Abstract
Growth hormone (GH) secretion is subject to complex regulation. How pre- and postmenopausal age (PRE, POST), estradiol (E2) availability, and abdominal visceral fat (AVF) jointly affect peptidyl-secretagogue drive of GH secretion is not known. To this end, healthy PRE (n = 20) and POST (n = 22) women underwent a low- vs. high-E2 clamp before receiving a continuous intravenous infusion of GH-releasing hormone (GHRH) or GH-releasing peptide (GHRP-2). According to analysis of covariance, PRE and POST women achieved age-independent hypo- and euestrogenemia under respective low- and high-E2 clamps. All four of age (P < 0.001), E2 status (P = 0.006), secretagogue type (P < 0.001), and an age × peptide interaction (P = 0.014) controlled pulsatile GH secretion. Independently of E2 status, POST women had lower GH responses to both GHRH (P = 0.028) and GHRP-2 (P < 0.001) than PRE women. Independently of age, GHRP-2 was more stimulatory than GHRH during low E2 (P = 0.011) and high E2 (P < 0.001). Stepwise forward-selection multivariate analysis revealed that computerized tomographic estimates of AVF explained 22% of the variability in GHRH action (P = 0.002), whereas age and E2 together explained 60% of the variability in GHRP-2 drive (P < 0.001). These data establish that age, estrogen status, and AVF are triple covariates of continuous peptide-secretagogue drive of pulsatile GH secretion in women. Each factor must be controlled for to allow valid comparisons of GH-axis activity.
Keywords: somatotropin, growth hormone-releasing hormone, growth hormone secretion, growth hormone-releasing peptide, human, estrogen
growth hormone (GH) secretion is predominantly (>85%) pulsatile in healthy young adults (20). The first day of infant life, Tanner stages IV-V of puberty, and the late-follicular phase of the menstrual cycle are associated with severalfold augmentation of the mass (size) of GH secretory bursts with no evident changes in GH secretory-burst frequency or GH half-life (54). Conversely, midchildhood, hypogonadism in young adults, older age, low aerobic capacity, and adiposity are accompanied by a marked diminution in pulsatile GH production (8, 17, 29, 30, 33, 44, 55).
Although the factors that control basal (nonpulsatile) GH secretion are not well known, pulsatile GH secretion is stimulated by fasting, aromatizable androgens, and estradiol (E2) but attenuated by factors associated with food intake, abdominal visceral fat (AVF), and aging (11, 19, 23, 29, 31, 39, 44, 45, 48). From a mechanistic vantage, the size of GH secretory bursts is determined by specific peptide signals that mediate feedforward [GH-releasing hormone (GHRH), GH-releasing peptide (GHRP)/ghrelin] and feedback [GH, insulin-like growth factor I (IGF-I), and somatostatin (SS)] (3, 5, 7, 9, 18, 22, 32, 38, 42, 47). In this context, what remains difficult to parse includes 1) which particular secretagogues are affected by age, estrogen, and adiposity; 2) the relative contributions that age, sex steroids, and AVF make to the regulation of GH secretion; 3) the degree to which the same three covariates interact pairwise or altogether to determine GH production; and 4) the differential impact of age, sex hormones, and AVF on pulsatile vis-à-vis basal GH secretion.
To examine these questions, the present study utilizes an experimental paradigm of continuous intravenous infusion of a submaximally stimulatory amount of GHRH or GHRP-2 combined with experimentally controlled eu- and hypoestrogenemia in premenopausal (PRE) and postmenopausal (POST) women with varying degrees of relative adiposity. Indirect markers of E2 action were also measured to verify estrogenic effects.
METHODS
Subjects.
Volunteers provided written informed consent and were compensated for time spent in the study according to Mayo Institutional Review Board-approved guidelines. The studies were approved by the Mayo Institutional Review Board. No woman had received birth control pills or hormone replacement for at least 4 wk before the first leuprolide injection. Exclusion criteria were any history, symptoms, or signs of ischemic or occlusive arteriovenous events; hepatic, renal, cardiac, pulmonary, malignant, or infectious disease; untreated triglyceride-predominant hyperlipidemia; untreated cholelithiasis; known or suspected breast neoplasm; acute illness; anemia (hemoglobin <11.8 g/dl); ongoing psychiatric treatment; concurrent drug or alcohol abuse; use of neuroactive drugs, such as antidepressant, antihypertensive, or anticonvulsant agents; >3 kg weight change in 6 wk; nightshift work; and unwillingness or inability to provide informed consent. Inclusion criteria were community-living, consenting, informed healthy women ages 18–30 or 50–80 yr. PRE status was defined by cyclic menses with a history of normal puberty. Pregnancy was excluded by blood human chorionic gonadotropin measurement. POST status was defined by amenorrhea for at least 2 yr, follicle-stimulating hormone (FSH) >45 IU/l, luteinizing hormone (LH) >20 IU/l, and E2 ≤35 pg/ml (multiply by 3.68 for pmol/l).
Computerized axial tomography at L4-L5 was used to estimate AVF, as described earlier (12).
Clinical protocol.
The study was a prospectively randomized, parallel-cohort comparison of the effects of age on secretagogue vs. saline stimulation of GH secretion during controlled estrogen depletion vs. repletion. To achieve estrogen depletion, depot leuprolide acetate (3.75 mg im) was administered two times 3 wk apart (13). Leuprolide was given to both POST and PRE subjects to obviate any potential confounding by the downregulation regimen. The first injection was given in young volunteers within 8 days of menses onset and 48 h of a negative blood pregnancy test, and in older women three or more weeks after withdrawal of any estrogen supplements. Graded transdermal E2 repletion via a medicated patch was accomplished on an outpatient basis, starting on the day of the second leuprolide injection (day 0). A placebo patch was provided by the manufacturer, identical to the active patch except lacking E2. The transdermal E2 dose was 0.05 mg/day, and then increased every 4 days to 0.10 mg, 0.15 mg, and 0.20 mg/day [Estraderm (Novartis, Basel, Switzerland)]. The highest E2 dose (0.2 mg/day) was administered for 12 days (days 12–23 inclusive). The transdermal paradigm was designed to elevate serum E2 concentrations into the late-follicular range of 115–165 pg/ml (12, 13). At the end of the study, micronized progesterone (100 mg orally) was administered for 12 days to women with an intact uterus according to standards of good medical care.
Infusion schedule.
Two randomly ordered, separate-day intravenous infusion sessions were undertaken at least 48 h apart in fasting subjects. Infusion studies were performed on days 17–23 of transdermal E2 or placebo patches. At 1800 the night before study, volunteers received a standardized outpatient meal of 8 kcal/kg distributed as 20% protein, 50% carbohydrate, and 30% fat. Subjects then remained fasting overnight and until the end of sampling. At 0700 the next morning, two intravenous catheters were placed in (contralateral) forearm veins to allow simultaneous secretagogue infusion and blood sampling (1 ml) every 10 min for 6 h from 0800 to 1400. The infusion sessions comprised intravenous saline (20 ml/h) from 0800 to 1000, followed by GHRH or GHRP-2 delivered continuously for 4 h from 1000 to 1400 at a constant rate of 0.33 μg·kg−1·h−1. The foregoing peptide dose represents ∼50% of maximal stimulatory dose (2, 50).
Hormone assays.
Plasma GH concentrations were measured in duplicate by automated double-monoclonal immunoenzymatic chemiluminescence assay using 22-kDa recombinant human GH as assay standard (Sanofi Diagnostics Pasteur Access, Chaska, MN) (13). All samples (n = 73) from any given subject were analyzed together. Sensitivity was 0.010 μg/l (defined as 3 SDs above the 0-dose tube). Interassay coefficients of variation (CVs) were 7.9 and 6.3%, respectively, at GH concentrations of 3.4 and 12 μg/l. Intra-assay CVs were 4.9% at 1.1 μg/l and 4.5% at 20 μg/l. No values fell below 0.020 μg/l. Cross-reactivity with 20-kDa GH is <5%.
Screening serum E2, LH, and FSH concentrations were quantified by automated chemiluminescence assay (ACS 180; Bayer, Norwood, MA), using as standards E2, and the First and Second International Reference Preparations, respectively. Procedural sensitivities for E2, LH, and FSH are 35 pg/ml (189 pmol/l) and 0.2 and 0.4 IU/l. Intra-assay CVs for LH were 4.7% and 3.5 and 3.8%, and interassay CVs 8, 3.7, and 4.7% at 4.4, 18, and 39 IU/l, respectively. For FSH measurements, intra-assay CVs were 5.6, 4.3, and 3.5% and interassay CVs 6, 4, and 2.8% at 4.6, 25, and 62 IU/L, respectively.
Study-day values of E2 and testosterone (T) were quantified by liquid-chromatography tandem mass spectrometry as described (53). Sex hormone-binding globulin (SHBG) and albumin were measured, and free and bioavailable E2 and T concentrations were calculated as reported earlier (43). Total IGF-I, IGF-binding protein (IGFBP)-1, and IGFBP-3 concentrations were assayed by immunoradiometric assay (Diagnostic Systems Laboratories, Webster, TX), as presented (12, 13, 49).
Ghrelin was assayed as described recently allowing estimation of both total and acylated peptides (36).
Model-free analysis.
Unstimulated (presecretagogue) GH concentrations were averaged over the 2-h saline-infusion intervals (0800–1000) in each subject.
Deconvolution analysis.
GH concentration time series (all 6 h) were analyzed using a recently developed automated deconvolution method, which was mathematically verified by direct statistical proof and empirically validated using hypothalamo-pituitary sampling and simulated pulsatile time series (6, 25). The Matlab-based algorithm first detrends the data and normalizes concentrations to the unit interval [0, 1] (24). Second, the program creates multiple successive potential pulse-time sets, each containing one fewer burst via a smoothing process (a nonlinear adaptation of the heat-diffusion equation). Third, a maximum-likelihood expectation estimation method computes all secretion and elimination parameters simultaneously conditional on each of the candidate pulse-time sets. Deconvolution parameters comprise basal secretion (β0), two half-lives (α1, α2), secretory-burst mass (η0, η1), random effects on burst mass (σA), procedural/measurement error (σɛ), and a three-parameter flexible Gamma secretory-burst waveform (β1, β2, β3). The fast half-life was represented as 3.5 min constituting 37% of the decay amplitude and the slow half-life as 20.8 min (15). Statistical model selection was performed to distinguish among the independently framed fits of the multiple candidate pulse-time sets using the Akaike information criterion (1). Outcomes evaluated here were basal and pulsatile GH secretion (concentration units/session), mass secreted per burst (concentration units), and waveform shape (mode, or time delay to maximal secretion after objectively estimated burst onset, min).
Statistical methods.
The design was a prospectively randomized, double-masked parallel-cohort comparison of the effects of age and E2 on specific secretagogue actions. There were two age groups, two estrogen states, and two secretagogues. For the primary outcome of pulsatile GH secretion during either GHRH or GHRP-2 infusion, prestudy power analyses predicted >85% statistical power to detect a 30% difference due to age in either the low- or the high-E2 milieu by unpaired two-tailed Student's t-test if a total of 40 subjects completed the study (35).
The effects of age, estrogen status, secretagogue type, and their interactions were evaluated using a three-way (age, estrogen, secretagogue) least-squares general-linear ANCOVA model (57). The covariate was the mean 2-h prestimulus baseline GH concentration in each subject. Departure of the variance-covariance matrix from compound symmetry was adjusted for using the Huynh-Feldt statistic. Wilk's lambda was applied to evaluate the significance of paired (and triple) interactions between (among) age, E2 status, and secretagogue type. According to the null hypothesis, age stratum, E2 condition, and secretagogue type do not individually or jointly (interactively) determine GH secretion. Results were considered significant for experimentwise P < 0.05. Post hoc contrasts were made using Tukey's honestly significantly different (HSD) test (16).
Regression.
Stepwise forward-selection multivariate linear regression analysis was used to relate pulsatile (and basal) GH secretion to age, E2 concentration, and/or AVF in the combined cohorts (n = 42 subjects). Computations were made using SYSTAT Version 11 (Point Richmond, CA).
RESULTS
Subject characteristics.
All 42 women completed both infusion sessions. Enrollment comprised 20 healthy PRE subjects with an age range of 18–29 yr and 22 POST volunteers with an age range of 55–74 yr [Supplemental Table 1 (Supplemental material for this article can be found on the American Journal of Physiology: Endocrinology and Metabolism website)]. Body mass index in PRE ranged from 18 to 30 and in POST from 19 to 32 kg/m2 (P = not significant). AVF estimated by computed tomography scan was higher in POST than PRE volunteers (P < 0.01).
Baseline hormone values.
Baseline screening (outpatient) concentrations of LH and FSH (measured at 0750 fasting before leuprolide injection) in POST women were higher and E2 lower than corresponding values in PRE women (each P < 0.001 for age effect) (supplemental Table 1). Concentrations of total T were 33% (P = 0.004), IGF-I 52% (P < 0.001), prolactin 36% (P = 0.008), albumin 6.5% (P < 0.001), and IGFBP-3 16% (P = 0.002) lower in POST than PRE subjects. In contrast, IGFBP-1 was 77% higher in POST than PRE individuals (P = 0.030).
Leuprolide/E2 (or placebo) clamp.
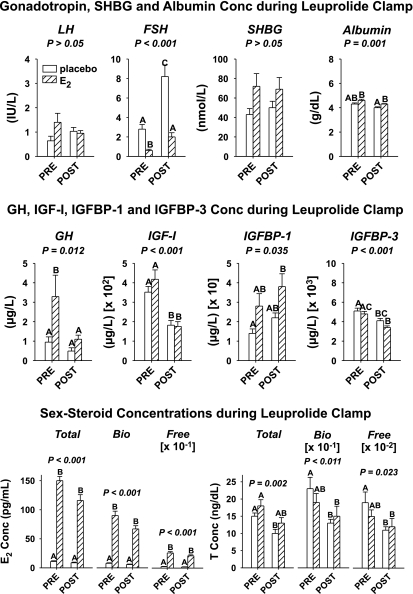
Indirect markers of E2 action were also measured in serum collected at 0750 fasting (10 min) before beginning saline/peptide infusions on the first of the two inpatient study sessions to verify estrogenic effects. Leuprolide suppressed LH concentrations to comparable values (P = 0.087) in PRE and POST women given placebo or E2 (grand mean for n = 42, 1.0 ± 0.20 IU/l) but reduced FSH (IU/l) concentrations to lower values in PRE + E2 (0.61 ± 1.2) than in the other three groups (P < 0.001) and to lower values in both PRE − E2 (2.8 ± 0.47) and POST + E2 (2.0 ± 0.43) than in POST − E2 (8.2 ± 1.2) (P < 0.001) (Fig. 1, top). Our earlier demonstration that an estrogen receptor (ER)-antagonist, which does not cross the blood-brain barrier, elevates FSH secretion during exogenous E2 supplementation in older women permits the hypothesis that E2 in part directly inhibits FSH secretion. The present data showing that E2 suppresses FSH concentrations further in the presence of leuprolide would be consistent with partial direct (gonadotropin-releasing hormone-independent) pituitary inhibition by exogenous E2.
Fig. 1.
Influences of premenopausal (PRE) and postmenopausal (POST) status on hormone concentrations (Conc) during leuprolide/placebo and leuprolide/estradiol (E2) clamp in 42 women. Top: luteinizing hormone (LH), follicle-stimulating hormone (FSH), sex hormone-binding globulin (SHBG), and albumin concentrations. Middle: growth hormone (GH), insulin-like growth factor (IGF)-I, IGF-binding protein (IGFBP)-I, and IGFBP-3. Bottom. E2 (left) and T (right) moieties. P values above each cluster of 4 columns were determined by 1-way ANOVA. Data are means ± SE (n = 10 in each cohort, except POST-E2 where n = 12). Bio, bioavailable. Means with different (unshared) alphabetic superscripts differ significantly by Tukey's honestly significantly different (HSD) post hoc multiple-comparison test at experiment-wise P < 0.05. Thus A differs from BC and C but not from AB.
During the leuprolide clamp, SHBG did not vary among the four cohorts (P = 0.077, grand mean 59 ± 9.4 nmol/l). Serum albumin (g/dl) was lower in POST − E2 (4.0 ± 0.77) than in both PRE + E2 and POST + E2 (mean 4.5 ± 0.065) but not than in PRE − E2 (4.3 ± 0.097) (P < 0.001). Total E2, free E2, and bioavailable (bio-) E2 were comparable in PRE + E2 and POST + E2 women, thus verifying efficacy of the high-E2 clamp (Fig. 1, middle). Likewise, E2 levels were similar in the low-E2 clamp in PRE − E2 and POST − E2 individuals. Each of total T, free T and bio T was decreased in POST − E2 compared with PRE − E2 (P = 0.002, P = 0.023, P = 0.011, respectively). There were no differences between PRE and POST T moieties (total, free, bio) during E2 addback. In addition, total, free, and bio T were similar in POST + E2 and POST − E2, whereas total T in PRE + E2 exceeded that in POST − E2 (P < 0.01). Therefore, POST women had lower T values than PRE women in the low-E2 but not in the high-E2 milieu.
GH, IGF-I, IGFBP-1, and IGFBP-3 during the leuprolide clamp tended to retain relative POST/PRE differences observed at baseline before the clamp (Fig. 1, bottom). In contrast, total and active ghrelin concentrations did not differ among the four study groups (respective grand means: 985 ± 98 and 16 ± 1.8 pg/ml).
Pulsatile GH secretion.
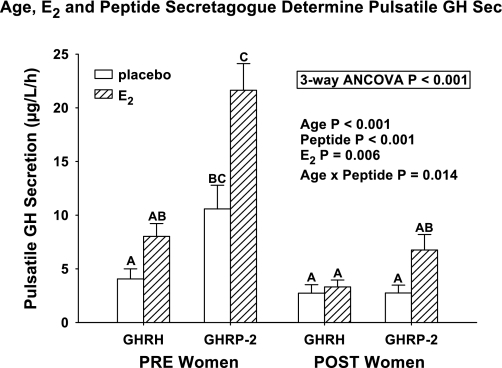
Three-way ANCOVA revealed that pulsatile GH secretion was statistically determined by each of age (P < 0.001), estrogen (P = 0.006), and secretagogue (P < 0.001) (Supplemental Table 2). The covariate (presecretagogue 2-h saline-infused mean GH concentration) was a determinant of pulsatile-GH responses (P = 0.002). Age and peptide type interacted significantly (P = 0.014). Primary outcomes of post hoc Tukey's HSD multiple-comparisons testing at experiment-wise P < 0.05 were as follows: 1) the highest pulsatile GH secretion occurred in PRE + E2 given GHRP-2 compared with all seven other conditions, except PRE − E2 given GHRP-2; 2) numerically lowest pulsatile GH secretion emerged in three of four POST conditions (GHRP-2 + E2 excepted) and in PRE − E2 during GHRH infusion; and 3) intermediate pulsatile GH output characterized POST + E2 + GHRP-2, PRE − E2 + GHRP-2, and PRE − E2 + GHRH (Fig. 2). For all 42 subjects, the descending numerical rank order of potency (assuming PRE + E2 + GHRP-2 = 130 ± 15 μg·l−1·6 h−1 = 100%) was as follows: PRE − E2 + GHRP-2 49%, PRE + E2 + GHRH 37%, POST + E2 + GHRP-2 32%, and PRE − E2 + GHRH 19% with POST + E2 + GHRH 15%, POST − E2 + GHRP-2 13%, and POST − E2 + GHRH 12%. Thus E2 doubled both the GHRP-2 and the GHRH effect in PRE women, but only amplified the GHRP-2 effect by 2.5-fold and the GHRH effect by 1.25-fold in POST women.
Fig. 2.
Triple influences of age (PRE vs. POST status), E2 clamp (low vs. high), and peptide secretagogue [GH-releasing hormone (GHRH) vs. GH-releasing peptide (GHRP)-2] on pulsatile GH secretion (Sec; μg·l−1·h−1) in 42 women. Format is described in Fig. 1. Means with unshared alphabetic superscripts differ significantly by multiple-comparison post hoc Tukey's HSD test.
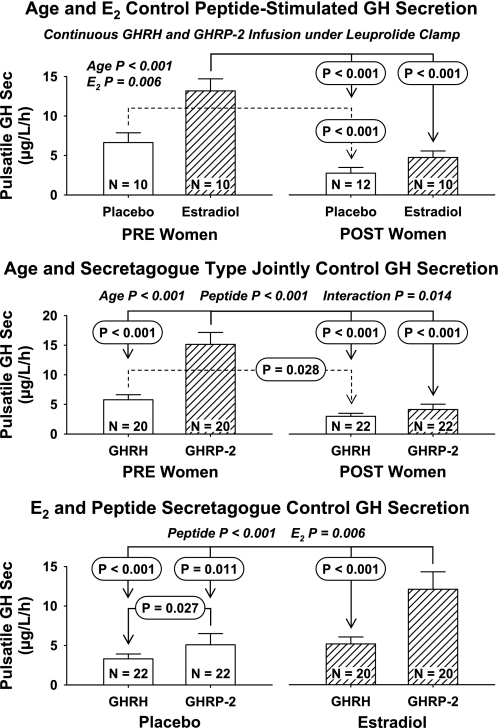
Figure 3 presents ANCOVA outcomes from the perspective of pairwise-factor effects. Combining responses to both peptidyl secretagogues, age (P < 0.001) and E2 stratum (P = 0.006), determined pulsatile GH secretion (top). Whereas age and E2 did not interact, PRE + E2 was associated with greater pulsatile GH secretion under peptide drive than both POST + E2 and POST − E2 (P < 0.001 by Tukey's HSD test). PRE − E2 responses were also higher than POST − E2 responses (P < 0.001). Considered independently of E2 status, age (P < 0.001), peptide type (P < 0.001), and their interaction (P = 0.014) controlled pulsatile GH secretion (middle). The PRE + GHRP-2 response exceeded that of PRE + GHRH, POST + GHRH, and POST + GHRP-2 (each P < 0.001). In addition, PRE + GHRH was greater than POST + GHRH (P = 0.028). The significant interaction term (P = 0.014) reflected conjoint effects of age and secretagogue types in defining pulsatile GH secretion. When viewed independently of age, peptidyl secretagogue (P < 0.001) and E2 level (P = 0.006) individually (but not interactively) modulated pulsatile GH secretion (bottom). GHRP-2 + E2 stimulated pulsatile GH secretion more than each of GHRH − E2 (P < 0.001), GHRH + E2 (P < 0.001), and GHRP-2 − E2 (P = 0.027). The collective data establish strong effects of age, E2 status, and secretagogue type as well as an interaction between age and secretagogue type in the determination of pulsatile GH secretion in women.
Fig. 3.
Three-way ANCOVA estimates of the effects on pulsatile GH secretion of age stratum and E2 concentration independently of secretagogue type (top), age and peptide secretagogue independently of E2 status (middle), and E2 level and peptide agonist independently of menopausal status (bottom). Data are otherwise presented as defined in Fig. 2.
Basal GH secretion.
Based upon two-way ANCOVA, basal (nonpulsatile) GH secretion was statistically determined by age (P = 0.016) and E2 status (P = 0.005), but not their interaction (P = 0.39). The main contrast was PRE + E2 > POST − E2 (P = 0.002). Secondarily, basal GH secretion in PRE + E2 tended to be greater than in PRE − E2 (P = 0.054).
Secretory-burst waveform (mode).
The mode of GH secretory bursts (time delay from burst onset to peak secretion rate) was invariant of secretagogue type (global mode 20 ± 1.7 min), according to three-way ANCOVA. There was an age × E2 interaction during GHRH and GHRP-2 stimulation (P = 0.020), wherein E2 supplementation in POST only was associated with more extended GH secretory bursts (mode 22.4 ± 1.2 min) than placebo addback (mode 17.6 ± 0.93 min) (P = 0.007) (Fig. 4).
Fig. 4.
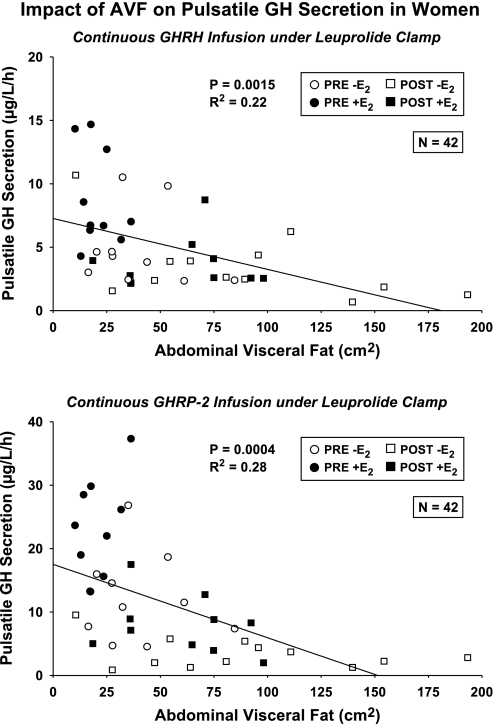
Negative influence of abdominal visceral fat (AVF) on pulsatile GH secretion during 4-h GHRH infusion (top) and 4-h GHRP-2 infusion (bottom). P and r2 values are univariate estimates (n = 42 subjects) based on Pearson's correlation coefficient.
Regression analyses.
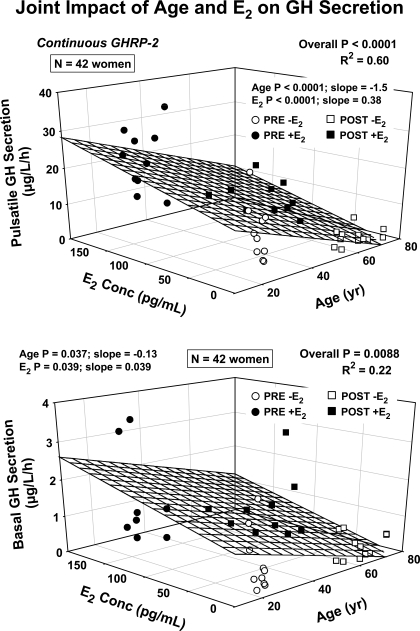
Univariate regression analysis revealed strongly negative effects of AVF on GHRH- and GHRP-2-stimulated pulsatile GH secretion (respective r2 = 0.22, P = 0.0015 and r2 = 0.28, P = 0.0004) (Fig. 5, top). Because age was a correlate of AVF (r2 = 0.29, P < 0.001), we next used stepwise forward-selection multivariate regression to distinguish possible individual and joint effects of age, AVF, and E2. In this analysis, neither age nor E2 remained significant, but there was still a negative correlation between GHRH-stimulated pulsatile GH secretion and AVF (multivariate r2 = 0.22 vs. univariate r2 = 0.22) (Supplemental Table 3). By the same analysis, AVF was no longer significant, but age (negative, P < 0.001) and E2 (positive, P < 0.001) together explained 60% of the variability in GHRP-2-stimulated pulsatile GH secretion (multi-r2 = 0.60, P < 0.001) (Fig. 5, bottom). In addition, age (P = 0.037) and E2 (P = 0.039) together determined basal (nonpulsatile) GH secretion (multi-r2 = 0.22, P = 0.0088) (bottom).
Fig. 5.
Three-dimensional plots depicting the joint linear effects of age and E2 concentrations on pulsatile GH secretion (top) and basal (nonpulsatile) GH secretion (bottom) in 42 women. Numerical values are multivariate P and r2, and partial (age or E2) P values with corresponding slope estimates. The 4 subgroups of women studied are defined by the indicated symbol types.
DISCUSSION
The combination of leuprolide plus transdermal E2 or placebo addback successfully enforced high- and low-estrogen milieus. This paradigm clearly demonstrated that E2 addback in POST women is unable to achieve the values of fasting GH, IGF-I, and IGFBP-3 concentrations demonstrated in E2-treated PRE women. Because GH administration in young and older adults does generate comparable IGF-I responses (54), these outcomes favor the interpretation that lower IGF-I and IGFBP-3 levels in POST than PRE women are due to lower GH drive per se. Lesser GH output in older than young women is unexpected in view of their lower IGF-I concentrations, which should decrease negative feedback and stimulate more GH secretion (47). Thus we propose that either IGF-I feedback efficacy is higher in POST than PRE women (an unstudied question) and/or that peptide-secretagogue drive of GH secretion is impaired in POST women. In examining the latter consideration, the present study disclosed that secretagogue type, PRE vs. POST menopausal status, E2 milieu, and AVF all determine pulsatile GH secretion in healthy women. In the case of GHRH stimulation, AVF was the dominant-negative regulator explaining 22% of response variability among the 42 women studied. In contrast, in the case of GHRP-2 stimulation, menopausal status and age emerged together as strong negative and positive modulators, respectively, which jointly accounted for 60% of GH-response variability.
Two-way ANCOVA demonstrated that E2 enhances the effects of both peptides studied (P = 0.006). The question arises how estrogen increases stimulation by GHRH and GHRP-2. Because the infused dose of GHRH was submaximally stimulatory (methods), in principle, E2 could augment the exogenous GHRH effect by: 1) decreasing hypothalamic SS release to (or action on) the pituitary gland; 2) increasing hypothalamic GHRH secretion to or actions of GHRH on somatotropes; and 3) amplifying the presumptive potentiating interaction between GHRH and endogenous ghrelin. A dose-response study in POST women showed that E2 does decrease the inhibitory potency (but not efficacy) of infused SS (4). Another dose-response analysis indicated that E2 doubles the stimulatory potency of infused GHRH (50). Whether E2 additionally reduces hypothalamic SS secretion and/or increases hypothalamic GHRH secretion in humans is not yet known. Indirect evidence points to a facilitative effect of E2 on endogenous GHRH release (46). Animal models allow for both possibilities, but neither has been proven by direct hypothalamopituitary portal-venous sampling (20, 54). Moreover, although E2 enhances submaximal ghrelin action in women (52), no studies have yet tested whether E2 can augment the interaction between exogenous GHRH and endogenous ghrelin (22). Finally, E2 supplementation increases overnight acylated ghrelin concentrations, but not values measured at 0800 (36), as confirmed here in both POST and PRE women.
Stepwise forward-selection multivariate regression analysis disclosed that POST vs. PRE status and E2 availability together explain 60% of overall intersubject variability in continuous GHRP-2-stimulated pulsatile GH secretion. By univariate regression analysis, AVF was a prominent negative predictor of GHRP-2 action (r2 = 0.28, P = 0.0004), but this effect vanished in stepwise forward-selection regression analysis probably because of the close positive association between AVF and age (r2 = 0.29, P < 0.001). Of the four variables studied (age, estrogen, secretagogue type, and visceral fat), visceral fat gives the weakest form of evidence, because its contribution was assessed by correlations rather than manipulations of body fat. Precisely how age or a low-E2 status impairs hypothalamopituitary responsiveness to GHRP-2 is not established (20, 54). However, the strong positive correlation between GHRP-2-stimulated pulsatile GH secretion and E2 concentrations (P < 0.001) is explicable by the capability of E2 to potentiate GHRP/ghrelin drive (2, 52). Potentiation might occur by estrogenic upregulation of the expression of the ghrelin receptor, which is subject to in vitro transcriptional activation by E2 (37). Model-based simulations using experimental data obtained in the rat further suggest the mechanistic hypotheses that E2 may reduce SS's inhibitory effects on the pituitary gland (26, 56) and/or augment arcuate-nucleus GHRH outflow (41). The notions arise because E2 downregulates the type 5 SS receptor (10, 26) and ER-α is expressed in GHRH neurons (40). Stimulation of endogenous GHRH release by E2 would be predicted to potentiate exogenous GHRP-2 actions (14).
Greater relative responsiveness to GHRP-2 than GHRH in older than young women was observed in the presence of E2. This was unexpected because brain GHRP-receptor expression is reportedly decreased in older humans (34). A possible explanation is that SSergic opposition declines more than GHRP responsivity in aging. This postulate is testable.
Little is known about the regulation of basal (unstimulated, nonpulsatile) GH secretion, except that knockout of the type 1 SS receptor increases basal (unstimulated) GH secretion in vitro (28) and that acromegaly is marked by elevated basal GH secretion in vivo (21). In the present analysis, E2 enhanced and age repressed basal GH release. Estrogen can stimulate GH secretion by pituitary cells in vitro and by ectopic pituitary tissue in vivo (23, 27), thus allowing the postulate of direct E2 drive of GH synthesis. How age decreases basal GH secretion is less clear. Possibilities involve increased SS outflow and/or increased systemic concentrations of putative GH-inhibiting cytokines, adipokines, free fatty acids, and/or insulin (54). Albeit statistically significant, basal GH secretion only represented 6–18% of total GH secretion.
Deconvolution analysis corroborated an earlier finding that E2 is able to prolong GHRH-stimulated (albeit not GHRP-stimulated) GH secretory-burst duration in POST women (51). Extended release of GH within individual secretory bursts could reflect a reduction in hypothalamic outflow of, or pituitary inhibition by, SS (54). Whereas the first point remains indeterminate (20, 54), a clinical dose-response study inferred that exogenous E2 does diminish the inhibitory potency of infused SS in POST women (4).
Caveats include the absence of data currently available on the dose-dependency of estrogenic effects; the possibility that leuprolide itself might influence GH secretion in some manner; and the need to extend the duration of low- and high-E2 clamps, replicate outcomes in larger cohorts of women, and assess similar mechanisms longitudinally.
In summary, a leuprolide-clamp paradigm maintains total, bioavailable, and free E2 concentrations at comparably low or high levels in PRE and POST women. Based on stepwise forward-selection multivariate analysis, AVF primarily determines pulsatile GH responses to continuous GHRH stimulation, whereas age and E2 together principally control pulsatile GH responses to continuous GHRP-2 drive. These three covariates explain 22–60% of the interindividual variability in sustained peptidyl-secretagogue actions in healthy women.
GRANTS
This work was supported in part via the Clinical Translational Research Center Grant MO1 RR-00585 to the Mayo Clinic and Foundation from the National Center for Research Resources (Rockville, MD) and R01 NIA AG-29362, R21 DK-072095, and DK-063609 from the National Institutes of Health (Bethesda, MD).
Supplementary Material
Acknowledgments
We thank Donna Scott for capable support of manuscript preparation, Ashley Bryant for excellent data analysis and graphics, the Mayo Immunochemical Laboratory for assay assistance, and the Mayo research nursing staff for implementing the protocol.
REFERENCES
- 1.Akaike H A new look at the statistical model identification. IEEE Trans Autom Control 19: 716–723, 1974. [Google Scholar]
- 2.Anderson SM, Shah N, Evans WS, Patrie JT, Bowers CY, Veldhuis JD. Short-term estradiol supplementation augments growth hormone (GH) secretory responsiveness to dose-varying GH-releasing peptide infusions in healthy postmenopausal women. J Clin Endocrinol Metab 86: 551–560, 2001. [DOI] [PubMed] [Google Scholar]
- 3.Arvat E, Ceda GP, Di Vito L, Ramunni J, Gianotti L, Ghigo E. Age-related variations in the neuroendocrine control, more than impaired receptor sensitivity, cause the reduction in the GH-releasing activity of GHRP's in human aging. Pituitary 1: 51–58, 1998. [DOI] [PubMed] [Google Scholar]
- 4.Bray MJ, Vick TM, Shah N, Anderson SM, Rice LW, Iranmanesh A, Evans WS, Veldhuis JD. Short-term estradiol replacement in postmenopausal women selectively mutes somatostatin's dose-dependent inhibition of fasting growth hormone secretion. J Clin Endocrinol Metab 86: 3143–3149, 2001. [DOI] [PubMed] [Google Scholar]
- 5.Calabresi E, Ishikawa E, Bartolini L, Delitala G, Fanciulli G, Oliva O, Veldhuis JD, Serio M. Somatostatin infusion suppresses GH secretory burst number and mass in normal men: a dual mechanism of inhibition. Am J Physiol Endocrinol Metab 270: E975–E979, 1996. [DOI] [PubMed] [Google Scholar]
- 6.Chattopadhyay S, Veldhuis JD, Keenan DM. Probabilistic recovery of pulsatile, secretory and kinetic structure: an alternating discrete and continuous schema. Quarterly Appl Math 66: 401–421, 2008. [Google Scholar]
- 7.Clark RG, Robinson ICAF. Growth hormone responses to multiple injections of a fragment of human growth hormone-releasing factor in conscious male and female rats. J Endocrinol 106: 281–289, 1985. [DOI] [PubMed] [Google Scholar]
- 8.Coiro V, Volpi R, Gramellini D, Cigarini C, Necchi GS, Capretti L, Caffarri G, Chiodera P. Altered neuroendocrine control of GH secretion in normal women of advanced reproductive age. J Gerontol A Biol Sci Med Sci 52: M254–M258, 1997. [DOI] [PubMed] [Google Scholar]
- 9.degli Uberti EC, Ambrosio MR, Cella SG, Margutti AR, Trasforini G, Rigamonti AE, Petrone E, Mueller EE. Defective hypothalamic growth hormone (GH)-releasing hormone activity may contribute to declining GH secretion with age in man. J Clin Endocrinol Metab 82: 2885–2888, 1997. [DOI] [PubMed] [Google Scholar]
- 10.Djordjijevic D, Zhang J, Priam M, Viollet C, Gourdji D, Kordon C, Epelbaum J. Effect of 17 beta-estradiol on somatostatin receptor expression and inhibitory effects on growth hormone and prolactin release in rat pituitary cell cultures. Endocrinology 139: 2272–2277, 1998. [DOI] [PubMed] [Google Scholar]
- 11.Eakman GD, Dallas JS, Ponder SW, Keenan BS. The effects of testosterone and dihydrotestosterone on hypothalamic regulation of growth hormone secretion. J Clin Endocrinol Metab 81: 1217–1223, 1996. [DOI] [PubMed] [Google Scholar]
- 12.Erickson D, Keenan DM, Farhy LS, Mielke K, Bowers CY, Veldhuis JD. Determinants of dual secretagogue drive of burst-like GH secretion in premenopausal women studied under a selective estradiol clamp. J Clin Endocrinol Metab 90: 1741–1751, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Erickson D, Keenan DM, Mielke K, Bradford K, Bowers CY, Miles JM, Veldhuis JD. Dual secretagogue drive of burst-like growth hormone secretion in postmenopausal compared with premenopausal women studied under an experimental estradiol clamp. J Clin Endocrinol Metab 89: 4746–4754, 2004. [DOI] [PubMed] [Google Scholar]
- 14.Farhy LS, Bowers CY, Veldhuis JD. Model-projected mechanistic bases for sex differences in growth-hormone regulation in humans. Am J Physiol Regul Integr Comp Physiol 292: R1577–R1593, 2007. [DOI] [PubMed] [Google Scholar]
- 15.Faria ACS, Veldhuis JD, Thorner MO, Vance ML. Half-time of endogenous growth hormone (GH) disappearance in normal man after stimulation of GH secretion by GH-releasing hormone and suppression with somatostatin. J Clin Endocrinol Metab 68: 535–541, 1989. [DOI] [PubMed] [Google Scholar]
- 16.Fisher LD, van Belle G. Descriptive statistics. In: Biostatistics: A Methodology for the Health Sciences. New York, NY: Wiley, 1996, p. 58–74.
- 17.Frantz AG, Rabkin MT. Effects of estrogen and sex difference on secretion of human growth hormone. J Clin Endocrinol Metab 25: 1470–1480, 1965. [DOI] [PubMed] [Google Scholar]
- 18.Frohman LA New insights into the regulation of somatotrope function using genetic and transgenic models. Metab 45: 1–3, 1996. [DOI] [PubMed] [Google Scholar]
- 19.Ghigo E, Gianotti L, Arvat E, Ramunni J, Valetto MR, Broglio F, Rolla M, Cavagnini F, Muller EE. Effects of recombinant human insulin-like growth factor I administration on growth hormone (GH) secretion, both spontaneous and stimulated by GH-releasing hormone or hexarelin, a peptidyl GH secretagogue, in humans. J Clin Endocrinol Metab 84: 285–290, 1999. [DOI] [PubMed] [Google Scholar]
- 20.Giustina A, Veldhuis JD. Pathophysiology of the neuroregulation of growth hormone secretion in experimental animals and the human. Endocr Rev 19: 717–797, 1998. [DOI] [PubMed] [Google Scholar]
- 21.Hartman ML, Pincus SM, Johnson ML, Matthews DH, Faunt LM, Vance ML, Thorner MO, Veldhuis JD. Enhanced basal and disorderly growth hormone secretion distinguish acromegalic from normal pulsatile growth hormone release. J Clin Invest 94: 1277–1288, 1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hataya Y, Akamizu T, Takaya K, Kanamoto N, Ariyasu H, Saijo M, Moriyama K, Shimatsu A, Kojima M, Kangawa K, Nakao K. A low dose of ghrelin stimulates growth hormone (GH) release synergistically with GH-releasing hormone in humans (Abstract). J Clin Endocrinol Metab 86: 4552, 2001. [DOI] [PubMed] [Google Scholar]
- 23.Jansson JO, Carlsson L, Seeman H. Estradiol - but not testosterone - stimulates the secretion of growth hormone in rats with the pituitary gland autotransplanted to the kidney capsule. Acta Endocrinol (Copenh) 103: 212–218, 1983. [Google Scholar]
- 24.Keenan DM, Chattopadhyay S, Veldhuis JD. Composite model of time-varying appearance and disappearance of neurohormone pulse signals in blood. J Theor Biol 236: 242–255, 2005. [DOI] [PubMed] [Google Scholar]
- 25.Keenan DM, Roelfsema F, Biermasz N, Veldhuis JD. Physiological control of pituitary hormone secretory-burst mass, frequency and waveform: a statistical formulation and analysis. Am J Physiol Regul Integr Comp Physiol 285: R664–R673, 2003. [DOI] [PubMed] [Google Scholar]
- 26.Kimura N, Tomizawa S, Arai KN, Kimura N. Chronic treatment with estrogen up-regulates expression of sst2 messenger ribonucleic acid (mRNA) but down-regulates expression of sst5 mRNA in rat pituitaries. Endocrinologt 139: 1573–1580, 1998. [DOI] [PubMed] [Google Scholar]
- 27.Komolov IS, Perez-Arce JA, Fedotov VP. The effects of estradiol on prolactin and growth hormone secretion in cultured pituitary cells from intact and ovariectomized rats. Endokrinologie 75: 278–284, 1980. [PubMed] [Google Scholar]
- 28.Kreienkamp HJ, Akgun E, Baumeister H, Meyerhof W, Richter D. Somatostatin receptor subtype 1 modulates basal inhibition of growth hormone release in somatotrophs. FEBS Lett 462: 464–466, 1999. [DOI] [PubMed] [Google Scholar]
- 29.Lang I, Schernthaner G, Pietschmann P, Kurz R, Stephenson JM, Templ H. Effects of sex and age on growth hormone response to growth hormone-releasing hormone in healthy individuals. J Clin Endocrinol Metab 65: 535–540, 1987. [DOI] [PubMed] [Google Scholar]
- 30.Lieman HJ, Adel TE, Forst C, von Hagen S, Santoro N. Effects of aging and estradiol supplementation on GH axis dynamics in women. J Clin Endocrinol Metab 86: 3918–3923, 2001. [DOI] [PubMed] [Google Scholar]
- 31.Loche S, Colao A, Cappa M, Bellone J, Aimaretti G, Farello G, Faedda A, Lombardi G, Deghenghi R, Ghigo E. The growth hormone response to hexarelin in children: reproducibility and effect of sex steroids. J Clin Endocrinol Metab 82: 861–864, 1997. [DOI] [PubMed] [Google Scholar]
- 32.Low MJ, Otero-Corchon V, Parlow AF, Ramirez JL, Kumar U, Patel YC, Rubinstein M. Somatostatin is required for masculinization of growth hormone-regulated hepatic gene expression but not of somatic growth. J Clin Invest 107: 1571–1580, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Martin FC, Yeo AL, Sonksen PH. Growth hormone secretion in the elderly: aging and the somatopause. Bailliere's Clin Endocrinol Metab 11: 223–250, 1997. [DOI] [PubMed] [Google Scholar]
- 34.Muccioli G, Ghe C, Ghigo MC, Papotti M, Arvat E, Boghen MF, Nilsson MHL, Deghenghi R, Ong H, Ghigo E. Specific receptors for synthetic GH secretagogues in the human brain and pituitary gland. J Endocrinol 157: 99–106, 1998. [DOI] [PubMed] [Google Scholar]
- 35.O'Brien PC The appropriateness of analysis of variance and multiple-comparison procedures. Biometrics 39: 787–794, 1983. [PubMed] [Google Scholar]
- 36.Paulo RC, Brundage R, Cosma M, Mielke KL, Bowers CY, Veldhuis JD. Estrogen elevates the peak overnight production rate of acylated ghrelin. J Clin Endocrinol Metab 93: 4440–4447, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Petersenn S, Rasch AC, Penshorn M, Beil FU, Schulte HM. Genomic structure and transcriptional regulation of the human growth hormone secretagogue receptor. Endocrinol 142: 2649–2659, 2001. [DOI] [PubMed] [Google Scholar]
- 38.Roelfsema F, Biermasz NR, Veldman RG, Veldhuis JD, Frolich M, Stokvis-Brantsma WH, Wit JM. Growth hormone (GH) secretion in patients with an inactivating defect of the GH-releasing hormone (GHRH) receptor is pulsatile: evidence for a role for non-GHRH inputs into the generation of GH pulses. J Clin Endocrinol Metab 86: 2459–2464, 2001. [DOI] [PubMed] [Google Scholar]
- 39.Shibasaki T, Shizume K, Nakahara M, Masuda A, Jibiki K, Demura H, Wakabayashi I, Ling N. Age-related changes in plasma growth hormone response to growth hormone-releasing factor in man. J Clin Endocrinol Metab 58: 212–214, 1984. [DOI] [PubMed] [Google Scholar]
- 40.Shimizu T, Kamegai J, Tamura H, Ishii S, Sugihara H, Oikawa S. The estrogen receptor (ER) alpha, but not ER beta, gene is expressed in hypothalamic growth hormone-releasing hormone neurons of the adult female rat. Neurosci Res 52: 121–125, 2005. [DOI] [PubMed] [Google Scholar]
- 41.Shirasu K, Stumpf WE, Sar M. Evidence for direct action of estradiol on growth hormone-releasing factor (GRF) in rat hypothalamus: localization of [3H] estradiol in GRF neurons. Endocrinology 127: 344–349, 1990. [DOI] [PubMed] [Google Scholar]
- 42.Shuto Y, Shibasaki T, Otagiri A, Kuriyama H, Ohata H, Tamura H, Kamegai J, Sugihara H, Oikawa S, Wakabayashi I. Hypothalamic growth hormone secretagogue receptor regulates growth hormone secretion, feeding, and adiposity. J Clin Invest 109: 1429–1436, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Takahashi PY, Votruba P, Abu-Rub M, Mielke K, Veldhuis JD. Age attenuates testosterone secretion driven by amplitude-varying pulses of recombinant human luteinizing hormone during acute gonadotrope inhibition in healthy men. J Clin Endocrinol Metab 92: 3626–3632, 2007. [DOI] [PubMed] [Google Scholar]
- 44.Vahl N, Jorgensen JO, Skjaerback C, Veldhuis JD, Orskov H, Christiansen J. Abdominal adiposity rather than age and sex predicts the mass and patterned regularity of growth hormone secretion in mid-life healthy adults. Am J Physiol Endocrinol Metab 272: E1108–E1116, 1997. [DOI] [PubMed] [Google Scholar]
- 45.van den Berg G, Veldhuis JD, Frolich M, Roelfsema F. An amplitude-specific divergence in the pulsatile mode of GH secretion underlies the gender difference in mean GH concentrations in men and premenopausal women. J Clin Endocrinol Metab 81: 2460–2466, 1996. [DOI] [PubMed] [Google Scholar]
- 46.Veldhuis JD, Anderson SM, Patrie JT, Bowers CY. Estradiol supplementation in postmenopausal women doubles rebound-like release of growth hormone (GH) triggered by sequential infusion and withdrawal of somatostatin: evidence that estrogen facilitates endogenous GH-releasing hormone drive. J Clin Endocrinol Metab 89: 121–127, 2004. [DOI] [PubMed] [Google Scholar]
- 47.Veldhuis JD, Bidlingmaier M, Anderson SM, Wu Z, Strassburger CJ. Lowering total plasma insulin-like growth factor I concentrations by way of a novel, potent, and selective growth hormone (GH) receptor antagonist, pegvisomant (B2036-peg), augments the amplitude of GH secretory bursts and elevates basal/nonpulsatile GH release in healthy women and men. J Clin Endocrinol Metab 86: 3304–3310, 2001. [DOI] [PubMed] [Google Scholar]
- 48.Veldhuis JD, Bowers CY. Three-peptide control of pulsatile and entropic feedback-sensitive modes of growth hormone secretion: modulation by estrogen and aromatizable androgen. J Pediatr Endocrinol Metab 16: 587–605, 2003. [PubMed] [Google Scholar]
- 49.Veldhuis JD, Erickson D, Mielke K, Farhy LS, Keenan DM, Bowers CY. Distinctive inhibitory mechanisms of age and relative visceral adiposity on GH secretion in pre- and postmenopausal women studied under a hypogonadal clamp. J Clin Endocrinol Metab 90: 6006–6013, 2005. [DOI] [PubMed] [Google Scholar]
- 50.Veldhuis JD, Evans WS, Bowers CY. Estradiol supplementation enhances submaximal feedforward drive of growth hormone (GH) secretion by recombinant human GH-releasing hormone-1,44-amide in a putatively somatostatin-withdrawn milieu. J Clin Endocrinol Metab 88: 5484–5489, 2003. [DOI] [PubMed] [Google Scholar]
- 51.Veldhuis JD, Keenan DM, Bowers CY. Peripheral estrogen receptor-α selectively modulates the waveform of GH secretory bursts in healthy women. Am J Physiol Regul Integr Comp Physiol 293: R1514–R1521, 2007. [DOI] [PubMed] [Google Scholar]
- 52.Veldhuis JD, Keenan DM, Iranmanesh A, Mielke K, Miles JM, Bowers CY. Estradiol potentiates ghrelin-stimulated pulsatile GH secretion in postmenopausal women. J Clin Endocrinol Metab 91: 3559–3565, 2006. [DOI] [PubMed] [Google Scholar]
- 53.Veldhuis JD, Mielke KL, Cosma M, Soares-Welch C, Paulo R, Miles JM, Bowers CY. Aromatase and 5-alpha-reductase inhibition during an exogenous testosterone clamp unveils selective sex-steroid modulation of somatostatin and growth-hormone secretagogue actions in healthy older men. J Clin Endocrinol Metab 94: 973–981, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Veldhuis JD, Roemmich JN, Richmond EJ, Bowers CY. Somatotropic and gonadotropic axes linkages in infancy, childhood, and the puberty-adult transition. Endocr Rev 27: 101–140, 2006. [DOI] [PubMed] [Google Scholar]
- 55.Weltman A, Weltman JY, Hartman ML, Abbott RD, Rogol AD, Evans WS, Veldhuis JD. Relationship between age, percentage body fat, fitness, and 24-hour growth hormone release in healthy young adults: effects of gender. J Clin Endocrinol Metab 78: 543–548, 1994. [DOI] [PubMed] [Google Scholar]
- 56.Werner H, Koch Y, Baldino F, Gozes I. Steroid regulation of somatostatin mRNA in the rat hypothalamus. J Biol Chem 263: 7666–7671, 1988. [PubMed] [Google Scholar]
- 57.Zar JH Biostatistical Analysis. Upper Saddle River, NJ: Prentice Hall, 1996.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.