Abstract
Sarcalumenin (SAR), a Ca2+-binding protein located in the longitudinal sarcoplasmic reticulum (SR), regulates Ca2+ reuptake into the SR by interacting with cardiac sarco(endo)plasmic reticulum Ca2+-ATPase 2a (SERCA2a). We have previously demonstrated that SAR deficiency induced progressive heart failure in response to pressure overload, despite mild cardiac dysfunction in sham-operated SAR knockout (SARKO) mice (26). Since responses to physiological stresses often differ from those to pathological stresses, we examined the effects of endurance exercise on cardiac function in SARKO mice. Wild-type (WT) and SARKO mice were subjected to endurance treadmill exercise training (∼65% of maximal exercise ability for 60 min/day) for 12 wk. After exercise training, maximal exercise ability was significantly increased by 5% in WT mice (n = 6), whereas it was significantly decreased by 37% in SARKO mice (n = 5). Cardiac function assessed by echocardiographic examination was significantly decreased in accordance with upregulation of biomarkers of cardiac stress in SARKO mice after training. After training, expression levels of SERCA2a protein were significantly downregulated by 30% in SARKO hearts, whereas they were significantly upregulated by 59% in WT hearts. Consequently, SERCA2 activity was significantly decreased in SARKO hearts after training. Furthermore, the expression levels of other Ca2+-handling proteins, including phospholamban, ryanodine receptor 2, calsequestrin 2, and sodium/calcium exchanger 1, were significantly decreased in SARKO hearts after training. These results indicate that SAR plays a critical role in maintaining cardiac function under physiological stresses, such as endurance exercise, by regulating Ca2+ transport activity into the SR. SAR may be a primary target for exercise-related adaptation of the Ca2+ storage system in the SR to preserve cardiac function.
Keywords: treadmill, calcium uptake, heart failure, excitation-contraction coupling
endurance exercise is one of the most common physiological stresses affecting the homeostasis of the whole body. Adaptations to chronic endurance exercise result in functional and structural changes in the heart (19, 31, 33); for example, after chronic endurance exercise training, it has been shown that resting heart rate is decreased and that maximal stroke volume is increased, since myocardial contractile function is enhanced and left-ventricular cavity dimension is augmented (2, 14, 25). A growing body of evidence has demonstrated that the regulation of intracellular Ca2+ through the sarcoplasmic reticulum (SR) plays a critical role in maintaining cardiac function under both physiological and pathological stresses (5, 7, 17). In particular, rapid transport of Ca2+ from the cytosol to the SR via the cardiac sarco(endo)plasmic reticulum Ca2+-ATPase 2a (SERCA2a) is a critical determinant for the maintenance of Ca2+ storage in the SR. Therefore, it is extremely important for us to understand the effect of endurance exercise training on SERCA2a function and thus on the Ca2+ storage system in the heart. In this regard, a considerable number of previous studies on animals have demonstrated that endurance exercise training increases the expression and/or activity of SERCA2a in the heart, resulting in enhanced cardiac function of the healthy (9, 10, 20, 22, 30, 35) or pathological heart (6, 15, 21, 24, 34, 39).
Sarcalumenin (SAR) is an SR luminal glycoprotein responsible for Ca2+ buffering in skeletal and cardiac muscles (13, 16). SAR is predominantly found in the longitudinal SR, where SERCA and phospholamban (PLN) are also located. Our laboratory's previous study has demonstrated that SAR interacts with SERCA2 to enhance the protein stability of SERCA2a, and that it facilitates Ca2+ sequestration into the cardiac SR (26). Although young sedentary SAR knockout (SARKO) mice exhibit only mild impairments in Ca2+ transient and cardiac function (38), we have recently demonstrated that SAR deficiency induced progressive heart failure in response to pressure overload (26), indicating that SAR plays a critical role in adapting to pathological stresses, such as pressure overload in the heart. We found that SAR is essential for maintaining SERCA2a expression and activity in the pressure-overloaded heart. However, it has recently been reported that skeletal muscle from SARKO mice is highly resistant to fatigue compared with that from wild-type (WT) mice (40); this fatigue resistance of SARKO skeletal muscle is likely due to enhanced store-operated Ca2+ entry (SOCE) induced by upregulated expression of mitsugumin 29 (MG29), a synaptophysin-related membrane protein that is not expressed in the heart. In addition, it is known that the heart often responds differently to physiological stresses, such as endurance exercise, than to pathological stresses, such as pressure overload. Therefore, it remains unknown whether SAR also plays a role in maintaining cardiac function when the heart is exposed to physiological stresses, such as endurance exercise. To clarify the mode of action of SAR in the heart under a physiological stress, such as endurance exercise training, we investigated the impact of SAR deficiency on the expression and activity of SERCA2a in the heart and on cardiac function after endurance exercise training.
MATERIALS AND METHODS
Animal preparation.
Generation of SARKO mice has been described previously (38). SARKO and C57BL/6J WT mice (8–10 wk of age) were bred at Yokohama City University. All mice used in the present study came from the same genetic background. All animal care and study protocols were approved by the Animal Ethics Committees of Yokohama City University School of Medicine and Waseda University, and the investigation conforms to the Guide for the Care and Use of Laboratory Animals published by the US National Institutes of Health (National Institutes of Health Publication No. 85–23, revised 1996).
Maximal exercise ability and treadmill endurance exercise training.
Mice were randomized into four groups: sedentary WT (SED-WT) and sedentary SARKO (SED-SARKO) mice, and WT (ET-WT) and SARKO (ET-SARKO) mice subjected to endurance exercise training.
Animals ran on a rodent motor-driven treadmill (MANUAL, LE 8700 series, Panlab, Barcelona, Spain) with adjustable belt speed (0–150 cm/s). The treadmill apparatus was equipped with adjustable-amperage (0–2 mA) shock bars at the rear of the belt, through which mild electrical stimulation (grid shock <1 mA) was applied to encourage the mice to run. A detector located above the shock grid measured the number of shock stimuli received by each mouse.
First, mice were acclimated to the treadmill via three 15-min running sessions with mild shock stimulation and a belt speed of 30 cm/s. After acclimation, all mice underwent a treadmill exercise test to determine their exercise ability before the endurance exercise training described below; a similar assessment was made during and after training for comparison purposes. The belt speed of the treadmill was set to 30 cm/s at the beginning of each test. It was then increased linearly by 2 cm/s every 30 s until the mice could not continue to run regularly on the treadmill, or until they had rested on the shock grid more than three times. The final belt speed achieved by each mouse was considered to be that mouse's maximal exercise ability. Maximal exercise ability was determined by averaging the maximal belt speeds of at least three measurements for each mouse; there was an intermission of at least 1 h between each measurement. Workloads of endurance exercise training were then adjusted for each mouse in accordance with its maximal exercise ability.
Before the start of each exercise training session, each mouse performed a 5-min warm-up at 40% of its maximal speed. ET-WT and ET-SARKO mice then ran on the treadmill (at 0° inclination) at 65% of their maximal speeds for 60 min/day, 5 days/wk, for 12 wk. Each mouse's maximal exercise ability was reevaluated every 4 wk, and each mouse's workload was adjusted again based on its current maximal speed (Supplemental Fig. 1). (The online version of this article contains supplemental data.) For sedentary mice, running skill was maintained by treadmill running for 15 min at 0° inclination at a belt speed of 30 cm/s, 3 days/wk.
Citrate synthase activity.
As a marker for endurance training, the myocardial citrate synthase (CS) activity was measured at 37°C in the presence of 0.2% Triton X-100 with 20 μg protein sample, as previously described (27, 32). CS activity was also measured in soleus muscle homogenates to assess the efficacy of endurance exercise training.
Cardiac function assessed by echocardiography.
Mice were anesthetized with an intraperitoneal injection of Avertin (250 μg/g) and subjected to echocardiography, as described in our laboratory's previous publications (28, 38). Since we have observed that the heart rates of mice decrease after intraperitoneal injection of Avertin, reaching stable minimal levels around 15–20 min after injection (Supplemental Fig. 2), we obtained the echocardiographic data around 15–20 min after injection of Avertin. After the final assessment of cardiac function after endurance training, heart and skeletal (soleus) muscles were immediately placed in chilled phosphate-buffered saline to remove all residual blood. Hearts were then weighed, and left ventricles were immediately frozen in liquid nitrogen and stored at −80°C.
Quantitative RT-PCR analysis.
Total RNA was isolated from various tissues using TRIzol reagent (Invitrogen, Carlsbad, CA), as recommended by the manufacturer. Generation of cDNA and RT-PCR analysis was performed as described previously (36, 37). The primers for PCR amplification were designed based on the mouse nucleotide sequences of atrial natriuretic factor (ANF) and brain natriuretic peptide (BNP). The mRNA levels of interest were normalized to mouse glyceraldehyde-3-phosphate dehydrogenase.
Immunoblot analysis.
We prepared protein samples from the left ventricular tissues of the sedentary and trained mice, which had been immediately frozen and stored at −80°C after death of the animals. Immunoblot analyses were performed as described previously (26, 36). Briefly, tissues were defrosted to 0°C and homogenized in a chilled homogenization buffer [in mM: 50 Tris (pH 8.0), 1 EDTA, 1 EGTA, 1 dithiothreitol, and 200 sucrose] with protease inhibitors (Complete Mini, Roche, Basel, Switzerland). Protein content was determined using the Coomassie Plus protein assay (Pierce Chemical, Rockford, IL), and BSA (0.1–1 mg/ml) was used as a standard. The protein samples (20 μg) were separated in the same gel by SDS-polyacrylamide gel electrophoresis and transferred to polyvinylidene difluoride membranes (Bio-Rad, Hercules, CA). When the molecular size of target proteins was different, polyvinylidene difluoride membranes were cut in accordance with their size. When the molecular size of target proteins was similar, we reused the same membrane for a different antibody after washing the membrane with a stripping buffer [in mM: 62.5 Tris (pH 8.0), 100 2-mercaptoethanol, and 2% SDS]. Antibodies used in the present study are shown in Supplemental Table 1. After application of a secondary antibody, quantification of the target signals was performed using the LAS-3000 imaging system (FUJIFILM, Tokyo, Japan). The protein levels of interest were normalized to rat β-actin. For reuse, a membrane was washed with a stripping buffer at 55°C for 10 min and was washed three times with 0.1% Tris buffered saline-Tween 20 buffer.
Table 1.
Cardiac function after endurance exercise training
|
SED-WT |
ET-WT
|
SED-SARKO
|
ET-SARKO
|
|||||
|---|---|---|---|---|---|---|---|---|
| Pre | Post | Pre | Post | Pre | Post | Pre | Post | |
| n | 10 | 10 | 6 | 6 | 10 | 10 | 6 | 5 |
| BW, g | 24.2±1.3 | 32.6±1.9 | 26.3±1.7 | 31.1±1.3 | 23.3±1.0 | 27.3±1.1 | 22.6±1.2 | 26.3±1.8 |
| HR, beats/min | 464±15 | 474±15 | 438±18 | 436±18 | 430±12 | 409±14 | 449±24 | 425±14 |
| LV weight, mg | 115±9 | 111±7 | 93±6 | 86±7 | ||||
| LV-to-BW weight ratio, mg/g | 3.55±0.25 | 3.58±0.15 | 3.38±0.12 | 3.26±0.15 | ||||
| LV FS, % | 35.6±1.1 | 35.5±1.0 | 34.7±1.2 | 34.9±2.6 | 37.5±1.3 | 34.0±1.2 | 38.6±2.7 | 28.4±1.1a,c,d |
| LVIDd, mm | 4.11±0.08 | 4.12±0.11 | 4.03±0.06 | 4.25±0.12 | 3.88±0.10 | 4.02±0.09 | 3.84±0.12 | 4.14±0.19a |
| IVSTd, mm | 0.77±0.03 | 0.79±0.05 | 0.77±0.02 | 0.71±0.05 | 0.76±0.02 | 0.67±0.01b | 0.76±0.02 | 0.66±0.02b |
| LVPWTd, mm | 0.76±0.03 | 0.75±0.04 | 0.76±0.04 | 0.76±0.06 | 0.72±0.07 | 0.65±0.04a | 0.69±0.02 | 0.67±0.02a,c |
| Ejection time, ms | 60±1 | 60±1 | 64±2 | 66±3 | 64±2 | 65±2 | 58±2 | 69±3a |
| Vcfc, circumferences/s | 2.14±0.06 | 2.13±0.07 | 2.12±0.10 | 2.14±0.17 | 2.21±0.10 | 2.04±0.14 | 2.44±0.18 | 1.56±0.05b,c,d |
Values are means ± SE; n, no. of mice. SED, sedentary; WT, wild-type mice; ET, endurance exercise training; SARKO, sarcalumenin-knockout mice; Pre, before ET; Post, after ET; BW, body weight; HR, heart rate; LV, left ventricle; FS, fractional shortening; LVIDd, LV internal dimensions at end diastole; IVSTd, interventricular septum thickness at end diastole; LVPWTd, LV posterior wall thickness at end diastole; Vcfc, corrected velocity of circumferential fiber shortening. Significant difference vs. Pre:
P < 0.05 and
P < 0.01; vs. WT:
P < 0.05; and vs. SED:
P < 0.05.
SR Ca2+-ATPase assay.
SR Ca2+-ATPase activity was measured in triplicate spectrophotometrically at 37°C, as described previously with some modifications (18). Briefly, using 5 μg of SR protein from mice heart tissues, the reaction was carried out at 37°C in a reaction medium [in mM: 30 TES, 100 KCl, 5 NaN3, 5 MgCl2, 0.5 EGTA, and 4 ATP, with or without 0.5 CaCl2]. The reaction medium was preincubated at 37°C for 5 min. The reaction was started at 37°C by adding SR protein to the medium. After 5 min, the reaction was stopped by adding 0.5 ml of ice-cold 10% trichloroacetic acid solution, and the mixture was placed on ice. Inorganic phosphate was measured by using U2001 (Hitachi), as described previously (8). Ca2+-ATPase activity was calculated by subtracting the ATPase activity in the presence of 0.5 mM EGTA (no added Ca2+) from the activity in the presence of 0.5 mM CaCl2.
Statistical analysis.
All values are expressed as means ± SE. Comparisons of data from multiple groups were performed by unpaired ANOVA followed by the Student Newman-Keuls post hoc test. Statistical significance was defined as P < 0.05.
RESULTS
Effects of endurance exercise training on exercise ability in SARKO mice.
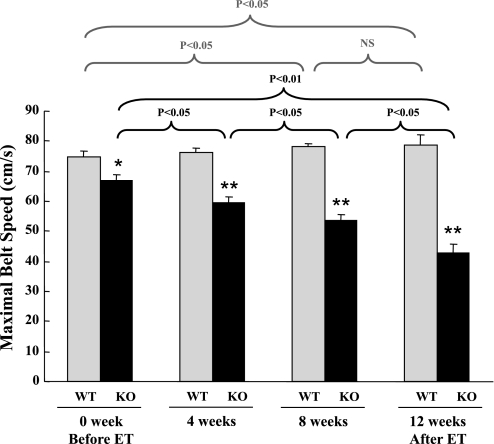
Before the start of endurance exercise training, exercise ability was examined in WT and SARKO mice by a treadmill-based exercise stress test, described above. Maximal exercise ability, as evaluated by maximal belt speed, was lower in SARKO mice (n = 16, 65.0 ± 3.6 cm/s) than in WT mice (n = 16, 74.1 ± 2.1 cm/s), although it did not reach a statistical significance (P = 0.059). As expected, maximal exercise ability in sedentary animals (SED-WT and SED-SARKO mice) did not significantly change during the 12-wk training period (data not shown). In ET-WT mice, maximal exercise ability gradually increased during endurance exercise training, whereas, in ET-SARKO mice, it gradually decreased (Fig. 1). Whenever a change in a mouse's maximal exercise ability was detected by a regular treadmill test, that mouse's training workload was adjusted based on its current maximal speed (Supplemental Fig. 1). Maximal exercise ability after endurance exercise training significantly increased by 5% in ET-WT mice, whereas it actually decreased by 37% in ET-SARKO mice compared with their ability measured before the training regime began (Fig. 1).
Fig. 1.
The effect of endurance exercise training (ET) on maximal exercise ability in sarcalumenin (SAR) knockout (SARKO) mice. Maximal exercise ability, as evaluated by maximal belt speed, is already lower in SARKO mice than in wild-type (WT) mice before ET. During ET, maximal exercise ability gradually increased in WT mice, whereas it actually decreased in SARKO mice in a time-dependent manner. Maximal exercise ability after ET was significantly increased in WT mice, whereas it was actually decreased in SARKO mice compared with their maximal exercise ability before the training. Values are means ± SE; n = 6 and 5 for WT and knockout (KO), respectively.
Exercise training did not improve CS activity in SARKO mice.
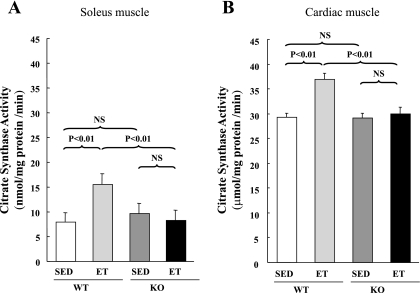
We observed no difference between WT and SARKO mice in terms of CS activity of skeletal or cardiac muscle at a basal condition. After the endurance exercise training, ET-WT mice exhibited increased CS activity of soleus muscle (Fig. 2A), indicating an appropriate effect of the training program on working muscles. In accordance, they also exhibited increased CS activity of cardiac muscle (Fig. 2B), which is consistent with several previous studies (1, 20), although most of previous studies have demonstrated that CS activity is not increased or little increased by endurance exercise in rodent hearts (4, 19). In ET-SARKO mice, on the other hand, CS activity was not increased in either soleus or cardiac muscle (Fig. 2).
Fig. 2.
Citrate synthase (CS) activity after ET. After ET, CS activity of soleus muscle (A) and cardiac muscle (B) was increased in ET-WT mice, but not in ET-SARKO mice. Values are means ± SE; n = 5 for each group. SED, sedentary; NS, not significant.
Endurance exercise training resulted in cardiac dysfunction in SARKO mice.
To examine the effect of endurance exercise training on cardiac function, we investigated it using transthoracic echocardiography. Before endurance exercise training, all parameters listed in Table 1 were similar between WT and SARKO mice, including body weight, heart rate, left ventricular fractional shortening, thickness of myocardial walls, and ejection time. After endurance exercise training, left ventricular fractional shortening was significantly decreased in ET-SARKO mice, whereas it was not changed in ET-WT mice (Table 1). As we expected, the diameter of the end-diastolic left ventricular chamber was significantly increased in ET-SARKO mice. Furthermore, ejection time was significantly prolonged in ET-SARKO mice, and their heart rate corrected velocity of circumferential fiber shortening was significantly lower (Table 1).
Biomarkers of cardiac stress were increased in ET-SARKO hearts.
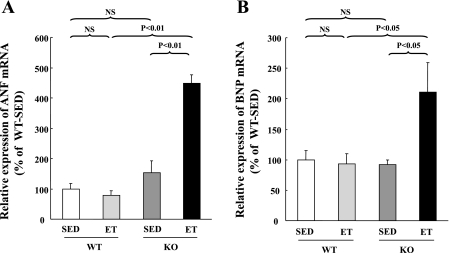
To examine the effect of endurance exercise training on the myocardium itself, we measured molecular markers of cardiac stress, such as ANF and BNP mRNAs. These were significantly upregulated in ET-SARKO mice (Fig. 3). Endurance training did not affect the expression of ANF and BNP mRNAs in ET-WT mice.
Fig. 3.
Upregulation of atrial natriuretic factor (ANF) and brain natriuretic peptide (BNP) mRNAs in ET-SARKO mice. Quantitative RT-PCR analyses revealed that the expression levels of ANF (A) and BNP (B) mRNAs were significantly upregulated in the ventricles of ET-SARKO mice. The expression levels observed in SED-WT mice were set as 100% as a control. mRNA expression was normalized by GAPDH. Values are means ± SE; n = 5 for each group.
Significant reductions in the expression of Ca2+ handling proteins in ET-SARKO mice.
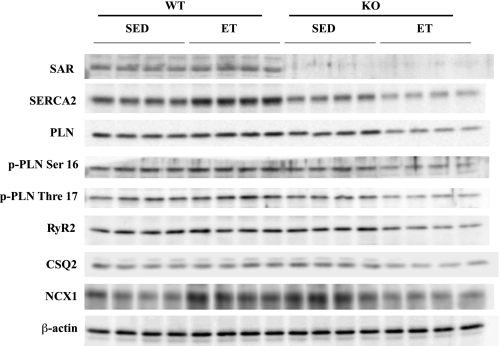
Since the expression levels of SERCA2a and other Ca2+ handling proteins are critical for the regulation of cardiac function, we examined them by Western blot analyses (Fig. 4, Table 2). Consistent with our laboratory's previous report (26, 38), the expression levels of SERCA2a and total PLN were significantly downregulated in SED-SARKO mice compared with those in SED-WT mice. After endurance exercise training, the expression level of SERCA2a protein was significantly increased by 59% in ET-WT mice, whereas it was reduced by 30% in ET-SARKO mice compared with sedentary mice of each group's respective genotype. Endurance exercise training also resulted in a further significant downregulation of both total and phosphorylated PLN proteins in ET-SARKO mice, but not in ET-WT mice. The SERCA2a-to-PLN protein ratio was significantly decreased in ventricular muscles of ET-SARKO mice (Table 2). The ratio of phosphorylated threonine 17 PLN to total PLN protein was significantly lower in ET-SARKO than in ET-WT, but that of serine 16 to total PLN protein was not (Table 2). It should be noted that intraperitoneal injection of Avertin did not affect the phosphorylation status of serine 16 and threonine 17 in PLN (Supplemental Fig. 2).
Fig. 4.
The expression of Ca2+ handling proteins after ET. The expression levels of sarcalumenin (SAR), sarco(endo)plasmic reticulum Ca2+-ATPase 2 (SERCA2), phospholamban (PLN), phosphorylated PLN (p-PLN), ryanodine receptor 2 (RyR2), calsequestrin 2 (CSQ2), and sodium/calcium exchanger 1 (NCX1) proteins were quantified in hearts isolated from SED and ET mice. Protein expression was normalized by β-actin.
Table 2.
The expression of calcium handling proteins after endurance exercise training
| SED-WT | ET-WT | SED-SARKO | ET-SARKO | |
|---|---|---|---|---|
| SAR | 100±5 | 108±9 | ||
| SERCA2 | 100±10 | 159±13§ | 74±4* | 52±6†‡ |
| PLN | 100±6 | 123±8 | 83±2* | 71±2†§ |
| p-PLN Ser 16 | 100±3 | 120±11 | 95±5 | 82±3†‡ |
| p-PLN Thre 17 | 100±4 | 112±7 | 92±6 | 78±4†‡ |
| SERCA2/PLN | 100±5 | 132±12‡ | 94±4 | 75±10†‡ |
| p-PLN Ser 16/PLN | 100±4 | 98±8 | 113±4 | 116±8 |
| p-PLN Thre 17/PLN | 100±1 | 98±4 | 93±2 | 88±2* |
| RyR2 | 100±5 | 100±10 | 97±6 | 68±8*‡ |
| CSQ2 | 100±7 | 101±4 | 99±3 | 83±6*‡ |
| NCX1 | 100±10 | 139±11‡ | 124±3* | 92±11*‡ |
Values are means ± SE; n = 5 mice for each group. The expression level in SED-WT mice was referred to 100% as a control. Protein expression was normalized by β-actin. SAR, sarcalumenin; SERCA2, sarco(endo)plasmic reticulum Ca2+-ATPase 2; PLN, phospholamban; p-PLN: phosphorylated phospholamban; RyR2, ryanodine receptor 2; CSQ2, calsequestrin 2; NCX1, sodium/calcium exchanger 1. Significant difference vs. WT:
P < 0.05 and
P < 0.01; vs. SED:
P < 0.05 and
P < 0.01.
The expression levels of calsequestrin 2 (CSQ2) and ryanodine receptor type 2 (RyR2) proteins in SED-SARKO mice were comparable to those in SED-WT mice, while those of sodium/calcium exchanger 1 (NCX1) protein were even higher in SED-SARKO mice than in SED-WT mice. After the endurance exercise training, all of these proteins were significantly downregulated in ET-SARKO mice, but not in ET-WT mice (Fig. 4, Table 2). Overall, in addition to SERCA2, all other Ca2+ handling proteins that we examined were downregulated in ET-SARKO mice after endurance exercise training.
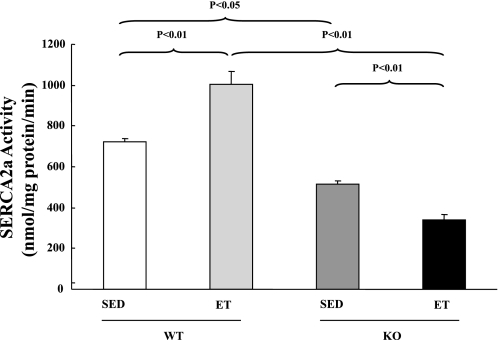
Significant reduction in SERCA2a activity in ET-SARKO mice.
As measured in myocardial homogenates, maximal Ca2+-ATPase activity was lower in SED-SARKO mice than in SED-WT mice (Fig. 5). After the endurance exercise training, maximal Ca2+-ATPase activity was further significantly decreased in ET-SARKO mice, whereas it was significantly increased in ET-WT mice. This result was consistent with the change in the ratio of SERCA2a to PLN protein expression shown in Table 2.
Fig. 5.
SERCA2a activity after ET. SERCA2a activity was increased in ET-WT, whereas it was decreased in ET-SARKO after ET. Values are means ± SE; n = 5 for each group.
DISCUSSION
The most striking finding in the present study is that long-term (12 wk) endurance exercise training induced a significant cardiac dysfunction in mice that harbor systemic ablation of the SAR gene. Along the same lines, we have recently demonstrated that SARKO mice failed to adapt to pressure-overloaded stress induced by transverse aortic constriction (26), whereas sedentary young SARKO mice exhibit mild cardiac dysfunction (38). Since exercise is one of the most common physiological stresses, the present data indicate that SAR plays an important role in preserving cardiac function during adaptation to not only pathological, but also physiological, stresses.
It should be noted that the absolute training intensity undertaken by SARKO mice was significantly lower than that undertaken by WT mice (Supplemental Fig. 1), because the intensity of each mouse's exercise regime was determined on the basis of that mouse' maximal exercise ability. Accordingly, CS activity in soleus muscle after endurance exercise training was significantly lower in ET-SARKO mice than in ET-WT mice (Fig. 2). Since skeletal muscle CS activity is a marker for mitochondrial content (a hallmark of endurance exercise) and muscle oxidative capacity, this result indicates that our exercise training program is sufficient to enhance the exercise ability of WT mice, but insufficient to enhance that of SARKO mice. Although this may explain a number of the negative effects on SARKO mice that were caused by exercise training in the present study, it is, nevertheless, very difficult to explain why ET-SARKO mice exhibited progressive cardiac dysfunction. We assume that inadequate adaptation to endurance exercise in ET-SARKO mice caused impaired cardiac function, the primary insult, which, secondarily, resulted in a number of negative effects on SARKO mice caused by training.
The mechanism by which endurance exercise induced progressive cardiac dysfunction in SARKO mice is a critical question. One observation that may be relevant to this question is the significant decrease in the expression and activity of SERCA2a in ET-SARKO mice. A number of previous studies have reported that endurance exercise training increased the expression and/or activity of SERCA2a in healthy (9, 10, 20, 22, 30, 35) or diseased rodents (6, 21, 24, 34, 39); similarly, we found that the expression and activity of SERCA2a increased after endurance exercise training in control mice. Yet other studies have demonstrated that endurance exercise training does not change the expression and/or activity of SERCA2a (3, 4) or Ca2+ transients (12) in rodents. It is worth noting that these conflicting results may have their origins in such factors as differences in species, exercise protocols, and/or condition of the subjects; few studies, however, have shown that endurance exercise decreases the expression and/or activity of SERCA2a. Therefore, our results found in ET-SARKO mice were so remarkable that it is very important to investigate why SAR deficiency caused the significant reduction in the expression and activity of SERCA2a under endurance exercise training.
Our laboratory's recent study has demonstrated that SAR interacts with SERCA2 to enhance the protein stability of SERCA2a (26). Since exercise training usually increases protein synthesis and degradation in muscle (11, 23), we assume that endurance exercise training also increased the turnover rate of SERCA2a protein. Then we postulate that SAR deficiency induced a progressive degradation of SERCA2a protein due to impaired protein stabilization under endurance exercise training and resulted in the significant decrease in the expression of SERCA2a in ET-SARKO mice. Importantly, the present study demonstrated that endurance exercise training slightly increased the expression levels of SAR protein in WT hearts, in accordance with a significant increase in the expression of SERCA2a protein. To our knowledge, this is the first report to show the effect of endurance exercise training on the expression of SAR protein. These data suggest that SAR is a key regulatory protein to maintain the expression level of SERCA2a protein under pathophysiological stresses. In addition, the ratios of SERCA2a to PLN protein and phosphorylated threonine 17 PLN to total PLN protein were significantly decreased in the ventricular muscles of ET-SARKO mice, indicating that SERCA2a activity was inhibited by PLN more in ET-SARKO mice than in other groups. Taken together, this evidence shows that SAR deficiency induced a significant reduction in SERCA2a activity and deterioration of the Ca2+ storage system in the SR under endurance exercise stress, which is very likely to play a primary role in the exercise-induced cardiac dysfunction exhibited by ET-SARKO mice.
Interestingly, in addition to the decreases in the SERCA2a and PLN proteins that interact with SAR in the longitudinal SR, other Ca2+ handling proteins, such as RyR2, CSQ2, and NCX1, were also significantly downregulated in ET-SARKO mice, which has not been investigated in pressure-overloaded SARKO hearts (26). These abnormalities probably contribute to the further impairment of cardiac function during endurance exercise training. We assume that the downregulation of RyR2, CSQ2, and NCX1 could be a secondary phenomenon that occurs under physiological stress conditions, as SAR does not directly interact with these proteins. The mechanism of these discrepant responses to different stresses in SARKO mice is currently not clear; it is an important question that should be addressed in future studies.
In one way, the results of the present study somehow contradict those of a recent report by Zhao et al. (40), which showed that skeletal muscles from SARKO mice are highly resistant to fatigue compared with those from WT mice. The same authors have also demonstrated that SOCE was promoted in SARKO skeletal muscle by the upregulation of MG29 (40). They proposed that the promotion of SOCE played a role in making skeletal muscle more fatigue resistant (40). In the present study, however, we did not detect any expression of MG29 protein in either WT or SARKO hearts, before or after exercise training, although we used the same membranes for our Western blot analyses (data not shown). This observation is consistent with a previous study (29). Currently, we cannot explain the exact reason for the disagreement between the results of Zhao et al. (40) and our own. A possible explanation is the difference in the exercise programs our two groups used to evaluate the exercise performance of SARKO mice. Further investigation is needed to clarify whether a defect of MG29 may cause the negative responses to exercise in SARKO cardiac muscle cells.
In conclusion, we found that cardiac function and maximal exercise ability were significantly impaired in SARKO mice after endurance treadmill exercise training. These impairments were due, at least in part, to a significant downregulation of SERCA2a and other Ca2+ handling proteins and to a deterioration of the Ca2+ storage system in the SARKO heart under endurance exercise. Thus present study indicates that SAR plays a critical role in maintaining cardiac function under physiological stresses, such as endurance exercise, by regulating Ca2+ transport activity into the SR. SAR may be a primary target for exercise-related adaptation of the Ca2+ storage system in the SR to preserve cardiac function.
GRANTS
This work was partly supported by grants from the Honjo International Scholarship Foundation (Q. Jiao), the Yokohama Foundation for Advanced Medical Science (S. Minamisawa, T. Akaike, Y. Ishikawa), the Ministry of Education, Science, Sports and Culture of Japan (S. Minamisawa, Y. Ishikawa), the Special Coordination Funds for Promoting Science and Technology, MEXT (S. Minamisawa), the “High-Tech Research Center” Project for Private Universities: matching fund subsidy from MEXT (S. Minamisawa), a Waseda University Grant for Special Research Projects (S. Minamisawa), the Mother and Child Health Foundation (S. Minamisawa), the Miyata Cardiology Research Promotion Funds (S. Minamisawa), the Takeda Science Foundation (S. Minamisawa), the Foundation for Growth Science (S. Minamisawa), the Japan Cardiovascular Research Foundation (S. Minamisawa), the Mitsubishi Pharma Research Foundation (S. Minamisawa), the Yokohama Academic Foundation (T. Akaike), the Inoue Foundation for Science (T. Akaike), the Naito Foundation (T. Akaike), the Japan Space Forum (Y. Ishikawa), and the National Institute of General Medical Sciences (RO1 GM067773) (Y. Ishikawa).
Supplementary Material
REFERENCES
- 1.Call JA, Voelker KA, Wolff AV, McMillan RP, Evans NP, Hulver MW, Talmadge RJ, Grange RW. Endurance capacity in maturing mdx mice is markedly enhanced by combined voluntary wheel running and green tea extract. J Appl Physiol 105: 923–932, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Charlton GA, Crawford MH. Physiologic consequences of training. Cardiol Clin 15: 345–354, 1997. [DOI] [PubMed] [Google Scholar]
- 3.de Waard MC, van der Velden J, Bito V, Ozdemir S, Biesmans L, Boontje NM, Dekkers DH, Schoonderwoerd K, Schuurbiers HC, de Crom R, Stienen GJ, Sipido KR, Lamers JM, Duncker DJ. Early exercise training normalizes myofilament function and attenuates left ventricular pump dysfunction in mice with a large myocardial infarction. Circ Res 100: 1079–1088, 2007. [DOI] [PubMed] [Google Scholar]
- 4.Delgado J, Saborido A, Moran M, Megias A. Chronic and acute exercise do not alter Ca2+ regulatory systems and ectonucleotidase activities in rat heart. J Appl Physiol 87: 152–160, 1999. [DOI] [PubMed] [Google Scholar]
- 5.Frank KF, Bolck B, Erdmann E, Schwinger RH. Sarcoplasmic reticulum Ca2+-ATPase modulates cardiac contraction and relaxation. Cardiovasc Res 57: 20–27, 2003. [DOI] [PubMed] [Google Scholar]
- 6.French JP, Quindry JC, Falk DJ, Staib JL, Lee Y, Wang KK, Powers SK. Ischemia-reperfusion-induced calpain activation and SERCA2a degradation are attenuated by exercise training and calpain inhibition. Am J Physiol Heart Circ Physiol 290: H128–H136, 2006. [DOI] [PubMed] [Google Scholar]
- 7.Houser SR, Piacentino V 3rd, Weisser J. Abnormalities of calcium cycling in the hypertrophied and failing heart. J Mol Cell Cardiol 32: 1595–1607, 2000. [DOI] [PubMed] [Google Scholar]
- 8.Hwang KJ Interference of ATP and acidity in the determination of inorganic phosphate by the Fiske and Subbarow method. Anal Biochem 75: 40–44, 1976. [DOI] [PubMed] [Google Scholar]
- 9.Iemitsu M, Miyauchi T, Maeda S, Tanabe T, Takanashi M, Matsuda M, Yamaguchi I. Exercise training improves cardiac function-related gene levels through thyroid hormone receptor signaling in aged rats. Am J Physiol Heart Circ Physiol 286: H1696–H1705, 2004. [DOI] [PubMed] [Google Scholar]
- 10.Kemi OJ, Ceci M, Condorelli G, Smith GL, Wisloff U. Myocardial sarcoplasmic reticulum Ca2+ ATPase function is increased by aerobic interval training. Eur J Cardiovasc Prev Rehabil 15: 145–148, 2008. [DOI] [PubMed] [Google Scholar]
- 11.Kumar V, Atherton P, Smith K, Rennie MJ. Human muscle protein synthesis and breakdown during and after exercise. J Appl Physiol 106: 2026–2039, 2009. [DOI] [PubMed] [Google Scholar]
- 12.Laughlin MH, Schaefer ME, Sturek M. Effect of exercise training on intracellular free Ca2+ transients in ventricular myocytes of rats. J Appl Physiol 73: 1441–1448, 1992. [DOI] [PubMed] [Google Scholar]
- 13.Leberer E, Charuk JH, Green NM, MacLennan DH. Molecular cloning and expression of cDNA encoding a lumenal calcium binding glycoprotein from sarcoplasmic reticulum. Proc Natl Acad Sci USA 86: 6047–6051, 1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Levy WC, Cerqueira MD, Abrass IB, Schwartz RS, Stratton JR. Endurance exercise training augments diastolic filling at rest and during exercise in healthy young and older men. Circulation 88: 116–126, 1993. [DOI] [PubMed] [Google Scholar]
- 15.Lu L, Mei DF, Gu AG, Wang S, Lentzner B, Gutstein DE, Zwas D, Homma S, Yi GH, Wang J. Exercise training normalizes altered calcium-handling proteins during development of heart failure. J Appl Physiol 92: 1524–1530, 2002. [DOI] [PubMed] [Google Scholar]
- 16.MacLennan DH, Wong PT. Isolation of a calcium-sequestering protein from sarcoplasmic reticulum. Proc Natl Acad Sci USA 68: 1231–1235, 1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Minamisawa S, Sato Y, Cho MC. Calcium cycling proteins in heart failure, cardiomyopathy and arrhythmias. Exp Mol Med 36: 193–203, 2004. [DOI] [PubMed] [Google Scholar]
- 18.Minamisawa S, Wang Y, Chen J, Ishikawa Y, Chien KR, Matsuoka R. Atrial chamber-specific expression of sarcolipin is regulated during development and hypertrophic remodeling. J Biol Chem 278: 9570–9575, 2003. [DOI] [PubMed] [Google Scholar]
- 19.Moore RL Cellular adaptations of the heart muscle to exercise training. Ann Med 30, Suppl 1: 46–53, 1998. [PubMed] [Google Scholar]
- 20.Moran M, Saborido A, Megias A. Ca2+ regulatory systems in rat myocardium are altered by 24 weeks treadmill training. Pflügers Arch 446: 161–168, 2003. [DOI] [PubMed] [Google Scholar]
- 21.Mou YA, Reboul C, Andre L, Lacampagne A, Cazorla O. Late exercise training improves non-uniformity of transmural myocardial function in rats with ischaemic heart failure. Cardiovasc Res 81: 555–564, 2009. [DOI] [PubMed] [Google Scholar]
- 22.Pierce GN, Sekhon PS, Meng HP, Maddaford TG. Effects of chronic swimming training on cardiac sarcolemmal function and composition. J Appl Physiol 66: 1715–1721, 1989. [DOI] [PubMed] [Google Scholar]
- 23.Pikosky MA, Gaine PC, Martin WF, Grabarz KC, Ferrando AA, Wolfe RR, Rodriguez NR. Aerobic exercise training increases skeletal muscle protein turnover in healthy adults at rest. J Nutr 136: 379–383, 2006. [DOI] [PubMed] [Google Scholar]
- 24.Rolim NP, Medeiros A, Rosa KT, Mattos KC, Irigoyen MC, Krieger EM, Krieger JE, Negrao CE, Brum PC. Exercise training improves the net balance of cardiac Ca2+ handling protein expression in heart failure. Physiol Genomics 29: 246–252, 2007. [DOI] [PubMed] [Google Scholar]
- 25.Seals DR, Hagberg JM, Spina RJ, Rogers MA, Schechtman KB, Ehsani AA. Enhanced left ventricular performance in endurance trained older men. Circulation 89: 198–205, 1994. [DOI] [PubMed] [Google Scholar]
- 26.Shimura M, Minamisawa S, Takeshima H, Jiao Q, Bai Y, Umemura S, Ishikawa Y. Sarcalumenin alleviates stress-induced cardiac dysfunction by improving Ca2+ handling of the sarcoplasmic reticulum. Cardiovasc Res 77: 362–370, 2008. [DOI] [PubMed] [Google Scholar]
- 27.Singh M, Brooks GC, Srere PA. Subunit structure and chemical characteristics of pig heart citrate synthase. J Biol Chem 245: 4636–4640, 1970. [PubMed] [Google Scholar]
- 28.Tadano M, Edamatsu H, Minamisawa S, Yokoyama U, Ishikawa Y, Suzuki N, Saito H, Wu D, Masago-Toda M, Yamawaki-Kataoka Y, Setsu T, Terashima T, Maeda S, Satoh T, Kataoka T. Congenital semilunar valvulogenesis defect in mice deficient in phospholipase C epsilon. Mol Cell Biol 25: 2191–2199, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Takeshima H, Shimuta M, Komazaki S, Ohmi K, Nishi M, Iino M, Miyata A, Kangawa K. Mitsugumin29, a novel synaptophysin family member from the triad junction in skeletal muscle. Biochem J 331: 317–322, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tate CA, Helgason T, Hyek MF, McBride RP, Chen M, Richardson MA, Taffet GE. SERCA2a and mitochondrial cytochrome oxidase expression are increased in hearts of exercise-trained old rats. Am J Physiol Heart Circ Physiol 271: H68–H72, 1996. [DOI] [PubMed] [Google Scholar]
- 31.Thomas DP Effects of acute and chronic exercise on myocardial ultrastructure. Med Sci Sports Exerc 17: 546–553, 1985. [PubMed] [Google Scholar]
- 32.Trounce IA, Kim YL, Jun AS, Wallace DC. Assessment of mitochondrial oxidative phosphorylation in patient muscle biopsies, lymphoblasts, and transmitochondrial cell lines. Methods Enzymol 264: 484–509, 1996. [DOI] [PubMed] [Google Scholar]
- 33.Ventura-Clapier R, Mettauer B, Bigard X. Beneficial effects of endurance training on cardiac and skeletal muscle energy metabolism in heart failure. Cardiovasc Res 73: 10–18, 2007. [DOI] [PubMed] [Google Scholar]
- 34.Wisloff U, Loennechen JP, Currie S, Smith GL, Ellingsen O. Aerobic exercise reduces cardiomyocyte hypertrophy and increases contractility, Ca2+ sensitivity and SERCA-2 in rat after myocardial infarction. Cardiovasc Res 54: 162–174, 2002. [DOI] [PubMed] [Google Scholar]
- 35.Wisloff U, Loennechen JP, Falck G, Beisvag V, Currie S, Smith G, Ellingsen O. Increased contractility and calcium sensitivity in cardiac myocytes isolated from endurance trained rats. Cardiovasc Res 50: 495–508, 2001. [DOI] [PubMed] [Google Scholar]
- 36.Yokoyama U, Minamisawa S, Adachi-Akahane S, Akaike T, Naguro I, Funakoshi K, Iwamoto M, Nakagome M, Uemura N, Hori H, Yokota S, Ishikawa Y. Multiple transcripts of Ca2+ channel α1-subunits and a novel spliced variant of the α1C-subunit in rat ductus arteriosus. Am J Physiol Heart Circ Physiol 290: H1660–H1670, 2006. [DOI] [PubMed] [Google Scholar]
- 37.Yokoyama U, Minamisawa S, Quan H, Ghatak S, Akaike T, Segi-Nishida E, Iwasaki S, Iwamoto M, Misra S, Tamura K, Hori H, Yokota S, Toole BP, Sugimoto Y, Ishikawa Y. Chronic activation of the prostaglandin receptor EP4 promotes hyaluronan-mediated neointimal formation in the ductus arteriosus. J Clin Invest 116: 3026–3034, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yoshida M, Minamisawa S, Shimura M, Komazaki S, Kume H, Zhang M, Matsumura K, Nishi M, Saito M, Saeki Y, Ishikawa Y, Yanagisawa T, Takeshima H. Impaired Ca2+ store functions in skeletal and cardiac muscle cells from sarcalumenin-deficient mice. J Biol Chem 280: 3500–3506, 2005. [DOI] [PubMed] [Google Scholar]
- 39.Zhang LQ, Zhang XQ, Ng YC, Rothblum LI, Musch TI, Moore RL, Cheung JY. Sprint training normalizes Ca2+ transients and SR function in postinfarction rat myocytes. J Appl Physiol 89: 38–46, 2000. [DOI] [PubMed] [Google Scholar]
- 40.Zhao X, Yoshida M, Brotto L, Takeshima H, Weisleder N, Hirata Y, Nosek TM, Ma J, Brotto M. Enhanced resistance to fatigue and altered calcium handling properties of sarcalumenin knockout mice. Physiol Genomics 23: 72–78, 2005. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.