Abstract
Net photosynthesis (Pn) is inhibited by moderate heat stress. To elucidate the mechanism of inhibition, we examined the effects of temperature on gas exchange and ribulose 1,5-bisphosphate carboxylase/oxygenase (Rubisco) activation in cotton and tobacco leaves and compared the responses to those of the isolated enzymes. Depending on the CO2 concentration, Pn decreased when temperatures exceeded 35–40°C. This response was inconsistent with the response predicted from the properties of fully activated Rubisco. Rubisco deactivated in leaves when temperature was increased and also in response to high CO2 or low O2. The decrease in Rubisco activation occurred when leaf temperatures exceeded 35°C, whereas the activities of isolated activase and Rubisco were highest at 42°C and >50°C, respectively. In the absence of activase, isolated Rubisco deactivated under catalytic conditions and the rate of deactivation increased with temperature but not with CO2. The ability of activase to maintain or promote Rubisco activation in vitro also decreased with temperature but was not affected by CO2. Increasing the activase/Rubisco ratio reduced Rubisco deactivation at higher temperatures. The results indicate that, as temperature increases, the rate of Rubisco deactivation exceeds the capacity of activase to promote activation. The decrease in Rubisco activation that occurred in leaves at high CO2 was not caused by a faster rate of deactivation, but by reduced activase activity possibly in response to unfavorable ATP/ADP ratios. When adjustments were made for changes in activation state, the kinetic properties of Rubisco predicted the response of Pn at high temperature and CO2.
Current models of global climate predict a gradual increase in the atmospheric concentrations of greenhouse gases over the next century and associated increases in global temperature (1). The resulting effects on net photosynthesis will have significant ecological and agricultural consequences. For temperate C3 plants, the rate of photosynthesis under current atmospheric conditions exhibits a broad temperature optimum, with the maximum rate occurring near the daytime temperature under which the plants are grown (2). The broad temperature optimum for photosynthesis has been attributed to differential changes in the solubility of CO2 and O2 with temperature (3, 4) and to specific changes in the kinetic properties of ribulose 1,5-bisphosphate carboxylase/oxygenase (Rubisco) (4–6). Increasing the CO2 concentration above ambient generally increases the temperature optimum for photosynthesis by several degrees (2, 4).
Models of photosynthesis have been developed to explain the response of photosynthesis to environmental conditions (7, 8). These models make a clear distinction between limitations attributable to Rubisco and those attributable to factors that limit the rate of ribulose-1,5-bisphosphate (RuBP) regeneration, but they usually do not include changes in the activation state of Rubisco among the factors that influence the rate of photosynthesis. The activation state of Rubisco, which is the proportion of Rubisco that is catalytically competent (9), determines the amount of Rubisco that can contribute to the overall rate of carboxylation by a leaf. Numerous studies have shown that the activation state of Rubisco varies in leaves depending on environmental conditions (9–14), but conflicting results have been reported (15) and the time course of activation state changes do not always correlate with changes in photosynthetic rate (13). Consequently, changes in photosynthesis in response to changing environmental conditions seldom are attributed to changes in Rubisco activation. Two exceptions are when photosynthesis is inhibited by moderate heat stress (10, 11, 16–18) and when photosynthesis is not in steady state after an increase in light intensity (19).
For a Rubisco active site to be catalytically competent, it must be carbamylated by CO2 and free of inhibitory sugar phosphates (20). Active sites that are not carbamylated bind RuBP very tightly to form a dead-end complex that carbamylates only after the very slow dissociation of RuBP (21). Carbamylated sites also form dead-end complexes, not with RuBP, but with other sugar phosphates including isomers and epimers of RuBP that are produced at the active site by misprotenation of RuBP (22). To facilitate the removal of sugar phosphates from Rubisco active sites, chloroplasts contain a AAA+ protein (23) called Rubisco activase (24, 25). Activase is an ATPase that loosens the binding of Rubisco for sugar phosphates (26). Thus, activase promotes “activation” of Rubisco by facilitating dissociation of sugar phosphates from either decarbamylated sites containing RuBP or analogs of RuBP, or carbamylated sites containing analogs of RuBP or the reaction intermediate (24, 25). Because of this important function, activase ultimately determines the proportion of Rubisco active sites that are catalytically competent. Because activase requires ATP and is inhibited by ADP, conditions that affect the ATP/ADP ratio of the chloroplast affect activase activity and, hence, the activation state of Rubisco (27).
Here, we examine the response of Rubisco activation and photosynthesis to moderate heat stress in cotton and tobacco leaves under photorespiratory and nonphotorespiratory conditions. Because the temperature response of photosynthesis changes with CO2 (2, 4), we also examined the effect of CO2 on Rubisco activation under control and heat stress conditions. By comparing the effects of temperature and CO2 on Rubisco activation in leaves with the behavior of isolated Rubisco and activase in a reconstituted activation assay, it was possible to elucidate the biochemical basis for changes in Rubisco activation that occur in response to moderate heat stress and high CO2. When adjusted for these changes, the kinetic properties of Rubisco were able to predict the measured rates of photosynthesis.
Materials and Methods
Plant Material.
Cotton (Gossypium hirsutum L. cv. Coker 100A-glandless) and tobacco (Nicotiana rusticum cv. Pulmila) plants were cultivated in a temperature-controlled greenhouse as described previously (18). Plants were maintained at a high-fertility level under natural lighting that reached a maximum of 2,000 μmol photons m−2 s−1 of photosynthetically active radiation on most days. Experiments were conducted on the fifth or sixth true leaf after the leaf had reached full expansion.
Gas Exchange.
Plants were transferred to a plant growth chamber for gas exchange measurements. Steady-state net photosynthesis (Pn) was determined with a Li-Cor 6400 portable photosynthesis system (Li-Cor, Lincoln, NE), using the built-in light source set at 1,800 μmol photons m−2 s−1. The level of CO2 supplied to the leaf was controlled by using the built-in CO2 injection system of the photosynthesis unit. For measurements under nonphotorespiratory conditions, gas containing 10 mbar O2, balance N2, was fed directly into the photosynthesis unit and mixed with CO2. Leaf temperature was controlled as described previously (18, 28), and the internal CO2 concentration (Ci) of the leaf was monitored to ensure that stomatal closure was not restricting the supply of CO2. After measuring steady-state Pn, the leaf chamber was removed and the leaf tissue contained within the chamber was frozen rapidly between pieces of metal cooled to liquid N2 temperature. The frozen leaf tissue was stored at −80°C for determination of Rubisco activation state.
Rubisco Activation State.
Frozen leaf tissue was extracted rapidly (i.e., ≈20 s) in CO2-free buffer as described previously (18) except that the buffer was 100 mM Na2B4O7, pH 7.6, instead of Tricine-NaOH, pH 8.0, to preserve enzyme activity (28). Preliminary experiments showed that Rubisco activity was constant with time for at least 15 min in crude leaf extracts prepared with this buffer (data not shown). Immediately after extraction, extracts were assayed for initial Rubisco activity by determining incorporation of 14CO2 into acid-stable products at 30°C (29). Total Rubisco activity was determined after incubating the extract in assay buffer without RuBP for 10 min at 30°C. The activation state of Rubisco or percent activation (9) was determined by the ratio of initial to total Rubisco activities. For each treatment, extracts from three different leaves were assayed, each in duplicate.
Isolation of Rubisco and Activase.
Rubisco was isolated from tobacco and cotton leaves. Deveined leaves were frozen at −80°C, powdered, and then extracted in a blender containing 50 mM Tris⋅HCl, pH 7.6, 20 mM MgCl2, 20 mM NaHCO3, 0.2 mM EDTA, 5 mM DTT, 1 mM PMSF, and 10 μM leupeptin (extraction buffer), plus 2% (wt/vol) polyvinyl polypyrrolidone and 10% (vol/vol) glycerol. For isolation of Rubisco from cotton leaves, the solution was buffered with 100 mM Na2B4O7, pH 7.6, instead of Tris. After centrifugation, the extract was fractionated with ammonium sulfate to obtain the protein fraction that precipitated between 40% and 55%. Protein was dissolved in Tris extraction buffer and the solution was layered on linear gradients containing 0.2–0.8 M sucrose in half-strength extraction buffer. After centrifugation (30), the gradients were fractionated and the fractions containing Rubisco were supplemented with 65% ammonium sulfate for storage at −80°C (29). Rubisco concentration was determined from the A280 (29). Recombinant tobacco activase was isolated after expression in Escherichia coli (30, 31), and the concentration was determined as described previously (29). Tobacco activase is composed of a single polypeptide type (30) that is not activated by thioredoxin-mediated reduction (32).
Assay of Isolated Rubisco and Rubisco Activase.
The response of Rubisco activity to temperature was determined by incubating the enzyme isolated from tobacco or cotton for 6 min at the indicated temperatures before initiation of the assays with RuBP (see above). To ensure that CO2 was saturating for carbamylation and activity even at the higher temperatures, a NaHCO3 concentration of 30 mM was used for these experiments. The ATPase activity of recombinant tobacco activase was determined at various temperatures by using a spectrophotometric assay (29). The temperature of the assay solution was measured by using a type T thermocouple. Reactions were initiated with activase and were monitored continuously for 6 min at the indicated temperatures. Preliminary experiments verified that the linking enzymes used in the assay were not limiting at any of the temperatures used in the study.
Activation and deactivation of Rubisco under catalytic conditions were determined in the presence and absence of activase by using a two-stage assay (29). Before assay, Rubisco was desalted and converted to the decarbamylated form complexed with RuBP (29) or fully carbamylated by incubating the enzyme for 30 min in buffer containing 10 mM NaHCO3 and 10 mM MgCl2. The first-stage reactions contained 100 mM Tricine-NaOH, pH 8.0, 10 mM MgCl2, 1 mM NaHCO3, 6 mM RuBP, 2 mM DTT, 5% (wt/vol) polyethylene glycol-3350, 5 mM ATP, 4.2 mM phosphocreatine, 83 units ml−1 phosphocreatine kinase, and 5,000 units ml−1 carbonic anhydrase. The reaction mixtures minus tobacco activase and tobacco Rubisco were incubated for 2 min at the indicated temperatures. The reactions were purged with humidified air containing 370 μbar CO2 during the incubation and throughout the course of the reaction to maintain a constant concentration of free CO2 in solution. For the high-CO2 experiments, the reactions contained 10 mM NaHCO3 and were purged with 3,700 μbar CO2. Activase, at the concentrations indicated in the text, was added to the reactions 30 s before the addition of Rubisco to a final concentration of 0.5 mg ml−1 (7.14 μM protomer). The amount of active Rubisco (i.e., catalytically competent sites) was determined at the times indicated in the text by measuring Rubisco activity and relating this activity to the specific activity of fully carbamylated enzyme (29). Calculations based on the maximum rates of carboxylation by Rubisco and ATP hydrolysis by activase indicated that RuBP and ATP consumption was not sufficient to decrease their levels significantly during the course of the experiment. In addition, the amount of ADP measured after the first-stage reaction was equivalent to less than 10% of the total adenine nucleotide pool. Thus, ATP regeneration by the phosphocreatine-based system was sufficient to maintain a high ratio of ATP/ADP during the entire course of the reaction.
Predicted Rates of Photosynthesis.
Net photosynthetic rates were calculated from the kinetic properties of Rubisco by using the full equations of Laing et al. (33) for vc and vo and the solubilities of CO2 and O2, assuming that Pn was limited only by Rubisco. The specific activity of fully carbamylated Rubisco from cotton leaves was determined experimentally at 25°C and used as the Vmax for carboxylation at this temperature. This value, which was nearly identical to the value calculated from the initial slope of the CO2 response curve for Pn (data not shown), was adjusted for temperature by using the experimentally determined response of cotton Rubisco activity to temperature (see above). The values of Jordan and Ogren (5) were used for the specificity factor and the kinetic constants for O2 and CO2 after correction for temperature. The Vmax for oxygenase activity was calculated as described (5). Net photosynthetic rates were corrected for activation where indicated by adjusting the Vmax for carboxylation and oxygenation. Net photosynthesis was not corrected for dark respiration.
Results
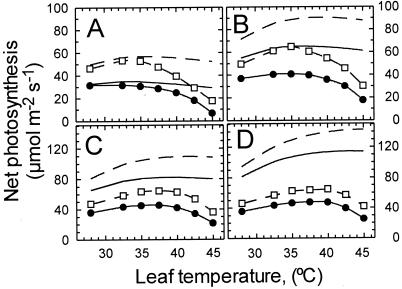
The response of Pn to leaf temperature varied depending on the concentration of CO2 and O2 (Fig. 1). At 210 mbar O2 and 280 μbar CO2, Pn decreased with temperature from 28°C to 45°C (Fig. 1A). At 550, 750, and 1,200 μbar CO2, Pn either increased or remained constant with temperatures up to about 35°C, but decreased above 40°C. The temperature optima for Pn at 210 mbar O2 were 28°C, 35°C, 37.5°C, and 40°C at 280, 550, 750, and 1,200 μbar CO2, respectively (Fig. 1).
Figure 1.
Measured and predicted response of Pn to leaf temperature in cotton leaves at different internal partial pressures of CO2 (Ci) and O2 concentrations. Pn was determined at 210 (●) or 10 mbar O2 (□) at 280 (A), 550 (B), 750 (C), and 1,200 (D) μbar Ci. For each leaf, steady-state Pn was attained at 28°C, 280 μbar Ci, and either 210 or 10 mbar O2, after which the Ci was increased incrementally to 550, 750, and 1,200 μbar. After readjusting the Ci to 280 μbar, leaf temperature was increased and the process was repeated. The response of Pn predicted from the kinetic properties of fully activated Rubisco at 210 (solid line) and 10 mbar O2 (dashed line) were determined as described in Materials and Methods.
Overall, the temperature response of Pn at 10 mbar (i.e., conditions that eliminated photorespiration) was similar to the response at 210 mbar O2, but minor quantitative and qualitative differences were evident between the responses at the two O2 levels (Fig. 1). For example, at both 280 and 550 μbar CO2, Pn increased when leaf temperature was increased from 28°C to 35°C at 10 mbar O2, but not at 210 mbar O2 (Fig. 1 A and B). Also, the relative decrease in Pn that occurred above 35°C at these CO2 concentrations was greater at 10 mbar O2 than at 210 mbar O2.
Under both photorespiratory and nonphotorespiratory conditions, the response of Pn to temperature predicted from the kinetics of fully activated Rubisco and taking into account temperature-dependent changes in the solubility of the gases differed markedly from the response measured for cotton leaves (Fig. 1). The kinetics of Rubisco predicted that Pn at both 210 and 10 mbar O2 should be relatively insensitive to temperature, except at the highest Ci, where the rates would increase with temperature. Instead, Pn measured for leaves eventually decreased with increasing temperature at all of the Ci examined (see above). Thus, the kinetics of Rubisco failed to predict the decrease in Pn that occurred with temperature. The predicted and measured Pn were similar at 280 μbar CO2 up to a temperature of about 35°C. At higher Ci, the measured rates were considerably lower than those predicted from the kinetics of Rubisco throughout the temperature range.
The activation state of Rubisco was determined at 28°C and 37°C in cotton and tobacco leaves exposed to a variety of atmospheric conditions (Table 1). Tobacco was included in the study because this species was the source of the recombinant activase (see below). The results showed that leaf temperature, Ci, and O2 level all affected the activation state of Rubisco in a similar manner for both cotton and tobacco. In general, the activation state of Rubisco decreased when temperature and Ci were increased and O2 was decreased. Activation states ranged from 91% at 28°C, 280 μbar CO2, and 210 mbar O2 to 39% at 37°C, 1,200 μbar CO2, and 10 mbar O2.
Table 1.
The response of Rubisco activation in cotton and tobacco to leaf temperature, Ci, and O2 concentration
| Species | Ci, μbar | O2, mbar | Rubisco activation, % ± SE
|
|
|---|---|---|---|---|
| 28°C | 37°C | |||
| Cotton | 280 | 210 | 91 ± 2 | 74 ± 4 |
| 280 | 10 | 77 ± 1 | 62 ± 6 | |
| 700 | 210 | ND | 62 ± 2 | |
| 700 | 10 | ND | 50 ± 3 | |
| 1,200 | 210 | 47 ± 2 | 49 ± 4 | |
| 1,200 | 10 | ND | 39 ± 3 | |
| Tobacco | 280 | 210 | 92 ± 2 | 77 ± 1 |
| 280 | 10 | 66 ± 5 | 53 ± 5 | |
| 950 | 210 | 65 ± 6 | 59 ± 2 | |
| 950 | 10 | ND | 39 ± 7 | |
ND, not determined.
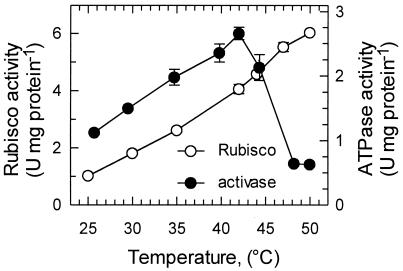
The temperature responses of Rubisco and activase activities were examined separately by using Rubisco isolated from tobacco and purified recombinant tobacco activase (Fig. 2). Rubisco activity increased linearly and by nearly 6-fold as the assay temperature was increased from 25°C to 50°C. The ATPase activity of activase also increased with temperature, but the increase was less than 3-fold and reached a maximum at 42°C. However, even at 50°C, activase was still active, capable of hydrolyzing ATP at a rate equivalent to 50% of the rate at 25°C (Fig. 2).
Figure 2.
Effect of assay temperature on the activities of isolated Rubisco (○) and recombinant activase (●).
Even though Rubisco activity increased with temperature in vitro, the activation state of the enzyme in both cotton and tobacco leaves decreased at 37°C (Table 1). These results suggest that the ability of activase to promote Rubisco activation decreases at high temperature. To test this hypothesis, a series of experiments was conducted to examine the effect of temperature on the ability of activase to maintain and/or promote activation of Rubisco in vitro under catalytic conditions.
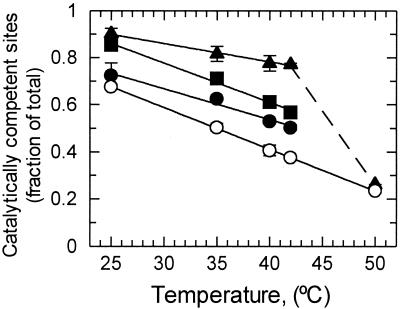
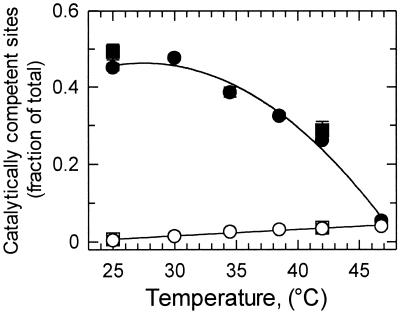
In the first set of experiments, fully carbamylated Rubisco deactivated within 1.5 min when incubated with RuBP at 370 μbar CO2 in the absence of activase (Fig. 3). The fraction of Rubisco sites that deactivated in the absence of activase increased linearly with temperature from 30% of the sites at 25°C to 75% of the sites at 50°C (Fig. 3). Increasing the CO2 concentration 10-fold had almost no effect on Rubisco deactivation (Table 2). However, addition of activase decreased Rubisco deactivation at temperatures between 25°C and 42°C (Fig. 3). This ability of activase to maintain Rubisco in an activated state was dependent on the concentration of activase. For example, at 42°C, 22% of the Rubisco sites lost activity after 1.5 min in the presence of 0.125 mg ml−1 activase compared with 53% with 0.025 mg ml−1. It should be noted that assays containing 0.125 mg ml−1 activase had an activase-to-Rubisco ratio 10-fold higher than the ratio in leaves (34). However, even this very high level of activase was totally ineffective in preventing Rubisco from deactivating when the temperature was 50°C.
Figure 3.
Effect of temperature and activase concentration on the activation state of Rubisco in vitro. Fully carbamylated Rubisco was incubated at the indicated temperatures in the absence (○) or presence of 0.025 (●), 0.05 (■), or 0.125 (▴) mg ml−1 activase under catalytic conditions. After 1.5 min, the fraction of catalytically competent sites was determined by measuring Rubisco activity. At all temperatures, Rubisco deactivation was linear with time for the first 2 min.
Table 2.
Effect of CO2 concentration and temperature on Rubisco deactivation
| Temperature, °C | Rubisco activation (fraction of sites
catalytically competent)
|
|
|---|---|---|
| 370 μbar CO2 | 3,700 μbar CO2 | |
| 25 | 0.80 ± 0.02 | 0.77 ± 0.06 |
| 35 | 0.74 ± 0.02 | 0.66 ± 0.04 |
| 42 | 0.51 ± 0.02 | 0.53 ± 0.05 |
Fully carbamylated Rubisco was incubated under catalytic conditions for 1.5 min at the indicated temperatures and CO2 levels.
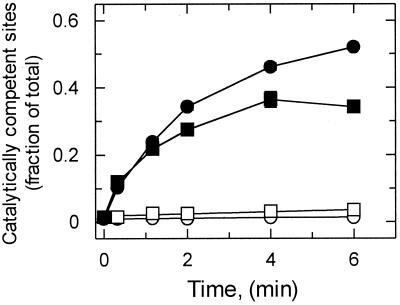
In the second set of experiments, the effect of temperature on the ability of activase to promote activation of Rubisco was determined. In a time course experiment, activase promoted activation of decarbamylated Rubisco complexed with RuBP at both 25°C and 42°C and the initial rate of activation was similar at the two temperatures (Fig. 4). However, as time progressed beyond 1 min, the rate of activation and the final level achieved after 6 min were considerably lower at 42°C than at 25°C. The level of activation achieved after 6 min with a constant amount of activase was examined over a range of temperatures from 25°C to 47°C (Fig. 5). Above 30°C, the level of activation promoted by activase decreased with temperature, and, at 47°C, activase was unable to promote Rubisco activation above the level attained spontaneously without activase. Increasing the CO2 concentration of the activation assay 10-fold produced only a slight increase in Rubisco activation at 25°C and 42°C.
Figure 4.
Effect of temperature on the time course of Rubisco activation by activase in vitro. Decarbamylated Rubisco complexed with RuBP was incubated under catalytic conditions in the absence (○, □) and presence (●, ■) of 0.07 mg ml−1 activase at 25°C (○, ●) and 42°C (□, ■). The fraction of catalytically competent sites was determined at the indicated times by measuring Rubisco activity.
Figure 5.
Effect of temperature and CO2 level on activation of Rubisco by activase in vitro. Decarbamylated Rubisco complexed with RuBP was incubated under catalytic conditions in the absence (○, □) and presence (●, ■) of 0.05 mg ml−1 activase. Reaction mixtures containing either 370 (○, ●) or 3,700 (□, ■) μbar CO2 were incubated at the indicated temperatures. The fraction of catalytically competent sites was determined after 6 min by measuring Rubisco activity.
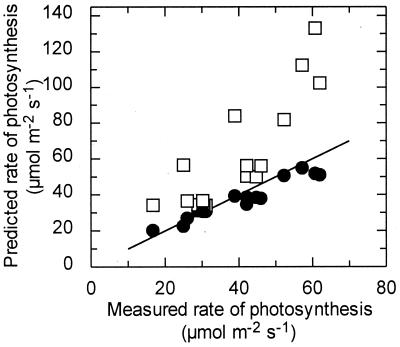
To determine whether changes in Rubisco activation in response to temperature, CO2, and O2 could account for the differences between the predicted and measured Pn, the measured rate of Pn under each condition was compared with the predicted rate either with or without adjustment for changes in the activation state (Fig. 6). Regardless of the temperature, Ci, and O2 level, the predicted Pn was similar to the measured Pn when adjustments were made for changes in activation state of Rubisco. If no adjustments were made for changes in activation state, the predicted Pn was generally much higher than the rates measured in leaves, particularly at high CO2 and high temperature. Rubisco activation was highest under conditions that most resembled the normal ambient environment (see Table 1). Interestingly, the predicted rates of Pn were similar to the measured rates under these conditions, even without adjusting for changes in the activation state of Rubisco.
Figure 6.
Effect of Rubisco activation on the relationship between the rates of Pn measured in cotton leaves and predicted from the kinetics of Rubisco. Pn and Rubisco activation were measured in leaves exposed to various combinations of leaf temperature (28°C or higher), Ci (280 μbar or higher), and O2 level (210 and 10 mbar). The kinetic properties of Rubisco were used to predict the rate of Pn under each condition either with (●) or without (□) adjustment for the activation state of Rubisco. The line indicates a 1:1 relationship between the predicted and measured rates.
Discussion
In cotton and tobacco leaves, Rubisco activation decreased at high temperature and Ci and low O2, i.e., under conditions in which measured Pn deviated from predicted Pn. This effect of temperature on Rubisco activation has been reported previously (10, 11, 16–18, 28) and includes evidence from gas exchange measurements, which showed that the maximum carboxylation rate, calculated from gas exchange parameters, decreased at high temperature (35). The effects of high CO2 and low O2 on Rubisco activation also have been reported previously (12–14), but an effect of CO2 is not always observed (15), possibly because of differences in experimental technique. In the present study, the activity of Rubisco was preserved in crude extracts for at least 15 min, longer than the time required to achieve maximum carbamylation for measurement of total Rubisco activity. Also, extraction of frozen tissue required no more than 20 s, thus ensuring that initial activities were always started within 30 s of extraction.
The activation state of Rubisco in leaves represents the equilibrium between the rate of deactivation and the rate of activase-promoted activation. Deactivation can involve the spontaneous loss of the carbamate and formation of the Rubisco–RuBP complex or formation of an inactive quaternary complex consisting of carbamylated Rubisco containing bound metal and a tightly bound sugar phosphate, the latter either formed at the active site or produced naturally by metabolism (22, 25). Likewise, activation of Rubisco can involve either dissociation of RuBP from decarbamylated sites and spontaneous carbamylation of the sites by CO2 or release of a tightly bound sugar phosphate from sites that are already carbamylated (22, 24, 25). Regardless of the carbamylation status, a decrease in activation state in response to environmental conditions indicates that the equilibrium has shifted toward deactivation and less of the sites are catalytically competent.
Deactivation of Rubisco under high CO2 and at high temperature could be related to a faster rate of catalytic turnover, which could accelerate the rate of Rubisco deactivation. However, when measured in vitro in the absence of activase, the rate of Rubisco deactivation increased with increasing temperature (Fig. 3; see also ref. 36) but not at a higher CO2 concentration (Table 2). Similarly, increasing the CO2 concentration from 370 to 3,700 μbar had little effect on the rate of Rubisco activation by activase in vitro (Fig. 5). Thus, different mechanisms apparently were responsible for the decrease in Rubisco activation that occurred in leaves in response to increases in temperature and CO2.
One possible explanation for the decrease in Rubisco activation that occurred in leaves under moderate heat stress is that the rate at which activase promoted Rubisco activation was not sufficient to offset a faster rate of Rubisco deactivation at higher temperatures. This possibility was confirmed experimentally by showing that the ability of activase to promote activation of Rubisco or to maintain Rubisco in an active state in vitro decreased above about 30°C. Because the ATPase activity of activase increased with temperature up to 42°C, the loss of ability to promote Rubisco activation as temperature increased from 25°C and 42°C was probably not caused by inhibition of activase activity per se. Rather, the faster rate of Rubisco deactivation at higher temperatures appeared to simply outpace the rate at which activase could promote Rubisco activation. In support of this idea was the observation that increasing the concentration of activase produced a higher level of Rubisco activation at a given temperature (Fig. 3).
Previous studies have documented the relatively low temperature optimum of activase and its marked lability at even moderately high temperatures (27, 37). In the present study, the ATPase activity of activase was inhibited at temperatures greater than about 42°C. Because Rubisco activation is dependent in an as yet undefined way on the rate of ATP hydrolysis (26, 27, 29, 31), the rate at which activase promotes Rubisco activation would be expected to decrease at temperatures that exceeded the temperature optimum for ATPase activity. A decrease in activase activity coupled with a much faster rate of Rubisco deactivation could explain why activase was ineffective at promoting or maintaining Rubisco activation above the spontaneous rate at temperatures above 47°C.
The above explanation does not preclude the possibility that high temperature perturbs subunit associations between Rubisco and activase, thereby slowing the rate at which activase promotes the conformation changes necessary to loosen Rubisco binding for sugar phosphates. In fact, intrinsic fluorescence measurements indicated that the decrease in ATPase activity that occurred at moderately high temperatures was associated with a change in the quaternary structure of activase from the more active associated state to the less active dissociation state (37). Also, the distribution of activase changed from soluble to insoluble in cotton leaves that were heated above 40°C, suggesting that the ability of activase to self-associate in planta was altered at temperatures below the temperature optimum for ATPase activity (17). Because ATP hydrolysis is required for but is not tightly coupled to Rubisco activation (27, 31), changes in the quaternary structure of activase could affect its ability to physically interact with Rubisco somewhat independent of the effect on ATP hydrolysis. Thus, the rate at which activase associates with Rubisco, the stability of the activase–Rubisco complex, and the rate at which activase changes the conformation of Rubisco could all be affected if the structure of activase changes with temperature. A direct effect of high temperature on one or more of these processes or an indirect effect caused by changes in the structure of activase could reduce the overall rate of Rubisco activation by activase below the level required to offset the faster rate of deactivation.
The activation state of Rubisco decreased in response to both high temperature and high CO2 in leaves, but only in response to high temperature in vitro. The discrepancy suggests that a factor that reduces activase activity in leaves under high CO2 was eliminated in the in vitro assay. A likely possibility is ADP, which, as a ratio with ATP, determines the activity of activase (27). Increasing the Ci of a leaf decreases the ratio of ATP/ADP in chloroplasts by increasing the rate of ATP consumption (38). In contrast, increasing the temperature of a leaf apparently stimulates ATP synthesis sufficiently to offset an increased rate of consumption (10), possibly by redirecting electron flow through cyclic photophosphorylation (39). These observations, together with the results of the activation assay, suggest that two different mechanisms, both of which are mediated by activase, underlie the decrease in Rubisco activation that occurs in response to high CO2 and moderate heat stress. Under conditions of moderate heat stress, the ATP/ADP ratio is probably adequate for maximal activase activity, but Rubisco activation cannot be maintained because activase activity is insufficient by itself or because of physical impairment to offset the faster rate of Rubisco deactivation. Under conditions of high CO2, and possibly low O2, the rate of Rubisco deactivation is unchanged, but Rubisco deactivates because activase activity is reduced by a lower ATP/ADP ratio.
When corrected for changes in activation state, the response of Pn predicted solely from the kinetics of Rubisco was very similar to the measured response (Fig. 6). Thus, changes in the activation state of Rubisco accounted for the changes in the rate of Pn in response to temperature, CO2, and O2. Because the activation state of Rubisco in vivo is determined by activase, conditions that affect activase activity, either directly or indirectly, influence Pn by altering the activation state of Rubisco. With regard to temperature, activase activity per se appears to limit the photosynthetic potential of leaves at elevated temperatures, even in the presence of high CO2. This involvement of activation state in limiting or colimiting photosynthesis should be considered in predicting photosynthetic performance in response to global climate changes or when devising strategies to optimize plant productivity in controlled environments.
Abbreviations
- Ci
internal CO2 concentration
- Rubisco
ribulose 1,5-bisphosphate carboxylase/oxygenase
- RuBP
ribulose-1,5-bisphosphate
- Pn
net photosynthesis
Footnotes
See commentary on page 12937.
Article published online before print: Proc. Natl. Acad. Sci. USA, 10.1073/pnas.230451497.
Article and publication date are at www.pnas.org/cgi/doi/10.1073/pnas.230451497
References
- 1.Rosenweig C, Hillel D. Climate Change and the Global Harvest: Potential Impacts of the Greenhouse Effect on Agriculture. Oxford: Oxford Univ. Press; 1998. [Google Scholar]
- 2.Berry J A, Björkman O. Annu Rev Plant Physiol. 1980;31:491–543. [Google Scholar]
- 3.Ku S-B, Edwards G E. Plant Physiol. 1977;59:986–990. doi: 10.1104/pp.59.5.986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Monson R K, Stidham M A, Williams G J, III, Edwards G E. Plant Physiol. 1982;69:921–928. doi: 10.1104/pp.69.4.921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jordan D B, Ogren W L. Planta. 1984;161:308–313. doi: 10.1007/BF00398720. [DOI] [PubMed] [Google Scholar]
- 6.Sage R F, Sharkey T D. Plant Physiol. 1987;84:658–664. doi: 10.1104/pp.84.3.658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Farquhar G D, von Caemmerer S, Berry J A. Planta. 1980;149:178–190. doi: 10.1007/BF00386231. [DOI] [PubMed] [Google Scholar]
- 8.Sharkey T D. Bot Rev. 1985;51:53–105. [Google Scholar]
- 9.Perchorowicz J T, Raynes D A, Jensen R G. Proc Natl Acad Sci USA. 1981;78:2985–2989. doi: 10.1073/pnas.78.5.2985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Weis E. Planta. 1981;151:33–39. doi: 10.1007/BF00384234. [DOI] [PubMed] [Google Scholar]
- 11.Weis E. FEBS Lett. 1981;129:197–200. [Google Scholar]
- 12.Sharkey T D, Seeman J R, Berry J A. Plant Physiol. 1986;81:788–791. doi: 10.1104/pp.81.3.788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sage R F, Sharkey T D, Seeman J R. Planta. 1988;174:407–416. doi: 10.1007/BF00959528. [DOI] [PubMed] [Google Scholar]
- 14.Sage R F, Sharkey T D, Seeman J R. Plant Physiol. 1989;89:590–596. doi: 10.1104/pp.89.2.590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Makino A, Mae T. Plant Cell Physiol. 1999;40:999–1006. [Google Scholar]
- 16.Kobza J, Edwards G E. Plant Physiol. 1987;83:69–74. doi: 10.1104/pp.83.1.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Feller U, Crafts-Brandner S J, Salvucci M E. Plant Physiol. 1998;116:539–546. doi: 10.1104/pp.116.2.539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Law R D, Crafts-Brandner S J. Plant Physiol. 1999;120:173–181. doi: 10.1104/pp.120.1.173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Woodrow I E, Kelly M E, Mott K A. Aust J Plant Physiol. 1996;23:141–149. [Google Scholar]
- 20.Badger M R, Lorimer G H. J Biol Chem. 1979;254:5599–5601. [PubMed] [Google Scholar]
- 21.Jordan D B, Chollet R. J Biol Chem. 1983;258:13752–13758. [PubMed] [Google Scholar]
- 22.Andrews T J. Nat Struct Biol. 1996;3:3–7. doi: 10.1038/nsb0196-3. [DOI] [PubMed] [Google Scholar]
- 23.Neuwald A F, Aravind L, Spouge J L, Koonin E V. Genome Res. 1999;9:27–43. [PubMed] [Google Scholar]
- 24.Salvucci M E, Ogren W L. Photosynth Res. 1996;47:1–11. doi: 10.1007/BF00017748. [DOI] [PubMed] [Google Scholar]
- 25.Portis A R., Jr J Exp Bot. 1995;46:1285–1291. [Google Scholar]
- 26.Wang Z Y, Portis A R., Jr Plant Physiol. 1992;99:1348–1353. doi: 10.1104/pp.99.4.1348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Robinson S P, Portis A R., Jr Arch Biochem Biophys. 1989;268:93–99. doi: 10.1016/0003-9861(89)90568-7. [DOI] [PubMed] [Google Scholar]
- 28.Crafts-Brandner, S. J. & Law, R. D. (2000) Planta, in press. [DOI] [PubMed]
- 29.Salvucci M E. Arch Biochem Biophys. 1992;298:688–696. doi: 10.1016/0003-9861(92)90467-b. [DOI] [PubMed] [Google Scholar]
- 30.Salvucci M E, Klein R R. Arch Biochem Biophys. 1994;314:178–185. doi: 10.1006/abbi.1994.1427. [DOI] [PubMed] [Google Scholar]
- 31.van de Loo F J, Salvucci M E. Biochemistry. 1996;35:8143–8148. doi: 10.1021/bi9604901. [DOI] [PubMed] [Google Scholar]
- 32.Zhang N, Portis A R., Jr Proc Natl Acad Sci USA. 1999;96:9438–9443. doi: 10.1073/pnas.96.16.9438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Laing W A, Ogren W L, Hageman R H. Plant Physiol. 1974;54:678–685. doi: 10.1104/pp.54.5.678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mate C J, Hudson G S, von Caemmerer S, Evans J R, Andrews T J. Plant Physiol. 1993;102:1119–1128. doi: 10.1104/pp.102.4.1119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bunce J A. Photosynth Res. 2000;63:59–67. doi: 10.1023/A:1006325724086. [DOI] [PubMed] [Google Scholar]
- 36.Lorimer G H. J Biol Chem. 1979;254:5599–5601. [PubMed] [Google Scholar]
- 37.Crafts-Brandner S J, van de Loo F J, Salvucci M E. Plant Physiol. 1997;114:439–444. doi: 10.1104/pp.114.2.439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gardeström P, Wigge B. Plant Physiol. 1988;88:69–76. doi: 10.1104/pp.88.1.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bukhov N G, Wiese C, Neimanis S, Heber U. Photosynth Res. 1999;59:81–93. doi: 10.1007/BF00014888. [DOI] [PubMed] [Google Scholar]