Abstract
We have produced mice in which expression of the rat cardiac Na+/Ca2+ exchanger (NCX1) transgene was switched on when doxycycline was removed from the feed at 5 wk. At 8 to 10 wk, NCX1 expression in induced (Ind) mouse hearts was 2.5-fold higher but protein levels of sarco(endo)plasmic reticulum Ca2+-ATPase, α1- and α2-subunits of Na+-K+-ATPase, phospholamban, ryanodine receptor, calsequestrin, and unphosphorylated and phosphorylated phospholemman were unchanged compared with wild-type (WT) or noninduced (non-Ind) hearts. There was no cellular hypertrophy since WT, non-Ind, and Ind myocytes had similar whole cell membrane capacitance. In Ind myocytes, NCX1 current amplitude was ∼42% higher, L-type Ca2+ current amplitude was unchanged, and action potential duration was prolonged compared with WT or non-Ind myocytes. Contraction and intracellular Ca2+ concentration ([Ca2+]i) transient amplitudes in Ind myocytes were lower at 0.6, not different at 1.8, and higher at 5.0 mM extracellular Ca2+ concentration ([Ca2+]o) compared with WT or non-Ind myocytes. Despite similar Ca2+ current amplitude and sarcoplasmic reticulum (SR) Ca2+ uptake, SR Ca2+ content at 5.0 mM [Ca2+]o was significantly higher in Ind compared with non-Ind myocytes, indicating that NCX1 directly contributed to SR Ca2+ loading. Echocardiography demonstrated that heart rate, left ventricular mass, ejection fraction, stroke volume, and cardiac output were similar among the three groups of animals. In vivo close-chest catheterization demonstrated similar contractility and relaxation among the three groups of mice, both at baseline and after stimulation with isoproterenol. We conclude that induced expression of NCX1 transgene resulted in altered [Ca2+]i homeostasis, myocyte contractility, and action potential morphology. In addition, heart failure did not occur 3 to 5 wk after NCX1 transgene was induced to be expressed at levels found in diseased hearts.
Keywords: tet-off, excitation-contraction, fura-2, isolated mouse myocytes, electrophysiology, sarcoplasmic reticulum, intracellular Ca2+ concentration
ca2+ ions are centrally involved in excitation-contraction (EC) in cardiac muscle. Among the many transporters and ion channels involved in Ca2+ fluxes in cardiac myocytes, Na+/Ca2+ exchanger (NCX1) is unique in that it mediates both Ca2+ efflux (forward mode, 3 Na+ in and 1 Ca2+ out) and Ca2+ influx (reverse mode, 3 Na+ out and 1 Ca2+ in) during an EC cycle (4). At physiological extracellular Ca2+ concentration ([Ca2+]o) and at rest, it is generally accepted that NCX1 primarily functions in the Ca2+ efflux mode. During systole, when the membrane potential (Em) exceeds the equilibrium potential of NCX1 (ENaCa), Ca2+ influx is favored (2, 38). The extent and duration of Ca2+ influx mediated by NCX1 is species dependent, likely due to differences in intracellular Na+ concentration ([Na+]i) and action potential morphology among species (31, 38). For example, in rodent myocytes, the relatively high [Na+]i (∼11 to 12 mM) compared with that present in rabbit myocytes (∼4.5 mM) (10, 31) would bias NCX1 to Ca2+ influx mode during the action potential most of the time (13, 38). Indeed, although NCX1 overexpression in isolated adult rabbit myocytes (by adenovirus-mediated gene transfer) results primarily in impaired myocyte contractility compared with control myocytes (25, 30), in adult rat myocytes NCX1 overexpression can result in either decreased, no change, or increased contraction and intracellular Ca2+ concentration ([Ca2+]i) transient amplitudes, depending on prevailing [Ca2+]o (45).
Over the last decade, Philipson and his colleagues have made major contributions toward our understanding of NCX1 function in vivo and in vitro by the generation of constitutively expressed NCX1 transgenic (1, 27, 28, 36, 40) and the cardiac-specific NCX1 knock-out (17, 22–24) mice. During the same period, it became increasingly clear that alterations in NCX1 expression and/or activity are associated with many models of cardiac hypertrophy and heart failure (for review, see Ref. 32). It remains controversial, however, whether the change in NCX1 expression and/or activity is a beneficial compensatory mechanism in response to contractile dysfunction or detrimental, leading to further deterioration of heart failure. One approach to differentiate between cause and compensatory response is the ability to switch on the expression of the NCX1 transgene concomitantly with the onset of the disease state. The purpose of the present study was to characterize a novel transgenic mouse model in which expression of the rat NCX1 transgene was under the control of a cardiac-specific promoter driving the expression of a tetracycline transactivator (tTA). When doxycycline (Dox) was removed from the feed, expression of NCX1 transgene was induced. Using this novel mouse model, we tested the hypotheses that 1) myocytes with induced NCX1 expression exhibited changes in Ca2+ homeostasis and contractility according to the thermodynamic driving force for the exchanger and 2) moderate NCX1 overexpression, by itself, did not cause contractile failure in vivo.
METHODS
Generation of inducible NCX1 transgenic mouse.
A cardiac-specific and inducible controlled vector composed of a modified mouse α-myosin heavy chain (MHC) minimal promoter fused with nucleotide binding sites for tTA (TREMHC) was used to provide robust expression when turned on in the absence of Dox and minimal leakage when turned off in the presence of Dox (29). Sequence-verified rat NCX1 gene (45) was cloned into TREMHC vector and microinjected into the nuclei of FVB mice for transgenic mice production. Eleven transgenic founders (7 males and 4 females) were generated. To quantify the number of transgenes inserted into the genome, genomic DNA from mouse-tail was isolated using the Qiagen DNAeasy kit. Genomic DNA (100 ng) was used for real-time PCR using NCX1-specific primer set (forward, CCCAATGTTTCAATGGGATT; and reverse, AGATGGGTCTTGGGGTTC) and GAPDH set (forward, AACGACCCCTTCATTGAC; and reverse, TCCACGACATACTCAGCAC). NCX1-specific primers were conserved between mouse and rat NCX1 and detected rat and mouse NCX1 gene with equal efficiency (data not shown). Real-time PCR was performed in a 20-μl reaction (100 ng of genomic DNA; 500 nM each primer; 1X SYBRE Green Master Mix). Each experimental sample was performed in triplicate. The ΔCT method was used to quantify the results, which are presented as relative fold changes to the GAPDH gene. Each primer set was designed to assume a melting temperature of 60°C and 50% GC content.
Since expression of the transgene required the presence of Dox-responsive transcription factor tTA, all founder mice were healthy and fertile. To determine phenotype, transgenic founders were crossed to the cardiac tTA transgenic mice in FVB background (MHC-tTA). Littermates that were heterozygous for tTA but negative for NCX1 transgene were used as wild-type (WT) controls. To prevent NCX1 expression, Dox (300 mg/kg mouse diet; Bio-Serv) was administered to all pregnant mothers and offsprings of WT and NCX1 transgenic groups. To induce NCX1 expression in adult mice, Dox was removed from the feed at 5 wk of age and mice were studied 3 to 5 wk later. The MHC-tTA mouse line expressed tTA at very low levels. Expression of tTA in WT mice did not affect mouse heart weight and function up to 12 wk. Similarly, Dox alone did not affect WT mouse heart size or function (data not shown).
Mice were housed and fed on a 12-h:12-h light-dark cycle at the Thomas Jefferson University Animal Facility and were supervised by veterinary staff members. Standard care was provided to all mice used for experiments. All protocols applied to the mice in this study were approved and supervised by the Institutional Animal Care and Use Committee at Thomas Jefferson University.
Echocardiographic and hemodynamic analysis of cardiac function.
Transthoracic two-dimensional echocardiography (TTE) was performed in anesthetized (2% inhaled isoflurane) WT, induced (Ind), and noninduced (non-Ind) mice with a 12-MHz probe (20). TTE in M mode was carried out in the parasternal short-axis to assess left ventricular (LV) diameter and function. For in vivo hemodynamic measurements, a 1.4 French micromanometer-tipped catheter (SPR-671; Millar Instruments) was inserted into the right carotid artery and then advanced into the LV of lightly anesthetized (tribromoethanol-amylene hydrate, Avertin; 2.5% wt/vol, 8 μl/g ip) mice with spontaneous respirations. Hemodynamic parameters including heart rate (beats per min−1), LV end-diastolic pressure, and maximal first time derivative of LV pressure rise (+dP/dt) and fall (−dP/dt) were recorded in closed-chest mode, both at baseline and in response to progressive doses of isoproterenol (0.1, 0.5, 1, 5, and 10 ng).
Isolation of adult murine cardiac myocytes.
Cardiac myocytes were isolated from the septum and LV free wall of WT, Ind, and non-Ind mice (∼27 g) according to the protocol of Zhou et al. (47) and modified by our laboratory (35, 37). Briefly, mice were heparinized (1,500 u/kg ip) and anesthetized (pentobarbital sodium, 50 mg/kg ip). The heart was excised, mounted on a steel cannula, and retrograde perfused (100 cmH2O, 37°C) with Ca2+-free bicarbonate buffer followed by enzymatic digestion (collagenases B and D, protease XIV). Isolated myocytes were plated on laminin-coated glass coverslips in a petri dish, and the Ca2+ concentration of the buffer was progressively increased from 0.05 to 0.125 to 0.25 to 0.5 mM in three steps (10-min interval each). The 0.5 mM Ca2+ buffer was then aspirated and replaced with minimal essential medium (MEM; Sigma M1018) containing 1.2 mM Ca2+, 2.5% fetal bovine serum (FBS), and antibiotics (1% penicillin-streptomycin). The pH was adjusted to 7.0 in 4% CO2 by the addition of NaHCO3 (0.57 g/l). After 1 h (4% CO2, 37°), media was replaced with FBS-free MEM containing 0.1 mg/ml bovine serum albumin and antibiotics. Myocytes were used within 2 to 8 h of isolation.
Myocyte shortening measurements.
Myocytes adherent to coverslips were bathed in 0.6 ml of air- and temperature-equilibrated (37°C), HEPES-buffered (20 mM, pH 7.4) medium 199 containing 0.6, 1.8, or 5.0 mM [Ca2+]o. Measurements of myocyte contraction (1 Hz) were performed as previously described (35, 37).
[Ca2+]i transient measurements.
Myocytes were exposed to 0.67 μM of fura-2 AM for 15 min at 37°C. Fura-2-loaded myocytes were field stimulated to contract (1 Hz, 37°C) in medium 199 containing 0.6, 1.8, or 5.0 mM [Ca2+]o. [Ca2+]i transient measurements, daily calibration of fura-2 fluorescent signals, and [Ca2+]i transient analyses were performed as previously described (35, 37).
Na+/Ca2+ exchange current measurements.
Whole cell patch-clamp recordings were performed at 30°C as previously described (35, 37, 42, 43). Briefly, fire-polished pipettes with resistances of 1–1.5 MΩ when filled with standard internal solution were used. For Na+/Ca2+ exchange current (INaCa) measurements, pipette solution contained (in mM) 100 Cs+-glutamate, 7.25 Na+-HEPES, 1 MgCl2, 12.75 HEPES, 2.5 Na2ATP, 10 EGTA, and 6 CaCl2 (pH 7.2). Free Ca2+ in the pipette solution was 205 nM. Myocytes were bathed in an external solution containing (in mM) 130 NaCl, 5 CsCl, 1.2 MgSO4, 1.2 NaH2PO4, 5 CaCl2, 10 HEPES, 10 Na+-HEPES, and 10 glucose (pH 7.4) (35, 37, 42). Verapamil (1 μM), ouabain (1 mM), and niflumic acid (10 μM) were used to block L-type Ca2+ currents, Na+-K+-ATPase currents, and Cl− currents, respectively. The myocyte was held at the calculated ENaCa of −73 mV for at least 5 min before current was elicited with a descending-ascending voltage ramp (from +100 to −120 and back to +100 mV, 500 mV/s). INaCa, defined as the difference current in the absence and presence of NiCl2 (1 mM), was normalized to whole cell capacitance (Cm) before comparisons. Our conditions for measuring INaCa were carefully chosen to minimize contamination by Na+-K+-ATPase activity (K+ free and the presence of ouabain) and ion fluxes through the NCX1 before the onset of voltage ramp (by holding the cell at the calculated ENaCa), thereby allowing [Na+]i and [Ca2+]i to equilibrate with those present in the pipette solution.
L-type Ca2+ current measurements.
Pipette solution consisted of (in mM) 100 CsCl, 10 NaCl, 20 triethylammonium chloride, 10 HEPES, 5 MgATP, and 10 EGTA (pH 7.2 with CsOH). External solution contained (in mM) 137 NaCl, 5.4 CsCl, 1.8 CaCl2, 1.3 MgSO4, 1.2 NaH2PO4, 20 HEPES, 4 4-aminopyridine, and 15 glucose (pH 7.4 with NaOH). Before myocyte stimulation was started, holding potential was changed from −70 to −40 mV to inactivate fast inward Na+ current. To ensure steady-state sarcoplasmic reticulum (SR) Ca2+ loading, six conditioning pulses (from −40 to 0 mV, 300 ms, 1 Hz) were delivered before arrival of each test pulse (from −30 to +40 mV, 10-mV increments, 400 ms). After the last test pulse at +40 mV, the myocyte was held at −40 mV for 1 s before being returned to holding potential of −70 mV. Leak-subtracted inward currents were used in analysis for L-type Ca2+ current (ICa) amplitudes and inactivation kinetics. ICa was normalized to Cm before comparison among WT, Ind, and non-Ind myocytes.
ICa inactivation kinetics were determined by least-square fitting of a two-exponential function [A1exp(-t/τ1) + A2exp(-t/τ2) + C], where t is time; τ1 and τ2 are the fast and slow inactivation time constants, respectively; and A1, A2, and C are arbitrary constants. ICa data for voltage dependence of steady-state activation were fitted to Boltzmann distribution of the form I/Imax = 1/[1 + exp(Vh − V)/k] where I and Imax are current and maximal current, respectively; V was the test activating potential; Vh was the test potential eliciting one-half Imax; and k was the slope factor. Similarly, voltage dependence of steady-state inactivation of ICa was determined by a voltage prepulse protocol (43), and ICa data were fitted to a Boltzmann function of the form I/Imax = 1/[1 + exp(V − Vh)/k], where V is the prepulse potential and Imax, Vh, and k have their usual meanings.
Action potential measurements.
Action potentials were recorded using current-clamp configuration at 1.5× threshold stimulus and 4-ms duration (37, 46). Pipette solution consisted of (in mM) 125 KCl, 4 MgCl2, 0.06 CaCl2, 10 HEPES, 5 K+-EGTA, 3 Na2ATP, and 5 Na2-creatine phosphate (pH 7.2). External solution consisted of (in mM) 132 NaCl, 5.4 KCl, 1.8 CaCl2, 1.8 MgCl2, 0.6 NaH2PO4, 7.5 HEPES, 7.5 Na+-HEPES, and 5 glucose (pH 7.4).
SR Ca2+ content measurements.
SR Ca2+ content was estimated by integrating forward INaCa induced by caffeine exposure as described previously (35, 37, 41). The pipette solution consisted of (in mM) 100 Cs+-glutamate, 1 MgCl2, 30 HEPES, and 2.5 MgATP (pH 7.2). The external solution contained (in mM) 130 NaCl, 5 CsCl, 1.2 MgSO4, 1.2 NaH2PO4, 5 CaCl2, 20 HEPES, and 10 glucose (pH 7.4; 30°C). Holding potential was −70 mV. At 200 ms after the 11th conditioning pulse (from −70 to 0 mV, 300 ms, 1 Hz), with Em held at −70 mV, caffeine (5 mM, 2.4 s) was applied by puffer superfusion. The resulting inward current was digitized at 1 kHz and collected for 5 s. To convert INaCa time integral (coulombs) to moles, charge was divided by Faraday's constant of 96,487 coulombs/equivalent, based on 3 Na+ being exchanged for each Ca2+. SR Ca2+ content was normalized to cell size and given as femtomole per femtofared.
Immunoblotting.
Crude membranes from mouse left ventricles were prepared using a two-step centrifugation protocol described previously (34). For detection of NCX1, sarco(endo)plasmic reticulum Ca2+-ATPase (SERCA2), calsequestrin, and α1- and α2-subunits of Na+-K+-ATPase, proteins in crude membranes were subjected to 7.5% SDS-PAGE under nonreducing (10 mM N-ethylmaleimide for NCX1) or reducing (5% β-mercaptoethanol for SERCA2, calsequestrin, and Na+-K+-ATPase) conditions. For detection of cardiac ryanodine receptor (RyR2) and phospholamban (PLB), proteins were subjected to 3–8% (Tris-acetate; reducing conditions) and 12% (Tris-glycine; both reducing and nonreducing conditions) SDS-PAGE, respectively. Primary antibodies used were as follows: for NCX1, π11-13 polyclonal antibody (1:500; Swant, Bellinzona, Switzerland); for SERCA2, MA3-919 antibody (1:2,500; Affinity BioReagents, Golden, CO); for RyR2, MA3-916 antibody (1:1,000; Affinity BioReagents); for PLB, MA3-922 antibody (1:500; Affinity BioReagents); for calsequestrin, rabbit anti-calsequestrin antibody (1:2,500; Swant); and polyclonal antibodies against either α1- (1:1,000; Upstate USA, Charlottesville, VA) or α2-subunit of Na+-K+-ATPase (1:1,200; Upstate USA). Secondary antibodies were donkey anti-rabbit or sheep anti-mouse IgG (Amersham). For phospholemman (PLM) immunoblotting, proteins in crude membranes were subjected to 12% SDS-PAGE under reducing conditions. Polyclonal C2 antibody (1:10,000) was used to detect the COOH terminus of endogenous mouse PLM (predominantly unphosphorylated form)(33). Polyclonal CP68 antibody (1:2,000) was used to detect PLM phosphorylated at serine68 (26). Immunoreactive proteins were detected with enhanced chemiluminescence Western blotting system. Protein band signal intensities were quantitated by scanning autoradiograms of the blots with a phosphorimager.
Statistics.
All results are expressed as means ± SE. For analysis of maximal contraction and [Ca2+]i transient amplitudes as a function of group and [Ca2+]o, +dP/dt and −dP/dt as a function of group and isoproterenol, INaCa, and ICa as a function of group and voltage, two-way ANOVA was used. For analysis of protein abundance, action potential parameters, Cm, echocardiographic data, ICa activation and inactivation parameters, one-way ANOVA was used. A commercial software package (JMP version 7; SAS Institute, Cary, NC) was used. In all analyses, P < 0.05 was taken to be statistically significant.
RESULTS
Inducible NCX1 transgenic mouse.
Eleven founders (7 males, 4 females) harboring the rat NCX1 transgene were generated. The transgenic mice were all heterozygous. Analysis of mouse genomic DNA showed that founder lines contained transgene copies that are 2 to 37 times above the endogenous mouse NCX1 gene copies (data not shown). We selected a transgenic line with 11 times more NCX1 transgene copies (line B) for expression studies. To control the timing of overexpression, we crossed the NCX1 transgenic founder with homozygous MHC-tTA mice in the presence of Dox. Mice that were heterozygous for both tTA and NCX1 transgenes were used as the experimental (Ind and non-Ind) groups, whereas littermates that were heterozygous for tTA only were used as WT controls.
We chose founder line B because preliminary experiments showed that 3 wk after induction, NCX1 protein levels were ∼2× that of WT hearts, assuming equal sensitivity of the polyclonal π11-13 antibody to detect rat and mouse NCX1. This magnitude of NCX1 increase in Ind hearts was similar to that found in models of heart failure (32). Another reason to choose to study a founder line with modest levels of NCX1 transgene expression is that high levels of transgenic expression of even seemingly innocuous proteins such as green fluorescent protein (18) or Cre-recombinase (6) can cause dilated cardiomyopathy.
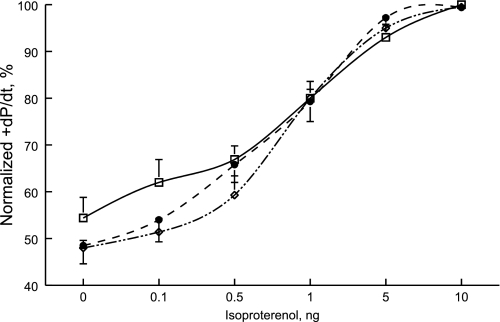
Both Ind and non-Ind mice had no readily observable altered phenotype in that both body weights and LV mass were similar to those measured in WT mice (Table 1). Indexes of cardiac performance, as determined by TTE and in vivo catheterization, were also similar among the three groups of animals (Table 1). Contractility responses (+dP/dt) to isoproterenol stimulation were similar among the three groups of animals (Fig. 1; group × isoproterenol interaction effect, P < 0.42). For example, in response to maximal doses of isoproterenol, +dP/dt increased by 86.6 ± 18.0%, 107.9 ± 7.9%, and 114.4 ± 20.3% in WT (n = 6), Ind (n = 7), and non-Ind (n = 3) hearts, respectively. Likewise, there were no significant differences in relaxation (−dP/dt) in response to maximal doses of isoproterenol in WT (77.7 ± 24.5% increase), Ind (86.9 ± 19.7% increase), and non-Ind (84.7 ± 4.2% increase) mice (group × isoproterenol interaction effect, P < 0.29).
Table 1.
Characteristics of WT and mice with induced NCX1 transgene
| WT | Non-Ind | Induced | |
|---|---|---|---|
| Body weight, g | 27.4±0.6 (5) | 26.2±0.4 (6) | 26.6±0.7 (3) |
| LV mass, mg | 53.0±2.6 | 53.5±2.0 | 56.7±2.0 |
| Heart rate, beats/min | 409±26 | 393±8 | 411±18 |
| Ejection fraction, % | 75.2±1.7 | 77.1±2.2 | 74.3±1.6 |
| Fractional shortening, % | 43.2±1.6 | 45.1±2.1 | 42.3±1.5 |
| Stroke volume, μl | 37.8±3.3 | 38.3±1.7 | 38.2±2.4 |
| Cardiac output, ml/min | 15.3±1.3 | 15.1±0.9 | 15.6±0.5 |
| +dP/dt, mmHg/s | 6,277±567 (7) | 6,356±228 (3) | 5,489±472 (7) |
| −dP/dt, mmHg/s | 6,314±633 | 5,708±248 | 5,133±442 |
Values are means ± SE; numbers in parentheses are numbers of mice. NCX1, cardiac Na+/Ca2+ exchanger; WT, wild-type; Non-Ind, noninduced; +dP/dt and −dP/dt, maximal first time derivative of left ventricular (LV) pressure rise and fall, respectively.
Fig. 1.
Induced expression of Na+/Ca2+ exchanger transgene does not affect contractility response to isoproterenol in vivo. Doxycycline (Dox) was administered to all mice from gestation to day of experiment with the exception of induced (Ind) mice in which Dox was removed from the diet at 5 wk of age. Mice were studied at 8 to 10 wk of age. In vivo catheterization was performed in anesthetized mice, and maximal first time derivative of left ventricular pressure rise (+dP/dt) and fall (−dP/dt) and heart rate were continuously monitored, both at baseline and increasing doses of isoproterenol. To construct dose-response curve for isoproterenol, +dP/dt was normalized to the maximal value measured in each mouse. There are 7 wild-type (□), 7 induced (•), and 3 noninduced (◊) mice. Error bars are not shown if they fall within the boundaries of the symbol. Composite results are shown in Table 1.
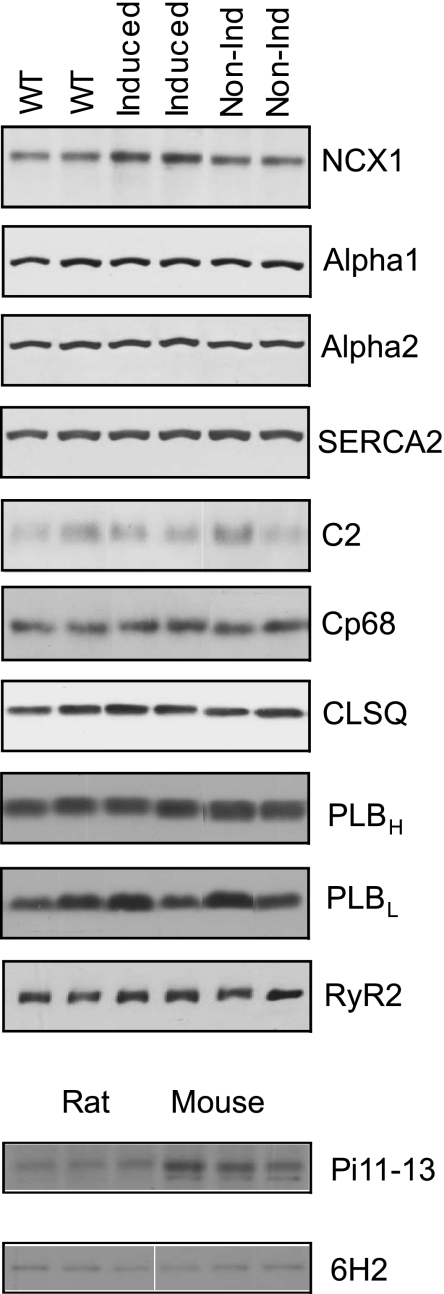
To quantify the fold increase in NCX1 expression in Ind compared with WT or non-Ind hearts, we evaluated the reactivity of π11-13 antibody (raised against canine NCX1 but epitope is unknown) against the rat NCX1 transgene and native mouse NCX1. Crude LV membranes were prepared from Spraque-Dawley rats and FVB mice (n = 4 each) and subjected to Western blotting. With the assumption of an equal number of NCX1 molecules per milligram of LV membrane between rat and mouse, the signals from π11-13 antibody were 1.70× higher for mouse (2,949 ± 110) than rat (1,732 ± 51 arbitrary units) NCX1 (Fig. 2; P < 0.0001). With the use of the monoclonal antibody 6H2 raised against the NH2 terminus of rabbit NCX1, the NCX1 signals from rat and mouse LV membranes were similar (Fig. 2), providing support for our assumption that NCX1 density was similar between mouse and rat LV membranes. Applying the correction factor of 1.7 suggests that protein levels of NCX1 in Ind hearts were ∼2.5-fold higher compared with WT or non-Ind hearts (Fig. 2 and Table 2). By contrast, expression of SERCA2, α1- and α2-subunits of Na+-K+-ATPase, RyR2, PLB, calsequestrin, and both unphosphorylated and phosphorylated forms of PLM was not altered by the induction of NCX1 transgene (Fig. 2 and Table 2). Ind myocytes did not undergo hypertrophy since Cm, a measure of cell surface membrane area, was similar among WT (154.8 ± 5.3 pF; n = 24), non-Ind (149.9 ± 7.5 pF; n = 17), and Ind (163.5 ± 9.0 pF; n = 18) myocytes.
Fig. 2.
Induced expression of Na+/Ca2+ exchanger transgene does not change the expression of selected proteins involved in excitation-contraction coupling. Top: crude membranes were prepared from wild-type (WT), induced, and noninduced (non-Ind) mouse left ventricle (LV) and subjected to SDS-PAGE followed by Western blot analysis. Primary antibodies (see methods) were used to detect cardiac Na+/Ca2+ exchanger (NCX1), α1 (alpha1)- and α2 (alpha2)- subunits of Na+-K+-ATPase, sarco(endo)plasmic reticulum Ca2+-ATPase (SERCA2), unphosphorylated phospholemman (C2), phospholemman phosphorylated at serine68 (Cp68), cardiac ryanodine receptor (RyR2), high and low molecular weight phospholamban (PLBH and PLBL, respectively), and calsequestrin (CLSQ). PLBH was detected under nonreducing conditions (only 1 band at ∼25 kDa), and PLBL was detected under reducing conditions (2 major bands at ∼25 and ∼5 KDa; only the low molecular weight PLB is shown for PLBL lanes). Composite results are shown in Table 2. Bottom: crude membranes prepared from rat and mouse LV were used to evaluate reactivity of polyclonal π11-13 antibody (1:500) against rat and mouse NCX1. To evaluate whether NCX1 density was similar between rat and mouse LV membranes, a monoclonal antibody (6H2; 1:1,000) raised against the NH2 terminus of NCX1 was used.
Table 2.
Effects of induced NCX1 transgene expression on levels of selected proteins
| WT | Non-Ind | Induced | |
|---|---|---|---|
| NCX1 | 28.4±0.8 (4) | 27.1±2.5 (5) | 54.2±3.7*(5) |
| SERCA2 | 89.2±1.8 | 85.3±2.2 | 81.4±2.4 |
| α1, Na+-K+-ATPase | 61.3±6.7 | 74.8±5.4 | 57.8±3.0 |
| α2, Na+-K+-ATPase | 42.8±2.7 | 49.9±3.0 | 49.7±0.8 |
| PLM, unphosphorylated | 16.8±2.5 | 13.9±3.8 | 11.0±1.4 |
| PLM, phosphorylated | 46.4±5.9 | 45.1±5.6 | 44.4±5.8 |
| Calsequestrin | 106.9±4.8 | 120.2±4.5 | 112.9±4.5 |
| RyR2 | 14.9±2.3 | 11.0±2.8 | 14.9±0.4 |
| PLBH | 43.9±8.8 | 56.4±11.9 | 40.4±4.6 |
| PLBL | 31.8±4.6 | 44.6±3.3 | 35.0±7.0 |
Values are means ± SE; numbers in parentheses are numbers of hearts used in crude membrane preparations. SERCA2, sarco(endo)plasmic reticulum Ca2+-ATPase; PLM, phospholemman; RyR2, cardiac ryanodine receptor; PLBH, high molecular weight phospholamban; PLBL, low molecular weight phospholamban.
P < 0.0005 compared with WT or Non-Ind. Since the π11-13 signal is 1.7× stronger for mouse than rat NCX1 (Fig. 2), the corrected NCX1 protein level is ∼2.54× in Induced when compared with WT or Non-Ind myocytes.
Effects of induced NCX1 transgene expression on Na+/Ca2+ exchange activity.
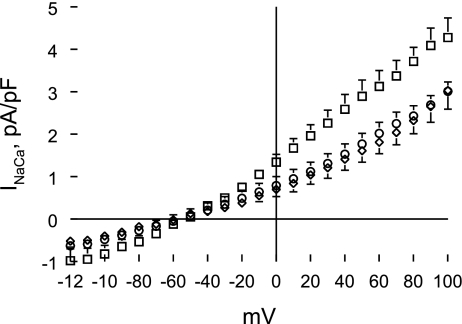
Na+/Ca2+ exchange activity measured as NCX1 current (INaCa) in all three groups of myocytes increased as voltage became more positive (Fig. 3; voltage effect, P < 0.0001). INaCa was significantly (group effect, P < 0.0001) increased in Ind myocytes compared with WT or non-Ind myocytes (Fig. 3). Our conditions were biased toward measuring outward (3 Na+ out and 1 Ca2+ in) rather than inward (3 Na+ in and 1 Ca2+ out) INaCa. This accounts for the significant group × voltage interaction effects (P < 0.0001), indicating that differences in INaCa magnitude between Ind and WT or non-Ind myocytes increased with positive voltages. At +100 mV, INaCa was ∼40% higher in Ind compared with WT or non-Ind myocytes. There were no differences in INaCa between WT and non-Ind myocytes (group effect, P < 0.48; group × voltage interaction effect, P < 0.23).
Fig. 3.
Induced expression of Na+/Ca2+ exchanger transgene increases Na+/Ca2+ exchange current (INaCa). INaCa was measured in WT, induced (Ind), and non-Ind myocytes at 30°C and 5.0 mM extracellular Ca2+ concentration ([Ca2+]o; see methods). Current-voltage relationships of INaCa (means ± SE) from WT (◊; n = 10), Ind (□; n = 7), and non-Ind (○; n = 8) myocytes are shown. The reversal potential of INaCa in all myocytes examined was ∼−60 mV. Error bars are not shown if they fall within the boundaries of the symbol.
Effects of induced NCX1 transgene expression on myocyte contraction.
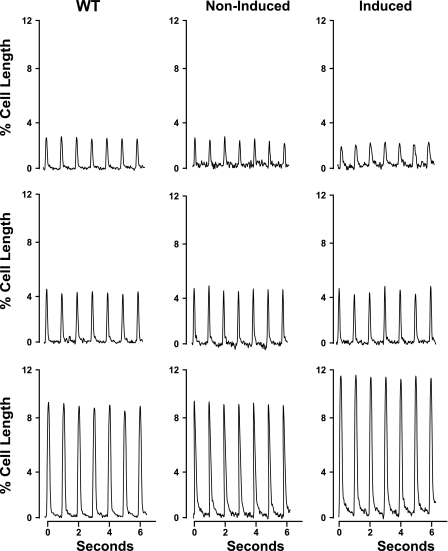
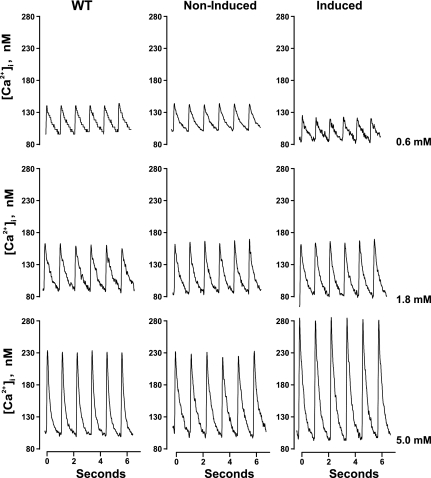
In all three groups of myocytes, elevating [Ca2+]o resulted in the expected increase in contraction amplitudes (Fig. 4 and Table 3; [Ca2+]o effect, P < 0.0001). However, the dynamic range in response to increasing [Ca2+]o was significantly increased in Ind myocytes. Specifically, at 0.6 mM [Ca2+]o, Ind myocytes shortened less than WT or non-Ind myocytes. By contrast, at 5.0 mM [Ca2+]o, Ind myocytes contracted more than WT or non-Ind myocytes. At 1.8 mM [Ca2+]o, contraction amplitudes were similar among WT, Ind, and non-Ind myocytes. These conclusions are supported by highly significant (P < 0.0001) group × [Ca2+]o interaction effects, indicating that the magnitude and/or direction of the effects of changing [Ca2+]o on cell shortening were different across the experimental groups.
Fig. 4.
Induced expression of Na+/Ca2+ exchanger transgene alters myocyte contractility. Myocytes isolated from LV of WT, noninduced, and induced mice were paced (1 Hz) to contract at 37°C and [Ca2+]o of 0.6 (top), 1.8 (middle), and 5.0 mM (bottom). Steady-state twitches are shown. Results are summarized in Table 3.
Table 3.
Effects of induced NCX1 transgene expression on myocyte shortening
| [Ca2+]o | WT | Non-Ind | Induced | ||
|---|---|---|---|---|---|
| Maximal contraction amplitude, % resting cell length | |||||
| 0.6 | 3.18±0.28 (16) | 3.28±0.38 (16) | 2.12±0.18*(16) | ||
| 1.8 | 5.42±0.32 (20) | 5.69±0.55 (11) | 5.71±0.28 (18) | ||
| 5.0 | 9.60±0.37 (21) | 9.95±0.43 (11) | 11.98±0.54*(20) | ||
| Maximal shortening velocity, cell length/s | |||||
| 0.6 | 0.44±0.05 | 0.60±0.07 | 0.32±0.04* | ||
| 1.8 | 0.82±0.05 | 0.93±0.10 | 0.98±0.04 | ||
| 5.0 | 1.41±0.08 | 1.54±0.10 | 1.78±0.10* | ||
| Maximal relengthening velocity, cell length/s | |||||
| 0.6 | 0.27±0.04 | 0.47±0.06 | 0.15±0.02* | ||
| 1.8 | 0.65±0.06 | 0.76±0.11 | 0.72±0.05 | ||
| 5.0 | 1.16±0.08 | 1.26±0.11 | 1.44±0.06* | ||
Values are means ± SE; numbers in parentheses are numbers of myocytes, without regard to the number of cells contributed by each heart (n = 5, 4, and 5 hearts for WT, Non-Ind, and Induced, respectively). [Ca2+]o, extracellular Ca2+ concentration.
P < 0.007 (group × [Ca2+]o interaction effects), Induced compared with WT or Non-Ind.
Both maximal shortening and relengthening velocities in Ind myocytes were significantly slower at 0.6 and faster at 5.0 mM [Ca2+]o, when compared with those measured in WT and non-Ind myocytes (Table 3; group × [Ca2+]o interaction effects, P < 0.007). There were no group × [Ca2+]o interaction effects in contraction amplitude, maximal shortening, and relaxation velocities when comparing WT and non-Ind myocytes.
Effects of induced NCX1 transgene expression on [Ca2+]i transients and SR Ca2+ uptake.
Differences in myocyte contractility between Ind and WT or non-Ind myocytes may be due to changes in [Ca2+]i associated with induced NCX1 overexpression. When compared with WT or non-Ind myocytes, systolic [Ca2+]i and [Ca2+]i transient amplitudes in Ind myocytes were lower at 0.6, similar at 1.8, and higher at 5.0 mM [Ca2+]o (Fig. 5 and Table 4; group × [Ca2+]o interaction effects, P < 0.004). There were no differences in diastolic [Ca2+]i among the three groups of myocytes.
Fig. 5.
Induced expression of Na+/Ca2+ exchanger transgene modulates intracellular Ca2+ concentration ([Ca2+]i) transients. Myocytes isolated from WT, noninduced, and induced murine LV were loaded with the Ca2+ indicator fura-2 and paced (1 Hz) to contract at 37°C and [Ca2+]o of 0.6 (top), 1.8 (middle), and 5.0 mM (bottom). Results are summarized in Table 4.
Table 4.
Effects of induced NCX1 transgene expression on [Ca2+]i transients, SR Ca2+ uptake, and SR Ca2+ contents
| [Ca2+]o | WT | Non-Ind | Induced | ||
|---|---|---|---|---|---|
| Systolic [Ca2+]i, nM | |||||
| 0.6 | 143±5 (8) | 144±7 (11) | 124±8*(7) | ||
| 1.8 | 165±7 (16) | 161±8 (13) | 157±7 (21) | ||
| 5.0 | 231±9 (19) | 233±10 (13) | 285±13*(21) | ||
| Diastolic [Ca2+]i, nM | |||||
| 0.6 | 105±4 | 101±6 | 96±6 | ||
| 1.8 | 91±5 | 87±4 | 83±4 | ||
| 5.0 | 98±5 | 100±4 | 100±3 | ||
| [Ca2+]i transient amplitude, % increase in fura-2 signal | |||||
| 0.6 | 8.5±0.8 | 7.8±0.6 | 5.7±0.6* | ||
| 1.8 | 17.0±1.0 | 17.3±1.1 | 17.1±1.0 | ||
| 5.0 | 28.0±1.2 | 28.2±1.2 | 37.2±2.0* | ||
| t1/2 of [Ca2+]i transient decline, ms | |||||
| 1.8 | 213±13 | 215±9 | 206±10 | ||
| SR Ca2+ content, fmol/fF | |||||
| 5.0 | ND | 12.5±1.4 (12) | 16.9±1.2† (10) | ||
Values are means ± SE; numbers in parentheses are numbers of myocytes, without regard to the number of cells contributed by each heart [for intracellular Ca2+ concentration ([Ca2+]i) transient measurements, n = 4 hearts each for WT, Non-Ind, and Induced; for sarcoplasmic reticulum (SR) Ca2+ content measurements, n = 2 and 4 hearts for Non-Ind and Induced, respectively]. ND, not done; t1/2, half-time.
P < 0.004 (group × [Ca2+]o interaction effects), Induced vs. WT or Non-Ind;
P < 0.03, Induced vs. Non-Ind.
SR Ca2+ uptake activity can be estimated from the half-time (t1/2) of [Ca2+]i transient decline (44). Since the kinetics of [Ca2+]i transient decline is dependent on peak [Ca2+]i (5), we focused on t1/2 of [Ca2+]i transient decline measured at 1.8 mM [Ca2+]o at which there were no differences in either systolic [Ca2+]i or [Ca2+]i transient amplitudes among the three groups of myocytes. There were no differences in t1/2 of [Ca2+]i transient decline among WT, non-Ind, and Ind myocytes (Table 4), indicating that induced expression of NCX1 transgene had no discernible effects on SR Ca2+ uptake.
Effects of induced NCX1 transgene expression on ICa.
Altered contractility and [Ca2+]i transients in Ind myocytes may partly be due to changes in ICa and its kinetics. In addition, in homozygous mice constitutively overexpressing NCX1 transgene, ICa was significantly increased (27), whereas in cardiac-specific NCX1 knockout mice, ICa was substantially decreased compared with that of control WT mice (24). Therefore, we measured ICa in all three groups of myocytes. Our initial attempts to measure ICa in FVB mouse myocytes using pipette and extracellular solutions previously designed for adult rat LV myocytes (43) and successfully applied to C57BL/6 mouse myocytes (37) were failures. For FVB mouse myocytes, we obtained consistent ICa results after we adopted the pipette and bathing solutions published by Zhou et al. (47) and detailed in methods.
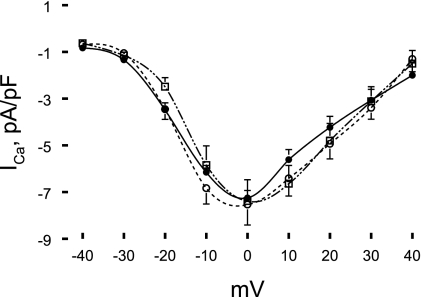
Maximal ICa density and current-voltage relationships were not different among WT (−7.53 ± 0.88 pA/pF; n = 14), non-Ind (−7.38 ± 0.91 pA/pF; n = 9), and Ind (−7.25 ± 0.32 pA/pF; n = 11) myocytes (Fig. 6). The test potential at which maximal ICa occurred was ∼0 mV for all three groups of myocytes (Fig. 6). Neither the fast (τ1) nor the slow (τ2) inactivation time constant measured at 0 mV was significantly (P < 0.07) different in Ind (τ1, 13.6 ± 1.9 ms; and τ2, 49.3 ± 6.6 ms) compared with WT (τ1, 15.4 ± 1.5 ms; and τ2, 72.7 ± 7.7 ms) or non-Ind myocytes (τ1, 13.7 ± 1.5 ms; and τ2, 76.2 ± 10.9 ms).
Fig. 6.
Induced expression of Na+/Ca2+ exchanger transgene has no effect on L-type Ca2+ current (ICa) density. ICa was measured in WT (○; n = 14), noninduced (□; n = 9), and induced (•; n = 11) myocytes at 30°C and 1.8 mM [Ca2+]o. Current density of peak ICa (means ± SE) at each test potential (−40 to + 40 mV) is shown. Error bars are not shown if they fall within the boundaries of a symbol.
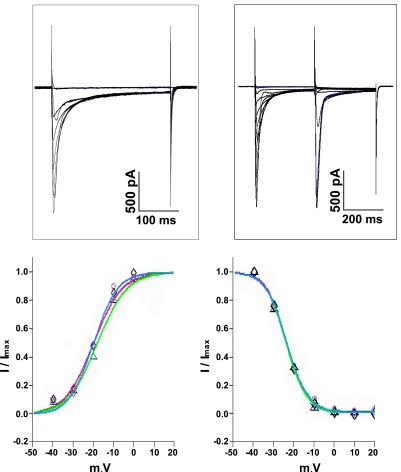
To further evaluate voltage-dependent properties of ICa, steady-state inactivation of ICa was determined using the classical two-pulse protocol (Fig. 7). The sigmoidal relationships between the extent of inactivation (f∞ = I/Imax) and prepulse potential were superimposable for all three groups of myocytes (Fig. 7). The test potential eliciting one-half maximal ICa, Vh, was not different among WT (−23.8 ± 0.5 mV; n = 8), non-Ind (−24.2 ± 0.5 mV; n = 6), and Ind (−23.9 ± 0.5 mV; n = 6) myocytes. Likewise, the slope factor k was similar among WT (5.1 ± 0.4 mV), non-Ind (5.0 ± 0.4 mV), and Ind (5.0 ± 0.4 mV) myocytes.
Fig. 7.
Induced expression of Na+/Ca2+ exchanger transgene does not affect voltage dependence of activation and inactivation of ICa. Top left: family of leak-subtracted ICa from a WT myocyte at 1.8 mM [Ca2+]o and 30°C (see methods). Bottom left: voltage dependence of activation (d∞) of ICa in WT (○, red line; n = 14), induced (◊, green line; n = 11), and noninduced (▵, blue line; n = 9) myocytes. Top right: family of leak-subtracted ICa from another WT myocyte at 1.8 mM [Ca2+]o and 30°C. Myocyte was held at −70 mV and switched to −40 mV immediately before prepulse (−30 to +20 mV, 10-mV increments, 300 ms), after which myocyte was returned to −40 mV for 3 ms before test pulse (−40 to 0 mV, 300 ms). Bottom right: voltage dependence of inactivation (f∞) of ICa in WT (○, red line; n = 8), induced (◊, green line; n = 6), and noninduced (▵, blue line; n = 6) myocytes.
Steady-state voltage dependence of ICa activation (d∞ = I/Imax) was also similar among the three groups of myocytes (Fig. 7). Vh was −20.2 ± 1.8 mV, −18.2 ± 2.1 mV, and −20.2 ± 2.0 mV in WT (n = 14), non-Ind (n = 9), and Ind (n = 11) myocytes, respectively. The slope factor k was 5.9 ± 1.8 mV, 7.2 ± 1.8 mV, and 7.0 ± 1.8 mV in WT, non-Ind, and Ind myocytes, respectively.
Effects of induced NCX1 transgene expression on SR Ca2+ content.
Altered twitch and [Ca2+]i transient amplitudes are likely related to changes in SR Ca2+ contents. Despite no significant differences in SERCA2, PLB and RyR2 expression (Fig. 2 and Table 2), and SR Ca2+ uptake activity (Table 4), SR Ca2+ content determined at 5.0 mM [Ca2+]o was significantly (P < 0.03) higher in Ind compared with non-Ind myocytes (Table 4). Given that ICa is not affected by induced NCX1 overexpression (Figs. 6 and 7), our findings suggest that NCX1 contributed directly to SR Ca2+ loading.
Effects of induced NCX1 transgene expression on action potential.
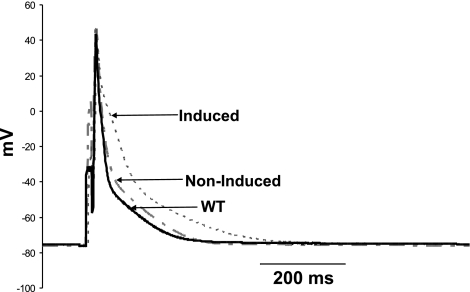
Increased NCX1 activity may alter action potential morphology (2, 31). We therefore measured action potential in WT, non-Ind, and Ind myocytes (Fig. 8). When compared with that of WT myocytes, resting Em was significantly (P < 0.01) higher in non-Ind but not in Ind myocytes (Table 5). Action potential amplitude was lower in WT compared with either non-Ind (P < 0.008) or Ind (P < 0.035) myocytes. The most dramatic finding is the prolongation of action potential duration (APD) at 50% (APD50) and 90% (APD90) repolarization (P < 0.005) in Ind myocytes compared with either WT or non-Ind myocytes.
Fig. 8.
Induced expression of Na+/Ca2+ exchanger transgene prolongs action potential duration. Action potentials were measured in WT (solid line), noninduced (dashed line), and induced (dotted line) myocytes at 30°C and 1.8 mM [Ca2+]o (see methods). Composite data are presented in Table 5.
Table 5.
Effects of induced NCX1 transgene expression on AP
| WT | Non-Ind | Induced | |
|---|---|---|---|
| Resting Em, mV | −69.3±1.3 (15) | −79.2±1.5† (14) | −72.5±1.9 (17) |
| AP amplitude, mV | 110.5±2.4 | 120.0±2.1† | 120.5±3.6 |
| APD50, ms | 4.53±0.76 | 3.29±0.22 | 8.85±0.78* |
| APD90, ms | 24.31±1.80 | 22.73±1.47 | 55.93±9.52* |
Values are means ± SE; numbers in parentheses are numbers of myocytes. Em, membrane potential; AP, action potential; APD50 and APD90, AP duration at 50% and 90% repolarization, respectively. Cells were paced at 1 Hz.
P < 0.01, WT vs. Non-Ind;
P < 0.003, Induced vs. WT or Non-Ind.
DISCUSSION
The first major finding is that we have engineered mice with inducible cardiac-specific expression of the rat cardiac NCX1. There are many similarities and some notable differences when comparing our current inducible mouse model with previously published mouse models that constitutively overexpress the canine NCX1 transgene (1, 27, 28, 36, 40). For comparison purposes, we would focus mainly on the heterozygous (Het) mice constitutively overexpressing NCX1 transgene (1, 28, 36, 40) since our inducible mouse model is also heterozygous for the NCX1 transgene. We would also discuss relevant features of the homozygous (Hom) mice constitutively overexpressing the canine NCX1 transgene (27, 28).
To achieve controlled expression of a transgene, investigators utilized a cardiac-specific promoter driving the expression of a tTA or of a reverse tTA in which four point mutations converted the system from a Tet-off (Dox is needed to keep transgene expression silent) to a Tet-on (transgene expression is active only in the presence of the drug) system (14). Unfortunately, early experience with these systems was not favorable because of heterogeneous expression throughout the ventricular and atrial cardiomyocyte populations, the potential for context-dependent expression because of the use of a virus-derived sequence, and leakiness or an inability to activate the gene in the presence or absence of Dox, respectively. The TREMHC vector we used allows robust expression when turned on in the absence of Dox and minimal leakage when turned off in the presence of Dox (29). Minimal leakage is supported by the observation of similar INaCa and NCX1 protein levels between WT and non-Ind myocytes, and inducible transgene expression is evidenced by increases in both INaCa amplitude and NCX1 protein levels in Ind myocytes compared with WT or non-Ind myocytes.
Similar to Het mice constitutively overexpressing NCX1 examined at 4 mo (28), mice with induced NCX1 overexpression (at 2 to 2.5 mo) had no differences in body weights, LV mass, heart rate, ejection fraction, fractional shortening, and baseline +dP/dt and −dP/dt compared with WT or non-Ind mice. The major difference between constitutive and induced NCX1 expression is that unlike HET mice constitutively overexpressing NCX1 that exhibited reduced contractility (+dP/dt) and relaxation (−dP/dt) in response to increasing doses of dobutamine compared with WT littermates (28), mice with induced NCX1 overexpression demonstrated similar inotropic and lusitropic responses to increasing doses of isoproterenol compared with WT or non-Ind controls. It is noteworthy that Hom mice constitutively overexpressing NCX1 developed cardiac hypertrophy, impaired LV fractional shortening, and markedly reduced contractility and relaxation in response to dobutamine compared with WT littermates (28).
Before comparing myocyte characteristics between Ind and Het mice, it is pertinent to point out that although myocytes were isolated from Ind mice at 8 to 10 wk of age, the ages of Het mice from which myocytes were derived were not specified (1, 36, 40). At the myocyte level, Na+-dependent Ca2+ uptake in sarcolemmal vesicles isolated from adult Het mice constitutively overexpressing NCX1 is 148% (1) or 2.34× (28) higher and INaCa (at +70 mV) is ∼80% larger (36) compared with WT controls. Our results in myocytes with induced NCX1 expression are comparable in that the NCX1 protein was 2.5-fold higher and INaCa was ∼40% larger compared with WT or non-Ind myocytes. In an attempt to account for the differences between NCX1 protein expression and activity increase in Ind myocytes, we measured expression of PLM, which is a known endogenous inhibitor of NCX1 (7). Neither the expression level nor fractional phosphorylation of PLM was altered by induced NCX1 overexpression. The slight variability in increases in NCX1 activity between Ind and Het myocytes is most likely due to different techniques to estimate NCX1 activity and the conditions employed in measuring INaCa. Similar to Het myocytes constitutively overexpressing NCX1 (28, 36), there were no alterations in the expression of SERCA2, PLB, calsequestrin, and Na+-K+-ATPase in myocytes with induced NCX1 overexpression. Similar to Ind myocytes overexpressing rat NCX1, Het myocytes constitutively overexpressing canine NCX1 also had no differences in ICa (1, 40) compared with WT myocytes.
In contrast with myocytes constitutively overexpressing NCX1 transgene in which absolute cell shortening magnitude (1.0 mM [Ca2+]o and 0.5 Hz) was reported to be similar to that observed in WT myocytes (36), we found altered contractility in Ind myocytes depending on the prevailing [Ca2+]o, which is known to be a relatively strong determinant of the exchanger equilibrium. At low [Ca2+]o (conditions that promote Ca2+ efflux), myocytes with induced NCX1 overexpression contracted less, whereas at high [Ca2+]o (conditions that promote Ca2+ influx), myocytes with induced NCX1 overexpression contracted more vigorously compared with their WT or non-Ind controls. This increased dynamic range in response to increased [Ca2+]o is characteristic of adult rat myocytes in which NCX1 was overexpressed by adenovirus-mediated gene transfer (45) and mouse myocytes in which NCX1 activity was increased by genetically eliminating PLM (37). The results from these three fundamentally different approaches [in vitro NCX1 gene transfer (45), induced NCX1 transgene expression in vivo (present study), and relief of NCX1 inhibition by PLM knockout (37)] support our conclusion that alterations in NCX1 expression and/or activity can significantly impact myocyte contractile behavior.
Changes in NCX1 activity in NCX1 overexpressed myocytes would be expected to alter Ca2+ fluxes during EC coupling. At intermediate [Ca2+]o (1 to 1.8 mM), systolic and diastolic [Ca2+]i, as well as [Ca2+]i transient amplitude in Het myocytes with either constitutive (1, 36, 40) or induced NCX1 overexpression, were not different than those measured in WT or non-Ind myocytes. When [Ca2+]o was increased to promote Ca2+ influx via NCX1, Ind myocytes exhibited higher [Ca2+]i transient amplitudes. Larger [Ca2+]i transient amplitudes in Ind myocytes studied under elevated [Ca2+]o conditions are most likely the result of increased SR Ca2+ contents due to enhanced NCX1 activity (35, 37, 45). Indeed, this is confirmed by increased SR Ca2+ contents in Ind myocytes compared with non-Ind myocytes. Conversely, when myocytes are studied under conditions that promote Ca2+ efflux (0.6 mM [Ca2+]o), increasing NCX1 expression (45) or enhancing NCX1 activity by genetically eliminating its endogenous inhibitor PLM (37, 42) results in lower [Ca2+]i transient and contraction amplitudes, as well as SR Ca2+ contents. It is the unique ability of NCX1 to drive Ca2+ in and pump Ca2+ out during an EC cycle, depending on the prevailing thermodynamic driving force, that explains the differential effects of low versus high [Ca2+]o on [Ca2+]i transient and contraction amplitudes and SR Ca2+ contents in myocytes in which either NCX1 expression or activity is altered.
In cardiac myocytes, NCX1 competes with SERCA2 for Ca2+ during decline of the [Ca2+]i transient. Therefore, theoretically, rate of [Ca2+]i transient decline would be expected to increase with NCX1 overexpression. In Het myocytes, Terracciano et al. (36) reported shorter t50 of [Ca2+]i transient decline, but this occurs even in the absence of Na+ and Ca2+, suggesting faster SR Ca2+ uptake. Yao et al. (40) reported faster time constant (τ) of [Ca2+]i transient decline in Het myocytes; the most prominent difference is in the terminal phase (t50–10%) of [Ca2+]i transient decline. In Ind myocytes, we did not detect differences in the early phase (t1/2) of [Ca2+]i transient decline compared with WT or non-Ind myocytes. The slight discrepancy in results may be due to differences in temperature (37° vs. 22° or 25°C) at which experiments were performed and the phase of [Ca2+]i transient decline (early vs. late) that was evaluated. It is also useful to recall that unlike rabbit myocytes in which NCX1 contributes some 28% of Ca2+ transport, the fraction of Ca2+ transported by NCX1 is only 7% in rodent myocytes (3). Indeed, NCX1 has been demonstrated to play only a minor role in relaxation in mouse myocytes compared with other species (36). Even in rabbit myocytes in which NCX1 plays a much bigger role in [Ca2+]i transient decline (3), Yao et al. (39) showed that abrupt inhibition of NCX1 does not affect the initial rate of decline of the [Ca2+]i transient. The weight of current evidence suggests that in rodent myocytes, the effects of NCX1 on [Ca2+]i transient decline are primarily manifest during the later rather than the early phase.
SR Ca2+ content is determined by Ca2+ influx via L-type Ca2+ channels and NCX1 operating in the reverse mode, SR Ca2+ uptake activity, SR Ca2+ release, SR volume, calsequestrin content, and competition between SR Ca2+ uptake and NCX1 operating in the forward mode. There were no differences in cell sizes (and presumably SR volume), ICa parameters, SERCA2, PLB, calsequestrin, and RyR2 protein levels, and SR Ca2+ uptake activity among WT, non-Ind, and Ind myocytes. Therefore, increased SR Ca2+ content in Ind myocytes cannot be ascribed to changes in SR transport proteins or SR Ca2+ uptake activity. Theoretically, prolonged APD in Ind myocytes may allow more Ca2+ to enter via L-type Ca2+ channels, thereby partly explaining the increased SR Ca2+ content. We think that this explanation is less likely. First, increased Ca2+ flux through ICa due to prolonged APD cannot account for the decreased SR Ca2+ content at 0.6 mM [Ca2+]o in myocytes in which NCX1 is either overexpressed (45) or its activity increased (37). Second, APD prolongation is most dramatic at the tail-end of the action potential (APD90), at which Em (−40 mV) ICa would be minimally activated. Finally, even with prolongation of early APD (APD50), Ca2+-induced inactivation of ICa would be expected to limit the amount of Ca2+ that can enter. Therefore, the most consistent and unifying hypothesis for altered SR Ca2+ contents in NCX1 overexpressed myocytes is due to enhanced reverse or forward NCX1 activity, depending on prevailing [Ca2+]o and hence the thermodynamic driving force of the exchanger.
The thermodynamic driving force for NCX1 is given by Em − ENaCa; the latter is determined by [Na+]i, [Ca2+]i, [Ca2+]o, and [Na+]o. In our experiments with intact myocytes, [Ca2+]o and [Na+]o were controlled while [Ca2+]i and action potential were measured. Theoretically, changes in [Na+]i brought on by NCX1 overexpression in Ind myocytes can alter NCX1 activity. Although we did not measure [Na+]i, some insights can be gleaned from [Na+]i measurements in resting and field-stimulated PLM knockout myocytes. In PLM knockout myocytes in which INaCa is ∼22% higher than WT myocytes (42), resting [Na+]i (12.0 ± 1.5 mM) is not different than that measured in WT myocytes (12.5 ± 1.8 mM)(9). When field-stimulated to contract at 2 Hz, steady-state [Na+]i in PLM knockout myocytes is 17.0 ± 1.5 mM and not significantly different than that measured in WT myocytes (15.2 ± 1.5 mM)(11). Since the increase in INaCa is ∼40% in Ind myocytes, comparable with the ∼22% increase in PLM knockout myocytes (as compared with their respective controls), changes in [Na+]i due to moderately increased NCX1 activity, if any, would be small.
Another major finding is that when compared with non-Ind myocytes, both APD50 and APD90 were dramatically prolonged in Ind myocytes, despite no changes in resting Em and action potential amplitude. Prolongation of action potential was not due to changes in ICa since neither the current density nor the inactivation time constants were affected by induced NCX1 transgene expression. Altered NCX1 activity may theoretically affect APD (2). In rat and mouse cardiac myocytes with short APD, there is a net Ca2+ efflux via NCX1 at the shoulder of the action potential. This is because the action potential is already repolarizing before the [Ca2+]i transient reaches its peak, and INaCa is predominantly inward during this phase of the action potential (31). Increase in NCX1 activity in Ind myocytes would therefore be expected to prolong APD, which is what we observed. In Het myocytes constitutively overexpressing NCX1, Yao et al. (40) reported decreased action potential amplitude and abbreviated APD50 but prolonged APD90 compared with WT myocytes. These authors hypothesized that decreased action potential amplitude and shortening of APD50 were due to increased outward current secondary to enhanced reverse NCX1 (3 Na+ out and 1 Ca2+ in) activity during early depolarization, whereas prolonged APD90 was due to more marked forward NCX1 (3 Na+ in and 1 Ca2+ out) activity during the [Ca2+]i transient. The differences between our action potential findings in Ind myocytes and Het myocytes constitutively overexpressing NCX1 are not clear but may be due to different genetic backgrounds (FVB vs. C57Bl/6xC3H1), animal models (induced vs. constitutive), pipette solutions (Ca2+ buffered vs. not buffered), temperature (30° vs. 25°C), and potential differences in other repolarizing currents such as transient outward current or other K+ currents. We do not know the reason for the observed differences in resting Em and action potential amplitude between WT and non-Ind myocytes. It is not due to Dox since it was administered to both WT and non-Ind mice from gestation up to the day of experiment.
In some, but not all, models of heart failure and hypertrophy, NCX1 expression has been reported to be increased (32) and hypothesized to be a major contributor to contractile dysfunction (19, 21). In heart failure, expression of many proteins involved in cardiac Ca2+ homeostasis such as SERCA2, PLB, RyR2, NCX1, etc., is known to be altered (8, 16). Our current observations clearly indicated that induction of NCX1 overexpression in adult mice (to simulate switching on NCX1 expression with onset of disease state), without concomitant changes in other proteins involved in Ca2+ transport, did not result in contractile dysfunction in vivo. In this light, it is interesting to note that NCX1 has been shown to significantly contribute to the maintenance of contractile function and [Ca2+]i transients in failing human ventricular myocytes (12, 15). Our novel model of inducible NCX1 transgenic expression is eminently suited for studies to dissect whether overexpression of NCX1 found in heart failure models is beneficial or harmful to the animal.
In summary, we have generated a novel mouse model in which cardiac-specific expression of rat NCX1 can be controlled. Despite no detectable differences in cardiac performance in vivo, LV myocytes with induced expression of NCX1 transgene exhibited prolonged APD, enhanced contractility, [Ca2+]i transient amplitude, and SR Ca2+ content at high [Ca2+]o, but no changes in ICa, SERCA2, RyR2, PLB, Na+-K+-ATPase, phospholemman, calsequestrin, and SR Ca2+ uptake. We conclude that NCX1 operates in both forward and reverse modes during an action potential, directly contributes to SR Ca2+ filling, and regulates myocyte contractility. In addition, induced NCX1 transgene expression at levels found in diseased hearts, by itself, was not associated with cardiac dysfunction in young adult mice.
GRANTS
This work was supported in part by National Heart, Lung, and Blood Institute Grants RO1-HL-58672 and RO1-HL-74854 (to J. Y. Cheung); RO1-HL-56205, RO1-HL-61690, RO1-HL85503, PO1-HL-75443, and PO1-HL-91799 (to W. J. Koch); and PO1-HL-91799 (Project 2; to A. M. Feldman); by the Pennsylvania Research Formulary Fund (to A. M. Feldman); and by American Heart Association Scientist Development Grant F64702 (to T. O. Chan).
REFERENCES
- 1.Adachi-Akahane S, Lu L, Li Z, Frank JS, Philipson KD, Morad M. Calcium signaling in transgenic mice overexpressing cardiac Na+-Ca2+ exchanger. J Gen Physiol 109: 717–729, 1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Armoundas AA, Hobai IA, Tomaselli GF, Winslow RL, O′Rourke B. Role of sodium-calcium exchanger in modulating the action potential of ventricular myocytes from normal and failing hearts. Circ Res 93: 46–53, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bassani JW, Bassani RA, Bers DM. Relaxation in rabbit and rat cardiac cells: species-dependent differences in cellular mechanisms. J Physiol 476: 279–293, 1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bers DM Cardiac excitation-contraction coupling. Nature 415: 198–205, 2002. [DOI] [PubMed] [Google Scholar]
- 5.Bers DM, Berlin JR. Kinetics of [Ca]i decline in cardiac myocytes depend on peak [Ca]i. Am J Physiol Cell Physiol 268: C271–C277, 1995. [DOI] [PubMed] [Google Scholar]
- 6.Buerger A, Rozhitskaya O, Sherwood MC, Dorfman AL, Bisping E, Abel ED, Pu WT, Izumo S, Jay PY. Dilated cardiomyopathy resulting from high-level myocardial expression of Cre-recombinase. J Card Fail 12: 392–398, 2006. [DOI] [PubMed] [Google Scholar]
- 7.Cheung JY, Rothblum LI, Moorman JR, Tucker AL, Song J, Ahlers BA, Carl LL, Wang J, Zhang XQ. Regulation of cardiac Na+/Ca2+ exchanger by phospholemman. Ann NY Acad Sci 1099: 119–134, 2007. [DOI] [PubMed] [Google Scholar]
- 8.de Tombe PP Altered contractile function in heart failure. Cardiovasc Res 37: 367–380, 1998. [DOI] [PubMed] [Google Scholar]
- 9.Despa S, Bossuyt J, Han F, Ginsburg KS, Jia LG, Kutchai H, Tucker AL, Bers DM. Phospholemman-phosphorylation mediates the beta-adrenergic effects on Na/K pump function in cardiac myocytes. Circ Res 97: 252–259, 2005. [DOI] [PubMed] [Google Scholar]
- 10.Despa S, Islam MA, Pogwizd SM, Bers DM. Intracellular [Na+] and Na+ pump rate in rat and rabbit ventricular myocytes. J Physiol 539: 133–143, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Despa S, Tucker AL, Bers DM. PLM-mediated activation of Na/K-ATPase limits [Na]i and inotropic state during B-adrenergic stimulation in mouse ventricular myocytes. Circulation 117: 1849–1855, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dipla K, Mattiello J, Margulies K, Jeevanandam V, Houser S. Sarcoplasmic reticulum and the Na+/Ca2+ exchanger both contribute to the Ca2+ transient of failing human ventricular myocytes. Circ Res 84: 435–444, 1999. [DOI] [PubMed] [Google Scholar]
- 13.Egan TM, Noble D, Noble SJ, Powell T, Spindler AJ, Twist VW. Sodium-calcium exchange during the action potential in guinea-pig ventricular cells. J Physiol 411: 639–661, 1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Freundlieb S, Schirra-Muller C, Bujard H. A tetracycline controlled activation/repression system with increased potential for gene transfer into mammalian cells. J Gene Med 1: 4–12, 1999. [DOI] [PubMed] [Google Scholar]
- 15.Gaughan J, Furukawa S, Jeevanadam V, Hefner C, Kubo H, Margulies K, McGowan B, Mattiello J, Dipla K, Piacentino III V, Li S, Houser S. Sodium/calcium exchange contributes to contraction and relaxation in failed human ventricular myocytes. Am J Physiol Heart Circ Physiol 277: H714–H724, 1999. [DOI] [PubMed] [Google Scholar]
- 16.Hasenfuss G Alteration of calcium-regulatory proteins in heart failure. Cardiovasc Res 37: 279–289, 1998. [DOI] [PubMed] [Google Scholar]
- 17.Henderson SA, Goldhaber JI, So JM, Han T, Motter C, Ngo A, Chantawansri C, Ritter MR, Friedlander M, Nicoll DA, Frank JS, Jordan MC, Roos KP, Ross RS, Philipson KD. Functional adult myocardium in the absence of Na+-Ca2+ exchange: cardiac-specific knockout of NCX1. Circ Res 95: 604–611, 2004. [DOI] [PubMed] [Google Scholar]
- 18.Huang WY, Aramburu J, Douglas PS, Izumo S. Transgenic expression of green fluorescence protein can cause dilated cardiomyopathy. Nat Med 6: 482–483, 2000. [DOI] [PubMed] [Google Scholar]
- 19.Litwin S, Bridge JH. Enhanced Na+-Ca2+ exchange in the infarcted heart. Implications for excitation-contraction coupling. Circ Res 81: 1083–1093, 1997. [DOI] [PubMed] [Google Scholar]
- 20.Most P, Seifert H, Gao E, Funakoshi H, Volkers M, Heierhorst J, Remppis A, Pleger ST, DeGeorge BR Jr, Eckhart AD, Feldman AM, Koch WJ. Cardiac S100A1 protein levels determine contractile performance and propensity toward heart failure after myocardial infarction. Circulation 114: 1258–1268, 2006. [DOI] [PubMed] [Google Scholar]
- 21.Pogwizd SM, Qi M, Yuan W, Samarel AM, Bers DM. Upregulation of Na+/Ca2+ exchanger expression and function in an arrhythmogenic rabbit model of heart failure. Circ Res 85: 1009–1019, 1999. [DOI] [PubMed] [Google Scholar]
- 22.Pott C, Philipson KD, Goldhaber JI. Excitation-contraction coupling in Na+-Ca2+ exchanger knockout mice: reduced transsarcolemmal Ca2+ flux. Circ Res 97: 1288–1295, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pott C, Ren X, Tran DX, Yang MJ, Henderson S, Jordan MC, Roos KP, Garfinkel A, Philipson KD, Goldhaber JI. Mechanism of shortened action potential duration in Na+-Ca2+ exchanger knockout mice. Am J Physiol Cell Physiol 292: C968–C973, 2007. [DOI] [PubMed] [Google Scholar]
- 24.Pott C, Yip M, Goldhaber JI, Philipson KD. Regulation of cardiac L-type Ca2+ current in Na+-Ca2+ exchanger knockout mice: functional coupling of the Ca2+ channel and the Na+-Ca2+ exchanger. Biophys J 92: 1431–1437, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ranu HK, Terracciano CM, Davia K, Bernobich E, Chaudhri B, Robinson SE, Bin Kang Z, Hajjar RJ, MacLeod KT, Harding SE. Effects of Na+/Ca2+-exchanger overexpression on excitation-contraction coupling in adult rabbit ventricular myocytes. J Mol Cell Cardiol 34: 389–400, 2002. [DOI] [PubMed] [Google Scholar]
- 26.Rembold CM, Ripley ML, Meeks MK, Geddis LM, Kutchai HC, Marassi FM, Cheung JY, Moorman JR. Serine 68 phospholemman phosphorylation during forskolin-induced swine carotid artery relaxation. J Vasc Res 42: 483–491, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Reuter H, Han T, Motter C, Philipson KD, Goldhaber JI. Mice overexpressing the cardiac sodium-calcium exchanger: defects in excitation-contraction coupling. J Physiol 554: 779–789, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Roos KP, Jordan MC, Fishbein MC, Ritter MR, Friedlander M, Chang HC, Rahgozar P, Han T, Garcia AJ, Maclellan WR, Ross RS, Philipson KD. Hypertrophy and heart failure in mice overexpressing the cardiac sodium-calcium exchanger. J Card Fail 13: 318–329, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sanbe A, Gulick J, Hanks MC, Liang Q, Osinska H, Robbins J. Reengineering inducible cardiac-specific transgenesis with an attenuated myosin heavy chain promoter. Circ Res 92: 609–616, 2003. [DOI] [PubMed] [Google Scholar]
- 30.Schillinger W, Janssen PM, Emami S, Henderson SA, Ross RS, Teucher N, Zeitz O, Philipson KD, Prestle J, Hasenfuss G. Impaired contractile performance of cultured rabbit ventricular myocytes after adenoviral gene transfer of Na+-Ca2+ exchanger. Circ Res 87: 581–587, 2000. [DOI] [PubMed] [Google Scholar]
- 31.Shattock M, Bers DM. Rat vs. rabbit ventricle: Ca flux and intracellular Na assessed by ion-selective microelectrodes. Am J Physiol Cell Physiol 256: C813–C822, 1989. [DOI] [PubMed] [Google Scholar]
- 32.Sipido K, Volders P, Vos M, Verdonck F. Altered Na/Ca exchange activity in cardiac hypertrophy and heart failure: a new target for therapy? Cardiovasc Res 53: 782–805, 2002. [DOI] [PubMed] [Google Scholar]
- 33.Song J, Zhang XQ, Carl LL, Qureshi A, Rothblum LI, Cheung JY. Overexpression of phospholemman alter contractility and [Ca2+]i transients in adult rat myocytes. Am J Physiol Heart Circ Physiol 283: H576–H583, 2002. [DOI] [PubMed] [Google Scholar]
- 34.Song J, Zhang XQ, Ahlers BA, Carl LL, Wang J, Rothblum LI, Stahl RC, Mounsey JP, Tucker AL, Moorman JR, Cheung JY. Serine 68 of phospholemman is critical in modulation of contractility, [Ca2+]i transients, and Na+/Ca2+ exchange in adult rat cardiac myocytes. Am J Physiol Heart Circ Physiol 288: H2342–H2354, 2005. [DOI] [PubMed] [Google Scholar]
- 35.Song J, Zhang XQ, Wang J, Cheskis E, Chan TO, Feldman AM, Tucker AL, Cheung JY. Regulation of cardiac myocyte contractility by phospholemman:Na+/Ca2+ exchange vs. Na+-K+-ATPase. Am J Physiol Heart Circ Physiol 295: H1615–H1625, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Terracciano C, DeSouza A, Philipson KD, MacLeod K. Na+-Ca2+ exchange and sarcoplasmic reticular Ca2+ regulation in ventricular myocytes from transgenic mice overexpression the Na+-Ca2+ exchanger. J Physiol (Lond) 512: 651–667, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tucker AL, Song J, Zhang XQ, Wang J, Ahlers BA, Carl LL, Mounsey JP, Moorman JR, Rothblum LI, Cheung JY. Altered contractility and [Ca2+]i homeostasis in phospholemman-deficient murine myocytes: role of Na+/Ca2+ exchange. Am J Physiol Heart Circ Physiol 291: H2199–H2209, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Weber C, Piacentino III V, Ginsburg K, Houser S, Bers DM. Na+-Ca2+ exchange current and submembrane [Ca2+] during the cardiac action potential. Circ Res 90: 182–189, 2002. [DOI] [PubMed] [Google Scholar]
- 39.Yao A, Matsui H, Spitzer K, Bridge J, Barry W. Sarcoplsmic reticulum and Na+/Ca2+ exchanger function in early and late relaxation in ventricular myocytes. Am J Physiol Heart Circ Physiol 273: H2765–H2773, 1997. [DOI] [PubMed] [Google Scholar]
- 40.Yao A, Su Z, Nonaka A, Zubair I, Lu L, Philipson KD, Bridge JH, Barry WH. Effects of overexpression of the Na+-Ca2+ exchanger on [Ca2+]i transients in murine ventricular myocytes. Circ Res 82: 657–665, 1998. [DOI] [PubMed] [Google Scholar]
- 41.Zhang XQ, Tillotson DL, Moore RL, Zelis R, Cheung JY. Na+/Ca2+ exchange currents and SR Ca2+ contents in postinfarction myocytes. Am J Physiol Cell Physiol 271: C1800–C1807, 1996. [DOI] [PubMed] [Google Scholar]
- 42.Zhang XQ, Ahlers BA, Tucker AL, Song J, Wang J, Moorman JR, Mounsey JP, Carl LL, Rothblum LI, Cheung JY. Phospholemman inhibition of the cardiac Na+/Ca2+ exchanger. Role of phosphorylation. J Biol Chem 281: 7784–7792, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zhang XQ, Moore RL, Tillotson DL, Cheung JY. Calcium currents in postinfarction rat cardiac myocytes. Am J Physiol Cell Physiol 269: C1464–C1473, 1995. [DOI] [PubMed] [Google Scholar]
- 44.Zhang XQ, Ng YC, Moore RL, Musch TI, Cheung JY. In situ SR function in postinfarction myocytes. J Appl Physiol 87: 2143–2150, 1999. [DOI] [PubMed] [Google Scholar]
- 45.Zhang XQ, Song J, Rothblum LI, Lun M, Wang X, Ding F, Dunn J, Lytton J, McDermott PJ, Cheung JY. Overexpression of Na+/Ca2+ exchanger alters contractility and SR Ca2+ content in adult rat myocytes. Am J Physiol Heart Circ Physiol 281: H2079–H2088, 2001. [DOI] [PubMed] [Google Scholar]
- 46.Zhang XQ, Zhang LQ, Palmer BM, Ng YC, Musch TI, Moore RL, Cheung JY. Sprint training shortens prolonged action potential duration in postinfarction rat myocyte: mechanisms. J Appl Physiol 90: 1720–1728, 2001. [DOI] [PubMed] [Google Scholar]
- 47.Zhou YY, Wang SQ, Zhu WZ, Chruscinski A, Kobilka BK, Ziman B, Wang S, Lakatta EG, Cheng H, Xiao RP. Culture and adenoviral infection of adult mouse cardiac myocytes: methods for cellular genetic physiology. Am J Physiol Heart Circ Physiol 279: H429–H436, 2000. [DOI] [PubMed] [Google Scholar]