Abstract
Sonic hedgehog (SHH) plays an important role in postnatal tissue repair. The present study tested the hypothesis that impaired SHH pathway results in delayed wound healing by suppressing cutaneous nitric oxide (NO) function in type 1 diabetes. Adult male C57/B6 mice and streptozotocin (STZ)-induced type 1 diabetic mice were used. Although cutaneous SHH and Patched-1 (Ptc-1 encoded by PTCH, PTCH 1) proteins were increased significantly on day 4 after wounding compared with day 0 in normal mice, both were decreased significantly in STZ-induced diabetic mice. Topical application of SHH restored wound healing delay in STZ-induced diabetic mice, with a concomitant augmentation of both cutaneous constitutive nitric oxide synthase (NOS) activity and nitrite level. The effects of SHH on wound healing and cutaneous NO function were markedly inhibited by SHH receptor inhibitor cyclopamine. After 24-h treatment in vitro, SHH (5–20 μg/ml) significantly increased cutaneous endothelial NOS protein expression, NOS activity and NO level in normal mice and STZ-induced diabetic mice in a concentration-dependent manner, an effect that was blunted by cyclopamine and NOS inhibitor Nω-nitro-l-arginine methyl ester. The phosphatidylinositol 3-kinase inhibitor LY-294002 significantly blunted the increase of NOS activity and NO level induced by SHH treatment in human umbilican vein endothelial cells. These results demonstrate that the SHH pathway is activated in a normal wound, and its reduction results in impaired NO function and wound healing in diabetes. Strategies aimed at augmenting the endogenous SHH pathway may provide an effective means in ameliorating delayed diabetic wound healing.
Keywords: wound healing, sonic hedgehog, nitric oxide, diabetes
wound healing impairment in diabetic patients represents a particularly challenging clinical problem in which there is currently no efficacious treatment regimens (15). The etiology of delayed wound healing in diabetes is multifaceted, whereby vasculopathy and neuropathy are major contributors (15). However, the cellular mechanisms underlying refractory wounds in diabetes are incompletely understood.
Hedgehog proteins act as morphogens in many tissues during embryonic development (13, 29). Sonic hedgehog (SHH) is a member of the hedgehog protein family consisting of SHH, Indian hedgehog, and desert hedgehog (2, 4, 8, 17, 22). The mature forms of hedgehog are 19-kDa proteins that interact with heparin through an NH2-terminal basic domain and are tethered to the cell membrane through cholesterol and fatty acyl modification (19, 27). Hedgehog acts upon mesoderm in epithelial-mesenchymal interactions that are crucial to the formation of limb, lung, gut, hair follicles, and bone (13, 20, 29). Among the three highly conserved mammalian hedgehog genes, SHH is the most widely expressed during development (17, 28). SHH deficiency in mice is embryonically lethal, leading to multiple defects beginning in early to midgestation (12, 21). Hedgehog signaling occurs through the interaction of hedgehog protein with its receptor, Patched-1 (Ptc-1 encoded by PTCH) (3). This leads to activation of a transcription factor, Gli, which induces expression of downstream target genes, including PTCH and Gli themselves (18).
There is growing evidence that hedgehog pathway plays a pivotal role in the maintenance of skin tissue homeostasis (1, 9, 11). SHH promotes proliferation of human keratinocytes in vitro and in situ (5, 14). The regulatory role of SHH in cell proliferation during development suggests that this molecule may also participate in modulation of tissue repair (6, 10). Indeed, the SHH/PTCH/Gli cascade underlies the tissue repair process of airway epithelium and epidermis (24, 25). Nuclear translocation of Gli-1 associated with SHH upregulation was detected in cells of airway epithelium at day 1 of healing after an epithelial injury caused by naphthalene inhalation. Recent studies have shown that SHH pathway was impaired in nerve tissues and in the penis of diabetic rats, and such impairment was responsible for neural and phallic dysfunction (8, 22). Furthermore, another has shown that SHH accelerated wound healing in diabetic mice by enhancing endothelial cell-mediated microvascular remodeling (2). However, how SHH signaling pathway regulates cutaneous tissue repair in diabetes is not completely understood.
There is increasing evidence for a functional role of nitric oxide (NO) in wound healing (19, 27). SHH is capable of inducing the activation of the phosphatidylinositol 3-kinase (PI 3-kinase) pathway, which is necessary for endothelial NO synthase (eNOS) activation (16). Therefore, in this study, we tested the hypothesis that impaired SHH pathway results in delayed wound healing by suppressing cutaneous NO function in type 1 diabetes, which may be rescued by topical SHH application. Our results demonstrate that: 1) normal wounding induces activation of the SHH pathway that is necessary for wound healing, 2) cutaneous SHH pathway is impaired in type 1 diabetes, and 3) administration of exogenous SHH accelerates diabetic wound healing by enhancing cutaneous NO function.
MATERIALS AND METHODS
Induction of type 1 diabetes.
Male C57/B6 mice (Charles River Breeding Laboratories, Portage, MI) of 8–12 wk of age were injected intraperitoneally with streptozotocin (STZ; Sigma Chemical, St. Louis, MO) dissolved in sterile citrate buffer (0.05 mol/l sodium citrate, pH 4.5, 45 mg/kg). STZ or citrate buffer (control) was administered for five consecutive days during the first week of the study (19, 23). Blood was collected from the dorsal vein of the mouse hindfoot using Microvette blood collection tubes (CB 300 LH; Microvette, Sarstedt, Germany). Whole blood glucose levels were measured by a glucose analyzer (2300 stat plus analyzer; YSI Incorporated, Yellow Springs, OH). Mice with a blood glucose level >280 mg/dl were considered diabetic and used for wound experiments (23).
Full-thickness excisional wound model.
Adult male C57/BL6 mice were anesthetized with ketamine (100 mg/kg) and xylazine (10 mg/kg) (Sigma Chemical) intraperitoneally, and the dorsum was clipped free of hair. A full-thickness excisional wound, including the panniculus carnosus, was created with fine scissors as described (19, 27). Wounds were dressed with a clear, bioclusive transparent dressing (Johnson & Johnson, Arlington, TX), and wound closure rates were measured by tracing the wound area every other day on the bioclusive dressing. The tracings were digitized, and the areas were calculated in a blind fashion using a computerized algorithm (Sigma Scan; Jandel Scientific, San Raphael, CA) (19, 27). To investigate the effect of SHH pathway on wound healing, solution of SHH (5–20 μg/ml; R&D System, Minneapolis, MN) or SHH receptor inhibitor cyclopamine (5 nmol/l; Toronto Research Chemicals, Toronto, Ontario, Canada) was directly placed on the wound surface for 30 min every other day. All experiments were approved by the Institutional Animal Care and Use Committees at the University of Pittsburgh and Guangzhou Medical University.
Western blot analysis.
Western blot analysis for proteins of SHH, PTCH 1, and eNOS were performed as described (19, 22). Briefly, skin tissues were homogenized in lysis buffer [0.5 mol/l Tris·HCl (pH 6.8), 10% SDS, 10% glycerol] with protease inhibitors [0.5 mmol/l phenylmethylsulfonyl fluoride, 10 μg/ml aprotinin, and 10 μg/ml leupeptin (all from Sigma Chemical)]. Homogenates were centrifuged (11,000 g for 10 min, 4°C), and the protein supernatants were measured. Equal amounts of protein (8.2 μg) were separated on SDS-polyacrylamide gels (10%) and electrotransferred to polyvinylidene difluoride membrane (Bio-Rad Laboratories, Hercules, CA). Membranes were incubated with either SHH (250 μl/ml; Santa Cruz Biotechnology, Santa Cruz, CA), PTCH 1 (250 μl/ml; Santa Cruz), eNOS(1:2,500; BD Transduction Laboratories, Two Oak Park, MA) or β-actin (Sigma Chemical) antibodies for 18 h at 4°C (22). Membranes were washed three times for 15 min each with PBS-Tween before incubation with mouse anti-goat antibody (1:2,000; BD Transduction Laboratories) for 4 h at room temperature. Blots were developed using supersignal west pico chemiluminescent substrate (Pierce Manufacturing). Molecular band intensity was determined by densitometry (Bio-Rad image software).
RIA of NOS activity.
The skin tissues were homogenized and centrifuged, and supernatants were subjected to NOS activity assay with a commercial NOS RIA kit (Calbiochem, San Diego, CA). Aliquots of supernatants were incubated with [3H]arginine (10 μmol/l final arginine, 62 Ci/mmol; Amersham) in the presence of 1 mmol/l NADPH, 3 μmol/l tetrahydrobiopterin, 600 μmol/l CaCl2, 1 μmol/l FAD, and 1 μmol/l FMN in a final volume of 50 μl for 60 min at room temperature (17, 19). The reaction was quenched with 400 μl of stop buffer (50 mmol/l HEPES and 5 mmol/l EDTA, pH 5.5). Experiments were also performed in the presence of either EGTA (5.0 mmol/l) or Nω-nitro-l-arginine methyl ester (l-NAME, 1 mmol/l) (17, 19). After resin was added, the reaction mixtures were transferred to spin cups and centrifuged. l-[3H]citrulline content in eluate was determined by liquid scintillation counting. Samples of buffer containing l-[3H]arginine in the absence of skin tissue were served as background counts, which were subtracted from all measurements. Constitutive NOS (eNOS and neuronal NOS) activity was determined by subtracting total counts from both l-NAME- and calcium chelator EGTA-blocked counts and normalized for protein content (measured by the Bradford assay).
Nitrite measurement.
The skin tissue was cut into small pieces that were used to assay the nitrite level with Griess reagents (Sigma Chemical). After incubation with Eagle's minimal essential medium (EMEM) in a CO2 incubator for 24 h, the wound tissue was weighed, and the NO stable metabolite nitrite concentration in EMEM was determined (19).
Statistical analysis.
Data were presented as means ± SE. Experimental means were subjected to unpaired Student's t-test and one-way ANOVA with the Newman-Keul's Multiple Comparison Test. A P value of <0.05 was considered statistically significant.
RESULTS
SHH signaling pathway was activated during wounding in normal mice.
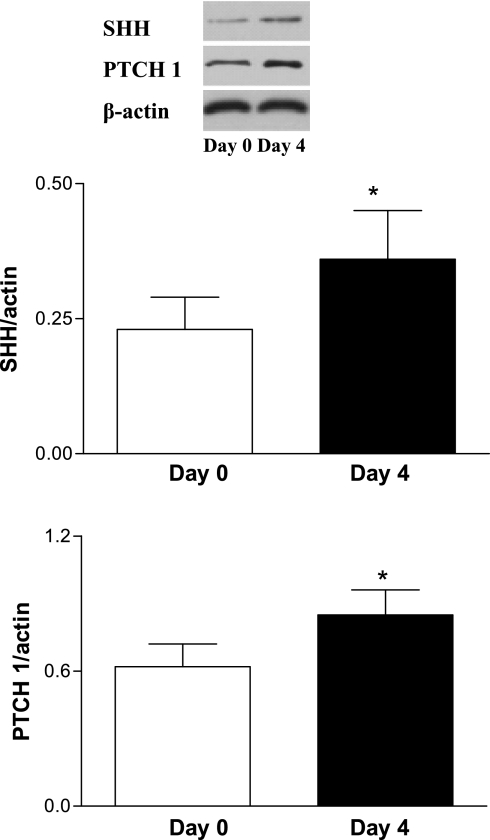
Western blot analysis for SHH and PTCH 1 was performed on day 0 and day 4 after wounding. Figure 1 showed that SHH and PTCH 1 protein levels in skin tissues were increased significantly on day 4 after wounding compared with day 0, reflecting activation of the SHH pathway.
Fig. 1.
Activation of wound sonic hedgehog (SHH) pathway in normal mice. A full-thickness excisional wound (1.5 × 1.5 cm) was made. Western blot analysis of cutaneous SHH and Patched-1 (PTCH 1) was performed on day 0 and day 4 after wounding. Values were presented as means ± SE; n = 5 mice in each group. *P < 0.05 vs. day 0.
Activation of SHH pathway contributed to wound healing in normal mice.
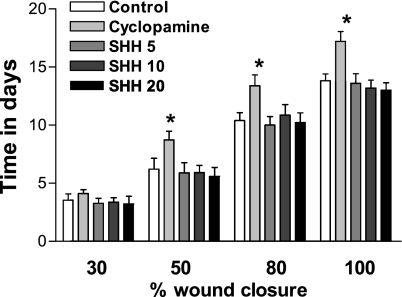
Next, we investigated the impact of blockade or enhancement of SHH pathway on wound healing. The solution of SHH (5–20 μg/ml) or SHH receptor inhibitor cyclopamine (5 nmol/l) was directly placed on the wound surface for 30 min every other day from day 1 after wounding, and wound closure rates were measured. Three doses (5, 10, and 20 μg/ml) of SHH had no effects on wound healing in normal mice. However, cyclopamine significantly decreased the wound healing in normal mice (Fig. 2). These results suggest that activation of SHH pathway is necessary for the wound healing in normal mice, and the physiological SHH pathway induced by wounding per se is sufficient for normal wound healing.
Fig. 2.
Blockade of SHH pathway on wound healing in normal mice. The solution of SHH (5–20 μg/ml) or selective SHH receptor inhibitor cyclopamine (5 nmol/l) was directly placed on the wound surface for 30 min every other day from day 1 after wounding. The rate of wound closure was monitored every other day by tracing the wound area. Values were presented as means ± SE; n = 5. *P < 0.05 vs. control.
Endogenous SHH and PTCH 1 proteins were decreased in STZ-induced diabetic mice.
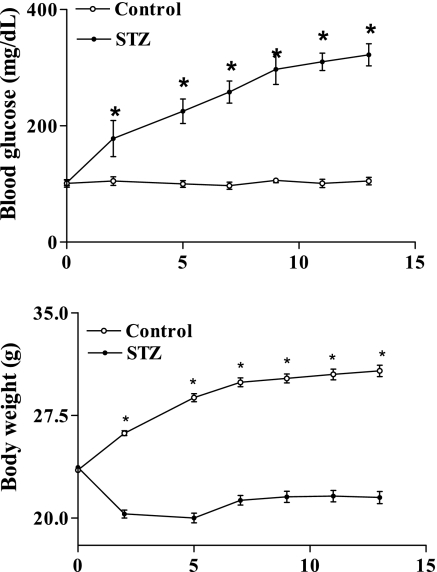
We used STZ-induced diabetic mice to study the role of SHH pathway in diabetic wound healing. A 5-day low-dose STZ injection regimen was used to ensure 13 wk of sustained hyperglycemia, which revealed impaired wound healing (19). With the use of such a regimen, STZ-treated mice maintained hyperglycemia and experienced body weight loss throughout the study (Fig. 3).
Fig. 3.
Whole blood glucose concentration (top) and body weight (bottom) in streptozotocin (STZ)-induced diabetic mice (•, 45 mg/kg, 5 days) and control mice (○, citrate buffer). Values were presented as means ± SE; n = 17 for STZ and n = 7 for control mice. *P < 0.05 vs. control.
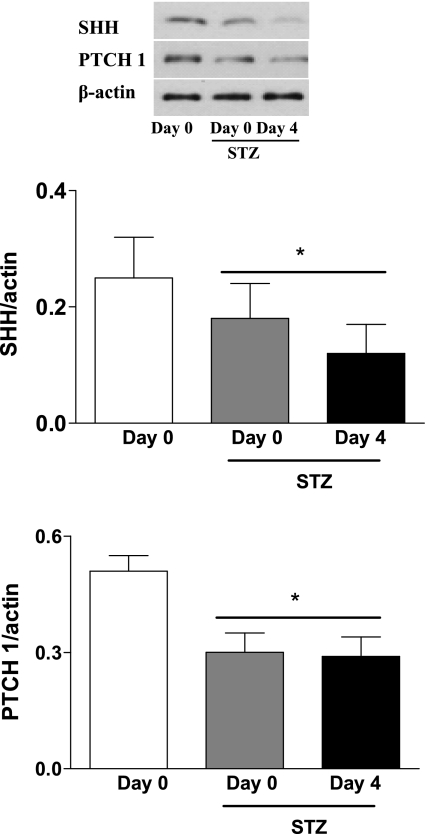
Endogenous SHH and PTCH 1 protein levels in skin tissues were decreased significantly in STZ diabetic mice compared with normal mice (Fig. 4). Specially, there was significant impairment of wound-induced endogenous SHH and PTCH 1 protein expressions during the wound healing process in STZ mice compared with normal controls. These data indicate that the cutaneous function of SHH pathway is reduced in STZ mice, which may be an important factor responsible for impaired wound healing.
Fig. 4.
Decrease of endogenous SHH and PTCH 1 protein expressions in STZ-induced diabetic mice. Western blot analysis of cutaneous SHH and PTCH 1 was performed on day 0 and day 4 after wounding. Values were presented as means ± SE; n = 5. *P < 0.05 vs. day 0 (control).
Exogenous SHH improved wound healing in STZ-induced diabetic mice.
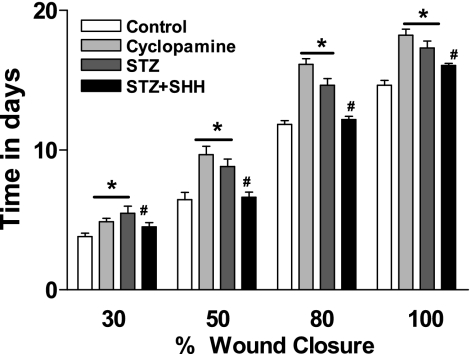
The rate of wound closure in STZ-induced diabetic mice to a 100% closure point was delayed by 29.6% compared with that of the control mice, as shown in Fig. 5. Direct application of SHH significantly accelerated the closure rate, in a dose-dependent manner, compared with the non-SHH treatment groups. In vivo application of the selective SHH receptor inhibitor cyclopamine significantly decreased the wound healing.
Fig. 5.
Exogenous SHH on wound healing in STZ-induced diabetic mice. After STZ treatment (10 wk), a full-thickness excisional wound (1.5 × 1.5 cm) was made. The solution of SHH (20 μg/ml) or the selective SHH receptor inhibitor cyclopamine (5 nmol/l) was directly placed on the wound surface for 30 min every other day from day 1 after wounding. The rate of wound closure was monitored every other day by tracing the wound area. Values were presented as means ± SE; n = 5. P < 0.05 vs. control (*) and vs. STZ (#).
SHH increased eNOS protein expression, NOS activity, and NO level in STZ-induced diabetic mice in vitro and in vivo.
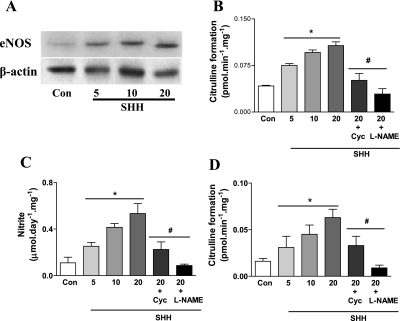
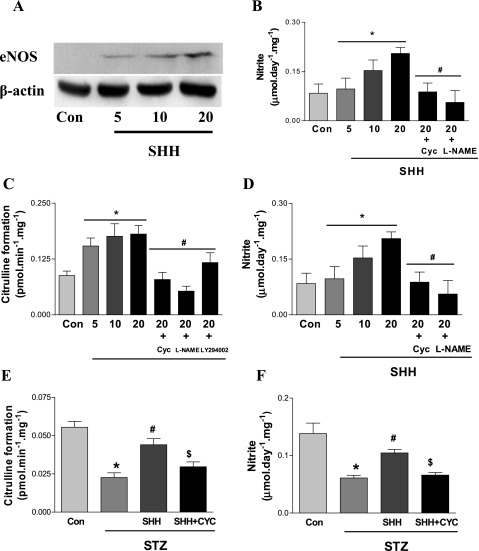
After 24-h treatment in vitro, SHH (5–20 μg/ml) significantly increased cutaneous eNOS protein expression, NOS activity, and NO level in a concentration-dependent manner (Fig. 6). These effects of SHH were abolished by SHH receptor inhibitor cyclopamine. The increase of cutaneous NOS activity and NO level was also blunted by NOS inhibitor l-NAME. As shown in Fig. 7, A–D, in vitro SHH treatment of skin tissues from STZ-induced diabetic mice increased cutaneous eNOS protein expression, NOS activity, and NO level in a concentration-dependent manner, an effect that was blunted by SHH receptor inhibitor cyclopamine (5 nmol/l) or NOS inhibitor l-NAME.
Fig. 6.
Induction of SHH on cutaneous endothelial nitric oxide synthase (eNOS) protein expression, nitric oxide synthase (NOS) activity, and nitric oxide (NO) level in normal mice in vitro. Skin tissues of normal mice were incubated with different concentration of SHH (5, 10, and 20 μg/ml). Some skin tissues incubated with 20 μg/ml of SHH were treated with SHH receptor inhibitor cyclopamine (Cyc, 5 nmol/l) or NOS inhibitor Nω-nitro-l-arginine methyl ester (l-NAME, 10−4 mol/l) for 24 h. eNOS protein expression (A and B), NOS activity (C), and NO level (D) were measured. Con, control. Values were presented as means ± SE; n = 5. P < 0.05 vs. control (*) and vs. 20 μg/ml SHH (#).
Fig. 7.
Induction of endogenous SHH on cutaneous eNOS protein expression, NOS activity, and NO level in STZ-induced diabetic mice in vitro and in vivo. After STZ treatment (10 wk), skin tissues of STZ mice were incubated with different concentrations of SHH (5, 10, and 20 μg/ml). Some skin tissues incubated with 20 μg/ml of SHH were treated with SHH receptor inhibitor cyclopamine (5 nmol/l) or NOS inhibitor l-NAME (10−4 mol/l) for 24 h. eNOS protein expression (A and B), NOS activity (C), and NO level (D) were measured. To determine the effects of SHH on cutaneous NOS pathway in vivo, 200 μl solution of SHH (20 μg/ml) or SHH receptor inhibitor cyclopamine (5 nmol/l) was placed directly on the wound surface for 30 min every other day from day 1 after wounding in STZ mice. The skin tissues of wound edge were cut on day 5 after wounding, and NOS activity (E) and NO level (F) were determined afterward. Values were presented as means ± SE; n = 5. A–D: P < 0.05 vs. control (*) and vs. 20 μg/ml SHH (#). E and F: P < 0.05 vs. control (*), vs. STZ (#), and vs. STZ + SHH ($).
To investigate the effects of SHH on cutaneous NOS pathway in vivo, the solution of SHH (20 μg/ml) or its receptor inhibitor cyclopamine (5 nmol/l) was directly placed on the wound surface for 30 min every other day from day 1 after wounding. The skin tissues of wound edge were cut on day 5 after wounding, and cutaneous NOS activity and NO level were determined afterward. SHH treatment significantly increased cutaneous NOS activity and NO level, which was significantly inhibited by cyclopamine (Fig. 7, E and F).
SHH stimulated eNOS protein expression, NOS activity, and NO level in endothelial cells.
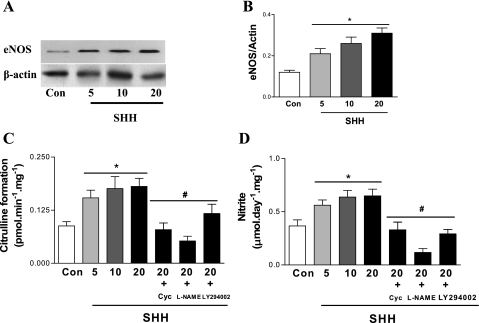
To ascertain that SHH was capable of increasing NOS function directly, its effects on NOS function were examined in human umbilical vein endothelial cells (HUVECs). In vitro SHH treatment of HUVECs increased eNOS protein expression, NOS activity, and NO level in a concentration-dependent manner (Fig. 8), an effect that was blunted by cyclopamine (5 nmol/l). NOS inhibitor l-NAME also inhibited the increase of NOS activity and NO level in HUVECs induced by SHH.
Fig. 8.
Induction of SHH on cutaneous eNOS protein expression, NOS activity, and NO level in human umbilical vein endothelial cells (HUVECs). HUVECs were incubated with different concentrations of SHH (5, 10, and 20 μg/ml). Some HUVECs incubated with 20 μg/ml of SHH were treated with SHH receptor inhibitor cyclopamine (5 nmol/l), NOS inhibitor l-NAME (10−4 mol/l), or phosphatidylinositol 3-kinase (PI 3-kinase) inhibitor LY-294002 (5 nmol/l) for 24 h. eNOS protein expression (A and B), NOS activity (C), and NO level (D) were measured. Values were presented as means ± SE; n = 5. P < 0.05 vs. control (*) and vs. 20 μg/ml SHH (#).
PI 3-kinase inhibitor LY-294002 abolished the effects of SHH on eNOS protein expression, NOS activity, and NO level in endothelial cells.
Because the activation of PI 3-kinase pathway is necessary for normal NO function, we determined whether activation of PI 3-kinase pathway is involved in NOS activation induced by SHH. Figure 8 showed that PI 3-kinase inhibitor LY-294002 significantly inhibited the increase of NOS activity and NO level induced by SHH treatment in HUVECs.
DISCUSSION
The present study provides the first evidence that 1) normal wounding induces activation of SHH pathway necessary for wound healing, 2) SHH pathway is impaired in type 1 diabetic mice, and 3) application of exogenous SHH accelerates diabetic wound healing by enhancing cutaneous NO function.
To directly demonstrate that SHH pathway participates in skin tissue repair, we detected the expression of SHH and PTCH 1 proteins and the effects of SHH pathway inhibitor cyclopamine on wound healing in normal mice. Our results showed that normal wounding per se was able to increase the expression of both SHH and PTCH 1 proteins in normal mice, the blockade of which by cyclopamine resulted in significant delay in wound repair. These strongly suggest that activation of SHH signaling pathway is necessary for normal wound healing. It is worth noting that topical application of exogenous SHH had no additional effect on normal wound closure, suggesting that endogenously activated SHH pathway upon wounding was sufficient for normal wound healing.
Diabetes is characterized by chronic hyperglycemia and associated with significant cardiovascular pathology (7). However, the detailed cellular mechanisms underlying refractory wounds in diabetes are not completely understood. Recent studies suggest that the hedgehog signaling pathway is impaired in nerve tissues and in the penis of diabetic rats (8, 17, 22). Treating diabetic rats with SHH restored motor- and sensory-nerve conduction velocities and maintained the axonal caliber of large myelinated fibers. To elucidate whether there is abnormal cutaneous SHH pathway in type 1 diabetic mice, we examined the expression of SHH and PTCH 1 proteins and tested the effects of topically applied SHH on wound healing in STZ diabetic mice. Our data revealed that the cutaneous expression of SHH and PTCH 1 proteins in STZ mice was significantly decreased in both the basal and stress status (wounding) compared with that of control mice. Topical application of exogenous SHH resulted in significant acceleration of wound healing in STZ-induced diabetic mice compared with that of control mice. These results suggest that an impaired SHH pathway contributes to wound healing delay in type 1 diabetes. Consistent with our findings, a recent study has demonstrated that topical SHH gene therapy results in significant local gene expression and acceleration of wound recovery in type 2 diabetic mice (db/db mice) (2). Hence, topical SHH gene therapy may improve wound healing in both type 1 and type 2 diabetes.
Emerging evidence indicates that NO plays an important role in normal wound healing (19, 27). Consistently, dysfunction of cutaneous NO is involved in impaired wound healing. Recent studies including ours have shown that both eNOS expression and NO level were declined in STZ-induced diabetes (19, 26). Importantly, cutaneous gene transfer of eNOS or manganese superoxide dismutase was able to restore eNOS expression and NO level, resulting in accelerated wound healing in STZ mice (19). Similarly, application of NO donor molsidomine or NO-releasing poly(vinyl alcohol) hydrogel dressing can partially restore the impaired wound healing in STZ rats (26). These studies indicate that impairment of cutaneous NO function is an important contributor to wound healing delay in type 1 diabetes.
However, the mechanism underlying SHH-induced augmentation of wound healing in diabetes was not clear. Because SHH can activate the PI 3-kinase pathway (16) downstream upon NOS activation, we tested the hypothesis that SHH improves wound healing by enhancing cutaneous NO function in STZ mice. Our results demonstrated that SHH significantly increased cutaneous eNOS protein expression, NOS activity, and NO level of normal and STZ-induced diabetic mice, in a concentration-dependent manner in vitro and in vivo. SHH also directly increased eNOS protein expression, NOS activity, and NO level in cultured endothelial cells in a concentration-dependent manner in vitro. The aforementioned effects of SHH were abolished by its receptor inhibitor cyclopamine. Furthermore, the increase of cutaneous NOS activity and NO level was also reversed by NOS inhibitor l-NAME. Finally, PI 3-kinase inhibitor LY-294002 significantly inhibited the increase in NOS activity and NO level induced by SHH in cultured endothelial cells. Together, these results suggest that SHH is able to activate NO pathway in cutaneous tissue and endothelial cells.
In summary, the present study demonstrates that normal wounding activates SHH pathway that is necessary for wound healing, and impaired cutaneous SHH signaling pathway contributes to impaired NO function and wound healing in diabetes. Delivery of exogenous SHH protein or its receptor agonists may provide an effective means in accelerating diabetic wound healing. Strategies aimed at augmenting endogenous SHH pathway may provide an effective means in ameliorating delayed diabetic wound healing.
GRANTS
This work was supported in part by National Institue of General Medical Sciences Grant R01 GM-077352, American Diabetes Association Research Award 7-08-RA-23, and National Natural Science Foundation of China (NSFC) Overseas Collaborative Grant No. 30728021 (to A. F. Chen) and by NSFC Grant no. 30572188, Natural Science Foundation of Guangdong Province in China Grant no. 040623, Scientific and Technological Program of Guangzhou in China Grant no. 2005J1-C0301, and Scientific and Technological Program of Guangzhou Education Bureau Grant no. 207336 (to J. D. Luo).
REFERENCES
- 1.Adolphe C, Narang M, Ellis T, Wicking C, Kaur P, Wainwright B. An in vivo comparative study of sonic, desert and Indian hedgehog reveals that hedgehog pathway activity regulates epidermal stem cell homeostasis. Development 131: 5009–5019, 2004. [DOI] [PubMed] [Google Scholar]
- 2.Asai J, Takenaka H, Kusano KF, Ii M, Luedemann C, Curry C, Eaton E, Iwakura A, Tsutsumi Y, Hamada H, Kishimoto S, Thorne T, Kishore R, Losordo DW. Topical sonic hedgehog gene therapy accelerates wound healing in diabetes by enhancing endothelial progenitor cell-mediated microvascular remodeling. Circulation 113: 2413–2424, 2006. [DOI] [PubMed] [Google Scholar]
- 3.Barnes EA, Kong M, Ollendorff V, Donoghue DJ. Patched1 interacts with cyclin B1 to regulate cell cycle progression. EMBO J 20: 2214–2223, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Beachy PA, Karhadkar SS, Berman DM. Tissue repair and stem cell renewal in carcinogenesis. Nature 432: 324–331, 2004. [DOI] [PubMed] [Google Scholar]
- 5.Bigelow RL, Jen EY, Delehedde M, Chari NS, McDonnell TJ. Sonic hedgehog induces epidermal growth factor dependent matrix infiltration in HaCaT keratinocytes. J Invest Dermatol 124: 457–465, 2005. [DOI] [PubMed] [Google Scholar]
- 6.Boukamp P Non-melanoma skin cancer: what drives tumor development and progression? Carcinogenesis 26: 1657–1667, 2005. [DOI] [PubMed] [Google Scholar]
- 7.Brownlee M Biochemistry and molecular cell biology of diabetic complications. Nature 414: 813–820, 2001. [DOI] [PubMed] [Google Scholar]
- 8.Calcutt NA, Allendoerfer KL, Mizisin AP, Middlemas A, Freshwater JD, Burgers M, Ranciato R, Delcroix JD, Taylor FR, Shapiro R, Strauch K, Dudek H, Engber TM, Galdes A, Rubin LL, Tomlinson DR. Therapeutic efficacy of sonic hedgehog protein in experimental diabetic neuropathy. J Clin Invest 111: 507–514, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chang CH, Yu M, Wu P, Jiang TX, Yu HS, Widelitz RB, Chuong CM. Sculpting skin appendages out of epidermal layers via temporally and spatially regulated apoptotic events. J Invest Dermatol 122: 1348–1355, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Daya-Grosjean L, Couve-Privat S. Sonic hedgehog signaling in basal cell carcinomas. Cancer Lett 225: 181–192, 2005. [DOI] [PubMed] [Google Scholar]
- 11.Ellis T, Smyth I, Riley E, Bowles J, Adolphe C, Rothnagel JA, Wicking C, Wainwright BJ. Overexpression of Sonic Hedgehog suppresses embryonic hair follicle morphogenesis. Dev Biol 263: 203–215, 2003. [DOI] [PubMed] [Google Scholar]
- 12.Gofflot F, Hars C, Illien F, Chevy F, Wolf C, Picard JJ, Roux C. Molecular mechanisms underlying limb anomalies associated with cholesterol deficiency during gestation: implications of Hedgehog signaling. Hum Mol Genet 12: 1187–1198, 2003. [DOI] [PubMed] [Google Scholar]
- 13.Hornstein E, Mansfield JH, Yekta S, Hu JK, Harfe BD, McManus MT, Baskerville S, Bartel DP, Tabin CJ. The microRNA miR-196 acts upstream of Hoxb8 and SHH in limb development. Nature 438: 671–674, 2005. [DOI] [PubMed] [Google Scholar]
- 14.Ikram MS, Neill GW, Regl G, Eichberger T, Frischauf AM, Aberger F, Quinn A, Philpott M. GLI2 is expressed in normal human epidermis and BCC and induces GLI1 expression by binding to its promoter. J Invest Dermatol 122: 1503–1509, 2004. [DOI] [PubMed] [Google Scholar]
- 15.Jeffcoate WJ, Harding KG. Diabetic foot ulcers. Lancet 361: 1545–1551, 2003. [DOI] [PubMed] [Google Scholar]
- 16.Kanda S, Mochizuki Y, Suematsu T, Miyata Y, Nomata K, Kanetake H. Sonic hedgehog induces capillary morphogenesis by endothelial cells through phosphoinositide 3-kinase. J Biol Chem 278: 8244–8249, 2003. [DOI] [PubMed] [Google Scholar]
- 17.Kusano KF, Pola R, Murayama T, Curry C, Kawamoto A, Iwakura A, Shintani S, Ii M, Asai J, Tkebuchava T, Thorne T, Takenaka H, Aikawa R, Goukassian D, von Samson P, Hamada H, Yoon YS, Silver M, Eaton E, Ma H, Heyd L, Kearney M, Munger W, Porter JA, Kishore R, Losordo DW. Sonic hedgehog myocardial gene therapy: tissue repair through transient reconstitution of embryonic signaling. Nat Med 11: 1197–1204, 2005. [DOI] [PubMed] [Google Scholar]
- 18.Lewis PM, Dunn MP, McMahon JA, Logan M, Martin JF, St-Jacques B, McMahon AP. Cholesterol modification of sonic hedgehog is required for long-range signaling activity and effective modulation of signaling by PTCH 1. Cell 105: 599–612, 2001. [DOI] [PubMed] [Google Scholar]
- 19.Luo JD, Wang YY, Fu WL, Wu J, Chen AF. Gene therapy of endothelial nitric oxide synthase and manganese superoxide dismutase restores delayed wound healing in type 1 diabetic mice. Circulation 110: 2484–2493, 2004. [DOI] [PubMed] [Google Scholar]
- 20.Maity T, Fuse N, Beachy PA. Molecular mechanisms of Sonic hedgehog mutant effects in holoprosencephaly. Proc Natl Acad Sci USA 102: 17026–17031, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pepicelli CV, Lewis PM, McMahon AP. Sonic hedgehog regulates branching morphogenesis in the mammalian lung. Curr Biol 8: 1083–1086, 1998. [DOI] [PubMed] [Google Scholar]
- 22.Podlasek CA, Zelner DJ, Harris JD, Meroz CL, Tang Y, McKenna KE, McVary KT. Altered Sonic hedgehog signaling is associated with morphological abnormalities in the penis of the BB/WOR diabetic rat. Biol Reprod 69: 816–827, 2003. [DOI] [PubMed] [Google Scholar]
- 23.Schatteman GC, Hanlon HD, Jiao C, Dodds SG, Christy BA. Blood-derived angioblasts accelerate blood-flow restoration in diabetic mice. J Clin Invest 106: 571–578, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Stewart GA, Hoyne GF, Ahmad SA, Jarman E, Wallace WA, Harrison DJ, Haslett C, Lamb JR, Howie SE. Expression of the developmental Sonic hedgehog (SHH) signalling pathway is up-regulated in chronic lung fibrosis and the SHH receptor patched 1 is present in circulating T lymphocytes. J Pathol 199: 488–495, 2003. [DOI] [PubMed] [Google Scholar]
- 25.Watkins DN, Berman DM, Burkholder SG, Wang B, Beachy PA, Baylin SB. Hedgehog signalling within airway epithelial progenitors and in small-cell lung cancer. Nature 422: 313–317, 2003. [DOI] [PubMed] [Google Scholar]
- 26.Witte MB, Kiyama T, Barbul A. Nitric oxide enhances experimental wound healing in diabetes. Br J Surg 89: 1594–1601, 2002. [DOI] [PubMed] [Google Scholar]
- 27.Yamasaki K, Edington HD, McClosky C, Tzeng E, Lizonova A, Kovesdi I, Steed DL, Billiar TR. Reversal of impaired wound repair in iNOS-deficient mice by topical adenoviral-mediated iNOS gene transfer. J Clin Invest 101: 967–971, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zardoya R, Abouheif E, Meyer A. Evolution and orthology of hedgehog genes. Trends Genet 12: 496–497, 1996. [DOI] [PubMed] [Google Scholar]
- 29.Zhang X, Stappenbeck TS, White AC, Lavine KJ, Gordon JI, Ornitz DM. Reciprocal epithelial-mesenchymal FGF signaling is required for cecal development. Development 133: 173–180, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]