Abstract
The effect of early intervention with a peroxisome proliferator-activated receptor-γ (PPARγ) agonist on skeletal muscle GLUT4 translocation and insulin signaling was examined in intrauterine (IUGR) and postnatal (PNGR) growth-restricted pregestational female rat offspring. Rosiglitazone [11 μmol/day provided from postnatal day (PN)21 to PN60] improved skeletal muscle insulin sensitivity and GLUT4 translocation in prenatal nutrient restriction [50% calories from embryonic day (e)11 to e21; IUGR] with (IUGR+PNGR) and without (IUGR) postnatal nutrient restriction (50% calories from PN1 to PN21; PNGR) similar to that of control (ad libitum feeds throughout; Con) (n = 6 each). This was accomplished by diminished basal and improved insulin-responsive GLUT4 association with the plasma membrane in IUGR, IUGR+PNGR, and PNGR mimicking that in Con (P < 0.005). While no change in p85-phosphatidylinositol 3-kinase (PI3-K) and phosphatase and tensin homolog deleted on chromosome 10 (PTEN) was observed, a decrease in protein tyrosine phosphatase 1B (PTP1B; P < 0.0002) and SH2-containing protein tyrosine phosphatase 2 (SHP2; P < 0.05) contributing to the rosiglitazone-induced insulin sensitivity was seen only in IUGR+PNGR. In contrast, an increase in phosphorylated 5′-adenosine monophosphate kinase (pAMPK; P < 0.04) and insulin responsiveness of phosphorylated phosphoinositide-dependent protein kinase-1 (pPDK1; P < 0.05), pAkt (P < 0.01), and particularly pPKCζ (P < 0.0001) and its corresponding enzyme activity (P < 0.005) were observed in all four experimental groups. We conclude that early introduction of PPARγ agonist improved skeletal muscle activation of AMPK and insulin signaling, resulting in insulin-independent AMPK and insulin-responsive GLUT4 association with plasma membranes in IUGR, IUGR+PNGR, and PNGR adult offspring, similar to that of Con. These findings support a role for insulin sensitizers in preventing the subsequent development of gestational or type 2 diabetes mellitus in intrauterine and postnatal growth-restricted offspring.
Keywords: fetal origins of adult disease, glucose transporter, metabolic programming, protein kinase Cζ
epidemiologic studies have linked low birth weight to the metabolic syndrome consisting of insulin resistance/type 2 diabetes mellitus, obesity, hypertension, and coronary artery disease during adult life (3, 23, 24, 41, 50, 54, 56). More recently, a slow growth rate during early childhood spanning from 0 to 2 yr superimposed on low birth weight was also associated with adult-onset type 2 diabetes mellitus and cardiovascular disease (4, 5, 14). Mimicking these human epidemiologic observations, we previously described (53) a rat model consisting of prenatal nutrient restriction that caused intrauterine growth restriction (IUGR). In this rat model, we previously demonstrated (53) aberrant skeletal muscle expression and insulin-induced translocation of the insulin-responsive glucose transporter isoform (GLUT4) in the IUGR newborn that persisted in the adult offspring. This mechanism contributed to the metabolic adaptations that mediated the subsequent dysregulation of glucose homeostasis and insulin sensitivity (19). In addition, introduction of various postnatal nutritional regimens in the IUGR were observed not to affect the skeletal muscle GLUT4 imprint acquired in utero (53). We (38, 53) and others (43) have shown that the observed insulin insensitivity of skeletal muscle GLUT4 translocation to the plasma membrane (PM) is due to aberrant PKCζ phosphorylation and enzyme activity.
PKCζ belongs to the atypical protein kinase C family of serine-threonine kinases that are calcium insensitive. PKCζ is distal to the phosphatidylinositol 3-kinase (PI3-K) and phosphorylated phosphoinositide-dependent protein kinase-1 (pPDK1) enzymatic steps of the insulin signaling pathway and mediates cellular glucose uptake by stimulating GLUT4 translocation to the PM (15, 34). This process of translocation is accomplished by PKCζ-mediated GLUT4 containing vesicular transport to the PM (17) with a diminution of phosphorylated (p)PKCζ noted in insulin-resistant states (33). Early nutritional deprivation has also decreased phosphorylation of PKCζ and its activity, thereby interfering with insulin signaling and leading to the subsequent emergence of insulin resistance in rats (38, 43) and humans (26, 42).
Thiazolidinediones (TZDs) are a class of insulin-sensitizing agents and are widely used as antidiabetic drugs (2, 44, 46). Their action is thought to be primarily on adipocytes by activation of peroxisome proliferator-activated receptor-γ (PPARγ) (48). Activation of PPARγ regulates the expression of numerous genes involved in glucose and lipid metabolism (29). Lower levels of PPARγ mRNA are also present in other tissues including the skeletal muscle (35). Skeletal muscle is the most important target tissue of insulin action, which also plays a predominant role in mediating TZD-induced improvement of glucose homeostasis (25). It has therefore been suggested that the insulin-sensitizing action of TZD may occur independent of adipose tissue (8). Furthermore, PPARγ-independent mechanisms have been proposed to mediate the effects of TZDs (7, 39, 40). TZDs have been shown to improve skeletal muscle insulin resistance of type 2 diabetic humans (37). TZDs can also enhance insulin-stimulated glucose transport by improving insulin signaling mechanisms even in the absence of changes in total glucose transporter concentrations (28, 55). TZD (rosiglitazone) administration improves insulin resistance in skeletal muscle and adipocyte of nonobese type 2 diabetic Goto-Kakizaki rats by ameliorating PKCζ enzyme activity (27, 28). However, the effect of rosiglitazone (Rosi) administration on skeletal muscle insulin signaling in intrauterine growth-restricted rat offspring has not been studied to date.
We recently showed (38) that skeletal muscle of adult female intrauterine growth-restricted offspring exhibits aberrant insulin signaling involving an imbalance in the kinase and phosphatase arms of the classical PI3-K pathway. We therefore hypothesized that early intervention by a PPARγ agonist in intrauterine growth-restricted female offspring will ameliorate aberrant skeletal muscle insulin signaling. To test this hypothesis, we employed the mid- to late-gestation and/or postnatal nutrient restriction rat model of intrauterine growth restriction (IUGR) and/or postnatal growth restriction (PNGR). This was accomplished by cross-fostering of animals, which generated four experimental groups. Thus the control mother with ad libitum access to nutrients fed the intrauterine semi-nutrient-restricted pups, which represented intrauterine nutrient restriction (IUGR), while the semi-nutrient-restricted mother fed the intrauterine growth-restricted pups, which represented intrauterine and postnatal nutrient restriction (IUGR+PNGR). These groups were compared with the control mother-fed control progeny (Con), which served as the “gold standard,” and the semi-nutrient-restricted mother-fed control pups, which represented postnatal nutrient restriction (PNGR) in the absence of IUGR. Rosiglitazone (Rosi) was administered daily to the offspring from all four experimental groups from postnatal day 21 to day 60. We then examined the effect of early chronic Rosi administration on skeletal muscle insulin signaling and GLUT4 protein under basal and insulin-stimulated states in the adult pregestational female intrauterine-growth restricted offspring.
EXPERIMENTAL PROCEDURES
Animals.
Sprague-Dawley rats (60 days old, 200–250 g; Charles River Laboratories, Hollister, CA) were housed in individual cages, exposed to 12:12-h light-dark cycles at 21–23°C, and allowed ad libitum access to standard rat chow. National Institutes of Health guidelines were followed in protocols approved by the Animal Research Committee of the University of California, Los Angeles.
Maternal semi-nutrient restriction model.
Pregnant rats received 50% of their daily food intake (11 g/day) beginning from day 11 through day 21 of gestation, which constitutes mid- to late gestation, compared with their control counterparts, which received ad libitum access to rat chow (∼22 g/day). Both groups had ad libitum access to drinking water (53).
Postnatal animal maintenance.
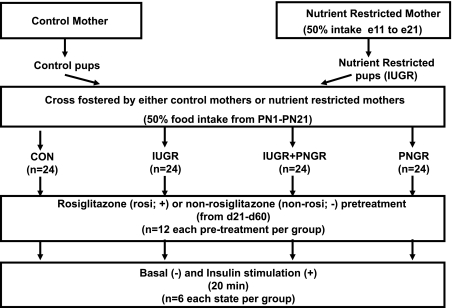
At birth, the litter size was culled to six. In addition, the newborn rats born to the semi-nutrient-restricted mothers were cross-fostered to be reared by either a mother who continued to be semi-nutrient restricted by receiving 20 g/day food intake through lactation (IUGR+PNGR) or a control mother with ad libitum access to rat chow (∼40 g/day) (IUGR). Similarly, newborn pups born to control mothers were cross-fostered to be reared by either a mother who continued to be semi-nutrient restricted through lactation (PNGR) or a control mother with ad libitum access to rat chow (Con). This food restriction scheme ensured that the semi-nutrient-restricted maternal rats received ∼50% of the ad libitum food intake through mid- to late pregnancy and lactation (1, 12, 22). Thus four groups were created, with control mothers rearing control (Con) or prenatal semi-nutrient-restricted pups (IUGR) and pre- and postnatal semi-nutrient-restricted mothers rearing prenatal semi-nutrient-restricted pups (IUGR+PNGR) or control pups (PNGR) (Fig. 1). At postnatal day 21, the pups from all four experimental groups were weaned from the mother and maintained in individual cages with ad libitum access to 0.1% Rosi mixed with standard rat chow. This ensured a daily consumption of 11 μmol Rosi/animal. For comparison, another group was weaned to receive only standard rat chow as previously described (38) without Rosi (non-Rosi).
Fig. 1.
Experimental design demonstrating the 4 experimental groups obtained by cross-fostering postnatal rat pups: Con, control mothers rearing control pups; IUGR, control mothers rearing in utero semi-nutrient-restricted pups; IUGR+PNGR, semi-nutrient-restricted mothers rearing in utero semi-nutrient-restricted pups; PNGR, semi-nutrient-restricted mothers rearing control pups. Semi-nutrient-restricted mothers received 50% of daily nutrient intake from mid- to late pregnancy [embryonic day (e)11 to e21] through lactation [postnatal day(PN)1 to PN21]. Female offspring were divided into 2 groups, non-Rosi and Rosi, based on receipt of 0.1% rosiglitazone pretreatment from PN1 to PN60. At PN60 the female offspring in each group received vehicle (basal, −) or insulin (insulin stimulated, +).
In vivo insulin administration.
At postnatal day 60 female animals pretreated with Rosi from all four experimental groups received either vehicle or insulin (8 U/kg) intraperitoneally (Fig. 1). After 20 min, the predetermined optimal time point (21, 53), the animals were deeply anesthetized with inhalational isoflurane to maintain organ blood flow, and skeletal muscle from the hindlimb was harvested.
Skeletal muscle preparation.
Skeletal muscle was rapidly separated from surrounding tissues, quickly snap frozen in liquid nitrogen, and stored at −70°C. Homogenates were prepared as previously described (53). Briefly, skeletal muscle was powdered under liquid nitrogen and suspended in three volumes of cell lysis buffer (Cell Signaling Technology, Beverly, MA). The samples were homogenized with a hand-held homogenizer for 1–2 min at half speed followed by 30-min incubation on ice. The samples were then subjected to further homogenization by 20 up- and downstrokes with a tight-fitting Potter-Elvenjhem homogenizer. These homogenates were then centrifuged at 10,000 rpm and 4°C for 10 min and stored at −70°C until Western blot analysis was undertaken.
Antibodies.
Rabbit polyclonal anti-Akt, anti-phosphorylated (p)Akt (Ser473), anti-PDK1, anti-pPDK1 (Ser241), anti-SH2-containing protein tyrosine phosphatase 2 (SHP2), anti-pPKCζ (Thr410/403), and anti-phosphorylated 5′-adenosine monophosphate kinase (pAMPK) collectively consisting of α1- and α2-AMPK were from Cell Signaling Technology (Danvers, MA). Anti-PI3-K p85 (p85PAN) antibodies were purchased from Upstate Biotechnology (Lake placid, NY). Rabbit polyclonal anti-phosphatase and tensin homolog deleted on chromosome 10 (PTEN) antibody was from Santa Cruz Biotechnology (Santa Cruz, CA). Anti-protein tyrosine phosphatase 1B (PTP1B) antibody was purchased from BD Transduction Laboratories (Lexington, KY). Anti-vinculin antibody was from Sigma (St. Louis, MO). Rabbit polyclonal anti-Akt2 antibody was a generous gift from Dr. M. J. Birnbaum (University of Pennsylvania School of Medicine).
Western blot analysis.
The homogenates were sonicated (60 sonic; Dismembrator, Fisher Scientific, Pittsburgh, PA) with two 50-s cycles of 5–7 W. The resulting suspension was centrifuged at 10,000 g and 4°C for 10 min, and the supernatant was subjected to Western blot analysis as previously described (53). Briefly, 50 μg of skeletal muscle homogenates was separated on SDS-PAGE and electroblotted onto nitrocellulose membranes. Membranes were then blocked in 5% nonfat dry milk in phosphate-buffered saline containing 0.1% Tween 20 (PBST) for 1 h, incubated with the specific primary antibody [1:1,000 dilution for p85 subunit of PI3-K, Akt, pAkt, PDK1, pPDK1, and SHP2, 1:1,500 for AMPK (over 1 h at room temperature), 1:500 for pAMPK, 1:100 for PKCζ, 1:200 for PTEN, 1:2,500 for PTP1B, 1:500 for pPKCζ (Thr410), and 1:4,000 for vinculin] overnight at 4°C with gentle agitation. The membranes were then washed with PBST three times for 15 min each. The membranes were then incubated with the appropriate secondary horseradish peroxidase-conjugated antibody for 1 h at room temperature. After the membranes were washed three times for 15 min each, protein bands were visualized with the enhanced chemiluminescence method (Amersham Biosciences, Piscataway, NJ). The quantification of protein bands was performed by densitometry with Scion Image software. The presence of linearity between the time of X-ray film exposure and the optical density of the various protein bands was initially ensured (53).
PKCζ activity assay.
PKCζ activity assay was performed as previously described (31, 36), with slight modifications (38). One milligram of skeletal muscle homogenate was precleared by incubation with 50 μl of a 50% slurry of protein A/G agarose (Santa Cruz Biotechnology) for 2 h at 4°C. Five micrograms of anti-PKCζ rabbit polyclonal antiserum raised against a peptide in the T loop of PKCζ was incubated with 50 μl of 50% slurry of protein A/G agarose for 2 h at 4°C on the rotor. The antibody-bound beads were washed three times with the cell lysis buffer and incubated with the precleared homogenate overnight at 4°C on the rotor. The precipitated agarose beads were then washed four times with the cell lysis buffer, followed by two washes with the kinase buffer, and resuspended in kinase buffer without myelin basic protein (MBP, substrate), and ATP in vitro kinase assay was carried out for 30 min at 25°C in 50 μl of buffer containing 35 mM Tris, pH 7.4, 10 mM MgCl2, 1 mM EGTA, 2 mM Na3VO4, 20 μg/ml leupeptin, 0.5 mM ATP, 4 μg MBP, and 0.4 μCi of [γ-32P]ATP. After incubation, 32P-labeled substrate was trapped on P81 filter papers. The P81 filter papers were then washed four times with 0.75% phosphoric acid and once with acetone and counted in a liquid scintillation counter. Samples that contained no antibody or no substrate were used as controls to account for background and endogenous phosphorylation, respectively.
Data analysis.
Data are expressed as means ± SD. The analysis of variance models were used to compare the various treatment groups and the F values obtained. Intergroup differences were determined by Fisher's protected least significant difference (PLSD) test when ANOVA revealed significance. Significance was assigned when the P value was ≤0.05.
RESULTS
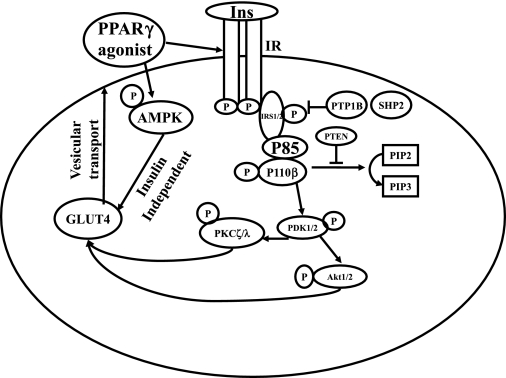
The insulin signaling pathway as it pertains to GLUT4 translocation is schematically represented in Fig. 2. Total protein concentrations of specific insulin signaling molecules were compared between non-Rosi- and Rosi-treated experimental groups. In contrast, basal and insulin-stimulated activation of these insulin signaling molecules are presented from the Rosi-pretreated experimental groups alone, since the non-Rosi-treated results from the same four experimental groups were similar to previous reports (38, 53). These previous non-Rosi-treated results are summarized in Table 1. The distinction between the present Rosi-treated versus previously described non-Rosi-treated four experimental groups is provided.
Fig. 2.
Schematic representation of the postreceptor insulin signaling pathway and insulin-independent 5′-adenosine monophosphate kinase (AMPK) beginning from insulin binding to its receptor to GLUT4 translocation via vesicular trafficking. Ins, insulin; IR, insulin receptor; IRS, insulin receptor substrate; P, phosphorylation; PPARγ, peroxisome proliferator-activated receptor-γ; PTP1B, protein tyrosine phosphatase 1B; SHP2, SH2-containing protein tyrosine phosphatase 2; PTEN, phosphatase and tensin homolog deleted on chromosome 10; PIP2, phosphatidylinositol 4,5-bisphosphate; PIP3, phosphatidylinositol 1,4,5-trisphosphate; PDK1, phosphoinositide-dependent protein kinase-1.
Table 1.
Summary of non-Rosi pretreatment of the four experimental groups
| Signaling Molecules | Con | IUGR | IUGR+PNGR | PNGR |
|---|---|---|---|---|
| PM GLUT4 | ||||
| Basal vs. Con | ↔ | ↑ | ↑ | Slight↑ |
| Insulin vs. basal | ↑ | ↔ | ↔ | Slight↑ |
| Insulin signaling, kinase arm | ||||
| pPKCζ, basal vs. Con | ↔ | ↑ | ↑ | ↔ |
| pPKCζ, insulin vs. basal | ↑ | ↔ | ↔ | ↔ |
| pAkt, basal vs. Con | ↔ | ↑ | ↑ | ↔ |
| pAkt, insulin vs. basal | ↑ | ↑ | ↑ | ↑ |
| pPDK1, basal vs. Con | ↔ | ↑ | ↑ | ↔ |
| pPDK1, insulin vs. basal | ↑ | ↑ | ↑ | ↑ |
| p85-PI3-K, basal vs. Con | ↔ | ↔ | ↔ | ↔ |
| Insulin signaling, phosphatase arm | ||||
| PTP1B, basal vs. Con | ↔ | ↑ | ↔ | ↔ |
| SHP2, basal vs. Con | ↔ | ↑ | ↔ | ↔ |
| PTEN, basal vs. Con | ↔ | ↔ | ↔ | ↔ |
| Non-insulin-dependent signaling | ||||
| pAMPK/AMPK, basal vs. Con | ↔ | ↔ | ↔ | ↔ |
Rosi, rosiglitazone; IUGR, intrauterine growth restricted; PNGR, postnatal growth restricted; Con, control; PM GLUT4, plasma membrane-associated GLUT4; PDK1, phosphoinositide-dependent protein kinase-1; PI3-K, phosphatidylinositol 3-kinase; PTP1B, protein tyrosine phosphatase 1B; SHP2, SH2-containing protein tyrosine phosphatase 2; PTEN, phosphatase and tensin homolog deleted on chromosome 10 (a tumor suppressor protein); AMPK, 5′-adenosine monophosphate kinase; p, phosphorylated; ↑, increase, ↔, no change vs. Con/basal state of the same experimental group. Con is the gold standard (38, 53).
GLUT4.
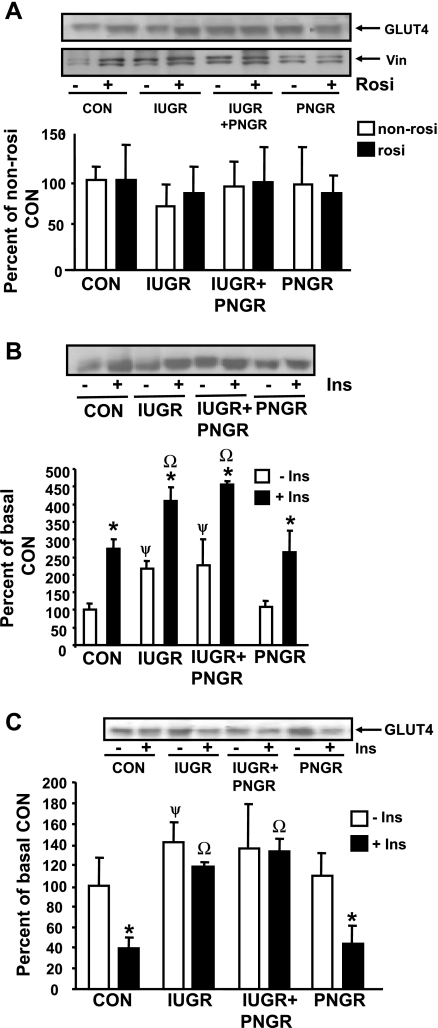
Figure 3A demonstrates total concentrations of GLUT4 in the four non-Rosi- and Rosi-pretreated experimental groups with no major differences observed. Figure 3B demonstrates the effect of Rosi on basal and insulin-stimulated PM-associated GLUT4 concentrations. The IUGR and IUGR+PNGR groups demonstrate a higher PM association of GLUT4 in the basal state compared with both Con and PNGR. However, all four experimental groups demonstrate an insulin sensitivity of GLUT4, with a similar response consisting of an increase in the PM-associated GLUT4 concentrations following insulin stimulation over the corresponding basal concentrations. Figure 3C demonstrates the reciprocal effect on the intracellular low-density microsomal (LDM) GLUT4 concentrations, with an increase in the basal concentrations in IUGR (P < 0.04) alone versus Con. While an insulin-sensitive decline in LDM-associated GLUT4 was noted in Con and PNGR (P < 0.005), a similar decline was not evident in IUGR and IUGR+PNGR. These observations gain significance compared with results in the non-Rosi group previously reported (53), where the IUGR and IUGR+PNGR demonstrated higher PM-associated GLUT4 versus Con and PNGR, with no further response following insulin stimulation in these two groups (53) (Table 1). Thus, unlike our present observations with Rosi pretreatment, the non-Rosi IUGR and IUGR+PNGR were insulin resistant and distinctly different from corresponding Con and PNGR (53) as summarized in Table 1.
Fig. 3.
Total, plasma membrane (PM), and low-density microsomal (LDM) GLUT4 protein concentrations. Top: representative Western blots of total GLUT4 with vinculin (Vin; internal control) in non-Rosi (−)- and Rosi (+)-pretreated groups (n = 6 per pretreatment and per experimental group) (A) and PM GLUT4 (B) and LDM GLUT4 (C) protein concentrations in the Rosi-pretreated groups under basal (−Ins) and insulin-stimulated (+Ins) states (n = 3 per state in each experimental group). Bottom: densitometric quantification of the protein band of interest shown as a ratio to vinculin (A) and as % of the corresponding either non-Rosi (A) or basal (B, C) Con; depicted as means ± SD. B: *P < 0.0005 vs. corresponding basal state (−Ins), ψP < 0.0025 vs. Con basal state (−Ins); ΩP < 0.0025 vs. Con insulin-stimulated (+Ins) state. C: *P < 0.005 vs. corresponding basal (−Ins) state; ψP < 0.04 vs. Con basal (−Ins) state; ΩP < 0.0005 vs. Con insulin-stimulated (+Ins) state.
To determine the mechanism by which Rosi altered the basal and insulin-stimulated PM association of GLUT4 toward that seen in Con without affecting total GLUT4 concentrations, the insulin signaling pathway was initially examined. The distal molecule (PKCζ) that was previously reported to demonstrate aberrant activity in the non-Rosi-pretreated state (38) (Table 1) to the most proximal step (p85 of PI3-K) that followed the insulin receptor and insulin receptor substrate activation were next investigated.
PKCζ.
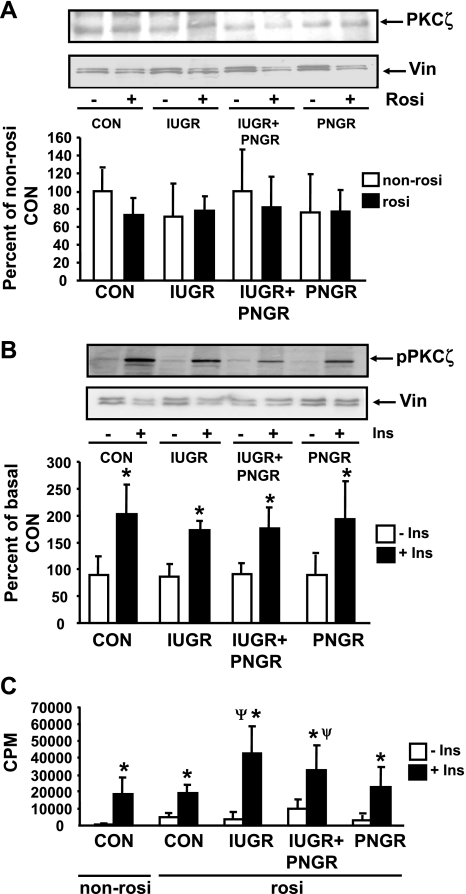
Figure 4A shows no change in total protein concentrations of PKCζ between the four non-Rosi- and Rosi-treated experimental groups. Figure 4B shows phosphorylation of PKCζ (pPKCζ) in basal and insulin-stimulated states of the four Rosi-treated experimental groups. In response to insulin stimulation, IUGR (P = 0.0007), IUGR+PNGR (P = 0.0009), and PNGR (P < 0.0001) groups demonstrate significantly higher pPKCζ concentrations compared with that of the corresponding basal state. This extent of insulin-stimulated phosphorylation of PKCζ in the three Rosi-pretreated experimental groups was comparable to that seen in the Rosi-treated Con group (P < 0.0001). This observation is different from the previously reported higher basal and insulin resistance of pPKCζ concentrations in the non-Rosi-treated IUGR and IUGR+PNGR groups, which was distinctly different from the insulin sensitivity of the non-Rosi-treated Con group (38) (Table 1). Figure 4C shows the kinase activity of PKCζ in the basal and insulin-stimulated states of the four Rosi-pretreated experimental groups. Paralleling the basal pPKCζ concentrations, all three nutrient-restricted groups show minimal basal kinase activity. After insulin stimulation, the Con (P < 0.0001), IUGR (P < 0.0001), IUGR+PNGR (P < 0.0001), and PNGR (P < 0.005) groups showed a significant increase in kinase activity of PKCζ compared with the corresponding basal kinase activity after insulin stimulation. The Rosi-pretreated IUGR (P < 0.0001) and IUGR+PNGR (P = 0.008) groups showed significantly higher insulin-stimulated PKCζ kinase activity compared with the Con group. These results are distinct from previously reported higher basal and insulin resistance of the kinase activity of PKCζ in the non-Rosi-pretreated IUGR and IUGR+PNGR groups versus that of the non-Rosi-treated Con group (31, 38) (Table 1).
Fig. 4.
Skeletal muscle total and phosphorylated (p) PKCζ concentrations and enzyme activity. Top: representative Western blots demonstrating total PKCζ and vinculin (internal control) in non-Rosi (−) and Rosi (+) pretreatments (A) and pPKCζ and vinculin (internal control) in the basal (−Ins) and insulin-stimulated (+Ins) states of the 4 Rosi-pretreated experimental groups (B). Bottom: densitometric quantification of the protein bands of interest/vinculin shown as % of either the non-Rosi-pretreated Con (A) or basal state of Con (B); presented as means ± SD (n = 6 per Rosi or non-Rosi treatments, per basal or insulin-stimulated states per experimental group in A and B). C: PKCζ activity in basal (−Ins) and insulin-stimulated (+Ins) states of non-Rosi and Rosi pretreatments is shown as cpm/min (means ± SD; n = 6 per basal or insulin-stimulated state and per experimental group). *P < 0.005 vs. corresponding basal state (−Ins) within the same experimental group; ψP < 0.02 vs. non-Rosi- and Rosi-pretreated Con insulin-stimulated state (+Ins).
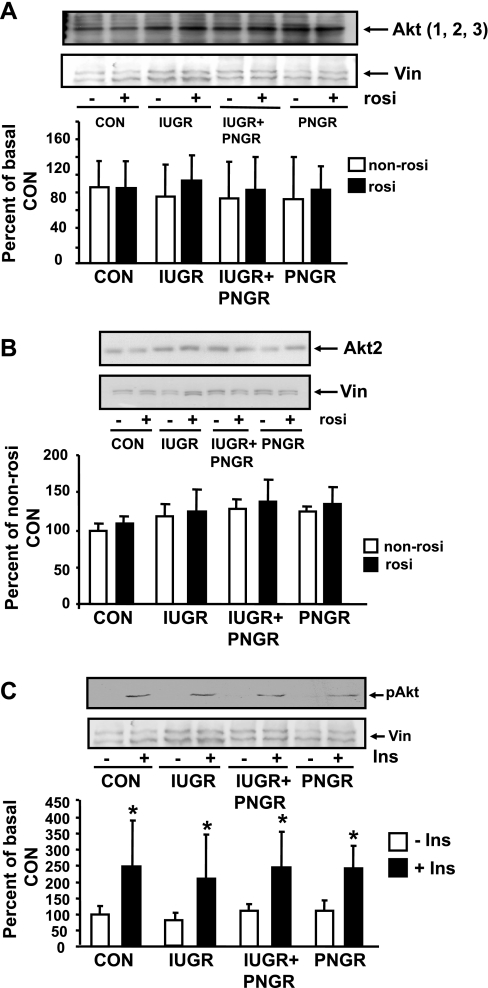
Akt.
Figure 5A demonstrates total Akt1, -2, and -3 proteins in the four non-Rosi- and Rosi-pretreated groups, which are no different from each other. Figure 5B depicts Akt2 alone and demonstrates no differences between Rosi and non-Rosi groups in all four experimental groups. Figure 5C demonstrates the phosphorylation status of Akt (pAktSer473) at basal and insulin-stimulated states of the Rosi-pretreated experimental groups. Minimal basal pAkt was noted in all four experimental groups. Insulin stimulation led to a similar increase from that of the corresponding basal pAkt concentrations in all four groups, namely Con (P = 0.005), IUGR (P = 0.01), IUGR+PNGR (P = 0.01), and PNGR (P = 0.01). Again these observations were different from the previously reported non-Rosi-pretreated groups, where the basal pAkt concentrations were higher in IUGR and IUGR+PNGR versus the corresponding Con group, although insulin sensitivity was maintained in all these non-Rosi-treated experimental groups (38) (Table 1).
Fig. 5.
Total (Akt1, 2, 3), Akt2, and phosphorylated (p)Akt. Top: representative Western blots demonstrating total Akt1, 2, and 3 and vinculin (internal control) in the non-Rosi (−)- and Rosi (+)-pretreated 4 experimental groups (n = 6 per treatment and per group) (A), Akt2 and vinculin (internal control) in the non-Rosi (−)- and Rosi (+)-pretreated 4 experimental groups (n = 3 per treatment and per group) (B), and pAkt and vinculin (internal control) in the basal (−) and insulin-stimulated (+)states of the Rosi-pretreated 4 experimental groups (n = 6 per state and per group) (C). Bottom: corresponding densitometric analyses of the protein bands of interest/vinculin shown as % of the non-Rosi-pretreated Con; presented as means ± SD. *P < 0.005 vs. corresponding basal (−Ins) state within the same experimental group.
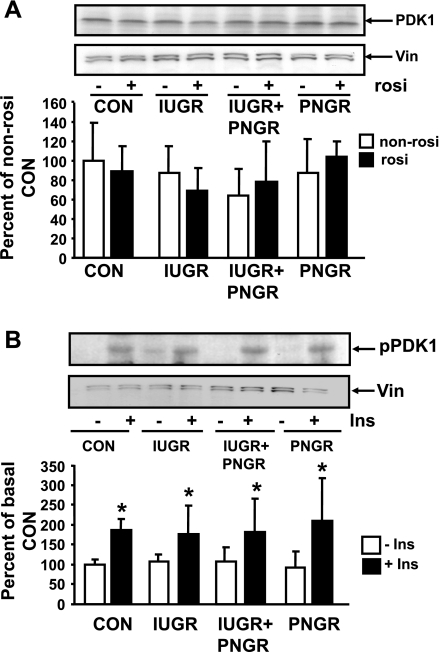
PDK1.
Figure 6A demonstrates total PDK1 protein concentrations in the non-Rosi- and Rosi-pretreated groups to be no different in the IUGR, IUGR+PNGR, and PNGR groups from the corresponding Con group. Figure 6B demonstrates the phosphorylation status of PDK1 (pPDK1) in basal and insulin-stimulated states of the Rosi-pretreated groups. No difference in the basal pPDK1 concentrations was evident between the IUGR, IUGR+PNGR, PNGR, and Con groups. All four experimental groups, namely, Con (P = 0.01), IUGR (P = 0.05), IUGR+PNGR (P = 0.03), and PNGR (P = 0.01), respond to insulin stimulation by a further increase in pPDK1 concentrations. Again these results are dissimilar to previously reported increase in basal pPDK1 in non-Rosi-pretreated IUGR and IUGR+PNGR groups versus the corresponding Con group, although insulin sensitivity was retained (38) (Table 1).
Fig. 6.
Total and phosphorylated PDK1. Top: representative Western blots demonstrating total PDK1 and vinculin (internal control) (A) and pPDK1 and vinculin (internal control) (B). Bottom: corresponding densitometric quantification of the protein bands of interest/vinculin shown as % of non-Rosi pretreatment or basal state of Con; presented as means ± SD (n = 6 per group and per pretreatment/state). *P < 0.05 vs. corresponding basal state (−Ins) within the same experimental group.
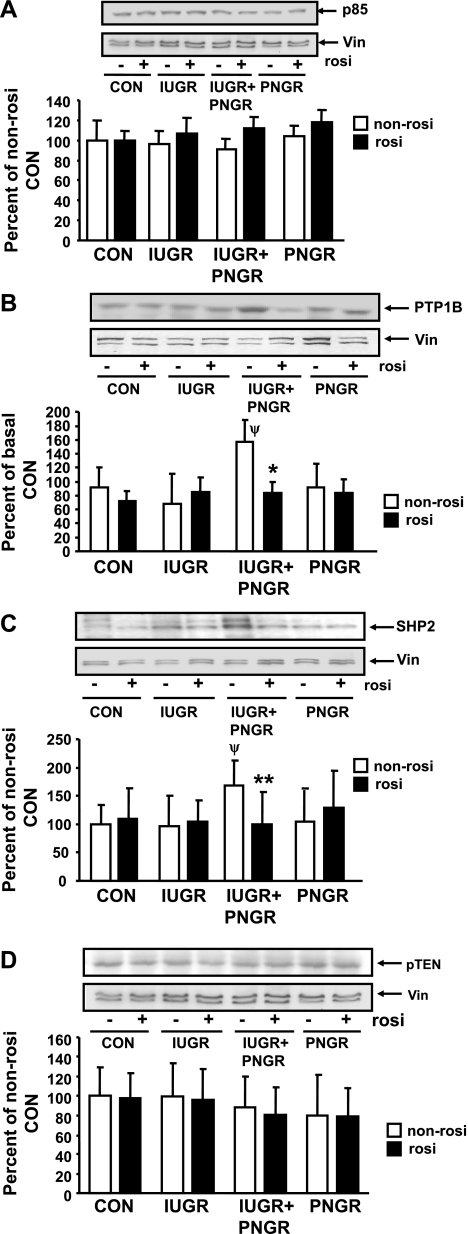
p85 component of PI3-K and PTP1B, SHP2, and PTEN.
Figure 7 demonstrates p85 subunit of PI3-K and phosphatases, namely, PTP1B, SHP2, and PTEN in the four non-Rosi- and Rosi-pretreated experimental groups. No intergroup differences were observed in the insulin-responsive p85 subunit of the PI3-K (Fig. 7A). Hence, some of the key phosphatases that counter the action of PI3-K were next examined. Only the IUGR+PNGR group demonstrates increased non-Rosi-treated baseline PTP1B (P < 0.0002, Fig. 7B) and SHP2 (P < 0.05, Fig. 7C) concentrations compared with the non-Rosi-treated Con, IUGR, and PNGR groups (Table 1). However, Rosi pretreatment led to reversal of this increased expression of PTP1B (P < 0.0001 vs. corresponding non-Rosi group; Fig. 7B) and SHP2 (P = 0.03 vs. corresponding non-Rosi group; Fig. 7C) in the IUGR+PNGR group comparable to the values of the Con group. PTP1B and SHP2 protein expression in the IUGR and PNGR groups are no different from those of the Con group with or without Rosi pretreatment (Fig. 7, B and C). PTEN concentrations were unaltered by Rosi treatment in the four experimental groups (Fig. 7D).
Fig. 7.
p85 subunit of phosphatidylinositol 3-kinase (PI3-K) and total PTP1B, SHP2, and PTEN concentrations. Top: representative Western blots demonstrating p85 subunit of the PI-3-K enzyme (A), PTP1B (B), SHP2 (C), PTEN (D), and vinculin (internal control) in non-Rosi and Rosi groups. Bottom: densitometric quantification of the protein bands of interest/vinculin shown as % of non-Rosi pretreatment of Con; presented as means ± SD (n = 6 per pretreatment and per experimental group). PTP1B: *P < 0.05 vs. corresponding non-Rosi pretreatment within the same experimental group; ψP < 0.05 vs. non-Rosi pretreated Con group. SHP2: **P < 0.03 vs. non-Rosi treatment within the same experimental group; ψP < 0.03 vs. non-Rosi-treated Con group.
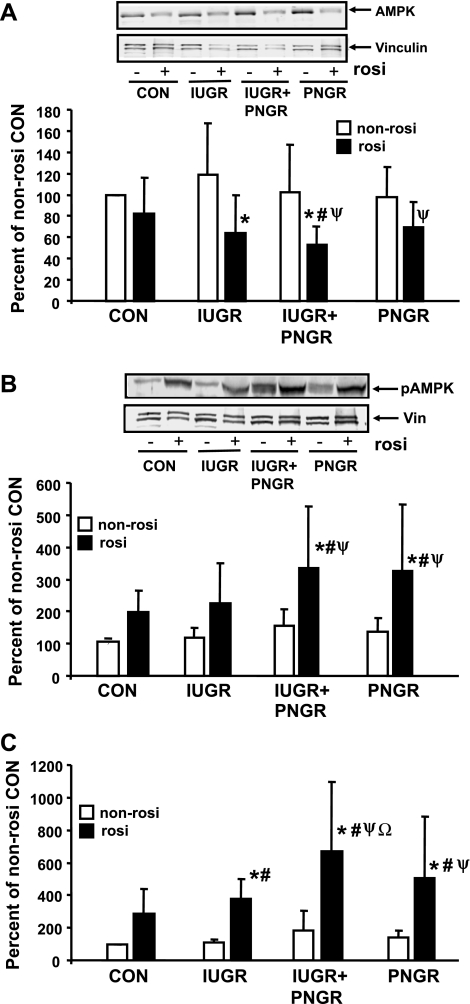
Total and phosphorylated AMPK.
Beyond the insulin signaling pathway, one of the insulin-independent mechanisms that mediates skeletal muscle GLUT4 translocation to the PM consists of AMPK activation (30). Rosi is known to activate AMPK (32); hence we next explored AMPK and pAMPK. Figure 8 demonstrates total AMPK (Fig. 8A) and pAMPK (Fig. 8B) concentrations in all four Rosi and non-Rosi pretreatment groups. While no inter-experimental group differences were evident with non-Rosi treatment, Rosi pretreatment reduced total AMPK mainly in the IUGR (P = 0.0059) and the IUGR+PNGR (P = 0.013) groups compared with their corresponding non-Rosi treatment groups. While the Rosi treatment of PNGR led to a decline (P = 0.012) from the non-Rosi treatment of IUGR, Rosi pretreatment of IUGR+PNGR reduced total AMPK from that of non-Rosi treatment of Con (P = 0.017), IUGR (P = 0.0012), and PNGR (P = 0.023) (Fig. 8A) groups. In contrast to total AMPK, Rosi pretreatment increased pAMPK, particularly in the IUGR+PNGR (P = 0.0087) and PNGR (P = 0.0067) groups compared with the corresponding non-Rosi pretreatment within the same experimental groups. This increase in these two groups was evident even on comparison to the non-Rosi pretreatment of Con (Rosi IUGR+PNGR vs. non-Rosi Con: P = 0.0009; Rosi PNGR vs. non-Rosi Con: P = 0.0015) and IUGR (Rosi IUGR+PNGR vs. non-Rosi IUGR: P = 0.002; Rosi PNGR vs. non-Rosi IUGR: P = 0.0034) experimental groups (Fig. 8A). Further Rosi pretreatment of the IUGR+PNGR group increased pAMPK over that increased by Rosi pretreatment of the Con group (P = 0.04). Since a change in opposite directions was seen in the case of total and phosphorylated AMPK by Rosi pretreatment, a ratio between the phosphorylated (active) form and total AMPK was calculated. A significant increase in the active form of AMPK was noted in the Rosi pretreatment of IUGR (P = 0.037), IUGR+PNGR (P = 0.0004), and PNGR (P = 0.0061) versus the respective non-Rosi treatment within the same experimental groups. In particular, Rosi treatment of IUGR+PNGR activated AMPK by a higher magnitude than seen in Con (P = 0.0039) and IUGR (P = 0.025).
Fig. 8.
Total and phosphorylated AMPK. Top: representative Western blots demonstrating total AMPK (A) and pAMPK (B) along with vinculin (internal control) in non-Rosi and Rosi groups. Bottom: corresponding densitometric quantification of the protein bands of interest/vinculin shown as % of non-Rosi pretreatment of Con; presented as means ± SD (n = 6 per pretreatment and per experimental group). *P < 0.01 (AMPK) or <0.008 (pAMPK) vs. non-Rosi pretreatment within the same experimental group; #P < 0.02 (AMPK) or <0.001 (pAMPK) vs. non-Rosi pretreatment of Con (AMPK and pAMPK) and PNGR (AMPK); ψP < 0.01 (AMPK) or <0.03 (pAMPK) vs. non-Rosi pretreatment of IUGR. C: ratio between pAMPK and total AMPK/vinculin each shown as % of non-Rosi pretreatment of Con and presented as means ± SD (n = 6 per pretreatment and per experimental group). *P < 0.04 vs. corresponding non-Rosi pretreatment of the same experimental group; #P < 0.04 vs. non-Rosi pretreatment of Cοn; ψP < 0.002 vs. non-Rosi pretreatment of IUGR and PNGR; ΩP < 0.02 vs. Rosi pretreatment of Con and IUGR.
All our present observations in response to Rosi pretreatment of the four experimental groups have also been summarized in Table 2.
Table 2.
Summary of Rosi treatment of the four experimental groups
| Signaling Molecules | Con | IUGR | IUGR+PNGR | PNGR |
|---|---|---|---|---|
| PM GLUT4 | ||||
| Basal vs. Con | ↔ | ↑ | ↑ | ↔ |
| Insulin vs. basal | ↑ | ⇈ | ⇈ | Slight↑ |
| Insulin signaling, kinase arm | ||||
| pPKCζ, basal vs. Con | ↔ | ↔ | ↔ | ↔ |
| pPKCζ, insulin vs. basal | ↑ | ↑ | ↑ | ↑ |
| pAkt, basal vs. Con | ↔ | ↔ | ↔ | ↔ |
| pAkt, insulin vs. basal | ↑ | ↑ | ↑ | ↑ |
| pPDK1, basal vs. Con | ↔ | ↔ | ↔ | ↔ |
| pPDK1, insulin vs. basal | ↑ | ↑ | ↑ | ↑ |
| p85-PI3-K, basal vs. Con | ↔ | ↔ | ↔ | ↔ |
| Insulin signaling, phosphatase arm | ||||
| PTP1B, basal vs. Con | ↔ | ↔ | ↔ | ↔ |
| SHP2, basal vs. Con | ↔ | ↔ | ↔ | ↔ |
| PTEN, basal vs. Con | ↔ | ↔ | ↔ | ↔ |
| Non-insulin-dependent signaling | ||||
| pAMPK/AMPK, basal vs. Con | ↑(trend) | ↑ | ↑ | ↑ |
↑, Increase, ⇈, further increase, ↔, no change vs. Con/basal state of the same experimental group. Con is the gold standard.
DISCUSSION
We previously described (53) perturbations in the skeletal muscle insulin signaling pathway that result in aberrant basal and insulin-induced GLUT4 translocation to the PM in adult pregestational IUGR and IUGR+PNGR offspring. This perturbation consisted of elevated basal PM-associated GLUT4, particularly in the IUGR and IUGR+PNGR groups compared with Con or PNGR. This change was noted as early as day 2 after birth and persisted to the adult stage of development. We referred to this phenomenon as an “in utero imprint,” established in response to the adverse nutritional and metabolic environment, toward maintaining basal skeletal muscle glucose supply. This increase in basal skeletal muscle PM GLUT4 association negated any further incremental response to insulin, a mechanism that perhaps offered further protection to the nutritionally compromised fetus (53). Our subsequent investigation demonstrated that the mechanism responsible for this aberrant basal and insulin-stimulated GLUT4 translocation to the PM was related to post-PDK1 signaling molecules. The basal increase in PM-associated GLUT4 in IUGR and IUGR+PNGR was related to increased pPDK1 and pAkt, which were both increased in these two experimental groups (38). In contrast, the inability to further increase PM-associated GLUT4 in response to insulin was not related to pAkt, since a further increase in phosphorylation of Akt in response to insulin was observed in all four experimental groups including the IUGR and IUGR+PNGR groups (38, 53). Instead, basal pPKCζ and PKCζ enzyme activity were increased and no further effect of insulin was noted on pPKCζ or the enzyme activity in IUGR and IUGR+PNGR (38). These findings established the mechanisms in the insulin signaling pathway that led to the aberrant GLUT4 translocation to the PM in these two experimental groups (38, 53).
In an attempt to reverse this “in utero imprint” on skeletal muscle GLUT4, we previously introduced (18) an early physiological intervention of chronic but moderate exercise. This lifestyle change was begun on postnatal day 21 and lasted until postnatal day 60. Such an intervention led to reversing the increase in basal PM-associated GLUT4 and further increased the insulin responsiveness of the GLUT4 protein only in the IUGR+PNGR group but not in the IUGR group. The IUGR group demonstrated resistance to the early exercise intervention (18). Given that exercise increases insulin sensitivity and thereby alters GLUT4 translocation, we began probing the role of early intervention with an insulin sensitizer. While the prescription of exercise is routinely met with considerable noncompliance in humans, rigorous adherence to daily exercise in rats did not ameliorate intrauterine changes in skeletal muscle GLUT4 that were encountered in the IUGR group (18, 53). Thus we undertook the present investigation by introducing an insulin sensitizer and PPARγ agonist (rosiglitazone) to examine the ability to reverse skeletal muscle GLUT4 changes, particularly in the IUGR group but also in the IUGR+PNGR group.
The reason we undertook early and sustained intervention with Rosi is because the IUGR female offspring develops pregnancy-induced glucose intolerance resulting in gestational diabetes (6). Gestational diabetes in the adult IUGR mother results in transgenerational propagation of dysregulated insulin sensitivity (6, 45, 57) inclusive of aberrant skeletal muscle GLUT4 translocation (6, 52). In fact, even in the face of embryo transfer on embryonic day 1 from the IUGR+PNGR mother to a control mother, the next generation demonstrates perturbed skeletal muscle GLUT4 subcellular distribution (52). Thus it was important to see whether early introduction of a sensitizer on a daily basis would reverse skeletal muscle GLUT4 subcellular distribution with concomitant changes in key insulin signaling molecules. Although insulin sensitizers are being used during human pregnancy (16), it was essential to introduce this intervention before conception and the development of maternal metabolic perturbations that adversely alter the milieu for the developing embryo/fetus (47). This thought process led to our present study design of Rosi treatment that began during the period of weaning and was sustained until the pregestational phase of life.
Unlike the early introduction of chronic and moderate exercise regimen (18), early Rosi led to a reversal of the basal and insulin-stimulated PM GLUT4 association in skeletal muscle. Thus Rosi treatment, while not affecting total GLUT4 concentrations, led to basal PM GLUT4 concentrations that were similar to those in the Con group. This is unlike observations previously reported in the absence of Rosi, where the basal PM-associated GLUT4 was much higher in the IUGR and IUGR+PNGR groups (53). Furthermore, insulin stimulation led to a response even in the IUGR and PNGR groups, resulting in a further increase in the PM association of GLUT4. Thus a PPARγ agonist led to changes in both IUGR and IUGR+PNGR groups that paralleled and superseded that of the Con group. In contrast to the PM findings, LDM-associated GLUT4 declined only in the Con and PNGR groups after insulin stimulation. The IUGR and IUGR+PNGR groups failed to demonstrate a similar decline, suggesting movement of GLUT4 from a different intracellular non-LDM and non-PM compartment to the PM in these two groups.
To determine the molecular mechanism behind this change with the early Rosi intervention, we noted that in IUGR and IUGR+PNGR phosphorylation of PDK1 and Akt in the basal state also mimicked that observed in the corresponding Con group. This observation is unlike that noted previously without any early intervention, where the basal concentrations of pPDK1, pAkt, and pPKCζ in IUGR and IUGR+PNGR were considerably higher than those of Con (38). This change may be responsible for the basal concentrations of PM GLUT4. With respect to the effect of insulin on PM GLUT4 concentrations, all three molecules, namely, pPDK1, pAkt, and pPKCζ, demonstrated insulin responsiveness paralleling that seen with Con. Thus it appears that the early introduction of Rosi reversed the previously reported inability to phosphorylate PKCζ in particular, which was deemed to be the critical step preventing insulin-induced PM GLUT4 association (38). This ability to phosphorylate PKCζ by the early Rosi intervention translated into increased PKCζ enzyme activity. This observation is particularly encouraging since adult humans born with a low birth weight also demonstrate aberrations in skeletal muscle GLUT4 and PKCζ (42, 26), setting the stage for future investigations targeted at the effect of PPARγ agonists on adult IUGR human skeletal muscle PKCζ and GLUT4 translocation.
Interrogation more proximally in the insulin signaling pathway did not yield a significant change at the PI3-K p85 subunit level. However, insulin sensitivity was perhaps established at the PI3-K level by a decline in key phosphatases that inhibit kinase activity, namely, PTP1B and SHP2 but not PTEN, mainly in the IUGR+PNGR group, excluding the IUGR group. This suggests that the phosphatases required the insulin sensitizer and postnatal nutrient restriction for a change, unlike the downstream insulin kinases such as PDK1, Akt, and PKCζ, which were also altered in the IUGR group. Thus, in the IUGR+PNGR group, an imbalance in the kinase and phosphatase arms of the PI3-K pathway occurred.
We also examined the energy-dependent pAMPK enzyme (20) since prior studies in the adult male rat demonstrated Rosi-induced changes in this moiety (10, 32) and since pAMPK has the ability to induce PM GLUT4 association in an insulin-independent manner (30). Unlike our prior investigations employing early exercise intervention (18), early Rosi intervention led to an increase in the pAMPK component of total AMPK enzyme. Activation of AMPK in turn could serve as another non-insulin-dependent mechanism that promotes PM GLUT4 association (30), as seen in Rosi-pretreated IUGR and IUGR+PNGR groups. Since Rosi pretreatment activated key signaling molecules in the insulin signaling and insulin-independent (AMPK) pathways, this dual activation led to enhancing GLUT4 translocation to the PM even in IUGR, thereby overcoming the previously observed resistance in response to exogenous insulin (53) or chronic exercise (18).
It is unlikely that Rosi will be introduced early in children given its other side effects, namely, the risk of dose-dependent hypoglycemia (11) and its negative impact on cognition (13), redistribution of white fat (9), increased risk of cardiovascular compromise (51), and hepatic toxicity (49). While newer generations of PPARγ agonists are being developed to overcome such side effects, our present study was undertaken purely as a proof of principle. Early introduction of insulin sensitizers has the ability to reverse the “in utero imprint” on skeletal muscle PM GLUT4 association. While we have deciphered the proximal and distal events in the insulin signaling and AMPK pathways culminating in this critical change, future studies will be designed to assess the functional effect of this GLUT4 subcellular redistribution on basal and insulin-stimulated skeletal muscle glucose uptake in pregestational female IUGR offspring.
In summary, early intervention with a PPARγ agonist in a pregestational adult IUGR offspring reduces basal and enhances insulin sensitivity of skeletal muscle PM GLUT4 association in the IUGR and IUGR+PNGR groups superseding that observed in the Con group. Potential targets in the insulin signaling pathway that may cause this change are downstream of PDK1 and involve activation of Akt (basal) and PKCζ (basal and insulin stimulated) along with activation of energy-dependent AMPK (basal). The insulin sensitizer alone in the IUGR failed to alter p85-PI3-K and the relevant phosphatases (PTP1B, SHP2, and PTEN) that act upstream of PDK1. However, along with postnatal nutrient restriction in the IUGR+PNGR group, a reduction in PTP1B and SHP2 further contributed toward enhanced insulin sensitivity. These studies set the stage for future investigations focused on the functional relevance of these observations in the pregestational and gestational stages of the IUGR offspring and their impact on halting the transgenerational progression of dysregulated insulin sensitivity.
GRANTS
This work was supported by National Institute of Child Health and Human Development Grants HD-41230, HD-25024, HD-33997, and HD-46979 (to S. U. Devaskar).
REFERENCES
- 1.Aerts L, Holemans K, Van Assche FA. Maternal diabetes during pregnancy: consequences for the offspring. Diabetes Metab Rev 6: 147–167, 1990. [DOI] [PubMed] [Google Scholar]
- 2.Aronoff S, Rosenblatt S, Braithwaite S, Egan JW, Mathisen AL, Schneider RL. Pioglitazone hydrochloride monotherapy improves glycemic control in the treatment of patients with type 2 diabetes: a 6-month randomized placebo-controlled dose-response study. The Pioglitazone 001 Study Group. Diabetes Care 23: 1605–1611, 2000. [DOI] [PubMed] [Google Scholar]
- 3.Barker DJ Fetal origins of coronary heart disease. Br Med J 311: 171–174, 1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Barker DJ, Osmond C, Forsen TJ, Kajantie E, Eriksson JG. Trajectories of growth among children who have coronary events as adults. N Engl J Med 353: 1802–1809, 2005. [DOI] [PubMed] [Google Scholar]
- 5.Bhargava SK, Sachdev HS, Fall CH, Osmond C, Lakshmy R, Barker DJ, Biswas SK, Ramji S, Prabhakaran D, Reddy KS. Relation of serial changes in childhood body-mass index to impaired glucose tolerance in young adulthood. N Engl J Med 350: 865–875, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Boloker J, Gertz SJ, Simmons RA. Gestational diabetes leads to the development of diabetes in adulthood in the rat. Diabetes 51: 1499–1506, 2002. [DOI] [PubMed] [Google Scholar]
- 7.Brunmair B, Gras F, Neschen S, Roden M, Wagner L, Waldhausi W, Furnsinn C. Direct thiazolidinedione action on isolated rat skeletal muscle fuel handling is independent of peroxisome proliferator-activated receptor-gamma mediated changes in gene expression. Diabetes 50: 2309–2315, 2001. [DOI] [PubMed] [Google Scholar]
- 8.Burant CF, Sreenan S, Hirano K, Tai TA, Lohmiller J, Lukens J, Davidson NO, Ross S, Graves RA. Troglitazone action is independent of adipose tissue. J Clin Invest 100: 2900–2908, 1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Campbell IW The clinical significance of PPAR gamma agonism. Curr Mol Med 5: 349–363, 2005. [DOI] [PubMed] [Google Scholar]
- 10.Ceolotto G, Gallo A, Papparella I, Franco L, Murphy E, Iori E, Pagnin E, Fadini GP, Albiero M, Semplicini A, Avogaro A. Rosiglitazone reduces glucose-induced oxidative stress mediated by NAD(P)H oxidase via AMPK-dependent mechanism. Arterioscler Thromb Vasc Biol 27: 2627–2633, 2007. [DOI] [PubMed] [Google Scholar]
- 11.Ching CK, Lai CK, Poon WT, Lui MC, Lam YH, Shek CC, Mak TW, Chan AY. Drug-induced hypoglycemia—new insight into an old problem. Hong Kong Med J 12: 334–338, 2006. [PubMed] [Google Scholar]
- 12.Cole HH, Hart GH. The effect of pregnancy and lactation on growth in the rat. Am J Physiol 123: 589–597, 1938. [Google Scholar]
- 13.Draelos MT, Jacobson AM, Weinger K, Widom B, Ryan CM, Finkelstein DM, Simpson DC. Cognitive function in patients with insulin-dependent diabetes mellitus during hyperglycemia and hypoglycemia. Am J Med 98: 135–144, 1995. [DOI] [PubMed] [Google Scholar]
- 14.Eriksson JG, Osmond C, Kajantie E, Forsén TJ, Barker DJ. Patterns of growth among children who later develop type 2 diabetes or its risk factors. Diabetologia 49: 2853–2858, 2006. [DOI] [PubMed] [Google Scholar]
- 15.Fecchi K, Volonte D, Hezel MP, Schmeck K, Galbiati F. Spatial and temporal regulation of GLUT4 translocation by flotillin-1 and caveolin-3 in skeletal muscle cells. FASEB J 20: 705–707, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Feig DS, Briggs GG, Koren G. Oral antidiabetic agents in pregnancy and lactation: a paradigm shift? Ann Pharmacother 41: 1174–1180, 2007. [DOI] [PubMed] [Google Scholar]
- 17.Fritzius T, Frey AD, Schweneker M, Mayer D, Moelling K. WD-repeat-propeller-FYVE protein, ProF, binds VAMP2 and protein kinase C zeta. FEBS J 274: 1552–1566, 2007. [DOI] [PubMed] [Google Scholar]
- 18.Garg M, Thamotharan M, Oak SA, Pan G, MacLaren DC, Lee PWN, Devaskar SU. Early exercise regimen improves insulin sensitivity in the intrauterine growth-restricted adult female rat offspring. Am J Physiol Endocrinol Metab 296: E272–E281, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Garg M, Thamotharan M, Rogers L, Bassilian S, Lee PWN, Devaskar SU. Glucose metabolic adaptations in the intrauterine growth-restricted adult female rat offspring. Am J Physiol Endocrinol Metab 290: E1218–E1226, 2006. [DOI] [PubMed] [Google Scholar]
- 20.Goodyear LJ, Hirshman MF, Horton ES. Exercise-induced translocation of skeletal muscle glucose transporters. Am J Physiol Endocrinol Metab 261: E795–E799, 1991. [DOI] [PubMed] [Google Scholar]
- 21.He J, Thamotharan M, Devaskar SU. Insulin-induced translocation of facilitative glucose transporters in fetal/neonatal rat skeletal muscle. Am J Physiol Regul Integr Comp Physiol 284: R1138–R1146, 2003. [DOI] [PubMed] [Google Scholar]
- 22.Holemans K, Van Bree R, Verhaeghe J, Meurrens K, Van Assche FA. Maternal semistarvation and streptozotocin-diabetes in rats have different effects on the in-vivo glucose uptake by peripheral tissues in their female adult offspring. J Nutr 127: 1371–1376, 1997. [DOI] [PubMed] [Google Scholar]
- 23.Holness MJ, Langdown ML, Sugden MC. Early-life programming of susceptibility to dysregulation of glucose metabolism and the development of Type 2 diabetes mellitus. Biochem J 349: 657–665, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hovi P, Andersson S, Eriksson JG, Järvenpää AL, Strang-Karlsson S, Mäkitie O, Kajantie E. Glucose regulation in young adults with very low birth weight. N Engl J Med 356: 2053–2063, 2007. [DOI] [PubMed] [Google Scholar]
- 25.Inzucchi SE, Maggs DG, Spollett GR, Page SL, Rife FS, Walton V, Shulman GI. Efficacy and metabolic effects of metformin and troglitazone in type II diabetes mellitus. N Engl J Med 338: 867–872, 1998. [DOI] [PubMed] [Google Scholar]
- 26.Jensen CB, Martin-Gronert MS, Storgaard H, Madsbad S, Vaag A, Ozanne SE. Altered PI3-kinase/Akt signaling in skeletal muscle of young men with low birth weight. PLoS ONE 3: e3736, 2008. [DOI] [PMC free article] [PubMed]
- 27.Kanoh Y, Bandyopadhyay G, Sajan MP, Standaert ML, Farese RV. Rosiglitazone, insulin treatment and fasting correct defective activation of protein kinase C-zeta/lambda by insulin in vastus lateralis muscles and adipocytes of diabetic rats. Endocrinology 142: 1595–1605, 2001. [DOI] [PubMed] [Google Scholar]
- 28.Kanoh Y, Bandyopadhyay G, Sajan MP, Standaert ML, Farese RV. Thiazolidinedione treatment enhances insulin effects on protein kinase C-zeta/lambda activation and glucose transport in adipocytes of nondiabetic and Goto-Kakizaki type II diabetic rats. J Biol Chem 275: 16690–16696, 2000. [DOI] [PubMed] [Google Scholar]
- 29.Kersten S, Mandard S, Tan NS, Escher P, Metzger D, Chambon P, Gonzalez FJ, Desvergne B, Wahli W. Characterization of the fasting-induced adipose factor FIAF, a novel peroxisome proliferator-activated receptor target gene. J Biol Chem 275: 28488–28493, 2000. [DOI] [PubMed] [Google Scholar]
- 30.Kurth-Kraczek DJ, Hirschman MF, Goodyear LJ, Winder WW. 5′ AMP-activated protein kinase activation causes GLUT4 translocation in skeletal muscle. Diabetes 48: 1667–1671, 1999. [DOI] [PubMed] [Google Scholar]
- 31.Laudanna C, Mochly-Rosen D, Liron T, Constantin G, Butcher EC. Evidence of zeta protein kinase C involvement in polymorphonuclear neutrophil integrin-dependent adhesion and chemotaxis. J Biol Chem 273: 30306–30315, 1998. [DOI] [PubMed] [Google Scholar]
- 32.Lessand SJ, Chen ZP, Watt MJ, Hashem M, Reid JJ, Febbraio MA, Kemp BE, Hawley JA. Chronic rosiglitazone treatment restores AMPK alpha2 activity in insulin-resistant rat skeletal muscle. Am J Physiol Endocrinol Metab 290: E251–E257, 2006. [DOI] [PubMed] [Google Scholar]
- 33.Li P, Koike T, Qin B, Kubota M, Kawata Y, Jia YJ, Oshida Y. A high-fructose diet impairs Akt and PKCzeta phosphorylation and GLUT4 translocation in rat skeletal muscle. Horm Metab Res 40: 528–532, 2008. [DOI] [PubMed] [Google Scholar]
- 34.Liu LZ, Zhao HL, Zuo J, Ho SK, Chan JC, Meng Y, Fang FD, Tong PC. Protein kinase Czeta mediates insulin-induced glucose transport through actin remodeling in L6 muscle cells. Mol Biol Cell 17: 2322–2330, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Loviscach M, Rehman N, Carter L, Mudaliar S, Mohadeen P, Ciaraldi TP, Veerkamp JH, Henry RR. Distribution of peroxisome proliferator-activated receptors (PPARs) in human skeletal muscle and adipose tissue: relation to insulin action. Diabetologia 43: 304–311, 2000. [DOI] [PubMed] [Google Scholar]
- 36.Maroni P, Bendinelli P, Piccoletti R. Intracellular signal transduction pathways induced by leptin in C2C12 cells. Cell Biol Int 29: 542–550, 2005. [DOI] [PubMed] [Google Scholar]
- 37.Miyazaki Y, He H, Mandarino LJ, De Fronzo RA. Rosiglitazone improves downstream insulin receptor signaling in type 2 diabetic patients. Diabetes 52: 1943–1950, 2003. [DOI] [PubMed] [Google Scholar]
- 38.Oak SA, Tran C, Pan G, Thamotharan M, Devaskar SU. Perturbed skeletal muscle insulin signaling in the adult female intrauterine growth-restricted rat. Am J Physiol Endocrinol Metab 290: E1321–E1330, 2006. [DOI] [PubMed] [Google Scholar]
- 39.Olefsky JM Treatment of insulin resistance with peroxisome proliferator-activated receptor gamma agonists. J Clin Invest 106: 467–472, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Olefsky JM, Saltiel AR. PPARgamma and the treatment of insulin resistance. Trends Endocrinol Metab 11: 362–368, 2000. [DOI] [PubMed] [Google Scholar]
- 41.Ozanne SE, Hales CN. Early programming of glucose-insulin metabolism. Trends Endocrinol Metab 13: 368–373, 2002. [DOI] [PubMed] [Google Scholar]
- 42.Ozanne SE, Jensen CB, Tingey KJ, Storgaard H, Madsbad S, Vaag AA. Low birthweight is associated with specific changes in muscle insulin-signalling protein expression. Diabetologia 48: 547–552, 2005. [DOI] [PubMed] [Google Scholar]
- 43.Ozanne SE, Olsen GS, Hansen LL, Tingey KJ, Nave BT, Wang CL, Hartil K, Petry CJ, Buckley AJ, Mosthaf-Seedorf L. Early growth restriction leads to down regulation of protein kinase Czeta and insulin resistance in skeletal muscle. J Endocrinol 177: 235–241, 2003. [DOI] [PubMed] [Google Scholar]
- 44.Phillips LS, Grunberger G, Miller E, Patwardhan R, Rappaport EB, Salzman A. Once- and twice-daily dosing with rosiglitazone improves glycemic control in patients with type 2 diabetes. Diabetes Care 24: 308–315, 2001. [DOI] [PubMed] [Google Scholar]
- 45.Pinheiro AR, Salvucci ID, Aguila MB, Mandarim-de-Lacerda CA. Protein restriction during gestation and/or lactation causes adverse transgenerational effects on biometry and glucose metabolism in F1 and F2 progenies of rats. Clin Sci (Lond) 114: 381–392, 2008. [DOI] [PubMed] [Google Scholar]
- 46.Rangwala SM, Lazar MA. Peroxisome proliferator-activated receptor gamma in diabetes and metabolism. Trends Pharmacol Sci 25: 331–336, 2004. [DOI] [PubMed] [Google Scholar]
- 47.Reece EA, Hornko CJ. Prepregnancy care and the prevention of fetal malformations in the pregnancy complicated by diabetes. Clin Obstet Gynecol 50: 990–997, 2007. [DOI] [PubMed] [Google Scholar]
- 48.Saltiel AR, Olefsky JM. Thiazolidinediones in the treatment of insulin resistance and type II diabetes. Diabetes 45: 1661–1669, 1996. [DOI] [PubMed] [Google Scholar]
- 49.Scheen AJ Thiazolidinediones and liver toxicity. Diabetes Metab 27: 305–313, 2001. [PubMed] [Google Scholar]
- 50.Sorensen HT, Thulstrup AM, Norgdard B, Engberg M, Madsen KM, Johnsen SP, Olsen J, Lauritzen T. Fetal growth and blood pressure in a Danish population aged 31–51 years. Scand Cardiovasc J 34: 390–395, 2000. [DOI] [PubMed] [Google Scholar]
- 51.Starner CI, Schafer JA, Heaton AH, Gleason PP. Rosiglitazone and pioglitazone utilization from January 2007 through May 2008 associated with five risk-warning events. J Manag Care Pharm 14: 523–531, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Thamotharan M, Garg M, Oak S, Rogers LM, Pan G, Sangiorgi F, Lee PW, Devaskar SU. Transgenerational inheritance of the insulin-resistant phenotype in embryo-transferred intrauterine growth-restricted adult female rat offspring. Am J Physiol Endocrinol Metab 292: E1270–E1279, 2007. [DOI] [PubMed] [Google Scholar]
- 53.Thamotharan M, Shin BC, Suddirikku DT, Thamotharan S, Garg M, Devaskar SU. Glut4 expression and subcellular localization in the intrauterine growth-restricted adult rat female offspring. Am J Physiol Endocrinol Metab 288: E935–E947, 2005. [DOI] [PubMed] [Google Scholar]
- 54.Van Assche FA, Holemans K, Aerts L. Fetal growth and consequences of later life. J Perinat Med 26: 337–346, 1998. [DOI] [PubMed] [Google Scholar]
- 55.Weinstein SP, Holand A, O'Boyle E, Habet RS. Effects of thiazolidinediones on glucocorticoid-induced insulin resistance and GLUT4 glucose transporter expression in rat skeletal muscle. Metabolism 42: 1365–1369, 1993. [DOI] [PubMed] [Google Scholar]
- 56.Weyer C, Pratley RE, Lindsay RS, Tataranni PA. Relationship between birth weight and body composition, energy metabolism and sympathetic nervous system activity later in life. Obes Res 8: 559–565, 2000. [DOI] [PubMed] [Google Scholar]
- 57.Zambrano E, Martinex-Samayoa PM, Bautista CJ, Deas M, Guillen L, Rodriquez-Gonzales GL, Guzman C, Larrea F, Nathanielsz PW. Sex differences in transgenerational alterations of growth and metabolism in progeny (F2) of female offspring (F1) of rats fed a low protein diet during pregnancy and lactation. J Physiol 566: 225–236, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]