Abstract
Erb’s palsy is initially frightening. The infant’s arm hangs limply from the shoulder with flexion of the wrist and fingers due to weakness of muscles innervated by cervical roots C5 and C6. Risk factors are macrosomia (large baby) and shoulder dystocia. However, Erb’s palsy may occur following cesarian section. The experience of the delivering physician may not influence the risk of Erb’s palsy (0.9 to 2.6 per 1000 live births). Differential diagnosis includes clavicular fracture, osteomyelitis and septic arthritis. Fortunately, the rate of complete recovery is 80% to 96%, especially if improvement begins in the first two weeks. Recommended treatment includes early immobilization followed by passive and active range of motion exercises (although there is no proof that any intervention is effective). For the few infants with no recovery by three to five months, surgical exploration of the brachial plexus may improve the outcome. Three infants with Erb’s palsy who illustrate variations in the evolution of this disorder are presented.
Keywords: Brachial plexus injury, Erb’s palsy, Review
Abstract
Au départ, le syndrome de Duchenne-Erb est effrayant. Le bras du nourrisson pend mollement, et le poignet et les doigts sont fléchis en raison de la faiblesse des muscles innervés par les racines cervicales C5 et C6. Les facteurs de risque sont la macrosomie (gros bébé) et la dystocie de l’épaule. De plus, un syndrome de Duchenne-Erb peut se produire après une césarienne. L’expérience du médecin accoucheur n’influe pas nécessairement sur le risque de syndrome de Duchenne-Erb (de 0,9 à 2,6 cas pour 1 000 naissances vivantes). Le diagnostic différentiel inclut une fracture de la clavicule, une ostéomyélite et une arthrite aiguë suppurée. Heureusement, le taux de guérison complète est de 80 % à 96 %, surtout lorsque des améliorations sont constatées au cours des deux premières semaines de vie. Le traitement recommandé inclut une immobilisation précoce suivie d’exercices passifs et actifs d’amplitude de mouvements (même si rien ne démontre l’efficacité d’une quelconque intervention). Pour les quelques enfants qui n’ont pas guéri en l’espace de trois à cinq mois, une exploration chirurgicale du plexus brachial peut améliorer l’issue. Est présenté le cas de trois nourrissons atteints du syndrome de Duchenne-Erb qui démontrent les variations de l’évolution de cette pathologie.
Erb’s palsy is initially frightening. When a newborn has a brachial plexus injury (BPI), it is often assumed that poor obstetrical technique is to blame. However, there is controversy about the role of the delivery ‘operator’ (1–4). The main risk factors for BPI are large fetal size (often from maternal diabetes) and shoulder dystocia.
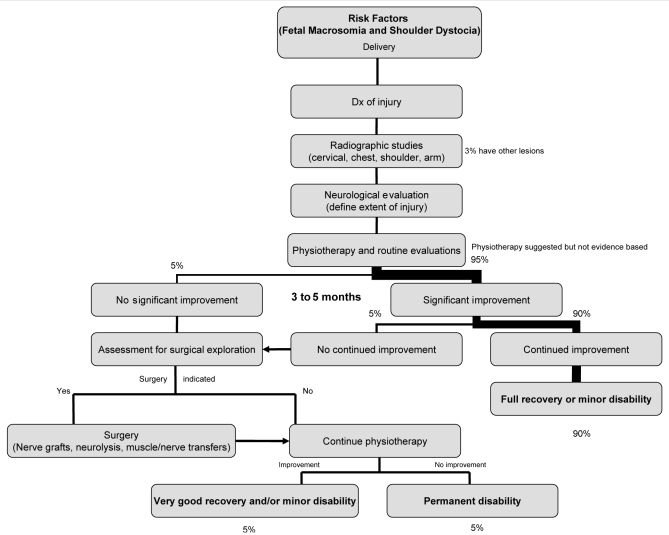
Fortunately, nearly all affected infants recover in the first few months. Surgical exploration may assist some infants with no recovery by five to six months. Only the rare child is left with a significant disability. In the present paper, we will focus on Erb’s palsy and only briefly mention other more severe (but very rare) forms of BPI (eg, Klumpke’s and total plexus injury). Figure 1 outlines our approach to the infant with BPI.
Figure 1).
Risk factors, investigations, treatment and prognosis for brachial plexus injury. Dx Diagnosis
CASE PRESENTATIONS
Case 1: Large baby, shoulder dystocia and complete rapid recovery
This 4398 g boy was born to a gravida (G)3 para (P)2 29-year-old woman. Labour was spontaneous with artificial rupture of membranes yielding meconium-stained fluid. Vaginal delivery was assisted with low forceps but shoulder dystocia led to a difficult extraction. Apgar scores were four (1 min) and eight (5 min). A right Erb’s palsy was immediately apparent with no deltoid and weak bicep function. By five weeks of age, he showed remarkable improvement with spontaneous deltoid contraction (90° of abduction). Also, his biceps flexed his arm against gravity. Wrist and finger extensors were both normal. By three months, recovery was complete.
Case 2: Large baby, shoulder dystocia and slow recovery
This 4200 g baby was delivered with low vacuum suction to a G1P0 mother. Labour was induced due to decreased fetal tone. Apgar scores were nine and nine. Moderate shoulder dystocia required suprapubic pressure, McRoberts maneuver and a ‘corkscrew’ maneuver. Severe right Erb’s palsy was immediately noted with little wrist extension and no bicep or deltoid function. By six weeks of age, the baby still had no bicep or deltoid function; however, the brachioradialis and triceps were normal. With the Moro reflex, there was only supraspinatus contraction with a few degrees of abduction. This infant may still recover; however, plastic surgery assessment is planned if there is no improvement by five months.
Case 3: Breech with ‘entrapped head’ and poor recovery
This 2216 g boy was delivered via vaginal breech at 40 weeks to a G2P1 mother. Following an episiotomy and gentle stretching, the hips and legs were delivered (followed by the arms and shoulders) but the head was ‘stuck’, requiring forceps. There was mild asphyxia. A left Erb’s palsy was noted with no contractions in triceps, biceps and deltoids, but good power in all wrist movements. Recovery was marginal and at eight months a magnetic resonance image (MRI) suggested avulsion of cervical roots C5 and C6. Only neurolysis of C5 and C6 was performed during exploratory surgery at 8.5 months. At six years of age, he has no function and marked wasting of the right deltoid and pectoralis major. At the time of writing, biceps strength was 3/5 and triceps strength was approximately 2.5/5. His grip was excellent but wrist extensors were mildly decreased. He was independent for virtually every activity but he liked to receive help putting his shirt on. He could not bring his left hand to the top of his head without ‘throwing’ the arm up. Unfortunately, he will always be mildly handicapped.
CLINICAL FEATURES
Erb’s palsy results from injury to nerve roots C5 and C6, with C7 also affected in 50% of instances. Muscles involved are listed in Table 1. The classical sign of Erb’s palsy is the ‘waiter’s tip hand’. The arm hangs limply from the shoulder with internal rotation of the forearm plus wrist and finger flexion. Sometimes there are sensory deficits over the lateral proximal upper extremity (5). C3 and C4 are injured in about 5% of patients leading to phrenic nerve dysfunction and paralysis of the hemidiaphragm (6). Horner’s syndrome occurs rarely when the stellate ganglion is disrupted (5).
TABLE 1.
Deficits as a result of brachial plexus injury
| Weak movement | Cord segment | Muscle | Resulting position |
|---|---|---|---|
| Shoulder abduction | C5 | Deltoid | Adducted |
| Shoulder external rotation | C5 | Supra- and infraspinatus | Internally rotated |
| Elbow flexion | C5, C6 | Biceps, brachioradialis | Extended |
| Supination | C5, C6 | Supinators | Pronated |
| Wrist extension | C6, C7 | Extensors of wrist | Flexed |
| Finger extension | C6, C7 | Extensors of fingers | Flexed |
| Diaphragmatic descent | C4, C5 | Elevated |
Data from reference 16
DIFFERENTIAL DIAGNOSIS
Clavicular fracture may lead to a pseudoparalysis, easily mistaken for BPI. In one review of 11,636 deliveries (7), 2.3% had clavicular fracture and 0.4% had Erb’s palsy. Eleven per cent of deliveries with clavicular fracture had Erb’s palsy, while 53% with Erb’s palsy had a clavicular fracture. Cervical ribs may predispose to Erb’s palsy by the stretching of nerves around the cervical rib or through concentrated pressure when the shoulder is forced against the cervical spine (8). However, most infants with cervical ribs do not have BPI.
Osteomyelitis of the humerus or clavicle and septic arthritis of the shoulder can occasionally lead to a BPI, although fewer than 10 cases have been reported (9–11). The pathophysiology appears to be thrombophlebitis of the vasa vasorum or an arterial embolism causing ischemic nerve damage (9).
NEUROPATHOLOGY
The injury is usually where nerve roots form the trunks of the brachial plexus. Erb’s palsy results from a lesion at Erb’s point where C5 and C6 unite to form the upper trunk of the brachial plexus. Klumpke’s palsy (injury in C8 and T1 roots) and total plexus palsy (injury to C5 to T1) are rare. The severity of BPI ranges from least (neuropraxia: permitting complete prompt recovery) to intermediate (axonotmesis: allowing gradual recovery) to severe, with avulsion of the roots from the spinal cord causing permanent injury (neurotmesis).
MECHANISM AND PATHOPHYSIOLOGY
In 1922, a study in cadavers (12) suggested that excessive widening of the angle between the head and shoulder resulted in an upper root injury, whereas abduction and backward rotation damaged lower roots. As reviewed by Jennet et al (13), studies from 1897 and 1916 in at least 476 stillborn cadavers came to identical conclusions.
The largest study of BPI and shoulder dystocia found a striking association between the two (206 dystocias among 323 BPIs). Vaginal breech delivery was also strongly associated with BPI (1). In contrast, Levine et al (14) studied 36 infants with BPI from approximately 14,000 deliveries. In the study, only 22% had shoulder dystocia. Others have suggested a nearly one to one correspondence between shoulder dystocia and Erb’s palsy, especially in cases with permanent injury (15). A major confounding issue is the lack of an objective definition of shoulder dystocia. A widely accepted definition is ‘difficulty’ in delivering the shoulders after the head has delivered (1). Dystocia is often associated with macrosomia (1,16). A study (1) of 776,618 deliveries in the United Kingdom from 1998 to 1999 found 323 cases of BPI (94% had shoulder dystocia and/or macrosomia).
When BPI is associated with dystocia (approximately two-thirds of cases), the position of the affected limb is most likely anterior while BPI without shoulder dystocia (approximately one-third of cases) tends to affect the posterior arm (2). The anterior shoulder gets caught behind the pubic symphysis while the posterior shoulder gets ‘hung up’ on the sacral promontory.
The duration of the second stage of labour may be another risk factor. BPI may be more common with either an extremely short or a prolonged second stage (2,14,17). Erb’s palsy may be more common in multiparous women than in nulliparous women, although this is not a universal finding (17). Fortunately, there is a very low risk of recurrence of shoulder dystocia in subsequent pregnancies. One study (17) noted only one recurrence in 93 subsequent cephalic vaginal deliveries in 80 women.
It is commonly believed that Erb’s palsy is caused by an inexperienced clinician applying too much lateral traction during delivery. The risk of Erb’s palsy is markedly reduced with caesarean section, although it does occur (five of 311 BPI cases in one series) (1,18). These cases could be caused by lateral traction during the caesarean but it is possible that an intrauterine insult is causative (4,19–24). Sandmire and DeMott (2) argued that maternal forces associated with an ultrashort second stage of labour result in a 4.7-fold increase in the rate of BPI (maternal forces may be four to nine times greater than the forces applied by the clinician). BPI could occur before the head is delivered.
Is BPI is the result of obstetrical maneuvers? In some studies (2), the rate of BPI is independent of the complexity of the maneuver used to resolve shoulder dystocia. Furthermore, there is a debate about which maneuvers have the highest risk of BPI. Baskett and Allen (17) claim that the McRoberts maneuver has a low risk of injury and that posterior arm maneuvers have a high risk of injury, while Allen and Edelberg (15) argue the opposite. Another study (2) found that the rate of BPI was independent of the experience of the physician delivering the baby. Therefore, there is a controversy about the mechanism of BPI. BPI continues to be of medical-legal concern with 28 legal actions over a five-year period brought against Canadian obstetricians for trauma to the infant during delivery, usually for BPI (approximately 1.3 million births) (personal communication, Canadian Medical Protective Association).
INVESTIGATIONS
Beyond the physical examination, few investigations are indicated. MRI of the brachial plexus and cervical cord is likely the best imaging technique, if required. There is no convincing evidence of the reliability of electromyography to predict outcome. However, absent fibrillations likely indicate neuropraxia (5). If electromyography denervation persists, spontaneous recovery may be unlikely. Absent sensory conductions may be diagnostic of root avulsion (6,19,25).
PROGNOSIS
Erb’s palsy resolves completely in the first year of life in 80% to 96% of patients and in nearly 100% if recovery begins within four weeks of birth (5).
The authors’ experience in Halifax, Nova Scotia resembles previous publications. Between 1980 to 1989, there were 50,838 deliveries at the Grace Maternity Hospital (routine and high risk) with 44 cases of BPI (0.9 per 1000 live births). By neonatal discharge, 16% completely resolved, 55% showed improvement and only 29% remained unchanged. Forty children with BPI were then followed to two years of age or older. Thirty-eight children (95%) recovered completely. The four lost to follow-up were improving by neonatal discharge. Only two had a permanent disability – a 10-year-old boy with mild to moderate supinator weakness and a six-year-old girl with mild weakness of supination and shoulder rotation. Neither had a significant functional deficit.
TREATMENT
There are no published, randomized, controlled studies addressing treatment for BPI. Immobilization of the affected limb is usually recommended during rest in the first week, followed by active and passive range of motion exercises. Splints may prevent flexion contractures of the wrist and fingers (5,19,26). Electrostimulation is of no proven benefit.
SURGERY
A multidisciplinary team is likely to provide optimal rehabilitation for those with residual deficits. Surgical exploration of the brachial plexus may lead to a better outcome, but controlled trials are needed (26–30). If an MRI does not show root avulsion and there is no bicep function by five months of age, then exploratory surgery may be considered. A flail arm or Horner’s syndrome at three months may justify earlier surgery (26). Overall, based on retrospective case studies, approximately two-thirds of patients undergoing surgical exploration show improvement (29).
Surgical or botulinum toxin injections to release shoulder or elbow contractures may permit major functional improvements (31). Muscle or nerve transfers and a spring-loaded extension splint may assist others with unsatisfactory outcome, especially with children unable to raise their hand to eat, button a shirt or comb their hair (31).
The authors did not find specific literature that indicates how often various complications of incomplete recovery occur. Issues such as shoulder dislocation and dwarfing of the affected limb may occur.
PSYCHOSOCIAL IMPACT
Although not carefully studied, a persistent Erb’s palsy may affect the child’s quality of life and self esteem. Sporting activities are affected, as well as activities of daily living (for example, dressing with a tight sweater).
Among a variety of resources for parents, we found the ‘United Brachial Plexus Network’ website <www.ubpn.org> to be informative (32). Erb’s palsy and treatment options are well explained, although the 90% spontaneous resolution rate is underemphasized. This Web site lists centres with BPI specialists, methods to reduce treatment costs and an extensive reference list. A discussion forum permits people from across the world to post their experiences and opinions and receive feedback.
Acknowledgments
The authors thank Dr Michael Van den Hof, Department of Obstetrics and Gynecology, Dalhousie University, for his careful review and comments on this manuscript.
REFERENCES
- 1.Evans-Jones G, Kay SP, Weindling AM, et al. Congenital brachial palsy: Incidence, causes and outcome in the United Kingdom and Republic of Ireland. Arch Dis Child Fetal Neonatal Ed. 2003;88:F185–9. doi: 10.1136/fn.88.3.F185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sandmire HF, DeMott RK. Erb’s palsy causation: A historical perspective. Birth. 2002;29:52–4. doi: 10.1046/j.1523-536x.2002.00156.x. [DOI] [PubMed] [Google Scholar]
- 3.Ouzounian JG, Korst LM, Phelan JP. Permanent Erb palsy: A traction-related injury? Obstet Gynecol. 1997;89:139–41. doi: 10.1016/s0029-7844(96)00312-2. [DOI] [PubMed] [Google Scholar]
- 4.Gherman RB, Ouzounian JG, Goodwin TM. Brachial plexus palsy: An in utero injury? Am J Obstet Gynecol. 1999;180:1303–7. doi: 10.1016/s0002-9378(99)70633-2. [DOI] [PubMed] [Google Scholar]
- 5.Volpe JJ. Neurology of the newborn. 3rd Edn. Philadelphia, Pennsylvania: WB Saunders; 1995. pp. 781–4. [Google Scholar]
- 6.Shenaq SM, Berzin E, Lee R, Laurent JP, Nath R, Nelson MR. Brachial plexus birth injuries and current management. Clin Plast Surg. 1998;25:527–36. [PubMed] [Google Scholar]
- 7.Peleg D, Hasnin J, Shalev E. Fractured clavicle and Erb’s palsy unrelated to birth trauma. Am J Obstet Gynecol. 1997;177:1038–40. doi: 10.1016/s0002-9378(97)70010-3. [DOI] [PubMed] [Google Scholar]
- 8.Becker MH, Lassner F, Bahm J, Ingianni G, Pallua N. The cervical rib. A predisposing factor for obstetric brachial plexus lesions. J Bone Joint Surg Br. 2002;84:740–3. doi: 10.1302/0301-620x.84b5.12446. [DOI] [PubMed] [Google Scholar]
- 9.Gabriel SR, Thometz JG, Jaradeh S. Septic arthritis associated with brachial plexus neuropathy. A case report. J Bone Joint Surg Am. 1996;78:103–5. doi: 10.2106/00004623-199601000-00014. [DOI] [PubMed] [Google Scholar]
- 10.Sharma RR, Sethu AU, Mahapatra AK, Pawar SJ, Nath A. Neonatal cervical osteomyelitis with paraspinal abscess and Erb’s palsy. A case report and brief review of the literature. Pediatr Neurosurg. 2000;32:230–3. doi: 10.1159/000028943. [DOI] [PubMed] [Google Scholar]
- 11.Clay SA. Osteomyelitis as a cause of brachial plexus neuropathy. Am J Dis Child. 1982;136:1054–6. doi: 10.1001/archpedi.1982.03970480020004. [DOI] [PubMed] [Google Scholar]
- 12.Adson AW. The gross pathology of brachial plexus injuries. Surg Gynecol Obstet. 1922;34:350–7. [Google Scholar]
- 13.Jennett RJ, Tarby TJ, Krauss RL. Erb’s palsy contrasted with Klumpke’s and total palsy: Different mechanisms are involved. Am J Obstet Gynecol. 2002;186:1216–20. doi: 10.1067/mob.2002.123743. [DOI] [PubMed] [Google Scholar]
- 14.Levine MG, Holroyde J, Woods JR, Siddiqi TA, Scott M, Miodovnik M. Birth trauma: Incidence and predisposing factors. Obstet Gynecol. 1984;63:792–5. [PubMed] [Google Scholar]
- 15.Allen RH, Edelberg SC. Erb’s palsy: Concepts of causation. Obstet Gynecol. 2000;96:801–2. doi: 10.1016/s0029-7844(00)01068-1. [DOI] [PubMed] [Google Scholar]
- 16.Boyd M, Usher R, Mclean F. Fetal macrosomia: Prediction, risks, proposed management. Obstet Gynecol. 1983;61:715–22. [PubMed] [Google Scholar]
- 17.Baskett TF, Allen AC. Perinatal implications of shoulder dystocia. Obstet Gynecol. 1995;86:14–7. doi: 10.1016/0029-7844(95)00099-D. [DOI] [PubMed] [Google Scholar]
- 18.al-Qattan MM, el-Sayed AA, al-Kharfy TM, al-Jurayyan NA. Obstetrical brachial plexus injury in newborn babies delivered by caesarean section. J Hand Surg. 1996;21B:263–5. doi: 10.1016/s0266-7681(96)80112-4. [DOI] [PubMed] [Google Scholar]
- 19.Dodds SD, Wolfe SW. Perinatal brachial plexus palsy. Curr Opin Pediatr. 2000;12:40–7. doi: 10.1097/00008480-200002000-00009. [DOI] [PubMed] [Google Scholar]
- 20.Gherman RB, Ouzounian JG, Miller DA, Kwok L, Goodwin TM. Spontaneous vaginal delivery: A risk factor for Erb’s palsy? Am J Obstet Gynecol. 1998;178:423–7. doi: 10.1016/s0002-9378(98)70413-2. [DOI] [PubMed] [Google Scholar]
- 21.Acker DB, Gregory DK, Sachs BP, Friedman EA. Risk factors for Erb-Duchenne Palsy. Obstet Gynecol. 1988;71:389–92. [PubMed] [Google Scholar]
- 22.Jennett RJ, Tarby TJ, Kreinick CJ. Brachial plexus palsy: An old problem revisited. Am J Obstet Gynecol. 1992;166:1673–7. doi: 10.1016/0002-9378(92)91555-o. [DOI] [PubMed] [Google Scholar]
- 23.Pecorari D. A guest editorial: Erb palsy without apparent shoulder dystocia. Obstet Gynecol Surv. 2002;57:547. doi: 10.1097/00006254-200209000-00001. [DOI] [PubMed] [Google Scholar]
- 24.Spellacy WN. Erb’s palsy without shoulder dystocia. Am J Obstet Gynecol. 1998;179:561–2. doi: 10.1016/s0002-9378(98)70403-x. [DOI] [PubMed] [Google Scholar]
- 25.Kwast O. Electrophysiological assessment of maturation of regenerating motor nerve fibres in infants with brachial plexus palsy. Dev Med Child Neurol. 1989;31:56–65. doi: 10.1111/j.1469-8749.1989.tb08412.x. [DOI] [PubMed] [Google Scholar]
- 26.Ramos LE, Zell JP. Rehabilitation program for children with brachial plexus and peripheral nerve injury. Semin Pediatr Neurol. 2000;7:52–7. doi: 10.1016/s1071-9091(00)80010-8. [DOI] [PubMed] [Google Scholar]
- 27.Waters P. Comparison of the natural history, the outcome of microsurgical repair, and the outcome of operative reconstruction in brachial plexus birth palsy. J Bone Joint Surg Am. 1999;81:649–59. doi: 10.2106/00004623-199905000-00006. [DOI] [PubMed] [Google Scholar]
- 28.Rust RS. Congenital brachial plexus palsy: Where have we been and where are we now? Semin Pediatr Neurol. 2000;7:58–63. doi: 10.1016/s1071-9091(00)80011-x. [DOI] [PubMed] [Google Scholar]
- 29.McNeely PD, Drake JM. A systematic review of brachial plexus surgery for birth-related brachial plexus injury. Pediatr Neurosurg. 2003;38:57–62. doi: 10.1159/000068045. [DOI] [PubMed] [Google Scholar]
- 30.Grossman JA. Early operative intervention for birth injuries to the brachial plexus. Semin Pediatr Neurol. 2000;7:36–43. doi: 10.1016/s1071-9091(00)80008-x. [DOI] [PubMed] [Google Scholar]
- 31.Price A, Tidwell M, Grossman JA. Improving shoulder and elbow function in children with Erb’s palsy. Semin Pediatr Neurol. 2000;7:44–51. doi: 10.1016/s1071-9091(00)80009-1. [DOI] [PubMed] [Google Scholar]
- 32.United Brachial Plexus Network < http://www.ubpn.org> (Version current at August 31, 2004).