Abstract
Muscle wasting is a critical feature of patients afflicted by acquired immune deficiency syndrome (AIDS), cancer, or chronic inflammatory diseases. In a mouse model of muscle wasting, TNF-α induces oxidative stress and nitric oxide synthase-2 (NOS2) and decreases myogenin, Jun-D, and creatinine kinase muscle isoform (CKM) expression. Here, we studied 12 patients with muscle wasting due to cancer (N = 10) or AIDS (N = 2) and 4 control subjects. We show that in skeletal muscle of cachectic patients there is 1) increased expression and activity of the TNF-α signaling, including TNF-α mRNA, activation of TNFR1, and TNF-α-associated to TNFR1; 2) increased oxidative stress, as determined by the presence of malondialdehyde-lysine adducts; 3) increased NOS2 mRNA and protein; 4) decreased expression of Jun-D, myogenin, myosin, and CKM mRNA and protein; 5) impaired CKM-E box binding activities, associated with decreased Jun-D/myogenin activities; and 6) oxidative modification and ubiquitination of Jun-D. These studies show that these molecular pathways are modulated in association with muscle wasting in patients with cancer or AIDS, and whether or not they cause muscle wasting remains to be determined.
Keywords: muscle atrophy, creatinine kinase, nitric oxide synthase-2, Ref-1
muscle wasting is a critical feature of patients afflicted by acquired immune deficiency syndrome (AIDS), cancer, or chronic inflammatory diseases (2, 12, 38). At present, there is no satisfactory treatment for this complication, which contributes significantly to the morbidity and mortality of patients with cachexia (12, 38). Using a murine model of muscle wasting, we found that TNF-α induces oxidative stress and expression of nitric oxide synthase-2 (NOS2) in skeletal muscle, leading to decreased creatinine kinase (CKM) expression and CKM-E box DNA binding activities, which are critical for CKM gene transcription (5). The impaired CKM-E box DNA binding activities of skeletal muscle nuclear extracts from TNF-α mice resulted from abnormal myogenin/Jun-D complexes and were normalized by the addition of Jun-D, dithiothreitol, or Ref-1 (5), a nuclear redox protein (46, 47). Moreover, treatment of TNF-α mice with the antioxidants d-α-tocopherol or BW755c, or the NOS2 inhibitor nitro-l-arginine, acting at a post-TNF-α receptor level, prevented the decreased body weight, muscle wasting, and skeletal muscle molecular abnormalities of cachexia (5). Although the TNF-α animal model of cachexia (6, 27, 39) provides a valuable system to study the mechanisms responsible for muscle wasting, its relevance to human cachexia remains to be determined.
The rationale for our study is that TNF-α is considered a critical inducer of cachexia in patients with cancer and AIDS (38). Because the levels of serum TNF-α detected in patients with cachexia and muscle wasting are comparable to those achieved in the mouse model of cachexia, which is induced with cells engineered to secrete human TNF-α (5), it is reasonable to postulate that the molecular mechanisms of muscle wasting in the animal model and humans could also be similar.
The central hypothesis of the study was that in patients with cancer or AIDS cachexia, muscle wasting initiated by TNF-α (and related cytokines) (2, 11, 12, 33, 36) would induce a typical signature cascade including 1) oxidative stress, 2) induction of NOS2, 3) decreased activities of myogenin/Jun-D (through decreased expression and oxidative modifications), and 4) decreased expression of CKM. Therefore, we analyzed the molecular pathways associated with muscle wasting in patients with cachexia.
MATERIALS AND METHODS
Human subjects.
We obtained anonymous, deidentified skeletal muscle samples from control and cachectic patients. The protocol was approved by the University of California, San Diego Human Protection Program. As depicted in Table 1, subjects were patients with cachexia (16 ± 6% decrease in ideal body weight over the preceding 6 mo) due to cancer (56 ± 5 yr; n = 10) [colorectal (n = 5), lung (n = 2), esophageal, laryngeal, and anal carcinomas] or AIDS (39 and 54 yr; n = 2). Control individuals were subjects of normal weight without cancer (59 ± 6 yr; n = 4). We obtained tissue from the rectus abdominis muscle during abdominal surgery or from the vastus lateralis muscle by needle biopsy in subjects and control individuals.
Table 1.
Characteristics of patients with muscle wasting
| Sample | Diagnosis | Sex | Age | Muscle | TNM |
|---|---|---|---|---|---|
| C1 | Control | F | 47 | RA | |
| C2 | Control | M | 60 | RA | |
| C3 | Control | M | 65 | VL | |
| C4 | Control | F | 63 | VL | |
| A1 | AIDS | M | 54 | VL | |
| A2 | AIDS | M | 39 | VL | |
| Ca1 | Colon Adeno CA | F | 59 | RA | T3N0MX |
| Ca2 | Colon Adeno CA | M | 56 | RA | T2N0MX |
| Ca3 | Colon Adeno CA | F | 55 | RA | T2N0MX |
| Ca4 | Colon Adeno CA | M | 56 | RA | na |
| Ca5 | Rectal Adeno CA | F | 54 | RA | T2N0MX |
| Ca6 | Lung CA | M | 65 | RA | na |
| Ca7 | Lung CA | M | 49 | VL | na |
| Ca8 | Esophageal CA | M | 53 | VL | na |
| Ca9 | Laryngeal CA | M | 57 | VL | na |
| Ca10 | Anal CA | M | 56 | RA | na |
The diagnosis (AIDS, acquired immune deficiency syndrome; CA, cancer), sex, age, TNM classification of the tumor and the muscle that was biopsied (RA, rectus abdominus; VL, vastus lateralis) are shown. The active therapy information and in some cancer patients the TNM classification was not available (na). T, extent of the primary tumor; N, absence or presence and extent of regional lymph node metastasis; M, absence or presence of distant metastasis; T2, T3, and T4, increasing size and/or local extent of the primary tumor; N0, no regional lymph node metastasis; MX, distant metastasis cannot be assessed.
Immunohistochemistry.
Human biopsies were obtained from the rectus abdominis muscle during abdominal surgery or from the vastus lateralis muscle by needle biopsy in subjects and control individuals. In each sample, half of the biopsy was frozen in liquid nitrogen for protein purification and analysis. The remaining sample (2-mm sections) was equilibrated in cryoprotectant (Tissue-Tek, no. 4583) and flash frozen on a metal block that has been cooled to −196°C by liquid nitrogen. It was then stored at −70°C, cut on a cryostat (Leica CM1900) to 7-μm sections at −20°C, and mounted onto polylysine-coated slides (Sigma PO425-72EA). After being cut and mounted on slides, the sections were allowed to air dry for 20 min at room temperature and then were fixed with 4% fresh paraformaldehyde (2 g to 25 ml heated to 60°C for 15 min in a fume hood. Buffer was neutralized with NaOH and allowed to cool, and volume was brought up to 50 ml with 2× PBS). Slides were fixed in 4% fresh paraformaldehyde for 10 min at room temperature. Washes were PBS for 5 min, 1% Triton X-100 in PBS for 2 min, and PBS wash 3 × 5 min, all at room temperature. For immunohistochemical staining slides were blocked with 3% BSA in PBS for 30 min at room temperature. Samples were incubated with the primary antibody diluted 1:100 in 3% BSA for 1 h at room temperature. They were then washed with PBS twice for 3 min at room temperature. The samples were incubated with the secondary antibody diluted 1:100 in 3% BSA for 1 h at room temperature. They were washed with PBS as above. The samples were counterstained with TO-PRO3 (Molecular Probes, Eugene, OR) 1:500 in 3% BSA for 20 min at room temperature. They were washed with PBS 3 times for 5 min each. Finally, samples were mounted with fade-retardant mount (Molecular Probes) and visualized with a confocal microscope (Bio-Rad). We performed immunohistochemical detection of malondialdehyde (MDA)-protein adducts, myosin, CKM, NOS2, and Jun-D in skeletal muscle sections as described previously (5, 8, 19), using the avidin-biotin-alkaline phosphatase system (Vector Laboratories, Burlingame, CA) or immunofluorescence by confocal scanning laser microscopy. We used antibodies against MDA-lysine epitopes (19), myosin (R & D, Minneapolis, MN), CKM (Vector Laboratories), and NOS2 (Transduction Laboratories, Franklin Lakes, NJ), ubiquitin, myogenin, Jun-D, TNF-α, phospho-TNFR1, and β-actin (Santa Cruz Biotechnology). Fluorochromes utilized were Alexa 488 and Alexa 594. We used TO-PRO-3 (Molecular Probes) to analyze nuclear morphology. The program that we used for immunohistochemistry quantitation was MetaMorph Offline, Universal Imaging Product version 6.1. The MetaMorph Offline software package is an accepted standard used to measure, analyze, and display data acquired from a confocal system. We used the MetaMorph Offline program to graph fluorescent intensities (integrated intensity; summed over all of the pixels in the region) in a defined area from the acquired confocal images. These data were exported directly to Excel (Microsoft) for the calculations. At least five random fields (×200) were analyzed per experimental point.
RT-PCR analysis.
We isolated total RNA from control and cachectic skeletal muscle samples using Trizol (Invitrogen). Samples were treated with DNase by use of Turbo DNA-free (Ambion) and precipitated with chloroform per the manufacturer's protocol. The cDNA synthesis was performed by using 250 ng of total RNA with the AffinityScript Reverse Transcriptase and Fermentas RNAse Inhibitor (Stratagene), with a random 9-mer (5′-NNNNNNNNN-3′) and 0.5–2.0 mg RNA with 2 pmol of gene-specific primer or random 9-mer, per manufacturer's protocol as described (3, 8). Specific sets of primers were utilized for RT-PCR amplification for TNF-α, NOS2, Jun-D, myogenin, and CKM, as described by the manufacturer (SuperArray, Frederick, MD). For each 25-μl PCR, the following components were mixed in a PCR tube: 12.5 μl 2× RT2 Real-Time SYBR Green PCR Master Mix; 1.0 μl First Strand cDNA template RT2 PCR; and 1.0 μl Primer Assay. They were brought to a final volume of 25 μl by use of double-distilled H2O as described by the manufacturer (SA Biosciences).
We have used the 2−ΔΔCt method to analyze the relative changes in gene expression between the control skeletal muscle and the cachectic muscle samples. GAPDH was utilized as an endogenous control because it was found to be internally consistent between the samples when measured as a consequence of equal amounts of RNA added to the cDNA reaction as measured by UV absorbance. Briefly, Poly(A)+ RNA was extracted from the cells and equivalent amounts were converted into cDNA. The amounts of GAPDH and two other housekeeping gene candidates (data not shown) were determined by real-time quantitative PCR with SYBR Green detection. The relative amounts of GAPDH were not statistically different between control skeletal and cachectic muscle samples when calculated by ΔCt = Ct,Time X − Ct,Time 0. 2−ΔΔCt was calculated from ΔΔCt = ΔCt(cachectic muscle) − ΔCt(control muscle), where ΔCt(cachectic muscle) = Ct(gene of interest) − Ct(GAPDH) and ΔCt(control muscle) = Ct(gene of interest) − Ct(GAPDH). Values are expressed as fold changes vs. control. Standard errors are derived from replicate samples (24). Ct is defined as the cycle threshold, and the parentheses define the objects of the calculations.
Immunoprecipitation and immunoblot.
Precleared skeletal muscle lysates were incubated for 2 h with purified specific antibodies followed by the addition of A/G+ agarose (Santa Cruz Biotechnology) for 12 h as described (3, 8). The immunoprecipitation reactions each contained 500 μg of total protein and 2 μg antibody (or purified IgG preimmune serum as negative control). Immunoprecipitates were washed three times in 500 ml of cell lysis buffer and resolved by SDS-PAGE, and NOS2, myogenin, CKM, Jun-D, ubiquitin, and MDA were detected by Western blot as described (3), following the chemiluminescence protocol (PerkinElmer, Shelton, CT) by using purified specific antibodies against TNF-α, myogenin, Jun-D, NOS2, myosin, CKM, ubiquitin, and β-actin (Santa Cruz Biotechnology) and MDA as described (9). Negative samples were performed by omitting the first antibody. The program that we used for quantifying the immunoblots was Alpha Ease FC, Alpha Innotech Version 3.2.2.
Nuclear extracts preparation and gel retardation assay.
We prepared nuclei by the procedure described previously (5, 8, 10). Tissues were homogenized in citric 0.5% NP-40, 10 mM NaF, 10 mM Na pyrophosphate, and 10 μM butylated hydroxytoluene with a glass Dounce homogenizer with a loose-fitting pestle. The homogenized cells were placed above a cushion consisting of 2.0 M sucrose. The nuclei were precipitated by a 4,000-g centrifugation at 4°C for 20 min, and frozen at −70°C. We performed gel retardation analysis of protein-DNA complexes in the presence of polydeoxyinosinic-deoxycytidylic acid, with an oligonucleotide spanning the E box of the CKM gene, as described previously (5, 8, 22, 41, 42). The sense oligonucleotides were CKM-E box (5′-CCCAACACCTGC TGCCTGAGCC-3′) and AP1 (5′-TGACATCATGGGCTGTCGACCATG-3′). We induced expression of recombinant Jun-D protein with isopropyl-β-d-thiogalactopyranoside, from the coding region of Jun-D cDNA (American Type Culture Collection) cloned into a pRSET vector and purified with a Probond Resin (Invitrogen). We oxidized recombinant Jun-D by exposure to air at room temperature for 18 h in the presence of leupeptin and aprotinin, but without reducing agents or antioxidants. We added purified recombinant Ref-1 (0.2 μg) (kindly provided by S. Xanthoudakis, Merck Frost, Kirkland, QC, Canada) or 10 mM dithiothreitol (DTT) to some samples as described (47). The program that we used for quantifying the gel retardation assay was Alpha Ease FC, Alpha Innotech Version 3.2.2.
Statistical methods.
Data from the RT-PCR analysis, performed on tissue samples from 4 control + 6 cachectic = 10 subjects, consist of three replicates per subject. We analyzed the data using mixed-effects models (21) fit with the nlme (30) package in R version 2.8.1 (31). The model included a fixed effect for group (cachectic vs. control), and a random effect for subject. Residual plots of fits of the untransformed data revealed heteroscedascity. We conducted a sensitivity analysis in which we log transformed the fold changes and found results that were consistent with the analysis of the raw fold changes but exhibited group differences of even greater statistical significance. We used a Holm (15) adjustment for multiple comparisons (Supplemental Table S1 for Figs. 1, 3, and 4). We also analyzed potential confounding patient characteristics (age, sex, muscle biopsy location). We found that MDA (P = 0.010) was elevated in vastus lateralis compared with rectus abdominis (Supplemental Fig. S1) and myosin (P = 0.041) was lower in vastus lateralis muscle compared with rectus abdominis (Supplemental Fig. S3). The group effect was still significant at P < 0.001 after controlling for muscle type in each case. Figures 2 and 5 and Supplemental Fig. S5 all had too little sample quantity (for the cachexia group) to perform meaningful statistical analysis.
The immunohistochemical data in Fig. 2 consisted of three replicates from 1 control + 1 cachectic = 2 subjects. The sample size was too small to support rigorous statistical analysis, so we summarize the means and standard errors of each subject's replicated log intensity.
As summarized in Supplemental Table S1, mixed-effects model estimates of the difference in group mean log intensities were significant (P < 0.05) for Jun-D RT-PCR, myogenin RT-PCR, CKM RT-PCR, TNF-α RT-PCR, NOS2 RT-PCR, TNF-α Western blot, Phos TNFR1 Western blot, NOS2 Western blot, myosin Western blot, CKM Western blot, myogenin Western blot, and Jun-D Western blot. TNFR1 and β-actin Western blots were not significant at the 0.05 level. All of these significant differences, except TNF-α RT-PCR, withstood a Holm correction for multiple comparisons. When the fold changes were log transformed, TNF-α RT-PCR was also significant at the 0.05 level after the Holm adjustment.
Supplemental Table S2 summarizes the analysis of Supplemental Fig. S1–S4 and S6. The immunohistochemical data in Supplemental Figs. S1–S4 and S6 were analyzed by using mixed-effects models (21) fit with the nlme (30) package in R version 2.8.1 (31), and a Holme (15) adjustment was used for multiple comparisons. The sample size was too small to support rigorous statistical analysis, so we summarize the means and standard errors of each subject's replicated log intensity for Supplemental Fig. S5.
RESULTS
Increased TNF-α, TNFR1, and NOS2 expression in skeletal muscle of cachectic patients.
The central hypothesis of the study was that, in patients with cancer or AIDS cachexia, muscle wasting initiated by TNF-α (and related cytokines) (2, 5, 11, 27) would induce a typical signature cascade including oxidative stress and induction of NOS2. Therefore, we studied TNF-α expression and its receptor as well as NOS2 expression in these patients.
We obtained anonymous, deidentified tissues from the rectus abdominis muscle during abdominal surgery or from the quadriceps (vastus lateralis) muscle by needle biopsy in patients and control individuals. The patients' characteristics are described in Table 1. We studied 16 patients, including 2 patients with AIDS (A1, A2) and 10 patients with cancer (Ca1–Ca10) as well as 4 control individuals (C1–C4). The TNM tumor classification was available only in four of the cancer patients; none of these patients had evidence of regional or distant metastasis. The subjects' active treatment information was not available to the investigators.
As summarized in Supplemental Table S1, mixed-effects model estimates of the difference in group mean log intensities were significant (P < 0.05) for Jun-D RT-PCR, myogenin RT-PCR, CKM RT-PCR, TNF-α RT-PCR, NOS2 RT-PCR, TNF-α Western blot, Phos TNFR1 Western blot, NOS2 Western blot, myosin Western blot, CKM Western blot, myogenin Western blot, and Jun-D Western blot. TNFR1 and β-actin Western blots were not significant at the 0.05 level, nor was TNF-α RT PCR after the Holm multiple-comparisons correction.
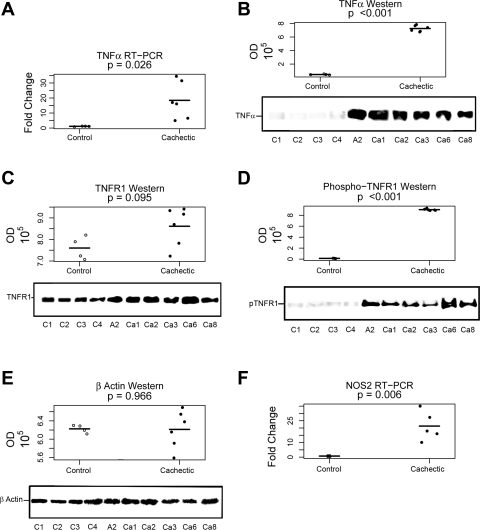
TNF-α mRNA (4 controls: C1–C4, 2 rectus abdominis and 2 vastus lateralis; 6 cachectic A2, Ca1–Ca3, Ca6, and Ca8, 2 rectus abdominis and 2 vastus lateralis, Table 1) levels were variably, but consistently, increased in cachectic patients compared with control individuals (P < 0.05) (Fig. 1A and Supplemental Table S1). We were not able to analyze the entire group of cachectic samples by this assay because of sample quantity limitations. We obtained additional confirmation of the increased expression of TNF-α in skeletal muscle of these patients by measuring the cytokine level associated with immunopurified phospho-TNFR1 by immunoblot (P < 0.001) (Fig. 1B and Supplemental Table S1). The expression of the TNF-α receptor TNFR1 was unchanged [not significant (NS)] (Fig. 1C and Supplemental Table S1), but the expression of the activated TNFR1, as determined by the presence of a specific phosphorylation, was increased in patients with muscle wasting compared with control (P < 0.001) (Fig. 1D and Supplemental Table S1). Expression of β-actin was unchanged in the skeletal muscle of cachectic patients (NS) (Fig. 1E and Supplemental Table S1). In addition, NOS2 mRNA (4 controls: C1–C4, 2 rectus abdominis and 2 vastus lateralis; 6 cachectic A2, Ca1–Ca3, Ca6, and Ca8, 4 rectus abdominis and 2 vastus lateralis, Table 1) was increased in skeletal muscle of cachectic patients compared with control (P < 0.05) (Fig. 1F and Supplemental Table S1).
Fig. 1.
Increased TNF-α and nitric oxide synthase-2 (NOS2) expression and activation of TNFR1 in skeletal muscle of cachectic patient. P values represent the significance of the mean group difference, as estimated by the mixed-effects model (see Supplemental Table S1). All 4 (C1–C4; from Table 1) control patients were analyzed (shown as open circles with a line indicating the mean), and 6 cachectic patients (A2, Ca1–Ca3, Ca6, and Ca8; from Table 1) were analyzed (shown as solid circles with a line indicating the mean). The entire group of cachectic patients could not be analyzed by this methodology because of insufficient tissue. Age, sex, and muscle biopsy location did not have statistical effects. All samples were run in triplicate, and replicate values were within an acceptable range of each other. Samples were 6 rectus abdominis and 4 vastus lateralis (Table 1). A: TNF-α mRNA was determined by RT-PCR as described in materials and methods. TNF-α mRNA was increased in skeletal muscle from cachectic patients (N = 6; samples described above) compared with control samples (N = 4; samples described above) (P < 0.05). B: TNF-α was determined by immunoblot in immunopurified TNFR1 as described in materials and methods. TNF-α was increased in skeletal muscle from cachectic patients (P < 0.001). Samples were those described above. Four representative control and 6 cachectic values are shown. IgG was used as a negative control. C: TNFR1 was determined by immunoblot in immunopurified TNFR1 as described in materials and methods. TNFR1 was unchanged in skeletal muscle from cachectic patients (P = 0.095). Samples were those described above. One representative control and 6 cachectic values are shown. IgG was used as a negative control. D: activated phosphorylated TNFR1 (pTNFR1) was determined by immunoblot in immunopurified TNFR1 as described in materials and methods. Phospho-TNFR1 was increased in skeletal muscle from cachectic patients (P < 0.001). Samples were those described above. Four representative control and 6 cachectic values are shown. IgG was used as a negative control. E: β-actin was determined by immunoblot in skeletal muscle lysates as described in materials and methods. β-Actin was unchanged in skeletal muscle from cachectic patients (not significant). Samples were those described above. Four representative control and 6 cachectic values are shown. F: NOS2 mRNA was determined by RT-PCR as described in materials and methods. NOS2 mRNA was increased in skeletal muscle from cachectic patients (N = 5; A2, Ca1–Ca3, and Ca6, Table 1) compared with control samples (N = 4, as described above) (P < 0.01). Samples were 6 rectus abdominis and 3 vastus lateralis (Table 1). Immunoblots quanified by optical density (OD).
Increased oxidative stress and decreased myosin, CKM, myogenin and Jun-D expression in skeletal muscle of cachectic patients.
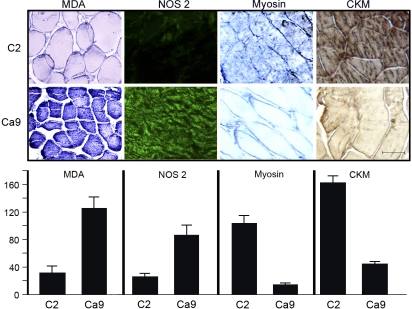
We performed immunohistochemical detection of MDA-protein adducts, NOS2, myosin and CKM, in matched skeletal muscle sections of control and cachectic subjects. We found a marked increase in MDA-protein adducts, a sensitive indicator of oxidative stress (5, 9, 16–19, 22, 33), in skeletal muscle of patients afflicted by cachexia, whereas skeletal muscle of control individuals without cachexia had negligible levels. The quantitative measurements of five random fields each (×200) showed significant increases in MDA and NOS2, and decreases in myosin and CKM in the muscle of a representative cachectic patient compared with a representative control subject (Fig. 2).
Fig. 2.
Increased oxidative stress and NOS2 and decreased myosin and muscle creatine kinase (CKM) by immunohistochemistry in the skeletal muscle of cachectic patients. Summary of the analysis of 2 subjects with 3 replicates each. All of the measures were completely differentiated between subjects. That is, all replicates from one subject were less than the replicates from the other subject (or vice versa). The sample size was too small to support rigorous statistical analysis. We were not able to analyze the entire group of cachectic samples by this assay because of sample quantity limitations. Top: representative examples of skeletal muscle (vastus lateralis) immunohistochemistry of a control individual (C2, Table 1) and a patient afflicted by cancer cachexia (Ca9). We performed immunohistochemistry for malondialdehyde-protein adducts (MDA), NOS2, myosin, and CKM as described in materials and methods. There was an increase in the expression of MDA and NOS2 and a decrease in the expression of myosin and CKM in skeletal muscle of the cachectic patient. Negligible staining was observed in all immunohistochemical staining when the first antibody was omitted. Size bar represents 100 μm. Bottom: these findings by immunohistochemistry were quantified by using the Metamorph Offline program, Universal Imaging, product version 6.1. The quantitative measurements of 5 random fields each (×200) showed significant increases in MDA and NOS2 and decreases in myosin and CKM in the muscle of the cachectic patient. The sample size was too small to support rigorous statistical analysis.
The additional immunofluorescent studies for MDA shown in Supplemental Fig. S1A support the findings of Fig. 2. As expected, NOS2 expression, which is stimulated by oxidative stress (5), was markedly stimulated in the skeletal muscle of cachectic patients, as detected by confocal microscopy (3, 8) (Fig. 2, Supplemental Fig. S2A). These findings indicate that, irrespective of the mechanisms responsible for initiating the cascade leading to muscle wasting in cachexia (2, 11, 12, 34, 35, 37), this cascade enhances oxidative stress and NOS2 expression in the skeletal muscle of cachectic patients.
Myosin is the major structural protein of skeletal muscle and in the presence of actin filaments, the ATPase activity of myosin is greatly stimulated (20). This process is essential for muscle contraction since the energy required for this process is derived from ATP hydrolysis (44). In turn, the function of CKM is critical in skeletal muscle homeostasis since it catalyzes the formation of ATP from phosphocreatine (20). As we found in cachectic TNF-α mice, the expression of myosin and CKM was decreased in the skeletal muscle of cachectic patients, as detected by immunofluorescence [Fig. 2 (control: C2; cachectic: Ca9), Supplemental Figs. S3A (control: C1, C2; cachectic: Ca2, Ca2–Ca3, Ca6, and Ca8) and S4A (control: C3, C4; cachectic: a1, Ca1, Ca4, and Ca7), Supplemental Table S2]. In contrast to the normal fibril arrangement, skeletal muscle from cachectic patients consisted of smaller fibrils (Fig. 2, Supplemental Fig. S3A, Supplemental Table S2). All of the measures were completely differentiated between subjects. That is, all replicates from one subject were less than the replicates from the other subject (or vice versa). The sample size for Fig. 2 was too small to perform meaningful statistical analysis. However, statistical analysis was performed in the companion samples for MDA (Supplemental Fig. S1, P = 0.001), NOS2 (Supplemental Fig. S2, P = 0.019), myosin (Supplemental Fig. S3, P < 0.001), and CKM (Supplemental Fig. S4, P < 0.001).
These findings by immunohistochemistry were quantified in five random fields each (×200) by use of the Metamorph Offline program, Universal Imaging Product version 6.1. The quantitative measurements showed significant increases in MDA (6 rectus abdominis and 2 vastus lateralis) (Supplemental Fig. S1B and Supplemental Table S2). MDA was elevated in vastus lateralis muscles compared with rectus abdominis (P = 0.010). NOS2 was also increased in cachectic patients (6 rectus abdominis and 2 vastus lateralis) (Supplemental Fig. S2B and Supplemental Table S2). Decreases in myosin (6 rectus abdominis and 2 vastus lateralis) (Supplemental Fig. S3B and Supplemental Table S2) and CKM (4 vastus lateralis and 2 rectus abdominis) (Supplemental Fig. S4B and Supplemental Table S2) were observed in the muscles of cachectic patients. Myosin was lower in vastus lateralis muscle compared with rectus abdominis (P = 0.041).
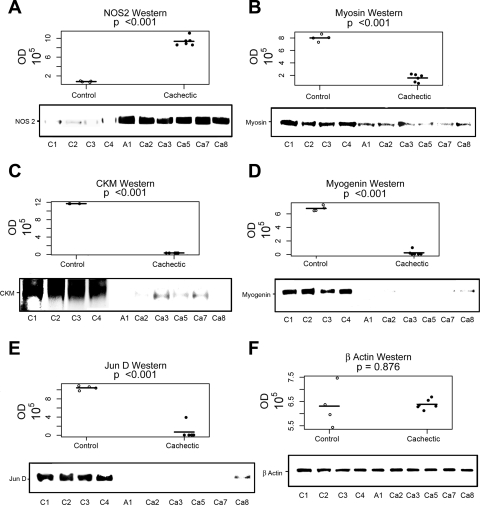
Also, immunopurification and immunoblot was performed for NOS2, myosin, CKM, myogenin, and Jun-D by using specific antibodies in control (N = 4; 2 rectus abdominis and 2 vastus lateralis) and cachectic (N = 6; 3 rectus abdominis and 3 vastus lateralis) skeletal muscle samples. NOS2 was increased, whereas myosin, CKM, myogenin, and Jun-D were decreased in cachectic skeletal muscle samples compared with control samples (Fig. 3 and Supplemental Table S1).
Fig. 3.
Increased NOS2 and decreased myosin, CKM, myogenin and Jun-D by immunoblot in the skeletal muscle of cachectic patients. P values represent the significance of the mean group difference, as estimated by the mixed-effects model. All 4 (C1–C4; from Table 1) control patients were analyzed, and 6 cachectic patients (A1, Ca2, Ca3, Ca5, Ca7, and Ca8; from Table 1) were analyzed (horizontal lines indicate the means for each group). The entire group of cachectic patients could not be analyzed by this methodology because insufficient tissue. Age, sex, and muscle biopsy location did not have statistical effects. All samples were run in triplicate, and replicate values were within an acceptable range of each other. Samples were 5 rectus abdominis and 5 vastus lateralis (Table 1). Immunopurification and immunoblot were performed for NOS2 (A), myosin (B), CKM (C), myogenin (D), Jun-D (E), and β-actin (F) (control for immunopurification) by using specific antibodies in control and cachectic samples as described above. NOS2 was increased, whereas myosin, CKM, myogenin, and Jun-D were decreased in cachectic skeletal muscle samples compared with control samples. Four representative control and 6 cachectic values are shown. IgG was used as a negative control. Additional quantitation of these muscle proteins was obtained from the immunoblot, using the Alpha Ease FC program, Alpha Innotech Version 3.2.2. The expression of NOS2 (P < 0.001) was increased whereas the expressions of myosin (P < 0.001), CKM (P < 0.001), myogenin (P < 0.001), and Jun-D (P < 0.001) were decreased. These are representative data from 4 independent experiments.
The program that we used for quantifying the immunoblots was Alpha Ease FC, Alpha Innotech Version 3.2.2. In agreement with the immunohistochemistry results, the expression of NOS2 was increased whereas the expression of myosin, CKM, myogenin, and Jun-D were decreased (P < 0.001 for all measurements) (Fig. 3, A–D and Supplemental Table S1). Expression of control β-actin was unchanged in the skeletal muscle of cachectic patients compared with controls (NS) (Fig. 3E and Supplemental Table S1).
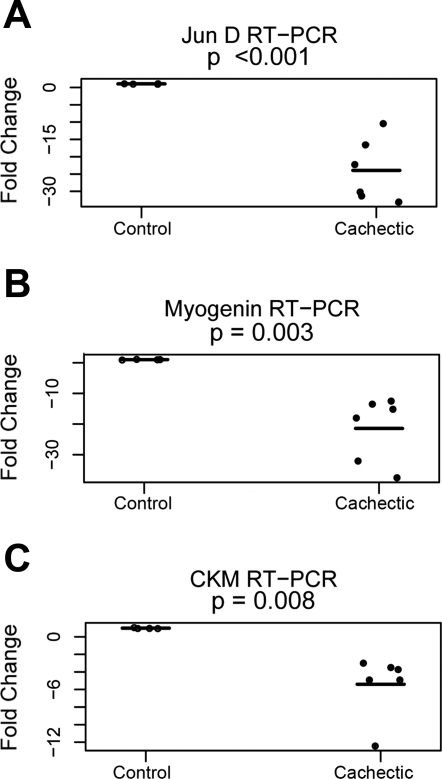
To further delineate the mechanisms responsible for the changes in skeletal muscle proteins, we also assessed the expression of the corresponding genes in muscle wasting of cachexia (N = 6; A1, Ca3–Ca6, and Ca9; 4 rectus abdominis and 2 vastus lateralis) compared with controls (N = 4; C1–C4; 2 rectus abdominis and 2 vastus lateralis) by RT-PCR using specific primers (3). Jun-D (P < 0.001), myogenin (P < 0.005), and CKM (P < 0.01) mRNA were decreased by RT-PCR (Fig. 4, A–C and Supplemental Table S1). Collectively, these results indicate a pretranslational inhibition of Jun-D, myogenin, and CKM gene expression in skeletal muscle of cachectic patients.
Fig. 4.
Decreased Jun-D, myogenin, and CKM mRNA in skeletal muscle from cachectic patients. P values represent the significance of the mean group difference, as estimated by the mixed-effects model. All 4 (C1–C4; from Table 1) control patients were analyzed, and 6 cachectic patients (A1, Ca3–Ca5, Ca6, and Ca9, from Table 1) were analyzed (horizontal lines indicate the means for each group). The entire group of cachectic patients could not be analyzed by this methodology because of insufficient tissue. Age, sex, and muscle biopsy location did not have statistical effects. All samples were run in triplicate, and replicate values were within an acceptable range of each other. Samples were 6 rectus abdominis and 4 vastus lateralis (Table 1). RT-PCR amplification for Jun-D (A), myogenin (B), and CKM (C) was performed as described in materials and methods. Jun-D, myogenin, and CKM mRNA were decreased in skeletal muscle from cachectic patients [N = 6; as described above) compared with controls (N = 4; as described above)] (P < 0.001 for Jun-D; P < 0.005 myogenin; and P < 0.01 CKM). These are representative data from 5 independent experiments.
CKM-E box binding activities are decreased in skeletal muscle from cachectic patients.
Next, as depicted in Supplemental Fig. S5 by a representative cancer patient (1 of 3 cachectic samples) and a control subject (1 of 2 controls) from three independent experiments, we analyzed the binding affinity of skeletal muscle nuclear proteins to the CKM DNA E-box, which is critical for the efficient transcription of the CKM gene. These samples were selected because they were the only ones that had sufficient quantity to isolate nuclear extracts. The sample size was too small to perform meaningful statistical analysis. To preserve nuclear proteins, nuclear extracts were obtained in the presence of antioxidants and protease and phosphatase inhibitors, as reported previously (7, 8, 10, 41). Skeletal muscle nuclear extracts from cachectic patients displayed a substantial decrease in DNA binding affinity to the CKM-E box (Supplemental Fig. S5, lane 3), compared with those from normal individuals (lane 2). Incubation of nuclear extracts from control patients with antibodies against Jun-D (lane 4) or myogenin (lane 5) disrupted the normal protein-DNA complex, as we reported with nuclear extracts from control animals (5).
Furthermore, the impaired CKM-E box binding activities of skeletal muscle nuclear extracts from cachectic patients were normalized by the addition of unoxidized recombinant Jun-D (Fig. S5, lane 6). Given that there is an marked increase in oxidative stress in the skeletal muscle of cachectic patients, and that Jun-D activity could be modulated by the nuclear redox factor Ref-1 (46), we studied whether Ref-1 would normalize the binding of skeletal muscle nuclear extracts from cachectic patients to the CKM-E box. The addition of either purified recombinant Ref-1 or the reducing agent DTT (5, 47) (Supplemental Fig. S5, lanes 7 and 8) normalized the DNA-protein complex, suggesting that the critical mechanism involves, at least in part, oxidation of a transcriptional factor, presumably the highly conserved cysteine domain (KC285R) of Jun-D (4, 46, 47). Neither Ref-1 nor DTT had any effect when incubated with probe alone (Supplemental Fig. S5).
In contrast to the impaired CKM-E box binding activities, skeletal muscle nuclear extracts from cachectic patients displayed a normal binding to NF-κB cognate DNA (data not shown). Nonetheless, nuclear extracts from cachectic patients had a decreased binding affinity to MEF-2 (TCTAAAAATAAC) (a cognate DNA from the skeletal muscle specific factor MEF-2A) (data not shown), suggesting that the oxidative stress cascade characteristic of muscle wasting affects the expression of various skeletal muscle-specific genes.
Jun-D is oxidatively modified, ubiquitinated, and decreased in skeletal muscle from cachectic patients.
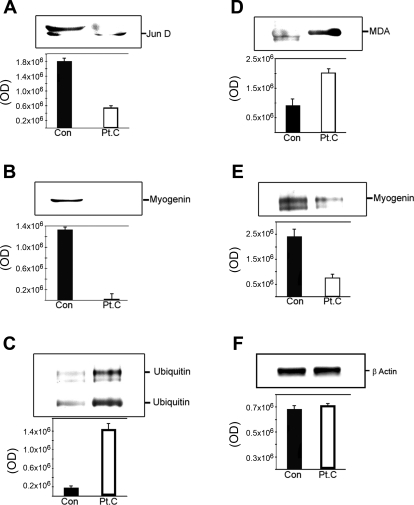
The expression of Jun-D protein was markedly decreased in skeletal muscle from cachectic patients (Figs. 3E, 5A, Supplemental Fig. S6, and Supplemental Table S2) and correlated with the decreased Jun-D mRNA expression (Fig. 4A and Supplemental Table S1) in muscle wasting of cachexia. Quantitation of Jun-D protein expression was obtained from the immunopurification and immunoblot in control (N = 2; C2, C3; 1 rectus abdominis and 1 vastus lateralis) and cachectic (N = 4; Ca6–Ca9; 1 rectus abdominis and 3 vastus lateralis) skeletal muscle samples representative of 3 independent experiments (Fig. 5A), and from immunofluorescent studies in control (N = 2; N1, N2; 2 rectus abdominis) and cachectic samples (N = 5; Ca1, Ca5, Ca6, Ca8, and Ca10; 4 rectus abdominis and 1 vastus lateralis) (Supplemental Fig. S6 and Supplemental Table S2), by using the programs described in materials and methods. Jun-D protein expression was decreased in skeletal muscle from cachectic patients (Fig. 3E, P < 0.001; Fig. 5A; Fig. S6B, P < 0.001; and Supplemental Table S2). Expression of myogenin (associated with Jun-D), indirectly immunopurified with Jun-D antibodies, was consistently observed in control skeletal muscles, but it was substantially decreased in cachectic skeletal muscle in control (N = 2; C2, C3; 1 rectus abdominis and 1 vastus lateralis) and cachectic samples (N = 4; Ca6–Ca9; 1 rectus abdominis and 3 vastus lateralis) representative from three independent experiments (Fig. 5B). No meaningful statistical analysis could be performed on the data from Fig. 5 because the sample size was too small.
Fig. 5.
Jun-D is oxidatively modified, ubiquitinated, and decreased in skeletal muscle from cachectic patients. P values represent the significance of the mean group difference, as estimated by the mixed-effects model. The sample size was too small to support rigorous statistical analysis. We were not able to analyze the entire group of cachectic samples by this assay because of sample quantity limitations. Representative immunoblots obtained using specific antibodies against Jun-D (A) and myogenin (B) in immunopurified Jun-D from skeletal muscle lysates from control (Con) and cachexia (Pt.C) subjects representative of Con (N = 2; C2, C3, 1 rectus abdominis and 1 vastus lateralis, Table 1) and cachectic (N = 4; A1, Ca7, Ca9, and Ca10, 1 rectus abdominis and 3 vastus lateralis, Table 1) skeletal muscle samples. IgG was used a negative Con. Additional quantitation of these muscle proteins was obtained from the immunoblot, by using the Alpha Ease FC program, Alpha Innotech Version 3.2.2. The expression of Jun-D and myogenin in Jun-D immunoprecipitates was decreased. These data are representative of 3 independent experiments. Representative immunoblots obtained by using specific antibodies against ubiquitin (C), MDA (D), myogenin (E), and β-actin (F) (Con for immunopurification) in immunopurified myogenin from skeletal muscle lysates from Con and Pt.C subjects representative of Con (N = 2; as described in A and B) and cachectic (N = 4; as described in A and B) skeletal muscle samples. The ubiquitin antibodies reacted against proteins running as myogenin (top band) and Jun-D (bottom band). The MDA band corresponded to the Jun-D band. IgG was used a negative Con. Additional quantitation of these muscle proteins was obtained from the immunoblot, by use of the Alpha Ease FC program, Alpha Innotech Version 3.2.2. The expression of ubiquitin and MDA was increased and the expression of myogenin was decreased in myogenin immunoprecipitates. These data are representative of 3 independent experiments.
In this context, another member of the Jun family, c-Jun, undergoes proteolysis via the ubiquitin system, and Jun-D is ubiquitinated in serum-stimulated fibroblasts (29, 43). Therefore, we immunoprecipitated Jun-D from skeletal muscle using specific antibodies that do not cross-react with c-Jun or Jun-B (5), and these immunoprecipitates were analyzed in a immunoblot with specific anti-ubiquitin antibodies. The degree of ubiquitination of Jun-D was negligible in skeletal muscle from control subjects (N = 2; C2, C3; 1 rectus abdominis and 1 vastus lateralis) but was increased in patients with cachexia (N = 4; Ca6–Ca9; 1 rectus abdominis and 3 vastus lateralis) representative from three independent experiments, as quantified by use of the program described in materials and methods (Fig. 5C). These findings suggest that the ubiquitination-mediated degradation of Jun-D may also contribute to the pathogenesis of muscle wasting and dedifferentiation of cachexia.
Posttranslational modifications of proteins such as some phosphorylations (40) or oxidations (23, 32) may determine both the degree of ubiquitination and subsequent degradation of these proteins (14, 28, 45). Because enhanced oxidative stress initiates a cascade leading to muscle wasting and dedifferentiation in TNF-α mice and cachectic patients, we assessed the extent of oxidative modifications of Jun-D. The level of MDA-lysine adducts, a sensitive indicator of oxidative stress (5, 16, 19, 33), was increased in immunopurified Jun-D from skeletal muscle of cachectic patients (N = 4; Ca6–Ca9; 1 rectus abdominis and 3 vastus lateralis) compared with control subjects (N = 2; C2, C3; 1 rectus abdominis and 1 vastus lateralis) representative from three independent experiments, as quantified by use of the program described in materials and methods (Fig. 5D).
Expression of myogenin in cachectic skeletal muscle (N = 4; Ca6–Ca9; 1 rectus abdominis and 3 vastus lateralis) compared with control skeletal muscle (N = 2; C2, C3; 1 rectus abdominis and 1 vastus lateralis) was also substantially decreased when directly immunopurified with myogenin antibodies, representative from three independent experiments as quantified by use of the program described in materials and methods (Fig. 5E). Expression of control β-actin was unchanged in the skeletal muscle of cachectic patients compared with controls (Fig. 5F). Thus we demonstrated that myogenin is physically associated with Jun-D in skeletal muscle from control individuals but that myogenin and Jun-D expression are decreased in muscle wasting of cachexia (Fig. 5, B and E).
DISCUSSION
The skeletal muscles of patients with cancer or AIDS had an increased expression of TNF-α mRNA and TNF-α associated with its cognate receptor, as well as activation by a specific phosphorylation of this receptor TNR1, indicating that this signaling cascade incriminated in experimental muscle wasting (5, 10, 11, 27, 33, 38) may also be relevant in human muscle wasting. Of interest, another TNF-α cognate receptor, TNFR2, was not activated in skeletal muscle of cachectic patients (unpublished observations), suggesting that TNFR1 is the critical pathway associated with muscle wasting in patients with cancer and AIDS. However, the precise mechanisms of these abnormalities in human muscle wasting of cachexia remain to be elucidated. Because of the anonymous nature of the skeletal muscle donors, we had no access to their blood samples and serum cytokine levels were not determined in these subjects.
Moreover, confounding factors may exist in the heterogeneous population that we have studied. Both the cancer and AIDS patients were likely on medications that could induce muscle atrophy. In addition, these patients may be malnourished to some extent, considering their recent weight loss, and probably physically inactive. All of these factors (medications, malnutrition, and muscle disuse) can induce muscle atrophy.
We also found an increased oxidative stress as detected by the sensitive and specific indicator MDA (15, 23, 30), as well as an increased NOS2 expression in skeletal muscle of cachectic patients. The increased oxidative stress in skeletal muscle and other tissues have been shown to be induced by TNF-α and related cytokines in cell culture systems and in experimental animal models of cachexia (4, 9), suggesting that a similar mechanism may occur in cachectic patients. Indeed, oxidative stress has been reported in the liver of cachectic animals and cachectic patients and correlated experimentally with decreased albumin synthesis of cachexia (9). NOS2 is also induced in the skeletal muscle and liver of cachectic animals resulting from oxidative stress, since lipophilic antioxidants blocked both oxidative stress and NOS2 induction (4, 9). It has been shown that NF-κB activation results in the induction of NOS2, suggesting that NO production stimulates MyoD mRNA loss (10).
The effects of cachexia were similar in rectus abdominis and vastus lateralis muscles in the different assays performed, but larger size samples will be required to analyze potential differences in the response of various types of skeletal muscles in patients with muscle wasting. MDA was elevated in vastus lateralis compared with rectus abdominis (Supplemental Fig. S1), and myosin was lower in vastus lateralis muscle compared with rectus abdominis (Supplemental Fig. S3). The group effect was still significant at P < 0.001 after controlling for muscle type in each case.
Similarly, there are studies indicating that oxidative stress causes skeletal muscle atrophy due to inactivity or denervation (1). In denervation-induced skeletal muscle atrophy, reactive oxygen species are generated mainly in the mitochondria (26). The relevance of oxidative stress in disuse muscle atrophy, as shown in cachectic animals (5), is emphasized by the prevention of muscle atrophy by antioxidant administration (24, 32).
Our data indicate that Jun-D expression is downregulated at the pretranslational level and that Jun-D undergoes oxidative modifications in muscle wasting of cachexia, possibly resulting in Jun-D ubiquitination. In addition, we demonstrated that myogenin expression is also decreased and that myogenin/Jun-D binding to the CKM-E box is impaired. Of interest, muscle mass of either mixed or oxidative muscles was dramatically decreased in CK−/− mice (25). Moreover, the structure of skeletal muscles is profoundly altered in CK-deficient mice. It has been suggested that the presence of fibers with central nuclei in these muscles may reflect degeneration and regeneration (25). We found a decreased CKM expression in muscle wasting of cachexia. However, it remains to be determined whether an acquired decrease in CKM during the development of cachexia contributes to the muscle mass loss characteristic of muscle wasting and whether it is associated with structural abnormalities in the skeletal muscles from cachectic patients.
Binding of skeletal muscle nuclear proteins from cachectic patients was impaired because of decreased Jun-D/myogenin activities. In turn, these decreased Jun-D/myogenin activities most likely resulted from lower content of these proteins as well as from their oxidation. Indeed, the addition of either purified recombinant Ref-1 or the reducing agent DTT (5, 47) normalized the DNA-protein complex, suggesting that the critical mechanism involves, at least in part, oxidation of a transcriptional factor, presumably the highly conserved cysteine domain (KC285R) of Jun-D (4, 46, 47). Congruent with these results, we found that immunopurified Jun-D was oxidatively modified in cachectic but not in control skeletal muscle. It is reasonable to postulate that these changes in the CKM-E box activities, which are critical for the efficient transcription of the CKM gene, are at least in part responsible for the decreased expression of CKM in cachectic skeletal muscles. However, DNA-protein binding assays could be performed only in three cancer patients and in two control subject because of unavailability of fresh skeletal muscle samples large enough to obtain nuclear extracts. Thus a more definitive analysis of the CKM-E box activities will require more comprehensive studies using larger skeletal muscle samples.
Alternatively, it has been suggested that muscle wasting in animals administered cytokines may result from a defective muscle repair process (36). In differentiated C2C12 myocytes, TNF-α-induced activation of NF-κB inhibited skeletal muscle differentiation by suppressing MyoD expression (13). It remains to be determined whether this mechanism plays a role in muscle wasting of patients with cachexia. The mechanism described herein and the one reported by Guttridge et al. (13) could act synergistically to accelerate muscle wasting in cachexia.
Collectively, this study and a previous report in a mouse model (5) demonstrate that irrespective of the initiating cascade (TNF-α in animals or cachexia in patients), the molecular pathways associated with muscle wasting of cachexia may be similar and perhaps correctable and/or preventable. These studies show that these molecular pathways are modulated in association with muscle atrophy in patients with cancer or AIDS, and whether they cause muscle atrophy remains to be determined.
GRANTS
This study was supported by grants from the National Institute of Health (DK-38652, CA-96932, and CA-128866). M. Buck was supported by the Howard Temin Award from the National Cancer Institute. These studies were carried out in part in the General Clinical Research Center, University of California San Diego, with funding provided by the National Center for Research Resources, M01RR 000827 United States Public Health Service.
Supplementary Material
Acknowledgments
We thank Barbara Andrews, NP, for assistance with the surgical samples and Daniela Traykova, Melissa Mitrou, Marcus Kouma, and Janel Suburu for technical assistance.
REFERENCES
- 1.Barker T, Traber MG. From animals to humans: evidence linking oxidative stress as a causative factor in muscle atrophy. J Physiol 583: 421–422, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Beutler B, Cerami A. Cachectin and tumour necrosis factor as two sides of the same biological coin. Nature 320: 584–588, 1986. [DOI] [PubMed] [Google Scholar]
- 3.Buck M, Chojkier M. A ribosomal S-6 kinase-mediated signal to C/EBP-beta is critical for the development of liver fibrosis. PLoS ONE 2: e1372, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Buck M, Chojkier M. In oxidative stress and muscle wasting of cachexia. In: Oxidative Stress in Skeletal Muscle, edited by Resnick AZ, Packer CK, Sen JO, Holloszy MJ, and Jackson MJ. Basel: Birkhauser Verlag, 1998, p. 277–286.
- 5.Buck M, Chojkier M. Muscle wasting and dedifferentiation induced by oxidative stress in a murine model of cachexia is prevented by inhibitors of nitric oxide synthesis and antioxidants. EMBO J 15: 1753–1765, 1996. [PMC free article] [PubMed] [Google Scholar]
- 6.Buck M, Houglum K, Chojkier M. Tumor necrosis factor α inhibits collagen α1(I) gene expression and wound healing in a murine model of cachexia. Am J Pathol 149: 195–204, 1996. [PMC free article] [PubMed] [Google Scholar]
- 7.Buck M, Poli V, van der Geer P, Chojkier M, Hunter T. Phosphorylation of rat serine 105 or mouse threonine 217 in C/EBPβ is required for hepatocyte proliferation induced by TGFα. Mol Cell 4: 1087–1092, 1999. [DOI] [PubMed] [Google Scholar]
- 8.Buck M, Turler H, Chojkier M. LAP (NF-IL6), a tissue-specific transcriptional activator, is an inhibitor of hepatoma cell proliferation. EMBO J 13: 851–860, 1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Buck M, Zhang L, Hunter T, Chojkier M. Nuclear export of phosphorylated C/EBPβ mediates the inhibition of albumin expression by TNF-α. EMBO J 20: 6712–6723, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Descombes P, Chojkier M, Lichtsteiner S, Falvey E, Schibler U. LAP, a novel member of the C/EBP gene family, encodes a liver-enriched transcriptional activator protein. Genes Dev 4: 1541–1551, 1990. [DOI] [PubMed] [Google Scholar]
- 11.Fong Y, Moldawer LL, Marano MA, Wei H, Barber A, Manogue K, Tracey KJ, Kuo G, Fischman DA, Cerami A, Lowry SF. Cachectin/TNF or IL-1 induces cachexia with redistribution of body proteins. Am J Physiol Regul Integr Comp Physiol 256: R659–R665, 1989. [DOI] [PubMed] [Google Scholar]
- 12.Grunfeld C, Feingold KR. Metabolic disturbances and wasting in the acquired immunodeficiency syndrome. N Engl J Med 327: 329–337, 1992. [DOI] [PubMed] [Google Scholar]
- 13.Guttridge DC, Mayo M, Madrid LV, Wang C, Baldwin A Jr. NF-κB-induced loss of MyoD messenger RNA: possible role in muscle decay and cachexia. Science 289: 2363–2365, 2000. [DOI] [PubMed] [Google Scholar]
- 14.Henkel T, Machleidt T, Alkalay I, Kronke M, Ben-Neriah Y, Baeuerle P. Rapid proteolysis of IκB-α is necessary for activation of transcription factor NF-κB. Nature 365: 182–185, 1993. [DOI] [PubMed] [Google Scholar]
- 15.Holm S A simple sequentially rejective multiple test procedure. Scand J Stat 6: 65–70, 1979. [Google Scholar]
- 16.Holvoet P, Perez G, Zhao Z, Brouwers E, Bernar H, Collen D. Malondialdehyde-modified low density lipoproteins in patients with atherosclerotic disease. J Clin Invest 95: 2611–2619, 1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Houglum K, Bedossa P, Chojkier M. TGF-β and collagen-α1(I) gene expression are increased in hepatic acinar zone 1 of rats with iron overload. Am J Physiol Gastrointest Liver Physiol 267: G908–G913, 1994. [DOI] [PubMed] [Google Scholar]
- 18.Houglum K, Buck M, Alcorn J, Contreras S, Bornstein P, Chojkier M. Two different cis-acting regulatory regions direct cell-specific transcription of the collagen α1(I) gene in hepatic stellate cells and in skin and tendon fibroblasts. J Clin Invest 96: 2269–2276, 1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Houglum K, Filip M, Witztum JL, Chojkier M. Malondialdehyde and 4-hydroxynonenal protein adducts in plasma and liver of rats with iron overload. J Clin Invest 86: 1991–1998, 1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kirchberger M Excitation and contraction of skeletal muscle. In: Physiological Basis of Medical Practice, edited by West JB. Baltimore, MD: Williams & Wilkins, 1991, p. 62–102.
- 21.Laird NM, Ware JH. Random-effects models for longitudinal data. Biometrics 38: 963–974, 1982. [PubMed] [Google Scholar]
- 22.Lee KS, Buck M, Houglum K, Chojkier M. Activation of hepatic stellate cells by TGFα and collagen type I is mediated by oxidative stress through c-myb expression. J Clin Invest 96: 2461–2468, 1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Levine RL, Garland D, Oliver CN, Amici A, Climent I, Lena A, Ahn B, Shaltiel S, Stadtman ER. Determination of carbonyl content in oxidatively modified proteins. Methods Enzymol 186: 464–478, 1990. [DOI] [PubMed] [Google Scholar]
- 24.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(−Delta Delta C(T)) method. Methods 25: 402–408, 2001. [DOI] [PubMed] [Google Scholar]
- 25.Momken I, Lechêne P, Koulmann N, Fortin D, Mateo P, Doan B, Hoerter J, Bigard X, Veksler V, Ventura-Clapier R. Impaired voluntary running capacity of creatine kinase-deficient mice. J Physiol 565: 951–964, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Muller FL, Song W, Jang YC, Liu Y, Sabia M, Richardson A, Van Remmen H. Denervation-induced skeletal muscle atrophy is associated with increased mitochondrial ROS production. Am J Physiol Regul Integr Comp Physiol 293: R1159–R1168, 2007. [DOI] [PubMed] [Google Scholar]
- 27.Oliff A, Defeo-Jones D, Boyer M, Martinez D, Kiefer D, Vuocolo G, Wolfe A, Socher SH. Tumors secreting human TNF-α/cachectin induce cachexia in mice. Cell 50: 555–563, 1987. [DOI] [PubMed] [Google Scholar]
- 28.Palombella VJ, Rando OJ, Goldberg AL, Maniatis T. The ubiquitin-proteasome pathway is required for processing the NF-κB1 precursor protein and the activation of NF-kB. Cell 78: 773–785, 1994. [DOI] [PubMed] [Google Scholar]
- 29.Pfarr C, Mechta F, Spyrou G, Lallemand D, Carillo S, Yaniv M. Mouse Jun-D negatively regulates fibroblast growth and antagonizes transformation by ras. Cell 76: 747–760, 1994. [DOI] [PubMed] [Google Scholar]
- 30.Pinheiro J, Bates D, DebRoy S, Sarkar D; R Development Core Team. nmle: Linear and Nonlinear Mixed Effects Models. R Package version 31-87. Vienna, Austria: R Foundation for Statistical Computing, 2008.
- 31.R Development Core Team. R: A Language and Environment for Statistical Computing. Vienna, Austria: R Foundation for Statistical Computing, 2008.
- 32.Reznick AZ, Packer L. Oxidative damage to proteins: spectrophotometric method for carbonyl assay. Methods Enzymol 233: 357–363, 1994. [DOI] [PubMed] [Google Scholar]
- 33.Schauenstein EH, Esterbauer H, Zollner H. Aldehydes in Biological Systems; Their Natural Occurrence and Biological Activities. London: Pion, 1977.
- 34.Spiegelman BM, Hotamisligil GS. Through thick and thin: wasting, obesity, and TNF-α. Cell 73: 625–627, 1993. [DOI] [PubMed] [Google Scholar]
- 35.Strassman G, Fong M, Kenney JS, Jacob CO. Evidence for the involvement of interleukin 6 in experimental cancer cachexia. J Clin Invest 89: 1681–1684, 1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tisdale M Protein loss in cancer cachexia. Science 289: 2293–2294, 2000. [DOI] [PubMed] [Google Scholar]
- 37.Todorov P, Carluk P, McDevitt T, Coles B, Fearon K, Tisdale M. Characterization of a cancer cachectic factor. Nature 379: 739–742, 1996. [DOI] [PubMed] [Google Scholar]
- 38.Tracey KJ, Cerami A. Tumor necrosis factor, other cytokines and disease. Annu Rev Cell Biol 9: 317–343, 1993. [DOI] [PubMed] [Google Scholar]
- 39.Tracey KJ, Morgello S, Koplin B, Fahey TJI, Fox J, Aledo A, Manogue KR, Cerami A. Metabolic effects of cachectin/tumor necrosis factor are modified by site of production. J Clin Invest 86: 2014–2024, 1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Traenckner EB, Wilk S, Baeuerle PA. A proteasome inhibitor prevents activation of NF-kB and stabilizes a newly phosphorylated form of IkB-α that is still bound to NF-kB. EMBO J 13: 5433–5441, 1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Trautwein C, Caelles C, van der Geer P, Hunter T, Karin M, Chojkier M. Transactivation by NF-IL6/LAP is enhanced by phosphorylation of its activation domain. Nature 364: 544–547, 1993. [DOI] [PubMed] [Google Scholar]
- 42.Trautwein C, van der Geer P, Karin M, Hunter T, Chojkier M. Protein kinase A and C site-specific phosphorylations of LAP (NF-IL6) modulate its binding affinity to DNA-recognition elements. J Clin Invest 93: 2554–2561, 1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Treier M, Staszewski LM, Bohmann D. Ubiquitin-dependent c-Jun degradation in vivo is mediated by the delta domain. Cell 78: 787–798, 1994. [DOI] [PubMed] [Google Scholar]
- 44.Vale RD Getting a grip on myosin. Cell 78: 733–737, 1994. [DOI] [PubMed] [Google Scholar]
- 45.Wing SS, Goldberg AL. Glucocorticoids activate the ATP-ubiquitin-dependent proteolytic system in skeletal muscle during fasting. Am J Physiol Endocrinol Metab 264: E668–E676, 1993. [DOI] [PubMed] [Google Scholar]
- 46.Xanthoudakis S, Curran T. Identification and characterization of Ref-1, a nuclear protein that facilitates AP-1 DNA-binding activity. EMBO J 11: 653–665, 1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Xanthoudakis S, Miao G, Wang F, Pan YC, Curran T. Redox activation of Fos-Jun-DNA binding activity is mediated by a DNA repair enzyme. EMBO J 11: 3323–3335, 1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.