Abstract
Elevated free fatty acids (FFA) are implicated with insulin resistance at the cellular level. However, the contribution of whole body lipid kinetics to FFA-induced insulin resistance is not well understood, and the effect of exercise and diet on this metabolic defect is not known. We investigated the effect of 12 wk of exercise training with and without caloric restriction on FFA turnover and oxidation (FFAox) during acute FFA-induced insulin resistance. Sixteen obese subjects with impaired glucose tolerance were randomized to either a hypocaloric (n = 8; −598 ± 125 kcal/day, 66 ± 1 yr, 32.8 ± 1.8 kg/m2) or a eucaloric (n = 8; 67 ± 2 yr, 35.3 ± 2.1 kg/m2) diet and aerobic exercise (1 h/day at 65% of maximal oxygen uptake) regimen. Lipid kinetics ([1-14C]palmitate) were assessed throughout a 7-h, 40 mU·m−2·min−1 hyperinsulinemic euglycemic clamp, during which insulin resistance was induced in the last 5 h by a sustained elevation in plasma FFA (intralipid/heparin infusion). Despite greater weight loss in the hypocaloric group (−7.7 ± 0.5 vs. −3.3 ± 0.7%, P < 0.001), FFA-induced peripheral insulin resistance was improved equally in both groups. However, circulating FFA concentrations (2,123 ± 261 vs. 1,764 ± 194 μmol/l, P < 0.05) and FFA turnover (3.20 ± 0.58 vs. 2.19 ± 0.58 μmol·kg FFM−1·min−1, P < 0.01) during hyperlipemia were suppressed only in the hypocaloric group. In contrast, whole body FFAox was improved in both groups at rest and during hyperlipemia. These changes were driven by increases in intracellular lipid-derived FFAox (12.3 ± 7.7 and 14.7 ± 7.8%, P < 0.05). We conclude that the exercise-induced improvement in FFA-induced insulin resistance is independent of the magnitude of weight loss and FFA turnover, yet it is linked to increased intracellular FFA utilization.
Keywords: lipid-induced insulin resistance, palmitate turnover, palmitate oxidation, diabetes, obesity, aging, physical activity
the inhibitory effect of free fatty acids (FFA) on glucose metabolism was first proposed by Randle et al. (37) in 1964. Subsequent experiments led to the hypothesis that elevated FFA concentrations may be responsible for the manifestation of insulin resistance in type 2 diabetes (5, 18, 36). More recent work in lean healthy individuals has demonstrated that elevation of plasma FFA levels using lipid-heparin infusions has a lipotoxic effect, impairing rates of whole body glucose disposal during hyperinsulinemic euglycemic clamps (13, 25, 28, 38, 49). Furthermore, experiments employing stable and radioisotope palmitate tracers show that, in healthy individuals, insulin suppresses FFA turnover and oxidation; yet during hyperinsulinemic hyperlipemic conditions, insulin is unable to suppress lipid metabolism (6, 31). Such effects have not been evaluated in clinically relevant obese, insulin-resistant groups.
Evidence also suggests that, compared with their lean counterparts, obese subjects have elevated basal FFA turnover, impaired basal FFA oxidation, and an attenuated insulin suppression of FFA turnover and oxidation, thus raising circulating FFA concentrations (8, 17). Therefore, obese individuals are described to be metabolically inflexible to the actions of insulin (27), and the underlying insulin resistance is likely due to lipotoxicity (34). Understanding the mechanism by which this phenomenon occurs is important due to the valuable outcome of improving insulin resistance in individuals at risk of developing diabetes and related macrovascular complications. An older, obese, impaired glucose-tolerant group is at great risk of such disease onset. Reducing plasma FFA concentrations using pharmaceutical agents (e.g., acipimox) has been shown to improve insulin sensitivity in healthy and diabetic individuals (33, 44). Furthermore, it has been demonstrated that weight loss and aerobic exercise have direct metabolic effects, improving not only insulin-stimulated glucose disposal but also whole body lipid oxidation (30, 32, 42). More specifically, exercise alone has been shown to elevate basal rates of lipolysis and lipid turnover in healthy individuals (15, 21), yet the effects of diet and exercise-induced weight loss in groups at high risk of developing diabetes-related complications are not fully understood. Certainly, hyperglycemia and hyperlipemia are key factors that contribute to disease onset; thus improving glycemic and lipemic control is a common therapeutic goal, particularly in older, obese, insulin-resistant populations. Here, we seek to investigate the effects of stimuli that are known to reduce insulin resistance on lipid kinetics (FFA turnover and oxidation) during basal, hyperinsulinemic, and hyperlipemic conditions. We hypothesized that additional weight loss via increases in physical activity and hypocaloric dietary intake would improve peripheral tissue insulin resistance more so than elevations in physical activity alone and that such changes would be driven by alterations in FFA availability and utilization.
EXPERIMENTAL PROCEDURES
Subject recruitment.
Sixteen obese, weight-stable volunteers (3 male and 13 female, age 66 ± 1 yr, body mass index 34.0 ± 1.4 kg/m2) were recruited into our ongoing studies investigating the role of lifestyle intervention in insulin resistance. Following medical screening, which included a 12-lead electrocardiogram, an oral glucose tolerance test, and a full blood profile, volunteers were randomized to one of two exercise training study arms: a eucaloric group (EX) or a hypocaloric group (EX-HYPO). Only obese volunteers who exhibited impaired glucose tolerance [2-h plasma glucose 140–199 mg/dl (2)] from oral glucose tolerance test screening were eligible to partake in the study. Volunteers were screened out if they demonstrated any evidence of renal, hepatic, cardiovascular, or hematological disease or if they exhibited normal glucose tolerance. None of the subjects were taking pharmaceutical agents known to interfere with our outcome variables. Contraindication to increased levels of activity was also screened via an exercise stress test. All volunteers provided their written informed consent to participate in the study, and all procedures were approved by the Institutional Review Board at MetroHealth Medical Center.
Intervention.
All subjects entered a lifestyle intervention involving 12 wk of aerobic exercise training and dietary counseling. The exercise component consisted of 60 min of exercise, 5 days/wk, performed at ∼65% of the individual's maximal oxygen uptake (V̇o2max) as dictated by a maximal exercise test. All exercise was fully supervised by an exercise physiologist; therefore, compliance was strictly monitored. These procedures are similar to those described in previous publications from our group (32, 42, 48). On the basis of indirect calorimetry measurements obtained during exercise training, we have determined that older obese subjects expend 400–500 kcal/h during these training bouts. The dietary component of the study was monitored via daily food consumption questionnaires plus weekly meetings with a registered dietician. Subjects randomized to the EX group were instructed to continue with their typical diet; subjects randomized to the EX-HYPO group were advised to reduce their typical caloric intake by ∼500 kcal/day to induce a negative energy balance and facilitate additional weight loss. Caloric requirements were estimated using the Harris-Benedict equation, multiplied by a sedentary (× 1.3) physical activity correction factor (19). Subjects' “typical” diets were thoroughly analyzed prior to the study by using dietary record questionnaires. Prior to and immediately following the intervention, metabolic testing was performed in a controlled 3-day inpatient setting in the General Clinical Research Center.
Participant characteristics.
Measurements of anthropometrics, whole body fat percentage (hydrostatic weighing), and maximal aerobic capacity during exhaustive exercise (V̇o2max) were determined according to standardized procedures. The details of these particular protocols may be viewed in Solomon et al. (42).
Insulin sensitivity and lipid kinetics.
Following an overnight (∼10 h) fast, a hyperinsulinemic euglycemic clamp was performed in combination with an intralipid/heparin infusion and a primed continuous infusion of [1-14C]palmitate. A retrograde catheter was inserted into a dorsal hand vein and warmed (∼60°C) in a heated box for arterialized venous sampling. An infusion site was identified in the antecubital fossa of the contralateral arm, and a second catheter was inserted into a median vein. A radiolabeled FFA infusate was prepared by complexing [1-14C]palmitate with human serum albumin to a final concentration of 10 mg/ml albumin, 0.5 μCi/ml [1-14C]palmitate, and 2.3 mg/ml sodium palmitate (47). At t = −90 min, a primed (2.5 μCi) continuous (0.1 μCi/min) infusion of [1-14C]palmitate was begun and proceeded until the end of the clamp. Simultaneously, a bolus (3.5 μCi) of NaH14CO3 was injected to prime the bicarbonate pool. At t = 0 min, a hyperinsulinemic euglycemic clamp began as described previously (3, 10, 42). A primed 40 mU·m2·min−1 infusion of insulin was started, and a variable rate 20% glucose infusion was used to titrate euglycemia (90 mg/dl). Glucose infusion rates were determined using the calculations of DeFronzo et al. (10) on the basis of arterialized glucose concentrations measured every 5 min throughout the procedure. At t = 120 min, to elevate plasma FFA concentrations, a continuous infusion of 20% intralipid (1.5 ml/min) and heparin (200 IU prime, then 15 IU/min) commenced, whereas hyperinsulinemia and euglycemia were maintained. This proceeded until the end of the clamp procedure at t = 420 min. Blood samples were collected at 10-min intervals during the last 30 min of the baseline (basal: t = −30 to 0 min), insulin-stimulated (INS; hyperinsulinemic stage: t = 90–120 min), and FFA-elevated periods (INS + FFA; hyperlipemic stage: t = 390–420 min) for the measurement of plasma FFA- and [1-14C]palmitate specific activity. Mean space-corrected glucose disposal rates (GDR) were also calculated during the last 30 min of the INS and INS + FFA periods. The clamped glucose concentrations for the hyperinsulinemic and hyperlipemic periods pre- and poststudy were as follows: 90.4 ± 0.8, 88.9 ± 1.2, 89.6 ± 0.5, and 89.2 ± 0.6 mg/dl. The glucose variation coefficients during these same periods were 4.1 ± 0.5, 3.2 ± 0.5, 2.7 ± 0.3, and 2.8 ± 0.3%. In addition, measurements of whole body substrate metabolism were estimated from indirect calorimetry through a ventilated hood. Expired air samples were analyzed via Hartmann-Braun (Frankfurt, Germany), differential paramagnetic O2 (Magnos 4G), and nondispersive infrared CO2 (Uras 4) analyzers (46). Samples were collected over a 30-min period at baseline and during the final 30 min of the INS and INS + FFA stages. At each of these stages, an expired air sample was also collected for analysis of 14CO2 specific activity.
Analytical methods.
Plasma insulin (Millipore, Billerica, MA) and leptin (Linco Research, St. Charles, MO) were assayed using commercially available radioimmunoassays. Plasma glucose concentrations were measured using an automated glucose oxidase method (Beckman Analyzer II). Triglycerides (TG) and total cholesterol were analyzed on an automated platform (Roche Modular Diagnostics, Indianapolis, IN). Plasma FFA levels were determined using an enzymatic colorimetric procedure (NEFA C kit; Wako Chemicals, Dallas TX). Plasma [1-14C]palmitate was extracted from plasma (1 ml) using heptane-isopropanol (4 ml; 3:7), and specific activity was measured by liquid scintillation counting, as described previously (9, 11, 35). Expired air 14CO2 specific activity was also measured as described previously (17).
Calculations.
Whole body lipid metabolism was calculated using the calculations of Frayn (14). Urinary nitrogen excretion rates were measured, and therefore, rates of carbohydrate and lipid oxidation were corrected for protein metabolism. Whole body lipid oxidation rates were divided by the molecular mass of a typical TG (palmitoyl-stearoyl-oleoyl-glycerol: Mr = 861 g/mol) and multiplied by three (the number of FFAs in a TG) to derive a rate of whole body FFA oxidation (net FFAox). FFA turnover was determined by dividing the [1-14C]palmitate infusion rate by the plasma [1-14C]palmitate specific activity (6, 35). The 14CO2 production rate was calculated as the product of the 14CO2 specific activity (disintegrations·min−1·mmol−1) and the CO2 excretion rate from indirect calorimetry (mmol/min) divided by 0.81 (which represents the correction factor for the amount of labeled CO2 not recovered from the bicarbonate pool in humans) (24, 35). Plasma lipid-derived FFA oxidation rate (plasma FFAox) was then determined by dividing the 14CO2 production rate by the specific activity of plasma [1-14C]palmitate (17, 35). Nonplasma lipid-derived FFA oxidation (i.e., intracellular lipid derived and plasma TG derived) was estimated as the difference between plasma FFAox and net FFAox (6, 17, 35). Due to the well-established effect of exercise training to elevate intramyocellular lipid utilization (see discussion), the changes in nonplasma lipid-derived FFA oxidation should reflect changes in intramuscular lipid oxidation. Rates of lipid kinetics (μmol/min) are expressed per unit of fat-free mass (FFM).
Statistical analyses.
A three-way (group × study time point × clamp time point) repeated-measures analysis of variance (ANOVA) was used to examine changes in lipid kinetics following the intervention. Two-way (group × study time point) ANOVA was used to analyze subject characteristics. In the event of a significant ANOVA F ratio, Bonferroni post hoc tests were applied to identify specific differences between means. Statistical significance was achieved if P < 0.05. All data are expressed as means ± SE.
RESULTS
Participant characteristics.
Table 1 describes the research volunteers' baseline (prestudy) characteristics. There were no differences between the EX or EX-HYPO groups for any variable at baseline (all comparisons, P > 0.05). The intervention-induced changes in body composition, metabolic status, and physical fitness variables are also highlighted in Table 1. Both groups demonstrated improvements in body weight and fat mass (FM). The changes were modest in the EX group (−3.3 ± 0.7% reduced body weight and −4.6 ± 2.4% reduced FM), whereas the effect was much greater in the EX-HYPO group (−7.7 ± 0.5% weight, −14.9 ± 4.1% FM, P < 0.01 vs. eucaloric). There were large, statistically significant decreases in fasting insulin, leptin, TG, and total cholesterol; however, changes in fasting plasma glucose were not significant. V̇o2max was similarly improved in both groups (all subjects: +8.6 ± 2.9%; effect of time, P < 0.05). In addition, during the study the EX group consumed the same daily caloric load as in their habitual diet (1,658 ± 96 vs. 1,684 ± 196 kcal/day, P > 0.05), whereas the EX-HYPO group reduced their caloric intake by −598 ± 125 kcal/day (1,891 ± 148 vs. 1,293 ± 63 kcal/day, P < 0.05). Prestudy diets did not differ in macronutrient composition between groups; however, during the intervention, the EX-HYPO group ingested a lower percentage of their caloric load from fat (22.1 ± 1.8 vs. 32.4 ± 3.1% kcal, P < 0.05).
Table 1.
Subject characteristics for the 16 volunteers who underwent a 12-wk exercise training intervention in combination with either a eucaloric or a hypocaloric diet
| Subject Characteristics (n = 16) |
EX (2 M, 6 F) |
EX-HYPO (1 M, 7 F)
|
||
|---|---|---|---|---|
| Prestudy | Poststudy | Prestudy | Poststudy | |
| Age,yr | 67±2 | 66±1 | ||
| Weight,kg | 96.0±6.1 | 92.8±6.0c | 88.4±4.6 | 81.7±4.4c** |
| BMI,kg/m2 | 35.3±2.1 | 34.0±1.9c | 32.8±1.8 | 30.2±1.7c** |
| FM,kg | 42.2±3.3 | 40.3±3.5b | 36.0±3.7 | 30.9±3.7b* |
| FFM,kg | 54.4±3.7 | 52.5±3.2a | 52.4±1.5 | 50.7±2.0a |
| FPG,mmol/l | 6.11±0.27 | 6.03±0.25 | 6.03±0.31 | 5.78±0.14 |
| FPI,μU/ml | 24.9±6.2 | 18.3±3.9b | 16.3±1.3 | 12.9±1.3b |
| Leptin,ng/ml | 25.8±5.6 | 23.3±5.0b | 18.5±4.8 | 13.6±3.0b |
| TG,mg/dl | 196.8±44.7 | 167.9±25.4a | 209.3±25.4 | 170.7±27.5a |
| Chol,mg/dl | 209.4±9.5 | 203.0±10.4b | 222.9±12.3 | 194.7±10.6c** |
| V̇o2max,l/min | 1.83±0.12 | 1.99±0.13a | 1.80±0.16 | 1.90±0.11a |
Data represent means ± SE. Older obese men and women (n = 16) underwent 12 wk of exercise training with [eucaloric group (EX-HYPO)] or without [hypocaloric group (EX)] caloric restriction. M, males; F, females; BMI, body mass index; FM, whole body fat mass; FFM, whole body fat-free mass; FPG, fasting plasma glucose; FPI, fasting plasma insulin; TG, fasting triglycerides; Chol, fasting total cholesterol; V̇o2max, maximal oxygen consumption during exhaustive exercise. Baseline characteristics were not different between groups for any variable.
Statistical differences between pre- and postintervention means are identified, representing the 5, 1, and 0.1% levels of significance.
Group × study time point ANOVA interactions, indicating significance at the 5 and 1% levels.
Insulin sensitivity.
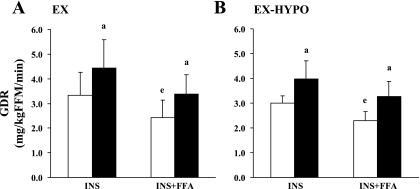
Figure 1 shows that insulin-stimulated GDRs (mg·kg FFM−1·min−1) were improved in both the EX (30.7 ± 12.2%) and EX-HYPO (31.5 ± 23.7%; effect of time: both P < 0.05) arms of the study. Prior to the intervention, intralipid/heparin infusion significantly reduced the insulin-stimulated GDR in both groups (P < 0.05). Following the study, GDR during the INS + FFA stage was increased but notably was no longer significantly different from the INS stage (P = 0.17). Improvements in insulin-stimulated GDR and FFA-induced insulin resistance were similar between groups (EX vs. EX-HYPO changes, group × study time, P > 0.05).
Fig. 1.
Changes in glucose disposal rate (GDR) during the hyperinsulinemic hyperlipemic euglycemic clamp following a 12-wk exercise/diet intervention. Older obese men and women (n = 16) underwent 12 wk of exercise training (1 h/day, 5 days/wk at 65% maximal oxygen uptake). Participants either maintained their habitual dietary intake [eucaloric group (EX): 1,684 ± 196 kcal/day; A] or were counseled to reduce caloric intake [hypocaloric group (EX-HYPO): 1,293 ± 63 kcal/day; B]. Error bars represent SE. Open bars indicate preintervention means; filled bars represent postintervention means. The x-axis indicates the insulin-stimulated (INS) and intralipid/heparin (INS + FFA) stages of the clamp. Postintervention GDR was significantly elevated above baseline during both INS and INS + FFA stages in both groups (apre- vs. postintervention, P < 0.05). Prior to the intervention, lipid infusion reduced GDR compared with insulin stimulation alone in both groups (eINS + FFA vs. INS, P < 0.05). Lipid-induced insulin resistance was no longer evident following the study (INS + FFA vs. INS, P = 0.17). FFM, fat-free mass.
Lipid kinetics.
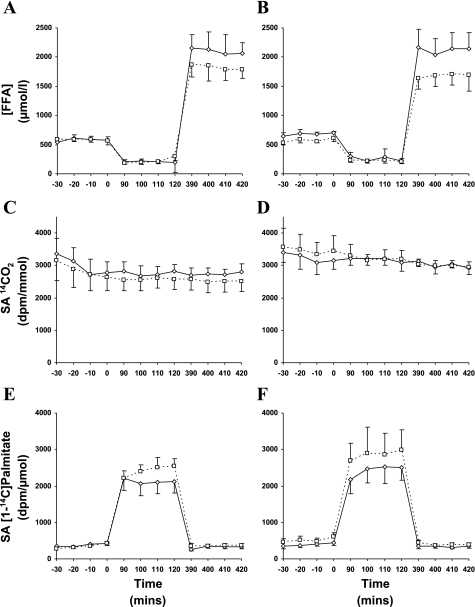
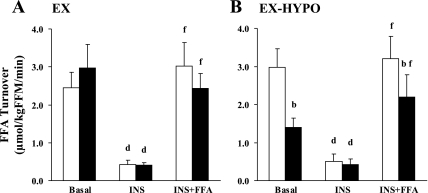
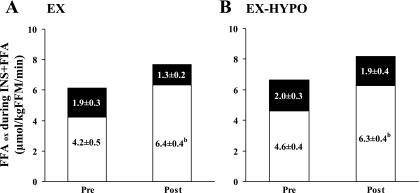
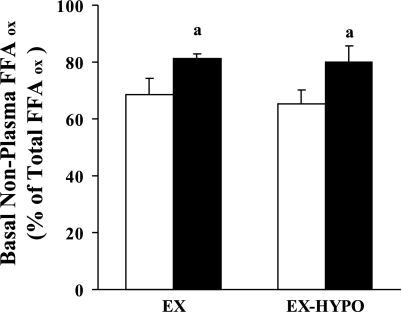
Steady-state profiles of plasma FFA, breath 14CO2, and plasma [1-14C]palmitate specific activity are shown in Fig. 2. Changes in circulating FFA concentrations ([FFA]) are shown in Table 2. Both prior to and following the study, insulin infusion alone suppressed [FFA] (P < 0.05), whereas [FFA] were elevated during the INS + FFA stage of the clamp (P < 0.05). However, in the EX-HYPO group, postintervention [FFA] during basal and INS + FFA stages were reduced compared with prestudy values (P < 0.05). Figure 3 illustrates changes in FFA turnover during basal, insulin-stimulated, and insulin hyperlipemic conditions. FFA turnover was effectively suppressed during INS in both groups pre- and poststudy (P < 0.01) but was elevated during the INS + FFA clamp stage (P < 0.01). Following the hypocaloric intervention, FFA turnover was significantly reduced during basal and INS + FFA conditions (EX-HYPO, P < 0.01; Fig. 3B); no change was seen in the EX group (P > 0.05). Net FFAox was increased following the intervention during basal and INS + FFA conditions in both the EX and EX-HYPO groups (P < 0.05; Table 2). Hyperinsulinemia effectively suppressed net FFAox in both groups, but only following the study (INS vs. basal: EX P < 0.05, EX-HYPO P < 0.01). Also, following both interventions, during the hyperlipemic clamp stages, net FFAox was significantly elevated above rates measured during hyperinsulinemic conditions (INS + FFA vs. INS: EX P < 0.01, EX-HYPO P < 0.001). No pre- vs. poststudy changes were identified in plasma lipid-derived FFAox, whereas significant increases in nonplasma lipid-derived FFAox during basal and INS + FFA stages were seen in both groups (pre- vs. poststudy: basal P < 0.05, INS + FFA P < 0.01). In addition, following the interventions, nonplasma lipid-derived FFAox during hyperlipemia was elevated above that measured during hyperinsulinemic conditions (INS + FFA vs. INS: EX P < 0.05, EX-HYPO P < 0.01). Figure 4 highlights the changes in FFAox during FFA-induced insulin-resistant conditions in the two dietary arms of the study; increases in net FFAox appeared to be driven by elevations in nonplasma lipid-derived FFAox (P < 0.01). The same trend was true for net FFAox during basal conditions (P < 0.05; Fig. 5).
Fig. 2.
Steady-state profiles during hyperinsulinemic hyperlipemic clamps performed before and after a 12-wk exercise/diet intervention. A and B: plasma free fatty acid concentrations ([FFA]) during the clamp. C and D: breath 14CO2 specific activities (SA). E and F: plasma [1-14C]palmitate SA. Time points: t = −30 to 0 min represents the basal clamp stage, t = 90–120 min represents the insulin-stimulated stage, and t = 390–420 min represents the insulin + intralipid/heparin stage. A, C, and E: EX group; B, D, and F: EX-HYPO group. Error bars represent SE.
Table 2.
Changes in lipid metabolism during the hyperinsulinemic hyperlipemic euglycemic clamp following the intervention
| Lipid Metabolism |
EX |
EX-HYPO
|
||
|---|---|---|---|---|
| Pre | Post | Pre | Post | |
| [FFA], μmol/l | ||||
| Basal | 583±53 | 584±40 | 682±41 | 574±38a |
| INS | 195±32c | 254±85c | 283±63c | 221±36c |
| INS + FFA | 2,012±220e | 1,866±168e | 2,123±261e | 1,764±194a,e |
| Net FFAox, μmol·kg FFM−1·min−1 | ||||
| Basal | 4.80±0.91 | 7.18±0.70a | 6.41±0.71 | 8.43±0.62a |
| INS | 4.51±0.62 | 5.58±0.76c | 5.26±0.91 | 5.78±0.36d |
| INS + FFA | 6.08±0.89 | 7.66±0.85a,f | 6.62±0.70 | 8.19±0.59a,g |
| Plasma-derived FFAox, μmol·kg FFM−1·min−1 | ||||
| Basal | 1.40±0.31 | 1.52±0.40 | 1.92±0.40 | 1.53±0.39 |
| INS | 0.27±0.06d | 0.20±0.03d | 0.32±0.06d | 0.37±0.11d |
| INS + FFA | 1.90±0.34g | 1.31±0.15f | 2.02±0.28f | 1.92±0.36g |
| Nonplasma lipid-derived FFAox, μmol·kg FFM−1·min−1 | ||||
| Basal | 3.40±0.51 | 5.66±0.38a | 5.39±0.51 | 5.93±0.45a |
| INS | 4.24±0.33 | 5.38±0.37 | 4.94±0.48 | 5.41±0.21 |
| INS + FFA | 4.17±0.54 | 6.35±0.42b,e | 4.60±0.42 | 6.27±0.41b,f |
Data represent means ± SE. Pre, preintervention; post, postintervention; [FFA], plasma free fatty acid concentration; FFAox, free fatty acid oxidation; plasma-derived, FFA entering cell from plasma; nonplasma, FFA derived from intracellular or plasma triglyceride sources. Older obese men and women (n = 16) underwent 12 wk of exercise training with (EX-HYPO) or without (EX) caloric restriction. The basal, insulin-stimulated, and lipid infusion stages of the clamp are represented by basal, INS, and INS + FFA, respectively. In the event of significant interactions between time and group, post hoc comparisons of means were performed.
P < 0.05 and
P < 0.01, significant differences between pre and post means;
P < 0.05 and
P < 0.01, differences between INS and basal means;
P < 0.05,
P < 0.01, and
P < 0.001, comparisons made between INS + FFA and INS means.
Fig. 3.
Changes in FFA turnover across the 3 stages of the hyperinsulinemic hyperlipemic euglycemic clamp following a 12-wk exercise/diet intervention. A and B represent the EX and EX-HYPO groups, respectively. Error bars represent SE. Open bars indicate preintervention means; filled bars represent postintervention means. Basal turnover was reduced in EX-HYPO subjects only (bpre- vs. postintervention, P < 0.01). Also, in the EX-HYPO group alone, FFA turnover was reduced during the hyperlipemic (INS + FFA) clamp stage (bpre- vs. postintervention, P < 0.01). dStatistical differences between INS and basal means at the 1% level; fstatistical differences between INS + FFA and basal means, also at the 1% level.
Fig. 4.
Changes in FFA oxidation (FFAox) during the hyperlipemic (INS + FFA) clamp stage following a 12-wk exercise/diet intervention. A and B represent the EX and EX-HYPO groups, respectively. Data indicate means ± SE. Total bar area represents net FFAox. Open bars represent nonplasma lipid-derived FFAox; filled bars represent plasma-derived FFAox. Net FFAox during INS + FFA was increased following the intervention in both groups (for full data, see Table 4). This was driven by increases in nonplasma FFAox only [bpre- (Pre) vs. postintervention (Post), P < 0.01].
Fig. 5.
Changes in basal FFAox derived from nonplasma lipid sources following a 12-wk exercise/diet intervention. Error bars represent SE. Open bars represent preintervention means; filled bars represent postintervention means. Basal nonplasma lipid-derived FFAox was increased following the study in both dietary groups (apre- vs. postintervention, P < 0.05).
DISCUSSION
This investigation demonstrates, for the first time, that reductions in circulating FFA concentrations during basal and hyperlipemic conditions may be attributed to decreased FFA turnover in older, obese individuals following weight loss (−8% body wt, −15% fat mass) induced by a long-term diet and exercise intervention. However, we have also demonstrated that improvements in peripheral tissue insulin sensitivity and FFA-induced insulin resistance are of equal magnitude following exercise training whether either a eucaloric or a hypocaloric diet is consumed. Therefore, these improvements are independent of the amount of weight loss. Additionally, improvements in whole body FFA oxidation that were found to be driven entirely by changes in nonplasma lipid-derived FFA metabolism were also identical in each study group. Thus, it would appear that a gain of physiological function with respect to peripheral insulin resistance is driven by elevations in FFA utilization rather than decreases in plasma FFA availability.
This study was designed so that a hypocaloric diet would complement a period of increased physical activity so as to induce weight loss. The hypocaloric group demonstrated reductions in body weight that were twice as great as the exercise-only group (6.7 vs. 3.2 kg), and reductions in body fat that were nearly three times larger (5.1 vs. 1.8 kg). The exercise-only group exhibited statistically significant decreases in body weight and whole body fat mass, yet these were modest in contrast to the EX-HYPO subjects. Insulin-stimulated glucose disposal was increased equally in both study groups, indicating that the exercise training-induced improvements in insulin sensitivity are not determined by the magnitude of change in body composition. Furthermore, both groups demonstrated identical attenuation in FFA-induced insulin resistance. A large body of evidence now indicates that FFAs are a key player in insulin resistance, causing lipotoxicity at elevated levels (8, 13, 17, 18, 25, 28, 36, 38, 49). The literature provides mechanistic insight for the direct and indirect effects of elevated FFA levels on insulin action. Fatty acids and their metabolites (e.g., diacylglycerol/ceramides/long-chain fatty acyl-CoAs) have been shown to activate the c-Jun NH2-terminal kinase (JNK) (1, 22) and IκB kinase-β (IKKβ) pathways (29), with each being involved in inflammation-induced insulin resistance (26, 41, 45). It has also been demonstrated that proinflammatory cytokines such as TNFα are increased during elevated FFA conditions (7) and that JNK and IKKβ activity is elevated with increased adiposity (7, 22, 50). In this study, plasma TNFα was increased during lipid infusion, yet no improvements were seen postintervention (data not shown), thus indicating a potentially minor effect of diet/exercise upon plasma cytokine involvement in FFA-induced insulin resistance. Our current study shows that basal circulating FFA concentrations and FFA turnover were reduced only in hypocaloric subjects, whereas elevations in lipid oxidation were common in both groups. In relation to the change we demonstrated in insulin sensitivity, this indicates that, indeed, elevations in FFA were inducing insulin resistance in these subjects at baseline but that such effects are not dictated simply by increased release into the circulation and therefore not by increased availability to peripheral tissue. It appears that improved insulin resistance is more likely driven by upregulation of lipid metabolism, specifically increased nonplasma, lipid-derived, FFA oxidation. Exercise training is known to upregulate mitochondrial biogenesis and capillarization as well as increase mitochondrial oxidative capacity in skeletal muscle (12, 23, 43). Exercise also elevates intramyocellular lipid metabolism in skeletal muscle (39), and therefore, our tracer-derived measurement of nonplasma lipid-derived FFA oxidation is likely a surrogate marker of increased utilization of intramyocellular lipid (IMCL). Intracellular accumulation of lipid metabolites (e.g., diacylglycerol/ceramides/long-chain fatty acyl CoAs) is known to have a lipotoxic effect by downregulating insulin action via mechanisms outlined above, and more recently, FFA-induced insulin resistance was shown to be associated with elevations in skeletal muscle diacylglycerol, protein kinase C activity, and IKKβ activity (25). Therefore, it is likely that the exercise component of our intervention has a profound effect on insulin resistance via reduction of such moieties. Indeed, we have recently shown that multiple plasma ceramide subspecies are increased in type 2 diabetes, and these increases correlate with the level of insulin resistance (20). In addition, Dube et al. (12) and Schenk and Horowitz (39) have shown that an exercise stimulus can reduce intramuscular levels of diacylglycerol and ceramide in relation to improvements in insulin action. Further investigation is warranted to explore these effects following weight loss in humans.
It is also notable that, although whole body and intracellular lipid metabolism were elevated following both interventions, FFA oxidation during conditions of increased circulating FFA concentrations was also augmented. Remarkably, no postintervention changes were evident in plasma lipid-derived FFA oxidation. It seems that the insulin suppression and lipemia-induced elevation of plasma-derived lipid metabolism were quite normal in these obese subjects; yet it was the lipid oxidation from nonplasma (likely intracellular) sources that was found to be impaired. Therefore, the improved response of whole body lipid metabolism during times of increased lipid availability was most likely due to improvements in intracellular lipid utilization. This observation is supported by the work of Blaak et al. (4), who also reported that plasma-derived FFA oxidation was unchanged following weight loss in obese individuals. Increased utilization of IMCL following exercise has been shown previously (40) and is found in endurance-trained athletes (16); however, increased IMCL utilization during periods of elevated plasma FFA concentrations is an issue that requires further investigation. These findings complement evidence from our previous work showing that exercise and dietary interventions decrease IMCL content in obese insulin-resistant humans and that the improvement in whole body lipid metabolism is related to decreases in IMCL (42). A further point of interest is that, when expressed per unit of fat mass, rates of FFA turnover showed no significant changes following the interventions. Fat mass (kg) was not related to absolute basal FFA turnover (μmol/min) prior to the study (ρ = −0.11, P = 0.68) but demonstrated an improved yet nonsignificant relationship poststudy (ρ = 0.43, P = 0.09). Although this trend is underpowered, it would certainly appear that, in obese insulin-resistant older adults, FFA release into the circulation does not occur in relation to the amount of whole body fat mass that is indicative of uncontrolled basal lipolysis. However, following the intervention, it appears that greater control of basal lipolysis is exhibited. Because these findings were independent of the study group, it is possible that exercise training per se influences FFA availability in relation to oxidative requirements of increased energy demands.
Our data also demonstrate significant improvements in plasma triglycerides and cholesterol, illustrating the beneficial effect of weight loss and increased physical activity on markers of cardiometabolic risk. To fully investigate in vivo lipid metabolism and understand such changes, one would require access to study hepatic lipid turnover and oxidation. Recent advances in magnetic resonance spectroscopy have reduced the methodological issues associated with studying the liver in humans. Future investigation should focus on hepatic metabolism since the liver is a key instigator of the underlying hyperglycemia common in obese, insulin-resistant patients. That said, skeletal muscle is the predominant site for glucose disposal in the adult human, and so understanding the mechanisms by which peripheral insulin resistance may arise will help clinical scientists develop therapeutic tools to combat the onset of hyperglycemia-related complications in individuals exhibiting high cardiometabolic risk.
These novel data indicate that although weight loss (via reduced caloric intake and increments in physical activity: EX-HYPO) may alleviate elevations in circulating lipids by reducing FFA turnover, this does not determine the magnitude of the gain in function with respect to peripheral tissue insulin sensitivity. In an exercising eucaloric group exhibiting modest changes in body composition, an identical gain of function with respect to insulin sensitivity was found. In conjunction with the finding that upregulation of intracellular lipid metabolism was comparable in both of our study groups, these data highlight the importance of increased physical activity for improving metabolic control. Therefore, the combination of exercise and caloric restriction is sensible because reductions in circulating FFA levels may have direct beneficial effects on lipotoxicity in other tissues not addressed by this investigation.
GRANTS
This research was funded by National Institute on Aging Grant AG-12834 (J. P. Kirwan) and General Clinical Research Center Grants MO1-RR-10732, RR-00080, and RR-018390.
Acknowledgments
We thank the nursing and dietary staff in the General Clinical Research Facility. Also, special thanks to our research volunteers for their hard work and determination in completing the study. We thank Todd Trappe, Raj Krishnan, and Dave Williamson for their assistance with the palmitate infusion studies and Luis del Aguila, Donal O'Gorman, Rebecca Lewis, Sakita Sistrun, and David Kris for their help with data collection.
REFERENCES
- 1.Aguirre V, Uchida T, Yenush L, Davis R, White MF. The c-Jun NH(2)-terminal kinase promotes insulin resistance during association with insulin receptor substrate-1 and phosphorylation of Ser(307). J Biol Chem 275: 9047–9054, 2000. [DOI] [PubMed] [Google Scholar]
- 2.American Diabetes Association. Diagnosis and classification of diabetes mellitus. Diabetes Care 31, Suppl 1: S55–S60, 2008. [DOI] [PubMed] [Google Scholar]
- 3.Avram AM, Patel V, Taylor HC, Kirwan JP, Kalhan S. Euglycemic clamp study in clozapine-induced diabetic ketoacidosis. Ann Pharmacother 35: 1381–1387, 2001. [DOI] [PubMed] [Google Scholar]
- 4.Blaak EE, Wolffenbuttel BH, Saris WH, Pelsers MM, Wagenmakers AJ. Weight reduction and the impaired plasma-derived free fatty acid oxidation in type 2 diabetic subjects. J Clin Endocrinol Metab 86: 1638–1644, 2001. [DOI] [PubMed] [Google Scholar]
- 5.Boden G Role of fatty acids in the pathogenesis of insulin resistance and NIDDM. Diabetes 46: 3–10, 1997. [PubMed] [Google Scholar]
- 6.Bonadonna RC, Groop LC, Zych K, Shank M, DeFronzo RA. Dose-dependent effect of insulin on plasma free fatty acid turnover and oxidation in humans. Am J Physiol Endocrinol Metab 259: E736–E750, 1990. [DOI] [PubMed] [Google Scholar]
- 7.Cai D, Yuan M, Frantz DF, Melendez PA, Hansen L, Lee J, Shoelson SE. Local and systemic insulin resistance resulting from hepatic activation of IKK-beta and NF-kappaB. Nat Med 11: 183–190, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Campbell PJ, Carlson MG, Nurjhan N. Fat metabolism in human obesity. Am J Physiol Endocrinol Metab 266: E600–E605, 1994. [DOI] [PubMed] [Google Scholar]
- 9.Chandler MP, Huang H, McElfresh TA, Stanley WC. Increased nonoxidative glycolysis despite continued fatty acid uptake during demand-induced myocardial ischemia. Am J Physiol Heart Circ Physiol 282: H1871–H1878, 2002. [DOI] [PubMed] [Google Scholar]
- 10.DeFronzo RA, Tobin JD, Andres R. Glucose clamp technique: a method for quantifying insulin secretion and resistance. Am J Physiol Endocrinol Metab Gastrointest Physiol 237: E214–E223, 1979. [DOI] [PubMed] [Google Scholar]
- 11.Dole VP A relation between non-esterified fatty acids in plasma and the metabolism of glucose. J Clin Invest 35: 150–154, 1956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dube JJ, Amati F, Stefanovic-Racic M, Toledo FG, Sauers SE, Goodpaster BH. Exercise-induced alterations in intramyocellular lipids and insulin resistance: the athlete's paradox revisited. Am J Physiol Endocrinol Metab 294: E882–E888, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ferrannini E, Barrett EJ, Bevilacqua S, DeFronzo RA. Effect of fatty acids on glucose production and utilization in man. J Clin Invest 72: 1737–1747, 1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Frayn KN Calculation of substrate oxidation rates in vivo from gaseous exchange. J Appl Physiol 55: 628–634, 1983. [DOI] [PubMed] [Google Scholar]
- 15.Friedlander AL, Casazza GA, Horning MA, Usaj A, Brooks GA. Endurance training increases fatty acid turnover, but not fat oxidation, in young men. J Appl Physiol 86: 2097–2105, 1999. [DOI] [PubMed] [Google Scholar]
- 16.Goodpaster BH, He J, Watkins S, Kelley DE. Skeletal muscle lipid content and insulin resistance: evidence for a paradox in endurance-trained athletes. J Clin Endocrinol Metab 86: 5755–5761, 2001. [DOI] [PubMed] [Google Scholar]
- 17.Groop LC, Bonadonna RC, Simonson DC, Petrides AS, Shank M, DeFronzo RA. Effect of insulin on oxidative and nonoxidative pathways of free fatty acid metabolism in human obesity. Am J Physiol Endocrinol Metab 263: E79–E84, 1992. [DOI] [PubMed] [Google Scholar]
- 18.Hales CN, Randle PJ. Effects of low-carbohydrate diet and diabetes mellitus on plasma concentrations of glucose, non-esterified fatty acid, and insulin during oral glucose-tolerance tests. Lancet 1: 790–794, 1963. [DOI] [PubMed] [Google Scholar]
- 19.Harris JA, Benedict FG. A biometric study of human basal metabolism. Proc Natl Acad Sci USA 4: 370–373, 1918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Haus JM, Kashyap SR, Kasumov T, Zhang R, Kelly KR, Defronzo RA, Kirwan JP. Plasma ceramides are elevated in obese subjects with type 2 diabetes and correlate with the severity of insulin resistance. Diabetes 58: 337–343, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hickner RC, Racette SB, Binder EF, Fisher JS, Kohrt WM. Effects of 10 days of endurance exercise training on the suppression of whole body and regional lipolysis by insulin. J Clin Endocrinol Metab 85: 1498–1504, 2000. [DOI] [PubMed] [Google Scholar]
- 22.Hirosumi J, Tuncman G, Chang L, Gorgun CZ, Uysal KT, Maeda K, Karin M, Hotamisligil GS. A central role for JNK in obesity and insulin resistance. Nature 420: 333–336, 2002. [DOI] [PubMed] [Google Scholar]
- 23.Holloszy JO, Coyle EF. Adaptations of skeletal muscle to endurance exercise and their metabolic consequences. J Appl Physiol 56: 831–838, 1984. [DOI] [PubMed] [Google Scholar]
- 24.Issekutz B Jr, Paul P, Miller HI, Bortz WM. Oxidation of plasma FFA in lean and obese humans. Metabolism 17: 62–73, 1968. [DOI] [PubMed] [Google Scholar]
- 25.Itani SI, Ruderman NB, Schmieder F, Boden G. Lipid-induced insulin resistance in human muscle is associated with changes in diacylglycerol, protein kinase C, and IkappaB-alpha. Diabetes 51: 2005–2011, 2002. [DOI] [PubMed] [Google Scholar]
- 26.Jones WK, Brown M, Wilhide M, He S, Ren X. NF-kappaB in cardiovascular disease: diverse and specific effects of a “general” transcription factor? Cardiovasc Toxicol 5: 183–202, 2005. [DOI] [PubMed] [Google Scholar]
- 27.Kelley DE, Mandarino LJ. Fuel selection in human skeletal muscle in insulin resistance: a reexamination. Diabetes 49: 677–683, 2000. [DOI] [PubMed] [Google Scholar]
- 28.Kelley DE, Mokan M, Simoneau JA, Mandarino LJ. Interaction between glucose and free fatty acid metabolism in human skeletal muscle. J Clin Invest 92: 91–98, 1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kim F, Tysseling KA, Rice J, Pham M, Haji L, Gallis BM, Baas AS, Paramsothy P, Giachelli CM, Corson MA, Raines EW. Free fatty acid impairment of nitric oxide production in endothelial cells is mediated by IKKbeta. Arterioscler Thromb Vasc Biol 25: 989–994, 2005. [DOI] [PubMed] [Google Scholar]
- 30.Kirwan JP, del Aguila LF, Hernandez JM, Williamson DL, O'Gorman DJ, Lewis R, Krishnan RK. Regular exercise enhances insulin activation of IRS-1-associated PI3-kinase in human skeletal muscle. J Appl Physiol 88: 797–803, 2000. [DOI] [PubMed] [Google Scholar]
- 31.Laville M, Rigalleau V, Riou JP, Beylot M. Respective role of plasma nonesterified fatty acid oxidation and total lipid oxidation in lipid-induced insulin resistance. Metabolism 44: 639–644, 1995. [DOI] [PubMed] [Google Scholar]
- 32.O'Leary VB, Marchetti CM, Krishnan RK, Stetzer BP, Gonzalez F, Kirwan JP. Exercise-induced reversal of insulin resistance in obese elderly is associated with reduced visceral fat. J Appl Physiol 100: 1584–1589, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Perseghin G, Scifo P, Pagliato E, Battezzati A, Benedini S, Soldini L, Testolin G, Del Maschio A, Luzi L. Gender factors affect fatty acids-induced insulin resistance in nonobese humans: effects of oral steroidal contraception. J Clin Endocrinol Metab 86: 3188–3196, 2001. [DOI] [PubMed] [Google Scholar]
- 34.Poitout V, Robertson RP. Glucolipotoxicity: fuel excess and beta-cell dysfunction. Endocr Rev 29: 351–366, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pratipanawatr T, Pratipanawatr W, Rosen C, Berria R, Bajaj M, Cusi K, Mandarino L, Kashyap S, Belfort R, DeFronzo RA. Effect of IGF-I on FFA and glucose metabolism in control and type 2 diabetic subjects. Am J Physiol Endocrinol Metab 282: E1360–E1368, 2002. [DOI] [PubMed] [Google Scholar]
- 36.Randle PJ, Garland PB, Hales CN, Newsholme EA. The glucose fatty-acid cycle. Its role in insulin sensitivity and the metabolic disturbances of diabetes mellitus. Lancet 1: 785–789, 1963. [DOI] [PubMed] [Google Scholar]
- 37.Randle PJ, Newsholme EA, Garland PB. Regulation of glucose uptake by muscle. 8. Effects of fatty acids, ketone bodies and pyruvate, and of alloxan-diabetes and starvation, on the uptake and metabolic fate of glucose in rat heart and diaphragm muscles. Biochem J 93: 652–665, 1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Roden M, Price TB, Perseghin G, Petersen KF, Rothman DL, Cline GW, Shulman GI. Mechanism of free fatty acid-induced insulin resistance in humans. J Clin Invest 97: 2859–2865, 1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Schenk S, Horowitz JF. Acute exercise increases triglyceride synthesis in skeletal muscle and prevents fatty acid-induced insulin resistance. J Clin Invest 117: 1690–1698, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Schrauwen-Hinderling VB, van Loon LJ, Koopman R, Nicolay K, Saris WH, Kooi ME. Intramyocellular lipid content is increased after exercise in nonexercising human skeletal muscle. J Appl Physiol 95: 2328–2332, 2003. [DOI] [PubMed] [Google Scholar]
- 41.Shoelson SE, Lee J, Goldfine AB. Inflammation and insulin resistance. J Clin Invest 116: 1793–1801, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Solomon TP, Sistrun SN, Krishnan RK, Del Aguila LF, Marchetti CM, O'Carroll SM, O'Leary VB, Kirwan JP. Exercise and diet enhance fat oxidation and reduce insulin resistance in older obese adults. J Appl Physiol 104: 1313–1319, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Toledo FG, Menshikova EV, Azuma K, Radikova Z, Kelley CA, Ritov VB, Kelley DE. Mitochondrial capacity in skeletal muscle is not stimulated by weight loss despite increases in insulin action and decreases in intramyocellular lipid content. Diabetes 57: 987–994, 2008. [DOI] [PubMed] [Google Scholar]
- 44.Vaag A, Skott P, Damsbo P, Gall MA, Richter EA, Beck-Nielsen H. Effect of the antilipolytic nicotinic acid analogue acipimox on whole-body and skeletal muscle glucose metabolism in patients with non-insulin-dependent diabetes mellitus. J Clin Invest 88: 1282–1290, 1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Werner ED, Lee J, Hansen L, Yuan M, Shoelson SE. Insulin resistance due to phosphorylation of insulin receptor substrate-1 at serine 302. J Biol Chem 279: 35298–35305, 2004. [DOI] [PubMed] [Google Scholar]
- 46.Williamson DL, Kirwan JP. A single bout of concentric resistance exercise increases basal metabolic rate 48 hours after exercise in healthy 59–77-year-old men. J Gerontol A Biol Sci Med Sci 52: M352–M355, 1997. [DOI] [PubMed] [Google Scholar]
- 47.Wolfe RR Radioactive and Stable Isotope Tracers in Biomedicine. Principles and Practice of Kinetic Analysis. New York: Wiley-Liss, 1992.
- 48.Yassine HN, Marchetti CM, Krishnan RK, Vrobel TR, Gonzalez F, Kirwan JP. Effects of exercise and caloric restriction on insulin resistance and cardiometabolic risk factors in older obese adults—a randomized clinical trial. J Gerontol A Biol Sci Med Sci 64: 90–95, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Yki-Jarvinen H, Puhakainen I, Koivisto VA. Effect of free fatty acids on glucose uptake and nonoxidative glycolysis across human forearm tissues in the basal state and during insulin stimulation. J Clin Endocrinol Metab 72: 1268–1277, 1991. [DOI] [PubMed] [Google Scholar]
- 50.Yuan M, Konstantopoulos N, Lee J, Hansen L, Li ZW, Karin M, Shoelson SE. Reversal of obesity- and diet-induced insulin resistance with salicylates or targeted disruption of Ikkbeta. Science 293: 1673–1677, 2001. [DOI] [PubMed] [Google Scholar]