Abstract
Voltage-dependent calcium (Ca2+, CaV1.2) channels are the primary Ca2+ entry pathway in smooth muscle cells of resistance-size (myogenic) arteries, but their molecular identity remains unclear. Here we identified and quantified CaV1.2 α1-subunit splice variation in myocytes of rat resistance-size (100–200 μm diameter) cerebral arteries. Full-length clones containing either exon 1b or the recently identified exon 1c exhibited additional primary splice variation at exons 9*, 21/22, 31/32, and ± 33. Real-time PCR confirmed the findings from full-length clones and indicated that the major CaV1.2 variant contained exons 1c, 8, 21, and 32+33, with ∼57% containing 9*. Exon 9* was more prevalent in clones containing 1c (72%) than in those containing 1b (33%), suggesting exon-selective combinatorial splicing. To examine the functional significance of this splicing profile, membrane currents produced by each of the four exon 1b/c/ ± 9* variants were characterized following transfection in HEK293 cells. Exon 1c and 9* caused similar hyperpolarizing shifts in both current-voltage relationships and voltage-dependent activation of currents. Furthermore, exon 9* induced a hyperpolarizing shift only in the voltage-dependent activation of channels containing exon 1b, but not in those containing exon 1c. In contrast, exon 1b, 1c, or +9* did not alter voltage-dependent inactivation. In summary, we have identified the CaV1.2 α1-subunit splice variant population that is expressed in myocytes of resistance-size arteries and the unique electrophysiological properties of recombinant channels formed by exon 1 and 9* variation. The predominance of exon 1c and 9* in smooth muscle cell CaV1.2 channels causes a hyperpolarizing shift in the voltage sensitivity of currents toward the physiological arterial voltage range.
Keywords: voltage-dependent calcium channel, myogenic artery, cloning, ribonucleic acid splicing
l-type, voltage-gated Ca2+ channels are expressed in multiple cell types, including neurons, cardiac myocytes, and smooth muscle cells (6). In vascular smooth muscle cells, L-type Ca2+ (Cav1.2) channels provide the major Ca2+ entry pathway (13, 22, 23). Ca2+ influx via Cav1.2 channels regulates multiple smooth muscle functions, including contractility and gene expression (26, 32). Indeed, Cav1.2 channels play a critical role in mediating myogenic tone development in small, resistance-size arteries and arterioles that regulate blood pressure and organ blood flow (4, 21). In systemic hypertension, arteries display elevated CaV1.2 channel expression and arterial smooth muscle cells exhibit an increase in L-type Ca2+ current density, consistent with increased contractility (24). In addition, because of their ability to block CaV1.2 channels and reduce Ca2+ influx, voltage-dependent Ca2+ channel blockers are effective for alleviating hypertension and other cardiovascular diseases associated with increased vascular tone, including some forms of angina (31). However, despite the importance of CaV1.2 channels in regulating arterial smooth muscle physiology and evidence that CaV1.2 channels contribute to human vascular disease (20, 24, 30, 34), the molecular identity of these channels in smooth muscle cells of myogenic arteries remains unclear.
L-type, voltage-gated Ca2+ channels are hetero-oligomeric protein complexes, consisting of a voltage-gated channel-forming α1- and regulatory (α2δ-, β-, γ-) subunits (3, 6). The CaV1.2 α1-subunit gene (CACNA1C) is subject to alternative splicing, which generates structural and functional diversity in channel proteins (1). For instance, 19 of the 55 exons present in the human CaV1.2 gene are subject to alternative splicing, providing a large potential variance in channel amino acid sequences that can modify the ion current phenotype (17, 27, 28).
Our laboratory has recently identified a novel Cav1.2 α1-subunit 5′ end (termed exon 1c), which is expressed in smooth muscle cells of rat resistance-size cerebral arteries that control brain blood pressure and regional flow (7). Earlier work from our laboratory determined that in cerebral artery smooth muscle cell CaV1.2 channel mRNA, exon 1c is predominant, with the residual mRNA containing exon 1b, and exon 1a being absent (7). Considering that the human CaV1.2 gene can undergo extensive alternative splicing and that two different 5′ ends, one of which has not been fully characterized, occur in rat arterial smooth muscle cell CaV1.2, we examined the molecular identities and functions of these channels in small cerebral arteries. In this study, we cloned full-length CaV1.2 subunits containing each 5′ end, identified CaV1.2 splice variation in these clones, and quantified splicing regions in smooth muscle cells of these arteries. Our data indicate that exon 1c and 9* undergo combinatorial splicing and that either of these variants causes a hyperpolarizing shift in Cav1.2 voltage-dependent activation. Our study suggests that CaV1.2 exon combinatorial splicing shifts the voltage sensitivity toward the physiological range found in arterial smooth muscle cells. Determining the molecular composition of CaV1.2 subunits that are expressed in smooth muscle cells of resistance-size arteries is a necessary first step to understanding the multiple functions of these channels in vascular physiology and disease and, more specifically, in controlling brain blood flow.
MATERIALS AND METHODS
Tissue preparation and cell isolation.
All procedures were approved by the Institutional Animal Care and Use Committee of the University of Tennessee Health Science Center (UTHSC). Sprague-Dawley rats (∼250 g) were euthanized by peritoneal injection of pentobarbital sodium solution (150 mg/kg). Small, resistance-size, myogenic, cerebral arteries (posterior and middle cerebral, cerebellar; <100–200 μm diameter) were dissected and cleaned of connective tissue using small forceps. Arterial smooth muscle cells were dissociated using an enzyme procedure similar to that previously described (15). Cardiac myocytes were kindly provided by Dr. P. A. Hofmann in the Department of Physiology at UTHSC. Arterial or cardiac myocytes were manually collected under an inverted microscope, yielding pure cell preparations (2, 7, 16).
RNA isolation and reverse transcription.
Cerebral arteries from 4 to 6 rats were combined, and total RNA was isolated using TRIzol reagent (Invitrogen). First-strand cDNA was synthesized using oligo d(T) and reverse transcriptase (SuperScript III; Invitrogen). For cerebral artery smooth muscle cells and cardiac myocytes, total RNA was extracted using the RNAqueous-4PCR kit (Ambion), followed by DNAase treatment. First-strand cDNA was synthesized using oligo d(T) and reverse transcriptase (SuperScript II; Invitrogen).
Amplification of full-length CaV1.2 cDNA using PCR.
Full-length CaV1.2 was amplified from cerebral artery cDNA with primers recognizing two different CaV1.2 5′ ends (exon 1b or 1c) using the Expand Long Template PCR System (Roche Applied Science). Sense 6 (5′- GCTCGCGGCTGTTGCTGCATTTCTTCC-3′) and antisense 4 (5′-AGGTCACGAGAACAGTGAGGCACTTCTGA-3′) were used for exon 1b-containing subunits, whereas sense 5 (5′- CCTGGGCTTGCTGTCTCCCGAGTTTCTG-3′) and antisense 4 were used for exon 1c-containing subunits (7). Antisense 4 was designed according to the highly conserved 3′ untranslated region of known CaV1.2 sequences (GenBank accession numbers: rat brain, M67515 and M67516; and rat aorta, M59786). PCR products were purified, ligated into pGEM-T easy vector (Promega), and transformed into JM109 cells (Promega). LB/ampicillin/IPTG/X-Gal plates were used to select plasmids, which were then cultured in 5 ml LB and purified with QIAprep Spin Miniprep kit. The presence of CaV1.2 cDNA in the plasmids was identified using restriction analysis on 0.8% agarose gels and confirmed DNA sequencing at the UTHSC Molecular Resource Center.
Real-time PCR.
TaqMan probes labeled with FAM and primers (Table 1) designed to detect exons 9/9*, 21/22, and 32/31+33/32+33 were obtained using Assays-by-Design (Applied Biosystems). Reaction mixtures were prepared using first-strand cDNA from either smooth muscle cells or cardiac myocytes, 2× TaqMan Universal PCR Master Mix (Applied Biosystems), and 20× Assays-by-Design Assay Mix (Applied Biosystems). PCR reactions were performed using an ABI PRISM 7700 Sequence Detection System (Applied Biosystems). Amplification was conducted for a period of 10 min at 95°C, followed by 40 PCR cycles of 15 s at 95°C and 1 min at 60°C. Each experiment was performed in triplicate, and the calculated mean was used for each experimental data point (or n). The amplification efficiency for exon 9 and exon 9+9* was 1.96, whereas the amplification efficiency of exons 21, 22, 32, 31+33, and 32+33 was 2. The relative ratio between spliced exons was calculated using XΔCt, where X is amplification efficiency and ΔCt is the difference in PCR cycle number.
Table 1.
Primer and probe sequences used for real-time PCR
| Variable | Primers and Probes |
|---|---|
| 9 | |
| Forward | AGGGCATGGATGAAGACAAACC |
| Reverse | TTTTCGGTGTTGACAGACTCAGT |
| FAM probe | TTGTGGGCATGCTCATGTT |
| 9 + 9* | |
| Forward | AGGGCATGGATGAAGACAAACC |
| Reverse | CCAAGCAAATTTCCCTTTCTTCTGAT |
| FAM probe | CTGGAGCGCCTCTGTTT |
| 21 | |
| Forward | ACATTGTTTTTACCACCATTTTCACCAT |
| Reverse | GCCCTTGTGCAGGAAAGC |
| FAM probe | CCGTAAGCAGTCATCTTT |
| 22 | |
| Forward | CAATGCAGACTATGTCTTCACTAGTATCT |
| Reverse | GCCCTTGTGCAGGAAAGC |
| FAM probe | CCGTAAGCAGTCATCTTAA |
| 32–33 | |
| Forward | GATTGTTGTGGGTAGCATTGTTGAT |
| Reverse | GATGGAGATGCGGGAGTTCT |
| FAM probe | CTCTGCACTGTGTACCTC |
| 31 + 33 | |
| Forward | CGTCATTGGGAGCATAATTGATGTC |
| Reverse | CACTCATAGAGGGAGAGCATTGG |
| FAM probe | ACTAATCCAGCTGAACATAC |
| 32 + 33 | |
| Forward | GATTGTTGTGGGTAGCATTGTTGAT |
| Reverse | CACTCATAGAGGGAGAGCATTGG |
| FAM probe | TACACCCAGCTGAACATA |
Cell culture and transient transfection.
HEK293 cells (ATCC) were maintained in DMEM (Cellgro) supplemented with 10% FBS and 1% penicillin-streptomycin under standard tissue culture conditions (21% O2-5% CO2; 37°C). Full-length Cav1.2 α1-subunits were subcloned from pGEM-T easy vector into pIRES-heGFP II vector (Stratagene). HEK293 cells were transfected with one of four different Cav1.2 α1-subunit splice variants: pIRES-Cav1.2e1b-hrGFPII (+exon 9), pIRES-Cav1.2e1b-hrGFPII (+exon 9+9*), pIRES-Cav1.2e1c-hrGFPII (+exon 9), and pIRES-Cav1.2e1c-hrGFPII (+exon 9+9*) with pcDNA3-α2δ-1 and pGW-β1b (1 μg of each). Transfection was done using the Ca2+ phosphate method. Transfected cells were grown on sterile glass coverslips. Electrophysiological experiments were performed 36 h after transfection. pGW-β1b and pcDNA3-α2δ-1 were kindly provided by Dr. Henry Colecraft (Johns Hopkins University School of Medicine) and Dr. Diane Lipscombe (Brown University), respectively.
Patch-clamp electrophysiology.
Whole cell patch-clamp recordings were obtained using an Axopatch 200B amplifier (Axon Instruments, Foster City, CA) and pCLAMP 8.2 or 9.2. Borosilicate glass electrodes (1–3 MΩ) were filled with a pipette solution containing (in mM) 135 CH3CsO3S, 5 CsCl, 5 EGTA, 4 MgATP, 0.25 Na2GTP, 10 HEPES, and 10 glucose (pH adjusted to 7.2 with CsOH). The extracellular bath solution contained (in mM) 130 NMDG, 1 MgCl2, 10 HEPES, 10 glucose, and 10 BaCl2 (adjusted to pH 7.4 with aspartic acid). HEK293 cells were visualized using a Nikon TS100 microscope using an epifluorescence attachment to identify GFP fluorescence. Fluorescent HEK cells that were not attached to neighboring cells were used to obtain whole cell patch-clamp recordings. Current-voltage (I-V) relationships and steady-state inactivation was measured every 15 s by providing 1-s conditioning pulses to between −80 mV and +30 mV in 10-mV increments before a 200-ms test pulse to 0 mV. I-V relationships were determined from the peak current evoked by each conditioning voltage. Steady-state inactivation was measured from the peak of the test pulse to 0 mV. To measure activation, tail currents were generated by repolarization to −80 mV after a series of 20-ms test pulses to between −60 mV and +60 mV in 10-mV increments. Whole cell currents were filtered at 1 to 2 kHz and digitized at 4–10 kHz. P/4 protocols were used to subtract leak and capacitive transients. Steady-state inactivation curves and tail currents were fit with a Boltzmann function: I/Imax = Rin + (Rmax − Rin)/[1 + exp(V − V1/2)/K], where I/Imax is the normalized peak current, V is the conditioning prepulse voltage, V1/2 is the half-inactivation voltage of steady-state inactivation or half-activation voltage for tail currents, K is the slope factor, Rin is the proportion of noninactivating current, and Rmax is the proportion of maximal current. Inactivation time constants (τ) were obtained using an exponential function: It = Ie−t/τ + I0, where It is the inward current at time t and I0 is the residual current.
Statistical analysis.
Electrophysiological data were analyzed using Clampfit 8.2 and 9.2 and Origin 6.5. Values are expressed as means ± SE. Statistical significance was calculated by using one-way ANOVA followed by Student-Newman-Keuls test for multiple comparisons, except for comparison of exon 21 versus 22, which was calculated using a paired Student's t-test. P < 0.05 was considered significant.
RESULTS
Multiple CaV1.2 splice variants are expressed in rat resistance-size cerebral arteries.
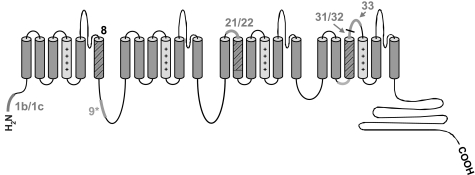
To determine CaV1.2 splice variation in smooth muscle cells of rat resistance-size cerebral arteries, we used RT-PCR to clone full-length CaV1.2 subunits containing either exon 1b or 1c. The entire coding region of CaV1.2 was amplified using one of two forward primers designed for either exon 1b or 1c and a reverse primer for the conserved 3′ untranslated region of CaV1.2. CaV1.2 subunits were then subcloned into the pGEM-T easy vector and sequenced. Twenty-seven plasmids containing full-length CaV1.2 cDNA were obtained, of which 18 contained exon 1c and nine contained exon 1b (Table 2). Sequencing revealed that in addition to exon 1 splicing, three common alternative splicing regions were also present at exon 9*, exon 21/22, and exon 31/32/33 (highlighted in green in Fig. 1). Exon 9* encodes a 25 amino acid sequence located within the intracellular linker between repeats I and II, exon 21/22 is responsible for segment 2 in repeat III (IIIS2), exon 31/32 generates segment 3 in repeat IV (IVS3), and exon 33 is part of the extracellular loop between IVS3 and IVS4 (Fig. 1). All transcripts contained exon 8, consistent with an earlier study that reported exon 8 is expressed in whole aorta, whereas exon 8a is expressed in cardiac myocytes (12). It should be noted that in Ref. 8, the nomenclature used for exon 8 and 8a is opposite to that used in both our study and another study (27). With the use of this nomenclature, exon 8 has a HaeIII restriction site that is absent in exon 8a.
Table 2.
Cav1.2 splice variants expressed in rat resistance-size cerebral arteries
| Transcripts | Exon 1 | Exon 8/8a | Exon 9* | Exon 21/22 | Exon 31/32 | Exon 33 |
|---|---|---|---|---|---|---|
| Cav1.2e1c | 1c | |||||
| C1, C2, C3, C4 | 8 | + | 21 | 32 | + | |
| C5, C6, C7, C8 | 8 | − | 21 | 32 | + | |
| C9 | 8 | + | 21 | 32 | − | |
| C10 | 8 | − | 21 | 32 | − | |
| C11, C12 | 8 | + | 21 | 31 | + | |
| C13, C14, C15, C16, C17 | 8 | + | 22 | 32 | + | |
| C18 | 8 | + | 22 | 31 | + | |
| Cav1.2e1b | 1b | |||||
| B1, B2 | 8 | + | 21 | 32 | + | |
| B3, B4, B5, B6, B7, B8 | 8 | − | 21 | 32 | + | |
| B9 | 8 | + | 22 | 31 + 71 nt | + |
Each clone has been arranged in the table according to the presence or absence of major splice variants. For exon 9* and 33: +presence; −absence. Individual clones are named to allow identification of the parent channel in which additional minor splice variants were found, as described in results. Cav1.2, voltage-dependent Ca2+ channel.
Fig. 1.
Major regions that undergo splicing in cerebral artery smooth muscle cell voltage-dependent Ca2+ (Cav1.2) channel α1-subunits. The 4 major regions are illustrated as bold lines or hatched cylinders. The presence of only exon 8 is also highlighted. The S4 segments that function as voltage sensors are shaded in light gray.
We then sought to determine whether exon 1 variation modifies the occurrence of downstream splice variants in arterial smooth muscle cell Cav1.2 α1-subunit mRNA. Sequencing of CaV1.2 clones containing exon 1c (termed CaV1.2e1c) revealed seven different exon combinations generated by splicing at exon 9*, 21/22, and 31/32/33 (Table 2). A majority of the transcripts contained exon 9* (72%), exon 21 (67%), exon 32 (83%), and exon 33 (89%). Thus data suggest that the major CaV1.2e1c isoform in rat cerebral arteries is the product of exon combination 8/9*/21/32+33. In contrast with the diversity of splicing found in CaV1.2e1c-derived isoforms, only three different exon combinations were found in CaV1.2 transcripts containing exon 1b (CaV1.2e1b). Furthermore, 67% of CaV1.2e1b clones exhibited the 21/32+33 combination with 9* absent. These data indicate that the major isoform of CaV1.2e1b in rat cerebral artery smooth muscle cells is likely to be derived from exons 8/21/32+33. Although exon 32+33 was the major exon combination found in both CaV1.2e1b and CaV1.2e1c transcripts, additional combinations also occurred, including exon 31+33 and exon 32 without 33 (Table 2). However, expression of exon 31 in the absence of exon 33 was not detected (Table 2).
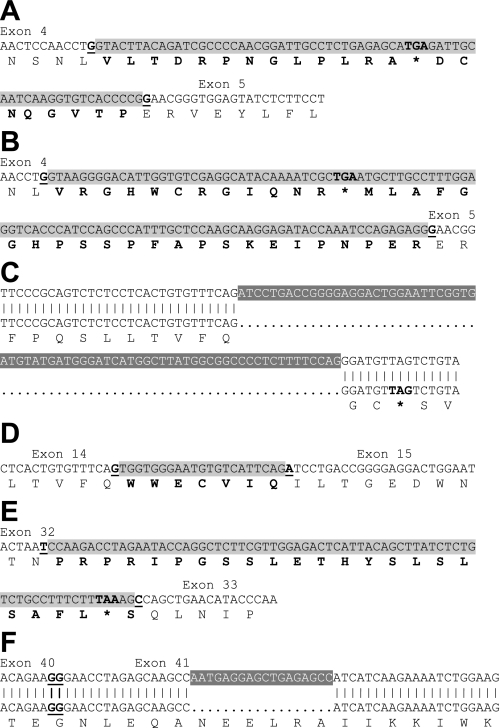
Several additional splicing sites were identified in Cav1.2 α1-subunit clones (Fig. 2). Transcripts C4 and C8 both contained an intron between exons 4 and 5, with a 66-nt insert in C24 and a 108-nt insert in C39 (Fig. 2, A and B). Both inserts result in a premature stop codon between exons 4 and 5. Transcript C5 contained a 21-nt insert between exons 14 and 15 that encodes the sequence WWECVIQ. This sequence is derived from a 5′ elongation of exon 15 due to RNA splicing near the 3′ intron end (Fig. 2C). Clone C17 contained a deletion within exon 15, which results in a stop codon downstream of the deletion site (Fig. 2D). Transcript B9 contained an additional 71-nt sequence due to 5′ elongation of exon 33, leading to a stop codon upstream of exon 33 (Fig. 2E). Finally, transcript C16 had an 18 nucleotide deletion within exon 41, which would result in deletion of the hexapeptide NEELRA (Fig. 2F). This sequence is located in the region that is involved in formation of the apocalmodulin binding pocket (11).
Fig. 2.
Additional splicing sites identified in cerebral artery CaV1.2 α1-subunits. Insertions are illustrated as black font shaded in light gray, with deletions as white font shaded with dark gray. *Stop codon. Amino acids introduced by insertions are indicated in bold font. Exon/exon junctions are presented as underlined bold font. A: 66-nt insert (155117087-155117024) between exons 4 and 5 in clone C4. B: 108-nt insert (155229797-155229690) between exons 4 and 5 in clone C8. C: 73-nt deletion (154984855-154984783) within exon 15 in clone C17. D: 21- nt insert (154984876-154984856) between exons 14 and 15 in clone C5. E: 71-nt insert (155174200-155174130) between exon 32 and 33 in clone B9. F: 18-nt deletion (154911308-154911291) within exon 41 in clone C16.
Quantification of alternative splicing of CaV1.2 exons in cerebral artery smooth muscle cells.
Having identified CaV1.2 splice variants that are expressed in small cerebral arteries, we next quantified relative expression of these variants in isolated cerebral artery smooth muscle cells using real-time PCR. For each experiment, we used ∼100 freshly isolated smooth muscle cells that were visualized under a microscope and individually aspirated into a micropipette to collect a pure cell population, as our laboratory has done previously (2, 7, 16).
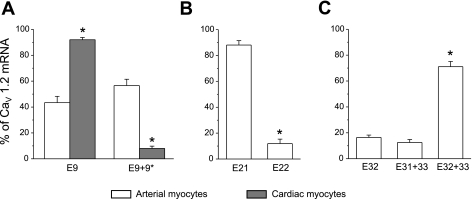
Data indicated that exon 9 and exon 9+9* exhibited similar expression levels (Fig. 3). Since exon 9* expression is considered to be relatively specific for smooth muscle cells, we compared the relative expression of exon 9 ± 9* in arterial smooth muscle cells with that in cardiac myocytes (12, 20). In contrast with cerebral artery smooth muscle cells, only 8% of CaV1.2 transcripts in rat cardiac myocytes contained exon 9*. These data support the idea that although exon 9* expression is relatively specific to arterial smooth muscle cells, arterial smooth muscle cells also express a significant proportion of Cav1.2 that is deficient in exon 9*. In contrast, in arterial smooth muscle cells, exon 21 expression was approximately ninefold higher than that of exon 22, or 81% of total. Furthermore, the combination of exons 32+33 was most prevalent, whereas exon 32 alone and exons 31+33 were present in less than 30% of total mRNA.
Fig. 3.
Real-time quantitative RT-PCR identifies relative expression of major CaV1.2 exon variants in isolated cerebral artery smooth muscle cells and cardiac myocytes. A: exon 9* expression is higher in cerebral artery smooth muscle cells (n = 6) than in cardiac myocytes (n = 5). *P < 0.05 when compared with exon 9 and 9* expression in arterial smooth muscle cells. B: in cerebral artery smooth muscle cells, CaV1.2 subunits preferentially express exon 21 (n = 4). *P < 0.05 when compared with exon 21 expression. C: exon 32+33 is the major splice variant in cerebral artery smooth muscle cells (n = 5). *P < 0.05 when compared with exon 32 and exon 31+33 expression. Each n is the calculated mean of real-time PCR experiments that were performed in triplicate.
Splicing of exon 1c and 9* generates Cav1.2 currents with unique electrophysiological properties.
A majority (72%) of full-length exon 1c-containing transcripts contained exon 9*, whereas a minority (33%) of exon 1b-containing transcripts contained exon 9* (Table 2). These data suggest that exon 1c and 9* undergo combinatorial splicing in arterial smooth muscle cell Cav1.2 α1-subunits. Such combinatorial splicing may produce channels with unique electrophysiological properties. To test this hypothesis, Cav1.2 α1-subunit clones containing each of the four possible splice variant combinations (e1b+9, e1b+9+9*, e1c+9, and e1c+9+9*) were individually expressed in HEK293 cells in combination with α2δ and β1b, a β-subunit that is expressed in cerebral artery smooth muscle cells (7). Currents generated by each variant were then characterized using patch-clamp electrophysiology.
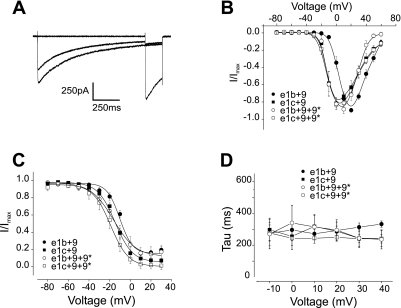
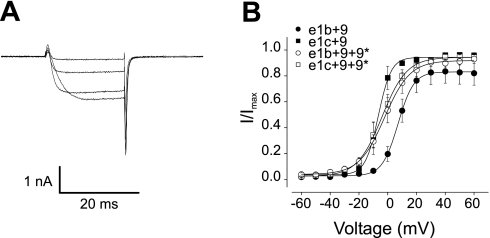
The I-V relationship produced by Cav1.2 α1-channels containing the e1b+9 combination was right-shifted when compared with the I-V of currents produced by the e1c+9 combination (Fig. 4B). The inclusion of exon 9* did not alter the current voltage-relationship of channels containing exon 1c (Fig. 4, A and B). In contrast, exon 9* caused a hyperpolarizing shift in the I-V relationship of Cav1.2 α1-subunits containing exon 1b+9 (Fig. 4B). This shift generated a I-V profile similar to that produced by e1c+9 and e1c+9+9* channels (Fig. 4B). A similar picture emerged for the voltage dependence of activation, where the V1/2 of channels containing 1c+9 was ∼12 mV more hyperpolarized than for channels containing 1b+9 (Fig. 5B and Table 3). In addition, exon 9* caused an ∼8 mV hyperpolarizing shift in the V1/2 of Cav1.2 α1-subunits containing exon 1b, but 9* did not alter the voltage dependence of activation of channels containing exon 1c (Fig. 5, A and B, and Table 3). In contrast, alternative splicing of exons 1b or 1c or the inclusion of exon 9* did not alter the voltage dependence of half-inactivation or τ, the rate of inactivation (Fig. 4, C and D, and Table 3).
Fig. 4.
Electrophysiological properties of currents produced by exon 1b/c and ± 9* Cav1.2 splice variants. A: representative current traces generated by the exon 1c+9+9* channel variant in response to 1-s conditioning pulses to −80, +10, or +30 mV followed by 200-ms test pulses to 0 mV. B: current-voltage relationships of Cav1.2 e1b+9 (n = 21), Cav1.2 e1c+9 (n = 10), Cav1.2 e1b+9+9* (n = 7), Cav1.2 e1c+9+9* (n = 21) variants. C: mean steady-state inactivation of all Cav1.2 splice variants [see Table 3 for half-inactivation voltage of steady-state inactivation (V1/2) values and experimental number]. D: mean current inactivation rate for all variants. I/Imax, normalized peak current.
Fig. 5.
Voltage-dependent activation of Cav1.2 splice variants. A: representative tail currents generated by the e1c+9+9* variant evoked by repolarization to −80 mV after depolarizing test pulses to −20, 0, +20, and +40 mV. B: mean voltage-dependent activation for all variants (see Table 3 for V1/2 values and experimental number).
Table 3.
Steady-state inactivation and activation of Cav1.2 α1-subunit splice variants
| CaV1.2 α1-variant |
V1/2, mV |
Window Current (I/Imax) Range at Half-maximal Amplitude, mV | |
|---|---|---|---|
| Steady-state inactivation | Activation | ||
| e1b+9 | −10.7±1.5 | 8.3±1.7 | −3.6 to N/A |
| n | 11 | 9 | |
| e1c+9 | −13.9±1.4 | −3.2±3.2* | −14.1 to +0.5 = 14.6 |
| n | 10 | 5 | |
| e1b+9+9* | −17.9±2.8 | −0.1±3.3* | −16.3 to +10.5 = 26.8 |
| n | 11 | 7 | |
| e1c+9+9* | −17.7±3.4 | −3.5±2.6* | −20.3 to −3.9 = 16.4 |
| n | 11 | 9 | |
Values are means ± SE.
P < 0.05 vs. e1b +9. V1/2, half-inactivation voltage of steady-state inactivation; I/Imax, normalized peak current; N/A, not applicable.
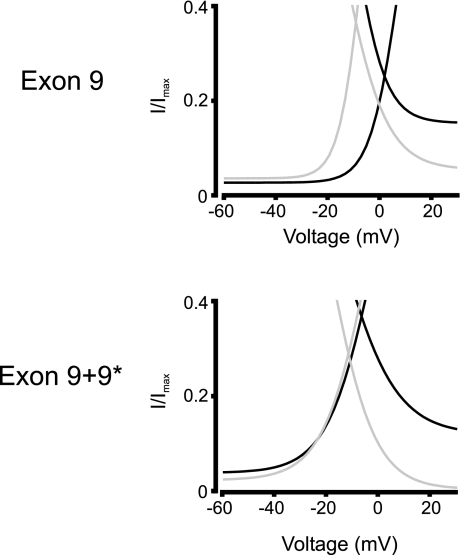
To further compare the voltage dependence of each splice variant combination, window currents were measured. The window current voltage range for each variant was measured at the half-maximal amplitude of the intersection of the activation and inactivation curves. Exon splicing from 1b to 1c caused an ∼11 mV hyperpolarizing shift in the activation of 9*-deficient channels, which was reduced to ∼3 mV in 9*-containing channels (Fig. 6 and Table 3). The voltage range of window currents produced by exon 1c+9+9*-containing channels was ∼10 mV narrower than for those containing exon 1b+9+9* (Fig. 6). The window current voltage range for e1b+9 currents could not be determined because a noninactivating component at positive voltages prevented determination of the half-maximal inactivating voltage.
Fig. 6.
Window currents of Cav1.2 splice variants. Graphs illustrate the voltage-dependent relative window current (I/Imax) produced by channels containing exon 1b (black lines) or exon 1c (gray lines) and 9 or 9+9* as indicated.
In summary, these data indicate that in the absence of exon 9*, alternative splicing of exon 1 modifies voltage-dependent activation of Cav1.2 α1-subunits. The data also indicate that the inclusion of exon 9* only alters the voltage-dependent activation of channels containing exon 1b, but not of those containing exon 1c. Furthermore, exon 1c causes a larger hyperpolarizing shift in window current activation of channels deficient in exon 9* than in those containing 9*.
DISCUSSION
Small, resistance-size cerebral arteries constrict in response to an elevation in intravascular pressure, a reaction termed the myogenic response (9). Myogenic tone sets a baseline diameter from which small arteries can either constrict or dilate in response to a wide variety of stimuli and, therefore, alter organ blood pressure and flow. L-type voltage-dependent Ca2+ channel activation is critical for the myogenic response, particularly in cerebral arteries, which control vascular pressure and regional blood flow within the brain. For the first time, this study has measured the voltage-dependent CaV1.2 channel splice variant population in smooth muscle cells of resistance-size arteries and determined the effects of combinatorial splicing at exons 1 and 9* on channel voltage sensitivity.
Alternative splicing of the CaV1.2 gene has been observed in several different tissues and species (12, 19, 33), but the complement of CaV1.2 splice variants expressed in small resistance-size arteries has not been examined. Recent work from our laboratory has identified a novel 5′ end (exon 1c) that is expressed in smooth muscle cells of rat resistance-size arteries (7). Although exon 1b is also expressed in these cells, exon 1c expression is predominant (7). Importantly, exon 1c is not restricted to the rat species. A Blast search using the rat exon 1c nucleotide sequence (Accession number AY974797), which generates the amino acid sequence MLCCALDCAC, reveals a highly homologous nucleotide sequence in the mouse genome that would produce the cysteine-rich amino acid sequence MLCCALACEY in Cav1.2 α1-subunits. Previous studies that have cloned full-length Cav1.2 α1-subunits used primers designed to recognize either exon 1a or 1b (27). Our earlier study that identified exon 1c raised the possibility that channels containing this splice variant might exhibit different downstream splicing profiles from those that contained either exon 1a or 1b. Using primers recognizing either exon 1b or 1c, we amplified full-length CaV1.2 cDNA from cerebral arteries and subcloned the PCR products into pGEM-T easy vector. Sequencing of cerebral artery CaV1.2 clones revealed three common alternative splicing regions besides exon 1, at exon 9*, 21/22, and 31/32/33. Given that 19 out of 55 CaV1.2 exons can undergo splicing (27), our data demonstrate that a relatively conservative population of CaV1.2 channels is expressed in smooth muscle cells of resistance-size cerebral arteries.
Real-time PCR using cerebral artery smooth muscle cell lysate determined that exon 21 and exons 32+33 are predominant, being present in 88% and 71% of all CaV1.2 transcripts, respectively. Full-length clones support the view that CaV1.2e1b and CaV1.2e1c have similar preferentiality for exon 21 and exon 32+33. We show that in cerebral artery smooth muscle cells, ∼57% of CaV1.2 transcripts contained exon 9*, compared with only 8% in cardiac myocytes. A previous study also indicated that exon 9* expression is similarly low in rat left ventricle (29). These data suggest preferential expression of exon 9* in smooth muscle cells of the cardiovascular system, consistent with the results of a previous study (20). However, our data indicate that approximately half of the arterial smooth muscle cell Cav1.2 channel mRNA is exon 9* deficient. Our data also suggest that in arterial myocytes, exon 1 variant selection may be linked to that of exon 9*. Seventy-two percent of all CaV1.2e1c clones contained exon 9*, whereas only 33% of CaV1.2e1b clones contained 9*. It could be argued that four of the 27 full-length clones contained internal stop codons, which would result in truncated, nonfunctional channels. However, when these four clones are excluded from the statistical analysis, a similar picture emerges in that 73% of CaV1.2e1c clones contained exon 9*, whereas only 25% of CaV1.2e1b clones contained exon 9*. Given that both exon 1c and 9* exhibit significantly higher expression in arterial myocytes than in cardiac myocytes (data here and in Refs. 7, 19, and 20), in the cardiovascular system this combinatorial expression profile may be highly selective for smooth muscle cells.
Alternative splicing of CaV1.2 enhances both structural and functional diversity of the channel (1, 17). Previous studies have been conducted to determine where CaV1.2 channel alternative splicing occurs (12, 30, 36). However, whether random or defined linkages occur between different exons is unclear. Studies that have investigated CaV1.2 splice variants have primarily scanned small regions of CaV1.2 message or used a combination of PCR and restriction enzyme analysis, which cannot derive splicing combination profiles. Recently, 41 linkages have been identified in full-length CaV1.2 mRNA isolated from rat whole aorta and heart (27). However, these CaV1.2 libraries were generated using primers designed from exon 1a and 1b. Exon 1c was not used to amplify Cav1.2 α1-message, because at that time this variant had not yet been discovered. Thus a complete CaV1.2 transcriptional profile could not be obtained. The lack of inclusion of exon 1c for primer generation and library construction may explain the discrepancy that only 24% of CaV1.2 transcripts from the rat aorta library expressed exon 9*, even though RT-PCR data indicated much higher exon 9* expression in the same vessel (20). Our identification of the third exon 1 sequence (e1c) provided the basis for future studies to determine splice variation and combinatorial splicing profiles in other cell types.
Substitution of a specific splice locus can alter electrophysiological and pharmacological properties of channels, including CaV1.2, and modify regulation by signaling proteins (19, 20). In agreement with our findings, exon 9* caused a hyperpolarizing shift in the activation curve of CaV1.2α1C,77 channels, which contain the exon combination 1b, 2-20, 22-30, 32-44, and 46-50 (20). A similar effect of exon 9* expression was observed with CaV1.2 channels with the exon combination 1b, 8, 21, 32, and 33 (27). Here we found that >40% of Cav1.2 α1-subunits in arterial smooth muscle cells did not contain exon 9*. Therefore, if exon 9* alone was essential for generating a Cav1.2 α1-subunit population with higher voltage sensitivity, almost half of these channels would be less voltage sensitive. However, data here indicate that exon 1c causes a significant hyperpolarizing shift in the voltage-dependent activation (determined from full activation curves and from window current analysis) of exon 9*-deficient channels. Thus exon 1c and 9* can both left-shift the voltage sensitivity of Cav1.2 α1-subunits. Interestingly, exon 1 switching in 9*-containing channels did not cause a hyperpolarizing shift in voltage dependence, consistent with a previous report from our laboratory (7). An explanation for these effects is that Cav1.2 α1-subunit voltage sensitivity may have already reached a ceiling, at least for the splice variant composition of the backbone channel. One additional possibility not studied here is that exon 1c and 9* shift the channel voltage sensitivity by interacting with the same regulatory molecule that does not interact with exon 1b or 9. A further finding was that window currents generated by exon 1c-containing channels were narrower than for 1b+9+9* channels. This appeared to be due primarily to different voltage-dependent inactivation of the 1b+9+9* window current. However, detailed analysis of this effect was not possible because e1b+9 currents did not inactivate below the half-maximal window current, precluding determination of the voltage range of this variant. In summary, based on our molecular and electrophysiological data, we suggest that ∼80% of Cav1.2 α1-subunits expressed in arterial smooth muscle cells will exhibit higher voltage sensitivity because of predominant exon 1c and 9* selection. This is an essential finding because the physiological steady-state voltage range of cerebral artery smooth muscle cells is approximately −60 to −40 mV (18). A hyperpolarized shift in Cav1.2 channel voltage sensitivity is important for controlling Ca2+ influx and contractility in smooth muscle cells under these physiological conditions.
CaV1.2 channels that contain the exon 8-encoded amino acid sequence are more sensitive to inhibition by dihydropyridines than channels containing exon 8a (35). In our study, all CaV1.2 clones obtained from cerebral arteries contained exon 8 rather than 8a. The data also indicate that most cerebral artery myocyte Cav1.2 α1-clones contain exon 21, whereas rat ventricle Cav1.2 α1-subunits contain primarily (∼59%) exon 22 (29). Therefore, the selection of exon 21 over 22 appears to be a major difference between cardiac and resistance-size arterial smooth muscle cell Cav1.2 α1-subunits. Alternative splicing of exon 21 with 22 did not modify CaV1.2 channel activation and inactivation, but channels expressing exon 22 were more sensitive to isradipine at a holding voltage at −90 mV than those containing exon 21 (25, 39). These data suggest that exon 8, but not 21, contributes to the higher dihydropyridine sensitivity of vascular smooth muscle cell Cav1.2 channels when compared with channels from cardiac myocytes. In addition, this finding may explain the effective and relatively selective clinical use of these drugs to reduce blood pressure and relieve vasospasm (31). In contrast with the tissue-selective occurrence of exon 8/8a and 21/22 in these cell types, arterial smooth muscle cell Cav1.2 α1-clones described here and in whole ventricle both primarily contain the exon 32+33 combination (29). Substitution of exon 31 with 32 did not alter channel electrophysiological properties (28, 39). However, in channels deficient in exon 9*, the absence of exon 33 shifts the activation curve in a hyperpolarizing direction with little effect on steady-state inactivation (27, 28). In contrast, in channels containing exon 9*, a lack of exon 33 did not alter activation properties but led to a hyperpolarizing shift in steady-state inactivation (27). Thus effects of exon 9* on channel properties appear to be linked not only to the exon 1 variant as demonstrated here but also to the presence or absence of exon 33.
Pathological states may modify Cav1.2 channel exon combinatorial profiles. Previous studies have shown that atherosclerosis and heart failure alter CaV1.2 exon expression (30, 38). Exons 9* and 41a are detected in normal human arterial smooth muscle cells but are absent in smooth muscle cells located in atherosclerotic regions (30). In contrast, exon 22 is absent in normal human arterial smooth muscle cells but is detected in cells from atherosclerotic areas (30). A switch between exons 31 and 32 has also been observed in heart failure, with the former being ∼2.5 times more abundant in nonfailing human ventricle and the latter being twice as abundant in failing hearts (38). Our determination of the combinatorial profiles of CaV1.2 splice variants in cerebral artery smooth muscle cells becomes important not only to determine contributions to tissue physiology and pharmacology but also as a first step in the identification of exon switches that may occur in disease. Disease-related CaV1.2 exon alterations could not only be used to better understand vascular pathophysiology but also as disease biomarkers.
Our study clearly indicates that when expressed with β1b- and α2δ-subunits, Cav1.2 α1-subunit exon 1c and 9* cause a hyperpolarizing shift in the voltage sensitivity of currents. However, the molecular identities and functional significance of Cav1.2 auxiliary subunits in arterial smooth muscle cells are poorly understood. We have shown that β1b and β3, but not β2a subunits are expressed in isolated cerebral artery smooth muscle cells (7). β1b associates with the plasma membrane through an acidic motif in the COOH terminus, whereas β3 is cytosolic (5, 8). α2δ is also a membrane-localized subunit. Here we characterized the electrophysiological properties of Cav1.2 α1-subunits when coexpressed with β1b to avoid intracellular dialysis of cytosolic auxiliary subunits, such as β3, during conventional whole-cell patch clamp. Thus effects on membrane current of exon 1 and 9* splicing that occur in the presence of membrane-bound β1b are also likely to occur with Cav1.2 currents in native arterial smooth muscle cells.
In native channels, interactions with membrane-associated proteins other than auxiliary subunits can also determine the current phenotype. Ca2+-dependent inactivation (CDI) of Cav1.2 occurs through Ca2+ binding to calmodulin that is associated with the channel COOH- and NH2-termini (10). Cloning did not reveal splice variation within arterial myocyte Cav1.2 α1-subunit NH2- or COOH-terminal regions responsible for mediating CDI through calmodulin, except for one clone (C16) out of 27 that had an 18 nucleotide deletion in exon 41. Therefore, CDI is likely to be similar between the four exon 1/9 ± 9* variants characterized here.
The electrophysiological properties of cerebral artery smooth muscle cell Cav1.2 currents have previously been measured using 10 mM Ba2+ as the charge carrier. In rat cerebral artery smooth muscle cells, half-maximal activation and inactivation voltages were −3.4 and −13.0 mV, respectively (37). In another species (rabbit), cerebral artery smooth muscle cell half-maximal activation and -inactivation voltages were 0.8 and −2.3 mV, respectively (14). The electrophysiological properties of rat arterial smooth muscle currents closely match those of the cloned rat channel currents, particularly those generated by channels containing exon 1c and/or 9*. Ultimately, it would be appropriate to compare in detail the electrophysiological properties of recombinant and smooth muscle cell Cav1.2 currents. However, this is not straightforward given that, as shown here, arterial myocyte currents occur due to the activation of a heterogeneous CaV1.2 splice variant population that is composed of many different subunits. Heterologously expressed currents are produced by a single clone and a defined set of auxiliary subunits, whereas arterial myocyte currents occur due to the activation of a heterogeneous CaV1.2 splice variant population that is composed of many different subunits. Furthermore, in arterial myocytes it is uncertain which β- and α2δ-subunit isoforms are expressed, the relative proportions of each isoform, the relative proportions of each auxiliary subunit to α1-subunits, and whether β- and α2δ-subunit isoforms differentially modulate α1-subunits containing different splice variants. The electrophysiological properties of arterial myocyte CaV1.2 currents will result from the combination of these multiple factors. Therefore, it is not yet possible to attempt to recapitulate the heterogeneous arterial myocyte Cav1.2 subunit population in an expression system. With the identification of the Cav1.2 α1-subunit splice variant population that is expressed in smooth muscle cells of myogenic cerebral arteries, one logical progression of the current study will be to compare the splice variant population that is expressed in smooth muscle cells of other vascular beds, in myogenic versus nonmyogenic vessels (conduit arteries, veins), and in arteries versus arterioles. Such studies may reveal important molecular variations in Cav1.2 that underlie functional differences in these tissues. In addition, our data provide the groundwork for a future comparison with Cav1.2 α1-splice variants that are expressed in other nonvascular smooth muscle cells and with nonsmooth muscle cell types. The identification of arterial smooth muscle cell-specific Cav1.2 α1-splice variants may provide the opportunity to target these channels in a cell-specific and therapeutic manner, e.g., by using gene targeting.
In summary, this study has identified the CaV1.2 channel splice variant population, quantified relative expression at the major regions subject to splicing, and found combinatorial splicing of exon 1c and 9* in smooth muscle cells of resistance-size arteries. We also show predominant exon 1c and 9* selection produces a Cav1.2 channel population with a voltage sensitivity that is nearer to the physiological arterial voltage range.
GRANTS
This study was supported by grants from the National Institutes of Health to J. H. Jaggar (HL-67061, HL-77678, and HL-94378) and A. M. Dopico (AA-11560 and HL-77424) and the American Heart Association National Center to J. H. Jaggar. X. Cheng and J. Liu are recipients of predoctoral and postdoctoral fellowships, respectively, from the Southeast Affiliate of the American Heart Association.
Acknowledgments
We thank Dr. John Bannister for critical reading of the manuscript. Nucleotide sequences of CaV1.2e1b and CaV1.2e1c are deposited in the GenBank data base (Accession numbers DQ538522 and AY974797, respectively).
REFERENCES
- 1.Abernethy DR, Soldatov NM. Structure-functional diversity of human L-type Ca2+ channel: perspectives for new pharmacological targets. J Pharmacol Exp Ther 300: 724–728, 2002. [DOI] [PubMed] [Google Scholar]
- 2.Adebiyi A, McNally EM, Jaggar JH. Sufonylurea receptor-dependent and -independent mechanisms mediate vasodilation induced by KATP channel openers. Mol Pharmacol 74: 736–743, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Arikkath J, Campbell KP. Auxiliary subunits: essential components of the voltage-gated calcium channel complex. Curr Opin Neurobiol 13: 298–307, 2003. [DOI] [PubMed] [Google Scholar]
- 4.Berridge MJ, Lipp P, Bootman MD. The versatility and universality of calcium signalling. Nat Rev Mol Cell Biol 1: 11–21, 2000. [DOI] [PubMed] [Google Scholar]
- 5.Bogdanov Y, Brice NL, Canti C, Page KM, Li M, Volsen SG, Dolphin AC. Acidic motif responsible for plasma membrane association of the voltage-dependent calcium channel β1b subunit. Eur J Neurosci 12: 894–902, 2000. [DOI] [PubMed] [Google Scholar]
- 6.Catterall WA, Perez-Reyes E, Snutch TP, Striessnig J. International Union of Pharmacology. XLVIII. Nomenclature and structure-function relationships of voltage-gated calcium channels. Pharmacol Rev 57: 411–425, 2005. [DOI] [PubMed] [Google Scholar]
- 7.Cheng X, Liu J, Asuncion-Chin M, Blaskova E, Bannister JP, Dopico AM, Jaggar JH. A novel CaV1.2 N-terminus expressed in smooth muscle cells of resistance size arteries modifies channel regulation by auxiliary subunits. J Biol Chem 282: 29211–29221, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chien AJ, Gao T, Perez-Reyes E, Hosey MM. Membrane targeting of L-type calcium channels. Role of palmitoylation in the subcellular localization of the β2a subunit. J Biol Chem 273: 23590–23597, 1998. [DOI] [PubMed] [Google Scholar]
- 9.Davis MJ, Hill MA. Signaling mechanisms underlying the vascular myogenic response. Physiol Rev 79: 387–423, 1999. [DOI] [PubMed] [Google Scholar]
- 10.Dick IE, Tadross MR, Liang H, Tay LH, Yang W, Yue DT. A modular switch for spatial Ca2+ selectivity in the calmodulin regulation of CaV channels. Nature 451: 830–834, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Erickson MG, Liang H, Mori MX, Yue DT. FRET two-hybrid mapping reveals function and location of L-type Ca2+ channel CaM preassociation. Neuron 39: 97–107, 2003. [DOI] [PubMed] [Google Scholar]
- 12.Fan QI, Vanderpool KM, Chung HS, Marsh JD. The L-type calcium channel alpha 1C subunit gene undergoes extensive, uncoordinated alternative splicing. Mol Cell Biochem 269: 153–163, 2005. [DOI] [PubMed] [Google Scholar]
- 13.Gollasch M, Nelson MT. Voltage-dependent Ca2+ channels in arterial smooth muscle cells. Kidney Blood Press Res 20: 355–371, 2000. [DOI] [PubMed] [Google Scholar]
- 14.Ishiguro M, Wellman TL, Honda A, Russell SR, Tranmer BI, Wellman GC. Emergence of a R-type Ca2+ channel (CaV 2.3) contributes to cerebral artery constriction after subarachnoid hemorrhage. Circ Res 96: 419–426, 2005. [DOI] [PubMed] [Google Scholar]
- 15.Jaggar JH Intravascular pressure regulates local and global Ca2+ signaling in cerebral artery smooth muscle cells. Am J Physiol Cell Physiol 281: C439–C448, 2001. [DOI] [PubMed] [Google Scholar]
- 16.Jaggar JH, Li A, Parfenova H, Liu J, Umstot ES, Dopico AM, Leffler CW. Heme is a carbon monoxide receptor for large-conductance Ca2+-activated K+ channels. Circ Res 97: 805–812, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jurkat-Rott K, Lehmann-Horn F. The impact of splice isoforms on voltage-gated calcium channel alpha1 subunits. J Physiol 554: 609–619, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Knot HJ, Nelson MT. Regulation of arterial diameter and wall [Ca2+] in cerebral arteries of rat by membrane potential and intravascular pressure. J Physiol 508: 199–209, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Liao P, Yong TF, Liang MC, Yue DT, Soong TW. Splicing for alternative structures of CaV1.2 Ca2+ channels in cardiac and smooth muscles. Cardiovasc Res 68: 197–203, 2005. [DOI] [PubMed] [Google Scholar]
- 20.Liao P, Yu D, Lu S, Tang Z, Liang MC, Zeng S, Lin W, Soong TW. Smooth muscle-selective alternatively spliced exon generates functional variation in CaV1.2 calcium channels. J Biol Chem 279: 50329–50335, 2004. [DOI] [PubMed] [Google Scholar]
- 21.Moosmang S, Schulla V, Welling A, Feil R, Feil S, Wegener JW, Hofmann F, Klugbauer N. Dominant role of smooth muscle L-type calcium channel CaV1.2 for blood pressure regulation. EMBO J 22: 6027–6034, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Navedo MF, Amberg GC, Westenbroek RE, Sinnegger-Brauns MJ, Catterall WA, Striessnig J, Santana LF. CaV1.3 channels produce persistent calcium sparklets, but CaV1.2 channels are responsible for sparklets in mouse arterial smooth muscle. Am J Physiol Heart Circ Physiol 293: H1359–H1370, 2007. [DOI] [PubMed] [Google Scholar]
- 23.Nelson MT, Patlak JB, Worley JF, Standen NB. Calcium channels, potassium channels, and voltage dependence of arterial smooth muscle tone. Am J Physiol Cell Physiol 259: C3–C18, 1990. [DOI] [PubMed] [Google Scholar]
- 24.Pratt PF, Bonnet S, Ludwig LM, Bonnet P, Rusch NJ. Upregulation of L-type Ca2+ channels in mesenteric and skeletal arteries of SHR. Hypertension 40: 214–219, 2002. [DOI] [PubMed] [Google Scholar]
- 25.Soldatov NM, Bouron A, Reuter H. Different voltage-dependent inhibition by dihydropyridines of human Ca2+ channel splice variants. J Biol Chem 270: 10540–10543, 1995. [DOI] [PubMed] [Google Scholar]
- 26.Sonkusare S, Palade PT, Marsh JD, Telemaque S, Pesic A, Rusch NJ. Vascular calcium channels and high blood pressure: pathophysiology and therapeutic implications. Vascul Pharmacol 44: 131–142, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tang ZZ, Hong X, Wang J, Soong TW. Signature combinatorial splicing profiles of rat cardiac- and smooth-muscle CaV1.2 channels with distinct biophysical properties. Cell Calcium 41: 417–428, 2007. [DOI] [PubMed] [Google Scholar]
- 28.Tang ZZ, Liang MC, Lu S, Yu D, Yu CY, Yue DT, Soong TW. Transcript scanning reveals novel and extensive splice variations in human L-type voltage-gated calcium channel, CaV1.2 α1 subunit. J Biol Chem 279: 44335–44343, 2004. [DOI] [PubMed] [Google Scholar]
- 29.Tang ZZ, Liao P, Li G, Jiang FL, Yu D, Hong X, Yong TF, Tan G, Lu S, Wang J, Soong TW. Differential splicing patterns of L-type calcium channel Cav1.2 subunit in hearts of spontaneously hypertensive rats and Wistar Kyoto rats. Biochim Biophys Acta 1783: 118–130, 2008. [DOI] [PubMed] [Google Scholar]
- 30.Tiwari S, Zhang Y, Heller J, Abernethy DR, Soldatov NM. Atherosclerosis-related molecular alteration of the human CaV1.2 calcium channel α1C subunit. Proc Natl Acad Sci USA 103: 17024–17029, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Triggle DJ Calcium channel antagonists: clinical uses—past, present and future. Biochem Pharmacol 74: 1–9, 2007. [DOI] [PubMed] [Google Scholar]
- 32.Wamhoff BR, Bowles DK, Owens GK. Excitation-transcription coupling in arterial smooth muscle. Circ Res 98: 868–878, 2006. [DOI] [PubMed] [Google Scholar]
- 33.Wang D, Papp AC, Binkley PF, Johnson JA, Sadee W. Highly variable mRNA expression and splicing of L-type voltage-dependent calcium channel alpha subunit 1C in human heart tissues. Pharmacogenet Genomics 16: 735–745, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wang WZ, Saada N, Dai B, Pang L, Palade P. Vascular-specific increase in exon 1B-encoded CaV1.2 channels in spontaneously hypertensive rats. Am J Hypertens 19: 823–831, 2006. [DOI] [PubMed] [Google Scholar]
- 35.Welling A, Ludwig A, Zimmer S, Klugbauer N, Flockerzi V, Hofmann F. Alternatively spliced IS6 segments of the alpha 1C gene determine the tissue-specific dihydropyridine sensitivity of cardiac and vascular smooth muscle L-type Ca2+ channels. Circ Res 81: 526–532, 1997. [DOI] [PubMed] [Google Scholar]
- 36.Wielowieyski PA, Wigle JT, Salih M, Hum P, Tuana BS. Alternative splicing in intracellular loop connecting domains II and III of the alpha 1 subunit of CaV1.2 Ca2+ channels predicts two-domain polypeptides with unique C-terminal tails. J Biol Chem 276: 1398–1406, 2001. [DOI] [PubMed] [Google Scholar]
- 37.Wilde DW, Furspan PB, Szocik JF. Calcium current in smooth muscle cells from normotensive and genetically hypertensive rats. Hypertension 24: 739–746, 1994. [DOI] [PubMed] [Google Scholar]
- 38.Yang Y, Chen X, Margulies K, Jeevanandam V, Pollack P, Bailey BA, Houser SR. L-type Ca2+ channel α1C subunit isoform switching in failing human ventricular myocardium. J Mol Cell Cardiol 32: 973–984, 2000. [DOI] [PubMed] [Google Scholar]
- 39.Zuhlke RD, Bouron A, Soldatov NM, Reuter H. Ca2+ channel sensitivity towards the blocker isradipine is affected by alternative splicing of the human alpha1C subunit gene. FEBS Lett 427: 220–224, 1998. [DOI] [PubMed] [Google Scholar]