasts. Am J Physiol Heart Circ Physiol 288: H2931–H2939, 2005. [DOI] [PubMed] [Google Scholar]
- 9.Desmouliere A, Geinoz A, Gabbiani F, Gabbiani G. Transforming growth factor-beta 1 induces alpha-smooth muscle actin expression in granulation tissue myofibroblasts and in quiescent and growing cultured fibroblasts. J Cell Biol 122: 103–111, 1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Elenes S, Martinez AD, Delmar M, Beyer EC, Moreno AP. Heterotypic docking of Cx43 and Cx45 connexons blocks fast voltage gating of Cx43. Biophys J 81: 1406–1418, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Entcheva E, Lu SN, Troppman RH, Sharma V, Tung L. Contact fluorescence imaging of reentry in monolayers of cultured neonatal rat ventricular myocytes. J Cardiovasc Electrophysiol 11: 665–676, 2000. [DOI] [PubMed] [Google Scholar]
- 12.Gaudesius G, Miragoli M, Thomas SP, Rohr S. Coupling of cardiac electrical activity over extended distances by fibroblasts of cardiac origin. Circ Res 93: 421–428, 2003. [DOI] [PubMed] [Google Scholar]
- 13.Gepstein L Electrophysiologic implications of myocardial stem cell therapies. Heart Rhythm 5: S48–S52, 2008. [DOI] [PubMed] [Google Scholar]
- 14.Goldsmith EC, Hoffman A, Morales MO, Potts JD, Price RL, McFadden A, Rice M, Borg TK. Organization of fibroblasts in the heart. Dev Dyn 230: 787–794, 2004. [DOI] [PubMed] [Google Scholar]
- 15.Heubach JF, Graf EM, Leutheuser J, Bock M, Balana B, Zahanich I, Christ T, Boxberger S, Wettwer E, Ravens U. Electrophysiological properties of human mesenchymal stem cells. J Physiol 554: 659–672, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Iravanian S, Nabutovsky Y, Kong CR, Saha S, Bursac N, Tung L. Functional reentry in cultured monolayers of neonatal rat cardiac cells. Am J Physiol Heart Circ Physiol 285: H449–H456, 2003. [DOI] [PubMed] [Google Scholar]
- 17.Jacquemet V, Henriquez CS. Loading effect of fibroblast-myocyte coupling on resting potential, impulse propagation, and repolarization: insights from a microstructure model. Am J Physiol Heart Circ Physiol 294: H2040–H2052, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jacquemet V, Henriquez CS. Modelling cardiac fibroblasts: interactions with myocytes and their impact on impulse propagation. Europace 9, Suppl 6: vi29–vi37, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jalife J, Sicouri S, Delmar M, Michaels DC. Electrical uncoupling and impulse propagation in isolated sheep Purkinje fibers. Am J Physiol Heart Circ Physiol 257: H179–H189, 1989. [DOI] [PubMed] [Google Scholar]
- 20.Kawara T, Derksen R, de Groot JR, Coronel R, Tasseron S, Linnenbank AC, Hauer RN, Kirkels H, Janse MJ, de Bakker JM. Activation delay after premature stimulation in chronically diseased human myocardium relates to the architecture of interstitial fibrosis. Circulation 104: 3069–3075, 2001. [DOI] [PubMed] [Google Scholar]
- 21.Klinger R, Bursac N. Cardiac cell therapy in vitro: reproducible assays for comparing the efficacy of different donor cells. IEEE Eng Med Biol Mag 27: 72–80, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kohl P, Camelliti P, Burton FL, Smith GL. Electrical coupling of fibroblasts and myocytes: relevance for cardiac propagation. J Electrocardiol 38: 45–50, 2005. [DOI] [PubMed] [Google Scholar]
- 23.Kreuzberg MM, Sohl G, Kim JS, Verselis VK, Willecke K, Bukauskas FF. Functional properties of mouse connexin30.2 expressed in the conduction system of the heart. Circ Res 96: 1169–1177, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lecanda F, Towler DA, Ziambaras K, Cheng SL, Koval M, Steinberg TH, Civitelli R. Gap junctional communication modulates gene expression in osteoblastic cells. Mol Biol Cell 9: 2249–2258, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lekgabe ED, Kiriazis H, Zhao C, Xu Q, Moore XL, Su Y, Bathgate RA, Du XJ, Samuel CS. Relaxin reverses cardiac and renal fibrosis in spontaneously hypertensive rats. Hypertension 46: 412–418, 2005. [DOI] [PubMed] [Google Scholar]
- 26.MacCannell KA, Bazzazi H, Chilton L, Shibukawa Y, Clark RB, Giles WR. A mathematical model of electrotonic interactions between ventricular myocytes and fibroblasts. Biophys J 92: 4121–4132, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Miragoli M, Gaudesius G, Rohr S. Electrotonic modulation of cardiac impulse conduction by myofibroblasts. Circ Res 98: 801–810, 2006. [DOI] [PubMed] [Google Scholar]
- 28.Miragoli M, Salvarani N, Rohr S. Myofibroblasts induce ectopic activity in cardiac tissue. Circ Res 101: 755–758, 2007. [DOI] [PubMed] [Google Scholar]
- 29.Moreno AP Biophysical properties of homomeric and heteromultimeric channels formed by cardiac connexins. Cardiovasc Res 62: 276–286, 2004. [DOI] [PubMed] [Google Scholar]
- 30.Moreno AP, Fishman GI, Spray DC. Phosphorylation shifts unitary conductance and modifies voltage dependent kinetics of human connexin43 gap junction channels. Biophys J 62: 51–53, 1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Moreno AP, Laing JG, Beyer EC, Spray DC. Properties of gap junction channels formed of connexin 45 endogenously expressed in human hepatoma (SKHep1) cells. Am J Physiol Cell Physiol 268: C356–C365, 1995. [DOI] [PubMed] [Google Scholar]
- 32.Nag AC Study of non-muscle cells of the adult mammalian heart: a fine structural analysis and distribution. Cytobios 28: 41–61, 1980. [PubMed] [Google Scholar]
- 33.Pedrotty DM, Klinger RY, Badie N, Hinds S, Kardashian A, Bursac N. Structural coupling of cardiomyocytes and noncardiomyocytes: quantitative comparisons using a novel micropatterned cell pair assay. Am J Physiol Heart Circ Physiol 295: H390–H400, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rohr S, Kucera JP, Kleber AG. Slow conduction in cardiac tissue, I: effects of a reduction of excitability versus a reduction of electrical coupling on microconduction. Circ Res 83: 781–794, 1998. [DOI] [PubMed] [Google Scholar]
- 35.Roy S, Khanna S, Bickerstaff AA, Subramanian SV, Atalay M, Bierl M, Pendyala S, Levy D, Sharma N, Venojarvi M, Strauch A, Orosz CG, Sen CK. Oxygen sensing by primary cardiac fibroblasts: a key role of p21(Waf1/Cip1/Sdi1). Circ Res 92: 264–271, 2003. [DOI] [PubMed] [Google Scholar]
- 36.Sachse FB, Moreno AP, Abildskov JA. Electrophysiological modeling of fibroblasts and their interaction with myocytes. Ann Biomed Eng 36: 41–56, 2008. [DOI] [PubMed] [Google Scholar]
- 37.Sathaye A, Bursac N, Sheehy S, Tung L. Electrical pacing counteracts intrinsic shortening of action potential duration of neonatal rat ventricular cells in culture. J Mol Cell Cardiol 41: 633–641, 2006. [DOI] [PubMed] [Google Scholar]
- 38.Shaner NC, Campbell RE, Steinbach PA, Giepmans BN, Palmer AE, Tsien RY. Improved monomeric red, orange and yellow fluorescent proteins derived from Discosoma sp. red fluorescent protein. Nat Biotechnol 22: 1567–1572, 2004. [DOI] [PubMed] [Google Scholar]
- 39.Shaw RM, Rudy Y. Electrophysiologic effects of acute myocardial ischemia. A mechanistic investigation of action potential conduction and conduction failure. Circ Res 80: 124–138, 1997. [DOI] [PubMed] [Google Scholar]
- 40.Shaw RM, Rudy Y. Ionic mechanisms of propagation in cardiac tissue. Roles of the sodium and L-type calcium currents during reduced excitability and decreased gap junction coupling. Circ Res 81: 727–741, 1997. [DOI] [PubMed] [Google Scholar]
- 41.Shibukawa Y, Chilton EL, Maccannell KA, Clark RB, Giles WR. K+ currents activated by depolarization in cardiac fibroblasts. Biophys J 88: 3924–3935, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Silva J, Rudy Y. Mechanism of pacemaking in I(K1)-downregulated myocytes. Circ Res 92: 261–263, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sun Y, Weber KT. Infarct scar: a dynamic tissue. Cardiovasc Res 46: 250–256, 2000. [DOI] [PubMed] [Google Scholar]
- 44.Tanaka K, Zlochiver S, Vikstrom KL, Yamazaki M, Moreno J, Klos M, Zaitsev AV, Vaidyanathan R, Auerbach DS, Landas S, Guiraudon G, Jalife J, Berenfeld O, Kalifa J. Spatial distribution of fibrosis governs fibrillation wave dynamics in the posterior left atrium during heart failure. Circ Res 101: 839–847, 2007. [DOI] [PubMed] [Google Scholar]
- 45.Wade MH, Trosko JE, Schindler M. A fluorescence photobleaching assay of gap junction-mediated communication between human cells. Science 232: 525–528, 1986. [DOI] [PubMed] [Google Scholar]
- 46.Wang J, Chen H, Seth A, McCulloch CA. Mechanical force regulation of myofibroblast differentiation in cardiac fibroblasts. Am J Physiol Heart Circ Physiol 285: H1871–H1881, 2003. [DOI] [PubMed] [Google Scholar]
- 47.Wu TJ, Ong JJ, Hwang C, Lee JJ, Fishbein MC, Czer L, Trento A, Blanche C, Kass RM, Mandel WJ, Karagueuzian HS, Chen PS. Characteristics of wave fronts during ventricular fibrillation in human hearts with dilated cardiomyopathy: role of increased fibrosis in the generation of reentry. J Am Coll Cardiol 32: 187–196, 1998. [DOI] [PubMed] [Google Scholar]
- 48.Zlochiver S, Munoz V, Vikstrom KL, Taffet SM, Berenfeld O, Jalife J. Electrotonic myofibroblast-to-myocyte coupling increases propensity to reentrant arrhythmias in two-dimensional cardiac monolayers. Biophys J 95: 4469–4480, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]