Abstract
Neuromedin U (NMU) is known to have potent actions on appetite and energy expenditure. Deletion of the NMU gene in mice leads to an obese phenotype, characterized by hyperphagia and decreased energy expenditure. Conversely, transgenic mice that overexpress proNMU exhibit reduced body weight and fat storage. Here, we show that central administration of NMU or the related peptide neuromedin S (NMS) dose-dependently decreases food intake, increases metabolic rate, and leads to significant weight loss in mice. The effects of NMU and NMS on both feeding and metabolism are almost completely lost in mice lacking the putative CNS receptor for NMU and NMS, NMUr2. However, NMUr2 knockout mice do not exhibit overt differences in body weight or energy expenditure compared with wild-type mice, suggesting that the dramatic phenotype of the NMU gene knockout mouse is not due simply to the loss of NMU/NMUr2 signaling. Putative proteolytic cleavage sites indicate that an additional peptide is produced from the NMU precursor protein, which is extremely well conserved between human, mouse, and rat. Here, we demonstrate that this peptide, proNMU104-136, has a pronounced effect on energy balance in mice. Specifically, central administration of proNMU104-136 causes a significant but transient (∼4 h) increase in feeding, yet both food intake and body weight are decreased over the following 24 h. proNMU104-136 administration also significantly increased metabolic rate. These results suggest that proNMU104-136 is a novel modulator of energy balance and may contribute to the phenotype exhibited by NMU knockout mice.
Keywords: metabolism, neuromedin S, neuromedin U receptor 2, obesity, energy expenditure
neuromedin u (NMU) is a highly conserved peptide, originally purified from porcine spinal cord and named for its ability to contract uterine smooth muscle (20, 21). However, expression of NMU has also been demonstrated in the pituitary, brain stem, gastrointestinal tract, thyroid gland, and ovary (3), suggesting biological functions of NMU beyond smooth muscle contraction. Several lines of evidence implicate NMU as a central regulator of energy balance. NMU mRNA expression within the brain is responsive to altered energy status, including fasting and obesity (7, 14, 16), and central administration of NMU results in decreased feeding concomitant with increased core body temperature, motor activity, and oxygen consumption (6, 11, 14, 16, 17, 24–26, 35).
The biological effects of NMU are mediated by two G protein-coupled receptors, NMUr1, which is expressed peripherally, and NMUr2, which is expressed primarily within the CNS. Expression of both NMU and NMUr2 is observed in brain sites that are important in regulating appetite and metabolism, including the paraventricular nucleus of the hypothalamus (PVN) (2, 4, 6–8, 14, 18, 29–31). In addition, pronounced expression of NMU mRNA is observed in the pars tuberalis of the pituitary, a structure that surrounds the hypophysial stalk (16), suggesting that NMU may be involved in hypothalamic-pituitary communication. In addition to NMU, NMUr1 and NMUr2 are activated by neuromedin S (NMS), a related neuropeptide localized exclusively to the suprachiasmatic nucleus of the hypothalamus (SCN) (15, 22), the primary circadian timing center of the brain. Like NMU, NMS is also anorexigenic when administered centrally to rats (15, 22).
An association has been demonstrated between NMU gene variants and human obesity (9), supporting a physiological role for NMU in body weight regulation. In line with this, overexpression of NMU in mice results in hypophagia, decreased body weight, and fat content (18). Furthermore, mice deficient in NMU become obese and hyperphagic and exhibit increased adiposity and decreased energy expenditure (10). Here, we demonstrate that, although the anorexic effects of NMU and NMS are absent in mice lacking NMUr2, these mice do not exhibit overt differences in body weight or metabolic rate compared with wild-type animals. We go on to show that the discrepancy between the phenotypic characteristics of mice lacking NMU expression and those lacking NMUr2 are likely to involve a second peptide derivative of the NMU gene, proNMU104-136. Our results show that this peptide, which is well conserved between human, mouse, and rat (Table 1), has a pronounced effect on energy balance in mice and may be a novel modulator of energy balance.
Table 1.
Primary peptide sequences
| Peptides | Sequences |
|---|---|
| NMU | |
| Human | FRVDEEFQSPFASQSRGYFLFRPRN-NH2 |
| Mouse | FKAEYQSPSVGQSKGYFLFRPRN-NH2 |
| NMS | |
| Human | ILQRGSGTAAVDFTKKDHTATWGRPFFLFRPRN-NH2 |
| Mouse | LPRLLRLDSRMATVDFPKKDPTTSLGRPFFLFRPRN-NH2 |
| proNMU104-136 | |
| Human | FLFHYSKTQKLGKSNVVSSVVHPLLQLVPHLHE |
| Mouse | FLFHYSKTQKLGNSNVVSSVVHPLLQLVPQLHE |
| Rat | FLFHYSKTQKLGNSNVVSSVVHPLLQLVPQLHE |
| proNMS70-103 | |
| Human | FLFHYSRTQEATHPVKTGFPPVHPLMHLAAKLAN |
| Mouse | FLFHYSRTRKPTHPVSAEFAPVHPLMRLAAKLAS |
Neuromedin (NM)U-8 is identified by the underlined region of the human NMU sequence. Boldface letters mark differences between human and rodent sequences.
MATERIALS AND METHODS
Animals and surgical procedures.
Male C57B6J mice (25–30 g) were obtained from Charles River Laboratories (Sandwich, UK). Mice with a targeted deletion of the NMUr2 gene (NMUr2−/−) and congenic wild-type mice (NMUr2+/+) were provided by Merck Research Laboratories and subsequently bred in the animal unit of the University of Manchester. All mice were provided with standard rodent chow (Beekay International, Hull, UK) and tap water ad libitum, unless stated otherwise, and housed at a constant ambient temperature of 22°C with a 12:12-h light-dark cycle.
For implantation of intracerebroventricular (icv) guide cannulae, mice were anesthetized with isoflurane (1–5% in O2) and placed in a stereotaxic frame. Guide cannulae were implanted above the lateral ventricle (0.4 mm posterior and 1.0 mm lateral to bregma) with the tip of the cannulae ∼1.0 mm ventral to the surface of the scull according to the mouse atlas of Paxinos and Franklin (27). Cannula placement was confirmed on brain sections collected at the termination of the experiments. Following surgery, animals were housed singly and allowed to recover for 7–10 days. All procedures were licensed under the UK Animals (Scientific Procedures) Act 1986 and approved by the local ethics committee.
Feeding experiments.
Mice were acclimated to handling and wire mesh-bottom cages leading up to all feeding experiments. During nocturnal feeding experiments, injections were carried out within 15 min of the commencement of the dark cycle (zeitgeber time 12; ZT12). During assessments of daytime (satiated) feeding, injections were carried out midmorning, ∼2 h after lights on (ZT2). Intracerebroventricular injections were performed on conscious animals by use of minimal restraint. Peptides, mouse NMU (0.5–2 nmol), mouse NMS (0.5–2 nmol), mouse/rat proNMU104-136 (0.5–2 nmol), all from Phoenix Pharmaceuticals Germany, or vehicle (isotonic saline, 0.9% NaCl) were delivered icv in a volume of 1 μl over a period of 30 s.
Indirect calorimetry.
For acute peptide injections, mice were acclimated to the indirect calorimetry cages (Columbus Instruments, Columbus, OH) for a minimum of 24 h, following which oxygen consumption (V̇o2), carbon dioxide production (V̇co2), and respiratory quotient (RQ) were measured every 10 min for a further 24 h. Mice were removed briefly from calorimetry cages for icv injections, which were carried out as described above. For assessment of metabolic rate in mice lacking NMUr2, age-matched NMUr2 wild-type and knockout mice were acclimated to the indirect calorimetry cages for 24 h, following which metabolic gases were sampled every 10 min for a further 4 days. Mean daily profiles of V̇o2, V̇co2, and RQ were determined for each animal on the basis of all four test days. Standard rodent chow and water were supplied ad libitum throughout these experiments.
Wheel running activity.
NMUr2−/− and NMUr2+/+ mice (n = 10 mice per genotype) were placed in cages fitted with running wheels and placed into individual light-tight boxes. Wheel running activity was monitored under 12-h light, 12-h dark (LD) lighting conditions for 7 days, at which point the animals were switched to constant darkness (DD). After ∼10 days in DD, mice were given a 1-h light pulse at circadian time 14 (CT14; 2 h into their subjective night). Diurnal and circadian wheel running activity was recorded using DataSciences software.
Immunohistochemistry.
In separate experiments, mice received either an icv injection of proNMU104-136 (2 nmol) or vehicle. Food was removed from the cages at the time of the injections. Ninety minutes after injection, mice were anesthetized with sodium pentobarbitone (0.5 g/kg; Rhone Merieux, Harlow, UK), and perfused transcardially with heparinized isotonic saline followed by 4% paraformaldehyde in 0.1 M phosphate buffer (PB). Brains were removed and postfixed for a further 24 h, equilibrated to 30% sucrose, and frozen on dry ice. Frozen 30-μm sections were cut using a sledge microtome and collected into 0.1 M PB. Free-floating sections were incubated overnight in rabbit polyclonal anti-c-Fos antibody (1:5,000; Calbiochem, Nottingham, UK), followed by biotin-conjugated goat anti-rabbit IgG antibody (1:500; Vector Laboratories, Burlingame, CA) and streptavidin-biotin-horseradish peroxidase complex (1:500; Amersham Biosciences, Little Chalfont, UK). Immunoreaction was visualized using nickel-intensified diaminobenzidine (DAB, Vector Laboratories). Sections were mounted and coverslipped and high-magnification digital pictures collected. The number of neurons expressing c-Fos immunoreactivity was counted bilaterally in brain nuclei according to the atlas of Paxinos and Franklin (27) by an observer blinded to the treatment groups. The mean number of neurons per section was determined for each animal by use of a minimum of four sections per animal for each area of interest.
Statistical analyses.
Data are presented as means ± SE. For studies involving C57B6J mice, data were analyzed using a one-way analysis of variation (ANOVA) with a Dunnet's post hoc test. A two-way ANOVA with Bonferroni's post hoc test was used in studies involving the transgenic mice.
RESULTS
NMU- and NMS-induced anorexia.
Intracerebroventricular administration of either NMU or NMS (0.5–2 nmol) at the onset of the dark period caused a significant reduction in ad libitum food consumption and body weight in C57B6J mice (Supplemental Fig. 1; n = 8–15 mice/group) (Supplementary material can be found in the online version of this article.). The COOH-terminal amino acids (FLFRPRN-NH2) are identical between NMU and NMS (Table 1) and have been shown to be required for binding of the peptides to NMUr1 and NMUr2 (12). However, administration of this segment of the peptides (NMU-8) alone did not alter food intake in the mice (data not shown).
Central administration of NMU or NMS during the day (ZT2) led to a significant increase in oxygen consumption (VO2) (Supplemental Fig. 1, D–G). The effects of NMU and NMS on metabolic rate were most pronounced in the first 4 h postadministration yet were normalised over the following 24 h. In contrast, when either peptide was administered at the start of the dark phase (ZT12), V̇o2 was not elevated above the normal nocturnal rise. However, a decrease was observed in RQ over ∼4 h postinjection, likely reflecting decreased food intake in these mice.
Interestingly, the anorexigenic and metabolic actions of NMU and NMS were attenuated during repeated administration (Supplemental Fig. 2). Specifically, both the acute (1 h postinjection) and longer-term (24 h) anorexic effects were reduced when either peptide was administered for three consecutive days (2 nmol/day delivered at ZT12). It is worth noting that 24-h food intake following repeated administration of NMS was significantly increased relative to repeated vehicle injection by day 3 and that this elevated intake was maintained on day 4, when no injections were made. Body weight of the mice paralleled food intake, with NMU- and NMS-treated mice being significantly heavier by day 4 than those treated with vehicle. The effects of the peptides on metabolic rate were also attenuated by repeat administration (data not shown).
NMUr2 knockout mouse.
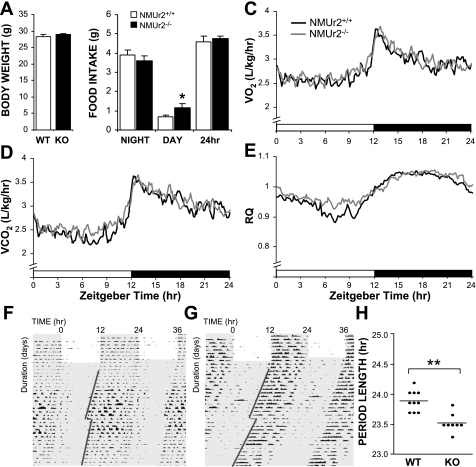
NMUr2−/− mice exhibited no overt phenotypic characteristics, and, as previously reported (36), body weights were similar between wild-type and knockout mice (body weight at 10 wk of age shown in Fig. 1A). No significant differences were observed in 24-h food intake between the mice (Fig. 1B). Nevertheless, the diurnal distribution of feeding was altered between the mice, with knockout mice consuming a greater amount of food during the light phase of the day. Daily profiles of metabolic rate were determined for both genotypes, and no pronounced differences were observed in V̇o2, V̇co2, or RQ (Fig. 1, C–E). An elevation in RQ was observed in knockout mice during the late day, possibly reflecting increased daytime food intake.
Fig. 1.
Metabolic and circadian phenotype of neuromedin U receptor 2 knockout (KO; NMUr2−/−) mice. NMUr2−/− mice exhibited normal body weight (A; body weight at 10 wk of age) and daily food intake (B) compared with congenic wild-type (WT) mice (n = 12 mice/genotype), although the KO mice did consume a greater amount of food during the light phase of the day (B; *P < 0.05, 2-way ANOVA with Bonferroni's post hoc test). Daily profiles of metabolic rate were determined for both genotypes, and no pronounced differences were observed in V̇o2 (C), V̇co2 (D), or respiratory quotient (RQ; E). Running wheel activity of WT (F) and NMUr2−/− (G) mice was monitored under both light-dark and constant dark conditions (n = 10 mice/genotype). The period of wheel running activity based on activity onset was significantly shorter in KO mice (F; **P < 0.01, Student's t-test.). No differences were observed in animals' phase-shift response to a 1-h light pulse delivered at circadian time 14 (CT14; F, G). F and G: shading in indicates dark period; red lines illustrate activity onsets from which circadian periods were calculated, with the break showing the day the light pulse was administered.
Increased diurnal feeding in mice lacking NMUr2 may reflect an alteration in circadian systems within these animals. To explore this possibility, running wheel activity was monitored under both light-dark and DD conditions (Fig. 1, G–H). Normal light entrainment was observed in both genotypes, yet under constant darkness the circadian period of knockout mice was shortened by ∼20 min (Fig. 1H). No differences were observed in the animals' phase-shift response to a 1-h light pulse delivered at CT14.
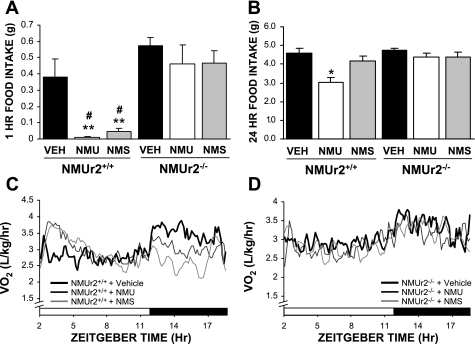
As NMUr2 is the primary receptor for NMU and NMS within the CNS, it has been assumed that the central effects of the two peptides are mediated through this receptor. This is indeed the case, as the actions of both peptides on food intake (Fig. 2, A and B) and metabolic rate (Fig. 2, C and D) were severely attenuated in mice lacking NMUr2. In light of this finding, it is surprising that NMUr2−/− mice did not exhibit a similar obese/hyperphagic phenotype to that reported for the NMU gene knockout mouse (10).
Fig. 2.
Effects of NMU and neuromedin S (NMS) on food intake and energy expenditure require NMUr2. Reduction of food intake in response to either NMU or NMS (2 nmol; n = 6 mice/group) was severely attenuated in NMUr2−/− mice (A–B). Similarly, neither peptide increased metabolic rate in NMUr2−/− mice following daytime administration (C–D). **P < 0.01 vs. vehicle-treated WT mice, #P < 0.01 vs. respective peptide-treated NMUr2−/− mice, 2-way ANOVA with Bonferroni's post hoc test.
Modification of energy balance by the NMU precursor protein-derived peptide proNMU104-136.
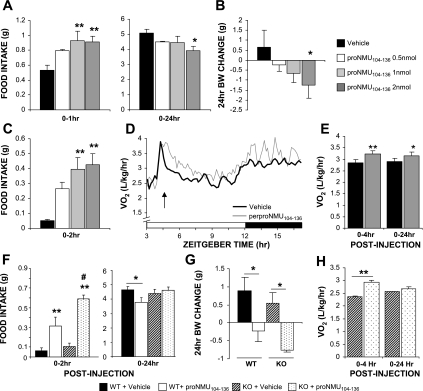
To explore the possibility that additional peptide products of the NMU gene also influence energy balance, proNMU104-136 was administered to mice. In contrast to NMU and NMS, central administration of proNMU104-136 (0.5–2 nmol) at the start of the night (ZT12) increased feeding over the short term (Fig. 3A, 1 h). The short-term orexigenic effects of proNMU104-136 were accentuated (relative to controls) when the peptide was administered to mice at ZT2, outside their normal feeding time (Fig. 3C). Nonetheless, proNMU104-136 administration ultimately reduced cumulative food intake over 24 h postinjection (Fig. 3A), which was accompanied by a significant loss in body weight (Fig. 3B). Body weight loss was similar in magnitude to that observed following NMU administration. In line with the reduction in body weight, proNMU104-136 administration caused a significant increase in V̇o2that was sustained over 24 h postinjection (Fig. 3, D and E). Short-term orexigenic effects of proNMU104-136 on food intake were maintained in NMUr2−/− mice, although no decrease in food intake was observed 24 h after injection in these mice (Fig. 3F; n = 5–6 mice/group, ZT2). Nevertheless, proNMU104-136 administration reduced body weight (Fig. 4G) and elevated metabolic rate (Fig. 3H) in the NMUr2−/− mice.
Fig. 3.
Actions of proNMU104-136 on food intake, body weight, and energy expenditure. Intracerebroventricular administration of proNMU104-136 (0.5–2 nmol; n = 6 mice/group) at zeitgeber time 12 (ZT12) caused a short-term increase in feeding; yet, over 24 h postinjection, food intake was significantly reduced (A). Decreased feeding following proNMU104-136 administration was accompanied by a significant loss of body weight (B). Short-term orexigenic effects of proNMU104-136 were maintained when the peptide was administered to mice at ZT2, outside their normal feeding time (C). proNMU104-136 administration also caused a significant increase in V̇o2, which was sustained over 24 h postinjection (D–E). *P < 0.05, **P < 0.01, 1-way ANOVA with Dunnet's post hoc test. Short-term orexigenic effects of proNMU104-136 on food intake were maintained in NMUr2−/− mice (F). Although no decrease in food intake was observed in KO mice 24 h after injection (F), proNMU104-136 administration did reduce body weight in these animals (G). proNMU104-136-induced elevation in metabolic rate was maintained in NMUr2−/− mice (H); n = 5–6 mice/group. **P < 0.01 vs. respective vehicle-treated group, #P < 0.01 vs. peptide-treated WT mice, 2-way ANOVA with Bonferroni's post hoc test.
Fig. 4.
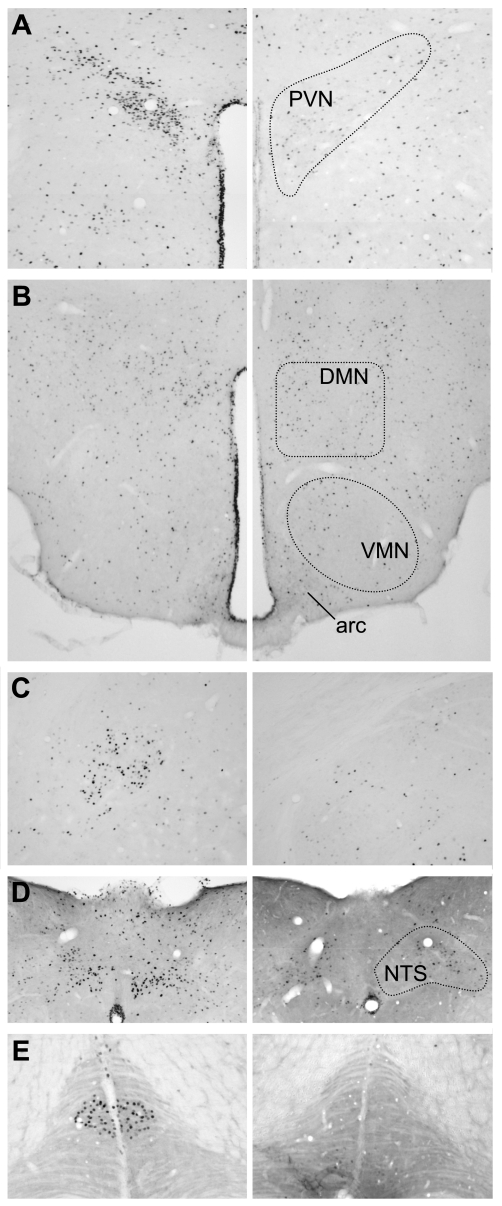
proNMU104-136-induced c-Fos expression. Representative photomicrographs of c-Fos immunoreactivity in mice treated with vehicle (right) or and proNMU104-136 (left) 90 min postinjection. Increased c-Fos immunoreactive profiles were observed in the PVN (A), DMN (B), central amygdala (C), and the lining of the 3rd ventricle (A–B) proNMU104-136 administration. Within the medulla, intense c-Fos expression was observed in the NTS (D) and inferior olive (E) of peptide-treated mice. No significant increases in c-Fos expression were observed in the arcuate, ventormedial, or lateral hypothalamus, locus coeruleus, or parabrachial nucleus; n = 5 mice/group.
Expression of c-Fos protein was used to determine the extent to which brain areas important in energy balance may be activated following proNMU104-136 administration. Increased numbers of c-Fos immunoreactive profiles were observed in the paraventricular (PVN; vehicle: 129 ± 15 cells/section, proNMU104-136: 516 ± 58) or dorsomedial (DMN; vehicle: 171 ± 17 cells/section, proNMU104-136: 304 ± 51) hypothalamic nuclei, central amygdala (vehicle: 60 ± 7 cells/section, proNMU104-136: 153 ± 34), and the lining of the third ventricle (Fig. 4), whereas no significant increases were observed in other areas of the hypothalamus, including the arcuate, ventromedial, and lateral hypothalamic areas. Within the medulla, intense peptide-induced c-Fos expression was observed in the nucleus of the tractus solitarius (NTS) and inferior olive (Fig. 4), both areas in which we have previously shown NMU to be expressed (16).
DISCUSSION
The actions of NMU and the related peptide NMS on appetite and energy balance are well established (14–17, 22, 24, 25, 35). As we show here, both peptides dramatically reduce food intake, elevate metabolic rate, and lead to body weight loss when administered centrally to mice. In addition, disruption of the NMU gene leads to hyperphagia, hypometabolism, and obesity (10), strongly suggesting a physiological role for NMU signaling in the regulation of energy balance and body weight. Signaling of NMU and NMS within the CNS is dependent on the receptor NMUr2 (5, 13, 14, 29, 30, 32). Therefore, one would expect the phenotype of NMUr2−/− mice to parallel that of the NMU−/−, yet this is not the case. As we show, NMUr2−/− mice exhibit relatively normal patterns of growth, food intake, and metabolic profiles compared with congenic wild-type mice. It was recently reported that NMUr2−/− mice eat slightly less over an 8-wk period and are partially resistant to diet-induced obesity (28), although the differences are only mild and were not observed in our study. The maintenance of normal body weight regulation in the NMUr2−/− mice does not appear to involve alternate NMU/NMS signaling pathways within the brain, as the anorexic and metabolic effects of both peptides are lost in the NMUr2−/− mice. In addition, specific binding of radiolabeled NMU is limited to the cerebellum in brain tissue derived from NMUr2−/− mice (34). It remains possible that other pathways compensate for the loss of NMUr2 signaling, although it is unclear why similar compensation would not occur in NMU−/− mice.
It is possible that the obese phenotype associated with the NMU−/− mouse may be due principally to a loss of NMU signaling via NMUr1 in the periphery. However, abnormal body weight profiles have not been reported in NMUr1−/− or NMUr1−/−/NMUr2−/− mice (1, 33).
The NMU precursor protein contains putative proteolytic processing sites that, in addition to NMU, generate the novel peptide proNMU104-136 (19). Our present studies demonstrate that proNMU104-136 is capable of modifying energy balance and body weight. Specifically, proNMU104-136 administration led to decreased food intake and body weight over 24 h postinjection, which was accompanied by an increase in V̇o2. This peptide is unrelated to NMU in sequence but has been identified in rat brain and small intestine extracts, suggesting that it constitutes a mature cleavage product (22). As we have shown, proNMU104-136 does not appear to signal through NMUr2, and no potential receptors for this peptide have been identified. Nevertheless, central administration of the peptide elicited an induction of c-Fos in a number of areas important in regulating energy balance, such as the PVN and NTS. The magnitude of weight loss following proNMU104-136 was similar to that of NMU or NMS, and together these results suggest that the loss of proNMU104-136 contributes to the obese phenotype of the NMU−/− mouse. An important physiological role for proNMU104-136 is indicated by the near-perfect sequence conservation between rat, mouse, and human (Table 1).
Interestingly, proNMU104-136 was also associated with a short-lived yet potent orexigenic action in the mice. This suggests that the two peptides (NMU and proNMU104-136) have opposing acute effects on feeding behavior. Such a counterbalance between the two NMU gene products may be important in limiting their effects in short-term aspects of feeding (such as in determination of meal duration/termination) yet reinforce long-lasting alterations to energy homeostasis, such as during situations of chronic positive or negative energy balance. NMU mRNA expression is responsive to energy status, being upregulated during positive energy balance (Zucker rats, ob/ob mice) and downregulated by fasting (7, 14, 16). Both NMU and proNMU104-136 exert a stimulatory effect on metabolic rate, which in the case of proNMU104-136 was maintained over 24 h postinjection. Therefore, modification of metabolic rate may be the primary action of the NMU-derived peptides. The effects of proNMU104-136 on energy expenditure and body weight were maintained in the NMUr2−/− mice; so perhaps in the NMUr2−/− mouse the loss of one (NMU signaling via NMUr2) is easily compensated for by the other (proNMU104-136 via an as yet unidentified receptor).
Circadian differences in NMUr2−/− mice were revealed in our studies. Specifically, NMUr2−/− mice exhibited a short period of wheel running activity under constant darkness and an increased proportion of daytime food intake. Arrhythmic feeding and locomotor behavior have been previously reported for the NMU knockout mouse (10). A role for NMU and NMS in circadian regulation of feeding behavior is plausible, and both NMU and NMS are capable of phase-shifting circadian rhythms in wheel running activity when administered in the subjective day (advance in activity onsets) or subjective night (delay in onsets) (22, 23). The increased feeding during the daytime in our NMUr2−/− mice is unlikely to be due to a widespread advancement of circadian behavior, since the diurnal patterns of energy expenditure were not similarly advanced. Nevertheless, it has been reported that both NMU and NMUr2 show diurnal rhythms in expression within the SCN (23), raising the possibility that NMU-mediated effects on energy balance may be under circadian control. This would be especially interesting should the receptor for proNMU104-136 exhibit some diurnal rhythm in expression.
In conclusion, we demonstrate for the first time that proNMU104-136 causes significant modifications of feeding behavior, body weight, and metabolic rate in mice. These results suggest that proNMU104-136 is a novel modulator of energy balance and, given the extreme sequence conservation of this peptide, may be an important factor in human metabolic regulation.
GRANTS
This work was supported by The Wellcome Trust and the Biotechnology and Biosciences Research Council.
Supplementary Material
Acknowledgments
We thank Prof. Andrew Loudon for the use of the wheel-running equipment, Jake Lebiecki for technical assistance, and Merck Research Laboratories for providing the NMUr2 knockout mice.
REFERENCES
- 1.Abbondanzo SJ, Manfra DJ, Chen SC, Pinzon-Ortiz M, Sun Y, Phillips JE, Laverty M, Vassileva G, Hu W, Yang S, Gustafson EL, Fine JS, Hedrick JA. Nmur1−/− mice are not protected from cutaneous inflammation. Biochem Biophys Res Commun 378: 777–782, 2009. [DOI] [PubMed] [Google Scholar]
- 2.Ballesta J, Carlei F, Bishop AE, Steel JH, Gibson SJ, Fahey M, Hennessey R, Domin J, Bloom SR, Polak JM. Occurrence and developmental pattern of neuromedin U-immunoreactive nerves in the gastrointestinal tract and brain of the rat. Neuroscience 25: 797–816, 1988. [DOI] [PubMed] [Google Scholar]
- 3.Brighton PJ, Szekeres PG, Willars GB. Neuromedin U and its receptors: structure, function, and physiological roles. Pharmacol Rev 56: 231–248, 2004. [DOI] [PubMed] [Google Scholar]
- 4.Domin J, Ghatei MA, Chohan P, Bloom SR. Neuromedin U—a study of its distribution in the rat. Peptides 8: 779–784, 1987. [DOI] [PubMed] [Google Scholar]
- 5.Fujii R, Hosoya M, Fukusumi S, Kawamata Y, Habata Y, Hinuma S, Onda H, Nishimura O, Fujino M. Identification of neuromedin U as the cognate ligand of the orphan G protein-coupled receptor FM-3. J Biol Chem 275: 21068–21074, 2000. [DOI] [PubMed] [Google Scholar]
- 6.Gartlon J, Szekeres P, Pullen M, Sarau HM, Aiyar N, Shabon U, Michalovich D, Steplewski K, Ellis C, Elshourbagy N, Duxon M, Ashmeade TE, Harrison DC, Murdock P, Wilson S, Ennaceur A, Atkins A, Heidbreder C, Hagan JJ, Hunter AJ, Jones DN. Localisation of NMU1R and NMU2R in human and rat central nervous system and effects of neuromedin-U following central administration in rats. Psychopharmacology 177: 1–14, 2004. [DOI] [PubMed] [Google Scholar]
- 7.Graham ES, Turnbull Y, Fotheringham P, Nilaweera K, Mercer JG, Morgan PJ, Barrett P. Neuromedin U and Neuromedin U receptor-2 expression in the mouse and rat hypothalamus: effects of nutritional status. J Neurochem 87: 1165–1173, 2003. [DOI] [PubMed] [Google Scholar]
- 8.Guan XM, Yu H, Jiang Q, Van Der Ploeg LH, Liu Q. Distribution of neuromedin U receptor subtype 2 mRNA in the rat brain. Brain Res 1: 1–4, 2001. [DOI] [PubMed] [Google Scholar]
- 9.Hainerova I, Torekov SS, Ek J, Finkova M, Borch-Johnsen K, Jorgensen T, Madsen OD, Lebl J, Hansen T, Pedersen O. Association between neuromedin U gene variants and overweight and obesity. J Clin Endocrinol Metab 91: 5057–5063, 2006. [DOI] [PubMed] [Google Scholar]
- 10.Hanada R, Teranishi H, Pearson JT, Kurokawa M, Hosoda H, Fukushima N, Fukue Y, Serino R, Fujihara H, Ueta Y, Ikawa M, Okabe M, Murakami N, Shirai M, Yoshimatsu H, Kangawa K, Kojima M. Neuromedin U has a novel anorexigenic effect independent of the leptin signaling pathway. Nat Med 10: 1067–1073, 2004. [DOI] [PubMed] [Google Scholar]
- 11.Hanada T, Date Y, Shimbara T, Sakihara S, Murakami N, Hayashi Y, Kanai Y, Suda T, Kangawa K, Nakazato M. Central actions of neuromedin U via corticotropin-releasing hormone. Biochem Biophys Res Commun 311: 954–958, 2003. [DOI] [PubMed] [Google Scholar]
- 12.Hashimoto T, Masui H, Uchida Y, Sakura N, Okimura K. Agonistic and antagonistic activities of neuromedin U-8 analogs substituted with glycine or d-amino acid on contractile activity of chicken crop smooth muscle preparations. Chem Pharmaceut Bull 39: 2319–2322, 1991. [DOI] [PubMed] [Google Scholar]
- 13.Hosoya M, Moriya T, Kawamata Y, Ohkubo S, Fujii R, Matsui H, Shintani Y, Fukusumi S, Habata Y, Hinuma S, Onda H, Nishimura O, Fujino M. Identification and functional characterization of a novel subtype of neuromedin U receptor. J Biol Chem 275: 29528–29532, 2000. [DOI] [PubMed] [Google Scholar]
- 14.Howard AD, Wang R, Pong SS, Mellin TN, Strack A, Guan XM, Zeng Z, Williams DL Jr Feighner SD, Nunes CN, Murphy B, Stair JN, Yu H, Jiang Q, Clements MK, Tan CP, McKee KK, Hreniuk DL, McDonald TP, Lynch KR, Evans JF, Austin CP, Caskey CT, Van der Ploeg LH, Liu Q. Identification of receptors for neuromedin U and its role in feeding. Nature 406: 70–74, 2000. [DOI] [PubMed] [Google Scholar]
- 15.Ida T, Mori K, Miyazato M, Egi Y, Abe S, Nakahara K, Nishihara M, Kangawa K, Murakami N. Neuromedin s is a novel anorexigenic hormone. Endocrinology 146: 4217–4223, 2005. [DOI] [PubMed] [Google Scholar]
- 16.Ivanov TR, Lawrence CB, Stanley PJ, Luckman SM. Evaluation of neuromedin U actions in energy homeostasis and pituitary function. Endocrinology 143: 3813–3821, 2002. [DOI] [PubMed] [Google Scholar]
- 17.Ivanov TR, Le Rouzic P, Stanley PJ, Ling WY, Parello R, Luckman SM. Neuromedin U neurones in the rat nucleus of the tractus solitarius are catecholaminergic and respond to peripheral cholecystokinin. J Neuroendocrinol 16: 612–619, 2004. [DOI] [PubMed] [Google Scholar]
- 18.Kowalski TJ, Spar BD, Markowitz L, Maguire M, Golovko A, Yang S, Farley C, Cook JA, Tetzloff G, Hoos L, Del Vecchio RA, Kazdoba TM, McCool MF, Hwa JJ, Hyde LA, Davis H, Vassileva G, Hedrick JA, Gustafson EL. Transgenic overexpression of neuromedin U promotes leanness and hypophagia in mice. J Endocrinol 185: 151–164, 2005. [DOI] [PubMed] [Google Scholar]
- 19.Lo G, Legon S, Austin C, Wallis S, Wang Z, Bloom SR. Characterization of complementary DNA encoding the rat neuromedin U precursor. Mol Endocrinol (Baltimore) 6: 1538–1544, 1992. [DOI] [PubMed] [Google Scholar]
- 20.Minamino N, Kangawa K, Matsuo H. Neuromedin U-8 and U-25: novel uterus stimulating and hypertensive peptides identified in porcine spinal cord. Biochem Biophys Res Commun 130: 1078–1085, 1985. [DOI] [PubMed] [Google Scholar]
- 21.Minamino N, Sudoh T, Kangawa K, Matsuo H. Neuromedins: novel smooth-muscle stimulating peptides identified in porcine spinal cord. Peptides 6, Suppl 3: 245–248, 1985. [DOI] [PubMed] [Google Scholar]
- 22.Mori K, Miyazato M, Ida T, Murakami N, Serino R, Ueta Y, Kojima M, Kangawa K. Identification of neuromedin S and its possible role in the mammalian circadian oscillator system. EMBO J 24: 325–335, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nakahara K, Hanada R, Murakami N, Teranishi H, Ohgusu H, Fukushima N, Moriyama M, Ida T, Kangawa K, Kojima M. The gut-brain peptide neuromedin U is involved in the mammalian circadian oscillator system. Biochem Biophys Res Commun 318: 156–161, 2004. [DOI] [PubMed] [Google Scholar]
- 24.Nakazato M, Hanada R, Murakami N, Date Y, Mondal MS, Kojima M, Yoshimatsu H, Kangawa K, Matsukura S. Central effects of neuromedin U in the regulation of energy homeostasis. Biochem Biophys Res Commun 277: 191–194, 2000. [DOI] [PubMed] [Google Scholar]
- 25.Niimi M, Murao K, Taminato T. Central administration of neuromedin U activates neurons in ventrobasal hypothalamus and brainstem. Endocrine 16: 201–206, 2001. [DOI] [PubMed] [Google Scholar]
- 26.Novak CM, Zhang M, Levine JA. Neuromedin U in the paraventricular and arcuate hypothalamic nuclei increases non-exercise activity thermogenesis. J Neuroendocrinol 18: 594–601, 2006. [DOI] [PubMed] [Google Scholar]
- 27.Paxinos G, Franklin KBJ. The Mouse Brain. San Diego, CA: Academic, 2001.
- 28.Peier A, Kosinski J, Cox-York K, Qian Y, Desai K, Feng Y, Trivedi P, Hastings N, Marsh DJ. The anti-obesity effects of centrally administered Neuromedin U and Neuromedin S are mediated predominantly by the Neuromedin U receptor 2 (NMUR2). Endocrinology 2009. Mar 26. [Epub ahead of print] PMID: 19324999. [DOI] [PMC free article] [PubMed]
- 29.Raddatz R, Wilson AE, Artymyshyn R, Bonini JA, Borowsky B, Boteju LW, Zhou S, Kouranova EV, Nagorny R, Guevarra MS, Dai M, Lerman GS, Vaysse PJ, Branchek TA, Gerald C, Forray C, Adham N. Identification and characterization of two neuromedin U receptors differentially expressed in peripheral tissues and the central nervous system. J Biol Chem 275: 32452–32459, 2000. [DOI] [PubMed] [Google Scholar]
- 30.Shan L, Qiao X, Crona JH, Behan J, Wang S, Laz T, Bayne M, Gustafson EL, Monsma FJ Jr, Hedrick JA. Identification of a novel neuromedin U receptor subtype expressed in the central nervous system. J Biol Chem 275: 39482–39486, 2000. [DOI] [PubMed] [Google Scholar]
- 31.Steel JH, Van Noorden S, Ballesta J, Gibson SJ, Ghatei MA, Burrin J, Leonhardt U, Domin J, Bloom SR, Polak JM. Localization of 7B2, neuromedin B, and neuromedin U in specific cell types of rat, mouse, and human pituitary, in rat hypothalamus, and in 30 human pituitary and extrapituitary tumors. Endocrinology 122: 270–282, 1988. [DOI] [PubMed] [Google Scholar]
- 32.Szekeres PG, Muir AI, Spinage LD, Miller JE, Butler SI, Smith A, Rennie GI, Murdock PR, Fitzgerald LR, Wu H, McMillan LJ, Guerrera S, Vawter L, Elshourbagy NA, Mooney JL, Bergsma DJ, Wilson S, Chambers JK. Neuromedin U is a potent agonist at the orphan G protein-coupled receptor FM3. J Biol Chem 275: 20247–20250, 2000. [DOI] [PubMed] [Google Scholar]
- 33.Torres R, Croll SD, Vercollone J, Reinhardt J, Griffiths J, Zabski S, Anderson KD, Adams NC, Gowen L, Sleeman MW, Valenzuela DM, Wiegand SJ, Yancopoulos GD, Murphy AJ. Mice genetically deficient in neuromedin U receptor 2, but not neuromedin U receptor 1, have impaired nociceptive responses. Pain 130: 267–278, 2007. [DOI] [PubMed] [Google Scholar]
- 34.Trivedi PG, Marsh DJ, Reitman ML, Guan XM, Hastings NB. Comparative distribution of [125I]-hNMU-25 binding sites in the rat and mouse brain: a receptor binding autoradiography study. Neuroscience Meeting Planner San Diego, CA: Society for Neuroscience Program No 524.7: Online, 2007.
- 35.Wren AM, Small CJ, Abbott CR, Jethwa PH, Kennedy AR, Murphy KG, Stanley SA, Zollner AN, Ghatei MA, Bloom SR. Hypothalamic actions of neuromedin U. Endocrinology 143: 4227–4234, 2002. [DOI] [PubMed] [Google Scholar]
- 36.Zeng H, Gragerov A, Hohmann JG, Pavlova MN, Schimpf BA, Xu H, Wu LJ, Toyoda H, Zhao MG, Rohde AD, Gragerova G, Onrust R, Bergmann JE, Zhuo M, Gaitanaris GA. Neuromedin U receptor 2-deficient mice display differential responses in sensory perception, stress, and feeding. Mol Cell Biol 26: 9352–9363, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.