Abstract
Cdc42GAP (GTPase-activating protein) has been implicated in the regulation of cell motility, adhesion, proliferation, and apoptosis. In this study, Cdc42GAP was cloned from smooth muscle tissues. Cdc42GAP, but not inactive R282A Cdc42GAP (alanine substitution at arginine-282), enhanced the GTP hydrolysis of Cdc42 in an in vitro assay. Furthermore, we developed an assay to evaluate the activity of Cdc42GAP in vivo. Stimulation of smooth muscle cells with 5-hydroxytryptamine (5-HT) resulted in the decrease in Cdc42GAP activity. The agonist-induced GAP suppression was reversed by reactive oxygen species inhibitors. Treatment with hydrogen peroxide also inhibited GAP activity in smooth muscle cells. Because the vimentin cytoskeleton undergoes dynamic changes in response to contractile activation, we evaluated the role of Cdc42GAP in regulating vimentin filaments. Smooth muscle cells were infected with retroviruses encoding wild-type Cdc42GAP or its R282A mutant. Expression of wild-type Cdc42GAP, but not mutant R282A GAP, inhibited the increase in the activation of Cdc42 upon agonist stimulation. Phosphorylation of p21-activated kinase (PAK) at Thr-423 (an indication of PAK activation), vimentin phosphorylation (Ser-56), partial disassembly and spatial remodeling, and contraction were also attenuated in smooth muscle cells expressing Cdc42GAP. Our results suggest that the activity of Cdc42GAP is regulated upon contractile activation, which is mediated by intracellular ROS. Cdc42GAP regulates the vimentin network through the Cdc42-PAK pathway in smooth muscle cells during 5-HT stimulation.
Keywords: p21-activated kinase, 5-hydroxytryptamine, smooth muscle cells, cytoskeleton
the vimentin network of smooth muscle undergoes dynamic changes in response to contractile stimulation, which substantially affects force development (5, 8, 15, 21, 35, 44, 45). The dynamic vimentin intermediate filament framework is regulated by protein phosphorylation (9, 30, 46). In smooth muscle cells/tissues, contractile stimulation triggers vimentin phosphorylation at Ser-56, partial disassembly, and spatial reorientation of vimentin filaments. Furthermore, alanine substitution at Ser-56 negatively modulates disassembly and the spatial reorganization of the vimentin network (21, 35, 45). Vimentin phosphorylation is mediated by p21-activated kinase (PAK) in various cell types including smooth muscle cells. Purified vimentin is phosphorylated by PAK in in vitro biochemical studies. Moreover, downregulation of PAK attenuates vimentin phosphorylation/disassembly, spatial reorganization, and force development in smooth muscle upon contractile activation (12, 21, 35, 45). PAK is a known downstream effector of the small GTPase Cdc42 (3).
Cdc42GAP is an intracellular regulator of cell signaling mediated by the Rho family small GTPase by enhancing intrinsic GTP hydrolysis of small GTPases (25, 42). GAP has been implicated in the regulation of cell motility, adhesion, and proliferation, and apoptosis in various cell types, including mouse embryonic fibroblasts, neutrophils, hematopoietic cells, and in some organs such as liver, heart, spleen, and stomach (32, 42, 43, 47, 48). Therefore, Cdc42GAP may serve as a regulator of the vimentin cytoskeleton in smooth muscle cells. Furthermore, it is largely unknown how Cdc42GAP is regulated in smooth muscle.
Reactive oxygen species (ROS) have been implicated in cell signaling in various cell types including smooth muscle cells. ROS have been shown to regulate the activity of protein kinases affecting cellular functions (18, 38, 39). The role of ROS in regulating GAP activity in mammalian cells in general and in smooth muscle cells in particular is not well understood.
In the present study, stimulation of smooth muscle cells with 5-hydroxytryptamine (5-HT, a well-known contractile agonist) attenuates the activity of Cdc42GAP, which is reversed by ROS inhibitors. This is the first evidence to suggest that Cdc42GAP is regulated by intracellular ROS. Moreover, the expression of Cdc42GAP inhibited Cdc42 activation, PAK phosphorylation, vimentin phosphorylation/partial disassembly, and the spatial reorganization of the vimentin framework in response to contractile stimulation.
MATERIALS AND METHODS
Cell culture, cDNA cloning, mutagenesis, and retroviral infections.
Canine tracheal smooth muscle cells were prepared and cultured using the method previously described (21, 35). All animal protocols were approved by the Institutional Animal Care and Usage Committee. cDNA of Cdc42GAP (accession number EF427641) was cloned from canine tracheal smooth muscle tissues using the standard molecular biology technique. PCR-mediated mutagenesis was carried out on wild-type Cdc42GAP cDNA to generate the inactive Cdc42GAP mutant R282A (alanine substitution at Arg-282). DNA sequencing was used to confirm the mutation of Cdc42GAP. Wild-type Cdc42GAP and its mutant cDNAs were subcloned into the MSCV-hph retroviral expression plasmid (a gift from Jean Y. J. Wang of University of California, San Diego, CA). Recombinant retroviruses were produced by transfecting murine stem cell virus (MSCV) plasmids into Bing cells (ATCC) using calcium phosphate transfection methods. After 48 h of transfection, supernatant containing recombinant virus particles was collected and filtered through a 0.45-μm filter to remove cells and debris. Cultured smooth muscle cells were infected by the stock of retroviruses encoding wild-type Cdc42GAP or R282A Cdc42GAP mutant. After 48 h of infection, cells were serum starved for 1 day before biochemical and immunofluorescent analysis.
Baculoviruses production, amplification, and recombinant protein expression and purification.
For in vitro experiments, recombinant wild-type and mutant Cdc42GAP, and Cdc42 were expressed in a baculovirus expression system. Briefly, cDNAs encoding these proteins were subcloned into the baculovirus expression vector pDEST10 (Invitrogen). Recombinant baculoviruses were generated in the monolayer of Sf21 cells (Invitrogen). Viral stocks recovered after two cycles of amplification (3 days culture/cycle) were used to produce high yields of proteins. For expression of recombinant proteins, High Five cells (5 × 106, Invitrogen) were infected with 0.25 ml (multiplicity of infection = 5) of viral stocks. Cells were harvested by centrifugation at 1,000 g for 5 min at 4°C after 3 days. The cell pellet was washed with PBS (pH 7.4) and then suspended in 1 ml of ice-cold lysis buffer [10 mM Tris·HCl, pH 7.5, 500 mM NaCl, 0.1% Nonidet P-40 (NP-40), 10% glycerol, 2 mM phenylmethylsulfonyl fluoride (PMSF), 15 mM imidazole, 2 mM β-mecaptoethanol, 20 μg/ml aprotinin, and 20 μg/ml leupeptin] and incubated on ice for 15 min. Cell debris was removed after centrifugation at 10,000 g for 10 min. The supernatant was loaded to preequilibrated Ni-NAT spin column (Qiagen). The loaded column was washed twice using wash buffer (10 mM Tris·HCl, pH 7.5, 200 mM NaCl, 0.2% NP-40, 10% glycerol, 2 mM PMSF, 15 mM imidazole, and 2 mM β-mercaptoethanol). Proteins were then eluted using buffer containing 10 mM Tris·HCl, pH 7.5, 100 mM NaCl, 0.1% NP-40, 10% glycerol, 2 mM PMSF, 250 mM imidazole, and 2 mM β-mecaptoethanol. The eluted protein was dialyzed against buffer (20 mM Tris·HCl, pH 7.5, 1 mM EDTA, 1 mM dithiothreitol, 1 mM NaN3, and 40% glycerol) and stored at −20°C.
Immunoprecipitation and immunoblot analysis.
Cells were treated with lysis buffer (20 mM Tris, pH 7.5, 150 mM NaCl, 1% Triton X-100, 1 mM EDTA, pH 8.3, 1 mM PMSF, 0.5 mM aprotinin, 2 mM benzamidine, 2 mM sodium pyrophosphate, 2 mM molybdate, and 2 mM sodium orthovanadate) at 4°C for 1 h. Supernatant was collected after centrifugation for 15 min at 13,600 g and then incubated with 50 μl of 10% protein A-Sepharose (Sigma) for 30 min. Precleared samples were incubated overnight at 4°C with Cdc42GAP antibody (Custom made by Biosynthesis, peptide sequence: SDDSKSSSPEP VTHLKWDD) followed by the addition of 10% protein A-Sepharose for 3 h. The entire immunoprecipitation procedure was performed at 4°C. Samples were washed three times in Tris-buffered saline. Immunoblot analysis was carried out using the procedures described previously (21, 35). Phospho-PAK1 (Thr-423)/PAK2 (Thr-403) antibody and PAK1 antibody were purchased from Cell Signaling. Phosphovimentin (Ser-56) antibody was custom made by SynPep. Vimentin antibody (clone, RV202) and Cdc42 antibody (clone, 21) were purchased from BD Biosciences (21, 35). Antibodies against Rac1 and RhoA were purchased from the Cytoskeleton.
Analysis of small GTPase activation.
Activation of Cdc42, Rac1, and Rho was determined by using the pull-down assay as described previously (36). Briefly, cells were mixed with lysis buffer containing 50 mM Tris, pH 7.5, 10 mM MgC2, 0.3 mM NaCl, and 2% NP-40. The mixtures were centrifuged in a microcentrifuge at 8,000 rpm, 5 min, at 4°C. The resulting supernatant was reacted with p21-activated kinase binding domain (PAK-PBD) (cytoskeleton) beads or Rhotekin-RBD beads in binding buffer (25 mM Tris, pH 7.5, 30 mM MgCl2, 40 mM NaCl, and 2% NP-40). GTP-bound Cdc42 or Rac1 (active) selectively binds to PAK-PBD tagged with GST, which can be affinity-precipitated by glutathione beads. Similarly, GTP-Rho binds to GST-fused Rhotekin-RBD, which is precipitated by glutathione beads. The beads were collected after centrifugation at 7,000 rpm for 3 min at 4°C and washed twice with wash buffer (25 mM Tris, pH 7.5, 30 mM MgCl2, and 40 mM NaCl). The beads were boiled in SDS sample buffer [1.5% dithiothreitol, 2% SDS, 80 mM Tris, pH 6.8, 10% (vol/vol) glycerol, and 0.01% bromphenol blue] to release GTP-bound small GTPases, which was separated 15% SDS-PAGE. Blots of the samples were probed by using antibodies against Cdc42, Rac, or RhoA.
Assessment of Cdc42GAP activity.
Recombinant Cdc42 (2.5 μg) was preloaded with GTP in 10 μl of loading buffer containing 2 mM GTP, 20 mM Tris·HCl, pH 7.5, 25 mM NaCl, 0.1 mM DTT, and 5 mM EDTA for 10 min at 30°C. MgCl2 (20 mM) was added into the mixture to stop the loading, and the GTP-loaded Cdc42 was kept on ice for future use. For in vitro GAP assay, the reaction was initiated by adding the preloaded GTP-Cdc42 into solution containing 0.6 μM Cdc42GAP, 20 mM Tris, pH 7.5, 100 mM NaCl, and 0.2 mg/ml BSA. After incubation for 5–15 min, mixtures were transferred into ice-cold buffer (50 mM Tris, pH 7.5, 50 mM NaCl, and 5 mM MgCl2) and incubated with PAK-PBD beads at 4°C on a rotator for 2 h. GTP-bound Cdc42 was released from the beads and assessed by immunoblot analysis as described above.
For in vivo GAP assay, Cdc42GAP was immunoprecipitated from cell lysates with Cdc42GAP antibody. Samples were then washed three times in wash buffer containing 20 mM Tris, pH 7.5, 150 mM NaCl, and 1% Triton X-100. The immune complex containing Cdc42GAP was resuspended in the reaction buffer (see above), and GTP-preloaded Cdc42 was added to the mixture at 20°C for 5 min. The amount of GTP-bound Cdc42 was then determined by the pull-down assay.
Immunofluorescence and fluorescence microscopy.
Immunofluorescence and fluorescence image analysis was performed using the experimental procedures as described previously (21, 35). Briefly, cells were plated in 35-mm dishes containing coverslips and incubated in serum-free media for 1 day. After different treatments, these cells were fixed for 15 min in 4% paraformaldehyde and were then washed three times in Tris-buffered saline (TBS) containing 50 mM Tris, 150 mM NaCl, and 0.1% NaN3 followed by permeabilization with 0.2% Triton X-100 dissolved in TBS for 5 min. These cells were immunofluorescently stained for vimentin using monoclonal vimentin antibody (1:100 dilution) followed by goat anti-mouse IgG conjugated to Alexa 488 (1:100 dilution, Molecular Probes, Eugene, OR). Hemagglutinin (HA) antibody (Covance, clone: 16B12) was used to visualize HA-tagged protein in cells. To visualize the nucleus, 4′,6′-diamidino-2-phenylindole (DAPI) was used. The cellular localization of fluorescently labeled proteins was viewed under a laser scanning confocal microscope (Zeiss LSM 510) using an Apo ×100 oil immersion objective. For each independent experiment, 20 cells were randomly selected for observation. The length of straight vimentin filaments and total cell length on cell images were assessed by use of Photoshop Measure Tool. Vimentin filaments with straightness longer than one-fourth cell length were considered as straight filaments (In these cells, the length of nucleus is approximately 1/3 cell length). Cells exhibiting more than four straight filaments were counted as cells with rearranged filaments. Percentage of cells with rearranged vimentin filaments was calculated as follows: numbers of cells with rearranged vimentin filaments/numbers of total cells observed × 100.
Assessment of ratios of soluble to insoluble vimentin.
Ratios of soluble to insoluble vimentin were determined using a fractionation assay as we previously described (21, 35).
Assessment of three-dimensional culture collagen lattice constriction (7, 22, 28).
Confluent smooth muscle cells were released from dishes with trypsination. Cells were resuspended in DMEM at a concentration of 6 × 105 cells/ml and mixed with neutralized collagen solutions (2 mg/ml) at 1:1 ratio to a final concentration of 3 × 105 cells/ml. The mixtures with 500 μl were distributed to precoated wells (24-well plate). Gels were polymerized at 37°C after 30–60 min, and the freshly made lattice was overlaid with DMEM with 10% FBS and cultured for 2 days. They were serum starved for 24 h before treatment with the contractile agonist for 2 h. Images of gels were taken using a Fuji LAS300 imaging system, and surface areas of lattices are calculated using Multigauge software. Relative smooth muscle contraction was calculated as follows: contraction (%) = 1 − (gel area after treatment)/(gel area before treatment) × 100%.
Statistical analysis.
All statistical analysis was performed using Prism 4 software (GraphPad Software, San Diego, CA). Comparison among multiple groups was performed by one-way analysis of variance followed by post-test (Tukey's multiple comparison test). Differences between pairs of groups were analyzed by Student-Newman-Keuls test or Dunn's method. Values of n refer to the number of experiments used to obtain each value. P < 0.05 was considered to be significant.
RESULTS
Characterization of recombinant Cdc42GAP in vitro.
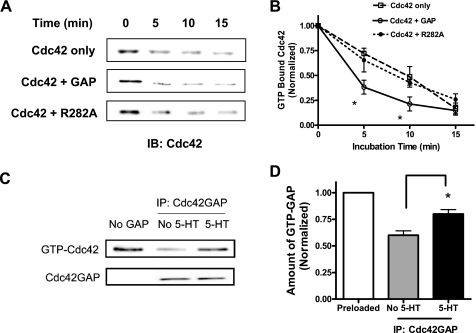
We cloned Cdc42GAP cDNA from canine tracheal smooth muscle tissues; its sequence was published in the GeneBank database, the National Center for Biotechnology Information (accession number EF427641). We assessed activity of recombinant Cdc42GAP in an in vitro study. Purified Cdc42 was preloaded with GTP and incubated in the absence or presence of Cdc42GAP for various time points. The activity of Cdc42GAP was determined by using the GAP assay. The amount Cdc42 remaining bound to GTP decreased over time in the absence of Cdc42GAP, indicating an intrinsic GTPase activity of Cdc42. However, the rate of GTP hydrolysis was faster when the small GTPase was treated with Cdc42GAP, indicating that GAP enhances the transition of GTP-bound state to GDP-bound state (Fig. 1, A and B).
Fig. 1.
Cdc42GAP, but not R282A Cdc42GAP mutant, accelerates the GTP hydrolysis of Cdc42 in vitro and in smooth muscle cells on stimulation with 5-hydroxytryptamine (5-HT). A: representative immunoblots illustrating the effects of Cdc42GAP or its mutant R282A on the GTP hydrolysis of Cdc42. Purified Cdc42 was preloaded with GTP and then incubated in the absence or presence of Cdc42GAP or mutant R282A Cdc42GAP (inactive). GAP activity was determined using the method described in materials and methods. B: amounts of Cdc42 remaining bound to GTP after incubation at different time points are normalized to the each level of GTP-Cdc42 before the incubation. *Significantly lower amounts of GTP-Cdc42 after treatment with Cdc42GAP than those of untreated samples or samples incubated with mutant R282A GAP at corresponding time points (P < 0.05). Values are means ± SE (n = 4–5). C: Cdc42GAP immunoprecipitated from 5-HT-stimulated cells or unstimulated cells was added to preloaded Cdc42 for 5 min. GAP activity was then determined. Representative blot showing that the amount of GTP-Cdc42 treated with Cdc42GAP precipitates from stimulated cells was higher than that from unstimulated cells, suggesting that 5-HT stimulation inhibits the activity of Cdc42GAP in smooth muscle cells. D: amount of GTP-Cdc42 treated with Cdc42GAP precipitates is normalized to the level of preloaded GTP-Cdc42 (without exposure to GAP immunoprecipitates). *Significantly higher GTP-Cdc42 levels after treatment with GAP precipitates from stimulated cells than the levels from unstimulated cells (P < 0.05). Values are the means ± SE (n = 4).
Arg-282 is critical for the binding of Cdc42GAP to the small GTPase; alanine substitution at Arg-282 generates an inactive GAP mutant (R282A), which loses the binding capability of GAP to the small GTPase and has no effects on the activation of Cdc42 (13, 20). We used the inactive Cdc42GAP mutant R282A to serve as a protein control. The rate of GTP hydrolysis in the presence of the R282A mutant Cdc42GAP was similar to that in the absence of recombinant proteins (Cdc42 only), indicating that the R282A mutant does not promote the intrinsic GTP hydrolysis of Cdc42 (Fig. 1, A and B).
Endogenous Cdc42GAP activity is attenuated in response to 5-HT stimulation.
To assess whether Cdc42GAP activity can be regulated in smooth muscle cells in response to chemical stimulation, we developed a sensitive assay to determine the endogenous activity of Cdc42GAP in vivo. Cultured smooth muscle cells were treated with 10 μM 5-HT for 10 min or left unstimulated. Cdc42GAP was immunoprecipitated from cells using Cdc42GAP antibody, and the activity of endogenous Cdc42GAP was evaluated using the method described in materials and methods.
Treatment of cells with 5-HT resulted in the decrease in Cdc42GAP activity. The amount of GTP-bound Cdc42 after exposure to Cdc42GAP immunoprecipitated from stimulated cells was higher than that from unstimulated cells (Fig. 1C). The level of Cdc42 remaining bound to GTP in response to treatment with GAP immunoprecipitates was significantly lower in unstimulated cells than in 5-HT stimulated cells (Fig. 1D, n = 4, P < 0.05).
Stimulation with 5-HT induces ROS production in smooth muscle cells.
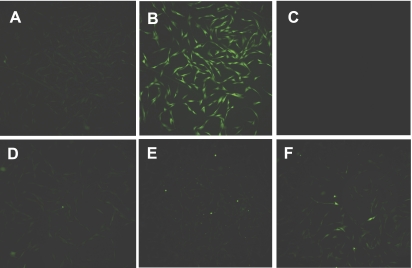
Because ROS have been implicated in signal transduction, we sought to understand whether activation with 5-HT initiates the generation of ROS in cells using the ROS fluorescent indicator 2′,7′-dichlorofluorescein diacetate (DCF-DA), which is cell permeable and not fluorescent until the acetate groups are removed by intracellular esterases and oxidation occurs within cells (38). Cells were loaded with 10 μM DCF-DA for 30 min followed by washing, and intensity of the DCF signal was monitored and analyzed by fluorescent microscopy. Stimulation with 5-HT (10 μM, 30 s) dramatically increases ROS production. Furthermore, pretreatment with the ROS inhibitors N-acetylcysteine (NAC, 10 mM) and diphenylene iodonium (DPI, an inhibitor of flavin-containing enzyme, 10 μM) for 30 min inhibited ROS production in response to activation of 5-HT (Fig. 2).
Fig. 2.
Effects of 5-HT on reactive oxygen species (ROS) production in the absence or presence of ROS inhibitors. Compared with unstimulated cells (A), activation of smooth muscle cells with 5-HT (10 μM, 30 s) increased the ROS production (B) as evidenced by an increase in intensity of the DCF signal. Pretreatment with N-acetylcysteine (NAC) (C and D) or diphenyl iodonium (DPI) (E and F) inhibited 5-HT-induced ROS generation in cells. C and E, unstimulated cells; D and F, stimulated cells. Images are representative of 3 identical experiments.
ROS inhibitors attenuate 5-HT-induced GAP suppression.
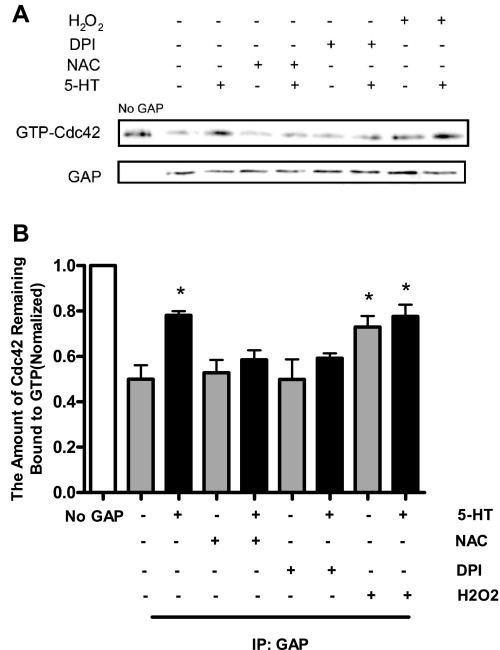
We evaluated the role of ROS in regulating GAP activity by determining the effects of the ROS inhibitors on 5-HT-induced GAP suppression. Cells were pretreated with 10 mM NAC, 10 μM DPI, or vehicle for 30 min, GAP activity of these cells in response to stimulation with 5-HT was then assessed. In vehicle-treated cells, GAP activity was suppressed in 5-HT-stimulated cells as evidenced by the increased amount of Cdc42 remaining bound to GTP after exposure to GAP immunoprecipitates from stimulated cells. In contrast, the pretreatment with NAC and DPI reversed 5-HT-mediated GAP suppression; GAP activity in response to 5-HT stimulation was not significantly attenuated in cells pretreated with the ROS inhibitors (Fig. 3, n = 4, P > 0.05).
Fig. 3.
Effects of ROS inhibitors and hydrogen peroxide on Cdc42GAP activity in smooth muscle cells. Smooth muscle cells were pretreated with the ROS inhibitors NAC and DPI (an inhibitor of flavin-containing enzyme) or vehicle for 30 min. GAP activity of these cells in response to stimulation with 5-HT (10 μM, 10 min) was then assessed using the method described under materials and methods. In addition, GAP activity in response to treatment with hydrogen peroxide (100 μM, 20 min) was also determined. A: representative immunoblots showing the effects of ROS inhibitors and hydrogen peroxide on GAP activity. B: amount of Cdc42 remaining bound to GTP from treated cells is normalized to the level of preloaded GTP-Cdc42. *Significantly higher GTP-Cdc42 levels after exposure to GAP precipitates from treated cells than the levels from untreated and unstimulated cells (P < 0.05). Values are the means ± SE (n = 4).
Treatment with hydrogen peroxide depresses GAP activity in smooth muscle cells.
We also evaluated whether exposure to hydrogen peroxide (a major ROS) affects GAP activity in smooth muscle cells. Smooth muscle cells were exposed to 100 μM hydrogen peroxide for 20 min. The activity of Cdc42GAP from these samples was determined using the GAP assay. GAP activity was lower in cells exposed to hydrogen peroxide compared with control cells (Fig. 3, n = 4). Interestingly, treatment with both hydrogen peroxide and 5-HT did not show synergic effects on GAP activity (Fig. 3), suggesting that the agonist-induced GAP suppression may be mediated by intracellular ROS.
Effects of ROS inhibitors and hydrogen peroxide on Cdc42 activation on 5-HT stimulation.
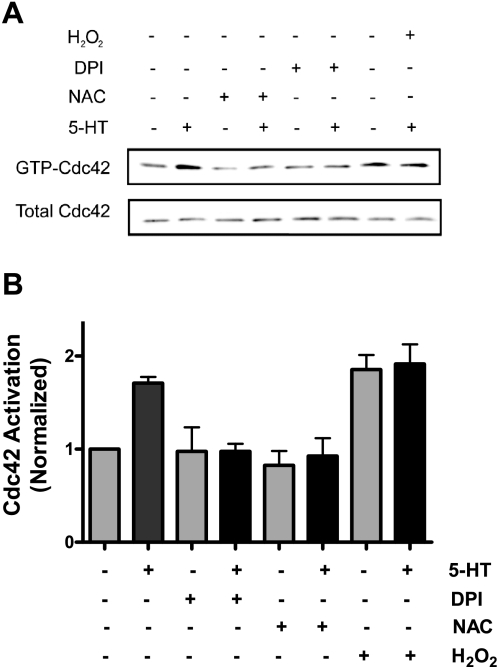
Because ROS inhibitors attenuate GAP activity in cells, we evaluated whether the inhibition of ROS production affects the activation of Cdc42, a major downstream effector of GAP. Pretreatment with 10 mM NAC or 100 μM DPI for 30 min attenuated Cdc42 activation in response to 5-HT stimulation as assessed by the pull-down assay. Moreover, treatment with hydrogen peroxide was able to enhance Cdc42 activation on stimulation with 5-HT (Fig. 4, n = 4 to 5).
Fig. 4.
Effects of ROS inhibitors and hydrogen peroxide on Cdc42 activation in smooth muscle cells. Pretreatment with NAC or DPI attenuated the activation of Cdc42 on 5-HT stimulation. Treatment with hydrogen peroxide increased Cdc42 activation. A: representatives immunoblots showing the effects of ROS inhibitors and hydrogen peroxide on Cdc42 activation. B: ratios of GTP-bound Cdc42/total Cdc42 (an index of Cdc42 activation) from treated cells are normalized to the ratio of untreated and unstimulated cells. *Significantly higher GTP-Cdc42 levels stimulated by 5-HT or hydrogen peroxide than the levels of untreated and unstimulated cells (P < 0.05). Values are the means ± SE (n = 4–5).
Expression of Cdc42GAP inhibits Cdc42 activation, but not Rac1 and Rho activation, in cells on 5-HT stimulation.
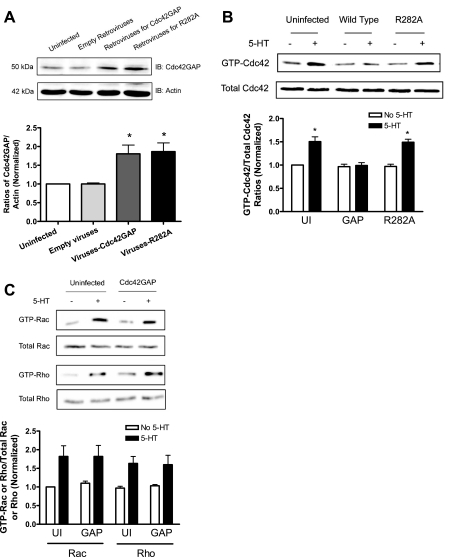
To determine the role of Cdc42GAP in smooth muscle cells, we assessed the effects of expression of Cdc42GAP on the activation of small GTPases. Cells were infected with retroviruses encoding wild-type Cdc42GAP or the R282A Cdc42GAP mutant. Protein expression was confirmed by immunoblot analysis (Fig. 5A). These cells were also stimulated with 10 μM 5-HT for 10 min or they were not stimulated. Activation of Cdc42, Rac1, and Rho was assessed by the pull-down assay.
Fig. 5.
Expression of Cdc42GAP attenuates the activation of Cdc42, but not Rac1 and RhoA, in cells in response to 5-HT stimulation. A: representative blots illustrating the expression of recombinant Cdc42GAP in cells infected with recombinant retroviruses. Ratios of Cdc42 over actin in cells infected with empty retroviruses, retroviruses encoding wild-type Cdc42GAP, or R282A mutant Cdc42GAP are normalized to the ratios from uninfected cells. *Significantly higher protein ratios from cells infected with recombinant retroviruses compared with the values from uninfected (UI) cells (P < 0.05, n = 7). B: representative immunoblots showing the reduced 5-HT-stimulated Cdc42 activation in cells expressing wild-type Cdc42GAP compared with UI cells or cells expressing mutant R282A GAP. Ratios of GTP-Cdc42 to total Cdc42 in response to 5-HT stimulation in cells expressing recombinant proteins are normalized to the ratios of unstimulated and uninfected cells. *Significantly lower ratios of GTP-Cdc42 to total Cdc42 in cells expressing wild-type Cdc42GAP than in uninfected cells or in cells expressing mutant R282A GAP in response to 5-HT stimulation (P < 0.05, n = 5–9). C: expression of GAP does not inhibit the activation of Rac1 and Rho on 5-HT stimulation. Ratios of GTP-Rac1/RhoA to total Rac1/RhoA stimulated by 5-HT in cells expressing GAP are not significantly different from those in uninfected cells (P > 0.05, n = 3).
Stimulation with 5-HT resulted in the increase in the amount of GTP-Cdc42 (active Cdc42) in uninfected cells. However, the amount of activated Cdc42 upon 5-HT stimulation was not increased in cells expressing wild-type Cdc42GAP (Fig. 5B), suggesting that the overexpression of GAP inhibits Cdc42 activation in stimulated cells. The ratios of GTP-Cdc42 to total Cdc42 in response to 5-HT stimulation were lower in cells expressing Cdc42GAP than in uninfected cells or in cells expressing mutant R282A GAP (Fig. 5B, n = 5–9, P < 0.05). Additionally, the activation of Rac1 and Rho on 5-HT stimulation was also increased in uninfected cells. However, the expression of GAP did not inhibit the activation of Rac1 or Rho in cells in response to 5-HT stimulation (Fig. 5C, n = 3, P > 0.05).
PAK1 phosphorylation at Thr-423 on 5-HT activation is reduced in cells expressing Cdc42GAP.
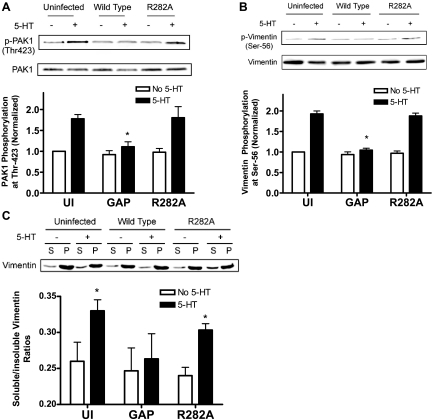
We evaluated whether Cdc42GAP affects PAK phosphorylation at Thr-423 (an indication of PAK activation) in cells. Blots of unstimulated or 5-HT stimulated cells that had been infected with retroviruses encoding wild-type Cdc42GAP or R282A inactive mutant were probed using phospho-PAK (Thr-423) antibody, stripped, and reprobed by use of PAK1 antibody.
In uninfected cells and cells expressing mutant R282A GAP, stimulation with 5-HT led to the enhancement of PAK1 phosphorylation on Thr-423. However, treatment with 5-HT did not lead to the increase in PAK phosphorylation in cells expressing wild-type Cdc42GAP. The phosphorylation level of PAK1 on 5-HT stimulation was increased by approximately twofolds in uninfected cells and cells expressing R282A mutant (Fig. 6A, n = 4, P < 0.05), whereas the phosphorylation level of PAK stimulated by 5-HT was not significantly increased in cells expressing wild-type Cdc42GAP compared with unstimulated cells (Fig. 6A, n = 3, P > 0.05). These results suggest that Cdc42GAP negatively modulates PAK1 activation in cells in response to agonist stimulation.
Fig. 6.
Expression of Cdc42GAP attenuates p21-activated kinase (PAK)1 phosphorylation, vimentin phosphorylation, and partial disassembly on stimulation with 5-HT. Smooth muscle cells expressing wild-type Cdc42GAP and mutant R282A Cdc42GAP were stimulated with 5-HT or left unstimulated. PAK and vimentin phosphorylation and ratios of soluble to insoluble vimentin were determined by immunoblot analysis and the fractionation assay, respectively. Protein phosphorylation or ratios of soluble to insoluble vimentin in response to 5-HT stimulation in cells expressing recombinant proteins are normalized to the corresponding levels in unstimulated and uninfected cells. A: PAK1 phosphorylation at Thr-423 is reduced in cells producing wild-type Cdc42GAP on 5-HT stimulation. *Significantly lower phosphorylation levels stimulated by 5-HT in cells expressing wild-type Cdc42GAP compared with cells infected with mutant R282A Cdc42GAP (P < 0.05). Values are means ± SE (n = 3–4). B: expression of Cdc42GAP attenuates the increase in vimentin phosphorylation in cells in response to 5-HT stimulation. *Significantly lower vimentin phosphorylation during 5-HT stimulation in cells expressing wild-type Cdc42GAP than in uninfected cells and in cells expressing mutant R282A Cdc42GAP (P < 0.05, n = 4). C: enhancement of soluble-to-insoluble vimentin ratios on 5-HT stimulation is inhibited in cells producing Cdc42GAP. Vimentin soluble (supernatant, S) and insoluble (pellet, P) fractions of these cells were evaluated by use of fractionation analysis. *Significantly higher ratios of soluble to insoluble vimentin in stimulated cells than corresponding soluble to insoluble vimentin ratios in unstimulated cells (P < 0.05). Values are means ± SE (n = 4).
Expression of Cdc42GAP in smooth muscle cells inhibits the vimentin-associated process on stimulation with 5-HT.
We have previously shown that contractile activation initiates vimentin phosphorylation at Ser-56, partial disassembly, and spatial remodeling in smooth muscle cells, which plays a critical role in vimentin cytoskeleton signaling (21, 35, 45). Vimentin remodeling also occurs in other cell types in response to external stimulation or during mitosis (9, 46). Vimentin phosphorylation at Ser-56 in smooth muscle is mediated by PAK, a known downstream effector of small GTPases (3, 21, 35, 45). To determine whether Cdc42GAP affects vimentin phosphorylation, we assessed the effects of wild-type Cdc42GAP and mutant R282A GAP on vimentin phosphorylation at Ser-56 in cells. Cells were infected with retroviruses encoding wild-type Cdc42GAP or mutant R282A Cdc42GAP. These cells were then stimulated with 10 μM 5-HT for 10 min or they were not stimulated. Blots of protein extracts from these cells were probed using the phospho-vimentin antibody, stripped, and reprobed by use of anti-vimentin antibody.
Stimulation with 5-HT led to increases in vimentin phosphorylation at Ser-56 in uninfected cells, consistent with our previous findings (21, 35). However, the phosphorylation levels in cells expressing wild-type Cdc42GAP was diminished during activation with 5-HT. Moreover, vimentin phosphorylation in response to agonist activation was not inhibited in cells expressing mutant R282A GAP (Fig. 6B). These results suggest a pivotal role for Cdc42GAP in regulating vimentin phosphorylation at Ser-56 in smooth muscle cells during agonist stimulation.
To determine the role of GAP in regulating vimentin disassembly, the ratios of soluble to insoluble vimentin of cells expressing recombinant GAP were determined by using the fractionation assay (9, 21, 35, 45, 46). Stimulation with 5-HT led to the increase in soluble-to-insoluble vimentin ratios in uninfected cells or in cells expressing mutant R282A GAP. In contrast, the enhancement of soluble-to-insoluble vimentin ratios in response to 5-HT stimulation was inhibited in cells expressing wild-type Cdc42GAP (Fig. 6C).
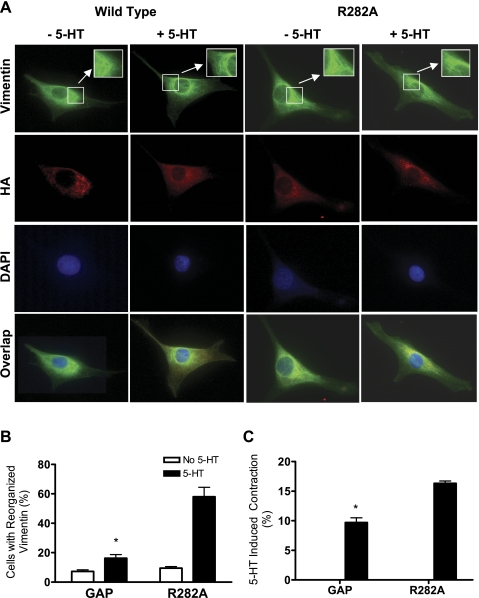
To evaluate the role of GAP in the modulation of vimentin spatial reorganization, cells were infected with recombinant retroviruses encoding HA-tagged Cdc42GAP or its R282A mutant. Cells were stimulated with 10 μM 5-HT for 10 min or left unstimulated and stained for vimentin, HA, and DAPI (to visualize the nucleus). Cell images were viewed and captured under a high-resolution fluorescent microscope.
Our previous studies show that vimentin filaments exhibit a curved structure in unstimulated smooth muscle cells, whereas they become straight in response to contractile stimulation (21, 35). In this report, vimentin filaments on 5-HT stimulation were not able to undergo spatial reorientation (curved filaments in unstimulated cells versus straight filaments in stimulated cells) in cells expressing wild-type Cdc42GAP. However, the treatment with 5-HT induced the spatial rearrangement of the vimentin network in cells expressing the mutant Cdc42GAP R282A (Fig. 7, A and B).
Fig. 7.
A and B: spatial reorganization of vimentin filaments in response to contractile activation is inhibited in cells expressing Cdc42GAP. Smooth muscle cells expressing hemagglutinin (HA)-tagged wild-type Cdc42GAP or mutant R282A Cdc42GAP were treated with 5-HT or left unstimulated. Cells were stained with antibodies against vimentin and HA and DAPI (for nuclear visualization). A: in cells expressing wild-type Cdc42GAP, vimentin filaments (green) displayed a curved network in the absence or presence of 5-HT. In contrast, stimulation with 5-HT induced the spatial reorientation of vimentin filaments from a curved structure to a straight structure in cells producing mutant R282A GAP. HA immunostaining (red) confirms the expression of recombinant Cdc42GAP in these cells. DAPI-stained nuclei are shown in blue. B: cell numbers with reorganized vimentin filaments are reduced in cells expressing wild-type Cdc42GAP. *Significantly lower numbers of reorganized cells on 5-HT stimulation in cells expressing wild-type Cdc42GAP than in cells expressing R282A Cdc42GAP inactive mutant (P < 0.05, n = 4). C: contraction was attenuated in cells producing wild-type GAP but not mutant R282A GAP. Cell contraction was evaluated by using the collagen gel constriction assay. Contraction is calculated as follows: contraction (%) = (1 − gel area after stimulation/gel area before stimulation) × 100. *Significantly lower 5-HT induced contraction in cells expressing GAP compared with that in cells expressing the GAP mutant (n = 3–4).
Expression of Cdc42GAP inhibits smooth muscle contraction stimulated by 5-HT.
We assessed the role of Cdc42GAP in regulating smooth muscle contraction during agonist stimulation. Uninfected cells or cells expressing recombinant proteins were stimulated with 5-HT or they were not stimulated. Cell contraction was evaluated by using the three-dimensional collage constriction assay. The expression of wild-type Cdc42GAP, but not mutant R282A GAP, inhibited cell contraction in response to 5-HT activation (Fig. 7C).
DISCUSSION
The physiological role and regulation of Cdc42GAP in the vimentin framework and smooth muscle are largely unknown. In the present study, we have demonstrated 1) contractile activation results in the decrease in the activity of Cdc42GAP in smooth muscle cells; 2) ROS mediates the agonist-induced GAP suppression; 3) expression of Cdc42GAP, but not its inactive mutant R282A GAP, inhibits the activation of Cdc42 on agonist stimulation; 4) the retrovirus-mediated expression of Cdc42GAP attenuates the activation of Cdc42 and PAK phosphorylation in smooth muscle cells on agonist stimulation. The expression of Cdc42GAP also inhibits vimentin phosphorylation and disassembly, and the spatial reorientation of vimentin filaments as well as cell contraction. We propose that ROS-sensitive Cdc42GAP may serve as a negative regulator of the vimentin filament framework and contraction in smooth muscle.
In this report, purified Cdc42GAP from the baculovirus expression system promoted the GTP hydrolysis of preloaded Cdc42. This observation is supported by previous studies from other investigators in which GAP is able to enhance the transition from GTP-bound state to GDP-bound state of small GTPases (13, 25). Cdc42GAP contains an NH2-terminal Sec-14 motif that is able to interact with Cdc42, a central SH3 domain, and a COOH-terminal GAP domain that is catalytically toward Cdc42 (42). In vitro biochemical studies indicate that the COOH-terminal GAP domain associates with the Sec-14 motif, forming an autoinhibitory conformation. Prenylated small GTPases bind to the Sec-14 motif, inducing a conformational change, exposing GAP domain to small GTPases and activating GAP activity (25). The conserved amino acid Arg-282 is believed to be essential for GAP activity. Alanine substitution at this position may impair the binding of GAP to the small GTPase, resulting in an inactive mutant, which does not affect the GTP hydrolysis of Cdc42 (Fig. 1) (13).
Small G proteins of the Rho family (Cdc42, Rac, and Rho) serve as molecular switches to regulate various biological responses, including actin cytoskaletal dynamics, cell motility, cell cycle progression, gene expression, and smooth muscle contraction (2, 11, 34, 36, 41). There is evidence that stimulation of endothelial cells with vascular endothelial growth factor activates guanine nucleotide exchange factors (GEFs), thereby enhancing activity of the small GTPases (6, 10, 31). Although the role of GAP in accelerating the GTP hydrolysis of small GTPases is well recognized, there is little information on whether GAP activity is regulated in cells in response to external stimulation. In this study, the activity of Cdc42GAP is relatively high in unstimulated cells, whereas its activity decreases upon contractile activation. These results suggest that Cdc42GAP possesses inherent activity and renders Cdc42 in an inactive state in quiescent cells. Agonist stimulation attenuates the activity of Cdc42GAP, facilitating the activation of Cdc42 in cells. Moreover, the expression of Cdc42GAP did not depress the activation of Rac1 and RhoA, which is consistent with previous observation (42). These results suggest that Cdc42GAP specifically enhances the GTP hydrolysis of Cdc42 but not Rac1 and RhoA.
ROS have been reported to regulate the activity of protein kinases in a variety of cell types. Angiotensin II-induced Abl tyrosine phosphorylation is mediated by ROS in arterial smooth muscle cells, which is thought to be critical for signaling induced by the activation of angiotensin subtype 1 receptor (39). In another study, the activity of cAMP-dependent protein kinase (PKA) is regulated by oxidation and reduction (18). In the present study, 5-HT-induced GAP suppression is reversed by the ROS inhibitors. Stimulation with hydrogen peroxide mimics GAP suppression induced by activation with 5-HT. Furthermore, stimulation with 5-HT triggers ROS production in cells. These results strongly suggest that 5-HT-induced GAP suppression is mediated by intracellular ROS. To best of our knowledge, this is the first evidence to suggest that GAP activity is regulated by ROS in mammalian cells. The mechanisms by which ROS regulate GAP activity are currently unknown. Since the activity of PKA is directly modulated by redox (18), it is possible that ROS may directly oxidize GAP and thus suppress its activity. Future studies are needed to test the hypothesis.
Cdc42GAP has been implicated in the regulation of cell motility, adhesion, and proliferation, and apoptosis in nonmuscle cells (32, 42, 43, 47, 48). In the present study, Cdc42GAP, but not its inactive mutant, is able to modulate phosphorylation and partial disassembly of vimentin and the spatial reorientation of vimentin filaments during agonist stimulation. Vimentin consists of an NH2-terminal head domain containing several phosphorylation sites, a central α-helical rod domain (forming the backbone of filaments) and a COOH-terminal tail domain (16, 26). Phosphorylation on the head domain may inhibit lateral association of intermediate filament proteins and eventually assembly and/or spatial rearrangement of vimentin filaments (17, 21, 30, 35, 46).
With regard to biological significance of vimentin dynamics, the spatial rearrangement and depolymerization of vimentin filaments have been shown to be associated with migration and mitosis of nonmuscle cells, including fibroblasts, BHK-21 cells, endothelial cells, REF-52 cells, promyelocytic cells, and COS-7 cells (4, 24, 40, 46). In smooth muscle cells/tissues, disassembly of the vimentin system may regulate the translocation of Crk-associated substrate (CAS) and Ca2+/calmodulin-dependent protein kinase II (CamKII), which may participate in the cellular processes that control force development (1, 21, 23, 27, 29, 35, 37, 45). In this report, the expression of Cdc42GAP, but not its inactive mutant, inhibits cell contraction as assessed by the collagen gel constriction assay. Because vimentin filaments connect to cytoplasmic dense bodies (to which actin filaments also attach) and to desmosomes (intercellular junctions), it is also possible that the spatial reorientation of vimentin filaments induced by contractile agonists may facilitate the reorganization of contractile elements and/or the intercellular and intracellular force transmission, which may be a part of the cellular processes that coordinate force development in smooth muscle (14, 19, 21, 33, 35, 44, 45).
Studies from our group and others have previously shown that the vimentin filament network is regulated by PAK, a known downstream effector of Cdc42 (3, 12, 21, 35, 45). Therefore, we assumed that the Cdc42GAP-regulated change in the vimentin framework during agonist stimulation may be mediated by Cdc42 and PAK. In this study, both Cdc42 activation and PAK phosphorylation stimulated by the agonist are depressed in cells producing wild-type Cdc42GAP but not in cells expressing the inactive Cdc42GAP mutant. It is generally accepted that Cdc42 activity is regulated by the balance between GEF and GAP; GEF enhances Cdc42 activity, whereas GAP inhibits its activation. Thus the overexpression of GAP may overcome the effects of GEF and accelerate the GTP hydrolysis of Cdc42 in these cells. In addition, PAK phosphorylation may be activated by both Cdc42 and Rac (3). In the present study, the inhibition of Cdc42 activation is sufficient to inhibit, but not abolish, PAK phosphorylation in cells overexpressing GAP, although Rac activity is not impaired. The results suggest that both Cdc42 and Rac are required for PAK activation in smooth muscle cells.
Simply put, our studies suggest that Cdc42GAP regulates the vimentin framework through the Cdc42-PAK pathway. As described earlier, Cdc42-PAK-regulated phosphorylation modulates assembly/disassembly and structural rearrangement of vimentin filament network.
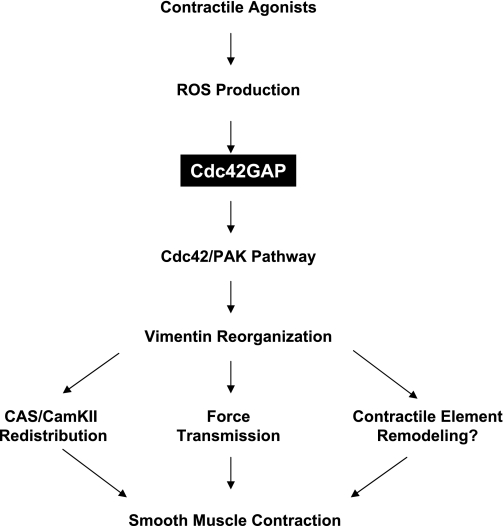
In conclusion, physiological properties and regulation of Cdc42GAP in smooth muscle are not well characterized. In the present study, the activity of Cdc42GAP is attenuated on agonist stimulation. Furthermore, contractile activation of smooth muscle cells induces ROS production, which may mediate agonist induced regulation of GAP activity. Finally, we demonstrate an essential role for Cdc42GAP in regulating vimentin phosphorylation, disassembly and spatial reorganization of the vimentin filament system, and contraction in smooth muscle. Cdc42GAP regulates the vimentin filament network and smooth muscle contraction by affecting the Cdc42/PAK pathway (Fig. 8).
Fig. 8.
Proposed mechanism by which Cdc42GAP modulates smooth muscle contractility. Contractile activation may suppress the activity of Cdc42GAP via the production of ROS, which in turn leads to the activation of Cdc42 and PAK. Activated PAK may mediate phosphorylation, disassembly, and spatial reorganization of the vimentin network, which may regulate smooth muscle contraction by affecting redistribution of Crk-associated substrate (CAS) and Ca2+/calmodulin-dependent protein kinase II (CamKII), remodeling of contractile elements, and intercellular/intracellular force transmission.
GRANTS
This work was supported by National Heart, Lung, and Blood Institute Grant HL-75388 (to D. D. Tang).
Acknowledgments
The authors thank Ruping Wang for technical assistance.
REFERENCES
- 1.Anfinogenova Y, Wang R, Li QF, Spinelli AM, Tang DD. Abl silencing inhibits CAS-mediated process and constriction in resistance arteries. Circ Res 101: 420–428, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Aspenstrom P Effectors for the Rho GTPases. Curr Opin Cell Biol 11: 95–102, 1999. [DOI] [PubMed] [Google Scholar]
- 3.Bokoch GM Biology of the p21-activated kinases. Annu Rev Biochem 72: 743–781, 2003. [DOI] [PubMed] [Google Scholar]
- 4.Bruel A, Paschke S, Jainta S, Zhang Y, Vassy J, Rigaut JP, Beil M. Remodeling of vimentin cytoskeleton correlates with enhanced motility of promyelocytic leukemia cells during differentiation induced by retinoic acid. Anticancer Res 21: 3973–3980, 2001. [PubMed] [Google Scholar]
- 5.Chan W, Kozma R, Yasui Y, Inagaki M, Leung T, Manser E, Lim L. Vimentin intermediate filament reorganization by Cdc42: involvement of PAK and p70 S6 kinase. Eur J Cell Biol 81: 692–701, 2002. [DOI] [PubMed] [Google Scholar]
- 6.Cote JF, Vuori K. GEF what? Dock180 and related proteins help Rac to polarize cells in new ways. Trends Cell Biol 17: 383–393, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Daya S, Loughlin AJ, Macqueen HA. Culture and differentiation of preadipocytes in two-dimensional and three-dimensional in vitro systems. Differentiation 75: 360–370, 2007. [DOI] [PubMed] [Google Scholar]
- 8.Eckes B, Dogic D, Colucci-Guyon E, Wang N, Maniotis A, Ingber D, Merckling A, Langa F, Aumailley M, Delouvee A, Koteliansky V, Babinet C, Krieg T. Impaired mechanical stability, migration and contractile capacity in vimentin-deficient fibroblasts. J Cell Sci 111: 1897–1907, 1998. [DOI] [PubMed] [Google Scholar]
- 9.Eriksson JE, He T, Trejo-Skalli AV, Harmala-Brasken AS, Hellman J, Chou YH, Goldman RD. Specific in vivo phosphorylation sites determine the assembly dynamics of vimentin intermediate filaments. J Cell Sci 117: 919–932, 2004. [DOI] [PubMed] [Google Scholar]
- 10.Garrett TA, Van Buul JD, Burridge K. VEGF-induced Rac1 activation in endothelial cells is regulated by the guanine nucleotide exchange factor Vav2. Exp Cell Res 313: 3285–3297, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gerthoffer WT Actin cytoskeletal dynamics in smooth muscle contraction. Can J Physiol Pharmacol 83: 851–856, 2005. [DOI] [PubMed] [Google Scholar]
- 12.Goto H, Tanabe K, Manser E, Lim L, Yasui Y, Inagaki M. Phosphorylation and reorganization of vimentin by p21-activated kinase (PAK). Genes Cells 7: 91–97, 2002. [DOI] [PubMed] [Google Scholar]
- 13.Graham DL, Eccleston JF, Lowe PN. The conserved arginine in rho-GTPase-activating protein is essential for efficient catalysis but not for complex formation with Rho. GDP and aluminum fluoride. Biochemistry 38: 985–991, 1999. [DOI] [PubMed] [Google Scholar]
- 14.Gunst SJ, Tang DD. The contractile apparatus and mechanical properties of airway smooth muscle. Eur Respir J 15: 600–616, 2000. [DOI] [PubMed] [Google Scholar]
- 15.Henrion D, Terzi F, Matrougui K, Duriez M, Boulanger CM, Colucci-Guyon E, Babinet C, Briand P, Friedlander G, Poitevin P, Levy BI. Impaired flow-induced dilation in mesenteric resistance arteries from mice lacking vimentin. J Clin Invest 100: 2909–2914, 1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Herrmann H, Aebi U. Intermediate filaments: molecular structure, assembly mechanism, and integration into functionally distinct intracellular Scaffolds. Annu Rev Biochem 73: 749–789, 2004. [DOI] [PubMed] [Google Scholar]
- 17.Herrmann H, Bar H, Kreplak L, Strelkov SV, Aebi U. Intermediate filaments: from cell architecture to nanomechanics. Nat Rev Mol Cell Biol 8: 562–573, 2007. [DOI] [PubMed] [Google Scholar]
- 18.Humphries KM, Pennypacker JK, Taylor SS. Redox regulation of cAMP-dependent protein kinase signaling: kinase versus phosphatase inactivation. J Biol Chem 282: 22072–22079, 2007. [DOI] [PubMed] [Google Scholar]
- 19.Ko KS, McCulloch CA. Intercellular mechanotransduction: cellular circuits that coordinate tissue responses to mechanical loading. Biochem Biophys Res Commun 285: 1077–1083, 2001. [DOI] [PubMed] [Google Scholar]
- 20.Li QF, Spinelli AM, Tang DD. Cdc42GAP (GTPase activating protein) regulates the activation of Cdc42 and PAK (p21-activated kinase) in tracheal smooth muscle cells upon 5-HT stimulation (Abstract). Am J Respir Crit Care Med 175: A348, 2007. [Google Scholar]
- 21.Li QF, Spinelli AM, Wang R, Anfinogenova Y, Singer HA, Tang DD. Critical role of vimentin phosphorylation at Ser-56 by p21-activated kinase in vimentin cytoskeleton signaling. J Biol Chem 281: 34716–34724, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Liebmann T, Rydholm S, Akpe V, Brismar H. Self-assembling Fmoc dipeptide hydrogel for in situ 3D cell culturing. BMC Biotechnol 7: 88, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Marganski WA, Gangopadhyay SS, Je HD, Gallant C, Morgan KG. Targeting of a novel Ca+2/calmodulin-dependent protein kinase II is essential for extracellular signal-regulated kinase-mediated signaling in differentiated smooth muscle cells. Circ Res 97: 541–549, 2005. [DOI] [PubMed] [Google Scholar]
- 24.Meriane M, Mary S, Comunale F, Vignal E, Fort P, and Gauthier-Rouviere C. Cdc42Hs and Rac1 GTPases induce the collapse of the vimentin intermediate filament network. J Biol Chem 275: 33046–33052, 2000. [DOI] [PubMed] [Google Scholar]
- 25.Moskwa P, Paclet MH, Dagher MC, Ligeti E. Autoinhibition of p50 Rho GTPase-activating protein (GAP) is released by prenylated small GTPases. J Biol Chem 280: 6716–6720, 2005. [DOI] [PubMed] [Google Scholar]
- 26.Mucke N, Wedig T, Burer A, Marekov LN, Steinert PM, Langowski J, Aebi U, Herrmann H. Molecular and biophysical characterization of assembly-starter units of human vimentin. J Mol Biol 340: 97–114, 2004. [DOI] [PubMed] [Google Scholar]
- 27.Ogden K, Thompson JM, Hickner Z, Huang T, Tang DD, Watts SW. A new signaling paradigm for serotonin: use of Crk-associated substrate in arterial contraction. Am J Physiol Heart Circ Physiol 291: H2857–H2863, 2006. [DOI] [PubMed] [Google Scholar]
- 28.Petroll WM, Ma L, Kim A, Ly L, Vishwanath M. Dynamic assessment of fibroblast mechanical activity during Rac-induced cell spreading in 3-D culture. J Cell Physiol 217: 162–171, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rokolya A, Singer HA. Inhibition of CaM kinase II activation and force maintenance by KN-93 in arterial smooth muscle. Am J Physiol Cell Physiol 278: C537–C545, 2000. [DOI] [PubMed] [Google Scholar]
- 30.Sihag RK, Inagaki M, Yamaguchi T, Shea TB, Pant HC. Role of phosphorylation on the structural dynamics and function of types III and IV intermediate filaments. Exp Cell Res 313: 2098–2109, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sternweis PC, Carter AM, Chen Z, Danesh SM, Hsiung YF, Singer WD. Regulation of Rho guanine nucleotide exchange factors by G proteins. Adv Protein Chem 74: 189–228, 2007. [DOI] [PubMed] [Google Scholar]
- 32.Szczur K, Xu H, Atkinson S, Zheng Y, Filippi MD. Rho GTPase CDC42 regulates directionality and random movement via distinct MAPK pathways in neutrophils. Blood 108: 4205–4213, 2006. [DOI] [PubMed] [Google Scholar]
- 33.Tang DD Invited review: intermediate filaments in smooth muscle. Am J Physiol Cell Physiol 294: C869–C878, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tang DD, Anfinogenova Y. Physiologic properties and regulation of the actin cytoskeleton in vascular smooth muscle. J Cardiovasc Pharmacol Ther 13: 130–140, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tang DD, Bai Y, Gunst SJ. Silencing of p21-activated kinase attenuates vimentin phosphorylation on Ser-56 and reorientation of the vimentin network during stimulation of smooth muscle cells by 5-hydroxytryptamine. Biochem J 388: 773–783, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tang DD, Gunst SJ. The small GTPase Cdc42 regulates actin polymerization and tension development during contractile stimulation of smooth muscle. J Biol Chem 279: 51722–51728, 2004. [DOI] [PubMed] [Google Scholar]
- 37.Tang DD, Tan J. Role of Crk-associated substrate in the regulation of vascular smooth muscle contraction. Hypertension 42: 858–863, 2003. [DOI] [PubMed] [Google Scholar]
- 38.Usatyuk PV, Romer LH, He D, Parinandi NL, Kleinberg ME, Zhan S, Jacobson JR, Dudek SM, Pendyala S, Garcia JG, Natarajan V. Regulation of hyperoxia-induced NADPH oxidase activation in human lung endothelial cells by the actin cytoskeleton and cortactin. J Biol Chem 282: 23284–23295, 2007. [DOI] [PubMed] [Google Scholar]
- 39.Ushio-Fukai M, Zuo L, Ikeda S, Tojo T, Patrushev NA, Alexander RW. cAbl tyrosine kinase mediates reactive oxygen species- and caveolin-dependent AT1 receptor signaling in vascular smooth muscle: role in vascular hypertrophy. Circ Res 97: 829–836, 2005. [DOI] [PubMed] [Google Scholar]
- 40.Valgeirsdottir S, Claesson-Welsh L, Bongcam-Rudloff E, Hellman U, Westermark B, Heldin CH. PDGF induces reorganization of vimentin filaments. J Cell Sci 111: 1973–1980, 1998. [DOI] [PubMed] [Google Scholar]
- 41.Villalonga P, Ridley AJ. Rho GTPases and cell cycle control. Growth Factors 24: 159–164, 2006. [DOI] [PubMed] [Google Scholar]
- 42.Wang L, Yang L, Burns K, Kuan CY, Zheng Y. Cdc42GAP regulates c-Jun N-terminal kinase (JNK)-mediated apoptosis and cell number during mammalian perinatal growth. Proc Natl Acad Sci USA 102: 13484–13489, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wang L, Yang L, Filippi MD, Williams DA, Zheng Y. Genetic deletion of Cdc42GAP reveals a role of Cdc42 in erythropoiesis and hematopoietic stem/progenitor cell survival, adhesion, and engraftment. Blood 107: 98–105, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wang R, Li Q, Tang DD. Role of vimentin in smooth muscle force development. Am J Physiol Cell Physiol 291: C483–C489, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wang R, Li QF, Anfinogenova Y, Tang DD. Dissociation of Crk-associated substrate from the vimentin network is regulated by p21-activated kinase on ACh activation of airway smooth muscle. Am J Physiol Lung Cell Mol Physiol 292: L240–L248, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yamaguchi T, Goto H, Yokoyama T, Sillje H, Hanisch A, Uldschmid A, Takai Y, Oguri T, Nigg EA, Inagaki M. Phosphorylation by Cdk1 induces Plk1-mediated vimentin phosphorylation during mitosis. J Cell Biol 171: 431–436, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Yang L, Wang L, Zheng Y. Gene targeting of Cdc42 and Cdc42GAP affirms the critical involvement of Cdc42 in filopodia induction, directed migration, and proliferation in primary mouse embryonic fibroblasts. Mol Biol Cell 17: 4675–4685, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zhou YT, Soh UJ, Shang X, Guy GR, Low BC. The BNIP-2 and Cdc42GAP homology/Sec14p-like domain of BNIP-Salpha is a novel apoptosis-inducing sequence. J Biol Chem 277: 7483–7492, 2002. [DOI] [PubMed] [Google Scholar]