Abstract
B-type natriuretic peptide (BNP) is an established first-line therapy for acute decompensated heart failure (HF), but its efficacy in preventing left ventricular (LV) remodeling after myocardial injury is unknown. The goal of this study was to evaluate the effects of BNP therapy on remodeling after ischemic injury in an awake canine model. Dogs were chronically instrumented for hemodynamics. Ischemia was created by daily coronary embolization (Embo; 3.1 × 104 beads/day) for 3 wk; 60 min after the first embolization, BNP (100 ng·kg−1·min−1; n = 6) or saline (control; n = 6) was continuously infused via a left atrial catheter for 3 wk. Hemodynamics and echocardiography were performed in an awake state at baseline, 3 wk after Embo + BNP infusion, and 4 wk after stopping Embo + BNP infusion. End-systolic elastance (Ees) and LV change in pressure over time (dP/dt) were preserved throughout Embo + BNP therapy versus control therapy (Ees: 3.76 ± 1.01 vs. 1.41 ± 0.16 mmHg/ml; LV dP/dt: 2,417 ± 96 vs. 2,068 ± 95 mmHg/s; both P < 0.05 vs. control). LV end-diastolic dimension was significantly smaller in BNP-treated dogs compared with control dogs (4.29 ± 0.10 vs. 4.77 ± 0.17 cm), and ejection fraction was maintained in treated dogs vs. control dogs (53 ± 1% vs. 46 ± 2%) (both P < 0.05 vs. control). Cyclooxygenase (COX)-2 expression in terminal LV tissue was significantly reduced after BNP therapy. Treatment with continuous infusion of BNP preserved LV geometry, improved systolic function, and prevented the progression of systolic HF after persistent ischemic injury.
Keywords: heart failure, myocardial function
recombinant b-type natriuretic peptide (BNP) has a wide array of pharmacological effects by affecting cardiovascular, renal, and neurohormonal pathways. These effects are mediated primarily through production of cGMP, which occurs after peptide binding to guanylyl cyclase-linked natriuretic peptide receptors. By producing both arterial and venous dilatation, BNP reduces left ventricular (LV) filling pressures, and myocardial work and oxygen consumption are reduced secondary to BNP-mediated coronary vasodilatation (29, 30). BNP also alters renal hemodynamics by increasing glomerular filtration rate, directly promoting natriuresis, and inhibiting aldosterone release (18). These acute hemodynamic changes cause a rapid reduction in clinical symptoms and have made it an effective first-line therapy in acutely decompensated congestive heart failure (CHF) (9, 31, 33).
An even more important clinical application of BNP lies in its potential ability to alter the natural history of heart failure following acute myocardial ischemia. Growing evidence suggests that natriuretic peptides affect multiple neurohormonal and antiproliferative pathways involved in pathological LV remodeling. BNP opposes genes involved in fibrosis, proliferation, and inflammation via cGMP-dependent protein kinase signaling (20). Likewise, studies in rats show that treatment decreased infarct size after coronary occlusion (34). Clinically, intravenous atrial natriuretic peptide (ANP) given during coronary angioplasty for 24 h improved LV ejection fraction and LV end-diastolic volume index at 1 mo (15); ANP administered for 48 h after acute myocardial infarction decreased cardiac sympathetic nerve activity and improved cardiac function by nuclear imaging after 2-wk follow-up (21).
These early studies imply that natriuretic peptide therapy may preserve LV geometry and enhance cardiac function after myocardial infarction. Currently, little animal or human data exists addressing the specific use of BNP therapy after myocardial infarction to prevent the development and progression of systolic heart failure. Clinical studies using ANP lack long-term follow-up and have not provided a systematic/quantitative analysis of cardiac function and biological mechanisms. Moreover, ANP differs in structure and function from BNP: BNP has a much longer half-life due to decreased affinity to neutral endopeptidases (24), greater potency generating higher cGMP levels, and increased expression in ventricular tissue compared with ANP (17). Finally, recombinant ANP is not approved for use in all countries, except Japan. As such, the primary purpose of the present study was to evaluate the long-term effect of continuous intravenous BNP on myocardial function and remodeling after sustained coronary embolization-induced ischemic myocardial injury in an awake canine model. Additionally, pathways specific to reverse remodeling, including inflammation and fibrosis, were examined in terminal myocardial samples.
METHODS
Study Design
The study consisted of three phases for a total period of over 7 wk: 1) an initial instrumentation surgery, 2) induction of CHF due to sustained myocardial ischemia through daily coronary embolization for 3 wk in addition to a continuous infusion of vehicle or BNP (Embo+Inf), and 3) a 4-wk observation period after stopping embolization and infusion (Obs). Twelve mongrel dogs of both sexes (age 2.0 ± 0.5 yr; weight 25 ± 6 kg) were chronically instrumented at the initial surgery and allowed to recover for 7–10 days. At the beginning of Embo+Inf, each dog was randomized to the control group (n = 6), which received a continuous infusion of vehicle (0.9% normal saline) or intravenous BNP (100 ng·kg−1·min−1) (Scios, Fremont, CA) (n = 6) at equivalent volumes starting 60 min after the first embolization with a portable infusion pump (CADD-Legacy PCA Pump model 6500, Smith Medical MD, St. Paul, MN) and continuing throughout the 3-wk Embo+Inf period. Hemodynamic measurements and echocardiograms were performed 10–12 days after instrumentation but immediately before Embo+Inf (baseline), after 3 wk of Embo+Inf, and after 4 wk of Obs in an awake state. At the end of 4 wk of Obs the animals were killed, and LV myocardial tissue was harvested for histology and immunohistochemistry. This study was approved by the Institutional Animal Care and Use Committee of Columbia University, which conforms to the Guide for the Care and Use of Laboratory Animals published by the National Institutes of Health (NIH Pub. No. 85-23, revised 1996).
Surgical Instrumentation
The surgical procedures involved in the initial instrumentation surgery have been described in full previously (8). In short, a Konigsberg solid-state pressure gauge (6.5, Konigsberg Instruments, Pasadena, CA) was placed through the LV apex for measurement of LV pressure, and fluid-filled catheters were placed in the ascending aorta and the LV for measurement of aortic pressure and calibration of the Konigsberg pressure gauge, respectively. A pneumatic cuff was placed around the inferior vena cava (IVC) to vary loading conditions postoperatively. A thin Silastic cannula was inserted into the left anterior descending coronary artery (LAD) for coronary embolization. Another Tygon catheter was chronically implanted into the right atrium, and the catheter was connected to a venous infusion pump for drug infusion. In addition, six sonomicrometric crystals (Sonometrics, London, ON, Canada) were placed on the anterior, posterior, apex, base, septal, and free walls of the LV. The catheters and wires were run subcutaneously and externalized through the skin, the chest was closed in layers, and a temporary chest tube was placed. Dogs were allowed to recover fully from surgery and were trained to lie quietly on a laboratory table before experiments.
Coronary Embolization-Induced Heart Failure
This model has been described previously in detail (8). In brief, CHF was produced by daily intracoronary microembolization with 25,000 polymer beads (90–120 μm in diameter) injected through the previously implanted LAD cannula for three consecutive weeks. To avoid bias in cardiac function and hemodynamics due to variable degrees of coronary embolization, daily and total doses of polymer beads during the 3-wk coronary embolization period were comparable among control and BNP-treated dogs. Embolization was begun after recovery from surgery and immediately after baseline measurements on the same day.
BNP Solution Preparation and Administration
BNP solution was prepared every day with normal saline. The concentration of BNP for pump infusion was varied and based on the animal's weight, as the infusion rate was kept constant at 1 ml/h. Thus the concentration of BNP solution (ng/ml) for a particular animal was calculated as follows: 100 (ng·kg−1·min−1) × body wt (kg) × 60 (min/h) × 1 (h/ml). At the start of infusion, the right atrial tunneled catheter was connected to the continuous infusion pump, which was attached to the back of the dogs with custom-made backpacks. New infusion pumps containing freshly prepared BNP solution or normal saline were connected every day to ensure stability of the solution.
The dose of continuous-infusion BNP was chosen, in part, based on canine pharmacokinetics of the drug (internal data, data not shown) and, in part, to provide maximal benefit in this pilot experiment and to cause significant increases in serum cGMP without obvious hemodynamic effects. Published clinical studies have utilized intravenous BNP doses ranging from 10 to 30 ng·kg−1·min−1 (9, 33). As such, the dose used in this experiment was more than double that in those studies, in order to ensure adequate dosing and maximum effect of downstream BNP signaling.
Hemodynamic Measurements and Echocardiography
Hemodynamics and echocardiography were measured as previously described (8). Aortic pressure was measured by attaching the previously implanted catheters to P23ID strain-gauge transducers (Statham Instruments, Oxnard, CA), and LV pressure was measured via a chronically implanted solid pressure gauge. Mean arterial pressure (MAP) was determined online by use of 3-Hz averaging filters (DA26, Medtron Engineering, Olivenhain, CA). Data were recorded on an eight-channel thermal writing chart recorder (30-V8808-10, Gould Electronics, East Rutherford, NJ), and periods of interest were digitized (Gateway 2000 486 computer equipped with an analog-to-digital conversion system, Sonometrics) for off-line analysis. Echocardiograms were performed with a Hewlett Packard Sonos 5500 at baseline, after 3 wk of Embo+Inf, and after 4 wk of Obs, as previously described (8), and analyzed off-line by a blinded observer. LV mass was calculated with the formula of Devereaux.
LV Pressure-Volume Relationship Analysis
LV pressure and LV volume were measured at baseline, after 3 wk of Embo+Inf, and after 4 wk of Obs via previously implanted solid-state pressure gauges in the LV and sonomicrometric crystals. As previously described (8), LV pressure-volume relationship loops were measured at rest and during a transient preload reduction induced by IVC occlusion; the end-systolic pressure-volume relationship (ESPVR) and end-diastolic pressure-volume relationship (EDPVR) were then calculated.
The area between ESPVR and EDPVR at steady state, known as pressure-volume area (PVA), is an afterload-independent index of pump function that is a predictor of myocardial consumption (39). LV stroke work (SW) was calculated as the integrated area of each pressure-volume loop at steady state. Both PVA and SW were normalized to echocardiographically determined LV mass, to remove any confounding differences in ventricular size. LV mechanical efficiency was calculated as the ratio of SW to PVA (4, 39). Arterial elastance (Ea) was calculated as stroke volume/end-systolic pressure. All calculations were made with custom software (Matlab, v6.5).
Assessment of Serum cGMP and BNP Levels
Blood samples via the implanted aortic catheter for cGMP levels were drawn from two BNP-treated dogs in order to verify BNP-mediated cGMP signaling. In these two dogs, BNP infusion was begun 1 h before embolization, unlike the remaining BNP group. Blood samples were drawn at baseline, 1 h after initial BNP infusion but before start of Embo, and then 1 h after Embo+Inf. Two samples were drawn for each animal per time point.
Blood samples for BNP levels were also drawn in all control and BNP-treated dogs at baseline (before Embo or Inf), after 1 h of Embo alone, after 1 h of Embo+Inf, after 1 wk of Embo+Inf, after 3 wk of Embo+Inf, and after 4 wk of Obs.
All blood samples were drawn while the dogs were lying quietly on the table, and serum was used for cGMP and BNP measurements. The levels of cGMP were measured with the cGMP EIA kit (Cayman Chemical), and BNP levels were measured with a radioimmunoassay kit (Phoenix Pharmaceuticals).
Immunohistochemistry and Histology: Fibrosis, Factor VIII, COX-2, and Macrophage Infiltration
For immunohistochemical staining, slides were deparaffined and rehydrated in PBS followed by blocking of endogenous peroxidase with 3% hydrogen peroxide. To avoid nonspecific reaction with primary antibody, slides were pretreated with 15% normal goat serum before incubation with primary antibodies. Slides were incubated with primary antibodies including anti-factor VIII (DAKO, Carpinteria, CA; 1:400), anti-Cox-2 (Novus Biologicals, Littleton, CO; 1:500) and anti-macrophage, respectively. Normal mouse IgG was used as a negative control. The immunoreactivities were visualized by ABC reagents (Vector, Burlingame, CA) and diaminobenzidine, followed by counterstaining with hematoxylin. The image analysis was performed with a Nikon E600 light microscope equipped with a Spot digital camera. Digital image software (Image Pro Plus 4.5.1, Silver Spring, MD) was used for image analysis of myocardial fibrosis, positive factor VIII-staining blood vessels, cyclooxygenase (COX)-2, as well as macrophage infiltration, measured in 10 fields per slide and normalized by the number of covered fields. The image analysis was blinded to the person who performed the histopathological evaluation.
Statistics
Values are reported as means ± SE. Continuous variables were compared by paired, two-tailed t-testing with Levene's test for equality of variances. A paired Student's t-test was used for subgroup comparisons made between measurements obtained at baseline, Embo+Inf, and after Obs. Significance was adjusted for multiple comparisons with one-way ANOVA with post hoc Bonferroni analysis, when necessary. Nonparametric analysis and analysis of covariance of ESPVR and EDPVR variables were performed with two-tailed Wilcoxon signed rank testing and analysis of covariance (ANCOVA), respectively, at the P < 0.05 significance level. Analysis was performed with SPSS v.11.5 (Chicago, IL).
RESULTS
Coronary Embolization
All 12 dogs survived the full study period. A trend toward a higher terminal heart weight was seen in control dogs versus BNP dogs (211 ± 5 vs. 194 ± 16 g; P = 0.13), although terminal mean body weight was similar (26.4 ± 8.7 vs. 23.4 ± 2.4 kg; P = 0.54), as was the ratio of terminal heart to body weight (0.95 ± 0.14 vs. 0.84 ± 0.18; P = 0.21). Embolization proceeded without mortality throughout the entire 3-wk Embo period in all control and BNP-treated dogs. An average of 31,000 beads was delivered daily to dogs; a slightly higher cumulative bead total was delivered to BNP-treated dogs compared with control dogs (BNP 6.6 × 105 vs. control 6.3 × 105 beads over 3-wk Embo period; P = 0.57).
Hemodynamic Measurements and Echocardiography
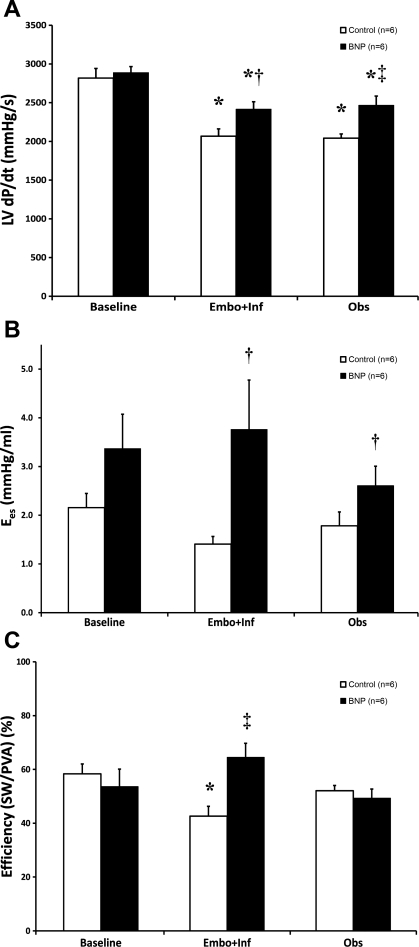
Hemodynamics at baseline, after 3 wk of Embo+Inf, and after 4 wk of Obs are summarized in Table 1. Baseline hemodynamics and echocardiographic function were comparable before embolization in BNP and Control dogs. After 3 wk of Embo+Inf, the heart failure state was effectively achieved by embolization in both BNP and control groups, as shown by a 13–14% reduction in MAP, a >50% increase in LV end-diastolic pressure, and significant decreases in LV maximal change in pressure over time (dP/dtmax; Fig. 1A) (all P < 0.05 vs. baseline).
Table 1.
Hemodynamics
| Baseline | Embo+Inf | Obs | ||||
|---|---|---|---|---|---|---|
| Heart rate, bpm | ||||||
| Control | 75.5±2.5 | 65.5±3.6 | 72.0±4.9 | |||
| BNP | 76.0±3.6 | 93.5±5.7† | 83.5±5.4 | |||
| MAP, mmHg | ||||||
| Control | 95.5±2.2 | 88±4.4* | 87±4.3 | |||
| BNP | 99.8±1.6 | 86.7±5.0* | 93.7±3.2 | |||
| LVESP, mmHg | ||||||
| Control | 119.5±2.4 | 111.7±2.4 | 113.6±4.4 | |||
| BNP | 124.0±1.9 | 107.3±4.8* | 114.3±3.9 | |||
| LVEDP, mmHg | ||||||
| Control | 12.9±1.8 | 20.4±1.3* | 18.3±0.9* | |||
| BNP | 11.3±1.7 | 17.5±2.9* | 17.6±2.7* | |||
Values are means ± SE for 6 dogs/group. Embo, embolization; Inf, infusion; Obs, observation; bpm, beats per minute; MAP, mean arterial pressure; LVESP, left ventricular (LV) end-systolic pressure; LVEDP, LV end-diastolic pressure; BNP, B-type natriuretic peptide.
P < 0.05 vs. baseline,
P < 0.005 vs. control.
Fig. 1.
Hemodynamics and pressure-volume analysis. In A, left ventricular (LV) maximal change in pressure over time (dP/dtmax) was significantly depressed from baseline after embolization + infusion (Embo+Inf) in both B-type natriuretic peptide (BNP) and control dogs; however, BNP-treated dogs experienced a significantly smaller decline throughout both Embo+Inf and the observation period (Obs). B depicts a significant improvement in end-systolic elastance (Ees) during Embo+Inf in BNP-treated dogs compared with control dogs. Finally, myocardial efficiency [stroke work (SW)/pressure-volume area (PVA)] was greatly enhanced during BNP therapy vs. control therapy (C). *P < 0.05 vs. baseline, †P < 0.05 vs. control, ‡P < 0.01 vs. control.
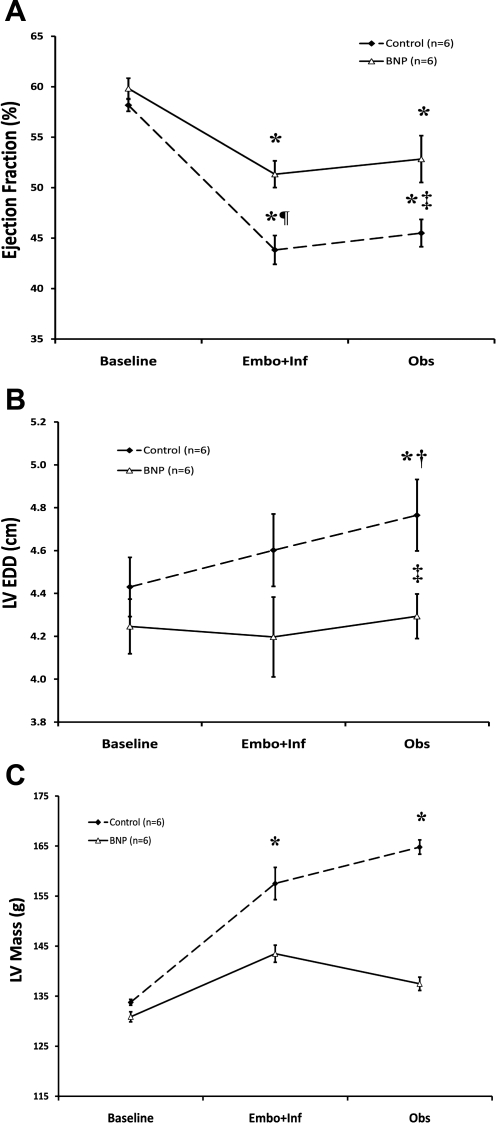
However, at the conclusion of both Embo+Inf and Obs periods, a consistent pattern of decreased myocardial injury was obvious in BNP-treated dogs. BNP-treated dogs recorded a significantly higher LV dP/dtmax and ejection fraction after Embo+Inf and Obs versus control dogs (2,466 ± 120 vs. 2,042 ± 54 mmHg/s and 53% vs. 46%, respectively; both P < 0.01 vs. control) (Fig. 1A and Fig. 2A). Recovery of ventricular function corresponded with lessened remodeling by echocardiography: ventricular dimensions [LV end-diastolic dimension (LVEDD)] did not change after embolization or observation after BNP therapy, unlike control dogs, which experienced an 8% increase in ventricular size (see Fig. 2B). At the end of Obs, the reverse remodeling effects were even more pronounced: LVEDD in BNP-treated dogs was significantly smaller compared with control dogs (4.29 ± 0.10 vs. 4.77 ± 0.17 cm; P < 0.05). Similar findings were present for end-systolic dimensions (data not shown). Finally, echocardiographically derived LV mass progressively increased from 134 ± 6 g to 165 ± 14 g in control dogs, suggestive of progressive pressure-volume overload stress (Fig. 2C). In contrast, BNP-treated dogs had no significant evidence of LV hypertrophy (LV mass: 131 ± 6 to 137 ± 8 g, baseline vs. Obs), despite the aforementioned gains in contractile function.
Fig. 2.
Echocardiographic changes before and after BNP therapy. In A, ejection fraction in the BNP cohort was preserved compared with the control cohort throughout the experiment. Similarly, in B, LV end-diastolic dimension (LVEDD) was smaller in BNP-treated dogs after Embo+Inf and Obs. LV mass, as shown in C, showed no change after BNP therapy during Embo+Inf and Obs, in contrast to control therapy, which demonstrated a significant increase in LV mass after embolization and after a period of observation. *P < 0.05 vs. baseline, †P < 0.05 vs. Embo+Inf, ‡P < 0.05 vs. control, ¶P < 0.005 vs. control.
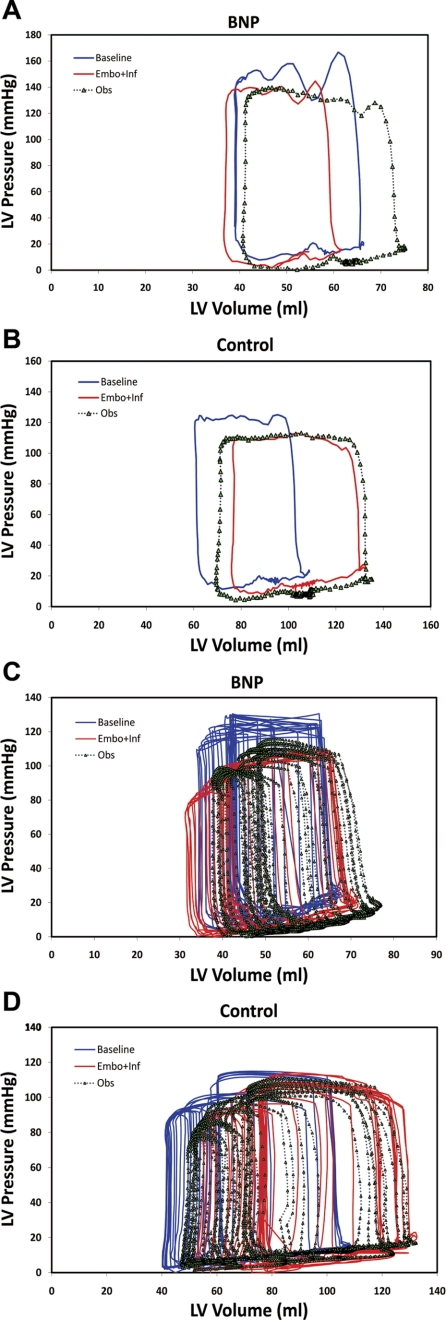
Pressure-Volume Analysis
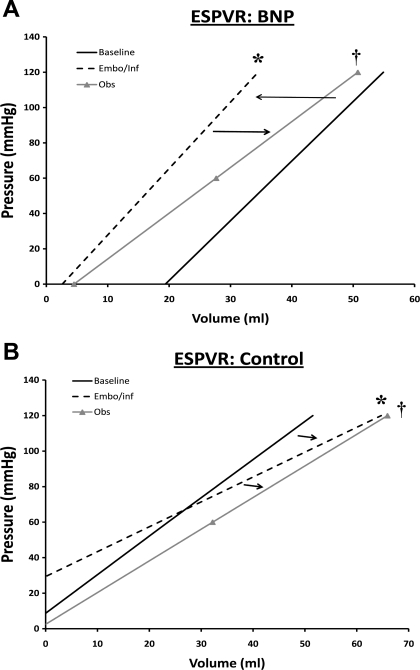
A complete summary of pressure-volume analyses is found in Table 2. Representative pressure-volume loops at steady state and after IVC occlusion are displayed in Fig. 3. BNP therapy was associated with a maintained ESPVR, as shown by an unchanged end-systolic elastance (Ees) in BNP-treated dogs versus control dogs after Embo+Inf and Obs (Fig. 1B) (P < 0.05 vs. control). ESPVR pressure-volume curves produced similar results, displaying an increase in systolic function in BNP dogs during Embo+Inf compared with a progressive decline in control dogs; a slight decline in ESPVR was seen after cessation of BNP therapy during Obs in BNP dogs (Fig. 4). BNP therapy was also associated with significantly improved myocardial efficiency during Embo+Inf compared with control therapy alone (P < 0.01 vs. control) (Fig. 1C). Analysis of myocardial energetics suggested improved cardiac performance with BNP therapy: trends to improved SW and reduced PVA at the end of Obs were seen. Finally, Ea was improved after BNP treatment versus control (P < 0.05 vs. control). EDPVR demonstrated no significant change.
Table 2.
Pressure-volume analysis
| Baseline | Embo+Inf | Obs | |
|---|---|---|---|
| V0, ml | |||
| Control | −4.32±5.06 | −21.24±4.22* | −1.50±8.78 |
| BNP | 19.39±18.48 | 2.64±6.65† | 4.58±5.25 |
| α | |||
| Control | 0.028±0.010 | 0.018±0.004 | 0.036±0.010 |
| BNP | 0.052±0.015 | 0.038±0.009 | 0.035±0.005 |
| β | |||
| Control | 2.74±1.60 | 2.86±1.00 | 0.49±0.36 |
| BNP | 2.54±2.10 | 5.70±4.36 | 0.50±0.12 |
| SW/LV mass, mmHg·ml·g−1 | |||
| Control | 28.4±2.0 | 23.3±4.9 | 25.2±2.3 |
| BNP | 24.8±4.5 | 32.6±13.0 | 22.8±2.0 |
| PVA/LV mass, mmHg·ml·g−1 | |||
| Control | 51.7±3.0 | 52.4±8.1 | 49.2±5.4 |
| BNP | 49.7±5.1 | 50.2±15.1 | 46.3±2.9 |
| Ea, mmHg·ml−1·m−2 | |||
| Control | 3.56±0.69 | 3.17±0.29 | 2.67±0.21 |
| BNP | 5.39±1.48 | 4.13±0.32† | 3.50±0.22† |
Values are means ± SE for 6 dogs/group. V0, volume intercept; α, myocardial stiffness; β, scaling constant; SW, stroke work; PVA, pressure-volume area; Ea, arterial elastance.
P < 0.05 vs. baseline,
P < 0.05 vs. control.
Fig. 3.
Representative pressure-volume (PV) loops at steady state (A and B) and after inferior vena cava occlusion (C and D) in BNP-treated and control dogs. After the Embo+Inf period, PV loops were shifted to the right and downward in control dogs along with an increase in cardiac dimensions, while PV loops after BNP therapy demonstrated a slight decrease in cardiac dimensions during embolization without shifting relative location.
Fig. 4.
Changes in end-systolic pressure volume relationship (ESPVR). In A, an increase in contractile function from baseline to Embo+Inf is seen in BNP dogs, as demonstrated by a shift to the left, while a slight decline is seen during Obs. B shows a progressive decline in ESPVR in control dogs from baseline to Embo+Inf through Obs, as shown by shifting of the curves downward and to the right. *P < 0.05 vs. baseline, †P < 0.05 vs. Embo+Inf.
Serum cGMP and BNP Levels
Summaries of serum cGMP and BNP levels are displayed in Tables 3 and 4. cGMP levels rose almost 10-fold (27.2 ± 8.8 to 187.1 ± 13.9 pmol/ml; P = 0.001) after 1 h of BNP infusion, signifying effective downstream cGMP-mediated signaling. After an hour of Embo+Inf, cGMP dropped slightly to 127.4 ± 15.9 pmol/ml (P = 0.45). Serum BNP levels also confirmed effective drug delivery: BNP levels more than doubled in BNP dogs (4.54 ± 0.06 to 9.66 ± 2.02 ng/ml) after 1 h of Embo from baseline but decreased to 5.20 ± 0.66 ng/ml by 3 wk of Embo+Inf (both P < 0.05). BNP levels dropped further in BNP dogs during 4 wk of Obs, in contrast to control dogs.
Table 3.
cGMP levels in BNP-treated animals
Values are means ± SE for 2 dogs/group.
P < 0.05 vs. baseline,
P < 0.05 vs. 1 h after Inf.
Table 4.
Plasma BNP
| Baseline | Embo 1 h | Embo+Inf 1 h | Embo+Inf 1 wk | Embo+Inf 3 wk | Obs 4 wk | |
|---|---|---|---|---|---|---|
| Control | 2.88±0.37 | 19.57±6.46† | 2.88±0.26 | 2.92±0.87 | 3.36±0.31 | |
| BNP | 4.54±0.06* | 9.66±2.02† | 9.33±2.61 | 9.83±0.72* | 5.20±0.66*‡ | 4.38±0.08* |
Values (in ng/ml) are means ± SE plasma BNP for 6 dogs/group.
P < 0.05 vs. control,
P < 0.05 vs. baseline,
P < 0.05 vs. Embo+Inf 1 wk.
Immunohistochemistry and Histology
Fibrosis.
Extensive ischemic myocardial scar in our study was confirmed on fibrosis staining of the ventricular region at risk for ischemia in both control and BNP dogs. No statistically significant difference was apparent between control and BNP dogs (20.6 ± 8.8% and 22.8 ± 16.3%, respectively, measured as % fibrosis of ischemic region; P = 0.81).
Factor VIII expression.
A strong trend toward increased factor VIII expression (important in the regulation of angiogenesis and measured as newly formed vessels per high-power field) in the ischemic region was seen in dogs treated with BNP versus control dogs (P = 0.07 vs. control).
COX-2 expression.
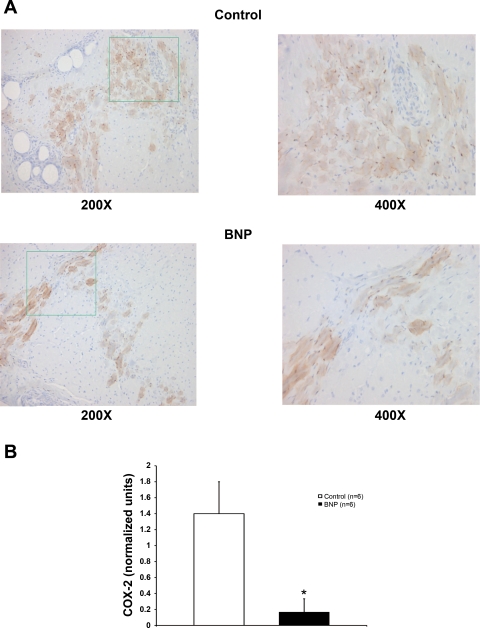
Expression of COX-2, a marker of systemic inflammation, was significantly lower in ischemic myocardium treated with BNP compared with myocardium from control therapy (P < 0.05 vs. control; Fig. 5). Similar to other histological variables, this effect was limited to the ischemic regions.
Fig. 5.
Cyclooxygenase (COX)-2 expression. A: control and BNP slides stained for COX-2 expression at ×200 and the area outlined in green at ×400 magnification. B: COX-2 expression in ischemic myocardium was significantly higher in BNP-treated dogs compared with control dogs. *P < 0.05 vs. control.
Macrophage infiltration.
No change in macrophage infiltration in ischemic myocardium was seen between BNP and control therapy dogs at experiment termination (50.2 ± 14.7 vs. 53.2 ± 23.1; P = 0.91).
DISCUSSION
Clinical studies thus far have focused on using BNP in the chronic heart failure population. The cardiovascular and renal effects after BNP administration in these studies, as well as its neurohormonal inhibitory properties, suggest that use after myocardial infarction may provide protection against the progression of heart failure. Accordingly, the purpose of the present experiment was to evaluate the long-term effects of continuous intravenous BNP infusion on myocardial performance and remodeling after sustained myocardial injury. We present strong evidence for the first time that short-term, continuous BNP infusion preserves systolic function, prevents LV remodeling, and improves cardiac performance specifically after sustained myocardial injury. BNP therapy also reduced expression of COX-2, thereby altering the inflammatory milieu. These study results justify further investigation addressing use after myocardial infarction. The application of BNP therapy to the acute myocardial infarction population could have important clinical implications on reducing the burden of heart failure on the health care system, currently estimated as 2% of health care expenditures (31).
Although concern has arisen recently regarding the safety of recombinant BNP (3), numerous earlier clinical reports have demonstrated its clinical benefit. In 2000, Colucci et al. (9) reported a reduction in pulmonary capillary wedge pressure, reduced dyspnea, and less fatigue in patients treated with 6 h of intravenous nesiritide; these findings were later confirmed by the Vasodilatation in the Management of Acute CHF (VMAC) trial (33). BNP after cardiac surgery has been studied extensively; short-term intravenous infusion increased cardiac output (14), decreased pulmonary artery pressures (37), and increased urine output (13) in separate small case series. The Nesiritide Administered Peri-Anesthesia in Patients Undergoing Cardiac Surgery (NAPA) trial studied 272 patients randomized to nesiritide or placebo (28). Contrary to previous studies (36), parameters of renal function were improved, and 180-day mortality was lower than placebo. To our knowledge, only ANP has been used immediately after acute myocardial infarction (15, 21): 10 days after a 24-h infusion of ANP administered after first anterior wall acute myocardial infarction, ejection fraction in treated patients increased while LV dimensions were not changed (15), and ANP reduced sympathetic activity 2 wk after myocardial infarction in a similar study (21). BNP offers a potentially superior alternative to ANP, because its slightly longer half-life (t1/2 22 min), peripheral intravenous route, easy dose adjustability, rapid onset of action, and documented safety in previous studies (9, 13, 14, 28, 33, 37) make it an attractive drug in post-acute myocardial infarction patients. Furthermore, BNP is a Food and Drug Administration (FDA)-approved drug, and its safety profile has been partially established already.
Effect of BNP on Myocardial Function
Our key findings with an extreme myocardial ischemia model are as follows. First, systolic function was preserved throughout the period of embolization and BNP infusion, as evidenced by the increase in Ees and relative preservation of LV dP/dtmax compared with control dogs. Ejection fraction throughout the experiment was higher in BNP-treated dogs. Second, SW, as measured by pressure-volume loop analysis, provided confirmation that BNP-treated hearts were capable of generating greater external work. Finally, and most importantly, these gains in systolic function were not at the expense of myocardial oxygen consumption, because there was no change in PVA; this is in direct contrast to dobutamine, which, augments contractility like BNP but increases mortality versus BNP (38). The consequence of these favorable inotropic findings of BNP was that myocardial efficiency (4), or ratio of SW to PVA, a measure of global cardiac performance, of the BNP-treated hearts was significantly greater than that of control hearts. These results are also similar to animal findings by Chen et al. (7) where subcutaneous BNP was given to dogs with pacing-induced heart failure, which showed that cardiac output, pulmonary capillary wedge pressure, and systemic vascular resistance were all improved after 10 days of therapy. Myocardial perfusion secondary to coronary vasodilatation and cGMP-generated nitric oxide (10, 23, 29) may also help account for gains in systolic function while lowering myocardial oxygen demand and salvaging at-risk myocardium, thus reducing infarct size (12).
An increase in heart rate was observed during the Embo+Inf period in BNP-treated animals, which may partially confound the true extent of BNP's effect on contractile function in this study. Conflicting reports exist as to whether intravenous BNP affects heart rate (43, 44), but high-dose therapy has consistently increased heart rate in most studies (24) and in the setting of ventricular dysfunction (27). We speculate that a concurrent fall in MAP, coupled with probable BNP-associated natriuresis, stimulated the baroreceptor reflex, thus driving an increase in heart rate (7, 16). Systemic vascular resistance likewise falls, and preload is decreased because of significant venodilatation; these results create favorable conditions for myocardial energetic and pump function, as reflected in the improvements in ejection fraction, LV dP/dtmax, and end-diastolic pressures. However, the effect on heart rate occurred only during BNP infusion, and blood pressure and heart rate equalized among groups after infusion cessation during the 4-wk observation period. Most importantly, the gains in cardiac function persisted after BNP infusion cessation, and indexes of ventricular remodeling in BNP dogs were, in many instances, less pronounced. Furthermore, Ees, a load-independent index, should not be altered with changes in volume status and was increased in animals treated with BNP, thus supporting the hypothesis that BNP sustains contractile function. Finally, the ability of BNP infusion to interrupt the cycle of heart failure and persist after cessation may suggest intact natriuretic receptor function, unlike the downregulation or unresponsiveness seen in CHF (32). The ability of BNP therapy to affect the course of heart failure before receptor desensitization occurs argues even more for its use after acute myocardial injury.
Effect of BNP on LV Remodeling
A substantial degree of LV remodeling was prevented by BNP therapy in the present study, and this is an important beneficial long-term clinical outcome associated with therapy after sustained myocardial injury. Although the myocardial and vascular pressure-volume unloading secondary to BNP partially contribute to long-term prevention of ventricular remodeling, the biological properties of BNP on inflammation, proliferation, and neurohormonal inhibition may also account for preservation of ventricular geometry. With microarray analysis, BNP was previously shown to oppose transforming growth factor-β (TGF-β), and genes involved in inflammation (COX2, IL6, TNF α-induced protein 6, TNF superfamily member 4), fibrosis (collagen I, fibronectin, CTGF, PAI-1, TIMP3), and proliferation (PDGF, IGF1, FGF18, IGFBP10), via cGMP-dependent protein kinase signaling (20). Our study confirmed a reduction in the inflammatory response mediator COX-2, which may help stabilize myocyte and vascular endothelium in response to ischemic stress. It is also known that BNP inhibits collagen synthesis, increases matrix metalloproteinase (MMP)-1, -2, and -3 (42), and limits cardiac fibroblast growth (6); this occurs likely as a mechanism to limit remodeling when secreted by cardiomyocytes and cardiac fibroblasts in response to stretch and infarction (5, 42). Mice with disruption of the BNP gene display exaggerated myocardial fibrosis, hypertension, and upregulation of fibrotic genes (20, 40). Although no change in fibrosis and only a trend to lower heart weight was seen in the present study after BNP therapy, antiproliferative pathways may promote recovery of cells at risk in infarct border and at-risk regions (34). Second, BNP has been shown to block the expression of a number of key neurohormones central to worsening heart failure, including endothelin-1, aldosterone, and norepinephrine (1, 2, 44), as well as inhibition of the renin-angiotensin-aldosterone system (25). Antifibrotic, antiproliferative, and neurohormonal sympathetic inhibitory mechanisms share common molecular pathways, dependent on BNP-related cGMP signaling. Modulation of endogenous cGMP by other pharmacological means, such as nitrates or bradykinin, has been shown to provide similar myocardial benefits in animal models, supporting the favorable biological actions of BNP (19, 26).
The clinical manifestation of attenuation of ventricular remodeling can be seen in our study results: cardiac dimensions did not change throughout embolization or 4 wk after BNP treatment, unlike control dog dimensions, which progressively increased even in the absence of embolization-induced injury in the observation period. Ea, representing ventriculo-vascular coupling and central arterial changes, demonstrated a beneficial effect after BNP therapy. Echocardiographically derived LV mass and ventricular dimensions were both lower in BNP-treated dogs, consistent with previous literature showing that natriuretic peptides decrease myocyte hypertrophy (41); in contrast, LV mass and ventricular dimensions significantly increased in control dogs, signifying LV hypertrophy and late dilatation in response to pressure-volume overload. In control dogs, as cardiac geometric dimensions increase, LV wall stress (governed by the law of Laplace), and thus myocardial oxygen consumption, should increase (4); this was also confirmed by pressure-volume analysis. This sustained increase in wall stress and stretch response feeds the vicious cycle of heart failure progression in control dogs, activating compensatory sympathetic pathways ultimately leading to decompensated hemodynamics. BNP counteracts these pathological mechanisms on multiple levels: hemodynamic, renal, and neurohormonal. As discussed above, improved hemodynamics maintain coronary and organ perfusion during acute injury. Renal mechanisms regulate volume status and reduce myocardial workload, and the specific actions of BNP on cardiomyocyte proliferation, fibrosis, inflammation, and sympathetic response mediate cardiac and vascular remodeling over a long-term period.
Coronary Embolization Model and Clinical Applicability
The coronary embolization model employed in the present experiment deserves mention. To create an extreme and sustained myocardial ischemia state and test whether BNP therapy can overcome the pathology associated with myocardial ischemia-induced heart failure, the daily coronary embolization model was chosen 1) to create hemodynamic derangement that is consistent with heart failure over a 3-wk time frame, 2) to use a well-known and established model of, specifically, ischemic injury with infarction (22, 35), and 3) to maintain constant and persistent stimulation for pathological remodeling through daily embolization. In our experience, simple coronary ligation is not sufficient to create the level of heart failure required to mimic large human myocardial infarction; extensive coronary collaterals aid myocardial recovery in a short time in canines. Furthermore, the specific method of injury, an ischemic insult, was necessary to mimic an appropriate human clinical setting, and was the reason pacing-induced heart failure was not used. The coronary embolization model fulfills these criteria, and awake measurements provide reliable and clinically relevant measurements. The study design was intended to replicate time from symptom onset to response by medical personnel, with induction of injury first, followed by administration of BNP after 60 min. The model does not replicate the physiology of reperfusion after revascularization or acute infarction—rather, it replicates remodeling that occurs after a severe prolonged infarction or repeated infarction without revascularization. The study findings, therefore, represent the most extreme form of acute ischemic myocardial injury, and it is plausible that improvement in this model also translates to improvement in less severe ischemic injury.
Conclusions
In conclusion, acute myocardial injury was created in awake dogs with daily coronary embolizations. Continuous intravenous BNP therapy preserved systolic function and attenuated LV remodeling, supported by both energetic and biochemical mechanisms. These results provide a rationale for prolonged intravenous BNP treatment after myocardial infarction with the goal of prevention of the chronic heart failure state.
GRANTS
J. Wang is partially supported by a grant (08431903002) from the Science and Technology Commission of Shanghai Municipality, People's Republic of China. This work was partially supported by National Heart, Lung, and Blood Institute Grant T32-HL-07854 (I. George).
DISCLOSURES
This work was supported by a research grant to J. Wang from Scios, Inc.
Acknowledgments
We sincerely acknowledge Yanping Cheng, Jordan Muraskin, Christian Soneru, Anguo Gu, and Geping Zhang for their contributions to this manuscript.
REFERENCES
- 1.Abraham WT, Lowes BD, Ferguson DA, Odom J, Kim JK, Robertson AD, Bristow MR, Schrier RW. Systemic hemodynamic, neurohormonal, and renal effects of a steady-state infusion of human brain natriuretic peptide in patients with hemodynamically decompensated heart failure. J Card Fail 4: 37–44, 1998. [DOI] [PubMed] [Google Scholar]
- 2.Aronson D, Burger AJ. Intravenous nesiritide (human B-type natriuretic peptide) reduces plasma endothelin-1 levels in patients with decompensated congestive heart failure. Am J Cardiol 90: 435–438, 2002. [DOI] [PubMed] [Google Scholar]
- 3.Arora RR, Venkatesh PK, Molnar J. Short and long-term mortality with nesiritide. Am Heart J 152: 1084–1090, 2006. [DOI] [PubMed] [Google Scholar]
- 4.Burkhoff D, Sagawa K. Ventricular efficiency predicted by an analytical model. Am J Physiol Regul Integr Comp Physiol 250: R1021–R1027, 1986. [DOI] [PubMed] [Google Scholar]
- 5.Cameron VA, Rademaker MT, Ellmers LJ, Espiner EA, Nicholls G, Richards AM. Atrial (ANP) and brain natriuretic peptide (BNP) expression after myocardial infarction in sheep: ANP is synthesized by fibroblasts infiltrating the infarct. Endocrinology 141: 4690–4697, 2000. [DOI] [PubMed] [Google Scholar]
- 6.Cao L, Garner D. Natriuretic peptides inhibit DNA synthesis in cardiac fibroblasts. Hypertension 25: 227–234, 1995. [DOI] [PubMed] [Google Scholar]
- 7.Chen HH, Grantham A, Schirger JA, Jougasaki M, Redfield MM, Burnett JC. Subcutaneous administration of brain natriuretic peptide in experimental heart failure. J Am Coll Cardiol 36: 1706–1712, 2000. [DOI] [PubMed] [Google Scholar]
- 8.Cheng Y, George I, Yi GH, Reiken S, Gu A, Tao YK, Muraskin J, Qin S, He KL, Hay I, Yu K, Oz MC, Burkhoff D, Holmes J, Wang J. Bradycardic therapy improves left ventricular function and remodeling in dogs with coronary embolization-induced chronic heart failure. J Pharmacol Exp Ther 321: 469–476, 2007. [DOI] [PubMed] [Google Scholar]
- 9.Colucci WS, Elkayam U, Horton DP, Abraham WT, Bourge RC, Johnson AD, Wagoner LE, Givertz MM, Liang CS, Neibaur M, Haught WH, LeJemtel TH. Intravenous nesiritide, a natriuretic peptide, in the treatment of decompensated congestive heart failure. N Engl J Med 343: 246–253, 2000. [DOI] [PubMed] [Google Scholar]
- 10.Costa MD, Bosc LV, Majowicz MP, Vidal NA, Balaszczuk AM, Arranz CT. Atrial natriuretic peptide modifies arterial blood pressure through nitric oxide pathways in rats. Hypertension 35: 1119–1123, 2000. [DOI] [PubMed] [Google Scholar]
- 11.Doering CW, Jalil JE, Janicki JS, Pick R, Aghili S, Abrahams C, Weber KT. Collagen network remodeling and diastolic stiffness of the rat left ventricle with pressure overload hypertrophy. Cardiovasc Res 22: 686–695, 1988. [DOI] [PubMed] [Google Scholar]
- 12.D'Souza SP, Yellon DM, Martin C, Schulz R, Heusch G, Onody A, Ferdinandy P, Baxter GF. B-type natriuretic peptide limits infarct size in rat isolated hearts via KATP channel opening. Am J Physiol Heart Circ Physiol 284: H1592–H1600, 2003. [DOI] [PubMed] [Google Scholar]
- 13.Feldman DS, Ikonomidis JS, Uber WE, Van Bakel AB, Pereira NL, Crumbley AJ 3rd, Tann SM. Human B-natriuretic peptide improves hemodynamics and renal function in heart transplant patients immediately after surgery. J Card Fail 10: 292–296, 2004. [DOI] [PubMed] [Google Scholar]
- 14.Gordon GR, Schumann R, Rastegar H, Khabbaz K, England MR. Nesiritide for treatment of perioperative low cardiac output syndromes in cardiac surgical patients: an initial experience. J Anesth 20: 307–311, 2006. [DOI] [PubMed] [Google Scholar]
- 15.Hayashi M, Tsutamoto T, Wada A, Maeda K, Mabuchi N, Tsutsui T, Horie H, Ohnishi M, Kinoshita M. Intravenous atrial natriuretic peptide prevents left ventricular remodeling in patients with first anterior acute myocardial infarction. J Am Coll Cardiol 37: 1820–1826, 2001. [DOI] [PubMed] [Google Scholar]
- 16.Holmes SJ, Espiner EA, Richards AM, Yandle TG, Frampton C. Renal, endocrine, and hemodynamic effects of human brain natriuretic peptide in normal man. J Clin Endocrinol Metab 76: 91–96, 1993. [DOI] [PubMed] [Google Scholar]
- 17.Hosoda K, Nakao K, Mukoyama M, Saito Y, Jougasaki M, Shirakami G, Suga S, Ogawa Y, Yasue H, Imura H. Expression of brain natriuretic peptide gene in human heart. Production in the ventricle. Hypertension 17: 1152–1155, 1991. [DOI] [PubMed] [Google Scholar]
- 18.Houben AJ, van der Zander K, de Leeuw PW. Vascular and renal actions of brain natriuretic peptide in man: physiology and pharmacology. Fundam Clin Pharmacol 19: 411–419, 2005. [DOI] [PubMed] [Google Scholar]
- 19.Jugdutt BI, Khan MI. Effect of prolonged nitrate therapy on left ventricular remodeling after canine acute myocardial infarction. Circulation 89: 2297–2307, 1994. [DOI] [PubMed] [Google Scholar]
- 20.Kapoun AM, Liang F, O'Young G, Damm DL, Quon D, White RT, Munson K, Lam A, Schreiner GF, Protter AA. B-type natriuretic peptide exerts broad functional opposition to transforming growth factor-β in primary human cardiac fibroblasts. Circ Res 94: 453–461, 2004. [DOI] [PubMed] [Google Scholar]
- 21.Kasama S, Toyama T, Hatori T, Sumino H, Kumakura H, Takayama Y, Ichikawa S, Suzuki T, Kurabayashi M. Effects of intravenous atrial natriuretic peptide on cardiac sympathetic nerve activity and left ventricular remodeling in patients with first anterior acute myocardial infarction. J Am Coll Cardiol 49: 667–674, 2007. [DOI] [PubMed] [Google Scholar]
- 22.Knecht M, Burkhoff D, Yi GH, Popiskis S, Homma S, Packer M, Wang J. Coronary endothelial dysfunction precedes heart failure and reduction of coronary reserve in awake dogs. J Mol Cell Cardiol 29: 217–227, 1997. [DOI] [PubMed] [Google Scholar]
- 23.Kubo M, Nakaya Y, Matsuoka S, Saito K, Kuroda Y. Atrial natriuretic factor and isosorbide dinitrate modulate the gating of ATP-sensitive K+ channels in cultured vascular smooth muscle cells. Circ Res 74: 471–476, 1994. [DOI] [PubMed] [Google Scholar]
- 24.Lainchbury JG, Richards AM, Nicholls MG, Espiner EA, Yandle TG. Brain natriuretic peptide and neutral endopeptidase inhibition in left ventricular impairment. J Clin Endocrinol Metab 84: 723–739, 1999. [DOI] [PubMed] [Google Scholar]
- 25.Levin ER, Gardner DG, Samson WK. Natriuretic peptides. N Engl J Med 339: 321–328, 1998. [DOI] [PubMed] [Google Scholar]
- 26.MacDonald KM, Mock J, D'Aloia A, Parrish T, Hauer K, Francis G, Stillman A, Cohn JN. Bradykinin antagonism inhibits the antigrowth effect of converting enzyme inhibition in the dog myocardium after discrete transmural myocardial necrosis. Circulation 91: 2043–2048, 1995. [DOI] [PubMed] [Google Scholar]
- 27.Marcus LS, Hart D, Packer M, Yushak M, Medina N, Danziger RS, Heitjan DF, Katz SD. Hemodynamic and renal excretory effects of human brain natriuretic peptide infusion in patients with congestive heart failure. A double-blind, placebo-controlled, randomized crossover trial. Circulation 94: 3184–3189, 1996. [DOI] [PubMed] [Google Scholar]
- 28.Mentzer RM, Oz MC, Sladen RN, Graeve AH, Hebeler RF, Luber JM Jr, Smedira NG, NAPA Investigators. Effects of perioperative nesiritide in patients with left ventricular dysfunction undergoing cardiac surgery: the NAPA trial. J Am Coll Cardiol 49: 716–726, 2007. [DOI] [PubMed] [Google Scholar]
- 29.Michaels AD, Klein A, Madden JA, Chatterjee K. Effects of intravenous nesiritide on human coronary vasomotor regulation and myocardial oxygen uptake. Circulation 107: 2697–2701, 2003. [DOI] [PubMed] [Google Scholar]
- 30.Moro C, Berlan M. Cardiovascular and metabolic effects of natriuretic peptides. Fundam Clin Pharmacol 20: 41–49, 2006. [DOI] [PubMed] [Google Scholar]
- 31.Munger MA Management of acute decompensated heart failure: treatment, controversy, and future directions. Pharmacotherapy 26: 131S–138S, 2006. [DOI] [PubMed] [Google Scholar]
- 32.Nakamura M, Arakawa N, Yoshida H, Makita S, Niinuma H, Hiramori K. Vasodilatory effects of B-type natriuretic peptide are impaired in patients with chronic heart failure. Am Heart J 135: 414–420, 1998. [DOI] [PubMed] [Google Scholar]
- 33.Publication Committee for the VMAC Investigators (Vasodilatation in the Management of Acute CHF). Intravenous nesiritide vs. nitroglycerin for treatment of decompensated congestive heart failure. JAMA 287: 1531–1540, 2002. [DOI] [PubMed] [Google Scholar]
- 34.Ren Y, Shen Y, Shao H, Qian J, Wu H, Jing H. Brain natriuretic peptide limits myocardial infarct size dependent of nitric oxide synthase in rats. Clin Chim Acta 377: 83–87, 2007. [DOI] [PubMed] [Google Scholar]
- 35.Sabbah HN, Stein PD, Kono T, Gheorghiade M, Levine TB, Jafri S, Hawkins ET, Goldstein S. A canine model of chronic heart failure produced by multiple sequential coronary microembolizations. Am J Physiol Heart Circ Physiol 260: H1379–H1384, 1991. [DOI] [PubMed] [Google Scholar]
- 36.Sackner-Bernstein JD, Skopicki HA, Aaronson KD. Risk of worsening renal function with nesiritide in patients with acutely decompensated heart failure. Circulation 111: 1487–1491, 2005. [DOI] [PubMed] [Google Scholar]
- 37.Salzberg SP, Filsoufi F, Anyanwu A, Harbou KV, Gass A, Pinney SP, Carpentier A, Adams DH. High-risk mitral valve surgery: perioperative hemodynamic optimization with nesiritide (BNP). Ann Thorac Surg 80: 502–506, 2005. [DOI] [PubMed] [Google Scholar]
- 38.Silver MA, Horton DP, Ghali JK, Elkayam U. Effect of nesiritide versus dobutamine on short-term outcomes in the treatment of patients with acutely decompensated heart failure. J Am Coll Cardiol 39: 798–803, 1997. [DOI] [PubMed] [Google Scholar]
- 39.Suga H, Goto Y, Futaki S, Kawaguchi O, Yaku H, Hata K, Takasago T. Systolic pressure-volume area (PVA) as the energy of contraction in Starling's law of the heart. Heart Vessels 6: 65–70, 1991. [DOI] [PubMed] [Google Scholar]
- 40.Tamura N, Ogawa Y, Chusho H, Nakamura K, Nakao K, Suda M, Kasahara M, Hashimoto R, Katsuura G, Mukoyama M, Itoh H, Saito Y, Tanaka I, Otani H, Katsuki M. Cardiac fibrosis in mice lacking brain natriuretic peptide. Proc Natl Acad Sci USA 97: 4239–4244, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tokudome T, Horio T, Soeki T, Mori K, Kishimoto I, Suga S, Yoshihara F, Kawano Y, Kohno M, Kangawa K. Inhibitory effect of C-type natriuretic peptide (CNP) on cultured cardiac myocyte hypertrophy: interference between CNP and endothelin-2 signaling pathways. Endocrinology 145: 2131–2140, 2004. [DOI] [PubMed] [Google Scholar]
- 42.Tsuruda T, Boerrigter G, Huntley BK, Noser JA, Cataliotti A, Costello-Boerrigter LC, Chen HH, Burnett JC Jr. Brain natriuretic peptide is produced in cardiac fibroblasts and induces matrix metalloproteinases. Circ Res 91: 1127–1134, 2002. [DOI] [PubMed] [Google Scholar]
- 43.Van der Zander K, Houben AJHM, Hofstra L, Kroon AA, de Leeuw PW. Hemodynamic and renal effects of low-dose brain natriuretic peptide infusion in humans: a randomized, placebo-controlled crossover study. Am J Physiol Heart Circ Physiol 285: H1206–H1212, 2003. [DOI] [PubMed] [Google Scholar]
- 44.Yoshimura M, Yasue M, Morita E, Sakaino N, Jougasaki Kurose M, Mukoyama M, Saito Y, Nakao K, Imura H. Hemodynamic, renal and hormonal responses to brain natriuretic peptide infusion in patients with congestive heart failure. Circulation 84: 1581–1588, 1991. [DOI] [PubMed] [Google Scholar]