Abstract
To determine the effect of an acute increase in hepatic glycogen on net hepatic glucose uptake (NHGU) and disposition in response to insulin in vivo, studies were performed on two groups of dogs fasted 18 h. During the first 4 h of the study, somatostatin was infused peripherally, while insulin and glucagon were replaced intraportally in basal amounts. Hyperglycemia was brought about by glucose infusion, and either saline (n = 7) or fructose (n = 7; to stimulate NHGU and glycogen deposition) was infused intraportally. A 2-h control period then followed, during which the portal fructose and saline infusions were stopped, allowing NHGU and glycogen deposition in the fructose-infused animals to return to rates similar to those of the animals that received the saline infusion. This was followed by a 2-h experimental period, during which hyperglycemia was continued but insulin infusion was increased fourfold in both groups. During the initial 4-h glycogen loading period, NHGU averaged 1.18 ± 0.27 and 5.55 ± 0.53 mg·kg−1·min−1 and glycogen synthesis averaged 0.72 ± 0.24 and 3.98 ± 0.57 mg·kg−1·min−1 in the saline and fructose groups, respectively (P < 0.05). During the 2-h hyperinsulinemic period, NHGU rose from 1.5 ± 0.4 and 0.9 ± 0.2 to 3.1 ± 0.6 and 2.5 ± 0.5 mg·kg−1·min−1 in the saline and fructose groups, respectively, a change of 1.6 mg·kg−1·min−1 in both groups despite a significantly greater liver glycogen level in the fructose-infused group. Likewise, the metabolic fate of the extracted glucose (glycogen, lactate, or carbon dioxide) was not different between groups. These data indicate that an acute physiological increase in the hepatic glycogen content does not alter liver glucose uptake and storage under hyperglycemic/hyperinsulinemic conditions in the dog.
Keywords: liver glycogen, hyperglycemia, hyperinsulinemia, hepatic insulin signaling
in response to a moderately sized oral glucose challenge or a mixed meal, approximately one-third of the ingested glucose is taken up by the liver, with the remaining two-thirds being metabolized by other tissues of the body (6, 25, 26). Previous research in humans, however, has indicated that in pathological conditions such as type 2 diabetes mellitus (T2DM) the ability of splanchnic tissues (which include the liver) to take up glucose from the blood and store it as glycogen is impaired (3–5, 22, 25). Such a manifestation of insulin resistance contributes significantly to glucose intolerance and the hyperglycemia seen during the postabsorptive period in individuals with T2DM and is a major impetus for the development of medications that would increase liver glycogen deposition in this population.
Hepatic glucose uptake has been shown to be regulated by a number of factors, including the glucose load to the liver, the sinusoidal plasma insulin level, and the arterial-portal glucose gradient. During the postabsorptive period, net hepatic glucose balance (NHGB) is such that glucose is produced at a rate of ∼2.0 mg·kg−1·min−1. However, infusion of glucose into a peripheral vein to double the load to the liver in the presence of basal insulin causes NHGB to become null or slightly negative (indicating slight uptake), because of a suppression of hepatic glucose output and a smaller increase in hepatic glucose uptake (34). Insulin is also well known to regulate hepatic glucose metabolism. While hyperinsulinemia within the physiological range fails to stimulate net hepatic glucose uptake (NHGU) under euglycemic conditions (27), it can stimulate NHGU and glycogen synthesis in a dose-response manner in the presence of hyperglycemia (33).
A number of papers have been published indicating an inverse relationship between the rate of insulin-mediated glucose uptake and the preexisting intramyocellular glycogen level (11, 20, 24). Furthermore, this relationship appears to be mediated, in part, by a similar inverse relationship between the glycogen synthetic rate and the glycogen pool size that may (11, 20, 24) or may not (18) be a consequence of impaired insulin signaling. Previous studies in the dog have shown that prolonging the fast duration, and thus reducing the hepatic glycogen content, does not alter NHGU during hyperglycemic/hyperinsulinemic clamp conditions (1), total parenteral nutrition (7), or intraduodenal glucose infusion (13). On the other hand, both Chen et al. (7) and Galassetti et al. (13) observed that lowering the liver glycogen level by virtue of a prolonged fast was associated with an increase in the proportion of NHGU converted to glycogen. However, it is difficult to attribute those changes to alterations in the glycogen mass per se given the various metabolic changes that are associated with fasting. The consequences of an acute rise in liver glycogen for hepatic glucose metabolism have not been studied, and they may not be the reciprocal of those associated with a decrease in liver glycogen. In the present study, we used hyperglycemia and catalytic amounts of fructose to stimulate NHGU and glycogen deposition in 18-h-fasted dogs, resulting in an acute physiological increase in the hepatic glycogen content. This was followed by a control period and a 2-h hyperglycemic/hyperinsulinemic period to determine whether the increase in glycogen would impair insulin's ability to stimulate NHGU and net glycogen synthesis.
DESIGN AND METHODS
Animals and Surgical Procedures
Studies were carried out on healthy, conscious 18-h-fasted mongrel dogs with a mean weight of 22.5 ± 0.5 kg. A fast of this duration was chosen because a longer fast would make it more difficult to elevate liver glycogen to the level desired. All animals were maintained on a diet of meat (Kal Kan, Vernon, CA) and chow (Purina Lab Canine Diet no. 5006; Purina Mills, St. Louis, MO) comprised of 34% protein, 14.5% fat, 46% carbohydrate, and 5.5% fiber based on dry weight (∼1,500 kcal/day). The animals were housed in a facility that met American Association for Accreditation of Laboratory Animal Care guidelines, and the protocol was approved by the Vanderbilt University Medical Center Animal Care and Use Committee.
Approximately 16 days before study, each dog underwent a laparotomy under general anesthesia (0.01 mg/kg buprenorphine and 5 mg/kg propofol before surgery and ∼1.0–2.5% isoflurane inhalation anesthetic during surgery), and silicone rubber catheters for sampling were inserted in a hepatic vein, the hepatic portal vein, and a femoral artery as described in detail elsewhere (33). Catheters for intraportal infusion were placed in a splenic and a jejunal vein (each of which empties into the portal vein), while ultrasonic flow probes (Transonic Systems, Ithaca, NY) were placed around the portal vein and the hepatic artery as described elsewhere (33).
Approximately 2 days before each study, blood was drawn to determine the leukocyte count and hematocrit for each animal. The dog was studied only if it had a leukocyte count <18,000/mm3, a hematocrit >35%, a good appetite (as evidenced by consumption of 75% of the entire daily ration), and normal stools.
On the morning of each study, the catheters and flow probe leads were exteriorized from subcutaneous pockets under local anesthesia (2% lidocaine, Hospira, Lake Forest, IL). The contents of each catheter were aspirated, and then they were flushed with saline. Catheters (Deseret Medical, Becton Dickinson, Sandy, UT) were then inserted into the cephalic and saphenous veins to allow infusions as described. The animals stood comfortably in a Pavlov harness throughout the experiment.
Experimental Design
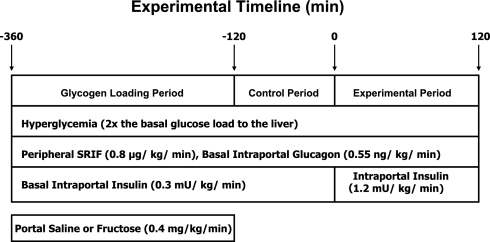
Each experiment consisted of a 4-h liver glycogen loading period (−360 to −120 min), a 2-h control period (−120 to 0 min), and a 2-h experimental period (0 to 120 min). Each experiment was initiated at −360 min by the infusion of somatostatin (SRIF; 0.8 μg·kg−1·min−1; Bachem, Torrance, CA) into a peripheral vein to disable the endocrine pancreas (Fig. 1). This was accompanied by the intraportal replacement of both insulin (0.3 mU·kg−1·min−1; Eli Lilly, Indianapolis, IN) and glucagon (0.55 ng·kg−1·min−1; Glucagen, Novo Nordisk, Bagsvaerd, Denmark) at basal rates. At the same time, the hepatic glucose load was doubled by the infusion of a 50% dextrose solution into a peripheral vein and fructose (n = 7; 0.4 mg·kg−1·min−1) or saline (n = 7; 0.9%) into the portal vein to stimulate NHGU and glycogen deposition (in the fructose group), creating a resultant difference in the hepatic glycogen content between groups. This 4-h glycogen loading period was followed by a 2-h hyperglycemic control period during which the portal vein infusions of saline and fructose were discontinued but the pancreatic clamp and hyperglycemic clamp were continued, allowing NHGU and glycogen deposition in the fructose-infused animals to return to rates similar to those of the saline-infused dogs. During the final 2-h period (i.e., 0–120 min), the intraportal infusion of insulin was increased to four times the basal rate (1.2 mU·kg−1·min−1) in both groups, while the elevated hepatic glucose load was maintained by the infusion of glucose into a peripheral vein as necessary. In all experiments a constant infusion of indocyanine green (ICG) dye (0.076 mg/min; Sigma Immunochemicals, St. Louis, MO) was begun at −210 min via a peripheral vein, and a constant infusion of [14C]glucose was begun at −90 min to measure hepatic glucose oxidation, allowing for equilibration of the tracer with blood glucose before the experimental period. At the conclusion of the study, animals were euthanized with an overdose of pentobarbital. Immediately thereafter, liver and muscle biopsies were taken with prechilled Wallenburger tongs. The positions of the catheter tips were then verified, and the biopsy samples were stored at −80°C until they were assayed.
Fig. 1.
Schematic representation of the study. SRIF, somatostatin.
Processing and Analysis of Samples
The collection and immediate processing of blood samples have been described previously (12). Four 10-μl aliquots of plasma from each sample were immediately analyzed for glucose with the glucose oxidase method (Beckman Instruments, Fullerton, CA). Plasma insulin, glucagon, cortisol, lactate, glycerol, and nonesterified fatty acid (NEFA) concentrations were measured as previously described (34). Liver samples were pulverized in liquid nitrogen and assayed for liver glycogen as described previously (21).
14C-labeled carbon dioxide was sequestered in an airtight 20-ml vial by treating a 500-μl aliquot of whole blood with 500 μl of 6 N HCl, to lyse the red cells and allow all gases to diffuse within the vial. The carbon dioxide was collected with Whatman chromatography paper (Whatman International; Maidstone, UK) treated with 400 μl of 1.0 M benzethonium hydroxide while suspended in a well above the blood sample to prevent contamination with tracer from glucose in the whole blood. Samples were allowed to incubate at room temperature overnight, after which the well and Whatman paper were transferred to a scintillation vial along with 2 ml of 0.5 M NaOH and 10 ml of scintillation cocktail (EcoLite) and agitated for 2 h at room temperature. Samples were then incubated in the dark for 2 wk before counting.
Protein Extraction, SDS-PAGE, and Immunoblotting
Electrophoretic separation, blotting, and immunodetection of proteins were performed as described previously (35). Frozen tissue samples were homogenized in buffer including 50 mM Tris·HCl pH 7.0, 100 mM sucrose, 10% (vol/vol) glycerol, 2 mM EDTA, 2 mM EGTA, 25 mM NaF, 10 μl/ml buffer of phosphatase inhibitor cocktail 1 and 2, and protease inhibitor cocktail (Sigma, St. Louis, MO). Homogenates were centrifuged at 10,000 g for 20 min, supernatants were removed, and soluble protein concentration was determined with the Bio-Rad protein assay (Bio-Rad, Hercules, CA). Aliquots of supernatant were mixed 1:1 (vol/vol) with freshly prepared 2× SDS-PAGE loading buffer [100 mM Tris·HCl, pH 6.8, 4% (wt/vol) SDS, 20% (vol/vol) glycerol, 0.2% (wt/vol) bromophenol blue, 10% (vol/vol) 2-mercaptoethanol], boiled for 10 min, and then frozen and stored at −20°C.
Samples were subjected to SDS-PAGE (12% resolving gel) followed by transfer to nitrocellulose membranes. Blocking was performed with 5% (wt/vol) bovine serum albumin in Tris-buffered saline containing Tween 20 [TBST: 10 mM Tris-base, pH 7.0, 150 mM NaCl, 0.1% (vol/vol) Tween 20] for 1 h at room temperature, and membranes were then incubated 2 h with the appropriate primary antibody (Cell Signaling, Danvers, MA). After three 5-min washes with TBST, membranes were incubated with horseradish peroxidase (HRP)-conjugated secondary antibody (Promega, Madison, WI) for 1 h at room temperature, followed by three 5-min washes in TBST. Proteins were visualized with ECL Plus Western detection reagents (GE Healthcare, Piscataway, NJ) and the ECL signal was detected after brief (5–30 s) exposure to X-ray film. Bands were quantified with ImageJ software (http://rsb.info.nih.gov/ij/), and the intensity of the phosphoprotein signal was normalized to the total protein signal, with β-actin used as a loading control.
Calculations and Data Analysis
Hepatic blood flow (HBF) was measured with ultrasonic flow probes and with the use of ICG dye according to the method of Leevy et al. (23). Plasma glucose levels were converted to whole blood values as described previously (19, 30). Net hepatic substrate balance, net hepatic fractional substrate extraction, sinusoidal insulin and glucagon levels, and the hepatic glucose load were calculated as described previously (32). Nonhepatic glucose uptake was calculated as the sum of NHGB and the exogenous glucose infusion rate after correcting for the change in glucose mass (28). The hepatic glucose oxidation rate was calculated by dividing the net hepatic balance of 14CO2 by the arterial [14C]glucose specific activity. Net hepatic glycogen synthesis was estimated as [NHGB] − [NHLO (in glucose equivalents) + hepatic glucose oxidation], where [NHGB] is the absolute value of NHGB, and NHLO is the net hepatic lactate output. Previous studies in humans have indicated that <2% of carbohydrate administered either orally or intravenously is converted to lipid in the liver (16), indicating its relative lack of importance in the measurement of glucose disposition. Furthermore, while acute consumption of fructose has been shown to increase the plasma triglyceride level, this has been ascribed to reduced clearance of triglycerides, not an increase in their production (8, 17).
Statistical Analysis
All data are presented as means ± SE, and statistical analyses were performed with SigmaStat (Aspire Software International; Ashburn, VA) software. Clamp data were analyzed with repeated-measures ANOVA (group × time), while unpaired Student's t-test was used to compare the glycogen levels between groups. Post hoc comparisons were made as appropriate with the Student-Newman-Keuls method. Regression analyses were performed via simple linear regression. Statistical significance was determined as P < 0.05.
RESULTS
Plasma Hormone and Glucose Concentrations and Hepatic Glucose and Lactate Metabolism
Glycogen loading period.
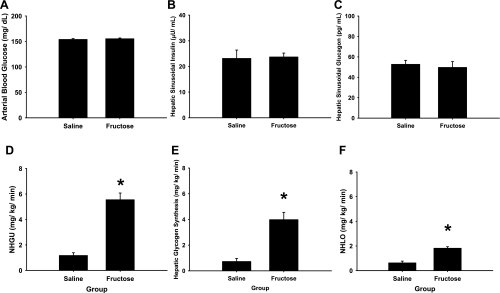
During the glycogen loading period the arterial plasma glucose level was 2 × basal in each group. On the other hand, the hepatic sinusoidal insulin and glucagon levels were basal and similar in both groups [P = not significant (NS); Fig. 2], as were the plasma cortisol levels (2.4 ± 0.3 μg/dl for saline and 2.8 ± 0.4 μg/dl for fructose). HBF was slightly higher during the glycogen loading period in the fructose group (P < 0.05 vs. saline; Table 1). NHGU, net hepatic glycogen synthesis, and net hepatic lactate output were much greater during the glycogen loading period in the fructose infusion group than in the saline infusion group (P < 0.001; Fig. 2).
Fig. 2.
Arterial blood glucose (A), hepatic sinusoidal insulin (B), hepatic sinusoidal glucagon (C), net hepatic glucose uptake (NHGU; D), net hepatic glycogen synthesis (E), and net hepatic lactate output (NHLO, in glucose equivalents; F) during the final 20 min of the 4-h glycogen loading period. *P < 0.05, fructose infusion group compared with saline infusion group.
Table 1.
Metabolic parameters and net substrate balances across the liver throughout the study
| Group |
Glycogen Loading Period |
Control Period
|
Experimental Period
|
|||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| −140 min | −120 min | −90 min | −60 min | −30 min | 0 min | 15 min | 30 min | 45 min | 60 min | 90 min | 120 min | |||||||||||||
| Total hepatic blood flow, ml·kg−1·min−1 | ||||||||||||||||||||||||
| Sal | 24±1 | 24±2 | 24±1 | 23±1 | 22±1 | 24±2 | 23±1 | 25±1 | 25±1 | 27±1 | 27±1 | 27±1 | ||||||||||||
| Fruc | 27±2* | 27±1* | 28±1* | 24±1 | 24±2 | 25±2 | 25±2 | 25±2 | 24±2 | 26±2 | 26±2 | 26±2 | ||||||||||||
| Arterial blood lactate, μmol/l | ||||||||||||||||||||||||
| Sal | 1,009±173 | 1,052±165 | 944±182 | 1,002±197 | 977±181 | 933±162 | 1,018±153 | 970±134 | 1,026±113 | 1,025±117 | 1,071±80 | 1,099±151 | ||||||||||||
| Fruc | 1,601±154* | 1,650±154* | 1,305±117* | 989±83 | 891±40 | 887±43 | 835±51 | 957±23 | 997±50 | 948±70 | 926±111 | 1,011±104 | ||||||||||||
| Net hepatic lactate production, μmol·kg−1·min−1 | ||||||||||||||||||||||||
| Sal | 6.9±1.5 | 6.7±2.1 | 6.4±1.7 | 4.1±2.1 | 5.7±1.4 | 7.6±1.4 | 8.2±1.4 | 9.0±1.0 | 7.8±1.1 | 8.1±1.5 | 4.0±1.0 | 3.8±1.6 | ||||||||||||
| Fruc | 19.8±1.7* | 20.0±1.7* | 9.2±1.3* | 4.0±1.2 | 2.6±1.2 | 5.4±1.6 | 6.9±1.6 | 8.7±1.9 | 7.3±1.0 | 6.5±1.3 | 4.0±1.2 | 2.5±1.1 | ||||||||||||
| Arterial FFA, μmol/l | ||||||||||||||||||||||||
| Sal | 411±77 | 441±84 | 448±43 | 449±77 | 194±41 | 123±48 | 113±36 | 73±22 | ||||||||||||||||
| Fruc | 446±74 | 433±70 | 562±84 | 581±123 | 249±78 | 146±42 | 134±47 | 150±51 | ||||||||||||||||
| Net hepatic FFA uptake, μmol·kg−1·min−1 | ||||||||||||||||||||||||
| Sal | 1.2±0.3 | 1.4±0.1 | 1.2±0.1 | 1.3±0.2 | 0.5±0.1 | 0.2±0.1 | 0.2±0.1 | 0.1±0.1 | ||||||||||||||||
| Fruc | 1.7±0.4 | 1.5±0.3 | 1.5±0.2 | 0.8±0.7 | 0.6±0.2 | 0.1±0.2 | 0.4±0.1 | 0.2±0.1 | ||||||||||||||||
| Arterial blood glycerol, μmol/l | ||||||||||||||||||||||||
| Sal | 57±8 | 62±13 | 64±13 | 59±12 | 56±7 | 60±7 | 43±6 | 37±4 | 29±3 | 31±5 | 32±4 | 29±5 | ||||||||||||
| Fruc | 51±9 | 45±7 | 50±10 | 64±9 | 60±10 | 63±10 | 41±6 | 31±7 | 30±5 | 31±8 | 33±7 | 30±6 | ||||||||||||
| Net hepatic glycerol uptake, μmol·kg−1·min−1 | ||||||||||||||||||||||||
| Sal | 1.0±0.2 | 1.2±0.2 | 1.2±0.3 | 1.1±0.3 | 0.9±0.2 | 1.1±0.2 | 0.7±0.1 | 0.7±0.1 | 0.5±0.1 | 0.6±0.1 | 0.7±0.1 | 0.5±0.1 | ||||||||||||
| Fruc | 0.6±0.6 | 0.9±0.2 | 0.8±0.2 | 1.1±0.2 | 1.0±0.3 | 1.2±0.3 | 0.7±0.2 | 0.6±0.2 | 0.5±0.2 | 0.7±0.3 | 0.7±0.2 | 0.7±0.3 | ||||||||||||
| Exogenous glucose infusion rate, mg·kg−1·min−1 | ||||||||||||||||||||||||
| Sal | 4.1±0.8 | 4.5±1.1 | 3.8±0.6 | 3.5±0.5 | 4.4±0.6 | 4.2±0.7 | 4.8±1.0 | 9.0±2.2 | 12.3±2.4 | 14.3±3.1 | 17.9±4.1 | 17.9±3.3 | ||||||||||||
| Fruc | 7.3±1.0* | 7.2±1.0* | 4.7±0.9 | 3.1±0.8 | 3.1±0.8 | 2.5±0.8 | 3.2±0.9 | 6.7±1.3 | 8.7±2.2 | 10.6±2.9 | 12.9±3.0 | 13.9±3.5 | ||||||||||||
| Nonhepatic glucose uptake, mg·kg−1·min−1 | ||||||||||||||||||||||||
| Sal | 3.1±1.0 | 2.3±.05 | 2.4±0.2 | 3.0±0.5 | 3.0±0.6 | 2.9±0.9 | 7.9±2.2 | 9.8±2.3 | 11.5±2.9 | 15.1±3.7 | 14.8±3.0 | |||||||||||||
| Fruc | 1.8±0.6 | 0.4±0.7 | 1.4±0.4 | 2.0±0.5 | 1.7±0.6 | 2.1±0.6 | 5.5±1.3 | 6.8±2.0 | 8.4±2.5 | 10.6±2.5 | 11.2±2.9 | |||||||||||||
Data are means ± SE. FFA, free fatty acids.
P < 0.05 for fructose infusion (Fruc) group compared with saline infusion (Sal) group.
Control and experimental periods.
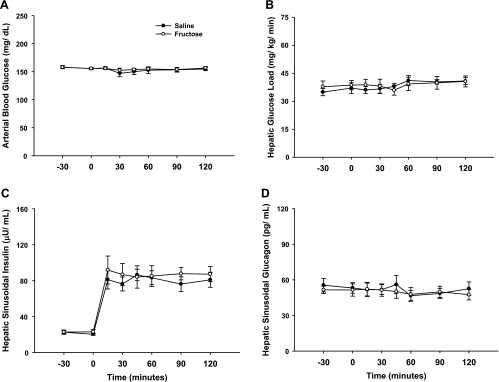
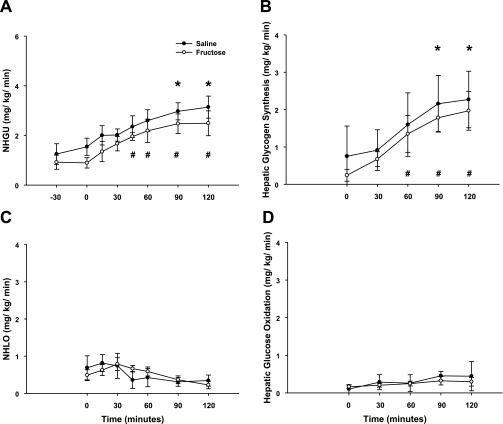
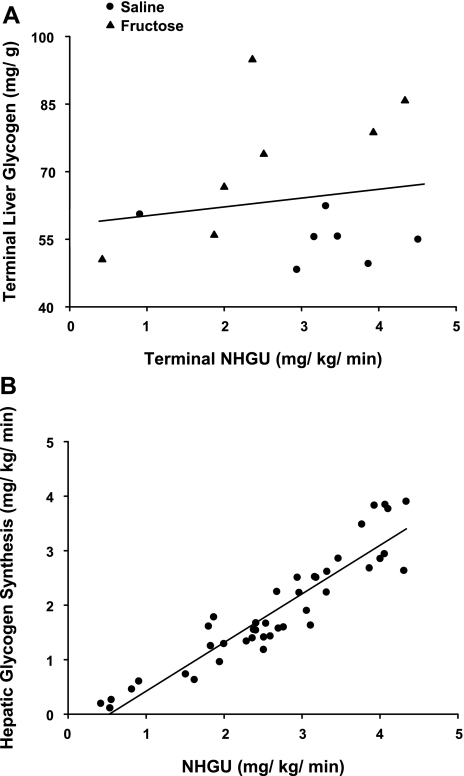
Total HBF, which was slightly elevated during fructose infusion, returned to a rate similar to that in the saline-infused group within 1 h of the withdrawal of fructose infusion (Table 1). Likewise, the arterial blood glucose levels and the hepatic glucose loads were similar between groups throughout these periods (Fig. 3). The hepatic sinusoidal insulin levels were increased fourfold, while the hepatic sinusoidal glucagon levels remained basal (Fig. 3) in both groups during the experimental period. As expected, NHGU was modest (∼1 mg·kg−1·min−1) during the last 30 min of the control period regardless of treatment. When insulin was raised, NHGU (Fig. 4) and the hepatic fractional extraction of glucose (data not shown) rose similarly in both groups, but it took 90 min and 45 min in the saline and fructose groups, respectively, for NHGU to increase significantly. The area under the curve for the change in NHGU from baseline (i.e., minute 0) over the entire 2-h experimental period was also similar (113 ± 14 and 133 ± 32 mg·kg−1·120 min in the 2 groups, respectively; P = NS between groups). This rise in NHGU was accompanied by a similar increase in net glycogen synthesis (Fig. 4) in both groups. Hepatic glucose oxidation and net hepatic lactate balance were also similar between groups (Fig. 4) and changed minimally over time. When expressed as a percentage of NHGU over the final hour of the experimental period, hepatic glucose oxidation, lactate production, and net glycogen synthesis accounted for 13 ± 3%, 20 ± 3%, and 67 ± 3%, respectively, of NHGU in the saline group, and 14 ± 4%, 24 ± 8%, and 62 ± 9%, respectively, in the fructose group. No significant relationship was detectable between the terminal (i.e., at minute 120 of the study) NHGU and the terminal hepatic glycogen content (r = 0.16, P = 0.57; Fig. 5), even when the groups were considered separately (data not shown), indicating that over the glycogen range studied the increase in hepatic glycogen did not diminish the response of NHGU to hyperinsulinemia. As expected, a significant positive relationship was observed between the rates of NHGU and net glycogen synthesis (r = 0.93, P < 0.001; Fig. 5) during the final hour of the experimental period.
Fig. 3.
Arterial blood glucose (A), glucose load to the liver (B), hepatic sinusoidal insulin (C), and hepatic sinusoidal glucagon (D) before and during the experimental period. No differences were detected between groups for any variable [P = not significant (NS)].
Fig. 4.
NHGU (A), net hepatic glycogen synthesis (B), NHLO (in glucose equivalents; C), and hepatic glucose oxidation (D) during the experimental period. No differences were detected between groups at any time point (P = NS). * and #Significantly different (P < 0.05) from minute 0 in saline and fructose groups, respectively.
Fig. 5.
Relationship between the terminal liver glycogen level and terminal NHGU (r = 0.16, P = NS; A) and the relationship between NHGU and net glycogen synthesis during the final hour of the experimental period (r = 0.93; P < 0.001; B).
Fat Metabolism
Arterial free fatty acids and glycerol were basal and similar between groups during the glycogen loading and control periods (Table 1). As expected, the fourfold rise in insulin levels lowered both. No differences in their concentrations or flux rates were detected between groups at any time during the study.
Whole Body and Nonhepatic Glucose Uptake
The total exogenous glucose infusion rate (GIR) was higher during fructose infusion compared with saline infusion (P < 0.05; Table 1). However, after fructose infusion was discontinued, GIR returned to a rate similar to that of the saline group during the control period. In response to insulin infusion, GIR rose similarly in both groups (Δ from baseline of 13.6 ± 2.7 and 11.3 ± 3.2 mg·kg−1·min−1 in saline and fructose groups, respectively). Nonhepatic glucose uptake (Table 1) was similar between groups during the fructose infusion and control periods and also rose steadily during the experimental period in both groups, reaching significance compared with the control period by minutes 30 and 60 in the saline and fructose groups, respectively (P < 0.05). As with GIR, nonhepatic glucose uptake rose similarly in both groups (Δ from baseline of 11.8 ± 2.6 and 9.5 ± 2.8 mg·kg−1·min−1 in saline and fructose groups, respectively), and no significant differences were observed between groups at any time point (P = NS).
Tissue Insulin Signaling and Liver Glycogen Levels
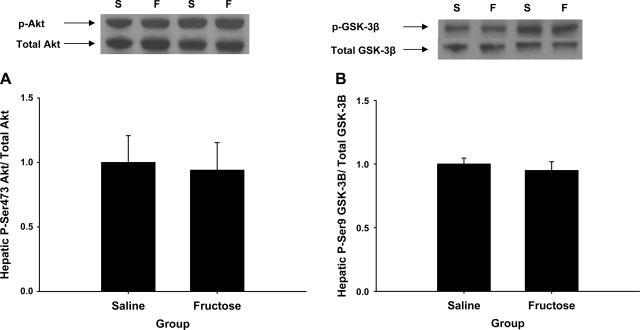
At the conclusion of the study the hepatic glycogen level was higher in the fructose infusion group than in the saline infusion group (72.3 ± 6.0 vs. 55.2 ± 2.0 mg/g liver, respectively; P < 0.02). On the other hand, the skeletal muscle glycogen level was similar in both groups (8.3 ± 0.9 vs. 8.0 ± 0.6 mg/g, respectively; P = 0.65). There were no differences in the phosphorylation state of hepatic Akt or GSK3β in the saline and fructose groups (P = NS; Fig. 6), indicating that insulin signaling was similar in both groups. Likewise, no differences in skeletal muscle insulin signaling were observed between groups (data not shown).
Fig. 6.
Postexperimental hepatic Akt (A) and GSK3β (B) signaling. Top: Western blot data representative of all animals (S and F, saline- and fructose-infused animals, respectively). No difference was observed between groups for either measure (P = NS).
DISCUSSION
The purpose of this study was to determine whether an acute physiological increase in the hepatic glycogen level would reduce the uptake and/or alter the disposition of glucose by the liver in response to hyperinsulinemia. Previous studies have measured NHGU and glucose disposition in the presence of moderate and low liver glycogen levels (1, 7, 13), but the prolonged fast employed to reduce liver glycogen undoubtedly produced many hepatic changes in addition to a reduction in glycogen, making interpretation of the data as they relate to the glycogen content problematic. Considering that efforts are underway to develop medications [e.g., glucokinase (GK) activators, phosphorylase inhibitors, glucagon receptor antagonists] that would increase the liver glycogen level, and may very well overfill the hepatic glycogen pool, it is important to understand the metabolic consequences associated with changes in liver glycogen. Our findings indicate that acutely raising the hepatic glycogen level by a large but physiological amount does not alter insulin signaling, insulin-mediated NHGU, or the disposition of glucose within the liver.
Although hyperinsulinemia could have been used to increase the hepatic glycogen level, we chose to use a small (∼2 g over a 4-h period) catalytic dose of fructose on the background of hyperglycemia. Fructose is almost exclusively metabolized by the liver and is converted to fructose-1-phosphate, which decreases the affinity of GK for its regulatory protein (GKRP), allowing GK to translocate from the nucleus to the cytosol where glucose is phosphorylated (2). The use of fructose was particularly attractive for our study because GK translocation and the stimulation of NHGU occur within 15 min (37), and this effect is rapidly reversed upon the cessation of fructose infusion (Shiota M and Cherrington AD, unpublished observations). Our data are in agreement with those observations, as evidenced by a fivefold increase in NHGU and net glycogen synthesis during fructose infusion and a rapid return of these parameters to rates no different from those in the saline group after the fructose infusion was stopped. Hyperinsulinemia was not as attractive as fructose for glycogen loading because it stimulates NHGU quite slowly in vivo (34), and this effect is also slowly reversed. In addition, insulin is not able to stimulate NHGU to the same extent as fructose and thus would not fill the glycogen pool to the same extent (33, 37).
In previous studies, our laboratory (1) and others (7, 13) have investigated the effect of differing fasting lengths (i.e., 18 h vs. 36 h), and thus a lowering of the hepatic glycogen level (albeit not the only change to occur) on hepatic glucose metabolism. Adkins-Marshall et al. (1), using conditions similar to those employed in our study (e.g., 2× basal glucose load, 4× basal insulin and basal glucagon), showed that lowering the glycogen level by prolonging the fast did not affect NHGU, with rates being 1.6 and 1.4 mg·kg−1·min−1 in the prolonged (36 h) and overnight (18 h) fasted groups, respectively. Furthermore, Chen et al. (7) provided 8 h of total parenteral nutrition to conscious dogs fasted for 18 or 42 h and showed no statistical difference in NHGU between groups. However, lactate release by the liver tended to be lower in the 42-h group, meaning that a greater percentage (86 ± 10%) of NHGU was stored as glycogen compared with the 18-h-fasted group (52 ± 9%). A finding of increased glycogen synthesis relative to NHGU after prolonged fasting was also reported by Galassetti et al. (13), who showed that despite similar rates of NHGU (∼2 mg·kg−1·min−1) during intraduodenal glucose infusion, 79% and 23% of NHGU were diverted to glycogen in 42- and 18-h-fasted dogs, respectively. However, the finding that only 23% of NHGU was converted to glycogen in 18-h-fasted dogs is surprising since our data show that ∼65% of glucose taken up by the liver was converted to glycogen. One explanation for this difference is the relatively low systemic levels of glucose and insulin reported by Galassetti et al., which translated into unusually low rates of NHGU during intraduodenal glucose infusion. Moore et al. (29), using an intraduodenal glucose infusion rate identical to that of Galassetti et al., observed NHGU rates as high as 4.6 mg·kg−1·min−1 in 42-h-fasted dogs, presumably due to markedly higher levels of glucose and insulin, with 57% of NHGU being disposed of as glycogen.
To study the impact of the glycogen level on hepatic glucose metabolism without the confounding effects of fasting, we acutely raised the hepatic glycogen level in 18-h-fasted dogs. Our data suggest that a physiological increase in the liver glycogen concentration does not affect insulin signaling, nor does it affect insulin-mediated NHGU. In fact, the maximal increase in NHGU from baseline during the 2-h insulin infusion period was 1.6 mg·kg−1·min−1 in both the saline- and fructose-infused groups despite the difference in hepatic glycogen content. Likewise, the disposition of glucose did not differ between groups.
It is worth noting that the increase in NHGU that was seen in response to hyperinsulinemia occurred quite slowly in both groups. Furthermore, it occurred in concert with a decline in plasma NEFA levels, consistent with previous studies in which hyperglycemia preceded hyperinsulinemia (31, 34). Thus one must question whether it is insulin per se that stimulated glucose uptake by the liver or the decline in NEFA. Previous data shed light on this question. In the presence of basal insulin and glucagon, and preexisting hyperglycemia, intralipid infusion to offset a nicotinic acid-mediated decline in NEFA (i.e., an increase in NEFA from ∼50 μmol/l to 914 μmol/l) reduced NHGU by ∼1.1 mg·kg−1·min−1, which was accounted for entirely by an increase in endogenous glucose production (32). This indirect effect of insulin would account for about two-thirds of the insulin-mediated increase in NHGU in our study, with the remaining third being due to a direct effect of the hormone on the liver and presumably the activation of glycogen synthase (34). While insulin signaling per se can cause the translocation of GK to the cytosol in the rat, its effect is not additive to that of hyperglycemia (9). Thus one might predict a slower rate of progression of NHGU when hyperinsulinemia is initiated after hyperglycemia. While future studies will be required to discriminate between the roles of insulin and glucose in hepatic glucose uptake, one can speculate that the primary role of insulin's direct action on the liver is to promote the incorporation of glucose into glycogen by activating glycogen synthase (36). Uptake of glucose by the liver would therefore appear to be primarily regulated by the glucose load and a signal associated with portal glucose delivery.
While previous studies have shown that the intracellular glycogen content can regulate insulin-mediated glucose uptake in skeletal muscle (11, 20, 24), the absence of this effect in liver can perhaps be explained by intrinsic differences between these tissues. First, insulin-mediated glucose uptake in muscle is regulated by the transport process, which involves the intracellular compartmentalization of Glut4. However, glucose transporters (i.e., Glut2) in the liver are indigenous to the membrane, and thus transport is not a site of NHGU regulation when the blood glucose level is within the normal physiological range. Rather, the relative activities of GK and glucose-6-phosphatase (G6Pase) determine NHGU in the liver. GK activity is acutely regulated in vivo not by insulin, but rather by its interaction with GKRP. Glucose dissociates GK and GKRP, allowing the translocation of GK to the cytosol, thereby allowing the transport and phosphorylation steps to remain intact, independent of insulin signaling. In turn, these steps lead to the accumulation of glucose 6-phosphate (G6P) in the liver, which complements the action of insulin to activate glycogen synthase (10, 14, 15) without inhibiting GK as is the case with hexokinase (HK) in muscle.
In summary, this study shows that a physiological increase in the hepatic glycogen level does not impair glucose uptake by the liver under hyperglycemic/hyperinsulinemic conditions. Likewise, it does not alter the fate of glucose after it has entered the hepatocyte, further indicating intact insulin action. Future studies will be required to determine whether this is also the case in humans. In addition, it remains to be determined whether the chronic administration of pharmacological therapies that increase glycogen deposition would lead to supranormal glycogen levels (i.e., >100 mg/g) and, if so, whether that would compromise the liver's ability to metabolize glucose.
GRANTS
This work was funded by National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) Grant RO1-DK-43706. Hormone analysis was performed by the Hormone Assay and Analytical Services Core, Vanderbilt Diabetes Research and Training Center, supported by NIDDK Grant DK-20593. J. J. Winnick was supported by the Vanderbilt Molecular Endocrinology Training Program (5T32-DK-07563; PI: Dr. Richard O'Brien) and an NIDDK postdoctoral fellowship award (F32-DK-080606). A. D. Cherrington is the Jacquelyn A. Turner and Dr. Dorothy J. Turner Chair in Diabetes Research.
Acknowledgments
We gratefully acknowledge the technical support provided by Margaret Lautz, Marta Smith, Jon Hastings, Wanda Snead, Patsy Raymer, and the Diabetes Research and Training Center Hormone Core in these studies. We also thank Phil Williams and Doss Neal for technical assistance and Drs. David Wasserman and Owen McGuinness for their valuable input during the preparation of this manuscript.
REFERENCES
- 1.Adkins-Marshall BA, Myers SR, Hendrick GK, Williams PE, Triebwasser K, Floyd B, Cherrington AD. Interaction between insulin and glucose-delivery route in regulation of net hepatic glucose uptake in conscious dogs. Diabetes 39: 87–95, 1990. [DOI] [PubMed] [Google Scholar]
- 2.Agius L, Peak M. Intracellular binding of glucokinase in hepatocytes and translocation by glucose, fructose and insulin. Biochem J 296: 785–796, 1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Basu A, Basu R, Shah P, Vella A, Johnson CM, Jensen M, Nair KS, Schwenk WF, Rizza RA. Type 2 diabetes impairs splanchnic uptake of glucose but does not alter intestinal glucose absorption during enteral glucose feeding: additional evidence for a defect in hepatic glucokinase activity. Diabetes 50: 1351–1362, 2001. [DOI] [PubMed] [Google Scholar]
- 4.Basu A, Basu R, Shah P, Vella A, Johnson CM, Nair KS, Jensen MD, Schwenk WF, Rizza RA. Effects of type 2 diabetes on the ability of insulin and glucose to regulate splanchnic and muscle glucose metabolism: evidence for a defect in hepatic glucokinase activity. Diabetes 49: 272–283, 2000. [DOI] [PubMed] [Google Scholar]
- 5.Basu R, Basu A, Johnson CM, Schwenk WF, Rizza RA. Insulin dose-response curves for stimulation of splanchnic glucose uptake and suppression of endogenous glucose production differ in nondiabetic humans and are abnormal in people with type 2 diabetes. Diabetes 53: 2042–2050, 2004. [DOI] [PubMed] [Google Scholar]
- 6.Capaldo B, Gastaldelli A, Antoniello S, Auletta M, Pardo F, Ciociaro D, Guida R, Ferrannini E, Sacca L. Splanchnic and leg substrate exchange after ingestion of a natural mixed meal in humans. Diabetes 48: 958–966, 1999. [DOI] [PubMed] [Google Scholar]
- 7.Chen SS, Torres-Sanchez CJ, Hosein N, Zhang Y, Lacy DB, McGuinness OP. Time course of the hepatic adaptation to TPN: interaction with glycogen depletion. Am J Physiol Endocrinol Metab 288: E163–E170, 2005. [DOI] [PubMed] [Google Scholar]
- 8.Chong MF, Fielding BA, Frayn KN. Mechanisms for the acute effect of fructose on postprandial lipemia. Am J Clin Nutr 85: 1511–1520, 2007. [DOI] [PubMed] [Google Scholar]
- 9.Chu CA, Fujimoto Y, Igawa K, Grimsby J, Grippo JF, Magnuson MA, Cherrington AD, Shiota M. Rapid translocation of hepatic glucokinase in response to intraduodenal glucose infusion and changes in plasma glucose and insulin in conscious rats. Am J Physiol Gastrointest Liver Physiol 286: G627–G634, 2004. [DOI] [PubMed] [Google Scholar]
- 10.Ciudad CJ, Carabaza A, Guinovart JJ. Glucose 6-phosphate plays a central role in the activation of glycogen synthase by glucose in hepatocytes. Biochem Biophys Res Commun 141: 1195–1200, 1986. [DOI] [PubMed] [Google Scholar]
- 11.Derave W, Hansen BF, Lund S, Kristiansen S, Richter EA. Muscle glycogen content affects insulin-stimulated glucose transport and protein kinase B activity. Am J Physiol Endocrinol Metab 279: E947–E955, 2000. [DOI] [PubMed] [Google Scholar]
- 12.Galassetti P, Chu CA, Neal DW, Reed GW, Wasserman DH, Cherrington AD. A negative arterial-portal venous glucose gradient increases net hepatic glucose uptake in euglycemic dogs. Am J Physiol Endocrinol Metab 277: E126–E134, 1999. [DOI] [PubMed] [Google Scholar]
- 13.Galassetti P, Hamilton KS, Gibbons FK, Bracy DP, Lacy DB, Cherrington AD, Wasserman DH. Effect of fast duration on disposition of an intraduodenal glucose load in the conscious dog. Am J Physiol Endocrinol Metab 276: E543–E552, 1999. [DOI] [PubMed] [Google Scholar]
- 14.Gomis RR, Favre C, Garcia-Rocha M, Fernandez-Novell JM, Ferrer JC, Guinovart JJ. Glucose 6-phosphate produced by gluconeogenesis and by glucokinase is equally effective in activating hepatic glycogen synthase. J Biol Chem 278: 9740–9746, 2003. [DOI] [PubMed] [Google Scholar]
- 15.Gomis RR, Ferrer JC, Guinovart JJ. Shared control of hepatic glycogen synthesis by glycogen synthase and glucokinase. Biochem J 351: 811–816, 2000. [PMC free article] [PubMed] [Google Scholar]
- 16.Hellerstein MK, Christiansen M, Kaempfer S, Kletke C, Wu K, Reid JS, Mulligan K, Hellerstein NS, Shackleton CH. Measurement of de novo hepatic lipogenesis in humans using stable isotopes. J Clin Invest 87: 1841–1852, 1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hirano T, Mamo J, Poapst M, Steiner G. Very-low-density lipoprotein triglyceride kinetics in acute and chronic carbohydrate-fed rats. Am J Physiol Endocrinol Metab 255: E236–E240, 1988. [DOI] [PubMed] [Google Scholar]
- 18.Hoy AJ, Bruce CR, Cederberg A, Turner N, James DE, Cooney GJ, Kraegen EW. Glucose infusion causes insulin resistance in skeletal muscle of rats without changes in Akt and AS160 phosphorylation. Am J Physiol Endocrinol Metab 293: E1358–E1364, 2007. [DOI] [PubMed] [Google Scholar]
- 19.Hsieh PS, Moore MC, Neal DW, Cherrington AD. Hepatic glucose uptake rapidly decreases after removal of the portal signal in conscious dogs. Am J Physiol Endocrinol Metab 275: E987–E992, 1998. [DOI] [PubMed] [Google Scholar]
- 20.Jensen J, Jebens E, Brennesvik EO, Ruzzin J, Soos MA, Engebretsen EM, O'Rahilly S, Whitehead JP. Muscle glycogen inharmoniously regulates glycogen synthase activity, glucose uptake, and proximal insulin signaling. Am J Physiol Endocrinol Metab 290: E154–E162, 2006. [DOI] [PubMed] [Google Scholar]
- 21.Keppler D, Decker K. Glycogen. In: Methods of Enzymatic Analysis, edited by Bergmeyer HU. New York: Verlag Chemie, 1984, p. 11–18.
- 22.Krssak M, Brehm A, Bernroider E, Anderwald C, Nowotny P, Dalla Man C, Cobelli C, Cline GW, Shulman GI, Waldhausl W, Roden M. Alterations in postprandial hepatic glycogen metabolism in type 2 diabetes. Diabetes 53: 3048–3056, 2004. [DOI] [PubMed] [Google Scholar]
- 23.Leevy CM, Mendenhall CL, Lesko W, Howard MM. Estimation of hepatic blood flow with indocyanine green. J Clin Invest 41: 1169–1179, 1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Litherland GJ, Morris NJ, Walker M, Yeaman SJ. Role of glycogen content in insulin resistance in human muscle cells. J Cell Physiol 211: 344–352, 2007. [DOI] [PubMed] [Google Scholar]
- 25.Ludvik B, Nolan JJ, Roberts A, Baloga J, Joyce M, Bell JM, Olefsky JM. Evidence for decreased splanchnic glucose uptake after oral glucose administration in non-insulin-dependent diabetes. J Clin Invest 100: 2354–2361, 1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ludvik B, Nolan JJ, Roberts A, Baloga J, Joyce M, Bell JM, Olefsky JM. A noninvasive method to measure splanchnic glucose uptake after oral glucose administration. J Clin Invest 95: 2232–2238, 1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.McGuinness OP, Myers SR, Neal D, Cherrington AD. Chronic hyperinsulinemia decreases insulin action but not insulin sensitivity. Metabolism 39: 931–937, 1990. [DOI] [PubMed] [Google Scholar]
- 28.Moore MC, Cardin S, Edgerton DS, Farmer B, Neal DW, Lautz M, Cherrington AD. Unlike mice, dogs exhibit effective glucoregulation during low-dose portal and peripheral glucose infusion. Am J Physiol Endocrinol Metab 286: E226–E233, 2004. [DOI] [PubMed] [Google Scholar]
- 29.Moore MC, Cherrington AD, Cline G, Pagliassotti MJ, Jones EM, Neal DW, Badet C, Shulman GI. Sources of carbon for hepatic glycogen synthesis in the conscious dog. J Clin Invest 88: 578–587, 1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Moore MC, Hsieh PS, Flakoll PJ, Neal DW, Cherrington AD. Differential effect of amino acid infusion route on net hepatic glucose uptake in the dog. Am J Physiol Endocrinol Metab 276: E295–E302, 1999. [DOI] [PubMed] [Google Scholar]
- 31.Moore MC, Hsieh PS, Neal DW, Cherrington AD. Nonhepatic response to portal glucose delivery in conscious dogs. Am J Physiol Endocrinol Metab 279: E1271–E1277, 2000. [DOI] [PubMed] [Google Scholar]
- 32.Moore MC, Satake S, Lautz M, Soleimanpour SA, Neal DW, Smith M, Cherrington AD. Nonesterified fatty acids and hepatic glucose metabolism in the conscious dog. Diabetes 53: 32–40, 2004. [DOI] [PubMed] [Google Scholar]
- 33.Myers SR, McGuinness OP, Neal DW, Cherrington AD. Intraportal glucose delivery alters the relationship between net hepatic glucose uptake and the insulin concentration. J Clin Invest 87: 930–939, 1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pagliassotti MJ, Holste LC, Moore MC, Neal DW, Cherrington AD. Comparison of the time courses of insulin and the portal signal on hepatic glucose and glycogen metabolism in the conscious dog. J Clin Invest 97: 81–91, 1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ramnanan CJ, Storey KB. Suppression of Na+/K+-ATPase activity during estivation in the land snail Otala lactea. J Exp Biol 209: 677–688, 2006. [DOI] [PubMed] [Google Scholar]
- 36.Satake S, Moore MC, Igawa K, Converse M, Farmer B, Neal DW, Cherrington AD. Direct and indirect effects of insulin on glucose uptake and storage by the liver. Diabetes 51: 1663–1671, 2002. [DOI] [PubMed] [Google Scholar]
- 37.Shiota M, Galassetti P, Monohan M, Neal DW, Cherrington AD. Small amounts of fructose markedly augment net hepatic glucose uptake in the conscious dog. Diabetes 47: 867–873, 1998. [DOI] [PubMed] [Google Scholar]