Abstract
This study was undertaken to determine whether the myocardial infarct-sparing effect of ATL-146e, a selective adenosine A2A receptor agonist, persists without a rebound effect for at least 48 h and to determine the optimal duration of ATL-146e treatment in anesthetized dogs. Reperfusion injury after myocardial infarction (MI) is associated with inflammation lasting 24–48 h that contributes to ongoing myocyte injury. We previously showed that an ATL-146e infusion, starting just before reperfusion, decreased inflammation and infarct size in dogs examined 2 h after MI without increasing coronary blood flow. In the present study, adult dogs underwent 90 min of left anterior descending coronary artery occlusion. Thirty minutes before reperfusion, ATL-146e (0.01 μg·kg−1·min−1; n = 21) or vehicle (n = 12) was intravenously infused and continued for 2.5 h (protocol 1) or 24 h (protocol 2). At 48 h after reperfusion hearts were excised and assessed for histological risk area and infarct size. Infarct size based on triphenyltetrazolium chloride (TTC) staining as a percentage of risk area was significantly smaller in ATL-146e-treated vs. control dogs (16.7 ± 3.7% vs. 33.3 ± 6.2%, P < 0.05; protocol 1). ATL-146e reduced neutrophil accumulation into infarcted myocardium of ATL-146e-treated vs. control dogs (30 ± 7 vs. 88 ± 16 cells/high-power field, P < 0.002). ATL-146e infusion for 24 h (protocol 2) conferred no significant additional infarct size reduction compared with 2.5 h of infusion. A 2.5-h ATL-146e infusion initiated 30 min before reperfusion results in marked, persistent (48 h) reduction in infarct size as a percentage of risk area in dogs with a reduction in infarct zone neutrophil infiltration. No significant further benefit was seen with a 24-h infusion.
Keywords: reperfusion injury, myocardial infarction
reperfusion injury after coronary ischemia is an important pathophysiological mechanism in myocardial infarction (MI) (19). It is a phenomenon of multifactorial etiology including intense inflammation, damage from reactive oxygen species, shifts in metabolic substrate consumption, and perturbations of mitochondrial and cytosolic calcium ion homeostasis (29). During reperfusion after prolonged ischemia, myocytes and endothelial cells release metabolic products and cytokines that are proinflammatory (24). Neutrophils accumulate early in tissue undergoing reperfusion injury (27), followed by macrophages and other leukocytes (5).
Adenosine infusion during reperfusion has been shown to reduce infarct size compared with placebo in clinical trials of patients suffering from MI with large areas of ischemic anterior myocardium at risk (16, 21). Perhaps because of limited sample size, this reduction in ischemic injury was not associated with a significant mortality reduction. Adenosine administration may lead to unwanted effects such as heart block or hypotension. These effects result from nonselective activation of four adenosine receptor subtypes (A1, A2A, A2B, and A3). Selective activation of the adenosine A2A receptor may provide cardioprotection against reperfusion injury via an anti-inflammatory mechanism without nonselective activation of the other receptor subtypes caused by adenosine. In fact, a 2.5-h infusion of a nonvasodilating yet anti-inflammatory dose of the A2A receptor agonist ATL-146e beginning 30 min before reperfusion in an open-chest, anesthetized canine model of MI has been shown to reduce infarct size as a percentage of risk area by 45% when assessed at 2 h after reperfusion (6). This low dose of ATL-146e did not cause increased regional myocardial blood flow as is observed with adenosine. Since the inflammatory response after MI and reperfusion may last at least 24 h (3), the primary objective of the present study was to determine whether the infarct reduction conferred by a 2.5-h infusion of a nonvasodilating, anti-inflammatory dose of ATL-146e persists at 48 h without a rebound increase in infarct size. A secondary objective was to determine whether a 24-h infusion reduced infarct size to a greater extent than a 2.5-h infusion when assessed at 48 h after MI. The data presented are critical in the justification and design of a randomized clinical trial to test whether ATL-146e therapy can attenuate reperfusion injury in patients suffering from acute MI.
MATERIALS AND METHODS
All animal experiments were performed with the approval of the University of Virginia Animal Care and Use Committee and were in compliance with the American Heart Association Position on Research Animal Use.
Experimental protocol.
Protocol 1 randomized 32 adult mongrel dogs of both sexes (mean weight 22.4 ± 2.6 kg, range 19.5–29.5 kg) to vehicle (n = 16) or ATL-146e (n = 16). Protocol 2 utilized 10 adult mongrel dogs of both sexes, all of which received ATL-146e for 24 h. Induction of general anesthesia was achieved with pentobarbital sodium (30 mg/kg iv). After endotracheal intubation, dogs were mechanically ventilated on room air (Harvard Apparatus, Holliston, MA). Anesthesia was maintained with an intravenous pentobarbital sodium infusion. One gram of cefazolin and 80 mg of gentamicin were administered before surgery. A 5-Fr catheter was placed in the right femoral artery with the Seldinger technique and flushed with heparinized saline in order to monitor central arterial pressure and to serially sample arterial blood.
The dogs were then placed in the right lateral decubitus position on a heating pad set to regulate and maintain a constant normal body temperature. Heart rate, electrocardiogram, blood pressure, oxygen saturation, expired Pco2, and body temperature were continuously monitored throughout the surgical procedure. In addition, arterial blood was periodically sampled and analyzed for pH, Po2, and Pco2 with a blood gas analyzer (Radiometer). After sterile skin preparation and draping, a thoracotomy was performed at the fifth intercostal space. The pericardium was divided, and the heart was suspended in a pericardial sling. All visible collaterals to the anterior wall of the left ventricle (LV) were ligated, followed by complete occlusion of the left anterior descending artery (LAD). We have previously demonstrated (6) that this technique produces a reproducible decrease in endocardial and transmural perfusion (i.e., risk area) during occlusion. After 60 min of occlusion (i.e., 30 min before reperfusion) intravenous infusion of ATL-146e (0.01 μg·kg−1·min−1) or vehicle (0.07% DMSO in saline) was randomly started in both the 2.5-h and 24-h infusion groups. The infusion was started 30 min before reflow in order to achieve a steady-state blood concentration before the ligatures were removed. The investigators performing the experiments were blinded to the identity of the solution being infused. Both groups of animals also received an intravenous 5-mg metoprolol bolus 30 min before reperfusion. If an animal was defibrillated or if an animal experienced >5 min of frequent nonsustained ventricular tachycardia, an additional 5 mg of intravenous metoprolol was administered at the time of the event. After 90 min of occlusion all coronary ligatures were removed to allow reperfusion. Arterial blood was sampled at the times depicted in Fig. 1. After 1 h of reperfusion, the chest was closed in layers. In the 24-h treatment group, the continuous infusion was maintained by subcutaneous implantation of an Alzet osmotic minipump (model 2001D; Durect, Cupertino, CA) that had been preloaded with the ATL-146e solution (4.4 μg/μl).
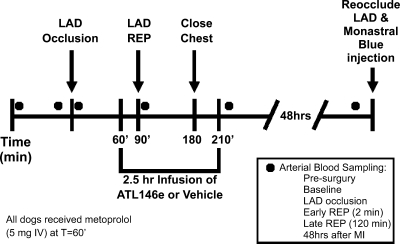
Fig. 1.
Time line of the experimental protocol. Sixty minutes after left anterior descending artery (LAD) occlusion (30 min before reperfusion) a 2.5-h intravenous infusion of ATL-146e was started. Forty-eight hours after reperfusion (Rep), histological assessment of risk area, infarct area, and neutrophil infiltration was performed as described in materials and methods. Circles indicate time points at which arterial blood was sampled for analysis.
After the pentobarbital sodium infusion was discontinued, animals were given morphine analgesia (buprenorphine 0.15 mg iv and 0.15 mg sc). Animals were transferred to a heated, oxygenated recovery chamber and extubated once spontaneous respiration commenced. All investigators involved in the surgical procedure and data analysis were blinded to the identity of the solution being infused (ATL-146e or vehicle) intravenously and via minipump until after the postmortem analysis was complete.
Serum laboratory values.
Troponin I concentration from arterial blood collected at the time points shown in Fig. 1 was analyzed on an automated blood chemistry machine (Architect CI8200, Abbott Diagnostics, Abbott Park, IL). Complete blood cell counts with differentials were performed on samples collected at the time points shown in Fig. 1. Analysis was performed on an automated hematology analysis machine (Cell Line 4000, Abbott Diagnostics).
Histology.
Forty-eight hours after MI, animals were placed under pentobarbital sodium anesthesia as described above. Risk area and infarct size were delineated with monastral blue dye and triphenyltetrazolium chloride (TTC), respectively, as previously described by our laboratory (6). The hearts were then harvested, and the LV was sectioned from base to apex into four short-axis slices, each ∼1 cm thick. These slices were stained with TTC (4). Sections were then digitally scanned. The infarct area, risk area, and normal myocardial area of each section were quantified with Sigmascan software (Systat, San Jose, CA) by a blinded operator. The average of the infarct area from each of the four sections was divided by the average of the risk area to arrive at the infarct area as a percentage of risk area as previously described by our laboratory (6). A priori, animals with a risk area <15% of the total myocardial area were excluded.
Myocardial neutrophil quantification.
Transmural heart fragments from the grossly infarcted zone were randomly sampled, excised, and fixed in 3.7% paraformaldehyde solution. After dehydration and clearing with xylene, tissue samples were embedded in molten paraffin at 60°C. The paraffin-embedded specimens were then sectioned at 5 μm and stained for neutrophils with a commercially available mouse anti-human antibody (MCA8T4GT, Serotec, Raleigh, NC). Sixteen photomicrographs (×20) were taken serially every 2 mm across each section. The number of neutrophils per field was quantified with ImagePro software (MediaCybernetics, Bethesda, MD). The investigator quantifying neutrophil counts was blinded to whether the specimens were from dogs given ATL-146e or vehicle.
Statistics.
One-way ANOVA with Tukey post hoc correction was used to compare infarct sizes between the groups. A two-tailed Student's t-test was used for comparisons of neutrophil counts between control and ATL-146e-treated animals. Two-way repeated-measures ANOVA was used to analyze the mean arterial pressure and heart rate responses between the groups over time. Fisher's exact test was used when comparing the number of dogs in each group that died from ventricular fibrillation (VF) and the number of dogs in each group that survived VF arrest. Statistical analysis was performed with SigmaStat v2.03 (SPSS, Chicago, IL).
RESULTS
Sample size.
Thirty-two dogs were originally randomized to protocol 1, control (n = 16) or ATL-146e (n = 16). The final study group included 25 dogs (control n = 12; ATL-146e n = 13). Four dogs in the control group and two dogs in the ATL-146e group died secondary to lethal arrhythmias. One dog in the ATL-146e group was excluded from the study because of a small risk area in accordance with predetermined criteria.
Protocol 2 (24-h infusion) included 10 adult mongrel dogs, with a final study group of 8. One dog in protocol 2 died from lethal arrhythmia. Another dog was excluded because of a small risk area.
Hemodynamics.
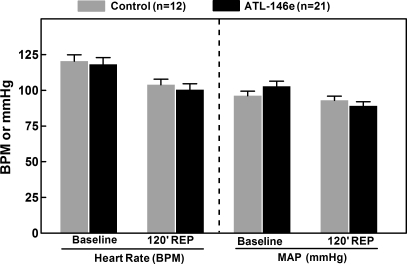
There was no difference in heart rate or mean arterial blood pressure between ATL-146e-treated or control animals at any monitored time point (Fig. 2). Importantly, no fall in systemic blood pressure was observed consequent to ATL-146e infusion.
Fig. 2.
Mean arterial pressure (MAP) and heart rate (HR) at baseline (before ATL-146e or vehicle infusion) and during the infusion at 120 min of reperfusion. There was no change in MAP or HR during the experimental protocol, and no differences were observed in these parameters between the animals receiving ATL-146e and those receiving vehicle. bpm, Beats per minute.
Ventricular fibrillation and metoprolol use.
In protocol 1, there was a trend toward a higher death rate from refractory VF in the control dogs (ATL-146e: 2/16 vs. control: 4/16); however, this trend did not reach statistical significance. Among the surviving animals there was no difference between groups in the number of dogs that experienced VF (ATL-146e: 7 vs. control: 6; P = 1.000). There was no difference in the mean metoprolol dose administered in order to suppress ventricular tachycardia/fibrillation between the two groups (ATL-146e: 6.9 ± 1.2 mg vs. control: 8.3 ± 1.4 mg; P = 0.530). In protocol 2, 1 of 10 ten dogs died from refractory VF.
Histological infarct size.
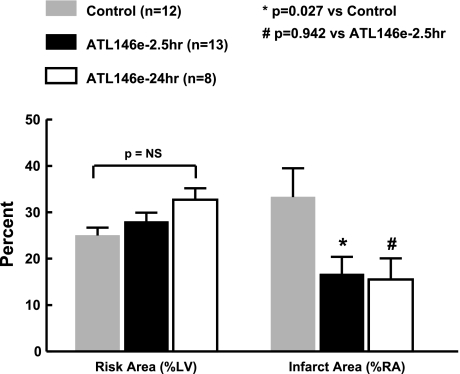
Risk areas were similar between treatment groups (Fig. 3). However, infarct size by TTC staining as a percentage of risk area at 48 h after reperfusion was reduced by 49% in dogs receiving the 2.5-h ATL-146e infusion compared with control animals (ATL-146e: 16.7 ± 3.7% vs. control: 33.3 ± 6.2%; P < 0.05). Infusion of ATL-146e for 24 h did not achieve a greater reduction in infarct size than that achieved by just a 2.5-h infusion (24 h: 17.1 ± 4.3% vs. 2.5 h: 16.7 ± 3.7%; P = 0.942).
Fig. 3.
Infarct size as % of risk area (%RA). Myocardial risk area (left) and infarct size (right) in the experimental model of myocardial infarction. There is no difference in risk area between the 3 groups as measured by monastral blue dye. However, the control animals had a larger infarct size compared with ATL-146e-treated animals. There was no difference in infarct size between the animals that received a 2.5-h infusion and a 24-h infusion of ATL-146e. NS, not significant.
Myocardial neutrophil infiltration.
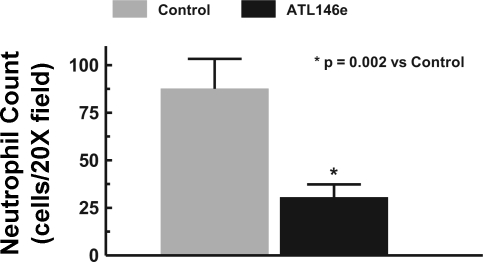
Infarct zone neutrophil infiltration (Figs. 4 and 5) differed markedly between the two groups at 48 h after MI (ATL-146e: 30.5 ± 6.8 vs. control: 87.6 ± 15.7 cells per field; P = 0.002). As shown, the ATL-146e-treated dogs had significantly fewer neutrophils in the reperfused infarct zone.
Fig. 4.
Infarct zone neutrophil count per ×20 field. The mean neutrophil count per ×20 field from infarct zone tissue was significantly lower in ATL-146e-treated dogs vs. control.
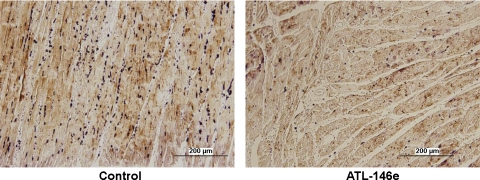
Fig. 5.
Photomicrographs (×20) from the infarct zone of a control animal (left) and an ATL-146e-treated animal (right). Neutrophils have been stained dark violet. Neutrophil infiltration of the infarct zone was visibly reduced in ATL-146e-treated dogs.
Serum laboratory values.
Troponin I concentration (in ng/ml) trended lower in the ATL-146e group versus control animals at the 2.5-h post-MI time point (ATL-146e 4.9 ± 4.7 vs. control 12.7 ± 13.4; P = 0.135) and the 48 h post-MI time point (ATL-146e 26.3 ± 22.7 vs. control 40.0 ± 39.2; P = 0.334). There was no difference in serum white blood cell count, neutrophil count, and lymphocyte count between the treated and control dogs at any time point.
DISCUSSION
The present study demonstrates that a brief, 2.5-h infusion of a nonvasodilating, anti-inflammatory dose of the adenosine A2A receptor agonist ATL-146e started 30 min before reperfusion confers a sustained reduction in infarct size as a percentage of risk area at 48 h after reflow. Histological infarct size in treated dogs was significantly smaller than observed after reperfusion with vehicle alone (16.7% vs. 37.3%) as assessed by blinded observers. This was associated with a substantial reduction in inflammation as measured by infarct zone neutrophil infiltration. The present findings are in accordance with previously reported data at 2 h after MI in which the radiolabeled leukotriene B4 antagonist RP517, an imaging agent that targets circulating neutrophils, showed reduced infarct zone neutrophil uptake in dogs treated with ATL-146e during reperfusion (6). There was no additional benefit from an extended 24-h infusion of ATL-146e over the short 2.5-h infusion with respect to infarct size reduction. Other potential benefits of a more prolonged infusion were not explored in this study. Our findings also support the hypothesized mechanism of benefit of ATL-146e, which is a reduction in the acute inflammatory response consequent to reperfusion, leading to a reduction in infarct size (25, 28, 29). The low ATL-146e dose used does not increase myocardial blood flow (7).
Because dogs have variable coronary anatomy, each animal may have a slightly different coronary anatomic distribution and collateralization. It is speculated that infarct size estimated by serial troponin I concentrations did not achieve statistical significance because these indexes were not controlled for variations in risk area as opposed to histological infarct size measurements, which were normalized to risk area. The importance of controlling for risk area is critical in human studies assessing reperfusion injury (17). Nevertheless, the serial troponin assessments were concordant with the histological data and the myocardial neutrophil assessments.
The present experimental model involved producing MI at the time of thoracotomy. As expected, surgical trauma to the chest wall caused a significant leukocytosis. Nonetheless, neutrophil counts from the infarct zone of ATL-146e-treated animals were significantly lower than those of control animals.
Mechanism of action of ATL-146e.
The adenosine receptors are G protein-coupled seven-transmembrane receptors. The A1 receptor produces the bradycardia and heart block associated with adenosine. Additionally, the A1 receptor has a proinflammatory effect on neutrophil function. The A2B receptor contributes to the vasodilatory and hypotensive response of adenosine. A2B (dogs and primates) and A3 (rodents) receptor activation can be proinflammatory in bronchial smooth muscle tissue and may facilitate allergic reactions in susceptible subjects. On the other hand, there have also been reports showing both anti-inflammatory (8, 26) and anti-infarct (1) effects of A3 adenosine receptor activation. Nevertheless, the cardioprotective effects of adenosine during reperfusion injury appear to be mediated at least in part by A2A receptor activation (2, 13, 15, 18). Activation of A2A receptors results in increased intracellular adenosine 3′,5′-cyclic monophosphate (cAMP) concentration, resulting in activation of protein kinase A and inhibition of multiple steps in the inflammatory cascade. A2A receptors are present on a variety of cells including various types of leukocytes, cardiac myocytes, and endothelial cells. ATL-146e is a highly selective A2A receptor agonist that has been shown to have more potent binding activity to recombinant human and canine A2A receptors than the commercially available A2A receptor agonist CGS-21680 (23). Data from Yang et al. (28) in A2A receptor-knockout mice demonstrated that the cardioprotective mechanism of ATL-146e is via interaction with the A2A receptor.
The leading hypothesis regarding the potent anti-inflammatory action of A2A receptor activation via ATL-164e in reducing reperfusion injury involves A2A receptor-mediated reduction of CD4+ lymphocyte activation. In the liver and kidney, NKT cells are the principal targets of A2A receptor activation. A2A receptor agonism on NKT cells has been shown to reduce neutrophil-mediated reperfusion injury in these two organs (11, 14). It is not yet known whether this is also the case in the heart; however, the data presented here demonstrate that ATL-164e treatment reduced inflammation in the reperfused zone, as evidenced by a reduction in neutrophil infiltration, compared with vehicle. In a recent study using an isolated, buffer-perfused heart model, Rork et al. (20) showed that, even in the absence of neutrophils, ATL-146e reduced tissue-resident mast cell degranulation and protected against postischemic myocardial necrosis. Thus current evidence demonstrates that the anti-inflammatory and infarct-sparing effects of A2A adenosine receptor activation result from inhibition of multiple inflammatory cell types including NKT cells, mast cells, and neutrophils. An alternative or complimentary, yet nonexclusive, mechanism may be the concept of postconditioning described by Kin et al. (10) as well as others that involves delayed washout of endogenous adenosine upon reperfusion, which exerts a cardioprotective effect.
Other investigators have also studied the role of the A2A receptor in reducing infarct size in acute models of reperfused MI in large animals. CGS-21680, a commercially available A2A receptor agonist, is ∼50 times less potent and less selective than ATL-146e. Schlack et al. (22) reported a 60-min LAD occlusion-reperfusion study in which intracoronary CGS-21680 was started 5 min before reperfusion. After 6 h of reperfusion, infarct size as a percentage of risk area was significantly smaller with CGS-21680. Jordan et al. (9) subsequently reported similar results in a canine model of LAD occlusion-reperfusion after 3 h of reperfusion. More recently, Lasley et al. (12) reported data from a porcine model of infarction and reperfusion with intracoronary CGS-21680. Infarct size as a percentage of risk area assessed after 3 h of reperfusion was reduced with intracoronary CGS-21680. The porcine model is interesting in that pigs are generally regarded as having less collateral myocardial perfusion than dogs.
Unlike adenosine, the dose of ATL-146e utilized in the present study conferred a beneficial effect on infarct size as a percentage of risk area without requiring any increase in myocardial blood flow (7). Furthermore, unlike the present study, none of the aforementioned studies evaluated the impact of adenosine receptor agonism in the presence of contemporary pharmacological treatment with metoprolol at the time of reperfusion.
Study limitations.
Coronary flow was not assessed during ATL-164e infusion in each of the experiments reported here. However, coronary flow during various infusions of ATL-146e, including the dose used here in the same experimental animal preparation, has been published previously by our group (7). In addition, our conclusions are based on experiments in which collateral flow was not measured. Therefore, we cannot completely discount the possibility that differences in infarct size between control and ATL-146e-treated groups can be attributed to differences in collateral flow and not the effect of the drug.
Clinical implications.
The present experimental study demonstrated a sustained reduction in infarct size after administration of a 2.5-h intravenous ATL-146e infusion beginning 30 min before reperfusion. It is hypothesized that ATL-146e administration to acute ST-elevation MI (STEMI) patients will be superior to the effect of adenosine since ATL-146e does not activate the A2B receptor causing hypotension and ATL-146e does not bind to the A1 receptor, which can be associated with bradycardia and atrioventricular block and with proinflammatory effects. In recent clinical trials (AMISTAD I and II) conducted to assess whether adenosine would limit myocardial reperfusion injury, patients in both studies assigned to adenosine treatment had an increased incidence of hypotension and heart block (16, 21). Additionally, patients assigned to adenosine in AMISTAD I had an increased incidence of bradycardia (16). Adverse hemodynamic effects such as hypotension consequent to the A2A agonist infusion were not observed in this canine experimental study (see Figs. 2 and 3). Furthermore, the benefit of ATL-146e occurred in the presence of beta-blocker administration. Thus the infarct-reducing effect of ATL-146e is complementary to any infarct-sparing effect of early intravenous metoprolol administration. This is important because early beta-blocker therapy is part of the standard of care in treating patients with MI. If ongoing chronic studies demonstrate no adverse effect of ATL-146e on infarct healing, as was seen with steroid administration after MI, then a randomized clinical trial assessing the efficacy of ATL-146e in reducing MI appears warranted.
In summary, the highly selective adenosine A2A receptor agonist ATL-146e limits reperfusion injury when given as a brief 2.5-h infusion starting 30 min before reperfusion. Despite the short time course of this infusion there is no rebound reperfusion injury when infarct size is assessed at 48 h after MI. No added benefit with regard to infarct size was observed with the more prolonged 24-h infusion of the drug. The beneficial effect of ATL-146e appears to be via its anti-inflammatory properties and persists in the presence of intravenous metoprolol given at the time of reperfusion. These data will be useful for the design of a randomized clinical trial assessing this novel treatment for attenuating reperfusion injury.
GRANTS
This work was supported by National Institutes of Health Grants RO1-HL-075320 and T32-EB-003841.
DISCLOSURES
D. K. Glover and J. Linden have equity interest/stock options in Adenosine Therapeutics; G. A. Beller has Founders Stock in and is a member of the advisory board of Adenosine Therapeutics.
Acknowledgments
We are grateful to Susan Ramos, who performed the histological preparation used for myocardial neutrophil counting and to the Adenosine Therapeutics Group, PGxHealth, LLC for supplying ATL-146e.
REFERENCES
- 1.Auchampach JA, Ge ZD, Wan TC, Moore J, Gross GJ. A3 adenosine receptor agonist IB-MECA reduces myocardial ischemia-reperfusion injury in dogs. Am J Physiol Heart Circ Physiol 285: H607–H613, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cargnoni A, Ceconi C, Boraso A, Bernocchi P, Monopoli A, Curello S, Ferrari R. Role of A2A receptor in the modulation of myocardial reperfusion damage. J Cardiovasc Pharmacol 33: 883–893, 1999. [DOI] [PubMed] [Google Scholar]
- 3.Dewald O, Ren G, Duerr GD, Zoerlein M, Klemm C, Gersch C, Tincey S, Michael LH, Entman ML, Frangogiannis NG. Of mice and dogs: species-specific differences in the inflammatory response following myocardial infarction. Am J Pathol 164: 665–677, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fishbein MC, Meerbaum S, Rit J, Lando U, Kanmatsuse K, Mercier JC, Corday E, Ganz W. Early phase acute myocardial infarct size quantification: validation of the triphenyl tetrazolium chloride tissue enzyme staining technique. Am Heart J 101: 593–600, 1981. [DOI] [PubMed] [Google Scholar]
- 5.Frangogiannis NG Targeting the inflammatory response in healing myocardial infarcts. Curr Med Chem 13: 1877–1893, 2006. [DOI] [PubMed] [Google Scholar]
- 6.Glover DK, Riou LM, Ruiz M, Sullivan GW, Linden J, Rieger JM, Macdonald TL, Watson DD, Beller GA. Reduction of infarct size and postischemic inflammation from ATL-146e, a highly selective adenosine A2A receptor agonist, in reperfused canine myocardium. Am J Physiol Heart Circ Physiol 288: H1851–H1858, 2005. [DOI] [PubMed] [Google Scholar]
- 7.Glover DK, Ruiz M, Takehana K, Petruzella FD, Riou LM, Rieger JM, Macdonald TL, Watson DD, Linden J, Beller GA. Pharmacological stress myocardial perfusion imaging with the potent and selective A2A adenosine receptor agonists ATL193 and ATL146e administered by either intravenous infusion or bolus injection. Circulation 104: 1181–1187, 2001. [DOI] [PubMed] [Google Scholar]
- 8.Jordan JE, Thourani VH, Auchampach JA, Robinson JA, Wang NP, Vinten-Johansen J. A3 adenosine receptor activation attenuates neutrophil function and neutrophil-mediated reperfusion injury. Am J Physiol Heart Circ Physiol 277: H1895–H1905, 1999. [DOI] [PubMed] [Google Scholar]
- 9.Jordan JE, Zhao ZQ, Sato H, Taft S, Vinten-Johansen J. Adenosine A2 receptor activation attenuates reperfusion injury by inhibiting neutrophil accumulation, superoxide generation and coronary endothelial adherence. J Pharmacol Exp Ther 280: 301–309, 1997. [PubMed] [Google Scholar]
- 10.Kin H, Zatta AJ, Lofye MT, Amerson BS, Halkos ME, Kerendi F, Zhao ZQ, Guyton RA, Headrick JP, Vinten-Johansen J. Postconditioning reduces infarct size via adenosine receptor activation by endogenous adenosine. Cardiovasc Res 67: 124–133, 2005. [DOI] [PubMed] [Google Scholar]
- 11.Lappas CM, Day YJ, Marshall MA, Engelhard VH, Linden J. Adenosine A2A receptor activation reduces hepatic ischemia reperfusion injury by inhibiting CD1d-dependent NKT cell activation. J Exp Med 203: 2639–2648, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lasley RD, Jahania MS, Mentzer RM Jr. Beneficial effects of adenosine A2a agonist CGS-21680 in infarcted and stunned porcine myocardium. Am J Physiol Heart Circ Physiol 280: H1660–H1666, 2001. [DOI] [PubMed] [Google Scholar]
- 13.Lasley RD, Kristo G, Keith BJ, Mentzer RM Jr. The A2a/A2b receptor antagonist ZM-241385 blocks the cardioprotective effect of adenosine agonist pretreatment in in vivo rat myocardium. Am J Physiol Heart Circ Physiol 292: H426–H431, 2007. [DOI] [PubMed] [Google Scholar]
- 14.Li L, Huang L, Sung SS, Lobo PI, Brown MG, Gregg RK, Engelhard VH, Okusa MD. NKT cell activation mediates neutrophil IFN-gamma production and renal ischemia-reperfusion injury. J Immunol 178: 5899–5911, 2007. [DOI] [PubMed] [Google Scholar]
- 15.Linden J Molecular approach to adenosine receptors: receptor-mediated mechanisms of tissue protection. Annu Rev Pharmacol Toxicol 41: 775–787, 2001. [DOI] [PubMed] [Google Scholar]
- 16.Mahaffey KW, Puma JA, Barbagelata NA, DiCarli MF, Leesar MA, Browne KF, Eisenberg PR, Bolli R, Casas AC, Molina-Viamonte V, Orlandi C, Blevins R, Gibbons RJ, Califf RM, Granger CB. Adenosine as an adjunct to thrombolytic therapy for acute myocardial infarction: results of a multicenter, randomized, placebo-controlled trial: the Acute Myocardial Infarction STudy of ADenosine (AMISTAD) trial. J Am Coll Cardiol 34: 1711–1720, 1999. [DOI] [PubMed] [Google Scholar]
- 17.Micari A, Belcik TA, Balcells EA, Powers E, Wei K, Kaul S, Lindner JR. Improvement in microvascular reflow and reduction of infarct size with adenosine in patients undergoing primary coronary stenting. Am J Cardiol 96: 1410–1415, 2005. [DOI] [PubMed] [Google Scholar]
- 18.Norton ED, Jackson EK, Turner MB, Virmani R, Forman MB. The effects of intravenous infusions of selective adenosine A1-receptor and A2-receptor agonists on myocardial reperfusion injury. Am Heart J 123: 332–338, 1992. [DOI] [PubMed] [Google Scholar]
- 19.Rezkalla SH, Kloner RA. No-reflow phenomenon. Circulation 105: 656–662, 2002. [DOI] [PubMed] [Google Scholar]
- 20.Rork TH, Wallace KL, Kennedy DP, Marshall MA, Lankford AR, Linden J. Adenosine A2A receptor activation reduces infarct size in the isolated, perfused mouse heart by inhibiting resident cardiac mast cell degranulation. Am J Physiol Heart Circ Physiol 295: H1825–H1833, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ross AM, Gibbons RJ, Stone GW, Kloner RA, Alexander RW. A randomized, double-blinded, placebo-controlled multicenter trial of adenosine as an adjunct to reperfusion in the treatment of acute myocardial infarction (AMISTAD-II). J Am Coll Cardiol 45: 1775–1780, 2005. [DOI] [PubMed] [Google Scholar]
- 22.Schlack W, Schafer M, Uebing A, Schafer S, Borchard U, Thamer V. Adenosine A2-receptor activation at reperfusion reduces infarct size and improves myocardial wall function in dog heart. J Cardiovasc Pharmacol 22: 89–96, 1993. [DOI] [PubMed] [Google Scholar]
- 23.Sullivan GW, Rieger JM, Scheld WM, Macdonald TL, Linden J. Cyclic AMP-dependent inhibition of human neutrophil oxidative activity by substituted 2-propynylcyclohexyl adenosine A2A receptor agonists. Br J Pharmacol 132: 1017–1026, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Taqueti VR, Mitchell RN, Lichtman AH. Protecting the pump: controlling myocardial inflammatory responses. Annu Rev Physiol 68: 67–95, 2006. [DOI] [PubMed] [Google Scholar]
- 25.Toufektsian MC, Yang Z, Prasad KM, Overbergh L, Ramos SI, Mathieu C, Linden J, French BA. Stimulation of A2A-adenosine receptors after myocardial infarction suppresses inflammatory activation and attenuates contractile dysfunction in the remote left ventricle. Am J Physiol Heart Circ Physiol 290: H1410–H1418, 2006. [DOI] [PubMed] [Google Scholar]
- 26.van der Hoeven D, Wan TC, Auchampach JA. Activation of the A3 adenosine receptor suppresses superoxide production and chemotaxis of mouse bone marrow neutrophils. Mol Pharmacol 74: 685–696, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Vinten-Johansen J Involvement of neutrophils in the pathogenesis of lethal myocardial reperfusion injury. Cardiovasc Res 61: 481–497, 2004. [DOI] [PubMed] [Google Scholar]
- 28.Yang Z, Day YJ, Toufektsian MC, Ramos SI, Marshall M, Wang XQ, French BA, Linden J. Infarct-sparing effect of A2A-adenosine receptor activation is due primarily to its action on lymphocytes. Circulation 111: 2190–2197, 2005. [DOI] [PubMed] [Google Scholar]
- 29.Yellon DM, Hausenloy DJ. Myocardial reperfusion injury. N Engl J Med 357: 1121–1135, 2007. [DOI] [PubMed] [Google Scholar]