Abstract
Background
The open artery hypothesis postulates that late opening of an infarct-related artery after myocardial infarction (MI) will improve clinical outcomes. The quality of life (QOL) and economic outcomes associated with this strategy have not been described.
Methods
The Occluded Artery Trial (OAT) compared percutaneous coronary intervention (PCI) plus stenting with medical therapy alone in stable, high-risk patients who had a totally occluded infarct-related artery at 3 to 28 days post-MI. In 951 patients (44% of those eligible), QOL was assessed by a battery that included two principal outcome measures, the Duke Activity Status Index (DASI) reflecting cardiac-related physical functioning, and the Rand Short-Form 36 Mental Health Inventory 5 reflecting psychological well-being. Structured QOL interviews were performed at baseline, 4, 12, and 24 months. Costs were measured in 458 of 469 U.S. patients (98%) and 2-year cost effectiveness was estimated.
Results
At 4 months, the medical therapy group showed a clinically marginal 3.4-point decline in DASI relative to the PCI group (p=0.007). At 1 and 2 years, the differences were smaller. No significant differences in psychological well-being were observed. In the 469 US OAT patients, cumulative 2-year costs were about $7,000 higher in the PCI group (p<0.0001) while quality-adjusted survival was marginally higher in the medical therapy group.
Conclusions
In this trial, PCI was associated with a marginal advantage in cardiac physical functioning at 4 months but not thereafter. At 2 years, medical therapy remained significantly less expensive than routine PCI and had higher quality-adjusted survival.
Keywords: Percutaneous coronary intervention, quality of life, costs, coronary artery disease, myocardial infarction
Introduction
Despite the widespread availability of several effective acute reperfusion strategies, at least one-third of hospitalized acute myocardial infarction (MI) patients have persistent occlusion of the infarct-related artery beyond 72 hours. Consequently, clinicians and researchers have had great interest in the possibility that some of the benefits seen with early opening of the infarct-related artery could be achieved with later opening. Evidence from experimental studies, observational studies, and small clinical trials has supported the “Open Artery Hypothesis,” which postulates that late opening of occluded infarct-related arteries following acute MI could improve survival (through less sudden cardiac death and less heart failure), ventricular function (through both revascularization of hibernating myocardium and improved post-MI remodeling), and quality of life.1
To assess this hypothesis, we performed a trial involving 2166 stable patients who had an occluded infarct-related artery 3 to 28 calendar days (minimum 24 hours) after an MI. Eligible patients had either their infarct-related artery occlusion in the proximal portion of a vessel with a large risk region or an ejection fraction below 50%.2 Subjects were randomly assigned to optimal medical therapy alone or to optimal medical therapy plus PCI with coronary stenting. We previously reported that the rate of the primary outcome of the trial—i.e. the composite of death, reinfarction, or hospital treatment for class IV heart failure— did not differ significantly between the PCI and medical therapy groups at 4 years (17.2% and 15.6%, respectively; hazard ratio for PCI 1.16, p=0.20).2 Significantly fewer PCI patients had angina (a secondary endpoint) at 4 months and 1 year; by 3 years, the rates in the two arms were similar. The purpose of the current report is to describe the effects of the two treatment strategies on economic and quality of life (EQOL) outcomes of OAT patients.1
Methods
Quality of Life Data Collection Methods
The initial OAT study plan was for all patients to be enrolled in North America; all U.S. patients were to be included in the economic study, and both U.S. and Canadian patients were to be included in the QOL analyses. Of the countries subsequently added to meet enrollment goals, 4 (Latvia, New Zealand, Poland, Russia) agreed to participate in the QOL substudy. Due to funding issues, EQOL enrollment stopped as planned at the end of 2004 when OAT enrollment had reached 1970 patients (Figure 1). Of those 1970 patients, 951 were enrolled in the QOL substudy. The parent OAT trial continued enrollment for one additional year, reaching a total sample of 2166 patients.2
Figure 1.
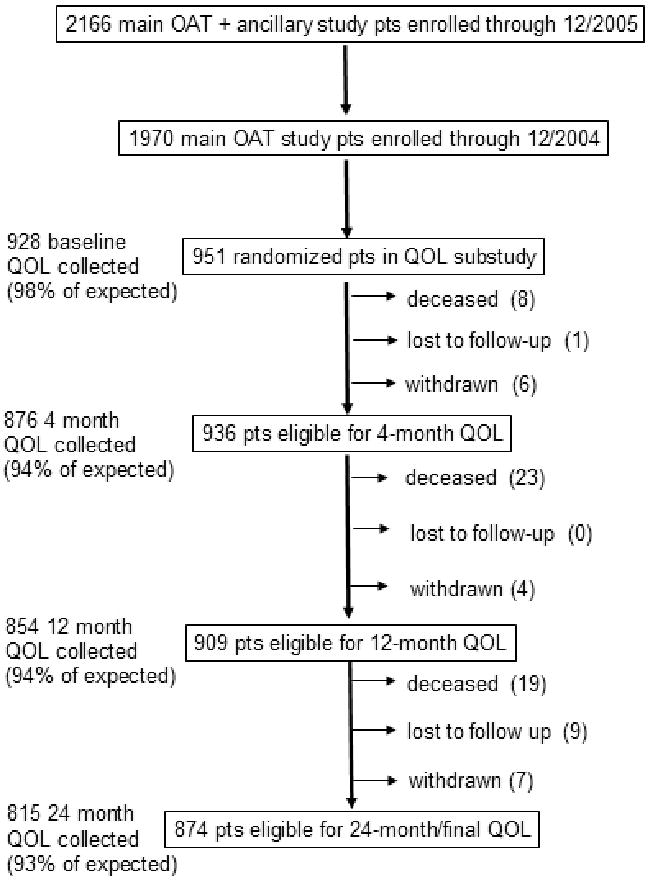
details the selection process that yielded the QOL Substudy cohort from the parent OAT cohort and provides information about the completeness of QOL data collection.
Baseline QOL questionnaires were administered after written informed consent was obtained and prior to randomization/treatment. Follow-up structured interviews were administered at 4 months, 12 months, and 24 months post-randomization. Site coordinators, who had been trained by the EQOL Coordinating Center, conducted all interviews during a clinic visit, or by telephone if a clinic visit was missed. From a total of 3670 expected patient contacts, 3473 (95%) QOL questionnaires were collected (Figure 1). Two percent of patients refused participation, 1% of questionnaires collected were incomplete, and 4% were shortened proxy forms used for patients who were unable to participate in the full interview, e.g., because of illness or incapacity.
Quality of Life Measures
Two principal QOL measures were prespecified: the Duke Activity Status Index (DASI) and the Short Form-36 (SF-36) Mental Health Inventory (MHI-5). The DASI is a questionnaire analog of the cardiopulmonary exercise test and is used to assess cardiac-related functional status.3 Scores range from 0 (worst) to 58 (best) and reflect the ability of patients to do physical activities in 12 domains without difficulty or assistance. A clinically significant difference is considered to be 4 points or more.4
The inclusion of non-English speaking patients outside North America (n=40) in the study made it necessary to identify a QOL instrument that already had validated translations; the SF-36 physical functioning (PF) scale was used in these patients rather than the DASI.5 This generic scale assesses the ability to engage in 10 sets of physical activities; these activities overlap substantially with those assessed in the DASI, although the scoring method is different. One-to-one imputation methods were used to transform the SF-36 PF subscales into corresponding DASI values. Since comparisons by treatment were unaffected by whether these imputed values were included or excluded, the imputed values are included in this paper.
The MHI-5 assesses psychological well-being, including both depression and anxiety.5 It is scored from 0 (worst) to 100 (best). A clinically significant difference is approximated by a ¼ SD difference, approximately 5 points.
Seven additional scales from the SF-36 were collected: role function-physical, role function-emotion, general health, bodily pain, social function, vitality, and health transitions. Each scale is scored separately and is customarily transposed to a 0 (worst) to 100 (best) scale. Differences of 5 points or more are considered clinically significant.
We collected the Rose Angina and Dyspnea scales to assess cardiac symptoms. 6 The Rose Angina scale consists of 7 questions that ask about the presence of chest pain and whether it is provoked by walking and relieved by rest. We classified typical angina as present when patients reported symptoms consistent with the classic features of that syndrome. The Rose Dyspnea scale consists of 4 questions asking about dyspnea induced by progressively lesser levels of exertion. We classified effort dyspnea as present if patients reported any effort dyspnea in response to these questions.
A short set of questions, originally derived for the Bypass Angioplasty Revascularization Investigation (BARI) randomized trial, assessed employment status at baseline and during follow-up.7
Health utilities were assessed using the time trade-off method, with each patient asked in a series of questions how much of a postulated life expectancy of 10 years in their current health state they would be willing to trade in order to live the remaining years in excellent health.8 We also asked patients to rate their health on a 0 to 100 scale where 0 was death and 100 was excellent health; a five point or more difference is considered clinically significant.
Statistical Analyses
Descriptive statistics include percentiles for discrete variables, and medians and interquartile ranges, means and standard deviations, or both for continuous variables. Comparisons were performed according to randomized treatment assignment. Univariate tests included the chi-square test for discrete variables and Wilcoxon rank-sum test for continuous variables.
P-values are reported without adjustment for multiple comparisons. We also calculated 95% confidence intervals on treatment differences at each time point (PCI minus medical therapy) based on the t-distribution.
Cost and Cost-Effectiveness Analyses
The economic substudy enrolled only U.S. OAT patients (n=469). For these patients, medical costs out to 2 years were estimated using a combination of hospital bills (for inpatient care) and Medicare fees (for physician fees and outpatient care). Hospital bill data were collected for 458 U.S. patients (98%). Hospital charges on the UB-92 hospital bill forms were converted to average hospital costs using the Medicare conversion factors reported each year in each hospital's Medicare Cost Report.9,10 Physician fees were estimated from counts of major physician services and the Medicare Fee Schedule, as previously described.11,12 Costs were expressed as 2005 U.S. dollars. Follow-up costs after the first year were discounted at a 3% annual rate.
Costs incurred at non-OAT hospitals prior to randomization were presumed balanced by random allocation and were not collected. By protocol, all enrolled patients had a diagnostic catheterization demonstrating angiographic eligibility.
Because the final results of OAT showed that PCI was not superior to medical therapy alone in reducing major cardiac events, but had a small advantage in freedom from angina in the early follow-up period, we performed a prespecified 2-year cost-effectiveness analysis to examine the relationship of incremental net health benefits [expressed as quality-adjusted life years (QALYs)] and incremental costs for PCI. For each arm, the observed survival was weighted with a utility or preference weight, derived from empirically assessed time-trade-off data.
As a sensitivity analysis, we used the mean time-trade-off weights for angina according to Canadian Cardiovascular Class (CCS) to assign patient utilities over the first two study years to surviving patients. Utility values were assigned at baseline and carried forward until the next available CCS re-classification (at 4, 12, and 24 months).
Cumulative 2-year QALYs and 2-year medical costs were aggregated by treatment assignment and the difference between treatments calculated. The ratio of these two incremental quantities constitutes the cost-effectiveness ratio. Bootstrap analysis (1000 samples with replacement) was used to generate a 95% confidence interval for the incremental 2-year costs and QALYs. Due to lack of any clear trends in the 2-year data and the lack of evidence that PCI reduced major events, longer term cost-effectiveness analyses were not performed.
All patients provided written informed consent. Study protocol approval was obtained from each site's institutional review board or ethics committee.
Results
Patient Population and Baseline Characteristics
Of the 2166 patients enrolled in OAT, 1970 were enrolled by the end of 2004, and 951 of these (48%) were enrolled in the QOL substudy (Figure 1). While the substudy patients were generally representative of the overall OAT population, the QOL sample had slightly higher proportions of minorities and patients with a history of infarction, heart failure and hypercholesterolemia (Table 1). Within the QOL substudy, the two treatment groups were well balanced, with only one of 18 baseline comparisons (history of cerebrovascular disease) yielding a nominally significant p-value (Table 2).
Table 1. Baseline Characteristics of Overall OAT Patient Population versus Quality of Life Subset.
| Overall OAT (n=1970) | Non-QOL OAT (n=1019) | QOL OAT Substudy (n=951) | P-value* | |
|---|---|---|---|---|
| Age, years | 58.8±11.0 | 58.3±10.9 | 59.2±11.1 | 0.06 |
| Median (25th, 75th) | 59.0 (51.0 to 67.0) | 59.0 (51.0 to 68.0) | 58.0 (50.0 to 67.0) | |
| Female | 21.9 | 22.2 | 21.7 | 0.78 |
| Non-white | 14.6 | 12.2 | 17.25 | <0.0001 |
| Medical History | ||||
| History of angina | 23.0 | 23.4 | 22.6 | 0.66 |
| Prior myocardial infarction | 11.7 | 9.8 | 13.8 | 0.006 |
| History of cerebrovascular disease | 3.6 | 3.0 | 4.3 | 0.11 |
| Prior stroke | 3.0 | 2.6 | 3.4 | 0.33 |
| History of peripheral vascular disease | 3.9 | 3.4 | 4.5 | 0.21 |
| History of renal insufficiency | 1.4 | 1.6 | 1.2 | 0.42 |
| History of ICD | 0.4 | 0.3 | 0.5 | 0.43 |
| History of congestive heart failure | 2.5 | 1.4 | 3.6 | 0.002 |
| Prior percutaneous coronary intervention | 4.9 | 4.0 | 5.9 | 0.06 |
| Prior coronary artery bypass grafting | 0.4 | 0.1 | 0.7 | 0.03 |
| Cardiac Risk Factors | ||||
| Diabetes | 20.8 | 19.2 | 22.6 | 0.06 |
| Hypercholesterolemia | 55.8 | 52.6 | 59.4 | 0.004 |
| Hypertension | 48.8 | 47.8 | 49.8 | 0.37 |
| Current smoker | 39.3 | 41.5 | 36.9 | 0.001 |
| NYHA Class | <0.001 | |||
| I | 83.3 | 89.0 | 85.3 | 0.02 |
| II | 16.8 | 10.2 | 14.7 | 0.02 |
Age values are presented as means (standard deviation) and medians (25th to 75th percentiles). All other values are N (percent). ICD = implantable cardioverter-defibrillator, NYHA = New York Heart Association.
P-values are comparing QOL versus non-QOL subsets.
Race was self reported on the clinical case report form.
Table 2. Baseline Characteristics of Patients in Quality of Life Substudy by Intention to Treat.
| PCI (n=477) | Medical therapy alone (n=474) | P-value | |
|---|---|---|---|
| Age, years | 59.5±10.9 | 59.0±11.3 | 0.50 |
| Median (25th, 75th) | 59.0 (52.0 to 68.0) | 59.0 (51.0 to 68.0) | |
| Female | 20.6 | 22.8 | 0.40 |
| Non-white | 16.8 | 17.7 | 0.13 |
| Medical History | |||
| History of angina | 22.3 | 22.8 | 0.86 |
| Prior myocardial infarction | 15.8 | 11.7 | 0.07 |
| History of cerebrovascular disease | 5.7 | 3.0 | 0.04 |
| Prior stroke | 4.5 | 2.4 | 0.09 |
| History of peripheral vascular disease | 5.7 | 3.2 | 0.07 |
| History of renal insufficiency | 1.7 | 0.6 | 0.13 |
| History of ICD | 0.8 | 0.2 | 0.18 |
| History of congestive heart failure | 4.0 | 3.2 | 0.51 |
| Prior percutaneous coronary intervention | 5.9 | 5.9 | 1.00 |
| Prior coronary artery bypass grafting | 0.8 | 0.6 | 0.71 |
| Cardiac Risk Factors | |||
| Diabetes | 20.6 | 24.6 | 0.14 |
| Hypercholesterolemia | 59.8 | 58.9 | 0.78 |
| Hypertension | 50.4 | 49.3 | 0.72 |
| Current smoker | 35.1 | 38.8 | 0.36 |
| NYHA Class | 0.53 | ||
| I | 88.2 | 86.7 | |
| II | 8.7 | 9.1 | |
| III | 1.3 | 2.5 | |
| IV | 1.9 | 1.7 | |
Age values are presented as means (standard deviation) and medians (25th to 75th percentiles). All other values are N (percent). ICD = implantable cardioverter-defibrillator, NYHA = New York Heart Association.
Race was self reported on the clinical case report form.
Quality of Life Outcomes
Principal Quality of Life Endpoints
Comparison of cardiac-related physical functioning by treatment group (Table 3) showed similar scores at baseline, and a 3.4-point lower DASI score (indicating more functional impairment) for the medical therapy group at 4 months (p=0.008). The mean difference in DASI was 1.0 (p=0.36) at 12 months and 1.7 (p=0.29) at 24 months, with PCI having the higher scores at both intervals.
Table 3. Selected QOL Measures by Intention to Treat.
| PCI | Medical therapy alone | Difference between PCI and medical therapy alone (95% CI) | ||
|---|---|---|---|---|
| DASI | ||||
| Baseline | N | 429 | 436 | |
| median | 42 (18 to 58) | 43 (19 to 58) | ||
| mean | 36.3±19.7 | 37.3±19.6 | -1.00 (-3.63 to 1.62) | |
| 4 months | N | 407 | 412 | |
| median | 41 (19 to 58) | 35 (16 to 51) | ||
| mean | 36.8±19.3 | 33.3±19.2 | 3.43 (0.79 to 6.07) | |
| 12 months | N | 400 | 409 | |
| median | 42 (19 to 58) | 41 (17 to 58) | ||
| mean | 37.0±20.0 | 36.0±20.1 | 1.01 (-1.76 to 3.77) | |
| 24 months | N | 384 | 392 | |
| median | 42 (20 to 58) | 40 (17 to 58) | ||
| mean | 37.1±19.5 | 35.4±20.2 | 1.74 (-1.06 to 4.54) | |
| Mental Health Inventory-5 | ||||
| Baseline | N | 443 | 446 | |
| median | 80 (64 to 88) | 76 (60 to 88) | ||
| mean | 74.7±18.6 | 72.6±19.3 | 2.08 (-0.41 to 4.58) | |
| 4 months | N | 413 | 420 | |
| median | 84 (64 to 92) | 80 (64 to 92) | ||
| mean | 77.2±18.5 | 75.7±18.9 | 1.53 (-1.01 to 4.07) | |
| 12 months | N | 408 | 406 | |
| median | 80 (68 to 92) | 84 (64 to 92) | ||
| mean | 77.4±18.5 | 77.0±18.9 | 0.38 (-2.19 to 2.95) | |
| 24 months | N | 386 | 387 | |
| median | (68 to 92) | 80 (65 to 92) | ||
| mean | 78.7±17.5 | 76.9±17.6 | 1.79 (-0.69 to 4.26) | |
| Exertional (Rose) Angina | ||||
| Baseline | N | 400 | 405 | |
| % yes | 26.3 | 26.9 | -0.66 (-6.77,5.44) | |
| 4 months | N | 398 | 405 | |
| % yes | 10.3 | 16.5 | -6.24 (-10.93,-1.55) | |
| 12 months | N | 395 | 393 | |
| % yes | 10.4 | 12.5 | -2.09 (-6.53,2.35) | |
| 24 months | N | 379 | 379 | |
| % yes | 7.1 | 11.9 | -4.75 (-8.91,-0.59) | |
| Exertional (Rose) Dyspnea | ||||
| Baseline | N | 422 | 423 | |
| % yes | 46.7 | 48.0 | -1.31 (-8.04,5.42) | |
| 4 months | N | 395 | 394 | |
| % yes | 32.4 | 39.6 | -7.19 (-13.87,-0.51) | |
| 12 months | N | 388 | 383 | |
| % yes | 30.7 | 40.2 | -9.54 (-16.26,-2.82) | |
| 24 months | N | 369 | 368 | |
| % yes | 28.5 | 35.3 | -6.87 (-13.58,-0.16) | |
Continuous variables shown as medians (25th to 75th percentiles) and means with standard deviations. Categorical variables are shown as percentages.
95% confidence intervals on treatment differences calculated as PCI minus medical therapy alone.
Comparison of the SF-36 MHI-5 did not demonstrate either statistically or clinically significant differences at any follow-up point (Table 3).
Additional Quality of Life Endpoints
No differences by treatment were found for the other SF-36 subscales measured (Appendix A).
At baseline, 26 to 27% of patients reported exertional angina in the month preceding the index myocardial infarction. At 4 months, 16.5% of the medical therapy patients and 10.3% of the PCI patients reported angina (p=0.01) but absolute differences narrowed subsequently (at 24 months 11.9% versus 7.1%, respectively; p=0.03). At baseline, 47-48% of patients reported exertional dyspnea. At 4 months, 39.6% of medical therapy patients and 32.4% of PCI patients reported exertional dyspnea (p=0.04). At 12 and 24 months, the corresponding rates were 40.2% and 30.7% (p=0.006) and 35.3% and 28.5% (p=0.05), respectively.
Resource Use and Costs
In the 458 OAT patients enrolled in the U.S. who had medical billing data collected (98% of 469 total U.S. enrollment), baseline characteristics were well-balanced by treatment assignment (data not shown). In the first 30 days following enrollment, PCI patients stayed in hospital on average 1.2 days longer than medical therapy patients (5.8 days vs. 4.6 days) with the difference reflecting differential ICU stay (3.4 days vs. 2.6 days) (Table 4). In the U.S. cohort, 98% of patients assigned to PCI underwent the procedure while 14% of medical therapy patients had a PCI prior to index hospital discharge (rate includes PCIs on both IRA and other vessels). Initial 30-day costs (hospital plus physician) for the PCI arm were $22,859 versus $12,683 for the medical therapy arm (p<0.0001).
Table 4. Resource Use in the U.S. Economic Substudy Cohort.
| PCI (N=232) | Medical therapy alone (N=226) | |
|---|---|---|
| Resource Use | ||
| 0-30 days | ||
| Length of stay (mean) | 5.8 days | 4.6 days |
| CABG (%) | 0.4 | 0.0 |
| ICD (%) | 2.6 | 0.9 |
| Cardiac catheterization (%) | 100 | 92.0 |
| PCI (%) | 97.8 | 14.2 |
| 31 days – 4 months | ||
| Length of stay (mean) | 0.6 days | 0.7 days |
| CABG (%) | 1.3 | 0.9 |
| ICD (%) | 1.7 | 1.3 |
| Cardiac catheterization (%) | 5.2 | 5.8 |
| PCI (%) | 2.6 | 5.3 |
| 4 months – 12 months | ||
| Length of stay (mean) | 0.5 | 1.2 |
| CABG (%) | 1.7 | 0.9 |
| ICD (%) | 0.9 | 0.0 |
| Cardiac catheterization (%) | 7.8 | 6.6 |
| PCI (%) | 3.9 | 4.4 |
| 12 months – 24 months | ||
| Length of stay (mean) | 0.5 | 0.8 |
| CABG (%) | 0.9 | 1.8 |
| ICD (%) | 0.9 | 0.9 |
| Cardiac catheterization (%) | 4.3 | 4.4 |
| PCI (%) | 1.3 | 1.8 |
CABG = coronary artery bypass grafting, ICD = implantable cardioverter-defibrillator, PCI = percutaneous coronary intervention.
From 30 days to 1 year, 7% of PCI and 10% of medical therapy patients had PCI performed. Mean follow-up hospital days during the first year were 1.1 days for PCI and 1.9 days for MED. Mean follow-up costs out to 12 months were $3,414 for PCI and $5,289 for MED (p<0.0001). Interval discounted costs for year 2 were $1,515 for PCI and $2,727 for MED (p=0.01).
Within-Trial Cost Effectiveness
The 2-year net costs for the PCI arm were $7,089. Using the observed follow-up utility weights at 4, 12, and 24 months (Appendix A), the 2-year quality-adjusted survival was 1.42 years for PCI versus 1.45 years for medical therapy. In 1,000 bootstrap repetitions, 89% of samples had either lower costs and higher QALYs for medical therapy (62%) or a cost-effectiveness ratio > $100,000 per QALY for PCI versus medical therapy (27%). When we applied time trade-off weights to the CCS empirical data for each treatment group and time point and recalculated the cost-effectiveness ratios, 94% of bootstrap samples had either both lower costs and higher QALYs for medical therapy (70%) or a cost-effectiveness ratio >$100,000 per QALY for PCI (24%).
As a sensitivity analysis, we used a regression model to develop cost weights from the US cost data for the entire 1970 OAT cohort. The estimated 2-year incremental cost of PCI in this calculation was $9,945, and only 1 out of 1000 bootstrap cost-effectiveness ratios for PCI versus medical therapy was < $100,000 per QALY.
Discussion
Our study found that, as compared with medical therapy alone, late PCI of occluded infarct arteries provided a marginal clinical advantage in cardiac physical functioning at 4 months that was not sustained. In addition, medical therapy alone resulted in both lower cumulative medical costs and higher quality-adjusted life expectancy out to 2 years, with no empirical trends suggesting that longer term follow-up might reveal a reversal of these patterns. Combined with the previously reported lack of advantage of PCI with respect to the primary endpoint of OAT, these data do not support the common practice of routine PCI in stable post-MI patients with an occluded IRA.2
Additional QOL comparisons showed only that PCI patients had less exertional angina and dyspnea at 4 months, and that the angina difference attenuated by 12 months, while the dyspnea difference was sustained through 24 months. The significance of these subjective differences is unclear since, except at the 4-month follow-up, they did not affect the activities patients reported they were able to do.
Comparison of our results with the Clinical Outcomes Utilizing Revascularization and Aggressive Drug Evaluation (COURAGE) trial is instructive.13 COURAGE found that routine PCI resulted in small incremental benefits in both angina symptoms and physical limitations at 3 months over medical therapy alone. By 12 months, the differences in physical limitations were no longer significant, and the differences in anginal symptoms had attenuated by about 50%. The Trial of Invasive versus Medical Therapy in Elderly Patients (TIME) also compared invasive therapy versus medicine, but enrolled elderly patients (mean age 80 years) with refractory angina.14 While both treatment groups improved over the first 6 months, the invasively treated group had larger improvements in angina and general health perceptions. However, at a median of 3.1 years, the advantage of the invasive treatment had disappeared.15
Thus, our study adds to the evidence that in a variety of clinical situations involving stabilized coronary artery disease patients, a strategy of routine revascularization adds only a modest early advantage in symptoms and functional status that is not maintained. Given that this modest QOL benefit was produced at a cost of over $7000 per patient, our cost-effectiveness analysis found that this strategy was not economically attractive. The COURAGE cost-effectiveness analysis came to a similar conclusion, estimating that the cost per additional quality-adjusted life-year with PCI versus medical therapy alone was around $288,000.16
Several caveats apply to our work. First, sample sizes for both the QOL and economic substudies were considerably smaller than initially planned, due to the enrollment in the parent study of patients outside North America. While the QOL substudy sample differed from the main OAT population in some baseline measures, the differences were clinically small and unlikely to have affected our conclusions. Sensitivity analysis for the economic substudy suggests that a substantively different result would have been unlikely even if we had included all OAT patients in the analysis. Because treatment was not masked, we cannot rule out biases in QOL responses and changes in patterns of care resulting from knowledge of treatment assignment.
In summary, PCI of a persistently occluded infarct artery at 3 to 28 days post infarction provided clinically marginal improvement in physical functioning relative to medical therapy alone but this effect was not sustained at 1 year or beyond. In addition, PCI was more expensive than medical therapy alone out to 2 years, and the small symptom benefits observed were insufficient to make PCI an economically attractive strategy in OAT-eligible patients.
Supplementary Material
Acknowledgments
We gratefully acknowledge Jason Blevins for the coordination of the EQOL study, Heather Read for the economic data collection, Judith Stafford for preparing the data for analysis, and Melanie R. Daniels for editing the manuscript. We are particularly indebted to the OAT site coordinators who worked extremely hard to collect the data for this study and the OAT patients who volunteered to be a part of the study.
The project described was supported by grants from the NHLBI (U01 HL062257 to Dr. Mark, U01 HL062509 to Dr. Hochman).
Disclosures: Dr. Mark reports having received lecture fees and grant support from Medtronic. Dr. Anstrom reports having received grant support from Eli Lilly, Pfizer, and Medtronic Vascular. Dr. Cowper reports having received grant support from CV Therapeutics, Eli Lilly, United Healthcare, and Pfizer. Dr. Hochman reports having received lecture fees from the Network for Continuing Medical Education (NCME receives funding from BMS/Sanofi) and grant support from Guidant, CV Therapeutics, Millenium, Schering Plough, Eli Lilly, and Merck.
Footnotes
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Heart, Lung, And Blood Institute or the National Institutes of Health.
References
- 1.Hochman JS, Lamas GA, Knatterud GL, Buller CE, Dzavik V, Mark DB, Reynolds HR, White HD. Design and methodology of the Occluded Artery Trial (OAT) Am Heart J. 2005;150:627–42. doi: 10.1016/j.ahj.2005.07.002. [DOI] [PubMed] [Google Scholar]
- 2.Hochman JS, Lamas GA, Buller CE, Dzavik V, Reynolds HR, Abramsky SJ, Forman S, Ruzyllo W, Maggioni AP, White H, Sadowski Z, Carvalho AC, Rankin JM, Renkin JP, Steg PG, Mascette AM, Sopko G, Pfisterer ME, Leor J, Fridrich V, Mark DB, Knatterud GL. Coronary intervention for persistent occlusion after myocardial infarction. N Engl J Med. 2006;355:2395–407. doi: 10.1056/NEJMoa066139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hlatky MA, Boineau RE, Higginbotham MB, Lee KL, Mark DB, Califf RM, Cobb FR, Pryor DB. A brief self-administered questionnaire to determine functional capacity (the Duke Activity Status Index) Am J Cardiol. 1989;64:651–4. doi: 10.1016/0002-9149(89)90496-7. [DOI] [PubMed] [Google Scholar]
- 4.Hlatky MA, Rogers WJ, Johnstone I, Boothroyd D, Brooks MM, Pitt B, Reeder G, Ryan T, Smith H, Whitlow P, Wiens R, Mark DB. Medical care costs and quality of life after randomization to coronary angioplasty or coronary bypass surgery. Bypass Angioplasty Revascularization Investigation (BARI) Investigators. N Engl J Med. 1997;336:92–9. doi: 10.1056/NEJM199701093360203. [DOI] [PubMed] [Google Scholar]
- 5.Ware JE, Jr, Snow KK, Kosinski M, Gandek B. SF-36 Health Survey: Manual & Interpretation Guide. Boston: Nimrod Press; 1993. [Google Scholar]
- 6.Rose GA, Blackburn H, Gillum RF, Prineas RJ. Cardiovascular Survey Methods. Geneva: World Health Organization; 1982. p. 162. [Google Scholar]
- 7.Hlatky MA, Charles ED, Nobrega F, Gelman K, Johnstone I, Melvin J, Ryan T, Wiens R, Pitt B, Reeder G, Smith H, Whitlow P, Zorn G, Mark DB Bari Study Group. Initial functional and economic status of patients with multivessel coronary disease randomized in the Bypass Angioplasty Revascularization Investigation (BARI) Am J Cardiol. 1995;75:34C–41C. [PubMed] [Google Scholar]
- 8.Torrance GW. Measurement of health state utilities for economic appraisal. A review J Health Econ. 1986;5:1–30. doi: 10.1016/0167-6296(86)90020-2. [DOI] [PubMed] [Google Scholar]
- 9.Mark DB, Hlatky MA. Medical economics and the assessment of value in cardiovascular medicine: Part I. Circulation. 2002;106:516–20. doi: 10.1161/01.cir.0000021407.93752.7b. [DOI] [PubMed] [Google Scholar]
- 10.Mark DB, Nelson CL, Anstrom KJ, Al-Khatib SM, Tsiatis AA, Cowper PA, Clapp-Channing NE, vidson-Ray L, Poole JE, Johnson G, Anderson J, Lee KL, Bardy GH. Cost-effectiveness of defibrillator therapy or amiodarone in chronic stable heart failure: results from the Sudden Cardiac Death in Heart Failure Trial (SCD-HeFT) Circulation. 2006;114:135–42. doi: 10.1161/CIRCULATIONAHA.105.581884. [DOI] [PubMed] [Google Scholar]
- 11.Mark DB, Anstrom KJ, Sun JL, Clapp-Channing NE, Tsiatis AA, vidson-Ray L, Lee KL, Bardy GH. Quality of life with defibrillator therapy or amiodarone in heart failure. N Engl J Med. 2008;359:999–1008. doi: 10.1056/NEJMoa0706719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mark DB, Harrington RA, Lincoff AM, Califf RM, Nelson CL, Tsiatis AA, Buell HE, Mahaffey KW, Davidson-Ray L, Topol EJ. Cost effectiveness of platelet glycoprotein IIb/IIIa inhibition with eptifibatide in patients with non-ST elevation acute coronary syndromes. Circulation. 2000;101:366–71. doi: 10.1161/01.cir.101.4.366. [DOI] [PubMed] [Google Scholar]
- 13.Weintraub WS, Spertus JA, Kolm P, Maron DJ, Zhang Z, Jurkovitz C, Zhang W, Hartigan PM, Lewis C, Veledar E, Bowen J, Dunbar SB, Deaton C, Kaufman S, O'Rourke RA, Goeree R, Barnett PG, Teo KK, Boden WE, Mancini GB. Effect of PCI on quality of life in patients with stable coronary disease. N Engl J Med. 2008;359:677–87. doi: 10.1056/NEJMoa072771. [DOI] [PubMed] [Google Scholar]
- 14.The TIME Investigators. Trial of invasive versus medical therapy in elderly patients with chronic symptomatic coronary-artery disease (TIME): a randomised trial. Lancet. 2001;358:951–7. doi: 10.1016/S0140-6736(01)06100-1. [DOI] [PubMed] [Google Scholar]
- 15.Pfisterer M. Long-term outcome in elderly patients with chronic angina managed invasively versus by optimized medical therapy: four-year follow-up of the randomized Trial of Invasive versus Medical therapy in Elderly patients (TIME) Circulation. 2004;110:1213–8. doi: 10.1161/01.CIR.0000140983.69571.BA. [DOI] [PubMed] [Google Scholar]
- 16.Weintraub WS, Zhang Z, Kolm P, Jurkovitz C, Hartigan PM, Culler S, Becker E, Lewis C, Veledar E, Bowen J, Dunbar SB, Deaton C, Kaufman S, Maron DJ, Teo K, O'Rourke RA, Goeree R, Barnett P, Spertus JA, Boden WE. Quality of life and economic outcomes in the Clinical Outcomes Utilizing Percutaneous Coronary Revascularization and Aggressive Guide-line-Driven Drug Evaluation (COURAGE) trial. American College of Cardiology 56th Annual Scientific Session, Late Breaking Clinical Trials; New Orleans, Louisiana, United States. Mar 27, 2007. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


