Abstract
The opposing actions of insulin and glucagon on hepatic carbohydrate metabolism are well documented. In contrast, relatively little is known about how the two hormones interact to regulate hepatic protein metabolism. Previously, we reported that glucagon in the absence of insulin represses signaling through the mammalian target of rapamycin complex 1 (mTORC1). In the present study, we sought to determine whether or not the action of one hormone would dominate over the other in the regulation of mTORC1 signaling. Livers were perfused in situ with medium containing either no added hormones (control), 10 nM insulin, 100 nM glucagon, or a combination of the hormones. Compared with control livers, insulin stimulated Akt phosphorylation and mTORC1 signaling, as assessed by increased phosphorylation of the mTORC1 targets eIF4E-binding protein (4E-BP)1 and ribosomal protein S6 kinase (S6K)1, and promoted assembly of the eIF4G·eIF4E complex. Glucagon alone had no effect on mTORC1 signaling but stimulated the activity of protein kinase A (PKA). In the presence of a combination of insulin and glucagon, Akt and TSC2 phosphorylation and PKA activity were all increased compared with controls. However, mTORC1 signaling was repressed compared with livers perfused with medium containing insulin alone, and this effect was associated with reduced assembly of the mTORC1·eIF3 complex. Overall, the results suggest that glucagon acts in a dominant manner to repress insulin-induced mTORC1 signaling, which is in contrast to previous studies showing a dominant action of insulin in the control of hepatic gluconeogenesis.
Keywords: eukaryotic initiation factor 3, ribosomal protein S6 kinase, eukaryotic initiation factor 4E-binding protein 1
the liver must adapt rapidly to large variations in nutrient availability and the associated fluctuations in anabolic and catabolic hormones to act in coordination with the other tissues of the body to maintain metabolic homeostasis. A change in the metabolic environment triggers responses within the liver, resulting in the activation or repression of biochemical pathways involved in carbohydrate, lipid, and protein metabolism. Two key hormones that play a prominent role in metabolic regulation in the liver are insulin and glucagon. Both hormones regulate diverse metabolic pathways through activation or repression of signaling pathways, including one that involves a Ser/Thr protein kinase that is referred to as the mammalian target of rapamycin (mTOR).
mTOR, functioning in a complex referred to as mTOR complex 1 (mTORC1), regulates an array of processes related to protein synthesis and cell growth, including mRNA translation, ribosome biogenesis, gene transcription, cell division, and autophagy through phosphorylation of downstream targets (reviewed in Refs. 1 and 11). Unquestionably, the best studied of the downstream actions of mTORC1 is the regulation of mRNA translation. mTORC1 directly phosphorylates at least two proteins, the eukaryotic initiation factor (eIF)4E-binding protein (4E-BP)1 and the ribosomal protein (rp)S6 kinase 1 (S6K1), both of which are involved in regulating the mRNA-binding step in the translation initiation stage of protein synthesis. Phosphorylation of 4E-BP1 releases eIF4E from the inactive 4E-BP1·eIF4E complex, thereby allowing eIF4E to bind to eIF4G and eIF4A to form the eIF4F complex. The eIF4F complex mediates the binding of mRNA to the 40S ribosomal subunit during translation initiation, and therefore changes in eIF4F complex assembly have important consequences in controlling the selection of mRNAs for translation. In addition to phosphorylating rpS6, S6K1 also phosphorylates other proteins that regulate mRNA translation, including eIF4B, an activator of the eIF4A component of the eIF4F complex. Of particular note, a recent study (8) shows that mTORC1-mediated phosphorylation of both 4E-BP1 and S6K1 depends on the binding of mTORC1 to eIF3. Thus, eIF3 acts as a scaffold not only for translation initiation factors such as eIF4A, eIF4B, and eIF4E but also for 4E-BP1, S6K1, and mTORC1.
Signaling through mTORC1 is positively regulated by inputs from the phosphatidylinositide 3-kinase (PI 3-kinase)/Akt and the extracellular-regulated protein kinase (ERK)/90-kDa rpS6 kinase (p90RSK) signaling pathways and negatively regulated by input from the AMP-activated protein kinase (AMPK) signaling pathway. Akt (10, 21), ERK (20), and p90RSK (27, 33) all phosphorylate and thereby repress the action of a protein referred to as the tuberous sclerosis complex (TSC)2 (also known as tuberin). In a complex with TSC1 (also known as hamartin), TSC2 acts as a GTPase-activating protein for the Ras homolog enriched in brain (Rheb) (9, 34, 36). Similarly to other Ras homologs, Rheb binds to GDP and GTP, and both the Rheb-GDP and Rheb-GTP complexes bind to mTORC1 (18, 19, 32). However, the GTP-bound form of Rheb activates mTORC1, whereas Rheb-GDP inhibits it (18, 19). Thus, by activating the PI 3-kinase/Akt and ERK/p90RSK signaling pathways, insulin acts to increase mTORC1 signaling through inhibition of TSC2 and thus elevation of the proportion of Rheb present in the active GTP complex. In contrast, AMPK acts to activate the GTPase-activating protein activity of TSC2 and thus repress signaling through mTORC1.
In the absence of other hormonal inputs, glucagon acts to repress mTORC1 signaling both in perfused rat liver (15) and in isolated hepatocytes (22). Moreover, in perfused liver, glucagon acts to attenuate the amino acid-induced activation of mTORC1 (15). In contrast, previous studies have shown that the glucagon-induced expression of genes encoding rate-limiting steps in gluconeogenesis such as phosphoenolpyruvate carboxykinase and glucose-6-phosphatase is overridden in a dominant manner by insulin (reviewed in Ref. 25). Thus, the question arises as to whether or not the action of one hormone would dominate over the other in regulating mTORC1 signaling when the liver is presented with a combination of insulin and glucagon. Therefore, the objective of the present study was to assess the individual and combined effects of insulin and glucagon on signaling through the mTORC1 pathway using the perfused rat liver as an experimental model.
MATERIALS AND METHODS
Materials.
Pentobarbital sodium was purchased from Abbot, bovine albumin, glucagon, and protease inhibitor mixture were purchased from Sigma, ECL Western blotting detection reagent was purchased from Pierce, and polyvinylidene difluoride membrane (BioTrace, 0.45 μm) was purchased from Pall Life Sciences. BioMag goat anti-rabbit and goat anti-mouse IgG beads were purchased from Qiagen, rabbit polyclonal antibodies raised against S6K1 and 4E-BP1 and the goat anti-rabbit IgG horseradish peroxidase-conjugated antibody were purchased from Bethyl Laboratories. Goat polyclonal antibody raised against eIF3 was purchased from Santa Cruz Biotechnology. All other antibodies were purchased from Cell Signaling Technology. Preparation of the eIF4G antibody has been described previously (14).
In situ liver perfusion.
Male Sprague-Dawley rats weighing ∼125 g were maintained on a 12:12-h light-dark cycle, with food (Harlan Teklad) and water provided ad libitum. The experimental protocol used for the studies described herein was reviewed and approved by the Institutional Animal Care and Use Committee of The Pennsylvania State University College of Medicine. Livers were perfused in situ with a nonrecirculating medium at a flow rate of 7 ml/min, as described previously (4, 5, 15). When present, glucagon [3.33 mM in 0.155 M NaCl (pH 3.0)], insulin [1.74 mM in 0.155 M NaCl (pH 2.0)], or a combination of both was infused directly into the inflow line at a rate of 0.2 ml/min for a final calculated concentration of 100 and 10 nM, respectively. Total perfusion time was 20 min. Immediately following perfusion, livers were excised and frozen between aluminum blocks that were precooled in liquid nitrogen, and the frozen liver was stored at −80°C for future analysis.
Measurement of PKA activity.
The phosphotransferase activity of PKA was measured using a PKA assay kit (Upstate Cell Signaling Solutions), as described previously (15). Briefly, frozen liver tissue was homogenized, and the resulting homogenate was centrifuged at 1,000 g for 3 min at 4°C. Supernatant containing 50 μg of protein was diluted to 5 μl with 1× assay dilution buffer, followed by the addition of 25 μl of assay mix. A duplicate sample of diluted supernatant was assayed using the same assay mix containing 1 μM PKA inhibitor peptide. Both assay mixtures were incubated at 30°C for 5 min, and then 15 μl of each of the assay mixtures was spotted onto P81 phosphocellulose filters. The phosphocellulose filters were washed, and the amount of radioactivity bound to the filters was measured by liquid scintillation spectrometry. PKA activity was calculated as the difference between the sample assayed in the absence of PKA inhibitor peptide and the sample assayed with the inhibitor.
Western blot analysis.
Rat livers (∼0.3 g) were homogenized in 7 volumes of buffer consisting of 20 mM HEPES, 2 mM EGTA, 50 mM NaF, 100 mM KCl, 0.2 mM EDTA, 50 mM β-glycerophosphate, 1 mM dithiothreitol, 1 mM benzamidine, 0.5 mM sodium vanadate, and 10 μl/ml Sigma protease inhibitor cocktail using a Polytron homogenizer. The homogenate was centrifuged at 1,000 g for 3 min at 4°C, and the resulting supernatant was subjected to SDS-polyacrylamide gel electrophoresis and Western blot analysis, as described previously (15).
Measurement of protein phosphorylation state.
Phosphorylation of S6K1, Akt, 4E-BP1, mTOR, and TSC2 was measured in the supernatants by Western blot analysis, as described previously (15).
Immunoprecipitations.
eIF4E was immunoprecipitated from 1,000-g supernatants of liver homogenates using a monoclonal antibody to eIF4E (14), and immunoprecipitates were subjected to Western blot analysis using a polyclonal antibody to eIF4G. mTOR was immunoprecipitated from supernatants of liver homogenates according to methods described by Kim et al. (13). Samples were then analyzed by Western blot analysis for mTOR and eIF3.
Statistics.
Data were analyzed with the Instat statistical software program (version 3.0b; GraphPad Software, La Jolla, CA) using an unpaired one-way AVOVA. If a statistical difference was detected by ANOVA, the data were subjected to post hoc Tukey analysis. P < 0.05 was considered significant.
RESULTS
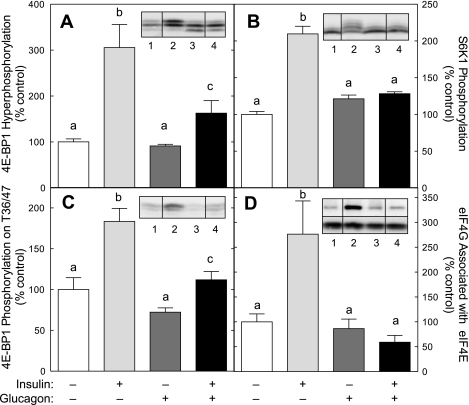
Previous studies have shown that mTORC1 is a common effector of both insulin- and glucagon-mediated regulation of mechanisms of mRNA translation in the liver. Therefore, changes in phosphorylation of the downstream targets 4E-BP1 and S6K1 were measured by Western blot analysis as an index of signaling through mTORC1. During electrophoresis, 4E-BP1 and S6K1 separate into multiple electrophoretic forms on the basis of their phosphorylation state, whereby more highly phosphorylated forms migrate more slowly. In livers perfused in the presence of insulin, the electrophoretic mobility of both 4E-BP1 (Fig. 1A) and S6K1 (Fig. 1B) was significantly reduced, suggesting that phosphorylation of both proteins, and thus signaling through mTORC1, was increased in response to insulin action. The presence of glucagon alone had little or no effect on the electrophoretic mobility of either 4E-BP1 or S6K1 but dramatically attenuated the insulin effect. To confirm that the changes in migration were associated with altered phosphorylation status, changes in phosphorylation of residues on 4E-BP1 known to be directly phosphorylated by mTORC1, i.e., Thr36/47 (6), were assessed by Western blot analysis. As shown in Fig. 1C, insulin acted to enhance 4E-BP1 phosphorylation on Thr36/47, and glucagon attenuated the insulin effect. To demonstrate that the changes in 4E-BP1 phosphorylation were sufficient in magnitude to alter eIF4F complex assembly, the association of eIF4G with eIF4E was measured. As shown in Fig. 1D, insulin acted to increase the amount of eIF4G associated with eIF4E >2.5-fold. Glucagon alone had no significant effect on eIF4G binding to eIF4E, but it prevented completely the insulin-induced assembly of the eIF4G·eIF4E complex. Overall, the results strongly support the conclusion that insulin acts to stimulate mTORC1 signaling in perfused rat liver, and glucagon acts in a dominant manner to override the stimulation.
Fig. 1.
Glucagon represses the insulin-induced activation of mammalian target of rapamycin (mTOR) complex 1 (mTORC1). Rat livers were perfused in situ for 20 min in the absence of hormones or in the presence of 100 nM glucagon and/or 10 nM insulin, as described in materials and methods. Phosphorylation of eukaryotic initiation factor (eIF)4E-binding protein (4E-BP1; A) and ribosomal protein S6 kinase 1 (S6K1; B) was measured as changes in electrophoretic mobility assessed by Western blot analysis, as described in materials and methods. C: phosphorylation of 4E-BP1 on Thr36/47 was measured by Western blot analysis using an antibody that specifically recognizes the protein only when it is phosphorylated on Thr36 and/or Thr47. D: the association of eIF4G with eIF4E was examined by immunoprecipitating eIF4E from liver homogenates and measuring the amount of eIF4E and eIF4G in the immunoprecipitates by Western blot analysis. Inserts show representative blots. The samples shown were analyzed on the same gel, but not necessarily in contiguous lanes. Lane 1, control liver; lane 2, livers perfused in the presence of insulin; lane 3, livers perfused in the presence of glucagon; lane 4, livers perfused in the presence of both glucagon and insulin. D, top insert: typical blot for eIF4G. D, bottom insert: typical blot for eIF4E. The results represent the mean ± SE for 6 (S6K1), 10 (4E-BP1 phosphorylation and eIF4G association with eIF4E), or 12 (4E-BP1 phosphorylation on Thr36/47) livers/condition. Values not sharing the same letter are significantly different (P < 0.05).
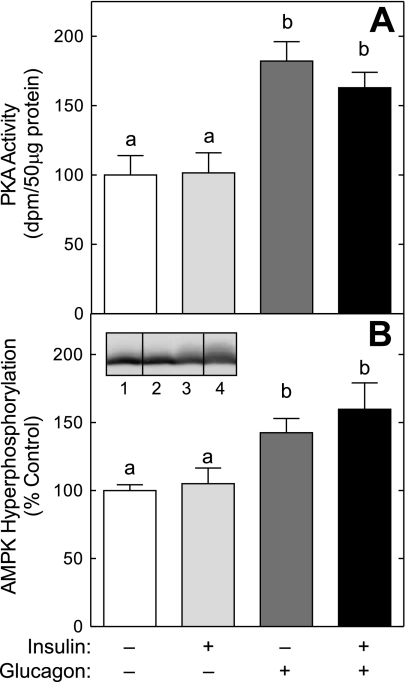
In a previous study (15), we showed that the glucagon-mediated repression of amino acid-induced mTORC1 signaling correlated with activation of PKA and AMPK. In confirmation of the previous study, the presence of glucagon alone caused PKA activation (Fig. 2A) and promoted a shift in the electrophoretic mobility of AMPK into slower migrating forms (Fig. 2B), suggesting that the hormone acted to increase AMPK phosphorylation. Insulin alone had no effect on either kinase and did not prevent the glucagon-induced activation of PKA or phosphorylation of AMPK. Also consistent with our previous work (15) is the finding that glucagon alone did not repress S6K1 phosphorylation (Fig. 1B). As noted previously (15), this finding is likely due to S6K1 being present largely in the hypophosphorylated form in the control condition, and therefore, it would be difficult to detect any further decrease that might occur in response to glucagon alone. Moreover, although glucagon alone did not reduce the proportion of 4E-BP1 in the hyperphosphorylated γ-form (Fig. 1A), glucagon alone promoted an increase in the amount of the protein in the hypophosphorylated α-form, suggesting that activation of AMPK by glucagon in the absence of insulin repressed mTORC1 signaling.
Fig. 2.
Glucagon, but not insulin, activates PKA and promotes AMP-activated protein kinase (AMPK) phosphorylation. Livers were perfused as described in the legend to Fig. 1. A: PKA activity was measured using a kit from Upstate Cell Signaling Solutions, as described previously (15). B: AMPK phosphorylation was measured as a decrease in migration during SDS-polyacrylamide gel electrophoresis, as described in materials and methods. Each sample was analyzed on the same blot, but not in contiguous lanes on the gel. Lane 1, control liver; lane 2, livers perfused in the presence of insulin; lane 3, livers perfused in the presence of glucagon; lane 4, livers perfused in the presence of both glucagon and insulin. The results represent the mean ± SE of 6 livers/condition. Values not sharing the same letter are significantly different (P < 0.05).
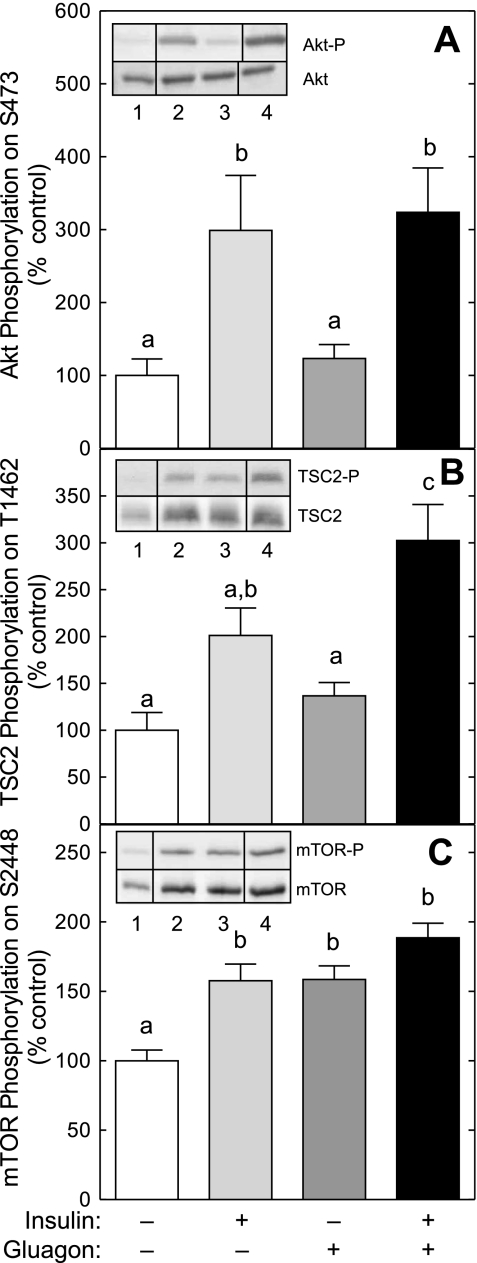
A possible mechanism through which glucagon might act to repress insulin-induced signaling through mTORC1 would be if it attenuated the insulin-mediated regulation of upstream activators of mTORC1, such as Akt and/or TSC2. To assess this possibility, the phosphorylation of Akt on Ser473 and TSC2 on Thr1462 was measured. As shown in Fig. 3, A and B, glucagon alone had no significant effect on phosphorylation of either Akt or TSC2, respectively. In contrast, insulin alone caused a significant increase in the phosphorylation of Akt and tended (P = 0.06) to increase TSC2 phosphorylation compared with controls. Importantly, glucagon did not attenuate insulin-induced phosphorylation of either Akt or TSC2, suggesting that glucagon acts downstream of both proteins to repress insulin-induced activation of mTORC1 signaling.
Fig. 3.
Glucagon does not prevent the insulin-induced increase in Akt, tuberous sclerosis complex 2 (TSC2), or mTOR phosphorylation. Livers were perfused as described in the legend to Fig. 1. Phosphorylation of Akt on Ser473 (A), TSC2 on Thr1462 (B), and mTOR on Ser2448 (C) was assessed by Western blot analysis using antibodies that specifically recognize the phosphorylated forms of the proteins. Inserts show representative blots. The samples shown were analyzed on the same gel, but not necessarily in contiguous lanes. Lane 1, control liver; lane 2, livers perfused in the presence of insulin; lane 3, livers perfused in the presence of glucagon; lane 4, livers perfused in the presence of both glucagon and insulin. The results were normalized for the total amounts of the respective protein and are presented as means ± SE of 5–6 (Akt and mTOR) or 12 (TSC2) livers/condition. Values not sharing the same letter are significantly different (P < 0.05). There is also a trend (P = 0.06) for insulin alone to increase TSC2 phosphorylation compared with glucagon alone.
A second mechanism through which glucagon might act to repress insulin-induced signaling through mTORC1 involves changes in mTOR phosphorylation. As shown in Fig. 3C, insulin alone caused a significant increase in the phosphorylation of mTOR on Ser2448 compared with controls. Glucagon alone also promoted mTOR phosphorylation and did not prevent insulin-induced phosphorylation, suggesting that glucagon does not act to attenuate insulin-induced mTORC1 signaling by preventing phosphorylation of mTOR on Ser2448.
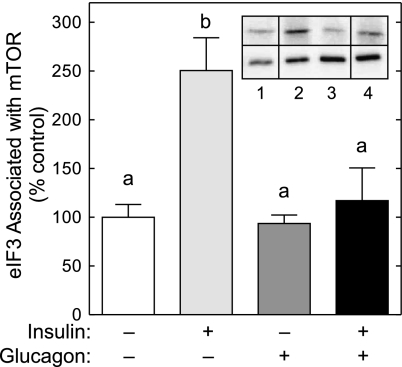
A third mechanism through which glucagon might act to repress insulin-induced signaling through mTORC1 would be if it attenuated assembly of the mTORC1·eIF3 complex. To assess this possibility, mTOR was immunoprecipitated and subjected to Western blot analysis for mTOR and eIF3. As shown in Fig. 4, the amount of eIF3 detected in mTOR immunoprecipitates from livers perfused with insulin alone was significantly increased compared with controls. Glucagon alone had no effect on the association of eIF3 with mTOR but prevented completely the insulin-induced increase in assembly of the mTORC1·eIF3 complex.
Fig. 4.
Glucagon prevents the insulin-stimulated association of eIF3 with mTOR. Livers were perfused as described in the legend to Fig. 1. The association of eIF3 with mTOR was examined by immunoprecipitating mTOR from liver homogenates and measuring the amount of mTOR and eIF3 in the immunoprecipitates by Western blot analysis. Insert shows representative blots. The samples shown were analyzed on the same gel, but not necessarily in contiguous lanes. Lane 1, control liver; lane 2, livers perfused in the presence of insulin; lane 3, livers perfused in the presence of glucagon; lane 4, livers perfused in the presence of both glucagon and insulin. Top blot: typical blot for eIF3; bottom blot: typical blot for mTOR. The results represent the mean ± SE for 6 livers/condition. Values not sharing the same letter are significantly different (P < 0.05).
DISCUSSION
The results of the present study confirm previous ones showing that, when present alone, insulin stimulates mTORC1 signaling in perfused livers (35). The present study extends the earlier one to demonstrate that glucagon acts in a dominant manner to repress insulin-induced activation of mTORC1 signaling. Thus, glucagon dramatically attenuated insulin-induced phosphorylation of the well-characterized mTORC1 substrates 4E-BP1 and S6K1 as well as blocked insulin-stimulated association of eIF4G with eIF4E. In part, insulin activates mTORC1 through a signaling pathway involving Akt (reviewed in Ref. 17). Therefore, one possible mechanism through which glucagon might act to repress insulin-induced activation of mTORC1 signaling is through repression of Akt phosphorylation. However, in the present study, glucagon was without effect on either basal or insulin-stimulated Akt phosphorylation. Moreover, in a previous study using isolated rat hepatocytes (22), glucagon did not promote Akt phosphorylation and did not prevent insulin-induced Akt phosphorylation.
Akt activates mTORC1 signaling through phosphorylation of several downstream effectors, including TSC2 (10, 21) and mTOR itself (23, 31). In the present study, TSC2 phosphorylation was significantly increased in livers perfused in the presence of insulin compared with controls. Glucagon did not prevent the insulin-stimulated increase in TSC2 phosphorylation, and in fact, the presence of both hormones led to a greater increase in TSC2 phosphorylation compared with either hormone alone. Together, the results of the present and previous studies suggest that glucagon acts downstream of Akt and TSC2 to repress mTORC1 signaling.
Besides mTORC1, mTOR also exists in a multiple-protein complex referred to as mTORC2. mTORC1 is distinguished from mTORC2 by both its protein composition and substrate specificity. For example, the mTORC1 complex contains the regulatory associated protein of mTOR (raptor), whereas mTORC2 instead contains the rapamycin-insensitive companion of mTOR (rictor) (12, 28). Raptor and rictor act to target substrates to the mTORC1 and -2 complexes, respectively. In this regard, raptor binds to a domain present on both 4E-BP1 and S6K1, referred to as a mTOR signaling motif, and thereby recruits them to the mTORC1 complex to be phosphorylated (24, 29). In addition to raptor, a recent study (8) suggests that mTORC1 must bind to the translation initiation factor eIF3 for optimal phosphorylation of S6K1 and 4E-BP1. In that study it was shown that, in serum-starved human embryonic kidney-293E cells, S6K1 and the 4E-BP1·eIF4E complex are bound to eIF3. Addition of insulin to the culture medium rapidly (within 2.5 min) promoted binding of mTORC1 to eIF3, which led to phosphorylation of S6K1 and 4E-BP1 and their release from the mTORC1·eIF3·eIF4E complex. Rapamycin prevented the binding of mTORC1 to eIF3 and the subsequent phosphorylation and release of S6K1 and 4E-BP1. The finding in the present study that insulin promotes the binding of eIF3 to mTORC1 in perfused rat liver corroborates in an intact tissue the findings of the results of the previous study using cells in culture. Moreover, the present study shows that glucagon represses in a dominant manner the insulin-induced assembly of the mTORC1·eIF3 complex. The mechanism through which insulin stimulates and glucagon represses the binding of mTOR to eIF3 will need to be addressed in future studies.
In response to consumption of a high-carbohydrate meal, plasma insulin levels increase rapidly, whereas glucagon levels fall. Plasma insulin levels also increase in response to consumption of a high-protein meal, although the magnitude of the response is less compared with carbohydrate alone (2, 3, 16, 30). However, in contrast to a high-carbohydrate meal, plasma glucagon levels increase rather than decrease in response to a high-protein meal (2, 3, 16, 30). Based on the results of the present study, it might therefore be expected that mTORC1 signaling would be repressed in the liver after consumption of a high-protein meal. However, the time course over which the two hormones change is typically distinct, with maximal insulin concentrations peaking prior to glucagon (3, 16, 30). Consequently, it is likely that mTORC1 signaling would initially be upregulated in the liver after consumption of a high-protein meal as a result of increased insulin and amino acid levels. Subsequent increases in glucagon levels would be expected to overcome the positive influence of insulin (present study) and amino acids (15), leading to downregulated mTORC1 signaling. The physiological consequences of transient activation of mTORC1 are unclear. However, acute inhibition of mTORC1 by rapamycin has no effect on global rates of protein synthesis in the liver but specifically decreases polysome association of mRNAs with a 5′-terminal oligopyrimidine tract, which are characteristic of ribosomal protein mRNAs (26). Although as yet unexplored, it is tempting to speculate that a transient increase in mTORC1 signaling induced by a high-protein meal might be sufficient to upregulate translation of 5′-terminal oligopyrimidine mRNAs, leading to a subsequent increase in ribosome number and the capacity to synthesize protein.
In summary, the present study demonstrates that, in contrast to the well-documented dominant activation of insulin over glucagon in the regulation of hepatic gluconeogenesis (reviewed in Ref. 25), the roles of the hormones are reversed with regard to the regulation of mTORC1 signaling, with the action of glucagon being dominant over that of insulin. The results are consistent with a model in which insulin acts to induce activation of mTORC1 signaling by enhancing the association of eIF3 with mTORC1. Glucagon, probably acting through PKA and AMPK, acts in a dominant manner to prevent the binding of eIF3 to mTORC1. Because AMPK is known to phosphorylate raptor and thereby inhibit mTORC1 signaling (7), it is tempting to speculate that raptor plays an important role in regulating association of eIF3 with mTORC1. However, a definitive explanation for the results will require future investigations.
GRANTS
This work was supported by Grant DK-13499 from the National Institute of Diabetes and Digestive and Kidney Diseases and a Mentor-Based Postdoctoral Fellowship Award from the American Diabetes Association.
Acknowledgments
We thank Drs. John Blenis and Marina K. Holz for advice concerning analysis of mTORC1·eIF3 association.
REFERENCES
- 1.Averous J, Proud CG. When translation meets transformation: the mTOR story. Oncogene 25: 6423–6435, 2006. [DOI] [PubMed] [Google Scholar]
- 2.Baum JI, Layman DK, Freund GG, Rahn KA, Nakamura MT, Yudell BE. A reduced carbohydrate, increased protein diet stabilizes glycemic control and minimizes adipose tissue glucose disposal in rats. J Nutr 136: 1855–1861, 2006. [DOI] [PubMed] [Google Scholar]
- 3.Calbet JA, MacLean DA. Plasma glucagon and insulin responses depend on the rate of appearance of amino acids after ingestion of different protein solutions in humans. J Nutr 132: 2174–2182, 2002. [DOI] [PubMed] [Google Scholar]
- 4.Exton JH The perfused rat liver. Methods Enzymol 39: 25–36, 1975. [DOI] [PubMed] [Google Scholar]
- 5.Feldhoff RC, Taylor JM, Jefferson LS. Synthesis and secretion of rat albumin in vivo, in perfused liver, and in isolated hepatocytes. Effects of hypophysectomy and growth hormone treatment. J Biol Chem 252: 3611–3616, 1977. [PubMed] [Google Scholar]
- 6.Gingras AC, Gygi SP, Raught B, Polakiewicz RD, Abraham RT, Hoekstra MF, Aebersold R, Sonenberg N. Regulation of 4E-BP1 phosphorylation: a novel two-step mechanism. Genes Dev 13: 1422–1437, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gwinn DM, Shackelford DB, Egan DF, Mihaylova MM, Mery A, Vasquez DS, Turk BE, Shaw RJ. AMPK phosphorylation of raptor mediates a metabolic checkpoint. Mol Cell 30: 214–226, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Holz MK, Ballif BA, Gygi SP, Blenis J. mTOR and S6K1 mediate assembly of the translation preinitiation complex through dynamic protein interchange and ordered phosphorylation events. Cell 123: 569–580, 2005. [DOI] [PubMed] [Google Scholar]
- 9.Inoki K, Li Y, Xu T, Guan KL. Rheb GTPase is a direct target of TSC2 GAP activity and regulates mTOR signaling. Genes Dev 17: 1829–1834, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Inoki K, Li Y, Zhu T, Wu J, Guan KL. TSC2 is phosphorylated and inhibited by Akt and suppresses mTOR signaling. Nat Cell Biol 4: 648–657, 2002. [DOI] [PubMed] [Google Scholar]
- 11.Inoki K, Ouyang H, Li Y, Guan KL. Signaling by target of rapamycin proteins in cell growth control. Microbiol Mol Biol Rev 69: 79–100, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jacinto E, Loewith R, Schmidt A, Lin S, Ruegg MA, Hall A, Hall MN. Mammalian TOR complex 2 controls the actin cytoskeleton and is rapamycin insensitive. Nat Cell Biol 6: 1122–1128, 2004. [DOI] [PubMed] [Google Scholar]
- 13.Kim DH, Sarbassov DD, Ali SM, King JE, Latek RR, Erdjument-Bromage H, Tempst P, Sabatini DM. mTOR interacts with raptor to form a nutrient-sensitive complex that signals to the cell growth machinery. Cell 110: 163–175, 2002. [DOI] [PubMed] [Google Scholar]
- 14.Kimball SR, Jurasinski CV, Lawrence JC Jr, Jefferson LS. Insulin stimulates protein synthesis in skeletal muscle by enhancing the association of eIF-4E and eIF-4G. Am J Physiol Cell Physiol 272: C754–C759, 1997. [DOI] [PubMed] [Google Scholar]
- 15.Kimball SR, Siegfried BA, Jefferson LS. Glucagon represses signaling through the mammalian target of rapamycin in rat liver by activating AMP-activated protein kinase. J Biol Chem 279: 54103–54109, 2004. [DOI] [PubMed] [Google Scholar]
- 16.Krezowski PA, Nuttall FQ, Gannon MC, Bartosh NH. The effect of protein ingestion on the metabolic response to oral glucose in normal individuals. Am J Clin Nutr 44: 847–856, 1986. [DOI] [PubMed] [Google Scholar]
- 17.Lee CH, Inoki K, Guan KL. mTOR pathway as a target in tissue hypertrophy. Annu Rev Pharmacol Toxicol 47: 443–467, 2007. [DOI] [PubMed] [Google Scholar]
- 18.Long X, Lin Y, Ortiz-Vega S, Yonezawa K, Avruch J. Rheb binds and regulates the mTOR kinase. Curr Biol 15: 702–713, 2005. [DOI] [PubMed] [Google Scholar]
- 19.Long X, Ortiz-Vega S, Lin Y, Avruch J. Rheb binding to mammalian target of rapamycin (mTOR) is regulated by amino acid sufficiency. J Biol Chem 280: 23433–23436, 2005. [DOI] [PubMed] [Google Scholar]
- 20.Ma L, Chen Z, Erdjument-Bromage H, Tempst P, Pandolfi PP. Phosphorylation and functional inactivation of TSC2 by Erk implications for tuberous sclerosis and cancer pathogenesis. Cell 121: 179–193, 2005. [DOI] [PubMed] [Google Scholar]
- 21.Manning BD, Tee AR, Logsdon MN, Blenis J, Cantley LC. Identification of the tuberous sclerosis complex-2 tumor suppressor gene product tuberin as a target of the phosphoinositide 3-kinase/Akt pathway. Mol Cell 10: 151–162, 2002. [DOI] [PubMed] [Google Scholar]
- 22.Mothe-Satney I, Gautier N, Hinault C, Lawrence JC Jr, Van Obberghen E. In rat hepatocytes glucagon increases mammalian target of rapamycin phosphorylation on serine 2448 but antagonizes the phosphorylation of its downstream targets induced by insulin and amino acids. J Biol Chem 279: 42628–42637, 2004. [DOI] [PubMed] [Google Scholar]
- 23.Navé BT, Ouwens M, Withers DJ, Alessi DR, Shepherd PR. Mammalian target of rapamycin is a direct target for protein kinase B: identification of a convergence point for opposing effects of insulin and amino-acid deficiency on protein translation. Biochem J 344: 427–431, 1999. [PMC free article] [PubMed] [Google Scholar]
- 24.Nojima H, Tokunaga C, Eguchi S, Oshiro N, Hidayat S, Yoshino K, Hara K, Tanaka N, Avruch J, Yonezawa K. The mammalian target of rapamycin (mTOR) partner, raptor, binds the mTOR substrates p70 S6 kinase and 4E-BP1 through their TOR signaling (TOS) motif. J Biol Chem 278: 15461–15464, 2003. [DOI] [PubMed] [Google Scholar]
- 25.Quinn PG, Yeagley D. Insulin regulation of PEPCK gene expression: a model for rapid and reversible modulation. Curr Drug Targets Immune Endocr Metabol Disord 5: 423–437, 2005. [DOI] [PubMed] [Google Scholar]
- 26.Reiter AK, Anthony TG, Anthony JC, Jefferson LS, Kimball SR. The mTOR signaling pathway mediates control of ribosomal protein mRNA translation in rat liver. Int J Biochem Cell Biol 36: 2169–2179, 2004. [DOI] [PubMed] [Google Scholar]
- 27.Roux PP, Ballif BA, Anjum R, Gygi SP, Blenis J. Tumor-promoting phorbol esters and activated Ras inactivate the tuberous sclerosis tumor suppressor complex via p90 ribosomal S6 kinase. Proc Natl Acad Sci USA 101: 13489–13494, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sarbassov DD, Ali SM, Kim DH, Guertin DA, Latek RR, Erdjument-Bromage H, Tempst P, Sabatini DM. Rictor, a novel binding partner of mTOR, defines a rapamycin-insensitive and raptor-independent pathway that regulates the cytoskeleton. Curr Biol 14: 1296–1302, 2004. [DOI] [PubMed] [Google Scholar]
- 29.Schalm SS, Fingar DC, Sabatini DM, Blenis J. TOS motif-mediated raptor binding regulates 4E-BP1 multisite phosphorylation and function. Curr Biol 13: 797–806, 2003. [DOI] [PubMed] [Google Scholar]
- 30.Schmid R, Schusdziarra V, Schulte-Frohlinde E, Maier V, Classen M. Role of amino acids in stimulation of postprandial insulin, glucagon, and pancreatic polypeptide in humans. Pancreas 4: 305–314, 1989. [DOI] [PubMed] [Google Scholar]
- 31.Sekulic A, Hudson CC, Homme JL, Yin P, Otterness DM, Karnitz LM, Abraham RT. A direct linkage between the phosphoinositide 3-kinase-Akt signaling pathway and the mammalian target of rapamycin in mitogen-stimulated and transformed cells. Cancer Res 60: 3504–3523, 2000. [PubMed] [Google Scholar]
- 32.Smith EM, Finn SG, Tee AR, Browne GJ, Proud CG. The tuberous sclerosis protein TSC2 is not required for the regulation of the mammalian target of rapamycin by amino acids and certain cellular stresses. J Biol Chem 280: 18717–18727, 2005. [DOI] [PubMed] [Google Scholar]
- 33.Tee AR, Anjum R, Blenis J. Inactivation of the tuberous sclerosis complex-1 and -2 gene products occurs by phosphoinositide 3-kinase/Akt-dependent and -independent phosphorylation of tuberin. J Biol Chem 278: 37288–37296, 2003. [DOI] [PubMed] [Google Scholar]
- 34.Tee AR, Manning BD, Roux PP, Cantley LC, Blenis J. Tuberous sclerosis complex gene products, Tuberin and Hamartin, control mTOR signaling by acting as a GTPase-activating protein complex toward Rheb. Curr Biol 13: 1259–1268, 2003. [DOI] [PubMed] [Google Scholar]
- 35.Ueno M, Carvalheira JB, Tambascia RC, Bezerra RM, Amaral ME, Carneiro EM, Folli F, Franchini KG, Saad MJ. Regulation of insulin signalling by hyperinsulinaemia: role of IRS-1/2 serine phosphorylation and the mTOR/p70 S6K pathway. Diabetologia 48: 506–518, 2005. [DOI] [PubMed] [Google Scholar]
- 36.Zhang Y, Gao X, Saucedo LJ, Ru B, Edgar BA, Pan D. Rheb is a direct target of the tuberous sclerosis tumour suppressor proteins. Nat Cell Biol 5: 578–581, 2003. [DOI] [PubMed] [Google Scholar]