Abstract
Stimulation of vascular endothelial cells with agonists such as acetylcholine (ACh) or bradykinin or with shear stress activates phospholipases and releases arachidonic acid (AA). AA is metabolized by cyclooxygenases, cytochrome P-450s, and lipoxygenases (LOs) to vasoactive products. In some arteries, a substantial component of the vasodilator response is dependent on LO metabolites of AA. Nitric oxide (NO)- and prostaglandin (PG)-independent vasodilatory responses to ACh and AA are reduced by inhibitors of LO and by antisense oligonucleotides specifically against 15-LO-1. Vasoactive 15-LO metabolites derived from the vascular endothelium include 15-hydroxy-11,12-epoxyeicosatrienoic acid (15-H-11,12-HEETA) that is hydrolyzed by soluble epoxide hydrolase to 11,12,15-trihydroxyeicosatrienoic acid (11,12,15-THETA). HEETA and THETA are endothelium-derived hyperpolarizing factors that induce vascular relaxations by activation of smooth muscle apamin-sensitive, calcium-activated, small-conductance K+ channels causing hyperpolarization. In other arteries, the 12-LO metabolite 12-hydroxyeicosatetraenoic acid is synthesized by the vascular endothelium and relaxes smooth muscle by large-conductance, calcium-activated K+ channel activation. Thus formation of vasodilator eicosanoids derived from LO pathways contributes to the regulation of vascular tone, local blood flow, and blood pressure.
Keywords: endothelium-derived hyperpolarizing factors, vascular relaxation, 15-lipoxygenase, 12-lipoxygenase, trihydroxyeicosatrienoic acids, hydroxyepoxyeicosatrienoic acids
vascular endothelial cells regulate the tone of the underlying vascular smooth muscle by releasing various relaxing and contracting factors. This was first demonstrated by the seminal work of Furchgott and Zawadzki (51) describing an endothelium-derived relaxing factor (EDRF). In response to chemical or physical stimuli such as acetylcholine (ACh), thrombin, bradykinin, and fluid sheer stress, the vascular endothelium produces several factors that relax the underlying smooth muscle and thus regulate the vascular tone (25, 72, 97, 112). These relaxing factors are prostacyclin (PGI2), nitric oxide (NO), and endothelium-derived hyperpolarizing factors (EDHFs). Unlike PGI2 and NO, numerous endothelium-derived factors function as EDHFs, and no single molecule or pathway has been identified that exhibits all of the characteristics of EDHF in different vascular beds, species, and diseases (19, 20, 22, 36, 41, 62, 90, 168). Among the compounds and pathways that have been proposed as mediators of EDHF activity are metabolites of arachidonic acid (AA).
Cytochrome P-450 (CYP) epoxygenases metabolize AA to epoxyeicosatrienoic acids (EETs). In the last decade, EETs have been shown by numerous laboratories to function as EDHFs in blood vessels from different species, including humans (19, 20, 44, 83). However, in rabbit aorta and mesenteric arteries as well as arteries of other species including dogs, rats, and pigs, EET production is absent under normal conditions (33, 93, 117, 166, 168). Yet, AA produces EDHF-like relaxations, suggesting that AA metabolites, other than the EETs, mediate the EDHF activity. These AA metabolites have been identified as products of the lipoxygenase (LO) pathways: the 15-LO-1 metabolites hydroxyepoxyeicosatrienoic acids (HEETAs) and their soluble epoxide hydrolase (sEH) hydrolytic products, the trihydroxyeicosatrienoic acids (THETAs) (21, 119, 120), and the 12-LO metabolite 12-hydroxyeicosatetraenoic acid (12-HETE) (93, 168). These LO pathways also occur in arteries of mice (55, 148), rats (93, 157), rabbits (21, 120), pigs (168), dogs (33, 94, 159), and humans (48, 82) and contribute to relaxation. The purpose of this review is to summarize the evidence that LO metabolites function as EDHFs in some arteries. Also, the mechanisms underlying vasorelaxation, the biosynthetic pathway, and the physiological and pathophysiological implications of alterations in the expression and activity of vascular 15-LO-1 are reviewed.
Endothelium-Derived Hyperpolarizing Factors
Bolton and colleagues (8, 9) first described endothelium-dependent hyperpolarization of vascular smooth muscle cells by a cholinergic agonist. They suggested that an endothelial factor might be responsible for this activity. Subsequent studies confirmed these findings and showed that this activity was due to an endothelium-derived transferable factor other than NO or PGI2 (25, 40, 77, 78, 156). The term EDHF was first introduced by Chen et al. (25). They demonstrated the presence of ACh-induced, endothelium-dependent hyperpolarizations of vascular smooth muscle that were associated with relaxation. These hyperpolarizations and relaxations to ACh occurred despite inhibition of NO activity and PGI2 synthesis. They concluded that ACh stimulated the endothelium to release an endogenous factor, in addition to PGI2 and NO, and they termed this factor EDHF. Subsequently, a number of investigators extended these findings, showing that relaxations to other agonists, cyclic stretch, and flow also were inhibited, but not blocked, by inhibition of NO and/or PGI2 production (30, 31, 95, 125). These treatments also induced endothelium-dependent hyperpolarization of smooth muscle cells, implicating EDHF as a mediator in their action.
ACh-induced hyperpolarizations are mediated by K+ channel activation. Relaxations and hyperpolarizations to agonists are blocked by high extracellular K+ concentrations ([K+]o) and K+ channel blockers; however, vessels differ in their sensitivity to blockers (20, 24, 26, 53, 115, 161, 169). Tetraethylammonium chloride, iberiotoxin, and charybdotoxin, but not glibenclamide, block relaxations to ACh in the coronary artery, whereas a combination of charybdotoxin and apamin are required for rat mesenteric and hepatic arteries. These data suggest that EDHF acts via Ca2+-dependent K+ (KCa) channels that are blocked by tetraethylammonium and charybdotoxin or KCa channels sensitive to the combination of charybdotoxin and apamin. ACh-induced hyperpolarizations and relaxations of the rabbit mesenteric artery are inhibited by apamin alone (100, 165).
The activity termed EDRF is mediated by a single compound: NO (112). In contrast, there is no single compound mediating EDHF activity but, rather, a family of endothelial factors that act by a common mechanism of K+ channel activation, smooth muscle membrane hyperpolarization, and relaxation (19, 28, 41, 62). Members of this family are chemically different and activate different K+ channels. Hence, a compound is considered an EDHF when 1) it produces vasorelaxation in the presence of cyclooxygenase (COX) and NO synthase (NOS) inhibitors that is blocked by a high [K+]o or by K+ channel inhibitors and 2) it produces endothelium-dependent hyperpolarization of vascular smooth muscle.
The chemical identity of EDHFs remains the focus of intense investigation. Several compounds and pathways mediate EDHF activity, including K+, AA metabolites, residual NO, hydrogen peroxide, C-type natriuretic peptide, and myoendothelial cell gap junctions (20–22, 27, 36, 62, 90). The presence of these compounds and pathways is not exclusive but can coexist in certain vascular beds (165).
Endothelial Signaling Pathways
An increase in free intracellular Ca2+ concentration ([Ca2+]i) in the endothelial cells is a crucial step in the production and release of NO, PGI2, and EDHFs (74, 107). Upon stimulation of muscarinic receptors, membrane-associated phospholipase C is activated, resulting in the production of inositol 1,4,5-trisphosphate (IP3) and diacylglycerol (DAG) (68, 153). Binding of IP3 to its receptor releases Ca2+ from the endoplasmic reticulum. The increase in the [Ca2+]i from the intracellular stores opens KCa channels, causing K+ efflux and hyperpolarization of the cell membrane, which increases the electrochemical driving force for Ca2+ influx through nonselective, Ca2+-permeable cation channels (68, 76, 87, 107).
Endothelial intracellular free Ca2+ binds to calmodulin, which activates the constitutively expressed endothelial NOS (eNOS). NOS catalyzes the conversion of l-arginine to l-citrulline and NO (15, 86). Once formed, NO diffuses to the underlying smooth muscle and causes relaxation.
Elevated endothelial cell [Ca2+]i also activates cytosolic phospholipase A2 to release free AA from the cell membrane by cleavage of the sn-2 bond of phosphatidylcholine and phosphatidylethanolamine (11). Free AA also can be derived from the phospholipase C pathway. DAG lipase converts DAG to 2-arachidonoylglycerol, from which free AA is released by monoacylglycerol lipase or fatty acid amidohydrolase (153). Free AA is metabolized by COX to PGI2 or by LOs and CYPs to AA-derived EDHFs. AA metabolites are then released, diffuse to the smooth muscle, and cause hyperpolarizations and relaxations (Fig. 1).
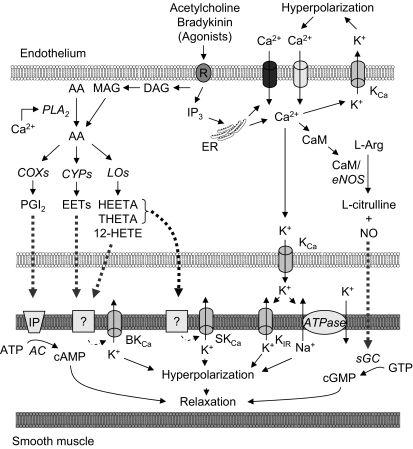
Fig. 1.
Schematic of the role of Ca2+ on relaxing factor production in vascular endothelial cells. Activation of muscarinic receptors leads to the production of inositol 1,4,5-trisphosphate (IP3), which releases Ca2+ from the endoplasmic reticulum (ER). Extracellular Ca2+ can enter the endothelial cell through nonselective Ca2+-permeable cation channels or store-operated Ca2+ channels, which are regulated by the ER Ca2+ store. An increase in Ca2+ concentration promotes K+ efflux through activated Ca2+-dependent K+ (KCa) channels, causing membrane hyperpolarization, which in turn increases Ca2+ influx. Intracellular Ca2+ binds to calmodulin (CaM) and activates nitric oxide synthase (NOS) and the production of nitric oxide (NO) or stimulates phospholipase A2 (PLA2), which results in the release of arachidonic acid (AA) from the cell plasma membrane. Free AA also can be obtained from the phospholipase C (PLC) pathway, in which diacylglycerol (DAG) is hydrolyzed at the sn-1 position by DAG lipase to monoacylglycerol (MAG), specifically 2-arachidonylglycerol (2-AG). MAG lipase or fatty acid amidohydrolase (FAAH) releases free AA from 2-AG. Free AA can be metabolized through cyclooxygenase (COX), cytochrome P-450 (CYP), and/or lipoxygenase (LO) pathways to produce relaxing factors. PGI2, prostacyclin; EET, epoxyeicosatrienoic acid; HEETA, hydroxyepoxyeicosatrienoic acid; THETA, trihydroxyeicosatrienoic acid; HETE, hydroxyeicosatetraenoic acid; BKCa, large-conductance KCa channel; SKCa, apamin-sensitive KCa channel; Kir, inward rectifying K+ channel; AC, adenylyl cyclase; sGC, soluble guanylate cyclase.
Role of Lipoxygenase Metabolites of AA in Endothelium-Dependent Relaxation
ACh induces potent endothelium-dependent vascular relaxations in most species, including rats, mice, cows, pigs, guinea pigs, and rabbits (28, 41). Several factors and pathways are proposed to mediate these relaxations to ACh. NO mediates part of the ACh-induced vascular relaxation, since the vascular responses are reduced, but not blocked, by NOS inhibitors. The contribution of PGI2 to ACh relaxation is variable. Several lines of evidence indicate that AA metabolites mediate endothelium-dependent vascular hyperpolarizations and a component of the endothelium-dependent relaxations to ACh and other stimuli. Furchgott and Zawadzki (51) first suggested that relaxations to ACh in the rabbit aorta were mediated by LO metabolites of AA. Endothelium-dependent relaxations to ACh were inhibited by the LO inhibitor eicosatetraynoic acid and the phospholipase A2 inhibitor meparcrine but not by COX inhibitors, aspirin, or indomethacin. The involvement of AA and its LO metabolites in ACh-induced relaxations was confirmed in arteries from a variety of vascular beds and species [skeletal muscle arterioles and mesenteric arteries of mice (55, 148), aorta and mesenteric arteries of rats (93, 157), aorta and mesenteric arteries of rabbits (21, 120, 165), coronary arteries of pigs (168), femoral and coronary arteries of dogs (33, 94, 133), and coronary arteries of humans (48, 82)]. All of these studies used pharmacological inhibitors to determine the contribution of AA metabolites. A large number of structurally different LO inhibitors, including nordihydroguaiaretic acid (NDGA), phenidone, BW755c, caffeic acid, diethylcarbamazine, AA861, TKM777, cinnanyl-dihydroxy-cyanocinnamate, ebselen, and others (21, 33, 45, 47, 50, 94, 116, 146), as well as inhibitors of phospholipases A2 and C (21, 33, 46, 85, 146, 153), inhibited relaxations to ACh. Interestingly, inhibitors of CPY also inhibited the endothelium-dependent relaxations to ACh (21, 45, 116, 147). Bradykinin, histamine, substance P, thrombin, and other agonists caused endothelium-dependent relaxations that were inhibited or blocked by inhibitors of phospholipases and LOs (33, 45, 47, 94, 158).
Moncada et al. (98) and Förstermann et al. (45) questioned the mechanism of inhibition by LO inhibitors and thus the role of LO metabolites as mediators of vascular relaxations. Endothelium-dependent relaxations to several agonists were inhibited by LO inhibitors; however, this inhibition was reversed by superoxide dismutase and oxidized cytochrome c, which inactivate or bind superoxide anion, respectively (98). Moncada et al. suggested that LO inhibitors block LO activity but also generate superoxide anion, which inactivates EDRF. This latter action was proposed as the primary mechanism for LO inhibitors for reduced endothelium-dependent relaxations. In a subsequent study, superoxide dismutase did not reverse the activity of all LO inhibitors that block ACh-induced relaxations (94). Despite the studies to the contrary, these findings questioned the specificity of LO inhibitors and the role of LO metabolites as mediators of ACh-induced relaxations. These concerns slowed the search and delayed the discovery of endothelium-derived LO metabolites that act as EDHFs.
Furchgott and Zawadzki (51) first showed that endothelial cells produce a diffusible, transferable relaxing factor. A detector vessel without endothelium did not relax to ACh; however, if a donor vessel with endothelium was placed next to the detector vessel, the detector relaxed. Other investigators have developed variations on this bioassay method by using either superfused cultured endothelial cells on beads or perfused arteries with an intact endothelium as the relaxing factor source, i.e., the donor (12, 56, 63, 69, 88, 116, 132, 133). The donor perfusate was used to superfuse or perfuse a detector artery without endothelium. Changes in tension or diameter were measured in the detector artery. In all cases, ACh or bradykinin added to the donor caused relaxation of the detector artery, but the agonists were without effect when added to detector directly or when added to a donor artery without endothelium. These results confirmed the existence of an endothelium-derived, transferable relaxing factor. Addition of NDGA to inhibit LO, metyrapone to inhibit CYP, or mepacrine to inhibit phospholipase to the donor inhibited agonist-induced relaxation of the detector artery (63, 88, 133). These studies also provided evidence for the existence of other endothelial factors (12, 69, 133, 160). ACh when added to the donor caused a biphasic concentration-related relaxation of the detector artery. Inhibitors of LO, CYP, and phospholipases inhibited the first phase of relaxations, occurring at low ACh concentrations, but not the second phase, occurring at high concentrations. Subsequent studies indicated that this factor was an EDHF, since ACh relaxations were inhibited by addition of high [K+]o to the detector artery (88). Addition of bradykinin to the donor caused membrane hyperpolarization of the smooth muscle cells of the detector artery (58, 96, 124). These studies suggest that endothelial cells release a transferable, hyperpolarizing factor that is a LO and/or CYP metabolite of AA.
Further evidence for a role of LO and CYP metabolites of AA was the ability of AA to induce endothelium-dependent vascular relaxations in arteries from various vascular beds of rabbits, dogs, rats, and pigs (33, 39, 50, 116, 131, 145, 147, 153, 166, 168). Relaxations to AA were inhibited by LO inhibitors and CYP inhibitors but not by phospholipase inhibitors, since AA bypasses the phospholipase step. Like ACh and bradykinin, addition of AA to a donor artery with endothelium caused relaxation of a detector artery without endothelium (116). This indicated that AA released a transferable relaxing factor. In rabbit aorta, AA also stimulated endothelium-dependent hyperpolarization of vascular smooth muscle that was blocked by LO inhibitors (21, 23, 57). Relaxations to AA also were inhibited by high [K+]o and blockade of K+ channels. Together, these data emphasize the role of LO metabolites of AA in endothelium-dependent relaxations and EDHF activity of some arteries (50, 116, 145, 166).
Lipoxygenases
LOs are a family of non-heme iron-containing enzymes that dioxygenate polyunsaturated fatty acids to hydroperoxyl metabolites. Three major LO isoforms include 5-LO (127), 12-LO (163), and 15-LO (79), which correspond to the carbon position of AA oxygenation. Depending on the isoform, each LO oxygenates AA to form a regio- and stereospecific hydroperoxyeicosatetraenoic acid (HPETE). HPETE is unstable and can be reduced by peroxidases to the corresponding hydroxyeicosatetraenoic acid (HETE). All three LO isoforms are found in vascular endothelial cells, and their products have various vasoactive properties, including both vasodilation and vasoconstriction (89, 93, 168). The 5-LO pathway is involved in the regulation of inflammation, and 5-LO is the initial enzyme in the synthesis of leukotrienes. Few reports have evaluated the role of 5-LOs in vascular tone regulation. 5-LO products were involved in the endothelium-dependent, ACh-induced constriction of the hypertensive rat aorta through the activation of cysteinyl leukotriene receptors (84). Also, 5-HETE inhibits the production of PGI2 in coronary artery endothelial cells, indirectly contributing to overall vascular constriction (60). 5-LO activity was not observed in rabbit or mouse arteries, and 5-HETE and leukotrienes were not detected when these arteries were incubated with AA (50, 118). In contrast, the 12-LO and 15-LO pathways are important in vasorelaxation in various blood vessels of pigs, rats, rabbits, dogs, mice, and humans (39, 50, 55, 82, 93, 120, 121, 145, 148, 157, 168).
The 12-LO pathway metabolizes AA to a variety of products with numerous biological activities. Major products of this pathway are 12(S)-HETE, hydroxyepoxy-containing hepoxilins, and trihydroxy-containing trioxilins (109). 12(S)-HETE is produced by arteries and the vascular endothelium (93, 99, 117, 120, 168). The role of 12(S)-HETE as an EDHF was reported in rat mesenteric arteries (93), rat basilar arteries (39), porcine coronary microvessels (168), and human coronary arteries (82). In these vessels, 12(S)-HETE induced vascular smooth muscle hyperpolarization through activation of large-conductance KCa (BKCa) channels, resulting in relaxation. In contrast, 12-HETE is a vasoconstrictor in dog renal arcuate arteries (89). 12(S)-HETE can induce vascular smooth muscle cell growth, matrix protein production (128), and migration (102), which may contribute to atherosclerosis, hypertension, and restenosis. Treatment of endothelial cells with 12(S)-HETE leads to increased ICAM-1 expression and monocyte binding (114, 129), suggesting this metabolite contributes to inflammation. 12(S)-HETE also may play a role in angiogenesis by inducing endothelial cell proliferation and migration (105, 152).
12(S)-HPETE is converted to the bioactive 8-hydroxy-11,12-epoxyeicosatrienoic acid (hepoxilin A3) and inactive 10-hydroxy-11,12-epoxyeicosatrienoic acid (hepoxilin B3) by hepoxilin A3 synthase activity of 12-LO (106, 111). The biological actions of hepoxilin A3 have been intensely studied in neutrophils (35), pancreatic islets (110), and neuronal synapses (123). However, little is known about the effect of hepoxilins on the regulation of vascular tone. Hepoxilin A3 stimulates intracellular Ca2+ release (35) and increases Ca2+ transport across the cell membrane (34); therefore, biological actions of hepoxilin A3 are, for the most part, Ca2+ dependent. In rat aorta and portal vein, hepoxilin A3 did not have a direct effect on the vascular tone but potentiated norepinephrine-induced vascular contraction in a Ca2+-dependent manner (80, 81). Rabbit aorta converted AA to 8,9,12-trihydroxyeicosatrienoic acid (trioxilin C3) when 12-LO was supplied exogenously, and trioxilin C3 produced concentration-dependent relaxations of rabbit aorta (121). The vascular actions of trioxilin A3 or B3 are unknown.
In humans, there are two types of 15-LO: reticulocyte type (15-LO-1) (144) and epidermis type (15-LO-2) (14). 15-LO-1 was first described in rabbit reticulocytes (139). The enzyme is a 75-kDa single-polypeptide chain with a two-domain structure (59). It contains one non-heme iron per molecule. 15-LO-2 was subsequently identified, and its expression was reported in human prostate, skin, and cornea (14). Both enzymes convert AA to 15(S)-HPETE. They share only 40% amino acid homology. There are two major differences between enzymes: 1) 15-LO-1 converts AA to 15(S)-HPETE (90%) and lesser amounts of 12(S)-HPETE (10%), whereas 15-LO-2 produces exclusively 15(S)-HPETE (14), and 2) although both AA and linoleic acid are preferred substrates for 15-LO-1 (73, 137), only AA is a substrate for 15-LO-2 (14).
Tang et al. (154) demonstrated the expression of 15-LO-1, but not 12-LO, mRNA and protein in rabbit aorta. Histologically, 15-LO is localized to the vascular endothelium (2, 155), and there is no metabolism of AA to LO products in arteries with the endothelium removed (2, 4). The kinetic characteristics of aortic 15-LO-1 are similar to those of purified reticulocyte 15-LO, supporting the conclusion that 15-LO-1 is responsible for the 15-LO activity in arteries (154). 15-LO-1 metabolizes AA to the chemically unstable 15-HPETE, which is reduced to its stable metabolite, 15-HETE. Supporting a vasodilatory role of 15-LO metabolites of AA, Uotila et al. (157) reported that endothelium-denuded rat aorta incubated with soybean 15-LO relaxes to AA. AA alone does not relax the aorta in the absence of the endothelium (33, 116, 145). On basal tone, 15-HETE and 15-HPETE cause slight relaxations in lower concentrations but contractions in higher concentrations (91, 157, 159). Thromboxane A2 receptor antagonists block the contractions to these LO metabolites. 15-HETE also causes concentration-dependent contraction of pulmonary arterial rings from rabbits exposed to hypoxia but not normoxia (167). In norepinephrine- or PGF2α-precontracted vessels, 15-HPETE causes endothelium-dependent relaxations that are not affected by COX inhibition. 15-HPETE does not relax arteries precontracted with KCl. However, 15-HETE does not cause relaxation of precontracted arteries (49, 117, 166). These observations that 15-HPETE causes relaxation of precontracted arteries, but 15-HETE does not, suggest that some other metabolite of 15-HPETE and the 15-LO pathway must mediate the relaxations to AA under these conditions.
Pharmacological LO inhibitors are nonspecific and block 5-LO, 12-LO, 15-LO, and possibly other pathways of AA metabolism (136). Many of the LO inhibitors also function as antioxidants (135). A more specific method of reducing 15-LO activity involved decreasing 15-LO-1 expression with antisense oligonucleotides (154). This decreased the production of LO metabolites by rabbit aorta and decreased the NO- and PGI2-independent relaxations to ACh (154). On the other hand, overexpression of 15-LO-1 with adenoviruses containing human 15-LO-1 cDNA increased the endothelial expression of the enzyme, increased the arterial production of LO metabolites, and enhanced relaxations to AA (3, 4). Together, these data suggest an important role of 15-LO-1 in the regulation of vascular tone.
Identification of 15-LO Metabolites
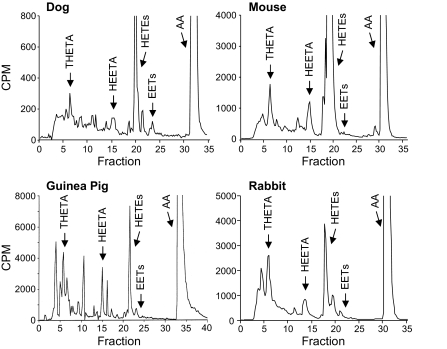
After the incubation of arteries with AA, metabolites were resolved by reverse-phase HPLC and coeluted with authentic THETAs, HEETAs, and 15-HETE (118, 120, 166) (Fig. 2). A similar pattern of AA metabolism was observed in arteries from rabbits, mice, dogs, and guinea pigs. No metabolism of AA was observed in arteries without endothelium. The THETA, HEETA, and HETE production in rabbit arteries was blocked by LO inhibitors and enhanced by indomethacin (119, 120). Biological THETA and HEETA peaks that were collected from HPLC relaxed preconstricted rabbit aortic rings (120). The biological HETE peak was without activity. Also, 15-HETE had no vasodilatory effect (49, 117, 166).
Fig. 2.
Metabolism of [14C]AA by rabbit aorta, mouse aorta, dog mesenteric arteries, and guinea pig carotid arteries. Arterial rings with an intact endothelium were incubated with indomethacin and [14C]AA. Metabolites were extracted and resolved by reverse-phase HPLC (21). Migration times of known standards are indicated by arrows above the chromatogram. CPM, counts per minute.
The THETAs produced by rabbit aorta were identified as 11,12,15- and 11,14,15-THETA (120). 11,12,15-THETA relaxed preconstricted mesenteric arteries and aorta in an endothelium-independent manner, and the relaxations were blocked by high [K+]o and by apamin (21, 165). 11,12,15-THETA relaxed rabbit aorta and hyperpolarized rabbit aortic smooth muscle cells through the opening of apamin-sensitive KCa (SKCa) channels (21, 57). In contrast, 11,14,15-THETA was inactive. The stereochemical configuration of 11,12,15-THETA was identified by comparing the vascular activities and chromatographic migration times of the biologically produced 11,12,15-THETA to eight chemically synthesized stereoisomers of 11,12,15-THETA (54). 11(R),12(S),15(S)-TH-5(Z),8(Z),13(E)-ETA was the only stereoisomer that comigrated with the biological 11,12,15-THETA in several chromatographic systems. In addition, it was the only stereoisomer that relaxed the rabbit aorta and activated SKCa channels. These findings indicate that 11(R),12(S),15(S)-TH-5(Z),8(Z),13(E)-ETA is the endothelium-derived active THETA. It also suggests that 11,12,15-THETA is the active isomer that mediates NO- and PGI2-independent relaxations to AA and ACh (21, 57).
15-Hydroxy-11,12-epoxyeicosatrienoic acid (15-H-11,12-EETA) was proposed as a biologically active precursor of 11,12,15-THETA (120). 15-H-11,12-EETA has an epoxide group in conjugation with a double bond and is rapidly converted to 11,12,15- and 11,14,15-THETA under acidic conditions. The half-life of 15-H-11,12-EETA in aqueous solution at pH 3.0 and pH 7.4 is ∼50 s and 33 h, respectively (66). To access the formation of the acid labile 15-H-11,12-EETA, aortic incubations with AA were terminated by acidification in an excess of methanol to trap the 15-H-11,12-EETA as the methoxy derivatives 12-methoxy-11,15-dihydroxy- and 14-methoxy-11,15-dihydroxyeicosatrienoic acids (MDHEs) (23). Pretreatment of aortic tissue with the sEH inhibitor 12-(3-adamantan-1-yl-ureido)-dodecanoic acid (AUDA) increased the formation of 15-H-11,12-EETA, measured as MDHEs, and decreased THETA production (23). Thus 15-H-11,12-EETA is a LO metabolite of AA and is metabolized by sEH to 11,12,15-THETA.
15-LO Biosynthetic Pathways
Pace-Asciak et al. (109) first reported that the rat lung produced 12-LO HEETA metabolites from AA. 12(S)-HPETE, formed by oxygenation of AA by 12-LO, undergoes reduction that is catalyzed by peroxidases to 12(S)-HETE or isomerization by hepoxilin synthase to hepoxilin A3 and B3. Hepoxilins are hydrolyzed by an epoxide hydrolase to the corresponding 8,11,12-THETA (trioxilin A3) and 10,11,12-THETA (trioxilin B3) (109). 15-LO-1 is the major LO expressed in rabbit aorta and other arteries (154), and it does not produce hepoxilins or trioxilins (121). Based on the biosynthetic pathway of hepoxilins and trioxilins, the 15-LO pathway in rabbit vascular endothelium was postulated (120). AA is first converted to 15(S)-HPETE by 15-LO-1. 15(S)-HPETE is reduced to 15(S)-HETE or metabolized to 15-H-11,12-EETA by a hydroperoxide isomerase. The epoxide group of 15-H-11,12-EETA is hydrolyzed by sEH and/or acid to 11,12,15-THETA.
Bifurcation of the 12-LO pathway occurs at the metabolism of HPETEs to either 12-HETE or hepoxilins. Cytosolic glutathione peroxidase (GPx) and membrane-bound phospholipid hydroperoxide GPx prevented the production of hepoxilins by shifting 12(S)-HPETE conversion to 12(S)-HETE (150). Inactivation of the GPx activity inhibited the formation of 12(S)-HETE by 80%, increased the accumulation of 12(S)-HPETE by two orders of magnitude, and increased hepoxilin production (150). The impact of GPx inhibition on the vascular synthesis or vascular effects of 12-HETE has not been investigated. This concept was tested in rabbit aorta to examine the role of GPxs on the regulation of HEETA production in 15-LO pathway. When GPxs were inhibited by depleting cellular glutathione with diethylmaleate, an increase in THETA and HEETA production was accompanied by a decrease in 15(S)-HETE production. This suggests a role of GPxs in regulating HEETA and THETA production.
In addition to the reduction of HPETEs to HETEs, several enzymes, collectively named hydroperoxide isomerases, metabolize fatty acid hydroperoxides to an array of products, including epoxy alcohols such as HEETAs. Transformation of an unsaturated fatty acid hydroperoxide containing the 1-hydroperoxy-2(E),4(Z)-pentadiene partial structure like that of HPETEs into epoxy alcohols occurs by two distinct pathways. These pathways differ with respect to the mode of formation and the origin of the epoxide group. One pathway involves homolytic cleavage of the hydroperoxide oxygen-oxygen bond, producing an alkoxyl radical that undergoes cyclization to an epoxide. The hydroxyl oxygen is derived from the terminal hydroperoxide oxygen, from O2, or from the solvent (52). The other pathway involves heterolytic cleavage of the hydroperoxide oxygen-oxygen bond and formation of an epoxide group by epoxidation of the double bonds. In this pathway, the epoxide group is derived from the terminal hydroperoxide oxygen, which can be transferred to the double bonds intra- or intermolecularly (65). The identity of hydroperoxide isomerase(s) that converts 15(S)-HPETE to HEETAs in arteries is unknown. However, from the chemical structures of the HEETAs, the mechanism of the synthesis and the enzymes involved can be speculated. The hydroperoxyl oxygen-oxygen bond of 15-HPETE can be cleaved heterolytically by a peroxygenase to form a hydroxyl group at the C-15 position. An epoxide group can then be formed by epoxidation of the 11,12 cis-double bonds, resulting in 15-H-11,12-EETA formation. The fact that 15-H-13,14-EETA has not been detected in arteries supports the role of a peroxygenase, since peroxygenases only epoxidize cis-, but not trans-, double bonds (7). CYP2J2, CYP2J5, CYP2J9, and CYP2C8 have been shown to be hydroperoxide isomerases in 11,12,15-THETA synthesis (122). Both CYP2J2 and CYP2C8 are present in rabbit aorta. Inhibitors of CYP2J, but not CYP2C, decrease THETA synthesis. In addition, other CYPs also have peroxygenase activity (92). Therefore, a CYP2J isoform in rabbit aorta may mediate the peroxygenase activity and 15-H-11,12-EETA formation. On the other hand, homolytic cleavage of the hydroperoxyl oxygen-oxygen bond will produce an alkoxyl radical, which can cyclize to form the 14,15-epoxide. Hydroxylation at the C-11 position results in 11-H-14,15-EETA formation. However, direct evidence of 11-H-14,15-EETA formation has not been observed in rabbit aorta.
Once formed, HEETAs can be hydrolyzed to their corresponding THETAs. The epoxide group of 15-H-11,12-EETA is hydrolyzed by sEH and/or acid to 11,12,15-THETA (23). sEH is present in rabbit aorta and other arteries (18, 23, 38). The nonspecific hydrolysis of 15-H-11,12-EETA by acidic conditions will produce both 11,12,15-THETA and 11,14,15-THETA.
There is no information about the metabolism of 11,12,15-THETA by vascular cells. Because there are strict structural and stereochemical requirements for vascular activity (54), metabolism of 11,12,15-THETA would result in a loss of activity. Many eicosanoids and prostanoids with a 15-hydroxyl group are inactivated by 15-hydroxyprostaglandin dehydrogenase, which converts the hydroxyl to a ketone, and subsequent reduction of the Δ13,14-olefin by a reductase (6, 67). β- and ω-oxidation are other inactivation pathways (29, 75). It is not known whether 11,12,15-THETA is a substrate for these enzymes. The proposed endothelial biosynthetic pathway of HEETAs and THETAs is described in Fig. 3.
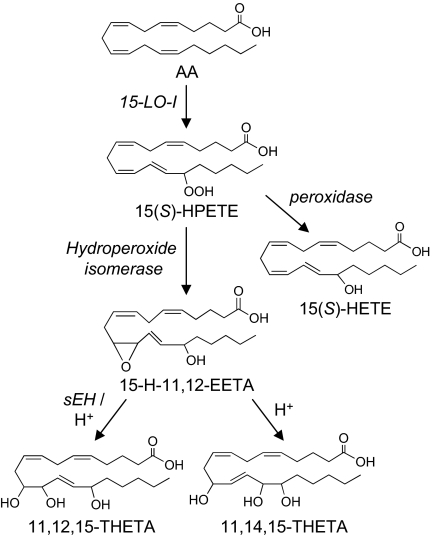
Fig. 3.
Proposed biosynthesis pathway of HEETAs and THETAs. AA is converted to 15(S)-hydroperoxyeicosatetraenoic acid [15(S)-HPETE] by 15-LO-1. 15(S)-HPETE is converted by hydroperoxide isomerases to the HEETAs. 15-Hydroxy-11,12-epoxyeicosatrienoic acid (15-H-11,12-EETA) can be hydrolyzed by either soluble epoxide hydrolase (sEH) or acid to THETAs. It is hydrolyzed to 11,12,15-THETA by sEH. However, with acid hydrolysis, 15-H-11,12-EETA is hydrolyzed to both 11,12,15- and 11,14,15-THETA. The exact stereochemistry of 15-H-11,12-EETAs is not known.
Roles of THETA and HEETAs in the Regulation of Vascular Tone
The roles of these metabolites as EDHFs were studied using synthetic analogs. 11(R),12(S),15(S)-TH-5(Z),8(Z),13(E)-ETA relaxed the preconstricted arteries of rabbits and mice (54, 55). These relaxations were blocked by apamin. With the use of whole cell patch clamp, 11(R),12(S),15(S)-TH-5(Z),8(Z),13(E)-ETA increased outward K+ current in isolated rabbit aortic smooth muscle cells (54). This current was also blocked by apamin. These studies suggest that 11(R),12(S),15(S)-TH-5(Z),8(Z),13(E)-ETA is an EDHF. The requirements for a specific structure and stereochemical configuration for 11,12,15-THETA suggest that a specific binding site or receptor is involved in mediating the SKCa channel activation, hyperpolarization, and vascular relaxations. In this regard, there are examples of membrane receptors for other trihydroxylated AA metabolites. Lipoxin A4, a trihydroxy metabolite of AA, also has strict structural requirements for activity, and its membrane receptor has been identified (43, 64).
The biological activity of 15-H-11,12-EETA requires further study. Because 15-H-11,12-EETA is easily hydrolyzed (23, 66), a stable analog is required to directly study its biological activity. At this time, such an analog is not available; therefore, there are no direct studies of its biological activity or comparisons of its activity to 11,12,15-THETA. However, by using the sEH inhibitor AUDA to prevent the hydrolysis of HEETAs to THETAs, the role of endogenous 15-H-11,12-EETA on the vascular tone has been indirectly tested. ACh- and AA-induced relaxations of rabbit aorta were enhanced by pretreatment with AUDA, and these enhanced responses to both agonists were blocked by the LO inhibitor NDGA and by high [K+]o (23). AA caused hyperpolarization of smooth muscle in endothelium-intact segments of aorta. The AA-induced hyperpolarizations also were enhanced by AUDA, and the enhanced hyperpolarizations were inhibited by NDGA. Thus the enhanced relaxations by sEH inhibition were mediated by a LO metabolite, K+ channel activation, and membrane hyperpolarization. This vasoactive HEETA is probably 15-H-11,12-EETA, since its production by rabbit aorta is enhanced by sEH inhibition. The HEETA causes relaxation by K+ channel activation, K+ efflux, and membrane hyperpolarization, since increasing [K+]o inhibits the AUDA-enhanced relaxations to AA and ACh (23). The ability of AUDA to increase vascular relaxation and hyperpolarization of smooth muscle suggests that the HEETA is more potent than the THETA. Nevertheless, these data must be interpreted with caution, since it is not known whether AUDA completely inhibited sEH under the experimental conditions used. The presence of THETA could complicate this interpretation.
In rabbit arteries, 11,12,15-THETA and 15-H-11,12-EETA both are produced by the endothelium and activate smooth muscle apamin-sensitive SKCa channels to cause membrane hyperpolarization and relaxation (23, 54, 57, 165, 166). This raises the question of how two structurally different AA metabolites activate the same SKCa channel, particularly when there is a strict structural requirement for 11,12,15-THETA (54). SKCa channels are voltage independent and activated by submicromolar concentrations of intracellular Ca2+ (10). The gating of SKCa channels is induced by Ca2+ binding to calmodulin, which is constitutively bound to each channel subunit. Ca2+ binding to calmodulin induces a conformational change, which leads to the opening of the channels (162). Therefore, 11,12,15-THETA and 15-H-11,12-EETA may not activate the channel directly but, instead, bind to their own receptors/binding sites and activate the same or different signaling pathways that open the SKCa channel.
SKCa and intermediate-conductance KCa (IKCa) channels are expressed on the vascular endothelium (16, 17, 37, 41, 104, 134). Specifically, endothelial cells express the SK2 and SK3 channel subtypes along with IK channels. SKCa channels of the SK2 subtype and BKCa channels are found on smooth muscle cells and are likely involved in smooth muscle hyperpolarization. Murphy and Brayden (100) reported in rabbit mesenteric arteries that the EDHF-mediated hyperpolarization was blocked by the SKCa inhibitor apamin and by other inhibitors of the apamin-sensitive K+ channel but not by the BKCa inhibitor iberiotoxin. Electrophysiological studies on vascular smooth muscle cells from rabbit aortas characterized a 24-pS-conductance K+ channel that is voltage and Ca2+ dependent and inhibited selectively by apamin (57). However, the subtype of this SKCa channel was not determined. Therefore, further characterization of the K+ channels, their regulation, the subtypes, and their cellular localization in the vasculature is needed.
LO Metabolites and Other EDHFs
In addition to AA metabolites, a small increase in [K+]o hyperpolarizes the smooth muscle cell membrane and causes relaxation (36, 104, 126). As discussed above, a crucial step in the EDHF pathway is a rise in endothelial [Ca2+]i, which is followed by the activation of endothelial IKCa and/or SKCa channels, allowing K+ efflux and hyperpolarization of the endothelial cell membrane. The K+ released by the endothelium into the myoendothelial intercellular space activates smooth muscle inward rectifier K+ (Kir) channels and/or Na+-K+-ATPase, causing smooth muscle hyperpolarization and relaxation in rat hepatic arteries (36). This observation is counterintuitive, since increasing [K+]o will change the equilibrium potential of K+, as predicted by the Nernst equation, and will favor smooth muscle membrane depolarization and contraction. However, the activation of Kir and the Na+-K+ ATPase by small increases in [K+]o (1 to 15 mM) overcomes the minor depolarizing effects caused by the increase in [K+]o. The net result is hyperpolarization and thus relaxation of the smooth muscle cells. Therefore, endothelium-derived K+ may mediate the EDHF response (36).
In rabbit mesenteric arteries, the corelease of endothelial cell K+ and AA metabolites of the 15-LO pathway synergistically function as EDHFs (165) (Fig. 4). With the use of whole cell patch clamp, ACh increased the outward K+ currents in endothelial cells. This outward current was blocked by charybdotoxin, an IKCa channel blocker. Thus ACh increases endothelial K+ release by activating charybdotoxin-sensitive IKCa channels. The relaxations to ACh were partially inhibited by apamin alone or charybdotoxin alone but were blocked by the combination. Barium blocked the smooth muscle cell Kir channels that were activated by K+ and inhibited a portion of ACh-induced relaxations. The combination of barium and apamin, like the combination of charybdotoxin and apamin, blocked the relaxations, indicating that they act in parallel on different pathways. In contrast, the combination of barium and charybdotoxin was only as effective as barium alone and charybdotoxin alone, indicating that these inhibitors act in series on the same pathway. These studies indicate that ACh stimulates endothelial IKCa channels to release K+ that activates Kir channels on smooth muscle cells. This mediates a portion of the hyperpolarization and relaxation response to the agonist. In parallel, ACh also stimulates the endothelial synthesis of 11,12,15-THETA (21). The THETA activates apamin-sensitive SKCa channels on smooth muscle to mediate a portion of the hyperpolarization and relaxation to ACh. Increasing the [K+]o from 5 to 10.9 mM caused partial relaxation of the artery and potentiated the relaxations to AA and 11,12,15-THETA (165). These studies indicate that ACh stimulates the corelease of K+ and 11,12,15-THETA, and these mediators act synergistically via different mechanisms to cause hyperpolarization and relaxation. It is not known whether a similar interaction occurs with other EDHFs such as 12-HETE or EETs.
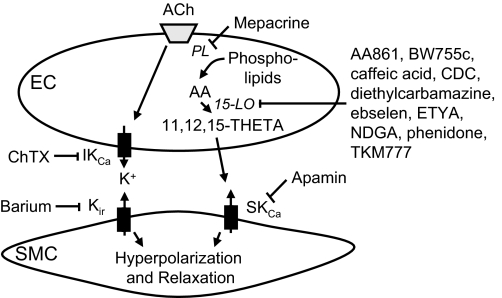
Fig. 4.
Endothelium-derived hyperpolarizing factor (EDHF)-mediated responses to acetylcholine (ACh) involve 2 mechanisms acting in parallel. ACh activates intermediate-conductance KCa (IKCa) channels on endothelial cells (EC), releasing K+ that activates Kir channels on smooth muscle. ACh also releases AA in endothelial cells that is metabolized by 15-LO to 15-H-11,12-EETA and 11,12,15-THETA. THETA activates SKCa channels on smooth muscle. These 2 pathways cause smooth muscle cell membrane hyperpolarization and relaxation. Inhibitors of these enzymes and channels are indicated. PL, phospholipase; CDC, cinnamyl-3,4-dihydroxy-α-cyanocinnamate; ETYA, 5,8,11,14-eicosatetraynoic acid; NDGA, nordihydroguaiaretic acid; ChTX, charybdotoxin; SMC, smooth muscle cells.
Role of LO Metabolites in Regulating Vascular Tone In Vivo
The role of LO metabolites on blood pressure regulation has been studied in normotensive and hypertensive animals. In normotensive rats and cholesterol-fed, normotensive rabbits, inhibition of LOs with phenidone, baicalein, or BW755c did not affect basal blood pressure or heart rate (2, 149). Similarly, the basal blood pressure was identical in wild-type and 12/15-LO knockout mice (5). Treatment of rabbits in vivo with an adenovirus containing the human 15-LO-1 cDNA increased the expression of the enzyme in the vascular endothelium and the vascular synthesis of 15-LO metabolites of AA (3). However, overexpression of 15-LO did not alter basal blood pressure or heart rate. These studies indicate that augmenting or inhibiting endothelial LOs does not change basal blood pressure.
LO metabolites contribute to ACh-mediated hypotension. In normal rabbits, ACh decreased blood pressure in a concentration-related manner (1–3). Treatment with inhibitors of COX and NOS reduced, but did not block, the ACh-induced hypotension (1). The non-PG, non-NO-mediated hypotension was inhibited by apamin and blocked by the combination of charybdotoxin and apamin. Thus ACh-induced hypotension has PG, NO, and EDHF components as described in isolated arteries (1, 21, 165). These hypotensive responses to ACh were significantly enhanced in rabbits overexpressing endothelial 15-LO-1 due to adenovirus treatment, age, or cholesterol treatment (1–3). ACh-induced hypotension was attributed to 15-LO metabolites of AA acting as EDHFs, since the LO inhibitor BW755c and K+ channel blocker apamin inhibited the responses to a similar extent. Similar results were obtained with wild-type mice and mice with genetic hypercholesterolemia (148). Although LO metabolites of AA do not contribute to basal blood pressure, they mediate a component of the hypotension to ACh.
In hypertensive models, the effects of LO inhibitors are complex. Stern et al. (149) first showed that the LO inhibitors phenidone, esculetin, and baicalein attenuate the contractile effects of angiotensin II in femoral arterial rings of rats and decrease the pressor response to angiotensin II. Responses to phenylephrine were unaltered by LO inhibition. These in vivo and in vitro findings were confirmed in a study using wild-type and 12/15-LO knockout mice (5). These data indicate that a vascular LO metabolite must mediate or modulate specifically angiotensin-induced vasoconstriction. This role of vascular smooth muscle LO differs from the role of endothelial LO and the synthesis of EDHFs. Similarly, LO inhibitors reduce blood pressure in rats with renovascular hypertension (103, 108), aortic coarctation-induced hypertension (32), spontaneous hypertension (138), and fructose-induced hypertension (61). However, in DOCA-salt hypertension, in which the renin-angiotensin system is suppressed, the LO inhibitor phenidone was without effect on blood pressure (108). Two mechanisms have been proposed for LO mediating or regulating angiotensin-induced vasoconstriction. Angiotensin II increases 12-HETE production by smooth muscle, and 12-HETE increases [Ca2+]i in smooth muscle (108, 138, 149). These findings imply that 12-HETE is an intracellular mediator of angiotensin vasoconstriction. In contrast, Nasjletti and colleagues (32, 103) have shown that 12-HPETE inhibits PGI2 synthase, decreasing the production of PGI2. As a result, less PGI2 is available to antagonize angiotensin-induced vasoconstriction. Inhibition of 12-HPETE production with LO inhibitors restores PGI2 synthase activity, and PGI2 antagonizes the action of angiotensin. Despite the mechanism or combination of mechanisms involved, these studies indicate that LO inhibitors reduce blood pressure in renin-dependent hypertension by reducing angiotensin-induced vasoconstriction. Additional studies are needed to determine the role of LO-derived EDHFs in hypertension.
Summary, Perspective, and Future Direction
15-LO-1 is expressed in a wide variety of human cells. The biological role of 15-LO-1, however, is unclear and sometimes contradictory. 15-LO-1 mediates inflammatory monocyte/endothelial interactions and atherosclerosis (129). On the other hand, the 15-LO-1-derived lipoxins are anti-inflammatory (142). The major AA metabolite from 15-LO-1 pathway is 15(S)-HETE. However, in some experimental conditions, micromolar concentrations of 15(S)-HETE are required to observe its activities (91, 159, 167). This concentration is unlikely a physiological concentration and suggests that other metabolites mediate the biological actions of 15-LO-1. In addition to 15(S)-HETE, the 15-LO-1 pathway also produces lipoxins (141), eoxins (42), 8(S),15(S)-diHETE (143), 5-oxo-15-hydroxy-6,8,11,13-eicosatetraenoic acid (140), HEETAs (23), and THETAs (21, 120), which possess several biological activities. The physiological or pathophysiological importance of 15-LO-1 has been shown by knockdown or overexpression studies (4, 5, 154). The HEETAs and THETAs may represent potential mediators of the actions of 15-LO-1.
15-LO metabolites function as novel EDHFs in both conduit arteries and resistance arteries (3, 21, 120, 165, 166). It is generally accepted that in large conduit arteries, such as the aorta, the contribution of EDHF to vascular relaxation is small (41, 71, 101). In contrast, in resistance arterioles (diameter <100 μm), which play a critical role in blood pressure control, EDHF is a predominant relaxing influence. The findings reviewed above are in agreement with this concept. PGI2 and PGE2 are active in mesenteric arteries (166), whereas in the aorta, they do not contribute to vasodilation (116). In conduit arteries such as rabbit aorta, 11(R),12(S),15(S)-TH-5(Z),8(Z),13(E)-ETA is the major EDHF that mediates non-NO, non-PGI2, ACh-induced relaxations and hyperpolarizations (21). In contrast, 11,12,15-THETA, possibly 15-H-11,12-EETA, and K+ all function as EDHFs in resistance arteries such as rabbit mesenteric arteries (165). 11,12,15-THETA and K+ act synergistically in this vascular bed to cause relaxation.
It is not uncommon for both HEETAs and their hydrolysis products, the THETAs, to have similar biological effects. Hepoxilin and its four trioxilin hydrolysis products activate peroxisome proliferator-activated receptor-α (PPARα) with a comparable efficiency (164). Therefore, the redundancy of vasodilatory functions of both 15-H-11,12-EETA and 11,12,15-THETA was not unexpected. Although the vascular activity of these 15-LO metabolites is the first to be characterized, it is likely that other biological functions of HEETAs and THETAs have yet to be discovered. In fact, we observed that 11(R),12(S),15(S)-THETA inhibits migration of aortic smooth muscle cells.
Cardiovascular disease is the major cause of death in the United States (130). EDHF plays an important role in cardiovascular physiology and pathology in various animal models as well as in humans (28, 41). For example, in patients with essential hypertension, the EDHF system becomes active when there is a defect in the endothelium-derived NO (113, 151), suggesting its role as an important mechanism to maintain endothelium-dependent vasodilation. In isolated aorta, mesenteric arteries, and renal arteries of hypercholesterolemic rabbits, an enhanced contribution of EDHF compensates for the decrease in NO-mediated relaxation (13, 70). Given this biological importance of EDHF, characterization of new members of the EDHF family will broaden our understanding, set a new direction for the research, and may lead to the development a new therapeutic target for the treatment of cardiovascular diseases.
GRANTS
These studies were supported by National Heart, Lung, and Blood Institute Grant HL-37981. Y. Chawengsub was supported by a predoctoral fellowship from the American Heart Association (Greater Midwest Affiliate), and K. M. Gauthier was supported by an American Heart Association Grant-in-Aid.
Acknowledgments
We thank Gretchen Barg for secretarial assistance.
REFERENCES
- 1.Aggarwal N, Gauthier KM, Campbell WB. 15-Lipoxygenase metabolites contribute to age-related reductions in acetylcholine-induced hypotension in rabbits. Am J Physiol Heart Circ Physiol 295: H89–H96, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Aggarwal N, Pfister SL, Campbell WB. Hypercholesterolemia enhances 15-lipoxygenase mediated vasorelaxation and acetylcholine-induced hypotension. Arterioscler Thromb Vasc Biol 28: 2209–2215, 2008. [DOI] [PubMed] [Google Scholar]
- 3.Aggarwal NT, Chawengsub Y, Gauthier KM, Viita H, Yla-Herttuala S, Campbell WB. Endothelial 15-lipoxygenase-1 overexpression increases acetylcholine-induced hypotension and vasorelaxation in rabbits. Hypertension 51: 246–251, 2008. [DOI] [PubMed] [Google Scholar]
- 4.Aggarwal NT, Holmes BB, Cui L, Viita H, Yla-Herttuala S, Campbell WB. Adenoviral expression of 15-lipoxygenase-1 in rabbit aortic endothelium: role in arachidonic acid-induced relaxation. Am J Physiol Heart Circ Physiol 292: H1033–H1041, 2007. [DOI] [PubMed] [Google Scholar]
- 5.Anning PB, Coles B, Bermudez-Fajardo A, Martin PWM, Levison BS, Hazen SL, Funk CD, Kuhn H, O'Donnell VB. Elevated endothelial nitric oxide bioactivity and resistance to angiotensin-dependent hypertension in 12/15-lipoxygenase knockout mice. Am J Pathol: 653–662, 2005. [DOI] [PMC free article] [PubMed]
- 6.Bergholte JM, Soberman RJ, Hayes R, Murphy RC, Okita RT. Oxidation of 15-hydroxyeicosatetraenoic acids and other hydroxy fatty acids by lung prostaglandin dehydrogenase. Arch Biochem Biophys 257: 444–450, 1987. [DOI] [PubMed] [Google Scholar]
- 7.Blee E, Wilcox AL, Marnett LJ, Schuber F. Mechanism of reaction of fatty acid hydroperoxides with soybean peroxygenase. J Biol Chem 268: 1708–1715, 1993. [PubMed] [Google Scholar]
- 8.Bolton TB, Clapp LH. Endothelial-dependent relaxant actions of carbachol and substance P in arterial smooth muscle. Br J Pharmacol 87: 713–723, 1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bolton TB, Lang RJ, Takewaki T. Mechanisms of action of noradrenaline and carbachol on smooth muscle of guinea-pig anterior mesenteric artery. J Physiol 351: 549–572, 1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bond CT, Maylie J, Adelman JP. Small-conductance calcium-activated potassium channels. Ann NY Acad Sci 868: 370–378, 1999. [DOI] [PubMed] [Google Scholar]
- 11.Bonventre JV Phospholipase A2 and signal transduction. J Am Soc Nephrol 3: 128–150, 1992. [DOI] [PubMed] [Google Scholar]
- 12.Boulanger C, Hendrickson H, Lorenz RR, Vanhoutte PM. Release of different relaxing factors by cultured porcine endothelial cells. Circ Res 64: 1070–1078, 1989. [DOI] [PubMed] [Google Scholar]
- 13.Brandes RP, Behra A, Lebherz C, Boger RH, Bode-Boger SM, Phivthong-Ngam L, Mugge A. NG-nitro-l-arginine- and indomethacin-resistant endothelium-dependent relaxation in the rabbit renal artery: effect of hypercholesterolemia. Atherosclerosis 135: 49–55, 1997. [DOI] [PubMed] [Google Scholar]
- 14.Brash AR, Beoglin WE, Chang MS. Discovery of a second 15S-lipoxygenase in humans. Proc Natl Acad Sci USA 94: 6148–6152, 1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bredt DS, Snyder SH. Isolation of nitric oxide synthetase, a calmodulin-requiring enzyme. Proc Natl Acad Sci USA 87: 682–685, 1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Burnham MP, Bychkov R, Feletou M, Richards GR, Vanhoutte PM, Weston AH, Edwards G. Characterization of an apamin-sensitive small-conductance Ca2+-activated K+ channel in porcine coronary artery endothelium: relevance to EDHF. Br J Pharmacol 135: 1133–1143, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bychkov R, Burnham MP, Richards GR, Edwards G, Weston AH, Feletou M, Vanhoutte PM. Characterization of a charybdotoxin-sensitive intermediate conductance Ca2+-activated K+ channel in porcine coronary endothelium: relevance to EDHF. Br J Pharmacol 137: 1346–1354, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Campbell WB, Deeter C, Gauthier KM, Ingraham RH, Falck JR, Li PL. 14,15-Dihydroxyeicosatrienoic acid relaxes bovine coronary arteries by activation of KCa channels. Am J Physiol Heart Circ Physiol 282: H1656–H1664, 2002. [DOI] [PubMed] [Google Scholar]
- 19.Campbell WB, Falck JR. Arachidonic acid metabolites as endothelium-derived hyperpolarizing factors. Hypertension 49: 590–596, 2007. [DOI] [PubMed] [Google Scholar]
- 20.Campbell WB, Gebremedhin D, Pratt PF, Harder DR. Identification of epoxyeicosatrienoic acids as endothelium-derived hyperpolarizing factors. Circ Res 78: 415–423, 1996. [DOI] [PubMed] [Google Scholar]
- 21.Campbell WB, Spitzbarth N, Gauthier KM, Pfister SL. 11,12,15-Trihydroxyeicosatrienoic acid mediates acetylcholine-induced relaxations in the rabbit aorta. Am J Physiol Heart Circ Physiol 285: H2648–H2656, 2003. [DOI] [PubMed] [Google Scholar]
- 22.Chauhan SD, Nilsson H, Ahluwalia A, Hobbs AJ. Release of C-type natriuretic peptide accounts for the biological activity of endothelium-derived hyperpolarizing factor. Proc Natl Acad Sci USA 100: 1426–1431, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chawengsub Y, Aggarwal NT, Nithipatikom K, Gauthier KM, Anjaiah S, Hammock BD, Falck JR, Campbell WB. Identification of 15-hydroxy-11,12-epoxyeicosatrienoic acid as a vasoactive 15-lipoxygenase metabolite in rabbit aorta. Am J Physiol Heart Circ Physiol 294: H1348–H1356, 2008. [DOI] [PubMed] [Google Scholar]
- 24.Chen G, Suzuki H. Some electrical properties of the endothelium-dependent hyperpolarization recorded from rat arterial smooth muscle cells. J Physiol 410: 91–106, 1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chen G, Suzuki H, Weston AH. Acetylcholine released endothelium-derived hyperpolarizing factor and EDRF from rat blood vessels. Br J Pharmacol 95: 1165–1174, 1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chen G, Yamamoto Y, Miwa K, Suzuki H. Hyperpolarization of arterial smooth muscle induced by endothelial humoral substances. Am J Physiol Heart Circ Physiol 260: H1888–H1892, 1991. [DOI] [PubMed] [Google Scholar]
- 27.Cohen RA, Plane F, Najibi S, Huk I, Malinski T, Garland CJ. Nitric oxide is the mediator of both endothelium-dependent relaxation and hyperpolarization of the rabbit carotid artery. Proc Natl Acad Sci USA 94: 4193–4198, 1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cohen RA, Vanhoutte PM. Endothelium-dependent hyperpolarization: beyond nitric oxide and cyclic GMP. Circulation 92: 3337–3349, 1995. [DOI] [PubMed] [Google Scholar]
- 29.Collins XH, Harmon SD, Kaduce TL, Berst KB, Fang X, Moore SA, Raju TV, Falck JR, Weintraub NL, Duester G, Plapp BV, Spector AA. Omega-oxidation of 20-hydroxyeicosatetraenoic acid (20-HETE) in cerebral microvascular smooth muscle and endothelium by alcohol dehydrogenase 4. J Biol Chem 280: 33157–33164, 2005. [DOI] [PubMed] [Google Scholar]
- 30.Cowan CL, Cohen RA. Two mechanisms mediate relaxation by bradykinin of pig coronary artery: NO-dependent and -independent responses. Am J Physiol Heart Circ Physiol 261: H830–H835, 1991. [DOI] [PubMed] [Google Scholar]
- 31.Cowan CL, Palacino JJ, Najibi S, Cohen RA. Potassium channel-mediated relaxation to acetylcholine in rabbit arteries. J Pharmacol Exp Ther 266: 1482–1489, 1993. [PubMed] [Google Scholar]
- 32.DelliPizzi A, Guan H, Tong X, Takizawa H, Nasjletti A. Lipoxygenase-dependent mechanisms in hypertension. Clin Exp Hypertens 22: 181–192, 2000. [DOI] [PubMed] [Google Scholar]
- 33.DeMey JG, Claeys M, Vanhoutte PM. Endothelium-dependent inhibitory effects of acetylcholine, adenosine triphosphate, thrombin and arachidonic acid in the canine femoral artery. J Pharmacol Exp Ther 222: 166–173, 1982. [PubMed] [Google Scholar]
- 34.Derewlany LO, Pace-Asciak CR, Radde IC. Hepoxilin A, hydroxyepoxide metabolite of arachidonic acid, stimulates transport of 45Ca across the guinea pig visceral yolk sac. Can J Physiol Pharmacol 62: 1466–1469, 1984. [DOI] [PubMed] [Google Scholar]
- 35.Dho S, Grinstein S, Corey EJ, Su WG, Pace-Asciak CR. Hepoxilin A3 induced changes in cytosolic calcium, intracellular pH and membrane potential in human neutrophils. Biochem J 266: 63–68, 1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Edwards G, Dora KA, Gardener MJ, Garland CJ, Weston AH. K is an endothelium-derived hyperpolarizing factor in rat arteries. Nature 396: 269–272, 1998. [DOI] [PubMed] [Google Scholar]
- 37.Eichler I, Wibawa J, Grgic I, Korr A, Brakemeier S, Priest AR, Hoyer J, Kohler R. Selective blockade of endothelial Ca2+-activated small- and intermediate-conductance K+-channels suppresses EDHF-mediated vasodilation. Br J Pharmacol 138: 594–601, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Fang X, Kaduce TL, Weintraub NL, Harmon S, Teesch LM, Morisseau C, Thompson DA, Hammock BD, Spector AA. Pathways of epoxyeicosatrienoic acid metabolism in endothelial cells: implications for the vascular effects of soluble epoxide hydrolase inhibition. J Biol Chem 276: 14867–14874, 2001. [DOI] [PubMed] [Google Scholar]
- 39.Faraci FM, Sobey CG, Chrissobolis S, Lund DD, Heistad DD, Weintraub NL. Arachidonate dilates basilar artery by lipoxygenase-dependent mechanism and activation of K+ channels. Am J Physiol Regul Integr Comp Physiol 281: R246–R253, 2001. [DOI] [PubMed] [Google Scholar]
- 40.Feletou M, Vanhoutte PM. Endothelium-dependent hyperpolarization of canine coronary smooth muscle. Br J Pharmacol 93: 515–524, 1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Feletou M, Vanhoutte PM. Endothelium-derived hyperpolarizing factor: where are we now? Arterioscler Thromb Vasc Biol 26: 1215–1225, 2006. [DOI] [PubMed] [Google Scholar]
- 42.Feltenmark S, Gautam N, Brunnstrom A, Griffiths W, Backman L, Edenius C, Lindbom L, Bjorkholm M, Claesson HE. Eoxins are proinflammatory arachidonic acid metabolites produced via the 15-lipoxygenase-1 pathway in human eosinophils and mast cells. Proc Natl Acad Sci USA 105: 680–685, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Fiore S, Maddox JF, Perez HD, Serhan CN. Identification of a human cDNA encoding a functional high affinity lipoxin A4 receptor. J Exp Med 180: 253–260, 1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Fisslthaler B, Popp R, Kiss L, Potente M, Harder DR, Fleming I, Busse R. Cytochrome P450 2C is an EDHF synthase in coronary arteries. Nature 401: 493–497, 1999. [DOI] [PubMed] [Google Scholar]
- 45.Förstermann U, Alheid U, Frolich JG, Mulsch A. Mechanisms of action of lipoxygenase and cytochrome P-450-mono-oxygenase inhibitors in blocking endothelium-dependent vasodilation. Br J Pharmacol 93: 569–578, 1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Forstermann U, Burgwitz K, Frolich JC. Effect of nonsteroidal phospholipase inhibitors and glucocorticoids on endothelium-dependent relaxations of rabbit aorta induced by different agents. J Cardiovasc Pharmacol 10: 356–364, 1987. [DOI] [PubMed] [Google Scholar]
- 47.Förstermann U, Hertting G, Neufang B. The role of endothelial and nonendothelial prostaglandins in the relaxation of isolated blood vessels of the rabbit induced by bradykinin and acetylcholine. Br J Pharmacol 87: 521–532, 1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Forstermann U, Mugge A, Frolich JC. Endothelium-dependent relaxations of human epicardial coronary arteries: frequent lack of effect of acetylcholine. Eur J Pharmacol 128: 277–281, 1986. [DOI] [PubMed] [Google Scholar]
- 49.Forstermann U, Neufang B. The endothelium-dependent relaxation of rabbit aorta: effects of antioxidants and hydroxylated eicosatetraenoic acids. Br J Pharmacol 82: 765–767, 1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Förstermann U, Neufang B. The endothelium-dependent vasodilator effect of acetylcholine: characterization of the endothelial relaxing factor with inhibitors of arachidonic acid metabolism. Eur J Pharmacol 103: 65–70, 1984. [DOI] [PubMed] [Google Scholar]
- 51.Furchgott RF, Zawadzki JW. The obligatory role of endothelial cells in the relaxation of arterial smooth muscle by acetylcholine. Nature 288: 373–376, 1980. [DOI] [PubMed] [Google Scholar]
- 52.Gardner HW Oxygen radical chemistry of polyunsaturated fatty acids. Free Radic Biol Med 7: 65–86, 1989. [DOI] [PubMed] [Google Scholar]
- 53.Garland CJ, McPherson GA. Evidence that nitric oxide does not mediate the hyperpolarization and relaxation to acetylcholine in the rat small mesenteric artery. Br J Pharmacol 105: 429–435, 1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Gauthier KM, Chawengsub Y, Goldman DH, Conrow RE, Anjaiah S, Falck JR, Campbell WB. 11(R),12(S),15(S)-trihydroxyeicosa-5(Z),8(Z),13(E)-trienoic acid: an endothelium-derived 15-lipoxygenase metabolite that relaxes rabbit aorta. Am J Physiol Heart Circ Physiol 294: H1467–H1472, 2008. [DOI] [PubMed] [Google Scholar]
- 55.Gauthier KM, Christian S, Goldman DH, Aggarwal N, Campbell WB. Lipoxygenase metabolites of arachidonic acid mediate acetylcholine-induced relaxations of mouse arteries (Abstract). Hypertension 52: e55, 2008. [Google Scholar]
- 56.Gauthier KM, Edwards EM, Falck JR, Reddy DS, Campbell WB. 14,15-Epoxyeicosatrienoic acid represents a transferable endothelium-dependent relaxing factor in bovine coronary arteries. Hypertension 45: 666–671, 2005. [DOI] [PubMed] [Google Scholar]
- 57.Gauthier KM, Spitzbarth N, Edwards EM, Campbell WB. Apamin-sensitive K+ currents mediate arachidonic acid-induced relaxations of rabbit aorta. Hypertension 43: 413–419, 2004. [DOI] [PubMed] [Google Scholar]
- 58.Gebremedhin D, Harder DR, Pratt PF, Campbell WB. Bioassay of an endothelium-derived hyperpolarizing factor from bovine coronary arteries: role of a cytochrome P450 metabolite. J Vasc Res 35: 274–284, 1998. [DOI] [PubMed] [Google Scholar]
- 59.Gillmor SA, Villasenor A, Fletterick R, Sigal E, Browner MF. The structure of mammalian 15-lipoxygenase reveals similarity to the lipases and the determinants of substrate specificity. Nat Struct Biol 4: 1003–1009, 1997. [DOI] [PubMed] [Google Scholar]
- 60.Gordon EE, Gordon JA, Spector AA. HETEs and coronary artery endothelial cells: metabolic and functional interactions. Am J Physiol Cell Physiol 261: C623–C633, 1991. [DOI] [PubMed] [Google Scholar]
- 61.Gowri MS, Reaven GM, Azhar S. Masoprocol lowers blood pressure in rats with fructose-induced hypertension. Am J Hypertens 12: 744–746, 1999. [DOI] [PubMed] [Google Scholar]
- 62.Griffith TM Endothelium-dependent smooth muscle hyperpolarization: do gap junctions provide a unifying hypothesis? Br J Pharmacol 141: 881–903, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Griffith TM, Edwards DH, Lewis MJ, Newby AC, Henderson AH. The nature of endothelium-derived vascular relaxant factor. Nature 308: 645–647, 1984. [DOI] [PubMed] [Google Scholar]
- 64.Gronert K, Gewirtz A, Madara JL, Serhan CN. Identification of a human enterocyte lipoxin A4 receptor that is regulated by interleukin (IL)-13 and interferon gamma and inhibits tumor necrosis factor alpha-induced IL-8 release. J Exp Med 187: 1285–1294, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Hamberg M Hydroperoxide isomerases. J Lipid Mediat Cell Signal 12: 283–292, 1995. [DOI] [PubMed] [Google Scholar]
- 66.Hamberg M, Herman CA, Herman RP. Novel biological transformations of 15-l-hydroperoxy-5,8,11,13-eicosatetraenoic acid. Biochim Biophys Acta 877: 447–457, 1986. [DOI] [PubMed] [Google Scholar]
- 67.Hansen HS 15-Hydroxyprostaglandin dehydrogenase: a review. Prostaglandins 12: 647–679, 1976. [DOI] [PubMed] [Google Scholar]
- 68.Himmel HM, Whorton AR, Strauss HC. Intracellular calcium, currents, and stimulus-response coupling in endothelial cells. Hypertension 21: 112–127, 1993. [DOI] [PubMed] [Google Scholar]
- 69.Hoeffner U, Feletou M, Flavahan NA, Vanhoutte PM. Canine arteries release two different endothelium-derived relaxing factors. Am J Physiol Heart Circ Physiol 257: H330–H333, 1989. [DOI] [PubMed] [Google Scholar]
- 70.Honda H, Moroe H, Fujii H, Arai K, Notoya Y, Kogo H. Short term hypercholesterolemia alters NG-nitro-l-arginine- and indomethacin-resistant endothelium-dependent relaxation by acetylcholine in rabbit renal artery. Jpn J Pharmacol 85: 203–206, 2001. [DOI] [PubMed] [Google Scholar]
- 71.Hwa JJ, Ghibaudi L, Williams P, Chatterjii M. Comparison of acetylcholine-dependent relaxation in large and small arteries of rat mesenteric vascular bed. Am J Physiol Heart Circ Physiol 266: H952–H958, 1994. [DOI] [PubMed] [Google Scholar]
- 72.Ignarro LJ, Buga GM, Wood KD, Byrns RE, Chaudhuri G. Endothelium-derived relaxing factor produced and released from artery and vein is nitric oxide. Proc Natl Acad Sci USA 84: 9265–9269, 1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Ivanov I, Schwarz K, Holzhutter HG, Myagkova G, Kuhn H. Omega-oxidation impairs oxidizability of polyenoic fatty acids by 15-lipoxygenases: consequences for substrate orientation at the active site. Biochem J 336: 345–352, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Johns A, Freay AD, Adams DJ, Lategan TW, Ryan US, van Breemen C. Role of calcium in the activation of endothelial cells. J Cardiovasc Pharmacol 12, Suppl 5: S119–S123, 1988. [PubMed] [Google Scholar]
- 75.Kaduce TL, Fang X, Harmon SD, Oltman CL, Dellsperger KC, Teesch LM, Gopal VR, Falck JR, Campbell WB, Weintraub NL, Spector AA. 20-Hydroxyeicosatetraenoic acid (20-HETE) metabolism in coronary endothelial cells. J Biol Chem 279: 2648–2656, 2004. [DOI] [PubMed] [Google Scholar]
- 76.Kamouchi M, Droogmans G, Nilius B. Membrane potential as a modulator of the free intracellular Ca2+ concentration in agonist-activated endothelial cells. Gen Physiol Biophys 18: 199–208, 1999. [PubMed] [Google Scholar]
- 77.Kauser K, Stekiel WJ, Rubanyi G, Harder DR. Mechanism of action of EDRF on pressurized arteries: effect on K conductance. Circ Res 65: 199–204, 1989. [DOI] [PubMed] [Google Scholar]
- 78.Komori K, Suzuki H. Electrical responses of smooth muscle cells during cholinergic vasolidation in the rabbit saphenous artery. Circ Res 61: 586–593, 1987. [DOI] [PubMed] [Google Scholar]
- 79.Kuhn H, Walther M, Kuban RJ. Mammalian arachidonate 15-lipoxygenases structure, function, and biological implications. Prostaglandins Other Lipid Mediat 68–69: 263–290, 2002. [DOI] [PubMed] [Google Scholar]
- 80.Laneuville O, Corey EJ, Couture R, Pace-Asciak CR. Hepoxilin A3 (HxA3) is formed by the rat aorta and is metabolized into HxA3-C, a glutathione conjugate. Biochim Biophys Acta 1084: 60–68, 1991. [DOI] [PubMed] [Google Scholar]
- 81.Laneuville O, Couture R, Pace-Asciak CR. Hepoxilins sensitize blood vessels to noradrenaline-stereospecificity of action. Br J Pharmacol 105: 297–304, 1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Larsen BT, Miura H, Campbell WB, Gutterman DD. 12- and 15-Hydroxyeicosatetraenoic acids (HETEs) function as endothelium-derived hyperpolarizing factors in human coronary arterioles (Abstract). FASEB J 23: 952.17, 2009. [Google Scholar]
- 83.Larsen BT, Miura H, Hatoum OA, Campbell WB, Hammock BD, Zeldin DC, Falck JR, Gutterman DD. Epoxyeicosatrienoic and dihydroxyeicosatrienoic acids dilate human coronary arterioles via BKCa channels: implications for soluble epoxide hydrolase inhibition. Am J Physiol Heart Circ Physiol 290: H491–H499, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Lefebvre B, Caron F, Bessard G, Stanke-Labesque F. Effect of 5-lipoxygenase blockade on blood pressure and acetylcholine-evoked endothelium-dependent contraction in aorta from spontaneously hypertensive rats. J Hypertens 24: 85–93, 2006. [DOI] [PubMed] [Google Scholar]
- 85.Long CJ, Sarau HM, Berkowitz BA. The inhibition of release of endothelium-derived relaxant factor by manoalide, a potent inhibitor of phospholipase A2. Br J Pharmacol 92: 843–849, 1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Lopez-Jaramillo P, Gonzalez MC, Palmer RM, Moncada S. The crucial role of physiological Ca2+ concentrations in the production of endothelial nitric oxide and the control of vascular tone. Br J Pharmacol 101: 489–493, 1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Luckhoff A, Busse R. Calcium influx into endothelial cells and formation of endothelium-derived relaxing factor is controlled by membrane potential. Pflügers Arch 416: 305–311, 1990. [DOI] [PubMed] [Google Scholar]
- 88.Luckhoff A, Busse R, Winter I, Bassenge E. Characterization of vascular relaxant factor released from cultured endothelial cells. Hypertension 9: 295–303, 1987. [DOI] [PubMed] [Google Scholar]
- 89.Ma YH, Harder DR, Clark JE, Roman RJ. Effects of 12-HETE on isolated dog renal arcuate arteries. Am J Physiol Heart Circ Physiol 261: H451–H456, 1991. [DOI] [PubMed] [Google Scholar]
- 90.Matoba T, Shimokawa H, Nakashima M, Hirakawa Y, Mukai Y, Hirano K, Kandaide H, Takeshita A. Hydrogen peroxide is an endothelium-derived hyperpolarizing factor in mice. J Clin Invest 106: 1521–1530, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Matsuda H, Miyatake K, Dahlen SE. Pharmacodynamics of 15(S)-hydroperoxyeicosatetraenoic (15-HPETE) and 15(S)-hydroxyeicosatetraenoic acid (15-HETE) in isolated arteries from guinea pig, rabbit, rat and human. J Pharmacol Exp Ther 273: 1182–1189, 1995. [PubMed] [Google Scholar]
- 92.McCallum GP, Weedon AC, Krug P, Bend JR. Microsomal cytochrome P450 peroxygenase metabolism of arachidonic acid in guinea pig liver. J Pharmacol Exp Ther 278: 1188–1194, 1996. [PubMed] [Google Scholar]
- 93.Miller AW, Katakam PVG, Lee HC, Tulbert CD, Busija DW, Weintraub NL. Arachidonic acid-induced vasodilation of rat small mesenteric arteries is lipoxygenase-dependent. J Pharmacol Exp Ther 304: 139–144, 2003. [DOI] [PubMed] [Google Scholar]
- 94.Minami Y, Toda N. Possible involvement of 5-lipoxygenase products in the generation of endothelium derived relaxing factor. J Pharmacol Exp Ther 250: 1055–1060, 1989. [PubMed] [Google Scholar]
- 95.Miura H, Wachtel RE, Liu Y, Loberiza J, FR, Saito T, Miura M, Gutterman DD. Flow-induced dilation of human coronary arterioles: important role of Ca2+-activated K+ channels. Circulation 103: 1992–1998, 2001. [DOI] [PubMed] [Google Scholar]
- 96.Mombouli JV, Bissiriou I, Agboton VD, Vanhoutte PM. Bioassay of endothelium-derived hyperpolarizing factor. Biochem Biophys Res Commun 221: 484–488, 1996. [DOI] [PubMed] [Google Scholar]
- 97.Moncada S Eighth Gaddum Memorial Lecture. University of London Institute of Education, December 1980. Biological importance of prostacyclin. Br J Pharmacol 76: 3–31, 1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Moncada S, Palmer RMJ, Gryglewski RJ. Mechanism of action of some inhibitors of endothelium-derived relaxing factor. Proc Natl Acad Sci USA 83: 9164–9168, 1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Moore SA, Spector AA, Hart MN. Eicosanoid metabolism in cerebromicrovascular endothelium. Am J Physiol Cell Physiol 254: C37–C44, 1988. [DOI] [PubMed] [Google Scholar]
- 100.Murphy ME, Brayden JE. Apamin-sensitive K channels mediate an endothelium-dependent hyperpolarization in rabbit mesenteric arteries. J Physiol 489: 723–734, 1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Nagao T, Illiano S, Vanhoutte PM. Heterogenous distribution of endothelium-dependent relaxations resistant to N-nitro-l-arginine in rats. Am J Physiol Heart Circ Physiol 263: H1090–H1094, 1992. [DOI] [PubMed] [Google Scholar]
- 102.Nakao J, Ooyama T, Ito H, Chang WC, Murota S. Comparative effect of lipoxygenase products of arachidonic acid on rat aortic smooth muscle cell migration. Atherosclerosis 44: 339–342, 1982. [DOI] [PubMed] [Google Scholar]
- 103.Nasjletti A The role of eicosanoids in angiotensin-dependent hypertension. Hypertension 31: 194–200, 1997. [DOI] [PubMed] [Google Scholar]
- 104.Nelson MT, Quayle JM. Physiological roles and properties of potassium channels in arterial smooth muscle. Am J Physiol Cell Physiol 268: C799–C822, 1995. [DOI] [PubMed] [Google Scholar]
- 105.Nie D, Tang K, Diglio C, Honn KV. Eicosanoid regulation of angiogenesis: role of endothelial arachidonate 12-lipoxygenase. Blood 95: 2304–2311, 2000. [PubMed] [Google Scholar]
- 106.Nigam S, Zafiriou MP, Deva R, Ciccoli R, Roux-Van der Merwe R. Structure, biochemistry and biology of hepoxilins: an update. FEBS J 274: 3503–3512, 2007. [DOI] [PubMed] [Google Scholar]
- 107.Nilius B, Droogmans G. Ion channels and their functional role in vascular endothelium. Physiol Rev 81: 1415–1459, 2001. [DOI] [PubMed] [Google Scholar]
- 108.Nozawa K, Tuck ML, Golub M, Eggena P, Nadler JL, Stern N. Inhibition of lipoxygenase pathway reduces blood pressure in renovascular hypertensive rats. Am J Physiol Heart Circ Physiol 259: H1774–H1780, 1990. [DOI] [PubMed] [Google Scholar]
- 109.Pace-Asciak CR, Granstrom E, Samuelsson B. Arachidonic acid epoxides: isolation and structure of two hydroxy epoxide intermediates in the formation of 8,11,12- and 10,11,12-trihydroxyeicosatrienoic acids. J Biol Chem 258: 6835–6840, 1983. [PubMed] [Google Scholar]
- 110.Pace-Asciak CR, Martin JM. Hepoxilin, a new family of insulin secretagogues formed by intact rat pancreatic islets. Prostaglandins Leukot Med 16: 173–180, 1984. [DOI] [PubMed] [Google Scholar]
- 111.Pace-Asciak CR, Reynaud D, Demin P, Nigam S. The hepoxilins. A review. Adv Exp Med Biol 447: 123–132, 1999. [PubMed] [Google Scholar]
- 112.Palmer RMJ, Ferrige AG, Moncada S. Nitric oxide release accounts for the biological activity of endothelium-derived relaxing factor. Nature 327: 524–526, 1987. [DOI] [PubMed] [Google Scholar]
- 113.Panza JA, Casino PR, Kilcoyne CM, Quyyumi AA. Role of endothelium-derived nitric oxide in the abnormal endothelium-dependent vascular relaxation of patients with essential hypertension. Circulation 87: 1468–1474, 1993. [DOI] [PubMed] [Google Scholar]
- 114.Patricia MK, Kim JA, Harper CM, Shih PT, Berliner JA, Natarajan R, Nadler JL, Hedrick CC. Lipoxygenase products increase monocyte adhesion to human aortic endothelial cells. Arterioscler Thromb Vasc Biol 19: 2615–2622, 1999. [DOI] [PubMed] [Google Scholar]
- 115.Petersson J, Zygmunt PM, Hogestatt ED. Characterization of the potassium channels involved in EDHF-mediated relaxation in cerebral arteries. Br J Pharmacol 120: 1344–1350, 1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Pfister SL, Campbell WB. Arachidonic acid- and acetylcholine-induced relaxations of rabbit aorta. Hypertension 20: 682–689, 1992. [DOI] [PubMed] [Google Scholar]
- 117.Pfister SL, Falck JR, Campbell WB. Enhanced synthesis of epoxyeicosatrienoic acids by cholesterol-fed rabbit aorta. Am J Physiol Heart Circ Physiol 261: H843–H852, 1991. [DOI] [PubMed] [Google Scholar]
- 118.Pfister SL, Schmitz JM, Willerson JT, Campbell WB. Characterization of arachidonic acid metabolism in Watanabe heritable hyperlipidemic (WHHL) and New Zealand White (NZW) rabbit aortas. Prostaglandins 36: 515–531, 1988. [DOI] [PubMed] [Google Scholar]
- 119.Pfister SL, Spitzbarth N, Edgemond W, Campbell WB. Vasorelaxation by an endothelium-derived metabolite of arachidonic acid. Am J Physiol Heart Circ Physiol 270: H1021–H1030, 1996. [DOI] [PubMed] [Google Scholar]
- 120.Pfister SL, Spitzbarth N, Nithipatikom K, Edgemond WS, Falck JR, Campbell WB. Identification of 11,14,15- and 11,12,15-trihydroxyeicosatrienoic acids as endothelium-derived relaxing factors of rabbit aorta. J Biol Chem 273: 30879–30887, 1998. [DOI] [PubMed] [Google Scholar]
- 121.Pfister SL, Spitzbarth N, Nithipatikom K, Falck JR, Campbell WB. Metabolism of 12-hydroperoxyeicosatetraenoic acid to vasodilatory trioxilin C3 by rabbit aorta. Biochim Biophys Acta 1622: 6–13, 2003. [DOI] [PubMed] [Google Scholar]
- 122.Pfister SL, Spitzbarth N, Zeldin DC, Lafite P, Mansuy D, Campbell WB. Rabbit aorta converts 15-HPETE to trihydroxyeicosatrienoic acids: potential role of cytochrome P450. Arch Biochem Biophys 420: 142–152, 2003. [DOI] [PubMed] [Google Scholar]
- 123.Piomelli D, Shapiro E, Zipkin R, Schwartz JH, Feinmark SJ. Formation and action of 8-hydroxy-11,12-epoxy-5,9,14-eicosatrienoic acid in Aplysia: a possible second messenger in neurons. Proc Natl Acad Sci USA 86: 1721–1725, 1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Popp R, Bauersachs J, Hecker M, Fleming I, Busse R. A transferable, beta-naphthoflavone-inducible, hyperpolarizing factor is synthesized by native and cultured porcine coronary endothelial cells. J Physiol 497: 699–709, 1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Popp R, Fleming I, Busse R. Pulsatile stretch in coronary arteries elicits release of endothelium-derived hyperpolarizing factor: a modulator of arterial compliance. Circ Res 82: 696–703, 1998. [DOI] [PubMed] [Google Scholar]
- 126.Prior HM, Webster N, Quinn K, Beech DJ, Yates MS. K+-induced dilation of a small renal artery: no role for inward rectifier K+ channels. Cardiovasc Res 37: 780–790, 1998. [DOI] [PubMed] [Google Scholar]
- 127.Radmark O Arachidonate 5-lipoxygenase. Prostaglandins Other Lipid Mediat 68–69: 211–234, 2002. [DOI] [PubMed] [Google Scholar]
- 128.Reddy MA, Thimmalapura PR, Lanting L, Nadler JL, Fatima S, Natarajan R. The oxidized lipid and lipoxygenase product 12(S)-hydroxyeicosatetraenoic acid induces hypertrophy and fibronectin transcription in vascular smooth muscle cells via p38 MAPK and cAMP response element-binding protein activation. Mediation of angiotensin II effects. J Biol Chem 277: 9920–9928, 2002. [DOI] [PubMed] [Google Scholar]
- 129.Reilly KB, Srinivasan S, Hatley ME, Patricia MK, Lannigan J, Bolick DT, Vandenhoff G, Pei H, Natarajan R, Nadler JL, Hedrick CC. 12/15-Lipoxygenase activity mediates inflammatory monocyte/endothelial interactions and atherosclerosis in vivo. J Biol Chem 279: 9440–9450, 2004. [DOI] [PubMed] [Google Scholar]
- 130.Rosamond W, Flegal K, Friday G, Furie K, Go A, Greenlund K, Haase N, Ho M, Howard V, Kissela B, Kittner S, Lloyd-Jones D, McDermott M, Meigs J, Moy C, Nichol G, O'Donnell CJ, Roger V, Rumsfeld J, Sorlie P, Steinberger J, Thom T, Wasserthiel-Smoller S, Hong Y. Heart disease and stroke statistics—2007 update: a report from the American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Circulation 115: e69-171, 2007. [DOI] [PubMed] [Google Scholar]
- 131.Rosolowsky M, Campbell WB. Role of PGI2 and EETs in the relaxation of bovine coronary arteries to arachidonic acid. Am J Physiol Heart Circ Physiol 264: H327–H335, 1993. [DOI] [PubMed] [Google Scholar]
- 132.Rubanyi GM, Lorenz RR, Vanhoutte PM. Bioassay of endothelium-derived relaxing factor(s): inactivation by catecholamines. Am J Physiol Heart Circ Physiol 249: H95–H101, 1985. [DOI] [PubMed] [Google Scholar]
- 133.Rubanyi GM, Vanhoutte PM. Nature of endothelium-derived relaxing factor: are there two relaxing mediators? Circ Res 61, Suppl II: 61–67, 1987. [PubMed] [Google Scholar]
- 134.Rusko J, Tanzi F, Van Breemen C, Adams DJ. Calcium-activated potassium channels in native endothelial cells from rabbit aorta: conductance, Ca2+ sensitivity and block. J Physiol 455: 601–621, 1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Sadik CD, Sies H, Schewe T. Inhibition of 15-lipoxygenases by flavonoids: structure-activity relations and mode of action. Biochem Pharmacol 65: 773–781, 2003. [DOI] [PubMed] [Google Scholar]
- 136.Salari H, Braquet P, Borgeat P. Comparative effects of indomethacin, acetylenic acids, 15-HETE, nordihydroguaiaretic acid and BW755C on the metabolism of arachidonic acid in human leukocytes and platelets. Prostaglandins Leukot Med 13: 53–60, 1984. [DOI] [PubMed] [Google Scholar]
- 137.Salzmann-Reinhardt U, Kuhn H, Wiesner R, Rapoport S. Metabolism of polyunsaturated fatty acids by rabbit reticulocytes. Eur J Biochem 153: 189–194, 1985. [DOI] [PubMed] [Google Scholar]
- 138.Sasaki M, Hori MT, Hino T, Golub MS, Tuck ML. Elevated 12-lipoxygenase activity in the spontaneously hypertensive rat. Am J Hypertens 10: 371–378, 1997. [PubMed] [Google Scholar]
- 139.Schewe T, Halangk W, Hiebsch C, Rapoport SM. A lipoxygenase in rabbit reticulocytes which attacks phospholipids and intact mitochondria. FEBS Lett 60: 149–152, 1975. [DOI] [PubMed] [Google Scholar]
- 140.Schwenk U, Morita E, Engel R, Schroder JM. Identification of 5-oxo-15-hydroxy-6,8,11,13-eicosatetraenoic acid as a novel and potent human eosinophil chemotactic eicosanoid. J Biol Chem 267: 12482–12488, 1992. [PubMed] [Google Scholar]
- 141.Serhan CH, Hamberg M, Samuelsson B. Lipoxins: novel series of biologically active compounds formed from arachidonic acid in human leukocytes. Proc Natl Acad Sci USA 81: 5335–5339, 1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Serhan CN, Savill J. Resolution of inflammation: the beginning programs the end. Nat Immunol 6: 1191–1197, 2005. [DOI] [PubMed] [Google Scholar]
- 143.Shak S, Perez HD, Goldstein IM. A novel dioxygenation product of arachidonic acid possesses potent chemotactic activity for human polymorphonuclear leukocytes. J Biol Chem 258: 14948–14953, 1983. [PubMed] [Google Scholar]
- 144.Sigal E, Grunberger D, Craik CS, Caughey GH, Nadel JA. Arachidonate 15-lipoxygenase (ω-6 lipoxygenase) from human leukocytes. J Biol Chem 263: 5328–5332, 1988. [PubMed] [Google Scholar]
- 145.Singer HA, Peach MJ. Endothelium-dependent relaxation of rabbit aorta. I. Relaxation stimulated by arachidonic acid. J Pharmacol Exp Ther 226: 790–795, 1983. [PubMed] [Google Scholar]
- 146.Singer HA, Peach MJ. Endothelium-dependent relaxation of rabbit aorta. II. Inhibition of relaxation stimulated by methacholine and A23187 with antagonists of arachidonic acid metabolism. J Pharmacol Exp Ther 226: 796–801, 1983. [PubMed] [Google Scholar]
- 147.Singer HA, Saye JA, Peach MJ. Effects of cytochrome P-450 inhibitors on endothelium-dependent relaxation in rabbit aorta. Blood Vessels 21: 223–230, 1984. [DOI] [PubMed] [Google Scholar]
- 148.Stapleton PA, Goodwill AG, James ME, Frisbee JC. Altered mechanisms of endothelium-dependent dilation in skeletal muscle arterioles with genetic hypercholesterolemia. Am J Physiol Regul Integr Comp Physiol 293: R1110–R1119, 2007. [DOI] [PubMed] [Google Scholar]
- 149.Stern N, Golub M, Nozawa K, Berger M, Knoll E, Yanagawa N, Natarajan R, Nadler JL, Tuck ML. Selective inhibition of angiotensin II-mediated vasoconstriction by lipoxygenase blockade. Am J Physiol Heart Circ Physiol 257: H434–H443, 1989. [DOI] [PubMed] [Google Scholar]
- 150.Sutherland M, Shankaranarayanan P, Schewe T, Nigam S. Evidence for the presence of phospholipid hydroperoxide glutathione peroxidase in human platelets: implications for its involvement in the regulatory network of the 12-lipoxygenase pathway of arachidonic acid metabolism. Biochem J 353: 91–100, 2001. [PMC free article] [PubMed] [Google Scholar]
- 151.Taddei S, Versari D, Cipriano A, Ghiadoni L, Galetta F, Franzoni F, Magagna A, Virdis A, Salvetti A. Identification of a cytochrome P450 2C9-derived endothelium-derived hyperpolarizing factor in essential hypertensive patients. J Am Coll Cardiol 48: 508–515, 2006. [DOI] [PubMed] [Google Scholar]
- 152.Tang DG, Renaud C, Stojakovic S, Diglio CA, Porter A, Honn KV. 12(S)-HETE is a mitogenic factor for microvascular endothelial cells: its potential role in angiogenesis. Biochem Biophys Res Commun 211: 462–468, 1995. [DOI] [PubMed] [Google Scholar]
- 153.Tang X, Edwards EM, Holmes BB, Falck JR, Campbell WB. Role of phospholipase C and diacylglyceride lipase pathway in arachidonic acid release and acetylcholine-induced vascular relaxation in rabbit aorta. Am J Physiol Heart Circ Physiol 290: H37–H45, 2006. [DOI] [PubMed] [Google Scholar]
- 154.Tang X, Holmes BB, Nithipatikom K, Hillard CJ, Kuhn H, Campbell WB. Reticulocyte 15-lipoxygenase-I is important in acetylcholine-induced endothelium-dependent vasorelaxation in rabbit aorta. Arterioscler Thromb Vasc Biol 26: 78–84, 2006. [DOI] [PubMed] [Google Scholar]
- 155.Tang X, Spitzbarth N, Kuhn H, Chaitidis P, Campbell WB. Interleukin-13 upregulates vasodilatory 15-lipoxygenase eicosanoids in rabbit aorta. Arterioscler Thromb Vasc Biol 23: 1768–1774, 2003. [DOI] [PubMed] [Google Scholar]
- 156.Taylor SG, Southerton JS, Weston AH, Baker JRJ. Endothelium-dependent effects of acetylcholine in rat aorta: a comparison with sodium nitroprusside and cromakalim. Br J Pharmacol 94: 853–863, 1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Uotila P, Vargas R, Wroblewska B, d'Alarcao M, Matsuda SP, Corey EJ, Cunard CM, Ramwell PW. Relaxing effects of 15-lipoxygenase products of arachidonic acid on rat aorta. J Pharmacol Exp Ther 242: 945–949, 1987. [PubMed] [Google Scholar]
- 158.Van de Voorde J, Leusen I. Role of the endothelium in the vasodilator response of rat thoracic aorta to histamine. Eur J Pharmacol 87: 113–120, 1983. [DOI] [PubMed] [Google Scholar]
- 159.VanDiest MJ, Verbeuren TJ, Herman AG. 15-Lipoxygenase metabolites of arachidonic acid evoke contractions and relaxations in isolated canine arteries: role of thromboxane receptors, endothelial cells and cyclooxygenase. J Pharmacol Exp Ther 256: 194–203, 1991. [PubMed] [Google Scholar]
- 160.Vanhoutte PM Other endothelium-derived vasoactive factors. Circulation 87: V-9–V-17, 1993. [Google Scholar]
- 161.Weston AH, Edwards G. Recent progress in potassium channel opener pharmacology. Biochem Pharmacol 43: 47–54, 1992. [DOI] [PubMed] [Google Scholar]
- 162.Xia XM, Fakler B, Rivard A, Wayman G, Johnson-Pais T, Keen JE, Ishii T, Hirschberg B, Bond CT, Lutsenko S, Maylie J, Adelman JP. Mechanism of calcium gating in small-conductance calcium-activated potassium channels. Nature 395: 503–507, 1998. [DOI] [PubMed] [Google Scholar]
- 163.Yoshimoto T, Takahashi Y. Arachidonate 12-lipoxygenases. Prostaglandins Other Lipid Mediat 68–69: 245–262, 2002. [DOI] [PubMed] [Google Scholar]
- 164.Yu Z, Schneider C, Boeglin WE, Brash AR. Epidermal lipoxygenase products of the hepoxilin pathway selectively activate the nuclear receptor PPARalpha. Lipids 42: 491–497, 2007. [DOI] [PubMed] [Google Scholar]
- 165.Zhang DX, Gauthier KM, Chawengsub Y, Campbell WB. ACh-induced relaxations of rabbit small mesenteric arteries: role of arachidonic acid metabolites and K+. Am J Physiol Heart Circ Physiol 293: H152–H159, 2007. [DOI] [PubMed] [Google Scholar]
- 166.Zhang DX, Gauthier KM, Chawengsub Y, Holmes BB, Campbell WB. Cyclooxygenase- and lipoxygenase-dependent relaxation to arachidonic acid in rabbit small mesenteric arteries. Am J Physiol Heart Circ Physiol 288: H302–H309, 2005. [DOI] [PubMed] [Google Scholar]
- 167.Zhu D, Medhora M, Campbell WB, Spitzbarth N, Baker JE, Jacobs ER. Chronic hypoxia activates lung 15-lipoxygenase, which catalyzes production of 15-HETE and enhances constriction in neonatal rabbit pulmonary arteries. Circ Res 92: 992–1000, 2003. [DOI] [PubMed] [Google Scholar]
- 168.Zink MH, Oltman CL, Lu T, Katakam PVG, Kaduce TL, Lee HC, Dellsperger KC, Spector AA, Myers PR, Weintraub NL. 12-Lipoxygenase in porcine coronary microcirculation: Implications for coronary vasoregulation. Am J Physiol Heart Circ Physiol 280: H693–H704, 2001. [DOI] [PubMed] [Google Scholar]
- 169.Zygmunt PM, Hogestatt ED. Role of potassium channels in endothelium-dependent relaxation resistant to nitroarginine in the rat hepatic artery. Br J Pharmacol 117: 1600–1606, 1996. [DOI] [PMC free article] [PubMed] [Google Scholar]