Abstract
Soluble epoxide hydrolase (Ephx2, sEH) is a bifunctional enzyme with COOH-terminal hydrolase and NH2-terminal phosphatase activities. sEH converts epoxyeicosatrienoic acids (EETs) to dihydroxyeicosatrienoic acids (DHETs), and the phosphatase activity is suggested to be involved in cholesterol metabolism. EETs participate in a wide range of biological functions, including regulation of vascular tone, renal tubular transport, cardiac contractility, and inflammation. Inhibition of sEH is a potential approach for enhancing the biological activity of EETs. Therefore, disruption of sEH activity is becoming an attractive therapeutic target for both cardiovascular and inflammatory diseases. To define the physiological role of sEH, we characterized a knockout mouse colony lacking expression of the Ephx2 gene. Lack of sEH enzyme is characterized by elevation of EET to DHET ratios in both the linoleate and arachidonate series in plasma and tissues of both female and male mice. In male mice, this lack of expression was also associated with decreased plasma testosterone levels, sperm count, and testicular size. However, this genotype was still able to sire litters. Plasma cholesterol levels also declined in this genotype. Behavior tests such as anxiety-like behavior and hedonic response were also examined in Ephx2-null and WT mice, as all can be related to hormonal changes. Null mice showed a level of anxiety with a decreased hedonic response. In conclusion, this study provides a broad biochemical, physiological, and behavioral characterization of the Ephx2-null mouse colony and suggests a mechanism by which sEH and its substrates may regulate circulating levels of testosterone through cholesterol biosynthesis and metabolism.
Keywords: cholesterol, fertility
the soluble epoxide hydrolase (sEH) gene Ephx2 encodes for a mature protein of 555 amino acid residues (28). The protein product is a bifunctional enzyme with an NH2-terminal phosphatase activity (8, 49) in addition to its name-bearing COOH-terminal EH activity (45, 46). Although the latter is responsible for the well-defined epoxide hydrolysis of endogenous fatty acid epoxides, no clear endogenous substrate for the NH2-terminal domain has been identified. Screening substrates by in vitro assays suggests that lipid phosphates and isoprenoid phosphates are good substrates for this enzymatic activity (18, 49, 59).
Endogenous substrates for the COOH-terminal sEH activity include arachidonate-derived epoxyeicosatrienoic acids (EETs). They are proposed to modulate blood pressure by their vasodilatory effects (6) and also have anti-inflammatory properties in several distinct models (51, 56). The EETs were also shown to have roles in angiogenesis, neurohormone release, cell proliferation, modulation of ion channel activity, and modulation of inflammatory pain (4, 10, 24, 34, 54). Interestingly, several human sEH polymorphisms were recently associated with increased plasma cholesterol levels in familial hypercholesterolemia (55). These observations are now further supported by Enayetallah et al. (15), who showed in vitro that cholesterol levels are regulated by EETs and their vicinal diols (DHETs) as modulated by sEH (15). Furthermore, recent reports link sEH and its substrate and product 11,12-EET and 14,15-DHET, respectively, to the activation of peroxisome proliferator-activated receptors (PPAR)α and -γ, which are thought to regulate lipid homeostasis (50). Regulation of sEH expression and activity was associated with hormonal development and reproduction, because sEH activity was enhanced with age and upregulated in males, potentially due to the regulation of sEH expression by testosterone (12, 26, 36, 53). Interestingly, leukotoxin diols, which are also endogenous products of sEH from linoleic acid (42), are proinflammatory mediators that can disrupt the estrous cycle in rats (41).
The Ephx2-null mouse model provides a valuable tool for investigating the basic biology of sEH and the bioactive lipids that it regulates. This tool may also provide insight regarding the safety of sEH inhibitors being investigated for clinical applications. For example, we (40) have shown a compensatory mechanism for homeostatic blood pressure regulation in Ephx2-gene-disrupted mice. In this study, we provide evidence showing a link among disruption of the Ephx2 gene expression, testosterone synthesis, and its precursor cholesterol. Using the Ephx2-null mice and their wild-type (WT) littermates, we broadly analyzed the colony for its reproductive ability, lipid composition, and anxiety-related behavior.
MATERIALS AND METHODS
Animals.
Mice with targeted disruption in exon 1 of the Ephx2 gene were originally obtained from Dr. Chris Sinal (57). These mice were back-crossed onto a C57BL6 (Jackson Laboratories, Bar Harbor, ME) genetic background an additional 5–10 generations prior to use in this study. Ephx2-null homozygotes or WT mice were paired for subsequent breeding. Animals were housed in a standard animal facility under controlled temperature (22°C) and photoperiod (12:12-h light-dark). They were maintained on a normal rodent diet (PicoLab rodent 20; LabDiet, Richmond, IN) and bottled water ad libitum. Animal handling, experimentation, and euthanasia were conducted in accordance with approved IACUC protocols.
EH assay.
Cytosolic and microsomal fractions were prepared from liver homogenates of sEH WT and null mice as described earlier (43). Protein concentration was quantified using the Pierce BCA assay with bovine serum albumin (BSA) as calibrating standard. EH activity was measured using 14,15-epoxyeicosatrienoic acid (14,15-EET) as substrate (48). Briefly, the cytosolic fraction was diluted with sodium phosphate buffer (0.1 M, pH 7.4), and the microsomal fraction was diluted with Tris·HCl buffer (0.1 M, pH 9.0) both supplemented with BSA (0.1 mg/ml). The assay was initiated by the addition of 1 μl of 14,15-EET (5 mM in DMSO) to 100 μl of protein extract ([S]final: 50 μM) to both fractions. For inhibitor treatment, the enzyme preparations were preincubated at 30°C for 5 min with 100 μM AUDA (sEH inhibitor, 12-[3-adamantane-1-yl-ureido]-dodecanoic acid) (44) or NTPA [microsomal (m)EH) inhibitor, 2-nonylthio-propiamide] (47) before substrate introduction. The mixture was incubated at 30°C for 5–60 min. The enzymatic reaction was then quenched by addition of ice-cold MeOH (400 μl). The sample was centrifuged and the supernatant transferred in vials for analysis. The remaining epoxide and resulting diol (14,15-DHET) were quantified by LC-MS-MS, as described earlier (40). The reactions were performed in triplicate. Results are means ± SD of three separate experiments.
Sample and tissue collection.
Following lethal injection of pentobarbital sodium (100 mg/kg body wt ip), blood was collected within an hour from null mice and their WT controls by cardiac puncture. Blood samples were collected into EDTA-rinsed syringes from the right ventricle of each animal, and plasma was separated (10 min, 400 g) and stored at −20°C until analysis. For hormone assay, mice were bled from the tail, and 10 μl of whole blood was collected. After recording body weight, testes and seminal vesicles from each mouse were removed and cleaned of fat, and peripheral blood vessels.
Reproductive parameters and hormone and sperm analysis.
Mating tests were carried out with mice at least 3 mo of age by placing a female in the male's home cage from the same genotype. Coital plug formation was recorded during 4 consecutive days, and pregnancy was assessed by palpitation of the abdomen. Offspring sex was determined by measuring anogenital distance of the pups at 2 and 21 days of age. Sperm analysis was assessed by isolating the epididymis from intact WT or Ephx2-deficient male mice. Sperm were allowed to swim up in 37°C prewarmed PBS (KCl, 2.7 mM; KH2PO4, 1.47 mM; NaCl, 137.9 mM; Na2HPO4, 8.1 mM; pH 7.4) solution for 15 min. Sperm (10 μl) were placed on a coverslip, and the percentage of sperm motility was evaluated by tracing the movement paths of sperm cells (n = 200). Morphological integrity was assessed by looking at 200 spermatozoa head, tail, and midpiece shapes (phase contrast-2 microscope, Nikon, Japan). No visible defects were seen in head shape, tail defects, or cytoplasmic droplets (39). At the end of the incubation, a 1:10 dilution was used for hemocytometric count after all tissue debris was removed. Plasma concentration of testosterone was measured by RIA following extraction with diethyl ether, as reported previously (60).
Histopathology.
Testis morphology was examined after use of a primary fixation in neutral buffer and hematoxylin staining of the 5-μm paraffin sections, as previously described (64). Lipids were identified through Oil red O (ORO) staining of 10-μm cryosections, as previously described (11). Photos of paraffin-sectioned testis were taken with an Olympus BH2RFCA microscope equipped with a Leica true colors camera and edited with Adobe Photoshop 7.0.1. Cryosection photos were captured using a digital system consisting of an Olympus BX40 fluorescent microscope with an Olympus DP71 digital color camera.
Anxiety-related behavioral tests.
For general observation of behavior, mice were placed in an open-field arena (40 × 40 × 30 cm) of a 16-square grid clean floor (23). Murine behavior and activity were monitored for 5 min (58). Activity was determined by the number of lines each mouse crossed as a function of time. Other parameters of behavior, such as cage-exploring behavior, grooming, rearing, head bobbing, urination, and hunched body posture were also examined (37).
Anxiety-related behavior was evaluated using a dark/light transition task. Mice were placed in the dark side of a box (7.5 × 4 × 4 cm) with a round opening of 5 cm in diameter. For a period of 5 min, the number of times mice poked their heads through the open window as well as the total amount of time they spent on the light side of the box were recorded (22).
Hedonic response.
WT or knockout (KO) male mice were first trained to consume a prewarmed condensed sweetened-milk solution (5% vol/vol). Training consisted of an initial daily exposure to sweetened-milk solution in place of water in their home cage at the same time of the day for 5 min over a weeklong period. At the end of the training period, the sweetened solution was introduced again for 20 min at the same time for an additional 7 days. Intake was measured by weighing preweighed bottles at the end of the test. During this time, mice were also recorded and monitored for any unusual behavior (9, 29). Sweetened solution was given to each cage (two mice) every day at the same time. Sweetened solution intake was measured after 20 min and recorded on a video camera.
Truemass lipomic profile.
Lipids from plasma were extracted in the presence of authentic internal standards by the method of Folch et al. (19), using chloroform-methanol (2:1 vol/vol). Individual lipid classes within each extract were separated by liquid chromatography as previously described (40). Each lipid class was transesterified in 1% sulfuric acid in methanol in a sealed vial under a nitrogen atmosphere at 100°C for 45 min. The resulting fatty acid methyl esters were extracted from the mixture with hexane containing 0.05% butylated hydroxytoluene and prepared for gas chromatography by sealing the hexane extracts under nitrogen. Fatty acid methyl esters were separated and quantified by capillary gas chromatography (Agilent Technologies model 6890) equipped with a 30-m DB-88MS capillary column (Agilent Technologies) and a flame ionization detector (61–63).
Total sterols.
To 50 μl of plasma samples a 10-μl mixture of deuterated surrogates was added for recovery assessment. Ethanolic potassium hydroxide (250 μl, 1 M) was added to all samples. Microtubes were purged with nitrogen, capped, and vortexed for 30 s. Each sample was then incubated at 70°C for 1 h and cooled for 15 min (4°C), and 250 μl of deionized water was added. The free sterols were then extracted with two 500-μl volumes of hexane-ethanol (20:1). The combined hexane layers were transferred to a GC vial, dried down under nitrogen, and reconstituted in 50 μl of decane. To each sample, 30 μl of Tri-Sil derivatizing reagent was added. Finally, silylated sterols were injected onto a 6890/5975 GC-MS (Agilent Technologies) with a 30 × 0.25-mm Rxi-5 MS column (Restek) with helium as the carrier gas. Mass spectrometric analysis was performed in the single ion monitoring (SIM) mode with electron ionization.
Statistic analysis.
Results were tested for statistical significance using a t-test or a Student-Newman-Keuls test after one-way repeated-measures ANOVA. Differences were considered significant if the P value was < 0.05.
RESULTS
In general, Ephx2-null mice developed normally and did not display obvious symptoms of disease or organ malformation. In a previous study (40), we showed that Ephx2-null mice lack the expression of sEH gene, protein, and activity. This strain also showed an increase in epoxyeicosatrienoic acids in both liver and renal homogenates as well as in plasma (40). The present study expands the phenotypic and metabolic characterization of the Ephx2-null mouse colony.
14,15-EET hydrolysis.
As expected, hydrolysis of 14,15-EET to its vicinal diol 14,15-DHET is almost abolished in the cytosolic fraction of Ephx2-null liver extracts (Table 1). In sEH-null mice, specific activity against 14,15-EET is reduced 200-fold in the cytosolic fraction and close to 10-fold in microsomal fraction compared with the WT liver homogenates. Inhibition by AUDA, a potent sEH inhibitor, in WT liver homogenates completely abolished specific 14,15-EET hydrolysis to levels seen in sEH-null cytosolic and microsomal samples (Table 1). In WT liver homogenates, inhibition by NTPA, a selective microsomal EH inhibitor, decreased 14,15-DHET production only in the microsomal fraction by 20% (Table 1). This suggests that only one-fifth of diol products in the microsomal fraction of WT liver homogenates is contributed by mEH and the rest is contributed by sEH-like activity or as a result of some cytosolic residues left along the microsomal preparation procedure, as previously observed (2). In the cytosol, sEH is the main enzyme that hydrolyzes epoxy fatty acids, and the contribution by mEH to EET hydrolysis accounts for only a small percentage of the total activity in the liver (Table 1). In null mice, possibly due to other enzymatic activity, 14,15-DHET formation is 0.5% in the cytosol and 9% in the microsomal fraction compared with the WT counterparts. The cytosolic activity was not significantly affected by AUDA, whereas the microsomal activity is almost abolished with treatment of NTPA (Table 1).
Table 1.
14,15-DHET production in liver homogenates from Ephx2-WT and null mice
|
sEH-WT |
sEH-Null
|
||||||
|---|---|---|---|---|---|---|---|
| Cytosol | Microsomes | Cytosol | Microsomes | ||||
| 14,15-EET specific activity, nmol·min−1·mg−1(tissue activity, nmol·min−1·g−1) | |||||||
| Control | 3.6±0.3 | 0.82±0.01 | 0.018±0.004 | 0.074±0.002 | |||
| (4,400±400) | (370±10) | (2.3±0.5) | (2.2±0.1) | ||||
| Boiled | 0.01±0.01* | 0.01±0.01* | 0.002±0.002* | 0.002±0.001* | |||
| (7±5) | (2±2) | (0.2±0.2) | (0.07±0.01) | ||||
| AUDA | 0.02±0.01* | 0.05±0.01* | 0.009±0.001* | 0.063±0.003* | |||
| (30±8) | (28±1) | (1.1±0.1) | (2.0±0.1) | ||||
| NTPA | 3.6±0.4 | 0.64±0.03* | 0.008±0.001* | 0.020±0.004* | |||
| (4,400±500) | (350±20) | (1.1±0.1) | (0.6±0.1) | ||||
Cytosolic and microsomal fractions from liver homogenates of Ephx2-WT and Ephx2-null male mice (n = 3) were analyzed for dihydroxyeicosatrienoic acid (14,15-DHET) production using mass spectrometry, as described in materials and methods. sEH, soluble epoxide hydrolase; AUDA, 12-[3-adamantane-1-yl-ureido]-dodecanoic acid (an sEH inhibitor); NTPA, 2-nonylthio-propiamide (a microsomal EH inhibitor); EET, epoxyeicosatrienoic acid. Results are presented as means ± SD of 3 separate experiments for specific activity per total mg protein (in bold) or for tissue activity per gram of wet liver weight (parentheses). AUDA (100 μM) and NTPA (100 μM) were incubated as described previously (44, 47). It should be noted that total protein and thus total sEH activity is far higher in the cytosolic fraction.
P < 0.01 vs. control samples (1-way ANOVA with Student-Newman-Keuls post hoc analysis).
Phenotypic and segregation analysis.
Homozygous Ephx2-null mice were viable and fertile. Neither Ephx2-null nor Ephx2-WT mice displayed any visible abnormalities at birth. Mice were of normal weight (Table 2). For the total number of offspring tested (167 null and 188 WT pups), no significant changes in number of pups per litter or pup size between the two genotypes were observed (Table 2). When offspring numbers were examined, of 167 offspring from the Ephx2-null colony, 93 were female and 74 were male (Table 2), while in the Ephx2-WT colony, of 188 offspring tested 88 were female and 100 were male (Table 2). Although the number of female litters seemed higher for the Ephx2-null mice, this was not statistically significant (unpaired t-test; P < 0.07). For further analysis, a higher number of pups should be evaluated.
Table 2.
Genotype and progeny analysis in Ephx2-WT and null mice
| Dam |
Litters |
Offsprings
|
||||
|---|---|---|---|---|---|---|
| No. of Dams Tested | No. of Litters/Dam | No. of Pups/Llitter | Weight of Pups (g) | No. of Females/Litter | No. of Males/Litter | |
| Ephx2-WT | 8 | 3.8±0.6 | 6.3±0.3 | 0.22±0.01 | 2.9±0.2 (47%) | 3.3±0.2 (53%) |
| Ephx2-null | 6 | 4.5±0.8 | 6.2±0.3 | 0.19±0.01 | 3.4±0.3 (56%) | 2.7±0.2 (44%) |
Litters and offspring measurements are presented as means ± SE from total of 167 and 188 offspring in the Ephx2-null or Ephx2-WT colony, respectively. Pup weight was measured 9 days after birth of pups from the 2 genotypes.
Reproductive rate, sperm analysis, testis size and plasma testosterone levels.
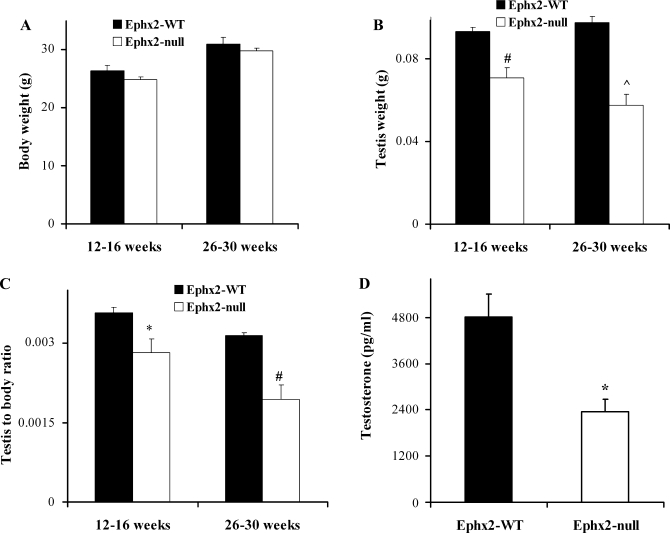
Reproductive parameters and behavior were further examined in both genotypes (Table 3). Within 4 days of placing females and males together, all females in Ephx2-WT and null mice were found to have coital plugs followed with a high pregnancy success rate (Table 3). Sperm analysis showed that, although sperm motility was similar in both genotypes, total sperm concentration was significantly reduced in the Ephx2-null mice (Table 3). Additionally, testis measurements showed a remarkable difference between the two genotypes (Supplementary Fig. S1A). (Supplementary materials, both figures and movies, are found in the online version of this article.). In both age populations examined (12–16 and 26–30 wk of age), testis size and weight were significantly lower in the Ephx2-null mice (Suppl. Fig. S1A and Fig. 1), whereas no significant differences in mean body weight were observed between WT and sEH-deficient mice (Fig. 1A). As expected, a slight increase in body weight was seen in older mice in both WT and Ephx2-null animals, but the testis weight was even lower in the latter (Fig. 1, A and B). Testis weight in relation to body mass in both age populations was also calculated, showing a significant decrease in testis weight in Ephx2-null male mice (Fig. 1, A–C). Seminal vesicle size also showed higher mass in WT male adult mice (Suppl. Fig. S1B).
Table 3.
Fertility data from breeding conducted with male and female mice of the same genotype
| Sperm Motility and Viability | Sperm Count, cells/ml | Total No. of Plugs, % | No. of Pregnancy, % | |
|---|---|---|---|---|
| Ephx2-WT | +++ | 9.1±1.1×106 | 100 | 100 |
| Ephx2-null | +++ | 4.3±0.8×106* | 100 | 100 |
Reproductive analysis in Ephx2-null and Ephx2-WT mice (n = 4, 16 wk old). Data are expressed as means ± SE.
P < 0.05 as measured by paired t-test. There were no obvious differences in motility or morphology in sperm samples from either Ephx2-null or Ephx2-WT standard animals.
Fig. 1.
Testis weight and plasma testosterone levels in sEH-WT (soluble epoxide hydrolase wild-type) and null (KO) mice. A: body weight from sEH-WT and null mice. B: testis weight and C: testis weight in relation to body weight were compared in 12- to 16-wk and 26- to 32-wk-old intact male mice. Data are expressed as means ± S from n = 12 and n = 6 mice (young and older mice, respectively). P values show significant differences between KO and WT colonies (paired t-test). *P < 0.05, #P < 0.001, ^P < 0.00001. D: testosterone was measured in plasma of 16-wk-old male mice from both genotypes (26 ± 1 and 25 ± 0.5 g body wt for sEH-WT and null, respectively). The hormone was measured by RIA as described in materials and methods. sEH-null mice have lower plasma testosterone. Data are expressed as means ± SE and were tested for significant differences by t-test, *P < 0.01 (n = 7).
These findings led us to measure plasma testosterone levels in both genotypes (Fig. 1D). Plasma concentration of testosterone was reduced twofold in the sEH-null mice (Fig. 1D). This observation may explain the reduction of testis size along with sperm count (Suppl. Fig. S1 and Table 3). Supportive evidence for the expression of sEH in testis is shown in previous studies where significant sEH activity was measured in mouse testis (13). This suggests that sEH may play a role in spermatogenesis.
Testis histology.
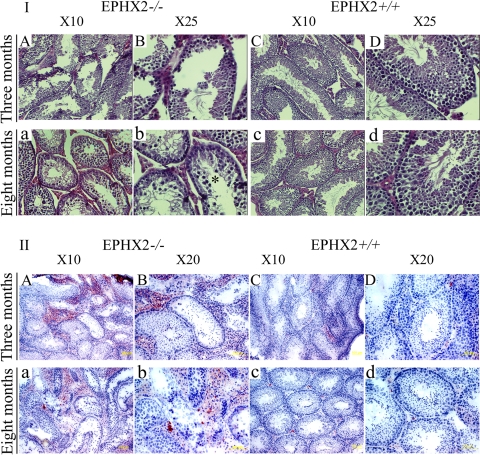
A close observation of the testis histology is shown in Fig. 2, detecting pathological alterations specifically in testes of 8-mo-old mice. Testes from 3-mo-old Ephx2-null mice show seminiferous tubules with apparently normal spermatogenesis and some increased region of the lumen (Fig. 2.I, A and B). Over time, the phenotype becomes more pronounced. In testes of 8-mo-old Ephx2-null male mice, seminiferous tubules displayed a severe impairment of spermatogenesis with severe vacuolization of most affected tubules (Fig. 2.I, a and b, vs. WT testis sections 2I, c and d).
Fig. 2.
Progressive depletion of germ cells in testes of Ephx2-null mice with advanced age. Each panel shows a representative seminiferous tubule from either a paraffin section stained with standard hematoxylin and eosin (I) or frozen testis sections stained with Oil Red O (II) in Ephx2-null (Ephx2−/−) or wild-type (WT; Ephx2+/+) mice at age 3 or 8 mo. Oil Red O stains lipids red (but does not distinguish between triglycerides and cholesterol) and therefore is used here to more clearly highlight lipid droplets within the seminiferous tubules and surrounding interstitium. Photos A and B and C and D are testis sections at different magnifications from Ephx2-null and WT 3-mo-old mice, respectively. Photos a and b and c and d are the same as above from older mice (8 mo old).
Interestingly, in some areas of sections from the 3-mo-old null testis, visible alterations already start to appear around the interstitial/Leydig cells (Fig. 2.I, a and b). A large gap between tubules is clearly seen in Fig. 2.I, A and B, that progresses dramatically in older mice (Fig. 2.I, a and b, asterisk). Since the method of paraffin fixation can cause changes in the interstitial area, we also confirmed it by cryosectioning the testes and stained them with ORO for lipid localization. ORO staining confirms this observation and also shows lipid content. Three- and eight-mo-old WT animals demonstrate weak ORO staining in Leydig cells (Fig. 2.II, C and D and c and d, respectively). Strikingly, there was an increase in the lipid content of Leydig cells in Ephx2-null mice in both ages (Fig. 2.II, A and B and a and b). Although no immunostaining specific for Leydig cells was done, it could be that the accumulation of ORO staining is due to the apparent increase in the number of Leydig cells populating the interstitial region (Fig. 2.II, Ephx2−/−).
Anxiety-related behavior.
Testosterone is required for the appropriate display of male patterns of behavior (20). In several affective behavior tests, administration of testosterone significantly reduced anxiety of aged mice in open-field and dark-light transition tests (21, 22). To determine whether the two genotypes are different in behavior, both open-field activity and dark-light transition tests were evaluated along with hedonic response measurements by scoring the daily uptake of sweetened condensed milk.
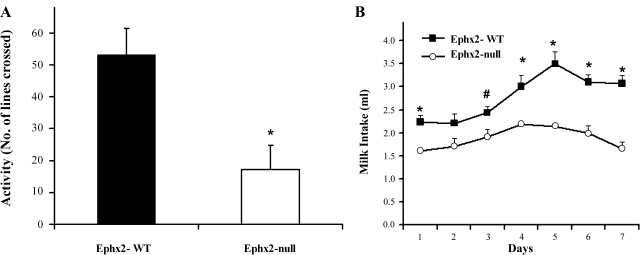
In the dark-light transition test, the number of exits into the open space was lower (16 ± 5, n = 6) in mice lacking sEH, whereas the WT mice spent more time in the light side (25 ± 4, n = 6). This may suggest that Ephx2-null male mice display a higher level of anxiety-related behavior. This observation was supported by a further open-activity test (Fig. 3A and Supplementary Movie S1). Again, Ephx2-null male mice displayed lower levels of exploratory behavior. The number of lines that sEH-KO mice crossed as a function of time was significantly lower than the WT counterparts (Fig. 3A and Suppl. Movie S1). Latency to the first grid crossing was also different between the two genotypes. When placed in an observation chamber, the WT animals were immediately active, whereas the null animals were immobile and did not move until an average of 3 min after placement (Suppl. Movie S1). Both the dark-light transition and open-field activity tests are generally considered complementary tests of affective behavior that can be modulated by hormones. The higher level of anxiety-related behavior of sEH-KO males observed in the dark-light transition test and open-field activity may reflect the lower levels of circulating testosterone observed.
Fig. 3.
Open-field activity test (A) and hedonic response (B) in both Ephx2-WT and null male mice. A: mice were placed in an observation chamber, and line crossing during a 5-min period was recorded. sEH-KO mice exhibit a significantly lower exploratory behavior in the open-field test (means ± SE of n = 6 *P < 0.05 in paired t-test). B: 2 males were placed together in each cage. For 7 consecutive days, warmed sweetened condensed milk was presented to mice every day at the same time for 5 min. At the end of training, mice were again presented with the same solution, and intake was measured for 20 min over 7 days. sEH-KO mice responded significantly less than their WT counterparts to the hedonic stimuli. Data are expressed as means ± SE of n = 6 amount of daily milk consumption per mouse over 7 days. #P < 0.05, * P < 0.01 (t-test).
Sweetened milk intake was also measured in both genotypes as a marker for hedonic response. As seen in Fig. 3B, the total consumption of milk was significantly lower in the male sEH-KO mice. On average, the WT counterparts consumed two ml per unit time more than the null-mice. Over time, the consumption of milk was increased in both genotypes in a proportional rate, but still the sEH-null mice consumed less sweetened solution (Fig. 3B). Behavior of the mice was also different (Suppl. Movie S2), in that the sEH-KO mice consumed the sweetened solution in intervals a few minutes apart while the WT mice showed a much greater enthusiasm toward the treat.
Metabolomic assessment of plasma lipids.
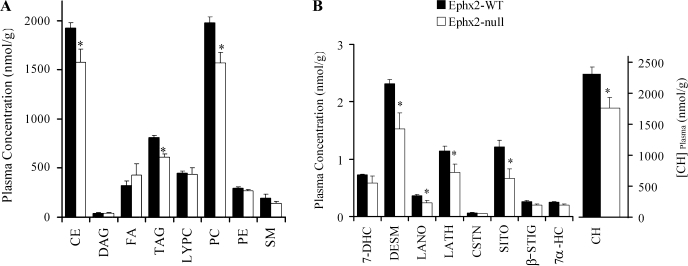
A broad analytical approach was taken to quantify the major lipid classes and the distribution of their fatty acid chains in plasma samples of Ephx2-null and WT male mice. Both absolute and relative amounts of eight lipid classes and their fatty acids content were measured (Fig. 4A). A significant decrease was observed in cholesterol ester (CE), phosphatidylcholine (PC), and triacylglycerol (TAG) in sEH-null mice (Fig. 4A). The TAG/DAG (diacylglycerol) ratio was not significantly different between the two genotypes (20 ± 3 vs. 17 ± 3 in WT and KO, respectively). Overall, besides a slight increase in free fatty acids, the sEH-deficient mice displayed lower plasma lipid content in CE, TAG, and PC (Fig. 4A).
Fig. 4.
Total plasma concentration of structural lipids (A) and sterol lipids (B) in Ephx2-WT and null male mice. Plasma samples of WT (n = 5) and null (n = 4) sEH mice (12–16 wk old) were subjected to lipid analysis. Values are presented as means of plasma lipid concentration ± SE. CE, cholesterol ester; DAG, diacylglycerol; FA, free fatty acids; TAG, triacylglycerol; LYPC, lysophosphatidylcholine; PC, phosphatidylcholine; PE, phosphatidylethanolamine; SM, sphingomyelin. 7-DHC, 7-dehydrocholesterol; DESM, desmosterol; LANO, lanosterol; LATH, lathosterol; CSTN, cholestanol; COPR, coprostanol; β-SITO, β-sitosterol; CAMP, campesterol; STIG, stigmasterol; 7α-HC, 7α-hydroxycholesterol. *P < 0.05, t-test.
Fatty acid chain composition was further analyzed for each lipid class (Suppl. Fig. S3). The reduction in esterified cholesterol is associated with a significant decrease of n-3 and n-6 polyunsaturated fatty acid chains (Suppl. Fig. S3A). A slight but insignificant increase was observed in free fatty acid (FFA) levels in null plasma samples (Suppl. Fig. S3B). While TAG levels were reduced in the sEH-KO plasma samples, the component composition of fatty acids of both TAG and its product DAG showed insignificant differences (Suppl. Fig. S3, C and D). PC significantly decreased in sEH-KO plasma samples (Fig. 4 and Suppl. Fig. S3F).
Cholesterol metabolic pathway.
The analyses shown in Fig. 4B were carried out to determine whether loss of sEH led to changes in de novo cholesterol synthesis. The cholesterol precursors lathosterol and desmosterol were significantly reduced in sEH-KO plasma samples (Fig. 4B). While cholesterol was slightly but significantly reduced in plasma samples of sEH-KO male mice (Fig. 4B), the phytosterols stigmasterol and campesterol were not significantly different between genotypes (Fig. 4B). In summary, we suggest that the lower level of plasma testosterone seen here is a result of decreased circulating cholesterol levels and that cholesterol biosynthesis rather than uptake is associated with this decrease.
DISCUSSION
The Ephx2-null mouse model provides a valuable tool for investigating the basic biology of sEH and the bioactive lipid functions that it regulates. EETs act as vasodilators and have anti-inflammatory properties; hence, sEH is proposed to be a novel therapeutic target for the treatment hypertension and inflammation in various disease models (33, 51, 56). This study addressed the potential consequences of the loss of sEH activity in mice by analyzing the physiological and behavioral characteristics of sEH KO mice and extending our knowledge regarding the role of sEH (40). Biological data from KO strains is notoriously strain dependent; however, the mice used here were extensively back-bred into C57BL6 animals by Sinal et al. (57) and further back-bred into C57BL6 at the University of California, Davis. This and the use of the corresponding WT animals out-bred from heterozygotes reduce the chance of breeding artifacts, although these results may be compounded by adaptive mechanisms.
We show here that gene deletion of Ephx2 results in a remarkable increase in epoxy fatty acids from both linoleate and arachidonate series, demonstrating the quantitative importance of sEH for normal fatty acid metabolism. We also observed insignificant production of diols in cytosolic fraction of null liver extracts. In the microsomal WT fraction, we and others (27, 30, 38) observed some diol production that is referred to as EH-like activity. Using both fatty acid epoxides and surrogate substrates, Borhan et al. (3) estimated that mEH accounts for little of the epoxide hydrolysis. Using the Ephx2 murine model and selective inhibitors for both sEH and mEH, we can say that only a small percentage of the 14,15-EET hydrolysis is due to the microsomal EH activity in WT animals, but it is responsible for much of the hydrolysis in the null mice.
Male mice carrying a homozygous null mutation for sEH exhibited a decrease in some components of masculine parameters. First, male KO mice exhibited lower plasma testosterone levels. Second, they also exhibited lower sperm concentration in the epididymis. Yet they were still able to sire litters, which suggests that spermatogenesis was not severely impaired. Third, the testes of Ephx2-null mice were smaller than those of their counterpart WT mice. Last, cholesterol and several of its precursor metabolites were reduced in plasma of KO mice. At this point, we cannot distinguish whether these effects are due to deletion of the phosphatase or EH activities coded by the Ephx2 gene.
A number of studies suggested that sEH activity is under a direct influence of sexual hormone regulation (35, 36). In rodents, liver and kidney sEH specific activity was elevated in males compared with females (35, 36, 53). Furthermore, castration of males decreased activity in both organs, which was restored by testosterone administration (19). Consistent with these observations, EH activity in male testes increased at the time of puberty with a corresponding rise in plasma testosterone levels (26, 52). The same pattern was also demonstrated in hepatic sEH (12). Although it is not known what the basis of these changes is, these studies suggest that sEH activity may be under the influence of androgenic hormonal regulation. Since sEH is present in all segments of epididymis and testis and to a less extent in sperm, it may have a role in sperm motility or functions (14). The detailed morphological analysis of the testis in this study revealed that the deletion of the Ephx2 gene resulted in a dramatic, time-dependent effect on germ cell synthesis and Leydig cell proliferation. Histological evaluation of testis sections in paraffin shows a clear region of the interstitial region, as null mice are getting older. Cryosectioning shows that this area is filled with ORO-stained Leydig cells. Although two different sectioning methods were used here, it is hard to conclude what the reason for that is. Clearly we can say that the majority of the tubules in older null mice showed impairment of spermatogenesis with formation of vacuoles that could be a result of dysfunction of Leydig cells.
As demonstrated here, it is unlikely that the sEH expression in sperm cells affects their functions, since sEH-null animals show apparently normal sperm motility, as was qualitatively measured. Furthermore, although the sperm concentration was lower in Ephx2-null mice, these mice were still able to sire litters. It is suggested, however, that lower circulating testosterone levels in plasma, as found in this study, can affect sperm count and testicular volume. A previous study found endocrine effects of the sEH inhibitor triclocarban (5). Although triclocarban is a potent inhibitor of the sEH, it does not appear to exert its endocrine effects through this mechanism (1).
Additional evidence that lower circulating testosterone is associated with this phenotype comes from behavioral tests. Analysis of the resulting phenotype and behavior is often difficult, because individual genes may have multiple functions. In the case of sEH this seems to be true because sEH is known to have two functional catalytic sites, i.e., the hydrolase and phosphatase activity. On the basis of this study, we can only speculate that higher-anxiety-like behavior seen by open-field activity and dark-light transmission are a result of lower levels of circulating testosterone. This hypothesis is supported by studies showing that testosterone has an antianxiety effect on animal behavior (20). Sweetened-milk consumption was used to evaluate the murine hedonic behavior and supports this conclusion. Alternatively, lower plasma levels of cholesterol may be the underlying cause of lower testosterone and the subsequent behavior. Earlier, we found that EETs selectively bind to the cannabinoid receptors cannabinoid receptor 2 (CB2) and peripheral-type benzodiazepine receptor (PBR) with moderate affinity (34). Although productive binding to these receptors should produce anti-anxiety-like outcomes, the effects of reduced cholesterol and testosterone might have overshadowed these effects here.
A striking finding of this study was the lower levels of cholesterol and its metabolites. This finding was also supported by an independent study showing lower levels of plasma cholesterol in Ephx2-null male mice (17). This was accompanied by a general decrease in serum lipids. Several possible hypotheses could be made to explain the hypocholesterolemia and hypolipidemia observed in Ephx2-null mice through the absence of the phosphatase activity. First, in vitro studies show that sEH phosphatase activity can hydrolyze lipid phosphates like isoprenoid phosphates, which are precursors of cholesterol biosynthesis and protein isoprenylation (18). Supporting this hypothesis, in humans, sEH polymorphism in Arg287Gln shows higher isoprenoid phosphatase activity in vitro (16). This finding has been associated with familial hypercholesterolemia (55). These results thus suggest a potential role for sEH in regulating cholesterol biosynthesis and metabolism. Squalene oxide and other terpene epoxides are slowly turned over by the sEH (32).
The hypocholesterolemia observed in Ephx2-null mice can also be linked to an indirect effect through the increase of EET levels. These endogenous sEH substrates were shown to bind to and activate PPARα, a known lipid-censoring nuclear receptor, resulting in the modulation of PPARα target genes (50). PPARα activators such as fibrates are generally effective in lowering elevated plasma triglycerides (7) and are strong inducers of the sEH in rodents (31). In addition to their hypolipidemic hepatic effect, activation of PPARα in Leydig cells resulted in the reduction of testis PBR mRNA levels and circulating testosterone levels (25). Considering the proposed role for PBR in cholesterol transport and steroidogenesis, this explains the buildup of cytoplasmic lipid droplets in Ephx2-null Leydig cells, suggesting that most of the cholesterol was not transported to the mitochondria for testosterone production but Instead was accumulated in the cytosolic droplets of Leydig cells.
In conclusion, this study shows that disruption of the sEH gene in mice results in phenotypic characteristics that are associated with the alteration of plasma androgen levels, behavior, and lipid levels. Since this study was designed to characterize a mouse colony lacking sEH expression, it is important to further investigate the role of sEH in cholesterol signaling pathways by using more direct molecular and cellular approaches. We speculate that the possible sources of the cholesterol substrate for testosterone production could be either reduced or redirected because of the effect of EETs on hepatic PPARα or through an effect on Leydig cells. Another option could be through phosphatase activity. Therefore, using inhibitors to block either the NH2 terminus or COOH terminus activities of sEH can be a useful therapeutic approach for controlling lipids and steroids levels.
GRANTS
This work was supported by the Tobacco-Related Disease Research Program (TRDRP), National Institute of Environmental Health Sciences (NIEHS) Superfund Basic Research Program P42 ES-004699, and NIEHS Grants R37 ES-02710 and NIH/NIEHS R01 ES-013933.
Supplementary Material
Acknowledgments
We are grateful to Drs. Marion G. Miller, John W. Newman, and Angela M. Zivkovic for critical review of the manuscript. We thank Dr. Laura S. Van Winkle for the use of the microscope. Dr. Jun-Yan Liu, Imelda Espiritu, and Anthony Lu helped with animals, and Leslie Finke and Media Lab at UCD helped in movie editing. We thank the Mouse Breeding Center at UC Davis and Polly Pearce for help in breeding and maintaining the Ephx2-WT and null mouse colonies.
REFERENCES
- 1.Ahn KC, Zhao B, Chen J, Cherednichenko G, Sanmarti E, Denison MS, Lasley B, Pessah IN, Kultz D, Chang DP, Gee SJ, Hammock BD. in vitro biologic activities of the antimicrobials triclocarban, its analogs, and triclosan in bioassay screens: receptor-based bioassay screens. Environ Health Perspect 116: 1203–1210, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bhathena SJ Relationship between fatty acids and the endocrine system. Biofactors 13: 35–39, 2000. [DOI] [PubMed] [Google Scholar]
- 3.Borhan B, Mebrahtu T, Nazarian S, Kurth MJ, Hammock BD. Improved radiolabeled substrates for soluble epoxide hydrolase. Anal Biochem 231: 188–200, 1995. [DOI] [PubMed] [Google Scholar]
- 4.Campbell WB, Gebremedhin D, Pratt PF, Harder DR. Identification of epoxyeicosatrienoic acids as endothelium-derived hyperpolarizing factors. Circ Res 78: 415–423, 1996. [DOI] [PubMed] [Google Scholar]
- 5.Chen J, Ahn KC, Gee NA, Ahmed MI, Duleba AJ, Zhao L, Gee SJ, Hammock BD, Lasley BL. Triclocarban enhances testosterone action: a new type of endocrine disruptor? Endocrinology 149: 1173–1179, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chiamvimonvat N, Ho CM, Tsai HJ, Hammock BD. The soluble epoxide hydrolase as a pharmaceutical target for hypertension. J Cardiovasc Pharmacol 50: 225–237, 2007. [DOI] [PubMed] [Google Scholar]
- 7.Chinetti G, Fruchart JC, Staels B. Peroxisome proliferator-activated receptors (PPARs): nuclear receptors with functions in the vascular wall. Z Kardiol 90, Suppl 3: 125–132, 2001. [DOI] [PubMed] [Google Scholar]
- 8.Cronin A, Mowbray S, Durk H, Homburg S, Fleming I, Fisslthaler B, Oesch F, Arand M. The N-terminal domain of mammalian soluble epoxide hydrolase is a phosphatase. Proc Natl Acad Sci USA 100: 1552–1557, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.D'Aquila PS, Brain P, Willner P. Effects of chronic mild stress on performance in behavioural tests relevant to anxiety and depression. Physiol Behav 56: 861–867, 1994. [DOI] [PubMed] [Google Scholar]
- 10.Davis BB, Thompson DA, Howard LL, Morisseau C, Hammock BD, Weiss RH. Inhibitors of soluble epoxide hydrolase attenuate vascular smooth muscle cell proliferation. Proc Natl Acad Sci USA 99: 2222–2227, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.De Taeye BM, Novitskaya T, McGuinness OP, Gleaves L, Medda M, Covington JW, Vaughan DE. Macrophage TNF-α contributes to insulin resistance and hepatic steatosis in diet-induced obesity. Am J Physiol Endocrinol Metab 293: E713–E725, 2007. [DOI] [PubMed] [Google Scholar]
- 12.Denlinger CL, Vesell ES. Hormonal regulation of the developmental pattern of epoxide hydrolases. Studies in rat liver. Biochem Pharmacol 38: 603–610, 1989. [DOI] [PubMed] [Google Scholar]
- 13.DiBiasio KW, Silva MH, Shull LR, Overstreet JW, Hammock BD, Miller MG. Xenobiotic metabolizing enzyme activities in rat, mouse, monkey, and human testes. Drug Metab Dispos 19: 227–232, 1991. [PubMed] [Google Scholar]
- 14.Du Teaux SB, Newman JW, Morisseau C, Fairbairn EA, Jelks K, Hammock BD, Miller MG. Epoxide hydrolases in the rat epididymis: possible roles in xenobiotic and endogenous fatty acid metabolism. Toxicol Sci 78: 187–195., 2004. [DOI] [PubMed] [Google Scholar]
- 15.Enayetallah AE, Cao L, Grant DF. Novel role of soluble epoxide hydrolase in regulating cholesterol in mammalian cells. Open Drug Metab J 1: 1–6, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Enayetallah AE, Grant DF. Effects of human soluble epoxide hydrolase polymorphisms on isoprenoid phosphate hydrolysis. Biochem Biophys Res Commun 341: 254–260, 2006. [DOI] [PubMed] [Google Scholar]
- 17.Enayetallah AE, Luria A, Luo B, Tsai HJ, Sura P, Hammock BD, Grant DF. Opposite regulation of cholesterol levels by the phosphatase and hydrolase domains of soluble epoxide hydrolase. J Biol Chem 283: 36592–36598, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.EnayetAllah AE, Sura P, Grant DF. Role of soluble epoxide hydrolase in regulating the isoprenoid biosynthesis pathway and its downstream effects. Drug Metab Rev 38: 202–203, 2006. [Google Scholar]
- 19.Folch J, Lees M, Stanley GHS. A simple method for the isolation and purification of total lipids from animal tissues. J Biol Chem 226: 497–509, 1957. [PubMed] [Google Scholar]
- 20.Frye CA, Edinger K, Sumida K. Androgen administration to aged male mice increases anti-anxiety behavior and enhances cognitive performance. Neuropsychopharmacology 33: 1049–1061, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Frye CA, Paris JJ, Rhodes ME. Engaging in paced mating, but neither exploratory, anti-anxiety, nor social behavior, increases 5 alpha-reduced progestin concentrations in midbrain, hippocampus, striatum, and cortex. Reproduction 133: 663–674, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Frye CA, Rhodes ME, Petralia SM, Walf AA, Sumida K, Edinger KL. 3 Alpha-hydroxy-5 alpha-pregnan-20-one in the midbrain ventral tegmental area mediates social, sexual, and affective behaviors. Neuroscience 138: 1007–1014, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Frye CA, Walf AA, Rhodes ME, Hamey JP. Progesterone enhances motor, anxiolytic, analgesic, and antidepressive behavior of wild-type mice, but not those deficient in type 15 alpha-reductase. Brain Res 1004: 116–124, 2004. [DOI] [PubMed] [Google Scholar]
- 24.Fulton D, McGiff JC, Quilley J. Pharmacological evaluation of an epoxide as the putative hyperpolarizing factor mediating the nitric oxide-independent vasodilator effect of bradykinin in the rat heart. J Pharmacol Exp Ther 287: 497–503, 1998. [PubMed] [Google Scholar]
- 25.Gazouli M, Yao ZX, Boujrad N, Corton JC, Culty M, Papadopoulos V. Effect of peroxisome proliferators on Leydig cell peripheral-type benzodiazepine receptor gene expression, hormone-stimulated cholesterol transport, and steroidogenesis: role of the peroxisome proliferator-activator receptor alpha. Endocrinology 143: 2571–2583, 2002. [DOI] [PubMed] [Google Scholar]
- 26.Gill SS, Hammock BD. Distribution and properties of a mammalian soluble epoxide hydrase. Biochem Pharmacol 29: 389–395, 1980. [DOI] [PubMed] [Google Scholar]
- 27.Gill SS, Hammock BD, Yamamoto I, Casida JE. Preliminary chromatographic studies on the metabolites and photodecomposition products of the juvenoid 1-(4′-ethylphenoxy-6,7-epoxy)-3,7-dimethyl-2-octene. In: Insect Juvenile Hormones: Chemistry and Action, edited by Menn JJ and Beroza M. New York: Academic, 1972, p. 177–189.
- 28.Grant DF, Storms DH, Hammock BD. Molecular cloning and expression of murine liver soluble epoxide hydrolase. J Biol Chem 268: 17628–17633, 1993. [PubMed] [Google Scholar]
- 29.Gronli J, Murison R, Fiske E, Bjorvatn B, Sorensen E, Portas CM, Ursin R. Effects of chronic mild stress on sexual behavior, locomotor activity and consumption of sucrose and saccharine solutions. Physiol Behav 84: 571–577, 2005. [DOI] [PubMed] [Google Scholar]
- 30.Guenthner TM, Hammock BD, Vogel U, Oesch F. Cytosolic and microsomal epoxide hydrolases are immunologically distinguishable from each other in the rat and mouse. J Biol Chem 256: 3163–3166, 1981. [PubMed] [Google Scholar]
- 31.Hammock BD, Ota K. Differential induction of cytosolic epoxide hydrolase, microsomal epoxide hydrolase, and glutathione S-transferase activities. Toxicol Appl Pharmacol 71: 254–265, 1983. [DOI] [PubMed] [Google Scholar]
- 32.Hammock BD, Gill SS, Mumby SM, Ota K. Comparison of epoxide hydrases in the soluble and microsomal fractions of mammalian liver. In: Molecular Basis of Environmental Toxicity, edited by Bhatnagar RS. Ann Arbor, MI: Ann Arbor Science Publishers, 1980, p. 229–272.
- 33.Imig JD, Zhao X, Zaharis CZ, Olearczyk JJ, Pollock DM, Newman JW, Kim IH, Watanabe T, Hammock BD. An orally active epoxide hydrolase inhibitor lowers blood pressure and provides renal protection in salt-sensitive hypertension. Hypertension 46: 975–981, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Inceoglu B, Schmelzer KR, Morisseau C, Jinks SL, Hammock BD. Soluble epoxide hydrolase inhibition reveals novel biological functions of epoxyeicosatrienoic acids (EETs). Prostaglandins Other Lipid Mediat 82: 42–49, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Inoue N, Fujiwara K, Iwata T, Imai K, Aimoto T. Involvement of pituitary-hormone in the sex-related regulation of hepatic epoxide hydrolase activity in mice. Biol Pharmaceut Bull 18: 536–539, 1995. [DOI] [PubMed] [Google Scholar]
- 36.Inoue N, Yamada K, Imai K, Aimoto T. Sex hormone-related control of hepatic epoxide hydrolase activities in mice. Biol Pharm Bull 16: 1004–1007, 1993. [DOI] [PubMed] [Google Scholar]
- 37.Long JM Analysis of neural cell function in gene knockout mice: behavior. Methods Enzymol 417: 66–79, 2006. [DOI] [PubMed] [Google Scholar]
- 38.Loury DN, Moody DE, Kim BW, Hammock BD. Effect of dietary clofibrate on epoxide hydrolase activity in tissues of mice. Biochem Pharmacol 34: 1827–1833, 1985. [DOI] [PubMed] [Google Scholar]
- 39.Luria A, Rubinstein S, Lax Y, Breitbart H. Extracellular adenosine triphosphate stimulates acrosomal exocytosis in bovine spermatozoa via P2 purinoceptor. Biol Reprod 66: 429–437, 2002. [DOI] [PubMed] [Google Scholar]
- 40.Luria A, Weldon SM, Kabcenell AK, Ingraham RH, Matera D, Jiang HP, Gill R, Morisseau C, Newman JW, Hammock BD. Compensatory mechanism for homeostatic blood pressure regulation in Ephx2 gene-disrupted mice. J Biol Chem 282: 2891–2898, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Markaverich BM, Alejandro M, Thompson T, Mani S, Reyna A, Portillo W, Sharp J, Turk J, Crowley JR. Tetrahydrofurandiols (THF-diols), leukotoxindiols (LTX-diols), and endocrine disruption in rats. Environ Health Perspect 115: 702–708, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Moghaddam MF, Grant DF, Cheek JM, Greene JF, Williamson KC, Hammock BD. Bioactivation of leukotoxins to their toxic diols by epoxide hydrolase. Nat Med 3: 562–566, 1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Morisseau C, Derbel M, Lane TR, Stoutamire D, Hammock BD. Differential induction of hepatic drug-metabolizing enzymes by fenvaleric acid in male rats. Toxicol Sci 52: 148–153, 1999. [DOI] [PubMed] [Google Scholar]
- 44.Morisseau C, Goodrow MH, Newman JW, Wheelock CE, Dowdy DL, Hammock BD. Structural refinement of inhibitors of urea-based soluble epoxide hydrolases. Biochem Pharmacol 63: 1599–1608, 2002. [DOI] [PubMed] [Google Scholar]
- 45.Morisseau C, Hammock BD. Epoxide hydrolase. In: Wiley Encyclopedia of Molecular Medicine, edited by Creighton TE. Hoboken, NJ: Wiley and Sons, 2002, p. 1194–1997.
- 46.Morisseau C, Hammock BD. Epoxide hydrolases: mechanisms, inhibitor designs, and biological roles. Annu Rev Pharmacol Toxicol 45: 311–333, 2005. [DOI] [PubMed] [Google Scholar]
- 47.Morisseau C, Newman JW, Wheelock CE, Hill T3rd, Morin D, Buckpitt AR, Hammock BD. Development of metabolically stable inhibitors of mammalian microsomal epoxide hydrolase. Chem Res Toxicol In press: 2008. [DOI] [PubMed]
- 48.Morisseau CaBDH Measurements of soluble epoxide hydrolase (sEH) activity. In: Techniques for Analysis of Chemical Biotransformation: Current Protocols in Toxicology, esdited by Bus JS, Costa LG, Hodgson E, Lawrence DA, and Reed DJ. Hoboken, NJ: Wiley & Sons, p. 4.23.21–24.23.18, 2007. [DOI] [PubMed]
- 49.Newman JW, Morisseau C, Harris TR, Hammock BD. The soluble epoxide hydrolase encoded by EPXH2 is a bifunctional enzyme with novel lipid phosphate phosphatase activity. Proc Natl Acad Sci USA 100: 1558–1563, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ng VY, Huang Y, Reddy LM, Falck JR, Lin ET, Kroetz DL. Cytochrome P450 eicosanoids are activators of peroxisome proliferator-activated receptor alpha. Drug Metab Disp 35: 1126–1134, 2007. [DOI] [PubMed] [Google Scholar]
- 51.Node K, Huo Y, Ruan X, Yang B, Spiecker M, Ley K, Zeldin DC, Liao JK. Anti-inflammatory properties of cytochrome P450 epoxygenase-derived eicosanoids. Science 285: 1276–1279, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.O'Shaughnessy PJ, Willerton L, Baker PJ. Changes in Leydig cell gene expression during development in the mouse. Biol Reprod 66: 966–975, 2002. [DOI] [PubMed] [Google Scholar]
- 53.Pinot F, Grant DF, Spearow JL, Parker AG, Hammock BD. Differential regulation of soluble epoxide hydrolase by clofibrate and sexual hormones in the liver and kidneys of mice. Biochem Pharmacol 50: 501–508, 1995. [DOI] [PubMed] [Google Scholar]
- 54.Potente M, Fisslthaler B, Busse R, Fleming I. 11,12-Epoxyeicosatrienoic acid-induced inhibition of FOXO factors promotes endothelial proliferation by down-regulating p27Kip1. J Biol Chem 278: 29619–29625, 2003. [DOI] [PubMed] [Google Scholar]
- 55.Sato K, Emi M, Ezura Y, Fujita Y, Takada D, Ishigami T, Umemura S, Xin Y, Wu LL, Larrinaga-Shum S, Stephenson SH, Hunt SC, Hopkins PN. Soluble epoxide hydrolase variant (Glu287Arg) modifies plasma total cholesterol and triglyceride phenotype in familial hypercholesterolemia: intrafamilial association study in an eight-generation hyperlipidemic kindred. J Hum Genet 49: 29–34, 2004. [DOI] [PubMed] [Google Scholar]
- 56.Schmelzer KR, Kubala L, Newman JW, Kim IH, Eiserich JP, Hammock BD. Soluble epoxide hydrolase is a therapeutic target for acute inflammation. Proc Natl Acad Sci USA 102: 9772–9777, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Sinal CJ, Miyata M, Tohkin M, Nagata K, Bend JR, Gonzalez FJ. Targeted disruption of soluble epoxide hydrolase reveals a role in blood pressure regulation. J Biol Chem 275: 40504–40510, 2000. [DOI] [PubMed] [Google Scholar]
- 58.Tang XD, Sanford LD. Home cage activity and activity-based measures of anxiety in 129P3/J, 129X1/SvJ and C57BL/6J mice. Physiol Behav 84: 105–115, 2005. [DOI] [PubMed] [Google Scholar]
- 59.Tran KL, Aronov PA, Tanaka H, Newman JW, Hammock BD, Morisseau C. Lipid sulfates and sulfonates are allosteric competitive inhibitors of the N-terminal phosphatase activity of the mammalian soluble epoxide hydrolase. Biochemistry 44: 12179–12187, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wang C, Leung A, Sinhahikim AP. Reproductive aging in the male brown-Norway rat—a model for the human. Endocrinology 133: 2773–2781, 1993. [DOI] [PubMed] [Google Scholar]
- 61.Watkins SM, German JB. Toward the implementation of metabolomic assessments of human health and nutrition. Curr Opin Biotechnol 13: 512–516, 2002. [DOI] [PubMed] [Google Scholar]
- 62.Watkins SM, Hammock BD, Newman JW, German JB. Individual metabolism should guide agriculture toward foods for improved health and nutrition. Am J Clin Nutr 74: 283–286, 2001. [DOI] [PubMed] [Google Scholar]
- 63.Watkins SM, Reifsnyder PR, Pan HJ, German JB, Leiter EH. Lipid metabolome-wide effects of the PPARgamma agonist rosiglitazone. J Lipid Res 43: 1809–1817, 2002. [DOI] [PubMed] [Google Scholar]
- 64.Yu B, Kodavanti UP, Takeuchi M, Witschi H, Pinkerton KE. Acute tobacco smoke-induced airways inflammation in spontaneously hypertensive rats. Inhal Toxicol 20: 623–633, 2008. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.