Abstract
Vascular smooth muscle cell (SMC) migration is an important mechanism in atherogenesis and postangioplasty arterial remodeling. Previously, we demonstrated that the proinflammatory cytokine interleukin (IL)-18 is a potent inducer of SMC migration. Since extracellular matrix metalloproteinase inducer (EMMPRIN) stimulates ECM degradation and facilitates cell migration, we investigated whether IL-18 and EMMPRIN regulate each other's expression, whether their cross talk induces SMC migration, and whether the phytoalexin resveratrol inhibits IL-18-EMMPRIN signaling and SMC migration. Our studies demonstrate that 1) IL-18 induces EMMPRIN mRNA and protein expressions and stimulates EMMPRIN secretion from human aortic SMCs; 2) IL-18 stimulates EMMPRIN expression via oxidative stress and phosphatidylinositol 3-kinase (PI3K)-Akt-ERK signaling; 3) IL-18-stimulated SMC migration is significantly blunted by EMMPRIN knockdown, EMMPRIN function-blocking antibodies, or adenoviral transduction of mutant EMMPRIN; 4) conversely, EMMPRIN stimulates IL-18 expression and secretion via PI3K, Akt, and ERK; and 5) resveratrol attenuates IL-18- and EMMPRIN-mediated PI3K, Akt, and ERK activations; blunts IL-18-mediated oxidative stress; blocks IL-18-EMMPRIN cross-regulation; and inhibits SMC migration. Collectively, our results demonstrate that the coexpression and regulation of IL-18 and EMMPRIN in the vessel wall may amplify the inflammatory cascade and promote atherosclerosis and remodeling. Resveratrol, via its antioxidant and anti-inflammatory properties, has the potential to inhibit the progression of atherosclerosis by blocking IL-18 and EMMPRIN cross-regulation and SMC migration.
Keywords: atherogenesis, restenosis, proinflammatory cytokines, signal transduction, extracellular matrix metalloproteinase inducer
inflammation is a critical mechanism in the development and progression of atherosclerosis (28, 36). Proinflammatory cytokines induce chemokine and adhesion molecule expression, attract activated immune and inflammatory cells to the site of injury and inflammation, and compromise plaque stability by promoting the breakdown of the extracellular matrix via the induction of matrix-degrading metalloproteinases (MMPs) (28, 36).
Interleukin (IL)-18 is a proatherogenic and proinflammatory cytokine that amplifies the inflammatory cascade by inducing the expression of proinflammatory cytokines, chemokines, and adhesion molecules implicated in atherogenesis (14). IL-18 has been shown to localize to human atherosclerotic lesions (19, 30), and levels of circulating IL-18 are reported to predict future cardiovascular events (57). In fact, when compared with control subjects, patients with acute coronary syndromes were shown to have significantly higher levels of IL-18 and significantly lower levels of IL-18-binding protein (IL-18BP; a natural inhibitor of IL-18) in the serum (32), suggesting an unopposed enhancement of IL-18 bioactivity. A strong correlation between serum IL-18 levels and carotid intimal-medial thickness has also been demonstrated. More importantly, specific polymorphisms in IL-18 correlate with disease severity (47). A role for IL-18 has also been demonstrated in animal models of atherosclerosis. The administration of IL-18 aggravates atherosclerosis in mice (53). In contrast, atherogenesis is reduced in IL-18-deficient apolipoprotein E-null mice (16), suggesting a causal role for IL-18 in the development and progression of atherosclerosis. Atherosclerosis and restenosis following percutaneous intervention are characterized by smooth muscle cell (SMC) proliferation and migration (29). In fact, we have recently demonstrated that IL-18 treatment stimulates SMC migration in vitro (7).
SMC migration follows ECM degradation, a process regulated by various MMPs. Extracellular MMP inducer (EMMPRIN), also known as basigin or CD147, is a highly glycosylated 58-kDa transmembrane protein that has been shown to induce MMP expression, ECM degradation, and plaque instability (4). EMMPRIN expression was originally identified in a number of tumors, where it was shown to activate diverse signal transduction pathways in peritumoral cells by transcellular homophilic EMMPRIN-EMMPRIN interaction (44). This interaction results in the induction of MMP-1, MMP-2, MMP-3, MMP-9, and membrane type 1 and type 2 MMPs (MT1-MMP and MT2-MMP) in stromal cells, proteolysis of matrix components, and tumor cell metastasis. EMMPRIN also regulates MMP expression during development and tissue remodeling under physiological conditions, as emphasized in EMMPRIN null mice, where diverse developmental defects, including small size, infertility, and blindness, are observed (23, 25).
EMMPRIN expression is also increased during injury and inflammation and is upregulated during atherosclerosis. Whereas normal vessels have low to undetectable levels of EMMPRIN, human coronary artery atherectomy specimens show increased EMMPRIN expression, localized predominantly to CD68-positive macrophage-rich atherosclerotic intima, and α-SMC-positive SMCs (37, 58). Because SMC migration and proliferation play critical roles in the initiation and progression of atheroma development and arteriosclerosis and since EMMPRIN stimulates ECM degradation and facilitates cell migration, we hypothesized that IL-18 induces SMC migration via EMMPRIN.
Resveratrol (trans-3,4′,5-trihydroxystilbene), a naturally occurring phytoalexin found largely in the skins of red grapes and other fruits, has been shown to exert both vascular and cardioprotective effects and is a potent antioxidant, anti-inflammatory, and antiproliferative agent (13). As an antioxidant, the stilbene derivative enhances intracellular glutathione levels, suppresses reactive oxygen species (ROS) generation, and protects low-density lipoproteins from oxidation (3, 27, 42). As an anti-inflammatory agent, resveratrol prevents platelet aggregation, blocks cyclooxygenase-2 activation and cyclooxygenase-2-dependent PGE2 synthesis, and inhibits the expression of proinflammatory cytokines such as IL-6 and IL-8 (12, 15, 52, 62). By suppressing adhesion molecule expression, it inhibits the infiltration of activated inflammatory and immune cells. As an anti-proliferative agent, resveratrol inhibits SMC proliferation (1). Since both IL-18 and EMMPRIN are expressed in atherosclerotic lesions and play critical roles in lesion development and progression (19, 30, 37, 58), we investigated whether resveratrol can block IL-18-EMMPRIN cross talk and inhibit SMC migration. Our results demonstrate that IL-18 and EMMPRIN regulate each other's expression in SMCs via PI3K, Akt, and ERK-dependent signaling; IL-18 mediates SMC migration in part via EMMPRIN; and resveratrol can block IL-18/EMMPRIN cross-regulation, and SMC migration.
MATERIALS AND METHODS
Materials.
Recombinant human (rh)IL-18, IL-18-neutralizing antibodies (α-IL-18Ab, D044-3; 10 μg/ml for 1 h), normal mouse IgG1 (MAB002), IL-18 antibodies used in immunoblotting (D043–3), IL-18BPa:fragment crystallizable region (Fc) chimera (119-BP-100; 10 μg/ml for 1 h), and Fc and rhEMMPRIN (No. 972-EMN-050) were purchased from R&D Systems (Minneapolis, MN). The efficacy of IL-18BPa:Fc for blocking IL-18-mediated gene regulation has been previously demonstrated both in vivo and in vitro (9, 40). Mouse anti-human EMMPRIN function-blocking antibodies (UM-8D6, No. 373-020) were from Ancell (Bayport, MN). The efficacy of these antibodies has been previously demonstrated (21, 37). Rabbit anti-EMMPRIN polyclonal antibodies used in immunoblotting were from Zymed (SKU No. 34-5600; South San Francisco, CA). Antibodies against Akt (No. 9272), phospho-Akt (Ser473, No. 9271), ERK1/2 (No. 9102), and phospho-ERK1/2 (No. 9101S) were obtained from Cell Signaling Technology (Beverly, MA). Anti-phosphatidylinositol 3-kinase (PI3K)p85 and actin antibodies were from Santa Cruz Biotechnology. The Akt inhibitor d-3-deoxy-2-O-methyl-myo-inositol 1-[(R)-2-methoxy-3-(octadecyloxy)propyl hydrogen phosphate] (SH-5) (Akt inhibitor II, No. 124008; 1 μM in DMSO for 1 h), the ERK1/2 inhibitor PD-98059 (10 μM in DMSO for 1 h), resveratrol (No. 554325), and DMSO were purchased from EMD Biosciences (San Diego, CA). At the indicated concentrations, SH-5 or PD-98059 did not affect cell viability (data not shown). 2′,7′-Dichlorofluorescein (DCF) diacetate (DCFH-DA) was obtained from Molecular Probes (Eugene, OR). Diphenyleneiodonium chloride (DPI) was purchased from Alexis Biochemicals (San Diego, CA). α-Tubulin polyclonal antibodies and all other chemicals were purchased from Sigma-Aldrich (St. Louis, MO).
Cell culture.
Human aortic SMCs (No. CRL-1999) were purchased from ATCC and grown as previously described in Ham's-F12 medium supplemented with 10% (vol/vol) FBS and endothelial cell growth supplement (0.03 mg/ml) (33). At 70–80% confluency, the complete medium was replaced with Ham's-F12/0.5% BSA. After 24 h, SMCs were incubated with IL-18 (10 ng/ml) or EMMPRIN for the indicated time periods. At the end of the experimental period, the culture supernatants were collected into slick tubes and snap frozen. Cells were harvested, snap frozen, and stored at −80°C. In studies involving resveratrol, SMCs were treated with resveratrol at the indicated concentrations for 1 h before the addition of IL-18 or EMMPRIN.
Adenoviral transduction and small interfering RNA-mediated knockdown.
Adenoviral vectors for dominant negative (dn)PI3Kp85 (Ad.dnPI3K), dnAkt (Ad.dnAkt), dnJNK1 (Ad.dnJNK), green fluorescent protein (Ad.GFP), mutant EMMPRIN (Ad.mEMMPRIN), and empty vector have been previously described (35, 41, 56). SMCs were grown to 60–70% confluency in complete medium. The medium was replaced with PBS, and the cells were infected at 100 multiplicity of infection for 1 h at 22°C. The infection medium was replaced with medium containing 0.5% BSA. After 24 h, the cells were treated with rhIL-18. The transfection efficiency with adenoviral vectors was nearly 100%, as evidenced by the expression of GFP in SMCs infected with Ad.GFP (data not shown). An infection with the adenoviral vectors had no affect on SMC viability or their adherence to the culture dishes.
Detection of intracellular ROS.
Intracellular ROS levels were determined as previously described (11, 51) using the cell-permeable, redox-sensitive fluorophore DCFH-DA. Following entry into the cells, it is converted to DCF by intracellular esterases and then to highly fluorescent DCF by ROS. SMCs were plated into clear-bottom, black-walled 96-well plates (Corning, Corning, NY) at 1,500 cells/well. SMCs were loaded for 20 min with 10 μM DCFH-DA in buffer containing (in mM) 137 NaCl, 1.2 MgSO4, 4.9 KCl, 1.2 NaH2PO4, 20 HEPES, 15 glucose, and 1.8 CaCl2 (pH 7.4); washed; treated with IL-18 (10 ng/ml); and read in a microplate fluorometer/luminometer with 485/20 excitation and 528/20 emission filters (FLx800i, Bio-Tek Instruments, Winooski, VT). DCF fluorescence was monitored for 15 min, stored on a microcomputer, and analyzed using KC4 software (Bio-Tek). The plates were then frozen at −80°C for 2 h and thawed and stained with the nucleic acid-sensitive CyQuant GR dye according to the manufacturer's protocol (Molecular Probes). This allowed the normalization of ROS generation rates to DNA content. The DCF fluorescence was normalized to CyQuant fluorescence, the ratio obtained in untreated samples was considered as 1, and the data were presented as fold increase from untreated samples.
Phosphatidylinositol 3-kinase.
PI3K activity was determined essentially as described previously (26), using a commercially available PI3K ELISA kit (Echelon Biosciences, Salt Lake City, UT). Quiescent SMCs were transduced with Ad.dnPI3K before the addition of IL-18 (10 ng/ml for 1 h), washed in ice-cold PBS, and lysed in 500 μl ice-cold lysis buffer containing 137 mM NaCl, 20 mM Tris·HCl (pH 7.4), 1 mM CaCl2, 1 mM MgCl2, 0.1 mM sodium orthovanadate, 1% Nonidet P-40, and 1 mM PMSF. PI3K was immunoprecipitated with 5 μl of anti-p85 antibody and 60 μl of protein A-Sepharose beads (Amersham Pharmacia). PI3K activity in the immunoprecipitates was assayed by PI3K ELISA according to the manufacturer's instructions. Briefly, immunoprecipitated enzyme and phosphatidylinositol 4,5-bisphosphate substrate were incubated for 1 h at room temperature in the reaction buffer. Kinase reactions were stopped by pelleting the beads by centrifugation and transferring the reaction mixture to the incubation plate and incubated overnight at 4°C with a phosphatidylinositol 3,4,5-trisphosphate [PI(3,4,5)P3] detector protein and then added to the PI(3,4,5)P3-coated microplate for 1 h for competitive binding. A peroxidase-linked secondary detection reagent and colorimetric detection (absorbance was measured at 450 nm) is used to detect PI(3,4,5)P3 detector protein binding to the plate. The colorimetric signal is inversely proportional to the amount PI(3,4,5)P3 produced by PI3K. The expression levels of the PI3K component p85α were detected by immunoblotting using pelleted beads.
Akt/protein kinase B.
Total and phospho-Akt levels in whole cell homogenates were analyzed by immunoblotting. The immunoreactive bands were detected by enhanced chemiluminescence (ECL Plus; GE Healthcare) and quantified by densitometry. Akt kinase activity was analyzed using a commercially available colorimetric assay kit (Cell Signaling Technology). The assay is based on Akt-induced phosphorylation of glycogen synthase kinase-3 (35).
Extracellular signal-regulated kinase.
ERK and phospho-ERK levels in whole cell homogenates were analyzed by immunoblotting. ERK enzyme activity was analyzed in whole cell homogenates using an immunecomplex kinase assay (p44/42 MAP Kinase Assay Kit; Cell Signaling Technology) (50). In brief, SMCs were treated with IL-18 for 1 h and then harvested and lysed in 1× lysis buffer provided by the manufacturer. The protein content in the lysates was determined by the Bradford method, and 200 μg of cleared cell lysate were incubated with 15 μl of immobilized phospho-p44/42 MAPK (Thr202/Tyr204) monoclonal antibody with gentle rocking at 4°C for 12 h. Immunecomplexes were collected by centrifugation at 8,000 g for 30 s and washed once with lysis buffer and twice with the kinase buffer containing (in mM) 25 Tris (pH 7.5), 5 glycerophosphate, 2 dithiothreitol, 0.1 Na3VO4, and 10 MgCl2. The complex was then incubated with 50 μl of kinase buffer containing 200 μM ATP and 2 μg of E-26-like protein-1 (Elk-1) fusion protein at 30°C for 30 min. The reaction was terminated by adding 12.5 μl of 5× SDS sample buffer. Samples were separated by 10% SDS-PAGE, transferred to PVDF membrane, and probed overnight with phosphospecific anti-Elk-1 (Ser383) antibodies diluted in 2% nonfat dry milk in Tris-buffered saline containing 0.05% Tween 20, followed by horseradish peroxidase-conjugated secondary antibodies for 1 h. Blots were developed in chemiluminescent substrate (SuperSignal Pico West; Pierce), supplemented with 5% SuperSignal Femto (Pierce), and exposed to film. Actin or α-tubulin served as a loading control.
IL-18 and EMMPRIN expression.
IL-18 and EMMPRIN mRNA expression was analyzed by reverse transcription followed by real-time quantitative PCR (RT-qPCR) as previously described (50). In brief, DNA-free total RNA was prepared using the RNAqueous-4PCR kit (Applied Biosystems/Ambion, Austin, TX). RNA quality was assessed by capillary electrophoresis using the Agilent 2100 Bioanalyzer (Agilent Technologies, Palo Alto, CA). All RNA samples used for qPCR had RNA integrity numbers >9 (scale = 1–10), as assigned by default parameters of the Expert 2100 Bioanalyzer software package (v. 2.02). IL-18 and EMMPRIN mRNA expressions were analyzed by RT-qPCR using SYBR Green as the detection fluorophore and the following primers: IL-18: sense, 5′-TTCGGGAAGAGGAAAGGAAC-3′, and antisense: 5′-AAGGATACAAAAAGTGACAT-3′; and EMMPRIN: sense, 5′-TTCAGCCTCTGGGTCTGAGT-3′, and antisense, 5′-GCCAAGAGGTCAGAGTCGTC-3′. Actin mRNA, which served as the internal reference control, was amplified using the following primers: sense, 5′-TCCTTCCTGGGCATGGAG-3′, and antisense 5′-AGGAGGAGCAATGATCTTGATCTT-3′. Samples analyzed without the RT step served as negative controls and gave no signal. Each sample was tested in triplicate. The results are expressed as a ratio of a specific gene to that of a corresponding actin mRNA expression. The expression of IL-18 or EMMPRIN in untreated cells was taken as 1, and their expression levels following treatment were presented as the fold induction from untreated samples.
IL-18 and EMMPRIN mRNA abundance was confirmed by Northern blot analysis, using cDNAs amplified from total RNA isolated from SMCs and the following primer pairs: IL-18: sense, 5′-GCTTCCTCTCGCAACAAAC-3′, and antisense, 5′-CACTTCACAGAGATAGTTACAGCC-3′; and EMMPRIN: sense, 5′-GTTCGTGCTGCTGGGATTCGCGCTG-3′, and antisense, 5′-CAGCGCGAATCCCAGCAGCACGAAC-3′. Actin, sense, 5′-ATCTGGCACCACACCTTCTACAATGAGCTGCG-3′, and antisense, 5′-CGTCATACTCCTGCTTGCTGATCCACATCTGC-3′ served as an internal control. Twenty-five micrograms of total RNA per lane were denatured, separated by agarose electrophoresis, transferred onto nitrocellulose membrane, UV cross-linked, and probed with 32P-labeled cDNA probes. The autoradiographic signals were semiquantified by videoimage analysis.
IL-18 protein levels in culture supernatants were quantified by ELISA (human IL-18 ELISA, No. 7620). The sensitivity of the assay is 12.5 pg/ml. EMMPRIN protein levels were analyzed by immunoblotting. The soluble EMMPRIN levels in culture supernatants were quantified by an ELISA and have been previously described (39). In brief, 96-well plates (Immulon No. 2; Dynatech, Chantilly, VA) were coated overnight at 22°C with 1 μg/ml goat anti-human EMMPRIN (R&D Systems). After being washed in PBS + 0.05% Tween-20 (wash buffer), the plate was blocked by PBS containing (in %) 1 BSA, 5 sucrose, and 0.05 NaN3 (at 22°C for 1 h). rhEMMPRIN was used to generate a standard curve. All assays were performed in duplicate, and the mean values were used.
Cell migration.
SMC migration was quantified as previously described (7) using BD BioCoat Matrigel invasion chambers (BD Biosciences Discovery Labware, No. 354481) and 8.0-μm pore polyethylene terephthalate membranes with a thin layer of Matrigel basement membrane matrix. Cultured SMCs were trypsinized and suspended in conditioned medium, and 1 ml containing 2.0 × 105 cells/ml was layered on the coated insert filters. The cells were stimulated with IL-18 (10 ng/ml). The medium in the lower chamber also contained IL-18 at identical concentrations. After incubation at 37°C for 12 h, the membranes were removed and washed with PBS, and the noninvading cells on the upper surface were removed with a cotton swab. The cells migrating to the lower surface of the membrane were quantified using 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenoltetrazolium bromide assay. To determine the role of EMMPRIN in IL-18-mediated SMC migration, EMMPRIN was targeted by RNA interference [human EMMPRIN small interfering (si)RNA: sense, 5′-GUACAAGAUCACUGACUCUUU-3′ and antisense, 5′-AGAGUCAGUGAUCUUGUACUU-3′]. SMCs were treated with EMMPRIN siRNA for 48 h before the addition of IL-18. The knockdown of EMMPRIN was confirmed by RT-PCR. The expression of actin served as a loading control. Neither the control siRNA nor the siRNA against GFP affected the expression of EMMPRIN or actin, demonstrating the specificity of the siRNA used and ruling out the off-target effects. EMMPRIN was also targeted using function-blocking antibodies. Normal IgG served as a control. In addition, Ad.mEMMPRIN was also used to target EMMPRIN signaling. An infection with empty virus served as control. To demonstrate that resveratrol blocks IL-18-mediated SMC migration, SMCs were treated with resveratrol (25 μM in DMSO for 1 h) before the addition of IL-18 or EMMPRIN. DMSO served as a vehicle control.
Statistical analysis.
Comparisons between controls and various treatments were performed by analysis of variance with post hoc Dunnett's t-tests. All assays were performed at least three times, and the error bars in the figures indicate means ± SE.
RESULTS
IL-18 stimulated SMC migration is EMMPRIN dependent.
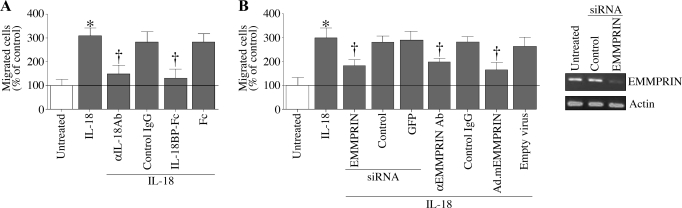
We and others (7, 14, 16, 19, 30, 32, 47, 53, 57) have previously demonstrated that the proinflammatory cytokine IL-18 plays a critical role in atherosclerosis in vivo and stimulates SMC migration in vitro. We have also demonstrated that IL-18-induced SMC migration is mediated in part via MMP-9 induction (7). EMMPRIN is a multifunctional glycosylated transmembrane protein that has been shown to induce MMP enzymes, including MMP-9 (4). Therefore, we investigated whether IL-18 stimulates SMC migration in an EMMPRIN-dependent manner. Confirming our earlier results (7), treatment with IL-18 stimulated SMC migration (Fig. 1A), an effect that was significantly inhibited by preincubating cells with IL-18-neutralizing antibodies or IL-18BP-Fc chimera. Normal IgG and the Fc, used as respective controls, did not inhibit IL-18-mediated SMC migration (Fig. 1A). We next examined the role of EMMPRIN in IL-18-induced SMC migration. EMMPRIN expression was targeted by three different approaches: function-blocking antibodies, siRNA-mediated knockdown, and transduction of an adenoviral vector expressing an inhibitory mutant of EMMPRIN (Ad.mEMMPRIN). Our results demonstrate that pretreatment with the function-blocking antibodies, siRNA-mediated knockdown (knockdown of EMMPRIN was confirmed by RT-PCR; Fig. 1B, right), and expression of mutant EMMPRIN all significantly inhibited IL-18-mediated SMC migration (Fig. 1B). Normal IgG, an irrelevant siRNA, GFP-specific siRNA, and adenoviral transduction of empty vector, all serving as respective controls, had no effect. Together, these results indicate that IL-18 is a potent inducer of SMC migration and mediates SMC migration in part via EMMPRIN (Fig. 1).
Fig. 1.
IL-18 stimulates smooth muscle cell (SMC) migration via extracellular matrix metalloproteinase inducer (EMMPRIN). A: IL-18 stimulates SMC migration. Cultured SMCs were trypsinized, suspended in Ham's F12 medium and 0.5% bovine serum albumin, and layered on Matrigel basement membrane matrix-coated filters. Cells were stimulated with IL-18 (10 ng/ml) in both upper and lower chambers. Plates were incubated at 37°C for 12 h to allow cell migration. Cells migrating to the other side of the membrane were quantified using 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenoltetrazolium bromide (MTT) assay. Specificity of IL-18 was verified by incubating the cells with IL-18-neutralizing antibodies or IL-18-binding protein (BP)-fragment crystallizable region (Fc) (10 μg/ml) for 1 h before IL-18 addition. Normal mouse IgG and Fc served as respective controls. *P < 0.001 vs. untreated; †P < 0.01 vs. IL-18 (n = 12 experiments). B: IL-18 stimulates SMC migration via EMMPRIN. SMCs were treated with anti-EMMPRIN function-blocking antibody (10 μg/ml for 1 h), EMMPRIN-specific small interfering (si)RNA (100 nM for 48 h), or transduced with adenoviral vector expressing an inhibitory mutant of EMMPRIN [Ad.mEMMPRIN; Δ208–269; 100 multiplicity of infection (MOI) for 48 h] before IL-18 addition (10 ng/ml for 24 h). Normal IgG, an irrelevant siRNA and green fluorescent protein (GFP)-specific siRNA and adenoviral transduction of empty vector served as respective controls. Knockdown of EMMPRIN was confirmed by RT-PCR (B, right). Actin served as a loading control. *P < 0.001 vs. untreated; †P < at least 0.05 vs. IL-18 + respective controls (n = 12 experiments).
IL-18 induces EMMPRIN expression in SMCs.
Having demonstrated a requirement for EMMPRIN in IL-18-stimulated SMC migration, we next investigated whether IL-18 can induce EMMPRIN expression in these cells. Quiescent SMCs were treated with rhIL-18 (10 ng/ml for 24 h), and EMMPRIN expression was quantified by RT-qPCR, Northern blot analysis, and immunoblotting. IL-18 stimulated EMMPRIN expression in SMCs in a time-dependent manner, with a maximal induction observed at 24 h (Fig. 2A). Even at 72 h, EMMPRIN expression remained high. The specificity of the response for IL-18 was investigated next. SMCs were incubated with IL-18-neutralizing antibodies or IL-18BP-Fc chimera before the addition of IL-18. EMMPRIN mRNA expression was analyzed at 24 h. The results in Fig. 2B show that the pretreatment with neutralizing antibodies and IL-18BP-Fc significantly attenuated IL-18-mediated EMMPRIN induction. In support of the RT-qPCR data, Northern blot analysis demonstrated IL-18-mediated EMMPRIN mRNA expression at 24 h in SMCs, an effect that was significantly attenuated by IL-18-neutralizing antibodies and IL-18BP-Fc chimera (Fig. 2C). Normal IgG and Fc served as respective controls and failed to modulate IL-18-mediated EMMPRIN induction. Furthermore, our results also demonstrate that IL-18 stimulates EMMPRIN protein expression (Fig. 2D) and secretion (Fig. 2E), indicating that IL-18 is a potent inducer of EMMPRIN expression in SMCs (Fig. 2).
Fig. 2.
IL-18 induces EMMPRIN expression. A: IL-18 induces EMMPRIN mRNA expression in a time-dependent manner. Quiescent SMCs were treated with recombinant human IL-18 (10 ng/ml for up to 72 h). At the indicated time periods, DNA-free total RNA was extracted and analyzed for EMMPRIN mRNA expression by RT-qPCR (n = 6 experiments). *P < 0.05 and **P < 0.001 vs. untreated control at 24 h. C, control. B: IL-18 induces EMMPRIN expression. Specificity of IL-18 was verified by preincubating cells with IL-18-neutralizing antibodies (10 μg/ml for 1 h) or IL-18BP:Fc chimera (10 μg/ml for 1 h) before IL-18 addition (10 ng/ml for 24 h). Normal mouse IgG and Fc served as respective controls. EMMPRIN mRNA expression was analyzed as in A (n = 6 experiments). *P < 0.001 vs. untreated; †P < 0.01 vs. IL-18 + control IgG or Fc. C: IL-18-mediated EMMPRIN mRNA expression was confirmed by Northern blot analysis. Total RNA obtained in B was analyzed for EMMPRIN mRNA expression by Northern blot analysis. Actin served as a loading control. A representative of 3 independent experiments is shown. D: IL-18 induces EMMPRIN protein expression. Quiescent SMCs treated as in A were analyzed for EMMPRIN protein levels in cleared cell lysates. α-Tubulin served as a loading control (n = 3 experiments). E: IL-18 stimulates EMMPRIN secretion. Culture supernatants from B were analyzed for soluble EMMPRIN by an ELISA (n = 6 experiments). *P < 0.01 vs. untreated; †P < 0.01 vs. IL-18 + control IgG or Fc.
IL-18 stimulates PI3K-dependent Akt activation in SMCs.
The PI3K family of lipid kinases regulates various cellular processes, including cell survival, growth, and migration, via the activation of diverse second messenger molecules, including Akt (5). We have previously demonstrated that IL-18 stimulates fibronectin in primary cardiac fibroblasts via PI3K/Akt signaling (35). Therefore, in the present study, we investigated whether IL-18 similarly stimulates EMMPRIN expression via PI3K and Akt. The results in Fig. 3A show that IL-18 induces a significant activation of PI3K in SMCs (Fig. 3A; ∼2.1-fold, P < 0.001 vs. untreated). Furthermore, IL-18 induced Akt phosphorylation (Fig. 3B) and stimulated its kinase activity (Fig. 3C), effects that were significantly attenuated by the adenoviral transduction of dnAkt (Fig. 3D) and by SH-5, a pharmacological Akt inhibitor (Fig. 3E), but not by the respective controls, Ad.GFP, or DMSO. Moreover, the adenoviral transduction of dnPI3Kp85 inhibited IL-18-mediated Akt phosphorylation (Fig. 3F), indicating that IL-18 is a potent inducer of PI3K and Akt activities in SMCs, and stimulates Akt activation in a PI3K-dependent manner (Fig. 3).
Fig. 3.
IL-18 stimulates phosphatidylinositol 3-kinase (PI3K)-dependent Akt activation. A: IL-18 induces PI3K activation. Quiescent SMCs were incubated with IL-18 (10 ng/ml) for 30 min. p85α-associated PI3K activities were analyzed by ELISA as described in materials and methods. *P < 0.01 vs. untreated (n = 6 experiments). PI3K ELISA was performed as described under materials and methods. A, top: immunoblot analysis of the same samples with anti-p85 antibody. B: IL-18 activates Akt. Quiescent SMCs were incubated with IL-18 for up to 2 h. Cleared cell lysates were immunoblotted with phospho-(p)Akt or Akt antibodies. A representative of 3 independent experiments is shown. C: IL-18 stimulates Akt kinase activity. Quiescent SMCs treated as in B, but for up to 1 h, were analyzed for Akt kinase activity using immunecomplex kinase assays. GSK3 served as a substrate (n = 3 experiments). D and E: adenoviral transduction of dominant negative (dn)Akt (D) or treatment with Akt inhibitor d-3-deoxy-2-O-methyl-myo-inositol 1-[(R)-2-methoxy-3-(octadecyloxy)propyl hydrogen phosphate] (SH-5; E) blocks IL-18 induced Akt activation. SMCs were transduced with Ad.dnAkt (100 MOI for 24 h; D) or treated with SH-5 (1 μM in DMSO for 1 h; E) before IL-18 addition (10 ng/ml for 1 h) and then processed for Akt activation as in B (n = 3 experiments). F: inhibition of PI3K blocks IL-18-mediated Akt activation. SMCs were transduced with Ad.dnPI3Kp85 (100 MOI for 24 h) before IL-18 addition (10 ng/ml for 1 h) and then processed for Akt activation as in B (n = 3 experiments).
IL-18 stimulates ERK activation via PI3K and Akt.
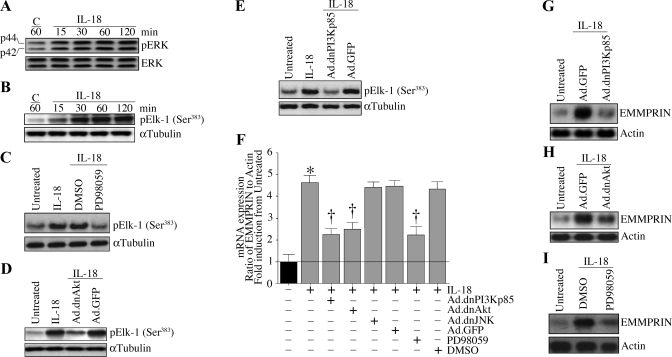
IL-18 is known to activate various stress-regulated kinases, including ERK. Therefore, we investigated whether IL-18 stimulates EMMPRIN expression via ERK. IL-18 stimulated ERK phosphorylation in a time-dependent manner (Fig. 4A). Whereas low levels of phospho-ERK were detectable under basal conditions, the treatment with IL-18 increased ERK phosphorylation at 15 min and reached peak levels around 30 min. However, the total ERK levels remained steady throughout the 2-h study period. Furthermore, IL-18 stimulated ERK activity (Fig. 4B), an effect that was significantly attenuated by the pretreatment with the pharmacological inhibitor PD-98059 (Fig. 4C). Moreover, the adenoviral transduction of dnAkt (Fig. 4D) and dnPI3Kp85 (Fig. 4E) significantly attenuated the IL-18-stimulated ERK activity. Importantly, RT-qPCR revealed that the inhibition of PI3K, Akt, and ERK blunted IL-18-mediated EMMPRIN mRNA expression (Fig. 4F). These results were confirmed by Northern blot analysis (Fig. 4, G–I). In contrast, the adenoviral transduction of dnJNK failed to modulate IL-18-mediated EMMPRIN expression (Fig. 4F), and the inhibition of PI3K, Akt, and ERK failed to induce cell death (data not shown). Together, these results indicate that IL-18 induces EMMPRIN expression in SMCs via PI3K, Akt, and ERK-dependent signaling (Fig. 4).
Fig. 4.
IL-18 stimulates EMMPRIN expression via PI3K-Akt-ERK signaling. A: IL-18 induces ERK activation. Quiescent SMCs were incubated with IL-18 (10 ng/ml) for up to 2 h. Cleared cell lysates were immunoblotted with pERK or ERK antibodies (n = 3 experiments). B: IL-18 stimulates ERK activity. Quiescent SMC treated as in A were analyzed for ERK activity using immunecomplex kinase assays. E-26-like protein (Elk) served as a substrate (n = 3 experiments). α-Tubulin served as a control. C: PD-98059 blocks IL-18-mediated ERK activity. Quiescent SMCs treated with PD-98059 (10 μM in DMSO for 1 h) before IL-18 addition were analyzed for ERK activity as described in B (n = 3 experiments). D: adenoviral transduction of dnAkt inhibits IL-18-mediated ERK activity. SMCs transduced with Ad.dnAkt (100 MOI for 24 h) and then treated with IL-18 (10 ng/ml for 1 h) were analyzed for ERK activity as described in B (n = 3 experiments). E: adenoviral transduction of dnPI3Kp85 inhibits IL-18-mediated ERK activity. SMCs transduced with Ad.dnPI3Kp85 (100 MOI for 24 h) and then treated with IL-18 (10 ng/ml for 1 h) were analyzed for ERK activity as described in B (n = 3 experiments). F: IL-18 induces EMMPRIN mRNA expression via PI3K, Akt, and ERK, but not via JNK. SMCs were transduced with Ad.dnPI3K, dnAkt, or dnJNK or treated with PD-98059 before IL-18 addition (10 ng/ml for 24 h). EMMPRIN mRNA expression was analyzed by RT-quantitative (q)PCR (F; n = 6 experiments) or Northern blot analysis (G, H, and I; n = 3 experiments). *P < 0.001 vs. untreated; †P < at least 0.05 vs. IL-18.
Resveratrol blocks IL-18-mediated PI3K-Akt-ERK-dependent EMMPRIN expression and SMC migration.
Resveratrol, a polyphenolic compound from grapes and red wine, exerts potent anti-inflammatory and anti-atherogenic effects (1, 3, 12, 13, 15, 27, 42, 52, 62). Therefore, we investigated whether resveratrol blocks IL-18 signaling, IL-18-mediated EMMPRIN induction, and EMMPRIN-dependent SMC migration. The results in Fig. 5A show that treatment with resveratrol blocks IL-18-mediated PI3K activity. The solvent control DMSO, on the other hand, had no effect on either basal or IL-18-mediated PI3K activation (Fig. 5A). Similarly, treatment with resveratrol attenuated IL-18-mediated Akt kinase activity (Fig. 5B), ERK activity (Fig. 5C), EMMPRIN mRNA expression (Fig. 5D, RT-qPCR; Fig. 5E, Northern blot analysis), and SMC migration (Fig. 5F). Together, these results demonstrate that resveratrol blocks IL-18 signaling, EMMPRIN induction, and SMC migration (Fig. 5).
Fig. 5.
Resveratrol blocks IL-18-mediated PI3K-Akt-ERK-dependent EMMPRIN expression. A: resveratrol blocks IL-18-mediated PI3K activation. Quiescent SMCs were incubated with resveratrol (25 μM in DMSO for 1 h) before IL-18 addition (10 ng/ml for 30 min). PI3K activations were analyzed by ELISA. PI3K ELISA was performed as described under materials and methods. A, top: immunoblot analysis of the same samples with anti-p85 antibody. *P < 0.01 vs. untreated; †P < 0.05 vs. IL-18 (n = 6 experiments). B: resveratrol blocks IL-18-mediated Akt kinase activity. Quiescent SMCs were incubated with resveratrol (25 μM in DMSO for 1 h) before IL-18 addition (10 ng/ml for 1 h). Akt kinase activity was analyzed by immune complex kinase assays. A representative of 3 independent experiments is shown. C: resveratrol blocks IL-18-mediated ERK activity. Quiescent SMCs were incubated with resveratrol (25 μM in DMSO for 1 h) before IL-18 addition. After 1 h, ERK activity was analyzed by immune complex kinase assays. A representative of 3 independent experiments is shown. D and E: resveratrol blocks IL-18-mediated EMMPRIN mRNA expression. Quiescent SMCs were incubated with resveratrol (25 μM in DMSO for 1 h) before IL-18 addition. EMMPRIN mRNA expression was analyzed by RT-qPCR (D; n = 6 experiments), and confirmed by Northern blot analysis (E; n = 3 experiments). *P < 0.001 vs. untreated; †P < 0.01 vs. IL-18. F: resveratrol attenuates IL-18-mediated SMC migration. Cultured SMCs were layered on Matrigel basement membrane matrix-coated filters as described in materials and methods. Cells were treated with resveratrol (25 μM in DMSO for 1 h) before IL-18 addition (10 ng/ml). IL-18 was added to the lower chamber at the same concentration. Plates were incubated at 37°C for 12 h to allow cell migration. Cells migrating to the other side of the membrane were quantified using an MTT assay. *P < 0.001 vs. untreated; †P < 0.05 vs. IL-18 (n = 6 experiments).
Resveratrol blunts IL-18-mediated ROS generation.
Since IL-18 enhances oxidative stress (55) and since resveratrol exerts potent antioxidant effects (13, 27), we investigated whether resveratrol blunts IL-18-mediated ROS generation and determined whether IL-18-mediated EMMPRIN expression is redox sensitive. The generation of ROS was analyzed by the conversion of DCFH-DA to highly fluorescent DCF. The results in Fig. 6A show that indeed the treatment with IL-18 induced a rapid increase in ROS generation, as evidenced by a significant increase in DCF fluorescence at 1 min. The fluorescence intensity increased further and appeared to reach a plateau at 5 min (Fig. 6A). Confirming its antioxidant properties, the treatment with resveratrol blunted IL-18-mediated ROS generation. The NADPH oxidase inhibitor DPI (10 μM) also inhibited IL-18-mediated ROS generation (Fig. 6B) and partially attenuated IL-18-mediated EMMPRIN expression (Fig. 6C). Together, these results indicate that IL-18 induces EMMPRIN expression in part via a ROS-sensitive mechanism that can be inhibited by both resveratrol and DPI (Fig. 6).
Fig. 6.
IL-18 induces EMMPRIN expression in part via reactive oxygen species (ROS) generation. A: IL-18 stimulates ROS generation in SMCs. SMCs were loaded with the fluorophore 2′,7′-dichlorofluorescein (DCF) diacetate (DCFH-DA), then stimulated with IL-18 as indicated, and monitored for up to 15 min in a microplate. DCF fluorescence was normalized to DNA content and represented as fold increase from untreated. *P < 0.05 and **P < 0.001 vs. control at 5 min (n = 12 experiments). B: IL-18-stimulated ROS generation is inhibited by resveratrol and the NADPH oxidase inhibitor diphenyleneiodonium chloride (DPI). SMCs were loaded with DCFH-DA as in A and treated with resveratrol (25 μM in DMSO for 1 h) or DPI (10 μM in DMSO for 30 min), followed by the addition of IL-18 (10 ng/ml). DCF fluorescence was quantified at 5 min. *P < 0.001 vs. untreated; †P < 0.05 and ††P <0.01 vs. IL-18 (n = 12 experiments). C: inhibition of ROS generation blunts IL-18-mediated EMMPRIN expression. SMCs were treated with resveratrol or DPI as in B and then treated with IL-18 (10 ng/ml for 24 h). EMMPRIN mRNA was analyzed by RT-qPCR. *P < 0.001 vs. untreated; †P < 0.05 and ††P <0.001 vs. IL-18 (n = 6 experiments).
EMMPRIN stimulates IL-18 expression in SMC.
EMMPRIN is also found in a soluble secreted form and therefore can act in a paracrine manner. Soluble EMMPRIN has been shown to stimulate VEGF expression in cancer cells via PI3K-Akt activation (45) and stimulates proinflammatory cytokine expression in monocytes (37), suggesting that in addition to MMP induction, EMMPRIN can also act as a proinflammatory molecule. Therefore, we investigated whether EMMPRIN stimulates IL-18 expression in SMCs. Using both RT-qPCR and Northern blot analysis, we observed that the treatment with EMMPRIN for 24 h induces IL-18 mRNA expression in SMCs in a dose-dependent manner, with peak levels detected at 5 μg/ml (RT-qPCR, Fig. 7A; and Northern blot analysis, Fig. 7B) and with no further increase at 10 μg/ml. Therefore, in all subsequent experiments, EMMPRIN was used at 5 μg/ml. At these concentrations, EMMPRIN had no affect on SMC viability for up to 48 h (data not shown). Time-course experiments revealed the peak IL-18 mRNA expression at 12 h following EMMPRIN treatment (Fig. 7C). The specificity of the EMMPRIN effects was verified by the incubation of the cells with function-blocking antibodies or by adenoviral transduction of mutant EMMPRIN. The results in Fig. 7D show that both the function-blocking antibodies and mutant EMMPRIN expression significantly attenuated EMMPRIN-mediated IL-18 mRNA expression. Similarly, EMMPRIN-induced IL-18 protein expression (Fig. 7E) and the treatment with function-blocking antibodies significantly attenuated both EMMPRIN-mediated IL-18 protein expression (Fig. 7F) and secretion (Fig. 7G), suggesting that EMMPRIN induces IL-18 expression in SMCs (Fig. 7).
Fig. 7.
EMMPRIN stimulates IL-18 expression in SMCs. A and B: EMMPRIN stimulates IL-18 expression in a dose-dependent manner. Quiescent SMCs were treated with rhEMMPRIN at the indicated concentrations for 24 h. IL-18 mRNA expression was analyzed by RT-qPCR (A, n = 6 experiments) and confirmed by Northern blot analysis (B, n = 3 experiments). Actin served as an internal control. *P < 0.05 and **P < 0.01 vs. untreated (n = 6 experiments). C: EMMPRIN induces IL-18 expression in a time-dependent manner. Quiescent SMCs were treated with EMMPRIN (5 mg/ml). At the indicated time periods, IL-18 mRNA expression was analyzed by RT-qPCR (n = 6 experiments). *P < at least 0.05 vs. control at 12 h. D: targeting EMMPRIN attenuates IL-18 mRNA expression. SMCs were incubated with EMMPRIN function-blocking antibodies (5 μg/ml for 1 h) or transduced with Ad.mEMMPRIN (100 MOI for 48 h) before EMMPRIN addition (5 μg/ml for 24 h). Normal mouse IgG and empty virus at similar concentrations served as respective controls. IL-18 mRNA expression was analyzed by RT-qPCR. Actin served as a control. *P < 0.001 vs. untreated; †P < 0.01 vs. EMMPRIN (n = 6 experiments). E: EMMPRIN induces IL-18 protein expression. Quiescent SMCs were treated with EMMPRIN (5 μg/ml for 24 h). Cleared cell lysates were analyzed for IL-18 protein expression by immunoblotting using antibodies against mature IL-18 (n = 3 experiments). α-Tubulin served as a loading control. F and G: targeting EMMPRIN attenuates IL-18 protein expression (F) and secretion (G). SMCs treated as in C, but for 24 h, were analyzed for IL-18 protein expression by immunoblotting (F, n = 3 experiments) and secretion by ELISA (G). *P < 0.01 vs. untreated; †P < 0.01 vs. EMMPRIN + control IgG (n = 6 experiments).
EMMPRIN stimulates IL-18 expression in SMCs via PI3K, Akt, and ERK.
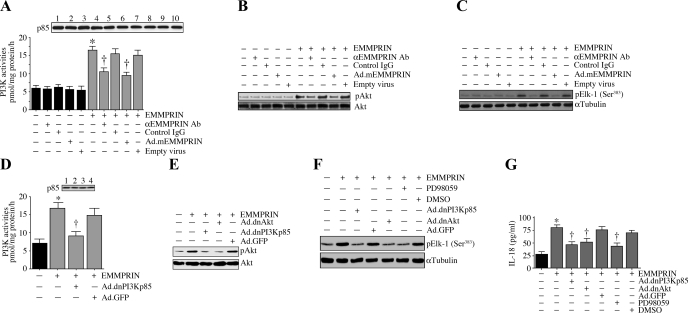
We have demonstrated that EMMPRIN stimulates IL-18 expression in SMCs (Fig. 7). We next investigated the underlying signaling mechanisms involved in EMMPRIN-mediated IL-18 expression. SMCs were treated with soluble EMMPRIN for 1 h, and the activation of PI3K, Akt, and ERK was analyzed as described in materials and methods. EMMPRIN potently activated PI3K activity in SMCs (Fig. 8A), an effect that was significantly attenuated by the incubation with function-blocking antibodies or by the adenoviral transduction of mutant EMMPRIN. Similarly, both the function-blocking antibodies and adenoviral transduction of mutant EMMPRIN significantly attenuated EMMPRIN-mediated Akt phosphorylation (Fig. 8B) and ERK activity (Fig. 8C). Furthermore, whereas the adenoviral transduction of dnPI3K attenuated EMMPRIN-mediated PI3K activities (Fig. 8D) and Akt phosphorylation (Fig. 8E), the adenoviral transduction of dnAkt inhibited EMMPRIN-mediated Akt phosphorylation (Fig. 8E) and ERK activity (Fig. 8F). Importantly, the adenoviral transduction of dnPI3K and dnAkt, as well as the treatment with PD-98059, attenuated EMMPRIN-mediated IL-18 secretion (Fig. 8G), indicating that EMMPRIN stimulates IL-18 expression in SMCs via activations of PI3K, Akt, and ERK (Fig. 8).
Fig. 8.
EMMPRIN stimulates IL-18 expression via PI3K, Akt, and ERK. A: EMMPRIN stimulates PI3K activation. Quiescent SMCs were incubated with function-blocking antibodies or transduced with Ad.mEMMPRIN before EMMPRIN (5 μg/ml for 30 min) addition. Normal IgG and empty virus served as respective controls. PI3K ELISA was performed as described under materials and methods. A, top: immunoblot analysis of the same samples with anti-p85 antibody. *P < 0.001 vs. untreated; †P < 0.05 vs. EMMPRIN (n = 6 experiments). B: EMMPRIN activates Akt. Quiescent SMCs were treated as A but for 1 h, and Akt activation was analyzed by immunoblotting using pAkt antibodies. A representative of 3 independent experiments is shown. C: EMMPRIN stimulates ERK activity. Quiescent SMCs treated as in A, but for 1 h, were analyzed for ERK activity using immunecomplex kinase assays. Elk served as a substrate (n = 3 experiments). α-Tubulin served as a control. D: adenoviral transduction of dnPI3Kp85 blocks EMMPRIN-mediated PI3K activation. SMCs were transduced with Ad.dnPI3K or Ad.GFP before EMMPRIN addition. PI3K ELISA was performed as described under materials and methods. D, top: immunoblot analysis of the same samples with anti-p85 antibody. *P < 0.01 vs. untreated; †P < 0.05 vs. EMMPRIN + Ad.GFP. E: adenoviral transduction of dnPI3K or dnAkt blunts EMMPRIN-mediated Akt activation. SMCs were transduced with Ad.dnPI3Kp85 or dnAkt before EMMPRIN addition. Akt activation was analyzed as in B (n = 3 experiments). F: EMMPRIN stimulates ERK activation via PI3K and Akt. SMCs were either traduced with Ad.dnPI3Kp85 or Ad.dnAkt or treated with PD-98059 (10 μM in DMSO for 1 h) before EMMPRIN addition. ERK activation was analyzed as in C (n = 3 experiments). G: EMMPRIN induces IL-18 expression via PI3K, Akt, and ERK. SMCs were transduced with Ad.nPI3Kp85, dnAkt, or GFP or treated with PD-98059 before EMMPRIN (5 μg/ml) or Fc (1.5 μg/ml) addition for 24 h. IL-18 levels in culture supernatants were analyzed by ELISA. *P < 0.001 vs. untreated; †P < at least 0.05 vs. EMMPRIN + respective controls (n = 6 experiments).
Resveratrol blocks EMMPRIN-mediated IL-18 expression and SMC migration.
Since resveratrol exerts anti-inflammatory and antiatherogenic effects (1, 3, 12, 13, 15, 27, 42, 52, 62), we next investigated whether resveratrol blocks EMMPRIN-mediated IL-18 induction. Confirming our earlier results (Fig. 7, A and B), both RT-qPCR (Fig. 9A) and Northern blot analysis (Fig. 9B) revealed that EMMPRIN stimulates IL-18 mRNA expression in SMCs, an effect that was significantly attenuated by resveratrol, but not by its solvent control DMSO. Similarly, resveratrol blocked the EMMPRIN-stimulated increase in intracellular (Fig. 9C) and secreted (Fig. 9D) IL-18 protein and SMC migration (Fig. 9E), indicating that resveratrol can also block EMMPRIN-mediated IL-18 induction and SMC migration (Fig. 9).
Fig. 9.
Resveratrol blocks EMMPRIN-mediated IL-18 expression and SMC migration. A and B: resveratrol blocks EMMPRIN-mediated IL-18 mRNA expression. Quiescent SMCs were incubated with resveratrol (25 μM in DMSO for 1 h) before EMMPRIN addition (5 μg/ml for 24 h). Fc (1.5 μg/ml) served as a control. IL-18 mRNA expression was analyzed by RT-qPCR (A) and confirmed by Northern blot analysis (B). Actin served as a control. *P < 0.01 vs. untreated; †P < 0.05 vs. EMMPRIN (n = 6 experiments). C and D: resveratrol attenuates EMMPRIN-mediated IL-18 protein expression (C) and secretion (D). Quiescent SMCs treated as in A were analyzed for IL-18 protein expression by immunoblotting (C) and IL-18 secretion (D) by ELISA. *P < 0.001 vs. untreated; †P < 0.05 vs. EMMPRIN + DMSO (n = 6 experiments). E: resveratrol attenuates EMMPRIN-mediated SMC migration. SMCs were layered on Matrigel basement-membrane matrix-coated filters as described in materials and methods. Cells were treated with resveratrol (25 μM in DMSO for 1 h) before EMMPRIN (5 μg/ml) or Fc (1.5 μg/ml) addition. Plates were incubated at 37°C for 12 h to allow cell migration. Cells migration was analyzed as in Fig. 1A. *P < 0.001 vs. untreated; †P < 0.05 vs. IL-18 (n = 12 experiments). F: schema showing that resveratrol blocks IL-18 and EMMPRIN cross-regulation and SMC migration.
DISCUSSION
Here we show for the first time that the proinflammatory cytokine IL-18 and the surface glycoprotein EMMPRIN regulate each other's expression in SMCs via a signal transduction pathway involving activations of PI3K, Akt, and ERK. Furthermore, our results also demonstrate that IL-18 stimulates ROS generation and induces EMMPRIN expression in part via a redox-sensitive pathway. Importantly, the phytoalexin resveratrol can inhibit IL-18-mediated oxidative stress; attenuate IL-18- and EMMPRIN-dependent activations of PI3K, Akt, and ERK; block IL-18/EMMPRIN cross-regulation; and blunt SMC migration (Fig. 9F). Since SMC migration is a critical mechanism in the development and progression of atherosclerotic vascular disease, and vascular remodeling following angioplasty, our results suggest a potential therapeutic role for resveratrol in these chronic inflammatory diseases.
Inflammation plays a critical role in all aspects of atherosclerotic lesion development and progression (28, 36). Atherosclerosis is characterized by an intimal accumulation of macrophages, immune cells, lipids, ECM components, and SMC proliferation and migration (28, 36). As the lesion progresses, the lumen narrows, and the resultant arteriosclerosis may lead to myocardial infarction. Restenosis that can occur after a percutaneous intervention or stent deployment may be considered as an overreaction of the wound-healing response after vascular injury and is also characterized by inflammation, ECM remodeling, and SMC proliferation and migration (17). Thus SMC proliferation and migration are critical events in both atherosclerosis and restenosis. Various proinflammatory cytokines stimulate SMC proliferation and migration. We have previously reported that IL-18 potently induces SMC migration in part via MMP-9 induction (7). The results from the present study demonstrate that IL-18 also induces EMMPRIN expression in SMCs and stimulates SMC migration in part via EMMPRIN, an MMP-9 inducer.
EMMPRIN is a potent inducer of various MMPs. MMP activities are a common denominator in the structural remodeling of tissues under both physiological and pathological conditions. MMP-2, MMP-9, and MT1-MMP in particular, are associated with vascular remodeling (34). MMP-1, MMP-2, and MMP-9 are also expressed in human atherosclerotic lesions, particularly in those enriched with macrophages and foam cells (58). Active MMPs contribute to matrix degradation, remodeling, weakening of atherosclerotic lesions, and rupture of vulnerable plaques. In addition to inducing MMP-1, EMMPRIN has also been shown to form a complex with MMP-1 at the tumor cell surface, and this complex may modify the tumor cell pericellular matrix and promote tumor cell invasion (20). Whether such a phenomenon occurs in atherosclerosis and whether EMMPRIN forms complexes with other MMPs in SMCs are not known. We have previously demonstrated that IL-18 induces SMC migration in part via MMP-9 (7). Since EMMPRIN also induces MMP-9 expression (37, 38) and as IL-18 and EMMPRIN regulate each other's expression in SMC, we hypothesize that IL-18-EMMPRIN signaling may be an important contributing factor in atherosclerosis and restenosis.
The results of our studies indicate that IL-18 and EMMPRIN regulate each other's expression in SMCs via a PI3K-Akt-ERK signaling pathway. Although we did not investigate the proximal events that led to PI3K activation, we have in previous studies shown that CXC chemokine ligand-16 expression in SMC (8), fibronectin expression in cardiac fibroblasts (35), and various proapoptotic genes in cardiac endothelial cells (10) are all induced by IL-18 through a myeloid differentiation factor-88/IL-1 receptor-associated kinase/TNF receptor-associated factor-6-dependent PI3K activation pathway. Therefore, we believe that a similar signal transduction pathway may be responsible for IL-18-mediated PI3K activation and EMMPRIN induction in SMCs. The upstream signaling events involved in EMMPRIN-mediated PI3K activation on the other hand are not known, and studies are needed to elucidate how EMMPRIN signaling complex upstream of PI3K is organized.
In the present investigation, we used IL-18 and soluble EMMPRIN at a relatively high concentration. We chose these concentrations based on previously published reports. We and others (6–8, 10, 54, 59) have previously used IL-18 between 100–500 ng/ml in vitro. Though the systemic levels of IL-18 varied between 200–1,000 pg/ml during various disease conditions (43, 60), it is highly possible, but technically difficult, to quantify IL-18 levels in intracellular and intercellular spaces, which we assume to be high. Therefore, serum/plasma levels may not truly reflect IL-18 levels in intra- or intercellular spaces. Furthermore, the dissociation constant (Kd) for IL-18 has been shown to be 18.5 nM (49), suggesting that even at 100 ng/ml, IL-18 will bind only 23% of the receptors and will not saturate all the receptors. It is also possible that IL-18 may act in concert with other cytokines/chemokines in vivo in exerting its biological effects. Pilot studies in our laboratory indicate that IL-18 can signal at relatively low (in pg/ml) concentrations when combined with TNF-α, and these observations are currently being examined. In contrast to IL-18, very few studies quantified serum/plasma levels of soluble EMMPRIN. In one report, serum EMMPRIN levels have been shown to range between almost undetectable to 9 ng/ml (61). However, EMMPRIN is released into extracellular spaces via shed vesicles (46), suggesting relatively high levels of EMMPRIN in intercellular spaces that are difficult to quantify. Furthermore, soluble EMMPRIN was previously used between 0.1 to 5 μg/ml in vitro in platelets and monocytes (37, 38). In the present investigation, we did perform dose-response studies (0.5 to 10 μg/ml; Fig. 6A) and found that moderate, but significant, IL-18 induction was detected when SMCs were incubated with 1 μg/ml soluble EMMPRIN. However, maximal induction was observed at 5 μg/ml. Therefore, EMMPRIN was used at 5 μg/ml. We did not, however, detect an enhanced IL-18 secretion (vs. basal levels) when SMCs were incubated with soluble EMMPRIN at concentrations below 1 μg/ml (data not shown).
An important observation in the present study is that treatment with resveratrol blocks IL-18 and EMMPRIN expressions, their cross talk, and SMC migration. Resveratrol blocks both IL-18 and EMMPRIN-mediated PI3K→Akt→ERK signaling. Though we have demonstrated that resveratrol inhibits PI3K activation, we did not investigate the underlying molecular mechanisms. However, in an elegant study, Fröjdö et al. (18) previously reported that resveratrol targets the class IA PI3K ATP-binding site in a competitive and reversible manner (18). These authors demonstrated that increasing ATP concentrations diminish the inhibitory action of resveratrol, implying that resveratrol acts as a reversible PI3K inhibitor by competing with ATP for the catalytic site.
Our results also show that resveratrol inhibits IL-18 and EMMPRIN-mediated PI3K-dependent Akt and ERK activation in SMCs. In fact, we have recently demonstrated that resveratrol blocks high glucose-induced PI3K-Akt-ERK-dependent IL-17 expression in cardiac fibroblasts (50). Resveratrol has also been shown to inhibit PMA-induced p38 and ERK1/2-dependent EMMPRIN expression in a monocytic cell line (22). Furthermore, resveratrol inhibits angiotensin II-induced ERK phosphorylation in SMCs via the activation of the silent information regulator 2 homolog 1 (SIRT1) (31). Since IL-18 and EMMPRIN cross talk involves ERK, it is possible that resveratrol might inhibit IL-18 and EMMPRIN expressions via SIRT1 activation.
Our studies also demonstrate that IL-18 stimulates oxidative stress in SMCs, an effect that is significantly inhibited by resveratrol. Similar with resveratrol treatment, the treatment with DPI, an NADPH oxidase inhibitor, attenuated IL-18-mediated oxidative stress and EMMPRIN mRNA expression. These studies suggest that resveratrol may attenuate IL-18 and EMMPRIN expression and their cross-regulation via its anti-inflammatory and antioxidant properties. Furthermore, it has been recently demonstrated that the signal-transducing subunit of IL-18, IL-18Rβ, physically interacts with the Rho family GTPase Rac-1 (24). Since Rac-1 is known to play a role in the activation of selective NADPH oxidases and induces oxidative stress (2), we hypothesize that IL-18 also induces EMMPRIN expression via a Rac-1-dependent signaling.
Resveratrol is also known to affect various cell types involved in atherosclerotic lesion development; it inhibits IL-18 and EMMPRIN expression in SMCs, induction and activation of MMPs in SMCs and monocytes, monocyte/macrophage differentiation, foam cell formation, endothelial and platelet activation, and their interaction (1, 3, 12, 13, 37). Platelets also contribute to plaque initiation and progression and are the key elements in the thromboembolic events that lead to myocardial infarction and stroke. Recently, EMMPRIN has been shown to be a novel receptor on platelets (37). In that study, the treatment with EMMPRIN induced platelet activation, as evidenced by increased CD40L and P-selectin surface expression (37). Furthermore, the coincubation of platelets and monocytes activated NF-κB-dependent inflammatory pathways in monocytes via EMMPRIN-EMMPRIN signaling. These authors also demonstrated that the coincubation of platelets and monocytes stimulated EMMPRIN-dependent κB-responsive MMP-9, IL-6, and TNF-α expression in monocytes (37). Since we show here that EMMPRIN stimulates IL-18 expression in SMCs via PI3K-Akt-ERK signaling and that IL-18 is a κB-responsive cytokine with κB-binding sites in both proximal and distal promoter regions (48), we hypothesize that EMMPRIN induces IL-18 transcription via NF-κB activation. Further studies are needed to examine this possibility.
In summary, our results show that IL-18 and EMMPRIN regulate each other's expression in SMCs via the activation of PI3K, Akt, and ERK. The coexpression and regulation of IL-18 and EMMPRIN in the vessel wall may amplify the inflammatory cascade and promote atherosclerosis and pathological remodeling. Resveratrol has the potential to inhibit atherogenesis by blocking IL-18/EMMPRIN cross-regulation and SMC migration.
GRANTS
This work was supported by National Heart, Lung, and Blood Institute Grant HL-86787 (to B. Chandrasekar) and a grant from the Biomedical Laboratory Research and Development Service of the Veterans Affairs Office of Research and Development (to B. Chandrasekar).
REFERENCES
- 1.Araim O, Ballantyne J, Waterhouse AL, Sumpio BE. Inhibition of vascular smooth muscle cell proliferation with red wine and red wine polyphenols. J Vasc Surg 35: 1226–1232, 2002. [DOI] [PubMed] [Google Scholar]
- 2.Baumer AT, Ten Freyhaus H, Sauer H, Wartenberg M, Kappert K, Schnabel P, Konkol C, Hescheler J, Vantler M, Rosenkranz S. Phosphatidylinositol 3-kinase-dependent membrane recruitment of Rac-1 and p47phox is critical for alpha-platelet-derived growth factor receptor-induced production of reactive oxygen species. J Biol Chem 283: 7864–7876, 2008. [DOI] [PubMed] [Google Scholar]
- 3.Belguendouz L, Fremont L, Linard A. Resveratrol inhibits metal ion-dependent and independent peroxidation of porcine low-density lipoproteins. Biochem Pharmacol 53: 1347–1355, 1997. [DOI] [PubMed] [Google Scholar]
- 4.Biswas C, Zhang Y, DeCastro R, Guo H, Nakamura T, Kataoka H, Nabeshima K. The human tumor cell-derived collagenase stimulatory factor (renamed EMMPRIN) is a member of the immunoglobulin superfamily. Cancer Res 55: 434–439, 1995. [PubMed] [Google Scholar]
- 5.Cain RJ, Ridley AJ. Phosphoinositide 3-kinases in cell migration. Biol Cell 101: 13–29, 2009. [DOI] [PubMed] [Google Scholar]
- 6.Chandrasekar B, Mummidi S, Claycomb WC, Mestril R, Nemer M. Interleukin-18 is a pro-hypertrophic cytokine that acts through a phosphatidylinositol 3-kinase-phosphoinositide-dependent kinase-1-Akt-GATA4 signaling pathway in cardiomyocytes. J Biol Chem 280: 4553–4567, 2005. [DOI] [PubMed] [Google Scholar]
- 7.Chandrasekar B, Mummidi S, Mahimainathan L, Patel DN, Bailey SR, Imam SZ, Greene WC, Valente AJ. Interleukin-18-induced human coronary artery smooth muscle cell migration is dependent on NF-kappaB- and AP-1-mediated matrix metalloproteinase-9 expression and is inhibited by atorvastatin. J Biol Chem 281: 15099–15109, 2006. [DOI] [PubMed] [Google Scholar]
- 8.Chandrasekar B, Mummidi S, Valente AJ, Patel DN, Bailey SR, Freeman GL, Hatano M, Tokuhisa T, Jensen LE. The pro-atherogenic cytokine interleukin-18 induces CXCL16 expression in rat aortic smooth muscle cells via MyD88, interleukin-1 receptor-associated kinase, tumor necrosis factor receptor-associated factor 6, c-Src, phosphatidylinositol 3-kinase, Akt, c-Jun N-terminal kinase, and activator protein-1 signaling. J Biol Chem 280: 26263–26277, 2005. [DOI] [PubMed] [Google Scholar]
- 9.Chandrasekar B, Valente AJ, Freeman GL, Mahimainathan L, Mummidi S. Interleukin-18 induces human cardiac endothelial cell death via a novel signaling pathway involving NF-kappaB-dependent PTEN activation. Biochem Biophys Res Commun 339: 956–963, 2006. [DOI] [PubMed] [Google Scholar]
- 10.Chandrasekar B, Vemula K, Surabhi RM, Li-Weber M, Owen-Schaub LB, Jensen LE, Mummidi S. Activation of intrinsic and extrinsic proapoptotic signaling pathways in interleukin-18-mediated human cardiac endothelial cell death. J Biol Chem 279: 20221–20233, 2004. [DOI] [PubMed] [Google Scholar]
- 11.Colston JT, de la Rosa SD, Strader JR, Anderson MA, Freeman GL. H2O2 activates Nox4 through PLA2-dependent arachidonic acid production in adult cardiac fibroblasts. FEBS Lett 579: 2533–2540, 2005. [DOI] [PubMed] [Google Scholar]
- 12.Culpitt SV, Rogers DF, Fenwick PS, Shah P, De Matos C, Russell RE, Barnes PJ, Donnelly LE. Inhibition by red wine extract, resveratrol, of cytokine release by alveolar macrophages in COPD. Thorax 58: 942–946, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Das S, Das DK. Resveratrol: a therapeutic promise for cardiovascular diseases. Recent Pat Cardiovasc Drug Discov 2: 133–138, 2007. [DOI] [PubMed] [Google Scholar]
- 14.Dinarello CA Interleukin-18, a proinflammatory cytokine. Eur Cytokine Netw 11: 483–486, 2000. [PubMed] [Google Scholar]
- 15.Donnelly LE, Newton R, Kennedy GE, Fenwick PS, Leung RH, Ito K, Russell RE, Barnes PJ. Anti-inflammatory effects of resveratrol in lung epithelial cells: molecular mechanisms. Am J Physiol Lung Cell Mol Physiol 287: L774–L783, 2004. [DOI] [PubMed] [Google Scholar]
- 16.Elhage R, Jawien J, Rudling M, Ljunggren HG, Takeda K, Akira S, Bayard F, Hansson GK. Reduced atherosclerosis in interleukin-18 deficient apolipoprotein E-knockout mice. Cardiovasc Res 59: 234–240, 2003. [DOI] [PubMed] [Google Scholar]
- 17.Forrester JS, Fishbein M, Helfant R, Fagin J. A paradigm for restenosis based on cell biology: clues for the development of new preventive therapies. J Am Coll Cardiol 17: 758–769, 1991. [DOI] [PubMed] [Google Scholar]
- 18.Fröjdö S, Cozzone D, Vidal H, Pirola L. Resveratrol is a class IA phosphoinositide 3-kinase inhibitor. Biochem J 406: 511–518, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gerdes N, Sukhova GK, Libby P, Reynolds RS, Young JL, Schonbeck U. Expression of interleukin (IL)-18 and functional IL-18 receptor on human vascular endothelial cells, smooth muscle cells, and macrophages: implications for atherogenesis. J Exp Med 195: 245–257, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Guo H, Li R, Zucker S, Toole BP. EMMPRIN (CD147), an inducer of matrix metalloproteinase synthesis, also binds interstitial collagenase to the tumor cell surface. Cancer Res 60: 888–891, 2000. [PubMed] [Google Scholar]
- 21.Hanata K, Yamaguchi N, Yoshikawa K, Mezaki Y, Miura M, Suzuki S, Senoo H, Ishikawa K. Soluble EMMPRIN (extra-cellular matrix metalloproteinase inducer) stimulates the migration of HEp-2 human laryngeal carcinoma cells, accompanied by increased MMP-2 production in fibroblasts. Arch Histol Cytol 70: 267–277, 2007. [DOI] [PubMed] [Google Scholar]
- 22.Huang Z, Wang C, Wei L, Wang J, Fan Y, Wang L, Wang Y, Chen T. Resveratrol inhibits EMMPRIN expression via P38 and ERK1/2 pathways in PMA-induced THP-1 cells. Biochem Biophys Res Commun 374: 517–521, 2008. [DOI] [PubMed] [Google Scholar]
- 23.Igakura T, Kadomatsu K, Kaname T, Muramatsu H, Fan QW, Miyauchi T, Toyama Y, Kuno N, Yuasa S, Takahashi M, Senda T, Taguchi O, Yamamura K, Arimura K, Muramatsu T. A null mutation in basigin, an immunoglobulin superfamily member, indicates its important roles in peri-implantation development and spermatogenesis. Dev Biol 194: 152–165, 1998. [DOI] [PubMed] [Google Scholar]
- 24.Kim J, Shao Y, Kim SY, Kim S, Song HK, Jeon JH, Suh HW, Chung JW, Yoon SR, Kim YS, Choi I. Hypoxia-induced IL-18 increases hypoxia-inducible factor-1alpha expression through a Rac1-dependent NF-kappaB pathway. Mol Biol Cell 19: 433–444, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kuno N, Kadomatsu K, Fan QW, Hagihara M, Senda T, Mizutani S, Muramatsu T. Female sterility in mice lacking the basigin gene, which encodes a transmembrane glycoprotein belonging to the immunoglobulin superfamily. FEBS Lett 425: 191–194, 1998. [DOI] [PubMed] [Google Scholar]
- 26.Lee TK, Man K, Ho JW, Sun CK, Ng KT, Wang XH, Wong YC, Ng IO, Xu R, Fan ST. FTY720 induces apoptosis of human hepatoma cell lines through PI3-K-mediated Akt dephosphorylation. Carcinogenesis 25: 2397–2405, 2004. [DOI] [PubMed] [Google Scholar]
- 27.Leiro J, Alvarez E, Arranz JA, Laguna R, Uriarte E, Orallo F. Effects of cis-resveratrol on inflammatory murine macrophages: antioxidant activity and down-regulation of inflammatory genes. J Leukoc Biol 75: 1156–1165, 2004. [DOI] [PubMed] [Google Scholar]
- 28.Libby P Inflammation in atherosclerosis. Nature 420: 868–874, 2002. [DOI] [PubMed] [Google Scholar]
- 29.Liu MW, Roubin GS, King SB 3rd. Restenosis after coronary angioplasty. Potential biologic determinants and role of intimal hyperplasia. Circulation 79: 1374–1387, 1989. [DOI] [PubMed] [Google Scholar]
- 30.Mallat Z, Corbaz A, Scoazec A, Besnard S, Leseche G, Chvatchko Y, Tedgui A. Expression of interleukin-18 in human atherosclerotic plaques and relation to plaque instability. Circulation 104: 1598–1603, 2001. [DOI] [PubMed] [Google Scholar]
- 31.Miyazaki R, Ichiki T, Hashimoto T, Inanaga K, Imayama I, Sadoshima J, Sunagawa K. SIRT1, a longevity gene, downregulates angiotensin II type 1 receptor expression in vascular smooth muscle cells. Arterioscler Thromb Vasc Biol 28: 1263–1269, 2008. [DOI] [PubMed] [Google Scholar]
- 32.Narins CR, Lin DA, Burton PB, Jin ZG, Berk BC. Interleukin-18 and interleukin-18 binding protein levels before and after percutaneous coronary intervention in patients with and without recent myocardial infarction. Am J Cardiol 94: 1285–1287, 2004. [DOI] [PubMed] [Google Scholar]
- 33.Patel DN, Bailey SR, Gresham JK, Schuchman DB, Shelhamer JH, Goldstein BJ, Foxwell BM, Stemerman MB, Maranchie JK, Valente AJ, Mummidi S, Chandrasekar B. TLR4-NOX4-AP-1 signaling mediates lipopolysaccharide-induced CXCR6 expression in human aortic smooth muscle cells. Biochem Biophys Res Commun 347: 1113–1120, 2006. [DOI] [PubMed] [Google Scholar]
- 34.Raffetto JD, Khalil RA. Matrix metalloproteinases and their inhibitors in vascular remodeling and vascular disease. Biochem Pharmacol 75: 346–359, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Reddy VS, Harskamp RE, van Ginkel MW, Calhoon J, Baisden CE, Kim IS, Valente AJ, Chandrasekar B. Interleukin-18 stimulates fibronectin expression in primary human cardiac fibroblasts via PI3K-Akt-dependent NF-kappaB activation. J Cell Physiol 215: 697–707, 2008. [DOI] [PubMed] [Google Scholar]
- 36.Ross R Atherosclerosis—an inflammatory disease. N Engl J Med 340: 115–126, 1999. [DOI] [PubMed] [Google Scholar]
- 37.Schmidt R, Bultmann A, Fischel S, Gillitzer A, Cullen P, Walch A, Jost P, Ungerer M, Tolley ND, Lindemann S, Gawaz M, Schomig A, May AE. Extracellular matrix metalloproteinase inducer (CD147) is a novel receptor on platelets, activates platelets, and augments nuclear factor kappaB-dependent inflammation in monocytes. Circ Res 102: 302–309, 2008. [DOI] [PubMed] [Google Scholar]
- 38.Schmidt R, Bultmann A, Ungerer M, Joghetaei N, Bulbul O, Thieme S, Chavakis T, Toole BP, Gawaz M, Schomig A, May AE. Extracellular matrix metalloproteinase inducer regulates matrix metalloproteinase activity in cardiovascular cells: implications in acute myocardial infarction. Circulation 113: 834–841, 2006. [DOI] [PubMed] [Google Scholar]
- 39.Shimada M, Yamabe H, Osawa H, Nakamura N, Kumasaka R, Murakami R, Fujita T, Osanai T, Okumura K. Extracellular matrix metalloproteinase inducer is expressed in the proximal tubular epithelial cells of the human kidney. Nephrology (Carlton) 14: 171–178, 2009. [DOI] [PubMed] [Google Scholar]
- 40.Sivakumar PV, Westrich GM, Kanaly S, Garka K, Born TL, Derry JM, Viney JL. Interleukin 18 is a primary mediator of the inflammation associated with dextran sulphate sodium induced colitis: blocking interleukin 18 attenuates intestinal damage. Gut 50: 812–820, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Siwik DA, Kuster GM, Brahmbhatt JV, Zaidi Z, Malik J, Ooi H, Ghorayeb G. EMMPRIN mediates beta-adrenergic receptor-stimulated matrix metalloproteinase activity in cardiac myocytes. J Mol Cell Cardiol 44: 210–217, 2008. [DOI] [PubMed] [Google Scholar]
- 42.Soleas GJ, Diamandis EP, Goldberg DM. Resveratrol: a molecule whose time has come? And gone? Clin Biochem 30: 91–113, 1997. [DOI] [PubMed] [Google Scholar]
- 43.Suchanek H, Mysliwska J, Siebert J, Wieckiewicz J, Hak L, Szyndler K, Kartanowicz D. High serum interleukin-18 concentrations in patients with coronary artery disease and type 2 diabetes mellitus. Eur Cytokine Netw 16: 177–185, 2005. [PubMed] [Google Scholar]
- 44.Sun J, Hemler ME. Regulation of MMP-1 and MMP-2 production through CD147/extracellular matrix metalloproteinase inducer interactions. Cancer Res 61: 2276–2281, 2001. [PubMed] [Google Scholar]
- 45.Tang Y, Nakada MT, Rafferty P, Laraio J, McCabe FL, Millar H, Cunningham M, Snyder LA, Bugelski P, Yan L. Regulation of vascular endothelial growth factor expression by EMMPRIN via the PI3K-Akt signaling pathway. Mol Cancer Res 4: 371–377, 2006. [DOI] [PubMed] [Google Scholar]
- 46.Taylor PM, Woodfield RJ, Hodgkin MN, Pettitt TR, Martin A, Kerr DJ, Wakelam MJ. Breast cancer cell-derived EMMPRIN stimulates fibroblast MMP2 release through a phospholipase A2 and 5-lipoxygenase catalyzed pathway. Oncogene 21: 5765–5772, 2002. [DOI] [PubMed] [Google Scholar]
- 47.Tiret L, Godefroy T, Lubos E, Nicaud V, Tregouet DA, Barbaux S, Schnabel R, Bickel C, Espinola-Klein C, Poirier O, Perret C, Munzel T, Rupprecht HJ, Lackner K, Cambien F, Blankenberg S. Genetic analysis of the interleukin-18 system highlights the role of the interleukin-18 gene in cardiovascular disease. Circulation 112: 643–650, 2005. [DOI] [PubMed] [Google Scholar]
- 48.Tone M, Thompson SA, Tone Y, Fairchild PJ, Waldmann H. Regulation of IL-18 (IFN-gamma-inducing factor) gene expression. J Immunol 159: 6156–6163, 1997. [PubMed] [Google Scholar]
- 49.Torigoe K, Ushio S, Okura T, Kobayashi S, Taniai M, Kunikata T, Murakami T, Sanou O, Kojima H, Fujii M, Ohta T, Ikeda M, Ikegami H, Kurimoto M. Purification and characterization of the human interleukin-18 receptor. J Biol Chem 272: 25737–25742, 1997. [DOI] [PubMed] [Google Scholar]
- 50.Venkatachalam K, Mummidi S, Cortez DM, Prabhu SD, Valente AJ, Chandrasekar B. Resveratrol inhibits high glucose-induced PI3K/Akt/ERK-dependent interleukin-17 expression in primary mouse cardiac fibroblasts. Am J Physiol Heart Circ Physiol 294: H2078–H2087, 2008. [DOI] [PubMed] [Google Scholar]
- 51.Venkatachalam K, Prabhu SD, Reddy VS, Boylston WH, Valente AJ, Chandrasekar B. Neutralization of interleukin-18 ameliorates ischemia/reperfusion-induced myocardial injury. J Biol Chem 284: 7853–7865, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wang Z, Zou J, Huang Y, Cao K, Xu Y, Wu JM. Effect of resveratrol on platelet aggregation in vivo and in vitro. Chin Med J (Engl) 115: 378–380, 2002. [PubMed] [Google Scholar]
- 53.Whitman SC, Ravisankar P, Daugherty A. Interleukin-18 enhances atherosclerosis in apolipoprotein E−/− mice through release of interferon-gamma. Circ Res 90: E34–E38, 2002. [DOI] [PubMed] [Google Scholar]
- 54.Woldbaek PR, Tonnessen T, Henriksen UL, Florholmen G, Lunde PK, Lyberg T, Christensen G. Increased cardiac IL-18 mRNA, pro-IL-18 and plasma IL-18 after myocardial infarction in the mouse; a potential role in cardiac dysfunction. Cardiovasc Res 59: 122–131, 2003. [DOI] [PubMed] [Google Scholar]
- 55.Wyman TH, Dinarello CA, Banerjee A, Gamboni-Robertson F, Hiester AA, England KM, Kelher M, Silliman CC. Physiological levels of interleukin-18 stimulate multiple neutrophil functions through p38 MAP kinase activation. J Leukoc Biol 72: 401–409, 2002. [PubMed] [Google Scholar]
- 56.Xia Z, Dickens M, Raingeaud J, Davis RJ, Greenberg ME. Opposing effects of ERK and JNK-p38 MAP kinases on apoptosis. Science 270: 1326–1331, 1995. [DOI] [PubMed] [Google Scholar]
- 57.Yamagami H, Kitagawa K, Hoshi T, Furukado S, Hougaku H, Nagai Y, Hori M. Associations of serum IL-18 levels with carotid intima-media thickness. Arterioscler Thromb Vasc Biol 25: 1458–1462, 2005. [DOI] [PubMed] [Google Scholar]
- 58.Yoon YW, Kwon HM, Hwang KC, Choi EY, Hong BK, Kim D, Kim HS, Cho SH, Song KS, Sangiorgi G. Upstream regulation of matrix metalloproteinase by EMMPRIN; extracellular matrix metalloproteinase inducer in advanced atherosclerotic plaque. Atherosclerosis 180: 37–44, 2005. [DOI] [PubMed] [Google Scholar]
- 59.Yoshimoto T, Mizutani H, Tsutsui H, Noben-Trauth N, Yamanaka K, Tanaka M, Izumi S, Okamura H, Paul WE, Nakanishi K. IL-18 induction of IgE: dependence on CD4+ T cells, IL-4 and STAT6. Nat Immunol 1: 132–137, 2000. [DOI] [PubMed] [Google Scholar]
- 60.Yuen CM, Chiu CA, Chang LT, Liou CW, Lu CH, Youssef AA, Yip HK. Level and value of interleukin-18 after acute ischemic stroke. Circ J 71: 1691–1696, 2007. [DOI] [PubMed] [Google Scholar]
- 61.Zhang W, Erkan M, Abiatari I, Giese NA, Felix K, Kayed H, Buchler MW, Friess H, Kleeff J. Expression of extracellular matrix metalloproteinase inducer (EMMPRIN/CD147) in pancreatic neoplasm and pancreatic stellate cells. Cancer Biol Ther 6: 218–227, 2007. [DOI] [PubMed] [Google Scholar]
- 62.Zhong M, Cheng GF, Wang WJ, Guo Y, Zhu XY, Zhang JT. Inhibitory effect of resveratrol on interleukin 6 release by stimulated peritoneal macrophages of mice. Phytomedicine 6: 79–84, 1999. [DOI] [PubMed] [Google Scholar]