Abstract
Actin polymerization has recently emerged as an important cellular process that regulates smooth muscle contraction. Abelson tyrosine kinase (Abl) has been implicated in the regulation of actin dynamics and force development in vascular smooth muscle. In the present study, the systolic blood pressure was lower in Abl−/− knockout mice compared with wild-type mice. The knockout of Abl diminished the tyrosine phosphorylation of p130 Crk-associated substrate (CAS, an adapter protein associated with smooth muscle contraction) in resistance arteries upon stimulation with phenylephrine or angiotensin II. The agonist-elicited enhancement of F-actin-to-G-actin ratios in arteries assessed by fluorescent microscopy was also reduced in Abl−/− mice. It has been known that vinculin is a structural protein that links actin filaments to extracellular matrix via transmembrane integrins, whereas paxillin is a signaling protein associated with focal contacts mediating actin cytoskeleton remodeling. The expression of vinculin and paxillin at protein and messenger levels was lower in arterial vessels from Abl knockout mice. However, the agonist-induced increase in myosin phosphorylation was not attenuated in arteries from Abl knockout mice. These results indicate that Abl differentially regulates Crk-associated substrate, vinculin, and paxillin in arterial vessels. The Abl-regulated cellular process and blood pressure are independent of myosin activation in vascular smooth muscle.
Keywords: Abelson tyrosine kinase, adapter protein, cytoskeletal proteins, actin cytoskeleton, contraction, vascular smooth muscle
vascular smooth muscle plays a key role in the regulation of blood pressure. Cross-bridge cycling regulated by regulatory myosin light chain (rMLC) phosphorylation has been recognized as a fundamental paradigm for the regulation of vascular smooth muscle contraction (14, 26, 32). Recent studies suggest that actin polymerization transpires in smooth muscle in response to contractile activation. The inhibition of actin polymerization by such inhibitors as cytochalasin D or latrunculin A attenuates force development during the activation with contractile stimuli without affecting rMLC phosphorylation (3, 20, 40, 41). These studies suggest that actin polymerization and myosin phosphorylation are independently regulated and that both the dynamic change in the actin cytoskeleton and myosin activation are required for force development during the contractile stimulation of smooth muscle (2, 20, 40–42).
The nonreceptor tyrosine kinase Abelson tyrosine kinase (Abl, c-Abl) has recently been implicated in the regulation of the actin cytoskeleton and force development in vascular smooth muscle (1). The activation with phenylephrine (PE) or angiotensin II (ANG II) induces Abl tyrosine phosphorylation (an indication of Abl kinase activity) in vascular smooth muscle tissues and cells (1, 44). The mechanisms by which Abl regulates the dynamics of the actin cytoskeleton in smooth muscle are not well understood.
The adapter protein p130 Crk-associated substrate (CAS) has been shown to modulate actin polymerization in smooth muscle cells, as well as in nonmuscle cells, including COS-7 cells and NIH3T3 cells (22, 31, 40). The depletion of CAS by antisense attenuates the constriction and actin polymerization in carotid arteries in response to contractile stimulation (40). Stimulation with ANG II, serotonin, or PE induces the tyrosine phosphorylation of CAS in vascular smooth muscle cells and tissues (1, 22, 33), as well as in nonmuscle cells in response to the activation with growth factors and cell adhesion (23, 31). It has been proposed that the phosphorylation of CAS is a key event regulating the actin cytoskeleton; phosphorylated CAS may enhance its binding to the adapter protein CrkII, which may facilitate the formation of multiprotein complexes including CrkII, neuronal Wiskott-Aldrich syndrome protein (N-WASP), and the actin-related protein-2/3 (Arp2/3) complex and initiating actin polymerization and branching mediated by the Arp2/3 complex. (1, 30, 38, 43, 45).
Vinculin and paxillin are the key components of focal adhesion sites of cultured smooth muscle cells, which are structurally analogous to dense plagues of smooth muscle tissues. At these sites, the structural protein vinculin primarily connects actin filaments to cytoplasmic domains of transmembrane β-integrins that physically bind matrix proteins with their extracellular motifs. The linkage of actin filaments to extracellular matrix at dense plaques is believed to be important for the transmission of mechanical force between the inside and outside of the smooth muscle. In contrast, paxillin at dense plaques of smooth muscle tissues mainly serves as a signaling protein regulating actin cytoskeleton remodeling (6, 36, 42).
The objective of the present study was to determine the functional role of Abl in regulating blood pressure, CAS, vinculin, and paxillin using an Abl knockout mouse model. Our results demonstrate that systolic blood pressure is lower in Abl knockout mice. Moreover, the contractile agonist-induced enhancement of CAS phosphorylation and actin polymerization, but not rMLC phosphorylation, is attenuated in blood vessels from Abl knockout mice. Abl knockout also inhibits the expression of vinculin and paxillin in the vasculature of mice.
MATERIALS AND METHODS
Measurement of blood pressure.
Systolic blood pressure was measured using the XBP1000 Noninvasive Tail Blood Pressure Measurement System (a computer automated system, Kent Scientific, Torrington, CT). Before measurement, the mice were trained in a container for at least 15 min at 37°C to obtain reliable parameters.
Tissue preparation.
All animal protocols were approved by the Institutional Animal Care and Use Committee. Abl knockout mice and wild-type mice were kindly provided by Stephen P. Goff (University of Columbia) (16). Male mice (30 ± 5 g) were euthanized in a CO2 chamber, and the resistance arteries (mesenteric artery, femoral artery, and carotid artery) and/or aorta were removed and placed at room temperature in physiological saline solution containing (in mM) 110 NaCl, 3.4 KCl, 2.4 CaCl2, 0.8 MgSO4, 25.8 NaHCO3, 1.2 KH2PO4, and 5.6 glucose. The solution was aerated with 95% O2-5% CO2 to maintain a pH of 7.4. After the removal of the endothelium and the connective tissue layer, segments of arteries were placed in test tubes containing physiological saline solution at 37°C. They were stimulated with contractile agonists before biochemical and microscopic analyses.
Immunoblot analysis.
Pulverized tissues were mixed with 40 μl of SDS sample buffer for 5 min and separated by SDS-PAGE. The proteins were transferred to a nitrocellulose membrane, after which the membrane was blocked with 2% bovine serum albumin for 1 h and probed with site-specific, state-dependent antibody for phospho-CAS (Tyr410, Cell Signaling), followed by horseradish peroxidase (HRP)-conjugated anti-rabbit Ig (ICN Biomedicals, Irvine, CA). The proteins were visualized by enhanced chemiluminescence (SuperSignal, Pierce) using Fuji Image System LAS3000. The membranes were stripped and reprobed with monoclonal antibodies against total CAS (clone 24, BD Biosciences), followed by the incubation with HRP-conjugated anti-mouse Ig (Amersham Life Sciences). The levels of phosphoprotein and total protein were quantified by scanning densitometry of immunoblots (Fuji Multigauge software). The changes in protein phosphorylation were expressed as a magnitude increase over the levels of phosphorylation in unstimulated arteries. Because a relatively large amount of proteins is needed for an assessment of CAS phosphorylation, the pooled tissues from the mesenteric artery, femoral artery, and carotid artery were used. Agonist-induced CAS phosphorylation was not different among the mesenteric artery, femoral artery, and carotid artery.
For protein expression analysis, the proteins from the mesenteric artery or aortae were separated by SDS-PAGE followed by a membrane transfer. The membranes were probed with vinculin antibody (Santa Cruz Biotechnology), paxillin antibody (clone 349, BD Biosciences), and smooth muscle α-actin antibody (clone 1A4, Sigma). The densitometric values of proteins were determined, and the ratios of these proteins were calculated. The luminescent signals from all immunoblots were within the linear range.
Determination of F-actin-to-G-actin ratios by fluorescence microscopy.
F-actin-to-G-actin (F/G-actin) ratios from the carotid artery of mice were evaluated using the method previously described with a minor modification (12). Carotid arteries from mice were placed in frozen tissue-embedding medium (Neg 52, Richard-Allen Scientific) and cryosectioned using Cryostats (Richard-Allen Scientific). Histological sections on glass slides were fixed for 15 min in 4% paraformaldehyde and were then washed three times in Tris-buffered saline (TBS) containing 50 mM Tris, 150 mM NaCl, and 0.1% NaN3, followed by the permeabilization with 0.2% Triton X-100 dissolved in TBS for 5 min. The samples were fluorescently stained for F-actin using rhodamine-labeled phalloidin (Invitrogen-Molecular Probes), followed by the staining with DNase I conjugated with Alexa 488 for G-actin (Invitrogen-Molecular Probes). Images of labeled F-actin and G-actin were viewed under a high-resolution fluorescent microscope (Leica). The time of image capturing, intensity gaining, and image black levels in both channels were optimally adjusted and kept constant for all experiments to standardize the fluorescence intensity measurements among the experiments. For each independent experiment, five regions were randomly selected for observation. Fluorescence intensities of rhodamine-phalloidin and Alexa 488-DNase I were simultaneously calculated using Multigauge software. Measurements from five areas were averaged for a single data point.
Analysis of real-time reverse transcription quantitative polymerase chain reaction.
Total RNA from mesenteric artery was extracted using TRIzol reagent (Invitrogen). The primers were designed as follows: paxillin 5′ primer, 5′-ACTACTGCAACGGACCCATC-3′; paxillin 3′ primer, 5′-TAGTGGACCTCACAGTACGG-3′; vinculin 5′ primer, 5′-GCCAAGCAGTGCACAGATAA-3′; vinculin 3′ primer, 5′-TTCCTTTCTGGTGTGTGAAGC-3′; GAPDH 5′ primer, 5′-ACTCCACTCACGGCAAATTC-3′; and GAPDH 3′ primer, 5′-ACTGTGGTCATGAGCCCTTC-3′. The reaction was performed using the iScript one-step RT-PCR kit with SYBR Green (Bio-Rad) on a MyiQ single color real-time PCR detection system (Bio-Rad). The quantitative RT-PCR condition was set as 10 min at 50°C for cDNA synthesis and 5 min at 95°C for RT inactivation, followed by 45 cycles of 10 s at 95°C and 30 s at 55°C for real-time PCR cycling and detection. A melting curve analysis was done by 1 min at 95°C, 1 min at 55°C, and then 10 s at 55°C, increasing each by 0.5°C at each cycle until the temperature reached 95°C. The levels of vinculin and paxillin mRNA were normalized to the level of the reference gene GAPDH.
Assessment of rMLC phosphorylation.
The mesenteric arteries were rapidly frozen in liquid nitrogen and immersed in precooled acetone containing 10% (wt/vol) trichloroacetic acid (TCA) and 10 mM dithiothreitol (DTT). Tissues were thawed in acetone-TCA-DTT at room temperature and then washed four times with acetone-DTT. The proteins were extracted for 4 h in 8 mM urea, 20 mM Tris base, 22 mM glycine, and 10 mM DTT. Myosin light chain was separated by glycerol-urea PAGE and transferred to nitrocellulose membranes. The membranes were blocked with 5% milk and incubated with myosin light chain 20 antibody. The primary antibody was reacted with HRP-conjugated anti-rabbit IgG (ICN Biomedicals). Unphosphorylated and phosphorylated bands of rMLC were visualized by enhanced chemiluminescence and quantified by scanning densitometry. The phosphorylation of rMLC was calculated as the ratio of phosphorylated rMLC to total rMLC.
Statistical analysis.
All statistical analysis was performed using Prism 4 software (GraphPad, San Diego, CA). Comparisons among multiple groups were performed by one-way analysis of variance followed by Tukey's multiple comparison test. Differences between pairs of groups were analyzed by Student-Newman-Keuls test or Dunn's method. Values of n refer to the number of experiments used to obtain each value. P < 0.05 was considered to be significant.
RESULTS
Systolic blood pressure is lower in Abl knockout mice.
Abl knockout mice and wild-type mice were provided by Dr. Goff of Columbia University (16). Most of the Abl−/− pups (94.7%) survived after birth and attained adulthood, which was similar to wild-type pups. A previous report showed that Abl−/− mice were osteoporotic and had defects in osteoblast maturation (16). These knockout mice did not display markedly abnormal behaviors compared with the wild-type mice.
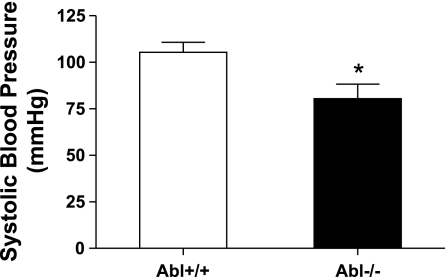
Our previous studies have shown that Abl silencing inhibits force development in isolated mesenteric artery during stimulation with PE or KCl (1). To evaluate the role of Abl in regulating blood pressure (which is largely regulated by vascular smooth muscle tone) in whole animals, we compared the blood pressures in Abl knockout mice and wild-type mice. Systolic blood pressure was significantly lower in Abl knockout mice than in wild-type mice (Fig. 1, n = 10, P < 0.05).
Fig. 1.
Systolic blood pressure in Abelson tyrosine kinase (Abl) knockout (KO) mice is lower than in wild-type (WT) mice. Systolic blood pressure was compared for Abl−/− mice and Abl+/+ mice using a computer-automated noninvasive tail blood pressure measurement system. Abl knockdown decreases systolic blood pressure in mice (*P < 0.05, n = 10). Values represent means ± SE; n, number of experiments used to obtain each value.
Increases in CAS phosphorylation upon contractile stimulation are diminished in Abl knockout mice.
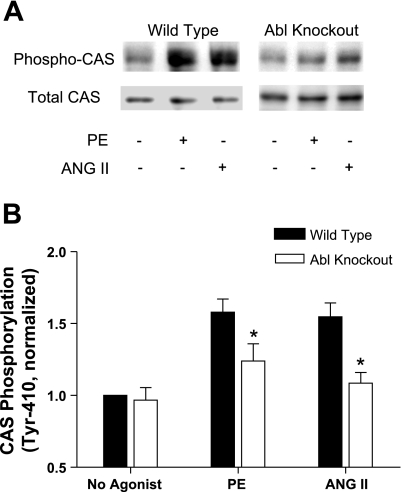
Because CAS has been implicated in the modulation of vascular smooth muscle (22, 40), we determined whether Abl knockout affects CAS phosphorylation in vascular smooth muscle. Pooled tissues from mesenteric artery, femoral artery, and carotid artery of Abl knockout mice or wild-type mice were treated with 10 μM PE for 5 min. PE is an agonist of α-receptor, which plays an important role in regulating vascular smooth muscle contractility and blood pressure. In addition, our previous studies showed that the contractile force reaches to a maximal level in resistance arteries in response to the stimulation with PE for 5 min (1). CAS phosphorylation was assessed by immunoblot analysis using phospho-CAS (Tyr410) antibody. The stimulation with PE resulted in an increase in CAS phosphorylation in arteries from wild-type mice. In contrast, CAS phosphorylation upon PE stimulation was reduced in the vascular tissues from Abl knockout mice (Fig. 2). The results demonstrate that Abl knockout attenuates the increase in PE-induced CAS phosphorylation in arterial tissues.
Fig. 2.
Increases in Crk-associated substrate (CAS) phosphorylation upon activation with phenylephrine (PE) or angiotensin II (ANG II) are attenuated in arteries from Abl KO mice. Unstimulated or stimulated resistance arteries from Abl−/− or Abl+/+ mice were frozen for biochemical analysis. A: representative immunoblots illustrating CAS phosphorylation (Tyr410) upon PE stimulation (10 μM, 5 min) or ANG II (1 μM, 2.5 min) in tissues from Abl KO and WT mice. B: the level of CAS phosphorylation elicited by PE or ANG II and the level of unstimulated CAS phosphorylation in tissues from KO mice are normalized to the value of unstimulated arteries of WT mice. *P < 0.05, significantly lower PE or ANG II induced CAS phosphorylation in arteries of Abl−/− mice compared with the level of corresponding WT mice. Values represent means ± SE; n = 5 to 6.
To determine whether the effects of Abl knockout on PE-induced CAS phosphorylation transpire at the receptor level, we examined CAS phosphorylation upon another vasoconstrictor ANG II in Abl−/− mice. ANG II is a major component of the renin-angiotensin system, which is an important hormone pathway to regulate blood pressure. Renin secreted from kidneys catalyses angiotensinogen to form angiotensin I (ANG I) in blood. ANG I is then converted to ANG II by angiotensin-converting enzyme. ANG II directly affects the vascular smooth muscle functions, such as stimulating vascular smooth muscle contraction (5, 28). Previous investigations show that a stimulation with 1 μM ANG II for 2.5 min induces maximal contractile responses (1, 5). CAS phosphorylation in response to a stimulation with 1 μM ANG II for 2.5 min was also reduced in vascular tissues of Abl knockout mice compared with wild-type mice (Fig. 2), suggesting that the effects of the Abl knockout on agonist-induced CAS phosphorylation is less likely attributed to changes in biological properties of α-receptor and ANG II receptor in Abl−/− mice.
Enhancement of F/G-actin ratios upon contractile activation is inhibited in Abl knockout mice.
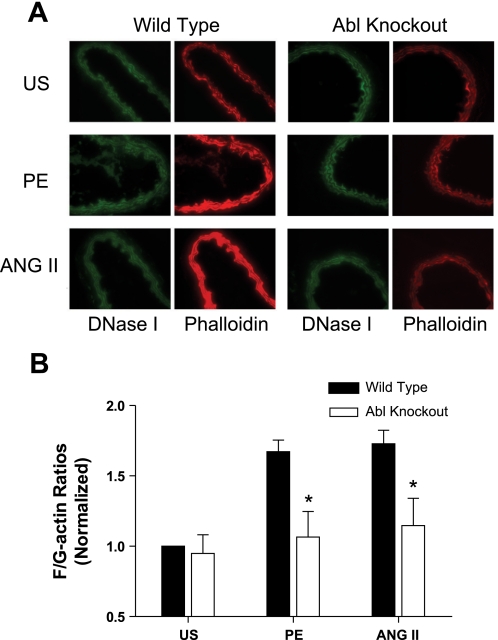
The adapter protein CAS has been shown to participate in the regulation of actin dynamics in vascular smooth muscle tissues. Because CAS phosphorylation is attenuated in arterial smooth muscle, we determined whether the actin filament assembly was altered in Abl−/− mice, the carotid arteries were isolated from knockout mice, or the wild-type mice were stimulated with PE or ANG II and stained with fluorescently labeled phalloidin (for F-actin) and DNase I (for G-actin). Images of labeled F-actin and G-actin were captured using a fluorescent microscope. The F/G-actin ratios were determined by using scanning densitometry of the images.
Ratios of F/G-actin in response to the activation with PE or ANG II were augmented in carotid arteries from wild-type mice. However, the agonist-induced increase in F/G-actin ratios was reduced in arterial tissues from the knockout mice (Fig. 3), indicating that Abl mediates actin filament polymerization upon contractile activation.
Fig. 3.
Enhancement of F-actin-to-G-actin (F/G-actin) ratios during PE or ANG II stimulation is inhibited in arteries from Abl−/− mice. A: representative images showing the effects of Abl deficiency on actin filament polymerization stimulated by agonists. Carotid arteries from Abl KO or WT mice were stimulated with PE (10 μM, 5 min) or ANG II (1 μM, 2.5 min), or they were unstimulated (US). Cryosections of these arteries were stained with Alexa 488-DNase I for G-actin (green) and with rhodamine-phalloidin for F-actin (red), and images were captured using a digital fluorescent microscope. B: F/G-actin ratios in response to agonist stimulation and the ratios of unstimulated tissues from KO mice are normalized to US values of arteries from WT mice. *P < 0.05, significantly lower F/G-actin ratios in Abl−/− arteries upon contractile stimulation compared with corresponding values obtained from Abl+/+ arteries. Values are means ± SE; n = 5 to 6.
Treatment with an Abl inhibitor attenuates increases in F/G-actin ratios upon contractile activation.
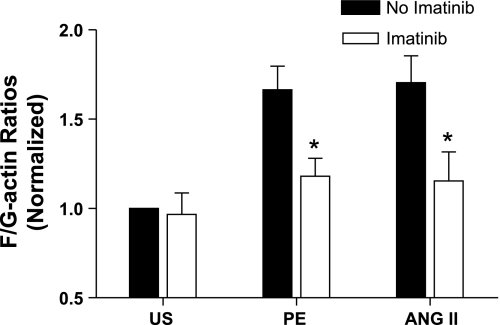
To further explore the role of Abl in regulating the actin architecture, carotid arteries harvested from wild-type mice were treated with the Abl inhibitor imatinib (Gleevec, Novartis) for 45 min. These arteries were then stimulated with PE or ANG II, or they were unstimulated. F/G-actin ratios were evaluated by fluorescent microscopy as described in materials and methods.
Exposure of arteries to imatinib did not affect F/G-actin ratios in unstimulated tissues. In contrast, increases in F/G-actin ratios upon stimulation with PE or ANG II were significantly inhibited by the treatment with the Abl inhibitor (Fig. 4, n = 4 to 5, P < 0.05). The results support our findings in isolated tissues from Abl knockout mice (Fig. 3), suggesting a critical role of Abl in the regulation of actin dynamics in vascular smooth muscle during contractile activation.
Fig. 4.
The increases in F/G-actin ratios upon PE or ANG II stimulation are attenuated by an Abl inhibitor. Carotid arteries from WT mice were treated with 20 μM imatinib for 45 min. These arteries were then stimulated with PE (10 μM, 5 min) or ANG II (1 μM, 2.5 min), or they were US. F/G-actin ratios in tissues were evaluated by fluorescent microscopy as described in materials and methods. F/G-actin ratios in response to various treatments are normalized to the ratio in US arteries not treated with imatinib. *P < 0.05, significantly lower F/G-actin ratios upon contractile stimulation in the presence of imatinib compared with corresponding values obtained from arteries not treated with imatinib. Values are means ± SE; n = 4 to 5.
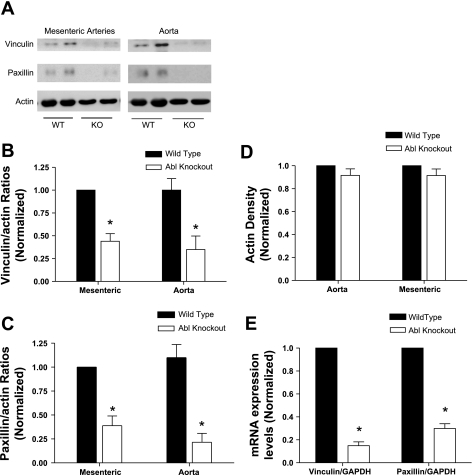
Focal adhesion-associated proteins vinculin and paxillin are downregulated in Abl knockout mice.
Vinculin and paxillin are associated with focal adhesions of cultured cells and have been shown to regulate actin dynamics and smooth muscle contraction (24, 27, 38, 42). Since agonist-induced actin polymerization is attenuated in Abl−/− mice, we assessed whether the expression of vinculin and paxillin is altered in these mice. The expression of these two proteins in arterial tissues from knockout and wild-type mice was assessed by immunoblot analysis.
As shown in Fig. 5A, the protein levels of vinculin and paxillin were lower in the mesenteric artery of knockout mice compared with wild-type mice. However, the amount of smooth muscle-specific α-actin was similar in tissues from both knockout and wild-type mice. The ratios of vinculin to actin and paxillin to actin were significantly lower in tissues of knockout mice compared with wild-type mice (Fig. 5, B and C, n = 7 to 8, P < 0.05). Furthermore, we also found that the pattern of protein expression was similar between the mesenteric arteries and the aorta, suggesting that Abl knockout affects the expression of vinculin and paxillin in both resistance and elastic arteries. The relative density of actin in vascular tissues was not significantly different between wild-type and Abl knockout mice (Fig. 5D, n = 7 to 8, P > 0.05). Our prior studies show that metavinculin, a splice variant of vinculin, is detected in airway smooth muscle tissues (38, 43). However, metavinculin was not detected in mouse arterial tissues (Fig. 5A). This could be due to the difference of tissue types (airway smooth muscle vs. vascular smooth muscle).
Fig. 5.
Expression of focal adhesion associated proteins vinculin and paxillin is lower in Abl KO mice. A: blots of extracts from mesenteric arteries and aortae from Abl KO and WT mice were detected with use of antibodies against vinculin, paxillin, or smooth muscle-specific α-actin. Two samples of each treatment are shown in the blots. Vinculin-to-actin (vinculin/actin) (B) and paxillin-to-actin (paxillin/actin) (C) ratios of each artery from KO mice are normalized to those of corresponding artery from WT mice. *P < 0.05 (n = 7 to 8), significantly lower proteins ratios from Abl−/− mice compared with Abl+/+ mice. D: the densitometric level of actin in vascular tissues from the KO mice is normalized to that in corresponding arteries from WT mice (n = 7 to 8). E: RT-quantitative PCR was used to assess mRNA levels of vinculin and paxillin of Abl−/− and Abl+/+ mice. GAPDH mRNA was also determined to serve as a control. Ratios of vinculin/GAPDH and paxillin/GAPDH from Abl−/− mice are normalized to corresponding ratios of Abl+/+ mice. *P < 0.01 (n = 5), significantly lower ratios from Abl−/− mice compared with Abl+/+ mice. Values are means ± SE.
To assess whether the transcription of the cytoskeletal proteins is inhibited, mRNA levels of vinculin and paxillin were analyzed using RT-quantitative PCR. Consistent with immunoblot analysis, the mRNA levels of vinculin and paxillin in mesenteric arteries were significantly lower in Abl−/− mice than in wild-type mice (Fig. 5E, P < 0.05, n = 5).
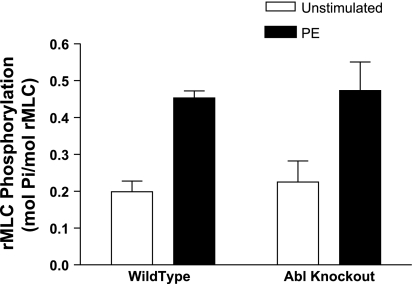
Increases in rMLC phosphorylation upon agonist stimulation are not reduced in Abl−/− mice.
Myosin phosphorylation has been thought to initiate cross-bridge cycling and smooth muscle contraction (34, 39). To determine whether the lower blood pressure in Abl−/− mice stem from the inhibition of rMLC phosphorylation, mesenteric arteries from wild-type or Abl−/− mice were stimulated with 10 μM PE for 5 min, or they were not stimulated. These arterial vessels were then frozen for the determination of rMLC phosphorylation.
Abl deficiency does not affect the agonist-induced increase in rMLC phosphorylation in the resistance arteries. The average increases in rMLC phosphorylation in segments from wild-type and Abl−/− mice were not significantly different after stimulation with PE (Fig. 6, P > 0.05, n = 4).
Fig. 6.
Effects of Abl knockdown on regulatory myosin light chain (rMLC) phosphorylation upon contractile stimulation. Increases in rMLC phosphorylation upon PE stimulation are similar in resistance arteries from Abl−/− and Abl+/+ mice (P > 0.05). Values shown are means ± SE; n = 4.
DISCUSSION
The role of the nonreceptor tyrosine kinase Abl in regulating blood pressure and cytoskeletal proteins in vascular smooth muscle is poorly understood. In this report, blood pressure is lower in Abl−/− mice compared with wild-type mice. Moreover, Abl knockout attenuates the agonist-induced enhancement of CAS phosphorylation and the expression of vinculin and paxillin in vascular smooth muscle tissues. However, myosin phosphorylation in response to agonist stimulation is not affected in arteries from Abl knockout mice. These results indicate that the Abl-regulated cellular process and blood pressure are independent of myosin activation. Abl differentially regulates CAS, vinculin, and paxillin in arterial vessels.
CAS is required for the agonist-elicited actin filament assembly and the active force development in arterial smooth muscle (40, 41). The stimulation with PE or serotonin induces CAS phosphorylation on tyrosine residues in vascular smooth muscle tissues (1, 22). In the present report, the systolic blood pressure was lower in Abl−/− mice compared with wild-type mice. PE- or ANG II-elicited CAS phosphorylation was attenuated in resistance arteries of Abl−/− mice. Furthermore, the agonist-induced enhancement of F/G-actin ratios was attenuated in arteries from Abl−/− mice and in arteries treated with the Abl inhibitor imatinib. Previous in vitro studies demonstrated that Abl directly catalyzes CAS phosphorylation (1, 18). These findings suggest that Abl may catalyze CAS phosphorylation in arterial smooth muscle, which may affect the actin dynamics and blood pressure in the animals.
It is well recognized that the focal adhesion-associated protein p130CAS and its downstream event are regulated by both receptor activation and the transmembrane integrins that are sensitive to mechanical environments (pressure and/or tension) surrounding vascular smooth muscle (7, 35, 36). In the present study, we determined CAS phosphorylation and actin polymerization in resistance arteries that were not under pressure or tension. Our biochemical analysis shows that the activation of α-adrenergic receptor by PE and the activation of AT receptor by ANG II lead to the increase in CAS phosphorylation and actin polymerization in the absence of pressure or tension in wild-type mice. However, the receptor-mediated process was impaired in Abl knockout mice. These results suggest that 1) receptor activation alone is sufficient to activate the CAS-mediated pathway in vascular smooth muscle and that 2) Abl is a key player in the receptor-mediated CAS activation in vascular smooth muscle when integrins are not activated by pressure or tension imposed to vascular tissues.
Tyr410 is one of the major phosphorylation sites on the substrate domain of CAS; phosphorylation on this residue exposes docking sites for the Src homology 2-containing signaling effector CrkII (4, 31). CrkII has been shown to regulate the activity of the actin-regulatory protein N-WASP in smooth muscle. When not activated, N-WASP forms an autoinhibitory conformation, preventing its engagement with the Arp2/3 complex. When bound to CrkII, N-WASP undergoes conformational changes, interacting with the Arp2/3 complex and initiating actin assembly and branching (1, 25, 38, 43, 45). CAS may also affect the association of the actin-regulatory protein profilin with G-actin, facilitating the transport of actin monomers to the barbed end of a growing filament (25, 40, 41).
Vinculin is a key structural protein that links actin filaments to extracellular matrix via integrins at focal adhesion sites of cultured smooth muscle cells. Paxillin associated with dense plaques participates in the signaling cascades that regulate actin dynamics in smooth muscle tissues (6, 36, 42). Because Abl knockout inhibits the agonist-induced increase in actin polymerization, we questioned whether Abl knockout influences the expression of vinculin and paxillin. The expression of vinculin and paxillin was reduced at the protein and messenger levels in arterial tissues from knockout mice. In addition, the expression of these two proteins was also reduced in tracheal smooth muscle tissues from Abl−/− mice (data not shown). The results suggest that Abl is an important component that regulates the expression of these two proteins in smooth muscle including arterial and airway smooth muscle tissues. The reduced expression of vinculin may impair the assembly of smooth muscle dense plaques, whereas paxillin deficiency may attenuate actin polymerization in the arterial tissues of Abl−/− mice. These changes may contribute to the mechanisms by which Abl knockout affects blood pressure.
The mechanisms where Abl modulates the expression of vinculin and paxillin in smooth muscle are currently unknown. Again, in the present study, Abl knockout impairs the agonist-induced enhancement of CAS phosphorylation in vascular smooth muscle. In another study, ANG II-induced CAS phosphorylation in vascular smooth muscle initiated the assembly of a multiprotein complex containing CAS, proline-rich tyrosine kinase 2, and phosphatidylinositol 3-kinase as determined by coimmunoprecipitation analysis. The complex formation between CAS, proline-rich tyrosine kinase 2, and phosphatidylinositol 3-kinase was associated with a rapid phosphorylation of the ribosomal p70 S6 kinase (an enzyme critical for protein synthesis) (29). Furthermore, agonist stimulation facilitates CAS/CrkII coupling that is able to activate MAPK via Rac1. MAPK has been shown to regulate the phosphorylation of the PHAS-1/elF4E complex, a key regulator of translation initiation (15, 29, 31). Thus it is possible that Abl regulates the expression of vinculin and paxillin by modulating CAS activation, which in turn affects PHAS-1/elF4E and p70 S6 kinase.
The actomyosin system regulated by rMLC phosphorylation has been considered a sole mechanism for the regulation of smooth muscle contractility (14, 26, 32). Although blood pressure was lower in Abl knockout mice, the increase in rMLC phosphorylation during agonist stimulation was not inhibited in arteries from Abl−/− mice. The results strongly suggest that 1) Abl knockout is sufficient to reduce blood pressure in these mice, 2) Abl is not involved in the regulation of myosin activation in resistance arteries, and 3) Abl may participate in the regulation of blood pressure by modulating CAS-mediated actin polymerization and the expression of focal adhesion-associated proteins.
Actin polymerization may regulate smooth muscle contraction by several mechanisms. First, the actin filaments of smooth muscle tissues connect to the extracellular matrix at dense plaques, facilitating the force transmission between the actin filaments and the matrix (7, 9, 38, 43). Cortical nascent actin polymerization regulated by the Arp2/3 complex may reinforce the linkage of actin filaments to integrins, strengthening the transduction of the mechanical force (30, 38, 43, 45). Second, actin polymerization may lead to an increase in numbers of contractile units and the length of actin filaments, providing more and efficient contractile elements for force development (3, 11). Third, the actin filament assembly may also be a part of the reorganization process that allows for the rapid adjustment of cytoskeletal organization elicited by external stimulation (6, 17, 24, 37, 43, 45). Fourth, actin polymerization may participate in the “latch” formation of contractile elements, supporting the force maintenance under the condition of lower cross-bridge phosphorylation (10, 19, 21, 26, 27).
Since Abl plays an important role in regulating the CAS-mediated signaling events and blood pressure, the development of a strategy to inhibit Abl kinase activity may be an intriguing approach to treat hypertension. Recent exciting studies support this theory; a selective inhibitor of Abl, imatinib (STI-571), is effective for the treatment of pulmonary hypertension in clinical studies (8, 13).
Summary.
The knockdown of Abl attenuates blood pressure, the tyrosine phosphorylation of the adapter protein CAS, and the actin polymerization in arterial smooth muscle upon contractile stimulation. The expression of focal adhesion proteins vinculin and paxillin is reduced in the blood vessels of Abl−/− mice. Abl knockout does not inhibit myosin activation in response to contractile activation. We conclude that Abl is an important biomolecule that orchestrates the regulation of blood pressure through a novel CAS-mediated cellular event.
GRANTS
This work was supported by National Heart, Lung, and Blood Institute Grant HL-75388 (to D. D. Tang).
Acknowledgments
We thank Yana Anfinogenova for technical assistance. S. Chen is a recipient of a scholarship from China Scholarship Council.
REFERENCES
- 1.Anfinogenova Y, Wang R, Li QF, Spinelli AM, Tang DD. Abl silencing inhibits CAS-mediated process and constriction in resistance arteries. Circ Res 101: 420–428, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Barany M, Barron JT, Gu L, Barany K. Exchange of the actin-bound nucleotide in intact arterial smooth muscle. J Biol Chem 276: 48398–48403, 2001. [DOI] [PubMed] [Google Scholar]
- 3.Cipolla MJ, Gokina NI, Osol G. Pressure-induced actin polymerization in vascular smooth muscle as a mechanism underlying myogenic behavior. FASEB J 16: 72–76, 2002. [DOI] [PubMed] [Google Scholar]
- 4.Defilippi P, Di SP, Cabodi S. p130Cas: a versatile scaffold in signaling networks. Trends Cell Biol 16: 257–263, 2006. [DOI] [PubMed] [Google Scholar]
- 5.Fortuno A, Muniz P, Ravassa S, Rodriguez JA, Fortuno MA, Zalba G, Diez J. Torasemide inhibits angiotensin II-induced vasoconstriction and intracellular calcium increase in the aorta of spontaneously hypertensive rats. Hypertension 34: 138–143, 1999. [DOI] [PubMed] [Google Scholar]
- 6.Gerthoffer WT Actin cytoskeletal dynamics in smooth muscle contraction. Can J Physiol Pharmacol 83: 851–856, 2005. [DOI] [PubMed] [Google Scholar]
- 7.Gerthoffer WT, Gunst SJ. Invited review: focal adhesion and small heat shock proteins in the regulation of actin remodeling and contractility in smooth muscle. J Appl Physiol 91: 963–972, 2001. [DOI] [PubMed] [Google Scholar]
- 8.Ghofrani HA, Seeger W, Grimminger F. Imatinib for the treatment of pulmonary arterial hypertension. N Engl J Med 353: 1412–1413, 2005. [DOI] [PubMed] [Google Scholar]
- 9.Gunst SJ, Tang DD. The contractile apparatus and mechanical properties of airway smooth muscle. Eur Respir J 15: 600–616, 2000. [DOI] [PubMed] [Google Scholar]
- 10.Gunst SJ, Tang DD, Opazo SA. Cytoskeletal remodeling of the airway smooth muscle cell: a mechanism for adaptation to mechanical forces in the lung. Respir Physiol Neurobiol 137: 151–168, 2003. [DOI] [PubMed] [Google Scholar]
- 11.Herrera AM, Martinez EC, Seow CY. Electron microscopic study of actin polymerization in airway smooth muscle. Am J Physiol Lung Cell Mol Physiol 286: L1161–L1168, 2004. [DOI] [PubMed] [Google Scholar]
- 12.Hirshman CA, Zhu D, Pertel T, Panettieri RA, Emala CW. Isoproterenol induces actin depolymerization in human airway smooth muscle cells via activation of an Src kinase and GS. Am J Physiol Lung Cell Mol Physiol 288: L924–L931, 2005. [DOI] [PubMed] [Google Scholar]
- 13.Hoeper MM, Rubin LJ. Update in pulmonary hypertension 2005. Am J Respir Crit Care Med 173: 499–505, 2006. [DOI] [PubMed] [Google Scholar]
- 14.Kamm KE, Stull JT. Regulation of smooth muscle contractile elements by second messengers. Annu Rev Physiol 51: 299–313, 1989. [DOI] [PubMed] [Google Scholar]
- 15.Lawrence JC Jr, Brunn GJ. Insulin signaling and the control of PHAS-I phosphorylation. Prog Mol Subcell Biol 26: 1–31, 2001. [DOI] [PubMed] [Google Scholar]
- 16.Li B, Boast S, de los SK, Schieren I, Quiroz M, Teitelbaum SL, Tondravi MM, Goff SP. Mice deficient in Abl are osteoporotic and have defects in osteoblast maturation. Nat Genet 24: 304–308, 2000. [DOI] [PubMed] [Google Scholar]
- 17.Li QF, Spinelli AM, Wang R, Anfinogenova Y, Singer HA, Tang DD. Critical role of vimentin phosphorylation at Ser-56 by p21-activated kinase in vimentin cytoskeleton signaling. J Biol Chem 281: 34716–34724, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mayer BJ, Hirai H, Sakai R. Evidence that SH2 domains promote processive phosphorylation by protein-tyrosine kinases. Curr Biol 5: 296–305, 1995. [DOI] [PubMed] [Google Scholar]
- 19.Meeks MK, Ripley ML, Jin Z, Rembold CM. Heat shock protein 20-mediated force suppression in forskolin-relaxed swine carotid artery. Am J Physiol Cell Physiol 288: C633–C639, 2005. [DOI] [PubMed] [Google Scholar]
- 20.Mehta D, Gunst SJ. Actin polymerization stimulated by contractile activation regulates force development in canine tracheal smooth muscle. J Physiol 519: 829–840, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Murphy RA, Rembold CM. The latch-bridge hypothesis of smooth muscle contraction. Can J Physiol Pharmacol 83: 857–864, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ogden K, Thompson JM, Hickner Z, Huang T, Tang DD, Watts SW. A new signaling paradigm for serotonin: use of Crk-associated substrate in arterial contraction. Am J Physiol Heart Circ Physiol 291: H2857–H2863, 2006. [DOI] [PubMed] [Google Scholar]
- 23.Ojaniemi M, Vuori K. Epidermal growth factor modulates tyrosine phosphorylation of p130Cas. Involvement of phosphatidylinositol 3′-kinase and actin cytoskeleton. J Biol Chem 272: 25993–25998, 1997. [DOI] [PubMed] [Google Scholar]
- 24.Opazo SA, Zhang W, Wu Y, Turner CE, Tang DD, Gunst SJ. Tension development during contractile stimulation of smooth muscle requires recruitment of paxillin and vinculin to the membrane. Am J Physiol Cell Physiol 286: C433–C447, 2004. [DOI] [PubMed] [Google Scholar]
- 25.Pollard TD Regulation of actin filament assembly by Arp2/3 complex and formins. Annu Rev Biophys Biomol Struct 36: 451–477, 2007. [DOI] [PubMed] [Google Scholar]
- 26.Rembold CM Force suppression and the crossbridge cycle in swine carotid artery. Am J Physiol Cell Physiol 293: C1003–C1009, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rembold CM, Tejani AD, Ripley ML, Han S. Paxillin phosphorylation, actin polymerization, noise temperature, and the sustained phase of swine carotid artery contraction. Am J Physiol Cell Physiol 293: C993–C1002, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rhoades RA, Tanner GA. Control mechanisms in circulatory function. In: Medical Physiology, edited by Rhoades RA and Tanner GA. Baltimore, MD: Lippincott Williams & Wilkins, 1995, p. 321–338.
- 29.Rocic P, Govindarajan G, Sabri A, Lucchesi PA. A role for PYK2 in regulation of ERK1/2 MAP kinases and PI 3-kinase by ANG II in vascular smooth muscle. Am J Physiol Cell Physiol 280: C90–C99, 2001. [DOI] [PubMed] [Google Scholar]
- 30.Rohatgi R, Nollau P, Ho HY, Kirschner MW, Mayer BJ. Nck and phosphatidylinositol 4,5-bisphosphate synergistically activate actin polymerization through the N-WASP-Arp2/3 pathway. J Biol Chem 276: 26448–26452, 2001. [DOI] [PubMed] [Google Scholar]
- 31.Shin NY, Dise RS, Schneider-Mergener J, Ritchie MD, Kilkenny DM, Hanks SK. Subsets of the major tyrosine phosphorylation sites in Crk-associated substrate (CAS) are sufficient to promote cell migration. J Biol Chem 279: 38331–38337, 2004. [DOI] [PubMed] [Google Scholar]
- 32.Somlyo AV, Khromov AS, Webb MR, Ferenczi MA, Trentham DR, He ZH, Sheng S, Shao Z, Somlyo AP. Smooth muscle myosin: regulation and properties. Philos Trans R Soc Lond B Biol Sci 359: 1921–1930, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Takahashi T, Kawahara Y, Taniguchi T, Yokoyama M. Tyrosine phosphorylation and association of p130Cas and c-Crk II by ANG II in vascular smooth muscle cells. Am J Physiol Heart Circ Physiol 274: H1059–H1065, 1998. [DOI] [PubMed] [Google Scholar]
- 34.Tang DC, Stull JT, Kubota Y, Kamm KE. Regulation of the Ca2+ dependence of smooth muscle contraction. J Biol Chem 267: 11839–11845, 1992. [PubMed] [Google Scholar]
- 35.Tang DD p130 Crk-associated substrate (CAS) in vascular smooth muscle. J Cardiovasc Pharmacol Ther 14: 89–98, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tang DD, Anfinogenova Y. Physiologic properties and regulation of the actin cytoskeleton in vascular smooth muscle. J Cardiovasc Pharmacol Ther 13: 130–140, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tang DD, Bai Y, Gunst SJ. Silencing of p21-activated kinase attenuates vimentin phosphorylation on Ser-56 and reorientation of the vimentin network during stimulation of smooth muscle cells by 5-hydroxytryptamine. Biochem J 388: 773–783, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tang DD, Gunst SJ. The small GTPase Cdc42 regulates actin polymerization and tension development during contractile stimulation of smooth muscle. J Biol Chem 279: 51722–51728, 2004. [DOI] [PubMed] [Google Scholar]
- 39.Tang DD, Gunst SJ. Depletion of focal adhesion kinase by antisense depresses contractile activation of smooth muscle. Am J Physiol Cell Physiol 280: C874–C883, 2001. [DOI] [PubMed] [Google Scholar]
- 40.Tang DD, Tan J. Role of Crk-associated substrate in the regulation of vascular smooth muscle contraction. Hypertension 42: 858–863, 2003. [DOI] [PubMed] [Google Scholar]
- 41.Tang DD, Tan J. Downregulation of profilin with antisense oligodeoxynucleotides inhibits force development during stimulation of smooth muscle. Am J Physiol Heart Circ Physiol 285: H1528–H1536, 2003. [DOI] [PubMed] [Google Scholar]
- 42.Tang DD, Wu MF, Opazo Saez AM, Gunst SJ. The focal adhesion protein paxillin regulates contraction in canine tracheal smooth muscle. J Physiol 542: 501–513, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tang DD, Zhang W, Gunst SJ. The adapter protein CrkII regulates neuronal Wiskott-Aldrich syndrome protein, actin polymerization, and tension development during contractile stimulation of smooth muscle. J Biol Chem 280: 23380–23389, 2005. [DOI] [PubMed] [Google Scholar]
- 44.Ushio-Fukai M, Zuo L, Ikeda S, Tojo T, Patrushev NA, Alexander RW. cAbl tyrosine kinase mediates reactive oxygen species- and caveolin-dependent AT1 receptor signaling in vascular smooth muscle: role in vascular hypertrophy. Circ Res 97: 829–836, 2005. [DOI] [PubMed] [Google Scholar]
- 45.Zhang W, Wu Y, Du L, Tang DD, Gunst SJ. Activation of the Arp2/3 complex by N-WASp is required for actin polymerization and contraction in smooth muscle. Am J Physiol Cell Physiol 288: C1145–C1160, 2005. [DOI] [PubMed] [Google Scholar]