Abstract
Postural tachycardia syndrome (POTS), a chronic form of orthostatic intolerance, has signs and symptoms of lightheadedness, loss of vision, headache, fatigue, and neurocognitive deficits consistent with reductions in cerebrovascular perfusion. We hypothesized that young, normocapnic POTS patients exhibit abnormal cerebral autoregulation (CA) that results in decreased static and dynamic cerebral blood flow (CBF) autoregulation. All subjects had continuous recordings of mean arterial pressure (MAP) and CBF velocity (CBFV) using transcranial Doppler sonography in both the supine supine position and during a 70° head-up tilt. During tilt, POTS patients (n = 9) demonstrated a higher heart rate than controls (n = 7) (109 ± 6 vs. 80 ± 2 beats/min, P < 0.05), whereas controls demonstrated a higher MAP than POTS (87 ± 2 vs. 77 ± 3 mmHg, P < 0.05). Also during tilt, mean CBFV decreased 19.5 ± 2.6% in POTS patients versus 10.3 ± 2.0% in controls (P < 0.05). We then used a transfer function analysis of MAP and CFBV in the frequency domain to quantify these changes. The low-frequency (LF; 0.04–0.15 Hz) component of CBFV variability increased during tilt in POTS patients (supine: 3 ± 0.9 vs. tilt: 9 ± 2, P < 0.02). In POTS patients, there was an increase in LF and high-frequency coherence between MAP and CBFV, an increase in LF gain, and a lack of significant change in phase. Static CA may be less effective in POTS patients compared with controls, since immediately after tilt CBFV decreased more in POTS patients and was highly oscillatory and autoregulation did not restore CBFV to baseline values until the subjects became supine. Dynamic CA may be less effective in POTS patients because MAP and CBFV during tilt became almost perfectly synchronous. We conclude that dynamic and static autoregulation of CBF are less effective in POTS patients compared with control subjects during orthostatic challenge.
Keywords: chronic orthostasis, coherence, transcranial Doppler
postural tachycardia syndrome (POTS) is a chronic form of orthostatic intolerance (OI). Upon standing upright, patients experience symptoms of OI, including lightheadedness, loss of vision, nausea, headache, fatigue, mental cloudiness, hyperpnea, and splanchnic blood pooling (29, 38). POTS often affects adolescents and young adults and is more often seen in females (1, 29). Symptoms are associated with an excessive postural tachycardia, which occurs within 10 min of becoming upright. In adults, this is defined by an increase in heart rate (HR) of >30 beats/min or a HR that exceeds 120 beats/min. Systolic blood pressure (SBP) is usually maintained. Symptoms of OI, such as lightheadedness, loss of vision, headache, fatigue, and neurocognitive deficits, are consistent with reductions of cerebral perfusion.
A decrease in cerebral blood flow (CBF) upon becoming upright may produce cerebral hypoperfusion (22, 27, 32, 50). It is controversial whether CBF velocity (CBFV) decreases or remains unchanged in POTS patients when they become upright (23, 27, 42). Furthermore, POTS patients represent a heterogeneous group of patients (29, 38, 41). The discrepancies between studies may be due to factors such as age, methodology, and levels of CO2 due to hyperventilation, all of which have been shown to affect CBFV (24, 34). Also, not all studies reported end-tidal CO2 (ETco2) values. Furthermore, a subset of POTS patients demonstrates symptoms of hyperventilation and hypocapnia, whereas another subset exhibits normal ventilation and eucapnia (47). Additional research controlling for these factors while critically delineating subject selection criteria is required to rule out the possibility of decreased CBFV.
If cerebral hypoperfusion does occur in POTS, it would suggest that cerebral autoregulation (CA) may be disrupted. CA describes the phenomenon in which cerebral blood vessels maintain a constant flow through a range of BPs by moderating resistance (15, 54). Static CA describes the response of the brain to long-term gradual alterations in BP; dynamic CA describes the response of the brain to rapid alterations in BP (15, 54). Altered static CA could mean that POTS patients have a different range of autoregulatory pressures. Altered dynamic CA would mean that POTS patients have difficulty appropriately compensating for quick fluctuations in pressure. Analyses of CA responses often use frequency domain transfer function methods to describe and relate interactions between mean arterial pressure (MAP) and CBFV (10, 15, 26, 27, 42, 59).
To obviate the influences of CO2 on CBF and CBFV, a study of normocapnic POTS patients is necessary. No prior studies have determined autoregulatory changes in the strictly defined subgroup of young, normocapnic POTS patients. Furthermore, cerebral hypoperfusion may be related to the neurocognitive symptoms experienced by POTS patients. Therefore, we hypothesized that young, normocapnic POTS patients exhibit abnormal CA that results in decreased static CBF (32) and reduced dynamic autoregulation and that changes may be quantified through transfer function analysis between MAP and CBFV.
METHODS
Subjects
As a way to control for influences of CO2, we only included subjects who maintained normocapnic CO2 levels (similar to control subjects) during supine and tilt conditions. This implied that subjects maintained unaltered respiratory rates during head-up tilt (HUT). Thus, a screening process using analysis of ETco2 and respiratory data occurred before the selection of our subject groups. Of 15 consecutive POTS subjects, 9 subjects [age: 15–29 yr (median age: 20.3 yr), 5 women and 4 men] met these inclusion criteria. Seven consecutive healthy control subjects met these criteria [age: 15–29 yr (median age: 24.4 yr), 4 women and 3 men].
POTS subjects were referred to our center for testing if they experienced symptoms of OI for at least 6 mo. POTS was identified during upright tilt table testing to 70° by signs and symptoms of OI and an excessive increase in HR of at least 30 beats/min and/or HR exceeding 120 beats/min within the first 10 min of HUT (1, 29, 38, 42). No other medical problems could explain these signs or symptoms. Normocapnia in POTS subjects was defined as an ETco2 between 35 and 45 mmHg both in the supine position and during 70° HUT.
Healthy control subjects were defined as individuals having no previously known medical conditions, free of systemic illness, having a normal physical exam and ECG, and a normal echocardiogram. Subjects had never experienced OI of any type, including POTS or syncope. Subjects with a history of OI were excluded. Normocapnia in healthy subjects has been previously defined in our laboratory; our range of ETco2 values are between 35 and 45 mmHg both in the supine postion and during 70° HUT.
Trained athletes, bed-ridden individuals, and individuals who used nicotine-containing products were excluded from enrollment. Subject with a history of asthma, congestive heart disease, renal disease, systemic hypertension, diabetes, acute or chronic inflammatory disease, neoplasm, immune-mediated disease, trauma, morbid obesity, congenital heart disease, peripheral vascular disease, respiratory disease, or other systemic medical problems were also excluded from enrollment. Subject who were pregnant or had been pregnant in the previous 3 mo were excluded. All subjects were required to refrain from all medications for at least 2 wk before the study; however, contraceptive medications were allowed. Seventy-two hours before the study, all subjects were required to stop the ingestion of xanthine-, caffeine-, or alcohol-containing substances. A light breakfast consisting of bread and water was permitted on the testing day if it could be eaten 2 h or more before the tests. All tests were performed in a single day.
The Institutional Review Board of New York Medical College reviewed and approved this protocol. Each subject received a detailed description of all protocols and was given an opportunity to have their questions answered. Signed informed consent was obtained from all participants.
Instrumentation
All subjects were instrumented in a similar fashion by the same operators. Height and weight were measured. During instrumentation, all subjects lay in a supine position on an electronic motorized tilt table (Colin Medical Instruments, San Antonio, TX) with a footboard.
Beat-to-beat BP was monitored using finger arterial plethysmography (Finometer, FMS, Amsterdam, The Netherlands) of the right middle or index fingers. These data were calibrated to brachial AP. The Finometer contains a sensor that corrects for height during positional changes, such as tilting. A single-lead ECG measured HR. A nasal cannula connected to a capnograph with a pulse oximeter (Smiths Medical, Waukesha, WI) measured ETco2. Respirations were measured using a RespiTrace device (NIMS, North Bay Village, FL). Transcranial Doppler (Neurovision, Multigon, Yonkers, NY) measured CBFV of the left middle cerebral artery (MCA) using a 2-MHz probe fixed to the subject's head by a custom-made headband.
Protocol
All subjects arrived at 9:30 AM. After instrumentation, subjects remained in the supine position for 30 min to acclimate. After acclimation, at least 5 min of continuous baseline data were recorded. With the completion of supine measurements, the 70° HUT test began. The tilt test continued for a maximum of 10 min. All subjects in both the POTS and control groups finished the full 10-min tilt test without any adverse events.
Data Analysis
All data were measured continuously and synchronously, and reported values are averages during the time period measured. Signals were converted with an analog-to-digital converter (DI-720 DataQ, Milwaukee, WI) connected to a personal computer and analyzed offline. After a tilt test, at least the first minute of data were omitted from the analysis until each subject stabilized. MAP was calculated by the following formula: MAP = (1/3 × systolic BP) + (2/3 × DBP), where DBP is diastolic BP. To estimate cerebral vascular resistance, the cerebral vascular resistance index (CVRi) was used, where CVRi = MAP/CBFV (4).
Variability Measures and Transfer Function Analyses
HR and BP variability and transfer function.
HR variability and BP variability were analyzed as previously described using autoregression (45, 50). At least 500 beats were acquired during both baseline and HUT measurements. Signals were analyzed for ectopic beats, which were removed using a polynomial curve-fitting routine as necessary. However, it was rarely necessary to correct for this; other types of arrhythmias were not seen. Data were digitalized at 200 Hz. A custom software package was used to analyze the R-R interval and MAP as previously described (45, 50). Briefly, an autoregressive model calculated spectral power for the R-R interval and MAP. The R-R interval and MAP were expressed as a sequence of discrete points that was transformed into an impulse train of equal intervals (equal to the mean R-R interval). Extended Yule-Walker equations calculated the digital power spectra, and Akaike's final prediction error chose the final order of the model. Spectral power was calculated by taking the power in the actual frequency band and dividing it into a very-low-frequency band (0.01–0.04 Hz), a low-frequency (LF) band (0.04–0.15 Hz), and a high-frequency (HF) band (0.15–0.40 Hz). Total power was also calculated. For this analysis, only LF and HF were used. The α-index, the ratio of LF R-R interval power to LF BP power, was calculated to express variations in cardiovagal baroreflex sensitivity (34, 39, 45).
The transfer function was calculated mathematically (3, 59) to obtain coherence, gain, and phase values. Minimum BP-HR coherence values of 0.5 were fulfilled for each subject and prevented the inclusion of excessively noisy signals.
BP and CBFV variability and transfer function.
The variation between BP and CBFV was measured with a similar method as stated in HR and BP variability and transfer function. CBFV variability was defined as the variation in the measured CBFV as seen in the frequency domain. MAP and CBFV were analyzed, and the transfer function was calculated. Minimum BP-CBFV coherence values of 0.5 were fulfilled for each subject and prevented the inclusion of excessively noisy signals. The αCBF-index, the ratio of LF MAP power to LF CBFV power, was also calculated.
Coherence, gain, and phase as a function of frequency were applied to describe dynamic CA in the frequency domain. Coherence describes the synchronization between oscillations in MAP and CBFV. It is the Fourier transform of the cross covariance. A low degree of synchronization implies strong autoregulation with low coherence because, while BP may change, blood flow remains constant. Conversely, an increase in synchronization and coherence implies weak autoregulation with a maximum of coherence of 1.0, signifying perfect synchrony between BP and CBF (54). Gain, or magnitude, describes the ratio between the oscillatory amplitudes of MAP and CBFV (54). Phase represents the time lag measured in fractions of an oscillation of MAP and CBFV, with oscillations in CBFV normally preceding changes in MAP (54). An increase in phase is expected upon standing and indicates that MAP and CBF may be falling out of step and thus signifies increasing CA, whereas a decrease in phase (approaching zero) is consistent with impaired autoregulation (54). Higher-frequency components of the transfer function represent rapid changes and thus dynamic CA. Lower frequencies of the transfer function may represent slowly varying cerebrovascular impedance (58).
Statistics
SPSS 13 software (Apache Software Federation) was used for statistical calculations. Data were compared both in the supine position and after HUT. SBP, DBP, MAP, HR, ETco2, respiration rate, R-R interval, CBFV, and CVRi were analyzed using ANOVA for repeated measures with a Bonferroni post test for multiple comparisons if findings were significant. The nonparametric Mann-Whitney U-test was used for to test variability between HR-BP and BP-CBFV because neither was normally distributed. Transfer function coherence, gain, and phase did not follow a normal distribution, and the nonparametric Friedman test was used. Subject age, height, and weight were analyzed using an independent two-tailed t-test. All measures are reported as means ± SE. Statistical significance was set at P < 0.05 for all tests.
RESULTS
Subject Demographic Data
No statistical differences existed in age, height, or weight between the POTS group and control group.
HR, BP, and CBFV
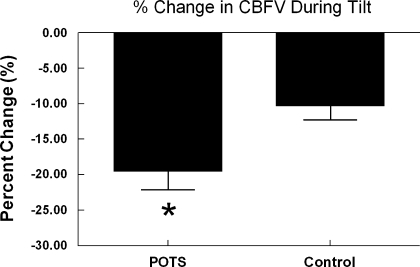
Table 1 shows the differences in hemodynamic and respiratory values in the supine position and during tilt. While in supine position, POTS subjects had a lower SBP and a higher HR than controls, although all values were within normal clinical ranges. During HUT, POTS subjects had a decreased SBP and an increased HR compared with controls, whereas control subjects demonstrated an increased MAP compared with POTS subjects. Figure 1 shows that during HUT, mean CBFV decreased 19.5 ± 2.6% in POTS subjects versus 10.3 ± 2.0% in controls subjects (P < 0.05). CVRi increased in both POTS and control subjects during tilt.
Table 1.
Hemodynamic and respiratory parameters in the supine position and during tilt in POTS and control subjects
| Measurement |
Supine |
70° Head-Up Tilt
|
||
|---|---|---|---|---|
| POTS subjects | Control subjects | POTS subjects | Control subjects | |
| Systolic BP, mmHg | 114±3† | 121±1 | 108±4† | 123±1 |
| Diastolic BP, mmHg | 59±2 | 61±3 | 62±2 | 68±3* |
| MAP, mmHg | 78±2 | 81±2 | 77±3† | 87±2* |
| HR, beats/min | 73±3† | 54±3 | 109±6*† | 80±4* |
| End-tidal CO2, mmHg | 43±1 | 43±1 | 39±1* | 41±1* |
| Respiration, breaths/min | 18±1 | 17±1 | 19±2 | 15±2 |
| R-R Interval, ms | 821±32† | 1,097±42 | 599±28*† | 759±42* |
| Mean CBFV, cm/s | 70±5 | 69±6 | 56±4* | 62±5* |
| Cerebral vascular resistance index, mmHg·cm−1·s−1 | 1.2±0.1 | 1.3±0.2 | 1.5±0.1* | 1.5±0.2* |
Values are means ± SE. POTS, postural tachycardia syndrome; BP, blood pressure, MAP, mean arterial pressure; HR, heart rate; CBFV, cerebral blood flow velocity.
P < 0.05 compared with supine values within the same group;
P < 0.05 compared with the control group.
Fig. 1.
Percent change in cerebral blood flow velocity (CBFV) during tilt. Postural tachycardia syndrome (POTS) subjects, during tilt, exhibited an ∼20% decrease in CBFV, whereas controls exhibited an ∼10% decrease. *P < 0.05 compared with controls.
Respiration Rate and ETco2
As shown in Table 1, the respiration rate did not change significantly in either POTS or control subjects while in the supine position or during HUT. HUT, however, resulted in a decrease in ETco2 in both POTS and control subjects to statistically equivalent levels, which remained in the normocapnic range. The range of supine ETco2 values for POTS subjects (39–45 mmHg) was not different from the range for controls (40–45 mmHg). Also, during HUT, the range of ETco2 values for POTS subjects (37–43 mmHg) was not different from the range for controls (36–43 mmHg, P > 0.05). The change in ETco2 when in upright position was not different between the two groups (POTS: 3.5 ± 0.6 vs. control: 2.2 ± 0.8, P = not significant). Because a decrease in 1 mmHg of CO2 corresponds to a decrease in CBFV of ∼3.5% (21, 57), the change in ETco2 in POTS subjects during HUT could account for an ∼12% decrease in CBFV, whereas in controls it could account for an ∼8% decrease in CBFV.
HR and BP Variability and BP-R-R Interval Transfer Function Analysis
Table 2 shows the values of HR variability and BP variability obtained while subjects were in the supine position and upright during HUT. In accordance with previous reports (45, 46), supine and tilt values for HR variability in POTS subjects were significantly lower than values in controls. In the supine position, the LF and HF components of HR variability were also lower in POTS subjects than in controls. The ratio of the LF to HF components increased in both groups during tilt. During HUT, only POTS subjects had a significant increase in BP variability. HUT decreased the α-index in both POTS and control subjects, but it remained significantly lower in POTS subjects compared with controls.
Table 2.
HR and BB variability measures
|
Supine |
70° Head-Up Tilt
|
|||
|---|---|---|---|---|
| POTS subjects | Control subjects | POTS subjects | Control subjects | |
| HR variability | 994±153† | 3450±520 | 707±216† | 2126±663 |
| LF component | 304±45† | 842±182 | 418±151 | 588±310 |
| HF component | 433±84† | 1555±395 | 138±42* | 416±223 |
| LF/HF | 0.9±0.2 | 0.5±0.1 | 4±0.8* | 4±1* |
| BP variability | 11±2 | 15±4 | 28±4* | 26±7 |
| LF component | 3±0.5 | 3±0.8 | 18±4* | 15±6* |
| HF component | 2±0.2 | 2±1.2 | 5±0.6* | 4±0.7 |
| α, ms/mmHg | 12±1† | 17±3 | 5±0.8*† | 9±1* |
| LF | ||||
| Coherence | 0.65±0.03 | 0.64±0.06 | 0.78±0.07* | 0.85±0.03* |
| Gain, ms/mmHg | 15±1 | 21±3 | 4±1*† | 8±1* |
| Phase, ° | 45±5 | 32±12 | 56±6 | 41±5 |
| HF | ||||
| Coherence | 0.83±0.03 | 0.79±0.03 | 0.64±0.06* | 0.77±0.04 |
| Gain, ms/mmHg | 15±2 | 27±5 | 5±1* | 9±2* |
| Phase, ° | 13±7 | 2±9 | 64±7*† | 37±11 |
Values are means ± SE. LF, low frequency; HF, high frequency.
P < 0.05 compared with supine values within the same group;
P < 0.05 compared with the control group.
As also shown Table 2, transfer function analysis demonstrated an increase in LF coherence in both groups during HUT, whereas HF coherence decreased significantly only in POTS subjects. HUT decreased the LF gain in both groups, but the decrease was significantly greater in POTS subjects than in controls. HF gain decreased in both groups upon tilt. HF phase during tilt was increased in both POTS subjects and controls but was significantly greater in POTS subjects.
BP Variability and CBFV Variability
Table 3 shows the changes in BP variability and CBFV variability in the supine position and during HUT. As previously mentioned, BP variability significantly increased during tilt only in POTS subjects. Likewise, the LF component of CBFV variability increased during tilt in POTS subjects only. In both POTS and control subjects, there was an increase in the HF component of CBFV during tilt. The αCBF-index increased significantly only in POTS subjects during tilt.
Table 3.
MAP and CBFV variability measures
|
Supine |
70° Head-Up Tilt
|
|||
|---|---|---|---|---|
| POTS subjects | Control subjects | POTS subjects | Control subjects | |
| MAP variability | 5±0.6 | 7±1 | 16±1* | 12±3 |
| LF component | 2±0.3 | 2±0.2 | 10±1* | 8±2* |
| HF component | 0.8±0.2 | 0.3±0.1 | 2±0.3* | 1±0.2* |
| CBFV variability | 10±2 | 10±2 | 17±4 | 14±3 |
| LF component | 3±0.9 | 3±0.6 | 9±2* | 6±1 |
| HF component | 1±0.3 | 1±0.2 | 3±0.7* | 2±0.5* |
| αCBF, ms/mmHg | 0.8±0.1 | 1±0.2 | 1±0.1* | 1±0.1 |
| LF | ||||
| Coherence | 0.53±0.04 | 0.54±0.08 | 0.90±0.02* | 0.81±0.05* |
| Gain, mmHg·cm−1·s−1 | 0.7±0.1 | 0.7±0.1 | 1.08±0.10* | 1.12±0.10 |
| Phase, ° | 39±5† | 64±6 | 42±4 | 48±7 |
| HF | ||||
| Coherence | 0.65±0.04 | 0.52±0.07 | 0.83±0.05* | 0.60±0.09 |
| Gain, mmHg·cm−1·s−1 | 0.8±0.1 | 0.7±0.1 | 0.9±0.1 | 1±0.2 |
| Phase, ° | 15±4 | −0.3±6 | 11±2 | 5±5 |
Values are means ± SE.
P < 0.05 compared with supine values within the same group;
P < 0.05 compared with the control group.
Static CA
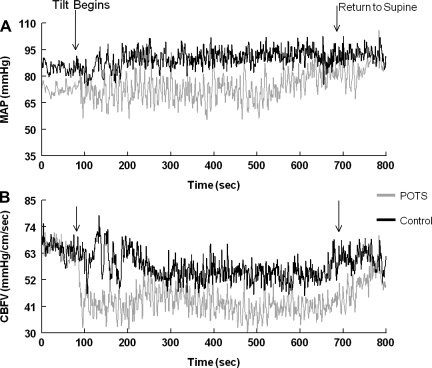
Figure 2 shows the effects of tilt on static CA during the entire HUT period in a representative POTS subject and a control subject. Quantization of data from all subjects showed that immediately after the tilt, CBFV decreased (Fig. 2B) in both the POTS subject and control subject. In the POTS subject, the decrease was larger, CBFV was highly oscillatory, and autoregulation did not restore CBFV to baseline values until the tilt was concluded. In contrast, autoregulation in the control subject brought CBFV back to baseline levels within ∼100 s, and CBFV remained stable at this level thereafter.
Fig. 2.
Static autoregulation in a representative POTS subject and a control subject during tilt. A: mean arterial pressure (MAP) oscillations during tilt. B: CBFV oscillations during tilt. Arrows represent the beginning and end of tilt (range: 600 s). In POTS subjects, CBFV initially fell upon tilt and did not recover until the return to the supine position. In control subjects, CBFV initially fell but soon recovered and remained relatively stable throughout the duration of the tilt.
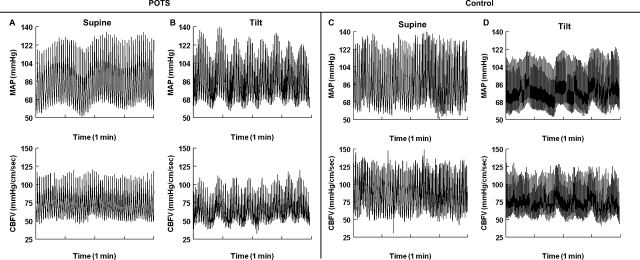
Dynamic CA
AP and CBFV were poorly synchronized in the representative POTS subject and control subject in the supine position, as shown in Fig. 3. AP and CBFV in POTS subjects were almost perfectly synchronous during HUT. Thus, beat-to-beat changes in CBFV passively and synchronously followed beat-to-beat changes in AP, as seen in Fig. 3B. The results shown in Fig. 3 demonstrate that dynamic CA is essentially absent and that changes in CBFV passively follow changes in AP.
Fig. 3.
Dynamic autoregulation in a representative POTS subject and a control subject in the supine position and during tilt. A: 1-min interval of a POTS subject's AP and CBFV while in the supine position. B: 1-min interval of a POTS subject's AP and CBFV during tilt. C: 1-min interval of a control subject's AP and CBFV while in the supine position. D: 1-min interval of a control subject's AP and CBFV during tilt. In the supine position, there did not appear to be a strong relationship between AP and CBFV in either POTS or control subjects. In POTS subjects during tilt, AP and CBFV became very synchronous, oscillations in CBFV passively followed oscillations in AP, and dynamic autoregulation was reduced. In control subjects during tilt, AP and CBFV were less synchronous, demonstrating intact dynamic autoregulation.
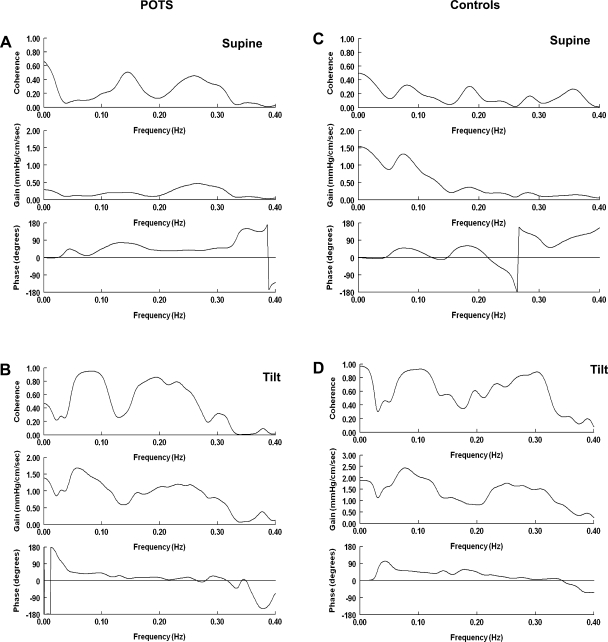
Dynamic CA was also determined through transfer function analysis of MAP-CBFV variability. Figure 4 shows a transfer function between MAP and CBFV for a representative POTS subject and a control subject in the supine position and during HUT. Table 3 shows average values for these measures. In the supine position, POTS subjects had a lower LF phase shift compared with controls. This suggests that while in the supine position, POTS subjects have a closer temporal relationship between MAP and CBFV, with a decrease in the time lag between the change in BP and the change in CBF, than controls. As shown in Fig. 5, both POTS and control subjects had a significant increase in LF coherence. Only POTS subjects had a significant increase in LF gain during tilt, and only POTS subjects exhibited a significant increase in HF coherence during HUT.
Fig. 4.
Frequency domain transfer function of MAP and CBFV for a representative POTS subject supine (A) and upright (B) and control subject supine (C) and upright (D). During tilt, there was an increase in low frequency (LF; 0.04–0.15 Hz) coherence between MAP and CBFV in both POTS and control subjects and an increase in high frequency (HF; 0.15–0.40 Hz) coherence between MAP and CBFV in POTS subjects. During tilt, POTS patients had a significant increase in LF gain. In POTS subjects during tilt, phase tended to decrease and approached zero as frequency increased into the HF range.
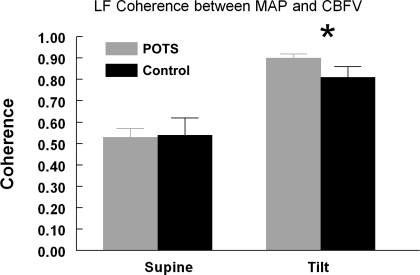
Fig. 5.
LF (0.04–0.15 Hz) coherence between MAP and CBFV in POTS and control subjects in the supine position and during tilt. Both POTS and control subjects exhibited an increase in LF coherence during tilt. *P < 0.05 compared with supine measurements.
Correlations
During HUT, only POTS subjects had significant correlations between HR and SBP (r = −0.74, P < 0.05), DBP (r = −0.85, P < 0.01), and MAP (r = −0.81, P < 0.01), whereas in controls HR did not significantly correlate with SBP (r = −0.45, P = 0.31), DBP (r = 0.68, P = 0.09), or MAP (r = 0.61, P = 0.14). Also, POTS subjects had significant correlations between MAP-CBFV LF coherence and SBP (r = −0.779, P < 0.05), DBP (r = −0.704, P < 0.05), and MAP (r = −0.748, P < 0.05). Control subjects did not have significant correlations between MAP-CBFV LF coherence and SBP (r = −0.07, P = 0.88), DBP (r = −0.28, P = 0.55), or MAP (r = −0.31, P = 0.49). These correlations are time domain counterparts of the frequency domain variability findings reported above.
DISCUSSION
Novel Findings
This study demonstrates new findings about CA in both POTS and healthy control subjects. First, we showed that in young, normocapnic POTS subjects, CBFV drops by 19.5% compared with only 10.3% in healthy controls during HUT. In POTS subjects, this could not be accounted for by a posturally induced change in ETco2 alone because that would only accounted for a 12% decrease. Static autoregulation (i.e., the average change in CBFV at a given AP) was, therefore, decreased in POTS subjects compared with control subjects and remained decreased throughout the tilt.
Second, although transfer function analysis of BP and CBFV implicitly involve the computation of CBFV variability (58, 59), we are, to our knowledge, first to make explicit use of CBFV variability measurements in relation to POTS. These are potentially important because they convey a tangible sense of how much and how rapidly CBF changes. CBFV variability, at LF, represents the overall effects of cerebrovascular transduction of BP, represented as Mayer waves (11), which appear with increased amplitude during tilt in POTS subjects (Fig. 3B). Mayer waves represent the increase in sympathetic baroreflex activity engendered by orthostasis (31, 35), and this increase in baroreflex activity is increased in POTS (Table 3).
We additionally demonstrated that during HUT, only POTS subjects show an increase in the LF component of CBFV variability, whereas both POTS and control subjects show a smaller increase in the HF component. Similarly, the LF gain (transfer function amplitude) and αCBF-index of MAP-CBFV variability increased during HUT only in POTS subjects. We demonstrated that the LF and HF coherence between MAP and CBFV increases during HUT in both POTS and control subjects but increased to a greater degree in POTS subjects. Also, the LF coherence between MAP and CBFV during HUT correlated with SBP, DBP, and MAP only in POTS subjects. The combination of increased Mayer wave amplitudes, increased gain, and increased coherence at LF accounts for the increase in CBFV variability. Corresponding observations were made in the time domain (Fig. 3), in which oscillations in AP were nearly synchronous with oscillations in CBFV. Also, we demonstrated how static and dynamic CA are ineffective in maintaining CBFV during tilt.
Implications for POTS Patients
Dynamic and static autoregulation are less effective in young, normocapnic POTS subjects compared with control subjects during HUT, as demonstrated by the decreased CBFV, increased LF (0.1 Hz) and HF (0.25 Hz) coherence between MAP and CBFV, and increased LF gain, with a lack of an associated change in phase differences, which remained low. This results in a lower CBFV with greater CBFV variability at LF, i.e., greater oscillations in the already reduced CBF, which are signs of both static and dynamic autoregulatory deficits. The frequency of 0.1 Hz converts to a time scale of 10 s, and half of an oscillatory period would be 5 s. This means that CBF is further decreased for 5 s and increased for 5 s compared with the reduced static baseline. As a result, substantially lowered CBF occurs 50% of the time in POTS subjects compared with control subjects when upright, which can impair cerebral perfusion and neurocognitive function (56). A decrease in perfusion of the brain may help to explain the symptoms of lightheadedness, dizziness, and mental confusion that are common in POTS patients (27, 33, 42). Dynamically, oscillations in CFBV coincide with oscillations in AP (see Fig. 3). Since POTS subjects exhibited lower MAP than controls, therapies that increase MAP may also increase CBFV, possibly alleviating the cognitive impairment.
Comparisons with the Literature
ETco2 and HUT.
It is well known that changes in arterial Pco2 levels affect brain blood flow and that CO2 levels decrease during tilt (9). Appropriately, we noted that POTS and control subjects exhibited a decrease in ETco2 during tilt. Because the respiratory rate does not change with tilt, the decrease in ETco2 has been ascribed to changes in the ventilation-perfusion relationship due to a wider expansion of the lower chest wall (9). Furthermore, decreases in ETco2 values may overestimate decreases in arterial Pco2 values and should be scrutinized when applied to changes in CBF (6, 43).
From our results, we show that the change in ETco2 cannot fully account for the changes in CBFV. Schondorf et al. (42) studied adult POTS patients and found that ETco2 values did not differ during early or late HUT and that the respiratory rates of POTS patients were similar to those of healthy control subjects. Thus, if ETco2 did accurately reflect changes in arterial Pco2 during the duration of the tilt, the maximum effect should be a 12% decrease. This is much less than the nearly 20% decrease we found, and our conclusion is consistent with the literature (43).
Static autoregulation: decreased CBFV during tilt.
We report a 19.5% static decrease in CBFV in POTS subjects during tilt compared with a 10.3% decrease in controls. These results contrast with those observed by Schondorf et al. (42), who reported that CBFV did not significantly differ between POTS and control subjects during tilt. This difference in findings may be due to the difference in age groups of each study, different durations of tilt timing, and the inclusion of hypocapnic test subjects as well as the heterogeneity between POTS patients. On the other hand, Jacob et al. (22) reported findings similar to ours, with a decrease in CBFV of 28 ± 10% in POTS patients and 10 ± 10% in controls during tilt. Their study included subjects who were older than ours (22–47 yr old) and they used varying degrees of tilt.
HR-BP variability.
We found that HR variability in POTS subjects was decreased while in the supine position and when upright but that BP variability was increased only when upright compared with control subjects. This is consistent with previous reports (45, 46). The decrease in LF and HF HR variability gain (see Table 2) in POTS subjects during HUT is consistent with vagal withdrawal (45). These data suggest that there is intact baroreflex and sympathetic function in young, normocapnic POTS patients. These sympathetic mechanisms may compensate for the decrease in BP in POTS subjects during HUT and may result in the oscillatory changes that were found (see Fig. 3).
Coherence and synchronization.
Due to the increased coherence and synchronization between MAP and CBFV, we suggest that increased baroreflex-mediated fluctuations in pressure may be translated into increased fluctuations (variability) of CBFV. Increased sympathetic activity during HUT in POTS is supported by a report (31) of increased muscle sympathetic nerve activity in POTS subjects compared with controls. The increased sympathetic baroreflex activity along with vagal withdrawal in POTS patients during tilt may play a role in the synchronization between CBFV and MAP (36).
CBFV variability.
CBFV variability describes the oscillatory changes that occur in CBFV during tilt. We found that the LF components of CBFV variability (Table 3) increased only in POTS subjects during HUT. LF components are associated with sympathetic baroreflex activity, whereas HF components are associated with parasympathetic-respiratory activity and cerebrovascular impedance and are less clearly defined physiologically.
The sympathetic-mediated constriction of the MCA is controversial: some investigators have found little sympathetic vascular control (4, 44, 48), whereas others have found a large sympathetic contribution to CBF regulation in humans and mammals (7, 55). Bondar et al. (7) stated that a sympathetic-mediated vasoconstriction could shift the autoregulatory curve to the right, and thus autoregulation would be efficient at high APs but inefficient at lower APs. If this occurred, the decrease in AP seen in POTS subjects during tilt would not be autoregulated, and CBFV would decrease in relation to the decrease in MAP. However, we are unable to say, nor do we intend to imply, that the sympathetic nervous system is the main control mechanism regulating CBF in POTS patients. However, the intact baroreflex in POTS patients mediates oscillations in MAP and CBFV. This was supported by Baumbach and Heistad (2), who stated that momentary changes in MAP produce similar changes in CBFV. Additional support comes from Birch et al. (5), who suggested that rapid alternation in MAP results in ineffective CA, allowing CBFV to oscillate directly with pressure. The oscillations of MAP are baroreflex mediated, but the responses of cerebral arterioles could relate to actions of the sympathetic nervous system or to other mechanisms, such as local, flow-mediated, myogenic, neural, cell signaling, and/or metabolic processes (8, 20, 25, 52).
Cerebral autoregulation.
CA is considered a high-pass filter of MAP, such that slow changes in MAP are dampened and rapid changes pass through, thereby causing oscillations in CBFV (26). The range of CA is not inflexible, and pathological states seen in humans, rats, and dogs, such as hypertension (49, 53) or hemorrhage (17), can shift it. Oscillations in CBFV during tilt in POTS subjects have previously been described by Hermosillo et al. (18), who suggested that they may be related to inefficient autoregulation and increased HR. Our data are in accordance with this.
To study static CA, we used the average change in CBFV during tilt (see Fig. 2 and Table 1). Others have used CVRi as an index of static CA (42) and reported a decrease in resistance during tilt. This is contrary to what we and others have found (7, 22). Physiologically, the assumption is that cerebral resistance should increase during tilt if cerebral perfusion pressure is being maintained and would decrease if CBF is being maintained. Therefore, CVRi may not be the best index of static CA, as these responses are contradictory and may oppose each other. Furthermore, during tilt, the brain is rostral to the heart, and systemic MAP may not be representative of cerebral MAP (58). The index has limitations to its accuracy. In relation to this, a normalized gain has been calculated by others based on CVRi (42). Due to the limitations of CVRi, we did not calculate normalized gain.
During tilt, dynamic CA appears to be impaired in POTS subjects. Dynamically, CBFV oscillations are nearly perfectly synchronous with the oscillations in AP in POTS subjects (see Fig. 3). This increase in synchronization is similar to what Birch et al. (5) reported during stand-squat maneuvers in healthy subjects. They also concluded that dynamic CA is ineffective if MAP changes quickly (5).
Additionally, we used transfer function analysis to describe dynamic CA. The increase in both LF and HF coherence (see Table 3 and Fig. 4) suggests that oscillations in MAP are directly influencing oscillations in CBFV. Thus, there is a decrease in dynamic autoregulation, and the cerebral vasculature is acting like a passive fixed Ohmic resistor. This conclusion is supported by Low et al. (27) and Diehl et al. (12), who also suggested a linear relationship between MAP and CBFV. Recent work by Zhang et al. (10) supports this claim in their study of dynamic autoregulation in healthy subjects. Increased gain suggests that the attenuation of dynamic CA is ineffectively dampening the effects of the oscillations of MAP on CBFV (15, 54). The lack of change in phase shift from the supine condition and the trend that phase decreases through the frequency range studied suggest that dynamic CA is not greatly influencing how oscillations in CBFV are responding to orthostatic conditions. Normally, CBFV leads MAP in phase (54). Phase shifts near zero degrees are considered to represent ineffective CA (26).
Coherence and tilt.
Why then is coherence seldom used as the index of (or lack of) dynamic autoregulation? A coherence of 1 implies a perfectly linear relationship between input and output, MAP and CBFV. Most investigators use a coherence of >0.5 to indicate the potential for any linear time-invariant relationship between two signals. This requirement can be explained by considering the reasons for deviation of coherence from a perfect 1.0. Coherence can be reduced by three main factors. The first is noise (3, 28, 37). The requirement for coherence of >0.5 is customarily used to avoid overly noisy systems. In our data, it is highly unlikely that noise predominates because upright coherence is so close to 1.0 and because we have no reason to believe that the act of tilting alters noise. Second, nonlinearity of a system can decrease coherence and make it an imperfect measure of synchronization (3, 16, 37). This could certainly be true, but, if so, nonlinearity is highly affected by posture, decreasing in the upright position. Finally, coherence can be decreased if there are other inputs that are not included in the calculation of coherence (37). We think this is most likely. We propose that in the supine position, certain input signals influence coherence and that these signals are essentially removed (or greatly lessened) as inputs when upright. Candidates for such input signals include the controversial sympathetic-mediated changes in CBF control, which may asymptotically reach a maximal value. However, it is unlikely that sympathetic activity is actually maximized in most POTS patients as this would result in upright HRs well in excess of those measured. We think a more likely possibility is vagal withdrawal, which is nearly complete in POTS patients (with HRs of >110 beats/min) and only partially present in control subjects (45). This is consistent with CBF dependence on parasympathetic activity. This remains speculative, and further work is needed to decipher the exact mechanism(s).
Limitations
Transcranial Doppler measures changes in MCA blood flow velocity instead of blood flow directly. Velocity and flow are not the same. This method assumes that the diameter of the MCA does not change during tilt. MRI studies in humans during orthostasis have demonstrated that the MCA diameter remains constant and is not affected by changing levels of CO2 (13, 44).
Transfer function analysis assumes linearity of a system. Our study assumes linearity between MAP and CFBV as well. This may not always be the case. Panerai et al. (37) suggested that linearity may not always hold, and coherence values may be higher if nonlinearity is taken into account. Mitsis et al. (30) described a nonlinear model of CA to illustrate changes in cerebral hemodynamics during lower body negative pressure experiments. Novak et al. (19) described a “multimodal pressure-flow model” to determine nonlinear CA between MAP and CBFV in supine subjects. They described CA as phase shifts between MAP and CBFV oscillations in control patients and also found a high correlation between the phase oscillations of MAP and cerebral perfusion pressure in traumatic brain injury patients (19).
Beat-to-beat continuous BP was monitored using finger arterial plethysmography. With this method, we assume that MAP in the finger is representative of MAP in the brain and that changes in intracranial pressure are insignificant.
CO2 is well known to affect CBF. In this study, we attempted to control for this factor by only using POTS and control subjects who exhibited CO2 levels in the normocapnic range while both in the supine position and during tilt. In both POTS and control subjects, ETco2 levels demonstrated small decreases upon tilt. We were limited by not measuring tidal volume or the overall volume of CO2 expired. Furthermore, we were limited by not measuring arterial Pco2 content.
Our subjects were allowed to eat a light breakfast 2 h or more before the experiment. Rowe et al. (40) demonstrated that feeding did not change CBF. On a related note, hypoglycemia does not appear to affect CBF until glucose levels are below a concentration (<2 mmol/l) that would cause noticeable cerebral cognitive impairment (51). Thus, we believe that neither feeding nor fasting confounded our results. On the other hand, we did not standardize blood glucose levels or monitor them, and, therefore, we state this limitation.
Our number of control subjects (n = 7) was small and may be questioned as limiting. We believe the small SEs may be interpreted as showing the uniformity of the physiological responses tested in these subjects.
Sympathetic nerve function was assessed using LF variability techniques. Respiration influences sympathetic activity (14), and we were limited by not measuring ventilation volumes.
Summary
In summary, we conclude that CBFV decreases more in POTS subjects than in control subjects during tilt. This may be due to altered and inefficient CA. Decreased CBF can impair consciousness and neurocognitive function.
GRANTS
This work was supported by National Heart, Lung, and Blood Institute Grants 5-R01-HL-074873-05, 1-R21-HL-091948-01, and 1-R01-HL-087803-01A1, American Heart Association Grant 0735603T, and a grant from the Chronic Fatigue and Immune Deficiency Syndrome Association of America.
Acknowledgments
The authors thank the members of the Division of Pediatric Cardiology, especially Dr. Michael H. Gewitz and Dr. Leonard Newman, for the support. The authors also thank our mentors, Dr. Thomas H. Hintze and Dr. Gabor Kaley, for the continual support and inspiration.
REFERENCES
- 1.Agarwal AK, Garg R, Ritch A, Sarkar P. Postural orthostatic tachycardia syndrome. Postgrad Med J 83: 478–480, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Baumbach GL, Heistad DD. Regional, segmental, and temporal heterogeneity of cerebral vascular autoregulation. Ann Biomed Eng 13: 303–310, 1985. [DOI] [PubMed] [Google Scholar]
- 3.Bendat JS, Piersol AG. Random Data Analysis and Measurement Procedures. New York: Wiley-Interscience, 1971.
- 4.Bevan RD, Dodge J, Nichols P, Penar PL, Walters CL, Wellman T, Bevan JA. Weakness of sympathetic neural control of human pial compared with superficial temporal arteries reflects low innervation density and poor sympathetic responsiveness. Stroke 29: 212–221, 1998. [DOI] [PubMed] [Google Scholar]
- 5.Birch AA, Dirnhuber MJ, Hartley-Davies R, Iannotti F, Neil-Dwyer G. Assessment of autoregulation by means of periodic changes in blood pressure. Stroke 26: 834–837, 1995. [DOI] [PubMed] [Google Scholar]
- 6.Bjurstedt H, Hesser CM, Liljestrand G, Matell G. Effects of posture on alveolar-arterial CO2 and O2 differences and on alveolar dead space in man. Acta Physiol Scand 54: 65–82. 1962. [DOI] [PubMed] [Google Scholar]
- 7.Bondar RL, Kassam MS, Stein F, Dunphy PT, Fortney S, Riedesel ML. Simultaneous cerebrovascular and cardiovascular responses during presyncope. Stroke 26: 1794–1800, 1995. [DOI] [PubMed] [Google Scholar]
- 8.Burnstock G Neurogenic control of cerebral circulation. Cephalalgia 5, Suppl 2: 25–33, 1985. [DOI] [PubMed] [Google Scholar]
- 9.Cencetti S, Bandinelli G, Lagi A. Effect of Pco2 changes induced by head-upright tilt on transcranial Doppler recordings. Stroke 28: 1195–1197, 1997. [DOI] [PubMed] [Google Scholar]
- 10.Claassen JA, Levine BD, Zhang R. Dynamic cerebral autoregulation during repeated squat-stand maneuvers. J Appl Physiol 106: 153–160, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Diehl RR, Linden D, Chalkiadaki A, Diehl A. Cerebrovascular mechanisms in neurocardiogenic syncope with and without postural tachycardia syndrome. J Auton Nerv Syst 76: 159–166, 1999. [DOI] [PubMed] [Google Scholar]
- 12.Diehl RR, Linden D, Lucke D, Berlit P. Spontaneous blood pressure oscillations and cerebral autoregulation. Clin Auton Res 8: 7–12, 1998. [DOI] [PubMed] [Google Scholar]
- 13.Djurberg HG, Seed RF, Evans DA, Brohi FA, Pyper DL, Tjan GT, al Moutaery KR. Lack of effect of CO2 on cerebral arterial diameter in man. J Clin Anesth 10: 646–651, 1998. [DOI] [PubMed] [Google Scholar]
- 14.Eckberg DL, Nerhed C, Wallin BG. Respiratory modulation of muscle sympathetic and vagal cardiac outflow in man. J Physiol 365: 181–196, 1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Franco FA Cerebral autoregulation and syncope. Prog Cardiovasc Dis 50: 49–80, 2007. [DOI] [PubMed] [Google Scholar]
- 16.Giller CA The frequency-dependent behavior of cerebral autoregulation. Neurosurgery 27: 362–368, 1990. [DOI] [PubMed] [Google Scholar]
- 17.Heistad DD, Marcus ML, Sandberg S, Abboud FM. Effect of sympathetic nerve stimulation on cerebral blood flow and on large cerebral arteries of dogs. Circ Res 41: 342–350, 1977. [DOI] [PubMed] [Google Scholar]
- 18.Hermosillo AG, Jauregui-Renaud K, Kostine A, Marquez MF, Lara JL, Cardenas M. Comparative study of cerebral blood flow between postural tachycardia and neurocardiogenic syncope, during head-up tilt test. Europace 4: 369–374, 2002. [DOI] [PubMed] [Google Scholar]
- 19.Hu K, Peng CK, Czosnyka M, Zhao P, Novak V. Nonlinear assessment of cerebral autoregulation from spontaneous blood pressure and cerebral blood flow fluctuations. Cardiovasc Eng 8: 60–71, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Iadecola C, Nedergaard M. Glial regulation of the cerebral microvasculature. Nat Neurosci 10: 1369–1376, 2007. [DOI] [PubMed] [Google Scholar]
- 21.Immink RV, Secher NH, Roos CM, Pott F, Madsen PL, van Lieshout JJ. The postural reduction in middle cerebral artery blood velocity is not explained by PaCO2. Eur J Appl Physiol 96: 609–614, 2006. [DOI] [PubMed] [Google Scholar]
- 22.Jacob G, Atkinson D, Jordan J, Shannon JR, Furlan R, Black BK, Robertson D. Effects of standing on cerebrovascular resistance in patients with idiopathic orthostatic intolerance. Am J Med 106: 59–64, 1999. [DOI] [PubMed] [Google Scholar]
- 23.Jordan J, Shannon JR, Black BK, Paranjape SY, Barwise J, Robertson D. Raised cerebrovascular resistance in idiopathic orthostatic intolerance: evidence for sympathetic vasoconstriction. Hypertension 32: 699–704, 1998. [DOI] [PubMed] [Google Scholar]
- 24.Jordan J, Shannon JR, Diedrich A, Black B, Costa F, Robertson D, Biaggioni I. Interaction of carbon dioxide and sympathetic nervous system activity in the regulation of cerebral perfusion in humans. Hypertension 36: 383–388, 2000. [DOI] [PubMed] [Google Scholar]
- 25.Koehler RC, Roman RJ, Harder DR. Astrocytes and the regulation of cerebral blood flow. Trends Neurosci 32: 160–169, 2009. [DOI] [PubMed] [Google Scholar]
- 26.Latka M, Turalska M, Glaubic-Latka M, Kolodziej W, Latka D, West BJ. Phase dynamics in cerebral autoregulation. Am J Physiol Heart Circ Physiol 289: H2272–H2279, 2005. [DOI] [PubMed] [Google Scholar]
- 27.Low PA, Novak V, Spies JM, Novak P, Petty GW. Cerebrovascular regulation in the postural orthostatic tachycardia syndrome (POTS). Am J Med Sci 317: 124–133, 1999. [DOI] [PubMed] [Google Scholar]
- 28.Marmarelis VZ Coherence and apparent transfer function measurements for nonlinear physiological systems. Ann Biomed Eng 16: 143–157, 1988. [DOI] [PubMed] [Google Scholar]
- 29.Medow MS, Stewart JM. The postural tachycardia syndrome. Cardiol Rev 15: 67–75, 2007. [DOI] [PubMed] [Google Scholar]
- 30.Mitsis GD, Zhang R, Levine BD, Marmarelis VZ. Cerebral hemodynamics during orthostatic stress assessed by nonlinear modeling. J Appl Physiol 101: 354–366, 2006. [DOI] [PubMed] [Google Scholar]
- 31.Muenter SN, Charkoudian N, Dotson RM, Suarez GA, Low PA. Baroreflex control of muscle sympathetic nerve activity in postural orthostatic tachycardia syndrome. Am J Physiol Heart Circ Physiol 289: H1226–H1233, 2005. [DOI] [PubMed] [Google Scholar]
- 32.Novak V, Novak P, Spies JM, Low PA. Autoregulation of cerebral blood flow in orthostatic hypotension. Stroke 29: 104–111, 1998. [DOI] [PubMed] [Google Scholar]
- 33.Novak V, Spies JM, Novak P, McPhee BR, Rummans TA, Low PA. Hypocapnia and cerebral hypoperfusion in orthostatic intolerance. Stroke 29: 1876–1881, 1998. [DOI] [PubMed] [Google Scholar]
- 34.Nowak JA, Ocon A, Taneja I, Medow MS, Stewart JM. Multiresolution wavelet analysis of time-dependent physiological responses in syncopal youths. Am J Physiol Heart Circ Physiol 296: H171–H179, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.O'Leary DD, Kimmerly DS, Cechetto AD, Shoemaker JK. Differential effect of head-up tilt on cardiovagal and sympathetic baroreflex sensitivity in humans. Exp Physiol 88: 769–774, 2003. [DOI] [PubMed] [Google Scholar]
- 36.Ogoh S, Brothers RM, Eubank WL, Raven PB. Autonomic neural control of the cerebral vasculature: acute hypotension. Stroke 39: 1979–1987, 2008. [DOI] [PubMed] [Google Scholar]
- 37.Panerai RB, Eames PJ, Potter JF. Multiple coherence of cerebral blood flow velocity in humans. Am J Physiol Heart Circ Physiol 291: H251–H259, 2006. [DOI] [PubMed] [Google Scholar]
- 38.Raj SR The postural tachycardia syndrome (POTS): pathophysiology, diagnosis and management. Indian Pacing Electrophysiol J 6: 84–99, 2006. [PMC free article] [PubMed] [Google Scholar]
- 39.Robbe HW, Mulder LJ, Ruddel H, Langewitz WA, Veldman JB, Mulder G. Assessment of baroreceptor reflex sensitivity by means of spectral analysis. Hypertension 10: 538–543, 1987. [DOI] [PubMed] [Google Scholar]
- 40.Rowe GG, Maxwell GM, Castillo CA, Freeman DJ, Crumpton CW. A study in man of cerebral blood flow and cerebral glucose, lactate and pyruvate metabolism before and after eating. J Clin Invest 38: 2154–2158, 1959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Schondorf R, Benoit J, Stein R. Cerebral autoregulation in orthostatic intolerance. Ann NY Acad Sci 940: 514–526, 2001. [DOI] [PubMed] [Google Scholar]
- 42.Schondorf R, Benoit J, Stein R. Cerebral autoregulation is preserved in postural tachycardia syndrome. J Appl Physiol 99: 828–835, 2005. [DOI] [PubMed] [Google Scholar]
- 43.Serrador JM, Hughson RL, Kowalchuk JM, Bondar RL, Gelb AW. Cerebral blood flow during orthostasis: role of arterial CO2. Am J Physiol Regul Integr Comp Physiol 290: R1087–R1093, 2006. [DOI] [PubMed] [Google Scholar]
- 44.Serrador JM, Picot PA, Rutt BK, Shoemaker JK, Bondar RL. MRI measures of middle cerebral artery diameter in conscious humans during simulated orthostasis. Stroke 31: 1672–1678, 2000. [DOI] [PubMed] [Google Scholar]
- 45.Stewart JM Autonomic nervous system dysfunction in adolescents with postural orthostatic tachycardia syndrome and chronic fatigue syndrome is characterized by attenuated vagal baroreflex and potentiated sympathetic vasomotion. Pediatr Res 48: 218–226, 2000. [DOI] [PubMed] [Google Scholar]
- 46.Stewart JM, Gewitz MH, Weldon A, Munoz J. Patterns of orthostatic intolerance: the orthostatic tachycardia syndrome and adolescent chronic fatigue. J Pediatr 135: 218–225, 1999. [DOI] [PubMed] [Google Scholar]
- 47.Stewart JM, Medow MS, Cherniack NS, Natelson BH. Postural hypocapnic hyperventilation is associated with enhanced peripheral vasoconstriction in postural tachycardia syndrome with normal supine blood flow. Am J Physiol Heart Circ Physiol 291: H904–H913, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Strandgaard S, Sigurdsson ST. Point:Counterpoint: Sympathetic activity does/does not influence cerebral blood flow. Counterpoint: Sympathetic nerve activity does not influence cerebral blood flow. J Appl Physiol 105: 1366–1367, 2008. [DOI] [PubMed] [Google Scholar]
- 49.Talman WT, Dragon DN, Ohta H. Baroreflexes influence autoregulation of cerebral blood flow during hypertension. Am J Physiol Heart Circ Physiol 267: H1183–H1189, 1994. [DOI] [PubMed] [Google Scholar]
- 50.Taneja I, Medow MS, Glover JL, Raghunath NK, Stewart JM. Increased vasoconstriction predisposes to hyperpnea and postural faint. Am J Physiol Heart Circ Physiol 295: H372–H381, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Thomas M, Sherwin RS, Murphy J, Kerr D. Importance of cerebral blood flow to the recognition of and physiological responses to hypoglycemia. Diabetes 46: 829–833, 1997. [DOI] [PubMed] [Google Scholar]
- 52.Thorin-Trescases N, Bartolotta T, Hyman N, Penar PL, Walters CL, Bevan RD, Bevan JA. Diameter dependence of myogenic tone of human pial arteries. Possible relation to distensibility. Stroke 28: 2486–2492, 1997. [DOI] [PubMed] [Google Scholar]
- 53.Troisi E, Attanasio A, Matteis M, Bragoni M, Monaldo BC, Caltagirone C, Silvestrini M. Cerebral hemodynamics in young hypertensive subjects and effects of atenolol treatment. J Neurol Sci 159: 115–119, 1998. [DOI] [PubMed] [Google Scholar]
- 54.Van Beek AH, Claassen JA, Rikkert MG, Jansen RW. Cerebral autoregulation: an overview of current concepts and methodology with special focus on the elderly. J Cereb Blood Flow Metab 28: 1071–1085, 2008. [DOI] [PubMed] [Google Scholar]
- 55.van Lieshout JJ, Secher NH. Point:Counterpoint: Sympathetic activity does/does not influence cerebral blood flow. Point: Sympathetic activity does influence cerebral blood flow. J Appl Physiol 105: 1364–1366, 2008. [DOI] [PubMed] [Google Scholar]
- 56.van Lieshout JJ, Wieling W, Karemaker JM, Secher NH. Syncope, cerebral perfusion, and oxygenation. J Appl Physiol 94: 833–848, 2003. [DOI] [PubMed] [Google Scholar]
- 57.Wilson TM, Strang R, MacKenzie ET. The response of the choroidal and cerebral circulations to changing arterial Pco2 and acetazolamide in the baboon. Invest Ophthalmol Vis Sci 16: 576–580, 1977. [PubMed] [Google Scholar]
- 58.Zhang R, Wilson TE, Witkowski S, Cui J, Crandall GG, Levine BD. Inhibition of nitric oxide synthase does not alter dynamic cerebral autoregulation in humans. Am J Physiol Heart Circ Physiol 286: H863–H869, 2004. [DOI] [PubMed] [Google Scholar]
- 59.Zhang R, Zuckerman JH, Giller CA, Levine BD. Transfer function analysis of dynamic cerebral autoregulation in humans. Am J Physiol Heart Circ Physiol 274: H233–H241, 1998. [DOI] [PubMed] [Google Scholar]