Abstract
In prosthetic loosening, bone resorption is induced by wear debris particles generated from the artificial joint articulation. Our prior work showed that synovial-like fibroblasts respond to titanium particles by producing receptor activator of NF-κB ligand (RANKL), a critical activator of osteoclastogenesis. While this effect occurs through a cyclooxygenase-2 (COX-2)-dependent pathway, the mechanism of COX-2 stimulation by titanium particles is not clear. Here we show that titanium particles induce COX-2 gene expression by activating NF-κB signaling. Inhibitor of NF-κB (IκBα) is degraded following particle treatment, permitting active NF-κB to translocate to the nucleus where it interacts with the COX-2 promoter and drives transcription. NF-κB activation is dependent on reactive oxygen species since antioxidants block the NF-κB signaling induced by particles. Surprisingly, IκBα degradation is independent of IKK (IκB kinase) and the 26S proteasome. Instead, calpain inhibitor can block the IκBα degradation induced by particles. Furthermore, the calpain-targeted COOH-terminal PEST sequence of IκBα is necessary for phosphorylation and degradation, consistent with a proteasome-independent mechanism of catabolism. Altogether, the data demonstrate a signaling pathway by which titanium particles induce oxidative stress, stimulate calpain-mediated NF-κB activation, and activate target gene expression, including COX-2. These findings define important targets for osteolysis but may also have importance in other diseases where fibroblasts respond to environmental particles, including pulmonary diseases.
Keywords: cyclooxygenase-2, osteolysis, inflammation, cell signaling, reactive oxygen species
increasing numbers of total joint replacements are performed annually, consistent with the aging of the population and increase in the burden of osteoarthritis. A devastating complication of joint replacement surgery is the development of bone resorption in the region surrounding the artificial implant which leads to the mechanical loosening of the implant (4). While the etiology of bone resorption is likely multifactorial, the generation of particulate wear debris and subsequent cell and tissue reactions contribute to this problem. Motion at the articulating surfaces of the artificial joint results in release of submicron-sized particles which become embedded within the synovial tissues lining the joint (14, 31). Membranes retrieved from around loose implants are composed of fibrovascular tissue containing ∼70% fibroblasts, 20% macrophages, and 10% lymphocytes and other inflammatory cells (13, 36). Thus, wear debris particles are in a position to trigger a variety of cellular responses that could lead to activation and survival of osteoclasts and result in bone resorption.
While macrophages have been extensively studied, the fibroblast-like synoviocytes (FLS) are the most dominant cell type forming the lining layer of the periarticular joint and have also been shown to have a critical role in the pathogenesis of inflammatory joint diseases like rheumatoid arthritis and, more recently, aseptic loosening (26, 40, 53, 56). We have recently shown that synovial-like fibroblasts exposed to titanium particles express receptor activator of NF-κB ligand (RANKL) and stimulate osteoclast formation (56). Additionally, we demonstrated that the effect is dependent on a cyclooxygenase-2 (COX-2)/prostaglandin E2 (PGE2) cascade transduced through the EP4 prostaglandin receptor, which is consistent with the observation that there is complete absence of inflammatory osteolysis in COX-2-deficient mice using an in vivo murine calvarial model of particle induced bone loss (56, 58). However, the molecular mechanism of COX-2 induction by particles in synovial-like fibroblasts remains unknown.
NF-κB is a transcription factor with an important role in numerous normal and pathologic conditions, including the innate immune response (1). Under basal conditions, active NF-κB heterodimers such as p50/p65 and p50/p52 are sequestered in the cytoplasm by inhibitor of NF-κB (IκB). Classical activation of NF-κB involves phosphorylation of IκB at serine 32 and 36, by IκB kinase (IKK), followed by ubiquitination and degradation in the 26S proteasome (19). This results in release of NF-κB, translocation to the nucleus, and activation of the transcription complex. Thus, NF-κB activation is downstream of IKKα/β kinases phosphorylation and activation (19). Among the various genes regulated by NF-κB is COX-2 (5, 16, 50).
Alternative pathways of IκBα degradation that are both proteasome dependent and independent have been described. One mechanism involves the proteasome-dependent metabolism of the p50 precursor, p105, which binds to the coactivator, p65 (2). The COOH-terminal portion of p105 acts like IκB and prevents nuclear translocation of the active complex (2, 34). Several kinases can target p105, including βTrCP, the IKKs, and GSK-3β (10, 27), resulting in ubiquitination and proteasome-dependent degradation of p105 which is independent of IκB metabolism.
Another mechanism of activation involves calpain, which degrades IκBα in a proteasome-independent manner (57). In humans there are at least 14 members of the calpain family of calcium-dependent cysteine proteases, and these target a variety of proteins for degradation and are involved in a number of disease processes (11, 57). Proteins targeted by calpain contain a PEST sequence. In IκBα, the PEST domain is located in the COOH-terminal region of the protein and has been shown to be required for calpain-mediated catabolism (11, 46).
The redox state of a cell is delicately balanced to maintain homeostasis. Whereas severe oxidative damage may be toxic or induce cell death, moderately high concentrations of reactive oxygen species (ROS) trigger cell signaling and regulate gene expression (51). ROS activate calpain, suggesting that NF-κB is one of the potential downstream effectors of altered redox state (21, 41, 42, 46). In fact, prior work has shown that NF-κB is activated in the T lymphocytic cell line (EL4) by oxidative stress through a calpain-dependent mechanism (46).
Here we define mechanisms involved in activation of COX-2 by titanium particles in FLS. Ti particles stimulated ROS and activated NF-κB. IκBα catabolism occurred in an IKK kinase and proteasome-independent manner. Instead, ROS-induced IκBα degradation was mediated by calpain and was dependent on the COOH-terminal PEST domain of IκBα. Finally, stimulation of COX-2 gene expression was found to be dependent on NF-κB. Since COX-2 induces RANKL and stimulates osteoclastogenesis (56), the findings provide a molecular mechanism through which wear debris particles target synovial-like fibroblasts, regulate gene expression, and contribute to osteolysis and prosthetic loosening.
EXPERIMENTAL PROCEDURES
Titanium particles.
Titanium particles (1–3 μm diameter) were obtained from Johnson Matthey Chemicals (Ward Hill, MA). Particles were prepared by alternately washing three times in nitric acid and sodium hydroxide solutions, and then washed three times in sterile PBS as described by Bi et al. (3). For use in cell culture, the particles were suspended in PBS at 3.1 × 108 particles/ml (∼0.02 g/ml) and autoclaved for sterilization.
Cell culture.
Murine FLS were previously harvested from the knee joint of mouse CBAxBL6 strain (28). These cells have previously been shown to express VCAM-1, and expression is increased in response to TNF-α (28). FLS were maintained in DMEM medium in the presence of 10% heat inactivated fetal bovine serum, 1% penicillin, and 5% CO2 at 37°C. mIκB FLS (mFLS) were generated previously by infecting the FLS with retroviral vector [pL(mIκB)SN], and G418-resistant cells were selected as a stable cell clone. For titanium particle stimulation, FLS were grown to subconfluence overnight at 37°C and then placed in serum-free DMEM for 5 h before titanium particle treatment (dose range of 1 × 105 particles/ml to 1 × 107 particles/ml in serum-free media). In selected experiments, butylated hydroxyanisole (BHA, 100 μM), MG-132 (20 μM), and calpain inhibitor I (CPI; 100 μM), all from Sigma (St. Louis, MO), were added 1 h before particle treatment. In some experiments, FLS were stimulated with PGE2 (Cayman Chemicals, Ann Arbor, MI); for these studies a dose of 1 μM was used which is consistent with our previous studies and within the accepted range that stimulates bone resorption in vitro (33, 56).
Quantitative real-time PCR.
Total RNA was isolated from cell cultures at various times using the RNeasy kit (Qiagen, Valencia, CA) and was reverse transcribed to complementary cDNAs using Superscript II according to the manufacturer's instructions (Invitrogen, Carlsbad, CA). Primers specific for murine COX-2 (5′-CAC AGC CTA CCA AAA CAG CCA-3′; 5′-GCT CAG TTG AAC GCC TTT TGA-3′) and GAPDH (5′-AAC GAC CCC TTC ATT GAC-3′; 5′-TCC ACG ACA TAC TCA GCAC-3′) were used. Duplicate PCR reactions were carried out in Rotor-Gene 3000 (Corbett Research, NSW, Australia) using n = 3 for each sample. SYBR Green dye was used for detection of the product using the SYBR Green PCR Master Mix assay (Applied Biosystems, Warrington, UK). The standard curve used a series of duplicate dilutions of cDNA from control samples.
Western blot analysis.
Total protein extracts were prepared from cells at various times in the presence of protease inhibitors as previously described (18). Fifteen micrograms of the protein extract was separated by SDS-PAGE. After transfer to a polyvinylidene difluoride membrane, the blots were probed overnight with antibodies specific for murine COX-2 (Cayman Chemicals), β-actin (Sigma), IκBα (Santa Cruz, Santa Cruz, CA), RANKL (Calbiochem, San Diego, CA), or α-Spectrin (Chemicon, Temecula, CA). After the blots were washed, horseradish peroxidase-conjugated secondary antibodies were used to detect the immune complexes using ECL plus (Amersham Biosciences, Piscataway, NJ). For phosphorylated IKKα/β or IκBα detection, cell proteins were collected in kinase-free lysis buffer (20 mM Tris·HCl, pH = 7.5; 100 mM NaCl; 0.5% Triton X-100; 80 mM β-glycerophosphate; 0.5 mM sodium orthovanadate; 1 mM EDTA; 1 mM phenylmethylsufonyl fluoride). Forty micrograms of protein extract was immunoblotted for phospho-IKKα/IKKβ (Ser176/180) or phospho-IκBα (Ser32/36) using a specific antibody (Cell Signaling, Beverly, MA) in a similar manner.
Transfection and reporter assay.
The reporter plasmids were gifts from Dr. Kenneth Wu (44). Briefly, human COX-2 5′-flanking DNA fragment (−891/+9) and its mutants were inserted in the luciferase reporter vector pGL3 basic (Promega, Madison, WI). M1 mutation of NF-κB is located at −477/−438 from GGGGATTCCC to attcATTCCC, M2 mutation of NF-κB is located at −222/−213 from GGGACTACCC to aattCTACCC and mutation of both sites (M1 + M2). Cells were seeded overnight in 12-well plates and transfected with 2 μg plasmid DNA per well by lipofectamine 2000 (Invitrogen). After 12 h recovery, cells were treated with titanium particles in serum-free media for 24 h and then collected for luciferase activity measurement by luminometer. Relative luciferase activity was normalized to total protein concentration of each sample.
Electrophoretic mobility shift assay.
Nuclear extracts (5 μg) were incubated with radioactive end-labeled double-stranded oligonucleotide probes containing the consensus binding sites for NF-κB in the mouse COX-2 promoter (5′-AGG TGA GGG GAT TCC CTT AGT-3′). Incubation was performed in a total of 10 μl of binding buffer [10 mM Tris·HCl, pH 7.5, 150 mM KCl, 1 mM EDTA, 0.1% Triton X-100, 10% glycerol, 1 μg poly(dI-dC), and 1 mM DTT] for 15–20 min at room temperature. For competition, unlabeled oligonucleotides of wild-type or mutant (5′-AGG TGA GGG ccT TCC CTT AGT-3′, mutation is underscored) were added in a 1:1 or 1:20 ratio. For supershift assay, the nuclear extract was incubated with specific antibodies for 30 min before addition of the labeled probe. After incubation with the labeled probe for an additional 20–30 min, samples were fractionated on a 4% native polyacrylamide gel and visualized by exposing the dry gel to X-ray film.
Immunofluorescence.
Cells were fixed with 4% paraformaldehyde for 10 min after a brief wash with phosphate-buffered saline. Cells were permeabilized with 0.2% Triton X-100 for 5 min at room temperature. After blocking with 10% goat serum for 1 h, cells were incubated with polyclonal antibody for p65 (Santa Cruz) at 4°C overnight. Tetramethyl isothiocyanate (TRITC)-conjugated secondary antibody (Sigma) was used to show the location of NF-κB p65, and DAPI (1 μg/ml, Calbiochem) was used to stain the nuclei. Images were obtained using a fluorescence microscope and digital camera (Axiovert 400 CFL, Zeiss, Gottingen, Germany).
Chromatin immunoprecipitation assay.
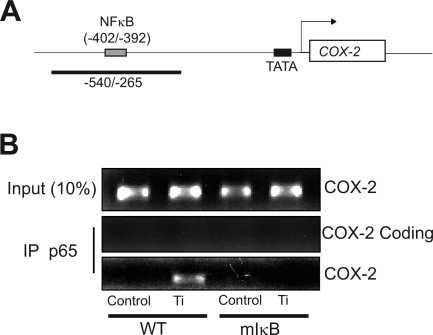
The interaction of NF-κB with COX-2 promoter was examined using a chromatin immunoprecipitation (ChIP) assay kit (Upstate Signaling, Lake Placid, NY) with minor changes. Cells were cross-linked with 1% formaldehyde for 10 min at 37°C and stopped with 125 mM glycine for 5 min. Cells were collected and resuspended in SDS lysis buffer (1% SDS, 10 mM EDTA, 50 mM Tris, pH = 8.1, and protease inhibitor). Cell lysates were sonicated on ice to shear chromatin into lengths of 200–1,000 bp and then incubated with p65 antibody overnight at 4°C. Antibody/DNA complexes were immunoprecipitated using a protein A agarose slurry with salmon sperm DNA. Immunoprecipitated DNA was reverse cross-linked and recovered by phenol/chloroform extraction and ethanol precipitation. PCR was performed to analyze the abundance of COX-2 promoter using specific primers for COX-2 (promoter, −540/−265), (5′-CTA ATT CCA CCA GTA CAG ATG-3′; 5′-TTC GCT GAG TCT GCG CCT AGT-3′); COX-2 coding region, (+5555 to +5654) as negative control, (5′-CAC AGC CTA CCA AAA CAG CCA-3′; 5′-GCT CAG TTG AAC GCC TTT TGA-3′); and genomic DNA as an input control.
Detection of ROS.
Cells plated in 96-well plates were washed with PBS and then incubated for 15 min with 5 μg/ml of 2′,7′-dichlorofluorescin diacetate (DCFDA; Sigma). Cells were then washed twice to remove free dye, and total fluorescence was assayed by microplate fluorometer (spectraMAX Gemini, Molecular Devices, Sunnyvale, CA) using excitation/emission wavelengths of 488 nm/525 nm.
Glutathione S-transferase fusion proteins and in vitro kinase assay.
Glutathione S-transferase (GST)-tagged protein constructs for the wild-type or PEST domain-mutated (MuF) IκB were described previously (48). For expression and purification of GST fusion proteins in vitro, plasmids were transformed into Escherichia coli [BL21(DE3)]. Two-hundred milliliter bacterial cultures were grown (until optical density at 600 nm = 1.0) and then induced for 4 h with 0.5 mM isopropyl-β-d-thiogalactoside. Cells were resuspended in 10 ml B-PER lysis buffer (Pierce Biotech, Rockford, IL) containing DTT (1 mM), phenylmethylsulfonyl fluoride (1 mM), and protease inhibitors (Calbiochem). The lysates were incubated with 200 μl 50% slurry of Glutathione Sepharose 4B beads (Amersham) and washed four times with PBS containing 0.1% Triton X-100 and 0.1% Tween 20. Final solutions (10% volume; 20 μl) containing GST fusion proteins were boiled in SDS buffer and then loaded in SDS-PAGE. Coomassie staining was performed to check the presence of proteins.
One-hundred micrograms cellular extract of FLS were incubated with glutathione-sepharose beads bound with GST fusion proteins (wild-type or MuF IκBα). After rotating for 1 h at 4°C, the beads were pelleted and washed with PBS containing 0.1% Triton X-100 and 0.1% Tween 20. The kinase assay was then performed at 30°C for 45 min in the kinase buffer (pH = 7.4, 20 mM HEPES-KOH, 10 mM MgCl2, 10 mM MnCl2, 1 mM DTT, 25 μM ATP, and 2 μCi of [γ-32P] ATP.) The phosphorylated proteins were then boiled in SDS sample buffer, resolved in SDS-PAGE, stained with Coomassie blue followed by autoradiography.
RESULTS
Titanium particles (Ti) induce COX-2 expression in mouse FLS.
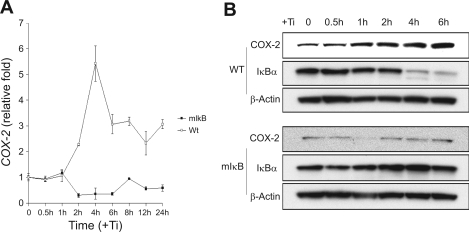
Murine FLS were cultured and treated with micron-size pure titanium particles (Ti) (5 × 106 particles/ml), and the expression of COX-2 was examined at various time points by quantitative real-time PCR and Western blot. COX-2 mRNA level increased at 2 h, peaked at 4 h where a 5- to 6-fold increase was observed, and declined by 6 h (Fig. 1A). A dramatic increase in COX-2 protein expression was noted by 2 h and sustained through 6 h following Ti treatment (Fig. 1B). These experiments show that the expression of COX-2 gene can be stimulated directly in mouse synovial fibroblasts by particles in vitro.
Fig. 1.
Titanium (Ti) induces cyclooxygenase-2 (COX-2) expression in mouse fibroblast-like synoviocytes (FLS) but not in cells stably expressing mIκB. FLS were treated with titanium particles (5 × 106 particles/ml), and total RNA or protein was harvested at various time points (0, 0.5, 1, 2, 4, 6, 8, 12, or 24 h). A: COX-2 expression was examined by quantitative real-time PCR. The relative fold induction compared with 0 time point was normalized by GAPDH expression. WT, wild type. B: COX-2 and inhibitor of NF-κB (IκBα) protein expression was examined by Western blot as described in experimental procedures. β-Actin was used as the loading control.
Ti induces NF-κB-dependent COX-2 promoter activity in FLS.
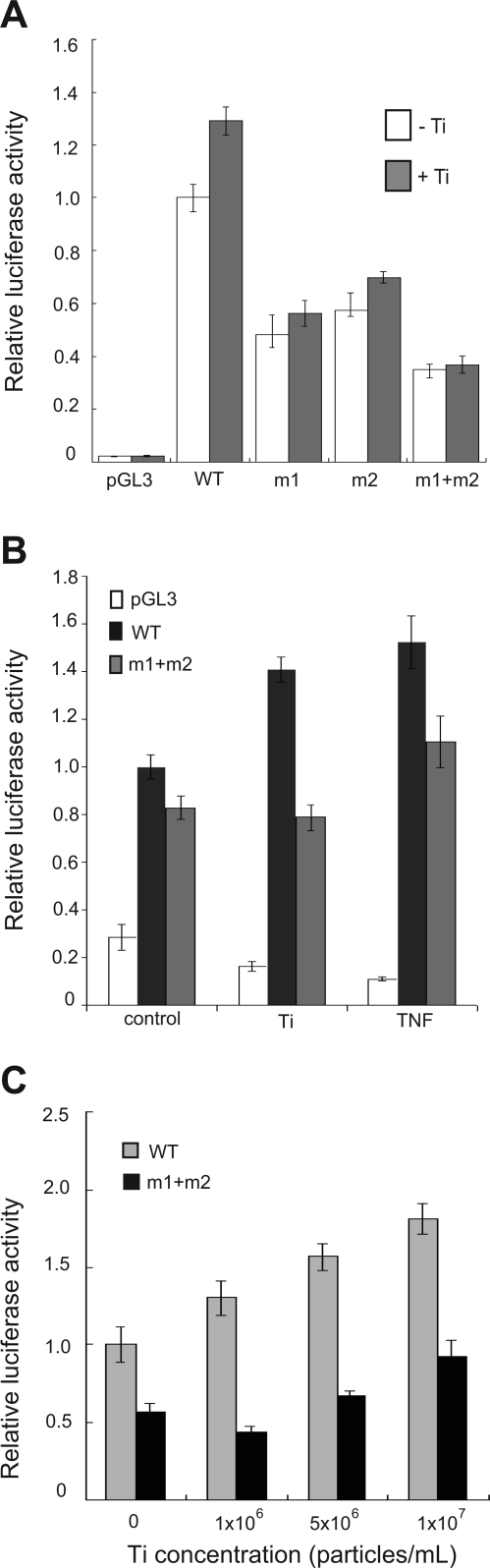
Human COX-2 promoter-luciferase reporter constructs were used to examine the role of NF-κB as an activator of COX-2 gene expression downstream of titanium particles. Human and mouse COX-2 promoter regions share 63% homology and include conserved transcription factor binding sites for NF-κB, AP-1, NF-IL6, and CRE-1/E-box in the same linear configuration within the upstream promoter region (25). The −890/+9 region of the COX-2 promoter contains two NF-κB consensus binding sequences (−447/−438 or −222/−213), and experiments were performed to examine the ability of titanium particles to activate wild-type (WT-COX-2-Luc) or a mutant promoter containing mutations of both NF-κB consensus binding sites (mt-COX-2-Luc) (Fig. 2A). Titanium particles stimulated wild-type COX-2 promoter activity in a dose-dependent manner (30–80% induction). In contrast, titanium effects were absent in cells transfected with mt-COX-2-Luc in which both NF-κB binding sites were mutated (Fig. 2A). Since TNF-α induces NF-κB and is known to stimulate COX-2 promoter activity and gene expression (5), the relative abilities of titanium and TNF-α to induce promoter activity were examined (Fig. 2B). Titanium (5 × 106 particles/ml) and TNF-α (10 ng/ml) induced similar levels of luciferase activity from the COX-2 promoter; this activity was reduced for both stimuli when the promoter was mutated (Fig. 2B). Finally, induction of luciferase activity from the COX-2 promoter by Ti particles was found to be dose dependent in a particle range between 1 × 106 particles/ml and 1 × 107 particles/ml (Fig. 2C). Together, these results point to NF-κB signaling as a major pathway responsible for the COX-2 gene induction by Ti.
Fig. 2.
Ti induces NF-κB-dependent COX-2 promoter activity in FLS. Transfection was performed with a control reporter plasmid (pGL3), a wild-type COX-2 reporter plasmid (WT), or COX-2 reporter plasmids with mutations of individual NF-κB-binding sites (m1) and (m2), or with a promoter having mutations at both sites (m1 + m2). A: luciferase activity from transfected FLS was measured 24 h after treatment with Ti particles (5 × 106 particles/ml) or control medium. B: FLS transfected with control reporter plasmid, or reporter plasmids expressing WT COX-2 or m1 + m2 COX-2 were treated with control medium, or medium containing Ti particles or TNF-α (10 ng/ml), and luciferase activity was measured. C: dose response of Ti particles in cultures of FLS transfected with either WT COX-2 or m1 + m2 COX-2 reporter plasmid.
Ti stimulates NF-κB signaling in FLS.
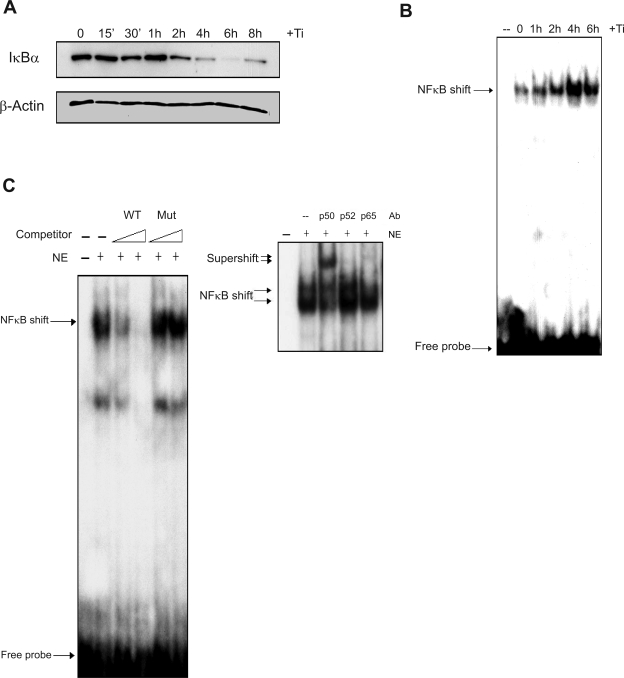
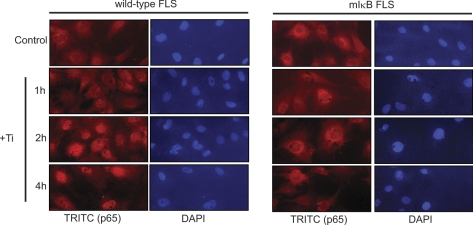
To directly examine the induction of NF-κB signaling in FLS by titanium particles, Western blot and EMSA were performed and fluorescent localization of NF-κB was examined. IκBα expression was analyzed by Western blot following Ti (5 × 106 particles/ml) treatment. IκBα levels were reduced 2 h following Ti treatment and remained suppressed after 8 h (Fig. 3A). To confirm activation of NF-κB, electrophoretic mobility shift assay (EMSA) was performed using nuclear extracts from FLS treated with titanium particles (Fig. 3B). Following Ti treatment, binding complexes were increased over basal levels between 1 and 2 h, peaked at 4 h, and remained elevated at 6 h. The specificity of the complex was examined using competition with unlabeled consensus and mutated NF-κB oligonucleotides and through supershift assays. An unlabeled consensus NF-κB binding sequence competed with the binding complex in a dose-dependent manner, while a mutated NF-κB oligonucleotide had no effect, confirming the specificity of the complex for the NF-κB binding site (Fig. 3C). Antibodies specific for p50, p52, or p65 were used to supershift the NF-κB binding complex to determine the factors participating in the binding complex and demonstrated the participation of p50 and p65 in the complex (Fig. 3C). Finally, immunofluorescence was used to examine the translocation of p65 to the nucleus following particle treatment. After 2 h culture in the presence of titanium particles, red fluorescent (TRITC) signal concentrated in nuclei (shown blue as DAPI dye) compared with control cultures, consistent with nuclear localization and activation of NF-κB (Fig. 4). These results confirm that Ti particles directly stimulate the activation of NF-κB in FLS cells.
Fig. 3.
Ti particles induce NF-κB signaling in FLS. FLS cell cultures were treated with control medium or medium containing titanium particles (5 × 106 particles/ml). A: cytoplasmic protein was harvested after 0 to 8 h. The protein extracts were separated by SDS-PAGE, and immunoblot was performed with either anti-IκBα antibody or anti-β-actin antibody. B: nuclear protein extracts were harvested between 0 and 6 h, and electrophoretic mobility shift assay was performed. C: the binding of nuclear protein extracts (NE) derived from FLS treated with Ti particles for 4 h to the NF-κB consensus sequence was subjected to competition using a 1- or 20-fold Molar excess of unlabeled wild-type or mutant (Mut) oligonucleotide (left). The same nuclear extracts were examined by EMSA in immunoshift assays using antibodies (Ab) for the p50, p52, and p65 subunits of NF-κB (right).
Fig. 4.
COX-2 induction by Ti is absent in mIκB FLS. Wild-type FLS and stably transfected mIκB FLS were treated with Ti particles (5 × 106 particles/ml) for 0, 1, 2, or 4 h. The translocation of NF-κB (p65) was detected by immunofluorescence using anti-p65 antibody and a tetramethyl isothiocyanate (TRITC)-conjugated secondary antibody. DAPI was used as a nuclear stain.
FLS overexpressing mutant IκB lose induction of COX-2 following Ti treatment.
Prior work in our laboratory has shown that titanium particles stimulate COX-2 gene expression and that this is essential for the ability of these cells to induce bone resorption (56). The relationship between COX-2 induction and NF-κB signaling in response to particles was examined in wild-type FLS and FLS stably overexpressing a dominant-negative IκBα (mIκB) with both NH2-terminal (S32, S36) and COOH-terminal (PEST domain) serine to alanine mutations (48, 54). Wild-type and mutant FLS were treated with Ti for 1, 2, or 4 h, and the intracellular localization of NF-κB (p65) was examined by immunofluorescence. While titanium treatment resulted in nuclear translocation of NF-κB in wild-type FLS after 2 and 4 h, nuclear translocation did not occur in mIκB FLS (Fig. 4). Furthermore, while titanium particles stimulated COX-2 gene and protein levels and IκBα degradation in wild-type FLS, this effect was absent in mIκB FLS following titanium particle treatment (Fig. 1). These data demonstrate that NF-κB signaling is required for the COX-2 induction stimulated by titanium particles.
Ti induces NF-κB binding to a consensus sequence in the native COX-2 promoter.
To further confirm that COX-2 is a target gene directly activated by NF-κB following Ti treatment, a ChIP assay was performed (Fig. 5). Both wild-type and mIκB FLS were treated with titanium particles (5 × 106 particles/ml) for 4 h at which time formaldehyde was added to cause protein-DNA cross-linking. NF-κB was immunoprecipitated with an anti-p65 antibody, and binding to the COX-2 promoter was detected by PCR using specific primers designed to generate a product in the mouse COX-2 promoter region (−540/−265) spanning the consensus NF-κB binding site located at −402/−392 (Fig. 5A). Under control conditions, no basal NF-κB binding to the promoter was observed. Treatment with titanium particles resulted in immunoprecipitation of the COX-2 promoter fragment in wild-type FLS cells. In contrast, no COX-2 promoter DNA was detected in mutant IκB FLS cells, consistent with lack of NF-κB activation of these in these cells and an absence of COX-2 gene induction (Fig. 5). Thus, the COX-2 gene is a direct target of transcription factor NF-κB in particle-stimulated FLS.
Fig. 5.
Ti particles stimulate NF-κB binding to the native COX-2 promoter. A: schematic of the COX-2 promoter showing the location of the NF-κB binding site and the expected PCR product in the chromatin immunoprecipitation assay. B: wild-type and mIκB FLS in subconfluent cultures were serum starved overnight and then treated with control medium or medium containing titanium (5 × 106 particles/ml) for 4 h. The protein-DNA complexes were cross-linked, and genomic DNA was extracted by immunoprecipitation (IP) with p65 antibody. PCR was performed using primers flanking the consensus NF-κB-binding site on the COX-2 gene as shown in A. The input control consisted of PCR amplification of the COX-2 promoter obtained from total genomic DNA before immunoprecipitation. The coding control used primers located in the coding region of the COX-2 promoter (+5555 to +5654).
Loss of RANKL induction in mIκB FLS following titanium particle stimulation.
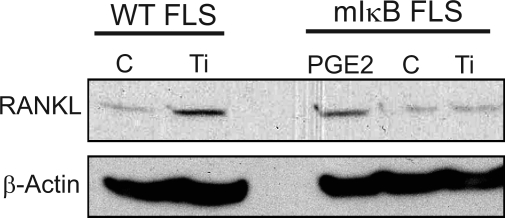
Previously, we showed that RANKL is a downstream target of COX-2 induction by titanium particles in FLS (56). To further examine the role of NF-κB/COX-2 in RANKL induction, wild-type and mIκB FLS were treated with titanium particles and RANKL expression was examined by Western blot. RANKL expression was increased by particles in wild-type FLS but not in mIκB FLS (Fig. 6). To verify that the RANKL induction is dependent on COX-2, we investigated whether RANKL expression could be rescued in mutant IκB FLS treated with PGE2, a downstream metabolite of COX-2 implicated in bone metabolism (23). Although titanium particles failed to increase RANKL in mIκB FLS, PGE2 resulted in an induction in RANKL levels in mutant cells that was similar to that observed in wild-type FLS treated with titanium particles (Fig. 6).
Fig. 6.
PGE2 rescues receptor-activated NF-κB ligand (RANKL) expression in mIκB FLS. Subconfluent wild-type and mIκB FLS were treated with control media (C), titanium particles (5 × 106 particles/ml), or PGE2 (1 μM) for 24 h. Total protein extracts were harvested, and RANKL expression was examined by Western blot as described in experimental procedures. β-Actin was used as the loading control.
Titanium particles activate NF-κB by stimulating ROS.
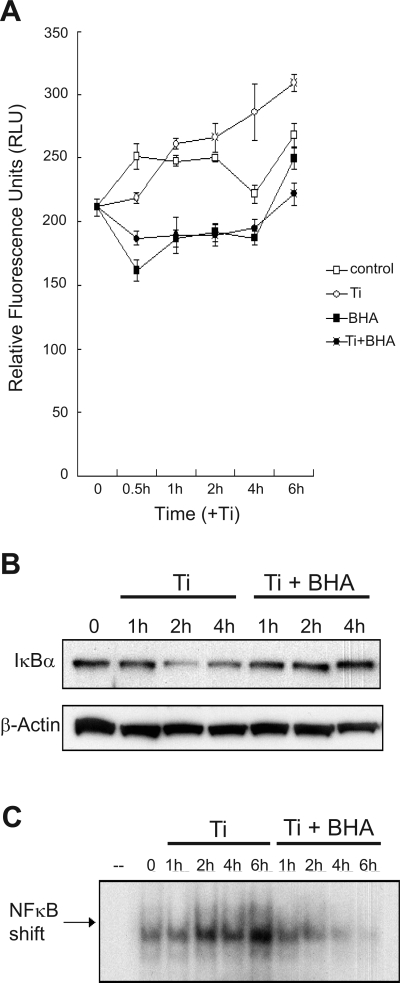
The generation of ROS was examined as a candidate linking titanium particles to NF-κB since ROS are induced by a variety of nonspecific stimuli and have been shown to affect G protein-coupled receptor and JNK signaling (29, 49). FLS were exposed to titanium particles in the presence or absence of the antioxidant BHA, for various times (0, 0.5, 1, 2, 3, and 4 h) and then loaded with the ROS-sensitive fluorescent dye H2DCFDA. FLS treated with titanium particles generated ROS, and this effect was inhibited by BHA (100 nM) (Fig. 7A). Subsequently, the role of ROS in NF-κB/COX-2 signaling in FLS was examined. Pretreatment of FLS with BHA blocked IκBα degradation following exposure to titanium particles Ti (Fig. 7B). BHA also inhibited the induction of NF-κB nuclear translocation (Fig. 7C). Altogether, the data suggest that that the induction of NF-κB signaling by titanium particles involves the generation of ROS.
Fig. 7.
Antioxidants block NF-κB induction in FLS treated with Ti particles. FLS were treated with control media or media containing Ti particles (5 × 106 particles/ml) in the presence or absence of 100 μM antioxidant butylated hydroxyanisole (BHA) for various times. A: 15 min before assaying, the cultures were washed with PBS and incubated with 2′,7′-dichlorofluorescin diacetate (5 μg/ml). Relative fluorescence units were detected using a microplate fluorometer after washing to remove free dye. B: total cell protein extracts were harvested at various times for Western blot for IκB. β-Actin was used as a loading control. C: nuclear protein extracts were obtained for use in gel mobility shift assays using an NF-κB consensus binding sequence.
Particles mediate IκBα degradation through the COOH-terminal PEST domain.
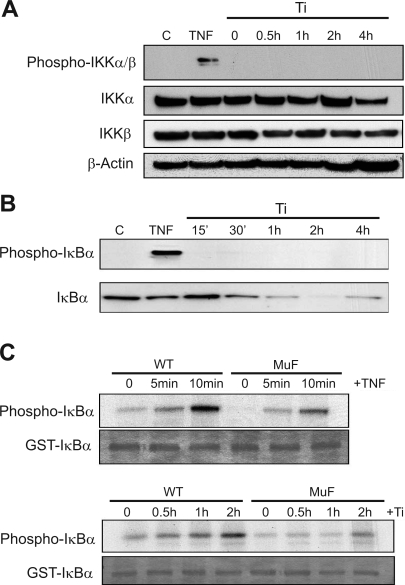
Phosphorylation of IκBα by IKK (IκB kinases) at serine 32 and 36 results in ubiquitination and degradation and is the classical pathway for IκB catabolism (19). IKK activation is dependent on phosphorylation, and this was assessed by Western blot in titanium-treated FLS. Surprisingly, FLS treated with titanium particles at various times (0, 0.5, 1, 2, and 4 h) had no phosphorylation of either IKKα or IKKβ proteins. In contrast, TNF-α resulted in strong phosphorylation within 5 min of treatment (Fig. 8A). Since IKK targets IκBα at serine 32 and 36, we further confirmed the absence of IKK activity by examination of IκBα phosphorylation at these sites using antibodies specific for serine 32 and 36 phosphorylation. While TNF-α treatment resulted in IκBα serine 32 and 36 phosphorylation, these sites were not phosphorylated following titanium treatment (Fig. 8B), despite the fact that IκBα was degraded over time in titanium-treated FLS (Figs. 1B, 3A, and 7B). These experiments indicate that titanium particles induce IκBα degradation independent of the IKK phosphorylation pathway.
Fig. 8.
Ti particles stimulate IκBα degradation independent of IKK and target the COOH-terminal PEST domain. FLS were treated with either TNF-α (10 ng/ml) for 5 min or Ti particles (5 × 106 particles/ml) for various times (0.5 to 4 h), and cell protein extracts were harvested and separated by SDS-PAGE. A: Western blot for IKKα and IKKβ, as well as the phosphorylated form of both IKKα and IKKβ. B: Western blot for S32/36 phosphorylated IκBα and unphosphorylated IκBα. C: in vitro kinase assays were performed using cell lysates from FLS treated with either TNF-α (10 ng/ml) or Ti particles (5 × 106 particles/ml). Glutathione S-transferase (GST) fusion proteins of IκBα and MuF PEST-mutated IκBα were used as substrates in buffer containing [γ-32P] ATP. The products were resolved by SDS-PAGE, and Coomassie staining was used to show equal amount of GST fusion proteins. Autoradiography was then performed to examine incorporation of 32P into the GST-fusion proteins.
Subsequent experiments focused on the COOH-terminal PEST domain since the mIκB used in earlier experiments (Fig. 4) that efficiently blocked NF-κB activation by titanium particles has serine/threonine to alanine inactivating mutations in both the NH2-terminal (S32/36) and COOH-terminal PEST (S283/288/293 and T291/296) domains. To distinguish the separate IκBα phosphorylation events in FLS following titanium particles, we used GST IκBα fusion proteins containing the wild-type sequence or serine/threonine to alanine mutations at all five PEST domain phosphorylation sites (MuF). However, MuF has normal serine phosphorylation sites at amino acids 32 and 36. In vitro kinase assays using FLS lysates following treatment with TNF-α or titanium particles showed IκBα protein phosphorylation of both wild-type and MuF IκBα proteins (Fig. 8C). The robust phosphorylation of MuF IκBα following TNF-α treatment is consistent with S32/36 as the classic IKK-mediated mechanism. However, the slightly reduced phosphorylation in the MuF IκBα GST-fusion protein suggests that TNF-α also targets the PEST domain phosphorylation sites. In contrast, in FLS treated with titanium particles over 2 h, phosphorylation of MuF IκBα was dramatically diminished compared with wild-type IκBα (Fig. 8C). These data suggested that IKK-associated S32/36 phosphorylation of IκBα is not primarily involved in the cellular response to particles. Instead, COOH-terminal phosphorylation of IκBα within the PEST domain is predominant.
Titanium particles induce IκBα degradation through a calpain-dependent pathway.
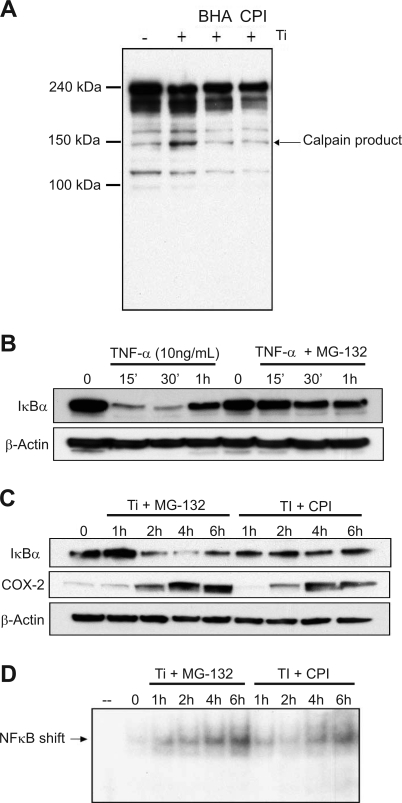
We next investigated the potential role of calpain, a cysteine protease previously shown to be activated by oxidative stress and to target IκBα degradation (6, 29, 45). The activation of calpain by titanium particles was determined by the catabolism of α-spectrin (9). Degradation of α-spectrin by calpain results in a characteristic increase in the appearance of a 145-kDa product (9). In FLS treated with titanium particles, the 145-kDa calpain cleavage product increased. Treatment of FLS with either CPI (100 μM) or the antioxidant BHA (100 nM) blocked the appearance of the 145-kDa product. The findings suggest that titanium particles activate calpain through induction of ROS (Fig. 9A).
Fig. 9.
Ti particles stimulate IκBα degradation through a calpain-dependent mechanism. A: FLS were treated with Ti particles (5 × 106 particles/ml) in the presence and absence of antioxidant BHA (100 μM) or calpain inhibitor I (CPI; 100 μM) for 2 h, and cell protein extracts were harvested and separated by SDS-PAGE. Western blot was performed using an antibody to detect the degradation products of α-spectrin. B: FLS were treated with TNF-α (10 ng/ml) in the absence or presence of proteasome inhibitor MG-132 (20 μM). Protein extracts were harvested between 0 and 1 h and assayed by Western blot for IκBα and β-actin. C: FLS were treated with Ti particles (5 × 106 particles/ml) in the absence or presence of proteasome inhibitor MG-132 (20 μM) or calpain inhibitor CPI (100 μM). Protein extracts were harvested between 0 and 6 h and assayed by Western blot for IκBα, COX-2, and β-actin. D: FLS were treated with Ti particles (5 × 106 particles/ml) in the absence or presence of proteasome inhibitor MG-132 (20 μM) or calpain inhibitor CPI (100 μM). Nuclear protein extracts were harvested between 0 and 6 h, and gel mobility shift assay was performed using a radiolabeled oligonucleotide probe containing an NF-κB consensus sequence.
Since calpain results in 26S proteasome-independent degradation, subsequent experiments were performed to confirm that particle-mediated catabolism of IκBα did not require the proteasome. Control experiments with TNF-α demonstrated the rapid degradation of IκB which is blocked in the presence of the proteasome inhibitor MG-132 (20 μM, Fig. 9B). The presence of an IκBα band 1 h following TNF-α treatment likely represents the de novo synthesis of IκBα following NF-κB activation which is critical in the negative feedback regulation of NF-κB activity. The baseline effect of titanium on IκBα degradation is previously shown in Figs. 1B, 3A, and 7B. Treatment with MG-132 failed to inhibit IκBα degradation in FLS treated with titanium, in contrast to the effect of MG-132 + TNF-α. However, CPI blocked IκBα degradation in titanium-treated FLS (Fig. 9C).
To further examine the role of calpain in NF-κB activation of FLS, EMSA was performed in titanium-treated FLS in the presence and absence of CPI (Fig. 9D). CPI reduced NF-κB binding in EMSA and reduced COX-2 expression (Fig. 9C). Altogether, the findings show that calpain is a critical component of the pathways leading to NF-κB activation following FLS exposure to titanium particles.
DISCUSSION
The formation of an aggressive inflammatory fibrovascular membrane in the joint capsule that invades the prosthesis-bone interface and stimulates bone resorption follows the accumulation of wear debris particles (13, 20, 36). Synovial fibroblasts have recently been recognized as a key target of particle-mediated inflammatory bone loss. FLS treated with particles in culture express RANKL and induce osteoclast formation from macrophage precursors (20, 30, 36, 38, 56). Similarly, expression of RANKL by synovial fibroblasts is implicated in the periarticular osteolysis that occurs in rheumatoid arthritis and other inflammatory joint diseases (39, 53).
The cyclooxygenase (COX) enzymes mediate a rate-limiting step in the metabolism of arachidonic acid to the prostaglandin compounds, of which PGE2 is a major metabolite (23). Previously, we showed that mice deficient in COX-2 have reduced inflammatory bone loss following implantation of Ti particles onto the calvaria, and we demonstrated that synovial fibroblasts produce RANKL following particle stimulation (56, 58). More recently, COX-2 inhibition reduced both inflammation and local bone loss in the hindfoot of rats with adjuvant-induced arthritis (22). The current findings are consistent with these prior reports and establish the importance of the NF-κB/COX-2 signaling pathway during RANKL induction in FLS. While it has recently been shown that silica particles induce COX-2 in fibroblasts (8, 17, 35), the current studies are the first to implicate a calpain-dependent mechanism.
Our results establish that titanium particles induce COX-2 expression in FLS through NF-κB and define key steps in the activation of this pathway. FLS exposed to titanium particles produce ROS, and treatment with the antioxidant BHA blocked NF-κB activation. ROS, including superoxide, hydrogen peroxide, hydroxyl radicals, and lipid hydroperoxides, are generated by a variety of cell responses and have an essential role in various signaling networks (43, 49, 51). The production of ROS in FLS by Ti particles is consistent with the induction of ROS by similar stimuli in other cells. THP-1 macrophages and avian osteoclasts exposed to particles produce reactive oxygen and nitrogen species in vitro (55). Lung fibroblasts exposed to cigarette smoke extract, air pollution particles, or silica particles also generate ROS (7, 51). In skeletal tissues, generation of ROS is associated with the inflammatory process leading to bone resorption (12, 24). In other tissues, including the lung, the process results in a fibrotic response (37). However, detailed information concerning the signaling pathways modulated by ROS has not been defined (7, 32, 51). In the current studies, generation of ROS was detected following Ti-treatment through the use of the reactive oxygen-sensitive fluorescent dye H2DCFDA. The antioxidant BHA blocked the generation of ROS, prevented IκBα degradation, inhibited formation of NF-κB binding complexes, and decreased the induction of COX-2 expression (Figs. 7 and 9). Together, the findings suggest the involvement of ROS in NF-κB/COX-2 activation by Ti particles.
Alternatively, ROS activity may also result in the production of nonenzymatic arachidonic acid metabolites such as prostaglandin isomers (isoprostanes). One example is 8-isoprostaglandin E2 which may act directly on the osteoclast cell (through EP2) to stimulate resorptive activity or indirectly on the osteoblast through EP1 to upregulate RANKL (52). However, since the isoprostane effect does not appear to involve EP4 (which we have previously shown to be the major receptor subtype involved in particle-mediated osteolysis), it is our working hypothesis that the increased oxidative stress primarily drives the activation of NF-κB and that isoprostane production may be coincidental.
mIκB is a strong dominant-negative regulator for NF-κB since it has mutations in the two major regions that regulate IκB degradation (two phosphorylation sites on NH2-terminal region and five sites within the COOH-terminal PEST domain) (54). mIκB FLS therefore express IκB proteins resistant to signals that ordinarily stimulate NF-κB signaling (28, 54). In mIκB FLS cells, Ti particles failed to induce IκB degradation, stimulate NF-κB nuclear translocation, or stimulate COX-2 and RANKL expression. ChIP assay confirmed that NF-κB binds to the COX-2 promoter and showed that the induction of NF-κB binding to the promoter in vivo by Ti particles is absent in cells overexpressing mIκB. Collectively, these experiments confirm NF-κB as an essential downstream target of Ti particles that is required for induction of COX-2 and RANKL.
Although NF-κB activation required IκBα degradation, catabolism was independent of the IKK activation, serine 32 and 36 phosphorylation, and the 26S proteasome. Instead, our experiments implicate the COOH-terminal PEST domain of IκBα as a critical target of Ti particle-mediated calpain-dependent degradation. Prior studies have shown that the COOH-terminal PEST domain has phosphorylation sites that facilitate calpain-dependent catabolism of IκBα (46). These phosphorylation sites are targeted by various kinases, including casein kinase II, which has a role in both in basal turnover and activated conditions (46, 48). While our cell culture experiments using mIκBα cells established IκBα as a target of Ti particles, the mIκBα has mutations at both NH2-terminal IKK-sensitive and PEST domain phosphorylation sites and thus cannot distinguish the relative importance of these domains (54). For this reason we used an in vitro kinase assay using FLS cell extracts that examined phosphorylation of both wild-type IκB and a MuF IκBα containing mutations limited to the PEST domain (48). These experiments confirmed a role for the PEST domain by showing that Ti-mediated IκBα phosphorylation was markedly reduced in MuF in which all five PEST domain phosphorylation sites are mutated.
The data suggest that the generation of ROS following Ti exposure is an upstream event in calpain-mediated IκB degradation. Several complementary approaches were used to confirm the role of calpain in this pathway, including use of calpain inhibition and detection of calpain-dependent degradation products. These results are consistent with prior findings showing that ROS induce NF-κB in lymphocytes via a mechanism involving the PEST domain and calpain-mediated catabolism of IκBα (46).
Taken together, the findings demonstrate that titanium particles cause oxidative stress which induces calpain-mediated NF-κB signaling, resulting in COX-2 expression and the subsequent signals leading to osteolysis. Our results support prior in vivo studies in knockout mice showing that NF-κB, COX-2, and RANKL are required for inflammatory bone resorption following Ti implantation on the calvarial surface (47, 58). The current study defines ROS generation, NF-κB activation through calpain, and COX-2 induction as an important signaling pathway in synovial fibroblasts in response to titanium particles. Because RANKL is a downstream target of these signals, this has critical importance for the understanding and potential treatment of osteolysis. The findings also suggest that similar pathways may be operant in other tissues, including the lung, where particles induce inflammatory processes that lead to scar formation and tissue dysfunction. Some of the many examples of therapeutic targets for osteolysis treatment include PGE synthase (COX-2), the EP4 receptor, and calpain. Indeed, nonsteroidal anti-inflammatory drugs (NSAIDs) have been successfully tested in several animal models of particle-mediated osteolysis with some efficacy (15, 58). Protection from oxidative stress may be a possibility as well; however, clinically, this has met with limited success even for conditions where ROS have been conclusively shown to play a critical role in the pathogenesis.
GRANTS
This work was supported by National Institutes of Health Public Health Services Award AR-46545.
Acknowledgments
The COX-2/luciferase reporter plasmids containing mutated NF-κB-binding sites were generously provided by Dr. Kenneth K. Wu.
Present address of H. Drissi: New England Musculoskeletal Institute, University of Connecticut Health Center, Farmington, CT 06034.
REFERENCES
- 1.Barnes PJ, Karin M. Nuclear factor-kappaB: a pivotal transcription factor in chronic inflammatory diseases. N Engl J Med 336: 1066–1071, 1997. [DOI] [PubMed] [Google Scholar]
- 2.Belich MP, Salmeron A, Johnston LH, Ley SC. TPL-2 kinase regulates the proteolysis of the NF-kappaB-inhibitory protein NF-kappaB1 p105. Nature 397: 363–368, 1999. [DOI] [PubMed] [Google Scholar]
- 3.Bi Y, Seabold JM, Kaar SG, Ragab AA, Goldberg VM, Anderson JM, Greenfield EM. Adherent endotoxin on orthopedic wear particles stimulates cytokine production and osteoclast differentiation. J Bone Miner Res 16: 2082–2091, 2001. [DOI] [PubMed] [Google Scholar]
- 4.Callaghan JJ, Forest EE, Olejniczak JP, Goetz DD, Johnston RC. Charnley total hip arthroplasty in patients less than fifty years old. A twenty to twenty-five-year follow-up note. J Bone Joint Surg Am 80: 704–714, 1998. [DOI] [PubMed] [Google Scholar]
- 5.Chen CC, Sun YT, Chen JJ, Chang YJ. Tumor necrosis factor-alpha-induced cyclooxygenase-2 expression via sequential activation of ceramide- dependent mitogen-activated protein kinases, and IkappaB kinase 1/2 in human alveolar epithelial cells. Mol Pharmacol 59: 493–500, 2001. [DOI] [PubMed] [Google Scholar]
- 6.Chen F, Lu Y, Kuhn DC, Maki M, Shi X, Sun SC, Demers LM. Calpain contributes to silica-induced I kappa B-alpha degradation and nuclear factor-kappa B activation. Arch Biochem Biophys 342: 383–388, 1997. [DOI] [PubMed] [Google Scholar]
- 7.Cho YJ, Seo MS, Kim JK, Lim Y, Chae G, Ha KS, Lee KH. Silica-induced generation of reactive oxygen species in Rat2 fibroblast: role in activation of mitogen-activated protein kinase. Biochem Biophys Res Commun 262: 708–712, 1999. [DOI] [PubMed] [Google Scholar]
- 8.Choi JK, Lee SG, Lee JY, Nam HY, Lee WK, Lee KH, Kim HJ, Lim Y. Silica induces human cyclooxygenase-2 gene expression through the NF-kappaB signaling pathway. J Environ Pathol Toxicol Oncol 24: 163–174, 2005. [DOI] [PubMed] [Google Scholar]
- 9.Czogalla A, Sikorski AF. Spectrin and calpain: a ‘target’ and a ‘sniper’ in the pathology of neuronal cells. Cell Mol Life Sci 62: 1913–1924, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Demarchi F, Bertoli C, Sandy P, Schneider C. Glycogen synthase kinase-3 beta regulates NF-kappa B1/p105 stability. J Biol Chem 278: 39583–39590, 2003. [DOI] [PubMed] [Google Scholar]
- 11.Franco SJ, Huttenlocher A. Regulating cell migration: calpains make the cut. J Cell Sci 118: 3829–3838, 2005. [DOI] [PubMed] [Google Scholar]
- 12.Garrett IR, Boyce BF, Oreffo RO, Bonewald L, Poser J, Mundy GR. Oxygen-derived free radicals stimulate osteoclastic bone resorption in rodent bone in vitro and in vivo. J Clin Invest 85: 632–639, 1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Goldring SR, Schiller AL, Roelke M, Rourke CM, O'Neil DA, Harris WH. The synovial-like membrane at the bone-cement interface in loose total hip replacements and its proposed role in bone lysis. J Bone Joint Surg Am 65: 575–584, 1983. [PubMed] [Google Scholar]
- 14.Hirakawa K, Bauer TW, Stulberg BN, Wilde AH, Secic M. Characterization and comparison of wear debris from failed total hip implants of different types. J Bone Joint Surg Am 78: 1235–1243, 1996. [DOI] [PubMed] [Google Scholar]
- 15.Im GI, Kwon BC, Lee KB. The effect of COX-2 inhibitors on periprosthetic osteolysis. Biomaterials 25: 269–275, 2004. [DOI] [PubMed] [Google Scholar]
- 16.Iniguez MA, Martinez-Martinez S, Punzon C, Redondo JM, Fresno M. An essential role of the nuclear factor of activated T cells in the regulation of the expression of the cyclooxygenase-2 gene in human T lymphocytes. J Biol Chem 275: 23627–23635, 2000. [DOI] [PubMed] [Google Scholar]
- 17.Inoue K, Takano H, Yanagisawa R, Ichinose T, Sadakane K, Yoshino S, Yamaki K, Uchiyama K, Yoshikawa T. Components of diesel exhaust particles differentially affect lung expression of cyclooxygenase-2 related to bacterial endotoxin. J Appl Toxicol 24: 415–418, 2004. [DOI] [PubMed] [Google Scholar]
- 18.Ionescu AM, Schwarz EM, Vinson C, Puzas JE, Rosier RN, Reynolds PR, O'Keefe RJ. PTHrP modulates chondrocyte differentiation through AP-1 and CREB signaling. J Biol Chem 276: 11639–11647, 2001. [DOI] [PubMed] [Google Scholar]
- 19.Israel A The IKK complex: an integrator of all signals that activate NF-kappaB? Trends Cell Biol 10: 129–133, 2000. [DOI] [PubMed] [Google Scholar]
- 20.Jiranek WA, Machado M, Jasty M, Jevsevar D, Wolfe HJ, Goldring SR, Goldberg MJ, Harris WH. Production of cytokines around loosened cemented acetabular components. Analysis with immunohistochemical techniques and in situ hybridization. J Bone Joint Surg Am 75: 863–879, 1993. [DOI] [PubMed] [Google Scholar]
- 21.Kaneko T, Yamashima T, Tohma Y, Nomura M, Imajoh-Ohmi S, Saido TC, Nakao M, Saya H, Yamamoto H, Yamashita J. Calpain-dependent proteolysis of merlin occurs by oxidative stress in meningiomas: a novel hypothesis of tumorigenesis. Cancer 92: 2662–2672, 2001. [DOI] [PubMed] [Google Scholar]
- 22.Katagiri M, Ogasawara T, Hoshi K, Chikazu D, Kimoto A, Noguchi M, Sasamata M, Harada S, Akama H, Tazaki H, Chung UI, Takato T, Nakamura K, Kawaguchi H. Suppression of adjuvant-induced arthritic bone destruction by cyclooxygenase-2 selective agents with and without inhibitory potency against carbonic anhydrase II. J Bone Miner Res 21: 219–227, 2006. [DOI] [PubMed] [Google Scholar]
- 23.Kawaguchi H, Pilbeam CC, Harrison JR, Raisz LG. The role of prostaglandins in the regulation of bone metabolism. Clin Orthop Relat Res: 36–46, 1995. [PubMed]
- 24.Koh JM, Lee YS, Kim YS, Kim DJ, Kim HH, Park JY, Lee KU, Kim GS. Homocysteine enhances bone resorption by stimulation of osteoclast formation and activity through increased intracellular ROS generation. J Bone Miner Res 21: 1003–1011, 2006. [DOI] [PubMed] [Google Scholar]
- 25.Kosaka T, Miyata A, Ihara H, Hara S, Sugimoto T, Takeda O, Takahashi E, Tanabe T. Characterization of the human gene (PTGS2) encoding prostaglandin-endoperoxide synthase 2. Eur J Biochem 221: 889–897, 1994. [DOI] [PubMed] [Google Scholar]
- 26.Kotake S, Udagawa N, Hakoda M, Mogi M, Yano K, Tsuda E, Takahashi K, Furuya T, Ishiyama S, Kim KJ, Saito S, Nishikawa T, Takahashi N, Togari A, Tomatsu T, Suda T, Kamatani N. Activated human T cells directly induce osteoclastogenesis from human monocytes: possible role of T cells in bone destruction in rheumatoid arthritis patients. Arthritis Rheum 44: 1003–1012, 2001. [DOI] [PubMed] [Google Scholar]
- 27.Lang V, Janzen J, Fischer GZ, Soneji Y, Beinke S, Salmeron A, Allen H, Hay RT, Ben-Neriah Y, Ley SC. betaTrCP-mediated proteolysis of NF-kappaB1 p105 requires phosphorylation of p105 serines 927 and 932. Mol Cell Biol 23: 402–413, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Li P, Sanz I, O'Keefe RJ, Schwarz EM. NF-kappa B regulates VCAM-1 expression on fibroblast-like synoviocytes. J Immunol 164: 5990–5997, 2000. [DOI] [PubMed] [Google Scholar]
- 29.Lombardi MS, Kavelaars A, Penela P, Scholtens EJ, Roccio M, Schmidt RE, Schedlowski M, Mayor F Jr, Heijnen CJ. Oxidative stress decreases G protein-coupled receptor kinase 2 in lymphocytes via a calpain-dependent mechanism. Mol Pharmacol 62: 379–388, 2002. [DOI] [PubMed] [Google Scholar]
- 30.Mandelin J, Li TF, Liljestrom M, Kroon ME, Hanemaaijer R, Santavirta S, Konttinen YT. Imbalance of RANKL/RANK/OPG system in interface tissue in loosening of total hip replacement. J Bone Joint Surg Br 85: 1196–1201, 2003. [DOI] [PubMed] [Google Scholar]
- 31.Margevicius KJ, Bauer TW, McMahon JT, Brown SA, Merritt K. Isolation and characterization of debris in membranes around total joint prostheses. J Bone Joint Surg Am 76: 1664–1675, 1994. [DOI] [PubMed] [Google Scholar]
- 32.Martin LD, Krunkosky TM, Voynow JA, Adler KB. The role of reactive oxygen and nitrogen species in airway epithelial gene expression. Environ Health Perspect 106, Suppl 5: 1197–1203, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Miyaura C, Inada M, Suzawa T, Sugimoto Y, Ushikubi F, Ichikawa A, Narumiya S, Suda T. Impaired bone resorption to prostaglandin E2 in prostaglandin E receptor EP4-knockout mice. J Biol Chem 275: 19819–19823, 2000. [DOI] [PubMed] [Google Scholar]
- 34.Moorthy AK, Ghosh G. p105 Ikappa Bgamma and prototypical Ikappa Bs use a similar mechanism to bind but a different mechanism to regulate the subcellular localization of NF-kappa B. J Biol Chem 278: 556–566, 2003. [DOI] [PubMed] [Google Scholar]
- 35.O'Reilly KM, Phipps RP, Thatcher TH, Graf BA, Van Kirk J, Sime PJ. Crystalline and amorphous silica differentially regulate the cyclooxygenase-prostaglandin pathway in pulmonary fibroblasts: implications for pulmonary fibrosis. Am J Physiol Lung Cell Mol Physiol 288: L1010–L1016, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Perry MJ, Mortuza FY, Ponsford FM, Elson CJ, Atkins RM. Analysis of cell types and mediator production from tissues around loosening joint implants. Br J Rheumatol 34: 1127–1134, 1995. [DOI] [PubMed] [Google Scholar]
- 37.Porter DW, Millecchia LL, Willard P, Robinson VA, Ramsey D, McLaurin J, Khan A, Brumbaugh K, Beighley CM, Teass A, Castranova V. Nitric oxide and reactive oxygen species production causes progressive damage in rats after cessation of silica inhalation. Toxicol Sci 90: 188–197, 2006. [DOI] [PubMed] [Google Scholar]
- 38.Ren W, Yang SY, Fang HW, Hsu S, Wooley PH. Distinct gene expression of receptor activator of nuclear factor-kappaB and rank ligand in the inflammatory response to variant morphologies of UHMWPE particles. Biomaterials 24: 4819–4826, 2003. [DOI] [PubMed] [Google Scholar]
- 39.Ritchlin CT, Haas-Smith SA, Li P, Hicks DG, Schwarz EM. Mechanisms of TNF-alpha- and RANKL-mediated osteoclastogenesis and bone resorption in psoriatic arthritis. J Clin Invest 111: 821–831, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sakai H, Jingushi S, Shuto T, Urabe K, Ikenoue T, Okazaki K, Kukita T, Kukita A, Iwamoto Y. Fibroblasts from the inner granulation tissue of the pseudocapsule in hips at revision arthroplasty induce osteoclast differentiation, as do stromal cells. Ann Rheum Dis 61: 103–109, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sanvicens N, Gomez-Vicente V, Masip I, Messeguer A, Cotter TG. Oxidative stress-induced apoptosis in retinal photoreceptor cells is mediated by calpains and caspases and blocked by the oxygen radical scavenger CR-6. J Biol Chem 279: 39268–39278, 2004. [DOI] [PubMed] [Google Scholar]
- 42.Sanvicens N, Gomez-Vicente V, Messeguer A, Cotter TG. The radical scavenger CR-6 protects SH-SY5Y neuroblastoma cells from oxidative stress-induced apoptosis: effect on survival pathways. J Neurochem 98: 735–747, 2006. [DOI] [PubMed] [Google Scholar]
- 43.Sauer H, Wartenberg M. Reactive oxygen species as signaling molecules in cardiovascular differentiation of embryonic stem cells and tumor-induced angiogenesis. Antioxid Redox Signal 7: 1423–1434, 2005. [DOI] [PubMed] [Google Scholar]
- 44.Saunders MA, Sansores-Garcia L, Gilroy DW, Wu KK. Selective suppression of CCAAT/enhancer-binding protein beta binding and cyclooxygenase-2 promoter activity by sodium salicylate in quiescent human fibroblasts. J Biol Chem 276: 18897–18904, 2001. [DOI] [PubMed] [Google Scholar]
- 45.Schaecher K, Goust JM, Banik NL. The effects of calpain inhibition on IkB alpha degradation after activation of PBMCs: identification of the calpain cleavage sites. Neurochem Res 29: 1443–1451, 2004. [DOI] [PubMed] [Google Scholar]
- 46.Schoonbroodt S, Ferreira V, Best-Belpomme M, Boelaert JR, Legrand-Poels S, Korner M, Piette J. Crucial role of the amino-terminal tyrosine residue 42 and the carboxyl-terminal PEST domain of I kappa B alpha in NF-kappa B activation by an oxidative stress. J Immunol 164: 4292–4300, 2000. [DOI] [PubMed] [Google Scholar]
- 47.Schwarz EM, Lu AP, Goater JJ, Benz EB, Kollias G, Rosier RN, Puzas JE, O'Keefe RJ. Tumor necrosis factor-alpha/nuclear transcription factor-kappaB signaling in periprosthetic osteolysis. J Orthop Res 18: 472–480, 2000. [DOI] [PubMed] [Google Scholar]
- 48.Schwarz EM, Van Antwerp D, Verma IM. Constitutive phosphorylation of IkappaBalpha by casein kinase II occurs preferentially at serine 293: requirement for degradation of free IkappaBalpha. Mol Cell Biol 16: 3554–3559, 1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Shen HM, Liu ZG. JNK signaling pathway is a key modulator in cell death mediated by reactive oxygen and nitrogen species. Free Radic Biol Med 40: 928–939, 2006. [DOI] [PubMed] [Google Scholar]
- 50.Singer CA, Baker KJ, McCaffrey A, AuCoin DP, Dechert MA, Gerthoffer WT. p38 MAPK and NF-κB mediate COX-2 expression in human airway myocytes. Am J Physiol Lung Cell Mol Physiol 285: L1087–L1098, 2003. [DOI] [PubMed] [Google Scholar]
- 51.Tao F, Gonzalez-Flecha B, Kobzik L. Reactive oxygen species in pulmonary inflammation by ambient particulates. Free Radic Biol Med 35: 327–340, 2003. [DOI] [PubMed] [Google Scholar]
- 52.Tintut Y, Parhami F, Tsingotjidou A, Tetradis S, Territo M, Demer LL. 8-Isoprostaglandin E2 enhances receptor-activated NFkappa B ligand (RANKL)-dependent osteoclastic potential of marrow hematopoietic precursors via the cAMP pathway. J Biol Chem 277: 14221–14226, 2002. [DOI] [PubMed] [Google Scholar]
- 53.Udagawa N, Kotake S, Kamatani N, Takahashi N, Suda T. The molecular mechanism of osteoclastogenesis in rheumatoid arthritis. Arthritis Res 4: 281–289, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Van Antwerp DJ, Martin SJ, Kafri T, Green DR, Verma IM. Suppression of TNF-alpha-induced apoptosis by NF-kappaB. Science 274: 787–789, 1996. [DOI] [PubMed] [Google Scholar]
- 55.Wang ML, Hauschka PV, Tuan RS, Steinbeck MJ. Exposure to particles stimulates superoxide production by human THP-1 macrophages and avian HD-11EM osteoclasts activated by tumor necrosis factor-alpha and PMA. J Arthroplasty 17: 335–346, 2002. [DOI] [PubMed] [Google Scholar]
- 56.Wei X, Zhang X, Zuscik MJ, Drissi MH, Schwarz EM, O'Keefe RJ. Fibroblasts express RANKL and support osteoclastogenesis in a COX-2-dependent manner after stimulation with titanium particles. J Bone Miner Res 20: 1136–1148, 2005. [DOI] [PubMed] [Google Scholar]
- 57.Zatz M, Starling A. Calpains and disease. N Engl J Med 352: 2413–2423, 2005. [DOI] [PubMed] [Google Scholar]
- 58.Zhang X, Morham SG, Langenbach R, Young DA, Xing L, Boyce BF, Puzas EJ, Rosier RN, O'Keefe RJ, Schwarz EM. Evidence for a direct role of cyclo-oxygenase 2 in implant wear debris-induced osteolysis. J Bone Miner Res 16: 660–670, 2001. [DOI] [PubMed] [Google Scholar]