Abstract
Altered cGMP signaling has been implicated in myocardial depression, morbidity, and mortality associated with sepsis. Previous studies, using inhibitors of soluble guanylate cyclase (sGC), suggested that cGMP generated by sGC contributed to the cardiac dysfunction and mortality associated with sepsis. We used sGCα1-deficient (sGCα1−/−) mice to unequivocally determine the role of sGCα1β1 in the development of cardiac dysfunction and death associated with two models of inflammatory shock: endotoxin- and TNF-induced shock. At baseline, echocardiographic assessment and invasive hemodynamic measurements of left ventricular (LV) dimensions and function did not differ between wild-type (WT) mice and sGCα1−/− mice on the C57BL/6 background (sGCα1−/−B6 mice). At 14 h after endotoxin challenge, cardiac dysfunction was more pronounced in sGCα1−/−B6 than WT mice, as assessed using echocardiographic and hemodynamic indexes of LV function. Similarly, Ca2+ handling and cell shortening were impaired to a greater extent in cardiomyocytes isolated from sGCα1−/−B6 than WT mice after endotoxin challenge. Importantly, morbidity and mortality associated with inflammatory shock induced by endotoxin or TNF were increased in sGCα1−/−B6 compared with WT mice. Together, these findings suggest that cGMP generated by sGCα1β1 protects against cardiac dysfunction and mortality in murine inflammatory shock models.
Keywords: soluble guanylate cyclase, left ventricular function, sepsis, mice, nitric oxide
in the united states, sepsis and septic shock develop in 750,000 people annually, of whom >210,000 die (1), thereby making sepsis a leading cause of death in intensive care units. Invading microorganisms (including gram-positive and -negative bacteria, viruses, fungi, and parasites) induce the release of a wide variety of proinflammatory mediators, causing a systemic inflammatory response syndrome. Systemic inflammatory response syndrome can develop independently of an infection, for example, in cases of pancreatitis or trauma, as well as after cardiopulmonary bypass. It is recognized that refractory hypotension and myocardial depression contribute significantly to the morbidity and mortality of sepsis and septic shock (33, 43, 57). Similarly, inflammatory shock induced by TNF is characterized by cardiovascular collapse (8, 34).
Abundant evidence suggests that nitric oxide (NO) has critical roles in the regulation of myocardial function and structure (5, 24, 48) and blood pressure (23, 50). NO plays a pivotal role in cytokine-induced myocardial dysfunction (17, 30, 52), either attenuating or exacerbating the adverse hemodynamic sequelae associated with systemic inflammation. NO is synthesized from l-arginine by a family of three enzymes referred to as NO synthases (NOSs). NOS1 and NOS3 are constitutively expressed in a variety of cells, including neuronal cells, endothelial cells, and cardiomyocytes. NOS2, or inducible NOS, was first identified in macrophages and has been detected in a wide variety of cells (including cardiomyocytes) exposed to endotoxin and cytokines, such as TNF, and produces high levels of NO in inflammatory settings (40). High levels of NO produced by NOS2 have been implicated in the development of cardiac dysfunction associated with inflammatory shock (26, 53). However, NOS2-derived NO has also been found to have protective effects in TNF-induced (9) and endotoxin-induced (59) shock models, curbing oxidative stress and apoptosis. Similarly, increased NO levels, associated with overexpression of NOS3 in cardiomyocytes, attenuated myocardial dysfunction in mice challenged with endotoxin (25). However, NOS3-derived NO was also shown to have adverse cardiovascular effects in endotoxin-induced (13) and anaphylactic (7, 10) shock models. Importantly, the serious adverse events in clinical trials studying the effect of NOS inhibitors on survival in patients with septic shock appeared to be primarily of cardiac origin (12, 20, 36, 46).
The multiple targets of NO contribute to the widely varying and often conflicting effects of this molecule. One of the primary targets of NO is soluble guanylate cyclase (sGC), a heme-containing heterodimeric enzyme, consisting of one α-subunit and one β-subunit, that generates the secondary messenger molecule cGMP (4). The regulatory effect of cGMP on myocardial contractile function (22, 27, 39, 49, 56) and ventricular myocyte contractility (21, 51) is well established. Although the sGCα1β1 heterodimer is considered to be the principal cardiovascular isoform (38), recent studies have suggested that low levels of cGMP generated by sGCα2β, are sufficient to mediate many of NO's cardiovascular effects (6, 18, 37, 42, 54).
In shock, detrimental (11, 14, 28, 32, 52, 58) and protective (2, 29, 31) effects on cardiac function have been attributed to NO-dependent cGMP signaling. These dual effects of cGMP appear to depend, at least in part, on the timing of sGC inhibition: cGMP might be detrimental in one phase of inflammatory shock but protective in another (15, 16). Alternatively, it cannot be excluded that the source of cGMP (sGCα1β1 or sGCα1β2) determines its impact on cardiac function. Furthermore, interpretation of these studies is complicated by the incomplete specificity and systemic effects of the pharmacological agents used to inhibit sGC activity, e.g., 1H-[1,2,4]oxadiazolo[4,3-a]quinoxalin-1-one, LY-83583, and methylene blue (MB).
In this study, we used sGCα1-deficient (sGCα1−/−) mice (6) to unequivocally determine the role of sGCα1β1 in the development of cardiac dysfunction associated with two inflammatory shock models: endotoxin- and TNF-induced shock. We found that sGCα1−/− mice are sensitized to the cardiac dysfunction and mortality associated with inflammatory shock, which suggests that sGCα1β1-derived cGMP is cardioprotective in murine inflammatory shock models.
MATERIALS AND METHODS
Mouse models.
sGCα1−/− mice were generated on the 129S6 background (sGCα1−/−S6) as previously described (6) and were back-crossed eight generations onto a C57BL/6 background (sGCα1−/−B6). sGCα1−/− mice carry a targeted deletion of the sixth exon of the gene encoding sGCα1, resulting in the expression of a mutant, catalytically inactive protein.
For the endotoxin-induced inflammatory shock model, male wild-type (WT) C57BL/6 mice and sGCα1−/−B6 mice were challenged with Escherichia coli 0111:B4 endotoxin (25 mg/kg ip). For TNF-induced shock, male WT and sGCα1−/− mice on a C57BL/6 background were challenged with recombinant murine TNF (0.35 mg/kg iv) produced in E. coli and purified to homogeneity (9). All animal procedures were conducted in accordance with guidelines published in the Guide for the Care and Use of Laboratory Animals (National Research Council, National Academy Press, Washington, DC, 1996) and were approved by the Massachusetts General Hospital Subcommittee on Research Animal Care and by the Animal Ethics Committee of Ghent University.
sGC enzyme activity.
sGC enzyme activity was measured as described elsewhere (6). Lung tissues were harvested from 12- to 14-wk-old mice and subsequently homogenized. Supernatants (containing 50 μg of protein) were incubated for 10 min at 37°C in a reaction mixture with or without 10 μmol/l of l2-(N,N-diethylamino)-diazenolate-2-oxide. cGMP in the reaction mixture was measured using a commercial radioimmunoassay (Biomedical Technologies, Stoughton, MA). sGC enzyme activity is expressed as picomoles of cGMP produced per minute per milligram of protein in lung extract supernatant.
Hemodynamic measurements.
sGCα1−/−B6 and WT mice, between 10 and 14 wk of age, were anesthetized by intraperitoneal injection with ketamine (100 mg/kg), fentanyl (50 μg/kg), and pancuronium (2 mg/kg), intubated, and mechanically ventilated (fraction of inspired O2 = 1, 10 μl/g, 120 breaths/min). A saline-filled catheter was inserted into the left carotid artery for infusion of saline (2 ml/h). For measurement of cardiac function, the chest was opened, and a pressure-volume conductance catheter (model SPR-839, Millar Instruments, Houston, TX) was introduced through the apex into the left ventricle (LV), as described previously (25). Heart rate (HR), LV end-systolic and end-diastolic pressures (LVESP and LVEDP, respectively), and LV volumes were measured. The maximum and minimum first derivative of developed LV pressure (dP/dtmax and dP/dtmin, respectively), stroke work (SW), maximal developed power (Pmax), time constant of isovolumic relaxation, cardiac output (CO), stroke volume (SV), and arterial elastance (Ea, defined as the ratio of LVESP to SV) were calculated. dP/dtmax divided by instantaneous pressure (IP) was calculated and is considered a relatively load-independent index of contractility.
In separate groups of sGCα1−/−B6 and WT mice (n = 8 and 9, respectively), sodium nitroprusside (SNP, 2.5, 5, and 10 μg/kg), BAY 41-2272 (100 μg/kg), or vehicle (PBS for SNP and 4% DMSO and 8% cremophor in PBS for BAY 41-2272) was administered intravenously (50-μl bolus in the jugular vein), and LVESP was measured.
Noninvasive blood pressure measurements.
Mean arterial blood pressure (MAP) was measured noninvasively in conscious WT and sGCα1−/−B6 mice (n = 5 each) with use of a tail-cuff system (Kent) warmed to 37°C. Mice were studied at 12–14 wk of age. Mice were habituated to the blood pressure measurement device for 7–10 days and underwent 2 cycles of 10 measurements per day for 10 days for blood pressure determination.
Echocardiographic measurements.
Transthoracic echocardiograms were obtained using a 13-MHz probe (Vivid 7, GE Medical Systems) in awake WT and sGCα1−/−B6 mice 14 h after a challenge with saline (n = 5 and 5, respectively) or 25 mg/kg endotoxin (n = 13 and 18, respectively), as described previously (48). Measurements were made by an observer who was blinded to the experimental group. LV ejection fraction was measured on two-dimensional images using the area-length method. HR, LV end-systolic internal diameter, and LV fractional shortening were measured using an M-mode echocardiogram obtained at the midpapillary level, as described previously (48).
Measurement of gene expression.
Total RNA was extracted from LV tissue using TRIzol reagent (Invitrogen), and cDNA was synthesized using Maloney's murine leukemia virus RT (Promega). NOS2, IL-6, and 18S rRNA transcript levels were measured by real-time PCR using a Mastercycler ep realplex 2 (Eppendorf). Primers were designed for 18S (5′-CGGCTACCACATCCAAGGAA-3′ and 5′-GCTGGAATTACCGCGGCT-3′). For NOS2 and IL-6, TaqMan primer sets (Applied Biosystems) were used. Changes in the relative gene expression normalized to levels of 18S rRNA were determined using the relative cycle threshold method.
Immunoblot analysis.
LV tissue samples were homogenized in 2 ml of RIPA buffer (Boston BioProducts) supplemented with 1% protease inhibitor cocktail (Sigma) and microcentrifuged for 20 min at 20,000 g. Supernatant proteins (15 μg) were fractionated on 10% SDS-polyacrylamide gels and transferred to polyvinylidene difluoride membranes. Membranes were blocked for 1 h in Odyssey blocking buffer (LI-COR) and incubated overnight with primary rabbit antibodies against sGCα1, sGCα2, or sGCβ1 (diluted 1:1,000; Abcam). Immunoblotting with a rabbit GAPDH antibody (Cell Signaling) was performed to confirm equal loading. Bound antibody was detected with anti-rabbit infrared dye (catalog no. 680, LI-COR) using an Odyssey infrared imaging system (LI-COR).
Cardiomyocyte isolation.
Cardiomyocytes were isolated as described previously (35). Briefly, mice were treated with heparin (200 U ip) and, 5 min later, were euthanized with pentobarbital sodium (10 mg ip). The chest was opened, and the heart was removed quickly and placed in cold (4°C) isolation buffer containing 137 mmol/l NaCl, 5.4 mmol/l KCl, 0.5 mmol/l MgCl2, 10 mmol/l HEPES, 10 mmol/l glucose, 5 mmol/l taurine, and 10 mmol/l 2,3-butanedione monoxime, with pH titrated to 7.4 with NaOH. The aorta was mounted on a cannula for retrograde perfusion at 3.5 ml/min. After cannulation, the heart was perfused with 3 ml of cold isolation buffer to clear blood from the coronary system. The arrested heart was subsequently mounted on a perfusion apparatus and perfused at 37°C with isolation buffer for 2 min and then with isolation buffer containing a mixture of digesting enzymes [0.5 mg/ml collagenase B and 0.5 mg/ml collagenase D (Roche Diagnostics) and 0.1 mg/ml type XIV protease (Sigma-Aldrich)]. The enzyme-containing solution was perfused for 7 min or until coronary pressure (measured continuously with a manometer) was <20 mmHg. At the end of perfusion, the heart was removed from the cannula, and the LV was isolated, chopped into small pieces in isolation buffer at room temperature, and then gently agitated for another 5 min. The cell suspension was filtered through nylon gauze (200-μm mesh) and sedimented in a 50-ml tube for 15 min, and the supernatant was replaced three times with a higher-Ca2+ (200, 500, and 1,000 mmol/l) solution. Cells were transferred in a physiological solution (see below) and kept at room temperature until use.
Measurement of contractility and Ca2+ handling in isolated cardiomyocytes.
LV cardiomyocytes were dissociated from WT and sGCα1−/−B6 mice 14 h after challenge with saline or endotoxin (n = 10 each). In studies of cardiac myocyte contractility and Ca2+ handling, only cells that had a rod shape and clear striations, were quiescent in the absence of stimulation, showed visible twitches at 6 Hz, and did not have spontaneous Ca2+ release events and waves were included. Furthermore, all cells with a resting sarcomere length <1.65 μm were excluded. Measurements of sarcomere shortening (cell shortening, as a percentage of diastolic length) and intracellular Ca2+ (ΔCai, measured as the difference between the peak systolic-to-diastolic fura 2 ratios) were performed simultaneously and as described previously (25). Briefly, isolated cardiac cells were loaded with the membrane-permeable Ca2+ indicator fura 2-AM (Molecular Probes; 1 μmol/l, 25 min) and superfused with a physiological solution containing (in mmol/l) 137 NaCl, 5.4 KCl, 1.2 Ca2+, 0.5 MgCl2, 10 HEPES, and 5.5 and 0.5 probenecid (to improve fura 2-AM retention), with pH titrated to 7.4 with NaOH. Cells were externally paced at 6 Hz (MyoPacer, Ionoptix) at 37°C, and sarcomere shortening and fura 2 ratiometric data were recorded and analyzed using IonWizard software (IonOptix). The integrated acquisition system (IonOptix) includes a Nikon TS100 inverted microscope, a MyoCam-S digital charge-coupled device video camera (with a 500-Hz acquisition rate), a HyperSwitch dual-excitation light source (with a paired 340-380-nm excitation rate of 250 Hz), and a photomultiplier tube. For statistical analysis, data were compared using an unpaired Student's t-test.
Survival analysis.
Survival after a challenge with endotoxin was studied in age-matched C57BL/6 WT and sGCα1−/−B6 mice. To avoid dehydration, saline (1 ml) was given intraperitoneally to all mice at time 0 and at 24 h after endotoxin challenge. Survival after a challenge with TNF was studied in age-matched WT and sGCα1−/− mice on the C57BL/6 background.
Statistical evaluation.
Unless stated otherwise, data were compared using multiple comparisons by two-way ANOVA. After ANOVA, Bonferroni's post hoc test was used to compare separate groups. Values are means ± SE. P < 0.05 was considered significant.
RESULTS
sGC enzyme activity, sGC expression levels, blood pressure, and cardiac function in sGCα1−/−B6 mice.
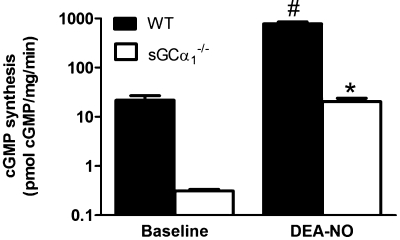
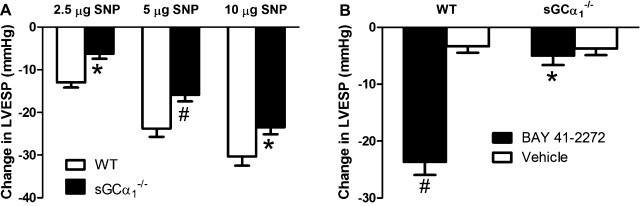
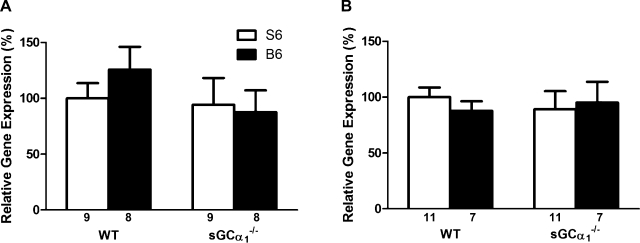
Similar to observations in sGCα1−/−S6 mice (6), NO-induced increases in sGC enzyme activity were severely attenuated in lung extracts from sGCα1−/−B6 mice compared with WT mice (Fig. 1). NO donor compounds reduced blood pressure in sGCα1−/−B6 mice, albeit to a lesser extent than in WT mice (Fig. 2A). BAY 41-2272 reduced blood pressure in WT, but not sGCα1−/−B6, mice (Fig. 2B). Expression of the sGCα2 gene in aortic and lung tissue did not differ in WT and sGCα1−/−B6 mice (Fig. 3). In contrast to male sGCα1−/−S6 mice, male sGCα1−/−B6 mice do not develop hypertension, measured invasively in anesthetized mice or noninvasively in awake mice (Table 1). At baseline, cardiac function parameters, measured invasively (Table 1) or noninvasively (Fig. 4), were similar between sGCα1−/−B6 and WT mice.
Fig. 1.
Soluble guanylate cyclase (sGC) enzyme activity. Unstimulated (baseline) and 2-(N,N-diethylamino)-diazenolate-2-oxide (DEA-NO)-stimulated sGC enzyme activity in lung extracts of 10- to 12-wk-old wild-type (WT) and sGC-deficient (sGCα1−/−B6) mice. Values are means ± SE (n = 6 for all groups). *P < 0.001 vs. WT DEA-NO. #P < 0.001 vs. baseline.
Fig. 2.
Acute effects on left ventricular (LV) end-systolic pressure (LVESP) of compounds acting on the nitric oxide (NO)-cGMP pathway. A: effect of sodium nitroprusside (SNP, 2.5, 5, or 10 μg/kg iv) on LVESP in WT (n = 9) and sGCα1−/−B6 (n = 8) mice. Values are means ± SE. *P < 0.05. #P < 0.01 vs. WT. B: sGC stimulator BAY 41-2272 decreased LVESP in WT, but not sGCα1−/−B6, mice. Values are means ± SE. *P < 0.001 vs. WT BAY 41-2272. #P < 0.001 vs. WT vehicle.
Fig. 3.
Levels of sGCα2 expression, as measured by quantitative RT-PCR, in extracts from aorta (A) and lung (B) of WT and sGCα1−/− mice on the 129S6 (S6) and the C57BL/6 (B6) backgrounds. Values are means ± SE of number of mice indicated below each bar.
Table 1.
LV function in WT and sGCα1−/−B6 mice 14 h after challenge with saline or endotoxin
|
WT |
sGCα1−/−B6
|
|||
|---|---|---|---|---|
| Control | LPS | Control | LPS | |
| n (invasive) | 15 | 11 | 11 | 10 |
| Body wt, g | 24±0 | 24±0 | 23±1 | 24±0 |
| HR, beats/min | 647±12 | 563±13* | 654±17 | 605±14† |
| LVESP, mmHg | 111±2 | 103±5 | 112±3 | 117±6‡ |
| LVEDP, mmHg | 6±1 | 3±0* | 6±1 | 3±1† |
| dP/dtmax, mmHg/s | 14,003±610 | 11,226±919† | 14,012±919 | 10,679±870† |
| dP/dtmin, mmHg/s | −13,690±513 | −11,578±977 | −13,642±730 | −12,204±644 |
| dP/dtmax/IP | 160±6 | 152±9 | 163±12 | 133±6† |
| SW, mmHg·ml | 1.9±0.1 | 1.8±0.3 | 2.0±0.1 | 1.1±0.2*‡ |
| Pmax, mW | 17±1 | 17±3 | 17±1 | 10±1*§ |
| τ, ms | 5.3±0.2 | 5.5±0.2 | 5.5±0.3 | 5.6±0.1 |
| CO, ml/min | 12±1 | 12±2 | 13±1 | 8±1*‡ |
| SV, μl | 19±1 | 21±3 | 20±1 | 13±1*§ |
| Ea, mmHg/μl | 6±0 | 6±1 | 6±0 | 10±1*§ |
| n (tail cuff) | 5 | 5 | ||
| MAP, mmHg | 107±1 | 108±1 | ||
Values are means ± SE. n, Number of 10- to 12-wk-old wild-type (WT) and soluble guanylate cyclase-α1-deficient (sGCα1−/−B6) mice; HR, heart rate; LVESP, left ventricular (LV) end-systolic pressure; LVEDP, LV end-diastolic pressure; dP/dtmax and dP/dtmin, maximum and minimum rates of developed LV pressure; IP, instantaneous pressure; SW, stroke work; Pmax, maximal power; τ, time constant of isovolumic relaxation; CO, cardiac output; SV, stroke volume; Ea, arterial elastance; MAP, mean arterial pressure.
P < 0.01;
P < 0.05 vs. control mice of the same genotype.
P < 0.05;
P < 0.01 vs. septic WT mice.
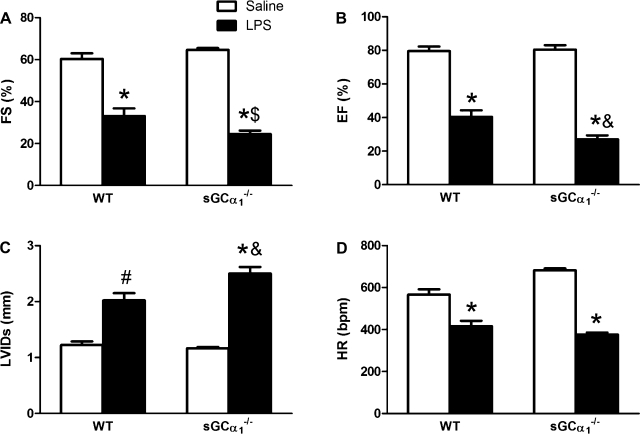
Fig. 4.
Echocardiographic analysis of LV function. LV fractional shortening (FS, A), ejection fraction (EF, B), LV end-systolic internal diameter (LVIDs, C), and heart rate (HR, D) were measured in WT and sGCα1−/−B6 mice 14 h after challenge with saline or 25 mg/kg endotoxin (LPS). Values are means ± SE. *P < 0.001; #P < 0.01 vs. saline. $P < 0.01; &P < 0.05 vs. WT LPS.
Impact of sGCα1 deficiency on myocardial dysfunction associated with endotoxin-induced inflammatory shock.
At 14 h after endotoxin challenge, sGCα1−/−B6 and WT mice developed hypothermia (27 ± 1°C and 29 ± 1°C) and bradycardia, measured noninvasively (Fig. 4D) and invasively (Table 1). The 14-h time point was chosen, because all mice were alive, and myocardial dysfunction was apparent. The decrease in LV fractional shortening, LV ejection fraction, and the increase in LV end-systolic internal diameter after endotoxin challenge, as measured by echocardiography, were more marked in sGCα1−/−B6 than in WT mice (Fig. 4, A–C). LVESP was higher in sGCα1−/−B6 than in WT mice after endotoxin challenge (Table 1). Although dP/dtmax, a measure of LV contractility that is relatively sensitive to afterload, was similarly depressed in WT and sGCα1−/−B6 mice after endotoxin challenge, dP/dtmax/IP, a load-independent parameter of LV contractility, was depressed to a greater extent in sGCα1−/−B6 than in WT mice. Similarly, SW and Pmax, both indexes of LV contractility, were decreased in sGCα1−/−B6, but not WT, mice after endotoxin challenge. The relaxation time constant, a measure of diastolic function, tended to be prolonged in WT and sGCα1−/−B6 mice after endotoxin challenge. Importantly, CO was decreased in sGCα1−/−B6, but not WT, mice after endotoxin challenge. This decrease in CO was accompanied by a lower SV. Finally, Ea, a measure of afterload, was increased in sGCα1−/−B6, but not WT, mice after endotoxin challenge.
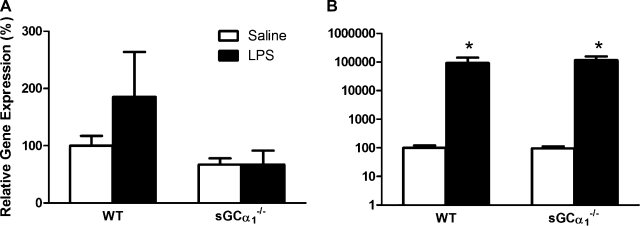
Effects of endotoxin on LV expression of genes encoding NOS2 and IL-6.
NOS2 and IL-6 mRNA levels in cardiac tissue extracts did not differ between WT and sGCα1−/−B6 mice at baseline (Fig. 5). NOS2 gene expression levels were not increased in the LV of WT or sGCα1−/−B6 mice 14 h after endotoxin challenge. IL-6 levels were similarly increased in WT and sGCα1−/−B6 mice 14 h after endotoxin injection (Fig. 5B).
Fig. 5.
Expression analysis of inflammatory markers. mRNAs encoding type 2 nitric oxide synthase (NOS2, A) and IL-6 (B) in the LV of WT and sGCα1−/−B6 mice were measured by quantitative RT-PCR 14 h after challenge with saline (n = 13 and 11, respectively) or 25 mg/kg endotoxin (LPS, n = 10 and 11, respectively). Values are means ± SE. *P < 0.01 vs. saline.
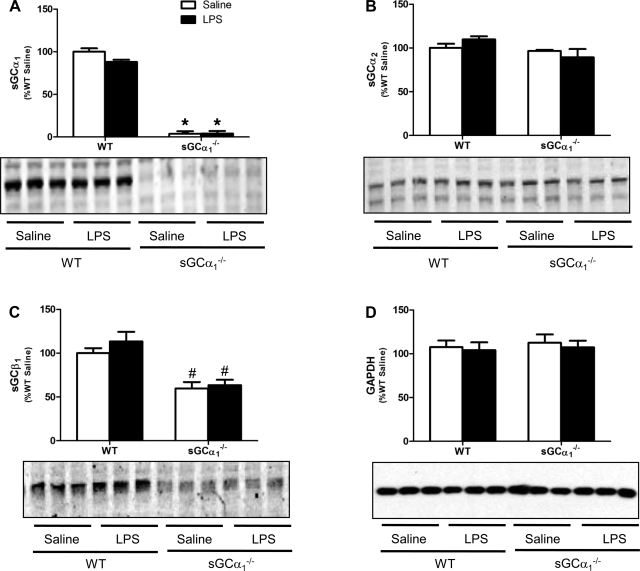
LV expression of sGC.
LV protein levels of sGCα1, sGCα2, and sGCβ1 did not differ between saline- and endotoxin-treated WT mice or between saline- and endotoxin-treated sGCα1−/−B6 mice (Fig. 6). Expression levels of sGCβ1 were lower in the LV of saline- and endotoxin-treated sGCα1−/−B6 than in WT mice (Fig. 6C).
Fig. 6.
Quantification of immunoblot analyses of sGCα1 (A), sGCα2 (B), sGCβ1 (C), and GAPDH (D) in LV protein extracts of WT and sGCα1−/−B6 mice 14 h after challenge with saline or 25 mg/kg endotoxin (LPS). Blots represent results from 3 mice in each group. Values are means ± SE (n = 5 per group). *P < 0.001 and #P < 0.05 vs. WT in the same treatment group.
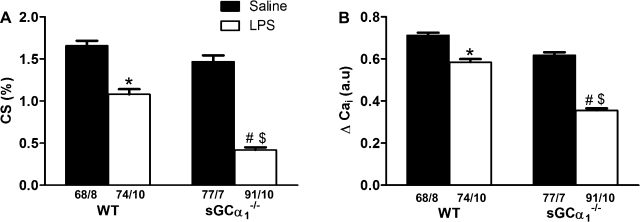
Effects of endotoxin on contractility and Ca2+ handling in cardiomyocytes isolated from WT and sGCα1−/−B6 mice.
At rest, sarcomere length was 1.77 ± 0.01 and 1.78 ± 0.01 μm for cardiomyocytes isolated from saline-treated WT and sGCα1−/−B6 mice, respectively, and 1.77 ± 0.01 and 1.79 ± 0.01 μm for cardiomyocytes isolated from LPS-treated WT and sGCα1−/−B6 mice, respectively. Cardiomyocyte contractility, as assessed by percent cell shortening (Fig. 7A), and Ca2+ handling, as measured by peak Ca2+ transient amplitude (ΔCai, Fig. 7B), were similar in cardiomyocytes isolated from saline-challenged WT and sGCα1−/−B6 mice paced at 6 Hz. Diastolic sarcomere length was similar in cardiomyocytes isolated from WT and sGCα1−/−B6 mice at baseline and after endotoxin challenge. However, the decrease in cell shortening was more marked in cardiomyocytes isolated from sGCα1−/− than from WT mice (Fig. 7A) 14 h after endotoxin challenge. Similarly, endotoxin challenge decreased ΔCai to a greater extent in sGCα1−/−B6 than in WT cardiomyocytes.
Fig. 7.
Percent cell shortening (CS, A) and amplitude of Ca2+ transient (ΔCai, B) in cardiomyocytes isolated from WT and sGCα1−/−B6 mice 14 h after challenge with saline or 25 mg/kg endotoxin (LPS) and paced at 6 Hz. Values are means ± SE of number of cells and mice (cells/mice) indicated below each bar. *P < 0.05; #P < 0.01 vs. saline-challenged mice of the respective genotype. $P < 0.05 vs. endotoxin-challenged WT mice.
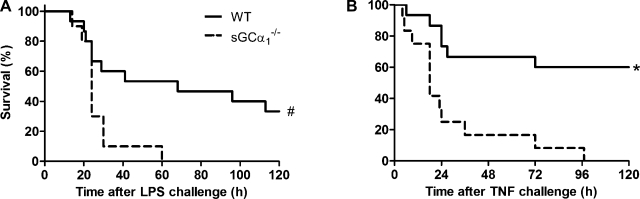
Effects of endotoxin on survival in WT and sGCα1−/−B6 mice.
Decreased LV function was accompanied by increased mortality in endotoxin-treated sGCα1−/−B6 mice, as demonstrated by Kaplan-Meier survival curves (Fig. 8A).
Fig. 8.
Survival analysis. Kaplan-Meier curve shows survival after challenge of WT and sGCα1−/−B6 mice with endotoxin (LPS, A) or TNF (B). #P < 0.05; *P < 0.01 vs. WT (by log-rank test).
Effects of TNF on survival in WT and sGCα1−/−B6 mice.
Rectal body temperatures were measured after a challenge with TNF, a second model of inflammatory shock. At 9 h after a challenge with TNF, hypothermia was more pronounced in sGCα1−/−B6 than in WT C57BL/6 mice (27 ± 3°C vs. 31 ± 4°C, P < 0.01). Similar to our observations in endotoxin-treated mice, TNF-induced inflammatory shock was associated with a higher mortality in sGCα1−/−B6 than in WT mice (Fig. 8B).
DISCUSSION
In this study, mice deficient in sGCα1 were used to investigate the function of cGMP generated by sGCα1β1 in murine models of endotoxin- and TNF-induced shock. The data suggest that cGMP generated by sGCα1β1 attenuates myocardial dysfunction and lethality associated with inflammatory shock in mice: mortality was higher and cardiac dysfunction was more pronounced in sGCα1−/−B6 than in WT mice. Furthermore, the adverse effects of endotoxin challenge on contractility and Ca2+ handling in cardiomyocytes were more marked in cardiomyocytes isolated from sGCα1−/−B6 than from WT mice. The use of genetically modified mice allowed us to avoid some of the limitations associated with studies focusing on the contribution of sGC to the pathogenesis of endotoxic shock with use of pharmacological inhibitors such as MB, 1H-[1,2,4]oxadiazolo[4,3-a]quinoxalin-1-one, and LY-83583, all of which modulate the activity of other enzymes in addition to sGC and none of which are sGC isoform specific. To assess the impact of sGCα2β1 on cardiac function in sepsis, a study of sGCα2-deficient mice is necessary.
We previously reported the generation and cardiovascular phenotype of sGCα1−/− mice on a mixed Swiss/129S6 or a 129S6 background (sGCα1−/−S6 mice). sGCα1−/−S6 mice develop gender-specific and testosterone-dependent hypertension (6). However, neither WT 129S6 nor sGCα1−/−S6 mice developed cardiac dysfunction when challenged by endotoxin, Staphylococcus aureus, or cecal ligation and puncture (data not shown), suggesting that 129S6 mice are particularly resistant to adverse effects of inflammatory shock on cardiac function. Because C57BL/6 strains are known to be sensitive to endotoxin, we back-crossed the sGCα1−/−S6 mice onto a C57BL/6 background (sGCα1−/−B6). Interestingly, although sGCα1−/−S6 and sGCα1−/− mice on a mixed Swiss/129S6 background develop hypertension (6), we found that sGCα1−/−B6 mice are normotensive, which is consistent with another report describing only modestly elevated blood pressures in independently generated sGCα1−/− mice on the C57BL/6 background (37). Together, these data strongly suggest the existence of genetic modifiers of the hypertensive effects of sGCα1 deficiency. Similarly, although increased cardiac contractility and Ea, as well as impaired ventricular relaxation, were observed in sGCα1−/−S6 mice (6), cardiac dysfunction was not evident in sGCα1−/−B6 mice at baseline. Importantly, although male sGCα1−/−S6 male mice, but not male sGCα1−/−B6 mice, develop hypertension, the blood pressure response to a low dose of an NO donor compound was similarly attenuated in sGCα1−/−S6 and sGCα1−/−B6 mice, suggesting that a difference in the hemodynamic response to NO does not underlie the different blood pressure phenotype. Furthermore, sGC enzyme activity, at baseline and activated by NO, was similarly attenuated in lung extracts from sGCα1−/−S6 and sGCα1−/−B6 mice compared with WT mice. Expression levels of sGCα2 did not differ between WT and sGCα1−/− mice on the 129S6 or the C57BL/6 background, indicating that a compensatory increase in expression levels of sGCα2 in sGCα1−/−B6 mice is not responsible for the absence of hypertension observed in sGCα1−/−B6 mice. Similar to observations in sGCα1−/−S6 mice (6), the effect of BAY 41-2272 on blood pressure was abolished in sGCα1−/−B6 mice. Together, these data demonstrate that sGCα2 is not able to mediate BAY 41-2272-induced decreases in blood pressure in sGCα1−/− mice, implying that BAY 41-2272 is an isoform-specific sGCα1β1 stimulator in vivo in mice.
Myocardial depression is a well-recognized manifestation of organ dysfunction in sepsis (47). Therefore, we studied cardiac function in WT and sGCα1−/−B6 mice after endotoxin challenge. Both echocardiographic parameters (fractional shortening and ejection fraction) measured in awake mice and invasive hemodynamic parameters (dP/dtmax/IP, SW, and Pmax) measured in anesthetized mice demonstrated that sGCα1 deficiency exacerbates contractile dysfunction associated with endotoxin-induced shock. SV was decreased in sGCα1−/−B6, but not WT, mice 14 h after a challenge with endotoxin, as reflected by a substantial decrease in CO. Together, these findings indicate that sGCα1β1-derived cGMP has a protective effect on myocardial function in a setting of endotoxic shock. Interestingly, Ea, an index of arterial vascular load, increased in sGCα1−/−B6, but not WT, mice after a challenge with endotoxin. This finding is consistent with a previous report of an endotoxin-induced increase in Ea in NOS3-deficient, but not WT, mice (25). Together, these findings suggest that, during inflammatory shock in mice, systemic vasoconstriction occurs in an NO-cGMP-dependent manner. More research is warranted to determine whether a higher afterload in sGCα1−/−B6 than in WT mice contributes to or is a consequence of increased heart failure associated with inflammatory shock in sGCα1−/−B6 mice.
To test the hypothesis that the worsened cardiac dysfunction associated with inflammatory shock in sGCα1−/−B6 mice is mediated by increased inflammation in sGCα1−/−B6 mice compared with WT mice, we measured gene expression levels of two markers of inflammation: IL-6 and NOS2. LV expression levels of NOS2 and IL-6 did not differ between saline-treated WT and sGCα1−/−B6 mice. IL-6 expression levels were similarly induced in the LV of endotoxin-treated WT and sGCα1−/−B6 mice, suggesting that a difference in inflammatory response in the LV to endotoxin challenge is not responsible for the observed difference in cardiac phenotype. In contrast to IL-6 mRNA levels, cardiac NOS2 gene expression did not differ before or 14 h after endotoxin challenge in WT or sGCα1−/−B6 mice. These findings are consistent with a prior report that endotoxin challenge increases cardiac NOS2 gene expression only transiently, returning to baseline after 12 h (19). A challenge with endotoxin did not alter LV protein levels of sGCα1, sGCα2, or sGCβ1 in WT or sGCα1−/−B6 mice. sGCβ1 protein levels were lower in saline-and endotoxin-treated sGCα1−/−B6 than in WT mice. These findings are in accordance with previously published findings suggesting that loss of sGCα1 results in decreased stability of sGCβ1 (6, 37).
sGC is expressed in multiple cell types, including cardiomyocytes (55), vascular smooth muscle cells, endothelial cells (3), and neuronal cells (38). Any of the cell types expressing sGC might be involved in modulating myocardial function during inflammatory shock. Studies of the cellular mechanisms mediating sepsis-induced myocardial depression in the intact animal are complicated by the multiple interacting cell types involved, as well as by the potentially confounding influences of neurohumoral factors. We studied Ca2+ handling and contractility of isolated cardiomyocytes in vitro. Cardiomyocyte contractility and Ca2+ handling were similar in cardiomyocytes isolated from saline-challenged WT and sGCα1−/−B6 mice, recapitulating the in vivo hemodynamic data showing no difference in cardiac function between saline-challenged WT and sGCα1−/−B6 mice. Contractility was impaired to a greater extent in cardiomyocytes isolated from endotoxin-treated sGCα1−/−B6 than from endotoxin-treated WT mice. Similarly, although endotoxin decreased ΔCai in cardiomyocytes isolated from both genotypes, the reduction was greater in cardiomyocytes isolated from sGCα1−/−B6 than from WT mice. Together, these data show that cardiomyocytes reproduced the greater contractile deficit induced by endotoxin in sGCα1−/−B6 than in WT mice. We recently reported that endotoxin induces marked impairment of Ca2+ handling in cardiomyocytes and that this impairment is attenuated by increasing myocardial NOS3 levels (25), suggesting that NO protects cardiomyocytes from endotoxin-induced dysfunction. In the present study, the observation that endotoxin-induced cardiomyocyte dysfunction is exacerbated in sGCα1−/− mice compared with WT mice suggests that the ability of NO to prevent contractile dysfunction of murine cardiomyocytes may be, at least partially, dependent on sGCα1β1. Taken together, our results indicate that the more marked endotoxin-induced myocardial dysfunction in sGCα1−/−B6 than in WT mice may be attributable to greater impairment of cardiomyocyte function.
Importantly, we observed that more pronounced cardiac dysfunction in endotoxin-induced inflammatory shock in sGCα1−/−B6 than in WT mice was associated with decreased survival. To unequivocally link decreased survival in sGCα1−/−B6 mice with greater cardiac dysfunction, a study of mice with cardiac specific sGCα1 deficiency is necessary. However, in patients with sepsis, cardiac dysfunction was identified to be an important predictor of mortality (41, 44, 45). Furthermore, increasing myocardial NO levels not only prevented myocardial dysfunction in murine models of inflammatory shock, but it also increased the survival rate (25). Our findings suggest that the ability of cardiac NO to protect against mortality in endotoxic shock may be dependent on sGCα1β1.
TNF is a pleiotropic cytokine that has a strong antitumor activity in vitro and in vivo. Systemic administration of TNF induces a systemic inflammatory response syndrome, characterized by bowel necrosis, liver damage, and severe hypotension leading to death. Previous studies suggested that TNF exerts its lethal effects via sGC activation: inhibition of sGC with MB or LY-83583 protected against TNF-induced lethality (11). However, similar to our finding in the endotoxin-induced shock model, mortality was more marked in sGCα1−/−B6 than in WT mice treated with TNF. Taken together, these findings demonstrate that cGMP generated by sGCα1β1 protects, at least partially, from myocardial dysfunction and death in endotoxin- and TNF-induced shock models.
In conclusion, inflammatory shock impaired Ca2+ handling and cell shortening to a greater extent in cardiomyocytes isolated from sGCα1−/−B6 than from WT mice. Ventricular dysfunction associated with inflammatory shock was more marked in sGCα1−/−B6 than in WT mice. Furthermore, mortality was higher in sGCα1−/−B6 than in WT mice in endotoxin-induced and TNF-induced inflammatory shock. These results identify a significant role for cGMP synthesized by sGCα1β1 in the modulation of ventricular function in a setting of inflammatory shock and identify activation of sGCα1β1 as a potential therapeutic strategy in septic shock.
GRANTS
This study was supported by a postdoctoral fellowship award of the American Heart Association Northeast Affiliate and a postdoctoral fellowship from the Massachusetts Biomedical Research Corporation (E. S. Buys), grants from the Fonds Wetenschappelijk Onderzoek-Vlaanderen (FWO-Vlaanderen) and the Geconcerteerde Onderzoeksacties (P. Brouckaert), and National Heart, Lung, and Blood Institute Grants HL-42397, HL-70896, and HL-71987. A. Cauwels is a postdoctoral fellow of the FWO Vlaanderen.
REFERENCES
- 1.Angus DC, Linde-Zwirble WT, Lidicker J, Clermont G, Carcillo J, Pinsky MR. Epidemiology of severe sepsis in the United States: analysis of incidence, outcome, and associated costs of care. Crit Care Med 29: 1303–1310, 2001. [DOI] [PubMed] [Google Scholar]
- 2.Avontuur JA, Bruining HA, Ince C. Inhibition of nitric oxide synthesis causes myocardial ischemia in endotoxemic rats. Circ Res 76: 418–425, 1995. [DOI] [PubMed] [Google Scholar]
- 3.Behrends S, Mietens A, Kempfert J, Koglin M, Scholz H, Middendorff R. The expression pattern of nitric oxide-sensitive guanylyl cyclase in the rat heart changes during postnatal development. J Histochem Cytochem 50: 1325–1332, 2002. [DOI] [PubMed] [Google Scholar]
- 4.Buechler WA, Nakane M, Murad F. Expression of soluble guanylate cyclase activity requires both enzyme subunits. Biochem Biophys Res Commun 174: 351–357, 1991. [DOI] [PubMed] [Google Scholar]
- 5.Buys ES, Raher MJ, Blake SL, Neilan TG, Graveline AR, Passeri JJ, Llano M, Perez-Sanz TM, Ichinose F, Janssens S, Zapol WM, Picard MH, Bloch KD, Scherrer-Crosbie M. Cardiomyocyte-restricted restoration of nitric oxide synthase 3 attenuates left ventricular remodeling after chronic pressure overload. Am J Physiol Heart Circ Physiol 293: H620–H627, 2007. [DOI] [PubMed] [Google Scholar]
- 6.Buys ES, Sips P, Vermeersch P, Raher MJ, Rogge E, Ichinose F, Dewerchin M, Bloch KD, Janssens S, Brouckaert P. Gender-specific hypertension and responsiveness to nitric oxide in sGCα1 knockout mice. Cardiovasc Res 79: 179–186, 2008. [DOI] [PubMed] [Google Scholar]
- 7.Cauwels A Nitric oxide in shock. Kidney Int 72: 557–565, 2007. [DOI] [PubMed] [Google Scholar]
- 8.Cauwels A, Brouckaert P. Survival of TNF toxicity: dependence on caspases and NO. Arch Biochem Biophys 462: 132–139, 2007. [DOI] [PubMed] [Google Scholar]
- 9.Cauwels A, Bultinck J, Brouckaert P. Dual role of endogenous nitric oxide in tumor necrosis factor shock: induced NO tempers oxidative stress. Cell Mol Life Sci 62: 1632–1640, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cauwels A, Janssen B, Buys E, Sips P, Brouckaert P. Anaphylactic shock depends on PI3K and eNOS-derived NO. J Clin Invest 116: 2244–2251, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cauwels A, Van Molle W, Janssen B, Everaerdt B, Huang P, Fiers W, Brouckaert P. Protection against TNF-induced lethal shock by soluble guanylate cyclase inhibition requires functional inducible nitric oxide synthase. Immunity 13: 223–231, 2000. [DOI] [PubMed] [Google Scholar]
- 12.Cobb JP Use of nitric oxide synthase inhibitors to treat septic shock: the light has changed from yellow to red. Crit Care Med 27: 855–856, 1999. [DOI] [PubMed] [Google Scholar]
- 13.Connelly L, Madhani M, Hobbs AJ. Resistance to endotoxic shock in endothelial nitric-oxide synthase (eNOS) knock-out mice: a pro-inflammatory role for eNOS-derived NO in vivo. J Biol Chem 280: 10040–10046, 2005. [DOI] [PubMed] [Google Scholar]
- 14.Daemen-Gubbels CR, Groeneveld PH, Groeneveld AB, van Kamp GJ, Bronsveld W, Thijs LG. Methylene blue increases myocardial function in septic shock. Crit Care Med 23: 1363–1370, 1995. [DOI] [PubMed] [Google Scholar]
- 15.Evgenov OV, Sager G, Bjertnaes LJ. Methylene blue reduces lung fluid filtration during the early phase of endotoxemia in awake sheep. Crit Care Med 29: 374–379, 2001. [DOI] [PubMed] [Google Scholar]
- 16.Fernandes D, Sordi R, Pacheco LK, Nardi GM, Heckert BT, Villela CG, Lobo AR, Barja-Fidalgo C, Assreuy J. Late, but not early, inhibition of soluble guanylate cyclase decreases mortality in a rat sepsis model. J Pharmacol Exp Ther 328: 991–999, 2009. [DOI] [PubMed] [Google Scholar]
- 17.Finkel MS, Oddis CV, Jacob TD, Watkins SC, Hattler BG, Simmons RL. Negative inotropic effects of cytokines on the heart mediated by nitric oxide. Science 257: 387–389, 1992. [DOI] [PubMed] [Google Scholar]
- 18.Friebe A, Mergia E, Dangel O, Lange A, Koesling D. Fatal gastrointestinal obstruction and hypertension in mice lacking nitric oxide-sensitive guanylyl cyclase. Proc Natl Acad Sci USA 104: 7699–7704, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gardiner SM, Kemp PA, March JE, Bennett T. Cardiac and regional haemodynamics, inducible nitric oxide synthase (NOS) activity and the effects of NOS inhibitors in conscious, endotoxaemic rats. Br J Pharmacol 116: 2005–2016, 1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Grover R, Zaccardelli D, Colice G, Guntupalli K, Watson D, Vincent JL. An open-label dose escalation study of the nitric oxide synthase inhibitor, NG-methyl-l-arginine hydrochloride (546C88), in patients with septic shock. Glaxo Wellcome International Septic Shock Study Group. Crit Care Med 27: 913–922, 1999. [DOI] [PubMed] [Google Scholar]
- 21.Hare JM Nitric oxide and excitation-contraction coupling. J Mol Cell Cardiol 35: 719–729, 2003. [DOI] [PubMed] [Google Scholar]
- 22.Hare JM, Colucci WS. Role of nitric oxide in the regulation of myocardial function. Prog Cardiovasc Dis 38: 155–166, 1995. [DOI] [PubMed] [Google Scholar]
- 23.Huang PL, Huang Z, Mashimo H, Bloch KD, Moskowitz MA, Bevan JA, Fishman MC. Hypertension in mice lacking the gene for endothelial nitric oxide synthase. Nature 377: 239–242, 1995. [DOI] [PubMed] [Google Scholar]
- 24.Ichinose F, Bloch KD, Wu JC, Hataishi R, Aretz HT, Picard MH, Scherrer-Crosbie M. Pressure overload-induced LV hypertrophy and dysfunction in mice are exacerbated by congenital NOS3 deficiency. Am J Physiol Heart Circ Physiol 286: H1070–H1075, 2004. [DOI] [PubMed] [Google Scholar]
- 25.Ichinose F, Buys ES, Neilan TG, Furutani EM, Morgan JG, Jassal DS, Graveline AR, Searles RJ, Lim CC, Kaneki M, Picard MH, Scherrer-Crosbie M, Janssens S, Liao R, Bloch KD. Cardiomyocyte-specific overexpression of nitric oxide synthase 3 prevents myocardial dysfunction in murine models of septic shock. Circ Res 100: 130–139, 2007. [DOI] [PubMed] [Google Scholar]
- 26.Ichinose F, Hataishi R, Wu JC, Kawai N, Rodrigues AC, Mallari C, Post JM, Parkinson JF, Picard MH, Bloch KD, Zapol WM. A selective inducible NOS dimerization inhibitor prevents systemic, cardiac, and pulmonary hemodynamic dysfunction in endotoxemic mice. Am J Physiol Heart Circ Physiol 285: H2524–H2530, 2003. [DOI] [PubMed] [Google Scholar]
- 27.Joe EK, Schussheim AE, Longrois D, Maki T, Kelly RA, Smith TW, Balligand JL. Regulation of cardiac myocyte contractile function by inducible nitric oxide synthase (iNOS): mechanisms of contractile depression by nitric oxide. J Mol Cell Cardiol 30: 303–315, 1998. [DOI] [PubMed] [Google Scholar]
- 28.Kirov MY, Evgenov OV, Evgenov NV, Egorina EM, Sovershaev MA, Sveinbjornsson B, Nedashkovsky EV, Bjertnaes LJ. Infusion of methylene blue in human septic shock: a pilot, randomized, controlled study. Crit Care Med 29: 1860–1867, 2001. [DOI] [PubMed] [Google Scholar]
- 29.Knotek M, Esson M, Gengaro P, Edelstein CL, Schrier RW. Desensitization of soluble guanylate cyclase in renal cortex during endotoxemia in mice. J Am Soc Nephrol 11: 2133–2137, 2000. [DOI] [PubMed] [Google Scholar]
- 30.Kojda G, Kottenberg K. Regulation of basal myocardial function by NO. Cardiovasc Res 41: 514–523, 1999. [DOI] [PubMed] [Google Scholar]
- 31.Kojda G, Kottenberg K, Nix P, Schluter KD, Piper HM, Noack E. Low increase in cGMP induced by organic nitrates and nitrovasodilators improves contractile response of rat ventricular myocytes. Circ Res 78: 91–101, 1996. [DOI] [PubMed] [Google Scholar]
- 32.Kumar A, Brar R, Wang P, Dee L, Skorupa G, Khadour F, Schulz R, Parrillo JE. Role of nitric oxide and cGMP in human septic serum-induced depression of cardiac myocyte contractility. Am J Physiol Regul Integr Comp Physiol 276: R265–R276, 1999. [DOI] [PubMed] [Google Scholar]
- 33.Kumar A, Haery C, Parrillo JE. Myocardial dysfunction in septic shock. I. Clinical manifestation of cardiovascular dysfunction. J Cardiothorac Vasc Anesth 15: 364–376, 2001. [DOI] [PubMed] [Google Scholar]
- 34.Kumar A, Thota V, Dee L, Olson J, Uretz E, Parrillo JE. Tumor necrosis factor-α and interleukin 1β are responsible for in vitro myocardial cell depression induced by human septic shock serum. J Exp Med 183: 949–958, 1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lim CC, Apstein CS, Colucci WS, Liao R. Impaired cell shortening and relengthening with increased pacing frequency are intrinsic to the senescent mouse cardiomyocyte. J Mol Cell Cardiol 32: 2075–2082, 2000. [DOI] [PubMed] [Google Scholar]
- 36.Lopez A, Lorente JA, Steingrub J, Bakker J, McLuckie A, Willatts S, Brockway M, Anzueto A, Holzapfel L, Breen D, Silverman MS, Takala J, Donaldson J, Arneson C, Grove G, Grossman S, Grover R. Multiple-center, randomized, placebo-controlled, double-blind study of the nitric oxide synthase inhibitor 546C88: effect on survival in patients with septic shock. Crit Care Med 32: 21–30, 2004. [DOI] [PubMed] [Google Scholar]
- 37.Mergia E, Friebe A, Dangel O, Russwurm M, Koesling D. Spare guanylyl cyclase NO receptors ensure high NO sensitivity in the vascular system. J Clin Invest 116: 1731–1737, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mergia E, Russwurm M, Zoidl G, Koesling D. Major occurrence of the new α2β1 isoform of NO-sensitive guanylyl cyclase in brain. Cell Signal 15: 189–195, 2003. [DOI] [PubMed] [Google Scholar]
- 39.Mery PF, Pavoine C, Belhassen L, Pecker F, Fischmeister R. Nitric oxide regulates cardiac Ca2+ current. Involvement of cGMP-inhibited and cGMP-stimulated phosphodiesterases through guanylyl cyclase activation. J Biol Chem 268: 26286–26295, 1993. [PubMed] [Google Scholar]
- 40.Moncada S, Higgs A. The l-arginine-nitric oxide pathway. N Engl J Med 329: 2002–2012, 1993. [DOI] [PubMed] [Google Scholar]
- 41.Munt B, Jue J, Gin K, Fenwick J, Tweeddale M. Diastolic filling in human severe sepsis: an echocardiographic study. Crit Care Med 26: 1829–1833, 1998. [DOI] [PubMed] [Google Scholar]
- 42.Nimmegeers S, Sips P, Buys E, Brouckaert P, Van de Voorde J. Functional role of the soluble guanylyl cyclase α1 subunit in vascular smooth muscle relaxation. Cardiovasc Res 76: 149–159, 2007. [DOI] [PubMed] [Google Scholar]
- 43.Parker MM, Shelhamer JH, Bacharach SL, Green MV, Natanson C, Frederick TM, Damske BA, Parrillo JE. Profound but reversible myocardial depression in patients with septic shock. Ann Intern Med 100: 483–490, 1984. [DOI] [PubMed] [Google Scholar]
- 44.Parker MM, Shelhamer JH, Natanson C, Alling DW, Parrillo JE. Serial cardiovascular variables in survivors and nonsurvivors of human septic shock: heart rate as an early predictor of prognosis. Crit Care Med 15: 923–929, 1987. [DOI] [PubMed] [Google Scholar]
- 45.Pathan N, Hemingway CA, Alizadeh AA, Stephens AC, Boldrick JC, Oragui EE, McCabe C, Welch SB, Whitney A, O'Gara P, Nadel S, Relman DA, Harding SE, Levin M. Role of interleukin 6 in myocardial dysfunction of meningococcal septic shock. Lancet 363: 203–209, 2004. [DOI] [PubMed] [Google Scholar]
- 46.Petros A, Lamb G, Leone A, Moncada S, Bennett D, Vallance P. Effects of a nitric oxide synthase inhibitor in humans with septic shock. Cardiovasc Res 28: 34–39, 1994. [DOI] [PubMed] [Google Scholar]
- 47.Rudiger A, Singer M. Mechanisms of sepsis-induced cardiac dysfunction. Crit Care Med 35: 1599–1608, 2007. [DOI] [PubMed] [Google Scholar]
- 48.Scherrer-Crosbie M, Ullrich R, Bloch KD, Nakajima H, Nasseri B, Aretz HT, Lindsey ML, Vancon AC, Huang PL, Lee RT, Zapol WM, Picard MH. Endothelial nitric oxide synthase limits left ventricular remodeling after myocardial infarction in mice. Circulation 104: 1286–1291, 2001. [DOI] [PubMed] [Google Scholar]
- 49.Shah AM, Spurgeon HA, Sollott SJ, Talo A, Lakatta EG. 8-Bromo-cGMP reduces the myofilament response to Ca2+ in intact cardiac myocytes. Circ Res 74: 970–978, 1994. [DOI] [PubMed] [Google Scholar]
- 50.Shesely EG, Maeda N, Kim HS, Desai KM, Krege JH, Laubach VE, Sherman PA, Sessa WC, Smithies O. Elevated blood pressures in mice lacking endothelial nitric oxide synthase. Proc Natl Acad Sci USA 93: 13176–13181, 1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Takimoto E, Champion HC, Belardi D, Moslehi J, Mongillo M, Mergia E, Montrose DC, Isoda T, Aufiero K, Zaccolo M, Dostmann WR, Smith CJ, Kass DA. cGMP catabolism by phosphodiesterase 5A regulates cardiac adrenergic stimulation by NOS3-dependent mechanism. Circ Res 96: 100–109, 2005. [DOI] [PubMed] [Google Scholar]
- 52.Tatsumi T, Keira N, Akashi K, Kobara M, Matoba S, Shiraishi J, Yamanaka S, Mano A, Takeda M, Nishikawa S, Asayama J, Fliss H, Nakagawa M. Nitric oxide-cGMP pathway is involved in endotoxin-induced contractile dysfunction in rat hearts. J Appl Physiol 96: 853–860, 2004. [DOI] [PubMed] [Google Scholar]
- 53.Ullrich R, Scherrer-Crosbie M, Bloch KD, Ichinose F, Nakajima H, Picard MH, Zapol WM, Quezado ZM. Congenital deficiency of nitric oxide synthase 2 protects against endotoxin-induced myocardial dysfunction in mice. Circulation 102: 1440–1446, 2000. [DOI] [PubMed] [Google Scholar]
- 54.Vermeersch P, Buys E, Pokreisz P, Marsboom G, Ichinose F, Sips P, Pellens M, Gillijns H, Swinnen M, Graveline A, Collen D, Dewerchin M, Brouckaert P, Bloch KD, Janssens S. Soluble guanylate cyclase-α1 deficiency selectively inhibits the pulmonary vasodilator response to nitric oxide and increases the pulmonary vascular remodeling response to chronic hypoxia. Circulation 116: 936–943, 2007. [DOI] [PubMed] [Google Scholar]
- 55.Wegener JW, Closs EI, Forstermann U, Nawrath H. Failure of 1H-[1,2,4]oxadiazolo[4,3-a]quinoxalin-1-one (ODQ) to inhibit soluble guanylyl cyclase in rat ventricular cardiomyocytes. Br J Pharmacol 127: 693–700, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wegener JW, Nawrath H, Wolfsgruber W, Kuhbandner S, Werner C, Hofmann F, Feil R. cGMP-dependent protein kinase I mediates the negative inotropic effect of cGMP in the murine myocardium. Circ Res 90: 18–20, 2002. [DOI] [PubMed] [Google Scholar]
- 57.Wheeler AP, Bernard GR. Treating patients with severe sepsis. N Engl J Med 340: 207–214, 1999. [DOI] [PubMed] [Google Scholar]
- 58.Zacharowski K, Berkels R, Olbrich A, Chatterjee PK, Cuzzocrea S, Foster SJ, Thiemermann C. The selective guanylate cyclase inhibitor ODQ reduces multiple organ injury in rodent models of Gram-positive and Gram-negative shock. Crit Care Med 29: 1599–1608, 2001. [DOI] [PubMed] [Google Scholar]
- 59.Zhu X, Zhao H, Graveline AR, Buys ES, Schmidt U, Bloch KD, Rosenzweig A, Chao W. MyD88 and NOS2 are essential for toll-like receptor 4-mediated survival effect in cardiomyocytes. Am J Physiol Heart Circ Physiol 291: H1900–H1909, 2006. [DOI] [PubMed] [Google Scholar]