Abstract
Adenosine protects the heart from adrenergic overstimulation. This adenoprotection includes the direct anti-adrenergic action via adenosine A1 receptors (A1R) on the adrenergic signaling pathway. An indirect A1R-induced attenuation of adrenergic responsiveness involves the translocation of PKC-ɛ to t-tubules and Z-line of cardiomyocytes. We investigated with sarcomere imaging, immunocytochemistry imaging, and coimmunoprecipitation (co-IP) whether A1R activation of PKC-ɛ induces the kinase translocation to receptor for activated C kinase 2 (RACK2) in isolated rat and mouse hearts and whether phospholipase C (PLC) is involved. Rat cardiomyocytes were treated with the A1R agonist chlorocyclopentyladenosine (CCPA) and exposed to primary PKC-ɛ and RACK2 antibodies with secondaries conjugated to Cy3 and Cy5 (indodicarbocyanine), respectively. Scanning confocal microscopy showed that CCPA caused PKC-ɛ to reversibly colocalize with RACK2 within 3 min. Additionally, rat and mouse hearts were perfused and stimulated with CCPA or phenylisopropyladenosine to activate A1R, or with phorbol 12-myristate 13-acetate to activate PKC. RACK2 was immunoprecipitated from heart extracts and resolved with SDS-PAGE. Western blotting showed that CCPA, phenylisopropyladenosine, and phorbol 12-myristate 13-acetate in the rat heart increased the PKC-ɛ co-IP with RACK2 by 186, 49, and >1,000%, respectively. The A1R antagonist 8-cyclopentyl-1,3-dipropylxanthine prevented the CCPA-induced co-IP with RACK2. In mouse hearts, CCPA increased the co-IP of PKC-ɛ with RACK2 by 61%. With rat cardiomyocytes, the β-adrenergic agonist isoproterenol increased sarcomere shortening by 177%. CCPA reduced this response by 47%, an action inhibited by the PLC inhibitor U-73122 and 8-cyclopentyl-1,3-dipropylxanthine. In conclusion, A1R stimulation of the heart is associated with PLC-initiated PKC-ɛ translocation and association with RACK2.
Keywords: adenosine A1 receptor, protein kinase C-ɛ, receptor for activated C kinase 2, rodent
adenosine is an important endogenous physiological modulator of heart function. A major role of this nucleoside in the myocardium is one of adenosine A1 receptor (A1R)-mediated adenoprotection, which is an attenuation of myocardial responsiveness to potentially toxic effects of adrenergic overstimulation. The manifestation of adenoprotection can involve the direct anti-adrenergic action of attenuating adrenergic signaling, involving the β-adrenergic catecholamine elicited increases in Gs protein cycling (17), adenylyl cyclase activity (24, 36, 37), cAMP formation (7), activation of PKA (8), protein phosphorylation (13), myocardial Ca2+ transient magnitude (14), and ventricular contractility (15). This anti-adrenergic property of adenosine limits the detrimental action of excessive levels of catecholamines that may occur with enhanced sympathetic drive. In this manner, adenosine curtails cardiotoxicity (38) of norepinephrine released during ischemia (39). We have reported that endogenous adenosine reduces the toxic effect of excessive catecholamine stimulation on contractile function (15).
Adenosine also attenuates catecholamine responsiveness independently of the anti-adrenergic action. The activation of adenosine receptors and PKC each alone decrease the maximum velocity of shortening (Vmax) of rat ventricular cardiomyocytes (25). This suggests contractile responsiveness to inotropic agents can be decreased independently of the anti-adrenergic action. Recently, we have reported that, upon activation of A1R by the agonist chlorocyclopentyl-adenosine (CCPA), PKC-ɛ translocates to the transverse tubules (t-tubules) of isolated rat cardiomyocytes (30). PKC-ɛ is abundantly expressed in the adult cardiomyocyte (39). This kinase in vitro has been determined to phosphorylate a wide variety of proteins, with little selectivity or specificity (32). However, the diversity of actions provided by the various isozymes of PKC suggests that mechanisms of recognition are present in the intact cell that would confer specificity to the PKC enzymatic activity (20). Receptors for activated C kinase (RACKs) have been identified as PKC binding proteins that enable the anchoring of the activated kinase, thereby conferring specificity of action (5). Evidence suggests that RACK2 serves as the specific intracellular anchor for PKC-ɛ (5, 21).
Activation of the A1R by the agonist R-(−)N6-(2-phenylisopropyl)-adenosine (PIA) has been reported to elevate inositol triphosphate levels in the myocardium (23). Others have reported, in avian ventricular myocytes, that the A1R agonist CCPA induces the accumulation of 1,2-diacylglycerol (DAG) and inositol phosphates, suggesting that the A1R is coupled to phospholipase C (PLC) (34). One objective of the present study was to investigate whether PKC-ɛ translocates to the RACK2 binding protein located at the t-tubules. Another objective was to determine whether the A1R-induced activation of PKC-ɛ in the rat heart involves the activation of PLC. Measurements of sarcomere shortening and immunocytochemical imaging and co-immunoprecipitation were approaches used with cardiomyocytes and extracts obtained from rat and mouse hearts.
METHODS
Animals used in this study were maintained and used in accordance with recommendations in the Guide for the Care and Use of Laboratory Animals, prepared by the Institute of Laboratory Animal Resources, National Research Council (DHEW Publication National Institutes of Health no. 85-23, Rev. 1996) and the guidelines and approval of the Institutional Animal Care and Use Committee of the University of Massachusetts Medical School. Male Sprague-Dawley rats (Harlan, Indianapolis, IN or Charles River, Wilmington, MA) or C57BL/6 mice (UMass colony) of 3–4 mo of age were housed in rooms with 12:12-h light-dark cycles and fed rodent chow and water ad libitum.
Isolated heart preparation.
Hearts were isolated and perfused as described previously by our laboratory (16). Hearts were rapidly removed after animals were guillotined and perfused via an aortic cannula at a constant rate with physiological saline (PS) containing the following (in mM): 118 NaCl, 4.7 KCl, 2.5 CaCl2, 25 NaHCO3, 1.2 KH2PO4, 1.2 MgSO4, and 10 glucose, with the pH maintained at 7.4 by gassing the PS with 95% O2-5% CO2. Flow rates ranged from 12 to 18 ml/min for rat hearts and 3.0 to 4.2 ml/min for mouse hearts, producing perfusion pressures ranging from 60 to 80 mmHg. Coronary perfusion pressures were measured by a pressure transducer attached to a side tube immediately above the aorta. Rat and mouse hearts were paced at 360 and 420 contractions/min, respectively. Water-filled latex (rat) or polyethylene (mouse) balloons were inserted into the left ventricular lumen via the mitral orifice and attached to a polygraph via a cannula to allow the hearts to develop tension against a load. Balloons were inflated to achieve a maximal developed force and maintained at constant volume thereafter. After instrumentation, hearts were allowed to stabilize for 15 min before initiating the experimental protocols. Agents were administered via infusion into the PS to achieve the desired concentrations. Durations and concentrations are described in results. The adenosine A2A receptor (A2AR) antagonist ZM-241385 was employed at 1 μM in all experiments to prevent the actions of A2AR in response to endogenous adenosine. Upon termination of the experimental periods, hearts were frozen with liquid N2-cooled aluminum clamps and stored in liquid N2 until assayed.
Isolation of ventricular myocytes.
Cardiomyocytes were isolated from rat hearts with the use of collagenase and hyaluronidase by procedures previously reported by us (10), only with the enzyme perfusion duration reduced to 3–4 min. Isolated cardiomyocytes were cultured for 3 h on poly-l-ornithine-coated glass coverslips to allow attachment. These myocytes were used in imaging experiments. Other myocytes were allowed to remain suspended for use in sarcomere shortening experiments.
Measurement of myocyte contractile function.
Cardiomyocyte contractile function was assessed as previously described (10). Briefly, an aliquot of ventricular myocytes was placed into a 506-μl chamber and suffused continuously at room temperature with suffusion solution containing the following (in mM): 136.4 NaCl, 4.7 KCl, 1.0 CaCl2, 10 HEPES, 1.0 NaHCO3, 1.2 MgSO4, 1.2 KH2PO4, 10 glucose, 0.6 ascorbate, and 1.0 pyruvate (pH 7.4). Myocytes were observed with an inverted microscope and stimulated to contract via platinum wire electrodes at 0.2 Hz. Sarcomere shortening was monitored using an IonOptix contractility system (Milton, MA). ZM-241385 was not included in experiments utilizing isolated cardiomyocytes, because cells were continuously suffused with fresh medium. Following an equilibration period of 15 min, reagents were added to the suffusion solution at the concentrations and durations indicated.
Immunocytochemistry and imaging.
After attachment, myocytes were either left untreated or treated with the adenosine A1R agonist CCPA at 1 μM for 5 min. In both cases, myocytes were then placed in fixation solution I [4% formaldehyde, 2 mM MgCl2, and 1 mM EGTA in phosphate-buffered saline (PBS); pH 7.4] and, after extensive washing, permeabilized with 0.1% Triton X-100 in PBS for 10 min. After permeabilization, the coverslips were washed twice with PBS and incubated for 30 min with 1% BSA in PBS. For double staining with PKC-ɛ and RACK2, the cells were first stained with rabbit PKC-ɛ (Santa Cruz Biotechnology) and mouse RACK2 (β′-COP; Sigma) antibodies for 16 h at 4°C overnight. After being washed with PBS three times, myocytes were incubated with Cy3 (indocarbocyanine)-conjugated anti-rabbit IgG secondary antibody for PKC-ɛ and Cy5 (indodicarbocyanine)-conjugated anti-mouse IgG secondary antibody (Jackson ImmunoResearch) for RACK2 at 28°C for 1 h. Excess secondary antibody was removed, and the coverslips were treated with 3% 1,4-diazabicyclo(2.2.2)octane (DABCO)-90% glycerol in PBS.
Images were observed using a Leica confocal microscope (Leica Microsystems, Germany). Differential interference contrast and fluorescence images were viewed using a Leica DM IRB laser scanning confocal microscope controlled by Leica TCS SP II confocal system (Leica Microsystems). The images were reconstructed using LCS three-dimensional software of Leica Microsystems. Images were processed using Photoshop 6.0 software (Adobe). Use of a PKC-ɛ antibody with fixed, permeabilized, rat ventricular cardiomyocytes allows the detection of endogenous PKC-ɛ. Because secondary antibodies were conjugated to Cy3-(indodicarbocyanine), the placement of the primary antibody was manifest as a red image. Localization of endogenous RACK2 using anti-RACK2 (anti-β′-COP) with secondary antibodies conjugated to Cy5 (indodicarbocyanine) results in a blue-green image.
Coimmunoprecipitation of PKC-ɛ and RACK2.
The effect of adenosine A1R stimulation on the translocation of PKC-ɛ to RACK2 was investigated using immunoprecipitation. One hundred milligrams of frozen rat or mouse heart were homogenized (PRO200 homogenizer, PRO Scientific, Oxford, CT) in 200 μl of homogenization buffer containing 20 mM HEPES, 0.3 mM MgCl2, and 0.2 mM EDTA (pH 7.4). After centrifugation for 30 min at 22,000 g, the pellet was resuspended in 100 μl of solubilization buffer composed of 100 mM Tris, 200 mM NaCl, 1 mM EGTA, and 0.05% CHAPS (pH 7.4). This mixture was incubated with 1.0 μg of TCP-1α antibody (anti-RACK2; 34) for 14 h at 4°C with shaking. After adding 10 μl of Protein A/G Plus Agarose, the mixture was incubated at room temperature for 90 min and centrifuged at 11,000 g for 5 min. The pellet was subsequently washed three times with a buffered phosphate solution containing 2 mM KH2PO4, 10 mM Na2HPO4, and 0.14 mM NaCl (pH 6.8), and resuspended in 100 μl of 2% SDS/10% glycerol. A sample was removed for protein determination, and the remainder was supplemented with 25% β-mercaptoethanol, 300 mM Tris, and 0.5% bromophenol blue. After boiling for 3–5 min, samples were centrifuged to pellet the agarose, and the supernatant was resolved on 10% SDS-PAGE. Resolved proteins were transferred to nitrocellulose membranes and blotted against primary rabbit anti-PKC-ɛ. The secondary antibody was goat anti-rabbit conjugated to horseradish peroxidase. The chemiluminescence was monitored with X-ray film and Western Lightning reagents (PerkinElmer LAS, Boston, MA). Film densities were quantified using UN-SCAN-IT software (Silk Scientific, Orem, UT).
Statistical methods.
Data were analyzed using StatMost (Dataxiom, Los Angeles, CA). After applying one-way ANOVA, additional analysis was conducted using Student-Newman-Keuls post hoc test. A P value of <0.05 was taken to indicate a statistically significant difference. All data are presented as means ± SE. Quantitative imaging analysis to assess the colocalization of the PKC-ɛ and RACK2 was performed using “Intensity Correlation Analysis” function in ImageJ (http://rsbweb.nih.gov) (27). The overlap coefficients generated by Pearson's correlation coefficient have values between 1 and 0 (1 indicating that 100% of both components of the two images overlap).
Materials.
Buffer salts, general laboratory reagents, DABCO, formaldehyde, and Triton X-100 were obtained from Fisher Scientific (Medford, MA) or Sigma (St. Louis, MO). SDS and all gel electrophoretic reagents were obtained from Bio-Rad (Richmond, CA). The PLC inhibitor U-73122 and its inactive analog U-73343 were purchased from Invitrogen (Camarillo, CA). 8-Cyclopentyl-1,3-dipropylxanthine (DPCPX) and PIA were obtained from Sigma (St. Louis, MO). ZM 241385 (ZM) and CCPA were purchased from Tocris (Ellisville, MO). Phorbol 12-myristate 13-acetate was obtained from Sigma (St. Louis, MO). PKC-ɛ polyclonal IgG and Protein A/G PLUS Agarose were obtained from Santa Cruz (sc-214; Santa Cruz, CA), and TCP-1α rat IgG (anti-RACK2) or β′-COP mouse IgG (anti-RACK2) were purchased from Stressgen (Ann Arbor, MI) or Sigma (St Louis, MO), respectively. Secondary antibodies Cy3 and Cy5 (indodicarbocyanine) conjugated anti-mouse IgG for RACK2 were obtained from Jackson ImmunoResearch (West Grove, PA). CCPA, DPCPX, and ZM were prepared as stock solutions in DMSO.
RESULTS
Immunocytochemistry, imaging, and PKC-ɛ translocation.
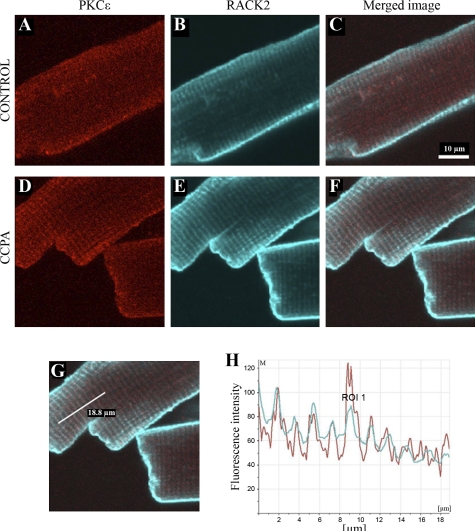
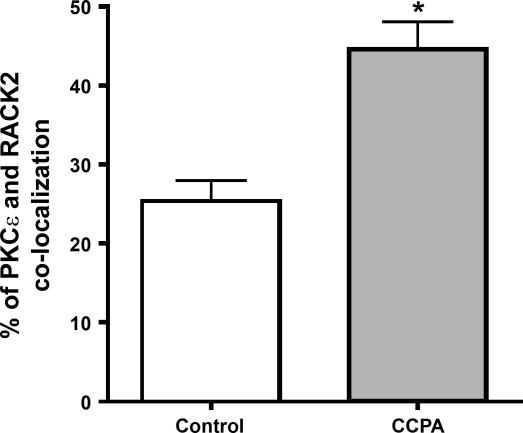
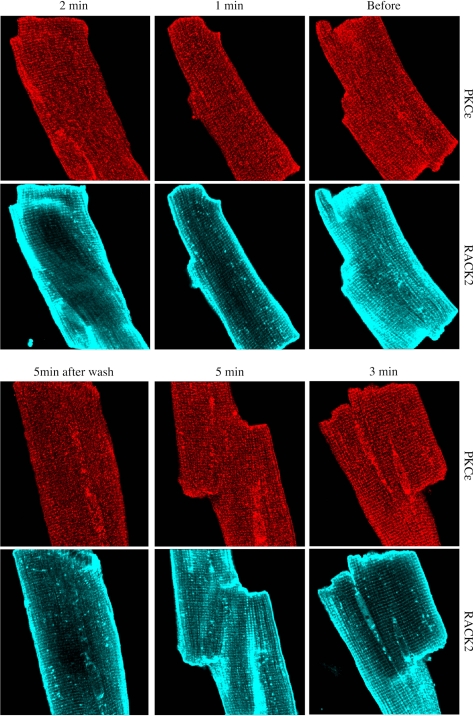
As indicated in methods, the placement of the primary PKC-ɛ antibody was imaged in red. In this manner, it was determined that stimulation of the A1R with CCPA elicited a translocation of the kinase to t-tubular structures in the cell (Fig. 1, A and D). Localization of endogenous RACK2 appearing as a blue-green image in the same cardiomyocytes indicated their presence in the t-tubular structures (Fig. 1, B and E). Images depicting localization of PKC-ɛ and RACK2 were merged for both control and CCPA-treated cells (Fig. 1, C and F). A linear fluorescent density scan was conducted over 18.8 μm perpendicular to the t-tubular structures, as depicted in Fig. 1G. Data shown in Fig. 1H reveal highly coincident traces for PKC-ɛ (red) and RACK2 (green). Quantitative image colocalization analysis indicated that the administration of CCPA significantly increased the colocalization of PKC-ɛ and RACK2 (Fig. 2). These data indicate that A1R stimulation results in a translocation of PKC-ɛ to the RACK2 protein that is a part of the ventricular myocyte t-tubule system. The timed administration of CCPA indicated that the localization of PKC-ɛ to the striated pattern characteristic of t-tubules reached a maximum intensity by 3 min of a 5-min exposure to CCPA and that the localization was reversible by 5 min (Fig. 3). For comparison, imaging of RACK2 was constant throughout the CCPA treatment period.
Fig. 1.
The effect of A1 receptor (A1R) stimulation of rat ventricular myocytes with chlorocyclopentyladenosine (CCPA) on the immunocytochemical localization of PKC-ɛ and receptor for activated C kinase 2 (RACK2). Cardiomyocytes were immunostained with antibodies against either PKC-ɛ or RACK2 (anti-β′-COP), and the endogenous proteins were detected by Cy3- or Cy5-conjugated secondary antibodies. Isolated rat ventricular myocytes were incubated either in the absence (control) or presence of 1 μM CCPA for 5 min. Images depict either PKC-ɛ (A and D), RACK2 (B and E), or both (C and F; merged image of PKC-ɛ and RACK2). A linear fluorescent density scan of F shown in G over 18.8 μm reveals highly coincident traces for PKC-ɛ (red) and RACK2 (blue-green) in H, indicating that A1R stimulation results in a translocation of PKC-ɛ to the RACK2 protein of the ventricular myocyte transverse (t)-tubule system. ROI, region of interest.
Fig. 2.
Effect of A1R stimulation on the colocalization of PKC-ɛ and RACK2 in isolated rat cardiomyocytes. Myocytes were stimulated with CCPA at 1 μM for 5 min and prepared for analysis, as described in methods. Colocalization was determined and statistics conducted as described in methods. Values are means ± SE for 3 cardiomyocytes. *Means are significantly different at P = 0.011.
Fig. 3.
Progressive effect of A1R stimulation on the association of PKC-ɛ with the t-tubules in isolated rat cardiomyocytes. Myocytes were fixed as described in methods after the administration of CCPA (1.0 μM), at 1, 2, 3, and 5 min of agent exposure, and at 5 min of washout following 5 min of exposure. Exposures depicting RACK2 are included for the same myocytes for comparison.
Coimmunoprecipitation of PKC-ɛ with RACK2.
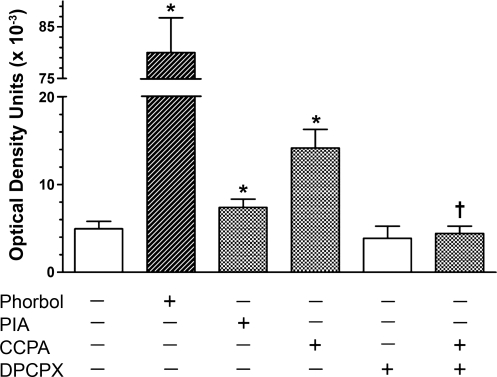
Additional information concerning the localization of PKC-ɛ in response to stimulation of A1R in the rat ventricular myocardium was obtained by first immunoprecipitating the RACK2 protein with anti-TCP-1α monoclonal IgG and Protein A/G Agarose, with subsequent Western blotting with anti-PKC-ɛ. Initial experiments were conducted with the nonspecific PKC activator phorbol myristate and the A1R agonist PIA at concentrations of 1 μM. The phorbol ester increased the coimmunoprecipitation of PKC-ɛ with RACK2 by greater than 1,500-fold (Fig. 4). Stimulation of A1R by PIA elicited a 49% increase in coimmunoprecipitation.
Fig. 4.
The effect in the isolated rat heart of the PKC activator phorbol myristate acetate (phorbol; 1 μM), the adenosine A1R agonists R-(−)N6-(2-phenylisopropyl)-adenosine (PIA; 1 μM) and CCPA (1 μM), and 8-cyclopentyl-1,3-dipropylxanthine (DPCPX; 0.1 μM) on the coimmunoprecipitation of PKC-ɛ with RACK2. Agents were administered to isolated hearts at the concentration indicated for 5 min in the continuous presence of the A2AR antagonist ZM-241385 (1 μM). RACK2 was immunoprecipitated using the RACK2 antibody anti-TCP-1α. Values are mean ± SE of 2–5 hearts. *Significant difference from control (P ≤ 0.05). †Significant difference from CCPA (P ≤ 0.05).
Experiments were repeated with the more specific A1R agonist CCPA separately and together with the A1R antagonist DPCPX. CCPA administered at 1 μM for 5 min elicited a 186% increase in the association of PKC-ɛ with RACK2 (Fig. 4). This increase was effectively inhibited with the administration of 0.1 μM DPCPX. DPCPX alone had no effect.
To determine whether the mouse myocardium responded similarly as the rat heart, coimmunoprecipitation of PKC-ɛ with RACK2 was determined with wild-type mouse hearts using the methodology employed for the rat heart above. Administration of CCPA in a concentration of 1 μM to the isolated heart was found to significantly enhance the coimmunoprecipitation by 61%. These data indicate that A1R stimulation results in an enhanced coimmunoprecipitation of PKC-ɛ with RACK2 in the mouse as well as the rat heart.
Inhibition of PLC and A1R function.
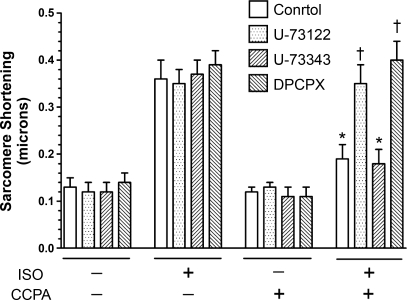
It has been suggested that PKC activity is enhanced in avian heart in response to elevated DAG levels elicited by A1R activation (34). We have reported that PKC-ɛ activation is involved in the anti-adrenergic action of A1R (30). Experiments were conducted to determine whether the inhibition of PLC attenuates the anti-adrenergic action of A1R. Basal sarcomere shortening in the absence of agents was 0.13 ± 0.02 μm. Administration of 2 nM isoproterenol to isolated rat cardiomyocytes for 3 min increased sarcomere shortening by 177% (Fig. 5). This effect was not altered by the presence of the PLC inhibitor U-73122 (10 μM), the inactive analog U-73343 (10 μM), or DPCPX (0.2 μM). CCPA for 5 min at 2 μM alone did not alter sarcomere shortening, but reduced the isoproterenol response by 47%. DPCPX and U-73122 inhibited the action of CCPA, while the inactive analog U-73343 had no effect. This suggests that A1R attenuates the contractile responsiveness to adrenergic stimulation involving activation of PLC.
Fig. 5.
The effect of the PLC inhibitor U-73122 on A1R attenuation of adrenergic-enhanced sarcomere shortening. Sarcomere shortening of isolated rat ventricular cardiomyocytes was determined as described in methods. The β1-adrenergic agonist isoproterenol (ISO) was administered at 2 nM for 3 min. The A1R agonist CCPA was administered at 2 μM, either alone or 5 min before ISO administration. DPCPX (0.2 μM), the PLC inhibitor U-73122 (10 μM), or the inactive analog U-73343 (10 μm) were applied during the 5 min before and during addition of CCPA to the suffusion solution. Control indicates absence of either U-73122 or U-73343. Values are means ± SE for 4–11 cells. All ISO values alone were significantly different from the values obtained in the absence of agents and in the presence of CCPA alone. *Significance from the appropriate ISO alone response. †Significance from the other two ISO + CCPA values.
DISCUSSION
The data presented in this study demonstrate that activation of PKC-ɛ on stimulation of the A1R in the rat or mouse heart elicits the translocation of the kinase to a RACK2 protein of the cardiomyocyte. Previously, we reported A1R activation promotes the translocation of PKC-ɛ, but not PKC-δ, to the t-tubules of the cardiomyocyte (30). The present data indicate that RACK2 was the target protein for this translocation. Our present observations include the measurement of contractile activity of isolated cardiomyocytes and the visualization with imaging (rat) and coimmunoprecipitation of the kinase and RACK2 (rat and mouse). Translocation of PKC-ɛ to RACK2 occurred whether the PKC-ɛ was activated nonspecifically by a phorbol ester, or by A1R activation with PIA, or with the selective agonist CCPA. The action induced by CCPA was selective for the A1R, as indicated by the inhibition elicited by the A1R antagonist DPCPX. Furthermore, PKC-ɛ translocation most likely results from an A1R-induced increase in PLC activity.
To date, studies of the effect of adenosine on contractile function in the heart have focused primarily on the direct effect of this nucleoside on β-adrenergic-induced elevations in cellular levels of cyclic AMP (9). This effect has been designated as the anti-adrenergic action of adenosine initiated by an A1R-induced reduction of Gs protein cycling in β-adrenergic-stimulated ventricular membranes (17). Such an action would manifest a reduced catecholamine-elicited activation of adenylyl cyclase (37), formation of cAMP (8), activation of PKA (8), and, ultimately, the phosphorylation of myocardial proteins important to contractile activity (13). By directly attenuating the β-adrenergic signaling, adenosine protects the heart from the toxic effects of adrenergic overstimulation, i.e., provides adenoprotection to the heart. It is apparent that the anti-adrenergic action of adenosine defines the direct effect of adenosine in reducing the signal transduction initiated by adrenergic stimulation.
Another mechanism of adenoprotection that does not involve the direct modulation of adrenergic signaling may involve PKC, a kinase associated with the phosphorylation of myofilament proteins, such as C protein, troponin I, myosin light chain, and others (3, 20). It has been reported that the activation of adenosine receptors and PKC each alone decreases the Vmax of rat ventricular cardiomyocytes in the absence of adrenergic stimulation (25). Lester and Hofmann (26) subsequently demonstrated that adenosine increased the turnover of inositol phosphates, induced PKC-ɛ activation, and reduced unloaded shortening velocities of ventricular myocytes. Therefore, adenosine can manifest adenoprotection by reducing the contractile responsiveness of the myocardium to adrenergic stimulation by a means other than the traditionally described anti-adrenergic mechanism. Activation of the PKC most likely results from the A1R-induced accumulation of DAG (34). It is thus likely that adenosine-induced adenoprotection of the heart is mediated, at least in part, by A1R activation of PLC. This is supported by our present observation that the A1R-induced reduction in sarcomere shortening stimulated by isoproterenol is prevented by the PLC inhibitor U-73122 (Fig. 5). Previously, we had shown that the inhibition of PKC-ɛ with inhibitor peptides also blocked the A1R-induced attenuation of β-adrenergic responses (10, 30). It is not entirely known at this point whether the two parallel mechanisms are operationally synergistic. However, it is interesting that inhibition of the A1R action by U-73122 was complete (Fig. 5). This is not entirely unexpected. Cardioprotection, for example, is provided by the process of ischemic preconditioning via multiple mechanisms, each of which achieves the same result (12). Such mechanisms involving adenosine, bradykinin, and opioids trigger parallel cardioprotective signal cascades involving PKC. These various mechanisms are not additive. With the process of adenoprotection, it is clear that common pathway elements may be shared. The complete inhibition of A1R action noted in Fig. 5 by blocking PLC activity suggests that signaling elements beyond PKC require delineation to learn how they interact with other mechanisms initiated by A1R. Of particular interest is the control of protein phosphatase (PP) activities by adenosine (44) and the modulation of endogenous inhibitors of PP that can be phosphorylated/activated by both PKA and PKC.
The present results reveal the importance of PKC in adenoprotection. PKC is an important inhibitory modulator of heart function (2, 11, 45). PKC-ɛ, the primary isoform found in the heart (39), is implicated in L-type Ca2+ regulation (22) and regulation of thin-filament Ca2+ sensitivity (18). PKC may also serve as a signal mediator for the preconditioning property of adenosine (1, 4, 34). However, while PKC-ɛ is undoubtedly important to the adenoprotection action of adenosine in the presence (10) or absence (30) of adrenergic stimulation, it remains to be elucidated how this is accomplished.
Mechanisms that allow direction to the movements of the various PKC isoforms to discrete localities within the cell have been discussed as essential for the appropriate and efficient manifestation of PKC activity (31, 41, 42). Such spatial redistribution of PKC on activation, described as “anchoring,” potentially allows the kinase activity to effectively phosphorylate the appropriate proteins required for a specific response (32). PKC-ɛ was reported to bind to the coatomer protein β′COP (RACK2) of Golgi membranes (5) at an 8-residue RACK-binding sequence, EAVSLKPT (11). Activated recombinant PKC-ɛ has been reported to bind reversibly and with high affinity to cardiac myofilaments free of t-tubuler membranes (19). Furthermore, when observed in skinned cardiomyocytes shown to be depleted of t-tubular membranes, the PKC-ɛ was found to localize in a cross-striated pattern, forming the Z-lines. Use of inhibitory synthetic peptides indicated that RACK2 (β′-COP) at the Z-line was the target anchoring protein (19). Our present and previous data (30), as well as reports of others (6), indicate that activated PKC-ɛ translocates to the Z-line and is colocalized with RACK2, as determined visually and by coimmunoprecipitation. This is also the location of t-tubules important to excitation-contraction coupling and sarcomeric actin filament tethering. Proteins phosphorylated by PKC-ɛ in the vicinity of RACK2 remain to be determined.
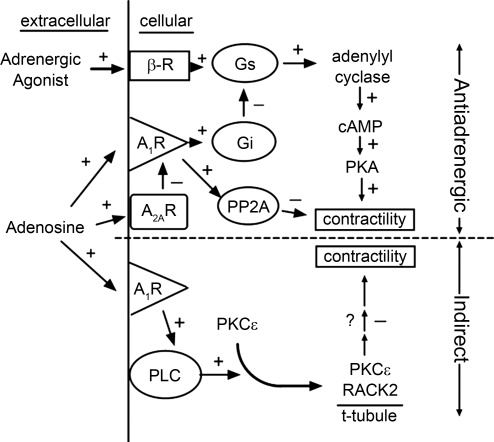
Suggested mechanisms by which adenosine can manifest an adenoprotective action in the heart are depicted in Fig. 6. As described above, β1-adrenergic receptor activation results ultimately in the phosphorylation of a number of proteins important to the modulation of heart contractility (28). In manifesting an anti-adrenergic action, adenosine acting via the adenosine A1R coupled to the Gi protein attenuates adrenergic signaling and contractile responses. A1R have also been found to enhance the cellular PP2A activity that would reduce the myocyte contractility by dephosphorylating the target proteins of PKA (29, 44) and other kinases that modulate cell function. Interestingly, the anti-adrenergic action of A1R is additionally modulated via the activity of adenosine A2A (33, 43). A recent report from our laboratory suggests a direct inhibitory action of A2AR on A1R function involving the modulation of G protein function (17). In addition to the anti-adrenergic action, a second component of adenoprotection, as described in this study, involves the activation of PKC-ɛ via A1R-induced PLC, followed by the translocation of the protein kinase to RACK2 anchoring protein at the cardiomyocyte Z-line.
Fig. 6.
Schematic depicting proposed mechanisms by which the nucleoside adenosine elicits an adenoprotective action in the myocardium. Adenosine affords adenoprotection against overstimulation by adrenergic agents via two mechanisms: 1) the direct attenuation of the adrenergic signaling pathway (anti-adrenergic action; top); and 2) the reduction of contractile responsiveness independently of this pathway (indirect; bottom). β-R, β1-adrenergic receptor; Gs and Gi, stimulatory and inhibitory G proteins, respectively; PP2A, protein phosphatase 2A; PLC, phospholipase C; + and − depict an activation and inhibition, respectively, in the subsequent event.
In conclusion, data are presented that delineates the involvement of PLC activation by adenosine in the manifestation of the nucleoside's adenoprotection of the myocardium. The signaling cascade includes the activation and translocation of PKC-ɛ to the RACK2 anchoring protein located in the region of Z-line and t-tubular system. In this fashion, adenosine induces adenoprotection of the myocardium independently of the well-known direct attenuation of the adrenergic signaling transduction pathway, which is the anti-adrenergic action.
GRANTS
This study was made possible by National Heart, Lung, and Blood Institute Grant HL-84160.
DISCLOSURES
The contents of this study are solely the responsibility of the authors and do not represent the official views of the National Institutes of Health.
REFERENCES
- 1.Albert CJ, Ford DA. Protein kinase C translocation and PKC-dependent protein phosphorylation during myocardial ischemia. Am J Physiol Heart Circ Physiol 276: H642–H650, 1999. [DOI] [PubMed] [Google Scholar]
- 2.Capogrossi MC, Kaku T, Filburn CR, Pelto DJ, Hansford RG, Spurgeon HA, Lakatta EG. Phorbol ester and dioctanoylglycerol stimulate membrane association of protein kinase C and have a negative inotropic effect mediated by changes in cytosolic Ca2+ in adult rat cardiac myocytes. Circ Res 66: 1143–1155, 1990. [DOI] [PubMed] [Google Scholar]
- 3.Clement O, Puceat M, Walsh MP, Vassort G. PKC enhances MLCK effects on force development and ATPase activity in rat single skinned cardiac cells. Biochem J 285: 311–317, 1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cohen MV, Downey JM. Adenosine: trigger and mediator of cardioprotection. Basic Res Cardiol 103: 203–215, 2008. [DOI] [PubMed] [Google Scholar]
- 5.Csukai M, Chen CH, DeMatteis MA, Mochly-Rosen D. The coatomer protein β′COP: a selective binding protein (RACK) for epsilon protein kinase C. J Biol Chem 272: 29200–29206, 1997. [DOI] [PubMed] [Google Scholar]
- 6.Disatnik MH, Buraggi G, Mochly-Rosen D. Localization of protein kinase C isozymes in cardiac myocytes. Exp Cell Res 210: 287–297, 1994. [DOI] [PubMed] [Google Scholar]
- 7.Dobson JG Jr. Reduction by adenosine of the isoproterenol-induced increase in cyclic adenosine 3′,5′-monophosphate formation and glycogen phosphorylase activity in rat heart muscle. Circ Res 43: 785–792, 1978. [DOI] [PubMed] [Google Scholar]
- 8.Dobson JG Jr. Mechanism of adenosine inhibition of catecholamine-induced elicited responses in heart. Circ Res 52: 151–160, 1983. [DOI] [PubMed] [Google Scholar]
- 9.Dobson JG Jr, Fenton RA. Cardiac physiology of adenosine. In: Cardiovascular Biology of Purines, edited by Burnstock G, Dobson JG, Jr, Liang BT, Linden J. Boston, MA: Kluwer, 1998, p. 21–39.
- 10.Dobson JG Jr, Shea LG, Fenton RA. Adenosine A2A and β-adrenergic calcium transient and contractile responses in rat ventricular myocytes. Am J Physiol Heart Circ Physiol 295: H2364–H2372, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dorn GW, Mochly-Rosen D. Intracellular transport mechanisms of signal transducers. Annu Rev Physiol 64: 407–429, 2002. [DOI] [PubMed] [Google Scholar]
- 12.Downey JM, Davis AM, Cohen MV. Signaling pathways in ischemic preconditioning. Heart Fail Rev 12: 181–188, 2007. [DOI] [PubMed] [Google Scholar]
- 13.Fenton RA, Dobson JG Jr. Adenosine and calcium alter adrenergic-induced intact heart protein phosphorylation. Am J Physiol Heart Circ Physiol 246: H559–H565, 1984. [DOI] [PubMed] [Google Scholar]
- 14.Fenton RA, Moore EDW, Fay FS, Dobson JG Jr. Adenosine reduces the Ca2+ transients of isoproterenol-stimulated rat ventricular myocytes. Am J Physiol Cell Physiol 261: C1107–C1114, 1991. [DOI] [PubMed] [Google Scholar]
- 15.Fenton RA, Galeckas KJ, Dobson JG Jr. Endogenous adenosine reduces depression of cardiac function induced by β-adrenergic stimulation during low flow perfusion. J Mol Cell Cardiol 27: 2373–2383, 1995. [DOI] [PubMed] [Google Scholar]
- 16.Fenton RA, Chung ES. Chronic ethanol enhances adenosine antiadrenergic actions in the isolated rat heart. Alcohol Clin Exp Res 25: 968–975, 2001. [PubMed] [Google Scholar]
- 17.Fenton RA, Dobson JG Jr. Adenosine A1 and A2A receptor effects on G-protein cycling in β-adrenergic stimulated ventricular membranes. J Cell Physiol 213: 785–792, 2007. [DOI] [PubMed] [Google Scholar]
- 18.Goldspink PH, Montgomery DE, Walker LA, Uroniene D, McKinney RD, Geenen DL, Solaro RJ, Buttrick PM. Protein kinase C epsilon overexpression alters myofilament properties and composition during the progression of heart failure. Circ Res 95: 424–432, 2004. [DOI] [PubMed] [Google Scholar]
- 19.Huang X, Walker JW. Myofilament anchoring of protein kinase C-epsilon in cardiac myocytes. J Cell Sci 117: 1971–1978, 2004. [DOI] [PubMed] [Google Scholar]
- 20.Jideama NM, Noland TA, Raynor RL, Blobe GC, Fabbro D, Kazanietz MG, Blumberg PM, Hannun YA, Kuo JF. Phosphorylation specificities of protein kinase C isozymes for bovine cardiac troponin I and troponin T and sites within these proteins and regulation of myofilament properties. J Biol Chem 271: 23277–23283, 1996. [DOI] [PubMed] [Google Scholar]
- 21.Johnson JA, Gray MO, Chen CH, Mochly-Rosen D. A protein kinase C translocation inhibitor as an isozyme-selective antagonist of cardiac function. J Biol Chem 271: 24962–24966, 1996. [DOI] [PubMed] [Google Scholar]
- 22.Kamp TJ, Hell JW. Regulation of cardiac L-type calcium channels by protein kinase A and protein kinase C. Circ Res 87: 1095–1102, 2000. [DOI] [PubMed] [Google Scholar]
- 23.Kohl C, Linck B, Schmitz W, Scholz H, Scholz J. Effects of carbachol and (-)-N6-phenylisopropyladenosine on myocardial inositol phosphate content and force contraction. Br J Pharmacol 101: 829–834, 1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.LaMonica DA, Frohloff N, Dobson JG Jr. Adenosine inhibition of catecholamine-stimulated cardiac membrane adenylate cyclase. Am J Physiol Heart Circ Physiol 248: H737–H744, 1985. [DOI] [PubMed] [Google Scholar]
- 25.Lester JW, Gannaway KF, Reardon RA, Koon LD, Hofmann PA. Effects of adenosine and protein kinase C stimulation on mechanical properties of rat cardiac myocytes. Am J Physiol Heart Circ Physiol 271: H1778–H1785, 1996. [DOI] [PubMed] [Google Scholar]
- 26.Lester JW, Hofmann PA. Role for PKC in the adenosine-induced decrease in shortening velocity of rat ventricular myocytes. Am J Physiol Heart Circ Physiol 279: H2685–H2693, 2000. [DOI] [PubMed] [Google Scholar]
- 27.Li Q, Lau A, Morris TJ, Guo L, Fordyce CB, Stanley EF. A syntaxin 1, Gαo, and N-type calcium channel complex at a presynaptic nerve terminal: analysis by quantitative immunocolocalization. J Neurosci 24: 4070–4081, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lindemann JP, Jones LR, Hathaway DR, Henry BG, Watanabe AM. β-Adrenergic stimulation of phospholamban phosphorylation and Ca2+-ATPase activity in guinea pig ventricles. J Biol Chem 258: 464–471, 1983. [PubMed] [Google Scholar]
- 29.Liu Q, Hofmann PA. Antiadrenergic effects of adenosine A1 receptor-mediated protein phosphatase 2a activation in the heart. Am J Physiol Heart Circ Physiol 283: H1314–H1321, 2002. [DOI] [PubMed] [Google Scholar]
- 30.Miyazaki K, Komatsu S, Ikebe M, Fenton RA, Dobson JG Jr. Protein kinase C epsilon and the antiadrenergic action of adenosine in rat ventricular myocytes. Am J Physiol Heart Circ Physiol 287: H1721–H1729, 2004. [DOI] [PubMed] [Google Scholar]
- 31.Mochly-Rosen D Localization of protein kinases by anchoring proteins: a theme in signal transduction. Science 268: 247–251, 1995. [DOI] [PubMed] [Google Scholar]
- 32.Newton AC Protein kinase C: structural and spatial regulation by phosphorylation, cofactors, and macromolecular interactions. Chem Rev 101: 2353–2364, 2001. [DOI] [PubMed] [Google Scholar]
- 33.Norton GR, Woodiwiss AJ, McGinn RJ, Lorbar M, Chung ES, Honeyman TW, Fenton RA, Dobson JG Jr, Meyer TE. Adenosine A1 receptor-mediated antiadrenergic effects are modulated by A2a receptor activation in rat heart. Am J Physiol Heart Circ Physiol 276: H341–H349, 1999. [DOI] [PubMed] [Google Scholar]
- 34.Parsons M, Young L, Lee JE, Jacobson KA, Liang BT. Distinct cardioprotective effects of adenosine mediated by differential coupling of receptor subtypes to phospholipase C and D. FASEB J 14: 1423–1431, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pass JM, Zheng Y, Wead WB, Zhang J, Li RCX, Bolli R, Ping P. PKC-ɛ activation induces dichotomous cardiac phenotypes and modulates PKC-ɛ-RACK interactions and RACK expression. Am J Physiol Heart Circ Physiol 280: H946–H955, 2001. [DOI] [PubMed] [Google Scholar]
- 36.Romano FD, MacDonald SG, Dobson JG Jr. Adenosine receptor coupling to adenylate cyclase of rat ventricular myocyte membranes. Am J Physiol Heart Circ Physiol 257: H1088–H1095, 1989. [DOI] [PubMed] [Google Scholar]
- 37.Romano FD, Dobson JG Jr. Adenosine modulates β-adrenergic signal transduction in guinea pig heart ventricular membranes. J Mol Cell Cardiol 22: 1359–1370, 1990. [DOI] [PubMed] [Google Scholar]
- 38.Rona G Catecholamine cardiotoxicity. J Mol Cell Cardiol 17: 291–306, 1985. [DOI] [PubMed] [Google Scholar]
- 39.Rybin VO, Steinberg SF. Protein kinase C isoform expression and regulation in the developing rat heart. Circ Res 74: 299–309, 1994. [DOI] [PubMed] [Google Scholar]
- 40.Schomig A, Haass M, Richardt G. Catecholamine release and arrhythmias in acute myocardial ischemia. Eur Heart J 124: F38–F47, 1991. [DOI] [PubMed] [Google Scholar]
- 41.Steinberg SF, Goldberg M, Rybin VO. The protein kinase C isoform diversity in the heart. J Mol Cell Cardiol 27: 141–153, 1995. [DOI] [PubMed] [Google Scholar]
- 42.Steinberg SF Structural basis of protein kinase C isoform function. Physiol Rev 88: 1341–1378, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tikh EI, Fenton RA, Dobson JG Jr. Contractile effects of adenosine A1 and A2A receptors in the isolated murine heart. Am J Physiol Heart Circ Physiol 290: H348–H356, 2006. [DOI] [PubMed] [Google Scholar]
- 44.Tikh EI, Fenton RA, Dobson JG Jr. Adenosine A1 and A2A receptor regulation of protein phosphatase 2A in the murine heart. J Cell Physiol 216: 83–90, 2008. [DOI] [PubMed] [Google Scholar]
- 45.Yuan S, Sunahara FA, Sen AK. Tumor-promoting phorbol esters inhibit cardiac functions and induce redistribution of protein kinase C in perfused beating rat heart. Circ Res 61: 372–378, 1987. [DOI] [PubMed] [Google Scholar]