Abstract
Both estrogen (E2) and EGF regulate lactotrophs, and we recently demonstrated that EGF phosphorylates S118 on estrogen receptor-α (ERα) and requires ERα to stimulate prolactin (PRL) release. However, the interactions between ligand-occupied ERα and activated ErbB1 and its impact on lactotroph function are unknown. Using rat GH3 lactotrophs, we found that both E2 and EGF independently stimulated proliferation and PRL gene expression. Furthermore, their combination resulted in an enhanced stimulatory effect on both cell proliferation and PRL gene expression. Inhibitors of ER as well as ErbB1 blocked the combined effects of E2 and EGF. Pretreatment with UO126 abolished the combined effects, demonstrating Erk1/2 requirement. Although bidirectionality in ER-ErbB1 cross-talk is a well-accepted paradigm, interestingly in lactotrophs, ErbB1 kinase inhibitor failed to block the effect of E2 on proliferation and stimulation of PRL gene expression, suggesting that ER does not require ErbB1 to mediate its effects. Furthermore, E2 did not affect the ability of EGF to induce c-Fos expression or modulate AP-1 activity. However, both E2 and EGF combine to enhance S118 phosphorylation of ERα, leading to enhanced E2-mediated estrogen response element transactivation. Taken together, our results suggest that, in lactotrophs, activated ErbB1 phosphorylates ERα to enhance the stimulatory effect of E2, thereby providing the molecular basis by which EGF amplifies the response of E2.
Keywords: lactotrophs, cell proliferation
estrogen (e2) exerts a wide range of biological activities, including rapid cell activation, reversible gene regulation, and chronic structural/proliferative alterations. Binding of E2 to the estrogen receptors (ERs) facilitates receptor binding to specific DNA target sequences called the estrogen response elements (ERE). The ability of ER to bind DNA is regulated by several coactivators or corepressors that enhance or suppress, respectively, the association of ER with the gene transcriptional apparatus (22, 26). The transcriptional activity of ER also depends on its phosphorylation, which takes place within minutes of E2 binding. The pituitary lactotroph is a well-characterized E2 target cell, with estrogens stimulating prolactin (PRL) gene expression and release, upregulating genes such as TGF-α, galanin, and VEGF, and increasing lactotroph proliferation (3, 11). The importance of ER(s) in lactotroph homeostasis is highlighted by the decreases in pituitary PRL content and lactotroph number in ovariectomized animals, whereas chronic exposure to E2 results in hyperprolactinemia and lactotroph hyperplasia (35).
In the absence of a human pituitary cell line, investigators have relied on the rat pituitary lactotroph GH3 cell line as a model for studying lactotroph function in response to E2. Although GH3 cells express both ERα and ERβ (3), ERα is considered the major receptor subtype that regulates lactotroph proliferation. This is supported by our finding that the anti-estrogen ICI 182780 (ICI) in the absence of E2 caused rapid degradation of ERα, but not ERβ, and potently inhibited GH3 cell proliferation (15). On the other hand, overexpression of ERβ in GH3 cells resulted in increased PRL production in response to E2, suggesting that ERβ may have a more prominent role on PRL production/release than in cell proliferation (23).
EGF is a potent mitogen that affects a wide variety of cell types via activation of the EGF receptor (EGFR/ErbB1). The ErbB family of receptor tyrosine kinases (RTK) includes EGFR/ErbB1, ErbB2/Her2, ErbB3, and ErbB4. Upon ligand binding, the RTKs undergo conformational changes that activate intrinsic tyrosine kinase. This leads to increased phosphorylation of specific tyrosine residues within the cytoplasmic domain of the receptor, thereby creating binding sites for adaptor proteins that propagate the signal (9, 18, 36). Although ErbB overexpression/hyperactivation has been the subject of intense investigations in several tumor models, its role in regulating lactotroph functions is not well characterized. EGF has been reported to stimulate PRL release, decrease GH production, and exert a stronger effect on GH3 cell differentiation than on their proliferation (27). Many human pituitary adenomas express EGF, its receptor, as well as ErbB2, another member of the ErbB family, suggesting that EGF and its signaling pathways play a role in tumor development/progression (4, 13, 19).
Given the apparent overlap between steroid receptors and peptide growth factor receptors in regulating cell proliferation and the utilization of common downstream signaling intermediaries, signal cross-talk between these receptors has been extensively studied. Two models have been proposed. The first stipulates that EGFR utilizes nuclear ER to mediate its biological effect. This notion is supported by the observations that anti-estrogens block the EGF-induced uterine cell proliferation, with EGF failing to stimulate proliferation of these cells in the ERα knockout mouse (8, 12). Interaction between the two receptors is believed to be facilitated by EGFR-activated Erk1/2, which phosphorylates ERα on S118 in the ligand-independent transactivation AF-1 domain (16). Several other phosphorylation sites within the AF-1 domain have also been implicated in the modulation of ER activation in the absence of E2 (5, 14, 17). The second model stipulates that ER utilizes the EGFR to mediate its biological effects. This signaling pathway involves activation of matrix metalloproteinases, which in turn cleave and release membrane-tethered growth factors that ultimately activate receptor tyrosine kinase. This model is supported by the observations that the ability of E2 to stimulate cell proliferation and Erk1/2 activation is blocked by inhibiting EGFR activation (20, 24).
A recent study demonstrated that GH3 cell-induced tumors in nude mice were significantly inhibited by Gefitinib, clearly implicating a role for EGFR in the pathogenesis of prolactinomas (33). Furthermore, we demonstrated that, even in the absence of E2, the ability of EGF to stimulate PRL release is ERα dependent, clearly establishing a cross-talk between ER- and EGFR-mediated signaling pathways (2). However, the interactions of the signaling pathways of ligand-occupied ER and activated EGFR and its impact on lactotroph function remains to be described. In this study we report that activated EGFR combined with ligand-occupied ERα, but not ERβ, to stimulate both cell proliferation and PRL gene expression. Whereas ER does not seem to require EGFR to mediate its effects, EGFR-mediated phosphorylation of S118 on ERα is critical for this cross-talk.
MATERIALS AND METHODS
Reagents.
Recombinant hEGF was purchased from Promega (Madison, WI). 17-β estradiol was bought from Sigma Chemical (St. Louis, MO). The isotype-specific agonists, ERα-specific agonist 4,4′,4′′-(4-Propyl-[1H] pyrazole-1,3,5-triyl) trisphenol (PPT) and ERβ-specific agonist 2,3-bis(4-hydroxyphenyl)-propionitrile diarylpropionitrile (DPN) were obtained from Tocris (Ellisville, MO). All solvents, buffers, and chemicals were of analytical grade.
Cell culture.
Routine maintenance of GH3 cells was done in DMEM:F-12 50:50 mix (Mediatech, Herndon, VA) containing 10% FBS (HyClone, Logan, UT) and 5 U/ml penicillin and 5 μg/ml streptomycin. Subculturing was performed as required, and cell culture medium was generally changed every 2–3 days.
Assessment of cell proliferation.
GH3 cells in log phase were washed three times with phenol red-free DMEM:F12 50:50 mix medium and seeded at 20,000–30,000 cells/well on protamine-coated 96-well plates in plating medium [DMEM: F12 (50:50) medium containing insulin, transferrin, selenious acid premix (BD Biosciences, San Jose, CA) and penicillin/streptomycin]. The next day, medium was replaced with plating medium containing treatments. Assessment of cell proliferation was done using the 3-[4,5-dimethylthiazole-2-yl]-2,5-diphenyltetrazolium bromide (MTT) (Sigma) assay as previously described (15). Briefly, 1 mg/ml MTT was added to the cells. After 2 h, 100 μl of a developing solution (50% vol/vol dimethylformamide; 20% wt/vol SDS; 0.24% vol/vol glacial acetic acid; 60 mM sodium acetate) was added, and optical density at 570 nm was read using a microplate reader (BioTek Instruments Inc, Winooski, VT). Data are presented as optical density or percentage of control. Our recent work shows that MTT assay has an excellent corelationship with BrdU incorporation assays in GH3 cells (15).
Western blotting.
After cell treatments were done, cell lysis was performed as described (15). Protein content in the supernatant was determined by the BCA protein assay (Pierce Chemical, Rockford, IL). Equal amounts of cell lysates were subjected to electrophoresis and then transferred onto PVDF membranes. After transfer, membranes were rinsed and incubated with blocking buffer (4–5% nonfat milk in PBST/TBST) for 1–2 h at room temperature. Membranes were then incubated overnight with primary antibodies at 4°C, followed by three 10-min washes with PBST/TBST, and then incubation with secondary antibodies at room temperature for 1 h. Per the manufacturer's instructions, enhanced chemiluminescence reagents (Pierce) were used to detect antibody-bound proteins.
Luciferase reporter assays.
After 24 h in culture, GH3 cells were transiently cotransfected with 0.8 μg of the 2.5-Kb rat PRL pA3 PRL/Luciferase plasmid (see Ref. 31; a kind gift from Dr. A. Gutierrez-Hartman, Denver, CO), or p3X ERE-luc reporter plasmid (containing 3 copies of the vitellogenin A2 ERE, kindly gifted from Dr. R. Bigsby, Indianapolis, IN), or AP-1 reporter plasmid (see Ref. 25; kindly provided by Dr. G. Chen, Milwaukee, WI), together with the control pGL4.70[hRLuc] Renilla plasmid (Promega), using Lipofectamine 2000 (Invitrogen) per the manufacturer's instructions. After an overnight incubation, cells were washed three times, and medium was replaced with plating medium containing the indicated treatments. Cells were lysed, and luciferase activity was determined using dual luciferase assay kit (Promega). After normalization to Renilla, fold change in luciferase activity was determined.
Data analysis.
For cell proliferation/MTT assays, data are expressed as optical density and are means ± SE of three to six determinations from a single experiment that was repeated three times. Statistical significance was determined using Student's t-test; a value of P <0.05 was considered significant.
RESULTS
EGF selectively enhances ERα- but not ERβ-stimulated lactotroph proliferation.
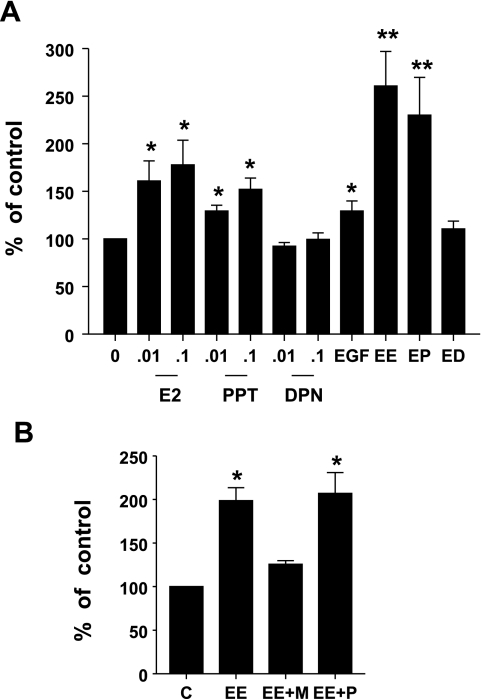
We first questioned the independent ability of EGF-, ERα-, and ERβ-specific ligands to stimulate lactotroph proliferation. GH3 cells were incubated with E2 (0.01 and 0.1 nM), the ERα-specific agonist PPT (0.01 and 0.1 nM), the ERβ-specific agonist DPN (0.01 and 0.1 nM), or EGF (5 ng/ml), or a combination (EE) of E2 (0.01 nM) and EGF (5 ng/ml), a combination (EP) of PPT (0.01 nM) and EGF (5 ng/ml), or a combination (ED) of DPN (0.01 nM) and EGF (5 ng/ml). Cell proliferation was assessed after 5 days using the MTT assay. A modest but significant stimulation of lactotroph proliferation was seen in response to E2 and EGF. This stimulation was mimicked by the ERα-specific agonist PPT, but not the ERβ-specific agonist DPN (Fig. 1A). We next questioned whether EGF could modulate cell proliferation of ligand-occupied ERs and, if so, whether it is ER subtype specific. Incubation with a combination of EGF and E2 resulted in a robust combined stimulatory effect on cell proliferation, which was mimicked by PPT but not DPN (Fig. 1A). To confirm receptor isotype participation in this combined stimulatory effect, we next treated GH3 cells with a combination (EE) of E2 (0.01 nM) and EGF (5 ng/ml), either by itself or in the presence of ERα-specific antagonist, 1,3-Bis(4-hydroxyphenyl)-4-methyl-5-[4-(2-piperidinylethoxy)phenol]-1H-pyrazoledihydrochloride (MPP) (100 nM), or the ERβ-specific antagonist 4-[2-Phenyl-5,7-bis (tri-fluoromethyl) pyrazolo [1,5-a]pyrimidin-3-yl]phenol (PHTPP) (100 nM). Our data show (Fig. 1B) that the combined stimulatory effect of E2 and EGF on lactotroph proliferation is significantly inhibited in the presence of the ERα antagonist MPP, whereas the ERβ antagonist failed to have any inhibitory effect. Taken together, these data clearly demonstrate that signaling pathways of activated EGFR and ligand-occupied ERα combine to stimulate lactotroph proliferation.
Fig. 1.
EGF selectively enhances estrogen receptor (ER)α- but not ERβ-stimulated lactotroph proliferation. A: GH3 cells were treated with estrogen (E2) (0.01 and 0.1 nM), 4,4′,4′′-(4-Propyl-[1H] pyrazole-1,3,5-triyl) trisphenol (PPT) (0.01 and 0.1 nM), 2,3-bis(4-hydroxyphenyl)-propionitrile diarylpropionitrile (DPN) (0.01 and 0.1 nM), EGF (5 ng/ml), a combination of E2 (0.01 nM) and EGF (5 ng/ml) (EE), a combination of PPT (0.01 nM) and EGF (5 ng/ml) (EP), or a combination of DPN (0.01 nM) and EGF (5 ng/ml) (ED). Cell proliferation was determined by 3-[4,5-dimethylthiazole-2-yl]-2,5-diphenyltetrazolium bromide (MTT) assay after 5 days. Data were calculated as percentages of vehicle control and expressed as means ± SE of 8 independent experiments, each performed with at least 4 replicates. *Significant differences from controls; **significant differences from E2, PPT, and EGF alone (P < 0.05). B: GH3 cells were treated with EE, or EE in the presence of 1,3-Bis(4-hydroxyphenyl)-4-methyl-5-[4-(2-piperidinylethoxy) phenol]-1H-pyrazoledihydrochloride (MPP) (100 nM) (EE + M); or EE in the presence of 4-[2-Phenyl-5,7-bis (tri-fluoromethyl) pyrazolo [1,5-a]pyrimidin-3-yl]phenol (PHTPP) (100 nM) (EE + P). Cell proliferation was determined as in A. Data were calculated as the percentages of vehicle control and expressed as means ± SE of 3 independent experiments, each performed with at least 5 replicates. *Significant differences from control (P < 0.05).
Differential stimulation of PRL gene expression by ERα and ERβ.
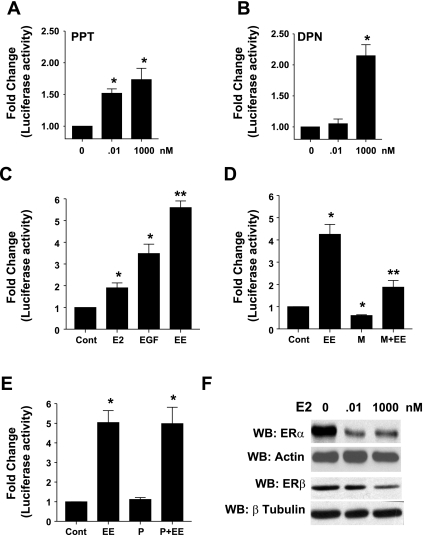
We next examined ER subtype ability to stimulate PRL gene expression. GH3 cells were transfected with pA3rPRL/Luc reporter plasmid and stimulated with the indicated concentrations of PPT or DPN. Normalized luciferase activity was determined as described in materials and methods. Our data show that both PPT and DPN are capable of stimulating PRL gene expression, with significant stimulation being observed with concentrations of PPT as low as 0.01 nM, whereas DPN only stimulated PRL gene expression at higher concentrations (1 μM) (Fig. 2, A and B).
Fig. 2.
EGF selectively enhances ERα-stimulated prolactin (PRL) gene expression. GH3 cells transiently cotransfected with PRL reporter gene and control reporter gene (Cont) were treated with the indicated concentrations of PPT (A), DPN (B), or E2 (0.01 nM), EGF (5 ng/ml), or a combination (EE) (C) for 24 h, and normalized luciferase activity was determined as described in materials and methods. Data were calculated as fold change over control (arbitrary value of 1). Each value is the mean ± SE of 3 separate experiments, each performed in triplicate. *Significant difference from control. **Significant differences from E2 and EGF alone (P < 0.05). GH3 cells, transiently cotransfected with PRL reporter gene and control reporter gene were treated with vehicle or a combination of E2 and EGF (EE) either by themselves or in presence of the ERα-specific antagonist, MPP (100 nM) (D), or the ERβ-specific antagonist, PHTPP (100 nM) (E), for 24 h, and normalized luciferase activity was determined as described in materials and methods. Data were calculated as fold change over control (arbitrary value of 1). Each value is the mean ± SE of 3 separate experiments, each performed in triplicate. *Significant difference from control. **Significant differences from EE (P < 0.05). F: GH3 cells were treated with the indicated concentrations of E2 for 24 h. An equal amount of cell lysate was subjected to Western blotting (WB) with anti-ERα antibody (Ab) or anti-ERβ Ab to ensure equal loading blots were stripped and reprobed with either anti-actin Ab or anti-β tubulin Ab. Results shown are from a single experiment and are representative of 3 separate experiments.
EGF cross-talks with ERα to enhance E2-stimulated PRL gene expression.
We next questioned whether EGF modulated E2-stimulated increases in PRL gene expression. GH3 cells were transfected with pA3rPRL/Luc reporter plasmid and treated with vehicle, E2 (0.01 nM), EGF (5 ng/ml), or a combination of E2 and EGF for 18–24 h, and normalized luciferase activity in cell lysates was determined as described in materials and methods. Our data show that both E2 and EGF independently stimulate PRL gene expression. In the presence of EGF, the ability of E2 to stimulate PRL gene expression is enhanced (Fig. 2C). To identify which ER subtype(s) is involved in the cross-talk with EGF, we used a pharmacological approach to address this issue. GH3 cells were transiently transfected with the PRL-luciferase reporter gene and treated with vehicle, a combination of E2 (0.01 nM) and EGF (5 ng/ml) (EE), EE in the presence of the ERα-specific antagonist MPP, 100 nM, or EE in the presence of ERβ-specific antagonist PHTPP, 100 nM, for 18–24 h. Cells were lysed, and luciferase activity was determined as described in materials and methods. Our data show that the combined effects of E2 and EGF on stimulation of PRL gene expression was significantly inhibited by the ERα-specific antagonist MPP (Fig. 2D) but not by the ERβ-specific antagonist PHTPP (Fig. 2E).
Ligand-induced degradation of ERα has been shown to play a positive role in modulating ERα transcriptional activity (32). GH3 cells were treated with vehicle or 0.01 or 1,000 nM E2 for 24 h, and equal amounts of cell lysates were used to examine ERα and ERβ levels. Our data show that, upon treatment with a physiologically relevant dose (0.01 nM) of E2, there is significant E2-induced degradation of ERα, but not of ERβ (Fig. 2F). Together, these results suggest the selective utilization of ERα at physiologically relevant concentrations of E2.
ErbB1 kinase inhibitor, AG1478, and anti-estrogen, ICI, block the combined effects of estrogen and EGF on cell proliferation as well as stimulation of PRL gene expression.
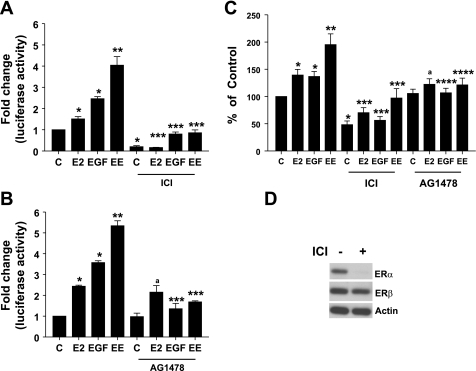
We next questioned whether inhibitors of ErbB1 kinase and anti-estrogen would block the combined effects of E2 and EGF on cell proliferation as well as PRL gene expression. To address this issue, GH3 cells were transiently cotransfected with the PRL-luciferase reporter gene and control gene and treated with vehicle, E2 (0.01 nM), EGF (5 ng/ml), or a combination of E2 and EGF (EE), either by itself or in presence of the anti-estrogen ICI (100 nM) (Fig. 3A), or the ErbB1 kinase inhibitor AG1478 (10 μM) (Fig. 3B). After 18–24 h, cells were lysed and luciferase activity was determined. Our data show that, although E2 and EGF were capable of stimulating PRL gene expression independently, their combination led to an amplification of the stimulatory response. In the presence of the anti-estrogen ICI, the ability of the independent effects of both E2 and EGF, as well as their combined effects, were completely blocked. However, in the presence of the ErbB1 kinase inhibitor, only the effects of EGF and a combination of E2 and EGF were inhibited by AG1478, whereas the ability of E2 to stimulate PRL gene expression was unaffected in the presence of the ErbB1 kinase inhibitor.
Fig. 3.
ErbB1 kinase inhibitor, AG1478, and anti-estrogen, ICI 182780 (ICI), block the combined effects of estrogen and EGF on cell proliferation and stimulation of PRL gene expression. GH3 cells transiently cotransfected with PRL reporter gene and control reporter gene were treated with vehicle (C), E2 (0.01 nM), EGF (5 ng/ml), or a combination of E2 and EGF (EE), either alone or in presence of ICI 182780 (100 nM) (A) or AG1478 (10 μM) (B) for 24 h, and normalized luciferase activity was determined as described in materials and methods. Data were calculated as fold change over control (arbitrary value of 1). Each value is the mean ± SE of 3 separate experiments, each performed in triplicate. *Significant difference from control; **significant difference from E2 and EGF alone; ***significant difference from treatments without ICI/AG1478; ano significant difference from E2 alone, (P < 0.05). C: GH3 cells were treated with vehicle (C), E2 (0.01 nM), EGF (5 ng/ml), or EE, either alone or in the presence of AG1478 (10 μM) or ICI 182780 (100 nM), and cell proliferation was determined by the MTT assay after 5 days. Data were calculated as percentages of vehicle control and presented as the means ± SE of 3 separate experiments. *Significant difference from control, **significant difference from E2 and EGF alone, ***significant difference from treatments without ICI, ****significant difference from treatments without AG1478; ano significant difference from E2 alone (P < 0.05). D: GH3 cells were treated with vehicle or ICI 100 nM for 18–24 h, and an equal amount of cell lysates was subjected to Western blotting with an anti-ERα antibody, followed by stripping and reprobing with anti-ERβ antibody, followed by stripping and reprobing with anti-actin antibody. Results shown are from a single experiment and are representative of 3 separate experiments.
We next examined the effects of receptor inhibitors on cell proliferation. GH3 cells were treated with vehicle, E2 (0.01 nM), EGF (5 ng/ml), or a combination of E2 and EGF (EE), either by itself or in presence of the anti-estrogen ICI (100 nM), or the ErbB1 kinase inhibitor AG1478 (10 μM). After 5 days, cell proliferation was assessed, and our results show (Fig. 3C) that, in the presence of either receptor inhibitor, the effect of a combined E2 and EGF stimulation on cell proliferation was abolished. Furthermore, like the effects on PRL gene stimulation, AG1478 failed to block the ability of E2 to stimulate cell proliferation. Together, these data clearly demonstrate the requirement of both ER and ErbB1 in mediating the combined stimulatory effect on cell proliferation as well as PRL gene expression. In addition, the failure of AG1478 to block E2 responses suggests that E2 does not require ErbB1 kinase activity to mediate its effect.
Anti-estrogen ICI-induced ERα degradation is well documented. To confirm our conclusion that EGF selectively cross-talks with ERα, but not ERβ, to modulate both lactotroph proliferation and stimulation of prolactin gene expression, we next questioned whether the ability of ICI to block the combined effects of E2 and EGF was attributable to ERα degradation. To address this issue, GH3 cells were treated overnight with vehicle or ICI (100 nM). Equal amounts of cell lysates were subjected to Western blotting with an anti-ERα antibody. Our results (Fig. 3D) show that ICI caused a robust degradation of ERα. Next, we stripped and reprobed the same membrane with antibodies to both actin and ERβ, and our results show (Fig. 3D) that ICI treatment had no effect on the levels of either ERβ or actin.
Erk1/2 inhibition blocks the combined stimulatory effect of EGF and E2 on cell proliferation and PRL gene expression.
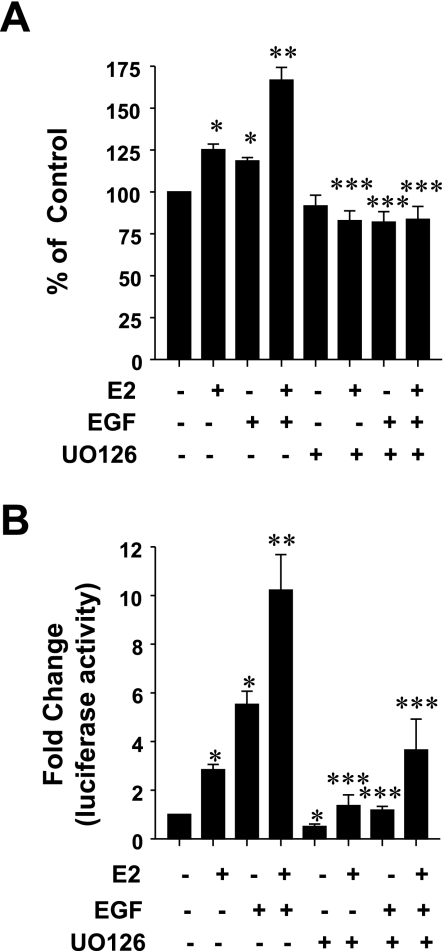
Given the utilization of Erk1/2 signaling pathway by both ErbB1 and ERα and the significant role played by Erk1/2 in signaling cross-talk between the two receptors, we questioned whether the stimulation of lactotroph proliferation, in response to a combination of E2 and EGF, was Erk1/2 dependent. GH3 cells were treated with either vehicle, EGF (5 ng/ml), E2 (0.01 nM), a combination of E2 and EGF, or the same treatments in the presence of the Mek1 inhibitor UO126 (10 μM). After 5 days, cell proliferation was assessed by the MTT assay. Figure 4A demonstrates that UO126 completely abolished the combined stimulatory effects of EGF and E2 on lactotroph proliferation. Next, we questioned whether Erk1/2 mediated the combined stimulatory effects of E2 and EGF on PRL gene expression. GH3 cells, transfected with PRL-luciferase reporter gene, were treated with either vehicle, EGF (5 ng/ml), E2 (0.01 nM), a combination of E2 and EGF, or the same treatments in the presence of the Mek1 inhibitor UO126 (10 μM). After 18–24 h of stimulation, luciferase activity was determined. Our data show (Fig. 4B) that GH3 cells pretreated with the Mek1 inhibitor failed to respond to the combined stimulatory effect of E2 and EGF. Furthermore, the independent ability of E2 and EGF to stimulate PRL gene expression is also blocked by UO126. A similar conclusion on Erk1/2 requirement for the combined stimulatory effects on PRL gene expression was verified by using a Mek1-specific inhibitor PD98059 (data not shown).
Fig. 4.
Erk1/2 inhibition blocks the combined stimulatory effect of estrogen and EGF on cell proliferation and PRL gene expression. A: GH3 cells were treated with E2 (0.01 nM), EGF (5 ng/ml), or their combination alone or in presence of UO126 (10 μM), and cell proliferation was determined by the MTT assay after 5 days. Data were calculated as percentages of vehicle control and presented as the means ± SE of 3 experiments. *Significant differences from control; **significant difference from E2 and EGF alone; ***significant difference from treatments without UO126 (P < 0.05). B: GH3 cells transiently cotransfected with PRL reporter gene and control reporter gene were treated with E2 (0.01 nM), EGF (5 ng/ml), or their combination, either alone or in presence of UO126 (10 μM), for 24 h, and normalized luciferase activity was determined as described in materials and methods. Data were calculated as fold change over control (arbitrary value of 1). Each value is the mean ± SE of 3 separate experiments, each performed in triplicate.
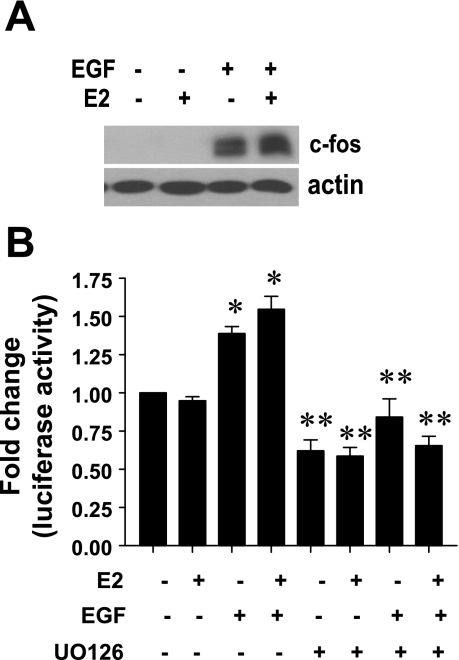
E2 does not modulate EGF-stimulated c-Fos expression and AP-1 activity.
Since the ErbB1 kinase inhibitor failed to block E2 responses in lactotrophs, we next questioned whether E2 could modulate EGF responses in these cells. To address this issue, GH3 cells were treated with E2 (0.01 nM), EGF (5 ng/ml), or their combination for 2 h, and equal amounts of cell lysates were analyzed for the EGF-induced early response gene, c-Fos. Our results demonstrate that, whereas EGF caused a robust induction of c-Fos, E2 had no effect. Furthermore, in the presence of E2, the ability of EGF to stimulate c-Fos induction was unaffected (Fig. 5A). To further examine whether E2 affected EGF signaling, we investigated the effects of E2 on EGF-induced AP-1 activity. GH3 cells cotransfected with an AP-1 luciferase reporter gene and a control reporter gene were treated with either vehicle, EGF (5 ng/ml), E2 (0.01 nM), a combination of E2 and EGF, or the same treatments in the presence of the Mek1 inhibitor UO126 (10 μM). After 18–24 h of stimulation, luciferase activity was determined. Our data show (Fig. 5B) that, consistent with c-Fos induction, EGF stimulated AP-1 transcriptional activity, and this was dependent on Erk1/2 activity but was unaffected by E2. Taken together, our results suggest that E2 has no effect on EGF-stimulated responses.
Fig. 5.
E2 does not modulate ErbB1 kinase activity in lactotrophs. A: GH3 cells were treated with E2 (0.01 nM), EGF (5 ng/ml), or their combination for 2 h, and equal amounts of cell lysates were subjected to Western blotting with anti c-Fos Ab (top) or anti-actin Ab (bottom). Data presented are from a single experiment and are representative of 2 separate experiments with similar results. B: GH3 cells transiently cotransfected with an AP-1 reporter gene and control reporter gene were treated with E2 (0.01 nM), EGF (5 ng/ml), or their combination, either alone or in presence of UO126 (10 μM) for 24 h, and normalized luciferase activity was determined as described in materials and methods. Data were calculated as fold change over control (arbitrary value of 1). Each value is the mean ± SE of 3 separate experiments, each performed in triplicates. *Significant difference from control; **significant difference from treatments without UO126 (P < 0.05).
EGF enhances E2-stimulated ERE activity.
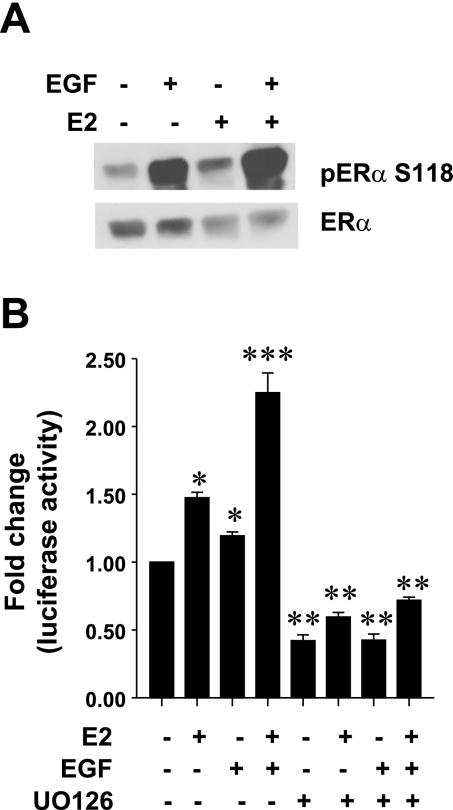
We have recently demonstrated that EGF phosphorylates ERα on S118 in an Erk1/2-dependent manner in GH3 cells (2). We next questioned whether this phosphorylation was critical to the cross-talk between E2 and EGF. Because our previous results suggest that E2 does not affect ErbB1-mediated signaling and that anti-estrogens did not affect ErbB1-mediated Erk1/2 activation (2), we hypothesized that both receptors activate signaling pathways that phosphorylate ERα on S118, and this is the point of intersection of the two signaling pathways. To address this issue, we stimulated GH3 cells with E2 (0.01 nM), EGF (5 ng/ml), or a combination of E2 and EGF for 10 min. Equal amounts of cell lysates were subject to Western blotting with an antibody that specifically detects S118-phosphorylated ERα. Our results (Fig. 6A) clearly show that, consistent with our previous report, EGF stimulation results in a robust phosphorylation of S118 on ERα (2). E2 by itself had a modest stimulatory effect; however, a combination of E2 and EGF results in enhanced phosphorylation of S118 on ERα. We next questioned whether this enhanced phosphorylation of S118 on ERα could modulate ER transcriptional activity and whether this would be sensitive to Erk1/2 inhibition. GH3 cells were transiently cotransfected with ERE-luciferase and control reporter gene and stimulated with either vehicle, EGF (5 ng/ml), E2 (0.01 nM), a combination of E2 and EGF, or the same treatments in the presence of the Mek1 inhibitor UO126 (10 μM). After 18–24 h of stimulation, luciferase activity was determined. Our data show (Fig. 6B) that both E2 and EGF stimulated ERE-luciferase activity, with their combination having an enhanced effect. However, in the presence of UO126, the ability of E2, EGF, and their combination to stimulate ERE-luciferase activity was abolished.
Fig. 6.
EGF enhances E2-stimulated estrogen response element (ERE) activity. A: GH3 cells were treated with E2 (0.01 nM), EGF (5 ng/ml), or their combination for 10 min, and equal amounts of cell lysates were subjected to Western blotting with anti-phospho-specific S118 ERα Ab (top) or ERα Ab (bottom). Data presented are from a single experiment and are representative of 4 separate experiments with similar results. B: GH3 cells transiently cotransfected with an ERE reporter gene and control reporter gene were treated with E2 (0.01 nM), EGF (5 ng/ml), or their combination, either alone or in presence of UO126 (10 μM) for 24 h, and normalized luciferase activity was determined as described in materials and methods. Data were calculated as fold change over control (arbitrary value of 1). Each value is the mean ± SE of 4 separate experiments, each performed in triplicate. *Significant difference from control; **significant difference from treatments without UO126; ***significant difference from E2 and EGF alone (P < 0.05).
DISCUSSION
Our data demonstrate for the first time that physiologically relevant concentrations of E2 and EGF cross-talk to positively modulate lactotroph cell proliferation and PRL gene expression. This cross-talk is mediated by Erk1/2 signaling. Although interactions between EGFR and ER have been studied extensively in the breast and the uterus, such a relationship is largely unknown in the pituitary. A single study previously suggested that interaction between the two receptors occurs in vivo and affects the development of prolactinomas (21). These authors reported that, when TGF-α, which exerts its biological effects through the EGFR/ErbB1, was selectively overexpressed in pituitary lactotrophs, hyperplasia and adenoma formation was observed only in female, but not in male, transgenic mice. Recently, a study highlighted the tumor-suppressive and serum PRL-lowering effects of ErbB kinase inhibitor Gefitinib, implicating an important role for ErbB signaling in the pathogenesis of prolactinomas (33). A study from our laboratory demonstrated that, even in the absence of E2, EGF requires ERα to stimulate PRL release in lactotrophs (2). Apart from these studies, there are no reports that have examined whether ligand-occupied ER can interact with activated EGFR signaling in the lactotrophs and how this interaction impacts lactotroph functions.
The exact mechanism by which E2 acts as a mitogen remains to be characterized. Work from several laboratories, including ours, has shown that GH3 cells express both ERα and ERβ (15, 23, 28, 29). Therefore, we first examined which ER subtypes are involved in the regulation of cell proliferation. Consistent with a previous report (6), we found that E2 at subnanomolar concentrations stimulates GH3 cell proliferation. Using commercially available ER isotype-specific agonists, we demonstrated that the mitogenic effect of E2 is mimicked by the ERα-specific agonist PPT, but not by the ERβ-specific agonist DPN (Fig. 1A). The inability of DPN to stimulate cell proliferation is not due to its ineffectiveness because, at higher concentrations, DPN does stimulate PRL gene expression (Fig. 2B). Therefore, we conclude that in lactotrophs ERα is the ER subtype that regulates cell proliferation. The above conclusion is supported by in vivo observations that the number of lactotrophs is significantly reduced in ERα knockout mice, whereas no reduction is seen in ERβ knockout animals (7, 30). In addition, when ERβ was overexpressed in GH3 cells, although it enhanced E2 stimulated PRL release, it had no significant effect on cell proliferation (23). Finally, we had previously demonstrated that even in the absence of ligand, ERα, but not ERβ, regulates lactotroph proliferation (15). Taken together, we conclude that in lactotrophs the ER subtype that plays a dominant role in regulating cell proliferation is ERα. In our in vitro assays, EGF had a modest stimulatory effect on cell proliferation (Fig. 1A). Our results suggest that lactotroph proliferation is positively regulated by ErbB signaling. Our conclusion would be consistent with previous observations that report expression of both receptors as well as ligands of the ErbB family in normal and adenomatous pituitary, suggesting a regulatory role for ErbB in the pituitary lactotrophs. When both E2 and EGF were used to stimulate GH3 cell proliferation, the combination resulted in a robust stimulation of cell proliferation, which was greater than either ligand alone (Fig. 1A). This suggested that in lactotrophs there exists a signal cross-talk between ErbB1 and ER. Our data demonstrating that PPT, but not DPN, mimics the effect of E2 clearly implicate ERα as the ER subtype involved in the cross-talk. This was further confirmed by a demonstration of the blockade of this stimulatory effect by the ERα-specific antagonist MPP and lack of inhibitory effect by the ERβ antagonist PHTPP (Fig. 1B).
To determine whether the combined effect of ligand-occupied ERα with activated ErbB1 signaling also affects PRL gene expression, we transiently transfected GH3 cells with a rat PRL/luciferase reporter gene. Interestingly, both ERα (PPT)- and ERβ (DPN)-specific agonists were capable of stimulating PRL gene expression (Fig. 2, A and B). Although clearly PPT was more potent at lower concentrations, at higher concentrations both PPT and DPN were equally effective. On the basis of the distinct dose profiles of PPT and DPN, it could be hypothesized that lower concentrations of E2 might selectively mediate its effect through ERα. To support this conclusion, our data (Fig. 2F) show that, at the physiologically relevant doses of E2 (0.01 nM), selective degradation of ERα but not ERβ is observed. This could be a mechanism by which the lactotrophs could effectively respond to E2 to maximize PRL production/release during certain physiological/pathological conditions. Indeed, it has been documented that ERβ is expressed in PRL-secreting tumors. Whether ERβ expression is increased in the lactotrophs during rising E2 levels remains to be determined.
Our data clearly demonstrate that both ER subtypes are functionally coupled to the PRL production/release mechanism. Furthermore, in the presence of EGF, the ability of E2 to stimulate PRL gene expression was significantly enhanced. Pharmacological utilization of the global anti-estrogen ICI, as well as the isotype-specific antagonists MPP and PHTPP, clearly demonstrates the involvement of ERα in the cross-talk with activated ErbB1 signaling. Finally, in the presence of ICI, our data show that ERα expression is significantly decreased, whereas ERβ expression is not altered (Fig. 3D). Collectively, both the proliferative response and stimulation of PRL gene expression by E2 are modulated by activated ErbB1 signaling through ERα.
We next questioned whether ligand-induced activation of both ERα and ErbB1 was required for the signal cross-talk. When GH3 cells were stimulated with a combination of E2 and EGF, both the anti-estrogen and the ErbB1 kinase inhibitor blocked the stimulatory effects on cell proliferation and PRL gene expression (Fig. 3, A, B, and C). Because our previous work had clearly demonstrated that, in the absence of E2, activated ErbB1 phosphorylates ERα on S118, we next questioned whether E2 was capable of transactivating ErbB1 (2). We observed that, in the presence of the potent ErbB1 kinase inhibitor, AG 1478, E2 was still capable of stimulating cell proliferation and PRL gene expression (Fig. 3, B and C). These results suggest that, in lactotrophs, the combined effect of E2 and EGF is not attributable to E2-mediated transactivation and amplification of ErbB1 signaling. Our conclusion is consistent with a previous report where a broad-spectrum tyrosine kinase inhibitor failed to block E2-mediated increase in PRL levels (34). Therefore, we hypothesized that both E2 and EGF independently activate their signaling cascades, which converge at a common downstream target.
We previously demonstrated that UO126 blocks the ability of EGF to phosphorylate ERα on S118 in GH3 cells (2). In the present study, our data show that the combined stimulatory effects of E2 and EGF on PRL gene expression and cell proliferation (Fig. 4, A and B) are also inhibited by UO126, suggesting that Mek1-mediated activation of Erk1/2 is essential for the cross-talk between these two signaling pathways. Although the IC50 for UO126 induced suppression of Mek1 and 2 are almost comparable, we confirmed the requirement of Mek1 by using an additional Mek1 specific inhibitor PD98059 (1, 10). Our data show that, like UO126, PD98059 blocks the combined stimulatory effect of E2 and EGF on PRL gene expression (data not shown).
ErbB1-activated Erk1/2 phosphorylates its downstream target ERα at S118, and in the presence of E2 this leads to increased ER activation. Indeed, we demonstrate that both E2 and EGF increase S118 phosphorylation of ERα (Fig. 6A), followed by an increase in ER transcriptional activity. The phosphorylation of ERα functions to prime the effects of E2. Although S118 phosphorylation appears to be central to this cross-talk, whether it is the exclusive mechanism by which ErbB1 amplifies E2 signaling cannot be concluded on the basis of our experiments. It is possible that E2 and EGF phosphorylate multiple sites, e.g., S167 or S104, and this remains to be examined. It is also possible that phosphorylation of S118 by EGF could enhance phosphorylation, by E2 or EGF at other residues leading to increased activation of ERα.
In conclusion, our studies reveal a cooperative action of two important regulators of lactotroph function, E2 and EGF. Their combined action amplified their mitogenic effects and their ability to induce PRL gene expression and could be the molecular basis by which enhanced cell proliferation is observed during lactotroph hyperplasia and pituitary adenoma development in humans.
GRANTS
This work was supported in part by start-up funds from the Medical College of Wisconsin (S. Kansra) and an American Cancer Society pilot research grant from the Medical College of Wisconsin-Cancer Center (S. Kansra). Support was also provided by NIH Grants ES012212, CA096613, P30-ES06096, and DOD BC050725, and Komen Foundation Grant BCRT87406 (N. Ben-Jonathan).
Acknowledgments
Portions of this study were presented at the 88th annual meeting of the Endocrine Society, Boston, MA, June, 2006.
REFERENCES
- 1.Alessi DR, Cuenda A, Cohen P, Dudley DT, Saltiel AR. PD 098059 is a specific inhibitor of the activation of mitogen-activated protein kinase kinase in vitro and in vivo. J Biol Chem 270: 27489–27494, 1995. [DOI] [PubMed] [Google Scholar]
- 2.Ben-Jonathan N, Chen S, Dunckley JA, Lapensee C, Kansra S. Estrogen receptor-alpha mediates the epidermal growth factor-stimulated prolactin expression and release in lactotrophs. Endocrinology 150: 795–802, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ben-Jonathan N, Hnasko RM. Dopamine as a prolactin inhibitor. Endocr Rev 22: 724–763, 2001. [DOI] [PubMed] [Google Scholar]
- 4.Chaidarun SS, Eggo MC, Sheppard MC, Stewart PM. Expression of epidermal growth factor (EGF), its receptor, and related oncoprotein (erbB-2) in human pituitary tumors and response to EGF in vitro. Endocrinology 135: 2012–2021, 1994. [DOI] [PubMed] [Google Scholar]
- 5.Chen D, Washbrook E, Sarwar N, Bates GJ, Pace PE, Thirunuvakkarasu V, Taylor J, Epstein RJ, Fuller-Pace FV, Egly JM, Coombes RC, Ali S. Phosphorylation of human estrogen receptor alpha at serine 118 by two distinct signal transduction pathways revealed by phosphorylation-specific antisera. Oncogene 21: 4921–4931, 2002. [DOI] [PubMed] [Google Scholar]
- 6.Chun Y, Gregg D, Sarkar D, Gorski J. Differential regulation by estrogens of growth and prolactin synthesis in pituitary cells suggests that only a small pool of estrogen receptors is required for growth. Proc Natl Acad Sci USA 95: 2325–2330, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Couse JF, Lindzey J, Grandien K, Gustafsson JA, Korach KS. Tissue distribution and quantitative analysis of estrogen receptor-a (ERa) and estrogen receptor-β (ERβ) messenger ribonucleic acid in the wild-type and ERa-knockout mouse. Endocrinology 138: 4613–4621, 1997. [DOI] [PubMed] [Google Scholar]
- 8.Curtis SW, Washburn T, Sewall C, DiAugustine R, Lindzey J, Couse JF, Korach KS. Physiological coupling of growth factor and steroid receptor signaling pathways: estrogen receptor knockout mice lack estrogen-like response to epidermal growth factor. Proc Natl Acad Sci USA 93: 12626–12630, 1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.De Luca A, Carotenuto A, Rachiglio A, Gallo M, Maiello MR, Aldinucci D, Pinto A, Normanno N. The role of the EGFR signaling in tumor microenvironment. J Cell Physiol 214: 559–567, 2008. [DOI] [PubMed] [Google Scholar]
- 10.Favata MF, Horiuchi KY, Manos EJ, Daulerio AJ, Stradley DA, Feeser WS, Van Dyk DE, Pitts WJ, Earl RA, Hobbs F, Copeland RA, Magolda RL, Scherle PA, Trzaskos JM. Identification of a novel inhibitor of mitogen-activated protein kinase kinase. J Biol Chem 273: 18623–18632, 1998. [DOI] [PubMed] [Google Scholar]
- 11.Freeman ME, Kanyicska B, Lerant A, Nagy G. Prolactin: structure, function, and regulation of secretion. Physiol Rev 80: 1523–1631, 2000. [DOI] [PubMed] [Google Scholar]
- 12.Ignar-Trowbridge DM, Nelson KG, Bidwell MC, Curtis SW, Washburn TF, McLachlan JA, Korach KS. Coupling of dual signaling pathways: epidermal growth factor action involves the estrogen receptor. Proc Natl Acad Sci USA 89: 4658–4662, 1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jaffrain-Rea ML, Petrangeli E, Lubrano C, Minniti G, Di Stefano D, Sciarra F, Frati L, Tamburrano G, Cantore G, Gulino A. Epidermal growth factor binding sites in human pituitary macroadenomas. J Endocrinol 158: 425–433, 1998. [DOI] [PubMed] [Google Scholar]
- 14.Joel PB, Smith J, Sturgill TW, Fisher TL, Blenis J, Lannigan DA. pp90rsk1 regulates estrogen receptor-mediated transcription through phosphorylation of Ser-167. Mol Cell Biol 18: 1978–1984, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kansra S, Yamagata S, Sneade L, Foster L, Ben-Jonathan N. Differential effects of estrogen receptor antagonists on pituitary lactotroph proliferation and prolactin release. Mol Cell Endocrinol 239: 27–36, 2005. [DOI] [PubMed] [Google Scholar]
- 16.Kato S, Endoh H, Masuhiro Y, Kitamoto T, Uchiyama S, Sasaki H, Masushige S, Gotoh Y, Nishida E, Kawashima H. Activation of the estrogen receptor through phosphorylation by mitogen-activated protein kinase. Science 270: 1491–1494, 1995. [DOI] [PubMed] [Google Scholar]
- 17.Lee H, Bai W. Regulation of estrogen receptor nuclear export by ligand-induced and p38-mediated receptor phosphorylation. Mol Cell Biol 22: 5835–5845, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lemmon MA, Schlessinger J. Regulation of signal transduction and signal diversity by receptor oligomerization. Trends Biochem Sci 19: 459–463, 1994. [DOI] [PubMed] [Google Scholar]
- 19.LeRiche VK, Asa SL, Ezzat S. Epidermal growth factor and its receptor (EGF-R) in human pituitary adenomas: EGF-R correlates with tumor aggressiveness. J Clin Endocrinol Metab 81: 656–662, 1996. [DOI] [PubMed] [Google Scholar]
- 20.Li X, Zhou L, Gorodeski GI. Estrogen regulates epithelial cell deformability by modulation of cortical actomyosin through phosphorylation of nonmuscle myosin heavy-chain II-B filaments. Endocrinology 147: 5236–5248, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.McAndrew J, Paterson AJ, Asa SL, McCarthy KJ, Kudlow JE. Targeting of transforming growth factor-alpha expression to pituitary lactotrophs in transgenic mice results in selective lactotroph proliferation and adenomas. Endocrinology 136: 4479–4488, 1995. [DOI] [PubMed] [Google Scholar]
- 22.McKenna NJ, O'Malley BW. An issue of tissues: divining the split personalities of selective estrogen receptor modulators. Nat Med 6: 960–962, 2000. [DOI] [PubMed] [Google Scholar]
- 23.Mitchner NA, Garlick C, Steinmetz RW, Ben-Jonathan N. Differential regulation and action of estrogen receptors a and b in GH3 cells. Endocrinology 140: 2651–2658, 1999. [DOI] [PubMed] [Google Scholar]
- 24.Nelson KG, Takahashi T, Bossert NL, Walmer DK, McLachlan JA. Epidermal growth factor replaces estrogen in the stimulation of female genital-tract growth and differentiation. Proc Natl Acad Sci USA 88: 21–25, 1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pramanik R, Qi X, Borowicz S, Choubey D, Schultz RM, Han J, Chen G. p38 isoforms have opposite effects on AP-1-dependent transcription through regulation of c-Jun. The determinant roles of the isoforms in the p38 MAPK signal specificity. J Biol Chem 278: 4831–4839, 2003. [DOI] [PubMed] [Google Scholar]
- 26.Rosenfeld MG, Glass CK. Coregulator codes of transcriptional regulation by nuclear receptors. J Biol Chem 276: 36865–36868, 2001. [DOI] [PubMed] [Google Scholar]
- 27.Schonbrunn A, Krasnoff M, Westendorf JM, Tashjian AH Jr. Epidermal growth factor and thyrotropin-releasing hormone act similarly on a clonal pituitary cell strain. J Cell Biol 85: 786–797, 1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Schreihofer DA, Resnick EM, Soh AY, Shupnik MA. Transcriptional regulation by a naturally occurring truncated rat estrogen receptor (ER), truncated ER Product-1 (TERP-1). Mol Endocrinol 13: 320–329, 1999. [DOI] [PubMed] [Google Scholar]
- 29.Schreihofer DA, Stoler MH, Shupnik MA. Differential expression and regulation of estrogen receptors (ERs) in rat pituitary and cell lines: estrogen decreases ERalpha protein and estrogen responsiveness. Endocrinology 141: 2174–2184, 2000. [DOI] [PubMed] [Google Scholar]
- 30.Scully KM, Gleiberman AS, Lindzey J, Lubahn DB, Korach KS, Rosenfeld MG. Role of estrogen receptor-a in the anterior pituitary gland. Mol Endocrinol 11: 674–681, 1997. [DOI] [PubMed] [Google Scholar]
- 31.Steinmetz R, Gutierrez-Hartmann A, Bigsby RM, Ben-Jonathan N. Activation of the prolactin promoter in transfected GH3 cells by posterior pituitary cells. Endocrinology 135: 2737–2741, 1994. [DOI] [PubMed] [Google Scholar]
- 32.Stenoien DL, Patel K, Mancini MG, Dutertre M, Smith CL, O'Malley BW, Mancini MA. FRAP reveals that mobility of oestrogen receptor-alpha is ligand- and proteasome-dependent. Nat Cell Biol 3: 15–23, 2001. [DOI] [PubMed] [Google Scholar]
- 33.Vlotides G, Siegel E, Donangelo I, Gutman S, Ren SG, Melmed S. Rat prolactinoma cell growth regulation by epidermal growth factor receptor ligands. Cancer Res 68: 6377–6386, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Watters JJ, Chun TY, Kim YN, Bertics PJ, Gorski J. Estrogen modulation of prolactin gene expression requires an intact mitogen-activated protein kinase signal transduction pathway in cultured rat pituitary cells. Mol Endocrinol 14: 1872–1881, 2000. [DOI] [PubMed] [Google Scholar]
- 35.Yin P, Arita J. Differential regulation of prolactin release and lactotrope proliferation during pregnancy, lactation and the estrous cycle. Neuroendocrinology 72: 72–79, 2000. [DOI] [PubMed] [Google Scholar]
- 36.Zandi R, Larsen AB, Andersen P, Stockhausen MT, Poulsen HS. Mechanisms for oncogenic activation of the epidermal growth factor receptor. Cell Signal 19: 2013–2023, 2007. [DOI] [PubMed] [Google Scholar]