Abstract
Muscular lymphatics use both phasic and tonic contractions to transport lymph for conducting their vital functions. The molecular mechanisms regulating lymphatic muscle contractions are not well understood. Based on the well-established finding that the phosphorylation of myosin light chain 20 (MLC20) plays an essential role in blood vessel smooth muscle contraction, we investigated if phosphorylated MLC20 (pMLC20) would modulate the tonic and/or phasic contractions of lymphatic muscle. The effects of ML-7, a MLC kinase inhibitor (1–10 μM), were tested on the contractile parameters of isolated and cannulated rat mesenteric lymphatics during their responses to the known modulators, pressure (1–5 cmH2O) and substance P (SP; 10−7 M). Immunohistochemical and Western blot analyses of pMLC20 were also performed on isolated lymphatics. The results showed that 1) increasing pressure decreased both the lymphatic tonic contraction strength and pMLC20-to-MLC20 ratio; 2) SP increased both the tonic contraction strength and phosphorylation of MLC20; 3) ML-7 decreased both the lymphatic tonic contraction strength and pMLC20-to-MLC20 ratio; and 4) the increase in lymphatic phasic contraction frequency in response to increasing pressure was diminished by ML-7; however, the phasic contraction amplitude was not significantly altered by ML-7 either in the absence or presence of SP. These data provide the first evidence that tonic contraction strength and phasic contraction amplitude of the lymphatics can be differentially regulated, whereby the increase in MLC20 phosphorylation produces an activation in the tonic contraction without significant changes in the phasic contraction amplitude. Thus, tonic contraction of rat mesenteric lymphatics appears to be MLC kinase dependent.
Keywords: ML-7, lymphatic contraction, tonic contraction, phasic contraction
the lymphatic system plays essential roles by returning the protein-rich interstitial fluid to the blood circulation for fluid homeostasis, transporting lipids and lipid-soluble vitamins absorbed from the intestines to the circulation for nutrition, and distributing immune cells to the lymph nodes for defense against diseases. All of these vital functions rely on the contractile activities of the muscle cells residing in the lymphatic vessel wall. The muscular lymphatic vessels are composed of many basic structural and functional units called lymphangions, which are capable of exhibiting intrinsic contractile activities to propel lymph flow through the network of the lymphatic vessels. Although the classical influence of pressure and flow on lymphatic contractility has been well documented (14–16, 24, 31, 37, 38, 51), the molecular mechanisms regulating the contractile activities of the lymphatic muscle are not well understood.
Cardiac muscles and most vascular smooth muscles (VSMs) exercise their physiological functions by phasic and tonic contractions, respectively, whereas lymphatic muscle accomplishes its functions using both tonic and phasic contractions. In general, an increase in the intracellular Ca2+ concentration ([Ca2+]i) is the primary mechanism that initiates muscle contraction, a mechanism shared by striated muscles and VSMs. In striated muscle, Ca2+ binding to troponin C initiates thin filament activation and myosin binding to actin cooperate the contractile activities. These mechanisms govern the phasic myocardial contraction, which is relatively short, fast, and powerful, to propel the blood. In VSM, the phosphorylation and dephosphorylation of myosin light chain 20 (MLC20) by MLC kinase (MLCK) and MLC phosphatase (MLCP), respectively, play central roles in the regulation of contraction (41). This mechanism controls the tonic VSM contraction, which is comparatively slow but sustained.
The molecular mechanisms regulating the contraction of lymphatic muscle are basically unknown. We (37) have previously shown that lymphatic muscle is structurally composed of both smooth and striated muscle contractile elements and functionally shares similarities with both cardiac muscle and VSM. This correlates well with the unique functional aspects of both tonic and phasic lymphatic contractions. Furthermore, our previous studies (16, 18) have shown that the tonic contraction of isolated rat mesenteric lymphatics is decreased as transmural pressure is increased, while the corresponding phasic contraction amplitude of the vessel is slightly increased. This observation suggests that lymphatic muscles share at least some regulatory mechanisms with VSM in the regulation of tonic contractions. However, there is no solid evidence showing the existence of the key elements that would regulate lymphatic phasic contractile activity. Thus, to understand the mechanisms regulating lymphatic muscle contraction, we raised the following questions in this study: 1) Is there a relationship between transmural pressure and MLC20 phosphorylation in lymphatic muscle? and 2) How does MLC20 phosphorylation modulate the contractile dynamics of lymphatic muscle? To address these questions, pressure-induced and substance P [SP, a stimulator of the Ca2+-calmodulin (CaM)-MLCK pathway]-induced alterations in contractile activity and the corresponding changes in MLC20 phosphorylation of rat mesenteric lymphatics were studied in the absence or presence of 1-(5-iodonaphthalene-1-sulfonyl)-1H-hexahydro-1, 4-diazepine hydrochloride (ML-7), a MLCK inhibitor.
MATERIALS AND METHODS
Isolated vessel preparation and functional analyses.
Male Sprague-Dawley rats weighing 290–330 g were used for these experiments. Rats were housed in an environmentally controlled vivarium approved by the American Association for Accreditation of Laboratory Animal Care. All animal protocols in this study were approved by Texas A&M University Laboratory Animal Care Committee. Animals were fasted for 24 h before each experiment, with water available ad libitum. Rats were anesthetized (0.3 ml/kg im) with a combination of a droperidol-fentanyl solution [droperidol (20 mg/ml) and fentanyl (0.4 mg/ml)] and diazepam (2.5 mg/kg im). Half-supplemental doses were given as needed. To isolate a rat mesenteric lymphatic vessel, a 6- to 7-cm long loop of the small intestine was exteriorized through a midline laparotomy. A section of the mesentery was gently positioned over a semicircular viewing pedestal on a vessel preparation board. A mesenteric lymphatic vessel was centered over an optical window on the preparation board. The exteriorized tissues were continuously suffused with Dulbecco's PBS (catalog no. 14040-133, Invitrogen). The lymphatic vessel (80–120 μm in diameter) was isolated under a dissecting microscope using extreme caution not to damage the vessel. A segment of the lymphatic vessel (0.8–1.0 cm) was cut and transferred to a bath chamber filled with an albumin-enriched physiological salt solution [APSS; containing (in mM) 145 NaCl, 4.7 KCl, 2.0 CaCl2, 1.17 MgSO4, 1.2 NaH2PO4, 5.0 dextrose, 2.0 sodium pyruvate, 0.02 EDTA, and 3.0 MOPS with 10 g/l BSA]. After the lymphatic vessel had been isolated, the animal was euthanized. The isolated lymphatic was cannulated and tied on to two glass pipettes (tip diameter: 80–100 μm). These glass pipettes were connected to independently adjustable pressure reservoirs. Slight positive pressure (2–3 cmH2O) was applied to the vessel to detect leaks and to ensure that the vessel was undamaged and untwisted. The vessel was set to its approximate in situ length and positioned just above the glass coverslip of the chamber bottom. The chamber with pipette assemblies was then transferred to the stage of a microscope (Zeiss ACM). The vessel was set to an equilibration pressure of 1 cmH2O and warmed to 37–38°C. Once tonic and phasic contractions were observed, the vessel was allowed to equilibrate at 1 cmH2O for 15 min. After the equilibration period, the vessel contractile function was determined with the replacement of bath solution in the following order in different experiments: APSS (as an experimental vehicle control), 0.01% DMSO (no. D8779, Sigma, as a solvent control for ML-7, a specific inhibitor for MLCK), SP (10−7 M, no. S6883, Sigma), ML-7 at 10−6 M and then 10−5 M (catalog no. 475880, Calbiochem) in APSS, the combination of ML-7 (10−6 M) and SP (10−7 M) in APSS, and the combination of ML-7 (10−5 M) and SP (10−7 M) in APSS. Finally, the passive diameter of the vessel at each transmural pressure was measured after the vessel was exposed to nominally Ca2+-free APSS (0 mM added Ca2+ and EDTA, 3.0 mM) for 15 min. Experimental data were acquired for the last 3 min of each 5-min interval at the different transmural pressures tested (1, 3, and 5 cmH2O). Vessels were rinsed and then equilibrated for 3 min after each replacement of the SP solution or for 15 min after each increasing concentration of ML-7 and thereafter for the addition of Ca2+-free APSS solution.
Experiments were dynamically monitored by a microscope-charge-coupled device video camera, and the video data were recorded to a video DVD for the functional analyses of lymphatic contractions after the experiment. Outer end-diastolic and outer end-systolic diameters were measured from the recorded images using computer-based software in which cardiac pump analogies were used to determine diastolic and systolic diameters during the lymphatic contraction cycle (7, 8). Lymphatic diameters were normalized to the corresponding passive diameter at that respective pressure. For experimental comparisons between groups, we used the tonic index and phasic contraction amplitude as the parameters to compare the alterations in the sustained contraction (tone) and phasic contraction strength of the lymphatics, respectively. The tonic index is the percent difference between passive outer lymphatic diameter in Ca2+-free APSS (normalized to 100%) at that pressure and outer end-diastolic diameter at the same pressure, expressed as a percentage of the passive outer lymphatic diameter. The phasic contraction amplitude was defined as the difference between outer end-diastolic diameter, expressed as a percentage of the passive diameter, minus the outer end-systolic diameter, expressed as a percentage of the passive diameter. This normalization procedure has been widely used in lymphatic studies (16, 17, 22, 27, 34, 46, 49) and other microcirculatory studies (20, 28, 32) to account for anatomic variations in vessel size.
Statistical differences were determined by ANOVA followed by Fisher's least-significant difference test. P values of <0.05 were considered significant.
Immunohistochemistry.
To determine the relative levels of phosphorylation of MLC20, rat mesenteric lymphatic vessels were prepared for immunohistochemical detection by the following procedure. Multiple adjacent lymphatic vessels were isolated as described above, cannulated, and then pressurized to 3 cmH2O from both the input and output ends followed by an incubation at 38°C in one of the following: 1) APSS as the vehicle control for 30 min, 2) APSS for 20 min and then SP (10−7 M) in APSS for 10 min, 3) ML-7 (10−5 M) in APSS for 30 min, 4) ML-7 (10−5 M) in APSS for 20 min, and then the combination of ML-7 (10−5 M) and SP (10−7 M) in APSS for 10 min. For each immunohistochemical experiment, APSS vehicle controls were included. After these treatments, vessels were rinsed three times in PBS, and pressurized vessels were fixed in 2% paraformaldehyde-PBS for 60 min at room temperature. After fixation, vessels were washed in PBS three times for 5 min each. Vessels were permeabilized for 5 min in −20°C methanol and washed three times in PBS for 5 min each. Fixed and permeablized vessels were incubated with the primary antibody, mouse anti-phospho-MLC (pMLC) monoclonal antibody (Cell Signaling Technology, Beverly, MA) at a concentration of 1:100 in blocking solution (1% BSA and 5% normal goat serum in PBS) or comparable concentrations of normal mouse IgG (for negative controls) overnight. Vessels were rinsed in PBS three times for 5 min each followed by an incubation with the secondary antibody, goat anti-mouse antibody conjugated to Oregon green 488 (Molecular Probes, Carlsbad, CA), for 60 min at room temperature. Vessels were finally washed with PBS three times for 5 min. Vessels (unknowns and the appropriate negative controls) were then secured to the stage of a confocal microscope (Leica TCS SP2) for immediate observation with a ×20 Leica objective. Stained vessels were scanned throughout the entire vessel diameter in 0.5-μm z-axis steps. Reconstructions on the three-dimensional (3-D) image stacks were performed using the Leica confocal software package, producing flat integrated projections. Negative controls for all experiments were produced and analyzed via similar procedures from one section of each vessel. The corresponding negative controls were scanned at the same instrument settings as the unknowns for the valid comparison of relative fluorescence intensities.
We quantified the fluorescence intensity of the MLC20 phosphorylation signal from the integrated projections using the following procedure: each 3-D dataset imaged two vessel sections from the same or nearby vessel segment, a section from a given treatment group, and its corresponding IgG negative control. The mean fluorescence intensities from the nonspecific background fluorescence were determined in the IgG negative control sample using thresholding techniques to outline the vessel. The integrated fluorescence intensities of the treated vessels were determined in a similar fashion. We analyzed vessel segments from the same animal (for both treatments and negative controls) in a pairwise fashion. The integrated value from the corresponding nonspecific background fluorescence vessel was subtracted from the integrated fluorescence intensity of the treated vessels, producing a background-subtracted fluorescence intensity value for each vessel. The integrated intensities (representing the total amount of pMLC20 in each vessel) of the APSS control and vessels from other treatment groups were determined in each experiment. We then determined the mean value of the integrated intensities for all of the APSS control vessels in each experimental group. The individual vessel background-subtracted signal was normalized to the mean value of the background-subtracted fluorescence intensities of the APSS control segments within that experiment. Thus, the fluorescent intensities of each experimental treatment group represent percentages of the fluorescent intensities of the MLC20 phosphorylation in their respective controls. Experiments were repeated in different animals (n = 3–8 animals) on different days.
Western blot analyses.
Lymphatic vessels were isolated, cannulated, and pressurized at different pressures, as previously described. At each pressure, vessels were equilibrated for 20 min, rapidly snap frozen in liquid nitrogen, and then stored at −80°C. Vessels treated with ML-7 were collected in a similar fashion. Lymphatic samples were sonicated in protein-solubilizing buffer and run on a 4–18% gradient SDS-polyacrylamide gel. Proteins were transferred to a nitrocellulose membrane with a Bio-Rad transblot apparatus. The transfer was verified with Ponceau-S staining. MLC20 phosphorylation was detected with mouse anti-pMLC monoclonal antibody (Cell Signaling Technology), while mouse monoclonal anti-MLC20 antibody (Sigma) was used for the detection of total MLC20. Antibody binding was revealed using the Pierce detection system (SuperSignal West Dura Extended Duration Substrate, Pierce). Densitometry on the resulting bands was performed using Multi-Analyst software (Bio-Rad). Membranes were stripped of the pMLC20 antibody using ImmunoPure IgG Elution Buffer (Pierce) and then reprobed with MLC20 antibody. The resulting pMLC20-to-MLC20 ratio was used for the quantitative analyses. Quantity One software (Bio-Rad) was used to quantify the intensities of the MLC20 bands obtained on the blots. Western blot analyses of lymphatic vessel proteins followed by quantification were performed three times for each sample, and the resulting mean values ± SE were calculated.
Detection of MLC20 phosphorylation by urea-glycerol gel electrophoresis.
MLC20 phosphorylation was also analyzed by urea glycerol gel electrophoresis, as previously described with few modifications (42). Protein samples were isolated from rat mesenteric lymphatic tissues that had been previously snap frozen in liquid nitrogen. Vessels (nonpressurized) had been either treated with SP or ML-7 or were untreated and used as controls. Samples were homogenized in cell lysis buffer (45) and sonicated. Protein was precipitated with trichloroacetic acid-acetone precipitation and solubilized in a sample buffer containing 8 M urea. Samples were electrophoresed in polyacrylamide gels at 400 V. Proteins were then transferred to a nitrocellulose membrane overnight. pMLC20 and nonphosphorylated MLC20 were detected by Western blot analysis using polyclonal MLC20 antibody (1:5,000, Sigma). The migration rate of pMLC20 was faster than the nonphosphorylated form (MLC20), and, hence, two bands (corresponding to the monophosphorylated and nonphophorylated forms) were detected on the blot. Quantification of the intensities of the MLC20 bands obtained on the blots was performed as described above. The percentage of phosphorylation of MLC20 from the urea-glycerol gels was calculated by the following formula: (IpMLC/IpMLC + IMLC) × 100, where IpMLC is the total intensity of the phosphorylated form and IMLC is the total intensity of the unphosphorylated form. Experiments were performed in triplicate, and mean values ± SE were calculated and plotted.
RESULTS
An increase in transmural pressure decreases tonic contraction and MLC20 phosphorylation.
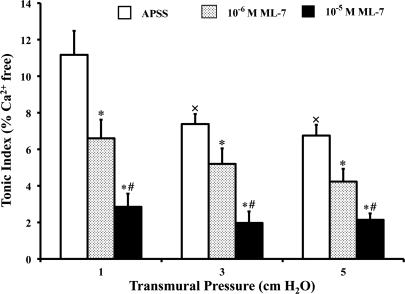
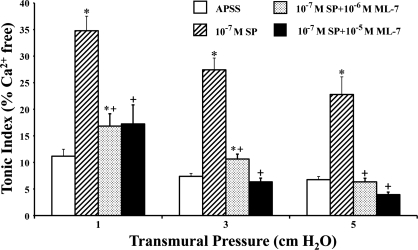
As transmural pressure was increased from 1 to 5 cmH2O, the tonic index of the lymphatics decreased, reflecting a decrease in the sustained contraction of the vessels. As shown in Fig. 1 (open bars), the tonic index fell 34% (from 11.2% at 1 cmH2O to 7.4% at 3 cmH2O, P < 0.01, n = 5) and 39% (from 11.2% at 1 cmH2O to 6.8% at 5 cmH2O, P < 0.004, n = 5) from its starting value as the transmural pressure was increased.
Fig. 1.
Increases in transmural pressure and ML-7 decrease the tonic contraction of the lymphatics. The tonic index was calculated as described in materials and methods. APSS, albumin-enriched physiological salt solution. Values are means ± SE for 5 experiments. ×P < 0.05 vs. 1 cmH2O of pressure; *P < 0.05 vs. control (APSS); #P < 0.05 vs. 10−6 M ML-7.
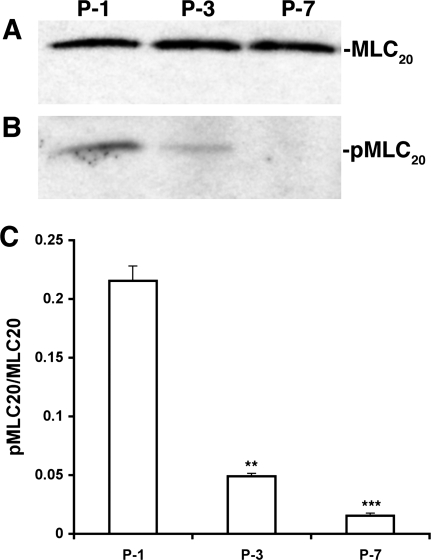
To determine the phosphorylation of MLC20 (pMLC20) under similar conditions, Western blot analyses were performed on proteins isolated from lymphatic vessels subjected to different pressures, and the ratios of pMLC20 to MLC20 were calculated. Figure 2, A and B, shows representative antibody reaction blots for the relative levels of MLC20 and pMLC20 in protein samples from lymphatic vessels held at 1, 3, or 7 cmH2O. The results demonstrate that there was very little change in the relative levels of MLC20 among the samples but a significant decrease in pMLC20 in lymphatic vessels subjected to pressures of 3 or 7 cmH2O (Fig. 2). Quantitative analyses indicated that, relative to the levels at 1 cmH2O, the pMLC20-to-MLC20 ratio was decreased by 77% at 3 cmH2O (from 0.215 ± 0.012 to 0.049 ± 0.002, P < 0.005, n = 3) and by 93% at 7 cmH2O (from 0.215 ± 0.012 to 0.016 ± 0.002, P < 0.001, n = 3; Fig. 2C). These data demonstrate that an increase in transmural pressure was accompanied by a large decrease in the phosphorylation of MLC20 in mesenteric lymphatics.
Fig. 2.
Increases in transmural pressure decrease myosin light chain 20 (MLC20) phosphorylation in pressurized rat mesenteric lymphatics. A: representative blot for the MLC20 reaction on lymphatic proteins isolated from lymphatics at different pressures. P-1, P-3, and P-7 indicate pressures of 1, 3, and 7 cmH2O, respectively. B: representative blot for phosphorylated MLC20 (pMLC20). C: quantitative analyses of the pMLC20-to-MLC20 ratio. Signal intensities for MLC20 and pMLC20 from three different blots were used for the quantitative analyses. **P < 0.005 between the values at P-1 to P-3 and ***P < 0.001 between the values at P-1 to P-7.
Effects of ML-7 on the contractile activity of isolated rat mesenteric lymphatic vessels.
To investigate the effects of MLC20 phosphorylation on lymphatic contractile activity, each vessel was exposed to ML-7, a selective MLCK inhibitor. ML-7 was dissolved in DMSO, and the ML-7-DMSO solution was then further diluted in APSS. The final concentration of DMSO in APSS was 0.01%. Control experiments showed that 0.01% DMSO did not significantly alter the contractile activity of the lymphatics compared with their function in APSS (data not shown). However, as shown in Fig. 1, with the addition of ML-7 to the APSS perfusion solution, the tonic index of the vessels treated with 10−6 M (shaded bars) and 10−5 M (solid bars) ML-7 was decreased in a dose-dependent manner by 41.1% and 78.6% at 1 cmH2O pressure, 29.7% and 73.0% at 3 cmH2O pressure, and 38.2% and 69.1% at 5 cmH2O pressure, respectively, compared with their corresponding control values (open bars for APSS alone). These results demonstrate that ML-7 significantly decreased the tonic contraction of the lymphatic vessels, presumably via the inhibition of MLC20 phosphorylation.
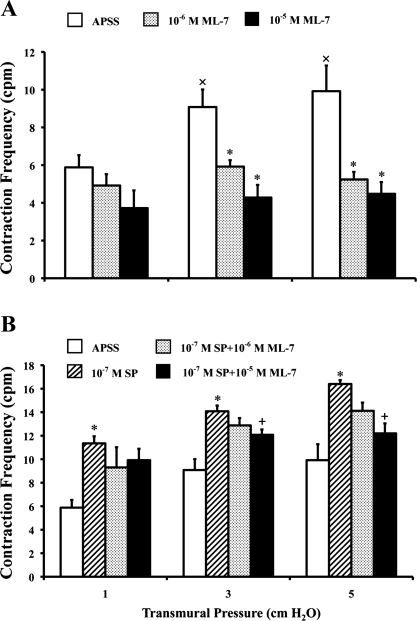
As shown in Fig. 3A, contraction frequencies of the APSS control lymphatics increased as the pressures increased (open bars) over a range of pressures from ∼1 to ∼5 cmH2O. Contraction frequencies at 3 and 5 cmH2O were significantly higher than those at 1 cmH2O. Interestingly, the lymphatic contraction frequency was decreased in the presence of ML-7 compared with the values obtained in APSS alone. In addition, the increase in frequency with increasing pressure normally observed in APSS was blunted in the presence of ML-7. Compared with their corresponding vehicle controls (Fig. 3A, open bars), both the lower (10−6 M) and higher (10−5 M) concentrations of ML-7 significantly decreased the contraction frequencies at 3 and 5 cmH2O. At 10−6 M ML-7 (Fig. 3A, shaded bars), the frequencies were decreased from 5.9 to 4.9 counts/min (16.3%) at 1 cmH2O, from 9.1 to 5.9 counts/min (34.8%) at 3 cmH2O, and from 9.9 to 5.2 counts/min (47.2%) at 5 cmH2O pressure. At 10−5 M ML-7 (Fig. 3A, solid bars), the values decreased from 5.9 to 3.7 counts/min (36.7%) at 1 cmH2O, from 9.1 to 4.3 counts/min (52.9%) at 3 cmH2O, and from 9.9 to 4.5 counts/min (54.8%) at 5 cmH2O pressure.
Fig. 3.
ML-7 decreases the phasic contraction frequency of the lymphatics. A: ML-7 decreases the contraction frequency [in counts/min (cpm)] of the lymphatics. B: ML-7 decreases the substance P (SP)-stimulated contraction frequency. Values are means ± SE for 5 experiments. *P < 0.05 vs. control; +P < 0.05 vs. SP; ×P < 0.05 vs. 1 cmH2O.
Furthermore, SP (10−7 M) significantly increased the contraction frequency of the lymphatics in a pressure-dependent manner (Fig. 3B, hatched bars) by 93.1% (from 5.9 to 11.4 counts/min) at 1 cmH2O, 55.1% (from 9.1 to 14.1 counts/min) at 3 cmH2O, and 65.3% (from 9.9 to 16.4 counts/min) at 5 cmH2O pressure compared with their corresponding vehicle controls (Fig. 3B, open bars) over a range of pressures from 1 to 5 cmH2O. In the presence of SP, the lower concentration of ML-7 (Fig. 3B, shaded bars) showed a trend to decrease the contraction frequency from 11.3 to 9.3 counts/min (18.1%) at 1 cmH2O, from 14.1 to 12.9 counts/min (8.52%) at 3 cmH2O, and from 16.4 to 14.1 counts/min (13.9%) at 5 cmH2O of pressure. The higher concentration of ML-7 (Fig. 3B, solid bars) significantly decreased the SP-induced contraction frequency from 14.1 to 12.1 counts/min (14.2%) at 3 cmH2O and from 16.4 to 12.2 counts/min (25.6%) at 5 cmH2O pressure; however, the value decrease from 11.4 to 9.9 counts/min (12.6%) at 1 cmH2O was not statistically significant.
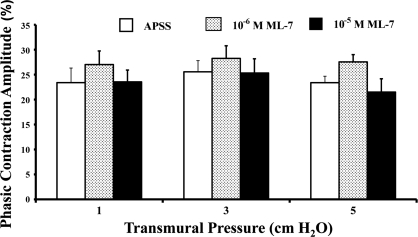
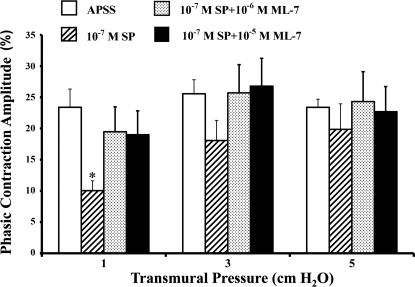
We also determined the phasic contraction amplitude of the lymphatics in the same vessels used for tonic index analyses. In the presence of APSS alone (vehicle control), the phasic contraction amplitudes were 23.4 ± 2.9% at 1 cmH2O, 25.6 ± 2.3% at 3 cmH2O, and 23.4 ± 1.3% at 5 cmH2O (Fig. 4, open bars). However, the corresponding phasic contraction amplitudes were not significantly altered by ML-7, even at the higher concentration of ML-7 (10−5 M; Fig. 4, shaded and solid bars).
Fig. 4.
ML-7 does not change the phasic contraction amplitude of the lymphatics. Effects of ML-7 on the phasic contraction amplitude were calculated as described in materials and methods. Values are means ± SE for 5 experiments.
To further understand the role of MLC20 phosphorylation in the regulation of contractile activity of lymphatic muscle, SP was used as an agonist to activate the Ca2+-CaM-MLCK pathway. Exposure of the lymphatic vessels to 10−7 M SP (Fig. 5, hatched bars), compared with the vehicle/APSS control (Fig. 5, open bars), significantly increased the tonic contraction of the lymphatic vessel by 186.6%, 270.3%, and 235.3% at 1, 3, and 5 cmH2O of pressure, respectively. Preincubation of the lymphatic vessels with ML-7 significantly inhibited the SP-induced increases in tonic contraction. The inhibition of SP-induced tone by 10−6 and 10−5 M ML-7 was 48.6% and 54.2% at 1 cmH2O, 61.3% and 77.0% at 3 cmH2O, and 72.4% and 82.9% at 5 cmH2O of pressure. These data demonstrate that the inhibition of MLC20 phosphorylation by ML-7 significantly blocked the SP-induced activation of tone.
Fig. 5.
ML-7 decreases SP-induced tonic contraction of the lymphatics. Values are means ± SE for 5 experiments. *P < 0.05 vs. control; +P < 0.05 vs. SP.
Figure 6 shows the phasic contraction amplitude of SP-treated vessels in the absence or presence of ML-7 and/or SP. The percentage of the phasic contraction amplitudes for the vehicle control (Fig. 6, open bars) versus SP-treated vessels (Fig. 6, shaded bars) were 23.4% versus 10.3% at 1 cmH2O, 25.6% versus 18.1% at 3 cmH2O, and 23.4% versus 19.9% at 5 cmH2O, respectively. However, SP significantly reduced the phasic contraction amplitude only at 1 cmH2O of pressure (P < 0.005, n = 5). The phasic contraction amplitude of the ML-7-treated vessels was not significantly different than that of the APSS controls even after SP activation. Thus, ML-7 significantly attenuated the SP-induced change in phasic contraction amplitude only at 1 cmH2O of pressure.
Fig. 6.
ML-7 does not significantly change the phasic contraction amplitude of the lymphatics in the presence of SP. Values are means ± SE for 5 experiments. *P < 0.05 vs. control.
ML-7 decreases the phosphorylation of MLC20.
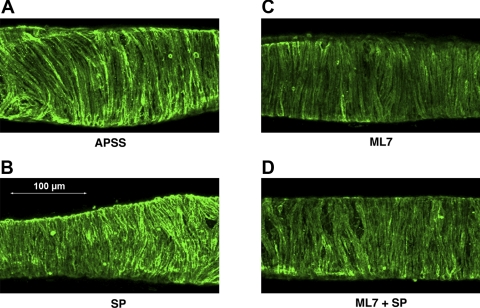
To verify the degree of inhibition of MLCK by ML-7, we used immunohistochemistry to compare the phosphorylation of MLC in isolated rat mesenteric lymphatics before and after incubation with ML-7, as demonstrated by the typical images shown in Fig. 7. Compared with the IgG negative control (data not shown), positive staining for pMLC20 was observed in all vessel treatment groups: APSS control (Fig. 7A), SP (10−7 M; Fig. 7B), ML-7 (10−5 M; Fig. 7C), or the combination of both ML-7 and SP (Fig. 7D). The positive staining was almost exclusively localized in the muscle layer and was consistent with the circumferential orientation of the muscle cells (Fig. 7, A–D). The fluorescent intensity of the pMLC20 staining was quantified and normalized to the APSS controls (normalized to 1.0) in the different treatment groups, as described in methods. SP-treated vessels (10−7 M, n = 23) showed significantly increased normalized pMLC20 staining (1.98 ± 0.28, mean ± SE) compared with that in paired control vessels exposed only to APSS. Vessels treated with ML-7 (10−5 M, n = 7) showed a significant decrease in the normalized staining intensity (0.51 ± 0.06) compared with the paired vessels treated with APSS. Furthermore, the average intensity of staining for pMLC20 in the vessels treated with a combination of 10−5 M ML-7 and 10−7 M SP (0.48 ± 0.21, n = 8) was not different than that seen with ML-7 treatment but was significantly weaker than that seen with either APSS or APSS + SP treatment.
Fig. 7.
Immunohistochemical staining analysis for pMLC20 in the lymphatics. pMLC20 in the isolated rat mesenteric lymphatics with the corresponding treatments described earlier were subjected to immunohistochemical detection. A: APSS-treated lymphatic vessel; B: 10−7 M SP-treated vessel; C: 10−5 M ML-7-treated vessel; D: 10−5 M ML-7 + 10−7 M SP-treated lymphatic vessel.
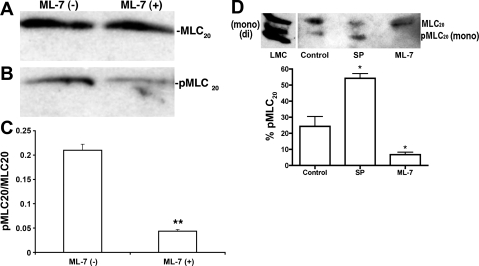
To further quantify the relative levels of MLC20 phosphorylation before and after ML-7 treatment, proteins from the lymphatic vessels were subjected to Western blot analyses, and the ratios of pMLC20 to MLC20 were calculated as previously described. Treatment of the vessels (pressurized at 1 cmH2O) with 10−5 M ML-7 decreased the phosphorylation of MLC20 significantly compared with the MLC20 phosphorylation levels in untreated vessels (Fig. 8). Figure 8, A and B, show representative blots for the relative levels of MLC20 and pMLC20, respectively, in protein samples from ML-7-treated or untreated lymphatic vessels. Quantitative analyses of three blots indicated that the pMLC20-to-MLC20 ratio significantly decreased by ∼80% [0.21 ± 0.012 (untreated) to 0.043 ± 0.004 (ML-7 treated), P < 0.001, n = 3] in the 10−5 M ML-7-treated samples compared with untreated samples (Fig. 8C). These results demonstrate a significant inhibition of MLC20 phosphorylation by ML-7 in isolated rat mesenteric lymphatics. We also quantified MLC20 phosphorylation levels using the urea-glycerol gel approach. As shown in Fig. 8D, the mono- and diphosphorylated forms (pMLC20) migrated faster than the nonphosphorylated form (MLC20). Interestingly, cultured lymphatic muscle cell extracts exhibited both mono- and diphosphorylated forms of MLC20, whereas only the monophosphorylated form was detectable in tissue samples. However, the results shown in Fig. 8D clearly demonstrate that the percentage of MLC20 phosphorylation was significantly increased in SP-treated nonpressurized vessels and that ML-7 treatment significantly decreased the MLC20 phosphorylation level in mesenteric lymphatics. The percentage of MLC20 phosphorylation significantly increased [from 24.4% ± 6.1 (untreated) to 54.1% ± 2.9 (SP treated), P < 0.011, n = 3] in samples treated with 10−6 M SP and significantly decreased [from 24.4% ± 6.1 (untreated) to 6.7% ± 1.5 (ML-7 treated), n = 3] in samples treated with 10−5 M ML-7 compared with untreated control samples (Fig. 8D). As the total phosphorylation for each lane was calculated separately, the percentages of MLC20 phosphorylation shown in Fig. 8D were independent of protein loading.
Fig. 8.
Quantitative analyses of pMLC20 to MLC20 in lymphatics. A and B: representative antibody reaction blots for the relative levels of MLC20 (A) and pMLC20 (B) in protein samples from ML-7 (10−5 M)-treated or untreated lymphatic vessels. C: signal intensities for MLC20 and pMLC20 from three different blots were used for the quantitative analysis. **P ≤ 0.001, significant difference between untreated and ML-7-treated samples. D: MLC20 phosphoprotein analysis by urea-glycerol gel. Un, mono, and di represent the unphosphorylated, monophosphorylated, and diphosphorylated forms of MLC20. Top, representative antibody reaction blot for the relative levels of MLC20 and pMLC20 in samples treated with either SP (10−7 M) or ML-7 (10−5 M) after urea-glycerol gel electrophoresis. LMC, lymphatic muscle cells. Bottom, quantitative analysis for the percentage of pMLC20, which was calculated as described in materials and methods from three different blots. *P ≤ 0.05, significant difference between untreated (control) and SP- or ML-7-treated samples.
DISCUSSION
Our results clearly demonstrate that the strength of tonic contractions of the isolated rat mesenteric lymphatics depend on MLC20 phosphorylation, whereas the phasic contraction amplitude does not appear to be regulated by the MLC20 phosphorylation status under these conditions. The mechanisms responsible for these two components of lymphatic contraction are poorly understood. In this study, we demonstrated that significant changes in tone, but not phasic contraction amplitude, were correlated with significant changes in MLC20 phosphorylation. An interesting observation was that the tonic component of contraction and MLC20 phosphorylation both decreased as transmural pressure increased. Finally, we unexpectedly observed that the MLCK inhibitor ML-7 significantly decreased phasic contraction frequency and blunted the normal rise in phasic contraction frequency that accompanies increasing pressure. These findings indicate that tonic contractions and the phasic contraction amplitude of the mesenteric lymphatics appear to be regulated by different pathways.
Although several kinases are involved in the phosphorylation of MLC20 in VSM, the major one that is widely documented is the Ca2+-CaM-MLCK pathway. Several studies (21, 35) have shown that the phosphorylation of MLC20 by MLCK is a crucial pivot through which different regulatory mechanisms are linked to modulate the contractile behavior of VSM. In our study, ML-7, a MLCK inhibitor, inhibited the tonic contraction of lymphatic vessels in a dose-dependent manner (Fig. 1), resulting in a significant increase in diastolic diameter. Similar results have been reported in studies (29, 36, 48) on the effects of the MLCK inhibitor on arteriolar, gastrointestinal, and uterine smooth muscle, suggesting that the tonic contraction of the lymphatic muscle may share some contractile regulatory mechanisms with smooth muscle from other organs.
Our data indicate that the basal level of pMLC20 at a pressure of 1 cmH2O decreased dramatically at higher transmural pressures (Fig. 2). We estimate that the percentage of MLC20 that is phosphorylated basally is ∼20–25%, based on the urea-glycerol Western blot data from untreated/unpressurized lymphatics (Fig. 8). Rat mesenteric lymphatics have been shown to exhibit optimal pumping over a diastolic pressure range from 2 to 5 cmH2O in vivo (4). Taken together, we propose that the phasic contractile activity of lymphatic muscle is operative with ∼20% or less of the MLC20 phosphorylated. This relative level of MLC20 phosphorylation in lymphatic muscle correlates well with findings in other smooth muscle (5, 44, 52). Furthermore, our immunohistochemical data clearly demonstrate that pMLC20 is localized in the muscle cells of lymphatics and that the treatments of ML-7 or SP alter the phosphorylation level of the protein without grossly altering protein localization.
SP induces contraction through the Ca2+-CaM-MLCK pathway in different muscle types, including lymphatics (6, 10). Our previous study (2) showed that SP stimulated the contractile activity of rat mesenteric lymphatic vessels in situ. However, in our recent isolated vessel study (9), 10 nM SP did not change the contraction amplitude significantly at lower pressure ranges tested (at 1, 3, or 5 cmH2O) but increased the frequency and tone. The present study supports our previous findings showing that SP potentiates the tonic contraction of isolated rat mesenteric lymphatic vessels and increases the frequency of contraction without a significant change in phasic contraction amplitude (Figs. 5, 3, and 6, respectively). In addition, our data showed an increase in MLC20 phosphorylation in the lymphatic muscle cell layer after SP treatment (Fig. 7B), suggesting that the MLCK pathway is activated in conjunction with SP-induced tonic contractile activity. Furthermore, the SP-induced tonic contraction of lymphatic vessels was significantly inhibited by preincubation of the vessels with ML-7, and the vessels showed a corresponding decrease in MLC20 phosphorylation when treated with the combination of ML-7 (10−5 M) and SP (10−7 M) (Fig. 7D). These results further support the view that the Ca2+-CaM-MLCK pathway is involved in the regulation of the tonic contractile mechanism of the lymphatic muscle.
In contrast, we found no significant difference in the phasic contraction amplitude of lymphatics treated with either ML-7 alone or ML-7 + SP compared with their APSS controls, suggesting little influence of MLCK on the phasic contraction strength of lymphatic muscle. In our Western blot experiments, the MLC20 phosphorylation level was inhibited by ∼80% in the presence of ML-7 (10−5 M). Thus, it is possible that the remaining MLC20 phosphorylation was sufficient to maintain the phasic contractile amplitude of lymphatics. It is also possible that some minimum level of MLC20 phosphorylation is sufficient for maintaining but not regulating the amplitude of phasic lymphatic contractions. Since pMLC20 levels are already reduced at higher pressures (Fig. 2), these observations suggest that the basal state of MLC20 phosphorylation may be critical in determining whether MLCK inhibitors (such as ML-7) selectively alter tonic versus phasic activity. Similar conclusions can be drawn from a study (23) in rat iliac lymphatics using the Rho kinase inhibitor Y-27632. In that study, the inhibition of MLC20 phosphorylation by Y-27632 led to an inhibition of lymphatic tone. The Rho-Rho kinase pathway is involved in Ca2+-independent regulation of MLC20 phosphorylation through its inhibition of MLCP and, thus, the regulation of smooth muscle contraction (13, 41). Those studies showed that receptor-mediated G protein-coupled mechanisms activate RhoA to modulate MLCP activity. In addition, several other mechanisms contribute to the inhibition of MLCP activity and Ca2+ sensitization in smooth muscle via the activation of PKC, Rho-associated kinase, or integrin-linked kinase (ILK) signaling cascades (13, 41, 25). Nevertheless, similar to the results of our studies, Hosaka et al. (23) found that the inhibition of Rho kinase by Y-27632 completely blocked the generation of phasic contractions at higher doses. While they attributed those effects to the inhibition of Rho-dependent Rho-associated protein kinase (ROCK)-I/II, the doses of Y-27632 used have also been shown to block PKC-related kinase (PRK)-2 (a different Rho-dependent kinase closely related to PRK 1, a known activator of CPI-17 and a smooth muscle Ca2+-sensitizing agent). The doses of Y-27632 used are also known to inhibit mitogen- and stress-activated protein kinase 1 (MSK1) and MAPK-activated protein kinase-1 (MAPKAP-K1b) (9). Finally, the complete cessation of phasic activity by high doses by Y-27632 may be due to blockade of the pacemaking activity of the lymphatic pump; however, this was not specifically evaluated. Unfortunately, that study did not examine pMLC20 levels under inhibitor-treated conditions, so it is difficult to directly correlate all of their results with ours.
Other possible explanations for the lack of effect of 10−5M ML-7 on phasic contraction amplitude in the present study could be related to the following. First, in addition to MLC20 phosphorylation, [Ca2+]i is also required for the development and maintenance of contractile activity of lymphatic muscle (19, 30, 46, 50). ML-7 has been reported to inhibit the tone of rat cremaster arterioles (52) in a dose-dependent manner but did not prevent pressure-induced increases in [Ca2+]i. Thus, it is possible that in our experiments, the ML-7 induced inhibition of MLCK but [Ca2+]i was not altered, which maintained the phasic contraction amplitude. Second, although MLCK in the Ca2+-CaM-MLCK pathway is thought to be responsible for the phosphorylation of MLC20, recent studies have shown that several other protein kinases might also contribute to the phosphorylation of the MLC20 in a Ca2+-independent manner, such as through CaM-dependent protein kinase II (39), MAPKAP (33), ROCK (23), ILK (47), or zipper-interacting protein kinase (11). Thus, it would be interesting to see whether these other kinases, acting through different mechanisms that are MLC20 phosphorylation independent, can modulate the phasic contractile activity of lymphatic muscle. Finally, the transient receptor potential (TRP) superfamily is a newly emerging gene family that encodes Ca2+-permeable nonselective cation channels/TRP canonical (TRPC) channels (1). A recent study (40) has demonstrated that ML-7 caused a rapid, reversible, and MLCK-independent enhancement of inward current in HEK-293 cells transfected with TRPC7 channel. This cation current may provide the minimum requirement for the contractile amplitude. Future studies using recombinant adenoviral approaches to specifically alter MLC20 phosphorylation by overexpressing nonphosphorylatable MLC20 into lymphatics may provide additional insights into the role of MLC20 phosphorylation in phasic contractile characteristics.
Furthermore, ML-7 significantly decreased the pressure-induced increase in phasic contraction frequency at both 10−6 and 10−5 M, both concentrations purportedly specific for MLCK inhibition (Fig. 3). Since MLC20 phosphorylation is decreased with increased pressure (Fig. 2) and ML-7 would have further inhibited MLC20 phosphorylation, it is possible that ML-7 might also have inhibited proteins other than MLCK to have an effect on phasic contraction frequency. It is reported that ML-7 is >30-fold more potent in inhibiting MLCK than the cation inward TRPC6 channel (40), indicating that ML-7 might have decreased [Ca2+]i, which would have subsequently decreased contraction frequency. As such, more experiments are warranted to address the effects of ML-7 on lymphatic contraction frequency. We did not use higher doses of ML-7 (10−4M) to keep the pharmacological level of ML-7 in the range that has been reported to be relatively selective for MLCK inhibition (3). The vehicle solution (APSS) that we used in this study contained 1% BSA for the purpose of maintaining a colloid osmolarity that is essential for functional lymphatics. However, albumin has a propensity to bind a wide variety of organic, inorganic, negatively, and positively charged ligands. The binding affinities of albumin for different ligands or peptides are often as high as those of the specific interactions (26, 43). Therefore, in initial experiments, we compared the influences of albumin-containing and nonalbumin-containing vehicle solution on the function of lymphatics. We found that data obtained from the experiments using albumin-containing vehicle solution were basically consistent with data obtained from the experiments using nonalbumin-containing vehicle solution except that the effects of ML-7 in nonalbumin-containing vehicle solution showed more obvious inhibitory effects on lymphatic contractile activity, with contractions stopping in nine of nine vessels, i.e., ML-7 reduced the contraction frequency to zero at a concentration of 10−5 M (data not shown).
In summary, this study presents the first evidence that the tonic and phasic components of mesenteric lymphatic contractions may be differentially regulated, in which the tonic component is strongly MLCK dependent. This information advances our understanding of the unique contractile mechanisms of lymphatic muscle and may eventually lead to therapeutic strategies for treating lymphatic dysfunction.
GRANTS
This work was supported by National Heart, Lung, and Blood Institute Grants HL-80526, KO2-HL-86650, and HL-75199. Z. Nepiyushchikh was the recipient of Lymphatic Research Foundation Postdoctoral Fellowship.
Acknowledgments
The authors thank Steve Greiner and E. Lynn Wink for the great efforts related to data analyses.
REFERENCES
- 1.Albert AP, Saleh SN, Large WA. Identifivation of canonical transient receptor potential (TRPC) channel proteins in native vascular smooth muscle cells. Curr Med Chem 16: 1158–1165, 2009. [DOI] [PubMed] [Google Scholar]
- 2.Amerini S, Ziche M, Greiner ST, Zawieja DC. Effect of substance P on mesenteric lymphatic contractility in the rat. Lymphat Res Biol 2: 2–10, 2004. [DOI] [PubMed] [Google Scholar]
- 3.Bain J, Mclauchlan H, Elliott M, Cohen M, Cohen P. The specificities of protein kinase inhibitors: an update. Biochem J 371: 199–204, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Benoit JN, Zawieja DC, Goodman AH, Granger HJ. Characterization of intact mesenteric lymphatic pump and its responsiveness to acute edemagenic stress. Am J Physiol Heart Circ Physiol 257: H2059–H2069, 1989. [DOI] [PubMed] [Google Scholar]
- 5.Chang S, Hypolite JA, DiSanto ME, Changolkar A, Wein AJ, Chacko S. Increased basal phosphorylation of detrusor smooth myosin in alloxan-induced diabetic rabbit is mediated by upregulation of Rho-kinase β and CPI-17. Am J Physiol Renal Physiol 290: F650–F656, 2006. [DOI] [PubMed] [Google Scholar]
- 6.Corson MA, Sellers JR, Adelstein RS, Schoenberg M. Substance P contracts bovine tracheal smooth muscle via activation of myosin light chain kinase. Am J Physiol Cell Physiol 259: C258–C265, 1990. [DOI] [PubMed] [Google Scholar]
- 7.Davis MJ An improved, computer-based method to automatically track internal and external diameter of isolated microvessels. Microcirculation 12: 361–372, 2005. [DOI] [PubMed] [Google Scholar]
- 8.Davis MJ, Zawieja DC, Gashev AA. Automated measurement of diameter and contraction waves of cannulated lymphatic microvessels. Lymphat Res Biol 4: 3–10, 2006. [DOI] [PubMed] [Google Scholar]
- 9.Davis MJ, Lane MM, Davis AM, Durtschi D, Zawieja DC, Muthuchamy M, Gashev AA. Modulation of lymphatic muscle contractility by the neuropeptide substance P. Am J Physiol Heart Circ Physiol 295: H587–H589, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Davies SP, Reddy H, Caivano M, Cohen P. Specificity and mechanism of action of some commonly used protein kinase inhibitors. Biochem J 351: 95–105, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.DeVane CL Substance P: a new era, a new role. Pharmacotherapy 21: 1061–1069, 2001. [DOI] [PubMed] [Google Scholar]
- 12.Endo A, Surks HK, Mochizuki S, Mochizuki N, Mendelsohn ME. Identification and characterization of zipper-interacting protein kinase as the unique vascular smooth muscle myosin phosphatase-associated kinase. J Biol Chem 279: 42055–42061, 2004. [DOI] [PubMed] [Google Scholar]
- 13.Fujihara H, Walker LA, Gong MC, Lemichez E, Boquet P, Somlyo AV, Somlyo AP. Inhibition of RhoA translocation and calcium sensitization by in vivo ADP-ribosylation with the chimeric toxin DC3B. Mol Biol Cell 8: 2437–2447, 1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gashev AA The pump function of the lymphangion and the effect on it of different hydrostatic conditions. Fiziol Zh SSSR Im I M Sechenova 75: 1737–1743, 1989. [PubMed] [Google Scholar]
- 15.Gashev AA Physiologic aspects of lymphatic contractile function: current perspectives. Ann NY Acad Sci 979: 178–187, 2002. [DOI] [PubMed] [Google Scholar]
- 16.Gashev AA, Davis MJ, Delp MD, Zawieja DC. Regional variations of contractile activity in isolated rat lymphatics. Microcirculation 11: 477–492, 2004. [DOI] [PubMed] [Google Scholar]
- 17.Gashev AA, Davis MJ, Zawieja DC. Inhibition of the active lymph pump by flow in rat mesenteric lymphatics and thoracic duct. J Physiol 540: 1023–1037, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gashev AA, Delp MD, Zawieja DC. Inhibition of active lymph pump by simulated microgravity in rats. Am J Physiol Heart Circ Physiol 290: H2295–H2308, 2006. [DOI] [PubMed] [Google Scholar]
- 19.Hill MA, Davis MJ, Song J, Zou H. Calcium dependence of indolactam- mediated contractions in resistance vessels. J Pharmacol Exp Ther 276: 867–874, 1996. [PubMed] [Google Scholar]
- 20.Hill CE, Gould DJ. Pathway-specific effects of calcitonin gene-related peptide on irideal arterioles of the rat. J Physiol 505: 797–809, 1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hirano K, Hirano M, Kanaide H. Regulation of myosin phosphorylation and myofilament Ca2+ sensitivity in vascular smooth muscle. J Smooth Muscle Res 40: 219–236, 2004. [DOI] [PubMed] [Google Scholar]
- 22.Hollywood MA, Cotton KD, Thornbury KD, McHale NG. Tetrodotoxin- sensitive sodium current in sheep lymphatic smooth muscle. J Physiol 503: 13–20, 1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hosaka K, Mizuno R, Ohhashi T. Rho-Rho kinase pathway is involved in the regulation of myogenic tone and pump activity in isolated lymph vessel. Am J Physiol Heart Circ Physiol 284: H2015–H2025, 2003. [DOI] [PubMed] [Google Scholar]
- 24.Johnston MG, Elias RM, Hayashi A, Nelson W. Role of the lymphatic circulatory system in shock. J Burn Care Rehabil 8: 469–474, 1987. [PubMed] [Google Scholar]
- 25.Kim HR, Appel S, Vetterkind S, Gangopadhyay SS, Morgan KG. Smooth muscle signaling pathways in health and disease. J Cell Mol Med 12: 2165–2180, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.King TP, Spencer M. Structural study and organic ligand-binding properties of bovine plasma albumin. J Biol Chem 245: 6134–6148, 1970. [PubMed] [Google Scholar]
- 27.Koller A, Mizuno R, Kaley G. Flow reduces the amplitude and increases the frequency of lymphatic vasomotion: role of endothelial prostanoids. Am J Physiol Regul Integr Comp Physiol 277: R1683–R1689, 1999. [DOI] [PubMed] [Google Scholar]
- 28.Kuo L, Davis MJ, Chilian WM. Myogenic activity in isolated subepicardial and subendocardial coronary arterioles. Am J Physiol Heart Circ Physiol 255: H1558–H1562, 1988. [DOI] [PubMed] [Google Scholar]
- 29.Longbottom ER, Luckas MJM, Kupittayanant S, Badrick E, Shmigol T, Wray S. The effects of inhibiting myosin light chain kinase on contraction and calcium signalling in human and rat myometrium. Pflügers Arch 440: 315–321, 2000. [DOI] [PubMed] [Google Scholar]
- 30.McHale NG, Allen JM. The effect of external Ca2+ concentration on the contractility of bovine mesenteric lymphatics. Microvasc Res 26: 182–192, 1983. [DOI] [PubMed] [Google Scholar]
- 31.McHale NG, Roddie IC. The effect of transmural pressure on pumping activity in isolated bovine lymphatic vessels. J Physiol 261: 255–269, 1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Meininger GA, Zawieja DC, Falcone JC, Hill MA, Davey JP. Calcium measurement in isolated arterioles during myogenic and agonist stimulation. Am J Physiol Heart Circ Physiol 261: H950–H959, 1991. [DOI] [PubMed] [Google Scholar]
- 33.Meloche S, Landry J, Houle J, Marceau F, Giasson E. p38 MAP kinase pathway regulates angiotensin II-induced contraction of rat vascular smooth muscle. Am J Physiol Heart Circ Physiol 279: H741–H751, 2000. [DOI] [PubMed] [Google Scholar]
- 34.Mizuno R, Ono N, Ohhashi T. Involvement of ATP-sensitive K+ channels in spontaneous activity of isolated lymph microvessels in rats. Am J Physiol Heart Circ Physiol 277: H1453–H1456, 1999. [DOI] [PubMed] [Google Scholar]
- 35.Murthy KS Signaling for contraction and relaxation in smooth muscle of the gut. Annu Rev Physiol 68: 345–374, 2006. [DOI] [PubMed] [Google Scholar]
- 36.Murthy TV, Spurrell BE, Hill MA. Mechanism underlying pervanadate-induced contraction of rat cremaster muscle arterioles. Eur J Pharmacol 442: 107–114, 2002. [DOI] [PubMed] [Google Scholar]
- 37.Muthuchamy M, Gashev AA, Boswell N, Dawson N, Zawieja DC. Molecular and functional analyses of the contractile apparatus in lymphatic muscle. FASEB J 17: 920–922, 2003. [DOI] [PubMed] [Google Scholar]
- 38.Ohhashi T, Azuma T, Sakaguchi M. Active and passive mechanical characteristics of bovine mesenteric lymphatics. Am J Physiol Heart Circ Physiol 239: H88–H95, 1980. [DOI] [PubMed] [Google Scholar]
- 39.Ozveren E, Korkmaz B, Buharalioglu CK, Tunctan B. Involvement of calcium/calmodulin-dependent protein kinase II to endotoxin-induced vascular hyporeactivity in rat superior mesenteric artery. Pharmacol Res 54: 208–218, 2006. [DOI] [PubMed] [Google Scholar]
- 40.Shi J, Takahashi Jin XH, Li YQ, Ito Y, Mori Y, Inoue R. Myosin light chain kinase independent inhibition by ML-9 of murine TRPC6 channels expression in HEK293 cells. Br J Pharmacol 152: 122–131, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Somlyo AP, Somlyo AV. Signal transduction and regulation in smooth muscle. Nature 372: 231–236, 1994. [DOI] [PubMed] [Google Scholar]
- 42.Srinivas SP, Satpathy M, Guo Y, Anandan V. Histamine induced phosphorylation of the regulatory light chain of the myosin II disrupts the barrier integrity of corneal endotheleial cells. Invest Ophthalmol Vis Sci 47: 4011–4018, 2006. [DOI] [PubMed] [Google Scholar]
- 43.Stopa B, Rybarska J, Drozd A, Konieczny L, Król M, Lisowski M, Piekarska B, Roterman I, Spólnik P, Zemanek G. Albumin binds self-assembling dyes as specific polymolecular ligands. Int J Biol Macromol 40: 1–8, 2006. [DOI] [PubMed] [Google Scholar]
- 44.Suematsu E, Resnick M, Morgan KG. Change of Ca2+ requirement for myosin phosphorylation by prostaglandin F2α. Am J Physiol Cell Physiol 261: C253–C258, 1991. [DOI] [PubMed] [Google Scholar]
- 45.Taylor DA, Stull JT. Calcium dependence of myosin light chain phosphorylation in smooth muscle cells. J Biol Chem 263: 14456–14462, 1988. [PubMed] [Google Scholar]
- 46.Von der Weid PY, Zawieja DC. Lymphatic smooth muscle: the motor unit of lymph drainage. Int Biochem Cell Biol 36: 1147–1153, 2004. [DOI] [PubMed] [Google Scholar]
- 47.Wilson DP, Sutherland C, Borman MA, Deng JT, MacDonald JA, Walsh MP. Integrin-linked kinase is responsible for Ca2+-independent myosin diphosphorylation and contraction of vascular smooth muscle. Biochem J 392: 641–648, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Word RA Myosin phosphorylation and the control of myometrial contraction/relaxation. Semin Perinatol 19: 3–14, 1995. [DOI] [PubMed] [Google Scholar]
- 49.Zawieja DC, Greiner ST, Davis KL, Hinds WM, Granger HJ. Reactive oxygen metabolites inhibit spontaneous lymphatic contraction. Am J Physiol Heart Circ Physiol 260: H1935–H1943, 1991. [DOI] [PubMed] [Google Scholar]
- 50.Zawieja DC, Gashev AA, Muthuchamy M. Lymphatic function and contractile proteins. In: 22nd Meeting of the European Society for Microcirculation. Devon, UK: Monduzzi Editore S, 2002, p. A.
- 51.Zawieja DC Lymphatic biology and the microcirculation: past, present, and future. Microcirculation 12: 141–150, 2005. [DOI] [PubMed] [Google Scholar]
- 52.Zou H, Ratz PH, Hill MA. Role of myosin phosphorylation and [Ca2+]i in myogenic reactivity and arteriolesr tone. Am J Physiol Heart Circ Physiol 269: H1590–H1596, 1995. [DOI] [PubMed] [Google Scholar]