Abstract
Matriptase, a transmembrane serine protease, is broadly expressed by, and crucial for the integrity of, the epithelium. Matriptase is synthesized as a zymogen and undergoes autoactivation to become an active protease that is immediately inhibited by, and forms complexes with, hepatocyte growth factor activator inhibitor (HAI-1). To investigate where matriptase is activated and how it is secreted in vivo, we determined the expression and activation status of matriptase in seminal fluid and urine and the distribution and subcellular localization of the protease in the prostate and kidney. The in vivo studies revealed that while the latent matriptase is localized at the basolateral surface of the ductal epithelial cells of both organs, only matriptase-HAI-1 complexes and not latent matriptase are detected in the body fluids, suggesting that activation, inhibition, and transcytosis of matriptase would have to occur for the secretion of matriptase. These complicated processes involved in the in vivo secretion were also observed in polarized Caco-2 intestinal epithelial cells. The cells target latent matriptase to the basolateral plasma membrane where activation, inhibition, and secretion of matriptase appear to take place. However, a proportion of matriptase-HAI-1 complexes, but not the latent matriptase, appears to undergo transcytosis to the apical plasma membrane for secretion. When epithelial cells lose their polarity, they secrete both latent and activated matriptase. Although most epithelial cells retain very low levels of matriptase-HAI-1 complex by rapidly secreting the complex, gastric chief cells may activate matriptase and store matriptase-HAI-1 complexes in the pepsinogen-secretory granules, suggesting an intracellular activation and regulated secretion in these cells. Taken together, while zymogen activation and closely coupled HAI-1-mediated inhibition are common features for matriptase regulation, the cellular location of matriptase activation and inhibition, and the secretory route for matriptase-HAI-1 complex may vary along with the functional divergence of different epithelial cells.
Keywords: hepatocyte growth factor activator inhibitor-1, secretion, protease
matriptase, a type ii transmembrane serine protease, is broadly expressed in most human epithelial tissue (28, 32). The protease is found in epithelium of all histological types, including columnar, cuboidal, squamous, and their complex forms. Distinct spatial distribution of matriptase in epithelial components has, however, been observed in several tissues. For example, ductal epithelia consistently express higher levels of matriptase than acinar secretory glandular epithelia. This expression pattern suggests that the protease may play a role common to the function of all epithelia. Furthermore, functional divergence of matriptase may be associated with the diverse functions of different epithelial tissues. Matriptase is coexpressed with its endogenous, Kunitz-type protease inhibitor, hepatocyte growth factor activator inhibitor (HAI)-1, in most epithelial tissues, suggesting a HAI-1-based regulatory mechanism for matriptase common to all these tissues. The functional linkage between matriptase and HAI-1 has been well documented by animal studies in which the mutant phenotypes associated with genetic ablation of HAI-1, such as embryonic lethality in mice and loss of epidermal integrity in zebrafish, can be corrected by the simultaneous deletion of matriptase (8, 23, 33). Similarly, the procarcinogenic effects produced by modest overexpression of matriptase in the skin of transgenic mice can be completely reverted by concomitant overexpression of HAI-1 (21).
Although both matriptase and HAI-1 are integral membrane proteins that are targeted to the basolateral plasma membrane of polarized epithelial cells (10, 30), these proteins have also been shown to be consistently secreted (19, 31). Epithelial cells grown as monolayers secrete matriptase, both as a latent form and as an activated form complexed with HAI-1. Similarly, epithelial cells in monolayer culture secrete HAI-1 as a free, noncomplexed form and in complexes with activated matriptase. While matriptase and HAI-1 are secreted at low levels by some epithelial/carcinoma cells, secretion is significantly enhanced when activation of the matriptase zymogen is induced. For example, 184 A1N4 immortal mammary epithelial cells do not activate or secrete matriptase in the absence of sphingosine 1-phosphate (S1P). Stimulation of the cells with this blood-borne lysophospholipid results in the rapid activation and shedding of matriptase (2, 3). HAI-1 appears to inhibit the newly activated matriptase almost immediately, and matriptase-HAI-1 complexes along with latent matriptase and free HAI-1 are rapidly shed into the extracellular milieu (15). This tight coupling of matriptase secretion with activation and inhibition is also observed in lymph node prostatic adenocarcinoma (LNCaP) cells in which these processes are, additionally, dependent on androgen stimulation (13). Matriptase and HAI-1 are also secreted into human milk by the lactating mammary gland (17). In contrast to the conditioned media of epithelial and carcinoma cells, human milk contains no latent matriptase. Activated matriptase is detected in human milk either in HAI-1 complexes or in complexes with serpins (35). Thus, there appear to be some similarities and differences in the ways in which matriptase is secreted in culture systems in vitro and in the lactating mammary gland in vivo.
To investigate how matriptase is secreted in vivo, we began the current study with the examination of matriptase expression and activation status in semen and urine as well as the prostate and the kidney, which secrete proteins into the body fluids, respectively. This survey reveals that for most polarized epithelial cells, matriptase secretion is a sequential event in a rapid kinetics involving zymogen activation of matriptase and HAI-1-mediated inhibition at the basolateral plasma membrane, transcytosis across the cells, and shedding from the apical surface into the lumen. An interesting exception was also observed in human stomach chief cells which activate matriptase and store matriptase-HAI-1 complex in the secretory granules. Many of the features associated with the in vivo secretion of matriptase appear to be retained and can be confirmed in vitro in cultured epithelial cells. This study further emphasizes the importance of subcellular localizations either at basolateral plasma membrane or in the secretory granules, only at which the relevant substrates could be processed by the short-lived, active matriptase which is quickly inhibited by HAI-1 right after its own activation.
MATERIALS AND METHODS
Chemicals and reagents.
CM-Sepharose and activated Sepharose beads were obtained from GE Healthcare (Piscataway, NJ). The anti-pepsinogen 2 antibody (sheep polyclonal, ab9013) was purchased from Abcam (Cambridge, UK), the FITC-conjugated anti-sheep antibody (313-096-003) and Texas Red- conjugated anti-mouse antibodies (115-076-003) were purchased from Jackson ImmunoResearch (Newmarket, UK). Diaminobenzidine (K3468) and the secondary antibody (K4063, EnVision+ Dual Link System Peroxidase) were from Dako (Glostrup, Denmark). Human body fluids were purchased from Lee BioSolutions (St. Louis, MO). All other chemical reagents were purchased from Sigma unless otherwise specified.
Monoclonal antibodies.
Human matriptase protein was detected using either the monoclonal antibody M32, which recognizes the third LDL receptor class A domain of matriptase in both the latent (one-chain) and activated (two-chain) forms of the protease, or using M69, a monoclonal antibody that recognizes an epitope present only in the activated (two-chain) form of the enzyme (2, 3). Human HAI-1 was detected using the HAI-1 specific monoclonal antibody M19 (17). The matriptase monoclonal antibody S5 was used in some immunohistochemical studies (27, 28). Rat monoclonal anti-matriptase antibody 21-9 also recognizes both latent and active forms of the enzyme (19).
Immobilization of mAbs.
Monoclonal antibodies M19, M69, and 21-9 were covalently coupled to Sepharose 4B at 5 mg/ml gel following the manufacturer's instructions (GE Healthcare). Briefly, the mAbs were purified and dialyzed against the coupling buffer (0.1 M sodium bicarbonate, 0.5 M sodium chloride) and incubated overnight at 4°C with CNBr-activated Sepharose 4B. Uncoupled antibody was removed by washing the beads with the coupling buffer, and the residual coupling sites on beads were quenched using 1 M Tris buffer. The mAb-Sepharose beads were stored in PBS at 4°C.
Western blot analysis.
Protein samples for Western blotting were diluted in 5× sample buffer. The sample buffer did not contain a reducing agent, and samples were not boiled before SDS-PAGE unless otherwise specified, since reducing agents may destroy the epitopes recognized by the monoclonal antibodies, and boiling disrupts matriptase/HAI-1 complexes. Proteins were resolved by 7.5% SDS-PAGE, transferred to Protran nitrocellulose membranes (Schleicher and Schuell, Keene, NH), and probed with the monoclonal antibodies M32, M69, and M19 or the polyclonal anti-serpin antibodies. The binding of the primary antibody was detected using horseradish peroxidase-conjugated secondary antibodies (Jackson ImmunoResearch Laboratories, West Grove, PA), and visualized using Western Lightening Chemiluminescence Reagent Plus (Perkin-Elmer, Boston, MA).
Immunodepletion and immunoprecipitation.
Samples of human body fluids were incubated with mAb 21-9 beads at 4°C for 2 h. Unbound proteins were separated from the beads by centrifugation at 2,000 rpm for 10 min, and the supernatants were collected. The beads were washed and the mAb-captured proteins were eluted from the immunoaffinity beads using glycine buffer, pH 2.4. The eluted proteins were neutralized by the addition of Tris buffer immediately after elution. All samples, including the crude fluids, unbound supernatants, and the materials eluted from the beads, were subjected to immunoblot analysis.
Immunohistochemistry.
Immunohistochemistry staining was performed using the manufacturer's standard protocol with minor modification (Dako). Sections were stained using the matriptase-specific mAbs, M32 S5 and M69, and the HAI-1-specific mAb, M19, at a concentration of 5 μg/ml. Mouse IgG1 at a concentration of 5 μg/ml was used as a negative control on duplicate sections. Colorimetric reactions for the control slides were developed for the same amount of time as experimental slides and did not stain.
Immunocytochemistry.
Caco-2 cells were originally obtained from American Type Culture Collection and were maintained in DMEM supplemented with 20% FBS. The cells were seeded at a density of 1 × 105 cells per well onto glass coverslips in 12-well plates and cultured in DMEM-20% FBS for 12 days with medium replacement every 3 days until the cells were well differentiated. Polarized cell monolayers were fixed and permeabilized in PBS containing 0.05% Triton X-100 and 3.7% formaldehyde for 20 min at room temperature. The cells were stained for matriptase using Alexa Fluor 594-conjugated mAb, M32. Tight junctions and adherens junctions were visualized using anti-ZO-1 and anti-E-cadherin antibodies conjugated with FITC (BD Biosciences, Palo Alto, CA). After fluorescent staining, the coverglasses were mounted with Prolong Antifade (Molecular Probes), and the fluorescent images were taken with a Nikon Confocal microscope.
Directional secretion assays.
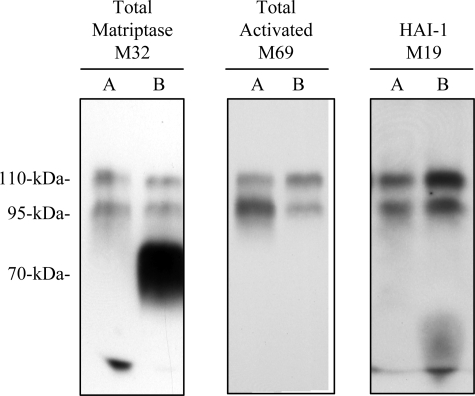
For the examination of directional secretion, Caco-2 cells were seeded in a transwell plate with polycarbonate membrane (0.4-μm pore size) and cultured until a well-differentiated monolayer was formed. The culture media in the top and bottom chambers were replaced with basal DMEM in the absence of fetal bovine serum. Twenty-four hours later, conditioned media were collected from the top and bottom chambers and assayed by immunoblot analysis for total matriptase using the matriptase mAb M32, activated matriptase using the activated matriptase mAb M69, and HAI-1 using the HAI-1 mAb M19.
Collection and processing of body fluids.
Human semen samples were purchased from Lee Biosolution (St. Louis, MO). The semen samples were centrifuged at 5,000 g for 10 min, and the seminal fluid was saved. Human urine was concentrated using Centriprep Centrifugal Filter Devices with YM-10.
RESULTS
Detection of activated matriptase in HAI-1 complexes in body fluids.
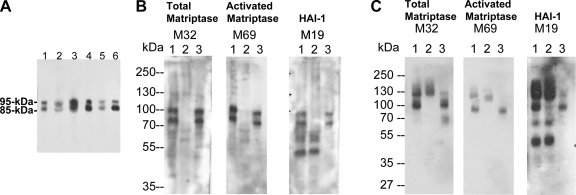
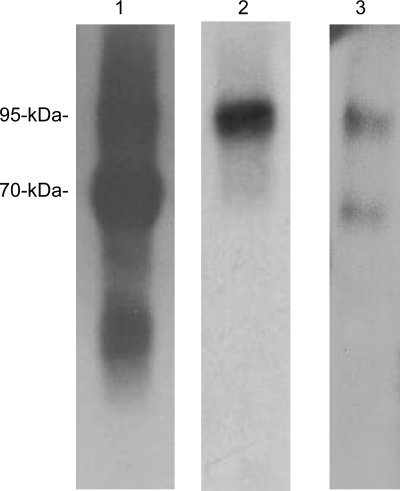
Matriptase has previously been shown to be present in human milk only in its activated form in complexes with either HAI-1 or serpins (17, 35). Despite the large amount of activated matriptase found in milk, no latent matriptase can be detected. Thus, secretion of matriptase by lactating mammary epithelial cells appears to be tightly associated with matriptase zymogen activation and inhibition of active matriptase by HAI-1. To investigate whether these three tightly coupled processes (activation, inhibition, and secretion) are a common phenomenon in the regulation of matriptase secretion in different epithelial tissues, we examined the forms of matriptase found in other body fluids, including seminal fluid and urine. We first examined the expression of matriptase in human seminal fluid from more than six healthy donors (Fig. 1A). Seminal fluid was subjected to Western blot analysis, and matriptase was consistently detected as bands of 95 and 85 kDa in all of these specimens using the matriptase mAb M32. We then confirmed that both the 95-kDa and 85-kDa species consisted of activated matriptase in HAI-1 complexes using a combination of immunoprecipitation and Western blotting (Fig. 1B). The seminal fluid was first incubated with matriptase mAb 21-9-Sepharose, which was shown to immunodeplete both the 95- and 85-kDa matriptase species (Fig. 1B, comparing lanes 2 with lanes 1). The captured matriptase complexes were then eluted from the beads with pH 2.4 buffer and immediately neutralized. The eluates were then analyzed by immunoblot for total matriptase using the mAb M32 (Fig. 1B, left, lane 3), activated matriptase using the mAb M69 (Fig. 1B, middle, lane 3), and HAI-1 using the mAb M19 (Fig. 1B, right, lane 3). All three mAbs interacted with both the 85- and 95-kDa species, suggesting that both bands contain activated matriptase complexed with HAI-1. These data demonstrate that seminal fluid contains only active matriptase in complex with HAI-1, but no latent matriptase. A 95-kDa matriptase-HAI-1 complex, containing 70-kDa active matriptase and a 45-kDa HAI-1 fragment, was previously detected in human milk and the conditioned media of epithelial and cancer cells (17). The 85-kDa complex may be a degradation product of the 95-kDa complex due to the presence of many active proteases in human seminal fluid. The 85-kDa complex may contain 70-kDa active matriptase and a 25-kDa HAI-1 fragment, a complex found in other systems and described in a previous study (17). While no latent matriptase was detected in the human seminal fluid (Fig. 1B, left), uncomplexed HAI-1 was detected (Fig. 1B, right, lanes 1 and 2).
Fig. 1.
Secretion of matriptase-hepatocyte growth factor activator inhibitor (HAI-1) complexes, but not latent matriptase in seminal fluid and urine. A: matriptase in human seminal fluid. The presence of matriptase in human seminal fluid from six healthy donors was characterized by immunoblotting using the M32 mAb. Two major bands at around 85 kDa and 95 kDa were observed in all six specimens. B: seminal fluid contains activated matriptase in HAI-1 complexes, but no latent matriptase. The seminal fluid (lanes 1) was subjected to immunoprecipitation with immobilized matriptase mAb 21-9. The unbound fractions were collected (lanes 2), and the matriptase mAb-captured proteins were eluted using acidic buffer, pH 2.4, and then immediately neutralized (lanes 3). All of these samples were analyzed by immunoblotting for total matriptase using the mAb M32 (left, total matriptase, M32), activated matriptase using the mAb M69 (middle, activated matriptase, M69), and HAI-1 using the mAb M19 (right, HAI-1, M19). Both the 85- and the 95-kDa matriptase species were recognized by the activated matriptase mAb and the HAI-1 mAb. Noncomplexed HAI-1 fragment at 50 kDa was also detected by the HAI-1 mAb M19 (right, lane 1) and was not precipitated by matriptase mAb 21–9 beads. C: human urine contains matriptase-HAI-1 complex, but no latent matriptase. The matriptase species present in human urine were assessed by immunoprecipitation followed by immunoblotting through the same procedures as in B. All three mAbs identified the same two proteins bands: one at 95 kDa and a smeared band of >120 kDa (lanes 1). The 95-kDa protein band was depleted by precipitation with matriptase mAb 21-9 beads (lanes 2) and was recognized by the activated matriptase mAb M69 (middle, lane 3) and the HAI-1 mAb M19 (right, lane 3), suggesting that the 95-kDa species is matriptase-HAI-1 complex. The smeared protein bands that were identified in all three immunoblots were not precipitated using the matriptase mAb 21–9 beads (lanes 2), suggesting that the bands do not contain matriptase. The smear of bands is most likely the result of human immunoglobulins in the sample that are identified through cross-reaction with the anti-mouse IgG secondary antibody. A relatively sharp band present at the lower end of the smear (left, lane 1) is, however, immunodepleted by the matriptase mAb 21–9 beads (left, lane 2) and was eluted (left, lane 3). This 110-kDa matriptase band is the matriptase-antithrombin III complex described in our previous study (35). The 50-kDa, free HAI-1 was also detected in human urine by the HAI-1 mAb M19 (right, lane 1) but was not precipitated by the matriptase mAb 21–9 beads, as expected (right, lane 2).
In addition to matriptase-HAI-1 complex, human seminal fluid also contains matriptase-antithrombin III complex with a size of 110-kDa, which was faintly visible (Fig. 1B, left, lane 3). The matriptase-antithrombin III complex was confirmed by immunoblot analysis using antithrombin III antibody (data not shown). Previously, we have purified matriptase-antithrombin III complex from human milk (35). The secretion of matriptase-serpin complexes into body fluid may involve nonepithelial cells in these organs, likely the migrating leukocytes, which do not express HAI-1 and may secrete active matriptase into the extracellular milieu at which the active matriptase encounters deposited antithrombin III. Since formation of matriptase-serpin complexes involving nonepithelial cells and is beyond the scope of the current study, we will address this aspect in the future study.
Human urine also contains matriptase-HAI-1 complexes. Two protein bands (95 kDa and a smeared band of around 130 kDa) were detected by the total matriptase mAb M32 (Fig. 1C, left). The 95-kDa species appears to be active matriptase in a HAI-1 complex since it is immunoprecipitated by the matriptase mAb 21–9 and is detected by both the activated matriptase mAb M69 (Fig. 1C, middle) and the HAI-1 mAb M19 (Fig. 1C, right). The 130-kDa smeared band is most likely the result of human immunoglobulins present in the urine which were recognized by anti-mouse IgG secondary antibody, but which were not immunoprecipitated by the 21-9 antibody beads (Fig. 1C, lanes 2). Similar to human milk and seminal fluid, there was no detectable latent matriptase present in the urine (Fig. 1C, left, lane 1). Urine did, however, contain the uncomplexed, 50-kDa HAI-1 fragment (Fig. 1C, right, lanes 1 and 2).
Latent matriptase is localized at the basolateral plasma membrane of the ductal epithelial cells of the prostate and kidney, but activated matriptase is rare.
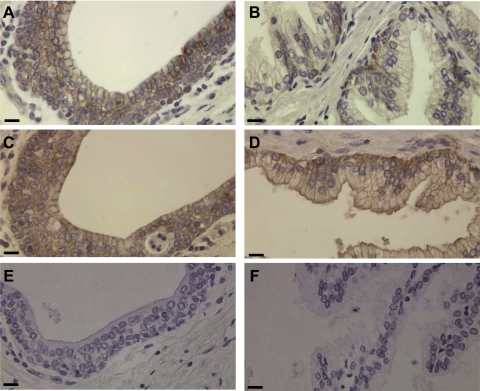
The presence of activated matriptase in complexes with HAI-1 in body fluids suggests that activation and HAI-1 binding of active matriptase must have taken place in those epithelial cells that contribute to the production of those body fluids. The prostate and the kidney are the main sources for the proteins present in the seminal fluid and urine, respectively. We, therefore, examined the expression and activation status of matriptase by immunohistochemical (IHC) staining of paraffin-embedded human prostate and kidney tissue sections. The glandular components of the human prostate are composed of ducts and acini. Matriptase was readily detected on the basolateral plasma membrane of the ductal epithelial cells (Fig. 2A). In the acini, matriptase was detected at low levels in the basal cell compartment but was not detected in the luminal cells (Fig. 2B). HAI-1 was also present at high levels in the ductal epithelial cells of the prostate, although interestingly, it was detected on both the basolateral and apical plasma membranes (Fig. 2C). In contrast to matriptase, HAI-1 was readily detected in both the luminal and basal epithelial cells of the prostates acini (Fig. 2D). In the luminal epithelial cells of the acini, HAI-1 was clearly observed at both basolateral and apical plasma membranes (Fig. 2D). HAI-1 was also detected in the basal cells with more intense staining than in the luminal cells (Fig. 2D). Although matriptase and HAI-1 are expressed at significant levels in prostate epithelial cells, activated matriptase was not detectable in the ductal or luminal epithelial cells by staining with the activated matriptase mAb M69 (Fig. 2, E and F). Very weak staining with this antibody was, however, detected in the basal cells of the acini.
Fig. 2.
Expression and distribution of matriptase and HAI-1 in the human prostate. Paraffin-embedded human prostate tissue sections were stained by immunohistochemistry using three mAbs: the M32 for total matriptase (A and B), the M69 for activated matriptase (E and F), and the M19 for HAI-1 (C and D). Positive staining was observed as brown precipitates (diaminobenzidine), and nuclei were counterstained with hematoxylin. The photomicrographs in A, C, and E were taken from the ductal portions of the prostate and in B, D, and F from the acinial portions. Bar = 25 μm.
In the human kidney, matriptase was also detected in the ductal epithelial cells. At high magnification, the protease is clearly observed at the basolateral plasma membrane of the ductal epithelial cells with stronger staining in the distal collecting ducts than in the proximal collecting ducts (Fig. 3A). HAI-1 was also detected in the ductal epithelial cells and at both the basolateral and apical plasma membrane, similar to the staining pattern seen in the prostate (Fig. 3B). Activated matriptase was essentially undetectable in any of the ductal epithelial cells of the kidney (Fig. 3C).
Fig. 3.
Distribution of matriptase and HAI-1 in human kidney. Paraffin-embedded human kidney specimens were stained by immunohistochemistry using mAbs against total matriptase (A), activated matriptase (C), or HAI-1 (B). Positive staining was observed as brown precipitates (diaminobenzidine), and nuclei were counterstained with hematoxylin. Bar = 20 μm.
The in vivo subcellular distribution seen for matriptase and HAI-1 appears to be consistent with their differential secretion, due to their relative proximity to the lumen. The apical localization of HAI-1 facilitates secretion into body fluids, whereas the basolateral localization of matriptase requires complicated mechanisms for secretion into the lumen. While the secretion of basolaterally located proteins into the lumen of glandular tissues occurs quite commonly, their secretion involves many steps. In order for basolaterally located proteins to reach the lumen during secretion, the proteins must undergo transcytosis, a process that involves internalization of the proteins from the basolateral plasma membrane, followed by intracellular trafficking to the apical plasma membrane for secretion (36). The absence of the latent form of matriptase in body fluids suggests that this form of the protein does not undergo transcytosis for secretion. In contrast, activated matriptase in HAI-1 complexes appears to reach the apical plasma membrane for secretion into the lumen, suggesting that transcytosis is involved.
In polarized epithelial cells, latent matriptase is secreted basolaterally, but matriptase-HAI-1 complexes can be secreted both apically and basolaterally.
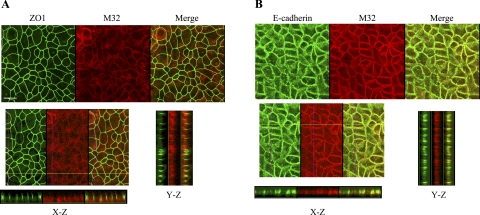
The secretion of matriptase-HAI-1 complexes might be achieved through multiple possible mechanisms that might involve many cellular processes, such as the activation of matriptase zymogen, inhibition of active matriptase by HAI-1, and transcytosis of matriptase-HAI-1 complexes, if the activation event takes place near the basolateral plasma membrane. Thus, to investigate the processes involved in the secretion of active matriptase-HAI-1 complexes and to determine the reason that latent matriptase is absent from body fluids in vivo, we employed a model system that uses Caco-2 human intestine epithelial cells. These cells, when grown to confluence, can be induced to undergo differentiation forming a polarized cell monolayer in which the plasma membrane of the cells is divided into an apical membrane and basolateral membrane by tight junctions between neighboring cells, a situation similar to that found in the epithelium in vivo. The barrier formed by the tight junctions prevents large molecules such as proteins moving freely between both plasma membrane domains, and, as a result, the activation and secretion of matriptase can be assessed at both basolateral and apical surfaces (22, 25, 26). We first examined the cellular distribution of matriptase in the differentiated Caco-2 cell system (Fig. 4). Confocal microscopy showed that matriptase was colocalized with E-cadherin, a marker for adherens junctions (Fig. 4B) and was detected underneath ZO-1, a marker for tight junctions (Fig. 4A). The relatively diffuse staining pattern of matriptase in Fig. 4A was due to the focal plane set on the apical plasma membrane but not the basolateral plasma membrane. When the focal plane was set on the basolateral membrane, both matriptase and E-cadherin were clearly observed on the cell-cell junction (Fig. 4B). These data show that matriptase is localized at the basolateral plasma membrane, consistent with the situation in vivo as demonstrated by IHC (Figs. 2 and 3). Next, we grew the cells in the transwell system, and conditioned media from both the basolateral and apical surfaces were collected. When these conditioned media were analyzed for the presence of secreted matriptase, both free latent matriptase and activated matriptase in HAI-1 complexes were detected in the conditioned media collected from the basolateral surface (Fig. 5, lanes B). The secretion of latent matriptase from the basolateral surface appears to be consistent with the finding that matriptase resides at the basolateral plasma membrane (Figs. 2, 3, and 4). The secretion of matriptase-HAI-1 complexes from the basolateral surface suggests that matriptase activation and inhibition by HAI-1 binding occurs at or near the basolateral plasma membrane. The conditioned media collected from the apical surface, however, only contained activated matriptase in HAI-1 complexes, and no latent matriptase (Fig. 5, lanes A). These data suggest that in polarized epithelial cells, some fraction of matriptase-HAI-1 complexes undergoes transcytosis and traffics to the apical surface for secretion, but that latent matriptase does not undergo transcytosis. This observation with the polarized Caco-2 cell system provides an explanation for the finding that while matriptase-HAI-1 complexes are present in body fluids such as milk, seminal fluid, and urine, latent matriptase is absent.
Fig. 4.
Subcellular localization of matriptase in polarized Caco-2 human intestinal epithelial cells. Caco-2 cells were grown and allowed to undergo differentiation on coverslips for 12 days. The polarized cells were fixed, permeabilized, and stained for matriptase with Alexa Fluor 594-conjugated mAb M32. The tight junction marker ZO-1 (A) and the adherens junctions marker E-cadherin (B) were costained with matriptase using FITC-conjugated antibodies to each protein. The staining was observed using a Nikon confocal microscope, and a series of images at different planes in the Z-axis were acquired. Representative images for matriptase, ZO-1, and E-cadherin from both staining studies are shown in the top panels, as indicated. The images in the X-Z- and Y-Z-axes were assembled from serial Z-section images and are shown in the bottom panels, as indicated. The scale bar represents 10 μm.
Fig. 5.
Differential secretion of matriptase in polarized Caco-2 human intestinal epithelial cells. Caco-2 cells were grown and allowed to differentiate in transwell chambers for 12 days. The cells were incubated with serum-free culture medium in the top and bottom chambers for 1 day, and the conditioned media from both sides were collected, concentrated, separated by SDS-PAGE, and analyzed by immunoblotting with the matriptase mAb M32, the activated matriptase mAb M69, or the HAI-1 mAb M19, respectively. A, apical surface; B, basolateral surface.
In contrast to the situation in polarized epithelial cells, when epithelial cells lose their polarity when grown in simple monolayer cultures, or when they become transformed, they secrete both latent matriptase and matriptase-HAI-1 complexes. In our previous studies (4, 13, 17, 19), we have detected both latent matriptase and matriptase-HAI-1 complexes in the conditioned media of many epithelial cells and carcinoma cells grown in monolayer culture. This includes immortal mammary epithelial cells isolated from mammary tissue, or isolated from milk, breast cancer cells, and prostate cancer cells. Conditioned media from all of these systems contain both latent and activated matriptase.
Activation of matriptase is rapidly followed by secretion.
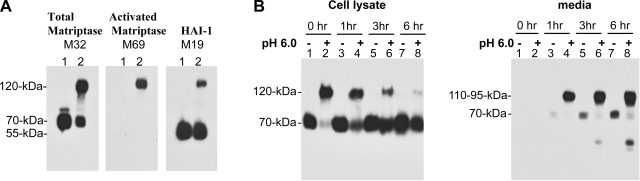
Detection of matriptase-HAI-1 complexes in semen and urine suggests that the activation of matriptase is occurring constitutively in the ductal epithelial cells of the prostate and the kidney. It is interesting, therefore, that activated matriptase was barely detectable in the ductal epithelial cells of these tissues (Figs. 2 and 3). We infer from this observation that matriptase-HAI-1 complexes are secreted very rapidly after activation has occurred. Two model systems have been developed in which matriptase activation can be induced by different exogenous stimuli, such as S1P and androgen, in a somewhat cell type-specific fashion (2, 3, 13). Matriptase activation was closely followed by shedding of matriptase-HAI-1 complexes in these two model systems. We have recently discovered that matriptase activation can be rapidly and robustly induced ubiquitously in all matriptase-expressing cells by exposing the cells to a mildly acidic extracellular milieu, with a pH optimum of approximately pH 6 (I.-C. Tseng, H. Xu, F.-P. Chou, M. Johnson, and C.-Y. Lin, unpublished observations). We made use of this mildly acidic pH-induced matriptase activation to study the detailed timing and dynamics of matriptase activation and shedding. Immortal mammary epithelial cells (184 A1N4) were grown overnight, which results in the level of matriptase activation dropping essentially to zero due to the depletion of available S1P in the medium (2, 3). In this condition, despite the fact that high levels of latent matriptase are expressed (Fig. 6A, total matriptase M32, lane 1), no activated matriptase was detected in cell lysate (Fig. 6A, activated matriptase M69, lane 1). HAI-1 was detected as a 55-kDa free form (Fig. 6A, HAI-1, M19). When the cells were incubated with pH 6.0 buffers, such as citric-phosphate buffer or phosphate buffer, for 20 min, robust matriptase activation was induced and the vast majority of 70-kDa latent matriptase was converted into 120-kDa activated matriptase-HAI-1 complex which was detected by these three mAbs (Fig. 6A, lanes 2), confirming that this 120-kDa species is composed of activated matriptase in complex with HAI-1. To investigate the kinetics of shedding of matriptase-HAI-1 complex and the latent matriptase, the pH 6.0-pretreated cells and the control cells were washed with PBS and incubated in basal culture medium. The medium and cells were then harvested immediately or after 1, 3, or 6 h. All samples were then analyzed by Western blot analysis using the M32 mAb (Fig. 6B). The 120-kDa matriptase-HAI-1 complex quickly disappeared from the cells (Fig. 6B, left, cell lysate, pH 6.0 +, lanes 2, 4, 6, and 8) and was shed into media as 110- and 95-kDa complexes (Fig. 6B, right, media, pH 6.0, lanes 2, 4, 6, and 8). One hour after the acid-treated cells were returned to the basal media, more than half of the 120-kDa cellular matriptase-HAI-1 (Fig. 6B, left, cell lysate, comparing lane 4 with lane 2) was shed to the conditioned media as 95- and 110-kDa complexes (Fig. 6B, right, lane 4). Most of the matriptase-HAI-1 complex was released into media after 3 h (Fig. 6B, left and right, lane 6). The 70-kDa latent matriptase was also shed by the nonactivation control cells, but at a much slower rate, suggesting that the shedding of matriptase is accelerated by activation (Fig. 6B, right, media, lanes 3, 5, and 7). Interestingly, the 70-kDa latent matriptase appeared to be quickly replenished in those cells in which matriptase activation had occurred, and matriptase-HAI-1 complex was rapidly shed (Fig. 6B, left, cell lysate, lanes 2, 4, 6, and 8). These data suggest that matriptase activation is rapidly followed by the secretion of the activated protein in complex with HAI-1, possibly explaining why we are unable to demonstrate the presence of activated matriptase by IHC in tissues such as the prostate and kidney that are apparently actively secreting activated matriptase into body fluids.
Fig. 6.
Matriptase activation is quickly followed by secretion. A: activation and HAI-1-mediated inhibition induced by exposure to a mildly acidic milieu. 184 A1N4 mammary epithelial cells were exposed to basal media or phosphate buffer, pH 6.0, for 20 min. Lysates from both treatments (lanes 1 for nonactivation control; lanes 2 for activation) were subjected to immunoblot analyses for total matriptase using the mAb M32 (left), activated matriptase using the mAb M69 (middle), or HAI-1 using the mAb M19 (right). B: kinetics of matriptase-HAI-1 complex shedding. Matriptase activation was induced by acid exposure, and the cells returned to the basal media for the indicated times. Cell lysates and conditioned media were harvested and analyzed by immunoblotting using the matriptase mAb M32, the activated matriptase mAb M69 (data not shown), and the HAI-1 mAb M19 (data not shown). Control cultures, not exposed to acid, were also examined with the same time course.
Matriptase-HAI-1 complex in the secretory granules of the human stomach chief cells.
While activated matriptase in most epithelial tissues studied was below the level of detection by IHC using mAb M69, which we hypothesize is due to rapid secretion after activation as described above, the human stomach appears to be an interesting exception.
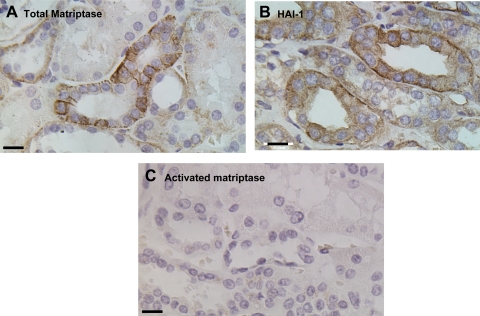
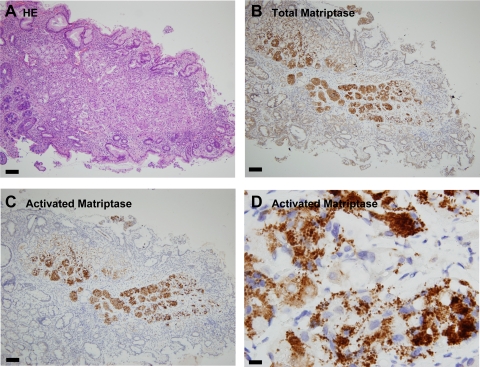
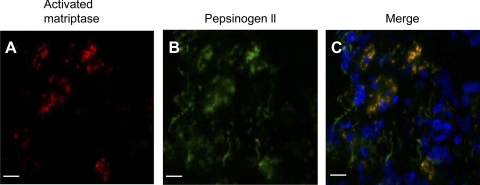
Although we had not assayed matriptase levels in gastric secretions as part of our survey of body fluids, we were interested in establishing the pattern of matriptase and HAI-1 expression in this tissue. Sections of human stomach were therefore stained using the matriptase mAb S5. Strong IHC staining was observed in both- parietal and chief cells, with the chief cells apparently expressing more matriptase than the parietal cells (Fig. 7B). When the activated matriptase mAb M69 was used, very strong staining of activated matriptase was observed in the chief cells (Fig. 7C). Interestingly, M69 staining was very weak or negative in the other cell types neighboring to the chief cells even though these cells express matriptase at high levels (Fig. 7C). The cognate matriptase inhibitor HAI-1 was also detected in the chief cells and other stomach cells (data not shown). Under higher magnification, both total matriptase (data not shown) and activated matriptase were observed to be in granule-like structures (Fig. 7D). The control staining with mouse IgG was negative (data not shown). The chief cells of the human stomach contain many secretory granules of pepsinogens. To investigate whether the granule-like staining of activated matriptase is colocalized with the pepsinogen granules, human stomach sections were simultaneously stained for pepsinogen II and activated matriptase using an immunofluorescence technique. In Fig. 8, merged images of the staining for activated matriptase (red) and pepsinogen II (green) clearly demonstrate the colocalization of these two antigens. These data suggest that activated matriptase is accumulated in the secretory granules of the human stomach chief cells.
Fig. 7.
Distribution of matriptase in the human stomach. Paraffin-embedded human stomach sections were stained with hematoxylin and eosin (HE; A) for total matriptase using the mAb S5 (B), and for activated matriptase using the mAb M69 (C and D). The bars are 100 μm in A, B, and C and 10 μm in D.
Fig. 8.
Colocalization of activated matriptase with pepsinogen 2 in the secretory granules of gastric chief cells. Paraffin-embedded human stomach sections were stained for activated matriptase (A) and pepsinogen 2 (B) using the mAb M69 and the sheep polyclonal antibody (ab9013), respectively. C: merged image of A and B to show the colocalization between the activated matriptase and pepsinogen 2. Bar = 30 μm.
In our previous studies (18), we have shown that matriptase can be detected in multiple species of different molecular mass due to its complicated life cycle: synthesized as 94-kDa full-length protein, followed by maturation through NH2-terminal processing to generate a short NH2-terminal fragment and a 70-kDa fragment which contains the bulk of extracellular domains, followed by an autoactivation process that generates a disulfide-linked, two-chain, active matriptase species that is inhibited by HAI-1 to form a 120-kDa matriptase-HAI-1 complex, and subsequent shedding from the lipid bilayer as 95- and/or 110-kDa matriptase-HAI-1 complexes (18). The 95-kDa matriptase-HAI-1 complex can subsequently be degraded into an 85-kDa species by a cleavage in the HAI-1 molecule. This 85-kDa species was seen in semen (Fig. 1) and milk (17). To determine which species of activated matriptase is recognized by the M69 mAb in the chief cell secretory granules, we prepared lysates from samples of human stomach and immunoprecipitated all matriptase species using the mAb 21-9-Sepharose. Immunoblot analysis revealed that multiple matriptase species were precipitated by the matriptase mAb beads, including bands at 95, 70, and 40 kDa (Fig. 9, lane 1). Of these species, the mAb M69 recognized only the 95-kDa matriptase-HAI-1 complex (Fig. 9, lane 2). The HAI-1 mAb M19 also recognized the 95-kDa matriptase species, confirming this 95-kDa protein band to be matriptase-HAI-1 complex (Fig. 9, lane 3). The presence of 95-kDa matriptase-HAI-1 complex in the secretory granules of the stomach chief cells suggests that matriptase activation and inhibition likely have occurred in the secretory granules.
Fig. 9.
Activated matriptase in HAI-1 complexes in human stomach. Normal human stomach tissue was homogenized using RIPA buffer. The lysate was incubated with the matriptase mAb 21-9-Sepharose beads to precipitate total matriptase. The eluted matriptase species were analyzed by immunoblotting for total matriptase using the mAb M32 (lane 1), activated matriptase using the mAb M69 (lane 2), and HAI-1 using the mAb M19 (lane 3).
DISCUSSION
In this study we characterized the in vivo and in vitro secretion of matriptase by epithelial cells. By examining the expression and activation status of matriptase in body fluids, their corresponding tissues, and cultured epithelial cells grown in simple monolayers or as differentiated polarized structures, we have developed a much clearer picture of the dynamics of matriptase activation and secretion. Secretion of matriptase in ductal epithelial cells appears to be a constitutive process that occurs as a consequence of the activation and inhibition of the protease, and that this secretion involves transcytosis of the activated complex from the basolateral surfaces of the epithelial cells. Body fluids, including milk, urine, and semen, contain matriptase, providing evidence that cells within the tissues that generate these fluids must secrete the protease. These body fluids share a common feature regarding the status of matriptase in that they only contain activated matriptase in complexes with protease inhibitors and are devoid of latent matriptase. Since polarized epithelial cells, such as the ductal epithelial cells of the prostate and kidney, sort matriptase to the basolateral plasma membrane (Figs. 2, 3, and 5), and because HAI-1 only binds to active matriptase and not to latent matriptase, secretion of matriptase in HAI-1 complexes into body fluids must involve matriptase zymogen activation, protease inhibition through HAI-1 binding, transcytosis, and subcellular targeting. The in vivo processes involved in matriptase secretion appear to be maintained in differentiated Caco-2 intestinal epithelial cells in which latent matriptase was detected in the basolateral surfaces and the matriptase-HAI-1 complexes are secreted from the apical surfaces (Figs. 4 and 5). Polarized epithelial cells likely activate matriptase zymogen and then rapidly inhibit the active matriptase through the action of HAI-1 near the basolateral surface. This hypothesis is supported not only by the presence of latent matriptase at the basolateral surface of these cells but also by the detection of matriptase-HAI-1 complexes in the conditioned media collected from the basolateral side of polarized Caco-2 cells (Fig. 5). To be secreted into body fluids, matriptase-HAI-1 complexes at the basolateral side must be internalized and undergo transcytosis to reach the apical plasma membrane, and are then shed into the lumen of these epithelial ducts. We summarized these processes in Fig. 10. Since polarized epithelial cells do not secrete latent matriptase from the apical surface, the protease likely does not contain the structural elements required for transcytosis. It is therefore likely that either HAI-1 or the matriptase-HAI-1 complexes per se bear the targeting signal for transcytosis. Indeed, HAI-1 was detected at both basolateral and apical surfaces in the current study and in a publication by the Vogel group (10). While Caco-2 cells secrete matriptase from the basolateral surface, basolateral secretion may be very limited in vivo at least in the prostate and kidney, due to the fact that despite the high levels of matriptase detected at the basal surface of the ductal epithelial cells of both organs, matriptase was not detected in the extracellular spaces underneath these ductal epithelial cells. The secretion of matriptase-HAI-1 complexes appears to be very efficient in the polarized ductal epithelial cells which results in the extremely low levels of activated matriptase present in these cells. Cultured epithelial and carcinoma cells apparently share this in vivo characteristic of matriptase secretion since activated matriptase was detected predominantly in the conditioned media with a very small proportion of the protein remaining in the cells. The secretion of matriptase-HAI-1 complexes may occur simultaneously with the activation and inhibition of the protease as shown previously (2, 13) and in the current study. Furthermore, the secretion may be enhanced by the activation.
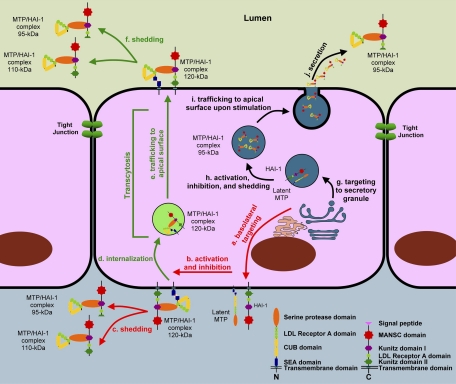
Fig. 10.
The models for matriptase activation, inhibition, and shedding. In most polarized epithelial cells, matriptase is synthesized as a latent zymogen and targeted to basolateral plasma membrane (a). Latent matriptase undergoes autoactivation to generate active matriptase, which is quickly inhibited by HAI-1 to form a 120-kDa complex (b). The 120-kDa matriptase-HAI-1 complex is either shed from the basal plasma membrane (c) or internalized (d) and then traffics to the apical plasma membrane (e) at which the 120-kDa complex is shed into the lumen as 95- and/or 110-kDa complexes by simultaneous proteolytic cleavages at both matriptase and HAI-1 (f). In specific types of epithelial cells, such as the gastric chief cells, matriptase may be synthesized and then targeted to secretory granules (g) within which matriptase undergoes autoactivation, HAI-1-mediated inhibition, and release as a 95-kDa complex with HAI-1, being liberated from the membrane through proteolytic cleavage (h). The 95-kDa matriptase-HAI-1 complex is stored in the secretory granules. Upon stimulation, the secretory granules traffic to apical surface (i), fuse with plasma membrane, and then release the 95-kDa matriptase-HAI-1 complex (j).
Most serine proteases are synthesized as zymogens and require a proteolytic cleavage at the activation motif to gain their full enzymatic activity. Thus, zymogen activation represents the most critical regulatory checkpoint for the vast majority of serine proteases. Zymogen activation is normally carried out by other active proteases. For few other proteases, particularly those on the top of protease cascades, the activation is carried out through the interaction between two zymogen molecules. Matriptase is one of these special proteases that undergoes autoactivation to gain their activities (29). In addition, the coupling of zymogen activation with HAI-1 inhibition and shedding makes matriptase an interesting case to study how membrane-associated proteases are regulated. While the activation and inhibition of other type II transmembrane serine proteases remain to be further investigated, the mechanism governing matriptase activation, inhibition, and secretion may provide insight into how these highly related membrane-associated serine proteases are regulated.
In our previous study (11), we showed that assembly of adherens junctions induced by S1P is required for the accumulation and activation of matriptase at the cell-cell junctions of 184 A1N4 mammary epithelial cells grown in monolayer culture. Here we show that in ductal epithelial cells and polarized Caco-2 cells, matriptase resides on the basolateral surfaces of the cells and is constitutively activated. These observations suggest that matriptase activation apparently depends on the fundamental architecture of the epithelium, the formation of tight cell-cell adhesion. Paradoxically, genetic ablation of matriptase results in loss of normal epithelial architecture, suggesting that matriptase is crucial for the maintenance of epithelial integrity (8, 33). This functional interrelationship between matriptase activation and assembly of cell-cell adhesion is consistent with our hypothesis that matriptase plays a role common to the function of all epithelia, based on its broad expression in the surface epithelial lining of nearly all tissues that is not limited to epithelia of any particular histological type (28). Despite its important role in epithelial biology, identification of the direct substrates of matriptase that are linked to the maintenance of epithelial integrity has been challenging. As a type II transmembrane serine protease whose serine protease domain is positioned toward the extracellular face of plasma membrane, matriptase has been proposed to activate substrates at the cell surface (7, 16, 20). The current study further suggests that the basolateral surface may be the functional location for matriptase for ductal epithelial cells. Since activation of matriptase is immediately followed by HAI-1-mediated inhibition, the vast majority of activated matriptase is, however, enzymatically inactive at any given time. One should not, therefore, predict that active matriptase is retained at the cell surface at meaningful levels to activate extracellular substrates. The extremely short life of active matriptase suggests that activation of downstream substrates is controlled by these very restricted spatial and temporal conditions and is closely coupled to matriptase activation itself. If matriptase substrates are located at the cell surface, the enzyme may be serving as a component in a signal transmission system. Matriptase is activated by the presence of upstream signals and then immediately passes the signal on by activating its substrates before downregulation of matriptase activity through inhibition by HAI-1. If the substrates are coming from the extracellular milieu, then the substrates would also have to serve as the signal to induce matriptase activation. Only through such a mechanism would activation of these matriptase substrates become possible.
The discovery of the accumulation of matriptase-HAI-1 complexes in the pepsinogen-secretory granules of the stomach chief cells shows that a different matriptase secretion mechanism is in operation in the chief cells compared with the ductal epithelial cells. The colocalization of matriptase-HAI-1 complexes with pepsinogens II in the secretory granules suggests that a regulated secretion process, rather than a constitutive one, may be at work. In addition, matriptase appears not to reside on the cell surface of the stomach chief cells (Fig. 7) since all the matriptase-HAI-1 complexes were seen in the secretory granules. These results suggest that activation of matriptase in the chief cells may take place in the secretory granules as an intracellular protease, and not at the basolateral surface as an extracellular protease. In our previous studies, we have shown that intracellular pools of matriptase are capable of undergoing activation, and that matriptase accumulates and becomes activated in organelle-like vesicle structures in response to treatment of cells with suramin (14, 15). Thus, while the basic mechanism for matriptase activation and inhibition appears to be the same in different cells, the chief cells apparently activate and regulated matriptase at a different cellular location from that found in ductal epithelial cells. The divergence in the regulation of matriptase between the ductal epithelial and the chief cell may simply be a facet of the functional divergence between both epithelial types. One of the main functions of ductal epithelial cells is the reabsorption of sodium ions from the tubular fluids, but the chief cells of the stomach are responsible for the secretion of zymogens and other hormones, such as leptin (9). The presence and activation of matriptase at the basolateral plasma membrane of the renal collecting ductal epithelial cells may allow the active matriptase to have direct access to one of its substrates, protease-activated receptor 2 (PAR-2), which also resides at the basolateral plasma membrane of renal collecting duct cells (5). Activation of PAR-2 by either trypsin or the agonist peptide has been shown to increase the maximal activity of Na+-K+-ATPase and sodium reabsorption (24). Matriptase in ductal epithelial cells could, therefore, play a role in sodium reabsorption through the activation of PAR-2 and ion channels. In contrast, the chief cells of the stomach may make use of matriptase as a pro-protein convertase due to the activation and inhibition of matriptase within the secretory granules.
The characterization of matriptase secretion in the current study reveals that matriptase activity is tightly regulated, not only by its cognate inhibitor HAI-1 with extremely rapid kinetics, but also by strict control of its cellular location, which may vary among different epithelial cell types. Several in vitro substrates for matriptase have been identified, including urokinase-type plasminogen activator, hepatocyte growth factor, macrophage-stimulating protein 1, matrix metalloproteinase 3, insulin-like growth factor binding protein-related protein-1, and components of extracellular matrix (1, 6, 12, 16, 30, 34). These suggest a role for the protease in matrix remodeling and the regulation of cell growth and survival, cell motility, and cellular morphogenesis. Genetic approaches in animal models have further identified prostasin and profilaggrin as matriptase substrates. An important future goal in the demonstration of in vivo cleavage and activation of these putative matriptase substrates had to address the accessibility of the substrates to the short-lived active matriptase within the appropriate cellular location.
GRANTS
This study was supported by National Cancer Institute Grants RO1-CA-096851 and RO1-CA-104944 (to C.-Y. Lin), Taiwan National Science Council Grant NSC 97-2320-B-002-052-MY3 (to M.-S. Lee), and Taiwan Department of Defense Grant DOD97-13-02 (to J.-K. Wang).
REFERENCES
- 1.Ahmed S, Jin X, Yagi M, Yasuda C, Sato Y, Higashi S, Lin CY, Dickson RB, Miyazaki K. Identification of membrane-bound serine proteinase matriptase as processing enzyme of insulin-like growth factor binding protein-related protein-1 (IGFBP-rP1/angiomodulin/mac25). FEBS J 273: 615–627, 2006. [DOI] [PubMed] [Google Scholar]
- 2.Benaud C, Dickson RB, Lin CY. Regulation of the activity of matriptase on epithelial cell surfaces by a blood-derived factor. Eur J Biochem 268: 1439–1447, 2001. [DOI] [PubMed] [Google Scholar]
- 3.Benaud C, Oberst M, Hobson JP, Spiegel S, Dickson RB, Lin CY. Sphingosine 1-phosphate, present in serum-derived lipoproteins, activates matriptase. J Biol Chem 277: 10539–10546, 2002. [DOI] [PubMed] [Google Scholar]
- 4.Benaud CM, Oberst M, Dickson RB, Lin CY. Deregulated activation of matriptase in breast cancer cells. Clin Exp Metastasis 19: 639–649, 2002. [DOI] [PubMed] [Google Scholar]
- 5.Bertog M, Letz B, Kong W, Steinhoff M, Higgins MA, Bielfeld-Ackermann A, Fromter E, Bunnett NW, Korbmacher C. Basolateral proteinase-activated receptor (PAR-2) induces chloride secretion in M-1 mouse renal cortical collecting duct cells. J Physiol 521: 3–17, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bhatt AS, Welm A, Farady CJ, Vasquez M, Wilson K, Craik CS. Coordinate expression and functional profiling identify an extracellular proteolytic signaling pathway. Proc Natl Acad Sci USA 104: 5771–5776, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bugge TH, List K, Szabo R. Matriptase-dependent cell surface proteolysis in epithelial development and pathogenesis. Front Biosci 12: 5060–5070, 2007. [DOI] [PubMed] [Google Scholar]
- 8.Carney TJ, von der HS, Sonntag C, Amsterdam A, Topczewski J, Hopkins N, Hammerschmidt M. Inactivation of serine protease Matriptase1a by its inhibitor Hai1 is required for epithelial integrity of the zebrafish epidermis. Development 134: 3461–3471, 2007. [DOI] [PubMed] [Google Scholar]
- 9.Cinti S, Matteis RD, Pico C, Ceresi E, Obrador A, Maffeis C, Oliver J, Palou A. Secretory granules of endocrine and chief cells of human stomach mucosa contain leptin. Int J Obes Relat Metab Disord 24: 789–793, 2000. [DOI] [PubMed] [Google Scholar]
- 10.Godiksen S, Selzer-Plon J, Pedersen ED, Abell K, Rasmussen HB, Szabo R, Bugge TH, Vogel LK. Hepatocyte growth factor activator inhibitor-1 has a complex subcellular itinerary. Biochem J 413: 251–259, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hung RJ, Hsu I, Dreiling JL, Lee MJ, Williams CA, Oberst MD, Dickson RB, Lin CY. Assembly of adherens junctions is required for sphingosine 1-phosphate-induced matriptase accumulation and activation at mammary epithelial cell-cell contacts. Am J Physiol Cell Physiol 286: C1159–C1169, 2004. [DOI] [PubMed] [Google Scholar]
- 12.Jin X, Yagi M, Akiyama N, Hirosaki T, Higashi S, Lin CY, Dickson RB, Kitamura H, Miyazaki K. Matriptase activates stromelysin (MMP-3) and promotes tumor growth and angiogenesis. Cancer Sci 97: 1327–1334, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kiyomiya KI, Lee MS, Tseng IC, Zuo H, Barndt RJ, Johnson MD, Dickson RB, Lin CY. Matriptase activation and subsequent shedding with HAI-1 is induced by steroid sex hormones in human prostate cancer cells, but not in breast cancer cells. Am J Physiol Cell Physiol 291: C40–C49, 2006. [DOI] [PubMed] [Google Scholar]
- 14.Lee MS, Tseng IC, Wang Y, Kiyomiya K, Johnson MD, Dickson RB, Lin CY. Autoactivation of matriptase in vitro: requirement for biomembrane and LDL receptor domain. Am J Physiol Cell Physiol 293: C95–C105, 2007. [DOI] [PubMed] [Google Scholar]
- 15.Lee MS, Kiyomiya K, Benaud C, Dickson RB, Lin CY. Simultaneous activation and HAI-1-mediated inhibition of matriptase induced at activation foci in immortal human mammary epithelial cells. Am J Physiol Cell Physiol 288: C932–C941, 2005. [DOI] [PubMed] [Google Scholar]
- 16.Lee SL, Dickson RB, Lin CY. Activation of hepatocyte growth factor and urokinase/plasminogen activator by matriptase, an epithelial membrane serine protease. J Biol Chem 275: 36720–36725, 2000. [DOI] [PubMed] [Google Scholar]
- 17.Lin CY, Anders J, Johnson M, Dickson RB. Purification and characterization of a complex containing matriptase and a Kunitz-type serine protease inhibitor from human milk. J Biol Chem 274: 18237–18242, 1999. [DOI] [PubMed] [Google Scholar]
- 18.Lin CY, Tseng IC, Chou FP, Su SF, Chen YW, Johnson MD, Dickson RB. Zymogen activation, inhibition, and ectodomain shedding of matriptase. Front Biosci 13: 621–635, 2008. [DOI] [PubMed] [Google Scholar]
- 19.Lin CY, Wang JK, Torri J, Dou L, Sang QA, Dickson RB. Characterization of a novel, membrane-bound, 80-kDa matrix-degrading protease from human breast cancer cells. Monoclonal antibody production, isolation, and localization. J Biol Chem 272: 9147–9152, 1997. [PubMed] [Google Scholar]
- 20.List K, Bugge TH, Szabo R. Matriptase: potent proteolysis on the cell surface. Mol Med 12: 1–7, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.List K, Szabo R, Molinolo A, Sriuranpong V, Redeye V, Murdock T, Burke B, Nielsen BS, Gutkind JS, Bugge TH. Deregulated matriptase causes ras-independent multistage carcinogenesis and promotes ras-mediated malignant transformation. Genes Dev 19: 1934–1950, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Martin-Belmonte F, Mostov K. Regulation of cell polarity during epithelial morphogenesis. Curr Opin Cell Biol 20: 227–234, 2008. [DOI] [PubMed] [Google Scholar]
- 23.Mathias JR, Dodd ME, Walters KB, Rhodes J, Kanki JP, Look AT, Huttenlocher A. Live imaging of chronic inflammation caused by mutation of zebrafish Hai1. J Cell Sci 120: 3372–3383, 2007. [DOI] [PubMed] [Google Scholar]
- 24.Morla L, Crambert G, Mordasini D, Favre G, Doucet A, Imbert-Teboul M. Proteinase-activated receptor 2 stimulates Na,K-ATPase and sodium reabsorption in native kidney epithelium. J Biol Chem 283: 28020–28028, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mostov K, Su T, ter Beest M. Polarized epithelial membrane traffic: conservation and plasticity. Nat Cell Biol 5: 287–293, 2003. [DOI] [PubMed] [Google Scholar]
- 26.Mostov KE Regulation of protein traffic in polarized epithelial cells. Histol Histopathol 10: 423–431, 1995. [PubMed] [Google Scholar]
- 27.Oberst M, Anders J, Xie B, Singh B, Ossandon M, Johnson M, Dickson RB, Lin CY. Matriptase and HAI-1 are expressed by normal and malignant epithelial cells in vitro and in vivo. Am J Pathol 158: 1301–1311, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Oberst MD, Singh B, Ossandon M, Dickson RB, Johnson MD, Lin CY. Characterization of matriptase expression in normal human tissues. J Histochem Cytochem 51: 1017–1025, 2003. [DOI] [PubMed] [Google Scholar]
- 29.Oberst MD, Williams CA, Dickson RB, Johnson MD, Lin CY. The activation of matriptase requires its noncatalytic domains, serine protease domain, and its cognate inhibitor. J Biol Chem 278: 26773–26779, 2003. [DOI] [PubMed] [Google Scholar]
- 30.Satomi S, Yamasaki Y, Tsuzuki S, Hitomi Y, Iwanaga T, Fushiki T. A role for membrane-type serine protease (MT-SP1) in intestinal epithelial turnover. Biochem Biophys Res Commun 287: 995–1002, 2001. [DOI] [PubMed] [Google Scholar]
- 31.Shimomura T, Denda K, Kitamura A, Kawaguchi T, Kito M, Kondo J, Kagaya S, Qin L, Takata H, Miyazawa K, Kitamura N. Hepatocyte growth factor activator inhibitor, a novel Kunitz-type serine protease inhibitor. J Biol Chem 272: 6370–6376, 1997. [DOI] [PubMed] [Google Scholar]
- 32.Szabo R, Hobson JP, List K, Molinolo A, Lin CY, Bugge TH. Potent inhibition and global co-localization implicate the transmembrane kunitz-type serine protease inhibitor hai-2 in the regulation of epithelial matriptase activity. J Biol Chem 2008. [DOI] [PMC free article] [PubMed]
- 33.Szabo R, Molinolo A, List K, Bugge TH. Matriptase inhibition by hepatocyte growth factor activator inhibitor-1 is essential for placental development. Oncogene 26: 1546–1556, 2007. [DOI] [PubMed] [Google Scholar]
- 34.Takeuchi T, Harris JL, Huang W, Yan KW, Coughlin SR, Craik CS. Cellular localization of membrane-type serine protease 1 and identification of protease-activated receptor-2 and single-chain urokinase-type plasminogen activator as substrates. J Biol Chem 275: 26333–26342, 2000. [DOI] [PubMed] [Google Scholar]
- 35.Tseng IC, Chou FP, Su SF, Oberst M, Madayiputhiya N, Lee MS, Wang JK, Sloane DE, Johnson M, Lin CY. Purification from human milk of matriptase complexes with secreted serpins: mechanism for inhibition of matriptase other than HAI-1. Am J Physiol Cell Physiol 295: C423–C431, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tuma PL, Hubbard AL. Transcytosis: crossing cellular barriers. Physiol Rev 83: 871–932, 2003. [DOI] [PubMed] [Google Scholar]